Significance
Amino acids are formed from simple organic precursors in iron oxyhydroxide mineral systems that contain geochemical gradients. Redox and pH gradients significantly impact reaction pathways: Amino acids only form when the mineral contains both oxidized and reduced iron, and when the surrounding solution is alkaline. This shows that aqueous, partially reducing iron mineral systems (which would have been common in early-Earth seafloor/vent environments) could have facilitated synthesis and concentration of prebiotic organic molecules relevant for the emergence of life. It also suggests that geochemical gradients in vent environments can drive product selectivity for prebiotic chemistry, perhaps leading to more complex organic reaction systems as these molecules continue to diffuse and react under different conditions within the gradients.
Keywords: life emergence, iron hydroxides, hydrothermal vents, early Earth, gradients
Abstract
Iron oxyhydroxide minerals, known to be chemically reactive and significant for elemental cycling, are thought to have been abundant in early-Earth seawater, sediments, and hydrothermal systems. In the anoxic Fe2+-rich early oceans, these minerals would have been only partially oxidized and thus redox-active, perhaps able to promote prebiotic chemical reactions. We show that pyruvate, a simple organic molecule that can form in hydrothermal systems, can undergo reductive amination in the presence of mixed-valence iron oxyhydroxides to form the amino acid alanine, as well as the reduced product lactate. Furthermore, geochemical gradients of pH, redox, and temperature in iron oxyhydroxide systems affect product selectivity. The maximum yield of alanine was observed when the iron oxyhydroxide mineral contained 1:1 Fe(II):Fe(III), under alkaline conditions, and at moderately warm temperatures. These represent conditions that may be found, for example, in iron-containing sediments near an alkaline hydrothermal vent system. The partially oxidized state of the precipitate was significant in promoting amino acid formation: Purely ferrous hydroxides did not drive reductive amination but instead promoted pyruvate reduction to lactate, and ferric hydroxides did not result in any reaction. Prebiotic chemistry driven by redox-active iron hydroxide minerals on the early Earth would therefore be strongly affected by geochemical gradients of Eh, pH, and temperature, and liquid-phase products would be able to diffuse to other conditions within the sediment column to participate in further reactions.
The synthesis of biomolecules, particularly amino acids and their condensation into peptides, from geochemical carbon and nitrogen sources is an important research topic for assessing the role of specific geochemical environments and mineral phases in the emergence of life. One mineral type of interest that would have been abundant in the mildly acidic, iron-rich oceans of the early Earth (1–3) is the iron oxyhydroxides, which can precipitate in a variety of stable or metastable redox states (4, 5). Iron oxides/oxyhydroxides are versatile reactive minerals that can drive redox reactions and concentrate phosphorus species, trace metals, organic molecules, and other anions (6–11). On the early Earth, iron oxyhydroxides and/or green rust would likely have been present in the water column as well as seafloor sediments, playing a fundamental role in elemental cycling and redox chemistry (4, 10). Iron oxyhydroxides would also have been a primary component in alkaline hydrothermal vent mounds and chimneys, which have been proposed as a possible environment for the emergence of metabolism due to their ambient pH, Eh, ion/chemical, and temperature gradients (12–15).
Seafloor hydrothermal sediments and chimneys are flow-through gradient systems that combine reactive minerals with organic compounds in a variety of possible reaction conditions. Alkaline vents produced by serpentinization on the early Earth could generate pH gradients from ∼5.5 to 6.5 in the ocean to ∼9 to 11 in the alkaline hydrothermal fluid, and temperatures could range from the cold seafloor to the warm ∼70 to 90 °C vent interior (2, 16). In modern alkaline vent systems the carbonate chimneys contain brucite Mg(OH)2 (16), but in a mildly acidic early-Earth ocean rich in dissolved Fe2+ (with minor Fe3+) chimneys as well as associated sediments would have contained a combination of iron oxyhydroxides that also adopt a brucite structure (along with sulfides) with varying Fe(II):Fe(III) ratios (1, 13, 17). Redox-active minerals such as iron or iron/nickel sulfides found in hydrothermal systems can abiotically reduce carbon-containing substrates to form formate, acetate, pyruvate, amino acids and peptides, α-hydroxy acids (αHAs), methanol, and/or methane (18–24). It has also been shown that iron oxides/oxyhydroxides and/or green rust are capable of reducing nitrate and nitrite to nitrous oxide or ammonia (8, 25, 26). Ammonia may thus have been present in early Earth’s oceans and hydrothermal vent systems, being ultimately sourced from mineral-catalyzed reduction of atmospheric N2 and/or gas-phase nitrogen oxides (i.e., NOx species) (27–30).
In this work we attempted reductive amination reactions in the presence of a precursor carbonyl compound and ammonia, driven by mixed-valence iron oxyhydroxides similar to those likely to be found in early-Earth oceans, hydrothermal vents, and sediments. We focused on reactions of pyruvate, which is of particular relevance for the emergence of metabolism since it is a key intermediate in various metabolic pathways. It has been shown that pyruvate and other α-keto acids can react in the presence of transition metal sulfide minerals to form a variety of products including lactate, β-hydroxy ketones, thiols (in the presence of sulfide), and the amino acid alanine (in the presence of ammonia) (31–33). Novikov and Copley (31) showed that, even when ammonia was present, the yield of alanine synthesis from pyruvate (and whether alanine formed at all in favor of some other product) was dependent on which mineral was present. Reductive amination of α-keto acids in the presence of reduced iron hydroxide was also reported for the production of phenylalanine (32); however, reductive amination of pyruvate driven by mixed valence or ferric iron hydroxide has not been reported.
