Abstract
A series of pyrazolo[1,5-a]pyridine-3-carboxamide (PPA) derivatives bearing diaryl side chain was designed and synthesized as new antituberculosis agents, aiming to improve the efficacy toward drug resistant Mycobacterium tuberculosis (Mtb) strains. Most of the substituted diphenyl and heterodiaryl PPAs exhibited excellent in vitro potency against the drug susceptive H37Rv strain (MIC < 0.002–0.381 μg/mL) and drug resistant Mtb strains (INH-resistant (rINH), MIC < 0.002–0.465 μg/mL; RMP-resistant (rRMP), MIC < 0.002–0.004 μg/mL). Noticeably, some compounds also showed very low cytotoxicity against Vero cells. Further, compound 6j displayed good pharmacokinetic profiles with oral bioavailability (F) of 41% and significantly reduced the bacterial burden in an autoluminescent H37Ra infected mouse model.
Keywords: Antituberculosis agents, INH-resistant Mtb strain, RMP-resistant Mtb strains, structure−activity relationship
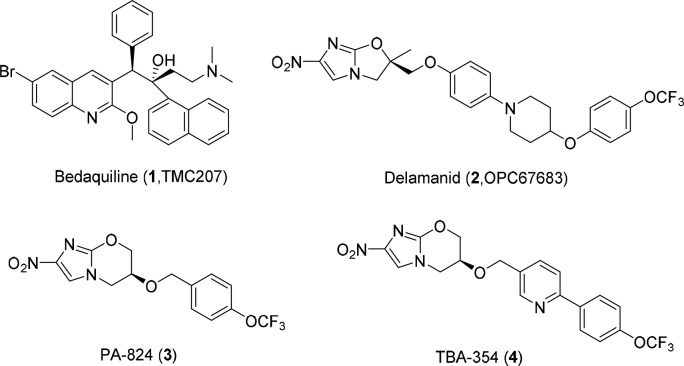
Tuberculosis (TB), caused by Mycobacterium tuberculosis (Mtb), remains the leading infectious cause of death worldwide. It was estimated that nearly 10.4 million people fell ill with TB and 1.7 million died of TB in 2016.1 A standard 6–9 months regimen recommended by World Health Organization (WHO) results in multidrug-resistant TB (MDR-TB) and extensively drug-resistant TB (XDR-TB) due to lack of patient adherence.2,3 At present, MDR-TB usually has to be treated with a combination of five to seven drugs lasting up to 18–24 months.4 Therefore, new drugs with the potential to shorten the duration of treatment or overcome drug-resistant TB are urgently needed. Encouragingly, bedaquiline (TMC207, 1)5,6 and delamanid (OPC67683, 2)7 were approved for the treatment of MDR-TB by FDA in 2012 and by EMA in 2014, respectively (Figure 1), even though the wide-scale use of 1 is restricted for safety risks.8 It is still imperative to identify new molecules with alternative scaffolds as effective agents to banish the scourge of TB.
Figure 1.
Chemical structures of antitubercular drugs bedaquiline, delamanid, PA-824, and TBA-354.
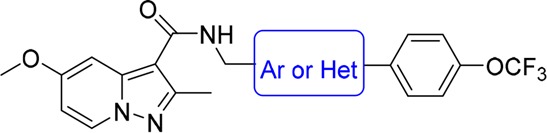
The other two nitroimidazole drugs PA-824 (3) and TBA-354 (4) are currently in clinical studies for drug-sensitive and drug-resistant TB (Figure 1).9,10 TBA-354, only with a 4-trifluoromethoxyphenyl-pyridine group to replace the 4-trifluoromethoxyphenyl in PA-824, is promising to shorten TB treatment as part of a regimen superior to that of PA-824 in terms of anti-TB potency and improved pharmacokinetic properties.11 Our previous studies had identified a series of pyrazolo[1,5-a]pyridine-3-carboxamides (PPAs) as antituberculosis agents (Figure 2), which exhibited good activities with low nanomolar MIC values against H37Rv strain (Figure 2).12,13 Considering PPAs linked with the N-benzylic moiety were crucial for the antitubercular activity, we first envisioned to make structural modifications on the side chain of lead 5 by introducing substituted diaryl (diphenyl or heterodiaryl) groups, which was similar to the optimization strategy of TBA354 and would possibly target drug resistant Mtb strains (Figure 2). In addition, our previous SAR study had suggested that the 2-methyl-5-methoxy-pyrazolo[1,5-a]pyridine scaffold-based compound displayed better potency than the related 2-methyl-5-methyl-pyrazolo[1,5-a]pyridine against drug resistant clinical Mtb isolates.12 Thus, a series of 2-methyl-5-methoxy-pyrazolo[1,5-a]pyridine-3-carboxamide diaryl derivatives were designed and synthesized.
