Significance
We employed photoexcitation as a unique control method to manipulate molecular aggregation and emission to advance luminescent probe techniques. This study harnesses dynamical and rhythmical photoluminescence to strengthen visualization in bioimaging. These advantages are based on the merit of a type of photocontrollable material, which can undergo fast and self-recoverable aggregation-induced phosphorescence while mostly avoiding possible side reactions and fatigue accumulation, compared with traditional bidirectional manipulations.
Keywords: photoexcitation, conformational regulation, aggregation-induced phosphorescence, self-recoverable, luminescent probe
Abstract
Chemical systems with external control capability and self-recoverability are promising since they can avoid additional chemical or energy imposition during the working process. However, it remains challenging to employ such a nonequilibrium method for the engineering of optoelectronic function and for visualization. Here, we report a functional molecule that can undergo intense conformational regulation upon photoexcitation. It enables a dynamical change in hydrophobicity and a follow-up molecular aggregation in aqueous media, accordingly leading to an aggregation-induced phosphorescence (AIP) behavior. This successive performance is self-recoverable, allowing a rapid (second-scale cycle) and long-standing (>103 cycles) flicker ability under rhythmical control of the AIP. Compared with traditional bidirectional manipulations, such monodirectional photocontrol with spontaneous reset profoundly enhances the operability while mostly avoiding possible side reactions and fatigue accumulation. Furthermore, this material can serve as a type of luminescent probe for dynamically strengthening visualization in bioimaging.
Artificial molecular switches continue to attract research attention due to their fascinating structures and smart control performances (1–5). Nevertheless, most of these chemical systems work between two or more stable states, and the rest of them requires at least a secondary chemical or energy stimuli, imposing additional inconvenience and the possibility of doubling fatigue accumulation (6–8). Inspired by the underlying mechanism of functional natural systems, scientists began to design and develop molecules with self-recoverability for nonequilibrium action control (9–11). Among the control methods, photocontrol is still a superior fashion because light stimuli are usually rapid and precise, and can be operated remotely (12, 13). In contrast to well-studied photochemical processes like photoreaction, photocyclization, and photoisomerization (14, 15), a photocontrol approach with self-recoverability largely connects to a photoexcitation principle. Thus, it may generally suffer from ultrafast energy relaxation and dissipation, and is extremely difficult to be utilized in materials. Engineering of optoelectronic function and visualization via such a photocontrol method is particularly challenging but also desirable.
While luminescent probe techniques enabled a significant scientific advancement in visualized analysis, sensing, and imaging (16–18), in this work, we expect to impose a photocontrol with self-recoverability into the advancing of operating methods for molecular luminescence. Aggregation-induced emission (AIE) is a type of approach where the molecules can exhibit high luminescence in condensed or constraint states by overcoming the aggregation-caused quenching effect (19–21). Controllable AIE probes that utilize specific chemical reactions have emerged to facilitate a series of frontier biological usage (22–25). In contrast, the necessity of spontaneous, repeatable, and rhythmic manipulation of the AIE effect for dynamically strengthening visualization desires a self-recoverability fashion for tuning the unique photophysical property. On this basis, this study proposes a strategy for achieving photoactivatable and self-recoverable AIE through a photoexcitation on a single emitter. The strategy is inspired by the fact that excitation of organic luminophores can normally lead to molecular conformational and energy change by electron–phonon coupling (26–28). However, it is difficult to sufficiently employ these tiny changes (10 ∼ 20 kJ/mol). This study employs a well-defined structure with a lifetime-prolonged excited state to cause a dynamical follow-up of molecular aggregation to address AIE.
