Abstract
Although quite rare, retroperitoneum can harbour malignant limphomas. On the grounds that the anatomical location is uncommon and the symptoms are scarce, the diagnosis is usually late and challenging. Imaging methods such as magnetic resonance imaging, computed tomography (CT) and positron emission tomography-computed tomography (PET-CT), can characterize and locate the tumor while endoscopic ultrasound fine needle aspiration (EUS-FNA) may provide pathological confirmation. We present the clinical case of a fifty-five-year-old female that is admitted to our hospital with epigastric discomfort, nausea and vomiting. CT showed a homogenously enhancing mass lesion that encased the pancreas, in contact with the portal vein, inferior vena cava, invading splenomesenteric confluence. To investigate further, EUS-FNA was decided and it revealed lymphocyte proliferation suggestive for the diagnosis of lymphoma. Hereinafter, surgical intervention was performed and immunohistochemical analysis and sub classification of lymphoma was obtained. The final diagnosis was non-Hodgkin lymphoma, Diffuse Large B-Cell Lymphoma (DLBCL). Poly-chemotherapy with R-CHOP was initiated. At the end of the treatment fluorodeoxyglucose positron emission tomography (FDG-PET) was performed and no pathological findings were found. A brief review of literature is also provided.
Keywords: Endoscopic ultrasound guided fine needle aspiration (EUS-FNA); lymph nodes biopsy, retroperitoneal lymphoma; contrast-enhanced computer tomography (CT)-guided; FDG-PET
Introduction
Non Hodgkin Lymphoma (NHL) primarily arising in the retroperitoneum represents a rare entity and has been the object of only a few case-reports [1, 2, 3, 4].
The most common histological subtype of NHL is represented by diffuse Large B-Cell Lymphoma (DLBCL) [5], accounting for 25% of all NHL cases. An extranodal involvement occurs in about 40% of the DLBCLs [6, 7] and the symptoms are highly dependent upon the tumor localization.
The anatomic location is uncommon, therefore the diagnosis and the management of these patients may be difficult, costly and time consuming.
Imaging methods such as magnetic resonance imaging, computed tomography (CT) and positron emission tomography-computed tomography (PET-CT) serve as a useful tool for diagnosis, although it can hinder the differentiation between inflammatory processes and malignancy.
Thus, a pathological confirmation is crucial for the diagnosis of lymphoma. Additionally, a precise pathological classification is critical for the choice of chemotherapeutic regimen in cases of lymphoma.
Image-guided needle biopsy (IGNB) and laparoscopic biopsy have been used to procure tissue from abdominal masses; nevertheless, these are risky and expensive procedures.
Due to ultrasound guidance in real time, Endoscopic ultrasound guided fine needle aspiration (EUS-FNA) enables the sampling of the target lesion and provides a minimally invasive access to the masses located nearby the gastrointestinal tract.
Despite its high accuracy, the application in the classification of lymphomas is limited due to the lack of sufficient tissue material needed for immunohistochemical analysis [8].
We present the clinical case of a female who was admitted to our hospital with epigastric discomfort, caused by a retroperitoneal lymphoma.
It encased the pancreas and it invaded the splenomesenteric confluence.
Case Report
A fifty-five-year-old female presented with a 7-day history of epigastric discomfort, nausea, and vomiting. On clinical examination she had a distended and dull abdomen with a palpable mass with smooth surface and firm consistency in the upper abdomen. The mass did not move with respiration, did not fall forward in the lateral positions nor did it have an intrinsic mobility. There were no enlarged and palpable lymph nodes. The patient did not have significant previous medical history. Eight years ago she underwent laparoscopic right adnexectomy for a benign pathology of the adnexa.
Laboratory work-up yielded a high lactate dehydrogenase value of 658U/L, CA 19-9 value of 3U/ml, CEA value of 2.25ng/ml without other abnormalities. Also, the serological screening for B and C hepatitis (HBsAg and anti-HCV) was negative. Transabdominal ultrasound revealed a thin walled heterogeneous hypoechoic mass lesion in the upper abdomen measuring 15cm x 11cm x 10cm.
An esophagogastroduodenoscopy (EGDS) was performed and it showed an extrinsic compression of the posterior gastric wall and duodenum. Colonoscopy indicated diverticular disease and colonic stricture at the hepatic flexure.