We also tested the effect of the pH, redox, and temperature gradients in iron oxyhydroxide precipitates, keeping in mind that in a chemical diffusive system like a seafloor sediment or hydrothermal mound, products formed in one location may diffuse to another location with different conditions to carry out further reactions (34–36). To simulate reactions at different locations in wet sediments subject to chemical gradients, we reacted pyruvate and ammonia in closed vials containing freshly precipitated iron hydroxide. We explored the effect of geochemical gradients by using the individual vial experiments to represent different points within a sediment column: varying the mole fraction of Fe(II) relative to total Fe in the mineral precipitate from 0 to 100%, the pH between 5 and 11, and temperature from 25 to 80 °C. We also investigated reactions that might occur in a hydrothermal chimney structure containing mixed-valence iron oxyhydroxides by growing simulated chimneys in the laboratory; this technique resulted in gradients being created within a single experiment (Fig. 1). Organic products were analyzed using liquid 1H NMR spectroscopy and mineral samples were analyzed by anaerobic X-ray diffraction and colorimetry. Under the conditions we explored—all of which contained ammonia but did not include sulfide—we identified two main reaction products, alanine and lactate (along with minor acetate concentrations) (Fig. 2). The relative consumption of pyruvate to produce lactate and/or alanine was dependent upon the oxidation state of the iron hydroxide mineral precipitate, the pH, and the temperature. These experiments provide a basis for understanding how reductive amination may be driven in seafloor systems by minerals, and how ambient geochemical gradients can drive selectivity for various products.
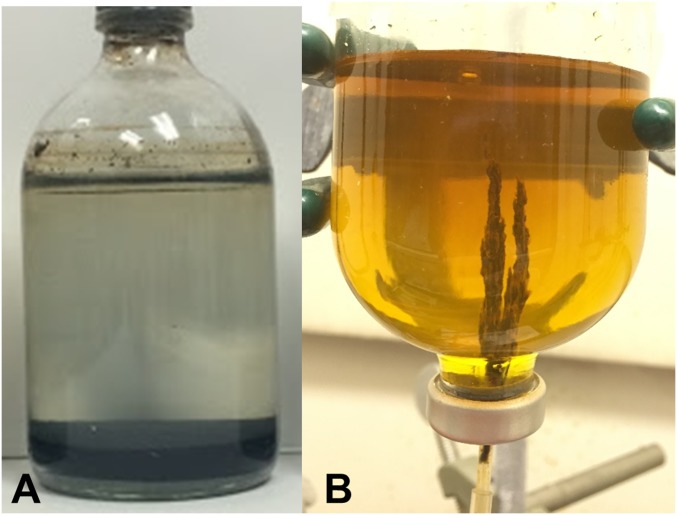
Fig. 1.
Iron hydroxide precipitates simulating seafloor sediment and hydrothermal chimneys. (A) Vial of freshly precipitated iron hydroxides [Fe(II):Fe(III) = 2:1] reacting with pyruvate and ammonia after 72 h. (B) Simulated hydrothermal chimney precipitated from a hydrothermal fluid simulant (containing pyruvate and NaOH) into an early-Earth ocean simulant containing Fe [Fe(II):Fe(III) = 2:1] and ammonia; photo taken after 4 h.
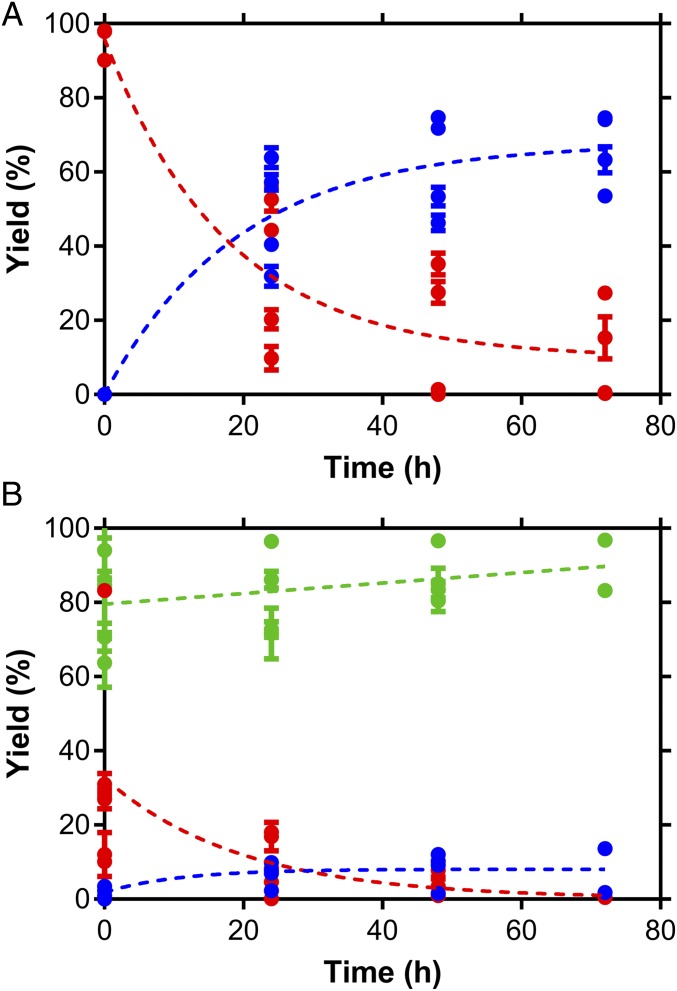
Fig. 2.
Reductive amination of pyruvate to alanine and/or reduction of pyruvate to lactate in the presence of iron hydroxides. The relative yields of each product depended on the pH and Fe(II):Fe(III) ratio in the iron hydroxide precipitate.
Experimental Considerations for Reacting Iron Oxyhydroxides at Gradient Conditions
Reacting Pyruvate with Ammonia in the Presence of Iron Oxyhydroxides.
