Abstract
A series of pleuromutilins modified by introduction of a boron-containing heterocycle on C(14) of the polycyclic core are described. These analogs were found to be potent anti-Wolbachia antibiotics and, as such, may be useful in the treatment of filarial infections caused by Onchocerca volvulus, resulting in Onchocerciasis or river blindness, or Wuchereria bancrofti and Brugia malayi and related parasitic nematodes resulting in lymphatic filariasis. These two important neglected tropical diseases disproportionately impact patients in the developing world. The lead preclinical candidate compound containing 7-fluoro-6-oxybenzoxaborole (15, AN11251) was shown to have good in vitro anti-Wolbachia activity and physicochemical and pharmacokinetic properties providing high exposure in plasma. The lead was effective in reducing the Wolbachia load in filarial worms following oral administration to mice.
Introduction
Parasitic nematodes of the family Filarioidea including Onchocerca volvulus, Wuchereria bancrofti, and Brugia malayi are responsible for significant disease burden in developing countries around the world.1 Specifically, O. volvulus is the parasite responsible for River Blindness, and W. bancrofti, B. malayi, and Brugia timori cause lymphatic filariasis (elephantiasis).2 Lymphatic filariasis is one of the leading causes of global disability and accounts for at least 2.8 million disability-adjusted life years. Treatment/control and elimination programs for these infections have been in place for many years, but fall short of full effectiveness due to the demanding treatment paradigm required to break disease transmission.3−5 More specifically, the long life span of adult worms (up to 15 years) requires annual to bi-annual mass drug administration of therapeutics such as ivermectin which only kills the juvenile microfilariae released by adult worms and sterilizes adult worms but is not macrofilaricidal.5 Very recently, the results of a triple-drug treatment (ivermectin, albendazole, and diethylcarbamazine) clinical trial demonstrated that this combination may require less frequent administration (perhaps once every 3 years), but only a marginal improvement of macrofilaricidal effects over a dual-drug therapy was noted.6 Consequently, new approaches to kill the adult worms (macrofilaricides) are required in order for elimination time-frames of both diseases to be radically reduced. A short course of treatment (7 days or fewer) would likely be required for ease of implementation in the field.
A unique feature of these parasitic worms is the presence of obligate symbiotic bacteria of the Wolbachia genus.7 It has been known for some time that classical antibacterial agents such as doxycycline can kill the bacteria present in the worms, which results in a reduced life span of the adult worm itself.8−10 Unfortunately, doxycycline presents challenges as a drug for mass administration, including the requirement for long treatment periods (4–6 weeks) and contraindications in pregnancy and in children.11 An anti-Wolbachia approach to the treatment of filarial infections has a number of patient benefits especially as the co-endemic eyeworm Loa loa does not harbor the endosymbiont and is therefore unaffected by treatment. It has been observed that concurrent killing of L. loa microfilariae by directly acting drugs in patients with >30 ;000 microfilariae per mL can have serious side effects including neurologic effects, coma, and death.12 In addition, recent work has suggested that depleting Wolbachia in worms can also diminish the number of microfilariae that are able to develop in the insect vector, thus providing transmission blocking activity.13 The underlying parasitology suggests that the discovery and development of new anti-Wolbachia drugs remains an attractive option for improving our ability to reduce the global burden of river blindness and elephantiasis and accelerate disease elimination goals.
As part of our ongoing effort to capitalize on the unique properties of benzoxaboroles in modifying pharmacologic, physicochemical, and pharmacokinetic properties of existing drug scaffolds, we prepared analogs of the antibiotic class known as pleuromutilins.14,15 This class of ribosomal protein synthesis inhibitors predominantly targets Gram-positive bacteria and had been extensively explored since the 1950s, but had not previously been shown to have activity against Wolbachia. The pleuromutilins have been shown to inhibit protein synthesis through binding to the peptidyl-transfer center of the ribosome, with a crystal structure of tiamulin (4) with the 50S subunit of Deinococcus radiodurans, providing the most direct evidence of this mechanism of action.16 Recently, Nabriva Therapeutics has been developing lefamulin (2), which is currently in clinical trials for community-acquired bacterial pneumonia, demonstrating the potential of this class of antibiotic.17 Furthermore, the starting material for our explorations, pleuromutilin (1), is readily available and inexpensive with synthetic modification, particularly of the hydroxyacetate at C(14), being synthetically straightforward. In addition to boronated analogs of the pleuromutilin core, we also obtained or prepared several clinically relevant non-boron analogs as summarized in Figure 1.18−21
Figure 1.
Pleuromutilin and clinically relevant derivatives.
Results
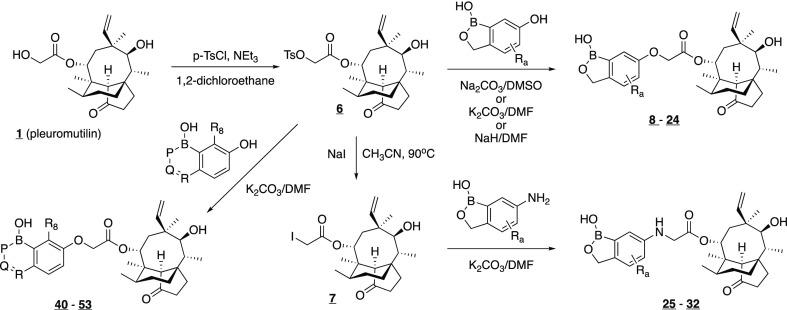
The boronpleuromutilins modified at the C(14) position of the pleuromutilin core were easily prepared via an SN2 displacement reaction of the pleuromutilin tosylate or iodide (Scheme 1). These intermediates were synthesized from the commercially available pleuromutilin. For compounds wherein the vinyl group at C(12) was modified, ozonolysis of the pleuromutilin tosylate with a reductive workup provided the C(12) aldehyde (Scheme 2). Displacement of the C(14) tosylate with 7-fluoro-6-hydroxybenzoxaborole afforded the boronpleuromutilin, which in turn was condensed with hydroxyl amines to provide the oximes, or with amines and sodium cyanoborohydride to provide the amines. Hydrogenation of the C(12) vinyl group of the boronpleuormutilin afforded the corresponding C(12) ethyl analog.
Scheme 1. General Route to C(14)-Modified Boronpleuromutilins.
Scheme 2. Route to C(12)-Modified Boronpleuromutilins.
The boronpleuromutilins with a C(14)-carbamate linker were prepared in two steps from 4-epi-pleuromutilin (72)22 in a manner analogous to that described in the literature (Scheme 3).23 For example, following conversion of 72 to the carbamoyl chloride 73, treatment with 6-aminobenzoxaborole 78 followed by a zinc chloride-mediated 1,5-hydride shift gave the C(14)-carbamate 74. Compounds 75–77 were also prepared in a similar manner.
Scheme 3. Route to C(14) Boronpleuromutilin Carbamates.
The boron-containing heterocycles were prepared by a variety of synthetic sequences that we have described in previous publications.24 As an example, 7-fluoro-6-hydroxybenzoxaborole was prepared by the route shown in Scheme 4. Protection of 3,4-difluorophenol (79) as the methoxymethyl ether was followed by ortho-metallation and trapping of the intermediate aryllithium with ethyl formate to provide the benzaldehyde derivative 81. Nucleophilic displacement of the para fluorine with benzyl alcohol under basic conditions provided 82. Deprotection of the methoxymethyl ether followed by conversion to triflate 84 permitted introduction of the boron via palladium-mediated borylation. Reduction of the aldehyde 85 with sodium borohydride followed by treatment with aqueous hydrochloric acid resulted in formation of the benzoxaborole 86. Finally, hydrogenolysis of the benzyl ether gave the desired 7-fluoro-6-hydroxybenzoxaborole intermediate 87.
Scheme 4. Synthesis of 7-Fluoro-6-hydroxybenzoxaborole.
We screened the pleuromutilin drug candidates in two primary in vitro assays that differed in the strain of Wolbachia used, the insect cell line used to host the bacteria, and in the method of detection.25,26 We used data from both assays to help ensure robust selection of compounds for progression to subsequent in vitro ADME and in vivo pharmacokinetics (PK) experiments. While the absolute potency of individual compounds varied between the two assays in some cases, the structure–-activity relationship (SAR) trends observed in these assays were generally similar. Initial screening revealed that several of the clinically relevant pleuromutilins, for example, retapamulin (3) and valnemulin (5), showed good activity in in vitro assays; significantly less activity was observed for lefamulin (2), tiamulin (4), or for the unadorned pleuromutilin core (1, Table 1). More interestingly, attachment of a benzoxaborole ring with either an oxygen (8), nitrogen (25), or sulfur (33) linker at the 6-position to the C(14) sidechain provided compounds (hereafter referred to as boronpleuromutilins) of similar in vitro potency to valnemulin and retapamulin. Introduction of a methylene spacer between the pleuromutilin core and the benzoxaborole ring as in 35 significantly reduced potency. Attachment of the benzoxaborole ring via the 5- position also provided compounds (37–39) with significantly reduced in vitro activity.
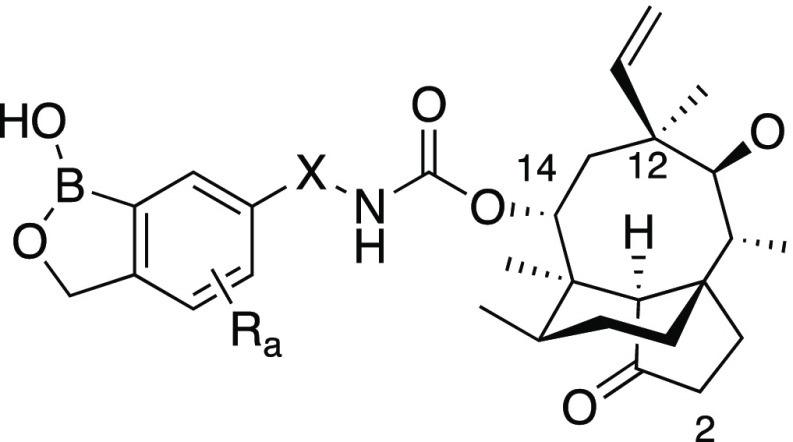
Table 1. Pleuromutilin Derivatives with Benzoxaboroles Linked via C(14).
| ID | link atom | X | R | Wolbachia infected C6/36 cells (wAlb) EC50 (nM) | Wolbachia infected LDW1 cells (wMel) EC50 (nM) |
|---|---|---|---|---|---|
| 1 (pleuromutilin) | NAa | OH | NA | >1000 | 6868 |
| 2 (lefamulin) | NA | NA | NA | 205 | 220 |
| 3 (retapamulin) | NA | NA | NA | NTb | 91 |
| 4 (tiamulin) | NA | NA | NA | 317 | 606 |
| 5 (valnemulin) | NA | NA | NA | NT | 6.1 |
| 8 | 6 | O | H | 6.3 | 1.3 |
| 9 | 6 | O | 3-Me (R,S) | 215 | 24 |
| 10 | 6 | O | 4-Me | 100 | 28 |
| 11 | 6 | O | 5-Me | 11 | 2.8 |
| 12 | 6 | O | 7-Me | 52 | 2.9 |
| 13 | 6 | O | 4-F | 158 | 19 |
| 14 | 6 | O | 5-F | 8.4 | NT |
| 15 | 6 | O | 7-F | 15 | 1.5 |
| 16 | 6 | O | 7-Cl | 32 | 14 |
| 17 | 6 | O | 7-OMe | NT | 3.7 |
| 18 | 6 | O | 5,7-F2 | NT | 1.2 |
| 19 | 6 | O | 3-CH2NH2 | 104 | 19 |
| 20 | 6 | O | 4-CH2NH2 | 64 | 18 |
| 21 | 6 | O | 7-Cl, 3-CH2NH2 | 197 | 49 |
| 22 | 6 | O | 7-F, 3-CH2NH2 | >1000 | 317 |
| 23 | 6 | O | 7-Cl, 4-CH2NH2 | 50 | 9 |
| 24 | 6 | O | 3,3-Me2 | >10 000 | 148 |
| 25 | 6 | NH | H | 5.0 | 4.1 |
| 26 | 6 | NH | 3-CH2NH2 | 229 | 17 |
| 27 | 6 | NH | 4-CH2NH2 | 409 | 44 |
| 28 | 6 | NH | 5-CH2NH2 | 302 | 1182 |
| 29 | 6 | NH | 7-CH2NH2 | 257 | 173 |
| 30 | 6 | NH | 3,3-Me2 | >1000 | 198 |
| 31 | 6 | NH | 5-F | 23 | NT |
| 32 | 6 | NH | 7-F | 106 | 13 |
| 33 | 6 | S | H | 123 | 17 |
| 34 | 6 | S | 7-F | NT | 15 |
| 35 | 6 | –CH2NH– | H | >1000 | 243 |
| 36 | 6 | –CH2NH– | 3,3-Me2 | >10 000 | 1418 |
| 37 | 5 | O | H | >10 000 | NT |
| 38 | 5 | O | 5-F | 298 | 39 |
| 39 | 5 | O | 7-F | 149 | NT |
NA = not applicable.
NT = not tested.
We next turned our attention to substitution of the benzoxaborole core, where we found that introduction of a fluoro substituent at the 4- (13), 5- (14) or 7- (15) positions was generally tolerated with the C(7) analog 15 exhibiting very potent activity. Other small substituents at C(7) such as methyl (12), chloro (16), or methoxy (17) were also tolerated, but did not improve activity beyond the fluoro analog. As the pleuromutilins are intrinsically very lipophilic, we also examined introduction of an aminomethyl substituent onto the benzoxaborole core (19–23, 26–29), but these analogs were generally less active than the corresponding neutral compounds. Finally, we found that addition of two methyl groups at C(3) of the benzoxaborole (24, 30), a strategy that had improved pharmacokinetic properties in other series,27 significantly reduced potency.
Initial results with other boron-containing heterocycles linked via C(14) of the pleuromutilin core were encouraging, as a number of these analogs were very potent in the in vitro Wolbachia assay (Table 2). In particular, the N-methanesulfonyl (40), N-acetyl (42), and N-methylcarbamoyl (45) derivatives of the diazaborine scaffold exhibited low nanomolar to picomolar potency. Unlike the benzoxaboroles, however, inclusion of a fluorine atom adjacent to the B–OH did not improve potency; in fact, these derivatives (47–51) were significantly less potent. The benzoxaborine 53, wherein the five-membered ring was expanded by one carbon to a six membered ring, was completely inactive.
Table 2. Pleuromutilins with Other Boron Heterocycles Linked via C(14).
| ID | P | Q–R | R8 | Wolbachia infected C6/36 cells (wAlb) EC50 (nM) | Wolbachia infected LDW1 cells (wMel) EC50 (nM) |
|---|---|---|---|---|---|
| 40 | CH3SO2N | N=CH | H | 9 | 0.8 |
| 41 | CH3N | N=CH | H | 14.8 | 1.5 |
| 42 | CH3C(=O)N | N=CH | H | 4 | 0.2 |
| 43 | Boc-N | N=CH | H | 0.7 | 0.2 |
| 44 | HN | N=CH | H | 3.5 | 2.7 |
| 45 | CH3OC(=O)N | N=CH | H | 1.5 | 0.3 |
| 46 | O | N=CH | H | 14.2 | 5.1 |
| 47 | CH3SO2N | N=CH | 8-F | 240 | 6.3 |
| 48 | CH3N | N=CH | 8-F | 172 | 11 |
| 49 | CH3C(=O)N | N=CH | 8-F | 107 | NTa |
| 50 | CH3OC(=O)N | N=CH | 8-F | 164 | 1.3 |
| 51 | O | N=CH | 8-F | 329 | 27 |
| 52 | CH3C(=O)N | N=CH | 8-CH3 | 26.1 | NT |
| 53 | O | CH2CH2 | H | >1000 | NT |
NT = not tested.
We also explored modification of the C(12) vinyl substituent (Table 3). Work in the classical pleuromutilin literature had shown that this double bond could be reduced to provide an ethyl group (54), converted to the epoxide (55) or oxidized to an aldehyde (58) that could be functionalized in a variety of ways.28 In particular, we sought to introduce small amines at C(12) to modify physicochemical and pharmacokinetic properties. Given the interesting properties of 15 (vide supra), this work was performed with the 7-fluoro-6-oxobenzoxaborole linked via to C(14) of the pleuromutilin core.
Table 3. Boronpleuromutilins Modified at C(12).
| ID | Rb | Wolbachia infected C6/36 cells (wAlb) EC50 (nM) | Wolbachia infected LDW1 cells (wMel) EC50 (nM) |
|---|---|---|---|
| 54 | CH3CH2– | 38 | 2.7 |
| 55 | epoxide | 148 | 13 |
| 58 | OHC– | 278 | 33 |
| 59 | HOCH2– | >1000 | 242 |
| 60 | HON=CH– | >1000 | 71 |
| 61 | CH3ON=CH– | 197 | 39 |
| 62 | iso-C3H7ON=CH– | NTa | 101 |
| 63 | H2NCH2– | >1000 | 6892 |
| 64 | CH3NHCH2– | >1000 | 1718 |
| 65 | C2H5NHCH2– | 474 | 1399 |
| 66 | n-C3H7NHCH2– | 357 | 478 |
| 67 | n-C4H9NHCH2 | 372 | 243 |
| 68 | cyclo-C3H5NHCH2 | 115 | 44 |
| 69 | (CH3)2NCH2– | 328 | 439 |
| 70 | CH3ONHCH2– | NT | 133 |
| 71 | CH3C(=O)NHCH2– | >1000 | 29 |
NT = not tested.
Disappointingly, only the ethyl (54) derivative exhibited potency close to the C(12) vinyl analog 15; oximes (60–62) and amines (63–70) were significantly less potent. Some potency was regained by acylation of the amine (71), but this was not perceived as being of sufficient advantage to pursue further.
As a final area of exploration, we prepared several boronpleuromutilins where the C(14)-hydroxyacetate linker was replaced by a shorter carbamate (Table 4). This strategy had previously been explored in the pleuromutilins as a means to improve oral bioavailability.23 The carbamates prepared (74–77) exhibited moderate-to-good in vitro potency.
Table 4. Boronpleuromutilin Carbamates.
| ID | X | Ra | Wolbachia infected C6/36 cells (wAlb) EC50 (nM) | Wolbachia infected LDW1 cells (wMel) EC50 (nM) |
|---|---|---|---|---|
| 74 | bond | H | 101 | 7.5 |
| 75 | bond | 5-F | >1000 | 14 |
| 76 | CH2 | H | 12 | 3.5 |
| 77 | CH2 | 7-F | 113 | 4.9 |
Concurrent with our evaluation of the in vitro potency of the boronpleuromutilins, we also selected a number of active analogs for characterization in both in vitro absorption, distribution, metabolism, excretion (ADME) and in vivo PK experiments. It has been suggested that one of the main limitations of the pleuromutilins is high metabolic instability leading to poor PK.29,30 In fact, the majority of the clinically relevant pleuromutilins are of limited oral bioavailability, which has hampered their utility for human applications. A notable exception is the recently described lefamulin (2), which is currently in phase 3 clinical trials as an oral treatment for a variety of bacterial infections.31,32 In order to calibrate our expectations for the boronpleuromutilins, we screened several of the clinically relevant analogs in our in vitro ADME assays as well (Table 5). As anticipated, tiamulin and retapamulin were found to be rapidly metabolized by mouse liver S9 fraction, whereas lefamulin was slowly metabolized. These classical pleuromutilins exhibited a range of binding to mouse plasma proteins, with free fraction (funbound) from 0.05–0.26. Slightly surprisingly, we found that lefamulin was not particularly permeable in our MDR1-MDCK assay, though the high stringency of this assay may underestimate the permeability of this compound through the gut. We had chosen the MDR1-MDCK assay to assess permeability over the related Caco2 assay due to throughput and the opportunity to more directly assess the impact of P-glycoprotein-mediated efflux in this cell line. In addition, it has also been reported that the MDR1-MDCK is an acceptable assay for evaluation of the permeability of compounds through the intestinal mucosa of humans.33
Table 5. In Vitro ADME Properties of Boronpleuromutilins.
| ID | mouse S9 Clint (μL/min/mg) | mouse protein binding (funbound @ 2 μM) | MDR1-MDCK Pappa |
|---|---|---|---|
| 2 | 4 | 0.218 | 0.2 |
| 3 | 41 | 0.154 | 6.7 |
| 4 | 131 | 0.26b | 22.1 |
| 5 | NT | 0.05b | 1.4 |
| 8 | 145 | 0.003 | 15.1 |
| 9 | 168 | 0.002 | NTc |
| 10 | 139 | 0.005 | NT |
| 11 | 77 | <0.001 | NT |
| 12 | 136 | 0.012 | NT |
| 14 | 117 | 0.003 | NT |
| 15 | 33 | 0.034 | 14.1 |
| 16 | 44 | 0.008 | NT |
| 19 | NT | 0.116 | 0.3 |
| 20 | 7 | 0.110 | 0.1 |
| 21 | NT | 0.119 | 0.5 |
| 23 | NT | 0.048 | 0.1 |
| 25 | 102 | 0.008 | 36.5 |
| 26 | 2 | 0.306 | 0.1 |
| 32 | NT | 0.005 | 22.6 |
| 33 | 142 | 0.008 | 9.0 |
| 40 | 226 | 0.058 | 6.6 |
| 41 | NT | 0.007 | NT |
| 42 | NT | 0.005 | NT |
| 44 | 80 | 0.008 | 2.3 |
| 45 | NT | 0.037 | NT |
| 46 | NT | <0.001 | 8.8 |
| 52 | 217 | 0.006 | NT |
| 53 | NT | <0.001 | NT |
| 55 | NT | NT | 8.7 |
| 59 | 53 | 0.033 | 3.7 |
Permeability measured in a Madin Darby canine kidney cell monolayer transfected with the multidrug resistance 1 (mdr1) gene encoding P-glycoprotein MDR1.
