Abstract
OBJECTIVES
We reviewed our combined clinical outcome in patients who underwent lung transplantation after ex vivo lung perfusion (EVLP) and compared it to the contemporary control group.
METHODS
At 2 Scandinavian centres, lungs from brain-dead donors, not accepted for donation but with potential for improvement, were subjected to EVLP (n = 61) and were transplanted if predefined criteria were met. Transplantation outcome was compared with that of the contemporary control group consisting of patients (n = 271) who were transplanted with conventional donor lungs.
RESULTS
Fifty-four recipients from the regular waiting list underwent transplantation with lungs subjected to EVLP (1 bilateral lobar, 7 single and 46 double). In the EVLP and control groups, arterial oxygen tension/inspired oxygen fraction ratio at arrival in the intensive care unit (ICU) was 30 ± 14 kPa compared to 36 ± 14 (P = 0.005); median time to extubation was 18 h (range 2–912) compared to 7 (range 0–2280) (P = 0.002); median ICU length of stay was 4 days (range 2–65) compared to 3 days (range 1–156) (P = 0.002); Percentage of expected forced expiratory volume at 1s (FEV1.0%) at 1 year was 75 ± 29 compared to 81 ± 26 (P = 0.18); and the 1-year survival rate was 87% [confidence interval (CI) 82–92%] compared to 83% (CI 81–85), respectively. Follow-up to a maximum of 5 years did not show any significant difference in survival between groups (log rank, P = 0.63).
CONCLUSIONS
Patients transplanted with lungs after EVLP showed outcomes comparable to patients who received conventional organs at medium-term follow-up. Although early outcome immediately after transplantation showed worse lung function in the EVLP group, no differences were observed at a later stage, and we consider EVLP to be a safe method for increasing the number of transplantable organs.
Keywords: Ex vivo lung perfusion , Lung transplantation , Lung reconditioning
INTRODUCTION
Lung transplantation (LTx) is an established treatment option for patients with end-stage pulmonary disease. The number of lung transplant procedures performed annually has consistently been increasing with encouraging and improving long-term results [1, 2].
The published rate of accepted organs from multiorgan donors varies from 15% to 50% [3], implying that in some regions or centres, up to 85% of available lungs are declined. Extended donor criteria and a more aggressive approach to optimize potential donor lungs in situ may increase organ availability in centres with a low acceptance rate.
Ex vivo lung perfusion (EVLP) has proven its potential by successfully differentiating between reversible and non-reversible lung pathology prior to transplantation [4–12]. Uncertainty over donor lung quality is often the reason for rejecting the organs. Some of these organs may not meet strict transplantation criteria at first evaluation, but in reality they may have the function that allows transplantation. In our setting, the evaluation of marginal organs ex vivo by the transplant team has increased the yield of organs available for transplantation. In the future, EVLP may also be a means of organ improvement by using different treatment strategies during EVLP.
The first successful LTx after EVLP was reported by Steen et al. [13] in 2001. EVLP has since gained increasing interest in transplant centres around the world. In 2008, Cypel et al. [14] in Toronto published an article on the extended EVLP assessment of lung function using a novel strategy, which was the starting point for the ‘Toronto protocol’. In 2011, the group published their first clinical study [15]. This was followed by short- to mid-term results in smaller series and case reports [4, 16–20], while publications on long-term results are still scarce [6, 21].
In our 2 Scandinavian centres, EVLP programmes were introduced in clinical practice in 2011 and 2012 [10, 22, 23]. Herein, we review the outcomes for patients who were transplanted with EVLP lungs and compare results to a control group of recipients who were transplanted with conventional lungs during the same period.
MATERIALS AND METHODS
Study design
The ethics committees of the University of Gothenburg and the University of Copenhagen approved this study. All patients were informed and consented to the possibility of receiving organs that had undergone EVLP when they were listed for transplantation. The organs were otherwise matched according to standard criteria.
At the outset, inclusion criteria for EVLP evaluation of rejected donor lungs were an arterial oxygen tension/inspired oxygen fraction (PaO2/FiO2) ratio <40 kPa and/or X-ray findings that were consistent with pulmonary oedema. Later, criteria were expanded to also include donor lungs for which it was not possible to properly evaluate in the donor (patient on veno-arterial extra corporeal membrane oxygenation (va-ECMO)), or ones with suspected lung injury (donors with pulmonary embolism or severe trauma as cause of death), or donor history, radiological or macroscopic findings suggesting severely impaired lung function that prevents the use of the organs. The decision to proceed to EVLP with lungs that were rejected for direct use was made after discussion between at least 2 transplant surgeons.
