Abstract
Cytochrome P450 (P450, CYP) enzymes have long been of interest due to their roles in the metabolism of drugs, pesticides, pro-carcinogens, and other xenobiotic chemicals. They have also been of interest due to their very critical roles in the biosynthesis and metabolism of steroids, vitamins, and certain eicosanoids. This review covers the 22 (of the total of 57) human P450s in Families 5–51 and their substrate selectivity. Also included is information and references regarding inducibility, inhibition, and (in some cases) stimulation by chemicals. We update and discuss important aspects of each of these 22 P450s and questions that remain open.
Keywords: cytochrome P450, xenobiotics, endogenous compounds, steroids, eicosanoids, vitamin D, vitamin A, retinoids, enzyme inhibition, enzyme induction
Introduction
The significance of the human cytochrome P450 (P450) enzymes in drug metabolism has been reviewed in detail in previous reviews (Guengerich and Rendic 2010; Rendic and Guengerich 2010, 2012; Guengerich 2015; Rendic and Guengerich 2015). In addition to a great number of compounds used as drugs or being found in the environment, and influencing the activity and/or expression of the cytochrome P450 enzymes (Guengerich and Rendic 2010; Rendic and Guengerich 2012), the effects of diseases and environmental factors—including ionizing radiation, UV, γ-rays, X-rays—are also of interest and have been reviewed (Semonin-Holleran 1991; Klammert et al. 2009; Guengerich and Rendic 2010; Rendic and Guengerich 2012). Such factors can have profound effects on enzyme activity and expression and therefore also on the final biological activity, efficacy, and safety of drugs and other chemicals. They can contribute to drug-drug, drug-chemical, or chemical-chemical interactions by modifying the disposition of xeno- and endobiotics and consequently their fate in the body. In some cases, analysis of the results on the effects of diseases and different environmental factors on human cytochrome P450 enzymes revealed inconsistency of the reported results, making it difficult to reach conclusions.
The scope of this article is the human P450s in Families 5–51. The topic follows two relevant reviews that one of us wrote in the past four years (Guengerich 2015, 2017) on the P450s involved in metabolism of endogenous compounds. Why are we focusing on these P450s in a journal that deals with drug metabolism? There are several reasons. One is that some of the steroids and vitamins are used as drugs. Another is that most of these P450s have important roles in physiology and are subject to induction and/or inhibition by drugs. Finally, several of these P450s are functional targets for drugs, e.g. 5A1, 11B1, 11B2, 17A1, 19A1. These enzymes synthesize important molecules but over-production may be an issue in some diseases. Families 5–51 were covered in a chapter several years ago (Guengerich 2015), but here we have focused on inhibitors and also updated the information.
Most of the P450 enzymes in Families 5–51 are mainly extrahepatic, with several exceptions (7A1, 8B1, 26A1, 27A1, 39A1, 51A1). In contrast to the “drug-metabolizing” P450s in Families 1–4, the levels of expression of these enzymes are highly regulated and do not vary among individuals as much and, in general, are not very inducible by xenobiotics.
Analyses of clinical tumor samples may link causality with changes in gene expression in some cases. For instance, increases/or downregulation in mRNA and/or protein expression of P450s 5A1, 7B1, 19A1, 26A1, 26B1, 26C1, 27A1, and 27B1 have been observed and suggested as markers of the aggressive biological potential of tumors and association with poor patient survival. It has been suggested that up-regulation of these enzymes might be useful as tumor markers in the diagnosis and prognosis of different malignancies. On the other hand, enhanced or lowered expression and/or activity of P450 enzymes in some diseases could result in clinically significant drug interaction potential, resulting in unfavorable clinical outcome or increased drug/chemical toxicity. Some examples include increased expression of P450 2E1 due to alcohol in healthy subjects and decreased enzyme expression in alcoholic liver disease, increased P450 2E1 expression in livers of transplant patients, high expression of P450 3A4 enzyme in lymphoid carcinoma (proposed as a useful predictor of poor response to the standard peripheral type lung cancer chemotherapy), and high expression of P450 3A enzymes in osteosarcomas (suggested as a predictor of metastasis and poor prognosis) (Guengerich and Rendic 2010).
For each of the 22 P450s we will review, the format will include a brief synopsis followed by a figure showing the main reaction and a table that includes a list of physiological substrates, function, and inhibitors and inducers. We have included references following the section on each P450 for the convenience of the reader. Collectively there is a total of 1,057 references for the entire review (not correcting for multiple entries in different sections).
We have divided the review into several sections, based on the substrates for these P450s. The four sections include eicosanoids, steroids, vitamin D and related secosteroids, and retinoids. A fifth section is for P450 20A1, for which no substrates or functions have yet been characterized. This P450 remains an “orphan,” in our sense of the word (Guengerich and Cheng 2011).
References
- Guengerich FP. 2015. Chapter 9, Human cytochrome P450 enzymes. In: Ortiz de Montellano PR, editor. Cytochrome P450: Structure, Mechanism, and Biochemistry 4th ed. New York: Springer; p. 523–785. [Google Scholar]
- Guengerich FP. 2017. Intersection of the roles of cytochrome P450 enzymes with xenobiotic and endogenous substrates: Relevance to toxicity and drug interactions. Chem Res Toxicol 30:2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guengerich FP, Cheng Q. 2011. Orphans in the human cytochrome P450 superfamily: approaches to discovering functions and relevance in pharmacology. Pharmacol. Rev 63:684–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guengerich FP, Rendic S. 2010. Update information on drug metabolism systems–2009, part I. Curr Drug Metab 11:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klammert U, Nickel J, Wurzler K, Klingelhoffer C, Sebald W, Kubler AC, Reuther T. 2009. Biological activity of a genetically modified BMP-2 variant with inhibitory activity. Head Face Med 5:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendic S, Guengerich FP. 2010. Update information on drug metabolism systems–2009, part II: Summary of information on the effects of diseases and environmental factors on human cytochrome P450 (CYP) enzymes and transporters. Curr Drug Metab 11:4–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendic S, Guengerich FP. 2012. Summary of information on the effects of ionizing and non-ionizing radiation on cytochrome P450 and other drug metabolizing enzymes and transporters. Curr Drug Metab 13:787–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendic S, Guengerich FP. 2015. Survey of human oxidoreductases and cytochrome P450 enzymes involved in the metabolism of xenobiotic and natural chemicals. Chem Res Toxicol 28:38–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semonin-Holleran R 1991. Pediatric trauma patients: differences and implications for emergency nurses. J Emergen Nurs 17:24–33. [PubMed] [Google Scholar]
Eicosanoid metabolism
Some of the Family 4 P450s are involved in ω- and ω−1 hydroxylation of leukotrienes and prostaglandins (Guengerich 2015), which are generally considered to be deactivating processes. The two P450s discussed here (P450s 5A1 and 8A1) catalyze rearrangements of prostaglandin H2 and related molecules (Fig. 1). P450s 8A1 and P450 5A1 are the only two human P450s that use an endoperoxide as a physiological substrate. These two enzymes isomerize prostaglandin endoperoxides without the use of molecular oxygen or any external electron donors (Li et al. 2008).
Fig. 1.
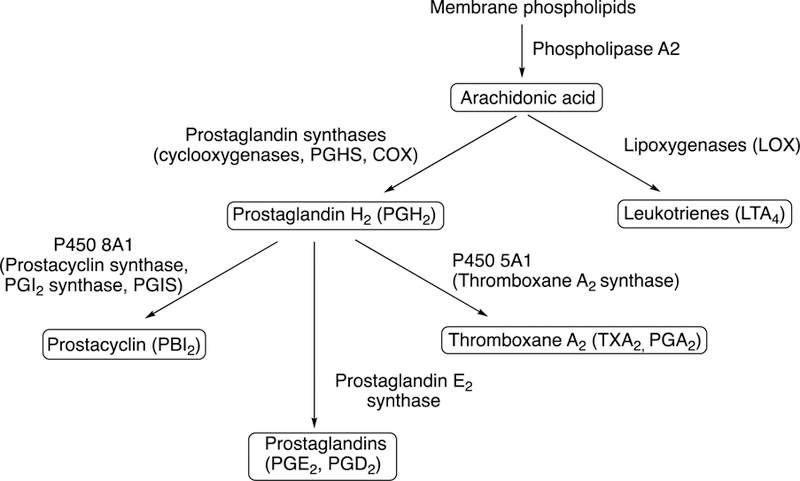
Metabolic pathways for arachidonic acid, with participation of P450s 5A1 and 8A1.
Both prostacyclin synthase (P450 8A1) and thromboxane synthase (P450 5A1) signaling affect a number of tumor cell survival pathways, e.g., cell proliferation, apoptosis, tumor cell invasion and metastasis, and angiogenesis. However, the effects of these respective synthases differ considerably with respect to the pathways described. Prostacyclin (prostaglandin I2 (PGI2)) is a potent inhibitor of vasoconstriction, platelet activation, and aggregation. Widely known for its vasoprotective activity, prostacyclin is synthesized mainly in the endothelial and smooth muscle cells via the described isomerization of prostaglandin H2, a reaction catalyzed by prostacyclin synthase (CYP8A1; also termed PGI2 synthase; PGIS). The balance of these oppositely-acting cyclooxygenase-derived prostanoids influences many processes throughout the body, e.g., blood pressure regulation, clotting, and inflammation. The prostacyclin/thromboxane ratio is important in vivo, with the corresponding synthases shown to be differentially regulated in a variety of disease states.
References
- Guengerich FP. 2015. Chapter 9, Human cytochrome P450 enzymes. In: Ortiz de Montellano PR, editor. Cytochrome P450: Structure, Mechanism, and Biochemistry 4th ed. New York: Springer; p. 523–785. [Google Scholar]
- Li YC, Chiang CW, Yeh HC, Hsu PY, Whitby FG, Wang LH, Chan NL. 2008. Structures of prostacyclin synthase and its complexes with substrate analog and inhibitor reveal a ligand-specific heme conformation change. J Biol Chem 283:2917–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
P450 5A1
P450 5A1 (also termed CYP5A1 and thromboxane synthase, TBXAS1) is expressed in platelets and some other cells (Yokoyama et al. 1991; Guengerich 2015). A 3-dimensional structure is still not available, to our knowledge. In contrast to most P450s, no external source of electrons or molecular oxygen is required. The reaction is an internal rearrangement of the endoperoxide prostaglandin H2, involving the interaction of the heme iron with the oxygen atoms and high valent intermediates. The most widely accepted mechanism is that proposed by Hecker and Ullrich (1989).
Arachidonic acid metabolites are key mediators involved in the pathogenesis of numerous cardiovascular, pulmonary, inflammatory, and thromboembolic diseases, and one of particular importance is thromboxane A2. It is produced by the action of thromboxane synthase (P450 5A1) on the prostaglandin endoperoxide H2 (PGH2), a product of the enzymatic transformation of arachidonic acid by the cyclooxygenases. Thromboxane A2 is a potent inducer of platelet aggregation, vasoconstriction, and bronchoconstriction, which are involved in a series of major pathophysiological conditions including myocardial infraction, unstable angina, pregnancy-induced hypertension and preeclampsia, thrombosis and thrombotic disorders, pulmonary hypertension, asthma, septic shock, atherosclerosis, lupus nephritis, and Raynaud’s phenomenon. Thromboxane A2 receptor antagonists, thromboxane synthase inhibitors, and drugs combining both properties have been developed since the 1980s (Dogne et al. 2006; Kontogiorgis and Hadjipavlou-Litina 2010). The activity of the enzyme (and/or transcription of the gene) can be affected by a number of drugs or drug-candidates (particularly azoles), by environmental factors and natural compounds, and even by physiological factors such as illnesses or hypoxia. In some cancers there is a significant increase of P450 5A1 mRNA/protein expression. Tumor progression can occur through modulation of cell motility (prostate cancer) (Nie et al. 2004), development and progression (pituitary tumor) (Onguru et al. 2004)), pathogenesis (papillary thyroid carcinoma) (Kajita et al. 2005), or the cancer cell proliferation (human colorectal carcinoma) (Sakai et al. 2006).
References
- Ackerley N, Brewster AG, Brown GR, Clarke DS, Foubister AJ, Griffin SJ, Hudson JA, Smithers MJ, Whittamore PR. 1995. A novel approach to dual-acting thromboxane receptor antagonist/synthase inhibitors based on the link of 1,3-dioxane-thromboxane receptor antagonists and -thromboxane synthase inhibitors. J Med Chem 38:1608–1628. [DOI] [PubMed] [Google Scholar]
- Casey MB, Zhang S, Jin L, Kajita S, Lloyd RV. 2004. Expression of cyclooxygenase-2 and thromboxane synthase in non-neoplastic and neoplastic thyroid lesions. Endocr Pathol 15:107–116. [DOI] [PubMed] [Google Scholar]
- Cathcart MC, Reynolds JV, O’Byrne KJ, Pidgeon GP. 2010. The role of prostacyclin synthase and thromboxane synthase signaling in the development and progression of cancer. Biochim Biophys Acta 1805:153–166. [DOI] [PubMed] [Google Scholar]
- Cimetiere B, Dubuffet T, Landras C, Descombes JJ, Simonet S, Verbeuren TJ, Lavielle G. 1998. New tetrahydronaphthalene derivatives as combined thromboxane receptor antagonists and thromboxane synthase inhibitors. Bioorg Med Chem Lett 8:1381–1386. [DOI] [PubMed] [Google Scholar]
- Davi G, Santilli F, Vazzana N. 2012. Thromboxane receptors antagonists and/or synthase inhibitors. Handbook of Experimental Pharmacology (210):261–286. [DOI] [PubMed] [Google Scholar]
- de Leval X, Benoit V, Delarge J, Julemont F, Masereel B, Pirotte B, Merville MP, David JL, Dogne JM. 2003. Pharmacological evaluation of the novel thromboxane modulator BM-567 (II/II). Effects of BM-567 on osteogenic sarcoma-cell-induced platelet aggregation. Prostagland Leukotrienes Essent Fatty Acids 68:55–59. [DOI] [PubMed] [Google Scholar]
- de Leval X, Dassesse T, Dogne JM, Waltregny D, Bellahcene A, Benoit V, Pirotte B, Castronovo V. 2006. Evaluation of original dual thromboxane A2 modulators as antiangiogenic agents. J Pharmacol Exp Ther 318:1057–1067. [DOI] [PubMed] [Google Scholar]
- Ding ZQ, Rowe J, Ng B, Sinosich MJ, Gallery ED. 2002. Modulation of prostacyclin and thromboxane secretion by cytotrophoblasts from normal and pre-eclamptic human pregnancies. Placenta 23:594–599. [DOI] [PubMed] [Google Scholar]
- Dogne JM, de Leval X, Delarge J, David JL, Masereel B. 2000. New trends in thromboxane and prostacyclin modulators. Curr Med Chem 7:609–628. [DOI] [PubMed] [Google Scholar]
- Dogne JM, de Leval X, Hanson J, Frederich M, Lambermont B, Ghuysen A, Casini A, Masereel B, Ruan KH, Pirotte B et al. 2004. New developments on thromboxane and prostacyclin modulators, part I: thromboxane modulators. Curr Med Chem 11:1223–1241. [DOI] [PubMed] [Google Scholar]
- Dogne JM, Hanson J, de Leval X, Pratico D, Pace-Asciak CR, Drion P, Pirotte B, Ruan KH. 2006. From the design to the clinical application of thromboxane modulators. Curr Pharmaceut Design 12:903–923. [DOI] [PubMed] [Google Scholar]
- Dogne JM, Rolin S, de Leval X, Benoit P, Neven P, Delarge J, Kolh P, Damas J, David JL, Masereel B. 2001. Pharmacology of the thromboxane receptor antagonist and thromboxane synthase inhibitor BM-531. Cardiovasc Drug Rev 19:87–96. [DOI] [PubMed] [Google Scholar]
- Dogne JM, Wouters J, Rolin S, Michaux C, Pochet L, Durant F, Delarge J, Masereel B. 2001. Design, synthesis and biological evaluation of a sulfonylcyanoguanidine as thromboxane A2 receptor antagonist and thromboxane synthase inhibitor. J Pharm Pharmacol 53(5):669–680. [DOI] [PubMed] [Google Scholar]
- Faull AW, Brewster AG, Brown GR, Smithers MJ, Jackson R. 1995. Dual-acting thromboxane receptor antagonist/synthase inhibitors: synthesis and biological properties of [2-substituted-4-(3-pyridyl)-1,3-dioxan-5-yl] alkenoic acids. J Med Chem 38:686–694. [DOI] [PubMed] [Google Scholar]
- Ford NF, Browne LJ,T, Gemenden C, Goldstein R, Gude C, Wasley JW 1985. Imidazo[1,5-a]pyridines: a new class of thromboxane A2 synthetase inhibitors. J Med Chem 28:164–170. [DOI] [PubMed] [Google Scholar]
- Ghuysen A, Dogne JM, Chiap P, Rolin S, Masereel B, Lambermont B, Kolh P, Tchana-Sato V, Hanson J, D’Orio V. 2005. Pharmacological profile and therapeutic potential of BM-573, a combined thromboxane receptor antagonist and synthase inhibitor. Cardiovasc Drug Rev 23:1–14. [DOI] [PubMed] [Google Scholar]
- Goerig M, Habenicht AJ. 1988. Effects of nicotine on eicosanoid synthesis of differentiating human promyelocytic leukemia cells. Klin Wochenschrift 66 Suppl 11:117–119. [PubMed] [Google Scholar]
- Goerig M, Ullrich V, Schettler G, Foltis C, Habenicht A. 1992. A new role for nicotine: Selective inhibition of thromboxane formation by direct interaction with thromboxane synthase in human promyelocytic leukaemia cells differentiating into macrophages. Clin Investigator 70:239–243. [DOI] [PubMed] [Google Scholar]
- Guengerich FP. 2015. Chapter 9, Human cytochrome P450 enzymes. In: Ortiz de Montellano PR, editor. Cytochrome P450: Structure, Mechanism, and Biochemistry 4th ed. New York: Springer; p. 523–785. [Google Scholar]
- Hanson J, Rolin S, Reynaud D, Qiao N, Kelley LP, Reid HM, Valentin F, Tippins J, Kinsella BT, Masereel B et al. 2005. In vitro and in vivo pharmacological characterization of BM-613 [N-n-pentyl-N´-[2-(4´-methylphenylamino)-5-nitrobenzenesulfonyl]urea], a novel dual thromboxane synthase inhibitor and thromboxane receptor antagonist. J Pharmacol Exp Ther 313:293–301. [DOI] [PubMed] [Google Scholar]
- Hartmann RW, Frotscher M. 1999. 1-Imidazolylcarbonyloxy-substituted tetrahydroquinolines and pyridines: synthesis and evaluation of P450 TxA2 inhibition. Archiv Pharm 332:358–362. [DOI] [PubMed] [Google Scholar]
- Hartmann RW, Frotscher M, Ledergerber D, Wachter GA, Grun GL, Sergejew TF. 1996. Synthesis and evaluation of azole-substituted tetrahydronaphthalenes as inhibitors of P450arom, P450 17, and P450 TxA2. Archiv Pharm 329:251–261. [DOI] [PubMed] [Google Scholar]
- Hecker M, Ullrich V. 1989. On the mechanism of prostacyclin and thromboxane A2 biosynthesis. J Biol Chem 264:141–150. [PubMed] [Google Scholar]
- Heinisch G, Holzer W, Kunz F, Langer T, Lukavsky P, Pechlaner C, Weissenberger H. 1996. On the bioisosteric potential of diazines: diazine analogues of the combined thromboxane A2 receptor antagonist and synthetase inhibitor Ridogrel. J Med Chem 39:4058–4064. [DOI] [PubMed] [Google Scholar]
- Hibi S, Okamoto Y, Tagami K, Numata H, Kobayashi N, Shinoda M, Kawahara T, Harada K, Miyamoto K, Yamatsu I. 1996. Structure-activity relationships of (E)-3-(1,4-benzoquinonyl)-2-[(3-pyridyl)-alkyl]-2-propenoic acid derivatives that inhibit both 5-lipoxygenase and thromboxane A2 synthetase. J Med Chem 39:3148–3157. [DOI] [PubMed] [Google Scholar]
- Hiraku S, Taniguchi K, Wakitani K, Omawari N, Kira H, Miyamoto T, Okegawa T, Kawasaki A, Ujiie A. 1986. Pharmacological studies on the TXA2 synthetase inhibitor (E)-3-[p-(1H-imidazol-1-ylmethyl)phenyl]-2-propenoic acid (OKY-046). Jpn J Pharmacol 41:393–401. [DOI] [PubMed] [Google Scholar]
- Howes LG, James MJ, Florin T, Walker C. 2007. Nv-52: A novel thromboxane synthase inhibitor for the treatment of inflammatory bowel disease. Expert Opin Invest Drugs 16:1255–1266. [DOI] [PubMed] [Google Scholar]
- Ihara H, Yokoyama C, Miyata A, Kosaka T, Nusing R, Ullrich V, Tanabe T. 1992. Induction of thromboxane synthase and prostaglandin endoperoxide synthase mRNAs in human erythroleukemia cells by phorbol ester. FEBS Lett 306:161–164. [DOI] [PubMed] [Google Scholar]
- Jacobs C, Frotscher M, Dannhardt G, Hartmann RW. 2000. 1-Imidazolyl(alkyl)-substituted di- and tetrahydroquinolines and analogues: Syntheses and evaluation of dual inhibitors of thromboxane A2 synthase and aromatase. J Med Chem 43:1841–1851. [DOI] [PubMed] [Google Scholar]
- Jarrar YB, Shin JG, Lee SJ. 2013. Expression of arachidonic acid-metabolizing cytochrome P450s in human megakaryocytic Dami cells. In Vitro Cell Dev Biol Anim 49:492–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajita S, Ruebel KH, Casey MB, Nakamura N, Lloyd RV. 2005. Role of COX-2, thromboxane A2 synthase, and prostaglandin I2 synthase in papillary thyroid carcinoma growth. Modern Pathol 18:221–227. [DOI] [PubMed] [Google Scholar]
- Kanda N, Kano R, Ishikawa T, Watanabe S. 2011. The antimycotic drugs itraconazole and terbinafine hydrochloride induce the production of human β-defensin-3 in human keratinocytes. Immunobiology 216:497–504. [DOI] [PubMed] [Google Scholar]
- Kanda N, Watanabe S. 2006. Suppressive effects of antimycotics on tumor necrosis factor-alpha-induced CCL27, CCL2, and CCL5 production in human keratinocytes. Biochem Pharmacol 72:463–473. [DOI] [PubMed] [Google Scholar]
- Kontogiorgis C, Hadjipavlou-Litina D. 2010. Thromboxane synthase inhibitors and thromboxane A2 receptor antagonists: a quantitative structure activity relationships (QSARs) analysis. Curr Med Chem 17:3162–3214. [DOI] [PubMed] [Google Scholar]
- Liu XJ. 2015. Design, synthesis and evaluation of antiplatelet aggregation inhibitory activities of the analogs of picotamide. Cardiovasc Hematol Agents Med Chem September 11 pii: CHAMC-EPUB-70310. [PubMed]
- Michaux C, Dogne JM, Rolin S, Masereel B, Wouters J, Durant F. 2003. A pharmacophore model for sulphonyl-urea (-cyanoguanidine) compounds with dual action, thromboxane receptor antagonists and thromboxane synthase inhibitors. Eur J Med Chem 38:703–710. [DOI] [PubMed] [Google Scholar]
- Michaux C, Rolin S, Dogne JM, Durant F, Masereel B, Delarge J, Wouters J. 2001. Structure determination and comparison of BM567, a sulfonylurea, with terbogrel, two compounds with dual action, thromboxane receptor antagonism and thromboxane synthase inhibition. Bioorg Med Chem Lett 11:1019–1022. [DOI] [PubMed] [Google Scholar]
- Moon CH, Jung YS, Kim MH, Lee SH, Baik EJ, Park SW. 2000. Mechanism for antiplatelet effect of onion: AA release inhibition, thromboxane A2 synthase inhibition and TXA2/PGH2 receptor blockade. Prostaglandins Leukotrienes Essent Fatty Acids 62:277–283. [DOI] [PubMed] [Google Scholar]
- Moon YJ, Zhang S, Brazeau DA, Morris ME. 2007. Effects of the flavonoid biochanin A on gene expression in primary human hepatocytes and human intestinal cells. Mol Nutr Food Res 51:317–323. [DOI] [PubMed] [Google Scholar]
- Moussa O, Riker JM, Klein J, Fraig M, Halushka PV, Watson DK. 2008. Inhibition of thromboxane synthase activity modulates bladder cancer cell responses to chemotherapeutic agents. Oncogene 27:55–62. [DOI] [PubMed] [Google Scholar]
- Muck S, Weber AA, Schror K. 1998. Effects of terbogrel on platelet function and prostaglandin endoperoxide transfer. Eur J Pharmacol 344:45–48. [DOI] [PubMed] [Google Scholar]
- Nie D, Che M, Zacharek A, Qiao Y, Li L, Li X, Lamberti M, Tang K, Cai Y, Guo Y et al. 2004. Differential expression of thromboxane synthase in prostate carcinoma: role in tumor cell motility. Am. J. Pathol 164:429–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oketani K, Nagakura N, Harada K, Inoue T. 2001. In vitro effects of E3040, a dual inhibitor of 5-lipoxygenase and thromboxane A2 synthetase, on eicosanoid production. Eur J Pharmacol 422:209–216. [DOI] [PubMed] [Google Scholar]
- Onguru O, Scheithauer BW, Kovacs K, Vidal S, Jin L, Zhang S, Ruebel KH, Lloyd RV. 2004. Analysis of Cox-2 and thromboxane synthase expression in pituitary adenomas and carcinomas. Endocr Pathol 15:17–27. [DOI] [PubMed] [Google Scholar]
- Pan ST, Xue D, Li ZL, Zhou ZW, He ZX, Yang Y, Yang T, Qiu JX, Zhou SF. 2016. Computational identification of the paralogs and orthologs of human cytochrome P450 superfamily and the implication in drug discovery. Int J Mol Sci June 28;17(7). pii: ijms17071020. doi: 10.3390/ijms17071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolin S, Dogne JM, Michaux C, Delarge J, Masereel B. 2001. Activity of a novel dual thromboxane A(2)receptor antagonist and thromboxane synthase inhibitor (BM-573) on platelet function and isolated smooth muscles. Prostaglandins Leukotrienes Essent Fatty Acids 65:67–72. [DOI] [PubMed] [Google Scholar]
- Rolin S, Dogne JM, Vastersaegher C, Hanson J, Masereel B. 2004. Pharmacological evaluation of both enantiomers of (R,S)-BM-591 as thromboxane A2 receptor antagonists and thromboxane synthase inhibitors. Prostaglandins Other Lipid Mediators 74:75–86. [DOI] [PubMed] [Google Scholar]
- Rowe J, Campbell S, Gallery ED. 2000. Effects of hypoxia on regulation of prostanoid production in decidual endothelial cells in normal and preeclamptic pregnancy. J Soc Gynecol Invest 7:118–124. [PubMed] [Google Scholar]
- Saareks V, Mucha I, Sievi E, Vapaatalo H, Riutta A. 1998. Nicotine stereoisomers and cotinine stimulate prostaglandin E2 but inhibit thromboxane B2 and leukotriene E4 synthesis in whole blood. Eur J Pharmacol 353:87–92. [DOI] [PubMed] [Google Scholar]
- Sakai H, Suzuki T, Takahashi Y, Ukai M, Tauchi K, Fujii T, Horikawa N, Minamimura T, Tabuchi Y, Morii M et al. 2006. Upregulation of thromboxane synthase in human colorectal carcinoma and the cancer cell proliferation by thromboxane A2. FEBS Lett 580(14):3368–3374. [DOI] [PubMed] [Google Scholar]
- Schuster I, Bernhardt R. 2007. Inhibition of cytochromes P450: Existing and new promising therapeutic targets. Drug Metab Rev 39:481–499. [DOI] [PubMed] [Google Scholar]
- Sekhar PN, Reddy LA, De Maeyer M, Kumar KP, Srinivasulu YS, Sunitha MS, Sphoorthi IS, Jayasree G, Rao AM, Kothekar VS et al. 2009. Genome wide analysis and comparative docking studies of new diaryl furan derivatives against human cyclooxygenase-2, lipoxygenase, thromboxane synthase and prostacyclin synthase enzymes involved in inflammatory pathway. J Mol Graph Model 28:313–329. [DOI] [PubMed] [Google Scholar]
- Soyka R, Guth BD, Weisenberger HM, Luger P, Muller TH. 1999. Guanidine derivatives as combined thromboxane A2 receptor antagonists and synthase inhibitors. J Med Chem 42:1235–1249. [DOI] [PubMed] [Google Scholar]
- Soyka R, Heckel A, Nickl J, Eisert W, Muller TH, Weisenberger H. 1994. 6,6-Disubstituted hex-5-enoic acid derivatives as combined thromboxane A2 receptor antagonists and synthetase inhibitors. J Med Chem 37:26–39. [DOI] [PubMed] [Google Scholar]
- Steinhilber D, Jaschonek K, Knospe J, Morof O, Roth HJ. 1990. Effects of novel antifungal azole derivatives on the 5-lipoxygenase and cyclooxygenase pathway. Arzneimittel-Forschung 40:1260–1263. [PubMed] [Google Scholar]
- Takeuchi K, Kohn TJ, True TA, Mais DE, Wikel JH, Utterback BG, Wyss VL, Jakubowski JA. 1998. Development of dual-acting agents for thromboxane receptor antagonism and thromboxane synthase inhibition. 3. Synthesis and biological activities of oxazolecarboxamide-substituted ω-phenyl-ω-(3-pyridyl)alkenoic acid derivatives and related compounds. J Med Chem 41:5362–5374. [DOI] [PubMed] [Google Scholar]
- Trochtenberg DS, Lefferts PL, King GA, Hwang YS, Christman BW, Snapper JR. 1992. Effects of thromboxane synthase and cyclooxygenase inhibition on PAF-induced changes in lung function and arachidonic acid metabolism. Prostaglandins 44:555–577. [DOI] [PubMed] [Google Scholar]
- Tubaro E, Belogi L, Mezzadri CM. 1996. Antiplatelet effect of a new inhibitor of thromboxane synthase and thromboxane A2 receptors. Arzneimittel-Forschung 46:35–41. [PubMed] [Google Scholar]
- Uematsu T, Kosuge K, Umemura K, Nakano M, Terakawa M, Nakashima M. 1996. Pharmacokinetic and pharmacodynamic properties of FK070 (KDI-792), a novel thromboxane receptor antagonist/thromboxane synthetase inhibitor, after single and multiple oral administrations to healthy volunteers. J Pharm Pharmacol 48:380–385. [DOI] [PubMed] [Google Scholar]
- Ullrich V, Hecker M. 1990. A concept for the mechanism of prostacyclin and thromboxane A2 biosynthesis. Adv Prostaglandin Thromboxane Leukotriene Res 20:95–101. [PubMed] [Google Scholar]
- Wachter GA, Hartmann RW, Sergejew T, Grun GL, Ledergerber D. 1996. Tetrahydronaphthalenes: Influence of heterocyclic substituents on inhibition of steroid enzymes P450arom and P45017. J Med Chem 39:834–841. [DOI] [PubMed] [Google Scholar]
- Wang LH, Matijevic-Aleksic N, Hsu PY, Ruan KH, Wu KK, Kulmacz RJ. 1996. Identification of thromboxane A2 synthase active site residues by molecular modeling-guided site-directed mutagenesis. J Biol Chem 271:19970–19975. [DOI] [PubMed] [Google Scholar]
- Yagi A, Kabash A, Mizuno K, Moustafa SM, Khalifa TI, Tsuji H. 2003. Radical scavenging glycoprotein inhibiting cyclooxygenase-2 and thromboxane A2 synthase from Aloe vera gel. Planta Med 69:269–271. [DOI] [PubMed] [Google Scholar]
- Yeh HC, Tsai AL, Wang LH. 2007. Reaction mechanisms of 15-hydroperoxyeicosatetraenoic acid catalyzed by human prostacyclin and thromboxane synthases. Arch Biochem Biophys 461:159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoyama C, Miyata A, Ihara H, Ullrich V, Tanabe T. 1991. Molecular cloning of human platelet thromboxane A synthase. Biochem Biophys Res Commun 178:1479–1484. [DOI] [PubMed] [Google Scholar]
- Yu SM, Wu TS, Teng CM. 1994. Pharmacological characterization of cinnamophilin, a novel dual inhibitor of thromboxane synthase and thromboxane A2 receptor. Brit J Pharmacol 111:906–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
P450 8A1
Prostacyclin synthase activity (P450 8A1) was first found in aorta, and the enzyme was later purified as a 50-kDa hemoprotein with spectroscopic characteristics of a cytochrome P450 (Graf et al. 1983). A P450 8A1 cDNA was cloned from aorta endothelial cells and heterologously expressed (Miyata et al. 1994; Wada et al. 2004). P450 8A1 mRNA is found expressed in many mammalian tissues including ovary, heart, skeletal muscle, lung, prostate (Miyata et al. 1994), umbilical cord, brain, and neurons (Guengerich 2015). Regulation involves a number of factors, including post-translational redox control. Peroxynitrite yields nitration of the Tyr-430 residue and thus steric hindrance to the active site (Bachschmid et al. 2005).
X-ray crystal structures of unliganded P450 8A1 and the enzyme containing a substrate analog (U51605) and inhibitor (minoxidil) have been published (Chiang et al. 2006; Li et al. 2008) (Protein Data Bank structures 3B6H, 3B98, 2IAG, 3B99). The most generally accepted reaction mechanism, initially proposed by Hecker and Ullrich (1989), involves the key step of an O–O homolytic scission of PGH2 to generate an alkoxyl radical from the substrate intermediate and a [FeIV–O–R] species from the enzyme.
Because of the physiology associated with prostacyclin, there is limited practical interest in synthesizing inhibitors of this enzyme. The field of P450 8A1 inhibition is dominated by efforts to avoid use of drugs that might inhibit this enzyme as a side effect. Many common P450 ligands, even imidazole or pyridine derivatives, do not bind P450 8A1. Only a few nitrogen-containing compounds bind P450 8A1 with notable affinity. Thus, the active site of P450 8A1 appears to have a very limited space and is accessible only for nitrogen compounds with side chains rather perpendicular to the Fe–N coordination axis. This hypothesis, which suggests a rigid active site, has not been tested with a wider range of heme ligands.
Prostacyclin synthase overexpression has been shown to be chemopreventive in a murine cancer model (Cathcart et al. 2010).
References
- Bachschmid M, Schildknecht S, Ullrich V. 2005. Redox regulation of vascular prostanoid synthesis by the nitric oxide-superoxide system. Biochem Biophys Res Commun 338:536–542. [DOI] [PubMed] [Google Scholar]
- Camacho M, Rodriguez C, Guadall A, Alcolea S, Orriols M, Escudero JR, Martinez-Gonzalez J, Vila L. 2011. Hypoxia upregulates PGI-synthase and increases PGI2 release in human vascular cells exposed to inflammatory stimuli. J Lipid Res 52:720–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camacho M, Rodriguez C, Salazar J, Martinez-Gonzalez J, Ribalta J, Escudero JR, Masana L, Vila L. 2008. Retinoic acid induces PGI synthase expression in human endothelial cells. J Lipid Res 49:1707–1714. [DOI] [PubMed] [Google Scholar]
- Cathcart MC, Reynolds JV, O’Byrne KJ, Pidgeon GP. 2010. The role of prostacyclin synthase and thromboxane synthase signaling in the development and progression of cancer. Biochim Biophys Acta 1805:153–166. [DOI] [PubMed] [Google Scholar]
- Chao WC, Lu JF, Wang JS, Yang HC, Chen HH, Lan YK, Yu YC, Chou PT, Wang LH. 2011. Probing the interaction between prostacyclin synthase and prostaglandin H2 analogues or inhibitors via a combination of resonance Raman spectroscopy and molecular dynamics simulation approaches. J Am Chem Soc 133:18870–18879. [DOI] [PubMed] [Google Scholar]
- Chiang CW, Yeh HC, Wang LH, Chan NL. 2006. Crystal structure of the human prostacyclin synthase. J Mol Biol 364:266–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding ZQ, Rowe J, Ng B, Sinosich MJ, Gallery ED. 2002. Modulation of prostacyclin and thromboxane secretion by cytotrophoblasts from normal and pre-eclamptic human pregnancies. Placenta 23:594–599. [DOI] [PubMed] [Google Scholar]
- Graf H, Ruf HH, Ullrich V. 1983. Prostacyclin synthase, a cytochrome P450 enzyme. Angew Chem Int Ed 22:487–488. [Google Scholar]
- Griffoni C, Spisni E, Strillacci A, Toni M, Bachschmid MM, Tomasi V. 2007. Selective inhibition of prostacyclin synthase activity by rofecoxib. J Cell Mol Med 11:327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guengerich FP. 2015. Chapter 9, Human cytochrome P450 enzymes. In: Ortiz de Montellano PR, editor. Cytochrome P450: Structure, Mechanism, and Biochemistry 4th ed. New York: Springer; p. 523–785. [Google Scholar]
- Hecker M, Ullrich V. 1989. On the mechanism of prostacyclin and thromboxane A2 biosynthesis. J Biol Chem 264:141–150. [PubMed] [Google Scholar]
- Korita D, Itoh H, Sagawa N, Yura S, Yoshida M, Kakui K, Takemura M, Fujii S. 2004. 17β-Estradiol up-regulates prostacyclin production in cultured human uterine myometrial cells via augmentation of both cyclooxygenase-1 and prostacyclin synthase expression. J Soc Gynecol Invest 11:457–464. [DOI] [PubMed] [Google Scholar]
- Korita D, Sagawa N, Itoh H, Yura S, Yoshida M, Kakui K, Takemura M, Yokoyama C, Tanabe T, Fujii S. 2002. Cyclic mechanical stretch augments prostacyclin production in cultured human uterine myometrial cells from pregnant women: possible involvement of up-regulation of prostacyclin synthase expression. J Clin Endocrinol Metab 87:5209–5219. [DOI] [PubMed] [Google Scholar]
- Li YC, Chiang CW, Yeh HC, Hsu PY, Whitby FG, Wang LH, Chan NL. 2008. Structures of prostacyclin synthase and its complexes with substrate analog and inhibitor reveal a ligand-specific heme conformation change. J Biol Chem 283:2917–2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao JT, Smoake J, Park HK, Lu QY, Xue B. 2016. Grape seed procyanidin extract mediates antineoplastic effects against lung cancer via modulations of prostacyclin and 15-HETE eicosanoid pathways. Cancer Prev Res (Philadelphia) 9:925–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata A, Hara S, Yokoyama C, Inoue H, Ullrich V, Tanabe T. 1994. Molecular cloning and expression of human prostacyclin synthase. Biochem Biophys Res Commun 200:1728–1734. [DOI] [PubMed] [Google Scholar]
- Nakayama T 2010. Genetic polymorphisms of prostacyclin synthase gene and cardiovascular disease. Int Angiol 29(2 Suppl):33–42. [PubMed] [Google Scholar]
- Okahara K, Sun B, Kambayashi J. 1998. Upregulation of prostacyclin synthesis-related gene expression by shear stress in vascular endothelial cells. Arteriosclerosis Thrombosis Vascul Biol 18:1922–1926. [DOI] [PubMed] [Google Scholar]
- Pan ST, Xue D, Li ZL, Zhou ZW, He ZX, Yang Y, Yang T, Qiu JX, Zhou SF. 2016. Computational identification of the paralogs and orthologs of human cytochrome P450 superfamily and the implication in drug discovery. Int J Mol Sci June 28;17(7). pii: ijms17071020. doi: 10.3390/ijms17071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharanek A, Burban A, Humbert L, Bachour-El Azzi P, Felix-Gomes N, Rainteau D, Guillouzo A. 2015. Cellular accumulation and toxic effects of bile acids in cyclosporine A-treated HepaRG hepatocytes. Toxicol Sci 147:573–587. [DOI] [PubMed] [Google Scholar]
- Skogastierna C, Björkhem-Bergman L, Bergman P, Eliasson E, Rane A, Ekstrom L. 2013. Influence of simvastatin on the thromboxane and prostacyclin pathways, in vitro and in vivo. J Cardiovasc Pharmacol 61:1–7. [DOI] [PubMed] [Google Scholar]
- Tan X, Poulose EM, Raveendran VV, Zhu BT, Stechschulte DJ, Dileepan KN. 2011. Regulation of the expression of cyclooxygenases and production of prostaglandin I2 and E2 in human coronary artery endothelial cells by curcumin. J Physiol Pharmacol 62:21–28. [PMC free article] [PubMed] [Google Scholar]
- Ullrich V, Hecker M. 1990. A concept for the mechanism of prostacyclin and thromboxane A2 biosynthesis. Adv Prostaglandin Thromboxane Leukotriene Res 20:95–101. [PubMed] [Google Scholar]
- Wada M, Yokoyama C, Hatae T, Shimonishi M, Nakamura M, Imai Y, Ullrich V, Tanabe T. 2004. Purification and characterization of recombinant human prostacyclin synthase. J Biochem (Tokyo) 135:455–463. [DOI] [PubMed] [Google Scholar]
- Wang J, Ikeda R, Che XF, Ooyama A, Yamamoto M, Furukawa T, Hasui K, Zheng CL, Tajitsu Y, Oka T et al. 2013. VEGF expression is augmented by hypoxiainduced PGIS in human fibroblasts. Int J Oncol 43:746–754. [DOI] [PubMed] [Google Scholar]
- Yeh HC, Hsu PY, Wang JS, Tsai AL, Wang LH. 2005. Characterization of heme environment and mechanism of peroxide bond cleavage in human prostacyclin synthase. Biochim Biophys Acta 1738:121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh HC, Tsai AL, Wang LH. 2007. Reaction mechanisms of 15-hydroperoxyeicosatetraenoic acid catalyzed by human prostacyclin and thromboxane synthases. Arch Biochem Biophys 461(2):159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Steroid hormone biosynthesis
The sole P450 involved in cholesterol formation, P450 51A1, is the lanosterol 14α-demethylase (Fig. 4A and Fig. 17, vide infra). There are three biologically important pathways from cholesterol catalyzed by P450 enzymes: (1) biosynthesis of steroid hormones, (2) formation of bile acids, and (3) vitamin D3 metabolism. All mammalian steroids, bile acids, and active forms of vitamin D are formed from cholesterol (Figs. 4A and 4B). Detailed reactions leading to the formation of bile acids and catalyzed by P450 enzymes are presented in Figs. 14A and 15, reactions related to biosynthesis and metabolism of vitamin D3 in Figs. 19 and 20, and thebiosynthesis of steroid hormomes in Figs. 7 to 13 and 14B. Clinical experience and studies with transgenic mice have shown the importance of all of these P450s (Nebert and Russell 2002; Auchus and Miller 2015; Auchus 2017). The only other major enzymes involved in steroid hormones biosynthesis are dehydrogenases (Fig. 4B) (plus conjugating enzymes that form methyl ethers, sulfate esters, and glucuronides).
