Significance
The projected increase in drought severity and duration worldwide poses a significant threat to the health of terrestrial ecosystems. We reveal that unique genetic features of desiccation sensing and protection in streptophyte algae not only distinguish them from chlorophyte algae, but also represent a crucial evolutionary step that may have facilitated colonization and subsequent diversification of terrestrial habitats. We demonstrate the evolutionary significance of a molecular mechanism underlying how plants sense drought stress via the coordination of chloroplast retrograde signaling to trigger the closure of stomata, protecting vital photosynthetic tissue. Our findings constitute a significant step forward in understanding the evolution of plant drought tolerance, contributing to the diversification of terrestrial plant communities through past global climate transitions.
Keywords: comparative genomics, water stress, green plant evolution, signal transduction, stomata
Abstract
Chloroplast retrograde signaling networks are vital for chloroplast biogenesis, operation, and signaling, including excess light and drought stress signaling. To date, retrograde signaling has been considered in the context of land plant adaptation, but not regarding the origin and evolution of signaling cascades linking chloroplast function to stomatal regulation. We show that key elements of the chloroplast retrograde signaling process, the nucleotide phosphatase (SAL1) and 3′-phosphoadenosine-5′-phosphate (PAP) metabolism, evolved in streptophyte algae—the algal ancestors of land plants. We discover an early evolution of SAL1-PAP chloroplast retrograde signaling in stomatal regulation based on conserved gene and protein structure, function, and enzyme activity and transit peptides of SAL1s in species including flowering plants, the fern Ceratopteris richardii, and the moss Physcomitrella patens. Moreover, we demonstrate that PAP regulates stomatal closure via secondary messengers and ion transport in guard cells of these diverse lineages. The origin of stomata facilitated gas exchange in the earliest land plants. Our findings suggest that the conquest of land by plants was enabled by rapid response to drought stress through the deployment of an ancestral SAL1-PAP signaling pathway, intersecting with the core abscisic acid signaling in stomatal guard cells.
Based on fossil evidence (1, 2), green plants (Viridiplantae) colonized land at least 450 million years ago (Mya), with molecular estimates of ∼500 Mya (3). This transition to land represents one of the most important steps in the evolution of life on Earth, ultimately leading to formation of current terrestrial ecosystems. Movement of plants to land significantly altered atmospheric oxygen concentration, facilitating the evolution of terrestrial animals (4, 5). However, crucial steps in green plant terrestrialization have not been fully understood (6–8). Viridiplantae comprise two clades: Streptophyta, which contain streptophyte algae and land plants (embryophytes) (SI Appendix, Fig. S1), and Chlorophyta, which include all the remaining green algae (9). Although there are many extant land-dwelling chlorophyte algae, a group of freshwater streptophyte algae represents the lineage that is sister to embryophytes. Environmental stress tolerance features of streptophyte algae may have facilitated their transition to land (6, 9).
Drought and excess light are inescapable threats to land plants due to the risk of desiccation, photooxidation and associated heat, and UV damage (9). Drought and excess light are key inducers of chloroplast-nucleus communication, which activate acclimatory cellular responses (6, 8, 10). The stress hormone abscisic acid (ABA), the synthesis of which is initiated in chloroplasts, is critical for the cellular response to drought and excess light. Streptophyte algae may possess all of the required features for ABA responsiveness and chloroplast-to-nucleus retrograde signaling (11, 12). The 3′-phosphoadenosine-5′-phosphate (PAP) retrograde signal has been demonstrated to function in ABA-regulated processes in drought stress and stomatal closure in the flowering plant Arabidopsis thaliana (13). Presumably, the functions and interrelationships of ABA and PAP signaling are present in both streptophyte algae and land plants (6). We hypothesize that chloroplast retrograde and ABA signaling played crucial roles in the process of terrestrialization, enabling ancestral land plants to sense and respond to fluctuating environmental conditions.
The evolution of stomata more than 400 Mya was a key innovation that enabled land plants to regulate gas exchange and hydration and to endure drought as they colonized land (14). As the gateways for terrestrial carbon and water fluxes, stomata have strongly influenced global water and carbon cycles over geological time and respond rapidly to drought (14–16). The ABA signaling network is likely to have evolved before the land plants (17–20), stomata, and stomatal guard cells. Drought-induced ABA production increases guard cell cytosolic Ca2+, hydrogen peroxide (H2O2), and nitric oxide (NO) to inhibit the K+in channel and to activate the Ca2+in, K+out, and anion channels, thereby triggering stomatal closure in A. thaliana (18, 19, 21). In contrast, the mechanisms by which chloroplasts sense drought and contribute to the signal cascade that ultimately triggers stomatal closure during drought are only beginning to be unraveled and have not yet been fully considered in an evolutionary context (6, 22).
