Significance
Identifying past hominin diets is a key to understanding adaptation and biological evolution. Bone collagen isotope studies have added much to the discussion of Neandertal subsistence strategies, providing direct measures of diet. Neandertals consistently show very elevated nitrogen isotope values. These values have been seen as the signature of a top-level carnivore diet, but this interpretation was recently challenged by a number of additional theories. We here apply compound-specific isotope analysis of carbon and nitrogen in bone collagen single amino acids of two Neandertals. These Neandertals had the highest nitrogen isotope ratios of bulk collagen measured so far, and our study confirms that these values can be most parsimoniously explained by a carnivorous diet.
Keywords: compound-specific isotope analyses, diet, late Neanderthals, Paleolithic, stable isotopes
Abstract
Isotope and archeological analyses of Paleolithic food webs have suggested that Neandertal subsistence relied mainly on the consumption of large herbivores. This conclusion was primarily based on elevated nitrogen isotope ratios in Neandertal bone collagen and has been significantly debated. This discussion relies on the observation that similar high nitrogen isotopes values could also be the result of the consumption of mammoths, young animals, putrid meat, cooked food, freshwater fish, carnivores, or mushrooms. Recently, compound-specific C and N isotope analyses of bone collagen amino acids have been demonstrated to add significantly more information about trophic levels and aquatic food consumption. We undertook single amino acid C and N isotope analysis on two Neandertals, which were characterized by exceptionally high N isotope ratios in their bulk bone or tooth collagen. We report here both C and N isotope ratios on single amino acids of collagen samples for these two Neandertals and associated fauna. The samples come from two sites dating to the Middle to Upper Paleolithic transition period (Les Cottés and Grotte du Renne, France). Our results reinforce the interpretation of Neandertal dietary adaptations as successful top-level carnivores, even after the arrival of modern humans in Europe. They also demonstrate that high δ15N values of bone collagen can solely be explained by mammal meat consumption, as supported by archeological and zooarcheological evidence, without necessarily invoking explanations including the processing of food (cooking, fermenting), the consumption of mammoths or young mammals, or additional (freshwater fish, mushrooms) dietary protein sources.
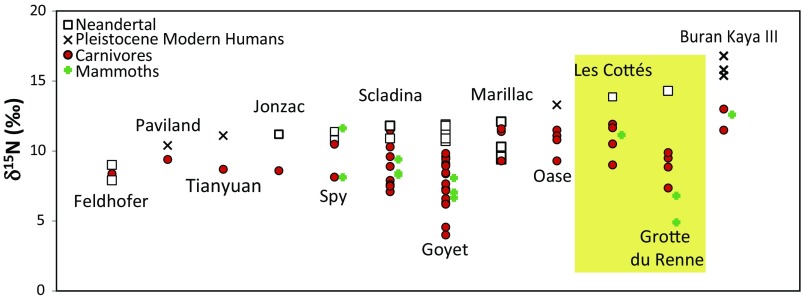
Since the first publication of bulk bone collagen carbon and nitrogen stable isotope ratios of Neandertals, (e.g., refs. 1–3), supported by more recent studies (4–8), an intriguing pattern has been observed: Neandertals are characterized by relatively high nitrogen isotope values, often higher than the carnivores from the same sites (Fig. 1). Nitrogen isotopes are usually employed as a tracer of trophic level (9), and this pattern was therefore, at first, interpreted as indicative of the top-level trophic position (TP) of Neandertals in Paleolithic food webs (2, 3, 10), specifically focused on the consumption of large herbivorous mammals (11). Early modern humans have a wider range of isotopic ratios (especially in carbon), and some individuals exhibit higher N isotope ratios than of the fauna associated with them and above the range generally observed in Neandertals (refs. 10 and 12–14 and Fig. 1). These elevated values in Pleistocene modern humans have been interpreted as the signature of freshwater fish consumption (10, 13) as N isotope ratios (δ15N) in fish or shell consumers are usually higher than in pure terrestrial carnivores (10). Recently, an exceptionally high N isotope ratio has been measured in an infant Neandertal (AR-14) from the site of Grotte du Renne (Arcy-sur-Cure, France; SI Appendix, Supporting Information 1). This individual had a δ15N value 3–5‰ above the associated carnivores (ref. 6 and Fig. 1). In this case, this very elevated ratio is likely to be due to breastfeeding (15), as the infant was ∼1 y old.
Fig. 1.
δ15N values of Neandertals, Pleistocene modern humans, and fauna from different Paleolithic European and Asian sites for which analyses on the associated fauna was performed. Data are from (Feldhofer) Richards and Trinkaus (10), (Paviland) Richards et al. (3) and Devièse et al. (56), (Tianyan) Hu et al. (13), (Jonzac) Richards et al. (8), (Spy) Naito et al. (37), (Scladina) Bocherens et al. (2), (Goyet) Wissing et al. (7), (Marillac) Fizet et al. (1, 57), Bocherens et al. (5), (Oase) Trinkaus et al. (12), (Les Cottés) this study, (Grotte du Renne) Welker et al. (6), and (Buran Kaya III) Drucker et al. (30).
