Significance
Disruptions in the circadian clock and its component proteins have been shown to be associated with disease states ranging from cancer to neurodegeneration. Herein, we identify the circadian clock protein Rev-erbα as a critical regulator of neuroinflammation. Rev-erbα deletion causes spontaneous microglial and astrocyte activation, increased microglial NF-κB signaling, and neuronal injury. Accordingly, pharmacological activation of Rev-erbα suppressed brain inflammation. Our results establish Rev-erbα as a link between the circadian clock, glial activation, and neuroinflammation.
Keywords: Rev-erbα, circadian, microglia, neuroinflammation
Abstract
Circadian dysfunction is a common attribute of many neurodegenerative diseases, most of which are associated with neuroinflammation. Circadian rhythm dysfunction has been associated with inflammation in the periphery, but the role of the core clock in neuroinflammation remains poorly understood. Here we demonstrate that Rev-erbα, a nuclear receptor and circadian clock component, is a mediator of microglial activation and neuroinflammation. We observed time-of-day oscillation in microglial immunoreactivity in the hippocampus, which was disrupted in Rev-erbα−/− mice. Rev-erbα deletion caused spontaneous microglial activation in the hippocampus and increased expression of proinflammatory transcripts, as well as secondary astrogliosis. Transcriptomic analysis of hippocampus from Rev-erbα−/− mice revealed a predominant inflammatory phenotype and suggested dysregulated NF-κB signaling. Primary Rev-erbα−/− microglia exhibited proinflammatory phenotypes and increased basal NF-κB activation. Chromatin immunoprecipitation revealed that Rev-erbα physically interacts with the promoter regions of several NF-κB–related genes in primary microglia. Loss of Rev-erbα in primary astrocytes had no effect on basal activation but did potentiate the inflammatory response to lipopolysaccharide (LPS). In vivo, Rev-erbα−/− mice exhibited enhanced hippocampal neuroinflammatory responses to peripheral LPS injection, while pharmacologic activation of Rev-erbs with the small molecule agonist SR9009 suppressed LPS-induced hippocampal neuroinflammation. Rev-erbα deletion influenced neuronal health, as conditioned media from Rev-erbα–deficient primary glial cultures exacerbated oxidative damage in cultured neurons. Rev-erbα−/− mice also exhibited significantly altered cortical resting-state functional connectivity, similar to that observed in neurodegenerative models. Our results reveal Rev-erbα as a pharmacologically accessible link between the circadian clock and neuroinflammation.
Circadian clocks allow organisms to precisely synchronize internal physiological processes with their external environment. A conserved transcriptional–translational feedback loop known as the core circadian clock controls cycles of protein expression that produce transcriptional and physiologic rhythms. This core circadian clock consists of the transcriptional activators BMAL1 and CLOCK, which drive transcription of their own transcriptional repressors, including CRYPTOCHROME (CRY), PERIOD (PER), and REV-ERB proteins (1). The circadian system regulates a variety of critical cellular processes, including aspects of metabolism, inflammation, and redox homeostasis (2). Disruptions of the clock or its associated proteins have been implicated in pathological conditions ranging from cancer to neurodegenerative diseases (2–4). However, the roles of cellular circadian clocks in brain health and neuroinflammation are still poorly understood.
Aberrant glial cell activation and neuroinflammation are hallmarks of many neurodegenerative conditions such as Alzheimer disease (AD) and Parkinson disease (PD) (5, 6). Circadian disruption is also a common symptom of these diseases (4). The circadian clock has been implicated in the regulation of peripheral inflammation (7–9), though its role in neuroinflammation is less clear. We have previously shown that deletion of Bmal1, which disrupts core clock transcriptional function, causes widespread glial activation, inflammation, and oxidative stress in the brain (10), though the downstream mechanisms have not been fully identified.
One potential mechanism by which BMAL1 could regulate neuroinflammation is via transcriptional regulation of Rev-erbα (gene name Nr1d1). Rev-erbα is a heme-responsive nuclear receptor protein as well as a circadian clock component which is directly regulated by BMAL1 (11–13). Rev-erbα is also a transcriptional repressor that inhibits Bmal1 expression and other targets at specific sites within the genome in a tissue-specific manner (13–16). Rev-erbα has been implicated in control of diverse processes, including metabolism, macrophage function, and inflammation (17, 18). Because it is a nuclear receptor, Rev-erbα is an attractive therapeutic target which can be modulated with available small-molecule agonists and antagonists (19, 20). While the function of Rev-erbα in neuroinflammation and neurodegeneration has not been directly examined, Rev-erb agonist drugs appear to prevent inflammation and cell death when infused into the brain (21). Thus, we have examined the role for Rev-erbα in the control of neuroinflammation, using genetic and pharmacological manipulations both in vivo and in vitro. Our results demonstrate a key role for Rev-erbα in the regulation of glial activation and neuroinflammation.
Results
Rev-erbα Deletion Induces Spontaneous Hippocampal Microgliosis.