Chloroplast retrograde signaling involves multiple signaling pathways necessary to coordinate chloroplast function and plant cell responses to environmental stimuli and to alter plant physiological responses (10). One such chloroplast retrograde signaling pathway involves the drought- and high light-induced phosphoadenosine PAP, a by-product of the reaction of sulfotransferase enzymes (SOTs) including tyrosyl protein sulfotransferases (TPSTs) (10, 23). The turnover of PAP in chloroplasts is primarily mediated by the nucleotide phosphatase SAL1/FRY1 (24, 25). Inactivation of SAL1 by transient silencing or loss-of-function led to improved drought tolerance in wheat (Triticum aestivum) and A. thaliana, respectively (26, 27). In A. thaliana, accumulation of PAP mediates large changes of the transcriptome during excess light and drought treatments, enhancing drought tolerance and stimulating stomatal closure with similar kinetics to ABA (13, 28, 29), but whether or not this occurs in other plants is unknown. These findings raise questions about the timing of the origin of retrograde PAP signaling and the evolution of its critical role in stress response and guard cell function.
Here, we address a critical knowledge gap with comparative genetic evidence of key features of drought sensing and protection mechanisms in streptophyte algae, including the sister group to land plants, Zygnematophyceae. We also trace the evolutionary conservation of SAL1 function throughout the streptophytes and demonstrate how the chloroplast retrograde signal PAP interacts with the ABA-signaling pathway to drive stomatal closure across a phylogenetically broad suite of land plants.
Results
Streptophyte Algae and the Movement of Plants to Land.
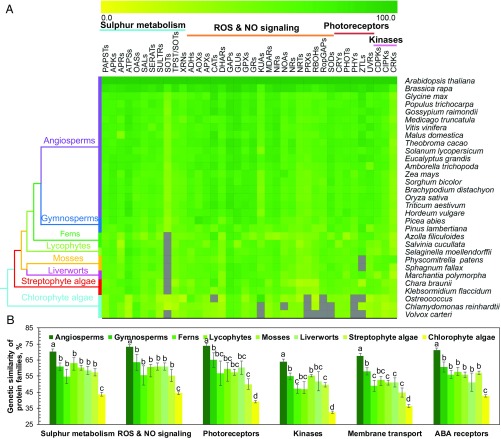
Using two methods, we conducted a comparative genetic similarity analysis (15, 30) of predicted sequences from 61 protein families from 31 species representing the major clades (31, 32) of land plants, streptophyte algae, and chlorophyte algae (Fig. 1 and SI Appendix, Tables S1–S3). These 61 protein families (Fig. 1 and SI Appendix, Tables S1–S3) were selected based on their key roles in chloroplast retrograde signaling of SAL1-PAP and sulfur metabolism (33), reactive oxygen species (ROS) and NO signaling (34), photoreceptors (35), Ca2+-dependent protein kinases (36), and membrane transport and ABA signaling (15, 30). The overall similarity in these conserved protein families among species of land plants and streptophyte algae is clear (Fig. 1A). Not surprisingly, across these protein families, the two streptophyte algae Klebsormidium flaccidum (K. nitens) and Chara braunii showed higher similarity to embryophytes than to the chlorophyte algae (Fig. 1B). Significantly, many components that subsequently form the key guard-cell–signaling pathways were present in streptophyte algae predating the origin of land plants and the evolutionary emergence of stomata (SI Appendix, Fig. S1).
Fig. 1.
Evolutionary similarity of protein families of the major clades of green plants. (A) Similarity heat map for the evolution of protein families of sulfur metabolism, ROS and NO signaling, photoreceptors, and protein kinases. A simplified tree is shown and color-coded to the main clades of Viridiplantae (green plants). (B) Comparative genetic similarity analysis was conducted with protein sequences of 31 plant and algal species that span the green plant kingdom from chlorophytes to angiosperms. Sulfur metabolism (n = 11), ROS and NO signaling (n = 19), membrane transporters (n = 20), ABA receptors (n = 3), photoreceptors (n = 5), protein kinases (n = 3). Different lowercase letters indicate statistical significance at P < 0.05. Genesis software (genome.tugraz.at/genesisclient/genesisclient_download.shtml) was used to estimate the similarity of proteins using A. thaliana sequences as the query with the criterion of E-value < 10−5. Colored squares: 0 (yellow), 100% (green), no proteins satisfied the selection criterion (gray). For abbreviations, see SI Appendix, Table S1.
Molecular Analyses Reveal a Broadly Conserved SAL1-PAP Pathway.