Protein binding measured at 1 μM.
NT = not tested.
The neutral boronpleuromutilins evaluated (8–12, 14, 25, 33) were generally metabolized quickly by mouse S9 (Clint > 50 μL/min/mg), were highly protein bound (funbound < 0.03), and were predicted to have good permeability based on the MDR1-MDCK assay (Papp > 5 × 10–6 cm/s). In contrast, boronpleuromutilins bearing an aminomethyl substituent (19–21, 23, 26) were less rapidly metabolized (Clint < 10 μL/min/mg), much less protein bound (funbound > 0.1), but were predicted to be of low permeability (Papp < 1 × 10–6 cm/s). Later compounds incorporating boron-containing heterocycles other than the benzoxaborole (40–42, 44–46, 52, 53) or modification of C(12) (55, 59) did not significantly change the overall in vitro ADME profile. One compound that stood out as having an attractive balance of properties was the 7-fluorobenzoxaborole derivative 15, which exhibited modest clearance (Clint = 33 μL/min/mg) and protein binding (funbound = 0.03), and good permeability (Papp = 14.1 × 10–6 cm/s).
Based on these in vitro profiles and due to resource constraints, we selected representative compounds from the various classes (e.g., neutral, aminomethyl), different linkers (e.g., O, N) and boron heterocycles (e.g., benzoxaboroles, diazaborines) for progression to in vivo PK experiments as summarized in Table 6. When the in vivo PK in mice of these representative compounds were measured, we observed that the PK parameters between them were variable following both IV and oral administration, consistent with the in vitro properties described above. Several observations were made from these studies. First, and as expected, clearance following intravenous dosing was generally quite high, with only compound 15 exhibiting low-to-modest clearance. Second, incorporation of an aminomethyl group on the benzoxaborole core (19) significantly reduced oral bioavailability, as did replacement of the benzoxaborole with a diazaborine (45, 52). Finally, oral exposure and bioavailability was generally good for the 7-fluorobenzoxaborole derivatives tested (15, 32, and 77).
Table 6. In Vivo Pharmacokinetic Properties of Lead Boronpleuromutilins in BALB/c Mice.
| IV at 5 mg/kg, vehicle 55/25/20 PEG/PG/H2O | PO at 10 mg/kg, vehicle 55/25/20 PEG/PG/H2O | ||||||
|---|---|---|---|---|---|---|---|
| Cmpd | Cmax (μg/mL) | CL (mL/hr/kg) | Vss (mL/kg) | AUC0–last (h μg/mL) | Cmax (μg/mL) | AUC0–last (h μg/mL) | % F |
| 8 | 4.13 | 2296 | 2716 | 2.18 | 0.673 | 1.54 | 35 |
| 15 | 3.21 | 505 | 4186 | 8.85 | 1.60 | 12.1 | 61 |
| 19 | 2.48 | 2120 | 10 435 | 2.26 | 0.003 | 0.02 | <1 |
| 32 | 5.91 | 1080 | 2231 | 4.60 | 1.66 | 11.0 | ∼100 |
| 45 | 7.40 | 1642 | 854 | 3.03 | 0.262 | 0.425 | 7 |
| 52 | 3.22 | 3273 | 4658 | 1.52 | 0.227 | 0.166 | 6 |
| 77 | 3.91 | 1338 | 2561 | 3.71 | 1.09 | 8.51 | ∼100 |
Given the relatively low clearance and high exposure exhibited by 15, coupled with its good biological potency, additional PK experiments were performed with this compound. These confirmed the attractive PK profile following oral administration to mice of 15, as systemic exposures at doses from 10 to 50 mg/kg were good, with Cmax values increasing dose-dependently, while AUC values remained relatively constant, suggesting dissolution-limited absorption of the compound from the gut (Table 7).
Table 7. In Vivo Pharmacokinetic Properties of 7-Fluorobenzoxaborole Analog 15 in BALB/c Mice.
| 15 IV | |
|---|---|
| 5 mg/kg (mean, n = 3) | |
| vehicle | 55/25/20 PEG/PG/H2O |
| Cmax (μg/mL) @ 5 min | 3.21 ± 1.3 |
| CL (mL/h/kg) | 505 |
| Vss (mL/kg) | 4186 |
| AUClast (h μg/mL) | 8.85 |
| AUC0–inf (h μg/mL) | 9.91 |
| terminal t1/2 (h) | 9.10 |
| 15 PO | ||||
|---|---|---|---|---|
| 10 mg/kg solution (mean, n = 3) | 10 mg/kg suspension (mean, n = 3) | 25 mg/kg suspension (mean, n = 3) | 50 mg/kg suspension (mean, n = 3) | |
| vehicle | 55/25/20 PEG/PG/H2O | 1% CMC, 0.1% Tween 80 in H2O | 1% CMC, 0.1% Tween 80 in H2O | 1% CMC, 0.1% Tween 80 in H2O |
| Cmax (μg/mL) | 1.60 | 2.81 | 4.25 | 6.38 |
| Tmax (h) | 0.083 | 0.50 | 0.25 | 0.50 |
| AUClast (h μg/mL) | 12.1 | 20.7 | 23.7 | 23.0 |
| AUC0–inf(h μg/mL) | 14.0 | 22.8 | 24.2 | NCa |
| terminal t1/2 (h) | 8.41 | 7.51 | 4.60 | NC |
| bioavailability (%) | 61.1 | NC | NC | NC |
NC = not calculated.
Based on this combination of in vitro potency and in vivo PK data, we next examined the ability of 15 to deplete the Wolbachia symbiont from filarial worms in vivo. In all in vivo efficacy assays, depletion of Wolbachia was determined through comparison to the Wolbachia load found in worms present in animals that were treated with only the dosing vehicle as described in the Experimental Section. For our initial in vivo screen, groups of three CB.17 SCID mice were inoculated with 50 L3-stage B. malayi larvae via the peritoneal cavity and treated from point of infection with 15 for 7 or 14 days with oral doses of 25 mg/kg, BID. At point of necropsy, on day 14, fourth stage (L4) B. malayi larvae were isolated from the peritoneal cavity. Larvae were pooled per group and intraworm Wolbachia titers were quantified from groups of 10 larvae using qPCR. Included in the same experiment were a vehicle control group and two groups of doxycycline-treated animals as positive controls. We were encouraged to find that 15 reduced the Wolbachia load in the B. malayi larvae by 75.4 and 98.8% in the 7- and 14-day treatment groups, respectively. In this experiment, doxycycline reduced Wolbachia load by 76.3 and 96.7% following 7- and 14-day treatment at 50 mg/kg, QD, respectively (Table 8).
Table 8. Summary of Efficacy of 15 and Doxycycline in Reduction of Wolbachia in a Larval B. malayi SCID Mouse Model.
| dosing duration | 7 days | 14 days |
| doxycycline, 50 mg/kg QD | 76.3% | 96.7% |
| 15, 25 mg/kg, BID | 75.4% | 98.8% |
Values represent percent (%) median reduction of Wolbachia versus the vehicle control, as measured by qPCR of Wolbachia surface protein single copy gene (wsp) per B. malayi L4 larva (n = 10 per group) obtained from necropsies undertaken 2 weeks after first dose.
Encouraged by the profile of 15, and in preparation for more extensive in vivo characterization of this molecule, we conducted a number of preliminary in vitro and in vivo studies to assess the safety of this molecule. In a non-GLP Ames assay (Bioreliance, Rockville, MD), 15 was determined to be nonmutagenic. Furthermore, 15 was found to be negative in its potential to induce micronuclei in human peripheral blood lymphocytes (Bioreliance, Rockville, MD). In a panel of over 50 mammalian receptors, enzymes and ion channels (Eurofins Cerep, France), 15 was found to be without any significant effect at 10 μM, and it was also shown to have no significant (<10%) effect on the hERG potassium channel expressed in HEK cells at a concentration of 30 μM (ChanTest, Cleveland, OH). Finally in a preliminary safety study (Anacor, Palo Alto, CA), no adverse effects were observed in BALB/c mice treated with 15 at doses up to 200 mg/kg/day for 7 days.
We next progressed 15 to chronic in vivo infection models of adult filarial parasitism (the target life cycle stage for an anti-Wolbachia indication); one using B. malayi and one using Litomosoides sigmodontis. We initially chose to use the B. malayi SCID mouse model34 as this worm species is a causative agent of LF in humans.2 In this B. malayi assay, 100 L3-stage larvae were inoculated via the peritoneal route and adult infections were allowed to develop. At 6-weeks post-infection, the mice were treated with 15 (25 mg/kg, BID) for 7, 14, or 28 days. At the end of the experiment (12-week post-infection), worms were recovered from the mice and Wolbachia load was determined by qPCR. In this experiment, vehicle and minocycline (25 mg/kg, BID × 28 days) groups were included as negative and positive controls, respectively. Minocycline was chosen as the positive control for this study based on concurrent results in an L. sigmondontis model (vide infra), which suggested that this tetracycline antibiotic exhibited superior efficacy to doxycycline.35 Slightly disappointingly, efficacy of 15 was not nearly as good in the adult model, with a 45.5% reduction of Wolbachia load observed for the 28 day treatment group. By comparison, minocycline provided 98.2% reduction in this experiment (Table 9). We hypothesize that the failure of 15 to show efficacy at the dose chosen in the B. malayi adult worm model is likely due to the increased washout period between treatment start and necropsy in comparison to the B. malayi L3 larval model, leading to a rebound of the Wolbachia with a suboptimal treatment regimen of 15.
Table 9. Summary of Efficacy of 15 and Minocycline Against Wolbachia in the Adult B. malayi SCID Mouse Model.
| dosing
duration |
|||
|---|---|---|---|
| 28 days | 14 days | 7 days | |
| 15 | |||
| 25 mg/kg, BID | 45.5 | 16.6 | 13.4 |
| Minocycline | |||
| 25 mg/kg, BID | 98.2 | ||
Values represent percent (%) median reduction of Wolbachia versus the vehicle control, as measured by qPCR of Wolbachia surface protein single copy gene (wsp) per adult female B. malayi (n = 10 per group) obtained from necropsies undertaken 6 weeks after first dose.
In a second efficacy model,35,36 we used mice infected with the rodent filarial nematode L. sigmodontis, another helminth species known to host Wolbachia, and treated these animals with 15 at a dose of 50 mg/kg, BID for 14 or 28 days. The L. sigmodontis model was chosen as an additional, confirmatory filarial model as it uses immunocompetent wild-type mice as host and initial studies demonstrated the efficacy of tetracycline against Wolbachia-containing filariae first in this model.37 In addition, the infection could be maintained in mice using a natural infection protocol.35,38−40 Upon completion of dosing, animals were maintained for a total of 64–77 days (to maintain a consistent total time following the final dose), at which time worms were recovered from the peritoneum and the thoracic cavity. Wolbachia load was determined by qPCR. Doxycycline was included as a positive control in this experiment. We were encouraged to find that 15 was able to reduce Wolbachia burden in L. sigmodontis by >99% at this dose and duration (Table 10). The stronger reduction of the Wolbachia load in the L. sigmodontis mouse model in comparison to the adult B. malayi mouse model is probably mostly due to the increased dose used for treatment with 15, although L. sigmodontis were previously reported to have an increased susceptibility for anti-Wolbachia drugs compared to B. malayi filariae.41
Table 10. Efficacy of 15 and Doxycycline against Wolbachia in the Adult L. sigmodontis Mouse Model.
| dosing
duration |
||
|---|---|---|
| 28 days | 14 days | |
| 15 | ||
| 50 mg/kg, BID | 99.2 | 99.7 |
| Doxycycline | ||
| 40 mg/kg, BID | 99.9 | 99.9 |
Values represent percent (%) reduction of Wolbachia versus the untreated control, as measured by qPCR FtsZ/actin per mouse, and were obtained from necropsies performed 1 month after first dose.
Discussion and Conclusions
Previous work from our laboratories has demonstrated that boron-containing heterocycles can be useful in the treatment of various diseases, including infectious diseases of the developing world such as malaria,42,43 human African trypanosomiasis,27,44 and tuberculosis.45,46 We have extended our work to look at modification of known classes of antibacterial agents as an approach to treat river blindness via the bacterial symbiont (Wolbachia) present in the worms that cause this disease. Clear clinical “proof of concept” for an anti-Wolbachia approach has been demonstrated with the tetracycline antibiotic doxycycline.9 The utility of doxycycline is limited, however, by the requirement that patients must be treated for 4–6 weeks in order to achieve significant reduction of Wolbachia in the O. volvulus parasite.8−10 More recently, it has been demonstrated that combination of doxycycline (200 mg/day for 3 weeks) with albendazole (800 mg/day for 3 days) may provide a shorter treatment course.47 While moving closer to the desired short course (7 day or fewer) treatment paradigm, this combination therapy is still not ideal and does not address tetracycline contraindicated groups (children of age ≤8 and pregnant women).
Our work started by evaluating representatives of various classes of antibiotics including several clinically relevant pleuromutilins. This initial screening revealed that some activity was present in this class of compounds, and we prepared several analogs containing our benzoxaborole core. The synthesis of these boronpleuromutilins was facilitated by the ready availability of the tosylate derivative of the hydroxyacetate ester at C(14) of the pleuromutilin core. Simple nucleophilic displacement reactions of this to afford C(14)-functionalized boronpleuromutilins with nitrogen, oxygen, or sulfur linker atoms proceeded in good yield. Early SARs revealed that direct attachment of the benzoxaborole ring to the pleuromutilin core provided compounds with good in vitro potency. In contrast, inclusion of a linker group such as present in the clinically relevant pleuromutilins between the core and benzoxaborole ring were of poor activity. Based on our previous work in the benzoxaboroles, we focused our attention on compounds linked through the 6-position, as these were both synthetically accessible and generally had been observed to have good ADME properties. Inclusion of a variety of substituents such as halogen, small alkyl, or aminoalkyl at positions 3, 4, 5, and 7 of the 6-O linked benzoxaboroles gave compounds of similar in vitro activity and allowed us to explore the effect of these substituents on ADME and PK. We were particularly pleased to find that the 7-fluoro analog (15) exhibited a good balance of potency and physicochemical and ADME properties. In particular, we were encouraged by the modest metabolic stability in mouse microsomes (Clint = 33 μL/min/mg) and good permeability through an MDR1-MDCK monolayer (Papp = 14.1 × 10–6 cm/s). We were slightly concerned about the high protein binding of 15 (funbound = 0.03), though this was predicted based on the calculated high lipophilicity (log D 4.23) of this compound. While other compounds, particularly those with an aminomethyl substituent on the benzoxaborole core (e.g. 20) exhibited better metabolic stability (Clint = 6 μL/min/mg), they were compromised by very low permeability (Papp < 0.1 × 10–6 cm/s).
When 15 was dosed to mice by the intravenous route (5 mg/kg), we were encouraged by the relatively low clearance (Clint = 505 mL/hr/kg), good exposure (AUC0–24h = 8.85 h/μg/mL). Following oral administration (10 mg/kg), 15 exhibited good exposure (AUC0–24h = 14.0 h/μg/mL) and bioavailability (F = 61%). Additional PK studies at higher oral doses demonstrated that exposure increased with dose, though not dose proportionally, to very high levels (AUC0–24h = 58.7 h/μg/mL at 400 mg/kg). In addition to the parent compound, we also tracked the major (and active) metabolite of 15 (15a, IC50 = 193 nM) where hydroxylation at C(2) of the pleuromutilin core had occurred, as this represented about 10–15% of the parent dose at most time points. Metabolism of pleuromutilins at this position of the core is well precedented.48,49
Based on these observations, 15 was selected for evaluation in several in vivo models that had been established for evaluation of anti-Wolbachia compounds in mice. In the first study, we found that 15, when dosed orally at 25 mg/kg, BID, to SCID mice infected with larval stage B. malayi, efficacy (defined as >99% reduction in Wolbachia load in worms) was achieved following 14 days of dosing; 7 days dosing did not achieve full efficacy. In a second B. malayi model employing worms that had been allowed to mature to the adult stage, we were disappointed to find that 15 did not demonstrate measureable efficacy when dosed at 25 mg/kg, BID for 28 days. More encouraging data were obtained in the L. sigmodontis BALB/c mouse model, where 15 was found to show excellent efficacy (>99% Wolbachia depletion) when dosed at 50 mg/kg, BID for 14 or 28 days. Additional in vivo studies to address this hypothesis are ongoing and will be reported in due course.
In conclusion, exploration of a series of pleuromutilin derivatives incorporating a novel boron-containing heterocycle linked to C(14) of pleuromutilin core as anti-Wolbachia agents has resulted in the identification of 15 as a potential preclinical candidate. SAR developed in this program has demonstrated that optimal activity was obtained by direct linkage of the benzoxaborole to the pleuromutilin core via C(6) of the benzoxaborole, and that small substituents on the benzoxaborole ring system had an impact on potency and pharmacokinetic properties. Good-to-excellent exposure following oral administration of 15 to mice was achieved, and this translated into high levels of Wolbachia depletion in several in vivo models. We were encouraged by the efficacy of 15 in these models, prompting more extensive evaluation of this compound, both alone and in combination with other known anti-Wolbachia drugs. The outcome of these studies will be reported in due course.
Experimental Section
General
All chemicals were purchased from commercial suppliers and used as received. 1H NMR spectra were recorded on a Bruker AVANCE 400 spectrometer. Chemical shifts are expressed in δ ppm referenced to an internal tetramethylsilane (δ = 0 ppm) standard. Abbreviations used in describing peak signals are br = broad, s = singlet, d = doublet, dd = doublet of doublets, t = triplet, q = quartet, m = multiplet. All final compounds were purified to have purity higher than 95% by reverse phase high-performance liquid chromatography (HPLC), supercritical fluid chromatography, normal phase flash chromatography, or crystallization. Preparative HPLC was accomplished using a Luna C18 250 × 30 mm, 10 μm column. The purity was assessed by reverse phase HPLC with a gradient of 5–95% acetonitrile in water (with or without acid modifier) and monitored by a diode array ultraviolet detector at 220 and 254 nm. Low-resolution mass spectra were recorded on a liquid chromatography–mass spectrometer in electrospray positive (ESI+) or negative (ESI−) modes.
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-(Tosyloxy)acetate (6)
p-Toluenesulfonyl chloride (19.1 g, 0.1 mol) in 1,2-dichloroethane (100 mL) was slowly added to a mixture of (3aR,4R,5R,7S,8S,9R,9aS,12R)-8-hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-hydroxyacetate (35.4 g, 0.1 mol), triethylamine (12.0 g, 0.1 mol), and pyridine (1 mL) in 1,2-dichloroethane (100 mL). The mixture was stirred at 10–15 °C for 20 h, washed with water (3 × 100 mL), and then concentrated to dryness. Purification was achieved by recrystallization from dichloromethane/petroleum ether (1:100) to afford (3aR,4R,5R,7S,8S,9R,9aS,12R)-8-hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-(tosyloxy)acetate 6 as a white solid (45.0 g, yield 90.0%). 1H NMR (DMSO-d6, 400 MHz): δ 7.80 (d, J = 8.0 Hz, 1H), 7.47 (d, J = 8.0 Hz, 1H), 6.05 (dd, J = 17.8, 11.2 Hz, 1H), 5.53 (d, J = 8.4 Hz, 1H), 5.09–4.96 (m, 2H), 4.81–4.59 (m, 2H), 3.40 (d, J = 5.6 Hz, 1H), 2.41 (s, 2H), 2.39 (br s, 1H), 2.24–1.95 (m, 5H), 1.75–1.41 (m, 4H), 1.30 (s, 4H), 1.27–1.18 (m, 4H), 1.03 (s, 3H), 0.99–0.92 (m, 2H), 0.81 (d, J = 7.2 Hz, 3H), 0.50 (d, J = 7.2 Hz, 3H).
Preparation of (3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-Iodoacetate (7)
To a solution of 6 (51.5 g, 96.7 mmol, 1.0 equiv) in acetonitrile (600.0 mL) was added sodium iodide (87.0 g, 580.1 mmol, 6.0 equiv). The mixture was stirred at 90 °C for 16 h at which time HPLC indicated the reaction was completed. The reaction mixture was concentrated under reduced pressure to remove acetonitrile. The residue was diluted with H2O (500 mL) and extracted with dichloromethane (3 × 500 mL). The combined organic layers were concentrated under reduced pressure to give a residue. The residue was washed with petroleum ether (200 mL). (3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-iodoacetate 7 (40.0 g, 81.9 mmol, 84.7% yield) was obtained as a yellow solid. 1H NMR (DMSO-d6, 400 MHz): δ 6.11 (dd, J = 11.2, 17.9 Hz, 1H), 5.52 (d, J = 7.9 Hz, 1H), 5.13–5.01 (m, 2H), 4.55 (d, J = 5.7 Hz, 1H), 3.82–3.75 (m, 1H), 3.73–3.66 (m, 1H), 3.43 (t, J = 5.3 Hz, 1H), 2.45–2.39 (m, 1H), 2.25–2.00 (m, 4H), 1.72–1.57 (m, 2H), 1.53–1.21 (m, 9H), 1.11–0.97 (m, 4H), 0.83 (d, J = 6.6 Hz, 3H), 0.64 (d, J = 7.1 Hz, 3H).