Since Rev-erbα has been shown to regulate macrophage function in the periphery (18), we first investigated the effect of Rev-erbα deletion on the tissue resident macrophages of the CNS: microglia. We placed 6- to 8-mo-old wild-type (WT) and Rev-erbα−/− mice (referred to as RKO throughout) in constant darkness, then performed microglial staining with Iba1 at CT4 and CT16, timepoints at which REV-ERB–regulated gene Fabp7 shows peak and trough expression, respectively (22). We observed that in the WT hippocampus, microglial volume varied by time of day, with increased Iba1 volume observed at CT16 (trough expression for Rev-erbα–regulated targets) (Fig. 1 A and B). This time-of-day effect was abrogated in the RKO mouse hippocampus, as microglial volume was similar to the WT CT16 timepoint at both CT4 and CT16 (Fig. 1 A and B). This finding suggested that oscillations in Rev-erbα may regulate genes which control basal microglial activation state. Accordingly, we observed markedly increased spontaneous microglial Iba1 staining in the hippocampus of 5-mo RKO mice (Fig. 1C), as well as increases in microglial-related inflammatory transcripts in the RKO hippocampus, including Il1β and Trem2, consistent with a proinflammatory response (Fig. 1D). Microglial activation can be characterized by morphologic changes, as well as increases in the phagocytic marker CD68 (23). We found a significant increase in CD68+ puncta within Iba1+ microglia by confocal imaging with 3D surface reconstruction in the hippocampus of RKO mice at 5–6 mo of age, indicating increased phagocytic activation [Fig. 1E and Movie S1 (WT cell) and Movie S2 (KO cell)]. We also observed decreased microglial branching morphologic changes in RKO microglia, consistent with activation (Fig. 1 F and G) (24). We also observed an increase in total number of Iba1+ microglia in the hippocampus of RKO mice (SI Appendix, Fig. S1A), as is observed in many neuroinflammatory conditions. However, this did not change with time of day and does not explain the microglial morphologic changes. Young (2–3 mo) RKO mice already had increased Iba1/Cd68 colocalization (SI Appendix, Fig. S1B), as well as increased Aif1 and Trem2 expression, though Il1b expression was age dependent (SI Appendix, Fig. S1C), suggesting that only some component of the phenotype is age dependent. Taken together, Rev-erbα appears to regulate microglial activation.
Fig. 1.
Rev-erbα deletion induces hippocampal microglial activation. (A) Images (60×) of Iba1 staining in 6- to 8-mo WT (Left) and RKO (Right) mouse hippocampus, with Iba1 volume quantification (B). Mice were killed at CT4 (AM, Top) and CT16 (PM, Bottom). n = 4–5 mice per genotype, three images per mouse. (C) Iba1 staining in 5-mo RKO hippocampus (Right) and WT littermates (Left). (D) qPCR analysis for microglial inflammatory genes from the hippocampus of WT and RKO mice. n = 6 mice per group. (E) Representative 100× 3D surface rendering showing CD68+ puncta (yellow) within Iba1+ microglia (red), in RKO hippocampus compared with WT, with quantification. n = 4 mice per genotype with three 40× fields of view each. (F) Representative skeletonized microglial reconstructions and (G) quantification of branches per cell. n = 3 mice, n = 35 microglia. ns, not significant; *P < 0.05, **P < 0.01, or ***P < 0.001 by two-tailed t test with Welch’s correction. (Scale bars, 50 µm for A, 120 µm for C.)
Enhanced NF-κB Signaling Is Associated with Immune Activation in RKO Microglia.
To further characterize the neuroinflammatory phenotype of RKO mice, we performed microarray-based transcriptomic analysis of hippocampal tissue from 5-mo WT or RKO mice. Raw data are deposited in the ArrayExpress database (25, 26). We observed significant increases in proinflammatory chemokines (Ccl2, Ccl5, Ccl8, and Cxcl5), astrocyte activation markers (Gfap, Timp1, S100a4, and S100A6), and microglial activation transcripts (Aif1, Cd68, Tyrobp, Trem2, and Csf1r) (Fig. 2A and Dataset S1). We confirmed several of these transcriptional changes by qPCR in a separate cohort of 5-mo mice, including Timp1, S100a6, and Cxcl5 (SI Appendix, Fig. S2A). Because Rev-erbα is a transcriptional repressor, we performed pathway analysis of transcripts which were significantly up-regulated (uncorrected P value <0.05, fold change >2) using DAVID software (27), which showed profound up-regulation of biological processes related to innate immune activation and inflammation (Fig. 2B), emphasizing the neuroinflammatory phenotype.
Fig. 2.
Rev-erbα–mediated neuroinflammation is associated with dysregulated microglial NF-κB signaling. (A) Relative expression of transcripts related to different aspects of neuroinflammation taken from microarray analysis performed on 5-mo RKO and WT mice (n = 3 per genotype). Colored bars on Left indicate hand-curated functional groupings. (B) Gene Ontology (GO) term analysis of microarray data for biological processes up-regulated in RKO hippocampus compared with WT (P > 0.05, fold change >2). (C) Representative images showing p65 nuclear translocation in RKO microglia compared with WT at baseline, 1 h and 3 h, following LPS stimulation. Quantification of the percent microglia with nuclear p65 staining (DAPI/p65 overlap) is shown (Right). n = 3 separate experiments. (D) qPCR showing mRNA expression for the proinflammatory mediators Il6 and Tnfa in primary WT and RKO microglia. (E) ChIP analysis of WT microglia for the Rev-erbα target Bmal1, NF-κB signaling components Traf2 as well as Nfkbib, a negative control S100a4 and a normalization control Gapdh. *P < 0.05 or **P < 0.01 by two-tailed t test with Welch’s correction. ****P < 0.001 by two-way ANOVA with Tukey’s multiple comparisons test.