Reactions were carried out in a nitrogen-purged glove box to simulate the anoxic conditions of the early Earth. Iron [Fe(II) and/or Fe(III)] was added as chloride salts in the desired mole fraction of Fe(II):Fe(III) along with pyruvate and ammonia into a glass vial of Milli-Q water (18.2 MΩ·cm) that had been sparged with argon to remove dissolved oxygen. Sodium hydroxide was then added slowly to precipitate with the dissolved Fe2+ and/or Fe3+ as iron hydroxides and to adjust the pH. Relative concentrations of reactants were chosen to be consistent with the experiments of Huber and Wächtershäuser (32), who previously attempted this reaction with a purely ferrous hydroxide precipitate. This coprecipitation method resulted in a ∼1- to 2-cm-thick layer of green, brown, or red precipitate (depending on the ratio of Fe2+ to Fe3+) at the bottom of the vial (Fig. 1 and SI Appendix, Fig. S1). The vials were left at room temperature or placed in a hot water bath for 72 h, and samples were taken at 24-h intervals. The reactions were allowed to proceed without external mixing, since this is analogous to seafloor processes where precipitates settle out of the water column to form layers of sediments through which fluids slowly percolate and gradients can persist. However, we observed that agitating the reaction mixture did not significantly affect the results. For sampling, the vial was agitated to ensure a constant liquid:solid ratio between samples; after sampling the precipitates were returned to the hot water bath. The samples were centrifuged and the supernatant was transferred to another tube, treated with additional NaOH to remove any remaining iron, and analyzed with liquid 1H NMR. The concentrations of pyruvate and its products are expressed as relative to the total concentration of all detectable organic species in the liquid phase (pyruvate + lactate + alanine + acetate), calculated using the area of the methyl region peaks. The identity of alanine, lactate, and pyruvate in a representative reaction mixture [1:1 Fe(II):Fe(III), pH = 10, temperature = 70 °C, t = 72 h] was confirmed by high-resolution mass spectrometry (SI Appendix).
We also performed experiments in which the pyruvate reaction was conducted in a vial containing a laboratory-grown hydrothermal chimney of iron hydroxides, to determine whether the self-assembling structure of the precipitate had any effect on the reaction. An inverted glass vial was used as the chimney vessel (37). An alkaline solution (representing the hydrothermal fluid) was injected with a syringe into a solution containing dissolved Fe2+ and/or Fe3+ (representing dissolved iron in the early ocean); pyruvate and ammonia were added to the ocean and hydrothermal solutions in different combinations. The alkaline solution was slowly injected over 24 to 48 h, and then the vial was drained and the liquid and the chimney precipitates were sampled. This chimney experiment was repeated at room temperature and in a hot water bath at 70 °C.
Control Reactions.
Control experiments were performed to confirm that synthesis of alanine and/or lactate was occurring from pyruvate only and that it required the presence of the mineral precipitate. In experiments where pyruvate was not included, or in experiments where only soluble Fe2+ was present along with pyruvate, alanine and lactate were not observed. In experiments where ammonia was not included, only lactate was observed. When alanine or lactate (instead of pyruvate) were added to an experiment containing minerals precipitated at various Fe(II):Fe(III) ratios, no reaction was observed, and significant adsorption of alanine or lactate into the mineral was not observed. In summary, a system where external pyruvate, ammonia, and iron-containing precipitate are all present is required to produce alanine.
Results and Discussion
Alanine and Lactate Synthesis from Pyruvate.
The reactivity of pyruvate over 72 h varied depending on both the experiment pH and the Fe(II):Fe(III) ratio in the mineral precipitate (Fig. 3). Under certain conditions no products were observed and only pyruvate was recovered at the end of the experiment. Under some conditions, only reduction of pyruvate to lactate was observed, and under other conditions synthesis of alanine was observed along with lactate. The condition that produced the highest yield of alanine (∼70%) along with ∼30% lactate was pH 10, Fe(II):Fe(III) = 1:1, and 70 °C, although high alanine yields were observed in various experiments around these values. At 70 °C, a reasonable condition for a warm alkaline vent environment, the reaction took between 48 and 72 h to reach completion under conditions where both alanine and lactate were synthesized. At lower temperatures (50 °C) the same reactions proceeded but more slowly, and at room temperature very little pyruvate reactivity was observed. Once the pyruvate was consumed and the alanine and lactate were formed, we did not observe any significant decrease of alanine or lactate over the rest of the sampling period; therefore, it is likely that these components, if formed in a prebiotic environment, would not be degraded rapidly and could become concentrated for subsequent reactions. Lactate synthesis, when it occurred, was highly efficient. When the precipitate was formed with 90 to 100% ferrous iron, most of the pyruvate had already converted to lactate even in the t = 0 sample at pH 9.2 (Fig. 3B). In another case (75% Fe(II), pH 7; SI Appendix, Fig. S5) no alanine was produced but ∼30% lactate was present in the t = 0 sample, eventually increasing to 30 to 50% lactate after 72 h. Meanwhile, alanine synthesis, when it occurred, was usually first observed at 24 h and was not complete until 48 h; alanine was not observed in the t = 0 sample of any experiment.
Fig. 3.
Pyruvate reactions in the presence of ammonia and freshly precipitated iron hydroxides as a function of time. Experiments conducted at 70 °C are shown; pH and Fe(II) mole fraction in the mineral were varied: (A) 66% Fe2+, pH 10 (lactate yield shown in SI Appendix, Fig. S3) and (B) 90% Fe2+, pH 9.2. The dotted line represents a least squares (ordinary) fit using a one phase decay model (i.e., first-order decay model; the exponential term can be used to estimate the half-life of the process). Each marker on the graph is the mean of three replicate measurements and the error bars consist of the SE on the mean.