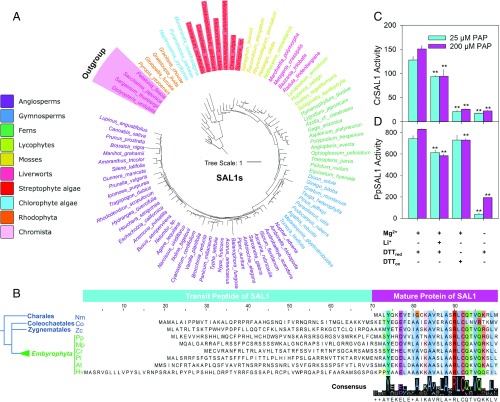
In the sulfur metabolism pathway in which PAP is produced and degraded, TPST and its related SOT19–SOT21 were evolutionarily conserved in all land plants and streptophyte algae, while most cytosolic SOTs (SOT1–SOT18) were not conserved in representative species of streptophyte algae, liverworts, mosses, lycophytes, and ferns (Fig. 1A and SI Appendix, Tables S2 and S3). Amino acid alignment and Logo analysis of the protein domains showed conserved sequence similarity for SAL1s and TPSTs, but not for SOT15s (a representative cytosolic SOT) in some species (SI Appendix, Fig. S2 A–C). Analysis using the 1000 Plant Transcriptome (1KP; www.onekp.com) database (37) showed that the presence and structure of SAL1s and TPSTs are highly conserved in sequences from streptophytes and chlorophytes (Fig. 2A and SI Appendix, Fig. S2 D and E and Table S4). Moreover, using the PlantOrDB (38), SAL proteins were identified in 35 land plants and 6 chlorophyte algae (SI Appendix, Fig. S3A). Gene structure comparison using the PIECE2 (39) software showed highly conserved protein domains and the exon and intron gene structure in SAL1s and TPSTs from angiosperms to chlorophytes, but these features are not conserved in SOT15s (SI Appendix, Figs. S2F and S3 and Table S4).
Fig. 2.
Bioinformatics analysis of SAL1s and their transit peptides in streptophyte species and cloning and functional analysis of SAL1s of C. richardii and P. patens. (A) Phylogenetic tree of SAL1s using key species of the major green plant and algal clades of the 1KP database (www.onekp.com). Phylogenetic tree with all of the SAL1s is in SI Appendix, Fig. S2D. (B) Alignment of the SAL1 transit peptides and partial mature protein in different species. Representative sequences are obtained from expressed mRNAs of different lineage groups. Alignment and sequence logos were conducted for only the first 30 amino acids of the SAL1 mature protein conserved region. At, A. thaliana; Co, C. orbicularis; Cr, C. richardii; Hv, H. vulgare; Mp, M. polymorpha; Nm, Nitella mirabilis; Pl, Pinus lambertiana; Pp, P. patens; Zc, Z. circumcarinatum. (C and D) CrSAL1 and PpSAL1 activity (pmol AMP⋅µg−1protein⋅min−1) assay against 25 and 200 μM PAP in the presence of different SAL1 activity regulators including cofactor (Mg2+), active site inhibitor (Li+), redox activator (DTTred), and redox inhibitor (DTTox) (n = 3). The “+” and “−” signs represent with or without the regulators, respectively, in the corresponding reactions. **P < 0.01.
We found predicted chloroplast transit peptides (cTPs), important for guiding and translocating the SAL1s into chloroplasts, in all tested land plants. Strong cTP predictions were recovered from analyses of ZcSAL1 and CoSAL1 sampled from Zygnema circumcarinatum (Zygnematales) and Coleochaete orbicularis (Coleochaetales), respectively, which are closely related to land plants. Interestingly, K. flaccidum (Klebsormidiales) was found to have SAL1 homologs (KfSAL1/KnSAL1) with putative mitochondrial transit peptide (mTP) as predicted by TargetP and a putative cTP recovered using ChloroP, albeit with different sequence characteristics relative to land plant SAL1 TPs (Fig. 2B and SI Appendix, Fig. S4 and Table S5).
We also experimentally validated the evolutionary conservation of SAL1 catalytic activity against PAP via the cloning and functional analysis of CrSAL1 from the model fern species Ceratopteris richardii and PpSAL1 from the model moss species Physcomitrella patens, respectively. CrSAL1 and PpSAL1 proteins showed PAP catalytic activity, but CrSAL1 activity was lower than PpSAL1 (Fig. 2 C and D). However, both proteins still exhibited enzymatic properties characteristic of AtSAL1 (28). Addition of Mg2+ as a cofactor or reduced DTT (DTTred) as a reductant increased the activity of CrSAL1 and PpSAL1, while known SAL1 inhibitors in A. thaliana, Li+ and oxidized DTT (DTTox) (13, 24), decreased their activity, with stronger inhibition observed in CrSAL1 compared with PpSAL1 (Fig. 2 C and D).