The NF-κB pathway is a critical regulator of innate immune activation which interacts with Rev-erbα (21, 28). We observed up-regulation of several genes involved in the positive regulation of NF-κB signaling, such as Nfkb2, Tlr4, Stat3, and Traf2, as well as down-regulation of two genes involved in inhibiting NF-κB signaling (Nfkbib and Usp31) (Fig. 2A). Of note, Nfkbib encodes the NF-κB pathway inhibitor IκBβ (29). We next examined basal and lipopolysaccharide (LPS)-induced p65 nuclear translocation in primary WT and RKO microglia. In WT microglia, nuclear p65 was minimal at baseline, increased markedly 1 h after LPS stimulation, then decreased by 3 h after LPS (Fig. 2C). However, RKO microglia showed significantly increased p65 nuclear translocation at baseline which remained elevated throughout the stimulation (Fig. 2C). Accordingly, primary RKO microglia showed increased basal cytokine production (Fig. 2D), though their response to LPS was similar to WT cells (Fig. 3C). We next performed chromatin immunoprecipitation (ChIP) assays in primary microglia using Rev-erbα antibody or IgG control, to identify direct targets of Rev-erbα. Using existing ChIP-seq databases for liver, adipose, and midbrain tissue (15), we identified Rev-erbα binding peaks located in the promoter regions of several genes which were altered in our transcriptomic data, including Traf2, Nfkb2, and Nfkbib. qPCR from WT microglial ChIP DNA revealed strong Rev-erbα binding to the promoters of Bmal1 (a known Rev-erb target), Traf2, and Nfkbib, with lesser but still significant signal for Nfkb2 (Fig. 2E). S100a4, an astrocyte gene included as a negative control, showed no signal, while DNA from RKO cells also showed no signal. These data suggest that loss of Rev-erbα directly regulates expression of multiple NF-κB–related genes to enhance basal NF-κB signaling in microglia and promote proinflammatory microglial activation.
Fig. 3.
Rev-erbs modulate LPS-induced neuroinflammation in vivo. (A) qPCR analysis of the hippocampal tissue from 5-mo RKO mice or WT littermates treated with i.p. LPS for 6 h. (B) ELISA for IL-1β levels in media from WT or RKO primary microglia treated with LPS alone or in combination with SR9009 as well as ATP for inflammasome maturation. (C) qPCR of WT or RKO primary microglia treated with LPS alone or in combination with SR9009 for Il6 mRNA expression. Gene expression is normalized to LPS-treated WT cells. (D) mRNA expression of Il6 in WT astrocytes treated with LPS and/or SR9009. (E) qPCR analysis of hippocampus from WT mice pretreated with SR9009 or vehicle (Veh) and then stimulated with i.p. LPS for 6 h. In both A and E, data are shown as fold increase from vehicle-treated WT mice (no LPS). *P < 0.05 by two-tailed t test.
Rev-erbα Deletion Exacerbates LPS-Induced Neuroinflammation in the Hippocampus.
Based on our in vitro findings, we next investigated the neuroinflammatory response of RKO mice to LPS. Notably, we observed an extremely high fatality rate specifically in RKO mice following intracerebroventricular LPS injection, and thus employed intraperitoneal (i.p.) LPS injections instead. i.p. LPS caused striking increases of inflammatory transcripts within the hippocampus of WT mice 6 h after injection (Fig. 3A). Mice were perfused before tissue harvest to minimize RNA contribution from inflammatory cells in blood. Rev-erbα deletion augmented LPS-induced expression of proinflammatory mediators in the hippocampus, including Il6 and Ccl2, the astrocyte activation marker Gfap, and the oxidative stress-responsive transcript Hmox1 (Fig. 3A). These data suggest that Rev-erbα deletion exacerbates the LPS-induced neuroinflammation in vivo.
Pharmacologic Activation of Rev-erbα Suppresses LPS-Induced Neuroinflammation.
We next sought to determine if activating Rev-erbα could mitigate LPS-induced neuroinflammation. Rev-erbα is an orphan nuclear receptor and thus can be activated using brain permeant small-molecule agonists such as SR9009 (19). To test the effect of Rev-erbα activation on inflammation, cells were stimulated with LPS with or without SR9009 for 8 h to activate NF-κB, with ATP added during the final 30 min to facilitate secretion of IL-1β into the media. In BV-2 immortalized microglial cells, SR9009 exhibited dose-dependent suppression of LPS+ATP-induced IL-1β protein secretion (SI Appendix, Fig. S4A). In primary WT microglia, SR9009 again inhibited LPS+ATP-induced IL-1β secretion (Fig. 3B), as well as Il6 mRNA expression (Fig. 3C). SR9009 did not significantly suppress cytokine expression in RKO microglia, suggesting some specificity of the drug for Rev-erbα (Fig. 3 B and C). SR9009 also significantly suppressed LPS-induced Il6, Tnfa, and Il1b expression in primary astrocyte-enriched cultures (Fig. 3D and SI Appendix, Fig. S4B), suggesting that this agent can mitigate inflammation across cell types in the brain. We next investigated the effects of SR9009 on LPS-mediated neuroinflammation in vivo in the hippocampus. WT mice were pretreated with twice-daily injections of SR9009 (100 mg/kg) or vehicle, at 7 AM and 7 PM for 2 d, then injected with LPS (2 mg/kg i.p.). WT mice pretreated with SR9009 showed a diminished response to LPS stimulation as evidenced by reduced levels of proinflammatory transcripts, including Il6 and Ccl2, in the hippocampus 6 h after LPS injection (Fig. 3E). Together, these data demonstrate that deletion of Rev-erbα exacerbates LPS-induced hippocampal inflammation, while Rev-erbα activation with SR9009 mitigates it.
Rev-erbα Deletion Induces Cell Nonautonomous Astrocyte Activation.