Figure 2.
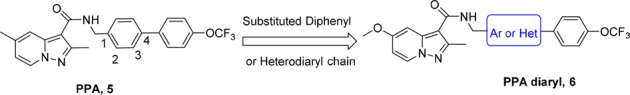
Design of pyrazolo[1,5-a]pyridine-3-carboxamide diaryl derivatives.
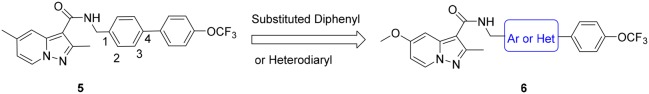
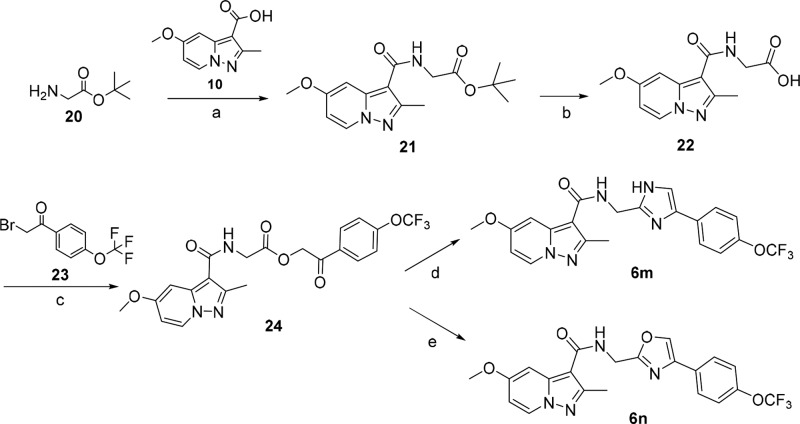
Compounds 6a–6l and 6o–6p were readily synthesized using a straightforward amidation of pyrazolo[1,5-a]pyridine-3-carboxylic acid 10(12) with different self-prepared primary amines 9, 13, 16, and 19 (Scheme 1). Different strategies were used to synthesize these amines. Briefly, the amines 8 were synthesized by Suzuki coupling bromo-substituted materials 7 and (4-(trifluoromethoxy)phenyl)boronic acid followed by nitrile reduction with lithium aluminum hydride (Supporting Information). The synthesis of (5-(4-(trifluoromethoxy)phenyl)thiophen-2-yl)methanamine 13 was started by Boc protection and bromination of thiophen-2-ylmethanamine 11 to give bromothiophene 12, which was followed by Suzuki coupling and deprotection reactions. The (1-(4-(trifluoromethoxy)phenyl)-1H-1,2,3-triazol-4-yl)methanamine 16 was obtained by diazo and click reactions starting from 4-(trifluoromethoxy)aniline 14. The (5-(4-(trifluoromethoxy)phenyl)-1,3,4-oxadiazol-2-yl)methanamine 19 was synthesized starting from 17 that was condensed with BOC-glycine, then followed by cyclization and deprotection reactions based on the published procedures.14
Scheme 1. Synthesis of Compounds 6a–6l and 6o–6p.