Acetate was observed in most experiments in <5% yield but did not increase or decrease with time and was also present in pyruvate control samples, so it was concluded to be a product of decarboxylation of the pyruvate reagent and not a reaction product. The SE of triplicate samples taken from the same experiment at a given time point was usually low, since samples were agitated to ensure a homogenous distribution of solid/liquid while sampling. However, the average yields of products observed in different experiments varied sometimes up to 10 to 15% between repeated experiments at the same conditions. The variability in reaction yield between repeated experiments likely depends on a number of unpredictable factors resulting from these nonequilibrium conditions such as particle size distribution and morphology of the aggregates. It is possible that this observation is transferrable to natural systems, such as sediments or vents, where factors like available mineral surface area and the permeability of the resulting sediments potentially affect reaction rates.
We also tested whether oxaloacetate in place of pyruvate could react with iron hydroxides to produce aspartic acid, which is more likely to concentrate in these minerals (38). However, at the conditions tested [70 °C, 66% Fe(II), pH 9] only pyruvate, alanine, and lactate were observed, indicating that oxaloacetate rapidly decarboxylated to pyruvate under these reaction conditions.
Effect of Iron Oxidation State of the Mineral Precipitate.
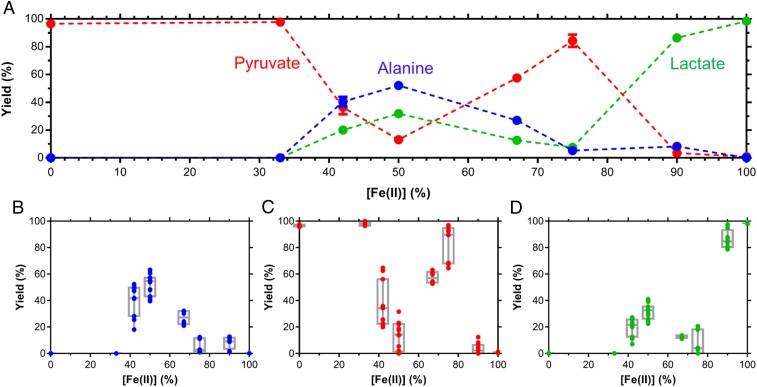
The ratio of Fe(II):Fe(III) in the iron hydroxide precipitate had a dramatic effect on the reactions of pyruvate in this system. Even though ammonia was present at the same concentration in all experiments, alanine was only produced when the iron hydroxide mineral was partially oxidized. Fig. 4 shows results from experiments at pH 9, 70 °C, at 48 h, for a range of iron hydroxide oxidation states. When the iron minerals were mostly oxidized [0 to 30% Fe(II)] pyruvate did not appear to react at all, and when the iron hydroxides were completely reduced [100% Fe(II)] all of the pyruvate was quickly consumed to form lactate. However, at intermediate mineral oxidation states alanine was produced as well as lactate, with alanine yield at its highest when the iron hydroxide mineral contained about 50% Fe(II). Interestingly, the alanine yield dropped off as the percent Fe(II) in the mineral increased from this point, until at 90% Fe(II) the pyruvate was almost all consumed in the t = 0 sample to form lactate, and the remaining pyruvate reacted to form alanine over the next 48 h. The competition between alanine and lactate production therefore is highly dependent on the Fe(II) composition in the simulated mineral.
Fig. 4.
Pyruvate reactions after 48 h at pH 9.2 and 70 °C as a function of the Fe(II) vs. Fe(III) mole fraction of the iron hydroxide precipitate. (A) Means and SEM for alanine (blue), pyruvate (red), and lactate (green) over the range of Fe(II) mole fraction tested. (B–D) Plots of analyte reaction yields (B: alanine; C: pyruvate; D: lactate) with values from individual experiments superimposed; the box extends from the 25th to 75th percentiles, with the horizontal line in the box representing the median; whiskers represent the lowest and highest datum.
The effect of the Fe(II) mole fraction in the hydroxide mineral on pyruvate reactivity can be explained by the variety of mineral phases that could form under these conditions. Depending on the specific chemical conditions, precipitates in our experiments may be composed of various iron minerals including ferrous hydroxide Fe(OH)2, goethite (α-FeOOH), akaganeite (β-FeOOH), magnetite (Fe3O4), chloride green rusts GR(Cl−), lepidocrocite (γ-FeOOH), and/or ferric oxide (39–41). The precipitation of magnetite would be expected from precipitation of Fe2+ and Fe3+ at Fe2+:Fe3+ = 1:2, which is also the beginning of our range of observed alanine synthesis; green rust can form at >33% Fe2+ but can also transform to magnetite (42, 43). We analyzed a precipitate from one of the experiments that gave the maximum yield of alanine [50% Fe(II)/pH 9.2/70 °C] and observed magnetite and ferric oxide (SI Appendix). However, these precipitates are extremely sensitive to even small amounts of oxygen and this result may not be representative of the actual mineralogy at the time of the reaction. The ratio of Fe(II):Fe(III) in an iron oxyhydroxide precipitate in an early-Earth ocean or seafloor system could vary, since even though the dissolved iron in the early anoxic ocean was likely mostly Fe2+, some oxidation to Fe3+ could occur (17). Early-Earth seafloor sediments or hydrothermal mounds could have contained any or all of these minerals [and green rusts in a natural system might have formed GR(CO32−) as well as GR(Cl−) due to dissolved oceanic CO2]. The conversion of pyruvate to alanine rather than lactate (or, whether any reaction occurs at all) would be highly dependent on the mineral oxidation state and other environmental factors. Thus, in a heterogeneous sediment column or hydrothermal mound subject to a redox gradient, both alanine and lactate products as well as unreacted pyruvate would be expected.
