Abstract
Introduction: Spondyloarthritis (SpA) is a group of chronic inflammatory disorders which includes ankylosing spondylitis (SA), psoriatic arthritis (PsA), reactive arthritis (ReA), arthritis associated with inflammatory bowel disease (IBD) and undifferentiated spondyloarthritis (uSpA). The enthesis, the area of insertion of the tendon, ligament or joint capsule onto the bone, can be characterized as a central feature in PsA. Material and methods: The study included a number of 28 patients, 18 females and 10 males, with PsA hospitalized during 2016-2018 in the Department of Rheumatology of the Emergency County Hospital of Craiova. All the patients were diagnosed with psoriatic arthritis according to CASPAR criteria and had a history of entheseal pain, mandatory criteria for inclusion in the study. All patients underwent clinical examination, laboratory tests and musculoskeletal ultrasonography (MSUS). Results: The Belgrade Ultrasound Enthesitis Score (BUSES) was not significantly associated either with ESR (p=0.536) or CRP (p=0.965) values. Furthermore, the clinical evaluation through the LEEDS enthesitis index (LEI) showed no significant association with ESR (p=0.067) or CRP (p=0.206). Despite MSUS and clinical findings, there was no significant correlation between disease activity in psoriatic arthritis (DAPSA) and BUSES (p=0,549) or LEI (p=0,197). However, clinical and echographic scores (BUSES and LEI) were significantly associated (p=0.001). Conclusions: Our study proved a significant correlation between LEI and BUSES, although in literature the evidence is contrasting. This is probably due to the fact that the majority of the patients had high disease activity which made the clinical assessment similar to the US. Further studies on more numerous groups of patients have to be conducted in order to debate the inconsistencies related to clinical and US examinations in patients with PsA.
Keywords: Psoriatic arthritis, enthesitis, ultrasound, disease activity, clinical index
Introduction
Spondyloarthritis (SpA) is a group of chronic inflammatory disorders which includes ankylosing spondylitis (SA), psoriatic arthritis (PsA), reactive arthritis (ReA), arthritis associated with inflammatory bowel disease (IBD) and undifferentiated spondyloarthritis (uSpA).
Certain clinical features such as chronic back pain, peripheral asymmetric oligoarthritis and enthesitis are common for all SpA [1].
Patients with SpA can be classified into two categories, following the criteria developed by the Assessment in Spondyloarthritis International Society: axial SpA with involvement of the spine and sacroiliac joints and peripheral SpA, in which case the peripheral manifestations such as arthritis, enthesitis or dactylitis are involved [2].
The enthesis, the area of insertion of the tendon, ligament or joint capsule onto the bone, can be characterized as a central feature in PsA Based on the type of tissue that forms the enthesis, it can be classified as either fibrocartilaginous or dense fibrous connective tissue [3].
The concept of enthesitis takes into account the enthesis, fibrocartilage, bursa, synovium and subchondral bone, particularly describing the “enthesis organ” [4].
Clinical involvement of the enthesis can be quantified using scoring systems such as Maastricht Ankylosing Spondylitis Enthesitis Score (MASES), Gladman enthesitis index, Spondyloarthritis Research Consortium of Canada Enthesitis Index (SPARCC) and LEEDS enthesitis index (LEI) [5].
In the past years, musculoskeletal ultrasonography (MSUS) has become an important tool in the diagnosis of several rheumatologic pathologies and has proven useful in diagnosing subclinical enthesitis [6].
There are several scoring systems developed for enthesitis evaluation in SpA such as Glasgow Enthesitis Scoring System (GUESS), D’Agostino scoring system, Spanish Enthesitis Index, Madrid Sonographic Enthesitis Index (MASEI) and Belgrade Ultrasound Enthesitis Score (BUSES) [7].
Materials and Methods
The study included a number of 28 patients, 18 females and 10 males, with PsA hospitalized between 2016-2018 in the Department of Rheumatology of the Emergency County Hospital of Craiova.
All the patients were diagnosed with psoriatic arthritis according to CASPAR criteria and had history of entheseal pain, mandatory criteria for inclusion in the study.
All patients expressed their agreement to be a part of this study.
All patients underwent clinical examination, laboratory tests and MSUS. The laboratory tests consisted of complete blood count (CBC), liver enzymes, serum creatinine, erythrocyte sedimentation rate (ESR) with a normal value <10mm/h, C reactive protein (CRP) with a normal value <5mg/L.
Entheseal involvement was determined using both a clinical score (LEI) and an echographic one (BUSES).
Ultrasound (US) examinations were performed on a MyLab25™Gold US system, using a multifrequency probe of 6-18MHz.
Grey-scale and power Doppler techniques in both longitudinal and transverse planes were used in order to assess the presence of increased thickness of the enthesis, hypoechogenicity, lack of normal fibrillar aspect, enthesophtytes, erosions and power Doppler signal at the enthesis.