The procurement of the donor lungs was performed according to the local standard protocol. During transport, the organs were stored cold on ice. EVLP was performed at the recipient hospital.
Patients from our 2 centres were prospectively and consecutively included in this study between January 2011 and December 2015, and they were followed up until the end of December 2016. Lungs from brain-dead donors that were primarily rejected for transplantation were considered for EVLP. Lungs that achieved acceptable lung function during EVLP were transplanted. In both centres, acceptable lung function was defined as follows: (i) PaO2/FiO2 ratio >40 kPa; (ii) pulmonary vascular resistance (PVR) and pulmonary compliance deemed normal under EVLP conditions (350–650 dyn⋅s/cm5 and >50 ml/cm H2O, respectively) and not deteriorating during EVLP; and (iii) macroscopic appearance and manual inspection without major pathology. Outcomes in recipients who were transplanted with lungs after EVLP or without prior EVLP were compared.
Ex vivo lung perfusion
EVLP at our 2 centres was performed based on a modified version of the procedure described by Steen et al. [24]. Equivalent protocols were applied at both institutions using the Vivoline LS1 device, in which lungs were perfused with Steen solution mixed with red blood cells to a haematocrit of 10–15%, 10 000 U of heparin and 100 mg of meropenem. Evaluation was performed at full perfusate flow. Acceptance criteria differed between our 2 centres with regard to Pao2/FiO2 (see below).
Gothenburg
The EVLP procedure has been described in detail previously [25]. Perfusion of the lungs was restricted to 70 ml/min/kg donor weight. The pressure limit of the pulmonary artery (PA) was increased to 20 mmHg during the evaluation phase. PA flow and thereby pressure was gradually increased. Mechanical ventilation with a positive end-expiratory pressure of 5 cm H2O and a tidal volume of 6–8 ml/kg were initiated at 32°C. At this stage, a bronchoscopy was performed to clear any secretions and to inspect the bronchial tree. At 36°C, a lung recruitment manoeuvre with increasing positive end-expiratory pressure levels under visual inspection was performed. Repeated samples for gas analysis were drawn from the left atrium and compared to simultaneous samples from the PA. The pO2 of the deoxygenated blood in the PA never exceeded 7 kPa. A physiological dead space fraction (calculated as PaCO2–EtCO2/PaCO2), static lung compliance and PVR were continuously monitored.
The acceptance of organs for transplantation was based on the following criteria: (i) a PaO2/FiO2 ratio >40 kPa during the evaluation phase; (ii) stable haemodynamic and respiratory variables (PVR, peak airway pressures and lung compliance) during EVLP. No absolute cut-off levels for these variables were used. A negative trend with deterioration of physiological variables during EVLP was considered a relative contraindication for transplantation; (iii) the absence of macroscopic signs of pneumonic infiltrates or lung infarctions; and (iv) a normal collapse test. Accepted lungs were surface cooled in the EVLP system awaiting transplantation.
Copenhagen
The EVLP procedure has been described in detail previously [23]. Lung protective ventilation was initiated at 32°C. At 36°C, blood gases were drawn to assess whether the evaluation phase could be entered. Evaluation was performed at 36°C, following lung recruitment manoeuvres and bronchoscopy. Lungs were approved if pCO2 < 6 kPa and if pO2 > 50 kPa at FiO2 = 1.0 or pO2 > 13 kPa at FiO2 = 0.21. The collapse test was performed to evaluate pulmonary oedema. Accepted lungs were surface cooled in the EVLP system awaiting transplantation.
Lung transplantation and outcome analyses
Recipient characteristics are reported in Table 1. Surgery was performed as to local preference and routine, either via bilateral sequential thoracotomy or sternotomy, either with or without extracorporeal circulation. After the operation, all patients received care and treatment according to standard protocols. The ventilator time was defined as time to extubation in hours. The time in intensive care unit (ICU) was defined as the number of days from ICU arrival to general ward discharge. If the recipient was re-intubated during the index procedure hospitalization, the ventilator time was defined as the total ventilator treatment time.
Table 1:
Recipient characteristics
| Recipient variables | EVLP (n = 54) | Conventional (n = 271) |
|---|---|---|
| Age (years), mean ± SD | 52 ± 12 | 51 ± 13 |
| Diagnosis (%) | ||
| IPF | 24 | 25 |
| PAH | 2 | 6 |
| COPD | 33 | 28 |
| Alfa-1-antitrypsin deficiency | 6 | 13 |
| CF | 20 | 12 |
| Other | 15 | 16 |
| Patients on preoperative mechanical ventilation, n (%) | 5 (9.3) | 12 (4.4) |
| ECMO, n (%) | 1 (1.9) | 16 (5.9) |
CF: cystic fibrosis; COPD: chronic obstructive pulmonary disease; ECMO: extracorporeal membrane oxygenation; EVLP: ex vivo lung perfusion; IPF: interstitial pulmonary fibrosis; PAH: pulmonary artery hypertension; SD: standard deviation.