Over the last few years, several studies have challenged the interpretation of high Neandertal bone collagen nitrogen isotope values as indicative of large herbivore consumption. It has been suggested that the systematic slightly higher δ15N values observed in Neandertal collagen, especially relative to that of the associated carnivores, could be explained by mammoth consumption (4), as mammoths generally exhibit higher δ15N values than other associated herbivores. Furthermore, recent studies carried on dental calculus highlight the existence of plant consumption (16–20), challenging the interpretation of a purely carnivorous diet of Neandertals. Other factors such as the consumption of putrid meat (21), mushrooms (16, 22, 23), freshwater fish (24), or cooked food (25, 26) have also been suggested as explanations for the elevated δ15N values of Neandertal collagen. The selective hunting of young animals by Neandertals and older animals by carnivores is another factor that would increase δ15N values, but the zooarcheological data generally suggest the opposite pattern (27). Bocherens et al. (28) also suggested that the difference between Pleistocene modern humans and Neandertals would not be explained by distinctive diets, but by a climate change during the Middle to Upper Paleolithic transition causing the elevation of the N isotope signature of the local plants, impacting the values in the whole food web and, therefore, temporally distinct populations. Most of the modern humans analyzed so far are indeed from Upper Paleolithic contexts while the Neandertals (24, 28), with the exception of the Grotte du Renne (AR-14) (6) and the Spy Cave individuals (29), were found in Middle Paleolithic contexts. Finally, Drucker et al. (30) demonstrated, using compound-specific isotope analyses (CSIA), that some elevated δ15N values of Paleolithic modern human bone collagen could mainly be explained by mammoth consumption, rather than freshwater fish. The above-mentioned factors (Table 1) are all likely to increase the δ15N of body tissues without dramatically influencing the δ13C values. It is therefore very difficult to estimate which one accounts for the pattern observed in bulk Neandertal bone collagen.
Table 1.
List of factors likely to cause elevated nitrogen isotope ratios in tooth collagen of the Les Cottés Neandertal
| Dietary adaptation | Output of the present study |
| Top-level carnivory | Yes |
| Mammoth consumption | Unknown |
| Young animal consumption | No/Minor |
| Freshwater fish consumption | No |
| Breastfeeding | Unlikely |
| Environmental factors triggering an elevation of the whole food web isotope values | No |
| Mushroom consumption | Unknown |
| Consumption of putrid meat | Unknown |
| Cooking | Unknown |
| Carnivore consumption | No/Minor |
The second column lists the relevant factors according to the results of this study. See text for more details.
Novel isotope techniques, such as compound-specific isotope analyses performed on single amino acids, could help identify the factor explaining this pattern. The CSIA are a very powerful technique, which consists in establishing the C and N isotope composition of the various amino acids in bone or tooth collagen. Carbon isotope values of individual amino acids can clearly distinguish terrestrial from freshwater food sources (31, 32). Honch et al. (31) demonstrated that animals from food webs based on terrestrial plants usually exhibit similar δ13CPhe and δ13CVal (Phe: Phenylalaline, Val: Valine) in their collagen, with higher values in C4 plant-based food webs than in C3 plant-based ones. Fish consumers have δ13CVal overlapping with the animals from C3 plants (for freshwater environment) and C4 plants (for marine environment)-based food webs, but much lower δ13CPhe values (31). Nitrogen isotope values of individual amino acids reveal TP in more detail than bulk isotopic data, especially using the data obtained on phenylalanine (Phe) and glutamic acid (Glu). δ15NPhe reflects the local baseline without being really impacted by the trophic level of the animal (trophic 15N-enrichment of 0.4 ± 0.4‰) (33) whereas the glutamic acid N isotope ratio (δ15NGlu), in addition to the local baseline, is strongly impacted by the TP (trophic 15N-enrichment of 8.0 ± 1.1‰) (34–36). The combination of the δ15N of these two amino acids therefore allows interpretations free of local baseline bias and allow more precisely assessing the TP of a specimen. For example, Naito et al. (37) documented the δ15N values of amino acids for the Spy Cave Neandertals (Fig. 1), revealing a possible contribution of plants into their diet, up to 20%. The use of CSIA of carbon and nitrogen can therefore potentially provide very detailed information on the diet of Neandertals and directly address the debate on the cause of the elevated nitrogen isotope signatures of their collagen.
Recently, we extracted the collagen of a Neandertal tooth from les Cottés (France; SI Appendix, Supporting Information 1 and Fig. S1, and ref. 38) for radiocarbon dating (ref. 39, SI Appendix, Supporting Information 2). In the frame of the assessment of collagen preservation, we measured the C and N isotope ratios in bulk collagen and found the second highest N isotope ratio ever seen in a Neandertal sample (SI Appendix, Table S1), knowing that the first one was the above mentioned breastfeeding infant from Grotte du Renne, AR-14 (6) (Fig. 1). The δ15N value of the Les Cottés Neandertal is about ∼7‰ higher than those of the associated herbivores previously measured in Talamo et al. (40), expected one-step trophic level enrichment (9). As there are a number of suggested reasons for the high Neandertal nitrogen isotope values (we list 10 possibilities in Table 1), we aimed to investigate which ones could account for the elevated value of the Les Cottés Neandertal. The tooth root analyzed belonged to a permanent maxillary lateral incisor, and thus, the dentine formed between the fourth and eighth years of age (SI Appendix, Supporting Information 3). If explained by breastfeeding, the δ15N value associated to the Les Cottés Neandertal would indicate a very late age of weaning, which would contradict previous findings (41, 42). At Les Cottés, hyena, wolf, and fox bones with cut-marks have been found in layer 06, layer 04/upper, and layer 02. Also, mammoth ivory transformed into beads have been found into layer 04/upper (43). Carnivore and mammoth consumption are two hypotheses that have to be considered. Fish remains are not present at les Cottés. No putrid meat or mushroom consumption has been reported for this site from the archeological evidence; however, these two types of consumption would unlikely leave any significant archeological traces. Concerning the environmental hypothesis suggested by Bocherens et al. (28), the tooth was dated to 43,740–42,720 cal. y BP (SI Appendix, Supporting Information 2) and the Neandertal should thus have lived before the hypothesized increased aridity, which was suggested to have impacted local isotope signatures of the whole food webs coming from transition layers in France according to Bocherens et al. (28).
We conducted N and C isotope analyses on bulk collagen and amino acids of the Les Cottés Neandertal and the associated fauna, which were identified using ZooMs (Dataset S1, Table S5), to determine which one of the abovementioned factors (Table 1) accounts for the exceptionally elevated δ15N value of the Les Cottés Neandertal. As no CSIA data are yet available for breastfeeding or suckling specimens, we also performed these analyses on (i) animal teeth from Les Cottés formed before the weaning age, and (ii) faunal and hominin remains from Grotte du Renne, including the breastfed Neandertal (AR-14). The C and N isotope data on bulk collagen of the Grotte du Renne food web are already published (6). The Grotte du Renne and Les Cottés Neandertals both have similar radiocarbon ages and come from Central France contexts, which reinforce the validity of the comparison of these two Neandertals (6, 39).