An abdominal contrast-enhanced CT scan indicates the presence of a homogenously enhancing mass lesion, centered retroperitoneally. Its size was 14cm x 11cm×12cm in contact with the pancreas, portal vein, inferior vena cava, also invading splenomesenteric confluence. The contrast-enhanced CT scans pointed out no cleavage limits between the mass, the spleen, and the pancreas. Pre-and para-aortic lymphadenopathies were revealed. (Fig. 1).
Figure 1.
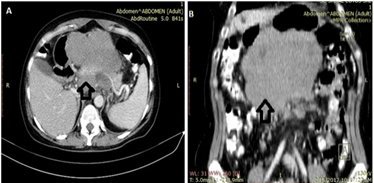
A,B. Axial contrast-enhanced CT showing a retroperitoneal mass with no cleavage limits between the mass, the spleen and the pancreas
The patient underwent EUS examination which revealed the retroperitoneal mass and encasement of the splenomesenteric confluence. EUS-FNA was performed with a 22 Gauge needle (3 passes) in order to obtain a tissue sample. Pathological examination revealed lymphocyte proliferation suggestive for the diagnosis of lymphoma. Immunohistochemical testing was not performed due to the insufficient tissue material (Fig. 2).
Figure 2.
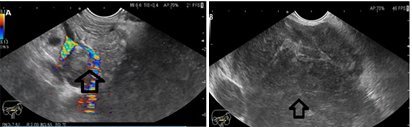
A. Color Doppler endoscopic ultrasound showing the retroperitoneal mass and the encasement of the splenomesenteric confluence; B. Endoscopic ultrasound showing the inhomogenous, hypoechoic retroperitoneal mass with irregular shape
CT scan of the brain and chest, ultrasonography of the neck and axilla and bone marrow biopsies were performed as part of the staging workup along with blood investigations such as complete blood counts with peripheral smear, renal function and liver function tests, Lactate Dehydrogenase (LDH), CEA, CA 19-9, Human Immunodeficiency (HIV), Hepatitis C Virus (HCV) and Hepatitis B surface Antigen (HBsAG).
The CT scan of the brain was unremarkable and CT of the chest revealed no mediastinal adenopathy, pleural or pericardial effusions.
Also, the bone marrow biopsies did not reveal any involvement.
Based on a multidisciplinary team decision the patient underwent surgical intervention with duodenopancreatectomy, cholecystectomy and splenectomy.
The results of pathological and immunohistochemical testing showed: CD20 (large cell+); Ki-67 (large cell 80%+); CD10(+); Bcl-2(+).
The diagnosis was: Non-Hodgkin lymphoma, diffuse large B-cell lymphoma.
The patient was started on rituximab+cyclophosphamide, doxorubicin, vincristine and prednisone (R-CHOP) regimen 6 cycles.
After the sixth course of treatment a total-body FDG-PET was performed and it did not show any pathological findings.
Discussion
Although it is quite rare, retroperitoneum can harbor malignant lymphomas, mostly Hodgkin lymphoma (HL) and non-Hodgkin lymphoma with B-cell lineage (NHL).
Only few cases in the literature described isolated lymphomatous involvement of the retroperitoneum.
The retroperitoneal lymphomatous involvement may be secondary to continuous spread from some other part of the gastrointestinal tract [9] or from abdominal lymph nodes [10].
A variety of underlying non-Hodgkin’s lymphoma can be incriminated for initial involvement of retroperitoneal lymphatic pathways: diffuse large B-cell lymphoma, follicular lymphoma, mantle cell lymphoma and marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT) type.
The most common histological subtype of NHL is DLBCL [11].
On the grounds that the anatomical location is uncommon and the symptoms are scarce for a long time, the diagnosis is late and challenging.
The symptoms are related to the extrinsic obstruction, the most common being abdominal pain, nausea, and postprandial bilious vomiting.
For the positive diagnosis cross-sectional imaging tests (contrast-enhanced CT or MRI) are necessary, thus allowing lesion characterization and localization.
CT represents the diagnostic test of choice for retroperitoneal lymphoma. However MRI in comparison with CT brings superior soft-tissue contrast [12].
In addition MRI can easily differentiate retroperitoneal fibrosis from lymphoma.
It seems that retroperitoneal fibrosis evolves to pelvic extension and medial ureteral bowing. In contrast, lymphoma is associated with retroperitoneal lymph nodes, larger size, suprarenal location, perirenal extension, contrast-enhanced images and heterogeneity on T2-weighted imaging [13].