Method A. Use of Pleuromutilin Tosylate 6. Preparation of (3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((1-Hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate (8)
A solution of 6 (1.8 g, 3.3 mmol), benzo[c][1,2] oxaborole-1,6(3H)-diol (0.5 g, 3.3 mmol), and K2CO3 (0.7 g, 5.0 mmol) in 20 mL of DMF was heated to 50 °C overnight, at which time LC/MS indicated the reaction was completed. Water was added and the mixture was adjusted to pH < 4 with 2 N HCl. The solid was filtered and the crude product was purified by prep HPLC to give (3aR,4R,5R,7S,8S,9R,9aS,12R)-8-hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl2-((1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy) acetate 8 (1.0 g, yield 57.0%). 1H NMR (DMSO-d6, 400 MHz): δ 7.30 (d, J = 8.4 Hz, 1H), 7.19 (d, J = 2.0 Hz, 1H), 7.04 (dd, J = 8.4, 2.4 Hz, 1H) 6.10 (dd, J = 17.6, 11.2 Hz, 1H), 5.59 (d, J = 8.4 Hz, 1H), 5.11–4.97 (m, 2H), 4.91 (s, 2H), 4.76–4.63 (m, 2H), 3.41 (d, J = 5.6 Hz, 1H), 2.40 (br s, 1H), 2.26–1.99 (m, 4H), 1.72–1.43 (m, 4H), 1.38 (d, J = 13.2 Hz, 1H), 1.34 (s, 3H), 1.29–1.15 (m, 4H), 1.03 (s, 3H), 1.01 (br s, 1H), 0.81 (d, J = 6.8 Hz, 3H), 0.63 (d, J = 6.8 Hz, 3H).
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((1-Hydroxy-3-methyl-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate (9)
Prepared from 6 by Method A. 1H NMR (DMSO-d6, 400 MHz): δ 9.00 (s, 1H), 7.28 (d, J = 9.2 Hz, 1H), 7.15 (d, J = 2.4 Hz, 1H), 7.02 (d, J = 8.0 Hz, 1H), 6.09 (dd, J = 17.6, 11.4 Hz, 1H), 5.59 (d, J = 8.0 Hz, 1H), 5.15–4.98 (m, 3H), 4.72–4.62 (m, 2H), 4.51 (d, J = 6.0 Hz, 1H), 3.40 (m, 1H), 2.40 (s, 1H), 2.26–1.98 (m, 4H), 1.67–1.26 (m, 14H), 1.07–1.01 (m, 3H), 0.81 (d, J = 6.8 Hz, 3H), 0.63 (d, J = 6.8 Hz, 3H). MS (ESI): mass calcd for C30H41BO7, 524.3; m/z: found, 523.2 [M – 1]−. HPLC: 93.3% (220 nm), 100% (254 nm).
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((1-Hydroxy-4-methyl-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate (10)
Prepared from 6 by Method A. 1H NMR (DMSO-d6, 400 MHz): δ 7.02 (d, J = 1.76 Hz, 1H), 6.83 (d, J = 1.8 Hz, 1H), 6.10 (dd, J = 17.6, 11.2 Hz, 1H), 5.60 (d, J = 8.4 Hz, 1H), 5.12–4.96 (m, 2H), 4.87 (s, 2H), 4.75–4.60 (m, 2H), 2.41 (br s, 1H), 2.17 (s, 3H), 2.00–2.13 (m, 5H), 1.72–1.55 (m, 2H), 1.54–1.39 (m, 2H), 1.35 (s, 3H), 1.32–1.20 (m, 3H), 1.04 (m, 4H), 0.82 (d, J = 6.4 Hz, 3H), 0.64 (d, J = 7.2 Hz, 3H). MS: 523 (M – 1)−.
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((1-Hydroxy-5-methyl-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate (11)
Prepared from 6 by Method A. 1H NMR (DMSO-d6, 400 MHz): δ 8.93 (br s, 1H), 7.19 (s, 1H), 7.09 (s, 1H), 6.08 (dd, J = 17.6, 11.2 Hz, 1H), 5.59 (d, J = 8.0 Hz, 1H), 5.12–4.95 (m, 2H), 4.87 (s, 2H), 4.71 (s, 2H), 3.40 (d, J = 4.8 Hz, 1H), 2.40 (s, 1H), 2.25 (s, 3H), 2.20–1.96 (m, 3H), 1.74–1.55 (m, 2H), 1.50–1.45 (m, 1H), 1.37 (s, 3H), 1.45–1.17 (m, 6H), 1.10–0.94 (m, 4H), 0.82 (d, J = 6.4 Hz, 3H), 0.62 (d, J = 6.4 Hz, 3H). MS: 523 (M – 1)−.
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((1-Hydroxy-7-methyl-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate (12)
Prepared from 6 by Method A. 1H NMR (DMSO-d6, 400 MHz): δ 7.09 (d, J = 8.0 Hz, 1H), 6.95 (d, J = 8.0 Hz, 1H), 6.11 (dd, J = 17.6, 11.2 Hz, 1H), 5.61 (d, J = 8.0 Hz, 1H), 5.09–4.98 (m, 2H), 4.87 (s, 2H), 4.72 (d, J = 2.0 Hz, 2H), 3.41 (d, J = 5.6 Hz, 1H), 2.36 (s, 1H), 2.33 (s, 3H), 2.20–2.10 (m, 1H), 2.10–1.95 (m, 4H), 1.72–1.55 (m, 2H), 1.54–1.36 (m, 2H), 1.34 (s, 3H), 1.31–1.15 (m, 3H), 1.03 (s, 4H), 0.82 (d, J = 6.8 Hz, 3H), 0.63 (d, J = 6.8 Hz, 3H). MS: 523 (M – 1)−.
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((4-Fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate (13)
Prepared from 6 by Method A. 1H NMR (DMSO-d6, 400 MHz): δ 9.29–9.24 (br s,1H), 7.08 (s, 1H), 6.93 (d, J = 11.2 Hz, 1H), 6.15–6.05 (m, 1H), 5.60–5.56 (d, J = 8.8 Hz, 1H), 5.05–4.99 (m, 4H), 4.77–4.75 (d, J = 8.0 Hz, 2H), 2.41 (s, 1H), 2.41–2.04 (m, 4H), 1.63–1.41 (m, 4H), 1.33–1.28 (m, 7H), 1.04–0.85 (m, 4H), 0.81 (d, J = 6.4 Hz, 3H), 0.63 (d, J = 6.4 Hz, 3H). MS (ESI): mass calcd for C29H38BFO7, 528.27; m/z: found, 527.2 [M – 1]−. HPLC: 96.0% (220 nm), 69.6% (254 nm).
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((5-Fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate (14)
Prepared from 6 by Method A. 1H NMR (DMSO-d6, 400 MHz): δ 9.10 (s, 1H), 7.35–7.26 (m, 2H), 6.08 (dd, J = 18.0, 11.2 Hz, 1H), 5.58 (d, J = 7.6 Hz, 1H), 5.09–4.96 (m, 2H), 4.90 (s, 2H), 4.85–4.74 (m, 2H), 4.52 (d, J = 6.0 Hz, 1H), 3.43–3.38 (m, 1H), 2.41 (s, 1H), 2.25–1.96 (m, 6H), 1.73–1.20 (m, 9H), 1.03 (s, 3H), 0.81 (d, J = 6.8 Hz, 3H), 0.61 (d, J = 6.8 Hz, 3H). MS: 527 (M – 1)−.
Method B. Use of Pleuromutilin Tosylate 7. Preparation of (3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((7-Fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate (15)
To a solution of 7-fluorobenzo[c][1,2]oxaborole-1,6(3H)-diol 80 (5.0 g, 29.77 mmol, 1.0 equiv) and 7 (18.9 g, 38.71 mmol, 1.3 equiv) in DMSO (60.00 mL) was added Na2CO3 (9.5 g, 89.32 mmol, 3.0 equiv). The mixture was stirred at 35 °C for 14 h under a nitrogen atmosphere, after which time HPLC indicated that starting material was consumed completely. The reaction mixture was quenched by addition H2O (200 mL) at 0 °C and then was adjusted to pH = 7 and then filtered to give a crude product. Four batches were combined together, and the crude product was purified by prep HPLC, then removed the acetonitrile, resulting aqueous phase was extracted by dichloromethane (3 × 1500 mL). The combined organic layers were concentrated under reduced pressure to give the product as a light yellow solid. This product was dissolved in dichloromethane, and then MTBE and petroleum ether was added until the product precipitated. The suspension was filtered and the filtrate was concentrated under reduced pressure to give additional product as a white solid. (3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((7-fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate 15 (15.0 g, 28.2 mmol, 24% yield, 99.0% purity) was obtained as a white solid. 1H NMR (DMSO-d6, 400 MHz): δ 9.26 (br s, 1H), 7.25–7.17 (m, 1H), 7.09 (d, J = 7.9 Hz, 1H), 6.11 (dd, J = 11.2, 17.9 Hz, 1H), 5.60 (d, J = 7.9 Hz, 1H), 5.10–4.98 (m, 2H), 4.92 (s, 2H), 4.86–4.74 (m, 2H), 4.52 (br s, 1H), 3.41 (br s, 1H), 2.41 (br s, 1H), 2.25–2.01 (m, 4H), 1.70–1.57 (m, 2H), 1.48–1.20 (m, 8H), 1.15–0.91 (m, 4H), 0.82 (d, J = 6.6 Hz, 3H), 0.62 (d, J = 7.1 Hz, 3H). MS (ESI): mass calcd for C29H38BFO7, 528.27; m/z: found, 527.3 [M – H]−. HPLC: 99.0% (220 nm), 100.0% (254 nm).
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((7-Chloro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate (16)
Prepared from 6 by Method A. 1H NMR (400 MHz, DMSO-d6): δ 9.06 (s, 1H), 7.18 (d, J = 8 Hz, 1H), 7.07 (d, J = 8.4 Hz, 1H), 6.04 (dd, J = 17.6, 11.2 Hz, 1H), 5.53 (d, J = 7.2 Hz, 1H), 5.02 (m, 2H), 4.84 (s, 2H), 4.79 (s, 2H), 4.49 (d, J = 6.4 Hz, 1H), 3.35 (m, 1H), 2.35 (s, 1H), 2.11–1.96 (m, 4H), 1.61–1.17 (m, 10H), 0.98–0.93 (m, 4H), 0.74 (d, J = 6.8 Hz, 3H), 0.58 (d, J = 6.8 Hz, 3H). MS (ESI): mass calcd For C29H38BO7Cl 544.87; m/z: found, 543.2 [M – H]−. HPLC: 100% (220 nm), 100% (254 nm).
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((1-Hydroxy-7-methoxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate (17)
Prepared from 6 by Method A. 1H NMR (DMSO-d6, 400 MHz): δ 9.20–8.94 (m, 1H), 7.03 (d, J = 8.4 Hz, 1H), 6.91 (d, J = 8.4 Hz, 1H), 6.12 (dd, J = 11.5, 17.6 Hz, 1H), 5.61 (d, J = 8.4 Hz, 1H), 5.10–5.00 (m, 2H), 4.89 (s, 2H), 4.74–4.60 (m, 2H), 3.93 (s, 3H), 3.42 (d, J = 6.2 Hz, 1H), 2.41 (br s, 1H), 2.24–1.99 (m, 4H), 1.71–1.20 (m, 11H), 1.12–0.94 (m, 4H), 0.82 (d, J = 7.1 Hz, 3H), 0.63 (d, J = 6.6 Hz, 3H). MS (ESI): mass calcd for C30H41BO8, 540.3; m/z: found, 539.3 [M – 1]−. HPLC: 100% (220 nm), 100% (254 nm).
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((5,7-Difluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate (18)
Prepared from 6 by Method A. 1H NMR (DMSO-d6, 400 MHz): δ 7.16 (d, J = 10.1 Hz, 1H), 6.05 (dd, J = 11.5, 17.6 Hz, 1H), 5.57 (d, J = 7.9 Hz, 1H), 4.97 (d, J = 6.6 Hz, 1H), 4.93 (s, 1H), 4.89 (s, 2H), 4.71 (d, J = 19.0 Hz, 2H), 3.36 (d, J = 6.2 Hz, 1H), 2.34 (br s, 1H), 2.20–2.09 (m, 1H), 2.08–1.94 (m, 3H), 1.67–1.51 (m, 3H), 1.41 (d, J = 17.2 Hz, 1H), 1.36–1.14 (m, 6H), 1.03–0.90 (m, 4H), 0.78 (d, J = 7.1 Hz, 3H), 0.53 (d, J = 6.6 Hz, 3H). MS (ESI): mass calcd For C29H37BF2NO7, 546.3; m/z: found, 545.2 [M – H]−. HPLC: 97.3% (220 nm), 94.0% (254 nm).
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((3-(Aminomethyl)-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate Hydrochloride (19)
Prepared from 7 by Method B. 1H NMR (DMSO-d6, 400 MHz): δ 9.47 (br s, 1H), 8.13 (br s, 3H), 7.44 (d, J = 8.4 Hz, 1H), 7.28 (d, J = 2.2 Hz, 1H), 7.09 (td, J = 2.4, 8.5 Hz, 1H), 6.10 (dd, J = 11.2, 17.9 Hz, 1H), 5.60 (d, J = 8.4 Hz, 1H), 5.28 (dd, J = 2.6, 9.3 Hz, 1H), 5.12–4.96 (m, 2H), 4.80–4.64 (m, 2H), 4.62–4.48 (m, 1H), 3.42 (d, J = 5.7 Hz, 2H), 2.78–2.63 (m, 1H), 2.42 (br s, 1H), 2.26–2.00 (m, 4H), 1.73–1.18 (m, 7H), 1.05 (s, 2H), 0.89–0.78 (m, 5H), 0.63 (d, J = 7.2 Hz, 3H). MS (ESI): mass calcd for C30H42BNO7, 539.31; m/z: found, 540.1 [M + H]+. HPLC: 99.2% (220 nm), 100.0% (254 nm).
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((4-(Aminomethyl)-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate Hydrochloride (20)
Prepared from 7 by Method B. 1H NMR (DMSO-d6, 400 MHz): δ 9.19 (s, 1H), 8.30 (br s, 3H), 7.26–7.17 (m, 2H), 6.11 (dd, J = 11.2, 17.9 Hz, 1H), 5.62 (d, J = 8.8 Hz, 1H), 5.15–4.95 (m, 4H), 4.79–4.65 (m, 2H), 4.55 (br s,1H), 3.93 (d, J = 5.3 Hz, 2H), 3.43 (br s, 2H), 2.44 (d, J = 7.9 Hz, 1H), 2.26–2.01 (m, 4H), 1.73–1.19 (m, 10H), 1.12–0.94 (m, 4H), 0.82 (d, J = 7.1 Hz, 3H), 0.65 (d, J = 7.1 Hz, 3H). MS (ESI): mass calcd for C30H43BClNO7, 575.3; m/z: found, 540.4 [M + H]+. HPLC: 96.1% (220 nm), 91.0% (weak absorption at 254 nm).
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((3-(Aminomethyl)-7-chloro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate Hydrochloride (21)
Prepared from 7 by Method B. 1H NMR (400 MHz, DMSO-d6): δ 9.31 (s, 1H), 8.13 (s, 3H), 7.40 (d, J = 8.4 Hz, 1H), 7.18 (t, J = 8.4 Hz, 1H), 6.15–6.08 (m, 1H), 5.60 (d, J = 7.2 Hz, 1H), 5.29 (d, J = 8.0 Hz, 1H), 5.14–4.57 (m, 5H), 3.47–3.38 (m, 3H), 2.87 (b, 1H), 2.42 (s, 1H), 2.23–2.02 (m, 4H), 1.67–1.05 (m, 14H), 0.82 (d, J = 6.8 Hz, 3H), 0.63 (d, J = 6.8 Hz, 3H). HPLC purity: 100% (220 nm); MS (ESI): mass calcd for C30H41BClNO7, 573.27; m/z: found, 574.2 [M + H]+.
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((3-(Aminomethyl)-7-fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate Hydrochloride (22)
Prepared from 7 by Method B. 1H NMR (DMSO-d6, 400 MHz): δ 9.51 (br s, 1H), 8.14 (br s, 3H), 7.27–7.22 (m, 2H), 6.11 (dd, J = 10.8, 18.0 Hz, 1H), 5.60 (d, J = 7.6 Hz, 1H), 5.30 (d, J = 8.8 Hz, 1H), 5.06 (d, J = 18.0 Hz, 1H), 5.03 (d, J = 10.8 Hz, 1H), 4.86–4.82 (m, 2H), 2.84–2.82 (m, 2H), 2.41 (s, 1H), 2.18–2.05 (m, 4H), 1.64–1.20 (m, 9H), 1.09–0.94 (m, 4H), 0.83–0.79 (d, J = 6.8 Hz, 3H), 0.63 (d, J = 6.8 Hz, 3H). MS (ESI): mass calcd for C30H42BClFNO7, 593.3; m/z: found, 558.0(M + H)+. HPLC: 99.0% (220 nm), 96.3% (254 nm).
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((4-(Aminomethyl)-7-chloro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate (23)
Prepared from 7 by Method B. 1H NMR (400 MHz, DMSO-d6): δ 9.04 (s, 1H), 7.17 (s, 1H), 6.16–6.13 (m, 1H), 5.61 (d, J = 8.0 Hz, 1H), 5.11–5.01 (m, 2H), 4.97 (s, 2H), 4.822 (d, J = 4.0 Hz, 2H), 4.54 (d, J = 6.0 Hz, 1H), 4.46–4.44 (m, 2H), 3.62 (s, 2H), 2.43 (s, 1H), 2.23–2.04 (m, 4H), 1.68–0.97 (m, 14H), 0.81 (d, J = 7.2 Hz, 3H), 0.66 (d, J = 6.8 Hz, 3H). HPLC purity: 100% (214 nm); MS (ESI): mass calcd For C30H41BClNO7, 573.27; m/z: found, 573.8 [M + H]+.
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((1-Hydroxy-3,3-dimethyl-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate (24)
Prepared from 7 by Method B. 1H NMR (DMSO-d6, 400 MHz): δ 8.92 (s, 1H), 7.29 (d, J = 8.4 Hz, 1H), 7.11 (d, J = 2.4 Hz, 1H), 7.00 (dd, J = 8.4, 2.4 Hz, 1H), 6.09 (dd, J = 17.6, 11.2 Hz, 1H), 5.59 (d, J = 8.4 Hz, 1H), 5.08–4.95 (m, 2H), 4.75–4.61 (m, 2H), 4.51 (d, J = 6.0 Hz, 1H), 3.45–3.38 (m, 1H), 2.40 (s, 1H), 2.25–1.98 (m, 5H), 1.73–1.55 (m, 3H), 1.52–1.15 (m, 12H), 1.08–0.98 (m, 4H), 0.81 (d, J = 7.0 Hz, 3H), 0.62 (d, J = 6.8 Hz, 3H). MS (ESI): mass calcd for C31H43BO73, 538.3; m/z: found, 537.2 [M – 1]−. HPLC: 99.3% (220 nm), 100% (254 nm).
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl (1-Hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)glycinate (25)
Prepared from 7 by Method B. 1H NMR (DMSO-d6, 400 MHz): δ 7.09 (d, J = 8.4 Hz, 1H), 6.83 (br s, 1H), 6.72 (d, J = 8.4 Hz, 1H), 6.12–5.96 (m, 1H), 5.53 (d, J = 8.0 Hz, 1H), 5.07–4.90 (m, 2H), 4.87–4.77 (m, 1H), 3.78 (d, J = 5.2 Hz, 2H), 3.37 (d, J = 5.2 Hz, 1H), 2.33 (br s, 1H), 2.22–2.12 (m, 1H), 2.10–1.91 (m, 3H), 1.70–1.52 (m, 3H), 1.43 (br s, 2H), 1.37–1.15 (m, 8H), 1.06–0.89 (m, 5H), 0.79 (d, J = 6.4 Hz, 3H), 0.61 (d, J = 6.4 Hz, 3H). MS (ESI): mass calcd for C29H41BClNO6, 545.27; m/z: found, 508.3 [M – H]−. HPLC: 100.0% (220 nm), 100.0% (254 nm).