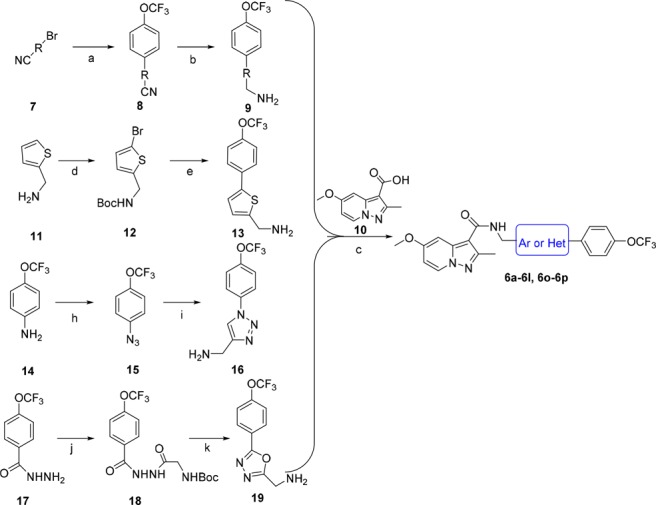
Reagents and conditions: (a) (4-(trifluoromethoxy)phenyl)boronic acid, Pd(PPh3)4, Na2CO3, toluene, 110°C, overnight, 83–92%; (b) LiAlH4, THF, −40°C, 3 h, 48–53%; (c) HATU, DIPEA, DCM, rt, overnight, 49–53%; (d) i. NaHCO3, Boc2O, THF, rt, 3 h, 97%; ii. NBS, DMF, rt, 5 h, 82%; (e) i. 4-(trifluoromethoxy)phenylboronic acid, K2CO3, Pd(PPh3)4, DME, 80°C, 4 h, 87%; ii. TFA, DCM, rt, 5 h, 82%; (h) hydrochloric acid, NaNO2, NaN3, H2O, 0 °C → rt, 2.5 h, 92%; (i) 2-propynylamine, CuI, THF, DIPEA, rt, overnight, 70%; (j) Boc-glycine, HATU, DIPEA, DMF, rt, overnight, 85%; (k) i. Et3N, PPh3, CCl4, DMF, rt, overnight, 68%; ii TFA, DCM, rt, 2 h, 98%.
The other two compounds 6m and 6n were synthesized in Scheme 2. The procedure was started by condensation of tert-butyl glycinate 20 and pyrazolo[1,5-a]pyridine-3-carboxylic acid 10, followed by hydrolysis in TFA to give intermediate 22. The intermediate 22 was then reacted with 2-bromo-1-(4-(trifluoromethoxy)phenyl)ethan-1-one 23 to give the ester 24, followed by cycloaddition with ammonium acetate and acetamide to produce compounds 6m and 6n, respectively.
Scheme 2. Synthesis of Compounds 6m and 6n.
Reagents and conditions: (a) HATU, DIEA, DCM, rt, overnight, 70%; (b) TFA, DCM, rt, 2 h, 98%; (c) Cs2CO3, EtOH, DMF, rt, overnight, 46%, (d) ammonium acetate, xylene, 140°C, 6 h, 56%; (e) acetamide, xylene, 140°C, 3 h, 45%.
The minimum inhibitory concentration (MIC) values of all the new compounds were preliminarily screened against Mtb H37Rv strain in microplate alamar blue assay (MABA) and low oxygen recovery assay (LORA) with the assay concentrations ranging from 0.02 to 5 μg/mL (Table 1).15 Compounds showing encouraging MICs (<0.02 μg/mL) in MABA were further tested against Mtb H37Rv, rRMP, and rINH with the assay concentrations ranging from 0.002 to 0.5 μg/mL and were also tested against Vero Cells to assess the compounds’ potential cytotoxicity (Table 2). Rifampicin (RMP), isoniazid (INH), linezolid (LIZ), and TMC207 were used as positive control drugs to support the reliability of our screening results.
Table 1. In Vitro Antitubercular Activity of Compounds 6a–6p against the Mtb Strains H37Rv in MABA and LORA with the Assay Concentrations Ranging from 0.02 to 5 μg/mL.
Calculated by MarvinSketch 5.4.1.1 version.