Below, we report unprecedented CSIA for carbon on collagen amino acids for Paleolithic collagen samples, as well as the second CSIA application for nitrogen on collagen amino acids for Neandertals. We suggest that the exceptionally high N isotope signature values of the Les Cottés Neandertal can simply be explained by a carnivorous diet, possibly preferentially relying on reindeer (Rangifer tarandus). We also confirm that the Grotte du Renne Neandertal (AR-14) was a suckling infant and exclude the possibility of freshwater fish consumption being an influence on the collagen isotope values at both sites.
Results
The raw data for taxonomic identification, bulk collagen and amino acid isotope analyses, as well as measurements on isotopic standards are given in the Supporting Tables (Dataset S1, Tables S5–S10).
Isotope Analyses of the Bulk Collagen.
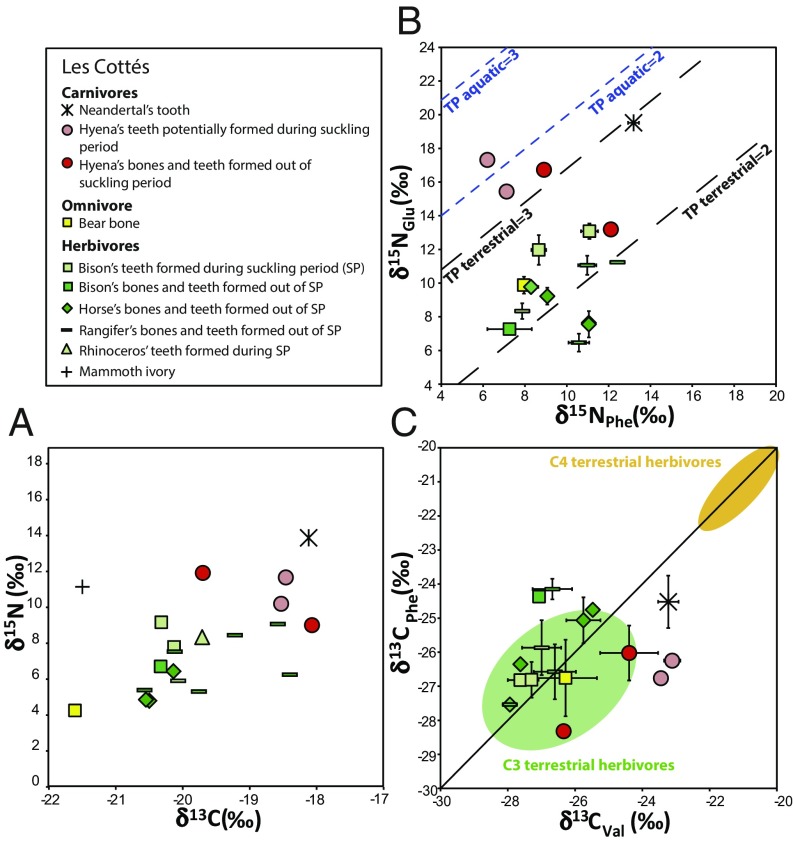
Carbon and nitrogen isotope analyses of bulk bone collagen are the classic method to estimate trophic relationship in food webs. C and N isotope analyses on the bulk collagen (δ13Cbulk and δ15Nbulk) for the AR-14 Neandertal and the associated fauna of Grotte du Renne were previously published in Welker et al. (6). The results for the Les Cottés food web are here presented. The collagen extracted from the Les Cottés samples is well preserved, with collagen yields of more than 1% and C:N ratios ranging from 3.1 to 3.3 (ref. 44 and Dataset S1, Tables S6 and S7). The herbivore bones and teeth from Les Cottés have δ13Cbulk ranging between −20.6 and −19.8‰, except for three Rangifer specimens exhibiting higher values, and the mammoth specimen exhibiting the lowest δ13Cbulk value (Fig. 2). The δ15Nbulk of the herbivores are comparable to what was observed in Grotte du Renne, except for the specimens with teeth, which would have formed during the suckling period, and that have, as expected, higher δ15Nbulk values. The mammoth specimen also has an elevated δ15Nbulk value, higher than in Grotte du Renne, but this pattern is generally observed for this species (7, 28, 30). The only carnivore species analyzed at Les Cottés beside the Neandertal is hyenas. Hyena teeth formed during or after suckling periods reveal a large range of δ15Nbulk values (9.0–11.9‰) and of δ13Cbulk values (−19.8 to −18.2‰). The hyena P3 teeth, possibly formed during the suckling period, do not show higher δ15Nbulk values than the other teeth. One of the hyenas has a δ15Nbulk comparable to that of the Rangifer teeth. The Neandertal tooth from Les Cottés falls in the range of that of the carnivore hyenas, both for C and N isotope ratios.
Fig. 2.
Isotope results for the site of Les Cottés. (A) C and N isotope ratios in bulk collagen. (B) N isotope ratios in phenylalanine and glutamic acid. The TP lines (B) are defined according to Chikaraishi et al. (35, 36), (C) C isotope ratios in valine and phenylalanine. The green and yellow areas (C) are defined according to Honch et al. (31).
Nitrogen Isotope Analyses of Amino Acids (Les Cottés and Grotte du Renne) and Associated TP.