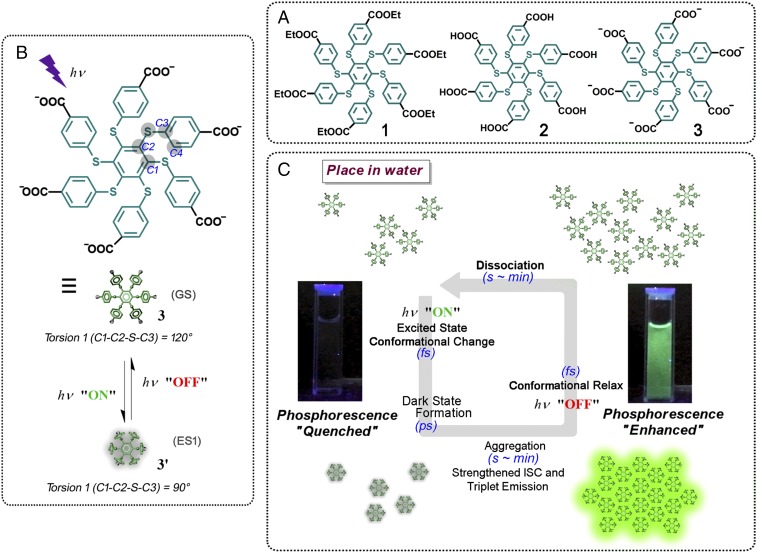
We herein present a hexathiobenzene skeleton linked with six carboxylate groups on the periphery (compound 3; see Fig. 1 for structure as well as for its precursor compounds 1 and 2). Persulfurated aromatic molecules are prone to serve as metal-free room-temperature phosphorescence emitters, since their intersystem crossing process is environmentally adjustable (29–31). In this study, we employed the long-excited state lifetime and the amphiphilic structural characteristic, and eventually found photoactivation and self-recovery behavior of the aggregation-induced phosphorescence (AIP) effect in water. This design can take advantage of the molecular conformational change from its ground-state (GS) structure to the first excited state (ES1) upon photoirradiation as a facile control method. The ES1 structure was more hydrophobic, and the formation was accompanied by a long-lifetime dark state. Thus, the system will have sufficient time against energy relaxation to ensure molecular aggregation as well as to exhibit AIP (Fig. 1). Via optimization, the photoactivation and self-recovery of the room-temperature phosphorescence can be rapidly and rhythmically operated. This material can also exhibit low cytotoxicity and high biocompatibility as exemplified by imaging studies, featuring a superior dynamically strengthened visualization at the cellular level. Such a flicker phosphorescence can be cycled for a long time since the utilization of the monodirectional photocontrol with spontaneous reset can avoid additional fatigue accumulation to the most extent for cycling.
Fig. 1.
Schematic illustration of the photoexcitation-controlled AIP. (A) Chemical structure of the related compounds. (B) The proposed conformational change upon photoexcitation of compound 3. The dihedral torsion as well as the related atoms were defined alongside the structure. (C) The successive action of the photoactivation and self-recovery cycle of the AIP behavior of compound 3 in aqueous media, based on the conformational interconversion accompanied by a long-lifetime dark state. ISC, intersystem crossing.
Results and Discussion
Molecular Conformational Change with Dark-State Formation upon Photoexcitation.
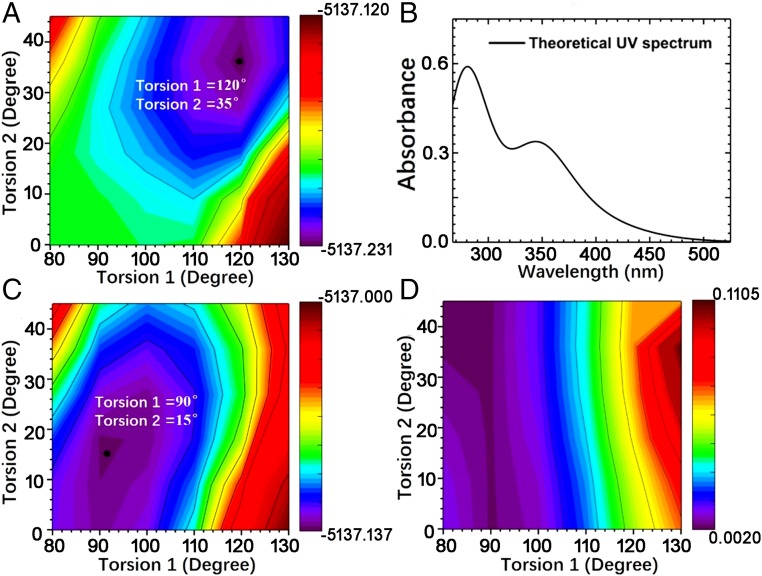
Since compound 3 has six benzene side chains linked to the central benzene ring with six sulfur atoms, obviously, both torsion C1-C2-S-C3 (torsion 1) and torsion C2-S-C3-C4 (torsion 2) determine the overall conjugation of the molecule (see atom labeling in Fig. 1). We therefore focused on the two torsional degrees of freedom and calculated the GS potential energy surface (Fig. 2A). The equivalent torsions in the six side chains were set to be identical during the computational scan. Both torsion 1 and torsion 2 were found to exert a strong impact on the GS energy, and the equilibrium geometry corresponds to torsion 1 = 120° and torsion 2 = 35° (SI Appendix, Fig. S2A). Based on this geometry, the time-dependent density-functional theory was utilized to calculate the excited states and to obtain the absorption spectrum, which agrees well with the experimental data (vide infra).
Fig. 2.
Theoretical study of the conformational change of compound 3 upon photoexcitation. (A) Calculated GS potential energy surface, where the black solid circle indicates the equilibrium geometry. (B) Absorption spectrum calculated based on the GS equilibrium geometry. (C) ES1 potential energy surface. (D) Scan of the ES1 oscillator strength.