Table 2. In Vitro Antitubercular Activity of Compounds 6a–6d, 6h–6j, and 6l against the Mtb Strains H37Rv, rRMP, and rINH with the Assay Concentrations Ranging from 0.002 to 0.5 μg/mL, and Vero Cellular Toxicity.
| MIC
(μg/mL) |
|||||
|---|---|---|---|---|---|
| compds | H37Rv | rRMP | rINH | VERO cell IC50 (μg/mL) | selectivity index (SI)a |
| 6a | 0.013 | <0.002 | <0.002 | 3.15 | 242 |
| 6b | 0.007 | <0.002 | 0.003 | 11.8 | 1685 |
| 6c | 0.029 | <0.002 | 0.003 | 11.6 | 402 |
| 6d | <0.002 | <0.002 | 0.004 | >50 | >25000 |
| 6h | 0.007 | <0.002 | 0.004 | >50 | >7142 |
| 6i | 0.053 | 0.458 | 0.004 | 28.4 | 535 |
| 6j | <0.002 | <0.002 | 0.002 | >50 | >25000 |
| 6l | <0.002 | 0.465 | 0.002 | >50 | >25000 |
| RMP | <0.008 | >2 | <0.008 | >100 | >12500 |
| INH | 0.239 | 0.438 | >8 | ||
| LIZ | 1.254 | 1.870 | 0.953 | ||
| TMC207 | 0.020 | <0.016 | <0.016 | >10 | >500 |
SI: IC50/MIC for H37Rv.
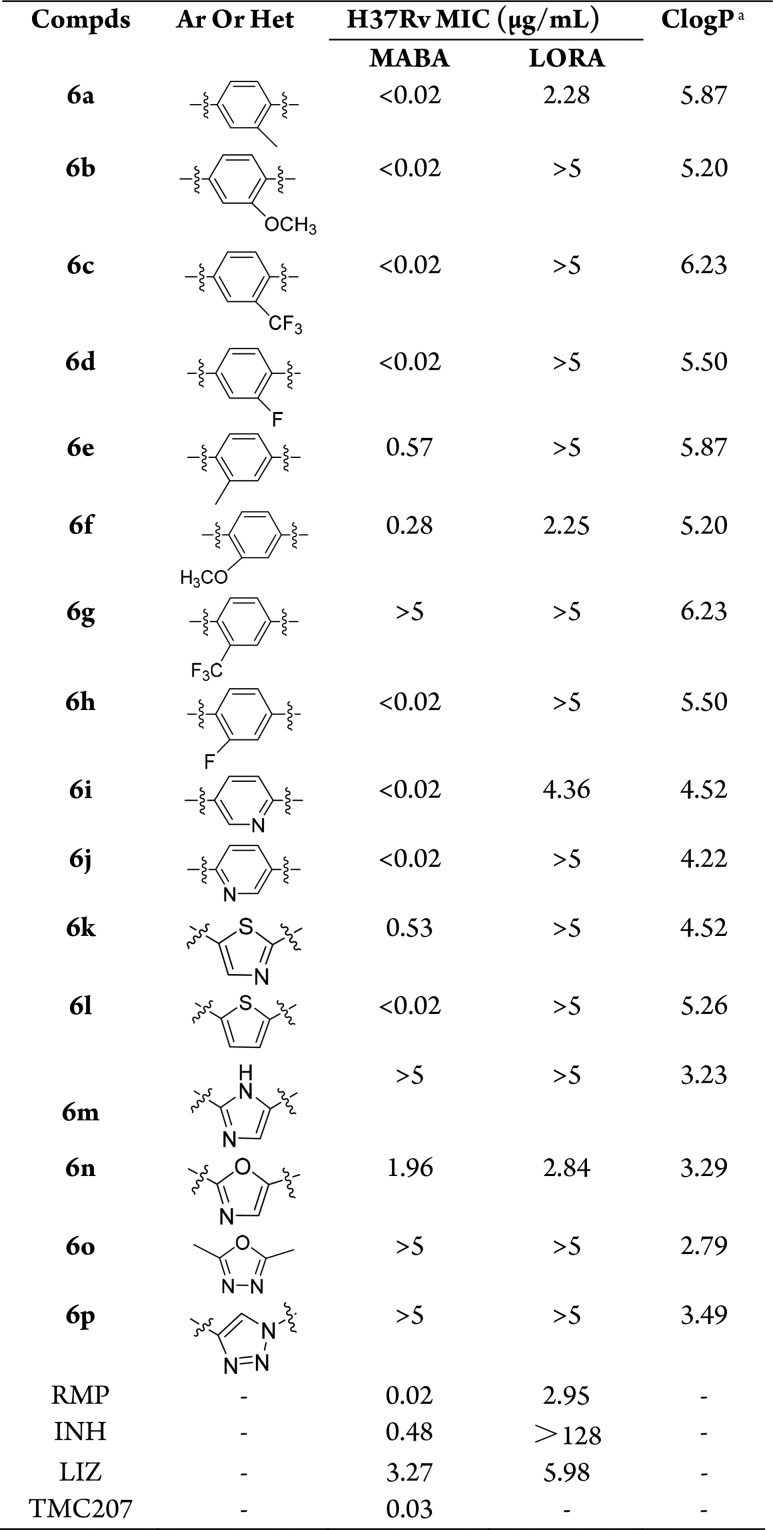
The first round of modifications was to investigate the role of the substituents at the 3-position of the phenyl group (Figure 2). Therefore, keeping intact the diphenyl chain, small functional groups (CH3, OCH3, CF3, F) were introduced at the 3-position of the phenyl group. The resulting compounds 6a–6d exhibited strong antitubercular activity against Mtb H37Rv with all MICs values of <0.02 μg/mL at the first round assay with concentrations ranging from 0.02 to 5 μg/mL (Table 1). This prompted us to gain further insights into the exact MICs at lower concentrations. Under the second screening concentrations (0.002–0.5 μg/mL), the MICs values of compounds 6a–6d were 0.013, 0.007, 0.029, and <0.002 μg/mL, respectively, which were comparable to that of RMP, TMC207, and the lead compound 5 (MIC = 0.006 μg/mL) (Table 2).12 These results suggested that this position is tolerated for substitution, especially for the OCH3 and F groups. Switching these substituents from the 3 to 2 position of the phenyl group, an almost 43- and 40-fold reduction in activity was noticed for compounds 6e (MIC = 