Iron sulfides have also been shown to be capable of driving reductive amination of α-keto acids to form amino acids (31, 32). However, Huber and Wächtershäuser (32) found that amino acid synthesis did not occur with freshly precipitated sulfides of other metals including Zn, Mn, Co, Cu, and Ni and that replacing the Fe(II) with Ni(II) in sulfide precipitates simply decreased the amino acid yield proportional to the decreased concentration of Fe. We also did an experiment in which half of the Fe(II) was replaced with Ni(II) (at a condition of [Fe(II) + Ni(II)]/[Fe(II) + Ni(II) + Fe(III)] = 75% or 66%, at 75 °C and pH 9). We observed a similar effect in that adding Ni did not increase the yield of alanine, and in fact no pyruvate reaction was observed at all. The remaining Fe(II) in this case would have been about 33 to 37% of the total [Fe + Ni], which is in the iron oxidation range where we typically did not observe any reaction of pyruvate (Fig. 4).
Effect of pH.
Alkaline conditions strongly favored the formation of alanine (along with lactate), whereas neutral conditions only favored lactate production. The effect of pH in the 7–11 range was tested at 66% Fe(II) and 75% Fe(II) at 70 °C. At 66% Fe(II), about a third of the pyruvate was rapidly consumed to form lactate and then no further reaction of pyruvate was observed. At 75% Fe(II), after the initial lactate production, slightly more pyruvate reacted to form lactate over 72 h (yielding up to ∼50% lactate eventually). Above pH 9 in experiments with 66% Fe(II), alanine was produced as well as lactate, with alanine relative concentrations increasing steeply between pH 9 and 10 (SI Appendix, Fig. S8). Above pH 10, the reaction proceeded more quickly and within 24 h nearly all of the pyruvate was consumed to form alanine and lactate. Similar trends were observed for 75% Fe(II), with an increase in alanine formation between pH 9 and 10, although at this Fe(II) content much of the pyruvate did not react at all. The increase in alanine formation with pH was anticipated given the pKa of ammonium at 9.24; above this pH more ammonia (NH3) than ammonium (NH4+) is present and would favor amino acid formation since ammonia is a strong nucleophile. Even if ammonia were present throughout a permeable sediment column or hydrothermal mound containing iron oxyhydroxides, amino acid formation from pyruvate would probably only be favored near a sufficiently alkaline condition such as a vent fluid.
Effect of Temperature.
Under conditions where alanine and lactate production were favored [50% Fe(II), pH 9.2], we tested the effect of temperature between room temperature and 80 °C (SI Appendix, Fig. S9). At room temperature, only about 15% of the pyruvate had reacted after 72 h (to form ∼4 to 7% alanine and lactate). However, at 50 °C and above, more pyruvate reacted and after 72 h ∼60% alanine had formed. As temperature increased above 50 °C, the yield of alanine did not change significantly; although the pyruvate was consumed faster at high temperature, by 72 h the yield was generally the same. However, unlike alanine, the total yield of lactate after 72 h did appear to increase slightly with temperature. These observations indicate that, in a seafloor system, only moderate to warm temperatures are required for pyruvate to react to form alanine and/or lactate, and the reaction could occur in various places in a thermal gradient between seawater and hydrothermal fluid as long as the temperature is above about 50 °C. The amount of pyruvate consumed in the experiment after 72 h increased significantly from 25 to 60 °C, and at higher temperatures it would probably all be reacted in under 72 h. It is reasonable to expect that pyruvate near a high-temperature, alkaline condition containing partially oxidized iron oxyhydroxides would all be converted to alanine and lactate.
Hydrothermal Chimney Simulations.
In natural vent systems, hydrothermal chimneys can form large and self-organized structures, containing networks of pores and/or redox-active minerals across which the gradients between vent fluid and seawater are focused. Previous work on simulating prebiotic hydrothermal chimneys has focused on factors such as carbon fixation reactions and incorporation and reactivity of organics in the chimney (19, 20, 37, 44). We attempted pyruvate reactions in experiments where iron hydroxides were precipitated in a hydrothermal chimney structure instead of in simulated sediment. Whereas sediment experiments were each at constant condition representing a point within a larger gradient, the chimney experiment maintains gradients across the precipitate in a single vial. Chimneys were grown via injection of an alkaline solution (representing a hydrothermal fluid) into an acidic solution containing dissolved Fe2+ and/or Fe3+ (representing the anoxic iron-rich early ocean) so that a chemical garden structure grew at the injection point (Fig. 1B). Various ocean and hydrothermal simulant configurations of fluid chemistry, injection rates, and volumes were tested in an attempt to synthesize chimneys which were analogous to the batch precipitation experiments in terms of precipitate composition and total precipitate volume, and which would generate observable products of lactate and/or alanine in 72 h (SI Appendix, Table S2). In chimney experiments, we sometimes observed lactate, but never alanine synthesis. This could be due to several factors, including that simulated chimneys are only a few centimeters high and there is not very much precipitate to react with pyruvate and ammonia compared with the much higher amount of solids present in the sediment experiments. Also, since the chimney is a flow-through system, pyruvate may have a shorter contact time with the minerals than in sediment experiments. Our simulated chimneys were small and quickly formed to perform experiments on reasonable laboratory timescales. However, hydrothermal chimneys in geological settings can persist for hundreds of thousands of years and grow to be tens of meters in height (45, 46). In a real chimney, there would be more mineral surface area interacting with reactants for longer periods of time and it is possible that reactions would occur that were not observable in our laboratory simulations.
Summary
Iron oxyhydroxide precipitates in aqueous systems are highly reactive for driving reactions of pyruvate, and partial oxidation of the iron hydroxide mineral was effective for promoting reductive amination to alanine. Redox, pH, and temperature gradients in the iron oxyhydroxide systems greatly affected the relative yield of products including amino acid and αHA formation. Alanine synthesis from pyruvate strongly depended on the mole fractions of Fe(II) and Fe(III) in the iron hydroxide precipitate, yielding a maximum of alanine production at a 1:1 Fe(II):Fe(III) proportion. No reaction of pyruvate was observed at all in cases where the iron hydroxide was completely oxidized, while only lactate formed in cases where it was completely reduced. In the pH gradient, only lactate formed at neutral pH, whereas alanine as well as lactate were formed at alkaline pH (at and above the pKa of ammonia at ∼9.25). Although some alanine and lactate formation occurred at room temperature (in the 72-h reaction period), at and above 50 °C substantial amounts of alanine and lactate were produced, indicating that pyruvate reactions would be accelerated in hydrothermally warmed settings. Reactive iron oxyhydroxide precipitates were required to stoichiometrically, and perhaps catalytically, drive the reactions.