Similarly, the potential energy surface of the ES1 state was calculated (Fig. 2C), and the equilibrium geometry was found to be torsion 1 = 90° and torsion 2 = 15° (SI Appendix, Fig. S2B). The corresponding scan of the ES1 oscillator strength is shown in Fig. 2D, which implies that the emission from ES1 is much more sensitive to torsion 1 than to torsion 2. Apparently, the ES1 of the equilibrium geometry is a dark state with almost zero oscillator strength. We found that the majority of the contributions to ES1 originate from the excitation of the highest occupied molecular orbital (HOMO) to the lowest unoccupied molecular orbital (LUMO). HOMO is localized on S atoms (SI Appendix, Fig. S2C), while LUMO is localized on the central benzene ring (SI Appendix, Fig. S2D). At the ES1 equilibrium geometry with torsion 1 = 90°, HOMO is symmetric in the vertical direction of the central benzene ring, while LUMO is antisymmetric. As a result, the overlap of HOMO and LUMO is almost zero, further featuring a dark-state characteristic. In fact, ES1 is a charge-transfer state with negligible oscillator strength.
Direct experimental evidence that can confirm the existence of a dark state and its lifetime was from transient absorption (SI Appendix, Fig. S3). Since the positive band around 400 nm is the ground-state bleaching, a remarkable broadband excited-state absorption signal (S1 → Sn) around 648 nm emerged during an ultrafast period (∼200 fs) upon photoexcitation. It can then rapidly decay with blue shift. Accompanied by the decay of this signal, a signal maximum at 458 nm grew during the first few ps, at almost the same rate as the decay of the 648-nm maximum (∼1.6 × 1011 s−1). Since there was no change of the molecular structure before and after irradiation, the absorption at 484 nm was attributed to be caused by a change in the molecular conformation with a charge-transfer process. This indicates the chance of a dark-state formation and is consistent with the computational results. The lifetime of the dark state can be seen to be at a time scale of ps ∼ ns.
Photoluminescence Study for the Photoactivation and Self-Recovery Behavior of the AIP.
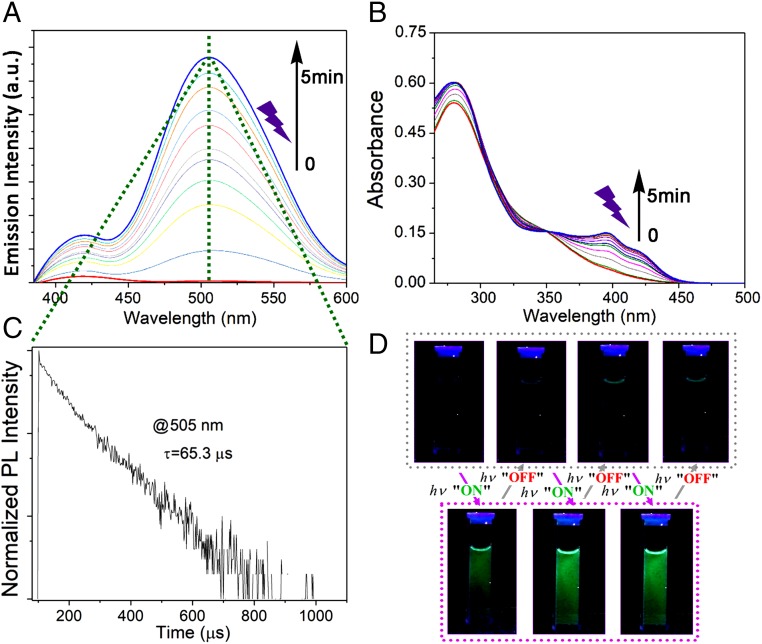
The absorption spectrum of compound 3 shows different bands, and that with the lowest energy has a maximum around 345 nm with a tail extending up to 450 nm (Fig. 3B), similar to the absorption behavior of compound 2 (SI Appendix, Fig. S4A). In this case, almost no photoluminescence (PL) signals were observed initially upon excitation at 365 nm at room temperature in solution (Fig. 3A and SI Appendix, Fig. S5B). In contrast, compound 3 showed intense emissions around 525 nm at 77 K, while it also exhibited a similar emission around 510 nm in the solid state at room temperature (SI Appendix, Fig. S5B). The temperature-dependent emission spectrum was also collected (SI Appendix, Fig. S4D). These results agree with similar findings in the literature that signify a typical AIP effect (31).
Fig. 3.
Photoactivation and self-recovery of the phosphorescence property of compound 3 in water (1 × 10−5 M) under irradiation by a commercial UV lamp (365 nm, 4 W). Continuous enhancement of the (A) PL spectra and (B) absorption spectra with prolonged irradiation duration. (C) PL lifetime at 505 nm after irradiation. (D) Image of photocontrolled phosphorescence on and off with different cycles. The light source was turned on for 1 min and off for 1 min during each cycle.