0.57 μg/mL) and 6f (MIC = 0.28 μg/mL), whereas the antitubercular activity of compound 6g was completely lost when the CF3 was introduced at the 2 position. Interestingly, introduction of F atom in the 2 position led to compound 6h (MIC = 0.007 μg/mL), which kept potent antitubercular activity compared to compound 6d. A plausible explanation of this result may be that F atom does not affect the activity due to its relatively small size (similar to that of H atom).
Like TBA-354, the pyridine ring was used to replace the phenyl group. The yielded compound 6i showed an MIC value of 0.053 μg/mL, which was better than that of the control drug INH and LIZ, and comparable to TMC207 (Table 2). Moving the N atom from the 3 to 2 position led to compound 6j, with improved antitubercular activity against H37Rv to <0.002 μg/mL. Inspirited by the activities of compounds 6i and 6j, we further investigated the five-membered heterocyclic ring instead of the phenyl group. However, only the thiophene derivative 6l (MIC < 0.002 μg/mL) had the MIC value comparable to 6j. The thiazole 6k (MIC = 0.53 μg/mL) and oxazole 6n (MIC = 1.96 μg/mL) had almost 265- and 980-fold reduction in activities compared to 6j (Table 1). The imidazole 6m, oxadiazole 6o, and triazole 6p completely lost the activities with all MICs values >5 μg/mL. Briefly, it was suggested that a five-membered heterocyclic ring such as thiophene can substitute the phenyl, but others led to loss of activity in varying degrees.
The compounds were also tested in LORA, which is an in vitro model for the assessment of activity against persistent Mtb. Among the current antitubercular drugs, only RMP and PZA have been reported to possess activity against dormant strain. From the results (Table 1), it was very remarkable that compounds 6a (MIC = 2.28 μg/mL), 6f (MIC = 2.25 μg/mL), and 6n (MIC = 2.84 μg/mL) exhibited good LORA MICs values comparable to that of RMP (MIC = 2.95 μg/mL). However, most of the other compounds were not very active in LORA with MIC values of >5 μg/mL.