Evolutionarily Conserved PAP-Induced Stomatal Closure, H2O2 and NO Signaling, and Ion Transport Are Key Features in Land Plants.
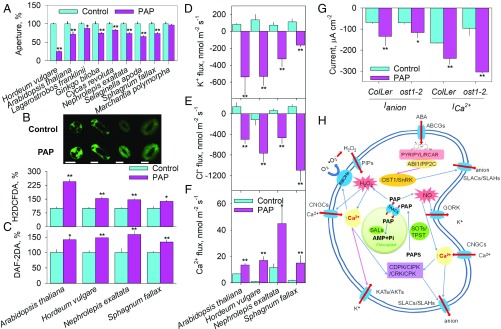
We investigated whether the physiological responses to PAP signaling are indeed evolutionarily conserved. Addition of 100 μM PAP resulted in significant closure of stomata in all tested plant species except Marchantia polymorpha, which has pores but no stomata (Fig. 3A and SI Appendix, Fig. S1). We then explored a possible evolutionary link between PAP retrograde signaling and H2O2 and NO, which are vital for abiotic and biotic stress response in most living organisms (40, 41). Treatment with 100 μM PAP led to significant increases in the H2O2 and NO levels in guard cells of two angiosperms (A. thaliana and Hordeum vulgare), a fern (Nephrolepis exaltata), and a moss (Sphagnum fallax) (Fig. 3 B and C).
Fig. 3.
PAP-induced stomatal closure, guard-cell ROS and NO signaling, and ion transport are evolutionarily conserved across plant clades. (A) Stomatal aperture (pore aperture for M. polymorpha) at 0 and 120 min after PAP treatment. Data are means ± SE (n = 5–7 biological replicates, 30–80 stomata/pores). (B and C) PAP induces H2O2 [2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA)] and NO [diaminofluorescein diacetate (DAF-2DA)] production in guard cells of plants from across three major clades measured in the control and after 50 min in 100 µM PAP. Data are means ± SE (n = 5 with 50–100 guard cells). (Scale bars, 10 μm.) (D–F) PAP regulates K+, Cl−, and Ca2+ fluxes from guard cells of three major clades. Data are averaged for control (0–10 min) and PAP (15–35 min). Data are means ± SE (n = 5–8). (G) PAP activates plasma membrane Ca2+ and anion channels in guard cells of A. thaliana. Average steady-state Ca2+ channel currents at −100 mV and anion channel currents at −200 mV in the control and 15 min after adding 100 μM PAP to the bath solution. Data are means ± SE (n = 4–10). *P < 0.05, **P < 0.01. (H) Schematic diagram of potential PAP-induced signal transduction in guard cells. Arrows: activation (blue), inhibition (purple), direction of ion and PAP movement (red), Ca2+ rise (green).
We further studied the extent of the conserved evolution of PAP action on guard cell ion transport using microelectrode ion flux measurement (MIFE). Across two angiosperms (A. thaliana and H. vulgare), a fern (N. exaltata), and a moss (S. fallax), net uptake of K+ and Cl− of guard cells in the control was reversed to net efflux by 100 μM PAP treatment (Fig. 3 D and E). Meanwhile, the net K+ and Cl− efflux was accompanied by an average of a 4.3-fold increase of net Ca2+ influx into guard cells of the four species (Fig. 3F). K+ and Cl− flux across the guard cell plasma membrane are regulated by the outwardly rectifying K+ channel (GORK), inwardly rectifying K+ channels (KATs), and slow anion channels (SLACs) (19). In situ PCR on guard cells of A. thaliana wild-type plants showed that GORK and SLAC1 are significantly up-regulated while KAT2 is significantly down-regulated by 100 μM PAP (SI Appendix, Fig. S5A). When expressing these genes in Xenopus laevis oocytes, none of the KAT1, KAT2, and SLAC1 channels were directly affected by PAP (SI Appendix, Fig. S5B). In A. thaliana, ICa2+ and Ianion in wild-type guard cells showed typical characteristics of anion and Ca2+ channels, and little change was observed over 15 min in the control (SI Appendix, Fig. S5 C and D). Addition of 100 μM PAP for 15 min led to a significant increase of ICa2+ and Ianion in guard cells (Fig. 3G).