The examined regions were the Achilles tendon, plantar fascia, distal and proximal patellar tendons, quadriceps tendon and common extensor tendon on the lateral epicondyle.
The clinical score quantified entheseal involvement of the following sites: Achilles tendon, medial femoral condyles and lateral epicondyles of the humerus.
Disease activity was monitored by calculating disease activity in psoriatic arthritis (DAPSA).
Statistical analysis of the data was performed using One-way ANOVA test within IBM SPSS Statistics 20. Values less than 0.05 for p were considered statistically significant.
Results
Our study included 28 patients with a mean age of 51.28 (±2.22), mean disease duration of 6.28 (±3.44) years and female to male ratio 18:10.
Out of 10 males, 7 (70%) were younger than 50 years and 3 (30%) were aged 50 or above.
Out of 18 females, 3 (16.66%) were younger than 50 years and 15 (83.33%) were aged 50 or above (Fig.1).
Figure 1.
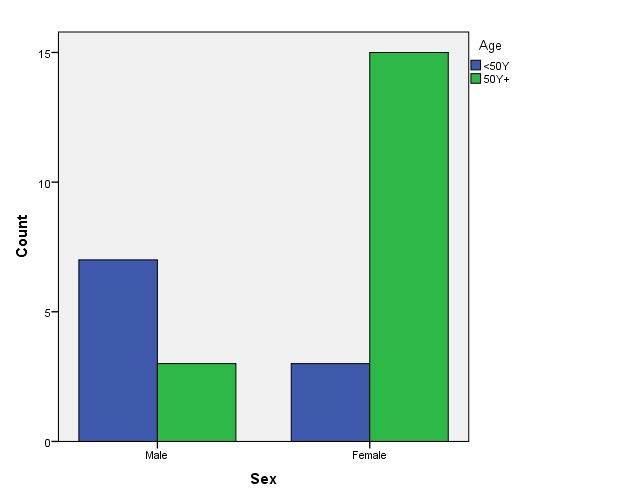
Patients’ age distributed on sexes
The study group included polyarticular disease (71.42%), oligoarticular disease (21.42%) and arthritis mutilans (7.16%).
The patients were undergoing treatment with Metotrexate (50%), Sulphasalazine (21.42%), Leflunomide (7.14%) or combination therapy with Metotrexate and Sulphasalazine (21.22%).
The patients had either moderate or high disease activity calculated with DAPSA.
Mean values of clinical, biological and ultrasonography parameters are illustrated in table 1 (Table 1).
Table 1.
Mean, standard deviation, minimum and maximum values of analyzed parameters
| Age | ESR (mm/h) | CRP (mg/L) | DAPSA | LEEDS | BUSES | |
| Mean | 51.28 | 45.5 | 24.35 | 50.32 | 3.96 | 26.14 |
| Standard deviation | 2.22 | 24.52 | 37.52 | 36.78 | 1.23 | 8.51 |
| Minimum | 25 | 10 | 1 | 21 | 2 | 12 |
| Maximum | 67 | 105 | 122 | 145 | 6 | 38 |
Clinical examination of the entheseal sites revealed involvement of Achilles tendons in 20 patients (71.42%), medial femoral condyles in 6 patients (21.42%) and lateral epicondyles of the humerus in 11 patients (39.28%).
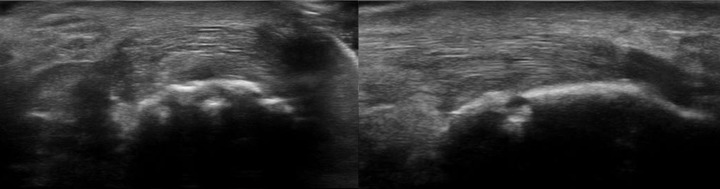
MSUS examination in grey-scale and power Doppler revealed structural abnormalities of Achilles tendons in 21 patients (75%), plantar fascia in 12 patients (42.85%), proximal patellar tendons in 5 cases (17.85%), distal patellar tendons in 4 patients (14.28%), quadriceps tendons in 8 patients (28.57%) and common extensor tendons on the lateral epicondyles in 13 cases (46.42%) (Fig.2,3,4).
Figure 2.