Encouraged by their strong potencies against the drug sensitive H37Rv strain, compounds 6a–6d, 6h–6j, and 6l were further evaluated against rRMP and rINH strains. It was shown that all of the compounds displayed excellent potencies against rRMP and rINH strains with MIC values <0.002–0.465 and <0.002–0.004 μg/mL (Table 2). Moreover, the in vitro Vero cell toxicity of these compounds was further determined. As it was shown in Table 2, the compounds had low or no cytotoxicity against VERO cells with IC50 values from 3.15 to >50 μg/mL. Particularly, compounds 6d, 6j, and 6l had no cytotoxicity against VERO cells, with all of the IC50 values of >50 μg/mL and the selectivity index (SI) values of >25000.
Combined with other factors such as ClogP, we selected compound 6j for further study. The pharmacokinetic (PK) properties of compound 6j were evaluated in rats by single intravenous injection (i.v. 2.5 mg/kg) and oral administration (p.o. 10 mg/kg). As shown in Table 3, 6j exhibited a half-life of 5.1 h and a relative high optimal plasma exposure with AUC value of 15638 h·μg/L. It also displayed a good oral bioavailability of 41%, suggesting its good absorption in rats.
Table 3. Pharmacokinetic Profile of Compound 6j in Rats.
| route | dose (mg/kg) | AUC0-∞ (h·μg/L) | Cmax (μg/L) | Tmax (h) | T1/2 (h) | MRT0-∞ (h) | F (%) |
|---|---|---|---|---|---|---|---|
| oral | 10 | 15638 | 1428 | 4.67 | 5.10 | 8.21 | 41 |
| iv | 2.5 | 9002 | 4152 | 0.08 | 3.49 | 4.02 |
Given the promising antitubercular activity against H37Rv and good PK properties in rats, 6j was evaluated for its in vivo antitubercular efficacy by using the mouse model infected with the selectable marker-free autoluminescent Mtb strain H37Ra.12,16 The animals were infected with log-phase autoluminescent Mtb H37Ra via intravenous injection (2 × 106 CFU per mouse) and then were repeatedly administered with 6j once daily via oral gavage for 5 consecutive days. The bacterial burden was measured by monitoring the bioluminescence intensity (relative light unit, RLU) from the same batch of live mice every day. As shown in Figure 3, with the two tested doses of 10 and 50 mg/kg/day, compound 6j exhibited dose-dependent in vivo antitubercular activity. The 6j 50 mg/kg/day group showed a significant reduction in lung RLU by ∼1.0 log10 compared with the untreated CMC-Na group (Figure 3A). The 6j 50 mg/kg/day group also reduced the spleen bacterial burdens by ∼0.61 log10 RLU relative to that of CMC-Na group. The RMP-treated group showed decreases by ∼1.42 log10 RLU and ∼1.34 log10 RLU in lung and spleen, respectively. These results strongly suggest the promising potential of compound 6j to serve as a lead compound for further antitubercular drug discovery.
Figure 3.
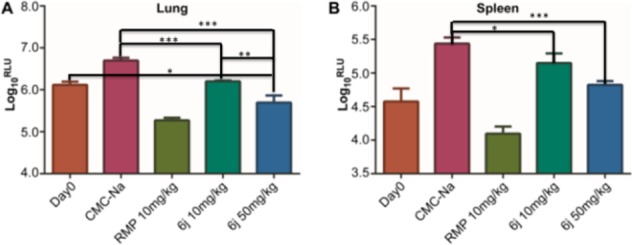
Mean RLU count (±SD) assessed at day 0 and the day after treatment completion (A) for lung and (B) for spleen. y-axis, corresponding Log10RLU/lung; x-axis, different treatment groups (ns, not significant; *P < 0.05; **P < 0.01; ***P < 0.001).
A series of PPA diaryl derivatives has been synthesized and evaluated for the antitubercular activity. Most of the derivatives exhibited excellent in vitro potency against the drug susceptive H37Rv strain (MIC < 0.002–0.381 μg/mL) and two rINH (MIC < 0.002–0.465 μg/mL) and rRMP (MIC < 0.002–0.004 μg/mL) Mtb strains. The representative compound 6j exhibited promising in vitro activities against Mtb H37Rv, rRMP, and rINH with MIC values ≤0.002 μg/mL and no toxicity against Vero cells. Further in vivo studies indicated that compound 6j displayed good pharmacokinetic profiles with an oral bioavailability of 41% and significantly reduced the mycobacterial burden in an H37Ra infected mouse model.