Statistical analyses
Continuous data are presented as mean and standard deviation or median and range. Categorical data are presented as frequency and/or percentage. Differences between groups were evaluated with the Mann–Whitney U-test or the Student’s t-test. A P-value of <0.05 was considered as statistically significant. Kaplan–Meier curves were used for survival plots and the log-rank test for comparison of proportional survival between the groups. The Shapiro–Wilk test was used for testing normality.
Cox proportional hazard regression was used to examine the relative risk of death between the studied groups. A test of the proportional hazards, which was a required assumption of Cox regression, was performed using a formal significance test based on the unscaled and scaled Schoenfeld residuals.
RESULTS
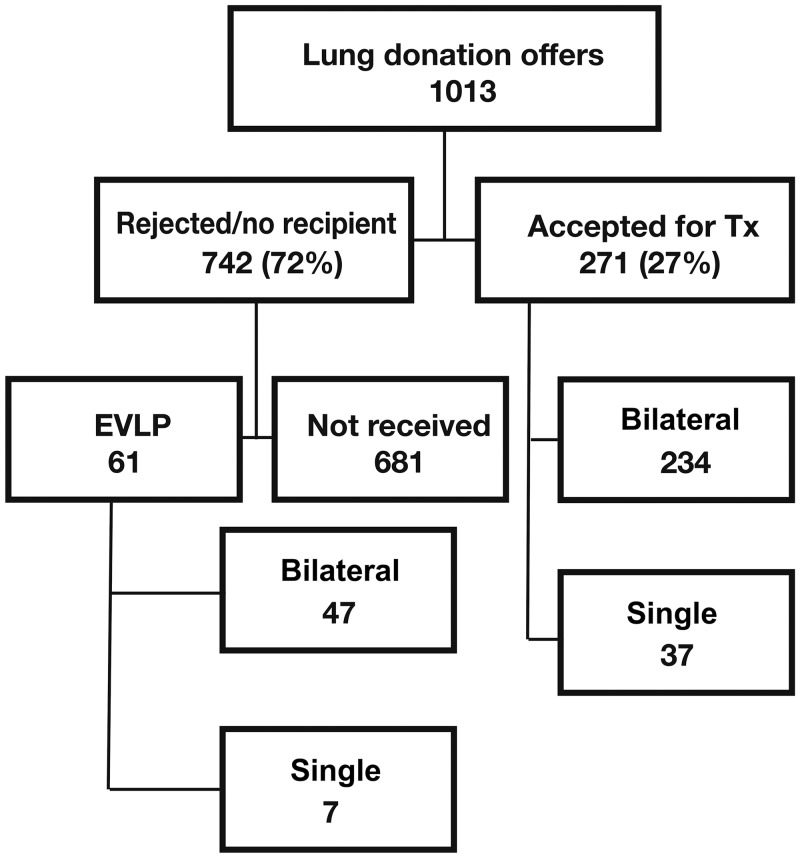
During the 4-year study period, from January 2011 to December 2015, with Gothenburg initiating its clinical EVLP programme in January 2011 and Copenhagen in May 2012, lungs from 1013 donors were offered to our 2 centres (Fig. 1). This number includes all brain-dead donors (donation after circulatory death (DCD) donation was not performed in our 2 countries during the study period), irrespective of donor age. Patients who underwent all other contemporary LTx (n = 271) procedures during the same time interval, with organs accepted according to standard selection criteria not requiring EVLP, were included as the control group. Retransplantations during the study period were excluded.
Figure 1:
All donor lungs offered to our 2 centres during the study period. Bilateral: bilateral sequential lung transplantation; EVLP: ex vivo lung perfusion; Single: single lung transplantation; Tx: transplantation.
Ex vivo lung perfusion
In our combined cohort, 61 patients with donor lungs underwent EVLP. The mean donor PaO2/FiO2 ratio was 30.6±12 kPa (excluding 2 donors on VA-ECMO). In cases where donor PaO2/FiO2 met standard acceptance criteria, reasons for EVLP were as follows: atelectasis unresponsive to ventilator lung recruitment manoeuvres (n = 4), infiltrates on pulmonary X-ray (n = 4), and donor on VA-ECMO (n = 2). No EVLP was performed for logistical reasons.