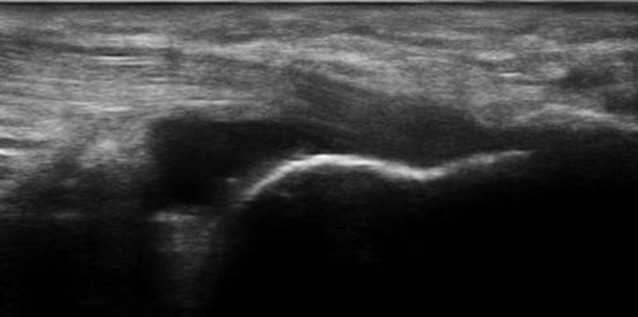
Longitudinal scan of Achilles tendon-preachillean bursitis with the presence of insertional enthesophytes
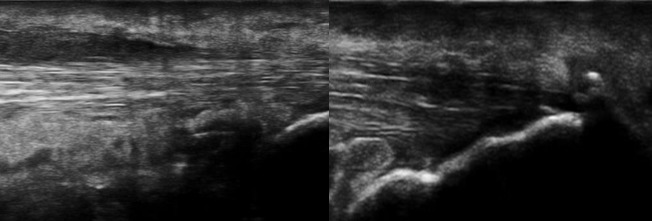
Figure 3.
Longitudinal and transverse scans of Achilles tendon with the presence of an erosion at the insertion of the tendon on the calcaneus
Figure 4.
Longitudinal scan-thickened, inhomogeneous Achilles tendon with hypoechogenicity and loss of fibrillar pattern, peritendinitis and insertional enthesophytes
The ultrasonography score BUSES was not significantly associated either with ESR (p=0.536) or CRP (p=0.965) values. Furthermore, the clinical evaluation through LEI showed no significant association with ESR (p=0.067) or CRP (p=0.206). Despite MSUS and clinical findings, there was no significant correlation between DAPSA and BUSES (p=0,549) or LEI (p=0,197). However, clinical and echographic scores (BUSES and LEI) were significantly associated (p=0.001) (Fig.5).
Figure 5.
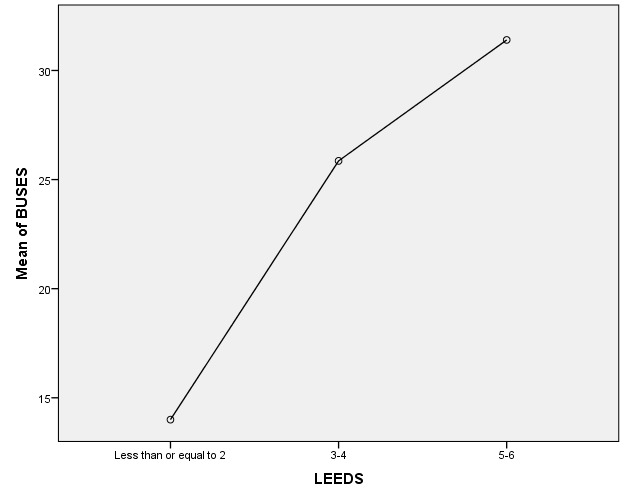
Significant association between BUSES and LEEDS
Results and Discussion
Enthesitis can be asymptomatic or cause tenderness and soft tissue swelling of the affected region. Achilles tendon and plantar fascia involvement are the most frequent findings after clinical examinations in patients with PsA. Entheseal involvement can be related to several conditions such as metabolic disorders (diabetes, gout, and hypercholesterolemia), mechanical stress due to sport related-injuries and degenerative pathologies so the development of specific clinical and ultrasound scoring systems plays an important role in differentiating certain diseases [8].
MSUS examination has proven its utility in evaluating enthesitis in patients with SpA due to the detection of abnormalities and inflammation at the entheseal region and the ability to discriminate between mechanical and inflammatory pathologies. Several studies have proven the utility of US in revealing more subclinical entheseal lesions than physical examination, emphasizing the important role of US in diagnosing PsA in early stages of the disease [9].
False positive entheseal pain may also be detected in clinical evaluation, possibly due to the fact that the pain may be referred from affected peripheral joints, leading to a discrepancy between US and clinical findings [10].
The association between BUSES and LEI has not been studied in literature, to our knowledge, probably due to the relatively recent development of the ultrasound score. However, studies regarding the clinical and ultrasound examination of enthesis in patients with SpA, particularly ankylosing spondylitis proved no correlation between BUSES, disease activity and inflammation markers [11].
A more recent study that evaluated patients using LEI as clinical assessment and an ultrasound score showed a moderate correlation between the clinical and US examination with emphasis on the strong correlation between LEI, hypoechogenicity and tendon thickness. However, there was no significant association between the presence of entesophytes, erosions and LEI, proving that more extensive studies have to be conducted in order to determine the positive association between scores [12].
Despite our findings, some studies have proven no association between clinical indexes and ultrasound scores, probably because the scores measured different entheseal sites [13].
In our study, disease activity score measured by DAPSA did not correlate with US evaluation of the enthesis. Recent PsA studies support our conclusion proving a discrepancy between DAPSA and US examination, the two assessments reflecting different aspects of disease activity [14].
Conclusions
Our study proved a significant correlation between LEI and BUSES, although in literature the evidence is contrasting. This is probably due to the fact that the majority of the patients had high disease activity which made the clinical assessment similar to the US.