Furthermore, the use of FDG-PET is required for the complete diagnostic evaluation. FDG-PET combined with computed tomography (PET-CT), represents standard pre-treatment imaging in DLBCL.
However the accumulation of FDG in the intestine or urinary tract can mimic involvement of pelvic and retroperitoneal lymph nodes.
Thus, the difficulty in interpretation of a specific pattern of FDG uptake may determine the need for invasive biopsy [14].
Also, in patients with aggressive lymphomas FDG-PET has recently become a useful prognostic tool.
Relapse risk can be predicted with this kind of investigation made after the chemotherapy. Poorer clinical outcomes were seen in patients with positive FDG-PET scans as compared to patients with negative scans.
Relapses were reported in 100% of the cases in patients where FDG-PET showed residual disease after treatment. In contrast patients with negative PET results had a long term survival in more than 80% of cases [15].
Considering the limited ability of these cross-sectional imaging tests to differentiate between inflammatory/reactive processes and malignancy, a histological evidence is needed to offer a definitive diagnosis.
Pathological evidence is useful not only for the diagnosis, but also for the classification of lymphomas, mandatory for the choice of chemotherapeutic regimen.
Image-guided needle biopsy (IGNB) and laparoscopic biopsy have been used to procure tissue from abdominal masses; nevertheless, these are risky and expensive procedures. CT-to ultrasound-guidance for retroperitoneal masses is usually preferred due to its clear visualization and wide field-of-view. A posterior approach with CT-guidance is preferable to the anterior approach with ultrasound guidance due to the lower risk of bowel perforation and bleeding.
Also, pain and breath motion are decreased with the posterior approach. Additionally, a safe puncture is difficult to perform when retroperitoneal masses are in contact or encase the aorta or inferior vena cava, ureters, kidneys and other structures.
Thus, contrast-enhanced CT-guidance is useful for identification and avoidance of these adjacent major structures [16].
A validated alternative method is represented by EUS-FNA, mostly due to its high accuracy. EUS-FNA offers minimally invasive access to the masses around the gastrointestinal tract enabling biopsy of the target lesion with ultrasound guidance. Although highly specific, EUS-FNA has limitations in order to provide adequate tissue material needed for immunohistochemical staining [17, 18, 19, 20].
An alternative to EUS-FNA represents the use of EUS-guided tru-cut biopsy (EUS-TCB) [21, 22], but with limited value in the classification of lymphoma. Nowadays, different EUS-Fine Needles Biopsy (FNB) are available with variable success rate. Recent studies have shown that FNB needles may be used in rescue procedures when EUS-FNA is non-diagnostic [23,24,25].
An alternative to non-diagnostic EUS-FNA for retroperitoneal NHL has been recently described in a case report.
Thus, EUS-assisted retroperitoneoscopy and lymph node biopsy was performed successfully without any complications [26].
In general, for the extranodal NHL the correct therapeutic approach is based on the combination of chemotherapy, radiotherapy and surgery, although it is the disease localization that may settle the choice of treatment.
The poly-chemotherapy with CHOP and rituximab represents the standard chemotherapy regimen for DLBCL treatment with a complete response in 45-53% of cases and long-term survival of 30-37% [27].
Conclusions
The prognosis of NHL has improved in recent years due to the advancement of various aggressive chemotherapeutic treatments according to the histological type and the stage of the tumor.
Thus, a definitive histological diagnosis is mandatory and minimally invasive techniques according to the location of NHL must be the first options.
Furthermore, EUS-FNA and more probably EUS-FNB need to be validated as standard diagnosis procedures for retroperitoneal masses.
Acknowledgments
This work was supported by a grant of Ministry of Research and Innovation, CNCS-UEFISCDI, project number PN-III-P4-ID-PCE-2016-0561, within PNCDI III.