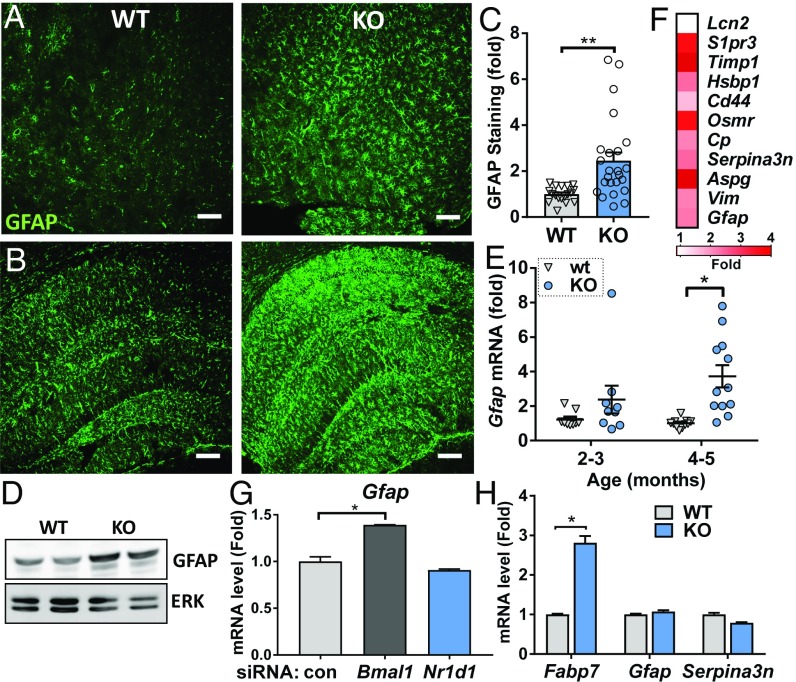
Since astrogliosis is associated with microglial activation and occurs in Bmal1−/− mice (10), we next examined astrocyte activation in RKO brain. We observed diffuse astrocyte activation, as assessed by increases in GFAP immunoreactivity (Fig. 4 A–C), protein (Fig. 4D), and mRNA (Fig. 4E) expression, throughout the cortex (Fig. 4A and SI Appendix, Fig. S3A) and hippocampus (Fig. 4B). Increases in mRNA expression is evident in 2- to 3-mo and 4- to 5-mo RKO mice (Fig. 4E). The degree of astrogliosis was variable between mice, though it was consistently observed beginning in the outer cortical layers and hippocampus by 3 mo (SI Appendix, Fig. S2A). We examined expression of pan-reactive astrocyte activation transcripts, as described previously (30), and found the majority of these to be significantly up-regulated in RKO hippocampus (Fig. 4F). We have recently demonstrated that BMAL1 can regulate astrocyte activation in a cell-autonomous manner (31). However, we observed no increase in Gfap expression in WT astrocytes following siRNA-mediated Nr1d1 knockdown (Fig. 4G) or in primary astrocyte cultures from RKO mice (Fig. 4H). Notably, RKO astrocyte cultures did show increased Il6 and Il1b expression in response to LPS (SI Appendix, Fig. S3D), suggesting that Rev-erbα may regulate NF-κB in multiple glial cell types. Thus, our results suggest that Rev-erbα deletion induces astrocyte activation indirectly, perhaps as a result of microglial activation, neuronal injury, or other factors.
Fig. 4.
Astrocyte activation in Rev-erbα−/− brain is cell nonautonomous. (A and B) GFAP staining in the piriform cortex (A) and hippocampus (B) of 5-mo RKO mice (KO, Right) compared with WT (Left). (C) Quantification of the percent area GFAP immunoreactivity in the cortex of RKO (KO) and WT littermates at 2–5 mo. n = 10–11 mice per genotype. (D) Western blot analysis for relative GFAP protein levels in WT and RKO mouse hippocampi as well as the associated ERK loading control. (E) qPCR analysis of Gfap mRNA expression in hippocampus from 2- to 3-mo and 4- to 5-mo RKO (KO) and WT mice. n = 10–12 mice per group. (F) Average expression fold changes for pan-reactive astrocyte activation markers in the hippocampus of 5-mo Rev-erbα−/−, compared with WT littermates, n = 3 mice per genotype. (G) qPCR for Gfap mRNA expression in primary WT astrocytes treated with control siRNA as well as siRNA targeting Bmal1 and Nr1d1. (H) Relative mRNA expression levels for the Rev-erbα target Fabp7, and astrocyte activation markers Gfap as well as Serpina3n in primary WT and RKO astrocytes. *P < 0.05 or **P < 0.01 by two-tailed t test with Holm–Sidak correction for multiple comparisons.
Glial Rev-erbα Deficiency Promotes Neuronal Death in a Coculture Model.
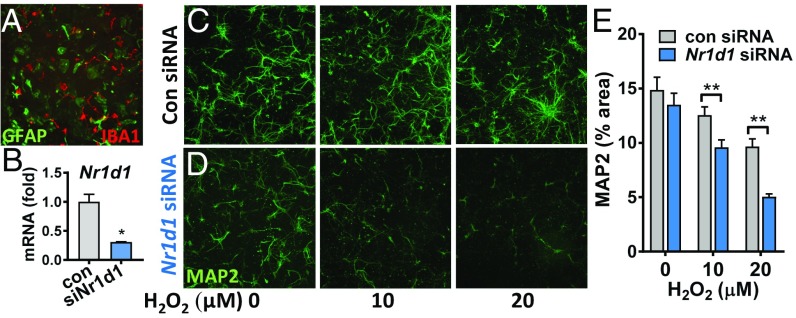
Aberrant glial activation can result in neuronal damage in the context of disease. Therefore, we explored the consequences of Rev-erbα deficiency on neuronal injury using a primary neuron-mixed glia coculture model. We prepared mixed glial cultures containing microglia and astrocytes from postnatal WT mice, then treated cultures with siRNA targeting Nr1d1 (siNr1d1) to knock down Rev-erbα, or with a nontargeting siRNA (siSCR) (Fig. 5 A and B). We removed the siRNA, then prepared conditioned media (CM) from these mixed cultures. We then grew WT primary cortical neurons in CM for 7 d, then treated neurons with hydrogen peroxide (H2O2) for 24 h to induce neuronal oxidative stress and death. WT neurons grown in siNr1d1 CM showed increased cell death at similar H2O2 concentrations compared with neurons grown in siSCR CM (Fig. 5 C–E). In a separate experiment, we depleted microglia by shaking (leaving 1–2% microglia) (31) from WT or RKO mixed glial cultures, leaving astrocyte-enriched cultures. WT neurons were grown on the astrocyte-enriched cultures and then stressed with H2O2. Here, we observed no significant differences between WT neurons grown on WT and RKO astrocytes (SI Appendix, Fig. S5). This illustrates the necessity of microglial Rev-erbα deficiency in promoting neuronal injury in vitro.