After EVLP, 47 pairs of lungs were deemed transplantable. In one of these cases, the lung pair was split and transplanted in 2 different recipients. In 1 case, bilateral bilobar transplantation was performed. In 5 cases, one of the lungs was used for single LTx, and the other discarded after EVLP. The conversion rate expressed as the total number of lungs transplanted to the total number of lungs placed on EVLP (99/122) was 81%. Another way of expressing conversion rate would be that in 85% of EVLP runs either one or both lungs were transplanted. EVLP data during evaluation are presented in Table 2.
Table 2:
EVLP data from lung pairs later transplanted (n = 53)
| EVLP variables | Mean | Median | Range |
|---|---|---|---|
| PaO2 (kPa)a | 58.5 ± 10.0 | 57.8 | 31.4–79.2 |
| Compliance (ml/cm H2O)a | 55 ± 28 | 52 | 19–151 |
| EVLP reconditioning time (min) | 200 ± 94 | 175 | 76–577 |
| EVLP weight change (g) | +49 ± 236 | 54 | −600 ± 500 |
Final measurement at EVLP evaluation forming the basis for the decision to accept the organs.
EVLP: ex vivo lung perfusion.
Transplantation
Forty-six bilateral, 1 bilobar and 7 single LTxs were performed after EVLP, compared to 246 bilateral and 37 single LTxs in the control group (Table 3). The use of intraoperative extracorporeal circulation or extracorporeal membrane oxygenation was similar in the 2 groups.
Table 3:
Intra- and postoperative characteristics
| Variables | EVLP | Control | P-value |
|---|---|---|---|
| Number of patients | 54 | 271 | |
| Single lung transplantation, n (%) | 7 (13) | 37 (14) | |
| Bilateral sequential lung transplantation (including one bilobar LTx), n (%) | 47 (87) | 234 (86) | |
| Intraoperative ECC/ECMO, n (%) | 29 (54) | 125 (46) | |
| PaO2/FiO2 at arrival in ICU (kPa), mean ± SD | 30 ± 14 | 36 ± 14 | 0.005 |
| Time to extubation (h), median (range) | 18 (2–912) | 7 (0–2280) | 0.002 |
| ICU stay (days), median (range) | 4 (2–65) | 3 (1–156) | 0.002 |
| Time to discharge (days), median (range) | 30 (17–112) | 28 (12–268) | 0.35 |
| FEV1% at 12 months, mean ± SD | 75 ± 29 | 81 ± 26 | 0.18 |
| 1-Year survival | 87% (CI 82–92%) | 83% (CI 81–85%) |
CI: confidence interval; ECC: extracorporeal circulation; ECMO: extracorporeal membrane oxygenation; EVLP: ex vivo lung perfusion; FEV1: forced expiratory ventilation in the 1 s; ICU: intensive care unit; LTx: lung transplantation; PaO2/FiO2: arterial oxygen tension/inspired oxygen fraction; SD: standard deviation.
Early postoperative results
One patient in the EVLP group and 4 in the conventional group died within the first 48 h after transplantation (Table 3). Death in none of these cases was attributable to insufficient lung function. PaO2/FiO2 at arrival in ICU was 30 ± 14 kPa in the EVLP group compared to 36 ± 14 in the control group (P = 0.005). When comparing the EVLP group to the control group, median time to extubation was 18 h (range 2–912) vs 7 h (range 0–2280 h) (P = 0.002), median ICU stay was 4 days (range 2–65) vs 3 days (range 1–156) (P = 0.002) and time to discharge to home or a rehabilitation facility was 30 days (range 17–112) vs 28 days (range 12–268), respectively (P = 0.35).