References
- 1.Jiménez MB, Pérez BM. Obstructive Jaundice by a Retroperitoneal Lymphoma Mimics a Pancreatic Cancer: A Case Report. J of Pancreas. 2018;19(3):151–156. [Google Scholar]
- 2.Pileri SA, Zinzani PL, Ascani S, Orcioni GF, Gamberi B, Piccioli M, Sabattini E, Poggi S, Piccaluga PP, Falini B. Diffuse large B-cell lymphoma with primary retroperitoneal presentation: Clinico-pathologic study of nine cases. Ann Oncol. 2001;12(10):1445–1453. doi: 10.1023/a:1012559725243. [DOI] [PubMed] [Google Scholar]
- 3.Guo J, Sun B, Wang S, Ge N, Wang G, Wu W, Liu X, Sun S. Diagnosis of lymphoma by endoscopic ultrasound-assisted transendoscopic direct retroperitoneal lymph node biopsy: A case report (with video) Endosc Ultrasound. 2015;4(1):69–72. doi: 10.4103/2303-9027.151368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fulignati C, Pantaleo P, Cipriani G, Turrini M, Nicastro R, Mazzanti R, Neri B. An uncommon clinical presentation of retroperitoneal non-Hodgkin lymphoma successfully treated with chemotherapy: A case report. World J Gastroenterol. 2005;11(20):3151–3155. doi: 10.3748/wjg.v11.i20.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaffe ES, Swerdlow SH, Vardiman JW. Haematopoietic and lymphoid malignancies. In: Stewart BW, Wild CP, editors. World cancer report 2014. Lyon: International Agency for Research on Cancer. World Health Organization; 2014. pp. 482–494. [Google Scholar]
- 6.Møller MB, Pedersen NT, Christensen BE. Diffuse large B-cell lymphoma: clinical implications of extranodal versus nodal presentation-a population-based study of 1575 cases. Br J Haematol. 2004;124(2):151–159. doi: 10.1046/j.1365-2141.2003.04749.x. [DOI] [PubMed] [Google Scholar]
- 7.Armitage JO, Mauch PM, Harris NL. Non-Hodgkin's lymphomas. In: de Vita., editor. Cancer: Principles and Practice of Oncology. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 2256–2316. [Google Scholar]
- 8.Ge N, Sun S. Endoscopic ultrasound: An all in one technique vibrates virtually around the whole internal medical field. J Transl Intern Med. 2014;2:104–106. [Google Scholar]
- 9.Lin YN, Chou JW, Chuang PH, Cheng KS, Peng CY, Chiang IP. Primary small intestinal natural killer/T cell lymphoma mimicking tuberculous peritonitis: report of a case and review of the literature. Intern Med. 2011;50(5):515–518. doi: 10.2169/internalmedicine.50.4435. [DOI] [PubMed] [Google Scholar]
- 10.Sharifah MI, Zamzami NA, Rafeah TN. Diffuse peritoneal lymphomatosis simulating peritoneal carcinomatosis. Medical Journal of Malaysia. 2011;66(3):270–272. [PubMed] [Google Scholar]
- 11.Ravindhran B, Prakash C, Govindharaj S, Shawnaz Bahnou, Pavithra B. An Aggressive Primary Retroperitoneal Diffuse Large B-Cell Lymphoma Mimicking a Pancreatic Neoplasm, Presenting as Duodenal Stenosis. J Clin Diagn Res. 2017;11(9):PD09–PD11. doi: 10.7860/JCDR/2017/27222.10611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vinnicombe SJ, Reznek RH. Computerised tomography in the staging of Hodgkin's disease and non-Hodgkin's lymphoma. Eur J Nucl Med Mol Imaging. 2003;1:S42–55. doi: 10.1007/s00259-003-1159-4. [DOI] [PubMed] [Google Scholar]
- 13.Rosenkrantz AB, Spieler B, Seuss CR, Stifelman MD, Kim S. Utility of MRI Features for Differentiation of Retroperitoneal Fibrosis and Lymphoma. AJR Am J Roentgenol. 2012;199(1):118–126. doi: 10.2214/AJR.11.7822. [DOI] [PubMed] [Google Scholar]
- 14.Tateishi U, Terauchi T, Inoue R, Tobinai K. Nodal status of malignant lymphoma in pelvic and retroperitoneal lymphatic pathways: PET/CT. Abdom Imaging. 2010;35(2):232–240. doi: 10.1007/s00261-009-9516-9. [DOI] [PubMed] [Google Scholar]