Fig. 4A.
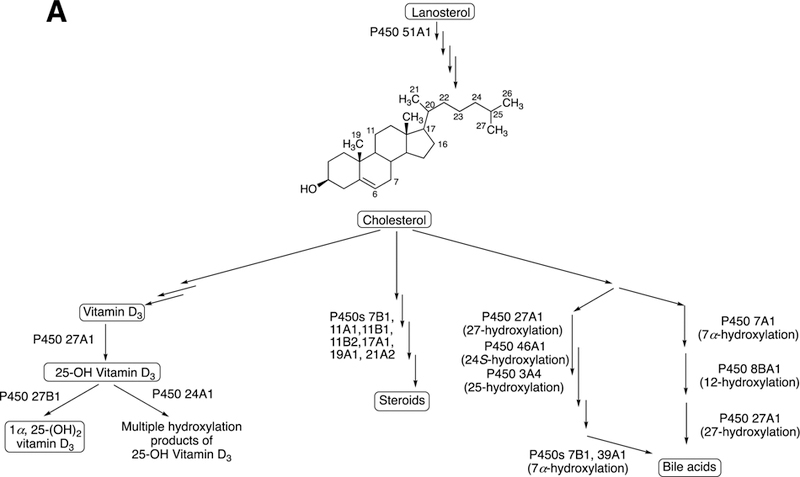
Formation of bile acids, steroids, and active form of vitamin D from cholesterol. All mammalian steroids, bile acids, and active form of vitamin D are formed from cholesterol.
Fig. 17.
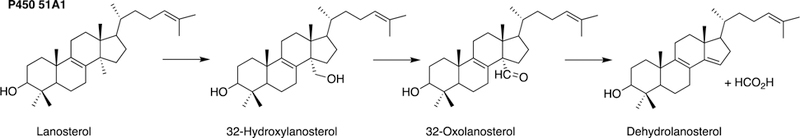
Three-step 14α-demethylation of lanosterol by P450 51A1.
Fig. 4B.
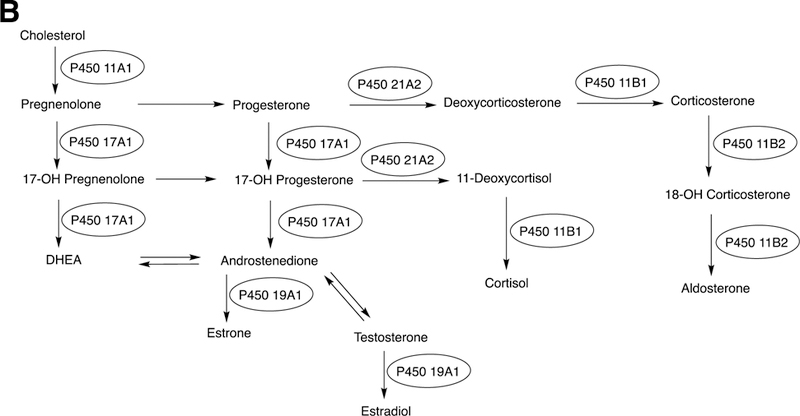
P450 enzymes involved in steroid biosythesis. P450 51A1, the lanosterol 14α-demethylase, is involved in the biosynthesis of cholesterol but is not shown. See Figs. 4A and 17.
Fig. 13.
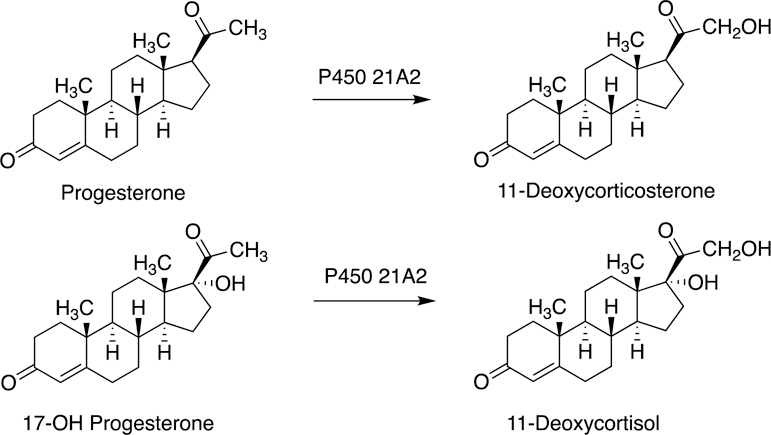
Hydroxylations catalyzed by P450 21A2.
Fig. 15.
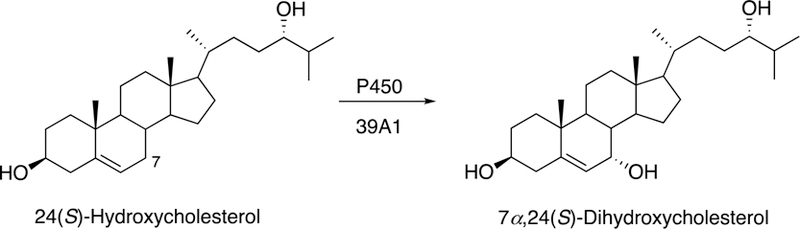
7α-Hydroxylation of 24(S)-hydroxycholesterol by P450 39A1.
Fig. 19.
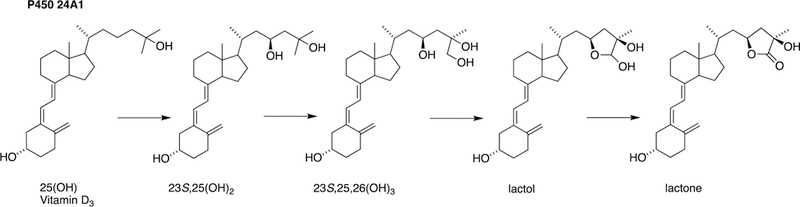
Oxidation of vitamin D3 catalyzed by P450 24A1.
Fig. 20.
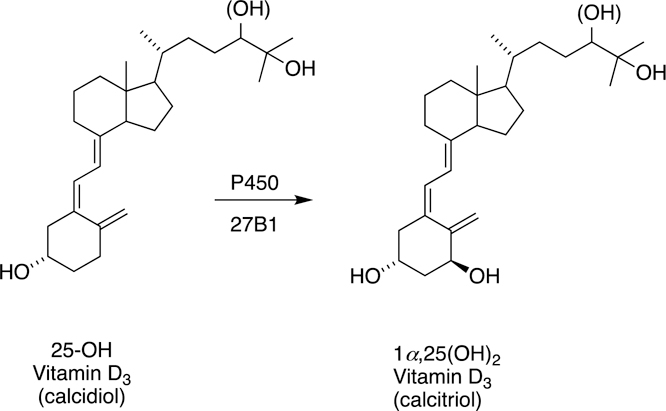
1α-Hydroxylation of 25-hydroxy vitamin D3 by P450 27B1.
Fig. 7.
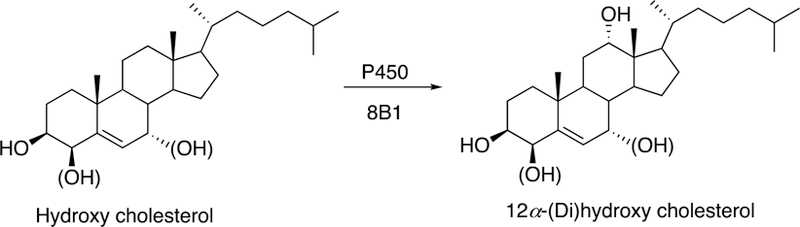
12α-Hydroxylation of 4β- or 7α-hydroxycholesterol by P450 8B1.
Fig. 14.
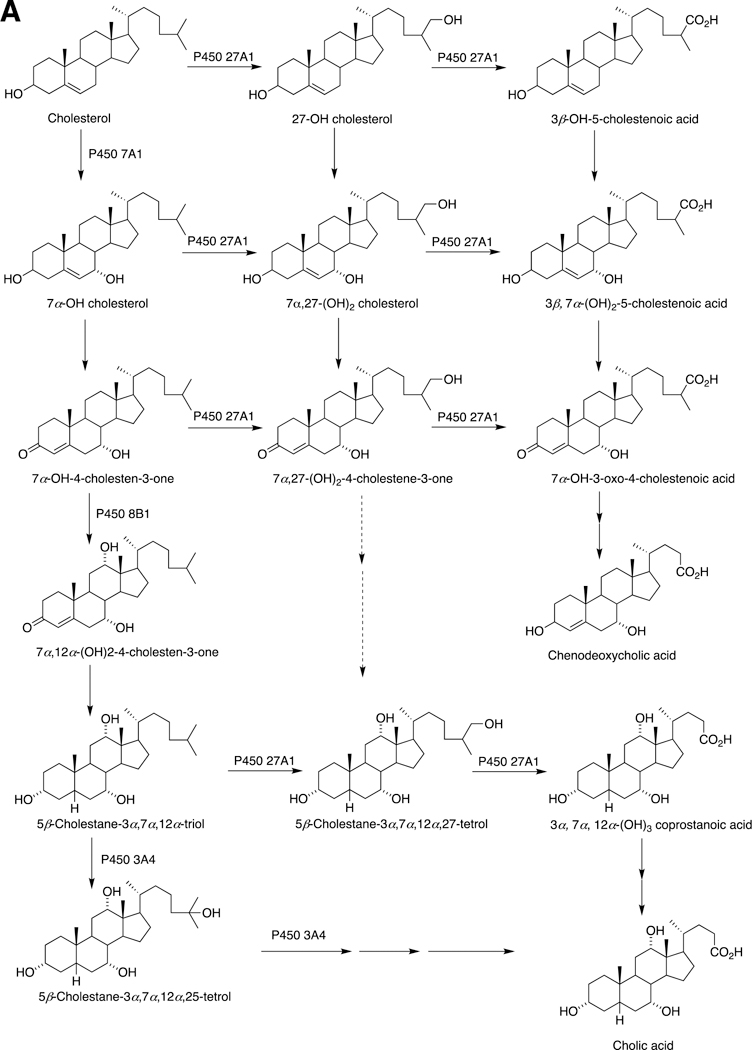
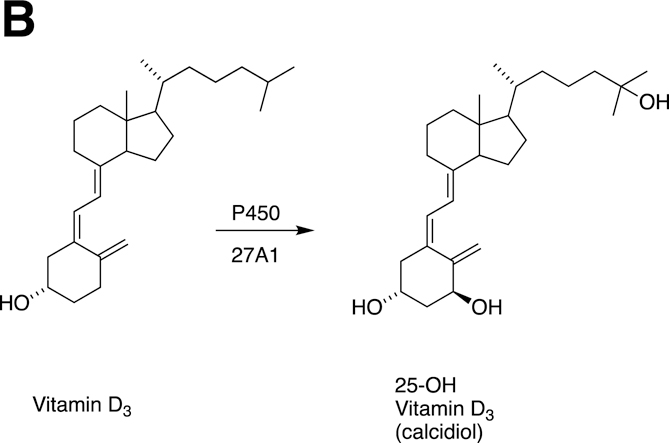
A, Sterol oxidations catalyzed by P450 27A1 in the synthesis of bile acids. B, 25-Hydroxylation of vitamin D3 by P450 27A1.
References
- Auchus RJ. 2017. Steroid 17-hydroxylase and 17,20-lyase deficiencies, genetic and pharmacologic. J Steroid Biochem Mol Biol 165:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auchus RJ, Miller WL. 2015. Chapter 12, P450 enzymes in steroid processing. In: Ortiz de Montellano PR, editor. Cytochrome P450: Structure, Mechanism, and Biochemistry 4th ed. New York: Springer; p. 851–879. [Google Scholar]
- Nebert DW, Russell DW. 2002. Clinical importance of the cytochromes P450. Lancet 360:1155–1162. [DOI] [PubMed] [Google Scholar]
P450 7A1
Primary bile acids are formed from cholesterol in the liver. The first and rate-limiting reaction in the pathway is 7α-hydroxylation by the cholesterol 7α-hydroxylase P450 7A1, an enzyme relatively selective for cholesterol and cholestanol (Ogishima et al. 1987; Jelinek et al. 1990). The conversion of cholesterol to bile acids in the liver (and its subsequent fecal excretion) represents a major route of elimination of cholesterol from the body. Bile acids can be synthesized via a ‘classical pathway’ (P450 7A1, Figs. 4A and 5) or an ‘alternate pathway’ utilizing a different sequence of initial steps (Fig. 4A, P450 39A1 or 7B1, vide infra). Compared with the alternative pathway, the classical pathway is more ‘flexible’.
Fig. 5.
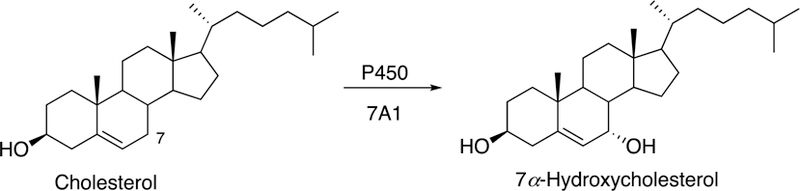
7α-Hydroxylation of cholesterol by P450 7A1.
Human P450 7A1, expressed in Escherichia coli, is active toward the substrates cholesterol, 20(S)-hydroxycholesterol, 25-hydroxycholesterol, 27-hydroxycholesterol, zymostenol, lathosterol, desmosterol, and 7-dehydrocholesterol (Li YC and Chiang 1991; Shinkyo et al. 2011; Acimovic et al. 2016).
The reaction with 7-dehydrocholesterol yields 7-ketocholesterol, an important oxysterol with detrimental properties (Shinkyo et al. 2011). The 7-keto product is formed without an epoxide intermediate, although some 7,8-epoxide is also formed. The reaction also occurs in vivo (in humans) (Björkhem et al. 2014) and may be of relevance in Smith-Lemli-Opitz syndrome, in which 7-dehydrocholesterol is elevated due to a genetic deficiency in the reductase.
The 7α-hydroxylation of cholesterol by human P450 7A1 is one of the faster reactions known with a mammalian P450, with kcat > 3 s−1 and specificity constant (kcat/Km) of 2.4 × 106 M−1 s−1 (Shinkyo et al. 2011). The kcat/Km for the oxidation of 7-dehydrocholesterol is considerably lower (2 × 104 M−1 s−1).
X-ray crystal structures of human P450 7A1 are available for the unliganded protein and with cholest-4-en-3-one and 7-ketocholesterol (Protein Data Bank (PDB) 3DZX, 3SN5, 3V8D, http://www.rscb.org) (Tempel et al. 2014).
The CYP7A1 gene is highly regulated, as might be expected for an enzyme with a central role in the clearance of an important sterol (Guengerich 2015, Zhang, Zhao, et al. 2017, Zhang, Jackson, et al. 2017, Zhang, Wang, et al. 2018, Lee et al. 2018). Transcription of the CYP7A1 gene is stimulated by dietary cholesterol in rodents (Horton et al. 1995). In mammals, however, dietary cholesterol does not stimulate hepatic P450 7A1 expression, resulting in accumulation of peripheral cholesterol and atherosclerosis (Chiang et al. 2001, Goodwin et al. 2003). The regulatory element FXR is responsive to bile acids and inhibits CYP7A1 gene transcription through the activation of SHP and inhibition of HNF4α transactivation. An A to C transversion mutation 278 bp upstream (of the CYP7A1 promoter) has been associated with variations in serum lipid levels in populations with hypertriglyceridemia, combined hyperlipidemia, familial dysbetalipoproteinemia, and familial hypercholesterolemia (Hofman et al. 2004).
Oxysterols are important degradation products of cholesterol and are intermediates in the biosynthesis of steroid hormones and bile acids. These compounds have biology of their own and a broad spectrum of effects, including modulation of the activity of enzymes involved in cholesterol homeostasis (Waterman et al. 1986; Axelson and Sjövall 1990; Björkhem 1992; Janowski et al. 1996; Lala et al. 1997; Lehmann et al. 1997; Janowski et al. 1999; Russell 1999; Schroepfer 2000).
References
- Acimovic J, Goyal S, Kosir R, Golicnik M, Perse M, Belic A, Urlep Z, Guengerich FP, Rozman D. 2016. Cytochrome P450 metabolism of the post-lanosterol intermediates explains enigmas of cholesterol synthesis. Sci Rep 6:28462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An S, Jang YS, Park JS, Kwon BM, Paik YK, Jeong TS. 2008. Inhibition of acyl-coenzyme A:cholesterol acyltransferase stimulates cholesterol efflux from macrophages and stimulates farnesoid X receptor in hepatocytes. Exp Mol Med 40:407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreou ER, Prokipcak RD. 1998. Analysis of human CYP7A1 mRNA decay in HepG2 cells by reverse transcription-polymerase chain reaction. Arch Biochem Biophys 357:137–146. [DOI] [PubMed] [Google Scholar]
- Axelson M, Sjövall J. 1990. Potential bile acid precursors in plasma–possible indicators of biosynthetic pathways to cholic and chenodeoxycholic acids in man. J Steroid Biochem 36:631–640. [DOI] [PubMed] [Google Scholar]
- Björkhem I 1992. Mechanism of degradation of the steroid side chain in the formation of bile acids. J Lipid Res 33:455–471. [PubMed] [Google Scholar]
- Björkhem I, Diczfalusy U, Lovgren-Sandblom A, Starck L, Jonsson M, Tallman K, Schirmer H, Ousager LB, Crick PJ, Wang Y et al. 2014. On the formation of 7-ketocholesterol from 7-dehydrocholesterol in patients with CTX and SLO. J Lipid Res 55:1165–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charoenteeraboon J, Nithipatikom K, Campbell WB, Piyachaturawat P, Wilairat P, Rongnoparut P. 2005. Induction of human cholesterol 7α-hydroxylase in HepG2 cells by 2,4,6-trihydroxyacetophenone. Eur J Pharmacol 515:43–46. [DOI] [PubMed] [Google Scholar]
- Chen W, Owsley E, Yang Y, Stroup D, Chiang JY. 2001. Nuclear receptor-mediated repression of human cholesterol 7α-hyoxylase gene transcription by bile acids. J Lipid Res 42:1402–1412. [PubMed] [Google Scholar]
- Chiang JY, Kimmel R, Weinberger C, Stroup D. 2000. Farnesoid X receptor responds to bile acids and represses cholesterol 7α-hydroxylase gene (CYP7A1) transcription. J Biol Chem 275:10918–10924. [DOI] [PubMed] [Google Scholar]
- Davalos A, Fernandez-Hernando C, Cerrato F, Martinez-Botas J, Gomez-Coronado D, Gomez-Cordoves C, Lasuncion MA. 2006. Red grape juice polyphenols alter cholesterol homeostasis and increase LDL-receptor activity in human cells in vitro. J Nutr 136:1766–1773. [DOI] [PubMed] [Google Scholar]
- Drover VA, Agellon LB. 2004. Regulation of the human cholesterol 7α-hydroxylase gene (CYP7A1) by thyroid hormone in transgenic mice. Endocrinology 145:574–581. [DOI] [PubMed] [Google Scholar]
- Drover VA, Wong NC, Agellon LB. 2002. A distinct thyroid hormone response element mediates repression of the human cholesterol 7α-hydroxylase (CYP7A1) gene promoter. Mol Endocrinol 16:14–23. [DOI] [PubMed] [Google Scholar]
- Ellis E, Axelson M, Abrahamsson A, Eggertsen G, Thorne A, Nowak G, Ericzon BG, Björkhem I, Einarsson C. 2003. Feedback regulation of bile acid synthesis in primary human hepatocytes: evidence that CDCA is the strongest inhibitor. Hepatology (Baltimore) 38:930–938. [DOI] [PubMed] [Google Scholar]
- Ellis EC. 2006. Suppression of bile acid synthesis by thyroid hormone in primary human hepatocytes. World J Gastroenterol 12:4640–4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan P, Zhang B, Kuroki S, Saku K. 2004. Pitavastatin, a potent hydroxymethylglutaryl coenzyme a reductase inhibitor, increases cholesterol 7α-hydroxylase gene expression in HepG2 cells. Circul J 68:1061–1066. [DOI] [PubMed] [Google Scholar]
- Gbaguidi GF, Agellon LB. 2004. The inhibition of the human cholesterol 7α-hydroxylase gene (CYP7A1) promoter by fibrates in cultured cells is mediated via the liver X receptor α and peroxisome proliferator-activated receptor α heterodimer. Nucl Acids Res 32:1113–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbod-Giannone MC, Del Castillo-Olivares A, Janciauskiene S, Gil G, Hylemon PB. 2002. Suppression of cholesterol 7alpha-hydroxylase transcription and bile acid synthesis by an α1-antitrypsin peptide via interaction with α1-fetoprotein transcription factor. J Biol Chem 277:42973–42980. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Cruz A, Ferrin G, Lopez-Cillero P, Briceno J, Gomez MA, Rufian S, Padillo J, De la Mata M, Marin JJ et al. 2011. Cytoprotective properties of rifampicin are related to the regulation of detoxification system and bile acid transporter expression during hepatocellular injury induced by hydrophobic bile acids. J Hepato-Biliary-Pancreatic Sci 18:740–750. [DOI] [PubMed] [Google Scholar]
- Gonzalez R, Cruz A, Ferrin G, Lopez-Cillero P, Fernandez-Rodriguez R, Briceno J, Gomez MA, Rufian S, Mata Mde L, Martinez-Ruiz A et al. 2011. Nitric oxide mimics transcriptional and post-translational regulation during α-tocopherol cytoprotection against glycochenodeoxycholate-induced cell death in hepatocytes. J Hepatol 55:133–144. [DOI] [PubMed] [Google Scholar]
- Guo J, Gao Y, Cao X, Zhang J, Chen W. 2017. Cholesterol-lowing effect of taurine in HepG2 cell. Lipids Health Dis 16:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guengerich FP. 2015. Chapter 9, Human cytochrome P450 enzymes. In: Ortiz de Montellano PR, editor. Cytochrome P450: Structure, Mechanism, and Biochemistry 4th ed. New York: Springer; p. 523–785. [Google Scholar]
- Hofman MK, Groenendijk M, Verkuijlen PJ, Jonkers IJ, Mohrschladt MF, Smelt AH, Princen HM. 2004. Modulating effect of the A-278C promoter polymorphism in the cholesterol 7α-hydroxylase gene on serum lipid levels in normolipidaemic and hypertriglyceridaemic individuals. Eur J Human Genet 12:935–941. [DOI] [PubMed] [Google Scholar]
- Honda A, Ikegami T, Nakamuta M, Miyazaki T, Iwamoto J, Hirayama T, Saito Y, Takikawa H, Imawari M, Matsuzaki Y. 2013. Anticholestatic effects of bezafibrate in patients with primary biliary cirrhosis treated with ursodeoxycholic acid. Hepatology (Baltimore) 57:1931–1941. [DOI] [PubMed] [Google Scholar]
- Jahan A, Chiang JY. 2005. Cytokine regulation of human sterol 12α-hydroxylase (CYP8B1) gene. Am J Physiol Gastrointest Liver Physiol 288:G685–695. [DOI] [PubMed] [Google Scholar]
- Janowski BA, Grogan MJ, Jones SA, Wisely GB, Kliewer SA, Corey EJ, Mangelsdorf DJ. 1999. Structural requirements of ligands for the oxysterol liver X receptors LXRα and LXRα. Proc Natl Acad Sci USA 96:266–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janowski BA, Willy PJ, Devi TR, Falck JR, Mangelsdorf DJ. 1996. An oxysterol signalling pathway mediated by the nuclear receptor LXRα. Nature 383:728–731. [DOI] [PubMed] [Google Scholar]
- Jelinek DF, Andersson S, Slaughter CA, Russell DW. 1990. Cloning and regulation of cholesterol 7α-hydroxylase, the rate-limiting enzyme in bile acid biosynthesis. J Biol Chem 265:8190–8197. [PMC free article] [PubMed] [Google Scholar]
- Jonkers IJ, Smelt AH, Princen HM, Kuipers F, Romijn JA, Boverhof R, Masclee AA, Stellaard F. 2006. Fish oil increases bile acid synthesis in male patients with hypertriglyceridemia. J Nutr 136:987–991. [DOI] [PubMed] [Google Scholar]
- Lala DS, Syka PM, Lazarchik SB, Mangelsdorf DJ, Parker KL, Heyman RA. 1997. Activation of the orphan nuclear receptor steroidogenic factor 1 by oxysterols. Proc Natl Acad Sci USA 94:4895–4900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammel Lindemann JA, Angajala A, Engler DA, Webb P, Ayers SD. 2014. Thyroid hormone induction of human cholesterol 7α-hydroxylase (Cyp7a1) in vitro. Mol Cell Endocrinol 388:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann JM, Kliewer SA, Moore LB, Smith-Oliver TA, Oliver BB, Su JL, Sundseth SS, Winegar DA, Blanchard DE, Spencer TA et al. 1997. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J Biol Chem 272:3137–3140. [DOI] [PubMed] [Google Scholar]
- Lee EJ, Kim MH, Kim YR, Park JW, Park WJ. 2018. Proteasome inhibition protects against diet-induced gallstone formation through modulation of cholesterol and bile acid homeostasis. Int J Mol Med 41:1715–1723. [DOI] [PubMed] [Google Scholar]
- Leng E, Xiao Y, Mo Z, Li Y, Zhang Y, Deng X, Zhou M, Zhou C, He Z, He J, Xiao L, Li J, Li W. 2018. Synergistic effect of phytochemicals on cholesterol metabolism and lipid accumulation in HepG2 cells. BMC Complement Altern Med 18:122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Yin W, Cai M, Liu Y, Hou H, Shen Q, Zhang C, Xiao J, Hu X, Wu Q et al. 2010. NO-1886 suppresses diet-induced insulin resistance and cholesterol accumulation through STAT5-dependent upregulation of IGF1 and CYP7A1. J Endocrinol 204:47–56. [DOI] [PubMed] [Google Scholar]
- Li T, Chanda D, Zhang Y, Choi HS, Chiang JY. 2010. Glucose stimulates cholesterol 7α-hydroxylase gene transcription in human hepatocytes. J Lipid Res 51:832–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Francl JM, Boehme S, Ochoa A, Zhang Y, Klaassen CD, Erickson SK, Chiang JY. 2012. Glucose and insulin induction of bile acid synthesis: mechanisms and implication in diabetes and obesity. J Biol Chem 287:1861–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Jahan A, Chiang JY. 2006. Bile acids and cytokines inhibit the human cholesterol 7α-hydroxylase gene via the JNK/c-jun pathway in human liver cells. Hepatology (Baltimore) 43:1202–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Kong X, Owsley E, Ellis E, Strom S, Chiang JY. 2006. Insulin regulation of cholesterol 7α-hydroxylase expression in human hepatocytes: Roles of forkhead box O1 and sterol regulatory element-binding protein 1c. J Biol Chem 281:28745–28754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li YC, Chiang JYL. 1991. The expression of a catalytically active cholesterol 7α-hydroxylase cytochrome P-450 in Escherichia coli. J Biol Chem 266:19186–19191. [PubMed] [Google Scholar]
- Liu D, Yang A, Wu C, Guo P, Proksch P, Lin W. 2014. Lipid-lowering effects of farnesylquinone and related analogues from the marine-derived Streptomyces nitrosporeus. Bioorg Med Chem Lett 24:5288–5293. [DOI] [PubMed] [Google Scholar]
- Liu J, Lu H, Lu YF, Lei X, Cui JY, Ellis E, Strom SC, Klaassen CD. 2014. Potency of individual bile acids to regulate bile acid synthesis and transport genes in primary human hepatocyte cultures. Toxicol Sci 141:538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrapodi M, Chiang JY. 2000. Peroxisome proliferator-activated receptor α (PPARα) and agonist inhibit cholesterol 7α-hydroxylase gene (CYP7A1) transcription. J Lipid Res 41:514–520. [PubMed] [Google Scholar]
- Morikawa K, Kondo I, Kanamaru Y, Nagaoka S. 2007. A novel regulatory pathway for cholesterol degradation via lactostatin. Biochem Biophys Res Commun 352:697–702. [DOI] [PubMed] [Google Scholar]
- Nguyen LB, Shefer S, Salen G, Tint SG, Batta AK. 1998. Competitive inhibition of hepatic sterol 27-hydroxylase by sitosterol: Decreased activity in sitosterolemia. Proc Assoc Am Physicians 110:32–39. [PubMed] [Google Scholar]
- Norlin M, Andersson U, Björkhem I, Wikvall K. 2000. Oxysterol 7α-hydroxylase activity by cholesterol 7α-hydroxylase (CYP7A). J Biol Chem 275:34046–34053. [DOI] [PubMed] [Google Scholar]
- Norlin M, Toll A, Björkhem I, Wikvall K. 2000. 24-Hydroxycholesterol is a substrate for hepatic cholesterol 7α-hydroxylase (CYP7A). J Lipid Res 41:1629–1639. [PubMed] [Google Scholar]
- Norlin M, Wikvall K. 2007. Enzymes in the conversion of cholesterol into bile acids. Curr Mol Med 7:199–218. [DOI] [PubMed] [Google Scholar]
- Ogishima T, Deguchi S, Okuda K. 1987. Purification and characterization of cholesterol 7α-hydroxylase from rat liver microsomes. J Biol Chem 262:7646–7650. [PubMed] [Google Scholar]
- Owsley E, Chiang JY. 2003. Guggulsterone antagonizes farnesoid X receptor induction of bile salt export pump but activates pregnane X receptor to inhibit cholesterol 7α-hydroxylase gene. Biochem Biophys Res Commun 304:191–195. [DOI] [PubMed] [Google Scholar]
- Pan ST, Xue D, Li ZL, Zhou ZW, He ZX, Yang Y, Yang T, Qiu JX, Zhou SF. 2016. Computational identification of the paralogs and orthologs of human cytochrome P450 superfamily and the implication in drug discovery. Int J Mol Sci June 28;17(7). pii: ijms17071020. doi: 10.3390/ijms17071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker RA, Garcia R, Ryan CS, Liu X, Shipkova P, Livanov V, Patel P, Ho SP. 2013. Bile acid and sterol metabolism with combined HMG-CoA reductase and PCSK9 suppression. J Lipid Res 54:2400–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roglans N, Vazquez-Carrera M, Alegret M, Novell F, Zambon D, Ros E, Laguna JC, Sanchez RM. 2004. Fibrates modify the expression of key factors involved in bile-acid synthesis and biliary-lipid secretion in gallstone patients. Eur J Clin Pharmacol 59:855–861. [DOI] [PubMed] [Google Scholar]
- Russell DW. 1999. Nuclear orphan receptors control cholesterol catabolism. Cell 97:539–542. [DOI] [PubMed] [Google Scholar]
- Schroepfer GJ Jr. 2000. Oxysterols: modulators of cholesterol metabolism and other processes. Physiol Rev 80:361–554. [DOI] [PubMed] [Google Scholar]
- Sharanek A, Burban A, Humbert L, Bachour-El Azzi P, Felix-Gomes N, Rainteau D, Guillouzo A. 2015. Cellular accumulation and toxic effects of bile acids in cyclosporine A-treated HepaRG hepatocytes. Toxicol Sci 147:573–587. [DOI] [PubMed] [Google Scholar]
- Shinkyo R, Guengerich FP. 2011. Cytochrome P450 7A1 cholesterol 7α-hydroxylation: Individual reaction steps in the catalytic cycle and rate-limiting ferric iron reduction. J Biol Chem 286:4632–4643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinkyo R, Xu L, Tallman KA, Cheng Q, Porter NA, Guengerich FP. 2011. Conversion of 7-dehydrocholesterol to 7-ketocholesterol is catalyzed by human cytochrome P450 7A1 and occurs by direct oxidation without an epoxide intermediate. J Biol Chem 286:33021–33028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smelt AH. 2010. Triglycerides and gallstone formation. Clin Chim Acta 411:1625–1631. [DOI] [PubMed] [Google Scholar]
- Song KH, Chiang JY. 2006. Glucagon and cAMP inhibit cholesterol 7α-hydroxylase (CYP7A1) gene expression in human hepatocytes: discordant regulation of bile acid synthesis and gluconeogenesis. Hepatology (Baltimore) 43:117–125. [DOI] [PubMed] [Google Scholar]
- Song Y, Xu C, Shao S, Liu J, Xing W, Xu J, Qin C, Li C, Hu B, Yi S et al. 2015. Thyroid-stimulating hormone regulates hepatic bile acid homeostasis via SREBP-2/HNF-4α/CYP7A1 axis. J Hepatol 62:1171–1179. [DOI] [PubMed] [Google Scholar]
- Taniguchi T, Chen J, Cooper AD. 1994. Regulation of cholesterol 7α-hydroxylase gene expression in HepG2 cells. Effect of serum, bile salts, and coordinate and noncoordinate regulation with other sterol-responsive genes. J Biol Chem 269:10071–10078. [PubMed] [Google Scholar]
- Tempel W, Grabovec I, MacKenzie F, Dichenko YV, Usanov SA, Gilep AA, Park HW, Strushkevich N. 2014. Structural characterization of human cholesterol 7α-hydroxylase. J Lipid Res 55:1925–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang DP, Stroup D, Marrapodi M, Crestani M, Galli G, Chiang JY. 1996. Transcriptional regulation of the human cholesterol 7α-hydroxylase gene (CYP7A) in HepG2 cells. J Lipid Res 37:1831–1841. [PubMed] [Google Scholar]
- Waterman MR, John ME, Simpson ER. 1986. Regulation of synthesis and activity of cytochrome P-450 enzymes in physiological pathways. In: Ortiz de Montellano PR, editor. Cytochrome P450: Structure, Mechanism and Biochemistry, 2nd Ed. New York: Plenum Press; p. 345–386. [Google Scholar]
- Zak A, Zeman M, Hrubant K, Vecka M, Tvrzicka E. 2007. [Effect of hypolipidemic treatment on the composition of bile and the risk or cholesterol gallstone disease]. Cas Lek Cesk 146:24–34. cze. [PubMed] [Google Scholar]
- Zhang JM, Wang XH, Hao LH, Wang H, Zhang XY, Muhammad I, Qi Y, Li GL, Sun XQ. 2017. Nrf2 is crucial for the down-regulation of Cyp7a1 induced by arachidonic acid in Hepg2 cells. Environ Toxicol Pharmacol 52:21–26. [DOI] [PubMed] [Google Scholar]
- Zhang T, Zhao M, Lu D, Wang S, Yu F, Guo L, Wen S, Wu B. 2018. REV-ERBα regulates CYP7A1 through repression of liver receptor homolog-1. Drug Metab Dispos 46:248–258. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Jackson JP, St Claire RL 3rd, Freeman K, Brouwer KR, Edwards JE. 2017. Obeticholic acid, a selective farnesoid X receptor agonist, regulates bile acid homeostasis in sandwich-cultured human hepatocytes. Pharmacol Res Perspect 5(4) doi: 10.1002/prp2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
P450 7B1
P450 7A1 catalyzes the first and rate-limiting step in bile acid synthesis, i.e. cholesterol 7α-hydroxylation (vide supra). P450 7B1 was first described in rodent brain tissue as a dehydroepiandosterone 7α-hydroxylase and was originally isolated from the hippocampus (Rose et al. 1997). Later it was identified as a hepatic oxysterol 7α-hydroxylase (Schwarz et al. 1997).
P450 7B1 mRNA is found not only in liver but also in the steroidogenic tissues testes, ovary, and prostate—as well as in brain, and in colon, kidney, and small intestine—but the tissue specificity is species specific. In humans the highest (mRNA) levels are in kidney and brain.
Much of the literature with this enzyme is based on rodent work. However, Yantsevich et al. (2014) characterized recombinant human P450 7B1 and reported several Ks values for ligand binding and (single-concentration) rates of 7α-hydroxylation of several steroids. The substrates with the highest rates of 7α-hydroxylation were 27- and 25-hydroxycholesterol, dehydroepiandrosterone, 5α-androstane-3β,17β-diol, 5-androsten-3β,17β-diol, and 5α-androstane-3β-ol-17-one (EpiA), with lower activity seen with pregnenolone and 21-hydroxypregnenolone.
No crystal structures are available but a homology model based on P450 7A1 has been proposed (Yantsevich et al. 2014).
Although the catalytic specificity is not immediately obvious regarding physiological issues, there are two major clinical issues: (i) liver failure in children due to genetic insufficiency and (ii) a neuropathy in adults, particularly the autosomal recessive disorder spastic paraplegia type 5 (Stiles et al. 2009).
Increased levels of the cholesterol metabolite 27-hydroxycholesterol in breast tissue tumor were correlated with diminished expression of P450 7B1. However, expression of the CYP7B1 gene in tumors is associated with poorer patient survival (Wu et al. 2013). The opposite pattern was found in prostatic cancer tissue, where P450 7B1 was overexpressed, and it was suggested that local methylation of the CYP7B1 promoter region may have a significant effect on gene transcription (Olsson et al. 2007). The latter results correspond to those presented for induction in prostate cancer tissue (Table 4).
Table 4.