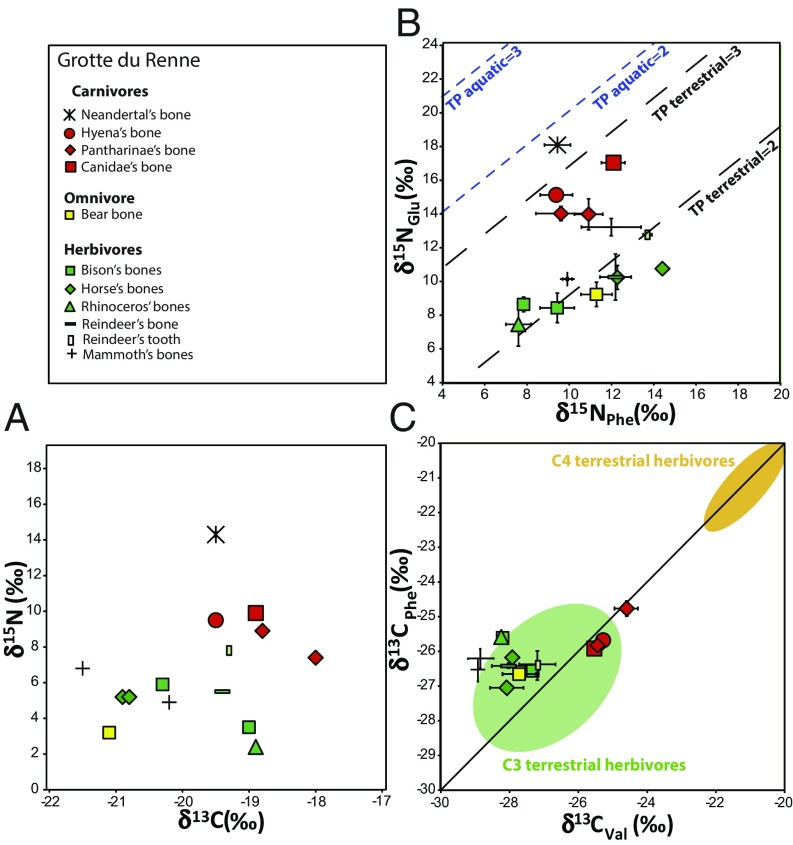
Nitrogen isotope analyses of single amino acids were conducted to estimate the TP of hominins and associated animals, and assess which species were hunted by the different carnivores. These analyses can unravel the presence of plants, fish, meat, and suckling in the diet of the different animals. Terrestrial herbivores have a wider range of δ15NPhe values in Grotte du Renne (7.6–14.4‰) than in Les Cottés (7.3–12.4‰). The δ15NPhe values of the herbivores do not correlate with the bulk collagen δ15N values, whereas the δ15NGlu values do. This correlation, both in Grotte du Renne and Les Cottés, indicates that most of the N isotopic variability among herbivores is due to a trophic level effect, likely caused by the sampling of tissues formed during the suckling period or shortly after weaning age. The δ15NPhe value of the Neandertal in les Cottés is clearly higher than that of the other carnivores, while the δ15NPhe value of the AR-14 Neandertal falls in the range of the other carnivores (Fig. 3). The TP of all of the animals has been assessed using δ15NGlu, δ15NPhe values and a β factor of −8.4‰ (refs. 33, 36, and 37 and Dataset S1, Table S6). The β factor corresponds to an enrichment factor between the δ15N in glutamic acid and phenylalanine (δ15NGlu − δ15NPhe). This factor therefore reflects the vertical shift between each line representing a different TP in Figs. 2 and 3. The uncertainty on the estimation of the TP is probably related to the variability of the β factor, which is around 0.1 (30), and therefore yields range between 1.9 and 2.1 for herbivores and between 2.9 and 3.1 for carnivores. Several herbivores have a TP above 2.1 and one hyena equals 3.1 while the other is above this value, which corresponds to the teeth (P3, M1) and bones possibly formed during the suckling period. However, some herbivores have a TP below 1.9 (two Equidae and one Rangifer at Les Cottés, and two Equidae and one cave bear at Grotte du Renne). Moreover, one of the four hyenas of les Cottés has a TP of 2.2, similar to what is expected for a herbivore, and three carnivores at Grotte du Rennes (two Pantherinae and a wolf) have, respectively, a TP of 2.5, 2.7, and 2.8 (Figs. 2 and 3 and SI Appendix, Table S6). The Les Cottés Neandertal has a TP of 2.9, indicating a pure carnivorous diet. The Grotte du Renne Neandertal has a TP of 3.2, slightly higher than the theoretical carnivore TP and much higher than the carnivores from this site.
Fig. 3.
Isotope results for the site of Grotte du Renne (Arcy-sur-Cure). (A) C and N isotope ratios in bulk collagen; data from Welker et al (6). (B) N isotope ratios in phenylalanine and glutamic acid. The TP lines (B) are defined according to Chikaraishi et al. (35, 36); (C) C isotope ratios in valine and phenylalanine. The green and yellow areas (C) are defined according to Honch et al. (31).
Carbon Isotope Analyses of Amino Acids (Les Cottés and Grotte du Renne).
Carbon isotope analyses of amino acids can help to identify the presence of freshwater or marine resources into the diet of past hominins. The carbon isotope composition of 9–12 amino acids (depending on the derivatization method used) has been measured for all of the specimens of les Cottés and Grotte du Renne, with the exception of the Neandertal from the second site for which not enough material was available. The data are given in Dataset S1, Table S8 and fully discussed in the SI Appendix, Supporting Information 4. The δ13CPhe (−24.4 to −28.3‰) and δ13CVal (−23.2 to −28.9‰) range of values are similar at both sites and generally fit with the range observed previously in food webs relying on C3 plants (31, 32), but slightly above the average food web values reported in a third study (40). These three publications represent the totality of the existing data on δ13CPhe and δ13CVal in mammal collagen. The pattern observed between the two sites is quite different: In Grotte du Renne, the carnivores exhibit similar δ13C values in the phenylalanine and valine, while the herbivores have enriched δ13CPhe values relative to δ13CVal (Figs. 3 and 4). In Les Cottés, most herbivores have also enriched δ13CPhe relative to δ13CVal, but to a lesser extent (Figs. 2 and 4). In this case, the carnivores have depleted δ13CPhe relative to δ13CVal, especially in the case of the two hyena teeth formed during the suckling period (Fig. 4).