- 15.Schot B, van Imhoff, Pruim J, Sluiter W, Vaalburg W, Vellenga E. Predictive value of early 18F-fluoro-deoxyglucosepositron emission tomography in chemosensitive relapsed lymphoma. Br J Haematol. 2003;123:282–287. doi: 10.1046/j.1365-2141.2003.04593.x. [DOI] [PubMed] [Google Scholar]
- 16.El-Badrawy A, Tawfik A, Abdelfattah A, Monir A, Salah-Eldin A, Azmy EE, El-Tantawy D, El-Karef A. Contrast-Enhanced CT-Guided Core Biopsy of Retroperitoneal Masses. Open Journal of Radiology. 2014;4:130–135. [Google Scholar]
- 17.Vishnu P, Wingerson A, Lee M, Mandelson MT, Aboulafia DM. Utility of Bone Marrow Biopsy and Aspirate for Staging of Diffuse Large B Cell Lymphoma in the Era of Positron Emission Tomography With 2-Deoxy-2-[Fluorine-18]fluoro-deoxyglucose Integrated With Computed Tomography. Clin Lymphoma Myeloma Leuk. 2017;17(10):631–636. doi: 10.1016/j.clml.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 18.Vilmann P, Jacobsen GK, Henriksen FW, et al. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc. 1992;38(2):172–173. doi: 10.1016/s0016-5107(92)70385-x. [DOI] [PubMed] [Google Scholar]
- 19.Eloubeidi MA, Wallace MB, Reed CE, Hadzijahic N, Lewin DN, Van Velse, Leveen MB, Etemad B, Matsuda K, Patel RS, Hawes RH, Hoffman BJ. The utility of EUS and EUS-guided fi ne needle aspiration in detecting celiac lymph node metastasis in patients with esophageal cancer: A single-center experience. Gastrointest Endosc. 2001;54(6):714–719. doi: 10.1067/mge.2001.119873. [DOI] [PubMed] [Google Scholar]
- 20.Levy MJ, Jondal ML, Clain J, Wiersema MJ. Preliminary experience with an EUS-guided trucut biopsy needle compared with EUS-guided FNA. Gastrointest Endosc. 2003;57(1):101–106. doi: 10.1067/mge.2003.49. [DOI] [PubMed] [Google Scholar]
- 21.Eloubeidi MA, Mehra M, Bean SM. EUS-guided 19-gauge trucut needle biopsy for diagnosis of lymphoma missed by EUS-guided FNA. Gastrointest Endosc. 2007;65(6):937–939. doi: 10.1016/j.gie.2006.08.036. [DOI] [PubMed] [Google Scholar]
- 22.Ribeiro A, Pereira D, Escalón MP, Goodman M, Byrne GE. EUS-guided biopsy for the diagnosis and classifi cation of lymphoma. Gastrointest Endosc. 2010;71(4):851–855. doi: 10.1016/j.gie.2009.12.033. [DOI] [PubMed] [Google Scholar]
- 23.Polkowski M, Jenssen C, Kaye P, Carrara S, Deprez P, Gines A, Fernández-Esparrach G, Eisendrath P, Aithal GP, Arcidiacono P, Barthet M, Bastos P, Fornelli A, Napoleon B, Iglesias-Garcia J, Seicean A, Larghi A, Hassan C, van Hooft, Dumonceau JM. Technical aspects of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline-March 2017. Endoscopy. 2017;49(10):989–1006. doi: 10.1055/s-0043-119219. [DOI] [PubMed] [Google Scholar]
- 24.Aadam AA, Wani S, Amick A, Shah JN, Bhat YM, Hamerski CM, Klapman JB, Muthusamy VR, Watson RR, Rademaker AW, Keswani RN, Keefer L, Das A, Komanduri S. A randomized controlled cross-over trial and cost analysis comparing endoscopic ultrasound fine needle aspiration and fine needle biopsy. Endosc Int Open. 2016;4(5):E497–505. doi: 10.1055/s-0042-106958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kandel P, Tranesh G, Nassar A, Bingham R, Raimondo M, Woodward TA, Gomez V, Wallace MB. EUS-guided fine needle biopsy sampling using a novel fork-tip needle: a case-control study. Gastrointest Endoscopy. 2016;84(6):1034–1039. doi: 10.1016/j.gie.2016.03.1405. [DOI] [PubMed] [Google Scholar]
- 26.Guo J, Sun B, Wang S, Ge N, Wang G, Wu W, Liu X, Sun S. Diagnosis of lymphoma by endoscopic ultrasound assisted transendoscopic direct retroperitoneal lymph node biopsy: A case report. Endosc Ultrasound. 2015;4(1):69–72. doi: 10.4103/2303-9027.151368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cai YL, Xiong XZ, Lu J, Lin YX, Cheng NS. Non-Hodgkin's lymphoma with uncommon clinical manifestations: A case report. Oncol Lett. 2015;10(3):1686–1688. doi: 10.3892/ol.2015.3493. [DOI] [PMC free article] [PubMed] [Google Scholar]