Follow-up
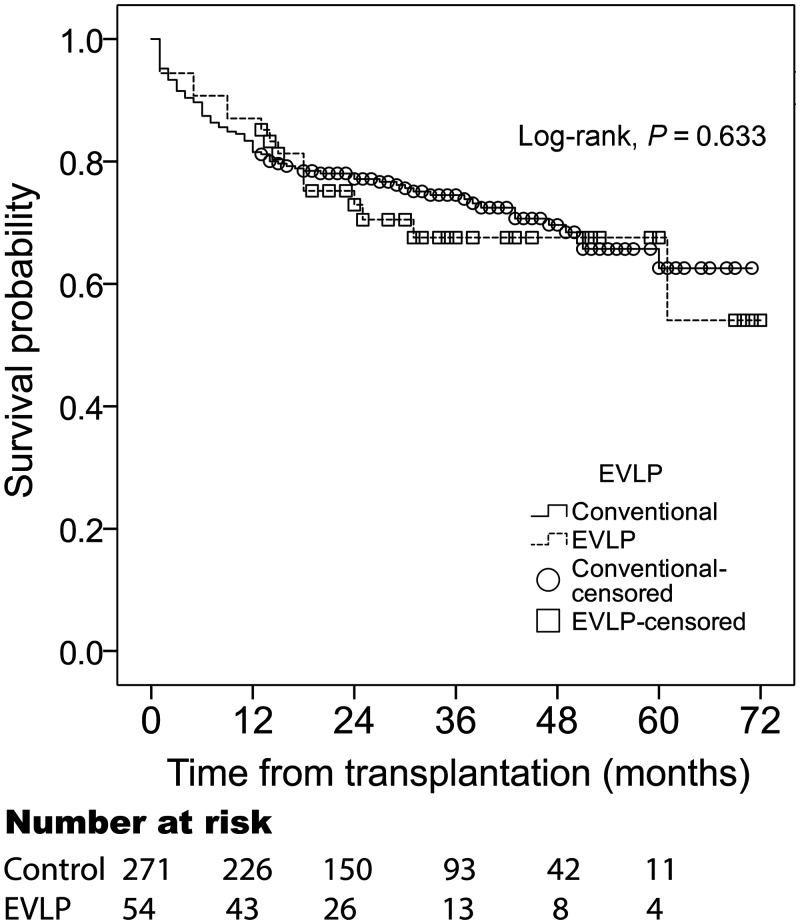
One-year retransplantation-free survival was 87% [confidence interval (CI) 82–92%] in the EVLP group and 83% (CI 81–85%) in the conventional group. Cumulative retransplantation-free survival did not differ significantly between the groups (log rank, P = 0.63) (Fig. 2) during the entire period in our combined cohorts. Causes of death in the EVLP group are reported in Table 4.
Figure 2:
Survival and freedom from retransplantation after lung transplantation. The combined EVLP and control groups had a similar survival and freedom from retransplantation after transplantation. EVLP: ex vivo lung perfusion.
Table 4:
Cause of death in EVLP recipients
| Diagnosis | Type of transplantation | Time after operation | Causes |
|---|---|---|---|
| PAH | Bilateral sequential | 31 months | Chronic graft failure |
| Fibrosis | Bilateral sequential | 24 months | Squamous cell carcionoma, bronchial stenosis after radiotherapy |
| Fibrosis | Bilateral sequential | 18 months | Graft failure secondary to pneumonia |
| α1AT deficiency | Bilateral sequential | 25 months | Graft failure secondary to recurrent pulmonary infections |
| COPD | Bilateral sequential | 18 months | Graft failure secondary to pneumonia |
| CF | Bilateral sequential | 5 months | Graft failure secondary to pneumonia |
| CF | Bilateral sequential | 13 months | Chronic graft failure, bronchial stenosis |
| COPD | Bilateral sequential | 36 months | Respiratory failure secondary to recurrent infections and rejection |
| COPD | Bilateral sequential | 5 months | PRES, CNI intolerance, rejection |
| Fibrosis | Bilateral sequential | 1 day | Bleeding, graft failure, ECMO |
| Fibrosis | Bilateral sequential | 1 day | Bleeding |
| Fibrosis | Bilateral sequential | 61 months | Unexpected death during sleep at home |
| Fibrosis | Bilateral sequential | 9 months | BOS |
| Fibrosis | Bilateral lobar | 14 months | Pulmonary embolism and BOS |
| Fibrosis | Single | 18 months | BOS |
α1AT: alpha 1-antitrypsin; BOS: bronchiolitis obliterans; CF: cystic fibrosis; CNI: calcineurin inhibitor; COPD: chronic obstructive pulmonary disease; ECMO: extracorporeal membrane oxygenation; PRES: posterior reversible encephalopathy syndrome; PAH: pulmonary artery hypertension.
Cox proportional hazards regression was performed. The hazard ratio for EVLP was 1.14 (95% CI 0.67–1.93), P = 0.62. The assumption of proportional hazards was tested as described in the statistics section and fulfilled. Cox regression did not show a significant difference in survival between groups. Data indicated a 14% higher relative risk for death or retransplantation in the EVLP group with a wide CI that did not reach significance.
Pulmonary function test
FEV1.0% was 75 ± 29% and 81 ± 26% at 1 year in the EVLP and control groups, respectively (P = 0.18).
DISCUSSION
The main finding of the present study in 2 Scandinavian centres was that the cumulative retransplantation-free survival for up to 5 years in patients transplanted with EVLP-evaluated lungs was comparable to a control group of all contemporary patients transplanted with non-EVLP organs. PaO2/FiO2 at arrival in ICU was lower, and time to extubation and time in ICU were significantly longer in the EVLP group. There was no difference in time to discharge from hospital, lung function at 1 year, or mortality or need for retransplantation. This indicates that the selection of donor organs for EVLP, as well as the selection of lungs for transplantation after EVLP, was adequate. This study is based exclusively on organs transplanted from brain-dead donors.