Next, a photoactivation phenomenon of phosphorescence was experimentally found during an irradiation experiment with compound 3 in water under room temperature. Compound 3 displayed almost no emission before irradiation under 365 nm using a hand-held UV lamp, whereas an immediate increase of the emission at 505 nm was observed after irradiation over time (Fig. 3A). The emission signal became stronger with extended irradiation time (SI Appendix, Fig. S6A). This phenomenon was also verified by the change of the UV-vis absorption spectra, in which two absorption bands at 396 and 421 nm emerged under irradiation (Fig. 3B). Interestingly, upon removal of the irradiation light, emission at 505 nm of compound 3 decreased quickly (SI Appendix, Fig. S6B), and such a reversible cycle could be repeated (Fig. 3D). In contrast, both compound 2 (SI Appendix, Fig. S4B) and compound 3 in DMF (SI Appendix, Fig. S4C) did not show this unique phenomenon along with photoirradiation.
To further confirm that this phenomenon is a photoactivation and self-recovery process of AIP, we collected the PL lifetimes of compound 3. The PL lifetimes of compound 3 for its powder state and in rigid matrices at 77 K were 336.2 μs (SI Appendix, Fig. S5C) and 3.9 ms (SI Appendix, Fig. S5D), respectively, due to the slowing of nonradiative deactivation of excited states when exposed to a rigid environment (32–34). The PL lifetime of compound 3 in water after irradiation was 65.3 μs at 505 nm (Fig. 3C), suggesting a phosphorescence band that likely originated from an aggregation factor that can inhibit nonradiative decay factors (e.g., molecular vibration and rotation) to strengthen the triplet emission. This unique phenomenon is a monostable process without molecular structural change, because there is no change in the fundamental characteristics before and after irradiation as verified by proton nuclear magnetic resonance (SI Appendix, Fig. S7A), carbon-13 nuclear magnetic resonance (SI Appendix, Fig. S7B), electrospray ionization mass spectrometry (SI Appendix, Fig. S7C), high performance liquid chromatography (SI Appendix, Fig. S7D), infrared (SI Appendix, Fig. S7E), and Raman spectra (SI Appendix, Fig. S7F). A possible photooxidation route can also be ruled out since the photoactivated phosphorescent signal can still be observed after a strict degassing pretreatment by three cycles of freeze–pump–thaw (SI Appendix, Fig. S5E and Fig. S5F) (35).
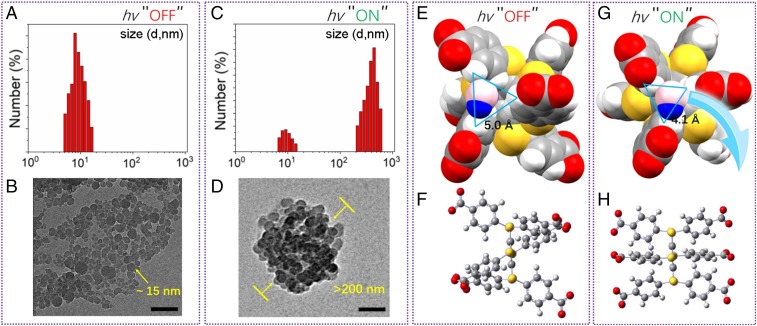
We speculated that the light-controlled aggregation of compound 3 in water played a significant role in producing the phosphorescence at room temperature. To further validate this hypothesis, transmission electron microscopy (TEM) and dynamic light scattering (DLS) experiments were performed. The TEM image showed that the well-dispersed nanometer-sized parts (about 15 nm in diameter) of compound 3 (Fig. 4B) aggregated and formed larger nanometer-sized aggregates (more than 200 nm in diameter) with a normal distribution after irradiation (Fig. 4D). These results were consistent with those obtained from DLS experiments before (Fig. 4A) and after (Fig. 4C) irradiation, with which a photoactivation and self-recovery process can also be monitored (SI Appendix, Fig. S8).
Fig. 4.
Nanoscale study (DLS results and TEM images of compound 3 in water) and computational data for confirming an AIP process. DLS results (A) before and (C) after irradiation for 5 min. TEM image (B) before and (D) after irradiation for 5 min. (Scale bars: 100 nm.) Equilibrium geometry of the (E and F) GS and (G and H) ES1. The van der Waals surface has been used in E and G, and a water molecule has been shown to guide the eyes. The triangles indicate the distances between corresponding hydrogen atoms.
Mechanism Study for the Phosphorescence Amplification by Photoexcitation.