Acknowledgments
We thank Guangdong Natural Science Funds (2016A030313106, 2015A030306042), National Natural Science Foundation of China (81673285), Guangzhou City Key Laboratory of Precision Chemical Drug and Chinese Academy of Sciences Grants (154144KYSB20150045, YJKYYQ20170036), and Guangzhou Municipal Industry and Research Collaborative Innovation Program (201508020248, 201604020019) for the financial support. We thank Jian Tang for establishing the synthetic method of intermediates 13 and 16 in Scheme 1.
Glossary
ABBREVIATIONS
- TB
tuberculosis
- PPA
pyrazolo[1,5-a]pyridine-3-carboxamide
- Mtb
Mycobacterium tuberculosis
- WHO
World Health Organization
- MDR-TB
multidrug-resistant TB
- XDR-TB
extensively drug-resistant TB
- MIC
minimum inhibitory concentration
- MABA
microplate alamar blue assay
- MRT
mean residence time
- LORA
low oxygen recovery assay
- RMP
rifampicin
- INH
isoniazid
- LIZ
linezolid
- RLU
relative light unit
- rINH
INH-resistant
- rRMP
RMP-resistant
- DIPEA
N,N-diisopropylethylamine
- HATU
2-(7-azabenzotriazol-1-yl)-N,N,N′,N′-tetramethyluronium hexafluorophosphate
- EMA
European Medicines Agency
- FDA
Food and Drug Administration
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.8b00410.
Chemistry procedures and analytical data for title compounds, and biological assay (PDF)
Author Contributions
# These authors contributed equally to this work.
The authors declare no competing financial interest.
Supplementary Material
References
- Global Tuberculosis Report 2017. https://www.who.int/tb/publications/global_report/archive/en/ (accessed December 2017). [Google Scholar]
- World Health Organization Treatment of Tuberculosis: Guidelines for National Programmes; WHO/HTM/TB/2009.420, 4th ed., 2009. [Google Scholar]
- Zumla A. I.; Gillespie S. H.; Hoelscher M.; Philips P. P.; Cole S. T.; Abubakar I.; McHugh T. D.; Schito M.; Maeurer M.; Nunn A. J. New antituberculosis drugs, regimens, and adjunct therapies: needs, advances, and future prospects. Lancet Infect. Dis. 2014, 14, 327–340. 10.1016/S1473-3099(13)70328-1. [DOI] [PubMed] [Google Scholar]
- Guidelines Approved by the Guidelines Review Committee. WHO Treatment Guidelines for Drug-Resistant Tuberculosis, 2016 Update; World Health Organization: Geneva, 2016. [Google Scholar]
- Andries K.; Verhasselt P.; Guillemont J.; Göhlmann H. W.; Neefs J. M.; Winkler H.; Van Gestel J.; Timmerman P.; Zhu M.; Lee E.; Williams P.; de Chaffoy D.; Huitric E.; Hoffner S.; Cambau E.; Truffot-Pernot C.; Lounis N.; Jarlier V. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. Science 2005, 307, 223–227. 10.1126/science.1106753. [DOI] [PubMed] [Google Scholar]
- Diacon A. H.; Donald P. R.; Pym A.; Grobusch M.; Patientia R. F.; Mahanyele R.; Bantubani N.; Narasimooloo R.; De Marez T.; van Heeswijk R.; Lounis N.; Meyvisch P.; Andries K.; McNeeley D. F. Randomized pilot trial of eight weeks of bedaquiline (TMC207) treatment for multidrug-resistant tuberculosis: long-term outcome, tolerability, and effect on emergence of drug resistance. Antimicrob. Agents Chemother. 2012, 56, 3271–3276. 10.1128/AAC.06126-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakare R.; Soni I.; Dasgupta A.; Chopra S. Delamanid for the treatment of pulmonary multidrug-resistant tuberculosis. Drugs Today 2015, 51, 117–123. 10.1358/dot.2015.51.2.2245645. [DOI] [PubMed] [Google Scholar]