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl (3-(Aminomethyl)-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)glycinate (26)
Prepared from 7 and 6-amino-1-hydroxy-3-nitromethyl-1,3-dihydrobenzo[c][1,2]oxaborole by Method B and reduction of the nitromethyl intermediate by hydrogenation. 1H NMR (DMSO-d6, 400 MHz): δ 8.12 (br s, 2H), 7.20 (d, J = 7.9 Hz, 1H), 6.91 (br s, 1H), 6.73 (dd, J = 1.6, 8.4 Hz, 1H), 6.08 (dd, J = 11.2, 17.6 Hz, 1H), 5.55 (d, J = 8.0 Hz, 1H), 5.19 (d, J = 7.6 Hz, 1H), 5.04 (d, J = 19.2 Hz, 1H), 4.95 (d, J = 11.2 Hz, 1H), 3.80 (d, J = 13.2 Hz, 2H), 3.41 (d, J = 5.6 Hz, 2H), 2.39 (br s, 1H), 2.22–2.14 (m, 1H), 2.12–1.98 (m, 3H), 1.69–1.57 (m, 2H), 1.54–1.39 (m, 2H), 1.38–1.32 (m, 5H), 1.31–1.18 (m, 3H), 1.08–0.94 (m, 4H), 0.81 (d, J = 6.4 Hz, 3H), 0.64 (d, J = 6.4 Hz, 3H). MS (ESI): mass calcd For C30H44BClN2O6, 574.9; m/z: found, 539.5 [M + H]+. HPLC: 98.9% (220 nm), 100.0% (254 nm).
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl (4-(Aminomethyl)-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)glycinate (27)
Prepared from 7 and 6-amino-4-(t-butyloxycarbonyl)aminomethyl-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborole by Method B and deprotection of the (t-butyloxycarbonyl)aminomethyl intermediate with trifluoracetic acid. 1H NMR (400 MHz, DMSO-d6): δ 8.84 (s, 1H), 6.71 (m, 2H), 6.09 (m, 1H), 5.92 (m, 1H), 5.54 (m, 1H), 4.89–5.07 (m, 4H), 4.52 (d, 1H), 3.78 (m, 2H), 3.58 (s, 4H), 3.41 (m, 1H), 2.41 (m, 1H), 2.02–2.09 (m, 5H), 1.34–1.67 (m, 7H), 1.24–1.28 (m, 4H), 1.01 (s, 4H). HPLC purity: 98.4% (214 nm), 100% (254 nm); MS (ESI): mass calcd for C30H43BN2O6, 538.32; m/z: found, 539.2 [M + H]+.
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl (5-(Aminomethyl)-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)glycinate (28)
Prepared from 7 and 6-amino-5-(t-butyloxycarbonyl)aminomethyl-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborole by Method B and deprotection of the (t-butyloxycarbonyl)aminomethyl intermediate with trifluoracetic acid. 1H NMR (400 MHz, DMSO-d6): δ 8.98 (s, 1H), 7.22 (s, 1H), 6.82 (s, 1H), 6.00–6.08 (dd, 1H), 5.56 (s, 1H), 5.53–5.57 (d, 1H), 4.88–5.02 (m, 3H), 4.43–4.54 (m, 1H), 3.85–4.04 (m, 2H), 3.04 (s, 1H), 2.07–2.50 (m, 7H), 1.31–1.66 (m, 13H), 1.05–1.24 (m, 4H), 0.50–0.60 (m, 3H), 0.22–0.37 (m, 3H). HPLC purity: 100% (214 nm), 100% (254 nm); MS (ESI): mass calcd for C30H43BN2O6, 538.32; m/z: found, 539.3 [M + H]+.
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl (7-(Aminomethyl)-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)glycinate (29)
Prepared from 7 and 6-amino-7-(t-butyloxycarbonyl)aminomethyl-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborole by Method B and deprotection of the (t-butyloxycarbonyl)aminomethyl intermediate with trifluoracetic acid. 1H NMR (500 MHz, added one drop of con. HCl, DMSO-d6): δ 8.2 (s, 3H), 7.19 (d, J = 8.0 Hz, 1H), 6.70 (d, J = 8.5 Hz, 1H), 6.08 (m, 1H), 5.55 (d, J = 8.5 Hz, 2H), 4.89 (s, 4H), 4.21 (m, 2H), 3.91 (m, 2H), 3.41 (m, 1H), 2.41 (s, 1H), 2.00–2.22 (m, 4H), 1.58–1.66 (m, 2H), 1.48 (m, 1H), 1.24–1.39 (m, 7H), 0.97–1.03 (m, 4H), 0.80 (d, J = 7.5 Hz, 3H), 0.64 (d, J = 7.0 Hz, 3H). HPLC purity: 100% (214 nm), 100% (254 nm); MS (ESI): mass calcd for C30H43BN2O6, 538.32; m/z: found, 539.4 [M + H]+.
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl (1-Hydroxy-3,3-dimethyl-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)glycinate (30)
Prepared from 7 by Method B. 1H NMR (400 MHz, DMSO-d6): δ 8.72 (s, 1H), 7.06 (d, J = 8.4 Hz, 1H), 6.75–6.65 (m, 2H), 6.11–6.03 (m, 2H), 5.55 (d, J = 8.4 Hz, 1H), 5.04–4.93 (m, 2H), 4.50 (d, J = 6 Hz, 1H), 3.83–3.75 (m, 2H), 3.40 (t, J = 7.8 Hz, 1H), 2.39 (s, 1H), 1.48–1.21 (m, 21H), 0.80 (d, J = 6.8 Hz, 3H), 0.63 (d, J = 8.8 Hz, 3H). MS (ESI): mass calcd For C31H44BNO6, 537.33; m/z: found, 538.4 [M + H]+. HPLC: 98.8% (220 nm), 99.5% (254 nm).
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl (5-Fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)glycinate (31)
Prepared from 7 by Method B. 1H NMR (DMSO-d6, 400 MHz): δ 8.88 (s, 1H), 7.11–7.09 (d, J = 8.0 Hz, 1H), 6.88–6.86 (d, J = 8.0 Hz, 1H), 6.09–6.04 (m, 1H), 5.75 (s, 1H), 5.54–5.42 (d, J = 8.0 Hz, 1H), 5.05–5.01 (m, 1H), 4.95–4.93 (m, 1H), 4.83 (s, 2H), 3.86–3.83 (m, 2H), 2.37–2.17 (m, 1H), 2.14–2.08 (m, 2H), 2.06–2.01 (m, 3H),1.66–1.62 (m, 3H),1.59–1.32 (m, 5H), 1.24–1.21 (m, 4H), 1.00 (s, 3H), 0.81–0.79 (d, J = 8.0 Hz, 3H), 0.62–0.61 (d, J = 4.0 Hz, 3H).
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl (7-Fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)glycinate (32)
Prepared from 7 by Method B. 1H NMR (DMSO-d6, 400 MHz): δ 9.10 (s, 1H), 6.94 (d, J = 7.9 Hz, 1H), 6.73 (t, J = 8.2 Hz, 1H), 6.09 (dd, J = 11.0, 17.6 Hz, 1H), 5.63 (br s, 1H), 5.55 (d, J = 7.9 Hz, 1H), 5.08–4.96 (m, 2H), 4.86 (s, 2H), 4.49 (d, J = 6.2 Hz, 1H), 3.91–3.81 (m, 2H), 3.38 (s, 1H), 2.38 (br s, 1H), 2.23–2.12 (m, 1H), 2.11–1.97 (m, 3H), 1.69–1.56 (m, 2H), 1.52–1.42 (m, 2H), 1.32 (s, 3H), 1.29–1.16 (m, 3H), 1.05–0.96 (m, 3H), 0.80 (d, J = 7.1 Hz, 3H), 0.63 (d, J = 6.6 Hz, 3H). MS (ESI): mass calcd For C29H39BFNO6, 527.4; m/z: found, 526.3 [M – H]−. HPLC: 96.7% (220 nm), 100.00% (254 nm).
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((1-Hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)thio)acetate (33)
Prepared from 7 by Method B. 1H NMR (DMSO-d6, 400 MHz): δ 9.18 (s, 1H), 7.72 (s, 1H), 7.49 (dd, J = 1.2, 8.0 Hz, 1H), 7.35 (d, J = 8.0 Hz, 1H), 6.03–5.94 (m, 1H), 5.47 (d, J = 8.8 Hz, 1H), 4.94 (s, 2H), 4.90 (d, J = 4.4 Hz, 1H), 4.47 (d, J = 6.0 Hz, 1H), 3.79 (q, J = 16.0 Hz, 2H), 3.37 (s, 1H), 2.37–2.31 (m, 1H), 2.22–1.87 (m, 4H), 1.68–1.55 (m, 2H), 1.50–1.14 (m, 7H), 1.08–0.93 (m, 6H), 0.79 (d, J = 6.4 Hz, 3H), 0.55 (d, J = 6.4 Hz, 3H). MS (ESI): mass calcd for C29H39BO6S 526.26; m/z: found, 549.3 [M + Na]+. HPLC: 96.5% (220 nm), 96.8% (254 nm).
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((7-Fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)thio)acetate (34)
Prepared from 7 by Method B. 1H NMR (DMSO-d6, 400 MHz): δ 9.34 (s, 1H), 7.61 (t, J = 7.2 Hz, 1H), 7.22 (d, J = 8.0 Hz, 1H), 6.04–5.94 (m, 1H), 5.46 (d, J = 8.0 Hz, 1H), 5.00–4.88 (m, 4H), 4.50 (d, J = 6.0 Hz, 1H), 3.88–3.71 (m, 2H), 3.41–3.36 (m, 2H), 2.38–2.35 (m, 1H), 2.24–1.87 (m, 4H), 1.69–1.55 (m, 2H), 1.50–1.40 (m, 1H), 1.29 (s, 5H), 1.06–0.93 (m, 5H), 0.80 (d, J = 6.8 Hz, 3H), 0.55 (d, J = 6.8 Hz, 3H). MS (ESI): mass calcd for C29H38BFO6S 544.25; m/z: found, 543.2 [M – H]−. HPLC: 100% (220 nm), 100% (254 nm).
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl ((1-Hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)methyl)glycinate (35)
Prepared from 7 by Method B. 1H NMR (400 M Hz, DMSO-d6): δ 0.60 (d, J = 7.2 Hz, 3H), 0.78 (d, J = 6.8 Hz, 3H), 0.97 (br s, 1H), 1.02 (d, 3H), 1.24 (d, 3H), 1.25 (s, 1H), 1.4, (m, 3H), 1.61–1.9 (m, 3H), 2.02 (m, 2H), 2.07 (m, 2H), 2.4 (s, 2H), 3.8 (m, 2H), 4.1 (m, 2H), 4.4 (br, s, 1H), 5.0–5.1 (m, 4H), 5.59 (d, J = 8.4 Hz, 1H), 6.06 (dd, J = 17.8, 11.2 Hz, 1H), 7.5 (m, 2H), 7.75 (s, 1H), 9.3 (br, s, 1H), 9.5 (br, s, 1H). MS (ESI): mass calcd For C30H42BNO6, 523.31; m/z: found, 524.3 [M + H]+. HPLC: 99.0% (220 nm).
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl ((1-Hydroxy-3,3-dimethyl-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)methyl)glycinate (36)
Prepared from 7 by Method B. 1H NMR (400 MHz, DMSO-d6): δ 8.96 (s, 1H), 7.57 (s, 1H), 7.38–7.32 (m, 2H), 6.24–6.17 (m, 1H), 5.62 (d, J = 8.0 Hz, 1H), 5.12–5.06 (m, 2H), 4.53 (d, J = 6.0 Hz, 1H), 3.71–3.68 (m, 2H), 3.43–3.17 (m, 3H), 2.42 (s, 2H), 2.16–2.08 (m, 4H), 1.70–1.03 (m, 21H), 0.83 (d, J = 6.8 Hz, 3H), 0.62 (d, J = 6.8 Hz, 3H). HPLC purity: 100% (214 nm), 100% (254 nm); MS (ESI): mass calcd for C32H46BNO6, 551.34; m/z: found, 552.2 [M + H]+.
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((1-Hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-5-yl)oxy)acetate (37)
Prepared from 7 by Method B. 1H NMR (400 MHz, DMSO-d6): δ 9.05 (s, 1H), 7.54 (d, J = 7.2 Hz, 1H), 6.82 (m, 2H), 6.03 (dd, J = 17.6, 11.2 Hz, 1H), 5.54 (d, J = 8.4 Hz, 1H), 5.00 (m, 2H), 4.81 (s, 2H), 4.70 (m, 2H), 4.51 (d, J = 6 Hz, 1H), 3.36 (m, 1H), 2.35 (s, 1H), 2.11–1.97 (m, 4H), 1.68–1.17 (m, 10H), 0.92 (m, 4H),0.74 (d, J = 7.2 Hz, 3H), 0.56 (d, J = 7.2 Hz, 3H). HPLC: 100% (220 nm), 100% (254 nm).
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((6-Fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-5-yl)oxy)acetate (38)
Prepared from 7 by Method B. 1H NMR (400 MHz, DMSO-d6): δ 9.28 (s, 1H), 7.39 (d, J = 10.4 Hz, 1H), 7.02 (d, J = 7.2 Hz, 1H), 6.03 (dd, J = 18.0, 11.2 Hz, 1H), 5.53 (d, J = 8.0 Hz, 1H), 4.96 (m, 2H), 4.81 (d, 2H), 4.78 (s, 2H), 4.50 (d, J = 6 Hz, 1H), 3.36 (m, 1H), 2.35 (s, 1H), 2.08–1.96 (m, 4H), 1.60–1.19 (m, 10H), 0.98–0.93 (m, 4H),0.74 (d, J = 6.8 Hz, 3H), 0.56 (d, J = 7.2 Hz, 3H). MS (ESI): mass calcd For C29H38BO7F 528.42; m/z: found, 527.2 [M – H]−. HPLC: 100% (220 nm), 100% (254 nm).
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((7-Fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-5-yl)oxy)acetate (39)
Prepared from 7 by Method B. 1H NMR (400 MHz, DMSO-d6): δ 9.20 (s, 1H), 6.73 (s, 1H), 6.60 (d, J = 10 Hz, 1H), 6.03 (dd, J = 18.0, 11.2 Hz, 1H), 5.54 (d, J = 8.4 Hz, 1H), 4.97 (m, 2H), 4.84 (s, 2H), 4.75 (m, 2H), 4.51 (d, J = 6 Hz, 1H), 3.36 (m, 1H), 2.35 (s, 1H), 2.13–1.96 (m, 4H), 1.61–1.17 (m, 10H), 0.98–0.91 (m, 4H),0.75 (d, J = 7.2 Hz, 3H), 0.57 (d, J = 6.8 Hz, 3H). MS (ESI): mass calcd For C29H38BO7F 528.42; m/z: found, 586.0 [M+59-H]-. HPLC: 100% (220 nm), 100% (254 nm).
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((1-Hydroxy-2-(methylsulfonyl)-1,2-dihydrobenzo[d][1,2,3]diazaborinin-7-yl)oxy)acetate (40)
Prepared from 7 by Method B. 1H NMR (DMSO-d6, 400 MHz): δ 8.18 (s, 1H), 8.80–8.78 (m, 1H), 8.66 (s, 1H), 7.41–7.39 (m, 1H), 6.12–6.08 (m, 1H), 5.12–5.08 (m, 1H), 5.01–4.98 (m, 2H), 4.88–4.86 (m, 2H),3.37 (s, 5H), 2.40 (s, 1H), 2.09–2.03 (m, 4H), 1.70–1.45 (m, 3H), 1.33–1.25 (m, 8H), 1.05–0.95 (m, 4H), 0.82–0.80 (m, 3H), 0.65–0.64 (m, 3H).
(3aR,4R,5R,7S,8S,9aS,12R)-8-Hydroxy-4,7,12-trimethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((1-Hydroxy-2-methyl-1,2-dihydrobenzo[d][1,2,3]diazaborinin-7-yl)oxy)acetate (41)
Prepared from 7 by Method B. 1H NMR (DMSO-d6, 400 MHz): δ 8.31 (s, 1H), 7.90 (s, 1H), 7.76 (br s, 1H), 7.68 (d, J = 8.8 Hz, 1H), 7.28 (d, J = 7.3 Hz, 1H), 6.10 (dd, J = 10.8, 17.2 Hz, 1H), 5.60 (d, J = 7.8 Hz, 1H), 5.14–4.93 (m, 2H), 4.88–4.71 (m, 2H), 4.54 (d, J = 5.8 Hz, 1H), 3.47 (s, 2H), 3.41 (br s, 1H), 3.07 (s, 3H), 2.41 (br s, 1H), 2.26–1.95 (m, 4H), 1.73–0.92 (m, 10H), 0.90–0.76 (m, 3H), 0.63 (d, J = 6.0 Hz, 3H). MS (ESI): mass calcd for C30H41BN2O6, 536.3; m/z: found, 535.3 (M – H)−. HPLC: 90.7% in 220 nm; 93.3% in 254 nm.
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((2-Acetyl-1-hydroxy-1,2-dihydrobenzo[d][1,2,3]diazaborinin-7-yl)oxy)acetate (42)
Prepared from 7 by Method B. 1H NMR (DMSO-d6, 400 MHz): δ 8.02 (s, 1H), 7.53 (d, J = 8.4 Hz, 1H), 7.07–6.94 (m, 2H), 6.16–6.03 (m, 1H), 5.56 (t, J = 8.0 Hz, 1H), 5.11–4.96 (m, 2H), 4.77 (t, J = 10.0 Hz, 2H), 4.53 (d, J = 5.8 Hz, 1H), 3.39 (d, J = 5.2 Hz, 1H), 2.36 (d, J = 1.4 Hz, 3H), 2.23–1.94 (m, 4H), 1.63 (br s, 2H), 1.49–1.09 (m, 7H), 1.00 (d, J = 17.8 Hz, 4H), 0.80 (d, J = 3.6 Hz, 3H), 0.61 (dd, J = 7.0, 12.0 Hz, 3H). MS (ESI): mass calcd for C31H41BN2O7, 564.3; m/z: found, 581.3 (M + H2O – H)-. HPLC: 94.9% in 220 nm; 100% in 254 nm.
tert-Butyl 1-Hydroxy-7-(2-(((3aR,4R,5R,7S,8S,9R,9aS,12R)-8-hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl)oxy)-2-oxoethoxy)benzo[d][1,2,3]diazaborinine-2(1H)-carboxylate (43)
Prepared from 7 by Method B. 1H NMR (DMSO-d6, 400 MHz): δ 8.57 (s, 1H), 8.10 (s, 1H), 7.75 (d, J = 8.2 Hz, 1H), 7.47 (br s, 1H), 7.38 (d, J = 9.2 Hz, 1H), 6.09 (dd, J = 11.0, 17.8 Hz, 1H), 5.60 (d, J = 8.6 Hz, 1H), 5.11–4.96 (m, 2H), 4.89 (d, J = 6.0 Hz, 1H), 4.53 (d, J = 6.0 Hz, 1H), 2.41 (s, 1H), 2.26–1.96 (m, 4H), 1.65–1.58 (m, 11H), 1.53–1.21 (m, 8H), 1.13–0.97 (m, 5H), 0.81 (d, J = 6.8 Hz, 3H), 0.66 (d, J = 6.8 Hz, 3H). MS (ESI): mass calcd for C34H47BN2O8, 622.3; m/z: found, 639.4 (M + H2O – H)−. HPLC: 96.4% in 220 nm; 96.5% in 254 nm.
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((1-Hydroxy-1,2-dihydrobenzo[d][1,2,3]diazaborinin-7-yl)oxy)acetate (44)
Prepared from 43 by deprotection with HCl/ethyl acetate in dichloromethane. 1H NMR (DMSO-d6, 400 MHz): δ 9.90 (s, 1H), 7.96 (s, 1H), 7.75–7.64 (m, 2H), 7.31 (d, J = 8.8 Hz, 1H), 6.09 (dd, J = 11.2, 17.8 Hz, 1H), 5.60 (d, J = 8.2 Hz, 1H), 5.13–4.95 (m, 2H), 4.89–4.74 (m, 2H), 3.41 (d, J = 5.6 Hz, 1H), 2.41 (s, 1H), 2.25–1.97 (m, 4H), 1.71–1.18 (m, 10H), 1.07–0.93 (m, 4H), 0.81 (d, J = 6.8 Hz, 3H), 0.64 (d, J = 6.8 Hz, 3H). MS (ESI): mass calcd for C29H39BN2O6, 522.3; m/z: found, 521.3 (M – H)−. HPLC: 99.7% in 220 nm; 100% in 254 nm.
Methyl 1-Hydroxy-7-(2-(((3aR,4R,5R,7S,8S,9R,9aS,12R)-8-hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl)oxy)-2-oxoethoxy)benzo[d][1,2,3]diazaborinine-2(1H)-carboxylate (45)
Prepared from 7 by Method B. 1H NMR (DMSO-d6, 400 MHz): δ 8.12 (s, 1H), 7.78 (d, J = 8.4 Hz, 1H), 7.48 (s, 1H), 7.39 (dd, J = 2.4, 8.4 Hz, 1H), 6.09 (dd, J = 11.2, 17.6 Hz, 1H), 5.60 (d, J = 7.8 Hz, 1H), 5.13–4.97 (m, 2H), 4.96–4.84 (m, 2H), 3.91 (s, 3H), 3.41 (d, J = 5.8 Hz, 1H), 2.41 (s, 1H), 2.25–1.99 (m, 4H), 1.74–1.20 (m, 10H), 1.10–0.94 (m, 4H), 0.81 (d, J = 6.8 Hz, 3H), 0.67 (d, J = 6.8 Hz, 3H). MS (ESI): mass calcd for C31H41BN2O8, 580.3; m/z: found, 597.4 (M + H2O – H)−. HPLC: 97.2% in 220 nm; 96.1% in 254 nm.