The theoretical investigation indicated that the equilibrium geometry of GS and ES1 differ significantly. In particular, torsion 1 changed from 120° to 90°, and torsion 2 changed from 35° to 15°, greatly improving molecular aggregation. As shown in Fig. 4 E and G, the GS equilibrium structure has a larger space between the side chains than the ES1 equilibrium structure. Water molecules may stay well between the side chains for GS, whereas it is very difficult in the ES1 case. Moreover, due to different torsions, the ES1 structure is much more regular than the GS structure (Fig. 4 F and H). Molecules are expected to be parallel to each other, preferring ordered stacking. Due to these reasons, water does not easily mix with molecules in the equilibrium geometry of ES1, resulting in a larger aggregation than in GS. On the other hand, the occurrence of this process is also because the ES1 is a long-lifetime dark state, enabling the dynamic possibility for a following aggregation behavior. The dark state (ps ∼ ns) upon photoexcitation sufficiently allows molecular collision (ps or sub-ps) (36, 37) and the formation of aggregates with small number of units (ns scale; see the time evolution of solvent-accessible surface area simulation in some persulfurated aromatic systems) (30, 38). Furthermore, referenced from similar findings (39, 40), we conclude that the aggregation state is more stable than the free state upon photoexcitation. In this way, the disassociation rate will be much slower than the aggregation rate upon photoexcitation, thus ensuring molecular collision to finally complete an entire aggregation for AIP. A possible photo-acid/base property can be ruled out since the same phenomenon of light-controllable aggregation can also be observed with increasing pH value. Removal of photoexcitation changed the conformation back from ES1 to GS, and hence, the self-recovery of AIP started along with the dissociation of compound 3, as illustrated in Fig. 1.
Bioimaging Studies.
Dynamic emission with time-resolution characteristics has received significant attention in photoluminescent probe techniques (41–43). After establishing such a photoresponsive system, we investigated its flicker emission imaging (44, 45) at the cellular level. Before endocytosis for imaging, cytotoxicity evaluation is an important parameter to consider. In this study, the accounting kit-8 (CCK8) assay was conducted to test the cytotoxicity. Through cell viability examination, no obvious cytotoxicity was observed with concentrations from 1 to 10 μM after 24 h of incubation (SI Appendix, Fig. S9A), demonstrating a low cytotoxicity of compound 3 in aqueous media. Then, we investigated the application of compound 3 in cellular imaging using confocal laser scanning microscopy (CLSM). After HeLa cells were incubated with aqueous solutions of compound 3 (1 × 10−5 M) and after irradiation, they can be excited by an FITC channel laser to emit strong luminescence (SI Appendix, Fig. S9D), whereas no emission signal was observed before irradiation (SI Appendix, Fig. S9C). The bright regions overlap at the cell position (SI Appendix, Fig. S9B), indicating that compound 3 has been successfully taken up and accumulated in cells.
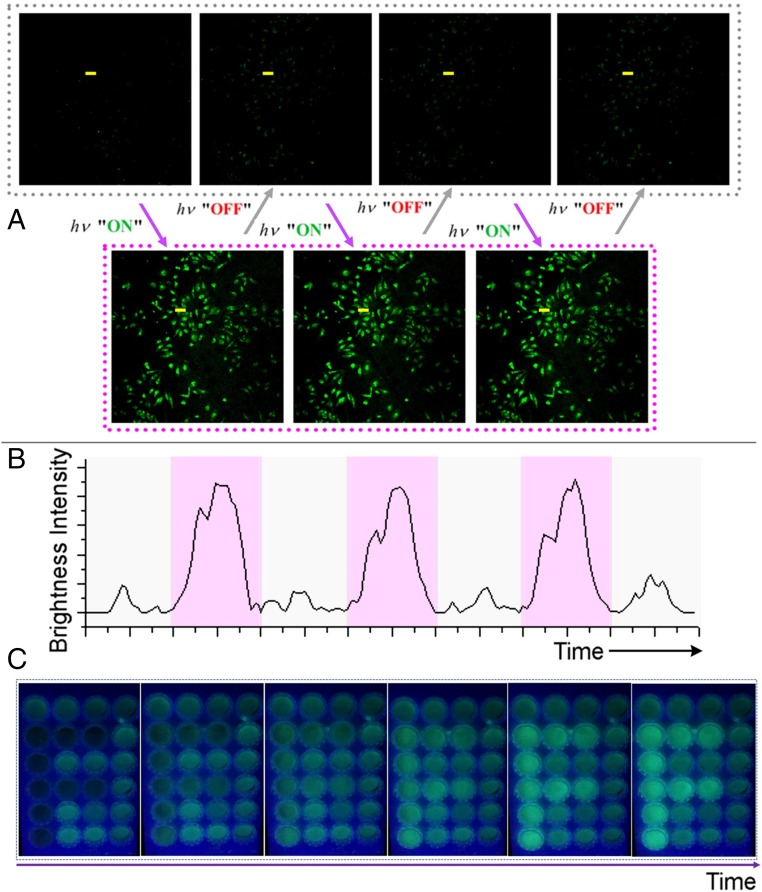
To conduct the flicker emission imaging experiment, the light source was optimized and a visible light beam at 405 nm with a relatively big power was applied instead of the traditional 365-nm UV lamp, for reducing harm to the biosample and achieving a faster photoresponse. By rhythmical irradiating for seconds and halting for a specific period of time, a dynamic flicker emission effect was observed from the CLSM images (Fig. 5A). Such a phenomenon is consistent with the PL signal alternation discussed above (Fig. 3D). This indicates that the photoactivation and self-recovery of AIP in each cycle can be observed in vivo. The brightness along the cellular profile can also be quantified (Fig. 5B), showing that a distinct signal alternation works well along with rhythmic irradiation. Although the current photoexcitation condition is limited for application in bulk biosamples or animals, a visualized patterning experiment in cell solution showed that such a strategy can utilize the dynamic emission signal to strengthen the visualization in the mapping (see an “F” character in a dynamic process, Fig. 5C and SI Appendix, Fig. S10). Our material can currently work at least 103 cycles, reflecting the merit of self-recoverable photocontrol.