Discussion
The streptophyte algae comprise a grade of lineages that share varying degrees of coancestry with land plants, including the land plant sister group Zygnematophyceae (32, 42). Transcriptomes of streptophyte algae have revealed that algal ancestors of land plants evolved stress-signaling pathways before colonization of terrestrial habitats by land plants (6, 22). Phytohormone-mediated signaling has been detected in streptophyte algae (12), which possessed a complex regulatory network that utilized many transcription factor families similar to those of land plants (43, 44). Thus, it might be expected that the sensing and signaling networks that land plants possess for acclimation and adaptation to the variable and harsh terrestrial environments existed in their algal ancestors (8). Importantly, our data show that SAL1s of the streptophyte algae are very similar to those of land plants. Our findings provide insights into the evolution of a suite of key protein families in the sister groups of land plants (Figs. 1 and 2 and SI Appendix, Fig. S1), with broad implications for understanding the evolution of plant abiotic stress responses and terrestrial plant life.
Here, SAL1 enzymatic capacity for PAP degradation and TPs for organellar targeting of SAL1 in C. richardii and P. patens (Fig. 2) suggest an evolutionarily conserved PAP-mediated signaling in seed-free land plant species. Approximately 95% of plastid proteins are nuclear-encoded, and their precursors contain N-terminal extension TPs (45), which direct the protein precursors into plastids through a conserved posttranslational mechanism (46). The identification of a putative TP in SAL1 of K. flaccidum and cTPs in SAL1s of C. orbicularis and Z. circumcarinatum demonstrates a possible stepwise evolution of TPs for SAL1 for plastidal targeting within the streptophyte algae (Fig. 2B and SI Appendix, Figs. S1 and S4 and Table S5). The acquisition of cTPs in SAL1s provides streptophyte algae with a fundamental functional advantage in gaining a chloroplastic oxidative stress sensor that potentially links the retrograde signaling SAL1-PAP pathway to plant tolerance to desiccation, which these algae frequently experience (6, 8, 22, 46). In this study, we have focused on the evolution of PAP signaling in relation to stomatal closure. This raises the question as to what other functions of SAL1-PAP signaling are conserved. In this context, it is worth noting that streptophyte algae lack guard cells and that PAP signaling has different roles in different cell types in angiosperms, such as having opposing effects on ROS accumulation in guard cells and bundle sheaths (13, 24). It would be of interest for future studies to investigate specific functions for PAP and SAL1 in a stretophyte alga and assess how the roles of PAP evolved and specialized during the evolution of multicellularity.
With regard to PAP synthesis, SOTs display significant differences and overlap in their substrate specificities and diversity (23, 47). TPST is exceptional in its size and structure, and the growth of A. thaliana tpst mutants is severely affected (23, 48), indicating an evolutionary significance of TPST in land plants. Conserved TPSTs (Fig. 1A) may have provided functional support for secondary sulfur metabolism in major lineages during green plant evolution. PAP induces stomatal closure in eight species of major plant lineages despite the absence of orthologs of SOT1–SOT18 in the moss, lycophyte, and fern examined. This suggests that PAP accumulation could be facilitated by the conserved orthologs of TPST and SOT19–SOT21 (Fig. 1A). Whether the explosion of SOT diversity in conifers and angiosperms (SI Appendix, Fig. S1 and Table S3) has any role in stomatal responses, or simply functions to facilitate specialized metabolism, remains to be determined.
We provide evidence that the evolutionary explanation for conservation of SAL1-PAP in land plants may be related to the role of PAP in stomatal regulation (Fig. 3). In angiosperms, ABA is a crucial phytohormone mediating plant abiotic stress responses (18), and ABA-mediated stomatal closure involves Ca2+-dependent and -independent signaling, regulating the secondary messengers H2O2 and NO and membrane transport (15, 19, 40, 41). Stomatal closure generally requires the elevation of cytosolic Ca2+ and the increase of K+ and anion release from guard cells (19). Conserved PAP-induced H2O2 and NO production (Fig. 3 B and C) and K+, Cl−, and Ca2+ fluxes (Fig. 3 D–F) from guard cells of angiosperms, ferns, and mosses demonstrate that these signaling and ion transport systems were acquired and used by guard cells of early land plants (15, 17, 49). We propose that guard cell H2O2 and NO signals (40, 41) merged into regulatory signaling cascades, which target ion transport for PAP-induced stomatal closure across major land plant lineages, acting alongside hormonal control by ABA (Fig. 3H).
In summary, we show that the shaping of the SAL-PAP retrograde signaling pathway predates the emergence of the first stomata, suggesting that intricate cellular communication networks were already in place to prime stomatal regulation. The evolutionary conservation and coordination of the SAL1-PAP pathway and ABA signaling during the diversification of land plants appear linked to the regulation of stomatal closure and adaptation to varying terrestrial habitats.
Materials and Methods
Evolutionary Bioinformatics.