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((1-Hydroxy-1H-benzo[d][1,2,6]oxazaborinin-7-yl)oxy)acetate (46)
Prepared from 7 by Method B. 1H NMR (DMSO-d6, 400 MHz): δ 9.29 (s, 1H), 8.54 (s, 1H), 7.71 (d, J = 8.2 Hz, 1H), 7.49 (s, 1H), 7.37 (d, J = 6.0 Hz, 1H), 6.13–6.04 (m, 1H), 5.60 (d, J = 7.8 Hz, 1H), 5.11–4.96 (m, 2H), 4.87 (d, J = 7.2 Hz, 1H), 4.53 (d, J = 6.2 Hz, 1H), 3.41 (m, 1H), 2.41 (s, 1H), 2.05 (m, 4H), 1.71–1.44 (m, 4H), 1.40–1.20 (m, 7H), 1.04 (s, 4H), 0.81 (d, J = 6.8 Hz, 3H), 0.65 (d, J = 7.0 Hz, 3H). MS (ESI): mass calcd for C29H38BNO7, 523.3; m/z: found, 522.3 (M – H)−. HPLC: 97.5% in 220 nm; 94.3% in 254 nm.
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((8-Fluoro-1-hydroxy-2-(methylsulfonyl)-1,2-dihydrobenzo[d][1,2,3]diazaborinin-7-yl)oxy)acetate (47)
Prepared from 7 by Method B. 1H NMR (DMSO-d6, 400 MHz): δ 7.90 (s, 1H), 7.50–7.28 (m, 2H), 6.13 (m, 1H), 5.61 (s, 1H), 5.19–4.84 (m, 3H), 4.54 (s, 1H), 3.24 (s, 3H), 2.41 (s, 1H), 2.07 (m, 4H), 1.73–1.19 (m, 11H), 1.06 (m, 4H), 0.82 (d, J = 6.8 Hz, 3H), 0.65 (d, J = 6.8 Hz, 3H). MS (ESI): mass calcd for C30H40BFN2O8S 618.3; m/z: found, 619.2 (M + H)+. HPLC: 95.2% in 220 nm; 98.0% in 254 nm.
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((8-Fluoro-1-hydroxy-2-methyl-1,2-dihydrobenzo[d][1,2,3]diazaborinin-7-yl)oxy)acetate (48)
Prepared from 7 by Method B. 1H NMR (DMSO-d6, 400 MHz): δ 7.93 (d, J = 2.4 Hz, 1H), 7.54–7.44 (m, 2H), 6.10 (dd, J = 11.2, 17.6 Hz, 1H), 5.60 (d, J = 8.4 Hz, 1H), 5.10–4.98 (m, 2H), 4.94 (d, J = 3.6 Hz, 1H), 3.49 (s, 3H), 3.40 (d, J = 6.4 Hz, 1H), 2.40 (br s, 1H), 2.24–1.97 (m, 5H), 1.72–1.19 (m, 10H), 1.04 (s, 4H), 0.81 (d, J = 7.2 Hz, 3H), 0.63 (d, J = 7.2 Hz, 3H). MS (ESI): mass calcd for C30H40BFN2O6, 554.5; m/z: found, 555.3 [M + H]+. HPLC: 100.00% (220 nm), 100.00% (254 nm).
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((2-Acetyl-8-fluoro-1-hydroxy-1,2-dihydrobenzo[d][1,2,3]diazaborinin-7-yl)oxy)acetate (49)
Prepared from 7 by Method B. 1H NMR (DMSO-d6, 400 MHz): δ 8.05 (s, 1H), 7.40 (d, J = 8.4 Hz, 1H), 7.17–7.12 (m, 1H), 6.15–6.07 (m, 1H), 5.61 (d, J = 7.6 Hz, 1H), 5.13–5.00 (d, J = 3.6 Hz, 2H), 4.97–4.83 (m, 2H), 4.56–4.50 (m, 1H), 2.44–2.38 (m, 2H), 2.23–2.00 (m, 7H), 1.70–1.19 (m, 11H), 1.05 (d, J = 3.2 Hz, 3H), 0.82 (d, J = 6.8 Hz, 3H), 0.65 (dd, J = 6.8, 17.6 Hz, 2H). MS (ESI): mass calcd for C31H40BFN2O7, 582.5; m/z: found, 583.3(M + H)+. HPLC: 90.0% (220 nm), 90.4% (254 nm).
Methyl 8-Fluoro-1-hydroxy-7-(2-(((3aR,4R,5R,7S,8S,9R,9aS,12R)-8-hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl)oxy)-2-oxoethoxy)benzo[d][1,2,3]diazaborinine-2(1H)-carboxylate (50)
Prepared from 7 by Method B. 1H NMR (DMSO-d6, 400 MHz): δ 8.53 (br s, 1H), 7.86 (br s, 1H), 7.48–7.29 (m, 2H), 6.11 (dd, J = 10.8, 17.6 Hz, 1H), 5.60 (d, J = 8.0 Hz, 1H), 5.13–4.99 (m, 2H), 4.98–4.86 (m, 2H), 4.54 (d, J = 6.4 Hz, 1H), 3.84 (s, 2H), 3.44–3.39 (m, 1H), 2.41 (br s, 1H), 2.29–2.00 (m, 3H), 1.73–1.19 (m, 10H), 1.11–0.94 (m, 4H), 0.81 (d, J = 6.4 Hz, 3H), 0.70–0.60 (m, 3H). MS (ESI): mass calcd for C31H40BFN2O8, 598.5; m/z: found, 599.3(M + H)+. HPLC: 89.1% (220 nm), 100.0% (254 nm).
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((8-Fluoro-1-hydroxy-1H-benzo[d][1,2,6]oxazaborinin-7-yl)oxy)acetate (51)
Prepared from 7 by Method B. 1H NMR (DMSO-d6, 400 MHz): δ 9.44–9.27 (m, 1H), 8.55 (br s, 1H), 7.54 (br s, 2H), 6.10 (dd, J = 11.2, 17.6 Hz, 1H), 5.59 (d, J = 8.8 Hz, 1H), 5.23–4.90 (m, 4H), 4.54 (br s, 1H), 2.40 (br s, 1H), 2.24–1.97 (m, 4H), 1.72–1.19 (m, 10H), 1.10–0.94 (m, 3H), 0.81 (d, J = 6.4 Hz, 3H), 0.64 (d, J = 7.2 Hz, 3H). MS (ESI): mass calcd for C29H37BFNO7, 541.4; m/z: found, 558.3(M + H2O – H)−. HPLC: 94.9% (220 nm), 95.8% (254 nm).
Methyl 1-Hydroxy-7-(2-(((3aR,4R,5R,7S,8S,9R,9aS,12R)-8-hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl)oxy)-2-oxoethoxy)-8-methylbenzo[d][1,2,3]diazaborinine-2(1H)-carboxylate (52)
Prepared from 7 by Method B. 1H NMR (DMSO-d6, 400 MHz): δ 9.15 (br s, 1H), 8.02 (br s, 1H), 7.59 (br s, 1H), 7.33 (br s, 1H), 6.11 (dd, J = 17.6, 11.2 Hz, 1H), 5.61 (d, J = 8.4 Hz, 1H), 5.09–4.98 (m, 2H), 4.90 (s, 1H), 4.52 (d, J = 2.4 Hz, 2H), 3.90 (s, 3H), 3.41 (d, J = 5.6 Hz, 1H), 2.33 (s, 3H), 2.20–2.05 (m, 4H), 1.72–1.55 (m, 2H), 1.54–1.36 (m, 2H), 1.34 (s, 3H), 1.31–1.15 (m, 3H), 1.03 (m, 4H), 0.82 (d, J = 6.8 Hz, 3H), 0.65 (d, J = 6.8 Hz, 3H). MS (ESI): mass calcd for C32H43BN2O8, 594.5; m/z: found, 595.4 [M + H]+. HPLC: 93.0% (220 nm), 88.6% (254 nm).
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((1-Hydroxy-3,4-dihydro-1H-benzo[c][1,2]oxaborinin-7-yl)oxy)acetate (53)
Prepared from 7 by Method B. 1H NMR (DMSO-d6, 400 MHz): δ 7.14 (s, 1H), 7.07 (d, J = 8.4 Hz, 1H), 6.90 (d, J = 8.4 Hz, 1H), 6.06 (dd, J = 11.2, 17.6 Hz, 1H), 5.56 (d, J = 8.0 Hz, 1H), 5.05–4.95 (m, 3H), 4.82–4.65 (m, 2H), 3.99 (t, J = 5.6 Hz, 2H), 3.42–3.37 (m, 1H), 2.74 (t, J = 5.6 Hz, 2H), 2.38–2.32 (m, 1H), 2.23–1.98 (m, 4H), 1.72–1.19 (m, 10H), 1.08–0.94 (m, 4H), 0.77 (d, J = 6.4 Hz, 3H), 0.60 (d, J = 6.4 Hz, 3H). MS (ESI): mass calcd for C30H41BO7, 524.29; m/z: found, 523.2 [M – H]−. HPLC: 99.9% (220 nm), 99.9% (254 nm).
(3aR,4R,5R,7R,8S,9R,9aS,12R)-7-Ethyl-8-hydroxy-4,7,9,12-tetramethyl-3-oxodecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((7-Fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate (54)
A suspension of 6 (500.0 mg, 938.6 μmol, 1.0 equiv) and Pd/C (300.0 mg, 938.6 μmol, 1.0 equiv) in THF (30.0 mL) was stirred at 25 °C for 12 h under 40 psi hydrogen atmosphere. The mixture was filtered and the filtrate was concentrated to give (3aR,4R,5R,7R,8S,9R,9aS,12R)-7-ethyl-8-hydroxy-4,7,9,12-tetramethyl-3-oxodecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-(tosyloxy)acetate (500.0 mg, 902.8 μmol, 96.2% yield, 96.5% purity) as a white foam. 1H NMR (CDCl3, 400 MHz): δ 7.83 (d, J = 8.4 Hz, 2H), 7.36 (d, J = 8.4 Hz, 2H), 5.66 (d, J = 7.9 Hz, 1H), 4.51 (s, 2H), 3.45–3.37 (m, 1H), 2.46 (s, 3H), 2.41–2.14 (m, 4H), 2.09 (br s, 1H), 1.85–1.05 (m, 15H), 0.99–0.91 (m, 6H), 0.72 (t, J = 7.5 Hz, 3H), 0.61 (d, J = 7.1 Hz, 2H).
A solution of (3aR,4R,5R,7R,8S,9R,9aS,12R)-7-ethyl-8-hydroxy-4,7,9,12-tetramethyl-3-oxodecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-(tosyloxy)acetate (300.0 mg, 561.1 μmol, 1.0 equiv), 80 (94.22 mg, 561.06 μmol, 1.00 equiv), and Na2CO3 (178.4 mg, 1.7 mmol, 3.0 equiv) in DMSO (15.0 mL) was heated to 30–40 °C for 12 h. Water (20 mL) was added to the mixture, white solid was precipitated. The mixture was filtered to give a crude product, which was purified by prep HPLC to give (3aR,4R,5R,7R,8S,9R,9aS,12R)-7-ethyl-8-hydroxy-4,7,9,12-tetramethyl-3-oxodecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((7-fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate 54 (95.00 mg, 179.10 μmol, 31.92% yield, 100% purity) as a white solid. 1H NMR (DMSO-d6, 400 MHz): δ 9.26 (s, 1H), 7.24–7.21 (m, 1H), 7.12–7.06 (m, 1H), 5.58 (d, J = 8.4 Hz, 1H), 4.92–4.83 (m, 2H), 4.84–4.80 (m, 2H), 4.40 (d, J = 5.6 Hz, 1H), 3.34–3.32 (m, 1H), 2.37–2.18 (m, 1H), 2.24–2.00 (m, 3H), 1.80–0.93 (m, 20H), 0.88–0.77 (m, 3H), 0.66–0.56 (m, 3H). MS (ESI): mass calcd for C29H40BFO7, 530.3; m/z: found, 529.3 [M – H]−. HPLC: 100% in 220 nm; 100% in 254 nm.
(3aR,4R,5R,7R,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-7-((R)-oxiran-2-yl)-3-oxodecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((7-Fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate (55)
A mixture of 6 (20.0 g, 37.5 mmol, 1.0 equiv) and m-chloroperoxybenzoic acid (8.9 g, 41.3 mmol, 1.1 equiv) in dichloromethane (200.0 mL) was degassed and purged with N2 for three times, and then the mixture was stirred at 25 °C for 12 h under an N2 atmosphere. The mixture was poured into ice–water (w/w = 1/1) (100 mL). The combined organic phase was washed with aq NaHCO3 (20 mL) and brine (100 mL), dried, filtered, and concentrated in vacuum. The residue was purified by silica gel chromatography (petroleum ether/ethyl acetate = 1/1) to give (3aR,4R,5R,7R,8S,9R,9aS,12R)-8-hydroxy-4,7,9,12-tetramethyl-7-((R)-oxiran-2-yl)-3-oxodecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-(tosyloxy)acetate (11.0 g, 20.0 mmol, 53.4% yield) as a white solid.
A mixture of (3aR,4R,5R,7R,8S,9R,9aS,12R)-8-hydroxy-4,7,9,12-tetramethyl-7-((R)-oxiran-2-yl)-3-oxodecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-(tosyloxy)acetate (2.0 g, 3.6 mmol, 1.0 equiv), 7-fluoro-1-hydroxy-3H-2,1-benzoxaborol-6-ol (612.9 mg, 3.6 mmol, 1.0 equiv), and Na2CO3 (1.2 g, 10.9 mmol, 3.0 equiv) in DMSO (30.0 mL) was degassed and purged with N2 for three times, and then the mixture was stirred at 30 °C for 12 h under an N2 atmosphere. The mixture was poured into ice–water (w/w = 1/1) (50 mL) and precipitating solid. The solid was collected to afford (3aR,4R,5R,7R,8S,9R,9aS,12R)-8-hydroxy-4,7,9,12-tetramethyl-7-((R)-oxiran-2-yl)-3-oxodecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((7-fluoro-1-hydroxy-1,3-dihydrobenzo[c] [1,2]oxaborol-6-yl)oxy)acetate 55 (1.7 g) as a white solid. 1H NMR (DMSO-d6, 400 MHz): δ 9.27 (s, 1H), 7.25–7.19 (m, 1H), 7.13 (d, J = 8.4 Hz, 1H), 5.6 (d, J = 8.4 Hz, 1H), 4.97–4.90 (m, 2H), 4.89–4.75 (m, 2H), 4.35 (d, J = 6.4 Hz, 1H), 3.48 (t, J = 6.4 Hz, 1H), 3.13–3.10 (m, 1H), 2.43–2.37 (m, 1H), 2.30–1.99 (m, 5H), 1.76–1.57 (m, 3H), 1.55–1.23 (m, 6H), 1.18–0.97 (m, 3H), 0.93–0.75 (m, 6H), 0.64 (d, J = 7.2 Hz, 3H) MS (ESI): mass calcd for C29H38BFO8, 544.26; m/z: found, 543.3 [M – H]−. HPLC: 96.6% (220 nm), 100.0% (254 nm).
The following compounds are derived from (3aR,4R,5R,7R,8S,9R,9aS,12R)-7-formyl-8-hydroxy-4,7,9,12-tetramethyl-3-oxodecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-(tosyloxy)acetate 57, which was prepared by reaction of (3aR,4R,5R,7R,8S,9R,9aS,12R)-7-formyl-8-hydroxy-4,7,9,12-tetramethyl-3-oxodecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-hydroxyacetate28 (56) and p-toluenesulfonyl chloride in pyridine.
(3aR,4R,5R,7R,8S,9R,9aS,12R)-7-formyl-8-hydroxy-4,7,9,12-tetramethyl-3-oxodecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((7-Fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate (58)
A solution of 57 (300.0 mg, 561.1 μmol, 1.0 equiv), 80 (94.2 mg, 561.1 μmol, 1.0 equiv), and Na2CO3 (178.41 mg, 1.68 mmol, 3.00 equiv) in DMSO (10.0 mL) was heated to 30–40 °C for 12 h. Water (20 mL) was added to the mixture, white solid was precipitated and filtered to give a crude product. The crude product was purified by prep HPLC to give (3aR,4R,5R,7R,8S,9R,9aS,12R)-7-formyl-8-hydroxy-4,7,9,12-tetramethyl-3-oxodecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((7-fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate 58 (81.0 mg, 152.7 μmol, 27.2% yield, 10% purity) as a white solid. 1H NMR (DMSO-d6, 400 MHz): δ 9.67 (s, 1H), 9.27 (s, 1H), 7.24 (t, J = 7.9 Hz, 1H), 7.11 (d, J = 8.4 Hz, 1H), 5.42 (d, J = 8.4 Hz, 1H), 4.92 (s, 2H), 4.86–4.75 (m, 3H), 3.57 (t, J = 6.6 Hz, 1H), 2.43–2.03 (m, 4H), 1.76–1.21 (m, 10H), 1.09 (s, 3H), 0.95 (d, J = 7.1 Hz, 3H), 0.89–0.80 (m, 2H), 0.62 (d, J = 7.1 Hz, 3H). MS (ESI): mass calcd for C28H36BFO8, 530.3; m/z: found, 529.3 [M – H]−. HPLC: 100% in 220 nm; 100% in 254 nm.
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-7-(Hydroxymethyl)-4,7,9,12-tetramethyl-3-oxodecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((7-Fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate (59)
A solution of 57 (300.0 mg, 561.1 μmol, 1.0 equiv) and NaBH(OAc)3 (237.8 mg, 1.1 mmol, 2.0 equiv) in dichloromethane (15.0 mL) was stirred at 20 °C for 2 h. Water (20 mL) was added to the mixture, the aqueous layer was treated with dichloromethane (10 mL × 3), the combined organic phase was treated with brine, dried and concentrated in vacuo to give crude (3aR,4R,5R,7S,8S,9R,9aS,12R)-8-hydroxy-7-(hydroxymethyl)-4,7,9,12-tetramethyl-3-oxodecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-(tosyloxy)acetate (300.0 mg, crude) as a colorless oil.
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-hydroxy-7-(hydroxymethyl)-4,7,9,12-tetramethyl-3-oxodecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-(tosyloxy)acetate (300.0 mg, 559.0 μmol, 1.0 equiv), 80 (93.9 mg, 559.0 μmol, 1.0 equiv), and Na2CO3 (177.7 mg, 1.7 mmol, 3.0 equiv) in DMSO (5.0 mL) were heated to 30–40 °C for 12 h. Water (20 mL) was added to the mixture; white solid was precipitated and filtered to give a crude product. The crude product was purified by prep HPLC to give (3aR,4R,5R,7S,8S,9R,9aS,12R)-8-hydroxy-7-(hydroxymethyl)-4,7,9,12-tetramethyl-3-oxodecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((7-fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate 59 (47.0 mg, 88.3 μmol, 15.8% yield) as a white solid. 1H NMR (DMSO-d6, 400 MHz): δ 9.26 (s, 1H), 7.22–7.20 (m, 1H), 7.10–7.00 (m, 1H), 5.55–5.53 (m, 1H), 5.17–5.15 (m, 1H), 4.92 (s, 1H), 4.83–4.72 (m, 3H), 4.56–4.48 (m, 2H), 3.90 (d, J = 11.0 Hz, 1H), 3.41 (d, J = 6.2 Hz, 1H), 3.32 (d, J = 11.0 Hz, 1H), 2.32–2.30 (m, 2H), 2.26–1.98 (m, 3H), 1.88–1.21 (m, 10H), 1.02 (s, 3H), 0.83 (d, J = 7.1 Hz, 3H), 0.61 (d, J = 6.6 Hz, 3H). MS (ESI): mass calcd for C28H38BFO8, 532.3; m/z: found, 531.3 [M – H]−. HPLC: 100% in 220 nm; 100% in 254 nm.
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-7-((hydroxyimino)methyl)-4,7,9,12-tetramethyl-3-oxodecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((7-Fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate (60)
A solution of 58 (400.0 mg, 754.2 μmol, 1.0 equiv) and hydroxylamine hydrochloride (62.9 mg, 905.0 μmol, 1.2 equiv) in H2O (5.0 mL) and ethanol (10.0 mL) were stirred at 15 °C for 12 h. HPLC and LCMS showed the desired product as major component and starting material consumed. Water (30 mL) was added to the mixture, white solid precipitated, the mixture was filtered to give a crude product. The crude product was purified by prep HPLC. The solvent was concentrated to about 15 mL and then lyophilized to give (3aR,4R,5R,7S,8S,9R,9aS,12R)-8-hydroxy-7-((hydroxyimino)methyl)-4,7,9,12-tetramethyl-3-oxodecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((7-fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate 60 (99.0 mg, 174.3 μmol, 23.1% yield, 96.0% purity) as a white solid. 1H NMR (DMSO-d6, 400 MHz): δ 10.41 (br s, 1H), 9.25 (br s, 1H), 7.51 (s, 1H), 7.23 (t, J = 7.9 Hz, 1H), 7.10 (d, J = 7.9 Hz, 1H), 5.55 (d, J = 7.9 Hz, 1H), 4.92 (s, 2H), 4.78 (s, 2H), 3.46 (d, J = 6.6 Hz, 1H), 2.41 (br s, 1H), 2.25–2.01 (m, 3H), 1.75–0.94 (m, 16H), 0.85 (d, J = 7.1 Hz, 3H), 0.62 (d, J = 6.6 Hz, 3H). MS (ESI): mass calcd for C28H37BFNO8, 545.26; m/z: found, 546.3 [M + H]+. HPLC: 96.2% in 220 nm; 100% in 254 nm.