Fig. 5.
Loss of Rev-erbα in glia impacts neuronal health in vitro. (A) GFAP/Iba1 costain of mixed glial culture used to generate the CM. (B) Nr1d1 mRNA levels in mixed glia following treatment with control or Nr1d1 siRNA. (C and D) Representative 10× images of MAP2 staining of neurons grown in CM from mixed glia treated with control (C) or Nr1d1 siRNA (D). After 7 d in CM, neurons were treated with H2O2 for 24 h. (E) Quantification of MAP2 staining in C and D. n = 51–60 wells per group from at least three separate experiments. *P < 0.05 by two-tailed t test and **P < 0.05 by two-tailed t test with Holm–Sidak correction for multiple comparisons.
Altered Resting-State Functional Connectivity in RKO Mice.
Given the effects on neuronal injury in vitro, we next asked if genetic deletion of Rev-erbα leads to impairment of systems-level brain function by examining cortical resting-state functional connectivity (FC) using optical intrinsic signal imaging (OISI) (32). Using OISI, changes in FC between different cortical regions can be observed in healthy mice (33, 34) or in models of neurodegeneration (10, 35). We performed OISI in anesthetized 5-mo WT and RKO (KO) mice, using a region-of-interest approach (Fig. 6A). Compared with WT mice, mice lacking Rev-erbα exhibited significantly reduced FC within the retrosplenial cortex (Fig. 6 B and C), a region which is selectively affected in other neurodegenerative models (35, 36). Interestingly, we also observed significantly increased FC within the somatosensory region of KO mice compared with WT mice (Bottom row, Fig. 6B and boxed blue region, Fig. 6C).We next examined quantitative, global FC differences between WT and KO mice, using spatial principal component analysis (PCA) of the group-averaged whole-cortex correlation difference matrix. The first two principal components, which explain 55% of the total variance between the groups, overlap remarkably with the retrosplenial (PC1, 39% variance) and somatosensory (PC2, 16% variance) regions examined above (Fig. 6D). Thus, Rev-erbα deletion causes in vivo changes in regional FC which resemble those observed in other mouse models of neurodegenerative disease.
Fig. 6.
Impaired cortical resting-state functional connectivity in Rev-erbα KO mice. (A) Functional parcellation of resting-stage networks using the average correlation structure across all mice studied. (B) Resting-state FC maps in WT and KO mice (n = 5–6 mice per genotype). Top row: Group-averaged retrosplenial FC. High correlation is indicated by reds; anticorrelation by blues. KO mice exhibit reduced interhemispheric FC as well as reduced anterior–posterior anticorrelations. Bottom row: Group-averaged somatosensory FC. WT mice exhibited lower interhemispheric FC compared with KO mice while both groups exhibited qualitatively similar ipsilateral FC. (C) Regional differences in correlation structure for each region-of-interest (ROI) pair. Maps of FC for each ROI examined in A were compressed into a difference matrix for quantitative evaluation of pairwise regional FC between WT and KO. WT mice exhibit significantly increased retrosplenial FC and significantly decreased FC in somatosensory regions compared with KO mice (black boxes). Cortical regions: C, cingulate; M, motor; R, retrosplenial; S, somatosensory. (D) Spatial PCA performed on the whole cortex correlation difference matrix assesses the topography of all pairwise correlation differences across groups. The first two PCs from this process explain 39% (PC1) and 16% (PC2) of the variance, respectively. This unbiased approach also reveals differences in KO mice involving retrosplenial (PC1) and somatosensory regions (PC2). Dotted black outlines of the ROIs are for reference.
Discussion
Here, we show that the circadian clock protein Rev-erbα regulates microglial activation in vitro and in vivo, and that loss of Rev-erbα function promotes spontaneous neuroinflammation and neuronal dysfunction in vivo. We observed a time-of-day effect on microglial immunoreactivity in the hippocampus which was lost following Rev-erbα deletion, suggesting that Rev-erbα may control circadian oscillation of microglial activation, and that loss of Rev-erbα may lock brain microglia in a proinflammatory state. ChIP experiments revealed that Rev-erbα can interact with promoter regions of transcripts involved in NF-κB signaling, with loss of Rev-erbα driving proinflammatory NF-κB activation in cultured microglia and in the mouse hippocampus. While astrocyte activation was observed in the RKO brain, Rev-erbα deletion did not induce Gfap expression in cultured astrocytes, suggesting that, in contrast to Bmal1 deletion (31), astrocyte activation in this model is noncell autonomous. Activation of Rev-erbs with the small-molecule agonist SR9009 suppressed inflammatory gene expression in cultured glia and in vivo. Taken together, these results implicate Rev-erbα as an important regulator of neuroinflammation.