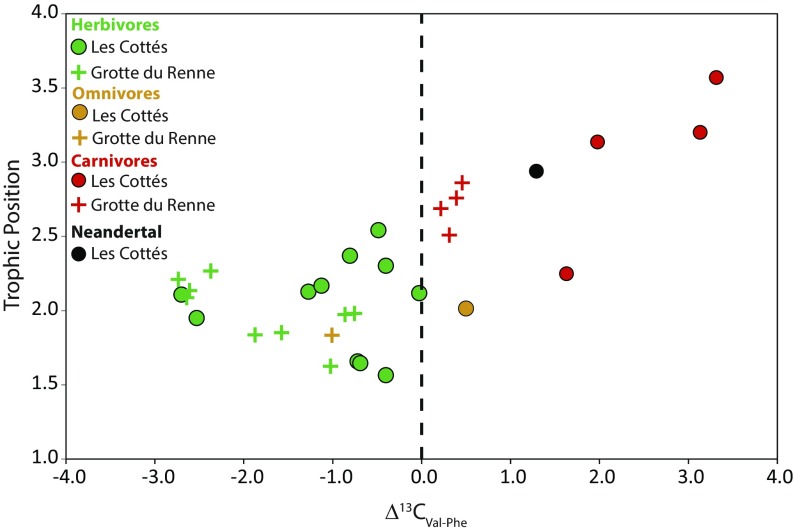
Fig. 4.
Difference (‰) between δ13Cval and δ13CPhe (Δ13CVal-Phe) in bone and tooth collagen and associated TP. The TP was estimated from the δ15NPhe and δ15NVal values. The C isotope ratios in amino acids of the Grotte du Renne Neandertal were not measured because not enough material was available. See text for more details.
Discussion
TP Uncertainties.
In Les Cottés, the estimated TPs of the animals are consistent with what is expected, except for three herbivores and one hyena. This could be explained by the consumption of plants with a peculiar β factor by these three herbivores. Most plants (50%) have a β factor of −8 ± 1‰, yet some reach a value of −12‰ and represent 3% of the reported values (45).
However, only 64 β values of plants have been analyzed so far, and there is therefore a need to better document those β factors in plants and the species associated to specific values (e.g., 3‰ in the case of tree flowers). With such a β value, the trophic level of the three herbivores goes up to 2.1–2.2 and to 2.8 for the associated hyena. In Grotte du Renne, this β moves back these herbivores (two Equidae and one Cave bear) as well as the Pantharinae to their expected TP. With the same β factor, the Neandertal from Grotte du Renne would then have a TP of 3.8, one trophic level above the carnivores. Such a TP could easily be explained by breastfeeding.
We observed difference between δ13CVal and δ13CPhe values, which can be explained by the existence of a trophic level effect, mostly affecting the δ13CVal (SI Appendix, Supporting Information 4) rather than freshwater fish consumption. This accounts for the fact that the Neandertal from Les Cottés and the hyenas are falling slightly off the range defined for terrestrial animals. As a result, the difference between δ13CVal and δ13CPhe (Δ13CVal-Phe) values correlate to the estimated trophic level (Fig. 4), and the Δ13CVal-Phe is systematically positive in carnivores and negative for herbivores. The report of this trophic level effect is unprecedented, since no other carnivore data are currently available for δ13CVal and δ13CPhe in mammal collagen. We also observed it for δ13CLeu and δ13CGly values (Gly = Glycine, Leu = Leucine; SI Appendix, Supporting Information 4). The combined use of carbon and nitrogen single amino acid isotope values could therefore help to account for the uncertainty on the TP estimation based on δ15N values only.
Neandertal Diets at Les Cottés and Grotte du Renne.
Compound-specific isotope analyses conducted on amino acids provide information on the sources of dietary protein over a number of years of life with a greater level of detail compared with bulk isotope analysis. However, as these CSIA studies are in their infancy, the exact relationship of δ15N and δ13C values of amino acids to TPs is not yet fully understood. Despite these caveats, these analyses shed light on the interpretation of Neandertal diets in Europe. We demonstrate here that exceptionally high δ15N values in bulk collagen of the Les Cottés Neandertal can still be explained by a carnivorous diet solely relying on herbivore meat without any need to call upon additional explanations such as consumption of putrid meat or cooked food. These types of consumption might, however, have existed but would mean that fermentation and cooking equally impact δ15NPhe and δ15NGlu values (Table 1). Freshwater fish and carnivore meat were not—or rarely—eaten by these Neandertals. Neandertals at les Cottés could have mostly hunted reindeer from arid environments (showing elevated δ15NPhe and δ13Cbulk) or horses (also characterized by elevated δ13CAsp, SI Appendix, Supporting Information 4), whereas hyenas would have been less specific in terms of their environments and prey. This is a similar interpretation to that proposed by the only other Neandertal CSIA study by Naito et al. (37), which also suggested different ecological niches between hyenas and Neandertals. The δ13Cbulk value of the Neandertal tooth does not support here the specific hunting of mammoths, which would generally yield low δ13C (as observed in Grotte du Renne, although only one individual could be analyzed). According to CSIA analysis, the Spy Neandertals (Neandertals dated circa 41,000 BP; ref. 46) show different hunting strategies: Spy 430-a and Spy 92b hunted reindeer, horse, and bovines, while Spy 92a seemed more opportunistic (37). Neandertal hunting of reindeer, rather than mammoths, is also generally supported by zooarcheological data (27, 47) including at Grotte du Renne and Les Cottés (38, 48, 49), even if some exceptions exist for Middle Paleolithic sites (47). In Grotte du Renne, both δ15NPhe and δ13C values of AR-14 bulk collagen falls in the variability of the other carnivores. The TP of the AR-14 Neandertal is clearly above that of the other carnivores, whatever the β factor is. This finding fits with the hypothesis of a suckling infant previously suggested by Welker et al. (6). This infant was therefore not weaned but could possibly already have eaten solid food, as its TP is below 4. According to the isotope data performed on bulk collagen and amino acids, the mother was probably sharing the same ecological niche as the other carnivores.