Comparative genetic similarity analysis of protein families and source of the genome dataset are described in refs. 15 and 30. The 1KP database (37) was used to retrieve the mRNA sequences of SAL1s, SOT15s, and TPSTs, and the Interactive Tree of Life resource (https://itol.embl.de/) was used to annotate phylogenetic trees. The protein secondary structure of cTPs and mTPs was predicted using Jnet (www.compbio.dundee.ac.uk/jpred/legacy/jnet/) and the JPred 4 server (50).
Plant Materials and Growth.
Plant species were selected to represent divergent lineages across land plant phylogeny (51). The growth conditions of plants are described in refs. 21, 30, and 52.
Stomatal Bioassay.
Stomatal aperture and air-pore aperture assays were carried out on epidermal peels (21, 30). The H2O2 and NO production in guard cells was determined according to refs. 21 and 30.
Molecular Biology.
Cloning of CrSAL1 of C. richardii and of PpSAL1 of P. patens, purification of SAL1 proteins, and AMP produced from degradation of PAP was conducted according to refs. 24 and 28.
Electrophysiology.
Net K+, Ca2+, and Cl− flux was measured using the noninvasive MIFE technique (52). Electrophysiological recordings of anion and Ca2+ channel currents in guard cells of ColLer and ost1-2 using double-barreled microelectrodes are described in ref. 21.
See SI Appendix for full details of the materials and methods.
Supplementary Material
Acknowledgments
We thank Dr. A. Sali, Dr. Y. Wang, Dr. S. Cai, Dr. B. Xu, Dr. X. Wang, Dr. Y. Huang, A. Athman, L. Westmoreland, and M. Mak for their technical support; Dr. F.-W. Li for the fern genome data; and Dr. G. Jin and Dr. F. Xu for bioinformatics. This project is supported by the Australian Research Council (Grants DE1401011143, CE140100008, DP150104007); National Natural Science Foundation of China (Grants 31571578, 31620103912); University of Florida Genetics Institute; and UK Biotechnology and Biological Sciences Research Council.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. H.M. is a guest editor invited by the Editorial Board.
Data deposition: The sequences reported in this paper have been deposited in the GenBank database [accession nos. MH686366 (CrSAL1) and MH686367 (PpSAL1)].
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1812092116/-/DCSupplemental.
References
- 1.Rubinstein CV, Gerrienne P, de la Puente GS, Astini RA, Steemans P. Early Middle Ordovician evidence for land plants in Argentina (eastern Gondwana) New Phytol. 2010;188:365–369. doi: 10.1111/j.1469-8137.2010.03433.x. [DOI] [PubMed] [Google Scholar]
- 2.Kenrick P, Crane PR. The origin and early evolution of plants on land. Nature. 1997;389:33–39. [Google Scholar]
- 3.Morris JL, et al. The timescale of early land plant evolution. Proc Natl Acad Sci USA. 2018;115:E2274–E2283. doi: 10.1073/pnas.1719588115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bateman RM, et al. Early evolution of land plants: Phylogeny, physiology, and ecology of the primary terrestrial radiation. Annu Rev Ecol Syst. 1998;29:263–292. [Google Scholar]
- 5.Berner RA. Atmospheric oxygen over Phanerozoic time. Proc Natl Acad Sci USA. 1999;96:10955–10957. doi: 10.1073/pnas.96.20.10955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Vries J, Stanton A, Archibald JM, Gould SB. Streptophyte terrestrialization in light of plastid evolution. Trends Plant Sci. 2016;21:467–476. doi: 10.1016/j.tplants.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 7.Turmel M, Otis C, Lemieux C. The chloroplast and mitochondrial genome sequences of the charophyte Chaetosphaeridium globosum: Insights into the timing of the events that restructured organelle DNAs within the green algal lineage that led to land plants. Proc Natl Acad Sci USA. 2002;99:11275–11280. doi: 10.1073/pnas.162203299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Vries J, Archibald JM. Plant evolution: Landmarks on the path to terrestrial life. New Phytol. 2018;217:1428–1434. doi: 10.1111/nph.14975. [DOI] [PubMed] [Google Scholar]
- 9.Becker B, Marin B. Streptophyte algae and the origin of embryophytes. Ann Bot. 2009;103:999–1004. doi: 10.1093/aob/mcp044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan KX, Phua SY, Crisp P, McQuinn R, Pogson BJ. Learning the languages of the chloroplast: Retrograde signaling and beyond. Annu Rev Plant Biol. 2016;67:25–53. doi: 10.1146/annurev-arplant-043015-111854. [DOI] [PubMed] [Google Scholar]