A growing literature supports the link between the circadian clock and mammalian innate and adaptive immune function (37). Rev-erbα regulates various aspects of peripheral innate immune activation and cytokine release in various tissues. Rev-erbα has been implicated in the regulation of pulmonary inflammation (38) and in the transcriptional suppression of inflammatory mediators, including IL-6 and CCL-2 (18, 39). Rev-erbα also controls circadian oscillations in the NLRP3 inflammasome, with Rev-erbα deletion exacerbating IL-1β production and toxin-induced inflammatory hepatitis in mice (43). Despite the wealth of information regarding Rev-erbα in peripheral immune function, little is known about its role in innate immune responses in the brain. Microglia exhibit robust circadian clock function and inflammatory activation of microglia varies in a circadian manner (41, 42). Thus, loss of rhythmic Rev-erbα–mediated transcriptional repression could play a key role in the regulation of the microglial activation state, as demonstrated by our finding that microglial Iba1 volume changes with time of day in a Rev-erbα–dependent manner. Of note, RKO mice display nearly normal wheel-running activity due to compensation by Rev-erbβ (13, 17).Thus, the inflammatory phenotypes observed are not due to gross circadian rhythm disruption. Future studies are needed to understand the circadian and noncircadian transcriptional functions of Rev-erbs in various cell types in the brain.
We observed activation of the NF-κB pathway in both our primary microglia experiments and RKO brain. This is consistent with the previously established links between NF-κB signaling and the circadian clock (43, 44), and a recent study using a Rev-erbα–targeted drug (21). Notably, our experiments showed enhanced basal NF-κB p65 nuclear accumulation in RKO microglia. Our data suggest that loss of Rev-erbα could promote NF-κB signaling by directly regulating transcription of several NF-κB–associated transcripts, including Traf2, Nfkbib, and Nfkb2. Traf2 conveys proinflammatory signals from the TNF receptor to NF-κB and can promote inflammation in glia (45). Rev-erbα deletion leads to loss of repression at the Traf2 promoter and increased Traf2 expression. Interestingly, Rev-erbα also binds to the Nfkbib promoter, but Rev-erbα deletion leads to decreased Nfkbib expression, suggesting that Rev-erbα binding may either act to somehow positively regulate Nfkbib transcription, or that the direct binding effects of Rev-erbα at the Nfkbib locus are minor and are counteracted by some indirect effect. Since Nfkbib acts as an inhibitor of NF-κB activation (29), loss of Nfkbib expression in Rev-erbα−/− cells could also promote inflammation. In macrophages, Rev-erbα/β integrate transcriptional signals from multiple damage-associated transcription factors, including NF-κB, Nrf2, and Smad3 (46), suggesting complex transcriptional regulation programs beyond canonical NF-κB signaling. Adding to the complexity, Rev-erbs can repress transcription by several distinct mechanisms, including DNA binding domain (DBD)-dependent binding to RORE sites (14), DBD-independent tethering by tissue-specific transcription factors (15), altering enhancer RNA expression (16), and by modulating chromatin looping (47). Notably, the Rev-erbα binding regions which we identified in Traf2 and Nfkbib did not contain canonical RORE sites, suggesting possible DBD-independent regulation. Future ChIP-seq experiments will be needed to fully elucidate the cell type-specific targets of Rev-erbα.
We demonstrate that Rev-erbα KO mice have impaired functional connectivity in the retrosplenial cortex, similar to other mouse models of neurodegeneration, including models of amyloid-β or tau aggregation, or Bmal1 deletion (10, 35, 36). It is unclear if this is due to damage related to neuroinflammation, or a direct effect of Rev-erbα in neurons. Rev-erbα is known to regulate dopaminergic neurotransmission via transcriptional control of tyrosine hydroxylase in the hippocampus and midbrain (48, 49), and Rev-erbα deletion alters cerebellar neuron development (50), suggesting that Rev-erbα could directly regulate cortical neuron function and/or health. Future studies using cell type-specific Rev-erbα deletion are needed to delineate the role of neuronal versus glial Rev-erbα in brain homeostasis.
Rev-erbα is a nuclear receptor that can be targeted pharmacologically with existing agonists (19). Previous studies show that Rev-erb agonists can suppress LPS-induced inflammatory cytokine production in C6 glioma cells and cultured microglia and in the murine brain following LPS infusion (21, 51). Our results are consistent with these previous studies, but greatly expand upon them by demonstrating the necessity of Rev-erbα for maintaining microglial homeostasis. Furthermore, we have demonstrated that the antiinflammatory effects of SR9009 are at least partially dependent on Rev-erbα, as we observed a blunted antiinflammatory response to SR9009 in Rev-erbα−/− microglia. Our studies use SR9009 as a proof-of-concept agent to demonstrate that development of more potent Rev-erb agonists may be a viable strategy for treatment of neurodegenerative pathology.
In summary, our data establish a role for Rev-erbα in the control of microglial activation and neuroinflammation. This work solidifies Rev-erbα as a potential therapeutic target for treatment of neuroinflammation and illustrates the need for future study of the role of Rev-erbα and Rev-erb–targeted therapies in neuroinflammatory and neurodegenerative diseases.
Methods
For detailed methods, see SI Appendix, SI Methods.
Mice.
All experiments were performed on RKO or WT littermate mice on a C57BL/6J background obtained from The Jackson Laboratory, and were approved by the Washington University Institutional Animal Care and Use Committee. Mice were housed on a 12/12 light/dark cycle unless noted. Drugs were administered by i.p. injection.
Cell Culture.
Astrocytes and microglia were isolated from postnatal day 2 (P2) mice. Mixed cultures of astrocytes and microglia were grown in GM-CSF (5 ng/mL) containing media for 1 wk and then shaken at 225 rpm at 37 °C for 2 h to isolate microglia. Primary microglia were kept in culture for 9–12 d, then treated with LPS (50 ng/mL) for a specified time.
Optical Intrinsic Signal Imaging.
OISI was performed in mice anesthetized with ketamine-xylazine using sequential illumination provided at four wavelengths by light emitting diodes, as previously described (32). See SI Appendix, SI Methods for details.