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-7-((methoxyimino)methyl)-4,7,9,12-tetramethyl-3-oxodecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((7-Fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate (61)
This compound was prepared in a manner similar to that described for 60, using O-methyl hydroxylamine in place of hydroxylamine. 1H NMR (DMSO-d6, 400 MHz): δ 9.25 (br s, 1H), 7.50 (s, 1H), 7.25 (t, J = 8.0 Hz, 1H), 7.10 (d, J = 8.4 Hz, 1H), 5.60 (d, J = 8.0 Hz, 1H), 4.92 (s, 2H), 4.78 (s, 2H), 3.62 (s, 3H), 3.48 (d, J = 5.6 Hz, 1H), 2.41 (br s, 1H), 2.24–1.97 (m, 4H), 1.72–1.40 (m, 5H), 1.35–1.19 (m, 5H), 1.15–0.96 (m, 5H), 0.85 (d, J = 6.4 Hz, 3H), 0.63 (d, J = 5.6 Hz, 3H) MS (ESI): mass calcd for C29H39BFNO8, 559.28; m/z: found, 560.3 [M + H]+. HPLC: 97.5% (220 nm), 100.0% (254 nm).
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-7-((isopropoxyimino)methyl)-4,7,9,12-tetramethyl-3-oxodecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((7-Fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate (62)
This compound was prepared in a manner similar to that described for 60, using O-isopropyl hydroxylamine in place of hydroxylamine. 1H NMR (DMSO-d6, 400 MHz): δ 9.26 (br s, 1H), 7.47 (s, 1H), 7.23 (t, J = 8.0 Hz, 1H), 7.10 (d, J = 8.0 Hz, 1H), 5.57 (d, J = 8.0 Hz, 1H), 4.92 (s, 1H), 4.76 (s, 1H), 4.16 (dt, J = 5.6, 12.1 Hz, 1H), 3.48 (d, J = 5.2 Hz, 2H), 2.41 (br s, 1H), 2.09 (d, J = 15.6 Hz, 4H), 1.73–1.21 (m, 12H), 1.19–0.97 (m, 10H), 0.86 (d, J = 6.4 Hz, 3H), 0.63 (d, J = 6.4 Hz, 3H). MS (ESI): mass calcd for C31H43BFNO8, 587.48; m/z: found, 586.3 [M – H]−. HPLC: 98.8% (220 nm), 85.1% (254 nm).
(3aR,4R,5R,7S,8S,9R,9aS,12R)-7-(Aminomethyl)-8-hydroxy-4,7,9,12-tetramethyl-3-oxodecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((7-Fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate (63)
To a solution of 60 (570.0 mg, 1.0 mmol, 1.0 equiv) in methanol (10.0 mL) was added Raney–nickel (0.5 g) under an N2 atmosphere. The suspension was degassed and purged with H2 for three times. The mixture was stirred under H2 (50 psi) at 25 °C for 2 h. The reaction mixture was filtered and the filter was concentrated to give a residue, which was purified by prep HPLC to give (3aR,4R,5R,7S,8S,9R,9aS,12R)-7-(aminomethyl)-8-hydroxy-4,7,9,12-tetramethyl -3-oxodecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((7-fluoro-1-hydroxy-1,3-dihydro benzo[c][1,2]oxaborol-6-yl)oxy)acetate 63 (300.0 mg, 464.8 μmol, 44.2% yield, TFA) as a light yellow solid. 1H NMR (DMSO-d6, 400 MHz): δ 9.27 (br s, 1H), 7.55 (br s, 2H), 7.25 (t, J = 7.6 Hz, 1H), 7.15–7.06 (m, 1H), 5.49–5.28 (m, 2H), 4.91 (br s, 1H), 4.78 (br s, 1H), 3.51 (br s, 1H), 3.18 (br s, 1H), 2.92 (br s, 1H), 2.42 (d, J = 8.4 Hz, 2H), 2.25–1.90 (m, 4H), 1.69–1.21 (m, 10H), 1.04 (br s, 3H), 0.84 (d, J = 5.6 Hz, 2H), 0.64 (d, J = 6.4 Hz, 3H) MS (ESI): mass calcd for C30H40BF4NO9, 531.28; m/z: found, 532.3 [M + H]+. HPLC: 97.1% (220 nm), 93.6% (254 nm).
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-7-((methylamino)methyl)-3-oxodecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((7-Fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate (64)
Sodium cyanoborohydride (142.2 mg, 2.3 mmol, 4.0 equiv) was added to a solution of 58 (300.0 mg, 565.6 μmol, 1.0 equiv) and methylamine (4.0 equiv) in methanol (10.0 mL) and acetic acid (1.0 mL). The mixture was stirred at 15 °C for 12 h. Water (30 mL) was added to the mixture, and the mixture was treated with dichloromethane (3 × 30 mL). The combined organic phase was concentrated to give a crude product. The crude product was purified by prep HPLC. The solvent was concentrated to about 15–20 mL of solution left and then was lyophilized to give (3aR,4R,5R,7S,8S,9R,9aS,12R)-8-hydroxy-4,7,9,12-tetramethyl-7-((methylamino)methyl)-3-oxodecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((7-fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate 64 (52.0 mg, 95.3 μmol, 16.8% yield) as a white TFA salt solid. 1H NMR DMSO-d6, (400 MHz): δ 9.30 (br s, 1H), 8.01 (br s, 2H), 7.31 (t, J = 8.0 Hz, 1H), 7.13 (d, J = 7.8 Hz, 1H), 5.36 (d, J = 8.0 Hz, 1H), 4.93 (s, 2H), 4.87–4.71 (m, 2H), 3.47 (d, J = 5.3 Hz, 1H), 3.27 (d, J = 8.8 Hz, 1H), 3.12 (d, J = 10.5 Hz, 1H), 2.62–2.57 (m, 3H), 2.26–1.93 (m, 6H), 1.71–1.21 (m, 10H), 1.14–0.94 (m, 4H), 0.84 (d, J = 6.3 Hz, 3H), 0.64 (d, J = 7.0 Hz, 3H). MS (ESI): mass calcd for C31H42BF4NO9, 545.3; m/z: found, 546.3 [M + H]+. HPLC: 96.4% in 220 nm; 100% in 254 nm.
The following compounds were prepared in a manner similar to that described for 64 from 58 and the appropriate amine.
(3aR,4R,5R,7S,8S,9R,9aS,12R)-7-((Ethylamino)methyl)-8-hydroxy-4,7,9,12-tetramethyl-3-oxodecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((7-Fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate (65)
1H NMR (DMSO-d6, 400 MHz): δ 9.28 (br s, 1H), 7.88 (br s, 1H), 7.73 (br s, 1H), 7.31 (t, J = 8.2 Hz, 1H), 7.12 (d, J = 8.4 Hz, 1H), 5.42 (br s, 1H), 5.36 (d, J = 8.4 Hz, 1H), 4.93 (s, 2H), 4.86–4.74 (m, 2H), 3.49 (d, J = 5.7 Hz, 1H), 3.33–3.22 (m, 1H), 3.14–2.94 (m, 3H), 2.42 (br s, 1H), 2.25–1.94 (m, 4H), 1.72–0.94 (m, 18H), 0.84 (d, J = 7.1 Hz, 2H), 0.64 (d, J = 7.1 Hz, 3H). MS (ESI): mass calcd for C32H44BF4NO9, 559.3; m/z: found, 560.3 [M + H]+. HPLC: 98.2% in 220 nm; 100% in 254 nm.
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-((propylamino)methyl)decahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((7-Fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate (66)
1H NMR (DMSO-d6, 400 MHz): δ 9.29 (br s, 1H), 7.96 (br s, 2H), 7.77 (br s, 1H), 7.31 (t, J = 7.9 Hz, 1H), 7.11 (d, J = 7.9 Hz, 1H), 5.48 (br s, 1H), 5.35 (d, J = 7.9 Hz, 1H), 4.93 (s, 2H), 4.87–4.75 (m, 2H), 3.49 (d, J = 5.7 Hz, 1H), 3.29 (t, J = 10.1 Hz, 1H), 3.17–3.04 (m, 1H), 2.97–2.82 (m, 2H), 2.41 (br s, 1H), 2.24–1.91 (m, 4H), 1.75–0.95 (m, 17H), 0.90–0.79 (m, 4H), 0.65 (d, J = 7.1 Hz, 3H). MS (ESI): mass calcd for C33H46BF4NO9, 573.3; m/z: found, 574.3 [M + H]+. HPLC: 98.9% in 220 nm; 100% in 254 nm.
(3aR,4R,5R,7S,8S,9R,9aS,12R)-7-((Butylamino)methyl)-8-hydroxy-4,7,9,12-tetramethyl-3-oxodecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((7-Fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate (67)
1H NMR (DMSO-d6, 400 MHz): δ 9.28 (br s, 1H), 7.97 (br s, 1H), 7.76 (br s, 1H), 7.31 (t, J = 8.2 Hz, 1H), 7.11 (d, J = 7.9 Hz, 1H), 5.35 (d, J = 8.4 Hz, 1H), 4.92 (s, 2H), 4.86–4.75 (m, 2H), 3.49 (d, J = 6.2 Hz, 1H), 3.28 (t, J = 9.9 Hz, 1H), 3.18–3.06 (m, 1H), 2.93 (br s, 2H), 2.42 (br s, 1H), 2.25–1.91 (m, 5H), 1.73–1.20 (m, 15H), 1.17–0.96 (m, 4H), 0.90–0.79 (m, 5H), 0.65 (d, J = 6.6 Hz, 3H). MS (ESI): mass calcd for C34H48BF4NO9, 587.3; m/z: found, 588.5 [M + H]+. HPLC: 98.6% in 220 nm; 91.6% in 254 nm.
(3aR,4R,5R,7S,8S,9R,9aS,12R)-7-((Cyclopropylamino)methyl)-8-hydroxy-4,7,9,12-tetramethyl-3-oxodecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((7-Fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate (68)
1H NMR (DMSO-d6, 400 MHz): δ 9.29 (br s, 1H), 8.29 (br s, 1H), 7.99 (br s, 1H), 7.32 (t, J = 8.2 Hz, 1H), 7.12 (d, J = 7.9 Hz, 1H), 5.42 (d, J = 8.4 Hz, 2H), 4.93 (s, 2H), 4.87–4.75 (m, 2H), 3.48 (d, J = 6.2 Hz, 1H), 3.39–3.22 (m, 2H), 2.73 (br s, 1H), 2.42 (br s, 1H), 2.25–1.91 (m, 5H), 1.74–1.22 (m, 10H), 1.13–0.92 (m, 6H), 0.89–0.68 (m, 6H), 0.65 (d, J = 7.1 Hz, 3H). MS (ESI): mass calcd for C33H44BF4NO9, 571.3; m/z: found, 572.3 [M + H]+. HPLC: 97.7% in 220 nm; 100% in 254 nm.
(3aR,4R,5R,7S,8S,9R,9aS,12R)-7-((Dimethylamino)methyl)-8-hydroxy-4,7,9,12-tetramethyl-3-oxodecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((7-Fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate (69)
1H NMR (DMSO-d6, 400 MHz): δ 9.29 (br s, 1H), 8.81 (br s, 1H), 7.27 (t, J = 7.7 Hz, 1H), 7.12 (d, J = 8.4 Hz, 1H), 5.94 (br s, 1H), 5.35 (d, J = 7.9 Hz, 1H), 4.93 (s, 2H), 4.83 (br s, 2H), 3.72–3.40 (m, 4H), 3.10 (d, J = 13.2 Hz, 1H), 2.86–2.84 (m, 4H), 2.41 (br s, 1H), 2.27–1.99 (m, 4H), 1.77–1.18 (m, 12H), 1.12–0.96 (m, 2H), 0.88 (d, J = 6.2 Hz, 3H), 0.65 (d, J = 5.7 Hz, 3H). MS (ESI): mass calcd for C32H44BF4NO9, 559.3; m/z: found, 560.3 [M + H]+. HPLC: 96.8% in 220 nm; 100% in 254 nm.
(3aR,4R,5R,7S,8S,9R,9aS,12R)-7-(Acetamidomethyl)-8-hydroxy-4,7,9,12-tetramethyl-3-oxodecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((7-Fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate (70)
A mixture of 63 (300.0 mg, 564.5 μmol, 1.0 equiv), acetic anhydride (63.4 mg, 620.9 μmol, 1.1 equiv), acetic acid (67.8 mg, 1.1 mmol, 2.0 equiv) in dichloromethane (20.0 mL) was degassed and purged with N2 for three times, and then the mixture was stirred at 60 °C for 12 h under an N2 atmosphere. LC/MS showed 63 was consumed completely and one main peak with desired MS was detected. The solvent was removed. The residue was purified by prep HPLC to give (3aR,4R,5R,7S, 8S,9R,9aS,12R)-7-(acetamidomethyl)-8-hydroxy-4,7,9,12-tetramethyl-3-oxodecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((7-fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate 70 (97.0 mg, 169.1 μmol, 29.9% yield) as a white solid. 1H NMR (DMSO-d6, 400MHz): δ 7.28 (t, J = 8.4 Hz, 1H), 7.09 (d, J = 8.4 Hz, 1H), 7.00–6.94 (m, 1H), 5.51 (d, J = 8.4 Hz, 1H), 4.90 (s, 2H), 4.85–4.74 (m, 2H), 3.60 (dd, J = 6.4, 13.8 Hz, 1H), 3.43 (d, J = 5.6 Hz, 1H), 3.00 (dd, J = 4.4, 13.8 Hz, 1H), 2.36 (br s, 1H), 2.31 (br s, 1H), 2.23–1.99 (m, 3H), 1.94–1.82 (m, 1H), 1.81–1.73 (m, 2H), 1.69–1.55 (m, 2H), 1.48 (br s, 1H), 1.40–1.20 (m, 6H), 1.05–0.87 (m, 4H), 0.83 (d, J = 6.4 Hz, 2H), 0.62 (d, J = 6.4 Hz, 3H) MS (ESI): mass calcd for C30H41BFNO8, 573.29; m/z: found, 574.3 [M + H]+. HPLC: 100.0% (220 nm), 100.0% (254 nm).
2-Tetramethyl-3-oxodecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((7-Fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)oxy)acetate (71)
To a solution of 70 (1.0 g, 1.8 mmol, 1.0 equiv) in ethanol (15.0 mL) was added pyridin-1-ium-1-ylboranuide (1.7 g, 17.9 mmol, 10.0 equiv). Subsequently, methanol/HCl (4 M, 8.0 mL, 17.9 equiv) was added under an N2 atmosphere at 0 °C. The mixture was stirred at 25 °C for 12 h. The mixture was adjusted to pH ≈ 6 with saturated NaHCO3 and extracted with ethyl acetate (20 mL × 2). After concentrated in vacuo, the residue was purified by prep HPLC to afford (3aR,4R,5R,7S,8S,9R,9aS,12R)-8-hydroxy-7-((methoxyamino)methyl)-4,7,9,12-tetramethyl-3-oxodecahydro-4,9a-propanocyclopenta[8]annulen-5-yl 2-((7-fluoro-1-hydroxy-1,3-dihydro-benzo[c][1,2]oxaborol-6-yl)oxy)acetate 71 (432.0 mg, 769.4 μmol, 43.0% yield) as a white solid. 1H NMR (DMSO-d6, 400 MHz): δ 7.28 (t, J = 8.0 Hz, 1H), 7.12 (d, J = 8.0 Hz, 1H), 5.45–5.36 (m, 1H), 4.93 (s, 2H), 4.81 (d, J = 2.4 Hz, 2H), 4.31 (d, J = 5.6 Hz, 1H), 3.77–3.71 (m, 4H), 3.50–3.44 (m, 1H), 3.26 (t, J = 13.2 Hz, 1H), 2.40 (br s, 1H), 2.23–1.93 (m, 4H), 1.77–1.23 (m, 10H), 1.19–0.97 (m, 4H), 0.87 (d, J = 6.4 Hz, 3H), 0.65 (dd, J = 3.2, 6.8 Hz, 3H). MS (ESI): mass calcd for C31H42BF4NO10, 561.29; m/z: found, 562.4 [M + H]+. HPLC: 95.4% (220 nm), 95.1% (254 nm).
(3aR,4R,5R,7S,9R,9aS,12R)-4,7,9,12-Tetramethyl-3,8-dioxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl (1-Hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)carbamate (74)
Trichloromethylchloroformate (215.0 mL, 1.5 mmol) and triethylamine (495.0 mL, 3.6 mmol) were added to an ice cooled solution of (3R,3aR,4R,5R,7S,9R,9aR,12R)-5-hydroxy-3-methoxy-4,7,9,12-tetramethyl-7-vinyloctahydro-4,9a-propanocyclopenta[8]annulen-8(9H)-one 72 (1.0 g, 3.0 mmol) in THF (10 mL). The mixture was stirred at room temperature for 2 h and then treated with further quantities of trichloromethylchloroformate (215.0 mL, 1.5 mmol) and triethylamine (495.0 mL, 3.6 mmol). After a further 2 h, the mixture was diluted with ethyl acetate and washed with brine. The organic phase was dried and concentrated to give (3R,3aR,4R,5R,7S,9R,9aR,12R)-3-methoxy-4,7,9,12-tetramethyl-8-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl carbonochloridate 73 without further purification (1.2 g, 100.0%).
To a solution of 6-aminobenzo[c][1,2]oxaborol-1(3H)-ol (200.0 mg, 1.4 mmol) in dichloromethane (15 mL) was added triethylamine (137.0 mg, 1.4 mmol) and 73 (1.2 g, 3.0 mmol). The reaction mixture was stirred at room temperature overnight, at which time TLC showed the starting materials were consumed completely. The mixture was extracted with ethyl acetate, washed with brine, dried, and concentrated in vacuo give the crude intermediate, to which was added a solution of zinc chloride in concentrated HCl. The mixture was stirred at room temperature for 0.5 h. Then, the mixture was added water and extracted with ethyl acetate. The crude product was purified by prep HPLC to give (3aR,4R,5R,7S,8S,9R, 9aS,12R)-8-hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl (1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)carbamate 74 (61.0 mg). 1H NMR (DMSO-d6, 400 MHz): δ 9.38 (s, 1H), 7.89 (s, 1H), 7.43 (d, J = 7.6 Hz, 1H), 7.27 (d, J = 8.0 Hz, 1H), 6.26 (m, 1H), 5.58 (s, 1H), 5.13 (m, 2H), 4.89 (s, 2H), 2.38 (m, 1H), 2.05 (m, 4H), 1.06–1.50 (m, 13H), 1.07 (m, 4H), 0.82 (d, 3H), 0.69 (d, 3H).
The following compounds were prepared from 73 in a similar manner:
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl (5-Fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)carbamate (75)
1H NMR (DMSO-d6, 400 MHz): δ 9.25 (s, 2H), 7.99 (s, 1H), 7.32 (d, J = 10.4 Hz, 1H), 6.74 (dd, J = 10.8, 17.6 Hz, 1H), 5.64 (d, J = 9.6 Hz, 1H), 5.29 (d, J = 10.8 Hz, 1H), 4.98 (m, 3H), 3.40 (s, 1H), 3.12 (s, 3H), 2.88 (m, 1H), 2.34 (m, 1H), 2.04 (s, 3H), 1.90 (m, 1H), 1.72 (m, 1H), 1.67 (d, 2H), 1.43 (m, 2H), 1.25–1.07 (m, 9H), 0.87–0.75 (m, 5H). MS (ESI): mass calcd for C29H39BNO6, 527.2; m/z: found, 526.2 [M – H]−. HPLC: 99.6% (220 nm), 100% (254 nm).
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl ((1-Hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)methyl)carbamate (76)
1H NMR (DMSO-d6, 400 MHz): δ 7.70 (t, J = 5.8 Hz, 1H), 7.61 (s, 1H), 7.38–7.33 (m, 2H), 6.74 (dd, J = 10.8, 17.6 Hz, 1H), 5.53 (d, J = 9.6 Hz, 1H), 5.24 (d, J = 10.4 Hz, 1H), 4.96–4.90 (m, 3H), 4.32–4.16 (m, 2H), 3.13–3.10 (m, 3H), 2.85 (d, J = 6.4 Hz, 1H), 2.36–2.33 (m, 1H), 2.07–1.61 (m, 5H), 1.41–1.38 (m, 2H), 1.18–1.01 (m, 11H), 0.90 (d, J = 6.4 Hz, 3H), 0.78 (d, J = 6.8 Hz, 3H). MS (ESI): mass calcd for C30H42BNO6, 523.3; m/z: found, 522.2 [M – H]−. HPLC: 99.0% (220 nm), 100% (254 nm).
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-4,7,9,12-tetramethyl-3-oxo-7-vinyldecahydro-4,9a-propanocyclopenta[8]annulen-5-yl ((7-Fluoro-1-hydroxy-1,3-dihydrobenzo[c][1,2]oxaborol-6-yl)methyl)carbamate (77)
1H NMR (DMSO-d6, 400 MHz): δ 9.23 (s, 1H), 7.50 (t, J = 6.0 Hz, 1H), 7.38 (t, J = 7.2 Hz, 1H), 7.14 (d, J = 8.0 Hz, 1H), 6.20 (dd, J = 11.2, 18.0 Hz, 1H), 5.41 (d, J = 8.8 Hz, 1H), 5.09–4.86 (m, 4H), 4.25–4.10 (m, 2H), 3.38 (d, J = 5.6 Hz, 1H), 2.29 (s, 1H), 2.22–1.95 (m, 4H), 1.69–1.14 (m, 10H), 1.06–0.88 (m, 4H), 0.78 (d, J = 6.6 Hz, 3H), 0.62 (d, J = 5.2 Hz, 3H). MS (ESI): mass calcd for C29H39BFNO6, 527.29; m/z: found, 526.3 [M – H]−. HPLC: 100% (220 nm), 100% (254 nm).