P450 7B1
| Properties | References |
|---|---|
| Physiological substrates: 25- and 27-Hydroxycholesterol, pregnenolone, dehydroepiandrosterone (DHEA), epiandrosterone, 5α-androstane-3β,17β-diol, estrone | (Rose et al. 1997; Steckelbroeck et al. 2002; Kim SB et al. 2004; Stiles et al. 2009; Yantsevich et al. 2014; Pan et al. 2016) |
|
Function: 25-Hydroxycholesterol 7α-hydroxylase, oxysterol and steroid 6α- or 7α-hydroxylase (Fig. 6); involved in metabolism of neurosteroids (brain), bile acid synthesis (liver), and metabolism of estrogen receptor ligands (in prostate) |
|
|
Inhibition: Possible liver failure and progressive neuropathy | |
|
Inhibitors: | |
|
Drugs: | |
|
Imidazole and triazole drugs (ketoconazole, bifoconazole, miconazole, clotrimazole, econazole, fluconazole, tioconazole, voriconazole) a (Kim SB et al. 2004; Yantsevich et al. 2014) | |
| Metyrapone a (Yantsevich et al. 2014) | |
|
Physiological compounds: | |
|
5α-Androstane-3β,17β-diol, estrone, testosterone, 17β-estradiol b (Kim SB et al. 2004; Tang et al. 2006; Pettersson et al. 2008) | |
| 5α-Dihydrotestosterone b (at high concentrations) (Pettersson et al. 2008) | |
| Estrogens (estrone, 17β-estradiol–in the absence of estrogen receptor α) b (Tang et al. 2006) | |
| Triiodothyronine c (T3) (Ellis 2006) | |
| β-Amyloid peptide (non-competitive inhibition) (Kim SB et al. 2004) | |
|
Physiological condition and illnesses: | |
|
Breast cancer d (Wu et al. 2013) | |
| Prostatic cancer d (in type 2 diabetes) (Lutz et al. 2018) | |
|
Other compounds: | |
|
Pesticides a (tebuconazole, propiconazole) (Yantsevich et al. 2014) | |
|
Induction: Immunostimulatory effect of 7α-hydroxydehydroepiandosterone (used in rheumatoid artritis) | |
|
Inducers: | |
|
Drugs: | |
|
Rifampicin (Kim B et al. 2013) | |
|
Physiological compounds: | |
|
17β-Estradiol e (in human embryonic kidney (HEK293) cells transfected with estrogen receptor α) (Dulos, van der Vleuten, et al. 2005; Tang et al. 2006) | |
| Interleukin-1 β interleukin-1α, interleukin-7, interleukin-17, activator protein-1, nuclear factor–κΒ, tumor necrosis factor-α e (Dulos, Kaptein, et al. 2005; Dulos, van der Vleuten, et al. 2005; Dulos and Boots 2006) | |
|
Physiological condition and illnesses: | |
|
Prostatic cancer f (Olsson et al. 2007) | |
| Psoriasis f (Sumantran et al. 2016) | |
|
Other compounds: | |
|
Oleic acid anilide e (An et al. 2008) | |
Footnotes:
Competitive inhibition, ligand binding
Decreased/suppressed/inhibited activity/product formation
Reduced/suppressed mRNA and/or protein level/expression and activity
Gene downregulated/suppressed
Increased transcription/mRNA/protein expression/levels /and/or catalytic activity
Up-regulation of biosynthesis, increased expression of protein
References
- An S, Jang YS, Park JS, Kwon BM, Paik YK, Jeong TS. 2008. Inhibition of acyl-coenzyme A:cholesterol acyltransferase stimulates cholesterol efflux from macrophages and stimulates farnesoid X receptor in hepatocytes. Exp Mol Med 40:407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulos J, Boots AH. 2006. DHEA metabolism in arthritis: A role for the P450 enzyme Cyp7b at the immune-endocrine crossroad. Ann New York Acad Sci 1069:401–413. [DOI] [PubMed] [Google Scholar]
- Dulos J, Kaptein A, Kavelaars A, Heijnen C, Boots A. 2005. Tumour necrosis factor-α stimulates dehydroepiandrosterone metabolism in human fibroblast-like synoviocytes: A role for nuclear factor-κB and activator protein-1 in the regulation of expression of cytochrome P450 enzyme 7b. Arthritis Res Ther 7:R1271–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulos J, van der Vleuten MA, Kavelaars A, Heijnen CJ, Boots AM. 2005. CYP7B expression and activity in fibroblast-like synoviocytes from patients with rheumatoid arthritis: regulation by proinflammatory cytokines. Arthritis Rheumatism 52:770–778. [DOI] [PubMed] [Google Scholar]
- Ellis EC. 2006. Suppression of bile acid synthesis by thyroid hormone in primary human hepatocytes. World J Gastroenterol 12:4640–4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Moon JY, Choi MH, Yang HH, Lee S, Lim KS, Yoon SH, Yu KS, Jang IJ, Cho JY. 2013. Global metabolomics and targeted steroid profiling reveal that rifampin, a strong human PXR activator, alters endogenous urinary steroid markers. J Proteome Res 12:1359–1368. [DOI] [PubMed] [Google Scholar]
- Kim SB, Chalbot S, Pompon D, Jo DH, Morfin R. 2004. The human cytochrome P450 7B1: catalytic activity studies. J Steroid Biochem Mol Biol 92:383–389. [DOI] [PubMed] [Google Scholar]
- Lutz SZ, Hennenlotter J, Scharpf MO, Sailer C, Fritsche L, Schmid V, Kantartzis K, Wagner R, Lehmann R, Berti L, Peter A, Staiger H, Fritsche A, Fend F, Todenhöfer T, Stenzl A, Häring HU, Heni M. 2018. Androgen receptor overexpression in prostate cancer in type 2 diabetes. Mol Metab 8:158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson M, Gustafsson O, Skogastierna C, Tolf A, Rietz BD, Morfin R, Rane A, Ekstrom L. 2007. Regulation and expression of human CYP7B1 in prostate: overexpression of CYP7B1 during progression of prostatic adenocarcinoma. Prostate 67:1439–1446. [DOI] [PubMed] [Google Scholar]
- Pan ST, Xue D, Li ZL, Zhou ZW, He ZX, Yang Y, Yang T, Qiu JX, Zhou SF. 2016. Computational identification of the paralogs and orthologs of human cytochrome P450 superfamily and the implication in drug discovery. Int J Mol Sci June 28;17(7). pii: ijms17071020. doi: 10.3390/ijms17071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson H, Holmberg L, Axelson M, Norlin M. 2008. CYP7B1-mediated metabolism of dehydroepiandrosterone and 5α-androstane-3β,17β-diol–potential role(s) for estrogen signaling. FEBS J 275:1778–1789. [DOI] [PubMed] [Google Scholar]
- Rose KA, Stapleton G, Dott K, Kieny MP, Best R, Schwarz M, Russell DW, Björkhem I, Seckl J, Lathe R. 1997. Cyp7b, a novel brain cytochrome P450, catalyzes the synthesis of neurosteroids 7α-hydroxy dehydroepiandrosterone and 7α-hydroxy pregnenolone. Proc Natl Acad Sci USA 94:4925–4930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steckelbroeck S, Watzka M, Lutjohann D, Makiola P, Nassen A, Hans VH, Clusmann H, Reissinger A, Ludwig M, Siekmann L et al. 2002. Characterization of the dehydroepiandrosterone (DHEA) metabolism via oxysterol 7α-hydroxylase and 17-ketosteroid reductase activity in the human brain. J Neurochem 83:713–726. [DOI] [PubMed] [Google Scholar]
- Stiles AR, McDonald JG, Bauman DR, Russell DW. 2009. CYP7B1: One cytochrome P450, two human genetic diseases, and multiple physiological functions. J Biol Chem 284:28485–28489. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumantran VN, Mishra P, Bera R, Sudhakar N. 2016. Microarray analysis of differentially-expressed genes encoding CYP450 and Phase II drug metabolizing enzymes in psoriasis and melanoma. Pharmaceutics 8(1) Feb 17;8(1) pii: pharmaceutics8010004. doi: 10.3390/pharmaceutics8010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang W, Eggertsen G, Chiang JY, Norlin M. 2006. Estrogen-mediated regulation of CYP7B1: A possible role for controlling DHEA levels in human tissues. J Steroid Biochem Mol Biol 100:42–51. [DOI] [PubMed] [Google Scholar]
- Wu Q, Ishikawa T, Sirianni R, Tang H, McDonald JG, Yuhanna IS, Thompson B, Girard L, Mineo C, Brekken RA et al. 2013. 27-Hydroxycholesterol promotes cell-autonomous, ER-positive breast cancer growth. Cell Rep 5:637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yantsevich AV, Dichenko YV, Mackenzie F, Mukha DV, Baranovsky AV, Gilep AA, Usanov SA, Strushkevich NV. 2014. Human steroid and oxysterol 7α-hydroxylase CYP7B1: Substrate specificity, azole binding and misfolding of clinically relevant mutants. FEBS J 281:1700–1713. [DOI] [PubMed] [Google Scholar]
P450 8B1
P450 8B1 is a liver enzyme and a sterol 12α-hydoxylase. Although it is most closely related to P450 8A1 in its primary sequence, its substrate specificity and function are not at all related (as in the case of P450 27C1 and its relatives, vide infra). While P450 8A1 is involved with eicosanoids, P450 8B1 utilizes sterols and is involved in bile acid synthesis. The enzyme controls the ratio of cholic acid to chenodeoxycholic acid. Substrates include 4β- and 7α-hydroxycholesterol and also 7α,24- and 7α,27-dihydroxycholesterol (Pikuleva 2006). The regulation of the enzyme is complex, and much of what has been reported in the literature is based on animal models.
P450 8B1 controls the balance between cholic acid and chenodeoxycholic acid, thus adjusting the hydrophobicity of bile (cholic acid is more hydrophilic), i.e. the neutral pathway and the acidic pathway (Russell 2003; Chiang 2004). This ratio between the bile acids is important for feedback regulation of bile acid synthesis (Ellis E et al. 2003). However, the ratio between these two bile acids does not appear to be sensitive to genetic variations in P450 8B1 (Pikuleva 2006). The enzyme plays a critical role in intestinal cholesterol absorption and pathogenesis of cholesterol gallstone, dyslipidemia, and diabetes (Pathak et al. 2013).
Interestingly, the CYP8B1 gene is devoid of introns, a unique phenomenon for eukaryotic P450s (Gafvels et al. 1999). No crystal structures have been reported yet and, despite some sequence similarity to P450 8A1, the functional differences make comparisons with homology modeling questionable.
References
- Andersson U, Yang YZ, Björkhem I, Einarsson C, Eggertsen G, Gafvels M. 1999. Thyroid hormone suppresses hepatic sterol 12α-hydroxylase (CYP8B1) activity and messenger ribonucleic acid in rat liver: failure to define known thyroid hormone response elements in the gene. Biochim Biophys Acta 1438:167–174. [DOI] [PubMed] [Google Scholar]
- Andreou ER, Prokipcak RD. 1998. Analysis of human CYP7A1 mRNA decay in HepG2 cells by reverse transcription-polymerase chain reaction. Arch Biochem Biophys 357(1):137–146. [DOI] [PubMed] [Google Scholar]
- Antherieu S, Bachour-El Azzi P, Dumont J, Abdel-Razzak Z, Guguen-Guillouzo C, Fromenty B, Robin MA, Guillouzo A. 2013. Oxidative stress plays a major role in chlorpromazine-induced cholestasis in human HepaRG cells. Hepatology (Baltimore) 57:1518–1529. [DOI] [PubMed] [Google Scholar]
- Chiang JY. 2004. Regulation of bile acid synthesis: Pathways, nuclear receptors, and mechanisms. J Hepatol 40:539–551. [DOI] [PubMed] [Google Scholar]
- Ellis E, Axelson M, Abrahamsson A, Eggertsen G, Thorne A, Nowak G, Ericzon BG, Björkhem I, Einarsson C. 2003. Feedback regulation of bile acid synthesis in primary human hepatocytes: evidence that CDCA is the strongest inhibitor. Hepatology (Baltimore) 38:930–938. [DOI] [PubMed] [Google Scholar]
- Ellis EC. 2006. Suppression of bile acid synthesis by thyroid hormone in primary human hepatocytes. World J Gastroenterol 12:4640–4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafvels M, Olin M, Chowdhary BP, Raudsepp T, Andersson U, Persson B, Jansson M, Björkhem I, Eggertsen G. 1999. Structure and chromosomal assignment of the sterol 12α-hydroxylase gene (CYP8B1) in human and mouse: eukaryotic cytochrome P-450 gene devoid of introns. Genomics 56:184–196. [DOI] [PubMed] [Google Scholar]
- Jahan A, Chiang JY. 2005. Cytokine regulation of human sterol 12α-hydroxylase (CYP8B1) gene. Am J Physiol Gastrointest Liver Physiol 288:G685–695. [DOI] [PubMed] [Google Scholar]
- Li Y, Mezei O, Shay NF. 2007. Human and murine hepatic sterol-12α-hydroxylase and other xenobiotic metabolism mRNA are upregulated by soy isoflavones. J Nutr 137:1705–1712. [DOI] [PubMed] [Google Scholar]
- Mörk LM, Strom SC, Mode A, Ellis EC. 2016. Addition of dexamethasone alters the bile acid composition by inducing CYP8B1 in primary cultures of human hepatocytes. J Clin Exp Hepatol 6:87–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan ST, Xue D, Li ZL, Zhou ZW, He ZX, Yang Y, Yang T, Qiu JX, Zhou SF. 2016. Computational identification of the paralogs and orthologs of human cytochrome P450 superfamily and the implication in drug discovery. Int J Mol Sci June 28;17(7). pii: ijms17071020. doi: 10.3390/ijms17071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker RA, Garcia R, Ryan CS, Liu X, Shipkova P, Livanov V, Patel P, Ho SP. 2013. Bile acid and sterol metabolism with combined HMG-CoA reductase and PCSK9 suppression. J Lipid Res 54:2400–2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pathak P, Li T, Chiang JY. 2013. Retinoic acid-related orphan receptor αregulates diurnal rhythm and fasting induction of sterol 12α-hydroxylase in bile acid synthesis. J Biol Chem 288:37154–37165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikuleva IA. 2006. Cytochrome P450s and cholesterol homeostasis. Pharmacol Therapeut 112:761–773. eng. [DOI] [PubMed] [Google Scholar]
- Russell DW. 2003. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem 72:137–174. [DOI] [PubMed] [Google Scholar]
- Sharanek A, Burban A, Humbert L, Bachour-El Azzi P, Felix-Gomes N, Rainteau D, Guillouzo A. 2015. Cellular accumulation and toxic effects of bile acids in cyclosporine A-treated HepaRG hepatocytes. Toxicol Sci 147:573–587. [DOI] [PubMed] [Google Scholar]
- Zhang M, Chiang JY. 2001. Transcriptional regulation of the human sterol 12α-hydroxylase gene (CYP8B1): Roles of heaptocyte nuclear factor 4α in mediating bile acid repression. J Biol Chem 276:41690–41699. [DOI] [PubMed] [Google Scholar]
P450 11A1
Cholesterol is the precursor of all steroid hormones and bile acids, and P450 11A1 is the main cholesterol side chain cleavage enzyme in steroidogenic tissues, yielding pregnenolone as a key intermediate (Fig. 4). The enzyme is localized in steroidogenic tissues (e.g. adrenal cortex, gonads, ovary) consistent with the reaction being the initiating step in steroid synthesis. The enzyme is synthesized on ribosomes in the cytosol and imported into mitochondria.
NADPH-adrenoredoxin reductase and adrenodoxin are used for electron delivery to mitochondrial P450 11A1. The principal intermediate in P450-mediated oxidations has been identified as Compound I, the high-valent FeO3+ species (McQuarters et al. 2014), including the case of P450 11A1 (Davydov et al. 2015). In addition, 18O labeling studies have demonstrated that Compound I reacts with one of the hydroxyl groups of 20(R), 22(R)-dihydroxycholesterol in the third step of the reaction, as opposed to hydrogen atom abstraction (from one of the alcohols) (Ortiz de Montellano 2015; Yoshimoto et al. 2016).
The substrate specificity of P450 11A1 is not so strict as once thought, i.e. it does not only act on cholesterol. Several other sterols are also substrates including 7-dehydrocholesterol, zymosterol, lathosterol, and desmosterol (Acimovic et al. 2016). Tuckey and his associates have found that vitamin D3 is hydroxylated on the side chain to multiple products (Guryev et al. 2003; Slominski A et al. 2006; Tuckey, Janjetovic, et al. 2008; Tuckey, Li, et al. 2008; Tuckey et al. 2011; Slominski A. T. et al. 2013). A Bristol-Myers Squibb drug candidate, BMS-A [(N-(4–1(1H-pyrrolo[2,3[b]pyridine-4-yl)oxy)-3-fluorophenyl)2-oxo-1,2-dihydropyridine-3-carboxamide, was also oxidized by rat and human P450 11A1 (Zhang et al. 2012), and this reaction was linked to adrenal toxicity of the compund.
Two groups have reported X-ray crystal structures, both with 22-hydroxycholesterol and one with 20,22-dihydroxycholesterol bound (Mast et al. 2011; Strushkevich et al. 2011) (PDB 3MZS, 3N9Y, 3N9Z, 3NA0, 3NA1).
Because of the importance of this enzyme and its role, there does not seem to be an impetus to develop inhibitory drugs. Deficiencies in the enzyme are debilitating. Another issue is the presence of autoantibodies to P450 11A1 in patients with autoimmune polyglandular syndrome types I and II and Addison’s disease, although no causal relationships have been shown (Chen et al. 1996; Seissler et al. 1999; Boe et al. 2004).
References
- Acimovic J, Goyal S, Kosir R, Golicnik M, Perse M, Belic A, Urlep Z, Guengerich FP, Rozman D. 2016. Cytochrome P450 metabolism of the post-lanosterol intermediates explains enigmas of cholesterol synthesis. Sci Rep 6:28462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asif AR, Ljubojevic M, Sabolic I, Shnitsar V, Metten M, Anzai N, Muller GA, Burckhardt G, Hagos Y. 2006. Regulation of steroid hormone biosynthesis enzymes and organic anion transporters by forskolin and DHEA-S treatment in adrenocortical cells. Am J Physiol Endocrinol Metab 291:E1351–1359. [DOI] [PubMed] [Google Scholar]
- Aumo L, Rusten M, Mellgren G, Bakke M, Lewis AE. 2010. Functional roles of protein kinase A (PKA) and exchange protein directly activated by 3´,5´-cyclic adenosine 5’-monophosphate (cAMP) 2 (EPAC2) in cAMP-mediated actions in adrenocortical cells. Endocrinology 151:2151–2161. [DOI] [PubMed] [Google Scholar]
- Babischkin JS, Grimes RW, Pepe GJ, Albrecht ED. 1997. Estrogen stimulation of P450 cholesterol side-chain cleavage activity in cultures of human placental syncytiotrophoblasts. Biol Reprod 56:272–278. [DOI] [PubMed] [Google Scholar]
- Boe AS, Bredholt G, Knappskog PM, Hjelmervik TO, Mellgren G, Winqvist O, Kampe O, Husebye ES. 2004. Autoantibodies against 21-hydroxylase and side-chain cleavage enzyme in autoimmune Addison’s disease are mainly immunoglobulin G1. Eur J Endocrinol 150:49–56. [DOI] [PubMed] [Google Scholar]
- Breckwoldt M, Selvaraj N, Aharoni D, Barash A, Segal I, Insler V, Amsterdam A. 1996. Expression of Ad4-BP/cytochrome P450 side chain cleavage enzyme and induction of cell death in long-term cultures of human granulosa cells. Mol Human Reprod 2:391–400. [DOI] [PubMed] [Google Scholar]
- Chen S, Sawicka J, Betterle C, Powell M, Prentice L, Volpato M, Rees Smith B, Furmaniak J. 1996. Autoantibodies to steroidogenic enzymes in autoimmune polyglandular syndrome, Addison’s disease, and premature ovarian failure. J Clin Endocrinol Metab 81:1871–1876. [DOI] [PubMed] [Google Scholar]
- Davydov R, Strushkevich N, Smil D, Yantsevich A, Gilep A, Usanov S, Hoffman BM. 2015. Evidence that Compound I is the active species in both the hydroxylase and lyase steps by which P450scc converts cholesterol to pregnenolone: EPR/ENDOR/cryoreduction/annealing studies. Biochemistry 54:7089–7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Blasio AM, Voutilainen R, Jaffe RB, Miller WL. 1987. Hormonal regulation of messenger ribonucleic acids for P450scc (cholesterol side-chain cleavage enzyme) and P450c17 (17α-hydroxylase/17,20-lyase) in cultured human fetal adrenal cells. J Clin Endocrinol Metab 65:170–175. [DOI] [PubMed] [Google Scholar]
- Ding L, Murphy MB, He Y, Xu Y, Yeung LW, Wang J, Zhou B, Lam PK, Wu RS, Giesy JP. 2007. Effects of brominated flame retardants and brominated dioxins on steroidogenesis in H295R human adrenocortical carcinoma cell line. Environ Toxicol Chem 26:764–772. [DOI] [PubMed] [Google Scholar]
- Dowie LJ, Smith JE, MacGilchrist AJ, Fraser R, Honour JW, Reid JL, Kenyon CJ. 1988. In vivo and in vitro studies of the site of inhibitory action of omeprazole on adrenocortical steroidogenesis. Eur J Clin Pharmacol 35:625–629. [DOI] [PubMed] [Google Scholar]
- Drewett JG, Adams-Hays RL, Ho BY, Hegge DJ. 2002. Nitric oxide potently inhibits the rate-limiting enzymatic step in steroidogenesis. Mol Cell Endocrinol 194:39–50. [DOI] [PubMed] [Google Scholar]
- Fassnacht M, Hahner S, Beuschlein F, Klink A, Reincke M, Allolio B. 2000. New mechanisms of adrenostatic compounds in a human adrenocortical cancer cell line. Eur J Clin Invest 30 (Suppl 3):76–82. [DOI] [PubMed] [Google Scholar]
- Garcia MM, Acquier A, Suarez G, Gomez NV, Gorostizaga A, Mendez CF, Paz C. 2012. Cisplatin inhibits testosterone synthesis by a mechanism that includes the action of reactive oxygen species (ROS) at the level of P450scc. Chem-Biol Interact 199:185–191. [DOI] [PubMed] [Google Scholar]
- Ghayee HK, Auchus RJ. 2007. Basic concepts and recent developments in human steroid hormone biosynthesis. Rev Endocr Metabol Disorders 8:289–300. [DOI] [PubMed] [Google Scholar]
- Gregoraszczuk EL, Ptak A, Karpeta A, Fiedor E, Wrobel A, Milewicz T, Falandysz J. 2014. Hexachlorobenzene and pentachlorobenzene accumulation, metabolism and effect on steroid secretion and on CYP11A1 and CYP19 expression in cultured human placental tissue. Reprod Toxicol (Elmsford, NY) 43:102–110. [DOI] [PubMed] [Google Scholar]
- Guo IC, Hu MC, Chung BC. 2003. Transcriptional regulation of CYP11A1. J Biomed Sci 10:593–598. [DOI] [PubMed] [Google Scholar]
- Guo IC, Shih MC, Lan HC, Hsu NC, Hu MC, Chung BC. 2007. Transcriptional regulation of human CYP11A1 in gonads and adrenals. J Biomed Sci 14:509–515. [DOI] [PubMed] [Google Scholar]
- Guryev O, Carvalho RA, Usanov S, Gilep A, Estabrook RW. 2003. A pathway for the metabolism of vitamin D3: Unique hydroxylated metabolites formed during catalysis with cytochrome P450scc (CYP11A1). Proc Natl Acad Sci USA 100:14754–14759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann RW, Muller U, Ehmer PB. 2003. Discovery of selective CYP11B2 (aldosterone synthase) inhibitors for the therapy of congestive heart failure and myocardial fibrosis. Eur J Med Chem 38:363–366. [DOI] [PubMed] [Google Scholar]
- Huang BM, Hsiao KY, Chuang PC, Wu MH, Pan HA, Tsai SJ. 2004. Upregulation of steroidogenic enzymes and ovarian 17α-estradiol in human granulosa-lutein cells by Cordyceps sinensis mycelium. Biol Reprod 70:1358–1364. [DOI] [PubMed] [Google Scholar]
- Johansson MK, Sanderson JT, Lund BO. 2002. Effects of 3-MeSO2-DDE and some CYP inhibitors on glucocorticoid steroidogenesis in the H295R human adrenocortical carcinoma cell line. Toxicol In Vitro 16:113–121. [DOI] [PubMed] [Google Scholar]
- Kau MM, Kan SF, Wang JR, Wang PS. 2005. Inhibitory effects of digoxin and ouabain on aldosterone synthesis in human adrenocortical NCI-H295 cells. J Cell Physiol 205:393–401. [DOI] [PubMed] [Google Scholar]
- Kisselev P, Tuckey RC, Woods ST, Triantopoulos T, Schwarz D. 1999. Enzymatic properties of vesicle-reconstituted human cytochrome P450SCC (CYP11A1) differences in functioning of the mitochondrial electron-transfer chain using human and bovine adrenodoxin and activation by cardiolipin. Eur J Biochem 260:768–773. [DOI] [PubMed] [Google Scholar]
- Kraugerud M, Zimmer KE, Ropstad E, Verhaegen S. 2011. Perfluorinated compounds differentially affect steroidogenesis and viability in the human adrenocortical carcinoma (H295R) in vitro cell assay. Toxicol Lett 205:62–68. [DOI] [PubMed] [Google Scholar]
- Lambeth JD. 1983. 22-Ketocholesterol: A potent competitive inhibitor of cytochrome P-450scc-dependent side-chain cleavage of cholesterol. Mol Pharmacol 23:743–747. [PubMed] [Google Scholar]
- Lan HC, Lin IW, Yang ZJ, Lin JH. 2015. Low-dose bisphenol a activates Cyp11a1 Gene expression and corticosterone secretion in adrenal gland via the JNK signaling pathway. Toxicol Sci 148:26–34. [DOI] [PubMed] [Google Scholar]
- Lin CW, Chang YH, Pu HF. 2012. Mitotane exhibits dual effects on steroidogenic enzymes gene transcription under basal and cAMP-stimulating microenvironments in NCI-H295 cells. Toxicology 298:14–23. [DOI] [PubMed] [Google Scholar]
- Mast N, Annalora AJ, Lodowski DT, Palczewski K, Stout CD, Pikuleva IA. 2011. Structural basis for three-step sequential catalysis by the cholesterol side chain cleavage enzyme CYP11A1. J Biol Chem 286:5607–5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mast N, Linger M, Pikuleva IA. 2013. Inhibition and stimulation of activity of purified recombinant CYP11A1 by therapeutic agents. Mol Cell Endocrinol 371:100–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauger JW. 1989. The impact of innovative drug delivery systems on drug toxicity. J Clin Toxicol 27:v–vii. [DOI] [PubMed] [Google Scholar]
- McQuarters AB, Wolf MW, Hunt AP, Lehnert N. 2014. 1958–2014: After 56 years of research, cytochrome P450 reactivity is finally explained. Angew Chem Int Ed 53:4750–4752. [DOI] [PubMed] [Google Scholar]
- Mesiano S, Katz SL, Lee JY, Jaffe RB. 1997. Insulin-like growth factors augment steroid production and expression of steroidogenic enzymes in human fetal adrenal cortical cells: implications for adrenal androgen regulation. J Clin Endocrinol Metab 82:1390–1396. [DOI] [PubMed] [Google Scholar]
- Milczarek R, Sokolowska E, Rybakowska I, Kaletha K, Klimek J. 2016. Paraquat inhibits progesterone synthesis in human placental mitochondria. Placenta 43:41–46. [DOI] [PubMed] [Google Scholar]
- Mosa A, Neunzig J, Gerber A, Zapp J, Hannemann F, Pilak P, Bernhardt R. 2015. 2α- and 16α-Hydroxylase activity of CYP11A1 and direct stimulatory effect of estrogens on pregnenolone formation. J Steroid Biochem Mol Biol 150:1–10. [DOI] [PubMed] [Google Scholar]
- Nelson-DeGrave VL, Wickenheisser JK, Cockrell JE, Wood JR, Legro RS, Strauss JF 3rd, McAllister JM. 2004. Valproate potentiates androgen biosynthesis in human ovarian theca cells. Endocrinology 145:799–808. [DOI] [PubMed] [Google Scholar]
- Ortiz de Montellano PR. 2015. Substrate oxidation. In: Ortiz de Montellano PR, editor. Cytochrome P450: Structure, Mechanism, and Biochemistry 4th ed. New York: Springer; p. 111–176. [Google Scholar]
- Oskarsson A, Ohlsson Andersson A. 2016. Suppressed sex hormone biosynthesis by alkylresorcinols: A possible link to chemoprevention. Nutr Cancer 68:978–987. [DOI] [PubMed] [Google Scholar]
- Pagotto MA, Roldan ML, Pagotto RM, Lugano MC, Pisani GB, Rogic G, Molinas SM, Trumper L, Pignataro OP, Monasterolo LA. 2011. Localization and functional activity of cytochrome P450 side chain cleavage enzyme (CYP11A1) in the adult rat kidney. Mol Cell Endocrinol 332:253–260. [DOI] [PubMed] [Google Scholar]
- Pan ST, Xue D, Li ZL, Zhou ZW, He ZX, Yang Y, Yang T, Qiu JX, Zhou SF. 2016. Computational identification of the paralogs and orthologs of human cytochrome P450 superfamily and the implication in drug discovery. Int J Mol Sci June 28;17(7). pii: ijms17071020. doi: 10.3390/ijms17071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picado-Leonard J, Voutilainen R, Kao LC, Chung BC, Strauss JF 3rd, Miller WL. 1988. Human adrenodoxin: Cloning of three cDNAs and cycloheximide enhancement in JEG-3 cells. J Biol Chem 263:3240–3244. [PubMed] [Google Scholar]
- Sawetawan C, Carr BR, McGee E, Bird IM, Hong TL, Rainey WE. 1996. Inhibin and activin differentially regulate androgen production and 17α-hydroxylase expression in human ovarian thecal-like tumor cells. J Endocrinol 148:213–221. [DOI] [PubMed] [Google Scholar]
- Schiffer L, Brixius-Anderko S, Hannemann F, Zapp J, Neunzig J, Thevis M, Bernhardt R. 2016. Metabolism of oral turinabol by human steroid hormone-synthesizing cytochrome P450 enzymes. Drug Metab Dispos 44:227–237. [DOI] [PubMed] [Google Scholar]
- Schuster I, Bernhardt R. 2007. Inhibition of cytochromes P450: Existing and new promising therapeutic targets. Drug Metab Rev 39:481–499. [DOI] [PubMed] [Google Scholar]
- Seissler J, Schott M, Steinbrenner H, Peterson P, Scherbaum WA. 1999. Autoantibodies to adrenal cytochrome P450 antigens in isolated Addison’s disease and autoimmune polyendocrine syndrome type II. Exp Clinical Endocrinol Diabetes 107:208–213. [DOI] [PubMed] [Google Scholar]
- Shih MC, Chiu YN, Hu MC, Guo IC, Chung BC. 2011. Regulation of steroid production: Analysis of Cyp11a1 promoter. Mol Cell Endocrinol 336:80–84. [DOI] [PubMed] [Google Scholar]
- Slominski A, Semak I, Wortsman J, Zjawiony J, Li W, Zbytek B, Tuckey RC. 2006. An alternative pathway of vitamin D metabolism. Cytochrome P450scc (CYP11A1)-mediated conversion to 20-hydroxyvitamin D2 and 17,20-dihydroxyvitamin D2. FEBS J 273:2891–2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Kim TK, Li W, Yi AK, Postlethwaite A, Tuckey RC. 2014. The role of CYP11A1 in the production of vitamin D metabolites and their role in the regulation of epidermal functions. J Steroid Biochem Mol Biol 144:28–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski AT, Li W, Kim T-K, Semak I, Wang J, Zjawiony JK, Tuckey RC. 2015. Novel activities of CYP11A1 and their potential physiological significance. J Steroid Biochem Mol Biol 151:25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R, He Y, Murphy MB, Yeung LW, Yu RM, Lam MH, Lam PK, Hecker M, Giesy JP, Wu RS et al. 2008. Effects of fifteen PBDE metabolites, DE71, DE79 and TBBPA on steroidogenesis in the H295R cell line. Chemosphere 71:1888–1894. [DOI] [PubMed] [Google Scholar]
- Strushkevich N, MacKenzie F, Cherkesova T, Grabovec I, Usanov S, Park HW. 2011. Structural basis for pregnenolone biosynthesis by the mitochondrial monooxygenase system. Proc Natl Acad Sci USA 108:10139–10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckey RC, Cameron KJ. 1993a. Catalytic properties of cytochrome P-450scc purified from the human placenta: comparison to bovine cytochrome P-450scc. Biochim Biophys Acta 1163:185–194. [DOI] [PubMed] [Google Scholar]
- Tuckey RC, Cameron KJ. 1993b. Side-chain specificities of human and bovine cytochromes P-450scc. Eur J Biochem 217:209–215. [DOI] [PubMed] [Google Scholar]
- Tuckey RC, Janjetovic Z, Li W, Nguyen MN, Zmijewski MA, Zjawiony J, Slominski A. 2008. Metabolism of 1α-hydroxyvitamin D3 by cytochrome P450scc to biologically active 1α,20-dihydroxyvitamin D3. J Steroid Biochem Mol Biol 112:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckey RC, Li W, Shehabi HZ, Janjetovic Z, Nguyen MN, Kim TK, Chen J, Howell DE, Benson HA, Sweatman T et al. 2011. Production of 22-hydroxy metabolites of vitamin D3 by cytochrome P450scc (CYP11A1) and analysis of their biological activities on skin cells. Drug Metab Dispos 39:1577–1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckey RC, Li W, Zjawiony JK, Zmijewski MA, Nguyen MN, Sweatman T, Miller D, Slominski A. 2008. Pathways and products for the metabolism of vitamin D3 by cytochrome P450scc. FEBS J 275:2585–2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckey RC, Nguyen MN, Slominski A. 2008. Kinetics of vitamin D3 metabolism by cytochrome P450scc (CYP11A1) in phospholipid vesicles and cyclodextrin. Int J Biochem Cell Biol 40:2619–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- sTuckey RC, Slominski AT, Cheng CY, Chen J, Kim TK, Xiao M, Li W. 2014. Lumisterol is metabolized by CYP11A1: Discovery of a new pathway. Int J Biochem Cell Biol 55:24–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Koetsveld PM, Vitale G, Feelders RA, Waaijers M, Sprij-Mooij DM, de Krijger RR, Speel EJ, Hofland J, Lamberts SW, de Herder WW et al. 2013. Interferon-α is a potent inhibitor of cell growth and cortisol production in vitro and sensitizes human adrenocortical carcinoma cells to mitotane. Endocr Related Cancer 20:443–454. [DOI] [PubMed] [Google Scholar]
- van Lier JE, Mast N, Pikuleva IA. 2015. Cholesterol hydroperoxides as substrates for cholesterol-metabolizing cytochrome P450 enzymes and alternative sources of 25-hydroxycholesterol and other oxysterols. Angew Chem Int Ed 54:11138–11142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CC, Mei S, Cheng J, Ding Y, Weidenhammer A, Garcia V, Zhang F, Gotlinger K, Manthati VL, Falck JR et al. 2013. Androgen-sensitive hypertension associates with upregulated vascular CYP4A12–20-HETE synthase. J Am Soc Nephrol 24:1288–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Su ZJ, Ge RS. 2011. Inhibitors of testosterone biosynthetic and metabolic activation enzymes. Molecules (Basel) 16:9983–10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimoto FK, Jung IJ, Goyal S, Gonzalez E, Guengerich FP. 2016. Isotope-labeling studies support the electrophilic Compound I iron active species, FeO3+, for the carbon-carbon bond cleavage reaction of the cholesterol side-chain cleavage enzyme, cytochrome P450 11A1. J Am Chem Soc 138:12124–12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Flint O, Wang L, Gupta A, Westhouse RA, Zhao W, Raghavan N, Caceres-Cortes J, Marathe P, Shen G et al. 2012. Cytochrome P450 11A1 bioactivation of a kinase inhibitor in rats: use of radioprofiling, modulation of metabolism, and adrenocortical cell lines to evaluate adrenal toxicity. Chem. Res. Toxicol 25:556–571. [DOI] [PubMed] [Google Scholar]
P450 11B1
Steroid production occurs primarily in the adrenal cortex and gonads, and cortisol production is localized mainly in the zona fasiculata region of the adrenal cortex. The production of cortisol is regulated through three major P450s—17A1, 21A2, and 11B1—in the zona glomurulosa and zona fasiculata regions. The last steps in aldosterone biosynthesis are mediated by the mitochondrial cytochrome P450 11B family (Fig. 4), which catalyze (subsequent) oxidation reactions on the C11, C18, and C19 carbons on the β-side of the steroid skeleton (Fig. 9). In cattle, swine, and frogs, aldosterone synthesis is performed by only one P450, 11B, but in humans (Fisher et al. 2001) and mice (Domalik et al. 1991) aldosterone formation involves two P450 genes, P450 11B1 (steroid 11β-hydroxylase) and 11B2 (aldosterone synthase) (rats have four P450 genes:11B1, 11B2, 11B3 (only expressed in neonates, similar activity as 11B2), and 11B4 (a pseudogene) (Nonaka and Okamoto 1991)). Human P450s 11B1 and 11B2 differ in only 32 residues. P450 11B1 catalyzes the 11β-hydroxylation of deoxycortisol to form cortisol, the major glucocorticoid in the body. Deficiencies result in congenital adrenal hyperplasia. In both humans and rats, only P450 11B2 can perform the final oxidation at C18 to produce aldosterone (Nonaka and Okamoto 1991; Fisher et al. 2001). C19 hydroxylation has been reported for rat P450 11B1 but not the human enzyme (Nonaka and Okamoto 1991), and P450 11B1 is known to play an important role in the biosynthesis of glucocorticoids. If an inhibitor of aldosterone synthesis is to be clinically useful, the biosynthesis of glucocorticoids should remain unaffected, indicating that the inhibition must be 11B2-selective (Roumen et al. 2007).
Fig. 9.
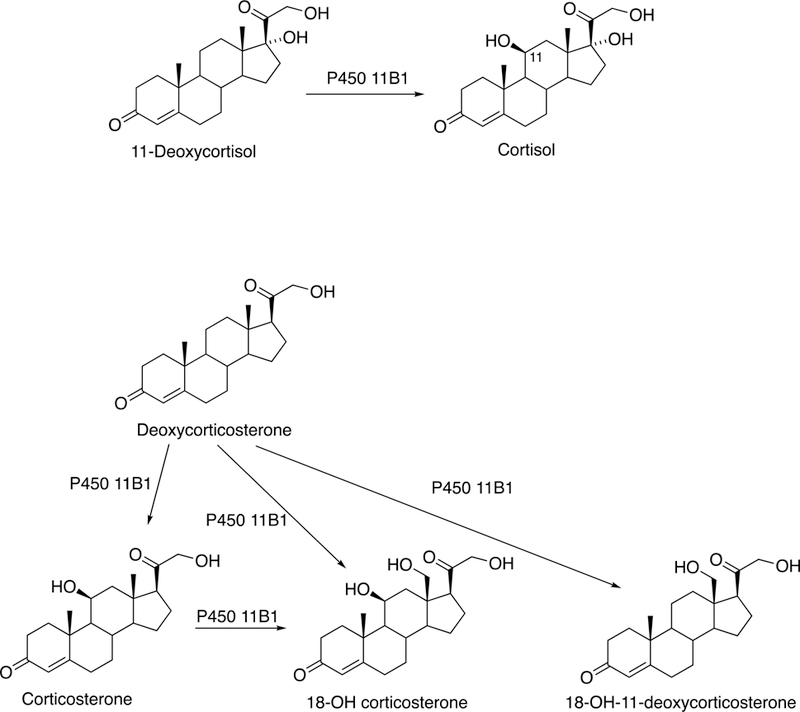
Steroid oxidations catalyzed by (human) P450 11B1.
The regulation of the CYP11B1 gene is very complex, and the animal models have several deficiencies in this regard. A large number of genetic variants in the CYP11B1 gene have been identified and linked to high 18β-hydroxycortisol levels and low 11β-hydroxylation, as well as congenital adrenal hyperplasia and hypertension (Guengerich 2015). No crystal structures of P450 11B1 have been published, to our knowledge.
Although there are medical issues related to P450 11B1 deficiency, there are also reasons to develop inhibitors. Cortisol dysregulation has been associated with diseases such as diabetes, heart failure, hypertension, stroke, obesity, renal failure, and prostate, breast, uterine, and ovarian cancers (Connolly and Wills 1967; Mitrunen et al. 2000; Chiodini et al. 2007; Barugh et al. 2014). In some cases, an excess amount of circulating cortisol is produced. High levels of cortisol are associated with Cushing’s syndrome (Bureik, Lisurek, et al. 2002). Metabolic syndrome is also linked to the overproduction of cortisol (Walker 2006).