Fig. 5.
Dynamic emission of compound 3 for imaging. (A) Confocal microphotographs (FITC channel) of HeLa cells incubated with compound 3 upon rhythmical irradiation at 405 nm. (B) Corresponding brightness intensity readout curves along the line highlighted in A. (C) Continuous enhancement of luminescence of the cell solution dyed with compound 3 with prolonged irradiation time in a visualized patterning experiment.
Conclusion
In summary, this study presents a unique AIP molecule powered by photoexcitation. This chemical system can perform AIP behavior with photoactivation and self-recovery in aqueous media. Two factors play key roles for such a successive process: (i) molecular conformational alteration mediated by photoexcitation changing the hydrophobicity of the compound; (ii) the dark lowest-excited state ensuring a dynamical follow-up of the molecular aggregation. Upon aggregation, AIP occurred and was self-resettable while photoexcitation was halted. By rhythmically controlling the AIP in our system, a flicker emission effect upon the delivery of the molecule into the cellular level was exhibited, featuring a dynamically strengthened visualization in bioimaging. All these advantages are based on the merit of a type of photocontrollable material which can undergo fast and self-recoverable AIP while avoiding possible side reactions and fatigue accumulation to the most extent, compared with traditional bidirectional manipulations. We believe the strategy demonstrated herein to be valuable for the interdisciplinary development of artificial molecular switches with lively controllable probing functions.
Materials and Methods
Full details of the materials and methods are given in SI Appendix. Briefly, compound 3 was synthesized by nucleophilic substitution and it was strictly purified. Computational work for 3 was done through Gaussian 09 program (46) and the polarizable continuum model (47). Information also shows descriptions of instruments, compounds synthesis, transient absorption measurement, additional photophysical experimental data and dynamics study, bioimaging study, visualized patterning experiment, computational details, and all fundamental characterization data with all of the compounds included.
Supplementary Material
Acknowledgments
This work was supported by 2017 Natural Science Foundation of Shanghai (Grant 17ZR1402400), and partially from the National Key Research and Development Program of China (Grant 2017YFA0207700). L.W. acknowledges support from the “Thousand Young Talents Plan” of China, the “Hundred Talents Plan” of Zhejiang University, and the National Natural Science Foundation of China (Grants 21703202 and 21873080).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1821991116/-/DCSupplemental.
References
- 1.Coskun A, Banaszak M, Astumian RD, Stoddart JF, Grzybowski BA. Great expectations: Can artificial molecular machines deliver on their promise? Chem Soc Rev. 2012;41:19–30. doi: 10.1039/c1cs15262a. [DOI] [PubMed] [Google Scholar]
- 2.Erbas-Cakmak S, Leigh DA, McTernan CT, Nussbaumer AL. Artificial molecular machines. Chem Rev. 2015;115:10081–10206. doi: 10.1021/acs.chemrev.5b00146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sauvage JP. From chemical topology to molecular machines (Nobel lecture) Angew Chem Int Ed Engl. 2017;56:11080–11093. doi: 10.1002/anie.201702992. [DOI] [PubMed] [Google Scholar]
- 4.De Bo G, et al. An artificial molecular machine that builds an asymmetric catalyst. Nat Nanotechnol. 2018;13:381–385. doi: 10.1038/s41565-018-0105-3. [DOI] [PubMed] [Google Scholar]
- 5.Feringa BL. The art of building small: From molecular switches to motors (Nobel lecture) Angew Chem Int Ed Engl. 2017;56:11060–11078. doi: 10.1002/anie.201702979. [DOI] [PubMed] [Google Scholar]
- 6.Petermayer C, Dube H. Indigoid photoswitches: Visible light responsive molecular tools. Acc Chem Res. 2018;51:1153–1163. doi: 10.1021/acs.accounts.7b00638. [DOI] [PubMed] [Google Scholar]