(3aR,4R,5R,7S,8S,9R,9aS,12R)-8-Hydroxy-7-((methoxyamino)methyl)-4,7,9,11,2-difluoro-3-(methoxymethoxy)benzene 80
To a solution of 2,3-difluorophenol (300.0 g, 2.3 mol, 1.0 equiv) in dichloromethane (1.5 L) was added methoxymethyl chloride (278.9 g, 3.5 mol, 1.5 equiv) and DIEA (597.1 g, 4.6 mol, 2.0 equiv). The mixture was stirred at 0 °C for 2 h. TLC indicated that starting material was consumed. The reaction mixture was quenched by addition of H2O (1500 mL) at 0 °C and then was adjusted to pH = 6. The combined organic layers were washed with saturation NH4Cl (3 × 500 mL), dried, filtered, and concentrated under reduced pressure to give a residue. 1,2-Difluoro-3-(methoxymethoxy)benzene (400.0 g, 2.3 mol, 99.4% yield) was obtained as yellow oil, which was used into the next step without further purification. 1H NMR (CDCl3, 400 MHz): δ 7.03–6.93 (m, 1H), 6.89–6.78 (m, 1H), 5.28–5.20 (m, 2H), 3.58–3.50 (m, 3H).
3,4-Difluoro-2-(methoxymethoxy)benzaldehyde 81
To a solution of 80 (100.0 g, 574.3 mmol, 1.0 equiv) in THF (1.5 L) was added n-butyllithium (2.5 M, 298.6 mL, 1.3 equiv). The mixture was stirred at −78 °C for 7 h. Then, ethyl formate (85.1 g, 1150 mmol, 2.0 equiv) was added and the mixture was stirred for another 1 h. TLC indicated that starting material was consumed completely. The reaction mixture was quenched by addition of H2O (1000 mL) at 0 °C and then was extracted with ethyl acetate (3 × 800 mL). The combined organic layers were washed with brine 1000 mL, dried, filtered, and concentrated under reduced pressure to give a residue. The 3,4-difluoro-2-(methoxymethoxy)benzaldehyde 81 (492.0 g, crude) as a yellow oil was used into the next step without further purification. 1H NMR (CDCl3, 400 MHz): δ 10.40–10.29 (m, 1H), 7.73–7.57 (m, 1H), 7.11–6.98 (m, 1H), 5.33 (s, 2H), 3.63–3.58 (m, 3H).
4-(Benzyloxy)-3-fluoro-2-(methoxymethoxy)benzaldehyde 82
To a solution of 81 (256.0 g, 1.27 mol, 1.0 equiv) in DMF (1.5 L) was added Cs2CO3 (618.9 g, 1.90 mol, 1.5 equiv) and benzyl alcohol (136.9 g, 1.3 mol, 1.0 equiv). The mixture was stirred at 70 °C for 10 h. TLC indicated that starting material was consumed completely. The reaction mixture was quenched by addition of H2O (1000 mL) at 0 °C, then diluted with ethyl acetate (1200 mL), and extracted with ethyl acetate (1200 mL × 2). The combined organic layers were washed with H2O (2 × 1000 mL), dried, filtered, and concentrated under reduced pressure to give a residue. 4-(Benzyloxy)-3-fluoro-2-(methoxymethoxy)benzaldehyde 82 (400.0 g, crude) was obtained as a yellow oil, which was used into the next step without further purification.
4-(Benzyloxy)-3-fluoro-2-hydroxybenzaldehyde 83
To a solution of 82 (760.0 g, 2.6 mol, 1.0 equiv) in CH3OH (600.0 mL) was added aq HCl (2 M, 200.0 mL). The mixture was stirred at 40 °C for 4 h. TLC indicated that starting material was consumed completely. The reaction was quenched by addition of H2O (300 mL) and the mixture was concentrated under reduced pressure to remove methanol. The residue was diluted with dichloromethane 500 mL and extracted with dichloromethane 1000 mL (2 × 500 mL). The combined organic layers were washed with brine (3 × 500 mL), dried, filtered, and concentrated under reduced pressure to give a residue. The residue was purified by column chromatography (SiO2, petroleum ether/ethyl acetate = 20:1 to 5:1). 4-(Benzyloxy)-3-fluoro-2-hydroxybenzaldehyde 83 (225.0 g, 913.8 mmol, 34.9% yield) was obtained as a black-brown solid. 1H NMR (DMSO-d6, 400 MHz): δ 10.97 (s, 1H), 10.06 (s, 1H), 7.34–7.52 (m, 6H), 6.90–6.98 (m, 1H), 5.28 (s, 2H).
3-(Benzyloxy)-2-fluoro-6-formylphenyl Trifluoromethanesulfonate 84
To a solution of 83 (117.0 g, 475.2 mmol, 1.0 equiv), pyridine (75.2 g, 950.3 mmol, 2.0 equiv) and 4-dimethylaminopyridine (5.8 g, 47.5 mmol, 0.1 equiv) in dichloromethane (1.5 L) was added Tf2O (201.1 g, 712.8 mmol, 1.5 equiv) dropwise. The mixture was stirred at 0 °C for 2 h. HPLC indicated that starting material was consumed completely. The reaction mixture was quenched by addition of H2O (1000 mL) and then extracted with dichloromethane (2 × 1000 mL). The combined organic layers were washed with brine (500 mL), dried, filtered, and concentrated under reduced pressure to give a residue. The residue was purified by column chromatography (SiO2, petroleum ether/ethyl acetate = 20:1 to 2:1). 3-(Benzyloxy)-2-fluoro-6-formylphenyl trifluoromethanesulfonate 84 (150.0 g, 396.5 mmol, 83.5% yield) was obtained as a yellow oil. 1H NMR (CDCl3, 400 MHz): δ 10.08 (s, 1H), 7.71 (d, J = 7.8 Hz, 1H), 7.49–7.36 (m, 5H), 7.19–7.13 (m, 1H), 5.28 (s, 2H).
4-(Benzyloxy)-3-fluoro-2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzaldehyde 85
To a solution of 84 (52.0 g, 137.5 mmol, 1.00 equiv), potassium acetate (40.5 g, 412.4 mmol, 3.0 equiv), and dipinacol diborane (104.7 g, 412.4 mmol, 3.0 equiv) in dioxane (1.0 L) was added Pd(dppf)Cl2 (2.0 g, 2.75 mmol, 0.02 equiv). The mixture was stirred at 70 °C for 16 h. HPLC indicated that starting material was consumed completely. The reaction mixture was filtered and concentrated under reduced pressure to give a residue. The residue was purified by column chromatography (SiO2, petroleum ether/ethyl acetate = 50:1 to 5:1). 4-(Benzyloxy)-3-fluoro-2-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzaldehyde 85 (37.0 g, 103.9 mmol, 75.6% yield) was obtained as a white solid. 1H NMR (DMSO-d6, 400 MHz): δ 9.81 (d, J = 3.0 Hz, 1H), 7.81 (d, J = 8.3 Hz, 1H), 7.54–7.33 (m, 7H), 5.31 (s, 2H), 1.39–1.28 (m, 12H).
6-(Benzyloxy)-7-fluorobenzo[c][1,2]oxaborol-1(3H)-ol 86
To a solution of 85 (20.0 g, 56.2 mmol, 1.00 equiv) in THF (50.0 mL) was added NaBH4 (3.2 g, 84.2 mmol, 1.5 equiv). The mixture was stirred at 0 °C for 1 h. TLC indicated that starting material was consumed completely. The reaction mixture was quenched by addition H2O (100 mL) at 0 °C, then adjusted pH = 5, removed the THF, then filtered, and concentrated under reduced pressure to give a residue. 6-(Benzyloxy)-7-fluorobenzo[c][1,2]oxaborol-1(3H)-ol 86 (43.0 g, 166.6 mmol, 98.9% yield) was obtained as a white solid. 1H NMR (DMSO-d6, 400 MHz): δ 7.31–7.48 (m, 5H), 7.17 (t, J = 7.78 Hz, 1H), 7.01 (d, J = 8.03 Hz, 1H), 5.18 (s, 2H), 5.04 (s, 2H), 4.89 (s, 1H).
7-Fluorobenzo[c][1,2]oxaborole-1,6(3H)-diol 87
To a solution of 86 (15.0 g, 58.1 mmol, 1.0 equiv) in ethyl acetate (400.0 mL) was added Pd/C (2.0 g). The mixture was stirred at 25 °C for 2 h under an H2 atmosphere (50 psi). TLC indicated that starting material was consumed completely. The reaction mixture was filtered and concentrated under reduced pressure to give a residue. 7-Fluorobenzo[c][1,2]oxaborole-1,6(3H)-diol 87 (7.0 g, 41.7 mmol, 71.7% yield) was obtained as a white solid. 1H NMR (DMSO-d6, 400 MHz): δ 9.59 (s, 1H), 9.14 (s, 1H), 7.05–7.12 (m, 1H), 6.97–7.03 (m, 1H), 4.88 (s, 2H). MS (ESI): mass calcd for C7H6BFO3, 168.04; m/z: found, 167.1 [M – H]−. HPLC: 92.5% (220 nm), 94.4% (254 nm).
Anti-wAlb High-Content in Vitro Assay
C6/36 cells (ECACC #89051705, derived from Aedes albopictus larvae) were infected with Wolbachia pipientis derived from the supernatant of cultured A. albopictus Aa23 cells to create a stably Wolbachia-infected cell line C6/36 (wAlbB). This cell line was subpassaged 6–8 days prior to plating out at a density of 2000 viable cells per well in a 384-well CellCarrier plate suspended in Liebovitz media supplemented with 20% fetal bovine serum, 2% tryptose phosphate broth, and 1% non-essential amino acids. Compounds were dissolved and diluted in DMSO, and compound solution was added to each well to provide a final DMSO concentration <1% and a total volume of 100 μL per well.
Following 7 days of sterile incubation at 26 °C, staining media-containing SYTO 11 DNA dye was added to each well. After 15 min, all media was removed from each well and fresh media (no stain) was added. Imaging of each well was accomplished using a PerkinElmer Operetta high-content imaging system. Five fields per well were imaged using a confocal 60× objective with the fluorescein filter (excitation filter: 460–490 nm; emission filter: 500–550 nm). Images were analyzed using the PerkinElmer Harmony software to score each intact cell on the basis of texture complexity of the cytoplasm. Full details of the image analysis have been published.25
Compound sample wells were analyzed and normalized (along with the positive controls) against the vehicle (untreated) control to give a percentage reduction of Wolbachia-infected cells. Using the cell number analysis, compounds with a host cell number amounting to less than 50% of the vehicle control were classified as toxic and retested at a reduced compound concentration. Dose–response curves were generated with percentage reduction of Wolbachia-infected cells versus compound concentration, using 5–10 compound serial dilutions. Data were analyzed and compound EC50’s determined using a 4-parameter logistic nonlinear regression model. EC50 is defined as the compound concentration producing a 50% reduction of Wolbachia in the C6/36 cell line.
Anti-wMel Wolbachia High-Content in Vitro Assay
The assay was developed based on previous work25 and carried out as previously described.50 Briefly, D. melanogaster LDW1 cells naturally infected with wMel strain of Wolbachia were maintained in Shields and Sang M3 (SSM3) Insect Medium (Sigma, S3652) supplemented with 10% heat-inactivated fetal bovine serum (FBS) (qualified, One Shot Format, Gibco, 26140-111) at 25 °C, in flasks with unvented caps. Greiner 1536-well assay plates (Part. no. 789091) were coated with 1 mg/mL solution of Concanavalin-A lectin (MP Biomedicals, 02195283) to facilitate cell adhesion. Compounds were acoustically transferred into plates using the Echo 555 Liquid Handler (Labcyte Inc.). Cells were seeded at 4000 cells/well in SSM3 medium supplemented with 2% FBS using the MultiFlo FX Multi-Mode Dispenser (Biotek). After incubating at 25 °C for 6 days, the cells were fixed with 4% paraformaldehyde (PFA) for at least 10 min and washed with phosphate buffered saline, pH 7, 0.1% Tween 20. Fluorescence in situ hybridization (FISH) was used to stain Wolbachia and 3 μM DAPI was used to stain DNA. The “bottle valve” dispenser with an angled-head (Kalypsys Inc, San Diego, CA) was used for fixation and staining. Stained cells were imaged using the CX5 Insight Cellomics high-content imaging instrument with a 10× objective (Thermo). Each well was analyzed using Compartmental Analysis in HCS Studio (Thermo) for cell number and Wolbachia content. Analysis outputs included (1) the total object number per well and (2) the total fluorescence intensity of all spots within each cell (averaged per well). Data were analyzed in Genedata Screener, Version 13.0.1-Standard by normalizing to DMSO (neutral) and inhibitor control-treated wells (12.5 μM doxycycline and 125 nM rifampicin). To determine the EC50’s, dose response curves (11′ point, 1:3 dilution) were fitted using the four parameter Hill equation. Each compound was tested in triplicate.
Ethics Statement on Animal Studies
All in vivo experiments involving B. malayi were conducted at the Liverpool School of Tropical Medicine (LSTM), were approved by the ethical committees of the University of Liverpool and LSTM, and were conducted according to Home Office (UK) requirements. All animal experiments with L. sigmodontis were conducted at the Institute for Medical Microbiology, Immunology and Parasitology (IMMIP), University Hospital of Bonn, in accordance to the European Union animal welfare guidelines. All protocols were approved by the local authorities (Landesamt für Natur, Umwelt und Verbraucherschutz, Cologne, Germany (AZ 84-02.04.2015.A507; 84-02.04.2012.A140). In vivo pharmacokinetic studies in mice were conducted either at Anacor Pharmaceuticals or Shanghai ChemPartner under appropriate review by Institutional Animal Care and Use Committees (IACUC) at each site.
B. malayi SCID Mouse Infection Models
A B. malayi SCID mouse infection model for anti-Wolbachia drug screening was used as previously described34,51−53 In brief, B. malayi L3 larvae were generated by membrane blood feeding Liverpool strain filarial susceptible Aedes aegypti mosquitoes with microfilariae isolated from patently infected Meriones unguiculatus gerbils. CB.17 SCID mice were purchased from Charles River UK. Groups of 3 or 5 SCID mice were infected with either 50 or 100 L3, ip, for larval or adult B. malayi efficacy testing, respectively. Drugs were dosed from point of infection for larval testing or after an incubation period of 6 weeks for adult-stage testing with variable length washout. Test drugs were administered orally at indicated doses and as detailed below. Doxycycline or minocycline positive controls were administered in water at indicated doses. At 2-weeks (larval testing) or 12-weeks post-infection (adult testing) motile B. malayi L4 or female parasites were isolated by peritoneal lavage, enumerated by microscopy and individually snap frozen. Total genomic DNA was isolated per worm, and QPCR quantifications of single copy B. malayiWolbachia were undertaken per group of 10 worms per treatment/control, as previously described.34,54 Efficacy of Wolbachia depletion is reported as median Wolbachia load per drug group expressed as a percentage of median vehicle control levels from animals treated only with vehicle.
L. sigmodontis in Vivo Mouse Model
Female BALB/c wild type mice were obtained from Janvier (Saint-Berthevin, France) and were housed at the animal facility of the IMMIP in individually ventilated cages on a 12 h light/dark cycle with food and water ad libidum. Mice were infected at 6–8 weeks of age via exposure to the natural mite vector Ornithonyssus bacoti containing infectious L. sigmodontis L3 larvae.36 To compare the infection, the same batch of mite-containing bedding was used to infect all animals of one experiment. Then, 35 days post infection, time point adult worms have developed within the thoracic cavity, and mice were treated per oral gavage with the test compounds at concentrations indicated in the result section. As a vehicle, 1% CMC + 0.1% Tween80 was used. Negative controls were treated with vehicle only, and as a positive control, mice were treated orally with doxycycline (Sigma) in 10% DMSO/1× PBS. Necropsies were performed at 64–77 days post-infection using an overdose of isoflurane (Baxter, Germany) and adult worms were isolated from the thoracic cavity and peritoneum and enumerated. Realtime PCR was performed from remaining female adult worms to quantify Wolbachia FtsZ and L. sigmodontis actin values by qRT-PCR as previously described.35
General PK Study Protocol
Animals were allowed to acclimate a minimum of 72 h prior to selection for study. On the morning of dosing, animals were weighed and grouped randomly. Animals received the test material by tail-vein injection (IV), or oral gavage (PO). After dosing, all clinical signs and observations were recorded, and if adverse effects were noted, the exact time that the animals returned to normal was also recorded. At prescribed timepoints, animals were anesthetized by isoflurane inhalation and blood samples were collected via retro-orbital bleed or cardiac puncture. After the blood sample collection, animals were euthanized by CO2 inhalation followed by cervical dislocation.
Blood samples were collected into microtainer tubes (K2EDTA) on ice and processed to provide plasma by centrifugation for 6 min at approximately 2000g or 6000 rpm. Processed samples were transferred to analysis tubes that were placed on dry ice. Samples were analyzed by LC/MS/MS.
Acknowledgments
The authors would like to thank the Bill & Melinda Gates Foundation for funding of this program through awards to Anacor (Contract number 23629), to the Liverpool School of Tropical Medicine (grant number OPP1054324), to the University of Bonn (grant number OPP1134310), and to Calibr (grant number OPP1107194). We thank Richard Elliott and Ken Duncan of the BMGF foundation for their support and guidance and Michael Xu and his team at WuXi AppTec for their contributions to the medicinal chemistry. We acknowledge the technical contributions of Bettina Dubben and Martina Fendler of University Hospital Bonn.
Glossary
Abbreviations
- NTDs
neglected tropical diseases
- DALYs
disability-adjusted life years
- MDA
mass drug administration
- PTC
peptidyl-transfer center
- MDR1-MDCK
multidrug resistance-1 transfected Madin Darby canine kidney cell line
- funbound
fraction unbound to plasma protein
- mouse S9
supernatant from 9000 g × 20 min fractionation of mouse liver preparation
- Cmax
maximal plasma concentration
- SCID
severe combined immune deficiency
- L3-stage
third stage larvae
- qPCR
quantitative polymerase chain reaction
- BID
twice daily
- QD
once daily
- Papp
apparent permeability
- Clint
intrinsic clearance
- wsp
Wolbachia surface protein
- FtsZ
prokaryotic homolog of mammalian tubulin
- BALB/c
Bagg albino strain mice
- br
broad
- s
singlet
- d
doublet
- dd
doublet of doublets
- t
triplet
- q
quartet
- m
multiplet
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jmedchem.8b01854.
Molecular formula strings (SMILES) and biochemical and in vitro ADME data (CSV)
Author Contributions
R.T.J., C.J.L., D.S.C., and Y.R.F. wrote the manuscript and contributed equally to the design and execution of the project. R.T.J., V.H., X.L., Y.X., D.S.C., and J.J.P. designed and coordinated synthesis of the compounds. R.T.J., Y.R.F., E.E., J.J.P., R.S., A.H., M.J.T., and S.A.W. provided scientific leadership and management of the project. P.W.B. and J.J. designed, coordinated, and interpreted in vitro and in vivo pharmacokinetics studies. L.F., K.L.J., D.A.N.C., R.C., A.C., L.M., H.T., J.G., A.F.G., A.S., F.L., A.E., S.J.F., M.K., and M.A.B. conducted in vitro and in vivo biological assays, which were designed and coordinated by Y.R.F., F.R., L.F., A.H., M.P.H., C.W.M., J.D.T., M.J.T., and S.A.W. The manuscript was edited by Y.R.F., P.W.B., A.H., M.P.H., J.D.T., M.J.T., and S.A.W.
The authors declare no competing financial interest.