When we then look at all of the bulk collagen isotope analyses of the 29 Neandertals that have been published so far [ranging in radiometric age from ∼90,000 BP (Scladina; ref. 46) to 36,840 BP (Grotte du Renne; ref. 6)], we can see a similar and very stable diet over time. This situation could be readily explained by Neandertals being top-level carnivores consuming herbivore meat, and this pattern even continues after the arrival of modern humans in Europe (50). Our work shows that even exceptionally high δ15N bulk collagen values of two late Neandertals still result from a terrestrial meat-based diet, the same diet that was interpreted for the other 27 Neandertals that have had bulk collagen C and N isotope measurements. Despite changes in climate, environment and associated faunal spectra, throughout the Middle Paleolithic, the focus remains on large herbivores.
Concluding Remarks.
Compound-specific isotope analyses are a very promising method for reconstructing aspects of past diets, especially by combining nitrogen and carbon isotope analyses on single amino acids to clearly distinguish aquatic and terrestrial sources of proteins, and to estimate with more precision the contribution of plants in individual diets. Our application of CSIA to two Neandertals has shown that both were high trophic level consumers, with large herbivores being the main protein source. Therefore, there is no reason to invoke myriad dietary interpretations such as the consumption of mushrooms, putrid animal flesh, mammoths, or freshwater fish to explain their high bulk bone collagen nitrogen isotope values of Neandertals in relation to carnivores from the same site. However, we acknowledge that our isotopic study does not rule out the occasional consumption of these foods. Instead, the differing high N values between Neandertals and associated carnivores is likely due to the consumption of different herbivores from different environments. When we conduct CSIA, which is not environmentally baseline sensitive like bulk collagen N values, Neandertals and carnivores clearly are at the same trophic level.
Methods
The bones and teeth were taxonomically identified using traditional zooarcheological methods and ZooMS (6, 49, 51) (Dataset S1, Table S5). The collagen extraction was performed using the protocol outlined in Talamo and Richards (44), and the bulk collagen isotope analyses were conducted at the Max Planck Institute for Evolutionary Anthropology, Leipzig, Germany. The collagen samples were then sent to the University of California, Los Angeles Isotope Lab Facility, where the compound-specific isotope analyses were performed using gas chromatography combustion isotope ratio mass spectrometry. The two first batches of samples were prepared with the following protocol: The amino acids were liberated using acid hydrolysis before the derivatization step to produce methoxycarbonyl methyl esters (MOC) for GC analysis, following the protocol established by Yarnes and Herzsage (52). The third batch of samples was prepared using the derivatization protocol (53) recommended by O’Connell and Collins (54), producing N-acetyl isopropyl esters, allowing the comparison of isotope signatures in hydroxyproline and proline (SI Appendix, Fig. S2). An external standard was analyzed with both protocols and exhibit similar isotope signatures for both protocols (Dataset S1, Table S10). All samples were analyzed in duplicate by GC-C-IRMS, and additional measurements were performed when the duplicates fell outside expected measurement error (55). Additional information is available in Dataset S1.
Supplementary Material
Acknowledgments
We thank Geoffrey Smith, Karen Ruebens, Chris Yarnes, and Nicolas Bourgon for helpful discussions; Lysann Klausnitzer and Sven Steinbrenner for technical help with the collagen extraction and bulk isotope analyses; J.-J. Cleyet-Merle (Musée National de Préhistoire) for providing samples; and Heiko Temming for µCT-scanning of the tooth prior to destructive sampling. This study was funded by the Max Planck Society.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1814087116/-/DCSupplemental.
References
- 1.Fizet M, et al. Effect of diet, physiology and climate on carbon and nitrogen stable isotopes of collagen in a late Pleistocene anthropic palaeoecosystem: Marillac, Charente, France. J Archaeol Sci. 1995;22:67–79. [Google Scholar]
- 2.Bocherens H, et al. Palaeoenvironmental and palaeodietary implications of isotopic biogeochemistry of last interglacial Neandertal and mammal bones in Scladina Cave (Belgium) J Archaeol Sci. 1999;26:599–607. [Google Scholar]
- 3.Richards MP, et al. Neanderthal diet at Vindija and Neanderthal predation: The evidence from stable isotopes. Proc Natl Acad Sci USA. 2000;97:7663–7666. doi: 10.1073/pnas.120178997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bocherens H. 2011. Diet and ecology of Neandertals: Implications from C and N isotopes. Neandertal Lifeways, Subsistence and Technology, Vertebrate Paleobiology and Paleoanthropology Series (Springer, Dordrecht, The Netherlands), pp 73–85.
- 5.Bocherens H, Drucker DG, Billiou D, Patou-Mathis M, Vandermeersch B. Isotopic evidence for diet and subsistence pattern of the Saint-Césaire I Neanderthal: Review and use of a multi-source mixing model. J Hum Evol. 2005;49:71–87. doi: 10.1016/j.jhevol.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Welker F, et al. Palaeoproteomic evidence identifies archaic hominins associated with the Châtelperronian at the Grotte du Renne. Proc Natl Acad Sci USA. 2016;113:11162–11167. doi: 10.1073/pnas.1605834113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wissing C, et al. Isotopic evidence for dietary ecology of late Neandertals in North-Western Europe. Quat Int. 2016;411:327–345. [Google Scholar]
- 8.Richards MP, et al. Isotopic dietary analysis of a Neanderthal and associated fauna from the site of Jonzac (Charente-Maritime), France. J Hum Evol. 2008;55:179–185. doi: 10.1016/j.jhevol.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Hedges REM, Reynard LM. Nitrogen isotopes and the trophic level of humans in archaeology. J Archaeol Sci. 2007;34:1240–1251. [Google Scholar]
- 10.Richards MP, Trinkaus E. Isotopic evidence for the diets of European Neanderthals and early modern humans. Proc Natl Acad Sci USA. 2009;106:16034–16039. doi: 10.1073/pnas.0903821106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bocherens H. 2009. Neanderthal dietary habits: Review of the isotopic evidence. The Evolution of Hominin Diets, Vertebrate Paleobiology and Paleoanthropology (Springer, Dordrecht, The Netherlands), pp 241–250.