References
- Asp V, Ulleras E, Lindstrom V, Bergstrom U, Oskarsson A, Brandt I. 2010. Biphasic hormonal responses to the adrenocorticolytic DDT metabolite 3-methylsulfonyl-DDE in human cells. Tox Appl Pharmacol 242:281–289. [DOI] [PubMed] [Google Scholar]
- Ayub M, Levell MJ. 1989. Inhibition of human adrenal steroidogenic enzymes in vitro by imidazole drugs including ketoconazole. J Steroid Biochem 32:515–524. [DOI] [PubMed] [Google Scholar]
- Barugh AJ, Gray P, Shenkin SD, MacLullich AM, Mead GE. 2014. Cortisol levels and the severity and outcomes of acute stroke: a systematic review. J Neurol 261:533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt R 2016. The potential of targeting CYP11B. Exp Opin Therapeut Targets 20:923–934. [DOI] [PubMed] [Google Scholar]
- Bureik M, Hubel K, Dragan CA, Scher J, Becker H, Lenz N, Bernhardt R. 2004. Development of test systems for the discovery of selective human aldosterone synthase (CYP11B2) and 11β-hydroxylase (CYP11B1) inhibitors. Discovery of a new lead compound for the therapy of congestive heart failure, myocardial fibrosis and hypertension. Mol Cell Endocrinol 217:249–254. [DOI] [PubMed] [Google Scholar]
- Bureik M, Lisurek M, Bernhardt R. 2002. The human steroid hydroxylases CYP11B1 and CYP11B2. Biol Chem 383:1537–1551. [DOI] [PubMed] [Google Scholar]
- Bureik M, Zeeh A, Bernhardt R. 2002. Modulation of steroid hydroxylase activity in stably transfected V79MZh11B1 and V79MZh11B2 cells by PKC and PKD inhibitors. Endocr Res 28:351–355. [DOI] [PubMed] [Google Scholar]
- Cheng SC, Suzuki K, Sadee W, Harding BW. 1976. Effects of spironolactone, canrenone and canrenoate-K on cytochrome P450, and 11β- and 18-hydroxylation in bovine and human adrenal cortical mitochondria. Endocrinology 99:1097–1106. [DOI] [PubMed] [Google Scholar]
- Chiodini I, Adda G, Scillitani A, Coletti F, Morelli V, Di Lembo S, Epaminonda P, Masserini B, Beck-Peccoz P, Orsi E et al. 2007. Cortisol secretion in patients with type 2 diabetes: Relationship with chronic complications. Diabetes Care 30:83–88. [DOI] [PubMed] [Google Scholar]
- Connolly CK, Wills MR. 1967. Plasma cortisol levels in heart failure. Brit Med J 2:25–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter CL, Jaffe RB. 1998. Functional maturation of the primate fetal adrenal in vivo: 3. Specific zonal localization and developmental regulation of CYP21A2 (P450c21) and CYP11B1/CYP11B2 (P450c11/aldosterone synthase) lead to integrated concept of zonal and temporal steroid biosynthesis. Endocrinology 139:5144–5150. [DOI] [PubMed] [Google Scholar]
- Denner K, Rainey WE, Pezzi V, Bird IM, Bernhardt R, Mathis JM. 1996. Differential regulation of 11β-hydroxylase and aldosterone synthase in human adrenocortical H295R cells. Mol Cell Endocrinol 121:87–91. [DOI] [PubMed] [Google Scholar]
- Denner K, Vogel R, Schmalix W, Doehmer J, Bernhardt R. 1995. Cloning and stable expression of the human mitochondrial cytochrome P45011B1 cDNA in V79 Chinese hamster cells and their application for testing of potential inhibitors. Pharmacogenetics 5:89–96. [DOI] [PubMed] [Google Scholar]
- Ding L, Murphy MB, He Y, Xu Y, Yeung LW, Wang J, Zhou B, Lam PK, Wu RS, Giesy JP. 2007. Effects of brominated flame retardants and brominated dioxins on steroidogenesis in H295R human adrenocortical carcinoma cell line. Environ Toxicol Chem 26:764–772. [DOI] [PubMed] [Google Scholar]
- Domalik LJ, Chaplin DD, Kirkman MS, Wu RC, Liu WW, Howard TA, Seldin MF, Parker KL. 1991. Different isozymes of mouse 11β-hydroxylase produce mineralocorticoids and glucocorticoids. Mol Endocrinol 5:1853–1861. [DOI] [PubMed] [Google Scholar]
- Ehmer PB, Bureik M, Bernhardt R, Muller U, Hartmann RW. 2002. Development of a test system for inhibitors of human aldosterone synthase (CYP11B2): Screening in fission yeast and evaluation of selectivity in V79 cells. J Steroid Biochem Mol Biol 81(2):173–179. [DOI] [PubMed] [Google Scholar]
- Emmerich J, van Koppen CJ, Burkhart JL, Engeli RT, Hu Q, Odermatt A, Hartmann RW. 2018. Accelerated skin wound healing by selective 11β-hydroxylase (CYP11B1) inhibitors. Eur J Med Chem 143:591–597. [DOI] [PubMed] [Google Scholar]
- Emmerich J, van Koppen CJ, Burkhart JL, Hu Q, Siebenburger L, Boerger C, Scheuer C, Laschke MW, Menger MD, Hartmann RW. 2017. Lead optimization generates CYP11B1 inhibitors of pyridylmethyl isoxazole type with improved pharmacological profile for the treatment of Cushing’s disease. J Med Chem 60:5086–5098. [DOI] [PubMed] [Google Scholar]
- Engelhardt D, Weber MM. 1994. Therapy of Cushing’s syndrome with steroid biosynthesis inhibitors. J Steroid Biochem Mol Biol 49:261–267. [DOI] [PubMed] [Google Scholar]
- Fassnacht M, Hahner S, Beuschlein F, Klink A, Reincke M, Allolio B. 2000. New mechanisms of adrenostatic compounds in a human adrenocortical cancer cell line. Eur J Clin Invest 30 (Suppl 3):76–82. [DOI] [PubMed] [Google Scholar]
- Fisher A, Friel EC, Bernhardt R, Gomez-Sanchez C, Connell JM, Fraser R, Davies E. 2001. Effects of 18-hydroxylated steroids on corticosteroid production by human aldosterone synthase and 11β-hydroxylase. J Clin Endocrinol Metab 86:4326–4329. [DOI] [PubMed] [Google Scholar]
- Gobbi S, Hu Q, Zimmer C, Belluti F, Rampa A, Hartmann RW, Bisi A. 2016. Targeting steroidogenic cytochromes P450 (CYPs) with 6-substituted 1-imidazolylmethylxanthones. ChemMedChem 11:1770–1777. [DOI] [PubMed] [Google Scholar]
- Gobbi S, Hu Q, Zimmer C, Belluti F, Rampa A, Hartmann RW, Bisi A. 2017. Drifting of heme-coordinating group in imidazolylmethylxanthones leading to improved selective inhibition of CYP11B1. Eur J Med Chem 139:60–67. [DOI] [PubMed] [Google Scholar]
- Grombein CM, Hu Q, Heim R, Rau S, Zimmer C, Hartmann RW. 2015. 1-Phenylsulfinyl-3-(pyridin-3-yl)naphthalen-2-ols: A new class of potent and selective aldosterone synthase inhibitors. Eur J Med Chem 89:597–605. [DOI] [PubMed] [Google Scholar]
- Guengerich FP. 2015. Chapter 9, Human cytochrome P450 enzymes. In: Ortiz de Montellano PR, editor. Cytochrome P450: Structure, Mechanism, and Biochemistry 4th ed. New York: Springer; p. 523–785. [Google Scholar]
- Hahner S, Stuermer A, Kreissl M, Reiners C, Fassnacht M, Haenscheid H, Beuschlein F, Zink M, Lang K, Allolio B et al. 2008. [123I]-Iodometomidate for molecular imaging of adrenocortical cytochrome P450 family 11B enzymes. J Clin Endocrinol Metab 93:2358–2365. [DOI] [PubMed] [Google Scholar]
- Hartmann RW, Muller U, Ehmer PB. 2003. Discovery of selective CYP11B2 (aldosterone synthase) inhibitors for the therapy of congestive heart failure and myocardial fibrosis. Eur J Med Chem 38:363–366. [DOI] [PubMed] [Google Scholar]
- Hu Q, Yin L, Ali A, Cooke AJ, Bennett J, Ratcliffe P, Lo MM, Metzger E, Hoyt S, Hartmann RW. 2015. Novel pyridyl substituted 4,5-dihydro-[1,2,4]triazolo[4,3-a]quinolines as potent and selective aldosterone synthase inhibitors with improved in vitro metabolic stability. J Med Chem 58:2530–2537. [DOI] [PubMed] [Google Scholar]
- Hu Q, Yin L, Jagusch C, Hille UE, Hartmann RW. 2010. Isopropylidene substitution increases activity and selectivity of biphenylmethylene 4-pyridine type CYP17 inhibitors. J Med Chem 53:5049–5053. [DOI] [PubMed] [Google Scholar]
- Johansson M, Larsson C, Bergman A, Lund BO. 1998. Structure-activity relationship for inhibition of CYP11B1-dependent glucocorticoid synthesis in Y1 cells by aryl methyl sulfones. Pharmacol Toxicol 83:225–230. [DOI] [PubMed] [Google Scholar]
- Johansson MK, Sanderson JT, Lund BO. 2002. Effects of 3-MeSO2-DDE and some CYP inhibitors on glucocorticoid steroidogenesis in the H295R human adrenocortical carcinoma cell line. Toxicol In Vitro 16:113–121. [DOI] [PubMed] [Google Scholar]
- Kau MM, Kan SF, Wang JR, Wang PS. 2005. Inhibitory effects of digoxin and ouabain on aldosterone synthesis in human adrenocortical NCI-H295 cells. J Cell Physiol 205:393–401. [DOI] [PubMed] [Google Scholar]
- Kau MM, Wang JR, Tsai SC, Yu CH, Wang PS. 2012. Inhibitory effect of bufalin and cinobufagin on steroidogenesis via the activation of ERK in human adrenocortical cells. Brit J Pharmacol 165:1868–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon CJ, Fraser R, Birnie GG, Connell JM, Lever AF. 1986. Dose related in vitro effects of ranitidine and cimetidine on basal and ACTH-stimulated steroidogenesis. Gut 27:1143–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Moon JY, Choi MH, Yang HH, Lee S, Lim KS, Yoon SH, Yu KS, Jang IJ, Cho JY. 2013. Global metabolomics and targeted steroid profiling reveal that rifampin, a strong human PXR activator, alters endogenous urinary steroid markers. J Proteome Res 12:1359–1368. [DOI] [PubMed] [Google Scholar]
- Lin CW, Chang YH, Pu HF. 2012. Mitotane exhibits dual effects on steroidogenic enzymes gene transcription under basal and cAMP-stimulating microenvironments in NCI-H295 cells. Toxicology 298:14–23. [DOI] [PubMed] [Google Scholar]
- Lin TC, Chien SC, Hsu PC, Li LA. 2006. Mechanistic study of polychlorinated biphenyl 126-induced CYP11B1 and CYP11B2 up-regulation. Endocrinology 147:1536–1544. [DOI] [PubMed] [Google Scholar]
- Mitrunen K, Jourenkova N, Kataja V, Eskelinen M, Kosma VM, Benhamou S, Vainio H, Uusitupa M, Hirvonen A. 2000. Steroid metabolism gene CYP17 polymorphism and the development of breast cancer. Cancer Epidemiol Biomarkers Prev 9:1343–1348. [PubMed] [Google Scholar]
- Muller-Vieira U, Angotti M, Hartmann RW. 2005. The adrenocortical tumor cell line NCI-H295R as an in vitro screening system for the evaluation of CYP11B2 (aldosterone synthase) and CYP11B1 (steroid-11β-hydroxylase) inhibitors. J Steroid Biochem Mol Biol 96:259–270. [DOI] [PubMed] [Google Scholar]
- Nonaka Y, Okamoto M. 1991. Functional expression of the cDNAs encoding rat 11β-hydroxylase [cytochrome P450(11β)] and aldosterone synthase [cytochrome P450(11β, aldo)]. Eur J Biochem 202:897–902. [DOI] [PubMed] [Google Scholar]
- Okahara K, Sun B, Kambayashi J. 1998. Upregulation of prostacyclin synthesis-related gene expression by shear stress in vascular endothelial cells. Arteriosclerosis Thrombosis Vascular Biol 18:1922–1926. [DOI] [PubMed] [Google Scholar]
- Otton SV, Schadel M, Cheung SW, Kaplan HL, Busto UE, Sellers EM. 1993. CYP2D6 phenotype determines the metabolic conversion of hydrocodone to hydromorphone. Clin. Pharmacol. Therapeut 54:463–472. [DOI] [PubMed] [Google Scholar]
- Pan ST, Xue D, Li ZL, Zhou ZW, He ZX, Yang Y, Yang T, Qiu JX, Zhou SF. 2016. Computational identification of the paralogs and orthologs of human cytochrome P450 superfamily and the implication in drug discovery. Int J Mol Sci June 28;17(7). pii: ijms17071020. doi: 10.3390/ijms17071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papillon JP, Adams CM, Hu QY, Lou C, Singh AK, Zhang C, Carvalho J, Rajan S, Amaral A, Beil ME et al. 2015. Structure-activity relationships, pharmacokinetics, and in vivo activity of CYP11B2 and CYP11B1 inhibitors. J Med Chem 58:4749–4770. [DOI] [PubMed] [Google Scholar]
- Parr MK, Zöllner A, Fussholler G, Opfermann G, Schlörer N, Zorio M, Bureik M, Schanzer W. 2012. Unexpected contribution of cytochrome P450 enzymes CYP11B2 and CYP21, as well as CYP3A4 in xenobiotic androgen elimination–insights from metandienone metabolism. Toxicol Lett 213:381–391. [DOI] [PubMed] [Google Scholar]
- Pinto-Bazurco Mendieta MA, Negri M, Jagusch C, Muller-Vieira U, Lauterbach T, Hartmann RW. 2008. Synthesis, biological evaluation, and molecular modeling of abiraterone analogues: novel CYP17 inhibitors for the treatment of prostate cancer. J Med Chem 51:5009–5018. [DOI] [PubMed] [Google Scholar]
- Roumen L, Sanders MP, Pieterse K, Hilbers PA, Plate R, Custers E, de Gooyer M, Smits JF, Beugels I, Emmen J et al. 2007. Construction of 3D models of the CYP11B family as a tool to predict ligand binding characteristics. J Comput Aided Mol Des 21:455–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffer L, Brixius-Anderko S, Hannemann F, Zapp J, Neunzig J, Thevis M, Bernhardt R. 2016. Metabolism of oral turinabol by human steroid hormone-synthesizing cytochrome P450 enzymes. Drug Metab Dispos 44:227–237. [DOI] [PubMed] [Google Scholar]
- Schiffer L, Müller AR, Hobler A, Brixius-Anderko S, Zapp J, Hannemann F, Bernhardt R. 2016. Biotransformation of the mineralocorticoid receptor antagonists spironolactone and canrenone by human CYP11B1 and CYP11B2: Characterization of the products and their influence on mineralocorticoid receptor transactivation. J Steroid Biochem Mol Biol 163:68–76. [DOI] [PubMed] [Google Scholar]
- Schroeder RL, Tram P, Liu J, Foroozesh M, Sridhar J. 2016. Novel functionalized 5-(phenoxymethyl)-1,3-dioxane analogs exhibiting cytochrome P450 inhibition: A patent evaluation WO2015048311 (A1). Exp Opin Therapeut Patents 26:139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmschneider S, Muller-Vieira U, Mitrenga M, Hartmann RW, Oberwinkler-Marchais S, Klein CD, Bureik M, Bernhardt R, Antes I, Lengauer T. 2005. Synthesis and evaluation of imidazolylmethylenetetrahydronaphthalenes and imidazolylmethyleneindanes: Potent inhibitors of aldosterone synthase. J Med Chem 48:1796–1805. [DOI] [PubMed] [Google Scholar]
- van Koetsveld PM, Vitale G, Feelders RA, Waaijers M, Sprij-Mooij DM, de Krijger RR, Speel EJ, Hofland J, Lamberts SW, de Herder WW et al. 2013. Interferon-β is a potent inhibitor of cell growth and cortisol production in vitro and sensitizes human adrenocortical carcinoma cells to mitotane. Endocrine Related Cancer 20:443–454. [DOI] [PubMed] [Google Scholar]
- Vermeulen A, Paridaens R, Heuson JC. 1983. Effects of aminoglutethimide on adrenal steroid secretion. Clin Endocrinol 19:673–682. [DOI] [PubMed] [Google Scholar]
- Voets M, Antes I, Scherer C, Muller-Vieira U, Biemel K, Barassin C, Marchais-Oberwinkler S, Hartmann RW. 2005. Heteroaryl-substituted naphthalenes and structurally modified derivatives: Selective inhibitors of CYP11B2 for the treatment of congestive heart failure and myocardial fibrosis. J Med Chem 48:6632–6642. [DOI] [PubMed] [Google Scholar]
- Voets M, Antes I, Scherer C, Muller-Vieira U, Biemel K, Marchais-Oberwinkler S, Hartmann RW. 2006. Synthesis and evaluation of heteroaryl-substituted dihydronaphthalenes and indenes: potent and selective inhibitors of aldosterone synthase (CYP11B2) for the treatment of congestive heart failure and myocardial fibrosis. J Med Chem 49:2222–2231. [DOI] [PubMed] [Google Scholar]
- Vukelic S, Stojadinovic O, Pastar I, Rabach M, Krzyzanowska A, Lebrun E, Davis SC, Resnik S, Brem H, Tomic-Canic M. 2011. Cortisol synthesis in epidermis is induced by IL-1 and tissue injury. J Biol Chem 286:10265–10275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker BR. 2006. Cortisol--cause and cure for metabolic syndrome? Diabetic Med 23:1281–1288. [DOI] [PubMed] [Google Scholar]
- Yin LN, Lucas S, Maurer F, Kazmaier U, Hu QZ, Hartmann RW. 2012. Novel imidazol-1-ylmethyl substituted 1,2,5,6-tetrahydropyrrolo-3,2,1-ij-quinolin-4-ones as potent and selective CYP11B1 inhibitors for the treatment of Cushing’s syndrome. J Med Chem 55:6629–6633. [DOI] [PubMed] [Google Scholar]
- Zhu W, Hu Q, Hanke N, van Koppen CJ, Hartmann RW. 2014. Potent 11β-hydroxylase inhibitors with inverse metabolic stability in human plasma and hepatic S9 fractions to promote wound healing. J Med Chem 57:7811–7817. [DOI] [PubMed] [Google Scholar]
P450 11B2
As mentioned earlier under P450 11B1, there are only 32 residues that differ between P450s 11B1 and 11B2, and their catalytic specificities overlap. The CYP11B2 gene is also highly regulated (Guengerich 2015). Many CYP11B2 genetic variants are known and related to a number of diseases (Guengerich 2015). “Crossovers” between the CYP11B1 and CYP11B2 genes are generally inactivating for both enzymes.
X-ray crystal structures of human P450 11B2 have been published, with the substrate deoxycorticosterone and also with the inhibitor fadrazole, as well as another inhibitor (Strushkevich et al. 2013 Martin, 2015, 59534) (PDB 4DVQ, 4ZGX, 4FDH).
P450 11B2 catalyzes the 3-step conversion of 11-deoxycorticosterone to aldosterone, with 11β-hydroxylation, 18-hydroxylation, and 2-electron oxidation of the 18-carbinol. Strushkevich et al. (2013) have proposed that this is a processive reaction, with no intermediates released. However, this proposal has not been examined more rigorously, e.g. with time course and pulse-chase experiments. The non-classical substrate methandienone is oxidized to the 11β- and 20β-norsteroid products (Parr et al. 2012).
Although a number of cases of genetic insufficiency are known P450 11B2 is a target for intervention in hypertension, with at least two major pharmaceutical firms screening for inhibitors.
P450 11B2 is involved in the biosynthesis of aldosterone, which has a critical role in blood pressure control (maintaining potassium and sodium homeostasis) and in cardiovascular and renal disease (Brown 2005; Cohn and Colucci 2006; Pimenta and Calhoun 2006; Funder and Mihailidou 2009). Excess aldosterone is linked to hypertension onset (Vasan et al. 2004) and is believed to play a critical role in mediating and aggravating resistant hypertension (Whaley-Connell et al. 2010), a common clinical problem for 20–28% of patients (Calhoun et al. 2008; Egan et al. 2011). Lowering aldosterone represents an attractive therapeutic approach to lower blood pressure and reducing the risk of cardiovascular events and end-organ damage.
An alternative approach to attenuating the effects of aldosterone is to decrease circulating levels by inhibiting its synthesis, preferably at the level of P450 11B2, which mediates the rate-limiting conversion of 11-deoxycorticosterone to aldosterone (Karns et al. 2013).
Despite the relatedness of P450s 11B1 and 11B2, it has been possible to identify compounds with > 500-fold selectivity (Voets et al. 2006).
References
- Amar L, Azizi M, Menard J, Peyrard S, Watson C, Plouin PF. 2010. Aldosterone synthase inhibition with LCI699: A proof-of-concept study in patients with primary aldosteronism. Hypertension 56:831–838. [DOI] [PubMed] [Google Scholar]
- Asp V, Ulleras E, Lindstrom V, Bergstrom U, Oskarsson A, Brandt I. 2010. Biphasic hormonal responses to the adrenocorticolytic DDT metabolite 3-methylsulfonyl-DDE in human cells. Tox Appl Pharmacol 242:281–289. [DOI] [PubMed] [Google Scholar]
- Azizi M, Amar L, Menard J. 2013. Aldosterone synthase inhibition in humans. Nephrol Dialysis Transplant 28:36–43. [DOI] [PubMed] [Google Scholar]
- Bernhardt R 2016. The potential of targeting CYP11B. Exp Opin Therapeut Targets 20:923–934. [DOI] [PubMed] [Google Scholar]
- Blaha L, Hilscherova K, Mazurova E, Hecker M, Jones PD, Newsted JL, Bradley PW, Gracia T, Duris Z, Horka I et al. 2006. Alteration of steroidogenesis in H295R cells by organic sediment contaminants and relationships to other endocrine disrupting effects. Environ Int 32:749–757. [DOI] [PubMed] [Google Scholar]
- Bogman K, Schwab D, Delporte ML, Palermo G, Amrein K, Mohr S, De Vera Mudry MC, Brown MJ, Ferber P. 2017. Preclinical and early clinical profile of a highly selective and potent oral inhibitor of aldosterone synthase (CYP11B2). Hypertension 69:189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown NJ. 2005. Aldosterone and end-organ damage. Curr Opin Nephrol Hypertens 14:235–241. [DOI] [PubMed] [Google Scholar]
- Brunssen C, Hofmann A, Peitzsch M, Frenzel A, Ziegler CG, Brown NF, Weldon SM, Eisenhofer G, Willenberg HS, Bornstein SR et al. 2017. Impact of aldosterone synthase inhibitor FAD286 on steroid hormone profile in human adrenocortical cells. Hormone Metab Res 49:701–706. [DOI] [PubMed] [Google Scholar]
- Bureik M, Hubel K, Dragan CA, Scher J, Becker H, Lenz N, Bernhardt R. 2004. Development of test systems for the discovery of selective human aldosterone synthase (CYP11B2) and 11β-hydroxylase (CYP11B1) inhibitors. Discovery of a new lead compound for the therapy of congestive heart failure, myocardial fibrosis and hypertension. Mol Cell Endocrinol 217:249–254. [DOI] [PubMed] [Google Scholar]
- Bureik M, Mion A, Kenyon CJ, Bernhardt R. 2005. Inhibition of aldosterone biosynthesis by staurosporine. Biol Chem 386:663–669. [DOI] [PubMed] [Google Scholar]
- Bureik M, Zeeh A, Bernhardt R. 2002. Modulation of steroid hydroxylase activity in stably transfected V79MZh11B1 and V79MZh11B2 cells by PKC and PKD inhibitors. Endocr Res 28:351–355. [DOI] [PubMed] [Google Scholar]
- Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D et al. 2008. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension 51:1403–1419. [DOI] [PubMed] [Google Scholar]
- Cohn JN, Colucci W. 2006. Cardiovascular effects of aldosterone and post-acute myocardial infarction pathophysiology. Am J Cardiol 97:4f–12f. [DOI] [PubMed] [Google Scholar]
- Condon JC, Pezzi V, Drummond BM, Yin S, Rainey WE. 2002. Calmodulin-dependent kinase I regulates adrenal cell expression of aldosterone synthase. Endocrinology 143:3651–3657. [DOI] [PubMed] [Google Scholar]
- Coulter CL, Jaffe RB. 1998. Functional maturation of the primate fetal adrenal in vivo: 3. Specific zonal localization and developmental regulation of CYP21A2 (P450c21) and CYP11B1/CYP11B2 (P450c11/aldosterone synthase) lead to integrated concept of zonal and temporal steroid biosynthesis. Endocrinology 139:5144–5150. [DOI] [PubMed] [Google Scholar]
- Denner K, Rainey WE, Pezzi V, Bird IM, Bernhardt R, Mathis JM. 1996. Differential regulation of 11β-hydroxylase and aldosterone synthase in human adrenocortical H295R cells. Mol Cell Endocrinol 121:87–91. [DOI] [PubMed] [Google Scholar]
- Ding L, Murphy MB, He Y, Xu Y, Yeung LW, Wang J, Zhou B, Lam PK, Wu RS, Giesy JP. 2007. Effects of brominated flame retardants and brominated dioxins on steroidogenesis in H295R human adrenocortical carcinoma cell line. Environ Toxicol Chem 26:764–772. [DOI] [PubMed] [Google Scholar]
- Egan BM, Zhao Y, Axon RN, Brzezinski WA, Ferdinand KC. 2011. Uncontrolled and apparent treatment resistant hypertension in the United States, 1988 to 2008. Circulation 124:1046–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehmer PB, Bureik M, Bernhardt R, Muller U, Hartmann RW. 2002. Development of a test system for inhibitors of human aldosterone synthase (CYP11B2): Screening in fission yeast and evaluation of selectivity in V79 cells. J Steroid Biochem Mol Biol 81:173–179. [DOI] [PubMed] [Google Scholar]
- Funder JW, Mihailidou AS. 2009. Aldosterone and mineralocorticoid receptors: Clinical studies and basic biology. Mol Cell Endocrinol 301:2–6. [DOI] [PubMed] [Google Scholar]
- Gennari-Moser C, Khankin EV, Escher G, Burkhard F, Frey BM, Karumanchi SA, Frey FJ, Mohaupt MG. 2013. Vascular endothelial growth factor-A and aldosterone: relevance to normal pregnancy and preeclampsia. Hypertension 61:1111–1117. [DOI] [PubMed] [Google Scholar]
- Gobbi S, Hu Q, Zimmer C, Belluti F, Rampa A, Hartmann RW, Bisi A. 2016. Targeting steroidogenic cytochromes P450 (CYPs) with 6-substituted 1-imidazolylmethylxanthones. ChemMedChem 11:1770–1777. [DOI] [PubMed] [Google Scholar]
- Grombein CM, Hu Q, Heim R, Rau S, Zimmer C, Hartmann RW. 2015. 1-Phenylsulfinyl-3-(pyridin-3-yl)naphthalen-2-ols: A new class of potent and selective aldosterone synthase inhibitors. Eur J Med Chem 89:597–605. [DOI] [PubMed] [Google Scholar]
- Guengerich FP. 2015. Chapter 9, Human cytochrome P450 enzymes. In: Ortiz de Montellano PR, editor. Cytochrome P450: Structure, Mechanism, and Biochemistry 4th ed. New York: Springer; p. 523–785. [Google Scholar]
- Hahner S, Stuermer A, Kreissl M, Reiners C, Fassnacht M, Haenscheid H, Beuschlein F, Zink M, Lang K, Allolio B et al. 2008. [123I]-Iodometomidate for molecular imaging of adrenocortical cytochrome P450 family 11B enzymes. J Clin Endocrinol Metab 93:2358–2365. [DOI] [PubMed] [Google Scholar]
- Hakki T, Hubel K, Waldmann H, Bernhardt R. 2011. The development of a whole-cell based medium throughput screening system for the discovery of human aldosterone synthase (CYP11B2) inhibitors: old drugs disclose new applications for the therapy of congestive heart failure, myocardial fibrosis and hypertension. J Steroid Biochem Mol Biol 125:120–128. [DOI] [PubMed] [Google Scholar]
- Hartmann RW, Muller U, Ehmer PB. 2003. Discovery of selective CYP11B2 (aldosterone synthase) inhibitors for the therapy of congestive heart failure and myocardial fibrosis. Eur J Med Chem 38:363–366. [DOI] [PubMed] [Google Scholar]
- Hoyt SB, Taylor J, London C, Ali A, Ujjainwalla F, Tata J, Struthers M, Cully D, Wisniewski T, Ren N et al. 2017. Discovery of indazole aldosterone synthase (CYP11B2) inhibitors as potential treatments for hypertension. Bioorg Med Chem Lett 27:2384–2388. [DOI] [PubMed] [Google Scholar]
- Hu Q, Jagusch C, Hille UE, Haupenthal J, Hartmann RW. 2010. Replacement of imidazolyl by pyridyl in biphenylmethylenes results in selective CYP17 and dual CYP17/CYP11B1 inhibitors for the treatment of prostate cancer. J Med Chem 53:5749–5758. [DOI] [PubMed] [Google Scholar]
- Hu Q, Yin L, Ali A, Cooke AJ, Bennett J, Ratcliffe P, Lo MM, Metzger E, Hoyt S, Hartmann RW. 2015. Novel pyridyl substituted 4,5-dihydro-[1,2,4]triazolo[4,3-a]quinolines as potent and selective aldosterone synthase inhibitors with improved in vitro metabolic stability. J Med Chem 58:2530–2537. [DOI] [PubMed] [Google Scholar]
- Karns AD, Bral JM, Hartman D, Peppard T, Schumacher C. 2013. Study of aldosterone synthase inhibition as an add-on therapy in resistant hypertension. J Clin Hypertens (Greenwich, CT) 15:186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kau MM, Kan SF, Wang JR, Wang PS. 2005. Inhibitory effects of digoxin and ouabain on aldosterone synthesis in human adrenocortical NCI-H295 cells. J Cell Physiol 205:393–401. [DOI] [PubMed] [Google Scholar]
- Kau MM, Wang JR, Tsai SC, Yu CH, Wang PS. 2012. Inhibitory effect of bufalin and cinobufagin on steroidogenesis via the activation of ERK in human adrenocortical cells. Brit J Pharmacol 165:1868–1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshava C, Whipkey D, Weston A. 2005. Transcriptional signatures of environmentally relevant exposures in normal human mammary epithelial cells: Benzo[a]pyrene. Cancer Lett 221:201–211. [DOI] [PubMed] [Google Scholar]
- Kim B, Moon JY, Choi MH, Yang HH, Lee S, Lim KS, Yoon SH, Yu KS, Jang IJ, Cho JY. 2013. Global metabolomics and targeted steroid profiling reveal that rifampin, a strong human PXR activator, alters endogenous urinary steroid markers. J Proteome Res 12:1359–1368. [DOI] [PubMed] [Google Scholar]
- Kobuke K, Oki K, Gomez-Sanchez CE, Gomez-Sanchez EP, Ohno H, Itcho K, Yoshii Y, Yoneda M, Hattori N. 2018. Calneuron 1 increased Ca2+ in the endoplasmic reticulum and aldosterone production in aldosterone-producing adenoma. Hypertension 71:125–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeHoux JG, Dupuis G, Lefebvre A. 2001. Control of CYP11B2 gene expression through differential regulation of its promoter by atypical and conventional protein kinase C isoforms. J Biol Chem 276:8021–8028. [DOI] [PubMed] [Google Scholar]
- LeHoux JG, Lefebvre A. 2004. On the control of the hCYP11B2 gene expressing cytochrome P450 aldosterone synthase. Endocr Res 30:807–812. [DOI] [PubMed] [Google Scholar]
- Lin TC, Chien SC, Hsu PC, Li LA. 2006. Mechanistic study of polychlorinated biphenyl 126-induced CYP11B1 and CYP11B2 up-regulation. Endocrinology 147:1536–1544. [DOI] [PubMed] [Google Scholar]
- Lucas S, Heim R, Negri M, Antes I, Ries C, Schewe KE, Bisi A, Gobbi S, Hartmann RW. 2008. Novel aldosterone synthase inhibitors with extended carbocyclic skeleton by a combined ligand-based and structure-based drug design approach. J Med Chem 51:6138–6149. [DOI] [PubMed] [Google Scholar]
- Lucas S, Heim R, Ries C, Schewe KE, Birk B, Hartmann RW. 2008. In vivo active aldosterone synthase inhibitors with improved selectivity: lead optimization providing a series of pyridine substituted 3,4-dihydro-1H-quinolin-2-one derivatives. J Med Chem 51:8077–8087. [DOI] [PubMed] [Google Scholar]
- Matsuda K, Uruno A, Kogure N, Sugawara K, Shimada H, Nezu M, Saito-Ito T, Iki Y, Kudo M, Shimizu K et al. 2014. Angiotensin II receptor blockers differentially affect CYP11B2 expression in human adrenal H295R cells. Mol Cell Endocrinol 383:60–68. [DOI] [PubMed] [Google Scholar]
- Meguro M, Miyauchi S, Kanao Y, Naito S, Suzuki K, Inoue S, Yamada K, Homma T, Chiba K, Nara F et al. 2017. 4-Anilino-pyrimidine, novel aldosterone synthase (CYP11B2) inhibitors bearing pyrimidine structures. Bioorg Med Chem Lett 27:1902–1906. [DOI] [PubMed] [Google Scholar]
- Moore TD, Nawarskas JJ, Anderson JR. 2003. Eplerenone: A selective aldosterone receptor antagonist for hypertension and heart failure. Heart Disease (Hagerstown, MD) 5:354–363. [DOI] [PubMed] [Google Scholar]
- Muller-Vieira U, Angotti M, Hartmann RW. 2005. The adrenocortical tumor cell line NCI-H295R as an in vitro screening system for the evaluation of CYP11B2 (aldosterone synthase) and CYP11B1 (steroid-11β-hydroxylase) inhibitors. J Steroid Biochem Mol Biol 96:259–270. [DOI] [PubMed] [Google Scholar]
- Pan ST, Xue D, Li ZL, Zhou ZW, He ZX, Yang Y, Yang T, Qiu JX, Zhou SF. 2016. Computational identification of the paralogs and orthologs of human cytochrome P450 superfamily and the implication in drug discovery. Int J Mol Sci June 28;17(7). pii: ijms17071020. doi: 10.3390/ijms17071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papillon JP, Adams CM, Hu QY, Lou C, Singh AK, Zhang C, Carvalho J, Rajan S, Amaral A, Beil ME et al. 2015. Structure-activity relationships, pharmacokinetics, and in vivo activity of CYP11B2 and CYP11B1 inhibitors. J Med Chem 58:4749–4770. [DOI] [PubMed] [Google Scholar]
- Parr MK, Zöllner A, Fussholler G, Opfermann G, Schlörer N, Zorio M, Bureik M, Schanzer W. 2012. Unexpected contribution of cytochrome P450 enzymes CYP11B2 and CYP21, as well as CYP3A4 in xenobiotic androgen elimination–insights from metandienone metabolism. Toxicol Lett 213:381–391. [DOI] [PubMed] [Google Scholar]
- Pimenta E, Calhoun DA. 2006. Aldosterone, dietary salt, and renal disease. Hypertension 48:209–210. [DOI] [PubMed] [Google Scholar]
- Pinto-Bazurco Mendieta MA, Negri M, Jagusch C, Muller-Vieira U, Lauterbach T, Hartmann RW. 2008. Synthesis, biological evaluation, and molecular modeling of abiraterone analogues: Novel CYP17 inhibitors for the treatment of prostate cancer. J Med Chem 51:5009–5018. [DOI] [PubMed] [Google Scholar]
- Roumen L, Sanders MP, Pieterse K, Hilbers PA, Plate R, Custers E, de Gooyer M, Smits JF, Beugels I, Emmen J et al. 2007. Construction of 3D models of the CYP11B family as a tool to predict ligand binding characteristics. J Comput Aided Mol Des 21:455–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roumen L, Van Hoof B, Pieterse K, Hilbers PA, Custers EM, Plate R, De Gooyer M, Beugels IP, Emmen JM, Leysen D et al. 2011. Application of a ligand-based theoretical approach to derive conversion paths and ligand conformations in CYP11B2-mediated aldosterone formation. J Computat Chem 32:2441–2448. [DOI] [PubMed] [Google Scholar]
- Schiffer L, Brixius-Anderko S, Hannemann F, Zapp J, Neunzig J, Thevis M, Bernhardt R. 2016. Metabolism of oral turinabol by human steroid hormone-synthesizing cytochrome P450 enzymes. Drug Metab Dispos 44:227–237. [DOI] [PubMed] [Google Scholar]
- Schiffer L, Muller AR, Hobler A, Brixius-Anderko S, Zapp J, Hannemann F, Bernhardt R. 2016. Biotransformation of the mineralocorticoid receptor antagonists spironolactone and canrenone by human CYP11B1 and CYP11B2: Characterization of the products and their influence on mineralocorticoid receptor transactivation. J Steroid Biochem Mol Biol 163:68–76. [DOI] [PubMed] [Google Scholar]
- Schuster I, Bernhardt R. 2007. Inhibition of cytochromes P450: Existing and new promising therapeutic targets. Drug Metab Rev 39:481–499. [DOI] [PubMed] [Google Scholar]
- Shimada H, Kogure N, Noro E, Kudo M, Sugawara K, Sato I, Shimizu K, Kobayashi M, Suzuki D, Parvin R et al. 2017. High glucose stimulates expression of aldosterone synthase (CYP11B2) and secretion of aldosterone in human adrenal cells. FEBS Open Biol 7:1410–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R, He Y, Murphy MB, Yeung LW, Yu RM, Lam MH, Lam PK, Hecker M, Giesy JP, Wu RS et al. 2008. Effects of fifteen PBDE metabolites, DE71, DE79 and TBBPA on steroidogenesis in the H295R cell line. Chemosphere 71:1888–1894. [DOI] [PubMed] [Google Scholar]
- Strushkevich N, Gilep AA, Shen L, Arrowsmith CH, Edwards AM, Usanov SA, Park HW. 2013. Structural insights into aldosterone synthase substrate specificity and targeted inhibition. Mol Endocrinol 27:315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki D, Saito-Hakoda A, Ito R, Shimizu K, Parvin R, Shimada H, Noro E, Suzuki S, Fujiwara I, Kagechika H et al. 2017. Suppressive effects of RXR agonist PA024 on adrenal CYP11B2 expression, aldosterone secretion and blood pressure. PloS One 12:e0181055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai YY, Rainey WE, Bollag WB. 2017. Very low-density lipoprotein (VLDL)-induced signals mediating aldosterone production. J Endocrinol 232:R115–R129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmschneider S, Muller-Vieira U, Mitrenga M, Hartmann RW, Oberwinkler-Marchais S, Klein CD, Bureik M, Bernhardt R, Antes I, Lengauer T. 2005. Synthesis and evaluation of imidazolylmethylenetetrahydronaphthalenes and imidazolylmethyleneindanes: Potent inhibitors of aldosterone synthase. J Med Chem 48:1796–1805. [DOI] [PubMed] [Google Scholar]
- Ulmschneider S, Negri M, Voets M, Hartmann RW. 2006. Development and evaluation of a pharmacophore model for inhibitors of aldosterone synthase (CYP11B2). Bioorg Med Chem Lett 16:25–30. [DOI] [PubMed] [Google Scholar]
- Vasan RS, Evans JC, Larson MG, Wilson PW, Meigs JB, Rifai N, Benjamin EJ, Levy D. 2004. Serum aldosterone and the incidence of hypertension in nonhypertensive persons. New Engl J Med 351:33–41. [DOI] [PubMed] [Google Scholar]
- Voets M, Antes I, Scherer C, Muller-Vieira U, Biemel K, Barassin C, Marchais-Oberwinkler S, Hartmann RW. 2005. Heteroaryl-substituted naphthalenes and structurally modified derivatives: selective inhibitors of CYP11B2 for the treatment of congestive heart failure and myocardial fibrosis. J Med Chem 48:6632–6642. [DOI] [PubMed] [Google Scholar]
- Voets M, Antes I, Scherer C, Muller-Vieira U, Biemel K, Marchais-Oberwinkler S, Hartmann RW. 2006. Synthesis and evaluation of heteroaryl-substituted dihydronaphthalenes and indenes: potent and selective inhibitors of aldosterone synthase (CYP11B2) for the treatment of congestive heart failure and myocardial fibrosis. J Med Chem 49:2222–2231. [DOI] [PubMed] [Google Scholar]
- Whaley-Connell A, Johnson MS, Sowers JR. 2010. Aldosterone: role in the cardiometabolic syndrome and resistant hypertension. Progress in cardiovascular diseases 52:401–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White WB, Carr AA, Krause S, Jordan R, Roniker B, Oigman W. 2003. Assessment of the novel selective aldosterone blocker eplerenone using ambulatory and clinical blood pressure in patients with systemic hypertension. Am J Cardiol 92:38–42. [DOI] [PubMed] [Google Scholar]
- Whitehead BR, Lo MM, Ali A, Park MK, Hoyt SB, Xiong Y, Cai J, Carswell E, Cooke A, MacLean J et al. 2017. Imidazopyridyl compounds as aldosterone synthase inhibitors. Bioorg Med Chem Lett 27:143–146. [DOI] [PubMed] [Google Scholar]
- Xing Y, Cohen A, Rothblat G, Sankaranarayanan S, Weibel G, Royer L, Francone OL, Rainey WE. 2011. Aldosterone production in human adrenocortical cells is stimulated by high-density lipoprotein 2 (HDL2) through increased expression of aldosterone synthase (CYP11B2). Endocrinology 152:751–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin L, Hu Q, Emmerich J, Lo MM, Metzger E, Ali A, Hartmann RW. 2014. Novel pyridyl- or isoquinolinyl-substituted indolines and indoles as potent and selective aldosterone synthase inhibitors. J Med Chem 57:5179–5189. [DOI] [PubMed] [Google Scholar]
P450 17A1
P450 17A1 functions at an important branch point in human steroidogenesis (Miller and Auchus 2011). P450 17A1 catalysis leads to either (i) precursors of glucocorticoids (e.g., cortisol) that regulate immune responses or (ii) androgens (e.g., testosterone) that drive the development and maintenance of male characteristics or are converted to estrogens (Gilep et al. 2011).