The precise mechanism of pyruvate reductive amination to alanine in these systems was not established. Products from reductive amination reactions vary greatly in diversity and yield depending on experimental conditions and the properties and selectivity of the catalyst (47, 48). The iron oxyhydroxide precipitates in our experiments are able to drive reductive amination under mild conditions, at relatively low temperatures, and without the addition of H2. In our sediment experiments the mineral surface area was measured to be ∼51 m2/g and particle size ∼1 to 2 μm (for a representative experiment at the conditions corresponding to the maximum alanine yield). Also, the total mass of iron precipitates in a typical experiment compared with the 2.5 mM pyruvate initially added means that carbon content of the precipitate would only be at trace levels (we measured ∼0.37% C in the solids in one representative experiment). In a natural sediment system or hydrothermal mound this would likely be true as well, with particle size and reactive surface area being factors that could affect organic content depending on locations within the geochemical gradients. Other likely parameters favoring the high yields of alanine in our experiments include the relative excess of ammonia to pyruvate, our use of water as the solvent, and the alkaline conditions, all of which have been shown in reductive amination studies to favor the formation of primary as opposed to secondary or tertiary amines (47). Ammonia in natural prebiotic seafloor/vent systems, being sourced by reduction of N2 or NOx species, may not have been in such excess compared with pyruvate as it is in our experiments and thus the yield of amino acids compared with other products under early-Earth conditions may have been lower.
There was an incomplete pyruvate mass balance in many of these experiments which may be trapped in the solid phase, perhaps as intermediates bound to surfaces. However, the reactants and products detected are soluble in water, meaning that in a permeable sediment column or hydrothermal mound they might be able to diffuse to another site within the gradient and undergo subsequent reactions under different conditions. The production of lactate in many of our experiments is also interesting for prebiotic chemistry since αHAs can enable peptide bond formation, and even form long polymers themselves (49, 50). Different amino acids/αHAs or their precursors may interact more strongly with hydrothermal mineral surfaces (38, 51, 52) and/or may have different stabilities at these moderate hydrothermal temperatures (53). For other amino acids, it is also possible that the selectivity of the iron oxyhydroxide minerals for different precursors or ammonia sources could affect the relative yield of the amine and/or other products; this, as well as the effect of ammonia concentration, remains to be investigated in future work. In summary, iron oxyhydroxide minerals precipitated in early-Earth conditions promote reactions of pyruvate including reductive amination to alanine and reduction to lactate. The geochemical redox, pH, and temperature gradients affect product selectivity and therefore must be considered in gauging the likelihood of amino acid synthesis from simple precursors in these systems, as well as their stability and retention for other prebiotic processes.
Materials and Methods
Pyruvate (as Na-pyruvate) and ammonia (as NH4Cl) were reacted with either freshly precipitated iron oxyhydroxides or simulated hydrothermal chimneys of iron hydroxide for 72 h. Iron oxyhydroxide precipitates were formed by precipitating dissolved FeCl2•4H2O and/or FeCl3•6H2O with NaOH; the sediment experiments were carried out at various ratios of Fe(II)/Fe(III) and titrated to various pH values and heated in a water bath. Pyruvate and its products were analyzed with liquid 1H NMR. Detailed descriptions of experimental and analytical methods are described in SI Appendix.
Supplementary Material
Acknowledgments
We thank Yeghegis Abedian, Kayo Kallas, Liz Miller, Ivria Doloboff, Vince Aguirre, Simonne Jocic, and Amalia E. Castonguay for assistance with experimentation and analysis, and Niklas Thompson for helpful discussions. This research was carried out at the Jet Propulsion Laboratory, California Institute of Technology, under a contract with NASA, supported by the NASA Astrobiology Institute Icy Worlds.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1812098116/-/DCSupplemental.