Supplementary Material
References
- Simonsen P. E. F.; Fischer P. U.; Hoerauf A.; Weil G. J.. The Filariases. In Manson’s Tropical Diseases, 23rd ed.; Farrar J. H., Junghanss T., Kang G., Lalloo D., White N. J., Eds.; Elsevier Saunders: Amsterdam, 2014; pp 737–765. [Google Scholar]
- Taylor M. J.; Hoerauf A.; Bockarie M. Lymphatic filariasis and onchocerciasis. Lancet 2010, 376, 1175–1185. 10.1016/s0140-6736(10)60586-7. [DOI] [PubMed] [Google Scholar]
- Ramaiah K. D.; Ottesen E. A. Progress and impact of 13 years of the global programme to eliminate lymphatic filariasis on reducing the burden of filarial disease. PLoS Neglected Trop. Dis. 2014, 8, e3319. 10.1371/journal.pntd.0003319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katiyar D.; Singh L. K. Filariasis: Current status, treatment and recent advances in drug development. Curr. Med. Chem. 2011, 18, 2174–2185. 10.2174/092986711795656234. [DOI] [PubMed] [Google Scholar]
- Geary T. Ivermectin 20 years on: maturation of a wonder drug. Trends Parasitol. 2005, 21, 530–532. 10.1016/j.pt.2005.08.014. [DOI] [PubMed] [Google Scholar]
- King C. L.; Suamani J.; Sanuku N.; Cheng Y.-C.; Satofan S.; Mancuso B.; Goss C. W.; Robinson L. J.; Siba P. M.; Weil G. J.; Kazura J. W. A trial of a triple-drug treatment for lymphatic filariasis. New Engl. J. Med. 2018, 379, 1801–1810. 10.1056/nejmoa1706854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slatko B. E.; Taylor M. J.; Foster J. M. The Wolbachia endosymbiont as an anti-filarial nematode target. Symbiosis 2010, 51, 55–65. 10.1007/s13199-010-0067-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoerauf A.; Mand S.; Volkmann L.; Büttner M.; Marfo-Debrekyei Y.; Taylor M.; Adjei O.; Büttner D. W. Doxycycline in the treatment of human onchocerciasis: Kinetics of Wolbachia endobacteria reduction and of inhibition of embryogenesis in female Onchocerca worms. Microbes Infect. 2003, 5, 261–273. 10.1016/s1286-4579(03)00026-1. [DOI] [PubMed] [Google Scholar]
- Hoerauf A.; Mand S.; Fischer K.; Kruppa T.; Marfo-Debrekyei Y.; Debrah A. Y.; Pfarr K. M.; Adjei O. Doxycycline as a novel strategy against bancroftian filariasis-depletion of Wolbachia endosymbionts from Wuchereria bancrofti and stop of microfilaria production. Med. Microbiol. Immunol. 2003, 192, 211–216. 10.1007/s00430-002-0174-6. [DOI] [PubMed] [Google Scholar]
- Hoerauf A.; Mand S.; Adjei O.; Fleischer B.; Büttner D. W. Depletion of Wolbachia endobacteria in Onchocerca volvulus by doxycycline and microfilaridermia after ivermectin treatment. Lancet 2001, 357, 1415–1416. 10.1016/s0140-6736(00)04581-5. [DOI] [PubMed] [Google Scholar]
- Goetze S.; Hiernickel C.; Elsner P. Phototoxicity of doxycycline: a systematic review on clinical manifestations, frequency, cofactors, and prevention. Skin Pharmacol. Physiol. 2017, 30, 76–80. 10.1159/000458761. [DOI] [PubMed] [Google Scholar]
- Gardon J.; Gardon-Wendel N.; Demanga-Ngangue N.; Kamgno J.; Chippaux J.-P.; Boussinesq M. Serious reactions after mass treatment of onchocerciasis with ivermectin in an area endemic for Loa loa infection. Lancet 1997, 350, 18–22. 10.1016/s0140-6736(96)11094-1. [DOI] [PubMed] [Google Scholar]
- Albers A.; Esum M.; Tendongfor N.; Enyong P.; Klarmann U.; Wanji S.; Hoerauf A.; Pfarr K. Retarded Onchocerca volvulus L1 to L3 larval development in the Simulium damnosum vector after anti-wolbachial treatment of the human host. Parasites Vectors 2012, 5, 12. 10.1186/1756-3305-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh F.; Hervey A.; Robbins W. J. Antibiotic substances from basidiomycetes: IX. Drosophila subtarata. (batsch ex fr.) quel. Proc. Natl. Acad. Sci. U.S.A. 1952, 38, 555–560. 10.1073/pnas.38.7.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh F.; Hervey A.; Robbins W. J. Antibiotic substances from basidiomycetes: viii. pleurotus multilus (fr.) sacc. and pleurotus passeckerianus pilat. Proc. Natl. Acad. Sci. U.S.A. 1951, 37, 570–574. 10.1073/pnas.37.9.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlünzen F.; Pyetan E.; Fucini P.; Yonath A.; Harms J. M. Inhibition of peptide bond formation by pleuromutilins: the structure of the 50S ribosomal subunit from Deinococcus radiodurans in complex with tiamulin. Mol. Microbiol. 2004, 54, 1287–1294. 10.1111/j.1365-2958.2004.04346.x. [DOI] [PubMed] [Google Scholar]
- Veve M. P.; Wagner J. L. Lefamulin: review of a promising novel pleuromutilin antibiotic. Pharmacotherapy 2018, 38, 935–946. 10.1002/phar.2166. [DOI] [PubMed] [Google Scholar]
- Dornhelm P.; Hogenauer G. The effects of tiamulin, a semisynthetic pleuromutilin derivative, on bacterial polypeptide chain initiation. Eur. J. Biochem. 1978, 91, 465–473. 10.1111/j.1432-1033.1978.tb12699.x. [DOI] [PubMed] [Google Scholar]
- Jones R. N.; Fritsche T. R.; Sader H. S.; Ross J. E. Activity of retapamulin (SB-275833), a novel pleuromutilin, against selected resistant gram-positive cocci. Antimicrob. Agents Chemother. 2006, 50, 2583–2586. 10.1128/aac.01432-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sader H. S.; Paukner S.; Ivezic-Schoenfeld Z.; Biedenbach D. J.; Schmitz F. J.; Jones R. N. Antimicrobial activity of the novel pleuromutilin antibiotic BC-3781 against organisms responsible for community-acquired respiratory tract infections (CARTIs). J. Antimicrob. Chemother. 2012, 67, 1170–1175. 10.1093/jac/dks001. [DOI] [PubMed] [Google Scholar]
- Aitken I. A.; Morgan J. H.; Dalziel R.; Burch D. G. S.; Ripley P. H. Comparative in vitro activity of valnemulin against porcine bacterial pathogens. Vet. Rec. 1999, 144, 128. 10.1136/vr.144.5.128. [DOI] [PubMed] [Google Scholar]
- Berner H.; Schulz G.; Schneider H. Synthese ab-trans-anellierter derivate des tricyclischen diterpens pleuromutilin durch intramolekulare 1,5-hydrid-verschiebung. Tetrahedron 1980, 36, 1807–1811. 10.1016/0040-4020(80)80078-0. [DOI] [Google Scholar]
- Brooks G.; Burgess W.; Colthurst D.; Hinks J. D.; Hunt E.; Pearson M. J.; Shea B.; Takle A. K.; Wilson J. M.; Woodnutt G.; Pleuromutilins Pleuromutilins. Part 1 The identification of novel mutilin 14-carbamates. Bioorg. Med. Chem. 2001, 9, 1221–1231. 10.1016/s0968-0896(00)00338-2. [DOI] [PubMed] [Google Scholar]
- Li X. L.; Jacobs R. T.; Hernandez V.; Xia Y.; Plattner J. J.; Cao K. J.. Boron Containing Small Molecules. WO 2006089067 A2, Feb 27, 2017.
- Clare R. H.; Cook D. A. N.; Johnston K. L.; Ford L.; Ward S. A.; Taylor M. J. Development and Validation of a High-Throughput Anti-WolbachiaWhole-Cell Screen. J. Biomol. Screening 2015, 20, 64–69. 10.1177/1087057114551518. [DOI] [PubMed] [Google Scholar]
- Serbus L. R.; Landmann F.; Bray W. M.; White P. M.; Ruybal J.; Lokey R. S.; Debec A.; Sullivan W. A cell-based screen reveals that the albendazole metabolite, albendazole sulfone, targets Wolbachia. PLoS Pathog. 2012, 8, e1002922. 10.1371/journal.ppat.1002922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs R. T.; Nare B.; Wring S. A.; Orr M. D.; Chen D.; Sligar J. M.; Jenks M. X.; Noe R. A.; Bowling T. S.; Mercer L. T.; Rewerts C.; Gaukel E.; Owens J.; Parham R.; Randolph R.; Beaudet B.; Bacchi C. J.; Yarlett N.; Plattner J. J.; Freund Y.; Ding C.; Akama T.; Zhang Y.-K.; Brun R.; Kaiser M.; Scandale I.; Don R. SCYX-7158, an orally-active benzoxaborole for the treatment of stage 2 human African trypanosomiasis. PLoS Neglected Trop. Dis. 2011, 5, e1151. 10.1371/journal.pntd.0001151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berner H. G.; Schulz H. Chemie der pleuromutiline-II. Tetrahedron 1981, 37, 915–919. 10.1016/s0040-4020(01)97660-4. [DOI] [Google Scholar]
- Novak R. Are pleuromutilin antibiotics finally fit for human use?. Ann. N.Y. Acad. Sci. 2011, 1241, 71–81. 10.1111/j.1749-6632.2011.06219.x. [DOI] [PubMed] [Google Scholar]
- Novak R.; Shlaes D. M. The pleuromutilin antibiotics: a new class for human use. Curr. Opin. Investig. Drugs 2010, 11, 182–191. [PubMed] [Google Scholar]
- Prince W. T.; Ivezic-Schoenfeld Z.; Lell C.; Tack K. J.; Novak R.; Obermayr F.; Talbot G. H. Phase II clinical study of BC-3781, a pleuromutilin antibiotic, in treatment of patients with acute bacterial skin and skin structure infections. Antimicrob. Agents Chemother. 2013, 57, 2087–2094. 10.1128/aac.02106-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitlinger M.; Schwameis R.; Burian A.; Burian B.; Matzneller P.; Müller M.; Wicha W. W.; Strickmann D. B.; Prince W. Simultaneous assessment of the pharmacokinetics of a pleuromutilin, lefamulin, in plasma, soft tissues and pulmonary epithelial lining fluid. J. Antimicrob. Chemother. 2016, 71, 1022–1026. 10.1093/jac/dkv442. [DOI] [PubMed] [Google Scholar]
- Tang F.; Horie K.; Borchardt R. T. Lingua::EN::Titlecase. Pharm. Res. 2002, 19, 765–772. 10.1023/a:1016140429238. [DOI] [PubMed] [Google Scholar]
- Halliday A.; Guimaraes A. F.; Tyrer H. E.; Metuge H.; Patrick C.; Arnaud K.-O.; Kwenti T.; Forsbrook G.; Steven A.; Cook D.; Enyong P.; Wanji S.; Taylor M. J.; Turner J. D. A murine macrofilaricide pre-clinical screening model for onchocerciasis and lymphatic filariasis. Parasites Vectors 2014, 7, 472. 10.1186/preaccept-4686600213964315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Specht S.; Pfarr K. M.; Arriens S.; Hübner M. P.; Klarmann-Schulz U.; Koschel M.; Sternberg S.; Martin C.; Ford L.; Taylor M. J.; Hoerauf A. Combinations of registered drugs reduce treatment times required to deplete Wolbachia in the Litomosoides sigmodontis mouse model. PLoS Neglected Trop. Dis. 2018, 12, e0006116. 10.1371/journal.pntd.0006116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmann L.; Fischer K.; Taylor M.; Hoerauf A. Antibiotic therapy in murine filariasis (Litomosoides sigmodontis): comparative effects of doxycycline and rifampicin on Wolbachia and filarial viability. Trop. Med. Int. Health 2003, 8, 392–401. 10.1046/j.1365-3156.2003.01040.x. [DOI] [PubMed] [Google Scholar]
- Hoerauf A.; Nissen-Pähle K.; Schmetz C.; Henkle-Dührsen K.; Blaxter M. L.; Büttner D. W.; Gallin M. Y.; Al-Qaoud K. M.; Lucius R.; Fleischer B. Tetracycline therapy targets intracellular bacteria in the filarial nematode Litomosoides sigmodontis and results in filarial infertility. J. Clin. Invest. 1999, 103, 11–18. 10.1172/jci4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajendra J.; Specht S.; Neumann A.-L.; Gondorf F.; Schmidt D.; Gentil K.; Hoffmann W. H.; Taylor M. J.; Hoerauf A.; Hübner M. P. ST2 deficiency does not impair type 2 immune responses during chronic filarial infection but leads to an increased microfilaremia due to an impaired splenic microfilarial clearance. PLoS One 2014, 9, e93072. 10.1371/journal.pone.0093072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris C. P.; Evans H.; Larsen S. E.; Mitre E. A comprehensive, model-based review of vaccine and repeat infection trials for filariasis. Clin. Microbiol. Rev. 2013, 26, 381–421. 10.1128/cmr.00002-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layland L. E.; Ajendra J.; Ritter M.; Wiszniewsky A.; Hoerauf A.; Hubner M. P. Development of patent Litomosoides sigmodontis infections in semi-susceptible C57BL/6 mice in the absence of adaptive immune responses. Parasites Vectors 2015, 8, 396. 10.1186/s13071-015-1011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong W. D.; Benayoud F.; Nixon G. L.; Ford L.; Johnston K. L.; Clare R. H.; Cassidy A.; Cook D. A. N.; Siu A.; Shiotani M.; Webborn P. J. H.; Kavanagh S.; Aljayyoussi G.; Murphy E.; Steven A.; Archer J.; Struever D.; Frohberger S. J.; Ehrens A.; Hübner M. P.; Hoerauf A.; Roberts A. P.; Hubbard A. T. M.; Tate E. W.; Serwa R. A.; Leung S. C.; Qie L.; Berry N. G.; Gusovsky F.; Hemingway J.; Turner J. D.; Taylor M. J.; Ward S. A.; O’Neill P. M. AWZ1066S, a highly specific anti-Wolbachia drug candidate for a short-course treatment of filariasis. Proc. Natl. Acad. Sci. U.S.A. 2019, 116, 1414–1419. 10.1073/pnas.1816585116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.-K.; Plattner J. J.; Freund Y. R.; Easom E. E.; Zhou Y.; Ye L.; Zhou H.; Waterson D.; Gamo F.-J.; Sanz L. M.; Ge M.; Li Z.; Li L.; Wang H.; Cui H. Benzoxaborole antimalarial agents. Part 2: Discovery of fluoro-substituted 7-(2-carboxyethyl)-1,3-dihydro-1-hydroxy-2,1-benzoxaboroles. Bioorg. Med. Chem. Lett. 2012, 22, 1299–1307. 10.1016/j.bmcl.2011.12.096. [DOI] [PubMed] [Google Scholar]
- Zhang Y.-K.; Plattner J. J.; Easom E. E.; Liu L.; Retz D. M.; Ge M.; Zhou H.-H. Benzoxaborole antimalarial agents. Part 3: design and syntheses of (carboxy-13C-3,3-2H2)-labeled and (3-14C)-labeled 7-(2-carboxyethyl)-1,3-dihydro-1-hydroxy-2,1-benzoxaboroles. J. Labelled Compd. Radiopharm. 2012, 55, 201–205. 10.1002/jlcr.2926. [DOI] [Google Scholar]
- Nare B.; Wring S.; Bacchi C.; Beaudet B.; Bowling T.; Brun R.; Chen D.; Ding C.; Freund Y.; Gaukel E.; Hussain A.; Jarnagin K.; Jenks M.; Kaiser M.; Mercer L.; Mejia E.; Noe A.; Orr M.; Parham R.; Plattner J.; Randolph R.; Rattendi D.; Rewerts C.; Sligar J.; Yarlett N.; Don R.; Jacobs R. Discovery of novel orally bioavailable oxaborole 6-carboxamides that demonstrate cure in a murine model of late-stage central nervous system african trypanosomiasis. Antimicrob. Agents Chemother. 2010, 54, 4379–4388. 10.1128/aac.00498-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palencia A.; Li X.; Bu W.; Choi W.; Ding C. Z.; Easom E. E.; Feng L.; Hernandez V.; Houston P.; Liu L.; Meewan M.; Mohan M.; Rock F. L.; Sexton H.; Zhang S.; Zhou Y.; Wan B.; Wang Y.; Franzblau S. G.; Woolhiser L.; Gruppo V.; Lenaerts A. J.; O’Malley T.; Parish T.; Cooper C. B.; Waters M. G.; Ma Z.; Ioerger T. R.; Sacchettini J. C.; Rullas J.; Angulo-Barturen I.; Pérez-Herrán E.; Mendoza A.; Barros D.; Cusack S.; Plattner J. J.; Alley M. R. K. Discovery of novel oral protein synthesis inhibitors of mycobacterium tuberculosis that target leucyl-trna synthetase. Antimicrob. Agents Chemother. 2016, 60, 6271–6280. 10.1128/aac.01339-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.; Hernandez V.; Rock F. L.; Choi W.; Mak Y. S. L.; Mohan M.; Mao W.; Zhou Y.; Easom E. E.; Plattner J. J.; Zou W.; Pérez-Herrán E.; Giordano I.; Mendoza-Losana A.; Alemparte C.; Rullas J.; Angulo-Barturen I.; Crouch S.; Ortega F.; Barros D.; Alley M. R. K. Discovery of a Potent and Specific M. tuberculosis Leucyl-tRNA Synthetase Inhibitor: (S)-3-(Aminomethyl)-4-chloro-7-(2-hydroxyethoxy)benzo[c][1,2]oxaborol-1(3H)-ol (GSK656). J. Med. Chem. 2017, 60, 8011–8026. 10.1021/acs.jmedchem.7b00631. [DOI] [PubMed] [Google Scholar]
- Klarmann-Schulz U.; Specht S.; Debrah A. Y.; Batsa L.; Ayisi-Boateng N. K.; Osei-Mensah J.; Mubarik Y.; Konadu P.; Ricchiuto A.; Fimmers R.; Arriens S.; Dubben B.; Ford L.; Taylor M.; Hoerauf A. Comparison of doxycycline, minocycline, doxycycline plus albendazole and albendazole alone in their efficacy against onchocerciasis in a randomized, open-label, pilot trial. PLoS Neglected Trop. Dis. 2017, 11, e0005156. 10.1371/journal.pntd.0005156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.; Shi W.; Hu D.; Zhang S.; Zhang H.; Wang Z.; Cheng L.; Sun F.; Shen J.; Cao X. In vitro and in vivo metabolite profiling of valnemulin using ultraperformance liquid chromatography-quadrupole/time-of-flight hybrid mass spectrometry. J. Agric. Food Chem. 2014, 62, 9201–9210. 10.1021/jf5012402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lykkeberg A. K.; Cornett C.; Halling-Sørensen B.; Hansen S. H. Isolation and structural elucidation of tiamulin metabolites formed in liver microsomes of pigs. J. Pharm. Biomed. Anal. 2006, 42, 223–231. 10.1016/j.jpba.2006.03.023. [DOI] [PubMed] [Google Scholar]
- Bakowski M. A.; Shiroodi R. K.; Liu R.; Olejniczak J.; Yang B.; Gagaring K.; Guo H.; White P. M.; Chappell L.; Debec A.; Landmann F.; Dubben B.; Lenz F.; Struever D.; Ehrens A.; Frohberger S.; Sjoberg H.; Pionnier N.; Murphy E.; Archer J.; Steven A.; Chunda V. C.; Fombad F. F.; Chounna P. W.; Njouendou A. J.; Metuge H. M.; Ndzeshang B. L.; Gandjui N. V.; Akumtoh D. N.; Kwenti T. D.; Woods A. K.; Joseph S. B.; Hull M. V.; Xiong W.; Kuhen K. L.; Taylor M.; Wanji S.; Turner J. D.; Hübner M. P.; Hoerauf A.; Roland J.; Tremblay M. S.; Schultz P. G.; Sullivan W.; Chu X.; Petrassi H. M.; McNamara C. W.. A single-dose oral therapy for the treatment of lymphatic filariasis and river blindness. Sci. Transl. Med., accepted. [Google Scholar]
- Sharma R.; Jayoussi G. A.; Tyrer H. E.; Gamble J.; Hayward L.; Guimaraes A. F.; Davies J.; Waterhouse D.; Cook D. A.; Myhill L. J.; Clare R. H.; Cassidy A.; Steven A.; Johnston K. L.; Ford L.; Turner J. D.; Ward S. A.; Taylor M. J. Minocycline as a re-purposed anti-Wolbachia macrofilaricide: superiority compared with doxycycline regimens in a murine infection model of human lymphatic filariasis. Sci. Rep. 2016, 6, 23458. 10.1038/srep23458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. D.; Sharma R.; Al Jayoussi G.; Tyrer H. E.; Gamble J.; Hayward L.; Priestley R. S.; Murphy E. A.; Davies J.; Waterhouse D.; Cook D. A. N.; Clare R. H.; Cassidy A.; Steven A.; Johnston K. L.; McCall J.; Ford L.; Hemingway J.; Ward S. A.; Taylor M. J. Albendazole and antibiotics synergize to deliver short-course anti-Wolbachiacurative treatments in preclinical models of filariasis. Proc. Natl. Acad. Sci. U.S.A. 2017, 114, E9712–E9721. 10.1073/pnas.1710845114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aljayyoussi G.; Tyrer H. E.; Ford L.; Sjoberg H.; Pionnier N.; Waterhouse D.; Davies J.; Gamble J.; Metuge H.; Cook D. A. N.; Steven A.; Sharma R.; Guimaraes A. F.; Clare R. H.; Cassidy A.; Johnston K. L.; Myhill L.; Hayward L.; Wanji S.; Turner J. D.; Taylor M. J.; Ward S. A. Short-course, high-dose rifampicin achieves wolbachia depletion predictive of curative outcomes in preclinical models of lymphatic filariasis and onchocerciasis. Sci. Rep. 2017, 7, 210. 10.1038/s41598-017-00322-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGarry H.; Egerton G. L.; Taylor M. J. Population dynamics of Wolbachia bacterial endosymbionts in Brugia malayi. Mol. Biochem. Parasitol. 2004, 135, 57–67. 10.1016/s0166-6851(04)00028-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.