In addition to 17α-hydroxylation, P450 17A1 also performs a carbon-carbon bond cleavage, which is unusual for cytochrome P450 enzymes (Zuber et al. 1985; Sohl and Guengerich 2010; Guengerich 2015; Guengerich and Yoshimoto 2018). For human P450 17A1, this 17,20-lyase reaction proceeds less efficiently for the Δ4,3-keto 17α-hydroxyprogesterone substrate than for its counterpart, the Δ5,3-ol 17α-hydroxypregnenolone (Fig. 11). As a result, the Δ5,3-ol 17α-hydroxypregnenolone 17,20-lyase product dehydroepiandrosterone is considered the more physiologically relevant intermediate in the formation of all human androgens and estrogens (Auchus R. J. and Miller 2015; Guengerich 2015), although this should not be assumed to be the case in all species.
Fig. 11.
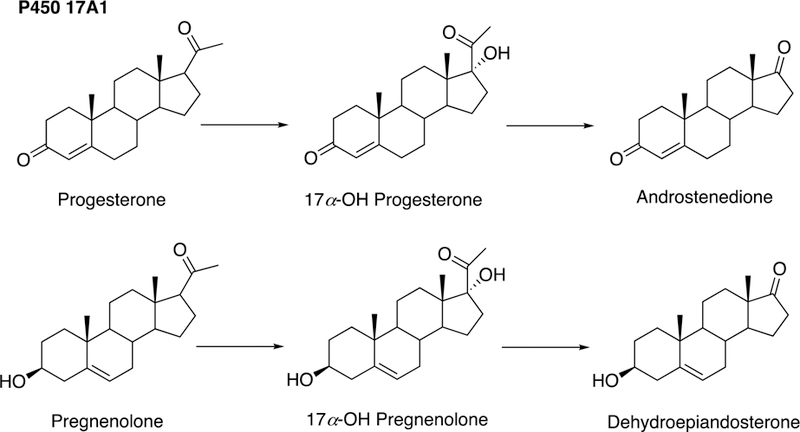
Two-step oxidations catalyzed by P450 17A1.
The addition of cytochrome b5 has a positive effect on the 17α-hydroxylation reactions, and it selectively facilitates the 17,20-lyase reaction (Fig. 11). Compartmentalization of cytochrome b5 and developmental changes in cytochrome b5 levels assist in controlling androgen production in humans, in that individuals with nonfunctional cytochrome b5 are impaired in performing the lyase reactions and produce sex steroids, although the hydroxylase reaction required for glucocorticoid synthesis is operational (Kok et al. 2010; Idkowiak et al. 2012). Facilitation of the lyase reaction by cytochrome b5 occurs without electron transfer (Auchus R.J. et al. 1998). Thus it has been suggested that cytochrome b5 might selectively stabilize the intermediate in the lyase reaction or cause substrates to assume orientations in the P450 17A1 active site more favorable for the lyase chemistry, but the details of the mechanism remain unresolved (Naffin-Olivos and Auchus 2006).
The structural basis for hydroxylase substrate regioselectivity, lyase reaction selectivity for the Δ4,3-keto versus Δ5,3-ol 17-hydroxylated substrate, and cytochrome b5 facilitation of the lyase versus hydroxylase reaction is still largely unknown. X-ray crystal structures of human P45017A1, in the presence of the four steroid substrates (Fig. 11) and the steroidal inhibitors abiraterone and TOK-001, have been published recently (DeVore and Scott 2012; Petrunak et al. 2014). The steroidal inhibitors both orient nearly perpendicular to the heme (DeVore and Scott 2012).
The CYP17A1 gene is highly regulated, in keeping with its critical role in androgen synthesis (Guengerich 2015). More than 50 deleterious genetic variants have been identified clinically. It is of interest that several of these strongly affect the lyase reaction but have only limited effects on the 17α-hydroxylation step (E305G, R347C, R347H, R358Q) (Geller et al. 1997; Van Den Akker et al. 2002; Sherbet et al. 2003). In addition, genetic variants that disrupt b5 activity show similar clinical responses (Kok et al. 2010; Idkowiak et al. 2012). However, understanding the basis of the selective loss of the lyase activity has been difficult. In teleost fish (including zebrafish), there are two genes, CYP17A1 and CYP17A2. The former yields a protein with the characteristics of human P450 17A1 (i.e., both 17α-hydroxylation and lyase activities) but P450 17A2 does only the former reaction, behaving like some of the human clinical variants. Structures of both zebrafish P450s (17A1, 17A2) have been obtained but look remarkably similar (Pallan et al. 2015) and switching the few differing residues near the heme periphery did not restore lyase activity (Gonzalez et al. 2018).
18O-labeling results are not unambiguous, in contrast to P450 19A1, but the lyase reaction can be supported by oxygen surrogates (iodosylbenzene and 17α-hydroperoxy steroids) (Yoshimoto et al. 2016; Gonzalez et al. 2018). Results with the 17α-hydroperoxy steroids support a dioxetane intermediate (Gonzalez et al. 2018).
One question is how processive the two sequential reactions are, i.e. whether the 17α-hydroxy product dissociates and rebinds (“distributive”) or remains bound to the enzyme through the lyase reaction (“processive”). Recent results indicate that human P450 17A1, in the presence of cytochrome b5, is distributive but that a fraction (~ ¼) of the 17α-hydroxypregnenolone remains bound to the enzyme for the lyase reaction, as revealed by pulse-chase and pre-steady-state kinetic modeling (Gonzalez and Guengerich 2017). Exactly how this model applies to the progesterone → androstenedione sequence has been addressed but more detail is needed (Gonzalez and Guengerich 2017). This information is highly relevant in efforts to develop selective inhibitors of the lyase step, which in principle might not be possible in a processive reaction.
Even the binding of substrate to P450 17A1 is complex and the data do not fit a simple diffusion-controlled 2-state model (Gonzalez and Guengerich 2017). There is evidence that multiple conformations of both unbound and inhibitor-bound exist, as judged by both NMR spectra and X-ray crystallographic data (Estrada et al. 2014; Petrunak et al. 2017). The kinetic data can be fit to a model in which both unbound and substrate-bound P450 17A1 have two conformations (Gonzalez and Guengerich 2017) but more details about what these conformations are and their relevance to catalysis remain obscure.
Androgens drive prostate cancer development, and estrogens are involved in hormone-responsive breast cancer (Edwards et al. 2014). Thus this enzyme has garnered substantial interest as a relatively new drug target, validated by successful use of the P450 17A1 inhibitor abiraterone in men with castration-resistant prostate cancer (de Bono et al. 2011; Auchus ML and Auchus 2012; Ferraldeschi and de Bono 2013) and its current evaluation in breast cancer patients. Several P450 17A1 inhibitors have been developed over the years, but only abiraterone has been approved by the FDA for treating prostate cancer. Some non-steroidal inhibitors (e.g., galaterone, orteronel, and VT-46411) have been evaluated in clinical trials. Abiraterone improves overall survival in men with metastatic castration-resistant prostate cancer (de Bono et al. 2011; Auchus ML and Auchus 2012; Loriot et al. 2013). The drug binds tightly to the P450 17A1 heme iron (DeVore and Scott 2012), preventing androgen production. However, this inhibition also increases the pool of mineralocorticoid precursors and blocks P450 17A1-mediated production of glucocorticoids. The situation is even more complex. Abiraterone is also an antagonist of the androgen receptor, and metabolites of P450 17A1 inhibitors can have their own biological activities (Alyamani et al. 2017). The resulting steroid imbalances in patients treated with abiraterone can frequently lead to hypertension, hypokalemia, and adrenocortical insufficiency, which must then be monitored and treated (Pia et al. 2013). Furthermore, there is some evidence that the increase in mineralocorticoids associated with complete inhibition of P450 17A1 (Fig. 4) may facilitate the flow of androgen precursors through a “back door” androgen biosynthesis pathway (Attard et al. 2012). Selective inhibition of the P450 17A1-mediated androgen biosynthetic step (second reaction in Fig. 11) has proven to increase overall survival, but sparing P450 17A1-mediated glucocorticoid biosynthesis (preventing corticosteroid imbalances) would ameliorate these issues for prostate cancer patients. P450 17A1 inhibition has also been associated with Cushing’s syndrome (Ogo et al. 1991), some forms of congenital adrenal hyperplasia (Maitra and Shirwalka2003), and polycystic ovary syndrome (Qin and Rosenfield 1998; Arlt et al. 2002; Strauss 2003).
References
- Ahmed S 1999. A novel molecular modelling study of inhibitors of the 17α-hydroxylase component of the enzyme system 17α-hydroxylase/17,20-lyase (P-45017α). Bioorg Med Chem 7:1487–1496. [DOI] [PubMed] [Google Scholar]
- Alyamani M, Li ZF, Berk M, Li JN, Tang JJ, Upadhyay S, Auchus RJ, Sharifi N. 2017. Steroidogenic metabolism of galeterone reveals a diversity of biochemical activities. Cell Chem Biol 24:825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arlt W, Martens JW, Song M, Wang JT, Auchus RJ, Miller WL. 2002. Molecular evolution of adrenarche: Structural and functional analysis of P450c17 from four primate species. Endocrinology 143:4665–4672. [DOI] [PubMed] [Google Scholar]
- Asif AR, Ljubojevic M, Sabolic I, Shnitsar V, Metten M, Anzai N, Muller GA, Burckhardt G, Hagos Y. 2006. Regulation of steroid hormone biosynthesis enzymes and organic anion transporters by forskolin and DHEA-S treatment in adrenocortical cells. Am J Physiol Endocrinol Metab 291:E1351–1359. [DOI] [PubMed] [Google Scholar]
- Attard G, Reid AH, Auchus RJ, Hughes BA, Cassidy AM, Thompson E, Oommen NB, Folkerd E, Dowsett M, Arlt W et al. 2012. Clinical and biochemical consequences of CYP17A1 inhibition with abiraterone given with and without exogenous glucocorticoids in castrate men with advanced prostate cancer. J Clin Endocrinol Metab 97:507–516. [DOI] [PubMed] [Google Scholar]
- Auchus ML, Auchus RJ. 2012. Human steroid biosynthesis for the oncologist. J Invest Med 60:495–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auchus RJ, Buschur EO, Chang AY, Hammer GD, Ramm C, Madrigal D, Wang G, Gonzalez M, Xu XS, Smit JW et al. 2014. Abiraterone acetate to lower androgens in women with classic 21-hydroxylase deficiency. J Clin Endocrinol Metab 99:2763–2770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auchus RJ, Lee TC, Miller WL. 1998. Cytochrome b5 augments the 17,20-lyase activity of human P450c17 without direct electron transfer. J Biol Chem 273:3158–3165. [DOI] [PubMed] [Google Scholar]
- Auchus RJ, Miller WL. 2015. Chapter 12, P450 enzymes in steroid processing. In: Ortiz de Montellano PR, editor. Cytochrome P450: Structure, Mechanism, and Biochemistry 4th ed. New York: Springer; p. 851–879. [Google Scholar]
- Ayub M, Levell MJ. 1989. Inhibition of human adrenal steroidogenic enzymes in vitro by imidazole drugs including ketoconazole. J Steroid Biochem 32:515–524. [DOI] [PubMed] [Google Scholar]
- Bird IM, Abbott DH. 2016. The hunt for a selective 17,20 lyase inhibitor; learning lessons from nature. J Steroid Biochem Mol Biol 163:136–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonomo S, Hansen CH, Petrunak EM, Scott EE, Styrishave B, Jorgensen FS, Olsen L. 2016. Promising tools in prostate cancer research: Selective non-steroidal cytochrome P450 17A1 inhibitors. Sci Rep 6:29468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canton RF, Sanderson JT, Nijmeijer S, Bergman A, Letcher RJ, van den Berg M. 2006. In vitro effects of brominated flame retardants and metabolites on CYP17 catalytic activity: A novel mechanism of action? Tox Appl Pharmacol 216:274–281. [DOI] [PubMed] [Google Scholar]
- Clement OO, Freeman CM, Hartmann RW, Handratta VD, Vasaitis TS, Brodie AM, Njar VC. 2003. Three dimensional pharmacophore modeling of human CYP17 inhibitors. Potential agents for prostate cancer therapy. J Med Chem 46:2345–2351. [DOI] [PubMed] [Google Scholar]
- de Bono JS, Logothetis CJ, Molina A, Fizazi K, North S, Chu L, Chi KN, Jones RJ, Goodman OB Jr., Saad F et al. 2011. Abiraterone and increased survival in metastatic prostate cancer. New Engl J Med 364:1995–2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Coster R, Caers I, Coene MC, Amery W, Beerens D, Haelterman C. 1986. Effects of high dose ketoconazole therapy on the main plasma testicular and adrenal steroids in previously untreated prostatic cancer patients. Clin Endocrinol 24:657–664. [DOI] [PubMed] [Google Scholar]
- DeVore NM, Scott EE. 2012. Structures of cytochrome P450 17A1 with prostate cancer drugs abiraterone and TOK-001. Nature 482:116–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Blasio AM, Voutilainen R, Jaffe RB, Miller WL. 1987. Hormonal regulation of messenger ribonucleic acids for P450scc (cholesterol side-chain cleavage enzyme) and P450c17 (17α-hydroxylase/17,20-lyase) in cultured human fetal adrenal cells. J Clin Endocrinol Metab 65:170–175. [DOI] [PubMed] [Google Scholar]
- Doi J, Takemori H, Ohta M, Nonaka Y, Okamoto M. 2001. Differential regulation of 3beta-hydroxysteroid dehydrogenase type II and 17α-hydroxylase/lyase P450 in human adrenocortical carcinoma cells by epidermal growth factor and basic fibroblast growth factor. J Endocrinol 168:87–94. [DOI] [PubMed] [Google Scholar]
- Edwards BK, Noone AM, Mariotto AB, Simard EP, Boscoe FP, Henley SJ, Jemal A, Cho H, Anderson RN, Kohler BA et al. 2014. Annual Report to the Nation on the Status of Cancer, 1975–2010, featuring prevalence of comorbidity and impact on survival among persons with lung, colorectal, breast, or prostate cancer. Cancer 120:1290–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhardt D, Weber MM. 1994. Therapy of Cushing’s syndrome with steroid biosynthesis inhibitors. J Steroid Biochem Mol Biol 49:261–267. [DOI] [PubMed] [Google Scholar]
- Estrada DF, Skinner AL, Laurence JS, Scott EE. 2014. Human cytochrome P450 17A1 conformational selection: Modulation by ligand and cytochrome b5. J Biol Chem 289:14310–14320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraldeschi R, de Bono J. 2013. Agents that target androgen synthesis in castration-resistant prostate cancer. Cancer J (Sudbury, MA) 19:34–42. [DOI] [PubMed] [Google Scholar]
- Furuta C, Noda S, Li C, Suzuki AK, Taneda S, Watanabe G, Taya K. 2008. Nitrophenols isolated from diesel exhaust particles regulate steroidogenic gene expression and steroid synthesis in the human H295R adrenocortical cell line. Tox Appl Pharmacol 229:109–120. [DOI] [PubMed] [Google Scholar]
- Geller DH, Auchus RJ, Mendonça BB, Miller WL. 1997. The genetic and functional basis of isolated 17,20-lyase deficiency. Nat Genetics 17:201–205. [DOI] [PubMed] [Google Scholar]
- Gilep AA, Sushko TA, Usanov SA. 2011. At the crossroads of steroid hormone biosynthesis: The role, substrate specificity and evolutionary development of CYP17. Biochim Biophys Acta 1814:200–209. [DOI] [PubMed] [Google Scholar]
- Glister C, Satchell L, Michael AE, Bicknell AB, Knight PG. 2012. The anti-epileptic drug valproic acid (VPA) inhibits steroidogenesis in bovine theca and granulosa cells in vitro. PloS One 7:e49553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez E, Guengerich FP. 2017. Kinetic processivity of the two-step oxidations of progesterone and pregnenolone to androgens by human cytochrome P450 17A1. J Biol Chem 292:13168–13185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez E, Johnson KM, Pallan PS, Phan TTN, Zhang W, Lei L, Wawrzak Z, Yoshimoto FK, Egli M, Guengerich FP. 2018. Inherent steroid 17α,20-lyase activity in defunct cytochrome P450 17A enzymes. J Biol Chem 293:541–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guengerich FP. 2015. Chapter 9, Human cytochrome P450 enzymes. In: Ortiz de Montellano PR, editor. Cytochrome P450: Structure, Mechanism, and Biochemistry 4th ed. New York: Springer; p. 523–785. [Google Scholar]
- Guengerich FP, Yoshimoto FK. 2018. Formation and cleavage of C-C bonds by enzymatic oxidation reactions. Chem Rev, in press. [DOI] [PMC free article] [PubMed]
- Handratta VD, Vasaitis TS, Njar VC, Gediya LK, Kataria R, Chopra P, Newman D Jr., Farquhar R, Guo Z, Qiu Y et al. 2005. Novel C-17-heteroaryl steroidal CYP17 inhibitors/antiandrogens: synthesis, in vitro biological activity, pharmacokinetics, and antitumor activity in the LAPC4 human prostate cancer xenograft model. J Med Chem 48:2972–2984. [DOI] [PubMed] [Google Scholar]
- Hasegawa E, Nakagawa S, Sato M, Tachikawa E, Yamato S. 2013. Effect of polyphenols on production of steroid hormones from human adrenocortical NCI-H295R cells. Biol Pharmaceut Bull 36:228–237. [DOI] [PubMed] [Google Scholar]
- Hille UE, Hu Q, Vock C, Negri M, Bartels M, Muller-Vieira U, Lauterbach T, Hartmann RW. 2009. Novel CYP17 inhibitors: Synthesis, biological evaluation, structure-activity relationships and modelling of methoxy- and hydroxy-substituted methyleneimidazolyl biphenyls. Eur J Med Chem 44:2765–2775. [DOI] [PubMed] [Google Scholar]
- Hsu CH, Yang YC, Lian ST, Lee SC, Shin SJ, Tsai JH, Lin SR. 2001. Significantly increased cortisol secretion in normal adrenocortical cells transfected with K-ras mutants derived from human functional adrenocortical tumors. DNA Cell Biol 20:231–238. [DOI] [PubMed] [Google Scholar]
- Hu Q, Yin L, Jagusch C, Hille UE, Hartmann RW. 2010. Isopropylidene substitution increases activity and selectivity of biphenylmethylene 4-pyridine type CYP17 inhibitors. J Med Chem 53:5049–5053. [DOI] [PubMed] [Google Scholar]
- Ideyama Y, Kudoh M, Tanimoto K, Susaki Y, Nanya T, Nakahara T, Ishikawa H, Yoden T, Okada M, Fujikura T et al. 1998. Novel nonsteroidal inhibitor of cytochrome P450(17α) (17α-hydroxylase/C17–20 lyase), YM116, decreased prostatic weights by reducing serum concentrations of testosterone and adrenal androgens in rats. Prostate 37:10–18. [DOI] [PubMed] [Google Scholar]
- Idkowiak J, Randell T, Dhir V, Patel P, Shackleton CH, Taylor NF, Krone N, Arlt W. 2012. A missense mutation in the human cytochrome b5 gene causes 46,XY disorder of sex development due to true isolated 17,20 lyase deficiency. J Clin Endocrinol Metab 97:E465–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson MK, Sanderson JT, Lund BO. 2002. Effects of 3-MeSO2-DDE and some CYP inhibitors on glucocorticoid steroidogenesis in the H295R human adrenocortical carcinoma cell line. Toxicol In Vitro 16:113–121. [DOI] [PubMed] [Google Scholar]
- Kim B, Moon JY, Choi MH, Yang HH, Lee S, Lim KS, Yoon SH, Yu KS, Jang IJ, Cho JY. 2013. Global metabolomics and targeted steroid profiling reveal that rifampin, a strong human PXR activator, alters endogenous urinary steroid markers. J Proteome Res 12:1359–1368. [DOI] [PubMed] [Google Scholar]
- Kisselev P, Tuckey RC, Woods ST, Triantopoulos T, Schwarz D. 1999. Enzymatic properties of vesicle-reconstituted human cytochrome P450SCC (CYP11A1) differences in functioning of the mitochondrial electron-transfer chain using human and bovine adrenodoxin and activation by cardiolipin. Eur J Biochem 260:768–773. [DOI] [PubMed] [Google Scholar]
- Kok RC, Timmerman MA, Wolffenbuttel KP, Drop SL, de Jong FH. 2010. Isolated 17,20-lyase deficiency due to the cytochrome b5 mutation W27X. J Clin Endocrinol Metab 95:994–999. [DOI] [PubMed] [Google Scholar]
- Leroux F 2005. Inhibition of P450 17 as a new strategy for the treatment of prostate cancer. Curr Med Chem 12:1623–1629. [DOI] [PubMed] [Google Scholar]
- Lin CJ, Cheng LC, Lin TC, Wang CJ, Li LA. 2014. Assessment of the potential of polyphenols as a CYP17 inhibitor free of adverse corticosteroid elevation. Biochem Pharmacol 90:288–296. [DOI] [PubMed] [Google Scholar]
- Loriot Y, Bianchini D, Ileana E, Sandhu S, Patrikidou A, Pezaro C, Albiges L, Attard G, Fizazi K, De Bono JS et al. 2013. Antitumour activity of abiraterone acetate against metastatic castration-resistant prostate cancer progressing after docetaxel and enzalutamide (MDV3100). Ann Oncol 24:1807–1812. [DOI] [PubMed] [Google Scholar]
- Lundqvist J, Norlin M, Wikvall K. 2010. 1α,25-Dihydroxyvitamin D3 affects hormone production and expression of steroidogenic enzymes in human adrenocortical NCI-H295R cells. Biochim Biophys Acta 1801:1056–1062. [DOI] [PubMed] [Google Scholar]
- Maitra A, Shirwalkar H. 2003. Congenital adrenal hyperplasia: Biochemical and molecular perspectives. Indian J Exp Biol 41:701–709. [PubMed] [Google Scholar]
- Malikova J, Brixius-Anderko S, Udhanea SS, Parween S, Dick B, Bernhardt R, Pandey AV. 2017. CYP17A1 inhibitor abiraterone, an anti-prostate cancer drug, also inhibits the 21-hydroxylase activity of CYP21A2. J Steroid Biochem Mol Biol 174:192–200. [DOI] [PubMed] [Google Scholar]
- Mesiano S, Katz SL, Lee JY, Jaffe RB. 1997. Insulin-like growth factors augment steroid production and expression of steroidogenic enzymes in human fetal adrenal cortical cells: implications for adrenal androgen regulation. J Clin Endocrinol Metab 82:1390–1396. [DOI] [PubMed] [Google Scholar]
- Miller WL, Auchus RJ. 2011. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocrin Rev 32:81–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naffin-Olivos JL, Auchus RJ. 2006. Human cytochrome b5 requires residues E48 and E49 to stimulate the 17,20-lyase activity of cytochrome P450c17. Biochemistry 45:755–762. [DOI] [PubMed] [Google Scholar]
- Nelson-DeGrave VL, Wickenheisser JK, Cockrell JE, Wood JR, Legro RS, Strauss JF 3rd, McAllister JM. 2004. Valproate potentiates androgen biosynthesis in human ovarian theca cells. Endocrinology 145:799–808. [DOI] [PubMed] [Google Scholar]
- Niwa T, Fujimoto M, Kishimoto K, Yabusaki Y, Ishibashi F, Katagiri M. 2001. Metabolism and interaction of bisphenol A in human hepatic cytochrome P450 and steroidogenic CYP17. Biol Pharm Bull 24:1064–1067. [DOI] [PubMed] [Google Scholar]
- Njar VC, Kato K, Nnane IP, Grigoryev DN, Long BJ, Brodie AM. 1998. Novel 17-azolyl steroids, potent inhibitors of human cytochrome 17α-hydroxylase-C17,20-lyase (P45017α): potential agents for the treatment of prostate cancer. J Med Chem 41:902–912. [DOI] [PubMed] [Google Scholar]
- Ogo A, Haji M, Ohashi M, Nawata H. 1991. Markedly increased expression of cytochrome P-450 17α-hydroxylase (P-450c17) mRNA in adrenocortical adenomas from patients with Cushing’s syndrome. Mol Cell Endocrinol 80:83–89. [DOI] [PubMed] [Google Scholar]
- Ohlsson A, Ulleras E, Oskarsson A. 2009. A biphasic effect of the fungicide prochloraz on aldosterone, but not cortisol, secretion in human adrenal H295R cells--underlying mechanisms. Toxicol Lett 191:174–180. [DOI] [PubMed] [Google Scholar]
- Pallan PS, Nagy LD, Lei L, Gonzalez E, Kramlinger VM, Azumaya CM, Wawrzak Z, Waterman MR, Guengerich FP, Egli M. 2015. Structural and kinetic basis of steroid 17α,20-lyase activity in teleost fish cytochrome P450 17A1 and its absence in cytochrome P450 17A2. J Biol Chem 290:3248–3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan ST, Xue D, Li ZL, Zhou ZW, He ZX, Yang Y, Yang T, Qiu JX, Zhou SF. 2016. Computational identification of the paralogs and orthologs of human cytochrome P450 superfamily and the implication in drug discovery. Int J Mol Sci June 28;17(7). pii: ijms17071020. doi: 10.3390/ijms17071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrunak EM, DeVore NM, Porubsky PR, Scott EE. 2014. Structures of human steroidogenic cytochrome P450 17A1 with substrates. J Biol Chem 289:32952–32964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrunak EM, Rogers SA, Aube J, Scott EE. 2017. Structural and functional evaluation of clinically relevant inhibitors of steroidogenic cytochrome P450 17A1. Drug Metab Dispos 45:635–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pia A, Vignani F, Attard G, Tucci M, Bironzo P, Scagliotti G, Arlt W, Terzolo M, Berruti A. 2013. Strategies for managing ACTH dependent mineralocorticoid excess induced by abiraterone. Cancer Treatment Rev 39:966–973. [DOI] [PubMed] [Google Scholar]
- Pikuleva IA, Mast N, Liao WL, Turko IV. 2008. Studies of membrane topology of mitochondrial cholesterol hydroxylases CYPs 27A1 and 11A1. Lipids 43:1127–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto-Bazurco Mendieta MA, Negri M, Jagusch C, Muller-Vieira U, Lauterbach T, Hartmann RW. 2008. Synthesis, biological evaluation, and molecular modeling of abiraterone analogues: novel CYP17 inhibitors for the treatment of prostate cancer. J Med Chem 51:5009–5018. [DOI] [PubMed] [Google Scholar]
- Qin KN, Rosenfield RL. 1998. Role of cytochrome P450c17 in polycystic ovary syndrome. Mol Cell Endocrinol 145:111–121. [DOI] [PubMed] [Google Scholar]
- Rege J, Nishimoto HK, Nishimoto K, Rodgers RJ, Auchus RJ, Rainey WE. 2015. Bone morphogenetic protein-4 (BMP4): A paracrine regulator of human adrenal C19 steroid synthesis. Endocrinology 156:2530–2540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawetawan C, Carr BR, McGee E, Bird IM, Hong TL, Rainey WE. 1996. Inhibin and activin differentially regulate androgen production and 17α-hydroxylase expression in human ovarian thecal-like tumor cells. J Endocrinol 148:213–221. [DOI] [PubMed] [Google Scholar]
- Schroeder RL, Tram P, Liu J, Foroozesh M, Sridhar J. 2016. Novel functionalized 5-(phenoxymethyl)-1,3-dioxane analogs exhibiting cytochrome P450 inhibition: A patent evaluation WO2015048311 (A1). Exp Opin Therapeut Patents 26:139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherbet DP, Tiosano D, Kwist KM, Hochberg Z, Auchus RJ. 2003. CYP17 mutation E305G causes isolated 17,20-lyase deficiency by selectively altering substrate binding. J Biol Chem 278:48563–48569. [DOI] [PubMed] [Google Scholar]
- Sohl CD, Guengerich FP. 2010. Kinetic analysis of the three-step steroid aromatase reaction of human cytochrome P450 19A1. J Biol Chem 285:17734–17743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R, He Y, Murphy MB, Yeung LW, Yu RM, Lam MH, Lam PK, Hecker M, Giesy JP, Wu RS et al. 2008. Effects of fifteen PBDE metabolites, DE71, DE79 and TBBPA on steroidogenesis in the H295R cell line. Chemosphere 71:1888–1894. [DOI] [PubMed] [Google Scholar]
- Stein MN, Patel N, Bershadskiy A, Sokoloff A, Singer EA. 2014. Androgen synthesis inhibitors in the treatment of castration-resistant prostate cancer. Asian J Androl 16:387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss JF 3rd. 2003. Some new thoughts on the pathophysiology and genetics of polycystic ovary syndrome. Ann New York Acad Sci 997:42–48. [DOI] [PubMed] [Google Scholar]
- Van Den Akker EL, Koper JW, Boehmer AL, Themmen AP, Verhoef-Post M, Timmerman MA, Otten BJ, Drop SL, De Jong FH. 2002. Differential inhibition of 17α-hydroxylase and 17,20-lyase activities by three novel missense CYP17 mutations identified in patients with P450c17 deficiency [Case Reports]. J Clin Endocrinol Metab 87:5714–5721. [DOI] [PubMed] [Google Scholar]
- van Duursen MB, Nijmeijer SM, Ruchirawat S, van den Berg M. 2010. Chemopreventive actions by enterolactone and 13 VIOXX-related lactone derivatives in H295R human adrenocortical carcinoma cells. Toxicol Lett 192:271–277. [DOI] [PubMed] [Google Scholar]
- van Koetsveld PM, Vitale G, Feelders RA, Waaijers M, Sprij-Mooij DM, de Krijger RR, Speel EJ, Hofland J, Lamberts SW, de Herder WW et al. 2013. Interferon-α is a potent inhibitor of cell growth and cortisol production in vitro and sensitizes human adrenocortical carcinoma cells to mitotane. Endocr Related Cancer 20:443–454. [DOI] [PubMed] [Google Scholar]
- Wickenheisser JK, Nelson-DeGrave VL, Hendricks KL, Legro RS, Strauss JF 3rd, McAllister JM. 2005. Retinoids and retinol differentially regulate steroid biosynthesis in ovarian theca cells isolated from normal cycling women and women with polycystic ovary syndrome. J Clin Endocrinol Metab 90:4858–4865. [DOI] [PubMed] [Google Scholar]
- Zuber MX, Simpson ER, Waterman MR. Regulation of cytochrome P-450(17)alpha activity and synthesis in bovine adrenocortical cells. 1985. Ann N Y Acad Sci 458:252–261. [DOI] [PubMed] [Google Scholar]
- Yadav R, Petrunak EM, Estrada DF, Scott EE. 2017. Structural insights into the function of steroidogenic cytochrome P450 17A1. Mol Cell Endocrinol 441:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaoka M, Hara T, Hitaka T, Kaku T, Takeuchi T, Takahashi J, Asahi S, Miki H, Tasaka A, Kusaka M. 2012. Orteronel (TAK-700), a novel non-steroidal 17,20-lyase inhibitor: Effects on steroid synthesis in human and monkey adrenal cells and serum steroid levels in cynomolgus monkeys. J Steroid Biochem Mol Biol 129:115–128. [DOI] [PubMed] [Google Scholar]
- Yin L, Hu Q. 2014. CYP17 inhibitors–abiraterone, C17,20-lyase inhibitors and multi-targeting agents. Nat Rev Urol 11:32–42. [DOI] [PubMed] [Google Scholar]
- Yoshimoto FK, Gonzalez E, Auchus RJ, Guengerich FP. 2016. Mechanism of 17α,20-lyase and new hydroxylation reactions of human cytochrome P450 17A1: 18O labeling and oxygen surrogate evidence for a role of a perferryl oxygen. J Biol Chem 291:17143–17164. [DOI] [PMC free article] [PubMed] [Google Scholar]
P450 19A1
P450 19A1 is the steroid aromatase and catalyzes the conversion of androstenedione and testosterone to estrone and estradiol, respectively (Figs. 4, 12). In mammals P450 19A1 is mainly expressed in the brain and the gonads, but it is also found in placental, adipose, and bone tissue (Conley and Hinshelwood 2001). While one single aromatase gene exists in humans, it contains at least ten different promoters (Bulun et al. 2003), with different promoters and ligands regulating estrogen synthesis across different tissue types (Means et al. 1991; Simpson et al. 2002; Simpson 2003; Clyne et al. 2004; Kamat et al. 2005; Mendelson et al. 2005). The balance between the different sex steroid hormones, androgens and estrogens, is crucial for the reproductive system as well as for the ontogenic differentiation of sexual phenotype. In mammals, differentiation of the male phenotype depends not only on testosterone but also on estradiol generated from testosterone by neuronal aromatase (in the central nervous system). In transgenic male mice overexpressing aromatase in the testis, the serum estradiol level was increased, and one-half of these animals were infertile and had larger testes and a significantly increased incidence of Leydig cell tumors in testes (Fisher et al. 1998; Robertson et al. 1999).
Fig. 12.
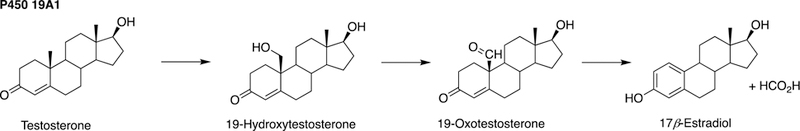
Three-step oxidation of testosterone to 17β-estradiol by P450 19A1.
X-ray crystal structures of P450 19A1 are now available. The first structure was obtained with a full-length protein purified from human placenta (Ghosh et al. 2009); subsequently other structures, with bound ligands, have been obtained with recombinant P450 19A1 (Ghosh et al. 2012; McCammon et al. 2016).
The mechanism of the third reaction in the 3-step sequence (Fig. 12) has been controversial. Multiple catalytic mechanisms were proposed in the 1970s and 1980s (Akhtar et al. 1976; Goto and Fishman 1977; Akhtar et al. 1982; Hahn and Fishman 1984; Caspi et al. 1986) and many of these have been ruled out. Some of the evidence has been equivocal, e.g. biophysical, theoretical, synthesis and testing of possible intermediates (Hackett et al. 2005; Khatri et al. 2014). For many years the most popular mechanism involved a ferric peroxide (FeIII–O2−) intermediate (Compound 0), proposed to attack the C19 formyl group (aldehyde) (Fig. 12). The strongest evidence for this mechanism was an 18O2-labeling study by the Akhtar group, who reported incorporation of a single 18O atom into formic acid (Akhtar et al. 1976; Akhtar et al. 1982). Similar findings were reported by Caspi et al. (1986), without presenting data. The 18O labeling study was repeated with both major substrates, testosterone and androstenedione, using purified P450 19A1, a new method of derivatizing the product formic acid, and higher resolution mass spectrometry. The opposite result was obtained, i.e., no 18O was incorporated into formic acid, leaving only the Compound I (FeO3+) mechanism as being viable (Yoshimoto and Guengerich 2014).
The multiple CYP19A1 promoters play a different role in benign as opposed to malignant breast tissue; although the 1.4 promoter is the main activator in normal breast tissue, promoters II, 1.3, and 1.7 have been shown to play a role (in addition to 1.4) in breast cancer tissue (Robertson et al. 1999). While circulating androstenedione (as well as testosterone) in postmenopausal women is considered to be of adrenal origin, the ovary seems to provide a minor contribution of circulating testosterone (Sluijmer et al. 1995; Couzinet et al. 2001).
Aromatase inhibitors have now become generally standard adjuvant endocrine therapy for postmenopausal breast cancer patients. The collective evidence advocates the use of aromatase inhibitors in the adjuvant treatment of postmenopausal women. There is no consensus in favor of either sequential or monotherapy compared with the alternative treatment strategy. Considering the cost per (quality-adjusted) life-year gained related with each strategy, this depends on the relapse riskand also patient age at diagnosis (Lønning and Eikesdal 2013).
P450 19A1 can be inhibited competitively and reversibly by azole compounds, as in the case of sterol 14α-demethylase (P450 51A1, vide infra). In a rat ovary model, the therapeutic drugs anastrozole, fadrozole, and letrozole exhibited IC50 values of 25, 7, and 7 nM, respectively (Odum and Ashby 2002). Not only azoles, including azole fungicides, but also natural plant constituents such as flavonoids are able to significantly inhibit P450 19A1 (Zarn et al. 2003).
Aromatase inhibitors can be divided into two groups, Types I and II. Type I inhibitors are steroidal derivatives of the substrate androstenedione and bind tightly to the heme prothetic group in the active site. Type II are nonsteroidal and also bind reversible to the heme group. The third-generation nonsteroidal aromatase inhibitors anastrozole and letrozole have longer half-lives and are the most effective blocking compounds (> 95%) in suppressing the formation of the estrogens (Boeddinghaus and Dowsett 2001). Due to the higher potency of letrozole, a greater increase in the concentrations of gonadotropins and testosterone has been reported in boys treated with letrozole when compared to the group treated with anastrozole (Mauras 2011).
Men with congenital aromatase deficiency have tall stature, associated with delayed fusion of the epiphyseal growth plate, osteoporosis, overweight, glucose intolerance, hyperlipidemia, and reduction of fertility. In girls, virilization of the external genitalia can result in atypical genitalia. Hypogonadism can also occur, with inappropriate mammary gland development and primary amenorrhea (Morishima et al. 1995; Carani et al. 1997; Diaz-Thomas and Shulman 2010). Aromatase excess syndrome, conversely, is caused by chromosomal recombination of the CYP19A1 gene (e.g., duplication, deletion, inversion), with recruitment of new promoters. The clinical presentation includes gynecomastia and bone age advancement, with potential reduction of final height (Shozu et al. 2014). Estrogens play a key role in bone maturation (Rochira et al. 2010) and the use of aromatase inhibitors, with the goal of reducing the estrogen-dependent skeletal maturation rate, has been proposed with boys receiving treatment for short stature (McGrath and O’Grady 2015; Ferris and Geffner 2017). These drugs reduce estrogen concentrations at the epiphyseal chondrocyte level and are associated with decreased circulating estrogen concentration and consequent reduction in the secretion of both growth hormone and insulin-like growth factor-1 (Juul et al. 1994; Shim 2015). Estrogen has limited relevance growth regulation in the prepubertal period but increases growth during puberty and, at high concentrations, determines maturation and closure of the epiphyses at the final stage of puberty (Wit et al. 2011).
References
- Abul-Hajj YJ. 1983. Studies on the mechanism of action of the aromatase inhibitor, 4-hydroxyandrostenedione. Steroids 41:783–790. [DOI] [PubMed] [Google Scholar]
- Adlercreutz H, Bannwart C, Wahala K, Makela T, Brunow G, Hase T, Arosemena PJ, Kellis JT Jr., Vickery LE. 1993. Inhibition of human aromatase by mammalian lignans and isoflavonoid phytoestrogens. J Steroid Biochem Mol Biol 44:147–153. [DOI] [PubMed] [Google Scholar]
- Ahmad I, Shagufta. 2015. Recent developments in steroidal and nonsteroidal aromatase inhibitors for the chemoprevention of estrogen-dependent breast cancer. Eur J Med Chem 102:375–386. [DOI] [PubMed] [Google Scholar]
- Ahmed S, Amanuel Y. 2000. Synthesis and biochemical evaluation of novel inhibitors of aromatase (AR) using an enhanced representation of the active site of AR derived from the consideration of the reaction mechanism. Biochem Biophys Res Commun 267:356–361. [DOI] [PubMed] [Google Scholar]
- Ahmed S, Smith JH, Nicholls PJ, Whomsley R, Cariuk P. 1995. Synthesis and biological evaluation of imidazole based compounds as cytochrome P-450 inhibitors. Drug Design Discov 13:27–41. [PubMed] [Google Scholar]
- Akhtar M, Calder MR, Corina DL, Wright JN. 1982. Mechanistic studies on C-19 demethylation in oestrogen biocynthesis. Biochem J 201:569–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar M, Corina D, Pratt J, Smith T. 1976. Studies on the removal of C-19 in oestrogen biosynthesis using 18O2. J Chem Soc Chem Commun 854–856.