- 11.Holzinger A, Becker B. Desiccation tolerance in the streptophyte green alga Klebsormidium: The role of phytohormones. Commun Integr Biol. 2015;8:e1059978. doi: 10.1080/19420889.2015.1059978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hori K, et al. Klebsormidium flaccidum genome reveals primary factors for plant terrestrial adaptation. Nat Commun. 2014;5:3978. doi: 10.1038/ncomms4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pornsiriwong W, et al. A chloroplast retrograde signal, 3′-phosphoadenosine 5′-phosphate, acts as a secondary messenger in abscisic acid signaling in stomatal closure and germination. eLife. 2017;6:e23361. doi: 10.7554/eLife.23361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berry JA, Beerling DJ, Franks PJ. Stomata: Key players in the earth system, past and present. Curr Opin Plant Biol. 2010;13:233–240. doi: 10.1016/j.pbi.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 15.Chen ZH, et al. Molecular evolution of grass stomata. Trends Plant Sci. 2017;22:124–139. doi: 10.1016/j.tplants.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 16.Hetherington AM, Woodward FI. The role of stomata in sensing and driving environmental change. Nature. 2003;424:901–908. doi: 10.1038/nature01843. [DOI] [PubMed] [Google Scholar]
- 17.Chater C, et al. Regulatory mechanism controlling stomatal behavior conserved across 400 million years of land plant evolution. Curr Biol. 2011;21:1025–1029. doi: 10.1016/j.cub.2011.04.032. [DOI] [PubMed] [Google Scholar]
- 18.Cutler SR, Rodriguez PL, Finkelstein RR, Abrams SR. Abscisic acid: Emergence of a core signaling network. Annu Rev Plant Biol. 2010;61:651–679. doi: 10.1146/annurev-arplant-042809-112122. [DOI] [PubMed] [Google Scholar]
- 19.Jezek M, Blatt MR. The membrane transport system of the guard cell and its integration for stomatal dynamics. Plant Physiol. 2017;174:487–519. doi: 10.1104/pp.16.01949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruszala EM, et al. Land plants acquired active stomatal control early in their evolutionary history. Curr Biol. 2011;21:1030–1035. doi: 10.1016/j.cub.2011.04.044. [DOI] [PubMed] [Google Scholar]
- 21.Chen ZH, et al. Nitrate reductase mutation alters potassium nutrition as well as nitric oxide-mediated control of guard cell ion channels in Arabidopsis. New Phytol. 2016;209:1456–1469. doi: 10.1111/nph.13714. [DOI] [PubMed] [Google Scholar]
- 22.de Vries J, Curtis BA, Gould SB, Archibald JM. Embryophyte stress signaling evolved in the algal progenitors of land plants. Proc Natl Acad Sci USA. 2018;115:E3471–E3480. doi: 10.1073/pnas.1719230115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hirschmann F, Krause F, Papenbrock J. The multi-protein family of sulfotransferases in plants: Composition, occurrence, substrate specificity, and functions. Front Plant Sci. 2014;5:556. doi: 10.3389/fpls.2014.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Estavillo GM, et al. Evidence for a SAL1-PAP chloroplast retrograde pathway that functions in drought and high light signaling in Arabidopsis. Plant Cell. 2011;23:3992–4012. doi: 10.1105/tpc.111.091033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiong L, et al. FIERY1 encoding an inositol polyphosphate 1-phosphatase is a negative regulator of abscisic acid and stress signaling in Arabidopsis. Genes Dev. 2001;15:1971–1984. doi: 10.1101/gad.891901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manmathan H, Shaner D, Snelling J, Tisserat N, Lapitan N. Virus-induced gene silencing of Arabidopsis thaliana gene homologues in wheat identifies genes conferring improved drought tolerance. J Exp Bot. 2013;64:1381–1392. doi: 10.1093/jxb/ert003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson PB, et al. The nucleotidase/phosphatase SAL1 is a negative regulator of drought tolerance in Arabidopsis. Plant J. 2009;58:299–317. doi: 10.1111/j.1365-313X.2008.03780.x. [DOI] [PubMed] [Google Scholar]
- 28.Chan KX, et al. Sensing and signaling of oxidative stress in chloroplasts by inactivation of the SAL1 phosphoadenosine phosphatase. Proc Natl Acad Sci USA. 2016;113:E4567–E4576. doi: 10.1073/pnas.1604936113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rossel JB, et al. A mutation affecting ASCORBATE PEROXIDASE 2 gene expression reveals a link between responses to high light and drought tolerance. Plant Cell Environ. 2006;29:269–281. doi: 10.1111/j.1365-3040.2005.01419.x. [DOI] [PubMed] [Google Scholar]
- 30.Cai S, et al. Evolutionary conservation of ABA signaling for stomatal closure. Plant Physiol. 2017;174:732–747. doi: 10.1104/pp.16.01848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Puttick MN, et al. The interrelationships of land plants and the nature of the ancestral embryophyte. Curr Biol. 2018;28:733–745.e2. doi: 10.1016/j.cub.2018.01.063. [DOI] [PubMed] [Google Scholar]