- 12.Trinkaus E, et al. Stable isotope evidence for early modern human diet in southeastern Europe: Peştera cu Oase, Peştera Muierii and Peştera Cioclovina Uscată. Mater Cercet Arheol. 2009;5:4–14. [Google Scholar]
- 13.Hu Y, et al. Stable isotope dietary analysis of the Tianyuan 1 early modern human. Proc Natl Acad Sci USA. 2009;106:10971–10974. doi: 10.1073/pnas.0904826106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fu Q, et al. Genome sequence of a 45,000-year-old modern human from western Siberia. Nature. 2014;514:445–449. doi: 10.1038/nature13810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fuller BT, Fuller JL, Harris DA, Hedges RE. Detection of breastfeeding and weaning in modern human infants with carbon and nitrogen stable isotope ratios. Am J Phys Anthropol. 2006;129:279–293. doi: 10.1002/ajpa.20249. [DOI] [PubMed] [Google Scholar]
- 16.Weyrich LS, et al. Neanderthal behaviour, diet, and disease inferred from ancient DNA in dental calculus. Nature. 2017;544:357–361. doi: 10.1038/nature21674. [DOI] [PubMed] [Google Scholar]
- 17.Henry AG, Brooks AS, Piperno DR. Microfossils in calculus demonstrate consumption of plants and cooked foods in Neanderthal diets (Shanidar III, Iraq; Spy I and II, Belgium) Proc Natl Acad Sci USA. 2011;108:486–491. doi: 10.1073/pnas.1016868108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henry AG, Brooks AS, Piperno DR. Plant foods and the dietary ecology of Neanderthals and early modern humans. J Hum Evol. 2014;69:44–54. doi: 10.1016/j.jhevol.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 19.Hardy BL, Moncel M-H. Neanderthal use of fish, mammals, birds, starchy plants and wood 125-250,000 years ago. PLoS One. 2011;6:e23768. doi: 10.1371/journal.pone.0023768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hardy K, et al. Neanderthal medics? Evidence for food, cooking, and medicinal plants entrapped in dental calculus. Naturwissenschaften. 2012;99:617–626. doi: 10.1007/s00114-012-0942-0. [DOI] [PubMed] [Google Scholar]
- 21.Speth JD. Putrid meat and fish in the Eurasian Middle and Upper Paleolithic: Are we missing a key part of Neandertal and modern human diet? Paleoanthropology. 2017;2017:44–72. [Google Scholar]
- 22.Hardy BL, et al. Impossible Neandertals? Making string, throwing projectiles and catching small game during marine isotope stage 4 (Abri du Maras, France) Quat Sci Rev. 2013;82:23–40. [Google Scholar]
- 23.O’Regan HJ, Lamb AL, Wilkinson DM. The missing mushrooms: Searching for fungi in ancient human dietary analysis. J Archaeol Sci. 2016;75:139–143. [Google Scholar]
- 24.Pearson J. Hunters, fishers and scavengers. Farming. 2007;2007:1–16. [Google Scholar]
- 25.Royer A, Daux V, Fourel F, Lécuyer C. Carbon, nitrogen and oxygen isotope fractionation during food cooking: Implications for the interpretation of the fossil human record. Am J Phys Anthropol. 2017;163:759–771. doi: 10.1002/ajpa.23246. [DOI] [PubMed] [Google Scholar]
- 26.DeNiro MJ, Schoeninger MJ, Hastorf CA. Effect of heating on the stable carbon and nitrogen isotope ratios of bone collagen. J Archaeol Sci. 1985;12:1–7. [Google Scholar]
- 27.Gaudzinski-Windheuser S, Roebroeks W. 2011. On Neanderthal subsistence in last interglacial forested environments in Northern Europe. Neanderthal Lifeways, Subsistence and Technology, Vertebrate Paleobiology and Paleoanthropology Series (Springer, Dordrecht, The Netherlands), pp 61–71.
- 28.Bocherens H, Drucker DG, Madelaine S. Evidence for a (15)N positive excursion in terrestrial foodwebs at the Middle to Upper Palaeolithic transition in south-western France: Implications for early modern human palaeodiet and palaeoenvironment. J Hum Evol. 2014;69:31–43. doi: 10.1016/j.jhevol.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 29.Semal P, et al. New data on the late Neandertals: Direct dating of the Belgian Spy fossils. Am J Phys Anthropol. 2009;138:421–428. doi: 10.1002/ajpa.20954. [DOI] [PubMed] [Google Scholar]
- 30.Drucker DG, et al. Isotopic analyses suggest mammoth and plant in the diet of the oldest anatomically modern humans from far southeast Europe. Sci Rep. 2017;7:6833. doi: 10.1038/s41598-017-07065-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Honch NV, McCullagh JS, Hedges RE. Variation of bone collagen amino acid δ13C values in archaeological humans and fauna with different dietary regimes: Developing frameworks of dietary discrimination. Am J Phys Anthropol. 2012;148:495–511. doi: 10.1002/ajpa.22065. [DOI] [PubMed] [Google Scholar]
- 32.Colonese AC, et al. Long-term resilience of late Holocene coastal subsistence system in Southeastern South America. PLoS One. 2014;9:e93854. doi: 10.1371/journal.pone.0093854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chikaraishi Y, Ogawa NO, Ohkouchi N. Earth, Life, Isotopes. Kyoto Univ Press; Kyoto, Japan: 2010. Further evaluation of the trophic level estimation based on nitrogen isotopic composition of amino acids; pp. 37–51. [Google Scholar]
- 34.McClelland JW, Montoya JP. Trophic relationships and the nitrogen isotopic composition of amino acids in plankton. Ecology. 2002;83:2173–2180. [Google Scholar]
- 35.Chikaraishi Y, et al. Determination of aquatic food-web structure based on compound-specific nitrogen isotopic composition of amino acids. Limnol Oceanogr Methods. 2009;7:740–750. [Google Scholar]
- 36.Chikaraishi Y, et al. High-resolution food webs based on nitrogen isotopic composition of amino acids. Ecol Evol. 2014;4:2423–2449. doi: 10.1002/ece3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Naito YI, et al. Ecological niche of Neanderthals from Spy Cave revealed by nitrogen isotopes of individual amino acids in collagen. J Hum Evol. 2016;93:82–90. doi: 10.1016/j.jhevol.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 38.Soressi M, et al. 2010. Les Cottés (Vienne). Nouveaux travaux sur l’un des gisements de référence pour la transition Paléolithique moyen/supérieur. Préhistoire entre Vienne et Charente: Hommes et société du Paléolithique (Association des Publications Chauvinoises, Chauvigny, France), pp 221–234.