Further studies on more numerous groups of patients have to be conducted in order to debate the inconsistencies related to clinical and US examinations in patients with PsA.
Conflict of interests
The authors declare that they have no conflict of interests.
Author contribution
Alesandra Florescu and Cristin Constantin Vere equally contributed to the manuscript.
References
- 1.Terenzi R, Monti S, Tesei G, Carli L. One year in review 2017: spondyloarthritis. Clin Exp Rheumatol. 2018;36(1):1–14. [PubMed] [Google Scholar]
- 2.D’Angelo S, Carriero A, Gilio M, Ursini F, Leccese P, Palazzi C. Safety of treatment options for spondyloarthritis: a narrative review. Expert Opin Drug Saf. 2018;17(5):475–486. doi: 10.1080/14740338.2018.1448785. [DOI] [PubMed] [Google Scholar]
- 3.Apostolakos J, Durant TJ, Dwyer CR, Russell RP, Weinreb JH, Alaee F, Beitzel K, McCarthy MB, Cote MP, Mazzocca AD. The enthesis: a review of the tendon-to-bone insertion. Muscles Ligaments Tendons J. 2014;(3):333–342. [PMC free article] [PubMed] [Google Scholar]
- 4.Micu MC, Fodor D. Concepts in monitoring enthesitis in patients with spondylarthritis-the role of musculoskeletal ultrasound. Med Ultrason. 2016;18(1):82–89. doi: 10.11152/mu.2013.2066.181.mcm. [DOI] [PubMed] [Google Scholar]
- 5.Healy PJ, Helliwell PS. Measuring clinical enthesitis in psoriatic arthritis: assessment of existing measures and development of an instrument specific to psoriatic arthritis. Arthritis Rheum. 2008;59(5):686–691. doi: 10.1002/art.23568. [DOI] [PubMed] [Google Scholar]
- 6.Spadaro A, Iagnocco A, Perrotta FM, Modesti M, Scarno A, Valesini G. Clinical and ultrasonography assessment of peripheral enthesitis in ankylosing spondylitis. Rheumatology (Oxford) 2011;50(11):2080–2086. doi: 10.1093/rheumatology/ker284. [DOI] [PubMed] [Google Scholar]
- 7.D'Agostino MA, Terslev L. Imaging Evaluation of the Entheses. Rheum Dis Clin North Am. 2016;42(4):679–693. doi: 10.1016/j.rdc.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Sakkas LI, Alexiou I, Simopoulou T, Vlychou M. Enthesitis in psoriatic arthritis. Semin Arthritis Rheum. 2013;43(3):325–334. doi: 10.1016/j.semarthrit.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Bandinelli F, Prignano F, Bonciani D, Bartoli F, Collaku L, Candelieri A, Lotti T, Matucci-Cerinic M. Ultrasound detects occult entheseal involvement in early psoriatic arthritis independently of clinical features and psoriasis severity. Clin Exp Rheumatol. 2013;31(2):219–224. [PubMed] [Google Scholar]
- 10.Zhang H, Liang J, Qiu J, Wang F, Sun L. Ultrasonographic evaluation of enthesitis in patients with ankylosing spondylitis. J Biomed Res. 2017;31(2):162–169. doi: 10.7555/JBR.31.20160088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Milutinovic S, Radunovic G, Veljkovic K, Zlatanovic M, Damjanov N. Construct validity and sensitivity to change of Belgrade Ultrasound Enthesitis Score in patients with spondyloarthritis: a pilot study. Rheumatol Int. 2018;38(3):383–391. doi: 10.1007/s00296-017-3898-8. [DOI] [PubMed] [Google Scholar]
- 12.Kristensen S, Christensen JH, Schmidt EB, Olesen JL, Johansen MB, Arvesen KB, Schlemmer A. Assessment of enthesitis in patients with psoriatic arthritis using clinical examination and ultrasound. Muscles Ligaments Tendons J. 2016;6(2):241–247. doi: 10.11138/mltj/2016.6.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ibrahim G, Groves C, Chandramohan M, Beltran A, Valle A, Reyes B, Healy P, Harrison A, Helliwell PS. Clinical and Ultrasound Examination of the Leeds Enthesitis Index in Psoriatic Arthritis and Rheumatoid Arthritis. ISRN Rheumatol. ISRN Rheumatol. 2011;2011:731917–731917. doi: 10.5402/2011/731917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puksic S, Bolton-King P, Sexton J, Michelsen B, Kvien TK, Berner Hammer. DAPSA and ultrasound show different perspectives of psoriatic arthritis disease activity: results from a 12-month longitudinal observational study in patients starting treatment with biological disease-modifying antirheumatic drugs. RMD Open. 2018;4(2):e000765–e000765. doi: 10.1136/rmdopen-2018-000765. [DOI] [PMC free article] [PubMed] [Google Scholar]