- 7.Wezenberg SJ, Feringa BL. Supramolecularly directed rotary motion in a photoresponsive receptor. Nat Commun. 2018;9:1984. doi: 10.1038/s41467-018-04249-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stoddart JF. Mechanically interlocked molecules (mims)-molecular shuttles, switches, and machines (Nobel lecture) Angew Chem Int Ed Engl. 2017;56:11094–11125. doi: 10.1002/anie.201703216. [DOI] [PubMed] [Google Scholar]
- 9.Liu R, Sen A. Autonomous nanomotor based on copper-platinum segmented nanobattery. J Am Chem Soc. 2011;133:20064–20067. doi: 10.1021/ja2082735. [DOI] [PubMed] [Google Scholar]
- 10.Venkataraman S, Dirks RM, Rothemund PW, Winfree E, Pierce NA. An autonomous polymerization motor powered by DNA hybridization. Nat Nanotechnol. 2007;2:490–494. doi: 10.1038/nnano.2007.225. [DOI] [PubMed] [Google Scholar]
- 11.Meng W, et al. An autonomous molecular assembler for programmable chemical synthesis. Nat Chem. 2016;8:542–548. doi: 10.1038/nchem.2495. [DOI] [PubMed] [Google Scholar]
- 12.Balzani V, Credi A, Venturi M. Light powered molecular machines. Chem Soc Rev. 2009;38:1542–1550. doi: 10.1039/b806328c. [DOI] [PubMed] [Google Scholar]
- 13.Xu L, Mou F, Gong H, Luo M, Guan J. Light-driven micro/nanomotors: From fundamentals to applications. Chem Soc Rev. 2017;46:6905–6926. doi: 10.1039/c7cs00516d. [DOI] [PubMed] [Google Scholar]
- 14.Yoon B, Lee S, Kim JM. Recent conceptual and technological advances in polydiacetylene-based supramolecular chemosensors. Chem Soc Rev. 2009;38:1958–1968. doi: 10.1039/b819539k. [DOI] [PubMed] [Google Scholar]
- 15.Nakashima T, et al. Self-contained photoacid generator triggered by photocyclization of triangle terarylene backbone. J Am Chem Soc. 2015;137:7023–7026. doi: 10.1021/jacs.5b02826. [DOI] [PubMed] [Google Scholar]
- 16.le Masne de Chermont Q, et al. Nanoprobes with near-infrared persistent luminescence for in vivo imaging. Proc Natl Acad Sci USA. 2007;104:9266–9271. doi: 10.1073/pnas.0702427104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu H, Fan J, Du J, Peng X. Fluorescent probes for sensing and imaging within specific cellular organelles. Acc Chem Res. 2016;49:2115–2126. doi: 10.1021/acs.accounts.6b00292. [DOI] [PubMed] [Google Scholar]
- 18.Liu JN, Bu W, Shi J. Chemical design and synthesis of functionalized probes for imaging and treating tumor hypoxia. Chem Rev. 2017;117:6160–6224. doi: 10.1021/acs.chemrev.6b00525. [DOI] [PubMed] [Google Scholar]
- 19.Mei J, Leung NL, Kwok RT, Lam JW, Tang BZ. Aggregation-induced emission: Together we shine, united we soar! Chem Rev. 2015;115:11718–11940. doi: 10.1021/acs.chemrev.5b00263. [DOI] [PubMed] [Google Scholar]
- 20.Wang J, et al. Ionization and anion-π+ interaction: A new strategy for structural design of aggregation-induced emission luminogens. J Am Chem Soc. 2017;139:16974–16979. doi: 10.1021/jacs.7b10150. [DOI] [PubMed] [Google Scholar]
- 21.Nicol A, et al. Ultrafast delivery of aggregation-induced emission nanoparticles and pure organic phosphorescent nanocrystals by saponin encapsulation. J Am Chem Soc. 2017;139:14792–14799. doi: 10.1021/jacs.7b08710. [DOI] [PubMed] [Google Scholar]
- 22.Zhang G, Palmer GM, Dewhirst MW, Fraser CL. A dual-emissive-materials design concept enables tumour hypoxia imaging. Nat Mater. 2009;8:747–751. doi: 10.1038/nmat2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu CY, et al. Mitochondrion-anchoring photosensitizer with aggregation-induced emission characteristics synergistically boosts the radiosensitivity of cancer cells to ionizing radiation. Adv Mater. 2017;29:160617. doi: 10.1002/adma.201606167. [DOI] [PubMed] [Google Scholar]
- 24.Ding D, Li K, Liu B, Tang BZ. Bioprobes based on AIE fluorogens. Acc Chem Res. 2013;46:2441–2453. doi: 10.1021/ar3003464. [DOI] [PubMed] [Google Scholar]
- 25.Feng G, Liu B. Aggregation-induced emission (AIE) dots: Emerging theranostic nanolights. Acc Chem Res. 2018;51:1404–1414. doi: 10.1021/acs.accounts.8b00060. [DOI] [PubMed] [Google Scholar]
- 26.Cheng X, Zhang Y, Jónsson E, Jónsson H, Weber PM. Charge localization in a diamine cation provides a test of energy functionals and self-interaction correction. Nat Commun. 2016;7:11013. doi: 10.1038/ncomms11013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vlček A, Jr, Kvapilová H, Towrie M, Záliš S. Electron-transfer acceleration investigated by time resolved infrared spectroscopy. Acc Chem Res. 2015;48:868–876. doi: 10.1021/ar5004048. [DOI] [PubMed] [Google Scholar]