- Almstrup K, Fernandez MF, Petersen JH, Olea N, Skakkebaek NE, Leffers H. 2002. Dual effects of phytoestrogens result in U-shaped dose-response curves. Environ Health Perspect 110:743–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen HR, Vinggaard AM, Rasmussen TH, Gjermandsen IM, Bonefeld-Jørgensen EC. 2002. Effects of currently used pesticides in assays for estrogenicity, androgenicity, and aromatase activity in vitro. Toxicol Appl Pharmacol 179:1–12. [DOI] [PubMed] [Google Scholar]
- Andrews WJ, Winnett G, Rehman F, Shanmugasundaram P, Hagen D, Schrey MP. 2005. Aromatase inhibition by 15-deoxy-prostaglandin J2 (15-dPGJ2) and N-(4-hydroxyphenyl)-retinamide (4HPR) is associated with enhanced ceramide production. J Steroid Biochem Mol Biol 94:159–165. [DOI] [PubMed] [Google Scholar]
- Ayub M, Levell MJ. 1988. Structure-activity relationships of the inhibition of human placental aromatase by imidazole drugs including ketoconazole. J Steroid Biochem 31:65–72. [DOI] [PubMed] [Google Scholar]
- Ayub M, Levell MJ. 1990. The inhibition of human prostatic aromatase activity by imidazole drugs including ketoconazole and 4-hydroxyandrostenedione. Biochem Pharmacol 40:1569–1575. [DOI] [PubMed] [Google Scholar]
- Barrera D, Avila E, Hernandez G, Halhali A, Biruete B, Larrea F, Diaz L. 2007. Estradiol and progesterone synthesis in human placenta is stimulated by calcitriol. J Steroid Biochem Mol Biol 103:529–532. [DOI] [PubMed] [Google Scholar]
- Bednarski PJ, Hartmann RW. 1993. Synthesis and evaluation of sulfur-containing glutethimide derivatives for aromatase and desmolase inhibitory activity. Archiv Pharm 326:391–394. [DOI] [PubMed] [Google Scholar]
- Benachour N, Moslemi S, Sipahutar H, Seralini GE. 2007. Cytotoxic effects and aromatase inhibition by xenobiotic endocrine disrupters alone and in combination. Toxicol Appl Pharmacol 222:129–140. [DOI] [PubMed] [Google Scholar]
- Blanco JG, Gil RR, Alvarez CI, Patrito LC, Genti-Raimondi S, Flury A. 1997. A novel activity for a group of sesquiterpene lactones: Inhibition of aromatase. FEBS Lett 409:396–400. [DOI] [PubMed] [Google Scholar]
- Boeddinghaus IM, Dowsett M. 2001. Comparative clinical pharmacology and pharmacokinetic interactions of aromatase inhibitors. J Steroid Biochem Mol Biol 79:85–91. [DOI] [PubMed] [Google Scholar]
- Bois FY, Golbamaki-Bakhtyari N, Kovarich S, Tebby C, Gabb HA, Lemazurier E. 2017. High-throughput analysis of ovarian cycle disruption by mixtures of aromatase inhibitors. Environ Health Perspect 125:077012. doi: 10.1289/EHP742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brueggemeier RW, Diaz-Cruz ES, Li PK, Sugimoto Y, Lin YC, Shapiro CL. 2005. Translational studies on aromatase, cyclooxygenases, and enzyme inhibitors in breast cancer. J Steroid Biochem Mol Biol 95:129–136. [DOI] [PubMed] [Google Scholar]
- Bulun SE, Sebastian S, Takayama K, Suzuki T, Sasano H, Shozu M. 2003. The human CYP19 (aromatase P450) gene: update on physiologic roles and genomic organization of promoters. J Steroid Biochem Mol Biol 86:219–224. [DOI] [PubMed] [Google Scholar]
- Campbell DR, Kurzer MS. 1993. Flavonoid inhibition of aromatase enzyme activity in human preadipocytes. J Steroid Biochem Mol Biol 46:381–388. [DOI] [PubMed] [Google Scholar]
- Cantón RF, Sanderson JT, Letcher RJ, Bergman A, van den Berg M. 2005. Inhibition and induction of aromatase (CYP19) activity by brominated flame retardants in H295R human adrenocortical carcinoma cells. Toxicol Sci 88:447–455. [DOI] [PubMed] [Google Scholar]
- Cantón RF, Scholten DE, Marsh G, de Jong PC, van den Berg M. 2008. Inhibition of human placental aromatase activity by hydroxylated polybrominated diphenyl ethers (OH-PBDEs). Toxicol Appl Pharmacol 227:68–75. [DOI] [PubMed] [Google Scholar]
- Caporuscio F, Rastelli G, Imbriano C, Del Rio A. 2011. Structure-based design of potent aromatase inhibitors by high-throughput docking. J Med Chem 54:4006–4017. [DOI] [PubMed] [Google Scholar]
- Carani C, Qin K, Simoni M, Faustini-Fustini M, Serpente S, Boyd J, Korach KS, Simpson ER. 1997. Effect of testosterone and estradiol in a man with aromatase deficiency. New Engl J Med 337:91–95. [DOI] [PubMed] [Google Scholar]
- Caspi E, Arunachalam T, Nelson PA. 1986. Biosynthesis of estrogens: aromatization of (19R)-, (19S)-, and (19S)-[19-3H,2H,1H]-3β-hydroxyandrost-5-en-17-ones by human placental aromatase. J Am Chem Soc 108:1847–1852. [Google Scholar]
- Chauhan B, Yu C, Krantis A, Scott I, Arnason JT, Marles RJ, Foster BC. 2007. In vitro activity of uva-ursi against cytochrome P450 isoenzymes and P-glycoprotein. Canad J Physiol Pharmacol 85:1099–1107. [DOI] [PubMed] [Google Scholar]
- Chen D, Reierstad S, Lin Z, Lu M, Brooks C, Li N, Innes J, Bulun SE. 2007. Prostaglandin E2 induces breast cancer related aromatase promoters via activation of p38 and c-Jun NH2-terminal kinase in adipose fibroblasts. Cancer Res 67:8914–8922. [DOI] [PubMed] [Google Scholar]
- Chen L, Chen X, Chen X, Hu Z, Li X, Su Y, Li X, Ge RS. 2017. Ziram inhibits aromatase activity in human placenta and JEG-3 cell line. Steroids 128:114–119. [DOI] [PubMed] [Google Scholar]
- Chen S, Oh SR, Phung S, Hur G, Ye JJ, Kwok SL, Shrode GE, Belury M, Adams LS, Williams D. 2006. Anti-aromatase activity of phytochemicals in white button mushrooms (Agaricus bisporus). Cancer Res 66:12026–12034. [DOI] [PubMed] [Google Scholar]
- Chen Y, Cai S, Wang J, Xu M. 2015. Valproic acid-induced histone acetylation suppresses CYP19 gene expression and inhibits the growth and survival of endometrial stromal cells. Int J Mol Med 36:725–732. [DOI] [PubMed] [Google Scholar]
- Chottanapund S, Van Duursen MB, Navasumrit P, Hunsonti P, Timtavorn S, Ruchirawat M, Van den Berg M. 2014. Anti-aromatase effect of resveratrol and melatonin on hormonal positive breast cancer cells co-cultured with breast adipose fibroblasts. Toxicol In Vitro 28:1215–1221. [DOI] [PubMed] [Google Scholar]
- Chottanapund S, Van Duursen MBM, Zwartsen A, Timtavorn S, Navasumrit P, Kittakoop P, Sureram S, Ruchirawat M, Van den Berg M. 2017. Depsidones inhibit aromatase activity and tumor cell proliferation in a co-culture of human primary breast adipose fibroblasts and T47D breast tumor cells. Toxicol Rep 4:165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu PW, Yang ZJ, Huang HH, Chang AA, Cheng YC, Wu GJ, Lan HC. 2018. Low-dose bisphenol A activates the ERK signaling pathway and attenuates steroidogenic gene expression in human placental cells. Biol Reproduct 98:250–258. [DOI] [PubMed] [Google Scholar]
- Clyne CD, Kovacic A, Speed CJ, Zhou J, Pezzi V, Simpson ER. 2004. Regulation of aromatase expression by the nuclear receptor LRH-1 in adipose tissue. Mol Cell Endocrinol 215:39–44. [DOI] [PubMed] [Google Scholar]
- Conley A, Hinshelwood M. 2001. Mammalian aromatases. Reproduction (Cambridge, UK) 121:685–695. [DOI] [PubMed] [Google Scholar]
- Conley A, Mapes S, Corbin CJ, Greger D, Graham S. 2002. Structural determinants of aromatase cytochrome P450 inhibition in substrate recognition site-1. Mol Endocrinol 16:1456–1468. [DOI] [PubMed] [Google Scholar]
- Couzinet B, Meduri G, Lecce MG, Young J, Brailly S, Loosfelt H, Milgrom E, Schaison G. 2001. The postmenopausal ovary is not a major androgen-producing gland. J Clin Endocrinol Metab 86:5060–5066. [DOI] [PubMed] [Google Scholar]
- Cui J, Wang Y, Dong Q, Wu S, Xiao X, Hu J, Chai Z, Zhang Y. 2011. Morphine protects against intracellular amyloid toxicity by inducing estradiol release and upregulation of Hsp70. J Neurosci 31:16227–16240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Nardo G, Cimicata G, Baravalle R, Dell’Angelo V, Ciaramella A, Catucci G, Ugliengo P, Gilardi G. 2018. Working at the membrane interface: Ligand-induced changes in dynamic conformation and oligomeric structure in human aromatase. Biotechnol Appl Biochem 65:46–53. eng. [DOI] [PubMed] [Google Scholar]
- Diaz-Cruz ES, Shapiro CL, Brueggemeier RW. 2005. Cyclooxygenase inhibitors suppress aromatase expression and activity in breast cancer cells. J Clin Endocrinol Metab 90:2563–2570. eng. [DOI] [PubMed] [Google Scholar]
- Diaz-Thomas A, Shulman D. 2010. Use of aromatase inhibitors in children and adolescents: What’s new? Curr Opin Pediatr 22:501–507. [DOI] [PubMed] [Google Scholar]
- Dieudonné MN, Sammari A, Dos Santos E, Leneveu MC, Giudicelli Y, Pecquery R. 2006. Sex steroids and leptin regulate 11β-hydroxysteroid dehydrogenase I and P450 aromatase expressions in human preadipocytes: Sex specificities. J Steroid Biochem Mol Biol 99:189–196. [DOI] [PubMed] [Google Scholar]
- Dong Y, Mao B, Li L, Guan H, Su Y, Li X, Lian Q, Huang P, Ge RS. 2016. Gossypol enantiomers potently inhibit human placental 3β-hydroxysteroid dehydrogenase 1 and aromatase activities. Fitoterapia 109:132–137. [DOI] [PubMed] [Google Scholar]
- Drenth HJ, Bouwman CA, Seinen W, Van den Berg M. 1998. Effects of some persistent halogenated environmental contaminants on aromatase (CYP19) activity in the human choriocarcinoma cell line JEG-3. Toxicol Appl Pharmacol 148:50–55. [DOI] [PubMed] [Google Scholar]
- Dunkel L 2006. Use of aromatase inhibitors to increase final height. Mol Cell Endocrinol 254–255:207–216. [DOI] [PubMed]
- Eng ET, Williams D, Mandava U, Kirma N, Tekmal RR, Chen S. 2001. Suppression of aromatase (estrogen synthetase) by red wine phytochemicals. Breast Cancer Res Treat 67:133–146. [DOI] [PubMed] [Google Scholar]
- Eng ET, Williams D, Mandava U, Kirma N, Tekmal RR, Chen S. 2002. Anti-aromatase chemicals in red wine. Ann New York Acad Sci 963:239–246. [DOI] [PubMed] [Google Scholar]
- Eng ET, Ye J, Williams D, Phung S, Moore RE, Young MK, Gruntmanis U, Braunstein G, Chen S. 2003. Suppression of estrogen biosynthesis by procyanidin dimers in red wine and grape seeds. Cancer Res 63:8516–8522. [PubMed] [Google Scholar]
- Enjuanes A, Garcia-Giralt N, Supervia A, Nogues X, Ruiz-Gaspa S, Bustamante M, Mellibovsky L, Grinberg D, Balcells S, Diez-Perez A. 2005. Functional analysis of the I.3, I.6, pII and I.4 promoters of CYP19 (aromatase) gene in human osteoblasts and their role in vitamin D and dexamethasone stimulation. Eur J Endocrinol 153:981–988. [DOI] [PubMed] [Google Scholar]
- Ertas M, Sahin Z, Berk B, Yurttas L, Biltekin SN, Demirayak S. 2018. Pyridine-substituted thiazolylphenol derivatives: Synthesis, modeling studies, aromatase inhibition, and antiproliferative activity evaluation. Archiv Pharm March 9. doi: 10.1002/ardp.201700272. [DOI] [PubMed] [Google Scholar]
- Ferris JA, Geffner ME. 2017. Are aromatase inhibitors in boys with predicted short stature and/or rapidly advancing bone age effective and safe? J Pediat Endocrinol Metab 30:311–317. [DOI] [PubMed] [Google Scholar]
- Filleur F, Le Bail JC, Duroux JL, Simon A, Chulia AJ. 2001. Antiproliferative, anti-aromatase, anti-17β-HSD and antioxidant activities of lignans isolated from Myristica argentea. Planta Med 67:700–704. [DOI] [PubMed] [Google Scholar]
- Fiorelli G, Picariello L, Martineti V, Tonelli F, Brandi ML. 1999. Estrogen synthesis in human colon cancer epithelial cells. J Steroid Biochem Mol Biol 71:223–230. [DOI] [PubMed] [Google Scholar]
- Fisher CR, Graves KH, Parlow AF, Simpson ER. 1998. Characterization of mice deficient in aromatase (ArKO) because of targeted disruption of the cyp19 gene. Proc Natl Acad Sci USA 95:6965–6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geelen JA, Deckers GH, van der Wardt JT, Loozen HJ, Tax LJ, Kloosterboer HJ. 1991. Selection of 19-(ethyldithio)-androst-4-ene-3,17-dione (ORG 30958): A potent aromatase inhibitor in vivo. J Steroid Biochem Mol Biol 38:181–188. [DOI] [PubMed] [Google Scholar]
- Ghosh D, Egbuta C, Lo J. 2018. Testosterone complex and non-steroidal ligands of human aromatase. J Steroid Biochem Mol Biol February 21 pii: S0960–0760(18)30105–5. doi: 10.1016/j.jsbmb.2018.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh D, Griswold J, Erman M, Pangborn W. 2009. Structural basis for androgen specificity and oestrogen synthesis in human aromatase. Nature 457:219–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh D, Lo J, Morton D, Valette D, Xi J, Griswold J, Hubbell S, Egbuta C, Jiang W, An J et al. 2012. Novel aromatase inhibitors by structure-guided design. J Med Chem 55:8464–8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glister C, Satchell L, Michael AE, Bicknell AB, Knight PG. 2012. The anti-epileptic drug valproic acid (VPA) inhibits steroidogenesis in bovine theca and granulosa cells in vitro. PloS One 7:e49553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Görlitzer K, Bonnekessel C, Jones PG, Palusczak A, Hartmann RW. 2006. [Derivatives of exemestane–synthesis and evaluation of aromatase inhibition]. Die Pharmazie 61:575–581. ger. [PubMed] [Google Scholar]
- Goto J, Fishman J. 1977. Participation of a nonenzymatic transformation in the biosynthesis of estrogens from androgens. Science 195:80–81. [DOI] [PubMed] [Google Scholar]
- Grube BJ, Eng ET, Kao YC, Kwon A, Chen S. 2001. White button mushroom phytochemicals inhibit aromatase activity and breast cancer cell proliferation. J Nutr 131:3288–3293. [DOI] [PubMed] [Google Scholar]
- Guo J, Yuan Y, Lu D, Du B, Xiong L, Shi J, Yang L, Liu W, Yuan X, Zhang G et al. 2014. Two natural products, trans-phytol and (22E)-ergosta-6,9,22-triene-3β,5α,8α-triol, inhibit the biosynthesis of estrogen in human ovarian granulosa cells by aromatase (CYP19). Toxicol Appl Pharmacol 279:23–32. [DOI] [PubMed] [Google Scholar]
- Hackett JC, Brueggemeier RW, Hadad CM. 2005. The final catalytic step of cytochrome P450 aromatase: A density functional theory study. J Am Chem Soc 127:5224–5237. [DOI] [PubMed] [Google Scholar]
- Hadizadeh F, Kalalinia F, Jouya M, Komachal AK, Mohammad AS, Karimi G, Behravan J, Abnous K, Etemad L, Kamali H. 2018. Design, synthesis, and biological evaluation of new azole derivatives as potent aromatase inhibitors with potential effects against breast cancer. Anticancer Agents Med Chem January 15. doi: 10.2174/1871520618666180116105858 [DOI] [PubMed] [Google Scholar]
- Hahn EF, Fishman J. 1984. Immunological probe of estrogen biosynthesis. Evidence for the 2β-hydroxylative pathway in aromatization of androgens. J Biol Chem 259:1689–1694. [PubMed] [Google Scholar]
- Hartmann RW, Bayer H, Grun G. 1994. Aromatase inhibitors. Syntheses and structure-activity studies of novel pyridyl-substituted indanones, indans, and tetralins. J Med Chem 37:1275–1281. [DOI] [PubMed] [Google Scholar]
- Hartmann RW, Bayer H, Grun G, Sergejew T, Bartz U, Mitrenga M. 1995. Pyridyl-substituted tetrahydrocyclopropa[a]naphthalenes: highly active and selective inhibitors of P450arom. J Med Chem 38:2103–2111. [DOI] [PubMed] [Google Scholar]
- Heneweer M, van den Berg M, Sanderson JT. 2004. A comparison of human H295R and rat R2C cell lines as in vitro screening tools for effects on aromatase. Toxicol Lett 146:183–194. [DOI] [PubMed] [Google Scholar]
- Hirani VN, Raucy JL, Lasker JM. 2004. Conversion of the HIV protease inhibitor nelfinavir to a bioactive metabolite by human liver CYP2C19. Drug Metab Dispos 32:1462–1467. [DOI] [PubMed] [Google Scholar]
- Hong Y, Yu B, Sherman M, Yuan YC, Zhou D, Chen S. 2007. Molecular basis for the aromatization reaction and exemestane-mediated irreversible inhibition of human aromatase. Mol Endocrinol 21:401–414. [DOI] [PubMed] [Google Scholar]
- Huang BM, Hsiao KY, Chuang PC, Wu MH, Pan HA, Tsai SJ. 2004. Upregulation of steroidogenic enzymes and ovarian 17β-estradiol in human granulosa-lutein cells by Cordyceps sinensis mycelium. Biol Reproduct 70:1358–1364. [DOI] [PubMed] [Google Scholar]
- Ibrahim AR, Abul-Hajj YJ. 1990. Aromatase inhibition by flavonoids. J Steroid Biochem Mol Biol 37:257–260. [DOI] [PubMed] [Google Scholar]
- Jacobsen NW, Halling-Sorensen B, Birkved FK. 2008. Inhibition of human aromatase complex (CYP19) by antiepileptic drugs. Toxicol In Vitro 22:146–153. [DOI] [PubMed] [Google Scholar]
- Jeong HJ, Chang LC, Kim HK, Kim IH, Kinghorn AD, Pezzuto JM. 2000. Aromatase inhibitors from Isodon excisus var. coreanus. Archiv Pharmacal Res 23:243–245. [DOI] [PubMed] [Google Scholar]
- Jeong HJ, Shin YG, Kim IH, Pezzuto JM. 1999. Inhibition of aromatase activity by flavonoids. Archiv Pharmacal Res 22:309–312. [DOI] [PubMed] [Google Scholar]
- Jiang B, Kamat A, Mendelson CR. 2000. Hypoxia prevents induction of aromatase expression in human trophoblast cells in culture: potential inhibitory role of the hypoxia-inducible transcription factor MASH-2 (mammalian achaete-scute homologous protein-2). Mol Endocrinol 14:1661–1673. [DOI] [PubMed] [Google Scholar]
- Jiang B, Mendelson CR. 2005. O2 enhancement of human trophoblast differentiation and hCYP19 (aromatase) gene expression are mediated by proteasomal degradation of USF1 and USF2. Mol Cell Biol 25:8824–8833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin UH, Kim KS, Park SY, Chung KH, Kim DS, Chang YC, Kim CH. 2006. Effect of Buthus martensi Karsch on aromatase activity and cytokine-inducted NOS and NO production in osteoblasts and leukaemic cell line FLG 29.1. Immunopharmacol Immunotoxicol 28:241–258. [DOI] [PubMed] [Google Scholar]
- Johnston JO, Wright CL, Holbert GW, Benson HD. 1990. Enzyme inactivation by potential metabolites of an aromatase-activated inhibitor (MDL 18,962). J Enz Inhib 4:137–142. [DOI] [PubMed] [Google Scholar]
- Juul A, Bang P, Hertel NT, Main K, Dalgaard P, Jorgensen K, Muller J, Hall K, Skakkebaek NE. 1994. Serum insulin-like growth factor-I in 1030 healthy children, adolescents, and adults: relation to age, sex, stage of puberty, testicular size, and body mass index. J Clin Endocrinol Metab 78:744–752. [DOI] [PubMed] [Google Scholar]
- Kamat A, Smith ME, Shelton JM, Richardson JA, Mendelson CR. 2005. Genomic regions that mediate placental cell-specific and developmental regulation of human Cyp19 (aromatase) gene expression in transgenic mice. Endocrinology 146:2481–2488. [DOI] [PubMed] [Google Scholar]
- Kang H, Xiao X, Huang C, Yuan Y, Tang D, Dai X, Zeng X. 2018. Potent aromatase inhibitors and molecular mechanism of inhibitory action. Eur J Med Chem 143:426–437. [DOI] [PubMed] [Google Scholar]
- Kellis JT Jr., Nesnow S, Vickery LE. 1986. Inhibition of aromatase cytochrome P-450 (estrogen synthetase) by derivatives of α-naphthoflavone. Biochem Pharmacol 35:2887–2891. [DOI] [PubMed] [Google Scholar]
- Kellis JT Jr., Vickery LE. 1984. Inhibition of human estrogen synthetase (aromatase) by flavones. Science 225:1032–1034. [DOI] [PubMed] [Google Scholar]
- Kelloff GJ, Lubet RA, Lieberman R, Eisenhauer K, Steele VE, Crowell JA, Hawk ET, Boone CW, Sigman CC. 1998. Aromatase inhibitors as potential cancer chemopreventives. Cancer Epidemiol Biomarkers Prev 7:65–78. [PubMed] [Google Scholar]
- Khatri Y, Luthra A, Duggal R, Sligar SG. 2014. Kinetic solvent isotope effect in steady-state turnover by CYP19A1 suggests involvement of Compound 1 for both hydroxylation and aromatization steps. FEBS Lett 588:3117–3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim B, Moon JY, Choi MH, Yang HH, Lee S, Lim KS, Yoon SH, Yu KS, Jang IJ, Cho JY. 2013. Global metabolomics and targeted steroid profiling reveal that rifampin, a strong human PXR activator, alters endogenous urinary steroid markers. J Proteome Res 12:1359–1368. [DOI] [PubMed] [Google Scholar]
- Kitawaki J, Yamamoto T, Urabe M, Tamura T, Inoue S, Honjo H, Okada H. 1990. Selective aromatase inhibition by pyridoglutethimide, an analogue of aminoglutethimide. Acta Endocrinol 122:592–598. [DOI] [PubMed] [Google Scholar]
- Kjeldsen LS, Ghisari M, Bonefeld-Jørgensen EC. 2013. Currently used pesticides and their mixtures affect the function of sex hormone receptors and aromatase enzyme activity. Toxicol Appl Pharmacol 272:453–464. [DOI] [PubMed] [Google Scholar]
- Kümler I, Knoop AS, Jessing CA, Ejlertsen B, Nielsen DL. 2016. Review of hormone-based treatments in postmenopausal patients with advanced breast cancer focusing on aromatase inhibitors and fulvestrant. ESMO Open 1:e000062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey M, Bohday J, Fonseka SM, Ullah AI, Whitehead SA. 2005. Dose-response effects of phytoestrogens on the activity and expression of 3β-hydroxysteroid dehydrogenase and aromatase in human granulosa-luteal cells. J Steroid Biochem Mol Biol 96:279–286. [DOI] [PubMed] [Google Scholar]
- Laville N, Balaguer P, Brion F, Hinfray N, Casellas C, Porcher JM, Ait-Aissa S. 2006. Modulation of aromatase activity and mRNA by various selected pesticides in the human choriocarcinoma JEG-3 cell line. Toxicology 228:98–108. [DOI] [PubMed] [Google Scholar]
- Le Bail JC, Champavier Y, Chulia AJ, Habrioux G. 2000. Effects of phytoestrogens on aromatase, 3β and 17β-hydroxysteroid dehydrogenase activities and human breast cancer cells. Life Sci 66:1281–1291. [DOI] [PubMed] [Google Scholar]
- Le Bail JC, Laroche T, Marre-Fournier F, Habrioux G. 1998. Aromatase and 17β-hydroxysteroid dehydrogenase inhibition by flavonoids. Cancer Lett 133:101–106. [DOI] [PubMed] [Google Scholar]
- Le Bail JC, Pouget C, Fagnere C, Basly JP, Chulia AJ, Habrioux G. 2001. Chalcones are potent inhibitors of aromatase and 17β-hydroxysteroid dehydrogenase activities. Life Sci 68:751–761. [DOI] [PubMed] [Google Scholar]
- Letcher RJ, van Holsteijn I, Drenth HJ, Norstrom RJ, Bergman A, Safe S, Pieters R, van den Berg M. 1999. Cytotoxicity and aromatase (CYP19) activity modulation by organochlorines in human placental JEG-3 and JAR choriocarcinoma cells. Toxicol Appl Pharmacol 160:10–20. [DOI] [PubMed] [Google Scholar]
- Li LA. 2007. Polychlorinated biphenyl exposure and CYP19 gene regulation in testicular and adrenocortical cell lines. Toxicol In Vitro 21:1087–1094. [DOI] [PubMed] [Google Scholar]
- Linardi A, Damiani D, Longui CA. 2017. The use of aromatase inhibitors in boys with short stature: what to know before prescribing? Arch Endocrinol Metab 61:391–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lønning PE, Eikesdal HP. 2013. Aromatase inhibition 2013: Clinical state of the art and questions that remain to be solved. Endocr Related Cancer 20:R183–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou YR, Murtola T, Tuohimaa P. 2005. Regulation of aromatase and 5α-reductase by 25-hydroxyvitamin D3, 1α,25-dihydroxyvitamin D3, dexamethasone and progesterone in prostate cancer cells. J Steroid Biochem Mol Biol 94:151–157. [DOI] [PubMed] [Google Scholar]
- Lu D, Yang L, Li Q, Gao X, Wang F, Zhang G. 2012. Egonol gentiobioside and egonol gentiotrioside from Styrax perkinsiae promote the biosynthesis of estrogen by aromatase. Eur J Pharmacol 691:275–282. [DOI] [PubMed] [Google Scholar]
- Lu DF, Yang LJ, Wang F, Zhang GL. 2012. Inhibitory effect of luteolin on estrogen biosynthesis in human ovarian granulosa cells by suppression of aromatase (CYP19). J Agri Food Chem 60:8411–8418. [DOI] [PubMed] [Google Scholar]
- Lu WJ, Desta Z, Flockhart DA. 2012. Tamoxifen metabolites as active inhibitors of aromatase in the treatment of breast cancer. Breast Cancer Res Treat 131:473–481. [DOI] [PubMed] [Google Scholar]
- Lu WJ, Xu C, Pei Z, Mayhoub AS, Cushman M, Flockhart DA. 2012. The tamoxifen metabolite norendoxifen is a potent and selective inhibitor of aromatase (CYP19) and a potential lead compound for novel therapeutic agents. Breast Cancer Res Treat 133:99–109. [DOI] [PubMed] [Google Scholar]
- Magistrato A, Sgrignani J, Krause R, Cavalli A. 2017. Single or multiple access channels to the CYP450s active site? An answer from free energy simulations of the human aromatase enzyme. J Phys Chem Lett 8:2036–2042. [DOI] [PubMed] [Google Scholar]
- Maiti A, Cuendet M, Croy VL, Endringer DC, Pezzuto JM, Cushman M. 2007. Synthesis and biological evaluation of (±)-abyssinone II and its analogues as aromatase inhibitors for chemoprevention of breast cancer. J Med Chem 50:2799–2806. [DOI] [PubMed] [Google Scholar]
- Marchand P, Le Borgne M, Palzer M, Le Baut G, Hartmann RW. 2003. Preparation and pharmacological profile of 7-(α-azolylbenzyl)-1H-indoles and indolines as new aromatase inhibitors. Bioorg Med Chem Lett 13:1553–1555. [DOI] [PubMed] [Google Scholar]
- Mauger JW. 1989. The impact of innovative drug delivery systems on drug toxicity. J Toxicol Clin Toxicol 27:v–vii. [DOI] [PubMed] [Google Scholar]
- Mauras N 2011. Strategies for maximizing growth in puberty in children with short stature. Ped Clinics North Amer 58:1167–1179. [DOI] [PubMed] [Google Scholar]
- McCammon KM, Panda SP, Xia C, Kim JJ, Moutinho D, Kranendonk M, Auchus RJ, Lafer EM, Ghosh D, Martasek P et al. 2016. Instability of the human cytochrome P450 reductase A287P variant is the major contributor to its Antley-Bixler Syndrome-like phenotype. J Biol Chem 291:20487–20502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath N, O’Grady MJ. 2015. Aromatase inhibitors for short stature in male children and adolescents. Cochrane Database System Rev (10):Cd010888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNulty J, Keskar K, Crankshaw DJ, Holloway AC. 2014. Discovery of a new class of cinnamyl-triazole as potent and selective inhibitors of aromatase (cytochrome P450 19A1). Bioorg Med Chem Lett 24:4586–4589. [DOI] [PubMed] [Google Scholar]
- McNulty J, Nair JJ, Bollareddy E, Keskar K, Thorat A, Crankshaw DJ, Holloway AC, Khan G, Wright GD, Ejim L. 2009. Isolation of flavonoids from the heartwood and resin of Prunus avium and some preliminary biological investigations. Phytochemistry 70:2040–2046. [DOI] [PubMed] [Google Scholar]
- Means GD, Kilgore MW, Mahendroo MS, Mendelson CR, Simpson ER. 1991. Tissue-specific promoters regulate aromatase cytochrome P450 gene expression in human ovary and fetal tissues. Mol Endocrinol 5:2005–2013. [DOI] [PubMed] [Google Scholar]
- Mendelson CR, Jiang B, Shelton JM, Richardson JA, Hinshelwood MM. 2005. Transcriptional regulation of aromatase in placenta and ovary. J Steroid Biochem Mol Biol 95:25–33. [DOI] [PubMed] [Google Scholar]
- Morinaga H, Yanase T, Nomura M, Okabe T, Goto K, Harada N, Nawata H. 2004. A benzimidazole fungicide, benomyl, and its metabolite, carbendazim, induce aromatase activity in a human ovarian granulose-like tumor cell line (KGN). Endocrinology 145:1860–1869. [DOI] [PubMed] [Google Scholar]
- Morishima A, Grumbach MM, Simpson ER, Fisher C, Qin K. 1995. Aromatase deficiency in male and female siblings caused by a novel mutation and the physiological role of estrogens. J Clin Endocrinol Metab 80:3689–3698. [DOI] [PubMed] [Google Scholar]
- Mu YM, Yanase T, Nishi Y, Hirase N, Goto K, Takayanagi R, Nawata H. 2000. A nuclear receptor system constituted by RAR and RXR induces aromatase activity in MCF-7 human breast cancer cells. Mol Cell Endocrinol 166:137–145. [DOI] [PubMed] [Google Scholar]
- Mu YM, Yanase T, Nishi Y, Waseda N, Oda T, Tanaka A, Takayanagi R, Nawata H. 2000. Insulin sensitizer, troglitazone, directly inhibits aromatase activity in human ovarian granulosa cells. Biochem Biophys Res Commun 271:710–713. [DOI] [PubMed] [Google Scholar]
- Muralimanoharan S, Kwak YT, Mendelson CR. 2018. Redox-sensitive transcription factor NRF2 enhances trophoblast differentiation via induction of miR-1246 and aromatase. Endocrinology en 2017–03024, 10.1210/en.2017-03024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nativelle-Serpentini C, Richard S, Séralini GE, Sourdaine P. 2003. Aromatase activity modulation by lindane and bisphenol-A in human placental JEG-3 and transfected kidney E293 cells. Toxicol In Vitro 17:413–422. [DOI] [PubMed] [Google Scholar]
- Neunzig J, Milhim M, Schiffer L, Khatri Y, Zapp J, Sanchez-Guijo A, Hartmann MF, Wudy SA, Bernhardt R. 2017. The steroid metabolite 16β-OH-androstenedione generated by CYP21A2 serves as a substrate for CYP19A1. J Steroid Biochem Mol Biol 167:182–191. [DOI] [PubMed] [Google Scholar]
- Nguyen DP, O’Malley P, Al Hussein Al Awamlh B, Furrer MA, Mongan NP, Robinson BD, Wang GJ, Scherr DS. 2017. Association of aromatase with bladder cancer stage and long-term survival: New insights into the hormonal paradigm in bladder cancer. Clin Genitourinary Cancer 15:256–262.e251. [DOI] [PubMed] [Google Scholar]
- Niinivehmas S, Postila PA, Rauhamäki S, Manivannan E, Kortet S, Ahinko M, Huuskonen P, Nyberg N, Koskimies P, Lätti S, Multamäki E, Juvonen RO, Raunio H, Pasanen M, Huuskonen J, Pentikäinen OT. 2018. Blocking oestradiol synthesis pathways with potent and selective coumarin derivatives. J Enzyme Inhib Med Chem 33:743–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning B, Dial S, Sun Y, Wang J, Yang J, Guo L. 2008. Systematic and simultaneous gene profiling of 84 drug-metabolizing genes in primary human hepatocytes. J Biomol Screen 13:194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M, Ohno S, Nakajin S. 2007. Mono-(2-ethylhexyl) phthalate (MEHP) induces nuclear receptor 4A subfamily in NCI-H295R cells: A possible mechanism of aromatase suppression by MEHP. Mol Cell Endocrinol 274:8–18. [DOI] [PubMed] [Google Scholar]
- Numazawa M, Ando M, Watari Y, Tominaga T, Hayata Y, Yoshimura A. 2005. Structure-activity relationships of 2-, 4-, or 6-substituted estrogens as aromatase inhibitors. J Steroid Biochem Mol Biol 96:51–58. [DOI] [PubMed] [Google Scholar]
- Numazawa M, Mutsumi A, Tachibana M, Yoshimura A. 2003. Kinetic analysis of reversible inhibition of 16α-hydroxyandrostenedione aromatization in human placental microsomes by suicide substrates of androstenedione aromatization. Biol Pharmaceut Bull 26:890–892. [DOI] [PubMed] [Google Scholar]
- Numazawa M, Shelangouski M, Nagasaka M. 2000. Probing the binding pocket of the active site of aromatase with 6-ether or 6-ester substituted androst-4-ene-3,17-diones and their diene and triene analogs. Steroids 65:871–882. [DOI] [PubMed] [Google Scholar]
- Numazawa M, Tachibana M, Mutsumi A, Yoshimura A, Osawa Y. 2002. Aromatization of 16alpha-hydroxyandrostenedione by human placental microsomes: effect of preincubation with suicide substrates of androstenedione aromatization. J Steroid Biochem Mol Biol 81:165–172. [DOI] [PubMed] [Google Scholar]
- Numazawa M, Yamada K. 1999. Studies of the time-dependent inactivation of aromatase by 4β,5β-epoxy-6-one and 5β 6β-epoxy-4-one steroids under various conditions. Biol Pharmaceut Bull 22:1207–1211. [DOI] [PubMed] [Google Scholar]
- Numazawa M, Yamaguchi S. 1998. 6-Phenylaliphatic-substituted androst-4-ene-3,17-diones as aromatase inhibitors: structure-activity relationships. J Steroid Biochem Mol Biol 67:41–48. [DOI] [PubMed] [Google Scholar]
- Numazawa M, Yamaguchi S. 1999. Synthesis and structure-activity relationships of 6-phenylaliphatic-substituted C19 steroids having a 1,4-diene, 4,6-diene, or 1,4,6-triene structure as aromatase inhibitors. Steroids 64:187–196. [DOI] [PubMed] [Google Scholar]
- Numazawa M, Yoshimura A, Oshibe M. 1998. Enzymic aromatization of 6-alkyl-substituted androgens, potent competitive and mechanism-based inhibitors of aromatase. Biochem J 329:151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odum J, Ashby J. 2002. Detection of aromatase inhibitors in vitro using rat ovary microsomes. Toxicol Lett 129:119–122. [DOI] [PubMed] [Google Scholar]
- Okada M, Yoden T, Kawaminami E, Shimada Y, Kudoh M, Isomura Y. 1997. Studies on aromatase inhibitors. IV. Synthesis and biological evaluation of N,N-disubstituted-5-aminopyrimidine derivatives. Chem Pharmaceut Bull 45:1293–1299. [DOI] [PubMed] [Google Scholar]
- Oskarsson A, Ohlsson Andersson A. 2016. Suppressed sex hormone biosynthesis by alkylresorcinols: A possible link to chemoprevention. Nutr Cancer 68:978–987. [DOI] [PubMed] [Google Scholar]
- Pan ST, Xue D, Li ZL, Zhou ZW, He ZX, Yang Y, Yang T, Qiu JX, Zhou SF. 2016. Computational identification of the paralogs and orthologs of human cytochrome P450 superfamily and the implication in drug discovery. Int J Mol Sci June 28;17(7). pii: ijms17071020. doi: 10.3390/ijms17071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pino AM, Rodriguez JM, Rios S, Astudillo P, Leiva L, Seitz G, Fernandez M, Rodriguez JP. 2006. Aromatase activity of human mesenchymal stem cells is stimulated by early differentiation, vitamin D and leptin. J Endocrinol 191:715–725. [DOI] [PubMed] [Google Scholar]
- Pogrmic-Majkic K, Samardzija D, Stojkov-Mimic N, Vukosavljevic J, Trninic-Pjevic A, Kopitovic V, Andric N. 2018. Atrazine suppresses FSH-induced steroidogenesis and LH-dependent expression of ovulatory genes through PDE-cAMP signaling pathway in human cumulus granulosa cells. Mol Cell Endocrinol 461:79–88. [DOI] [PubMed] [Google Scholar]
- Pokhrel M, Ma E. 2011. Synthesis and screening of aromatase inhibitory activity of substituted C19 steroidal 17-oxime analogs. Molecules (Basel, Switzerland) 16:9868–9885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu W, Yuan Y, Lu D, Wang X, Liu H, Wang C, Wang F, Zhang G. 2016. 2-Phenylbenzo[b]furans: Synthesis and promoting activity on estrogen biosynthesis. Bioorg Med Chem Lett 26:5497–5500. [DOI] [PubMed] [Google Scholar]
- Punetha A, Shanmugam K, Sundar D. 2011. Insight into the enzyme-inhibitor interactions of the first experimentally determined human aromatase. J Biomol Struct Dyn 28:759–771. [DOI] [PubMed] [Google Scholar]
- Purohit A, Ghilchik MW, Leese MP, Potter BV, Reed MJ. 2005. Regulation of aromatase activity by cytokines, PGE2 and 2-methoxyoestrone-3-O-sulphamate in fibroblasts derived from normal and malignant breast tissues. J Steroid Biochem Mol Biol 94:167–172. [DOI] [PubMed] [Google Scholar]
- Raobaikady B, Parsons MF, Reed MJ, Purohit A. 2007. Tibolone and its Δ4,7α-methyl norethisterone metabolite are reversible inhibitors of human aromatase. J Steroid Biochem Mol Biol 104:154–160. [DOI] [PubMed] [Google Scholar]
- Rice S, Mason HD, Whitehead SA. 2006. Phytoestrogens and their low dose combinations inhibit mRNA expression and activity of aromatase in human granulosa-luteal cells. J Steroid Biochem Mol Biol 101:216–225. [DOI] [PubMed] [Google Scholar]
- Rice S, Pellatt L, Ramanathan K, Whitehead SA, Mason HD. 2009. Metformin inhibits aromatase via an extracellular signal-regulated kinase-mediated pathway. Endocrinology 150:4794–4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieke S, Koehn S, Hirsch-Ernst K, Pfeil R, Kneuer C, Marx-Stoelting P. 2014. Combination effects of (tri)azole fungicides on hormone production and xenobiotic metabolism in a human placental cell line. Int J Environ Res Publ Health 11:9660–9679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson KM, O’Donnell L, Jones ME, Meachem SJ, Boon WC, Fisher CR, Graves KH, McLachlan RI, Simpson ER. 1999. Impairment of spermatogenesis in mice lacking a functional aromatase (cyp19) gene. Proc Natl Acad Sci USA 96:7986–7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochira V, Zirilli L, Maffei L, Premrou V, Aranda C, Baldi M, Ghigo E, Aimaretti G, Carani C, Lanfranco F. 2010. Tall stature without growth hormone: Four male patients with aromatase deficiency. J Clin Endocrinol Metab 95:1626–1633. [DOI] [PubMed] [Google Scholar]
- Saarinen N, Joshi SC, Ahotupa M, Li X, Ammala J, Makela S, Santti R. 2001. No evidence for the in vivo activity of aromatase-inhibiting flavonoids. J Steroid Biochem Mol Biol 78:231–239. [DOI] [PubMed] [Google Scholar]
- Saberi MR, Vinh TK, Yee SW, Griffiths BJ, Evans PJ, Simons C. 2006. Potent CYP19 (aromatase) 1-[(benzofuran-2-yl)(phenylmethyl)pyridine, -imidazole, and -triazole inhibitors: synthesis and biological evaluation. J Med Chem 49:1016–1022. [DOI] [PubMed] [Google Scholar]
- Sanderson JT, Boerma J, Lansbergen GW, van den Berg M. 2002. Induction and inhibition of aromatase (CYP19) activity by various classes of pesticides in H295R human adrenocortical carcinoma cells. Toxicol Appl Pharmacol 182:44–54. [DOI] [PubMed] [Google Scholar]
- Sanderson JT, Hordijk J, Denison MS, Springsteel MF, Nantz MH, van den Berg M. 2004. Induction and inhibition of aromatase (CYP19) activity by natural and synthetic flavonoid compounds in H295R human adrenocortical carcinoma cells. Toxicol Sci 82:70–79. [DOI] [PubMed] [Google Scholar]
- Sanderson JT, Letcher RJ, Heneweer M, Giesy JP, van den Berg M. 2001. Effects of chloro-s-triazine herbicides and metabolites on aromatase activity in various human cell lines and on vitellogenin production in male carp hepatocytes. Environ Health Perspect 109:1027–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson JT, Seinen W, Giesy JP, van den Berg M. 2000. 2-Chloro-S-triazine herbicides induce aromatase (CYP19) activity in H295R human adrenocortical carcinoma cells: a novel mechanism for estrogenicity? Toxicol Sci 54:121–127. [DOI] [PubMed] [Google Scholar]
- Sanderson T, Renaud M, Scholten D, Nijmeijer S, van den Berg M, Cowell S, Guns E, Nelson C, Mutarapat T, Ruchirawat S. 2008. Effects of lactone derivatives on aromatase (CYP19) activity in H295R human adrenocortical and (anti)androgenicity in transfected LNCaP human prostate cancer cells. Eur J Pharmacol 593:92–98. [DOI] [PubMed] [Google Scholar]
- Satoh K, Sakamoto Y, Ogata A, Nagai F, Mikuriya H, Numazawa M, Yamada K, Aoki N. 2002. Inhibition of aromatase activity by green tea extract catechins and their endocrinological effects of oral administration in rats. Food Chem Toxicol 40:925–933. [DOI] [PubMed] [Google Scholar]