During the study period, the acceptance rate at our 2 institutions for conventional lungs without prior EVLP was 27%, a number higher than what many international centres report [1, 2]. Of the organs not fulfilling standard criteria, 61 were selected for EVLP evaluation. In contrast to others, while applying wider indications for EVLP [6] we have only accepted lungs with poor gas exchange or other clear contraindications for transplantation for EVLP. Fifty-four patients were transplanted with EVLP lungs, in which the conversion rate for EVLP-evaluated lungs was 81–85%. The selection for EVLP is still mainly based on clinical judgement, but the criteria for the selection of donor lungs suitable for EVLP will need to be explored further in future studies.
Implementing an EVLP programme can significantly increase the number of organs available for transplantation, possibly even utilizing up to as many as 50% of donor lungs. Centres with an already high acceptance rate can expect to have a significant addition of available organs by the introduction of EVLP.
EVLP strategies at our 2 institutions have been described previously [22, 23] and are based on the protocol initially developed by Steen et al. [13], using a cellular perfusate with banked blood added to a haematocrit of 10–15%, an open left atrium and evaluation of the lungs at relatively high pressure and flow. Since the introduction of EVLP, this protocol has been implemented at our institutions after minor modifications. No study has so far presented a validated algorithm for which lungs to accept for transplantation following EVLP. P/F-ratio and macroscopic appearance are still the main determinants of whether to proceed to transplantation, while other variables such as compliance, PVR dynamics and dead space fraction may provide supporting evidence.
The optimal yield of transplantable organs after EVLP depends on several factors, including selection criteria for considering EVLP, the experience of the transplant team and thresholds for proceeding to transplantation. At our two centres, the decision to use initially non-acceptable marginal donor lungs was initially made by junior retrieval surgeons in the donor hospital, followed by more experienced surgeons and anaesthesiologists after EVLP. It could be argued that sending more experienced senior surgeons for organ retrievals could further increase the acceptance rate, thereby avoiding the quite substantial costs associated with EVLP evaluations in selected cases.
During the introduction of the EVLP programme at our 2 centres, funding was provided by research grants. However, after being adopted as a clinical routine procedure, it is now financed via the tax-based, general public health care system, in line with other medical procedures.
In this study, the conversion rate was 81%, which is high, but in agreement with most previous studies reporting a conversion rate between 46% and 100% [4, 6, 22]. The DEVELOP-UK study stands out in this aspect, reporting a conversion rate of only 34% [26]. It was an ambitious, multicentre observational study that compared EVLP to conventional lungs, which was terminated early due to slow recruitment and concerns about high levels of the use of ECMO in the EVLP arm. One could speculate that one reason for the low conversion rate may be that several of the participating centres had little experience in EVLP and consequently adopted a more conservative approach in accepting organs.
It could be argued that donor lungs that other centres would have considered for conventional transplantation were exposed to unnecessary EVLP in our centre, i.e. selecting very good lungs for EVLP. However, as our acceptance rate is already comparatively high, in combination with the fact that lungs subjected to EVLP in this study were first declined by multiple centres for conventional transplantation, we do not believe that other centres would have used any of these lungs for conventional transplantation. The high conversion rate may indicate that even more lungs should undergo EVLP, hopefully resulting in more lungs for transplantation. Martens et al. [27] published a retrospective database analysis of unused lung donors, identifying a large potential for EVLP to further increase the donor pool in transplant centres, even when the majority were already extended criteria donor lungs.
Different strategies for EVLP have been suggested and reported [14]. This study indicates that good long-term results can be achieved with more than 1 EVLP protocol. In a recently published study, where 2 EVLP strategies were compared in a porcine experimental setup, we could not show any significant difference in lung performance [28].
Our results indicate a significantly longer time on ventilator and a longer length of stay in ICU in patients receiving EVLP-treated lungs. This is not a surprise, because functionally less optimal organs were selected for EVLP and later transplanted. The time on EVLP is relatively short with a median of 175 min (range 76–577 min). It has been suggested that longer EVLP time could reduce oedema. Our clinical data show that lungs might lose or gain weight, i.e. fluid, during EVLP, and EVLP does not necessarily decrease oedema content in a specific pair of lungs. This has further been evaluated by our group in a recent publication [29]. Hemofiltration during EVLP, to increase perfusate oncotic pressure and thereby optimize oedema reduction, may be a way forward [25].
Our data show a comparable outcome in patients who were transplanted with EVLP lungs and controls. One-year retransplantation-free survival was close to 90%. There is no statistically significant difference between groups in our study with regard to long-term survival or freedom from retransplantation; however, numbers at risk are very low beyond 3 years after transplantation.