- 28.Coppens P, Novozhilova I, Kovalevsky A. Photoinduced linkage isomers of transition-metal nitrosyl compounds and related complexes. Chem Rev. 2002;102:861–884. doi: 10.1021/cr000031c. [DOI] [PubMed] [Google Scholar]
- 29.Fermi A, Bergamini G, Roy M, Gingras M, Ceroni P. Turn-on phosphorescence by metal coordination to a multivalent terpyridine ligand: A new paradigm for luminescent sensors. J Am Chem Soc. 2014;136:6395–6400. doi: 10.1021/ja501458s. [DOI] [PubMed] [Google Scholar]
- 30.Wu H, et al. Helical self-assembly-induced singlet-triplet emissive switching in a mechanically sensitive system. J Am Chem Soc. 2017;139:785–791. doi: 10.1021/jacs.6b10550. [DOI] [PubMed] [Google Scholar]
- 31.Fermi A, et al. Molecular asterisks with a persulfurated benzene core are among the strongest organic phosphorescent emitters in the solid state. Dyes Pigm. 2014;110:113–122. [Google Scholar]
- 32.Kwon MS, et al. Suppressing molecular motions for enhanced room-temperature phosphorescence of metal-free organic materials. Nat Commun. 2015;6:8947. doi: 10.1038/ncomms9947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baroncini M, Bergamini G, Ceroni P. Rigidification or interaction-induced phosphorescence of organic molecules. Chem Commun (Camb) 2017;53:2081–2093. doi: 10.1039/c6cc09288h. [DOI] [PubMed] [Google Scholar]
- 34.Cardolaccia T, Li Y, Schanze KS. Phosphorescent platinum acetylide organogelators. J Am Chem Soc. 2008;130:2535–2545. doi: 10.1021/ja0765316. [DOI] [PubMed] [Google Scholar]
- 35.Chen P, et al. Intramolecular electron transfer in the reductive chromophore-quencher complex [bpy)Re(CO)3(py-PTZ)]+ Inorg Chem. 1987;26:1116–1126. [Google Scholar]
- 36.Bjorkas C, Nordlund K, Caturla MJ. Influence of the picosecond defect distribution on damage accumulation in irradiated α-Fe. Phys Rev B Condens Matter Mater Phys. 2012;85:024105. [Google Scholar]
- 37.Ree J, Kim YH, Shina HK. Collision-induced intramolecular energy flow and C–H bond dissociation in excited toluene. J Chem Phys. 2002;116:4858–4870. [Google Scholar]
- 38.Wu H, et al. Molecular stacking dependent phosphorescence-fluorescence dual emission in a single luminophore for self-recoverable mechanoconversion of multicolor luminescence. Chem Commun (Camb) 2017;53:2661–2664. doi: 10.1039/c6cc04901j. [DOI] [PubMed] [Google Scholar]
- 39.Yang J, et al. The influence of the molecular packing on the room temperature phosphorescence of purely organic luminogens. Nat Commun. 2018;9:840. doi: 10.1038/s41467-018-03236-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gu L, et al. Dynamic ultralong organic phosphorescence by photoactivation. Angew Chem Int Ed Engl. 2018;57:8425–8431. doi: 10.1002/anie.201712381. [DOI] [PubMed] [Google Scholar]
- 41.Zhao Q, Huang C, Li F. Phosphorescent heavy-metal complexes for bioimaging. Chem Soc Rev. 2011;40:2508–2524. doi: 10.1039/c0cs00114g. [DOI] [PubMed] [Google Scholar]
- 42.Botchway SW, et al. Time-resolved and two-photon emission imaging microscopy of live cells with inert platinum complexes. Proc Natl Acad Sci USA. 2008;105:16071–16076. doi: 10.1073/pnas.0804071105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhen X, et al. Ultralong phosphorescence of water-soluble organic nanoparticles for in vivo afterglow imaging. Adv Mater. 2017;29:1606665. doi: 10.1002/adma.201606665. [DOI] [PubMed] [Google Scholar]
- 44.Zhou Y, et al. High-contrast flicker luminescence on dynamic covalent structure based nanoaggregates. Sci China Chem. 2019;62:220–225. [Google Scholar]
- 45.Xu G, et al. Autonomous fluorescence regulation in responsive polymer systems driven by a chemical oscillating reaction. Polym Chem. 2016;7:3211–3215. [Google Scholar]
- 46.Frisch MJ, et al. 2010. Gaussian 09 (Gaussian Inc., Wallingford, CT), Revision C.01.
- 47.Cossi M, Rega N, Scalmani G, Barone V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J Comput Chem. 2003;24:669–681. doi: 10.1002/jcc.10189. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.