- Schmidt M, Löffler G. 1997. RU486 is a potent inhibitor of aromatase induction in human breast adipose tissue stromal cells. J Steroid Biochem Mol Biol 60:197–204. [DOI] [PubMed] [Google Scholar]
- Seralini G, Moslemi S. 2001. Aromatase inhibitors: past, present and future. Mol Cell Endocrinol 178:117–131. [DOI] [PubMed] [Google Scholar]
- Sgrignani J, Bon M, Colombo G, Magistrato A. 2014. Computational approaches elucidate the allosteric mechanism of human aromatase inhibition: a novel possible route to small-molecule regulation of CYP450s activities? J Chem Inform Modeling 54:2856–2868. [DOI] [PubMed] [Google Scholar]
- Shim KS. 2015. Pubertal growth and epiphyseal fusion. Ann Ped Endocrinol Metab 20:8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shozu M, Fukami M, Ogata T. 2014. Understanding the pathological manifestations of aromatase excess syndrome: lessons for clinical diagnosis. Expert Rev Endocrinol Metab 9:397–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon V, Avet C, Grange-Messent V, Wargnier R, Denoyelle C, Pierre A, Dairou J, Dupret JM, Cohen-Tannoudji J. 2017. Carbon black nanoparticles inhibit aromatase expression and estradiol secretion in human granulosa cells through the ERK1/2 pathway. Endocrinology 158:3200–3211. [DOI] [PubMed] [Google Scholar]
- Simpson ER. 2003. Sources of estrogen and their importance. J Steroid Biochem Mol Biol 86:225–230. [DOI] [PubMed] [Google Scholar]
- Simpson ER, Clyne C, Rubin G, Boon WC, Robertson K, Britt K, Speed C, Jones M. 2002. Aromatase–a brief overview. Annu Rev Physiol 64:93–127. [DOI] [PubMed] [Google Scholar]
- Sluijmer AV, Heineman MJ, De Jong FH, Evers JL. 1995. Endocrine activity of the postmenopausal ovary: the effects of pituitary down-regulation and oophorectomy. J Clin Endocrinol Metab 80:2163–2167. [DOI] [PubMed] [Google Scholar]
- Song R, He Y, Murphy MB, Yeung LW, Yu RM, Lam MH, Lam PK, Hecker M, Giesy JP, Wu RS et al. 2008. Effects of fifteen PBDE metabolites, DE71, DE79 and TBBPA on steroidogenesis in the H295R cell line. Chemosphere 71:1888–1894. [DOI] [PubMed] [Google Scholar]
- Stillman SC, Evans BA, Hughes IA. 1991. Androgen dependent stimulation of aromatase activity in genital skin fibroblasts from normals and patients with androgen insensitivity. Clin Endocrinol 35:533–538. [DOI] [PubMed] [Google Scholar]
- Su B, Diaz-Cruz ES, Landini S, Brueggemeier RW. 2006. Novel sulfonanilide analogues suppress aromatase expression and activity in breast cancer cells independent of COX-2 inhibition. J Med Chem 49:1413–1419. [DOI] [PubMed] [Google Scholar]
- Su B, Diaz-Cruz ES, Landini S, Brueggemeier RW. 2008. Suppression of aromatase in human breast cells by a cyclooxygenase-2 inhibitor and its analog involves multiple mechanisms independent of cyclooxygenase-2 inhibition. Steroids 73:104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su B, Hackett JC, Diaz-Cruz ES, Kim YW, Brueggemeier RW. 2005. Lead optimization of 7-benzyloxy 2-(4´-pyridylmethyl)thio isoflavone aromatase inhibitors. Bioorg Med Chem 13:6571–6577. [DOI] [PubMed] [Google Scholar]
- Sureram S, Wiyakrutta S, Ngamrojanavanich N, Mahidol C, Ruchirawat S, Kittakoop P. 2012. Depsidones, aromatase inhibitors and radical scavenging agents from the marine-derived fungus Aspergillus unguis CRI282–03. Planta Med 78:582–588. [DOI] [PubMed] [Google Scholar]
- Takemura Y, Osuga Y, Yoshino O, Hasegawa A, Hirata T, Hirota Y, Nose E, Morimoto C, Harada M, Koga K et al. 2007. Metformin suppresses interleukin (IL)-1β-induced IL-8 production, aromatase activation, and proliferation of endometriotic stromal cells. J Clin Endocrinol Metab 92:3213–3218. [DOI] [PubMed] [Google Scholar]
- Tam TW, Liu R, Arnason JT, Krantis A, Staines WA, Haddad PS, Foster BC. 2009. Actions of ethnobotanically selected Cree anti-diabetic plants on human cytochrome P450 isoforms and flavin-containing monooxygenase 3. J Ethnopharmacol 126:119–126. [DOI] [PubMed] [Google Scholar]
- Tan T, Wang L, Wang B. 2015. Collagen and prostaglandin E2 regulate aromatase expression through the PI3K/AKT/IKK and the MAP kinase pathways in adipose stromal cells. Mol Med Rep 12:4766–4772. [DOI] [PubMed] [Google Scholar]
- Trösken ER, Fischer K, Volkel W, Lutz WK. 2006. Inhibition of human CYP19 by azoles used as antifungal agents and aromatase inhibitors, using a new LC-MS/MS method for the analysis of estradiol product formation. Toxicology 219:33–40. [DOI] [PubMed] [Google Scholar]
- Trösken ER, Scholz K, Lutz RW, Volkel W, Zarn JA, Lutz WK. 2004. Comparative assessment of the inhibition of recombinant human CYP19 (aromatase) by azoles used in agriculture and as drugs for humans. Endocr Res 30:387–394. [DOI] [PubMed] [Google Scholar]
- van Meeuwen JA, Korthagen N, de Jong PC, Piersma AH, van den Berg M. 2007. (Anti)estrogenic effects of phytochemicals on human primary mammary fibroblasts, MCF-7 cells and their co-culture. Toxicol Appl Pharmacol 221:372–383. [DOI] [PubMed] [Google Scholar]
- van Meeuwen JA, Nijmeijer S, Mutarapat T, Ruchirawat S, de Jong PC, Piersma AH, van den Berg M. 2008. Aromatase inhibition by synthetic lactones and flavonoids in human placental microsomes and breast fibroblasts–a comparative study. Toxicol Appl Pharmacol 228:269–276. [DOI] [PubMed] [Google Scholar]
- Vanden Bossche HV, Moereels H, Koymans LM. 1994. Aromatase inhibitors–mechanisms for non-steroidal inhibitors. Breast Cancer Res Treat 30:43–55. [DOI] [PubMed] [Google Scholar]
- Vinggaard AM, Hnida C, Breinholt V, Larsen JC. 2000. Screening of selected pesticides for inhibition of CYP19 aromatase activity in vitro. Toxicol In Vitro 14:227–234. [DOI] [PubMed] [Google Scholar]
- Vinh TK, Nicholls PJ, Kirby AJ, Simons C. 2001. Evaluation of 7-hydroxy-flavones as inhibitors of oestrone and oestradiol biosynthesis. J Enz Inhib 16:417–424. [DOI] [PubMed] [Google Scholar]
- Wang C, Mäkelä T, Hase T, Adlercreutz H, Kurzer MS. 1994. Lignans and flavonoids inhibit aromatase enzyme in human preadipocytes. J Steroid Biochem Mol Biol 50:205–212. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhao H, Xiang Q, Ju H, Han SM, Wang LY, Xu B. 2009. Effect of Yikun Neiyi Wan on the expression of aromatase P450, COX-2, and ER related receptor in endometrial cells in vitro from patients with endometriosis. J Trad Chinese Med 29:296–300. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chan FL, Chen S, Leung LK. 2005. The plant polyphenol butein inhibits testosterone-induced proliferation in breast cancer cells expressing aromatase. Life Sci 77:39–51. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lee KW, Chan FL, Chen S, Leung LK. 2006. The red wine polyphenol resveratrol displays bilevel inhibition on aromatase in breast cancer cells. Toxicol Sci 92:71–77. [DOI] [PubMed] [Google Scholar]
- Wang Y, Leung LK. 2007. Pharmacological concentration of resveratrol suppresses aromatase in JEG-3 cells. Toxicol Lett 173:175–180. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Noda M, Nakajin S. 2006. Effect of epidermal growth factor and prostaglandin on the expression of aromatase (CYP19) in human adrenocortical carcinoma cell line NCI-H295R cells. J Endocrinol 188:59–68. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Ohno S, Nakajin S. 2005. Forskolin and dexamethasone synergistically induce aromatase (CYP19) expression in the human osteoblastic cell line SV-HFO. Eur J Endocrinol 152:619–624. [DOI] [PubMed] [Google Scholar]
- White EL, Ross LJ, Steele VE, Kelloff GJ, Hill DL. 1999. Screening of potential cancer preventing chemicals as aromatase inhibitors in an in vitro assay. AntiCancer Res 19:1017–1020. [PubMed] [Google Scholar]
- Whitehead SA, Lacey M. 2003. Phytoestrogens inhibit aromatase but not 17β-hydroxysteroid dehydrogenase (HSD) type 1 in human granulosa-luteal cells: evidence for FSH induction of 17β-HSD. Hum Reprod 18(3):487–494. [DOI] [PubMed] [Google Scholar]
- Wit JM, Hero M, Nunez SB. 2011. Aromatase inhibitors in pediatrics. Nat Rev Endocrinol 8:135–147. [DOI] [PubMed] [Google Scholar]
- Wójtowicz AK, Milewicz T, Gregoraszczuk EL. 2007. DDT and its metabolite DDE alter steroid hormone secretion in human term placental explants by regulation of aromatase activity. Toxicol Lett 173:24–30. [DOI] [PubMed] [Google Scholar]
- Wu S, Ye J, Wang Z, Lin SX, Lu M, Liang Y, Zhu X, Olumi AF, Zhong WD, Wu CL. 2018. Expression of aromatase in tumor related stroma is associated with human bladder cancer progression. Cancer Biol Ther 19:175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahiaoui S, Pouget C, Fagnere C, Champavier Y, Habrioux G, Chulia AJ. 2004. Synthesis and evaluation of 4-triazolylflavans as new aromatase inhibitors. Bioorg Med Chem Lett 14:5215–5218. [DOI] [PubMed] [Google Scholar]
- Yanase T, Mu YM, Nishi Y, Goto K, Nomura M, Okabe T, Takayanagi R, Nawata H. 2001. Regulation of aromatase by nuclear receptors. J Steroid Biochem Mol Biol 79:187–192. [DOI] [PubMed] [Google Scholar]
- Yanase T, Suzuki S, Goto K, Nomura M, Okabe T, Takayanagi R, Nawata H. 2003. Aromatase in bone: Roles of vitamin D3 and androgens. J Steroid Biochem Mol Biol 86:393–397. [DOI] [PubMed] [Google Scholar]
- Yoshimoto FK, Guengerich FP. 2014. Mechanism of the third oxidative step in the conversion of androgens to estrogens by cytochrome P450 19A1 steroid aromatase. J Am Chem Soc 136:15016–15025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yumoto T, Ando K, Okamoto K, Okamoto S, Yoshida Y. 1976. [Studies on immunoblastic lymphadenopathy in the New Zealand Black strain mice (author’s transl)]. Nihon Ketsueki Gakkai zasshi 39:170–181. jpn. [PubMed] [Google Scholar]
- Zarn JA, Bruschweiler BJ, Schlatter JR. 2003. Azole fungicides affect mammalian steroidogenesis by inhibiting sterol 14α-demethylase and aromatase. Environ Health Perspect 111:255–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, He Y, Liu C, Liu C, Li S. 2018. In vitro screening and isolation of human aromatase inhibitors from Cicer arietinum by a novel continuous online method combining chromatographic techniques. J Sep Sci 41:483–492. [DOI] [PubMed] [Google Scholar]
- Zhao Y, Nichols JE, Valdez R, Mendelson CR, Simpson ER. 1996. Tumor necrosis factor-alpha stimulates aromatase gene expression in human adipose stromal cells through use of an activating protein-1 binding site upstream of promoter 1.4. Mol Endocrinol 10:1350–1357. [DOI] [PubMed] [Google Scholar]
- Zharikova OL, Deshmukh SV, Kumar M, Vargas R, Nanovskaya TN, Hankins GD, Ahmed MS. 2007. The effect of opiates on the activity of human placental aromatase/CYP19. Biochem Pharmacol 73:279–286. [DOI] [PubMed] [Google Scholar]
- Zharikova OL, Deshmukh SV, Nanovskaya TN, Hankins GD, Ahmed MS. 2006. The effect of methadone and buprenorphine on human placental aromatase. Biochem Pharmacol 71:1255–1264. [DOI] [PubMed] [Google Scholar]
- Zhong S, Ye WP, Xu PP, Feng E, Li H, Lin SH, Liu JY, Ma C, Lin YC. 2010. Aromatase expression in leptin-pretreated human breast pre-adipocytes is enhanced by zeranol and suppressed by (−)-gossypol. AntiCancer Res 30:5077–5084. [PubMed] [Google Scholar]
- Zhou DJ, Pompon D, Chen SA. 1990. Stable expression of human aromatase complementary DNA in mammalian cells: a useful system for aromatase inhibitor screening. Cancer Res 50:6949–6954. [PubMed] [Google Scholar]
- Zhu SJ, Li Y, Li H, Wang YL, Xiao ZJ, Vihko P, Piao YS. 2002. Retinoic acids promote the action of aromatase and 17β-hydroxysteroid dehydrogenase type 1 on the biosynthesis of 17β-estradiol in placental cells. J Endocrinol 172:31–43. [DOI] [PubMed] [Google Scholar]
P450 21A2
The CYP21A2 gene is one of the most frequently mutated genes in medicine and has consequences in steroid insufficiency; i.e., the lack of 21-hydroxylation lowers levels of all steroids downstream of the process (Fig. 4B). The hyperplasia can be one of several forms, depending upon the severity of the disease, either salt-wasting (most severe), simple virilizing (intermediate), or non-classical (least severe).
The (wild-type) enzyme is very efficient and has a specificity constant (kcat/Km) of 1.3 × 107 M-1 s-1 (Pallan, Wang, et al. 2015). The two steps that contribute to rate-limitation are substrate binding and the C-H bond-breaking step. It is of interest that the high kinetic deuterium isotope effect is persistent even in the very low activity variants (Wang et al. 2017). There is a general correlation between the catalytic activities of the mutants and the severity of the disease (Wang et al. 2017). However, it is not perfect and there is the possibility that protein stability may be an additional factor (Wang et al. 2017).
Our laboratory first reported a structure for bovine P450 21A2 (Zhao et al. 2012) and subsequently a structure for (wild-type) human P450 21A2 (Pallan, Wang, et al. 2015). Others (Haider et al. 2013) had modeled the human structure based on the bovine enzyme but a careful analysis shows that some critical features may be in error in such an approach (Pallan, Lei, et al. 2015). In analyzing the structure, there is a tendency for the most severe mutations to be located inside of the protein and the changes associated with the least severe disease on the outside of the protein (Pallan, Lei, et al. 2015).
In reviewing the disease and the contribution of the single nucleotide variations (i.e., single amino acid substitutions) (Wang et al. 2017), it is surprising that these seemingly small changes have such dramatic functional effects. Some of this can be attributed to protein stability and some to the fact that, using the Eyring equation, even a 6.4 kcal mol−1 difference (e.g., one of two H-bonds) can be linked to a 50,000-fold change in enzyme activity (Wang et al. 2017).
To date we have been unsuccessful in crystallizing any of the low-activity clinically-reported variants.
References
- Asif AR, Ljubojevic M, Sabolic I, Shnitsar V, Metten M, Anzai N, Muller GA, Burckhardt G, Hagos Y. 2006. Regulation of steroid hormone biosynthesis enzymes and organic anion transporters by forskolin and DHEA-S treatment in adrenocortical cells. Am J Physiol Endocrinol Metab 291:E1351–1359. [DOI] [PubMed] [Google Scholar]
- Auchus RJ, Sampath Kumar A, Andrew Boswell C, Gupta MK, Bruce K, Rath NP, Covey DF. 2003. The enantiomer of progesterone (ent-progesterone) is a competitive inhibitor of human cytochromes P450c17 and P450c21. Arch Biochem Biophys 409:134–144. [DOI] [PubMed] [Google Scholar]
- Ayub M, Levell MJ. 1990. The inhibition of human prostatic aromatase activity by imidazole drugs including ketoconazole and 4-hydroxyandrostenedione. Biochem Pharmacol 40:1569–1575. [DOI] [PubMed] [Google Scholar]
- Blaha L, Hilscherova K, Mazurova E, Hecker M, Jones PD, Newsted JL, Bradley PW, Gracia T, Duris Z, Horka I et al. 2006. Alteration of steroidogenesis in H295R cells by organic sediment contaminants and relationships to other endocrine disrupting effects. Environ Int 32:749–757. [DOI] [PubMed] [Google Scholar]
- Coulter CL, Jaffe RB. 1998. Functional maturation of the primate fetal adrenal in vivo: 3. Specific zonal localization and developmental regulation of CYP21A2 (P450c21) and CYP11B1/CYP11B2 (P450c11/aldosterone synthase) lead to integrated concept of zonal and temporal steroid biosynthesis. Endocrinology 139:5144–5150. [DOI] [PubMed] [Google Scholar]
- Ding L, Murphy MB, He Y, Xu Y, Yeung LW, Wang J, Zhou B, Lam PK, Wu RS, Giesy JP. 2007. Effects of brominated flame retardants and brominated dioxins on steroidogenesis in H295R human adrenocortical carcinoma cell line. Environ Toxicol Chem 26:764–772. [DOI] [PubMed] [Google Scholar]
- Dowie LJ, Smith JE, MacGilchrist AJ, Fraser R, Honour JW, Reid JL, Kenyon CJ. 1988. In vivo and in vitro studies of the site of inhibitory action of omeprazole on adrenocortical steroidogenesis. Eur J Clin Pharmacol 35:625–629. [DOI] [PubMed] [Google Scholar]
- Dragan CA, Hartmann RW, Bureik M. 2006. A fission yeast-based test system for the determination of IC50 values of anti-prostate tumor drugs acting on CYP21. J Enzyme Inhib Med Chem 21:547–556. [DOI] [PubMed] [Google Scholar]
- Haider S, Islam B, D’Atri V, Sgobba M, Poojari C, Sun L, Yuen T, Zaidi M, New MI. 2013. Structure-phenotype correlations of human CYP21A2 mutations in congenital adrenal hyperplasia. Proc Natl Acad Sci USA 110:2605–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa E, Nakagawa S, Sato M, Tachikawa E, Yamato S. 2013. Effect of polyphenols on production of steroid hormones from human adrenocortical NCI-H295R cells. Biol Pharmaceut Bull 36:228–237. [DOI] [PubMed] [Google Scholar]
- Johansson MK, Sanderson JT, Lund BO. 2002. Effects of 3-MeSO2-DDE and some CYP inhibitors on glucocorticoid steroidogenesis in the H295R human adrenocortical carcinoma cell line. Toxicol In Vitro 16:113–121. [DOI] [PubMed] [Google Scholar]
- Lin CJ, Cheng LC, Lin TC, Wang CJ, Li LA. 2014. Assessment of the potential of polyphenols as a CYP17 inhibitor free of adverse corticosteroid elevation. Biochem Pharmacol 90:288–296. [DOI] [PubMed] [Google Scholar]
- Lin CW, Chang YH, Pu HF. 2012. Mitotane exhibits dual effects on steroidogenic enzymes gene transcription under basal and cAMP-stimulating microenvironments in NCI-H295 cells. Toxicology 298:14–23. [DOI] [PubMed] [Google Scholar]
- Lundqvist J, Norlin M, Wikvall K. 2010. 1α,25-Dihydroxyvitamin D3 affects hormone production and expression of steroidogenic enzymes in human adrenocortical NCI-H295R cells. Biochim Biophys Acta 1801:1056–1062. [DOI] [PubMed] [Google Scholar]
- Malikova J, Brixius-Anderko S, Udhanea SS, Parween S, Dick B, Bernhardt R, Pandey AV. 2017. CYP17A1 inhibitor abiraterone, an anti-prostate cancer drug, also inhibits the 21-hydroxylase activity of CYP21A2. J Steroid Biochem Mol Biol 174:192–200. [DOI] [PubMed] [Google Scholar]
- Ohlsson A, Ulleras E, Oskarsson A. 2009. A biphasic effect of the fungicide prochloraz on aldosterone, but not cortisol, secretion in human adrenal H295R cells–underlying mechanisms. Toxicol Lett 191:174–180. [DOI] [PubMed] [Google Scholar]
- Oskarsson A, Ohlsson Andersson A. 2016. Suppressed sex hormone biosynthesis by alkylresorcinols: A possible link to chemoprevention. Nutr Cancer 68:978–987. [DOI] [PubMed] [Google Scholar]
- Pallan PS, Lei L, Wang C, Waterman MR, Guengerich FP, Egli M. 2015. Research Resource: Correlating human cytochrome P450 21A2 crystal structure and phenotypes of mutations in congenital adrenal hyperplasia. Mol Endocrinol 29:1375–1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallan PS, Wang C, Lei L, Yoshimoto FK, Auchus RJ, Waterman MR, Guengerich FP, Egli M. 2015. Human cytochrome P450 21A2, the major steroid 21-hydroxylase: Structure of the enzyme•progesterone substrate complex and rate-limiting C-H bond cleavage. J Biol Chem 290:13128–13143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan ST, Xue D, Li ZL, Zhou ZW, He ZX, Yang Y, Yang T, Qiu JX, Zhou SF. 2016. Computational identification of the paralogs and orthologs of human cytochrome P450 superfamily and the implication in drug discovery. Int J Mol Sci June 28;17(7). pii: ijms17071020. doi: 10.3390/ijms17071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parr MK, Zöllner A, Fussholler G, Opfermann G, Schlörer N, Zorio M, Bureik M, Schanzer W. 2012. Unexpected contribution of cytochrome P450 enzymes CYP11B2 and CYP21, as well as CYP3A4 in xenobiotic androgen elimination–insights from metandienone metabolism. Toxicol Lett 213:381–391. [DOI] [PubMed] [Google Scholar]
- Schroeder RL, Tram P, Liu J, Foroozesh M, Sridhar J. 2016. Novel functionalized 5-(phenoxymethyl)-1,3-dioxane analogs exhibiting cytochrome P450 inhibition: a patent evaluation WO2015048311 (A1). Expert Opin Therapeut Patents 26:139–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song R, He Y, Murphy MB, Yeung LW, Yu RM, Lam MH, Lam PK, Hecker M, Giesy JP, Wu RS et al. 2008. Effects of fifteen PBDE metabolites, DE71, DE79 and TBBPA on steroidogenesis in the H295R cell line. Chemosphere 71:1888–1894. [DOI] [PubMed] [Google Scholar]
- Wang C, Pallan PS, Zhang W, Lei L, Yoshimoto FK, Waterman MR, Egli M, Guengerich FP. 2017. Functional analysis of human cytochrome P450 21A2 variants involved in congenital adrenal hyperplasia. J Biol Chem 292:10767–10778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel J, Grabinski N, Knopp CA, Dendorfer A, Ramanjaneya M, Randeva HS, Ehrhart-Bornstein M, Dominiak P, Johren O. 2009. Hypocretin/orexin increases the expression of steroidogenic enzymes in human adrenocortical NCI H295R cells. Am J Physiol Regl Integrative Comp Physiol 297:R1601–1609. [DOI] [PubMed] [Google Scholar]
- Yoshimoto FK, Desilets MC, Auchus RJ. 2012. Synthesis of halogenated pregnanes, mechanistic probes of steroid hydroxylases CYP17A1 and CYP21A2. J Steroid Biochem Mol Biol 128:38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Lei L, Kagawa N, Sundaramoorthy M, Banerjee S, Nagy LD, Guengerich FP, Waterman MR. 2012. Three-dimensional structure of steroid 21-hydroxylase (cytochrome P450 21A2) with two substrates reveals locations of disease-associated variants. J Biol Chem 287:10613–10622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zöllner A, Parr MK, Dragan CA, Dras S, Schlorer N, Peters FT, Maurer HH, Schanzer W, Bureik M. 2010. CYP21-catalyzed production of the long-term urinary metandienone metabolite 17β-hydroxymethyl-17α-methyl-18-norandrosta-1,4,13-trien-3-one: A contribution to the fight against doping. Biol Chem 391:119–127. [DOI] [PubMed] [Google Scholar]
P450 27A1
This is another mitochondrial P450, expressed primarily in liver and kidney. It can be regarded as being at an intersection of sterol and vitamin D metabolism, in that it has defined roles in both areas (Fig. 14). The enzyme acts on cholesterol in extrahepatic tissues, bile acid intermediates in the liver, and vitamin D3 in the kidneys (Wikvall 1984; Masumoto et al. 1988). Despite its broad specificity for sterols, P450 27A1 is also a highly regio- and stereo-specific enzyme producing all of the 27-hydroxycholesterol in humans. Cholesterol 27-hydroxylation enables a means of cholesterol transport from extrahepatic tissues to the liver (Meaney et al. 2002), and 27-hydroxycholesterol is also a bioactive molecule interacting with different regulatory proteins, including liver X receptors (Kalaany and Mangelsdorf 2006).
The regulation of the gene is highly complex, with several pathways involved (Guengerich 2015).
No crystal structures have yet been published.
Clinical issues have been considered with changes in the enzyme in both the areas of vitamin D and sterol metabolism (Guengerich 2015). Complete deficiency of P450 27A1 activity leads to cerebrotendinous xanthomatosis, a progressive autosomal recessive disease characterized by deposition of cholesterol and cholestanol in the brain and other tissues, neurologic dysfunction, and ocular problems. Cerebrotendinous xanthomatosis patients usually have normal or sub-normal levels of plasma cholesterol but often develop premature atherosclerosis and osteoporosis (CY27A1+/− heterozygous individuals do not present with cerebrotendinous xanthomatosis (Björkhem 2013)).
27-Hydroxycholesterol, the reaction product, binds to estrogen receptors and acts as a selective estrogen receptor modulator (SERM), eliciting system-specific adverse effects. The response can take several forms. In the vascular wall 27-hydroxycholesterol can function as an estrogen receptor antagonist and inhibit estrogen-related cardioprotection (Umetani et al. 2007; Umetani and Shaul 2011; Umetani et al. 2014). Conversely, in breast tumors, 27-hydroxycholesterol can serve as a partial estrogen receptor agonist and stimulate tumor growth in mouse breast cancer models of (Nelson et al. 2013). Through it activity with liver X receptors, 27-hydroxycholesterol can also increase breast tumor metastasis (Nelson et al. 2013). In bone, 27-hydroxycholesterol attenuated estrogen action and bone mineralization (DuSell et al. 2010).
Epidemiology studies are consistent with the role of 27-hydroxycholesterol as an SERM. Menopause was accompanied by elevated plasma levels of 27-hydroxycholesterol (Burkard et al. 2007) and is known to dramatically increase the risk of coronary heart disease and ER-positive breast cancer (Lloyd-Jones et al. 2009).
References
- Abe D, Sakaki T, Kusudo T, Kittaka A, Saito N, Suhara Y, Fujishima T, Takayama H, Hamamoto H, Kamakura M et al. 2005. Metabolism of 2α-propoxy-1α,25-dihydroxyvitamin D3 and 2α-(3-hydroxypropoxy)-1α,25-dihydroxyvitamin D3 by human CYP27A1 and CYP24A1. Drug Metab Dispos 33:778–784. [DOI] [PubMed] [Google Scholar]
- Acimovic J, Goyal S, Kosir R, Golicnik M, Perse M, Belic A, Urlep Z, Guengerich FP, Rozman D. 2016. Cytochrome P450 metabolism of the post-lanosterol intermediates explains enigmas of cholesterol synthesis. Sci Rep 6:28462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaqih MA, Nelson ER, Liu W, Safi R, Jasper JS, Macias E, Geradts J, Thompson JW, Dubois LG, Freeman MR et al. 2017. CYP27A1 loss dysregulates cholesterol homeostasis in prostate cancer. Cancer Res 77:1662–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An S, Jang YS, Park JS, Kwon BM, Paik YK, Jeong TS. 2008. Inhibition of acyl-coenzyme A:cholesterol acyltransferase stimulates cholesterol efflux from macrophages and stimulates farnesoid X receptor in hepatocytes. Exp Mol Med 40:407–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya Z, Tang W, Wikvall K. 2003. Hormonal regulation of the human sterol 27-hydroxylase gene CYP27A1. Biochem J 372:529–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkhem I 2013. Cerebrotendinous xanthomatosis. Curr Opin Lipidol 24:283–287. [DOI] [PubMed] [Google Scholar]
- Burkard I, von Eckardstein A, Waeber G, Vollenweider P, Rentsch KM. 2007. Lipoprotein distribution and biological variation of 24S- and 27-hydroxycholesterol in healthy volunteers. Atherosclerosis 194:71–78. [DOI] [PubMed] [Google Scholar]
- Chen W, Chiang JY. 2003. Regulation of human sterol 27-hydroxylase gene (CYP27A1) by bile acids and hepatocyte nuclear factor 4α (HNF4α). Gene 313:71–82. [DOI] [PubMed] [Google Scholar]
- Diesing D, Cordes T, Fischer D, Diedrich K, Friedrich M. 2006. Vitamin D–metabolism in the human breast cancer cell line MCF-7. AntiCancer Res 26:2755–2759. [PubMed] [Google Scholar]
- DuSell CD, Nelson ER, Wang X, Abdo J, Modder UI, Umetani M, Gesty-Palmer D, Javitt NB, Khosla S, McDonnell DP. 2010. The endogenous selective estrogen receptor modulator 27-hydroxycholesterol is a negative regulator of bone homeostasis. Endocrinology 151:3675–3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis E, Axelson M, Abrahamsson A, Eggertsen G, Thorne A, Nowak G, Ericzon BG, Björkhem I, Einarsson C. 2003. Feedback regulation of bile acid synthesis in primary human hepatocytes: evidence that CDCA is the strongest inhibitor. Hepatology (Baltimore) 38:930–938. [DOI] [PubMed] [Google Scholar]
- Ellis EC. 2006. Suppression of bile acid synthesis by thyroid hormone in primary human hepatocytes. World J Gastroenterol 12:4640–4645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federspiel JD, Codreanu SG, Goyal S, Albertolle ME, Lowe E, Teague J, Wong H, Guengerich FP, Liebler DC. 2016. Specificity of protein covalent modification by the electrophilic proteasome inhibitor carfilzomib in human cells. Mol Cell Proteomics 15:3233–3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueguen Y, Ferrari L, Souidi M, Batt AM, Lutton C, Siest G, Visvikis S. 2007. Compared effect of immunosuppressive drugs cyclosporine A and rapamycin on cholesterol homeostasis key enzymes CYP27A1 and HMG-CoA reductase. Basic Clin Pharmacol Toxicol 100:392–397. [DOI] [PubMed] [Google Scholar]
- Guengerich FP. 2015. Chapter 9, Human cytochrome P450 enzymes. In: Ortiz de Montellano PR, editor. Cytochrome P450: Structure, Mechanism, and Biochemistry 4th ed. New York: Springer; p. 523–785. [Google Scholar]
- Honda A, Ikegami T, Nakamuta M, Miyazaki T, Iwamoto J, Hirayama T, Saito Y, Takikawa H, Imawari M, Matsuzaki Y. 2013. Anticholestatic effects of bezafibrate in patients with primary biliary cirrhosis treated with ursodeoxycholic acid. Hepatology (Baltimore) 57:1931–1941. [DOI] [PubMed] [Google Scholar]
- Kalaany NY, Mangelsdorf DJ. 2006. LXRS and FXR: the yin and yang of cholesterol and fat metabolism. Annu Rev Physiol 68:159–191. [DOI] [PubMed] [Google Scholar]
- Kimbung S, Chang CY, Bendahl PO, Dubois L, Thompson JW, McDonnell DP, Borgquist S. 2017. Impact of 27-hydroxylase (CYP27A1) and 27-hydroxycholesterol in breast cancer. Endocr Related Cancer 24:339–349. [DOI] [PubMed] [Google Scholar]
- Lam M, Mast N, Pikuleva IA. 2018. Drugs and scaffold that inhibit cytochrome P450 27A1 in vitro and in vivo. Mol Pharmacol 93:101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Chen W, Chiang JY. 2007. PXR induces CYP27A1 and regulates cholesterol metabolism in the intestine. J Lipid Res 48:373–384. [DOI] [PubMed] [Google Scholar]
- Llaverias G, Rebollo A, Pou J, Vazquez-Carrera M, Sanchez RM, Laguna JC, Alegret M. 2006. Effects of rosiglitazone and atorvastatin on the expression of genes that control cholesterol homeostasis in differentiating monocytes. Biochem Pharmacol 71:605–614. [DOI] [PubMed] [Google Scholar]
- Lloyd-Jones DM, Walsh JA, Prineas RJ, Ning H, Liu K, Daviglus ML, Shea S, Detrano RC, Tandri H, Greenland P. 2009. Association of electrocardiographic abnormalities with coronary artery calcium and carotid artery intima-media thickness in individuals without clinical coronary heart disease (from the Multi-Ethnic Study of Atherosclerosis [MESA]). Am J Cardiol 104:1086–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz SZ, Hennenlotter J, Scharpf MO, Sailer C, Fritsche L, Schmid V, Kantartzis K, Wagner R, Lehmann R, Berti L, Peter A, Staiger H, Fritsche A, Fend F, Todenhöfer T, Stenzl A, Häring HU, Heni M. 2018. Androgen receptor overexpression in prostate cancer in type 2 diabetes. Mol Metab 8:158–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons MA, Brown AJ. 2001. Metabolism of an oxysterol, 7-ketocholesterol, by sterol 27-hydroxylase in HepG2 cells. Lipids 36:701–711. [DOI] [PubMed] [Google Scholar]
- Mast N, Lin JB, Pikuleva IA. 2015. Marketed drugs can inhibit cytochrome P450 27A1, a potential new target for breast cancer adjuvant therapy. Mol Pharmacol 88:428–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masumoto O, Ohyama Y, Okuda K. 1988. Purification and characterization of vitamin D 25-hydroxylase from rat liver mitochondria. J Biol Chem 263:14256–14260. [PubMed] [Google Scholar]
- Meaney S, Bodin K, Diczfalusy U, Björkhem I. 2002. On the rate of translocation in vitro and kinetics in vivo of the major oxysterols in human circulation: critical importance of the position of the oxygen function. J Lipid Res 43:2130–2135. [DOI] [PubMed] [Google Scholar]
- Nelson ER, Wardell SE, Jasper JS, Park S, Suchindran S, Howe MK, Carver NJ, Pillai RV, Sullivan PM, Sondhi V et al. 2013. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science 342:1094–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen LB, Shefer S, Salen G, Tint SG, Batta AK. 1998. Competitive inhibition of hepatic sterol 27-hydroxylase by sitosterol: decreased activity in sitosterolemia. Proc Assoc Am Physicians 110:32–39. [PubMed] [Google Scholar]
- Norlin M, von Bahr S, Björkhem I, Wikvall K. 2003. On the substrate specificity of human CYP27A1: implications for bile acid and cholestanol formation. J Lipid Res 44:1515–1522. [DOI] [PubMed] [Google Scholar]
- Norlin M, Wikvall K. 2007. Enzymes in the conversion of cholesterol into bile acids. Curr Mol Med 7:199–218. [DOI] [PubMed] [Google Scholar]
- Pan ST, Xue D, Li ZL, Zhou ZW, He ZX, Yang Y, Yang T, Qiu JX, Zhou SF. 2016. Computational identification of the paralogs and orthologs of human cytochrome P450 superfamily and the implication in drug discovery. Int J Mol Sci June 28;17(7). pii: ijms17071020. doi: 10.3390/ijms17071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson H, Norlin M, Andersson U, Pikuleva I, Björkhem I, Misharin AY, Wikvall K. 2008. Metabolism of a novel side chain modified △8(14)-15-ketosterol, a potential cholesterol lowering drug: 28-hydroxylation by CYP27A1. Biochim Biophys Acta 1781:383–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pikuleva IA, Mast N, Liao WL, Turko IV. 2008. Studies of membrane topology of mitochondrial cholesterol hydroxylases CYPs 27A1 and 11A1. Lipids 43:1127–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn CM, Jessup W, Wong J, Kritharides L, Brown AJ. 2005. Expression and regulation of sterol 27-hydroxylase (CYP27A1) in human macrophages: a role for RXR and PPARγ ligands. Biochem J 385:823–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaki T, Kagawa N, Yamamoto K, Inouye K. 2005. Metabolism of vitamin D3 by cytochromes P450. Frontiers Biosci 10:119–134. [DOI] [PubMed] [Google Scholar]
- Schuster I, Egger H, Herzig G, Reddy GS, Schmid JA, Schussler M, Vorisek G. 2006. Selective inhibitors of vitamin D metabolism--new concepts and perspectives. AntiCancer Res 26:2653–2668. [PubMed] [Google Scholar]
- Sharanek A, Burban A, Humbert L, Bachour-El Azzi P, Felix-Gomes N, Rainteau D, Guillouzo A. 2015. Cellular accumulation and toxic effects of bile acids in cyclosporine A-treated HepaRG hepatocytes. Toxicol Sci 147:573–587. [DOI] [PubMed] [Google Scholar]
- Szanto A, Benko S, Szatmari I, Balint BL, Furtos I, Ruhl R, Molnar S, Csiba L, Garuti R, Calandra S et al. 2004. Transcriptional regulation of human CYP27 integrates retinoid, peroxisome proliferator-activated receptor, and liver X receptor signaling in macrophages. Mol Cell Biol 24:8154–8166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taban IM, Zhu J, DeLuca HF, Simons C. 2017. Analysis of the binding sites of vitamin D 1α-hydroxylase (CYP27B1) and vitamin D 24-hydroxylase (CYP24A1) for the design of selective CYP24A1 inhibitors: Homology modelling, molecular dynamics simulations and identification of key binding requirements. Bioorg Med Chem 25:5629–5636. [DOI] [PubMed] [Google Scholar]
- Tang W, Norlin M, Wikvall K. 2007. Regulation of human CYP27A1 by estrogens and androgens in HepG2 and prostate cells. Arch Biochem Biophys 462:13–20. [DOI] [PubMed] [Google Scholar]
- Tieu EW, Li W, Chen J, Baldisseri DM, Slominski AT, Tuckey RC. 2012. Metabolism of cholesterol, vitamin D3 and 20-hydroxyvitamin D3 incorporated into phospholipid vesicles by human CYP27A1. J Steroid Biochem Mol Biol 129:163–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokar EJ, Webber MM. 2005. Cholecalciferol (vitamin D3) inhibits growth and invasion by up-regulating nuclear receptors and 25-hydroxylase (CYP27A1) in human prostate cancer cells. Clin Exp Metastasis 22:275–284. [DOI] [PubMed] [Google Scholar]
- Umetani M, Domoto H, Gormley AK, Yuhanna IS, Cummins CL, Javitt NB, Korach KS, Shaul PW, Mangelsdorf DJ. 2007. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nat Med 13:1185–1192. [DOI] [PubMed] [Google Scholar]
- Umetani M, Ghosh P, Ishikawa T, Umetani J, Ahmed M, Mineo C, Shaul PW. 2014. The cholesterol metabolite 27-hydroxycholesterol promotes atherosclerosis via proinflammatory processes mediated by estrogen receptor α. Cell Metab 20:172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umetani M, Shaul PW. 2011. 27-Hydroxycholesterol: the first identified endogenous SERM. Trends Endocrinol Metab 22:130–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Lier JE, Mast N, Pikuleva IA. 2015. Cholesterol hydroperoxides as substrates for cholesterol-metabolizing cytochrome P450 enzymes and alternative sources of 25-hydroxycholesterol and other oxysterols. Angew Chem Int Ed 54:11138–11142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikvall K 1984. Hydroxylations in biosynthesis of bile acids. Isolation of a cytochrome P-450 from rabbit liver mitochondria catalyzing 26-hydroxylation of C27-steroids. J Biol Chem 259:3800–3804. [PubMed] [Google Scholar]
- Xu Y, Hutchison SM, Hernandez-Ledezma JJ, Bogan RL. 2018. Increased 27-hydroxycholesterol production during luteolysis may mediate the progressive decline in progesterone secretion. Mol Human Reprod 24:2–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin K, You Y, Swier V, Tang L, Radwan MM, Pandya AN, Agrawal DK. 2015. Vitamin D protects against atherosclerosis via regulation of cholesterol efflux and macrophage polarization in hypercholesterolemic swine. Arteriosclerosis Thrombosis Vasc Biol 35:2432–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
P450 39A1
The available evidence that the function of this enzyme is the 7α-hydroxylation of 24(S)-hydroxycholesterol, which is a product of oxidation of cholesterol by P450 46A1 (vide infra). Almost all of the information about P450 39A1 has come from animal models, mainly mice (Li-Hawkins, Lund, Bronson, et al. 2000; Li-Hawkins, Lund, Bronson, et al. 2000). The enzyme is hepatic. No crystal structures have been reported.