In our experience, although short-term outcome was inferior in the group transplanted with lungs after EVLP compared to conventional lungs, the medium-term follow-up showed similar results between groups. EVLP seems to be a safe method of increasing the availability of transplantable organs.
ACKNOWLEDGEMENTS
The authors thank the Lung Transplant Teams at the Sahlgrenska University Hospital and Rigshospitalet for their dedicated work.
Funding
This work was supported by the Swedish Heart and Lung Foundation, the Jan Elgqvist Foundation and the Sahlgrenska University Hospital.
Conflict of interest: Göran Dellgren has research grants from Astellas A/S for a study in immunosuppression after lung transplantation (the ScanCLAD study) and from Abbott/St Jude regarding a destination therapy study on LVAD (the SweVAD study), none of which are relevant for the content of this study. Michael Perch has a research grant from Roche for a study in Pirfinidone for chronic rejection after lung transplantation (the EPOS study). All other authors declared no conflict of interest.
REFERENCES
- 1.The U.S. Organ Procurement and Transplantation Network and the Scientific Registry of Transplant Recipients 2012. https://srtr.transplant.hrsa.gov/annual_reports/2012/Default.aspx (1 February 2018, date last accessed). [DOI] [PubMed]
- 2.Annual Report/Eurotransplant International Foundation.http://www.eurotransplant.org/cms/mediaobject.php?file=AR_ET_2015.pdf (1 February 2018, date last accessed).
- 3. Snell GI, Griffiths A, Levvey BJ, Oto T.. Availability of lungs for transplantation: exploring the real potential of the donor pool. J Heart Lung Transplant 2008;27:662–7. [DOI] [PubMed] [Google Scholar]
- 4. Aigner C, Slama A, Hotzenecker K, Scheed A, Urbanek B, Schmid W. et al. Clinical ex vivo lung perfusion–pushing the limits. Am J Transplant 2012;12:1839–47. [DOI] [PubMed] [Google Scholar]
- 5. Bennett DT, Reece TB, Smith PD, Grandhi MS, Yu Rove JA, Justison GA. et al. Ex vivo lung perfusion allows successful transplantation of donor lungs from hanging victims. Ann Thorac Surg 2014;98:1051–6. [DOI] [PubMed] [Google Scholar]
- 6. Cypel M, Yeung JC, Machuca T, Chen M, Singer LG, Yasufuku K. et al. Experience with the first 50 ex vivo lung perfusions in clinical transplantation. J Thorac Cardiovasc Surg 2012;144:1200–6. [DOI] [PubMed] [Google Scholar]
- 7. Pego-Fernandes PM, de Medeiros IL, Mariani AW, Fernandes FG, Unterpertinger FD, Samano MN. et al. Ex vivo lung perfusion: early report of Brazilian experience. Transplant Proc 2010;42:440–3. [DOI] [PubMed] [Google Scholar]
- 8. Sage E, Mussot S, Trebbia G, Puyo P, Stern M, Dartevelle P. et al. Lung transplantation from initially rejected donors after ex vivo lung reconditioning: the French experience. Eur J Cardiothorac Surg 2014;46:794–9. [DOI] [PubMed] [Google Scholar]
- 9. Valenza F, Rosso L, Coppola S, Froio S, Palleschi A, Tosi D. et al. Ex vivo lung perfusion to improve donor lung function and increase the number of organs available for transplantation. Transpl Int 2014;27:553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wallinder A, Ricksten SE, Silverborn M, Hansson C, Riise GC, Liden H. et al. Early results in transplantation of initially rejected donor lungs after ex vivo lung perfusion: a case-control study. Eur J Cardiothorac Surg 2014;45:40–4; discussion 44–5. [DOI] [PubMed] [Google Scholar]
- 11. Wallinder A, Riise GC, Ricksten SE, Silverborn M, Dellgren G.. Transplantation after ex vivo lung perfusion: a midterm follow-up. J Heart Lung Transplant 2016;35:1303–10. [DOI] [PubMed] [Google Scholar]
- 12. Andreasson AS, Dark JH, Fisher AJ.. Ex vivo lung perfusion in clinical lung transplantation—state of the art. Eur J Cardiothorac Surg 2014;46:779–88. [DOI] [PubMed] [Google Scholar]
- 13. Steen S, Sjoberg T, Pierre L, Liao Q, Eriksson L, Algotsson L.. Transplantation of lungs from a non-heart-beating donor. Lancet 2001;357:825–9. [DOI] [PubMed] [Google Scholar]