Data Analysis.
Unless otherwise stated, values are presented as mean ± SEM. Statistical analysis was performed using GraphPad Prism 7 or Matlab. A two-tailed Student’s t test was used when a single variable was compared between genotypes (with Welch’s correction when appropriate), and a two-way ANOVA with Tukey’s posttest was used when multiple variables were compared between genotypes.
Confocal Imaging and 3D Reconstructions.
Z stacks were obtained from 50-μm-thick sections using a Nikon A1Rsi confocal microscope. Colocalization analyses and 3D reconstructions were created using Imaris 9 software (Bitplane).
Supplementary Material
Acknowledgments
This work was supported by an award from the Coins for Alzheimer’s Research Trust fund (to E.S.M.) and NIH Grants R01AG054517 (to E.S.M.) and R01MH093429 (to T.P.B.). P.G. was supported by National Science Foundation Grant DGE-1745038. Imaging was performed with support from the Washington University Center for Cellular Imaging (which is funded by Washington University), the Children’s Discovery Institute (Grant CDI-CORE-2015-505), and the Foundation for Barnes-Jewish Hospital (Grant 3770).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: Microarray data have been deposited in the EMBL-EBI ArrayExpress database, accession no. E-MTAB-7590.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1812405116/-/DCSupplemental.
References
- 1.Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci. 2012;35:445–462. doi: 10.1146/annurev-neuro-060909-153128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330:1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kondratov RV, Antoch MP. The clock proteins, aging, and tumorigenesis. Cold Spring Harb Symp Quant Biol. 2007;72:477–482. doi: 10.1101/sqb.2007.72.050. [DOI] [PubMed] [Google Scholar]
- 4.Musiek ES, Holtzman DM. Mechanisms linking circadian clocks, sleep, and neurodegeneration. Science. 2016;354:1004–1008. doi: 10.1126/science.aah4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heneka MT, et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015;14:388–405. doi: 10.1016/S1474-4422(15)70016-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: A target for neuroprotection? Lancet Neurol. 2009;8:382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- 7.Keller M, et al. A circadian clock in macrophages controls inflammatory immune responses. Proc Natl Acad Sci USA. 2009;106:21407–21412. doi: 10.1073/pnas.0906361106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Druzd D, et al. Lymphocyte circadian clocks control lymph node trafficking and adaptive immune responses. Immunity. 2017;46:120–132. doi: 10.1016/j.immuni.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen KD, et al. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science. 2013;341:1483–1488. doi: 10.1126/science.1240636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Musiek ES, et al. Circadian clock proteins regulate neuronal redox homeostasis and neurodegeneration. J Clin Invest. 2013;123:5389–5400. doi: 10.1172/JCI70317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yin L, et al. Rev-erbalpha, a heme sensor that coordinates metabolic and circadian pathways. Science. 2007;318:1786–1789. doi: 10.1126/science.1150179. [DOI] [PubMed] [Google Scholar]
- 12.Raghuram S, et al. Identification of heme as the ligand for the orphan nuclear receptors REV-ERBalpha and REV-ERBbeta. Nat Struct Mol Biol. 2007;14:1207–1213. doi: 10.1038/nsmb1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Preitner N, et al. The orphan nuclear receptor REV-ERBalpha controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell. 2002;110:251–260. doi: 10.1016/s0092-8674(02)00825-5. [DOI] [PubMed] [Google Scholar]
- 14.Yin L, Lazar MA. The orphan nuclear receptor Rev-erbalpha recruits the N-CoR/histone deacetylase 3 corepressor to regulate the circadian Bmal1 gene. Mol Endocrinol. 2005;19:1452–1459. doi: 10.1210/me.2005-0057. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, et al. GENE REGULATION. Discrete functions of nuclear receptor Rev-erbα couple metabolism to the clock. Science. 2015;348:1488–1492. doi: 10.1126/science.aab3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lam MT, et al. Rev-Erbs repress macrophage gene expression by inhibiting enhancer-directed transcription. Nature. 2013;498:511–515. doi: 10.1038/nature12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho H, et al. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature. 2012;485:123–127. doi: 10.1038/nature11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gibbs JE, et al. The nuclear receptor REV-ERBα mediates circadian regulation of innate immunity through selective regulation of inflammatory cytokines. Proc Natl Acad Sci USA. 2012;109:582–587. doi: 10.1073/pnas.1106750109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solt LA, et al. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature. 2012;485:62–68. doi: 10.1038/nature11030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kojetin D, Wang Y, Kamenecka TM, Burris TP. Identification of SR8278, a synthetic antagonist of the nuclear heme receptor REV-ERB. ACS Chem Biol. 2011;6:131–134. doi: 10.1021/cb1002575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guo DK, et al. Pharmacological activation of REV-ERBα represses LPS-induced microglial activation through the NF-κB pathway. Acta Pharmacol Sin. 2019;40:26–34. doi: 10.1038/s41401-018-0064-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gerstner JR, et al. Brain fatty acid binding protein (Fabp7) is diurnally regulated in astrocytes and hippocampal granule cell precursors in adult rodent brain. PLoS One. 2008;3:e1631. doi: 10.1371/journal.pone.0001631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schafer DP, et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nayak D, Roth TL, McGavern DB. Microglia development and function. Annu Rev Immunol. 2014;32:367–402. doi: 10.1146/annurev-immunol-032713-120240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Musiek ES. 2018 Microarray of hippocampus from 5mo wt, Bmal1 KO and Nr1d1 (Rev-Erb-alpha) KO mice at a single timepoint. EMBL-EBI ArrayExpress database. Available at https://www.ebi.ac.uk/arrayexpress/experiments/E-MTAB-7590. Deposited December 21, 2018.