- 32.Wickett NJ, et al. Phylotranscriptomic analysis of the origin and early diversification of land plants. Proc Natl Acad Sci USA. 2014;111:E4859–E4868. doi: 10.1073/pnas.1323926111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rausch T, Wachter A. Sulfur metabolism: A versatile platform for launching defence operations. Trends Plant Sci. 2005;10:503–509. doi: 10.1016/j.tplants.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 34.Jeandroz S, et al. Occurrence, structure, and evolution of nitric oxide synthase-like proteins in the plant kingdom. Sci Signal. 2016;9:re2. doi: 10.1126/scisignal.aad4403. [DOI] [PubMed] [Google Scholar]
- 35.Li F-W, et al. Phytochrome diversity in green plants and the origin of canonical plant phytochromes. Nat Commun. 2015;6:7852. doi: 10.1038/ncomms8852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamel LP, Sheen J, Séguin A. Ancient signals: Comparative genomics of green plant CDPKs. Trends Plant Sci. 2014;19:79–89. doi: 10.1016/j.tplants.2013.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matasci N, et al. Data access for the 1,000 Plants (1KP) project. Gigascience. 2014;3:17. doi: 10.1186/2047-217X-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li L, et al. PlantOrDB: A genome-wide ortholog database for land plants and green algae. BMC Plant Biol. 2015;15:161. doi: 10.1186/s12870-015-0531-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, et al. PIECE 2.0: An update for the plant gene structure comparison and evolution database. Nucleic Acids Res. 2017;45:1015–1020. doi: 10.1093/nar/gkw935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gupta KJF, Fernie AR, Kaiser WM, van Dongen JT. On the origins of nitric oxide. Trends Plant Sci. 2011;16:160–168. doi: 10.1016/j.tplants.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 41.Møller IM, Sweetlove LJ. ROS signalling: Specificity is required. Trends Plant Sci. 2010;15:370–374. doi: 10.1016/j.tplants.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 42.Ruhfel BR, Gitzendanner MA, Soltis PS, Soltis DE, Burleigh JG. From algae to angiosperms: Inferring the phylogeny of green plants (Viridiplantae) from 360 plastid genomes. BMC Evol Biol. 2014;14:23. doi: 10.1186/1471-2148-14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Catarino B, Hetherington AJ, Emms DM, Kelly S, Dolan L. The stepwise increase in the number of transcription factor families in the Precambrian predated the diversification of plants on land. Mol Biol Evol. 2016;33:2815–2819. doi: 10.1093/molbev/msw155. [DOI] [PubMed] [Google Scholar]
- 44.Wilhelmsson PKI, Mühlich C, Ullrich KK, Rensing SA. Comprehensive genome-wide classification reveals that many plant-specific transcription factors evolved in streptophyte algae. Genome Biol Evol. 2017;9:3384–3397. doi: 10.1093/gbe/evx258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karlin-Neumann GA, Tobin EM. Transit peptides of nuclear-encoded chloroplast proteins share a common amino acid framework. EMBO J. 1986;5:9–13. doi: 10.1002/j.1460-2075.1986.tb04170.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bruce BD. Chloroplast transit peptides: Structure, function and evolution. Trends Cell Biol. 2000;10:440–447. doi: 10.1016/s0962-8924(00)01833-x. [DOI] [PubMed] [Google Scholar]
- 47.Varin L, DeLuca V, Ibrahim RK, Brisson N. Molecular characterization of two plant flavonol sulfotransferases. Proc Natl Acad Sci USA. 1992;89:1286–1290. doi: 10.1073/pnas.89.4.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Komori R, Amano Y, Ogawa-Ohnishi M, Matsubayashi Y. Identification of tyrosylprotein sulfotransferase in Arabidopsis. Proc Natl Acad Sci USA. 2009;106:15067–15072. doi: 10.1073/pnas.0902801106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lind C, et al. Stomatal guard cells co-opted an ancient ABA-dependent desiccation survival system to regulate stomatal closure. Curr Biol. 2015;25:928–935. doi: 10.1016/j.cub.2015.01.067. [DOI] [PubMed] [Google Scholar]
- 50.Drozdetskiy A, Cole C, Procter J, Barton GJ. JPred4: A protein secondary structure prediction server. Nucleic Acids Res. 2015;43:W389–W394. doi: 10.1093/nar/gkv332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cantino PD, et al. Towards a phylogenetic nomenclature of Tracheophyta. Taxon. 2007;56:1E–44E. [Google Scholar]
- 52.Chen Z, et al. Screening plants for salt tolerance by measuring K+ flux: A case study for barley. Plant Cell Environ. 2005;28:1230–1246. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.