- 14. Cypel M, Yeung JC, Hirayama S, Rubacha M, Fischer S, Anraku M. et al. Technique for prolonged normothermic ex vivo lung perfusion. J Heart Lung Transplant 2008;27:1319–25. [DOI] [PubMed] [Google Scholar]
- 15. Cypel M, Yeung JC, Liu M, Anraku M, Chen F, Karolak W. et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. N Engl J Med 2011;364:1431–40. [DOI] [PubMed] [Google Scholar]
- 16. Lindstedt S, Hlebowicz J, Koul B, Wierup P, Sjogren J, Gustafsson R. et al. Comparative outcome of double lung transplantation using conventional donor lungs and non-acceptable donor lungs reconditioned ex vivo. Interact CardioVasc Thorac Surg 2011;12:162–5. [DOI] [PubMed] [Google Scholar]
- 17. Zych B, Popov AF, Stavri G, Bashford A, Bahrami T, Amrani M. et al. Early outcomes of bilateral sequential single lung transplantation after ex vivo lung evaluation and reconditioning. J Heart Lung Transplant 2012;31:274–81. [DOI] [PubMed] [Google Scholar]
- 18. Warnecke G, Van Raemdonck D, Smith MA, Massard G, Kukreja J, Rea F. et al. Normothermic ex vivo preservation with the portable Organ Care System Lung device for bilateral lung transplantation (INSPIRE): a randomised, open-label, non-inferiority, phase 3 study. Lancet Respir Med 2018;6:357–67. [DOI] [PubMed] [Google Scholar]
- 19. Cypel M, Aigner C, Sage E, Machuca T, Slama A, Stern M. et al. Three center experience with clinical normothermic ex vivo lung perfusion. J Heart Lung Transplant 2013;32:S16. [Google Scholar]
- 20. Sanchez PG, Davis RD, D’Ovidio F, Weyan MJ, Camp PC, Cantu E. et al. Normothermic ex vivo lung perfusion as an assessment of marginal donor lungs—the NOVEL lung trial. J Heart Lung Transplant 2013;32:S16–7. [Google Scholar]
- 21. Yeung JC, Krueger T, Yasufuku K, de Perrot M, Pierre AF, Waddell TK. et al. Outcomes after transplantation of lungs preserved for more than 12 h: a retrospective study. Lancet Respir Med 2017;5:119–24. [DOI] [PubMed] [Google Scholar]
- 22. Wallinder A, Ricksten SE, Hansson C, Riise GC, Silverborn M, Liden H. et al. Transplantation of initially rejected donor lungs after ex vivo lung perfusion. J Thorac Cardiovasc Surg 2012;144:1222–8. [DOI] [PubMed] [Google Scholar]
- 23. Henriksen IS, Moller-Sorensen H, Moller CH, Zemtsovski M, Nilsson JC, Seidelin CT. et al. First Danish experience with ex vivo lung perfusion of donor lungs before transplantation. Dan Med J 2014;61:A4809.. [PubMed] [Google Scholar]
- 24. Steen S, Ingemansson R, Eriksson L, Pierre L, Algotsson L, Wierup P. et al. First human transplantation of a nonacceptable donor lung after reconditioning ex vivo. Ann Thorac Surg 2007;83:2191–4. [DOI] [PubMed] [Google Scholar]
- 25. Wallinder A, Hansson C, Dellgren G.. Hemoconcentration in ex vivo lung perfusion: a case report of a novel technique used in clinical lung transplantation. J Thorac Cardiovasc Surg 2013;145:e76–7. [DOI] [PubMed] [Google Scholar]
- 26. Fisher A, Andreasson A, Chrysos A, Lally J, Mamasoula C, Exley C. et al. An observational study of Donor ex vivo Lung Perfusion in UK lung transplantation: DEVELOP-UK. Health Technol Assess 2016;20:1–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martens A, Van Raemdonck DE, Smits J, Verleden SE, Vos R, Vanaudenaerde BM. et al. A retrospective database analysis to evaluate the potential of ex vivo lung perfusion to recruit declined lung donors. Transpl Int 2017;30:1002.. [DOI] [PubMed] [Google Scholar]
- 28. Nilsson T, Gielis JF, Slama A, Hansson C, Wallinder A, Ricksten SE. et al. Comparison of two strategies for ex vivo lung perfusion. J Heart Lung Transplant 2018;37:292–8. [DOI] [PubMed] [Google Scholar]
- 29. Nilsson T, Hansson C, Wallinder A, Malm CJ, Silverborn M, Ricksten SE. et al. Hemofiltration in ex vivo lung perfusion-a study in experimentally induced pulmonary edema. J Thorac Cardiovasc Surg 2016;151:570–5.e1. [DOI] [PubMed] [Google Scholar]