References
- 1.MacLeod G, McKeown C, Hall AJ, Russell MJ. Hydrothermal and oceanic pH conditions of possible relevance to the origin of life. Orig Life Evol Biosph. 1994;24:19–41. doi: 10.1007/BF01582037. [DOI] [PubMed] [Google Scholar]
- 2.Halevy I, Bachan A. The geologic history of seawater pH. Science. 2017;355:1069–1071. doi: 10.1126/science.aal4151. [DOI] [PubMed] [Google Scholar]
- 3.Tosca NJ, Guggenheim S, Pufahl PK. An authigenic origin for Precambrian greenalite: Implications for iron formation and the chemistry of ancient seawater. Geol Soc Am Bull. 2016;128:511–530. [Google Scholar]
- 4.Halevy I, Alesker E, Schuster EM, Popovitz-Biro R, Feldman Y. A key role for green rust in the Precambrian oceans and the genesis of iron formations. Nat Geosci. 2017;10:135–139. [Google Scholar]
- 5.Jolivet JP, Chanéac C, Tronc E. Iron oxide chemistry. From molecular clusters to extended solid networks. Chem Commun (Camb) 2004:481–487. doi: 10.1039/b304532n. [DOI] [PubMed] [Google Scholar]
- 6.Arrhenius GO. Crystals and life. Helv Chim Acta. 2003;86:1569–1586. [Google Scholar]
- 7.Barthélémy K, Naille S, Despas C, Ruby C, Mallet M. Carbonated ferric green rust as a new material for efficient phosphate removal. J Colloid Interface Sci. 2012;384:121–127. doi: 10.1016/j.jcis.2012.06.038. [DOI] [PubMed] [Google Scholar]
- 8.Hansen HCB, Borggaard OK, Sorensen J. Evaluation of the free energy of formation of Fe(II)-Fe(III) hydroxide-sulphate (green rust) and its reduction of nitrite. Geochim Cosmochim Acta. 1994;58:2599–2608. [Google Scholar]
- 9.Chaves LHG, Curry JE, Stone DA, Chorover J. Fate of nickel ion in (II-III) hydroxysulphate green rust synthesized by precipitation and coprecipitation. Rev Bras Ciênc Solo. 2007;31:813–818. [Google Scholar]
- 10.Zegeye A, et al. Green rust formation controls nutrient availability in a ferruginous water column. Geology. 2012;40:599–602. [Google Scholar]
- 11.Trolard F, Bourrié G. Fougerite a natural layered double hydroxide in clay soil: Habitus, structure, and some properties. In: Valaškova M, Martynkova GS, editors. Clay Minerals in Nature: Their Characterization, Modification and Application. InTech, Rijeka; Croatia: 2012. pp. 171–188. [Google Scholar]
- 12.Martin W, Baross J, Kelley D, Russell MJ. Hydrothermal vents and the origin of life. Nat Rev Microbiol. 2008;6:805–814. doi: 10.1038/nrmicro1991. [DOI] [PubMed] [Google Scholar]
- 13.Russell MJ, Hall AJ. The onset and early evolution of life. In: Kesler SE, Ohmoto H, editors. Evolution of Early Earth’s Atmosphere, Hydrosphere, and Biosphere—Constraints from Ore Deposits. Vol 198. Geological Soc of America; Boulder, CO: 2006. pp. 1–32. [Google Scholar]
- 14.Russell MJ, et al. The drive to life on wet and icy worlds. Astrobiology. 2014;14:308–343. doi: 10.1089/ast.2013.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sojo V, Herschy B, Whicher A, Camprubí E, Lane N. The origin of life in alkaline hydrothermal vents. Astrobiology. 2016;16:181–197. doi: 10.1089/ast.2015.1406. [DOI] [PubMed] [Google Scholar]
- 16.Kelley DS, et al. A serpentinite-hosted ecosystem: The Lost City hydrothermal field. Science. 2005;307:1428–1434. doi: 10.1126/science.1102556. [DOI] [PubMed] [Google Scholar]
- 17.Shibuya T, Russell MJ, Takai K. Free energy distribution and hydrothermal mineral precipitation in Hadean submarine alkaline vent systems: Importance of iron redox reactions under anoxic conditions. Geochim Cosmochim Acta. 2016;175:1–19. [Google Scholar]
- 18.Roldan A, et al. Bio-inspired CO2 conversion by iron sulfide catalysts under sustainable conditions. Chem Commun (Camb) 2015;51:7501–7504. doi: 10.1039/c5cc02078f. [DOI] [PubMed] [Google Scholar]
- 19.Yamaguchi A, et al. Electrochemical CO2 reduction by Ni-containing iron sulfides: How is CO2 electrochemically reduced at bisulfide-bearing deep-sea hydrothermal precipitates? Electrochim Acta. 2014;141:311–318. [Google Scholar]
- 20.Herschy B, et al. An origin-of-life reactor to simulate alkaline hydrothermal vents. J Mol Evol. 2014;79:213–227. doi: 10.1007/s00239-014-9658-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cody GD, et al. Primordial carbonylated iron-sulfur compounds and the synthesis of pyruvate. Science. 2000;289:1337–1340. doi: 10.1126/science.289.5483.1337. [DOI] [PubMed] [Google Scholar]
- 22.Huber C, Wächtershäuser G. Activated acetic acid by carbon fixation on (Fe,Ni)S under primordial conditions. Science. 1997;276:245–247. doi: 10.1126/science.276.5310.245. [DOI] [PubMed] [Google Scholar]
- 23.Huber C, Wächtershäuser G. Peptides by activation of amino acids with CO on (Ni,Fe)S surfaces: Implications for the origin of life. Science. 1998;281:670–672. doi: 10.1126/science.281.5377.670. [DOI] [PubMed] [Google Scholar]
- 24.Huber C, Wächtershäuser G. α-Hydroxy and α-amino acids under possible Hadean, volcanic origin-of-life conditions. Science. 2006;314:630–632. doi: 10.1126/science.1130895. [DOI] [PubMed] [Google Scholar]
- 25.Buchwald C, Grabb K, Hansel CM, Wankel SD. Constraining the role of iron in environmental nitrogen transformations: Dual stable isotope systematics of abiotic NO2− reduction by Fe(II) and its production of N2O. Geochim Cosmochim Acta. 2016;186:1–12. [Google Scholar]
- 26.Grabb KC, Buchwald C, Hansel C, Wankel S. A dual nitrite isotopic investigation of chemodenitrification by mineral-associated Fe(II) and its production of nitrous oxide. Geochim Cosmochim Acta. 2017;196:388–402. [Google Scholar]
- 27.Brandes JA, et al. Abiotic nitrogen reduction on the early Earth. Nature. 1998;395:365–367. doi: 10.1038/26450. [DOI] [PubMed] [Google Scholar]