- Cox E.; Laessig K. FDA approval of bedaquiline--the benefit-risk balance for drug-resistant tuberculosis. N. Engl. J. Med. 2014, 371, 689–691. 10.1056/NEJMp1314385. [DOI] [PubMed] [Google Scholar]
- Singh R.; Manjunatha U.; Boshoff H. I.; Ha Y. H.; Niyomrattanakit P.; Ledwidge R.; Dowd C. S.; Lee I. Y.; Kim P.; Zhang L.; Kang S.; Keller T. H.; Jiricek J.; Barry C. E. PA-824 kills nonreplicating Mycobacterium tuberculosis by intracellular NO release. Science 2008, 322, 1392–1395. 10.1126/science.1164571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kmentova I.; Sutherland H. S.; Palmer B. D.; Blaser A.; Franzblau S. G.; Wan B.; Wang Y.; Ma Z.; Denny W. A.; Thompson A. M. Synthesis and structure-activity relationships of aza- and diazabiphenyl analogues of the antitubercular drug (6S)-2-nitro-6-{[4-(trifluoromethoxy)benzyl]oxy}-6,7-dihydro-5H-imidazo[2,1-b][1,3]oxazine (PA-824). J. Med. Chem. 2010, 53, 8421–8439. 10.1021/jm101288t. [DOI] [PubMed] [Google Scholar]
- Upton A. M.; Cho S.; Yang T. J.; Kim Y.; Wang Y.; Lu Y.; Wang B.; Xu J.; Mdluli K.; Ma Z.; Franzblau S. G. In vitro and in vivo activities of the nitroimidazole TBA-354 against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2015, 59, 136–144. 10.1128/AAC.03823-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J.; Wang B. X.; Wu T.; Wan J. T.; Tu Z. C.; Njire M.; Wan B. J.; Franzblauc S. G.; Zhang T. Y.; Lu X. Y.; Ding K. Design, synthesis and biological evaluation of pyrazolo[1,5-a]pyridine-3-carboxamides as novel antitubercular agents. ACS Med. Chem. Lett. 2015, 6, 814–818. 10.1021/acsmedchemlett.5b00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X. Y.; Tang J.; Cui S. Y.; Wan B. J.; Franzblauc S. G.; Zhang T. Y.; Zhang X. T.; Ding K. Pyrazolo[1,5-a]pyridine-3-carboxamide hybrids: Design, synthesis and evaluation of anti-tubercular activity. Eur. J. Med. Chem. 2017, 125, 41–48. 10.1016/j.ejmech.2016.09.030. [DOI] [PubMed] [Google Scholar]
- van der Westhuyzen R.; Winks S.; Wilson C. R.; Boyle G. A.; Gessner R. K.; Soares de Melo C.; Taylor D.; de Kock C.; Njoroge M.; Brunschwig C.; Lawrence N.; Rao S. P. S.; Sirgel F.; van Helden P.; Seldon R.; Moosa A.; Warner D. F.; Arista L.; Manjunatha U. H.; Smith P. W.; Street L. J.; Chibale K. Pyrrolo[3,4-c]pyridine-1,3(2H)-diones: a novel antimycobacterial class targeting mycobacterial respiration. J. Med. Chem. 2015, 58, 9371–9381. 10.1021/acs.jmedchem.5b01542. [DOI] [PubMed] [Google Scholar]
- Cho S.; Lee H. S.; Franzblau S. Microplate alamar blue assay (MABA) and low oxygen recovery assay (LORA) for mycobacterium tuberculosis. Methods Mol. Biol. 2015, 1285, 281–292. 10.1007/978-1-4939-2450-9_17. [DOI] [PubMed] [Google Scholar]
- Zhang T.; Li S. Y.; Nuermberger E. L. Autoluminescent mycobacterium tuberculosis for rapid, real-time, non-invasive assessment of drug and vaccine efficacy. PLoS One 2012, 7, e29774 10.1371/journal.pone.0029774. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.