- 39.Hajdinjak M, et al. Reconstructing the genetic history of late Neanderthals. Nature. 2018;555:652–656. doi: 10.1038/nature26151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Talamo S, Soressi M, Roussel M, Richards M, Hublin J-J. A radiocarbon chronology for the complete Middle to Upper Palaeolithic transitional sequence of Les Cottés (France) J Archaeol Sci. 2012;39:175–183. [Google Scholar]
- 41.Skinner M. Dental wear in immature late Pleistocene European hominines. J Archaeol Sci. 1997;24:677–700. [Google Scholar]
- 42.Smith TM, et al. Dental evidence for ontogenetic differences between modern humans and Neanderthals. Proc Natl Acad Sci USA. 2010;107:20923–20928. doi: 10.1073/pnas.1010906107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rigaud S, et al. Les pratiques ornementales à l’Aurignacien ancien dans le Centre-Ouest de la France: L’apport des fouilles récentes aux Cottés (Vienne) Bull Soc Préhist Fr. 2014;111:19–38. [Google Scholar]
- 44.Talamo S, Richards M. A comparison of bone pretreatment methods for AMS dating of samples >30,000 BP. Radiocarbon. 2011;53:443–449. [Google Scholar]
- 45.Naito YI, et al. Reply to “Comment on ‘Ecological niche of Neanderthals from Spy Cave revealed by nitrogen isotopes of individual amino acids in collagen.’ [J. Hum. Evol. 93 (2016) 82–90]” [J. Hum. Evol. 117 (2018) 53–55] J Hum Evol. 2017;117:56–60. doi: 10.1016/j.jhevol.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 46.Pirson S, Toussaint M, Bonjean D, Di Modica K. Landscapes and Landforms of Belgium and Luxembourg. Springer; Berlin: 2018. Spy and Scladina caves: A Neandertal’s story; pp. 357–383. [Google Scholar]
- 47.Smith GM. Neanderthal megafaunal exploitation in Western Europe and its dietary implications: A contextual reassessment of La Cotte de St Brelade (Jersey) J Hum Evol. 2015;78:181–201. doi: 10.1016/j.jhevol.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 48.David F, et al. Le Châtelperronien de la grotte du Renne à Arcy-sur-Cure (Yonne). Données sédimentologiques et chronostratigraphiques. Bull Soc Préhist Fr. 2001;98:207–230. [Google Scholar]
- 49.Welker F, Soressi M, Rendu W, Hublin J-J, Collins M. Using ZooMS to identify fragmentary bone from the late Middle/early Upper Palaeolithic sequence of Les Cottés, France. J Archaeol Sci. 2015;54:279–286. [Google Scholar]
- 50.Higham T, et al. The earliest evidence for anatomically modern humans in northwestern Europe. Nature. 2011;479:521–524. doi: 10.1038/nature10484. [DOI] [PubMed] [Google Scholar]
- 51.Buckley M, Collins M, Thomas-Oates J, Wilson JC. Species identification by analysis of bone collagen using matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry. Rapid Commun Mass Spectrom. 2009;23:3843–3854. doi: 10.1002/rcm.4316. [DOI] [PubMed] [Google Scholar]
- 52.Yarnes CT, Herszage J. The relative influence of derivatization and normalization procedures on the compound-specific stable isotope analysis of nitrogen in amino acids. Rapid Commun Mass Spectrom. 2017;31:693–704. doi: 10.1002/rcm.7832. [DOI] [PubMed] [Google Scholar]
- 53.Styring AK, et al. Practical considerations in the determination of compound-specific amino acid δ15N values in animal and plant tissues by gas chromatography combusition-isotope ratio mass spectrometry, following derivatisation to their N-acetylisopropyl esters. Rapid Commun Mass Spectrom. 2012;26:2328–2334. doi: 10.1002/rcm.6322. [DOI] [PubMed] [Google Scholar]
- 54.O’Connell TC, Collins MJ. Comment on “Ecological niche of Neanderthals from Spy Cave revealed by nitrogen isotopes of individual amino acids in collagen” [J. Hum. Evol. 93 (2016) 82–90] J Hum Evol. 2017;117:53–55. doi: 10.1016/j.jhevol.2017.05.006. [DOI] [PubMed] [Google Scholar]
- 55.Docherty G, Jones V, Evershed RP. Practical and theoretical considerations in the gas chromatography/combustion/isotope ratio mass spectrometry δ(13)C analysis of small polyfunctional compounds. Rapid Commun Mass Spectrom. 2001;15:730–738. doi: 10.1002/rcm.270. [DOI] [PubMed] [Google Scholar]
- 56.Devièse T, et al. Direct dating of Neanderthal remains from the site of Vindija Cave and implications for the Middle to Upper Paleolithic transition. Proc Natl Acad Sci USA. 2017;114:10606–10611. doi: 10.1073/pnas.1709235114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fizet M, Lange-Badre B, Vandermeersch B, Borel JP, Bellon G. Isotopic biogeochemistry (13C, 15N) of fossil vertebrate collagen: Application to the study of a past food web including Neandertal man. J Hum Evol. 1991;20:481–492. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.