- 28.Stirling A, Rozgonyi T, Krack M, Bernasconi M. Prebiotic NH3 formation: Insights from simulations. Inorg Chem. 2016;55:1934–1939. doi: 10.1021/acs.inorgchem.5b02911. [DOI] [PubMed] [Google Scholar]
- 29.Summers DP, Chang S. Prebiotic ammonia from reduction of nitrite by iron (II) on the early Earth. Nature. 1993;365:630–633. doi: 10.1038/365630a0. [DOI] [PubMed] [Google Scholar]
- 30.Wong ML, Charnay BD, Gao P, Yung YL, Russell MJ. Nitrogen oxides in early earth’s atmosphere as electron acceptors for life’s emergence. Astrobiology. 2017;17:975–983. doi: 10.1089/ast.2016.1473. [DOI] [PubMed] [Google Scholar]
- 31.Novikov Y, Copley SD. Reactivity landscape of pyruvate under simulated hydrothermal vent conditions. Proc Natl Acad Sci USA. 2013;110:13283–13288. doi: 10.1073/pnas.1304923110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huber C, Wächtershäuser G. Primordial reductive amination revisited. Tetrahedron Lett. 2003;44:1695–1697. [Google Scholar]
- 33.Hafenbradl D, Keller M, Wächtershäuser G, Stetter KO. Primordial amino acids by reductive amination of α-oxo acids in conjunction with the oxidative formation of pyrite. Tetrahedron Lett. 1995;36:5179–5182. [Google Scholar]
- 34.Ding Y, Batista B, Steinbock O, Cartwright JHE, Cardoso SSS. Wavy membranes and the growth rate of a planar chemical garden: Enhanced diffusion and bioenergetics. Proc Natl Acad Sci USA. 2016;113:9182–9186. doi: 10.1073/pnas.1607828113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baaske P, et al. Extreme accumulation of nucleotides in simulated hydrothermal pore systems. Proc Natl Acad Sci USA. 2007;104:9346–9351. doi: 10.1073/pnas.0609592104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Priye A, Yu Y, Hassan YA, Ugaz VM. Synchronized chaotic targeting and acceleration of surface chemistry in prebiotic hydrothermal microenvironments. Proc Natl Acad Sci USA. 2017;114:1275–1280. doi: 10.1073/pnas.1612924114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barge LM, et al. Chemical gardens as flow-through reactors simulating natural hydrothermal systems. J Vis Exp. 2015;2015:53015, e53015. doi: 10.3791/53015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grégoire B, et al. Insights into the behaviour of biomolecules on the early Earth: The concentration of aspartate by layered double hydroxide minerals. Geochim Cosmochim Acta. 2016;176:239–258. [Google Scholar]
- 39.Génin JMR, et al. Thermodynamic equilibria in aqueous suspensions of synthetic and natural Fe(II)−Fe(III) green rusts: Occurrences of the mineral in hydromorphic soils. Environ Sci Technol. 1998;32:1058–1068. [Google Scholar]
- 40.Inoue K, et al. Analysis of iron oxyhydroxides and oxides converted from green rust in aqueous solution. ISIJ Int. 2007;47:453–457. [Google Scholar]
- 41.Ahn T, Kim JH, Yang H-M, Lee JW, Kim J-D. Formation pathways of magnetite nanoparticles by coprecipitation method. J Phys Chem C. 2014;116:6069–6076. [Google Scholar]
- 42.Peternele WS, et al. Experimental investigation of the coprecipitation method: An approach to obtain magnetite and maghemite nanoparticles with improved properties. J Nanomater. 2014;2014:1–10. [Google Scholar]
- 43.Sumoondur A, Shaw S, Ahmed I, Benning LG. Green rust as a precursor for magnetite: An in situ synchrotron based study. Mineral Mag. 2008;72:201–204. [Google Scholar]
- 44.Burcar BT, et al. RNA oligomerization in laboratory analogues of alkaline hydrothermal vent systems. Astrobiology. 2015;15:509–522. doi: 10.1089/ast.2014.1280. [DOI] [PubMed] [Google Scholar]
- 45.Ludwig KA, Kelley DS, Butterfield DA, Nelson BK, Früh-Green G. Formation and evolution of carbonate chimneys at the Lost City hydrothermal field. Geochim Cosmochim Acta. 2006;70:3625–3645. [Google Scholar]
- 46.Früh-Green GL, et al. 30,000 years of hydrothermal activity at the lost city vent field. Science. 2003;301:495–498. doi: 10.1126/science.1085582. [DOI] [PubMed] [Google Scholar]
- 47.Gomez S, Peters JA, Maschmeyer T. The reductive amination of aldehydes and ketones and the hydrogenation of nitriles: Mechanistic aspects and selectivity control. Adv Synth Catal. 2002;344:1037–1057. [Google Scholar]
- 48.Domine ME, Hernández-Soto MC, Pérez Y. Development of metal nanoparticles supported materials as efficient catalysts for reductive amination reactions using high-throughput experimentation. Catal Today. 2011;159:2–11. [Google Scholar]
- 49.Chandru K, et al. Simple prebiotic synthesis of high diversity dynamic combinatorial polyester libraries. Nat Commun Chem. 2018;1:30. [Google Scholar]
- 50.Forsythe JG, et al. Ester‐mediated amide bond formation driven by wet–dry cycles: A possible path to polypeptides on the prebiotic Earth. Angew Chem Int Ed Engl. 2015;54:9871–9875. doi: 10.1002/anie.201503792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Estrada C, Sverjensky DA, Pelletier M, Razafitianamaharavo A, Hazen RM. Interaction between l-aspartate and the brucite [Mg(OH)2]–water interface. Geochim Cosmochim Acta. 2015;155:172–186. [Google Scholar]
- 52.Farias AP, Carneiro CE, de Batista Fonseca IC, Zaia CT, Zaia DA. The adsorption of amino acids and cations onto goethite: A prebiotic chemistry experiment. Amino Acids. 2016;48:1401–1412. doi: 10.1007/s00726-016-2191-6. [DOI] [PubMed] [Google Scholar]
- 53.Kitadai N. Energetics of amino acid synthesis in alkaline hydrothermal environments. Orig Life Evol Biosph. 2015;45:377–409. doi: 10.1007/s11084-015-9428-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.