- 26.Kolesnikov N, et al. ArrayExpress update: Simplifying data submissions. Nucleic Acids Res. 2015;43:D1113–6. doi: 10.1093/nar/gku1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang DW, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 28.Migita H, Morser J, Kawai K. Rev-erbalpha upregulates NF-kappaB-responsive genes in vascular smooth muscle cells. FEBS Lett. 2004;561:69–74. doi: 10.1016/S0014-5793(04)00118-8. [DOI] [PubMed] [Google Scholar]
- 29.McKenna S, Wright CJ. Inhibiting IκBβ-NFκB signaling attenuates the expression of select pro-inflammatory genes. J Cell Sci. 2015;128:2143–2155. doi: 10.1242/jcs.168351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liddelow SA, et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature. 2017;541:481–487. doi: 10.1038/nature21029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lananna BV, et al. Cell-autonomous regulation of astrocyte activation by the circadian clock protein BMAL1. Cell Rep. 2018;25:1–9.e5. doi: 10.1016/j.celrep.2018.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.White BR, et al. Imaging of functional connectivity in the mouse brain. PLoS One. 2011;6:e16322. doi: 10.1371/journal.pone.0016322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bumstead JR, Bauer AQ, Wright PW, Culver JP. Cerebral functional connectivity and Mayer waves in mice: Phenomena and separability. J Cereb Blood Flow Metab. 2017;37:471–484. doi: 10.1177/0271678X16629977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kura S, et al. Intrinsic optical signal imaging of the blood volume changes is sufficient for mapping the resting state functional connectivity in the rodent cortex. J Neural Eng. 2018;15:035003. doi: 10.1088/1741-2552/aaafe4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bero AW, et al. Bidirectional relationship between functional connectivity and amyloid-β deposition in mouse brain. J Neurosci. 2012;32:4334–4340. doi: 10.1523/JNEUROSCI.5845-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Musiek ES, et al. Nmnat1 protects neuronal function without altering phospho-tau pathology in a mouse model of tauopathy. Ann Clin Transl Neurol. 2016;3:434–442. doi: 10.1002/acn3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scheiermann C, Gibbs J, Ince L, Loudon A. Clocking in to immunity. Nat Rev Immunol. 2018;18:423–437. doi: 10.1038/s41577-018-0008-4. [DOI] [PubMed] [Google Scholar]
- 38.Pariollaud M, et al. Circadian clock component REV-ERBα controls homeostatic regulation of pulmonary inflammation. J Clin Invest. 2018;128:2281–2296. doi: 10.1172/JCI93910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sato S, et al. A circadian clock gene, Rev-erbα, modulates the inflammatory function of macrophages through the negative regulation of Ccl2 expression. J Immunol. 2014;192:407–417. doi: 10.4049/jimmunol.1301982. [DOI] [PubMed] [Google Scholar]
- 40.Pourcet B, et al. Nuclear receptor subfamily 1 group D member 1 regulates circadian activity of NLRP3 inflammasome to eeduce the severity of fulminant hepatitis in mice. Gastroenterology. 2018;154:1449–1464.e20. doi: 10.1053/j.gastro.2017.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fonken LK, et al. Microglia inflammatory responses are controlled by an intrinsic circadian clock. Brain Behav Immun. 2015;45:171–179. doi: 10.1016/j.bbi.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nakazato R, et al. The intrinsic microglial clock system regulates interleukin-6 expression. Glia. 2017;65:198–208. doi: 10.1002/glia.23087. [DOI] [PubMed] [Google Scholar]
- 43.Spengler ML, et al. Core circadian protein CLOCK is a positive regulator of NF-κB-mediated transcription. Proc Natl Acad Sci USA. 2012;109:E2457–E2465. doi: 10.1073/pnas.1206274109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hong HK, et al. Requirement for NF-κB in maintenance of molecular and behavioral circadian rhythms in mice. Genes Dev. 2018;32:1367–1379. doi: 10.1101/gad.319228.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Su D, et al. Sphk1 mediates neuroinflammation and neuronal injury via TRAF2/NF-κB pathways in activated microglia in cerebral ischemia reperfusion. J Neuroimmunol. 2017;305:35–41. doi: 10.1016/j.jneuroim.2017.01.015. [DOI] [PubMed] [Google Scholar]
- 46.Eichenfield DZ, et al. Tissue damage drives co-localization of NF-κB, Smad3, and Nrf2 to direct Rev-erb sensitive wound repair in mouse macrophages. eLife. 2016;5:e13024. doi: 10.7554/eLife.13024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim YH, et al. Rev-erbα dynamically modulates chromatin looping to control circadian gene transcription. Science. 2018;359:1274–1277. doi: 10.1126/science.aao6891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jager J, et al. Behavioral changes and dopaminergic dysregulation in mice lacking the nuclear receptor Rev-erbα. Mol Endocrinol. 2014;28:490–498. doi: 10.1210/me.2013-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chung S, et al. Impact of circadian nuclear receptor REV-ERBα on midbrain dopamine production and mood regulation. Cell. 2014;157:858–868. doi: 10.1016/j.cell.2014.03.039. [DOI] [PubMed] [Google Scholar]
- 50.Chomez P, et al. Increased cell death and delayed development in the cerebellum of mice lacking the rev-erbA(alpha) orphan receptor. Development. 2000;127:1489–1498. doi: 10.1242/dev.127.7.1489. [DOI] [PubMed] [Google Scholar]
- 51.Morioka N, et al. Stimulation of nuclear receptor REV-ERBs regulates tumor necrosis factor-induced expression of proinflammatory molecules in C6 astroglial cells. Biochem Biophys Res Commun. 2016;469:151–157. doi: 10.1016/j.bbrc.2015.11.086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.