References
- Li-Hawkins J, Lund EG, Bronson AD, Russell DW. 2000. Expression cloning of an oxysterol 7α-hydroxylase selective for 24-hydroxycholesterol. J Biol Chem 275:16543–16549. [DOI] [PubMed] [Google Scholar]
- Li-Hawkins J, Lund EG, Turley SD, Russell DW. 2000. Disruption of the oxysterol 7α-hydroxylase gene in mice. J Biol Chem 275:16536–16542. [DOI] [PubMed] [Google Scholar]
- Oscarson M, Zanger UM, Rifki OF, Klein K, Eichelbaum M, Meyer UA. 2006. Transcriptional profiling of genes induced in the livers of patients treated with carbamazepine. Clin Pharmacol Therapeut 80:440–456. [DOI] [PubMed] [Google Scholar]
- Pan ST, Xue D, Li ZL, Zhou ZW, He ZX, Yang Y, Yang T, Qiu JX, Zhou SF. 2016. Computational identification of the paralogs and orthologs of human cytochrome P450 superfamily and the implication in drug discovery. Int J Mol Sci June 28;17(7). pii: ijms17071020. doi: 10.3390/ijms17071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumantran VN, Mishra P, Bera R, Sudhakar N. 2016. Microarray analysis of differentially-expressed genes encoding CYP450 and Phase II drug metabolizing enzymes in psoriasis and melanoma. Pharmaceutics 8(1). February 17;8(1). pii: E4. doi: 10.3390/pharmaceutics8010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
P450 46A1
P450 46A1 catalyzes cholesterol 24S-hydroxylation, the first step in cholesterol elimination from the brain (Lutjohann et al. 1996; Björkhem et al. 1998; Lund et al. 1999), where the concentration of cholesterol is even higher than in liver. This enzymatic reaction produces a membrane-permeable form of cholesterol, 24(S)-hydroxycholesterol, that diffuses across cellular membranes and the blood-brain barrier to the systemic circulation and moves to the liver for degradation (Björkhem et al. 1998; Meaney et al. 2002). P450 46A1 is considered to be important for memory and learning, in that Cyp46a1 knock-out (KO) mice show severe deficiencies in spatial, associative, and motor learning and have impaired cognitive performance (Kotti et al. 2006).
Numerous investigations have been done to assess a possible link between CYP46A1 genetic variations and neurodegenerative disease, including Alzheimer’s disease. The results on an association of CYP46A1 variants and dementias are still in conflict (for review, see Russell et al. (2009)). Reduced cholesterol biosynthesis does not, however, affect amyloid plaque deposition in Cyp46a1 KO mice cross-bred with one of the mouse models of Alzheimer disease but does extend animal life span (Halford and Russell 2009). Cyp46a1-expressing transgenic animals also had improved spatial memory (Hudry et al. 2010).
P450 46A1, in addition to using drugs as substrates and being inhibited by drugs (Mast N. et al. 2003; Shafaati et al. 2010), also shows an unexpected phenomenon of enzyme stimulation by drugs (Mast N. et al. 2012; Anderson et al. 2016; Mast N. et al. 2017). These are not inducers; they directly stimulate enzyme activity when added in vitro. Hydrogen-deuterium exchange studies (Mast N. et al. 2008), coupled with the X-ray crystal structure, show that the ‘allosteric’ site is distinct from the substrate binding site (Mast N. et al. 2014; Anderson et al. 2016). Not only drugs but also some physiological compounds in the brain show this effect (Mast N. et al. 2017). These compounds can enhance rates of reduction by NADPH-P450 reductase, although reduction is not rate-limiting and other factors are probably involved (Mast N. et al. 2017).
The specificity constant (kcat/Km) for cholesterol 24S-hydroxylation is very low (140 M−1 s−1) (Goyal et al. 2014), although the significance of the (mouse) enzyme was clearly shown in the behavioral studies by Russell and his associates (Kotti et al. 2006; Russell et al. 2009). Other substrates are zymostenol, lathosterol, 7-dehydrocholesterol, and desmosterol (Goyal et al. 2014; Acimovic et al. 2016). In some cases with these alternate substrates, 25- and 27-hydroxylations occur, as well as epoxidation of desmosterol (Acimovic et al. 2016).
References
- Acimovic J, Goyal S, Kosir R, Golicnik M, Perse M, Belic A, Urlep Z, Guengerich FP, Rozman D. 2016. Cytochrome P450 metabolism of the post-lanosterol intermediates explains enigmas of cholesterol synthesis. Sci Rep 6:28462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KW, Mast N, Hudgens JW, Lin JB, Turko IV, Pikuleva IA. 2016. Mapping of the allosteric site in cholesterol hydroxylase CYP46A1 for efavirenz, a drug that stimulates enzyme activity. J Biol Chem 291:11876–11886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkhem I, Lutjohann D, Diczfalusy U, Stahle L, Ahlborg G, Wahren J. 1998. Cholesterol homeostasis in human brain: turnover of 24S-hydroxycholesterol and evidence for a cerebral origin of most of this oxysterol in the circulation. J Lipid Res 39:1594–1600. [PubMed] [Google Scholar]
- Goyal S, Xiao Y, Porter NA, Xu L, Guengerich FP. 2014. Oxidation of 7-dehydrocholesterol and desmosterol by human cytochrome P450 46A1. J Lipid Res 55:1933–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford RW, Russell DW. 2009. Reduction of cholesterol synthesis in the mouse brain does not affect amyloid formation in Alzheimer’s disease, but does extend lifespan. Proc Natl Acad Sci USA 106:3502–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudry E, Van Dam D, Kulik W, De Deyn PP, Stet FS, Ahouansou O, Benraiss A, Delacourte A, Bougneres P, Aubourg P et al. 2010. Adeno-associated virus gene therapy with cholesterol 24-hydroxylase reduces the amyloid pathology before or after the onset of amyloid plaques in mouse models of Alzheimer’s disease. Mol Therapy 18:44–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotti TJ, Ramirez DM, Pfeiffer BE, Huber KM, Russell DW. 2006. Brain cholesterol turnover required for geranylgeraniol production and learning in mice. Proc Natl Acad Sci USA 103:3869–3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao WL, Dodder NG, Mast N, Pikuleva IA, Turko IV. 2009. Steroid and protein ligand binding to cytochrome P450 46A1 as assessed by hydrogen-deuterium exchange and mass spectrometry. Biochemistry 48:4150–4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund EG, Guileyardo JM, Russell DW. 1999. cDNA cloning of cholesterol 24-hydroxylase, a mediator of cholesterol homeostasis in the brain. Proc Natl Acad Sci USA 96:7238–7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutjohann D, Breuer O, Ahlborg G, Nennesmo I, Siden A, Diczfalusy U, Björkhem I. 1996. Cholesterol homeostasis in human brain: evidence for an age-dependent flux of 24S-hydroxycholesterol from the brain into the circulation. Proc Natl Acad Sci USA 93:9799–9804. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mast N, Anderson KW, Johnson KM, Phan TTN, Guengerich FP, Pikuleva IA. 2017. In vitro cytochrome P450 46A1 (CYP46A1) activation by neuroactive compounds. J Biol Chem 292:12934–12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mast N, Charvet C, Pikuleva IA, Stout CD. 2010. Structural basis of drug binding to CYP46A1, an enzyme that controls cholesterol turnover in the brain. J Biol Chem 285:31783–31795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mast N, Li Y, Linger M, Clark M, Wiseman J, Pikuleva IA. 2014. Pharmacologic stimulation of cytochrome P450 46A1 and cerebral cholesterol turnover in mice. J Biol Chem 289:3529–3538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mast N, Linger M, Clark M, Wiseman J, Stout CD, Pikuleva IA. 2012. In silico and intuitive predictions of CYP46A1 inhibition by marketed drugs with subsequent enzyme crystallization in complex with fluvoxamine. Mol Pharmacol 82:824–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mast N, Norcross R, Andersson U, Shou M, Nakayama K, Björkhem I, Pikuleva IA. 2003. Broad substrate specificity of human cytochrome P450 46A1 which initiates cholesterol degradation in the brain. Biochemistry 42:14284–14292. [DOI] [PubMed] [Google Scholar]
- Mast N, White MA, Björkhem I, Johnson EF, Stout CD, Pikuleva IA. 2008. Crystal structures of substrate-bound and substrate-free cytochrome P450 46A1, the principal cholesterol hydroxylase in the brain. Proc Natl Acad Sci USA 105:9546–9551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mast N, Zheng W, Stout CD, Pikuleva IA. 2013. Antifungal azoles: Structural insights into undesired tight binding to cholesterol-metabolizing CYP46A1. Mol Pharmacol 84:86–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney S, Bodin K, Diczfalusy U, Björkhem I. 2002. On the rate of translocation in vitro and kinetics in vivo of the major oxysterols in human circulation: critical importance of the position of the oxygen function. J Lipid Res 43:2130–2135. [DOI] [PubMed] [Google Scholar]
- Nunes MJ, Moutinho M, Milagre I, Gama MJ, Rodrigues E. 2012. Okadaic acid inhibits the trichostatin A-mediated increase of human CYP46A1 neuronal expression in a ERK1/2-Sp3-dependent pathway. J Lipid Res 53:1910–1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan ST, Xue D, Li ZL, Zhou ZW, He ZX, Yang Y, Yang T, Qiu JX, Zhou SF. 2016. Computational identification of the paralogs and orthologs of human cytochrome P450 superfamily and the implication in drug discovery. Int J Mol Sci June 28;17(7). pii: ijms17071020. doi: 10.3390/ijms17071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell DW, Halford RW, Ramirez DM, Shah R, Kotti T. 2009. Cholesterol 24-hydroxylase: an enzyme of cholesterol turnover in the brain. Annu Rev Biochem 78:1017–1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafaati M, Mast N, Beck O, Nayef R, Heo GY, Björkhem-Bergman L, Lutjohann D, Björkhem I, Pikuleva IA. 2010. The antifungal drug voriconazole is an efficient inhibitor of brain cholesterol 24S-hydroxylase in vitro and in vivo. J Lipid Res 51:318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa G, Staurenghi E, Zerbinati C, Gargiulo S, Iuliano L, Giaccone G, Fanto F, Poli G, Leonarduzzi G, Gamba P. 2016. Changes in brain oxysterols at different stages of Alzheimer’s disease: Their involvement in neuroinflammation. Redox Biol 10:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
P450 51A1
Although the liver is generally considered to be the major organ involved in cholesterol synthesis, CYP51A1 mRNA was found to be high in testis, ovary, adrenal, prostate, kidney, and lung as well as liver (Stromstedt et al. 1996). In mouse testis, mRNA levels were high and localized in both round and elongated spermatids (Cotman et al. 2001). This high level has been proposed to lead to the production of steroids for signaling (Rozman and Waterman 1998; Rozman et al. 2002). Cyp51a1-knockout mice show a phenotype reminiscent of the Antley-Bixler Syndrome (Keber et al. 2011).
Several X-ray crystal structures of human P450 51A1 have now been published, both ligand-free and with ligands (Strushkevich et al. 2010; Hargrove et al. 2016) (PDB 3JUS, 3JUV, 3LD6, 4UHI, 4UHL). In addition, several X-ray crystal structures of various parasite CYP51 enzymes have also been published (Lepesheva et al. 2010; Hargrove et al. 2015; Hargrove et al. 2017). These structures have potential value in directing the synthesis of inhibitors towards the parasitic 14α-demethylases and avoiding interactions with human P450 51A1.
The specificity constant (kcat/Km) for human P450 51A1 has been reported to be only ~ 300 M−1 s−1, which is relatively low among P450s and also enzymes in general (Lamb et al. 1998). Nevertheless, the significance of this enzyme is clear, as further demonstrated by the transgenic mouse results (Keber et al. 2011).
An open question is the catalytic mechanism of the third step of the 14α-demethylase reaction. Obviously this has similarity to the last step of the P450 19A1 reaction (vide supra). Although 18O-labeling and other evidence has been presented in favor of a ferric peroxide (FeO2−) nucleophilic attack on the C-32 formyl group (Fischer et al. 1991; Shyadehi et al. 1996) instead of the general Compound I (FeO3+) mechanism, the same limitations pointed out in the reassessment of the P450 19A1 mechanism (Yoshimoto and Guengerich 2014) apply (Guengerich and Yoshimoto 2018) and a reassessment with modern mass spectrometry methods is in order.
The oxidative removal of the 14α-methyl group from sterol precursors by sterol 14α-demethylase (CYP51 Family genes) is a universal step in the biosynthesis of membrane sterols and steroid hormones. Thus, P450 51A is a primary target in treatment of fungal infections in organisms ranging from humans to plants, and development of more potent and selective inhibitors is an important biological objective in many therapeutic fields. Currently the CYP51 gene family is found in at least 82 organisms, spread over all biological kingdoms. Several plants and fungi contain multiple CYP51 genes (e.g. rice (10), black oats (2), tobacco (2), Arabidopsis thaliana (2), Fuzarium graminearum (3), and several of the Aspergillus species: e.g., Aspergillus fumigatus (2), A. nidulans (2), A. orizae (3)). As a result, the number of known CYP51 gene sequences is >100. All mammalian genomes examined contain only a single CYP51A1 gene but some have nonfunctional CYP51 pseudogenes (e.g., three in humans and one in rats, http://drnelson.uthsc.edu/cytochromeP450.html).
Azoles (imidazoles and triazoles) play a pivotal role in the treatment of systemic and dermal mycoses. The substrate analogs 7-oxo, 15-keto, 15-oxime, 15-hydroxy, 26-oxo derivatives of lanosterol are effective in blocking cholesterol biosynthesis in humans. 14α-Amino derivatives of lanosterol were found effective to inhibit Candida albicans and Trypanasoma cruzi P450 51 orthologs and are effective to cure murine models of leishmania and Chagas disease (Lepesheva and Waterman 2007).
Sterol 14α-demethylase is one of the hypothetical targets an alternative to classic statin therapy (Kaluzhsiy et al. 2014). Most commercial and experimental inhibitors of microbial P450 51 orthologs (including all clinical antifungals) weakly inhibit human P450 51A1 activity. Only one relatively potent compound, (R)-N-(1-(3,4´-difluorobiphenyl-4-yl)-2-(1H-imidazol-1-yl)ethyl)-4-(5-phenyl-1,3,4-oxadiazol-2-yl)benzamide (VFV), was identified, and it was also found to decrease proliferation of different cancer cell types (Hargrove et al. 2016).
References
- Arlt W 2007. P450 oxidoreductase deficiency and Antley-Bixler syndrome. Rev Endocr Metabol Disorders 8:301–307. [DOI] [PubMed] [Google Scholar]
- Burton PM, Swinney DC, Heller R, Dunlap B, Chiou M, Malonzo E, Haller J, Walker KA, Salari A, Murakami S et al. 1995. Azalanstat (RS-21607), a lanosterol 14α-demethylase inhibitor with cholesterol-lowering activity. Biochem Pharmacol 50:529–544. [DOI] [PubMed] [Google Scholar]
- Cotman M, Rozma D, Banek L, Jezek D. 2001. Localisation of lanosterol 14α-demethylase in round and elongated spermatids of the mouse testis: an immunoelectron microscopic and stereological study. Pflugers Arch 442(6 Suppl 1):R167–168. [DOI] [PubMed] [Google Scholar]
- Fischer RT, Trzaskos JM, Magolda RL, Ko SS, Brosz CS, Larsen B. 1991. Lanosterol 14α-methyl demethylase: Isolation and characterization of the third metabolically generated oxidative demethylation intermediate. J Biol Chem 266:6124–6132. [PubMed] [Google Scholar]
- Frye LL, Leonard DA. 1999. Lanosterol analogs: dual-action inhibitors of cholesterol biosynthesis. Crit Rev Biochem Mol Biol 34):123–140. [DOI] [PubMed] [Google Scholar]
- Guengerich FP. 2017. Intersection of the roles of cytochrome P450 enzymes with xenobiotic and endogenous substrates: Relevance to toxicity and drug interactions. Chem. Res. Toxicol 30:2–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guengerich FP, Yoshimoto FK. 2018. Formation and cleavage of C-C bonds by enzymatic oxidation reactions. Chem Rev, submitted 12 January 2018, in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrove TY, Friggeri L, Wawrzak Z, Qi A, Hoekstra WJ, Schotzinger RJ, York JD, Guengerich FP, Lepesheva GI. 2017. Structural analyses of Candida albicans sterol 14α-demethylase complexed with azole drugs address the molecular basis of azole-mediated inhibition of fungal sterol biosynthesis. J Biol Chem 292:6728–6743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrove TY, Friggeri L, Wawrzak Z, Sivakumaran S, Yazlovitskaya EM, Hiebert SW, Guengerich FP, Waterman MR, Lepesheva GI. 2016. Human sterol 14α-demethylase as a target for anticancer chemotherapy: Towards structure-aided drug design. J Lipid Res 57:1552–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrove TY, Wawrzak Z, Lamb DC, Guengerich FP, Lepesheva GI. 2015. Structure-functional characterization of cytochrome P450 sterol 14α-demethylase (CYP51B) from Aspergillus fumigatus and molecular basis for the development of antifungal drugs. J Biol Chem 290:23916–23934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrove TY, Wawrzak Z, Liu J, Waterman MR, Nes WD, Lepesheva GI. 2012. Structural complex of sterol 14α-demethylase (CYP51) with 14α-methylenecyclopropyl-△7-24,25-dihydrolanosterol. J Lipid Res 53:311–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood HJ Jr., Petras SF, Hoover DJ, Mankowski DC, Soliman VF, Sugarman ED, Hulin B, Kwon Y, Gibbs EM, Mayne JT et al. 2005. Dual-action hypoglycemic and hypocholesterolemic agents that inhibit glycogen phosphorylase and lanosterol demethylase. J Lipid Res 46:547–563. eng. [DOI] [PubMed] [Google Scholar]
- Kaluzhsiy LA, Gnedenko OV, Gilep AA, Strushkevich NV, Shkel TV, Chernovetsky MA, Ivanov AS, Lisitsa AV, Usanov AS, Stonik VA et al. 2014. [The screening of the inhibitors of the human cytochrome P450(51) (CYP51A1): the plant and animal structural lanosterol’s analogs]. Biomeditsinskaia khimiia 60:528–537. rus. [DOI] [PubMed] [Google Scholar]
- Keber R, Motaln H, Wagner KD, Debeljak N, Rassoulzadegan M, Acimovic J, Rozman D, Horvat S. 2011. Mouse knockout of the cholesterogenic cytochrome P450 lanosterol 14α-demethylase (Cyp51) resembles Antley-Bixler syndrome. J Biol Chem 286:29086–29097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krone N, Dhir V, Ivison HE, Arlt W. 2007. Congenital adrenal hyperplasia and P450 oxidoreductase deficiency. Clin Endocrinol 66:162–172. [DOI] [PubMed] [Google Scholar]
- Lamb DC, Kelly DE, Kelly SL. 1998. Molecular diversity of sterol 14α-demethylase substrates in plants, fungi and humans. FEBS Lett 425:263–265. [DOI] [PubMed] [Google Scholar]
- Lepesheva GI, Park HW, Hargrove TY, Vanhollebeke B, Wawrzak Z, Harp JM, Sundaramoorthy M, Nes WD, Pays E, Chaudhuri M et al. 2010. Crystal structures of Trypanosoma brucei sterol 14α-demethylase and implications for selective treatment of human infections. J Biol Chem 285:1773–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepesheva GI, Waterman MR. 2007. Sterol 14α-demethylase cytochrome P450 (CYP51), a P450 in all biological kingdoms. Biochim Biophys Acta 1770:467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuura K, Yoshioka S, Tosha T, Hori H, Ishimori K, Kitagawa T, Morishima I, Kagawa N, Waterman MR. 2005. Structural diversities of active site in clinical azole-bound forms between sterol 14α-demethylases (CYP51s) from human and Mycobacterium tuberculosis. J Biol Chem 280:9088–9096. [DOI] [PubMed] [Google Scholar]
- Mayer RJ, Adams JL, Bossard MJ, Berkhout TA. 1991. Effects of a novel lanosterol 14α-demethylase inhibitor on the regulation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase in Hep G2 cells. J Biol Chem 266:20070–20078. [PubMed] [Google Scholar]
- Nakamura T, Iwase A, Bayasula B, Nagatomo Y, Kondo M, Nakahara T, Takikawa S, Goto M, Kotani T, Kiyono T et al. 2015. CYP51A1 induced by growth differentiation factor 9 and follicle-stimulating hormone in granulosa cells is a possible predictor for unfertilization. Reprod Sci (Thousand Oaks, CA) 22:377–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan ST, Xue D, Li ZL, Zhou ZW, He ZX, Yang Y, Yang T, Qiu JX, Zhou SF. 2016. Computational identification of the paralogs and orthologs of human cytochrome P450 superfamily and the implication in drug discovery. Int J Mol Sci June 28;17(7). pii: ijms17071020. doi: 10.3390/ijms17071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pont A, Williams PL, Loose DS, Feldman D, Reitz RE, Bochra C, Stevens DA. 1982. Ketoconazole blocks adrenal steroid synthesis. Ann Int Med 97:370–372. [DOI] [PubMed] [Google Scholar]
- Rozman D, Cotman M, Frangez R. 2002. Lanosterol 14α-demethylase and MAS sterols in mammalian gametogenesis. Mol Cell Endocrinol 187:179–187. [DOI] [PubMed] [Google Scholar]
- Rozman D, Waterman MR. 1998. Lanosterol 14α-demethylase (CYP51) and spermatogenesis. Drug Metab Dispos 26:1199–1201. [PubMed] [Google Scholar]
- Schuster I, Bernhardt R. 2007. Inhibition of cytochromes P450: Existing and new promising therapeutic targets. Drug Metab Rev 39:481–499. [DOI] [PubMed] [Google Scholar]
- Shyadehi AZ, Lamb DC, Kelly SL, Kelly DE, Schunck WH, Wright JN, Corina D, Akhtar M. 1996. Mechanism of the acyl-carbon bond cleavage reaction catalyzed by recombinant sterol 14αdemethylase of Candida albicans (other names are: lanosterol 14α-demethylase, P-45014DM, and CYP51). J Biol Chem 271:12445–12450. [DOI] [PubMed] [Google Scholar]
- Stromstedt M, Rozman D, Waterman MR. 1996. The ubiquitously expressed human CYP51 encodes lanosterol 14α-demethylase, a cytochrome P450 whose expression is regulated by oxysterols. Arch Biochem Biophys 329:73–81. [DOI] [PubMed] [Google Scholar]
- Strushkevich N, Usanov SA, Park HW. 2010. Structural basis of human CYP51 inhibition by antifungal azoles. J Mol Biol 397:1067–1078. [DOI] [PubMed] [Google Scholar]
- Swinney DC, So OY, Watson DM, Berry PW, Webb AS, Kertesz DJ, Shelton EJ, Burton PM, Walker KA. 1994. Selective inhibition of mammalian lanosterol 14α-demethylase by RS-21607 in vitro and in vivo. Biochemistry 33:4702–4713. [DOI] [PubMed] [Google Scholar]
- Trösken ER, Adamska M, Arand M, Zarn JA, Patten C, Volkel W, Lutz WK. 2006. Comparison of lanosterol-14α-demethylase (CYP51) of human and Candida albicans for inhibition by different antifungal azoles. Toxicology 228:24–32. [DOI] [PubMed] [Google Scholar]
- Trösken ER, Straube E, Lutz WK, Volkel W, Patten C. 2004. Quantitation of lanosterol and its major metabolite FF-MAS in an inhibition assay of CYP51 by azoles with atmospheric pressure photoionization based LC-MS/MS. J Am Soc Mass Spectrom 15:1216–1221. [DOI] [PubMed] [Google Scholar]
- Trzaskos JM. 1995. Oxylanosterols as modifiers of cholesterol biosynthesis. Prog Lipid Res 34:99–116. [DOI] [PubMed] [Google Scholar]
- Trzaskos JM, Magolda RL, Favata MF, Fischer RT, Johnson PR, Chen HW, Ko SS, Leonard DA, Gaylor JL. 1993. Modulation of 3-hydroxy-3-methylglutaryl-CoA reductase by 15α-fluorolanost-7-en-3α-ol. A mechanism-based inhibitor of cholesterol biosynthesis. J Biol Chem 268:22591–22599. [PubMed] [Google Scholar]
- Yoshimoto FK, Guengerich FP. 2014. Mechanism of the third oxidative step in the conversion of androgens to estrogens by cytochrome P450 19A1 steroid aromatase. J Am Chem Soc 136:15016–15025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vitamin D metabolism
P450s are the major enzymes involved in the conversion of vitamin D3 to more active products and also in the breakdown of vitamin D and its products (Fig. 18). Because of the many roles of vitamin D, the balance is important and these enzymes are generally highly regulated.
Fig. 18.
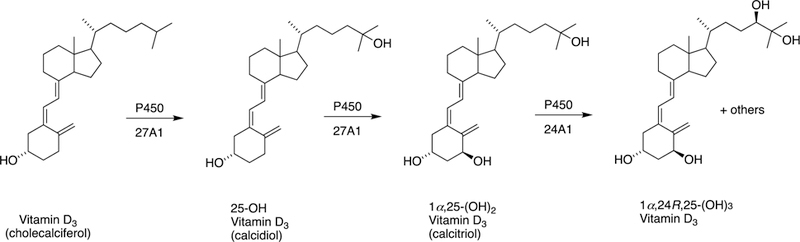
P450s involved in the metabolism of vitamin D3.
P450 24A1
P450 24A1 is one of the seven human P450s normally localized in mitochondria, and accordingly it receives electrons from NADPH-adrenodoxin reductase and adrenodoxin. This P450 is localized in the kidney, plus a few other places (Guengerich 2015). Its regulation is highly complex, as might be expected for an enzyme involved in a delicate regulation of the amount of an active vitamin (Guengerich 2015).
An X-ray crystal structure of rat P450 24A1 has been published (Annalora et al. 2010) (PDB 3K9V, 3K9Y). No substrate is present, only a molecule of either steroidal detergent 3-[(3-cholamidopropyl)dimethylammonion]-1-propane sulfonate (CHAPS) or the totally synthetic detergent CYMAL-5. Some of the reactions catalyzed by P450 24A1 are shown in Fig. 19, but the human enzyme will also oxidize a number of substrate analogs.
The degradation of 1α,25-dihydroxy vitamin D3 by P450 24A1 occurs through multiple, sequential oxidation reactions targeting specific carbon atoms in the side chain. These multicatalytic activities of P450 24A1 (Makin et al. 1989; Reddy and Tserng 1989; Akiyoshi-Shibata et al. 1994; Beckman et al. 1996) can be divided into two regioselective oxidation pathways, involving an initial attack at C24 to yield a side-chain truncated product, calcitroic acid (Makin et al. 1989; Reddy and Tserng 1989), or at C23 to give 1α,25-dihydroxy vitamin D3-26,23-lactone (Sakaki et al. 2000) (Fig. 19). The proportion of these catabolic products is species-dependent, with calcitroic acid production predominating in rodents (Hamamoto et al. 2006), 26,23-lactone predominating in the opossum and guinea pig (Pedersen et al. 1988; Horiuchi et al. 1995), and a mixture of the two products being formed in humans (Prosser and Jones 2004). Substrate contact with a species-specific residue in the I-helix adjacent to the heme group of the cytochrome P450 is proposed to control the pathway selection, with Ala-326 directing substrate into the C24 pathway in humans and Gly-326 driving 23-hydroxylation in opossums (Prosser et al. 2007). Other amino acids having a lesser influence on regioselectivity in rat include Thr-416 (Met-416 in humans) and Ile-500 (Thr-500 in humans) (Hamamoto et al. 2006). Thus, the failure of the enzyme to oxidize certain substrates may result from an inability to correctly position the side chain rather than an inherent inability to bind the substrate (Kaufmann et al. 2011).
In melanocytic tumors the level of P450 24A1 was higher than in the normal epidermis, with the highest mean P450 24A1 level found in nevi and early stage melanomas. During melanoma progression P450 24A1 levels decreased and in the advanced stages was comparable to the normal epidermis and metastases. Lower P450 24A1 levels correlated with the presence of ulceration, necrosis, nodular type, and amelanotic phenotypes. A lack of detectable P450 24A1 expression was related to disease-free survival. Thus, an elevated level of CYP24A1 appears to have an important impact on the formation of melanocytic nevi and melanomagenesis, or progression, in the early stages of tumor development (Brozyna et al. 2014).
References
- Abe D, Sakaki T, Kusudo T, Kittaka A, Saito N, Suhara Y, Fujishima T, Takayama H, Hamamoto H, Kamakura M et al. 2005. Metabolism of 2α-propoxy-1α,25-dihydroxyvitamin D3 and 2α-(3-hydroxypropoxy)-1α,25-dihydroxyvitamin D3 by human CYP27A1 and CYP24A1. Drug Metab Dispos 33:778–784. [DOI] [PubMed] [Google Scholar]
- Aboraia AS, Makowski B, Bahja A, Prosser D, Brancale A, Jones G, Simons C. 2010. Synthesis and CYP24A1 inhibitory activity of (E)-2-(2-substituted benzylidene)- and 2-(2-substituted benzyl)-6-methoxy-tetralones. Eur J Med Chem 45:4427–4434. [DOI] [PubMed] [Google Scholar]
- Akiyoshi-Shibata M, Sakaki T, Ohyama Y, Noshiro M, Okuda K, Yabusaki Y. 1994. Further oxidation of hydroxycalcidiol by calcidiol 24-hydroxylase: A study with the mature enzyme expressed in Escherichia coli. Eur J Biochem 224:335–343. [DOI] [PubMed] [Google Scholar]
- Amano Y, Cho Y, Matsunawa M, Komiyama K, Makishima M. 2009. Increased nuclear expression and transactivation of vitamin D receptor by the cardiotonic steroid bufalin in human myeloid leukemia cells. J Steroid Biochem Mol Biol 114:144–151. [DOI] [PubMed] [Google Scholar]
- Anderson MG, Nakane M, Ruan X, Kroeger PE, Wu-Wong JR. 2006. Expression of VDR and CYP24A1 mRNA in human tumors. Cancer Chemother Pharmacol 57:234–240. [DOI] [PubMed] [Google Scholar]
- Annalora AJ, Goodin DB, Hong WX, Zhang Q, Johnson EF, Stout CD. 2010. Crystal structure of CYP24A1, a mitochondrial cytochrome P450 involved in vitamin D metabolism. J Mol Biol 396:441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila E, Diaz L, Barrera D, Halhali A, Mendez I, Gonzalez L, Zuegel U, Steinmeyer A, Larrea F. 2007. Regulation of Vitamin D hydroxylases gene expression by 1,25-dihydroxyvitamin D3 and cyclic AMP in cultured human syncytiotrophoblasts. J Steroid Biochem Mol Biol 103:90–96. [DOI] [PubMed] [Google Scholar]
- Beckman MJ, Tadikonda P, Werner E, Prahl J, Yamada S, Deluca HF. 1996. Human 25-hydroxyvitamin D3-24-hydroxylase, a multicatalytic enzyme. Biochemistry 35:8465–8472. [DOI] [PubMed] [Google Scholar]
- Brozyna AA, Jochymski C, Janjetovic Z, Jozwicki W, Tuckey RC, Slominski AT. 2014. CYP24A1 expression inversely correlates with melanoma progression: Clinic-pathological studies. Int J Mol Sci 15:19000–19017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno RD, Njar VC. 2007. Targeting cytochrome P450 enzymes: A new approach in anti-cancer drug development. Bioorg Med Chem 15:5047–5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi M, Yamada S, Makishima M. 2011. Dynamic and ligand-selective interactions of vitamin D receptor with retinoid X receptor and cofactors in living cells. Mol Pharmacol 80:1147–1155. [DOI] [PubMed] [Google Scholar]
- Cozzolino M, Vidal M, Arcidiacono MV, Tebas P, Yarasheski KE, Dusso AS. 2003. HIV-protease inhibitors impair vitamin D bioactivation to 1,25-dihydroxyvitamin D. AIDS (London) 17:513–520. [DOI] [PubMed] [Google Scholar]
- Farhan H, Cross HS. 2002. Transcriptional inhibition of CYP24 by genistein. Ann New York Acad Sci 973:459–462. [DOI] [PubMed] [Google Scholar]
- Ferla S, Gomaa MS, Brancale A, Zhu J, Ochalek JT, DeLuca HF, Simons C. 2014. Novel styryl-indoles as small molecule inhibitors of 25-hydroxyvitamin D-24-hydroxylase (CYP24A1): Synthesis and biological evaluation. Eur J Med Chem 87:39–51. [DOI] [PubMed] [Google Scholar]
- Ge N, Chu XM, Xuan YP, Ren DQ, Wang Y, Ma K, Gao HJ, Jiao WJ. 2017. Associations between abnormal vitamin D metabolism pathway function and non-small cell lung cancer. Oncol Lett 14:7538–7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao XH, Dwivedi PP, Omdahl JL, Morris HA, May BK. 2004. Calcitonin stimulates expression of the rat 25-hydroxyvitamin D3–24-hydroxylase (CYP24) promoter in HEK-293 cells expressing calcitonin receptor: identification of signaling pathways. J Mol Endocrinol 32:87–98. [DOI] [PubMed] [Google Scholar]
- Guengerich FP. 2015. Chapter 9, Human cytochrome P450 enzymes. In: Ortiz de Montellano PR, editor. Cytochrome P450: Structure, Mechanism, and Biochemistry 4th ed. New York: Springer; p. 523–785. [Google Scholar]
- Hamamoto H, Kusudo T, Urushino N, Masuno H, Yamamoto K, Yamada S, Kamakura M, Ohta M, Inouye K, Sakaki T. 2006. Structure-function analysis of vitamin D 24-hydroxylase (CYP24A1) by site-directed mutagenesis: amino acid residues responsible for species-based difference of CYP24A1 between humans and rats. Mol Pharmacol 70:120–128. [DOI] [PubMed] [Google Scholar]
- Horiuchi N, Saikatsu S, Akeno N, Abe M, Kimura S, Yamada S. 1995. Synthesis of 25-hydroxyvitamin D3–26,23-lactone but not 24,25-dihydroxyvitamin D3 from 25-hydroxyvitamin D3 in opossum kidney cells treated with 1α,25-dihydroxyvitamin D3. Hormone Metabol Res 27:83–89. [DOI] [PubMed] [Google Scholar]
- Horvath E, Lakatos P, Balla B, Kosa JP, Tóbiás B, Jozilan H, Borka K, Horváth HC, Kovalszky I, Szalay F. 2012. Marked increase of CYP24A1 mRNA level in hepatocellular carcinoma cell lines following vitamin D administration. Anticancer Res 32:4791–4796. [PubMed] [Google Scholar]
- Ikezoe T, Bandobashi K, Yang Y, Takeuchi S, Sekiguchi N, Sakai S, Koeffler HP, Taguchi H. 2006. HIV-1 protease inhibitor ritonavir potentiates the effect of 1,25-dihydroxyvitamin D3 to induce growth arrest and differentiation of human myeloid leukemia cells via down-regulation of CYP24. Leuk Res 30:1005–1011. [DOI] [PubMed] [Google Scholar]
- Inouye K, Sakaki T. 2001. Enzymatic studies on the key enzymes of vitamin D metabolism; 1α-hydroxylase (CYP27B1) and 24-hydroxylase (CYP24). Biotechnol Annu Rev 7:179–194. [DOI] [PubMed] [Google Scholar]
- Ishizawa M, Matsunawa M, Adachi R, Uno S, Ikeda K, Masuno H, Shimizu M, Iwasaki K, Yamada S, Makishima M. 2008. Lithocholic acid derivatives act as selective vitamin D receptor modulators without inducing hypercalcemia. J Lipid Res 49:763–772. [DOI] [PubMed] [Google Scholar]
- Jones G, Prosser DE, Kaufmann M. 2012. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): Its important role in the degradation of vitamin D. Arch Biochem Biophys 523:9–18. [DOI] [PubMed] [Google Scholar]
- Jones G, Prosser DE, Kaufmann M. 2014. Cytochrome P450-mediated metabolism of vitamin D. J Lipid Res 55:13–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann M, Prosser DE, Jones G. 2011. Bioengineering anabolic vitamin D-25-hydroxylase activity into the human vitamin D catabolic enzyme, cytochrome P450 CYP24A1, by a V391L mutation. J Biol Chem 286:28729–28737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmis CM, Salvador SM, Smith KM, Welsh J. 2006. Human mammary epithelial cells express CYP27B1 and are growth inhibited by 25-hydroxyvitamin D-3, the major circulating form of vitamin D-3. J Nutr 136:887–892. [DOI] [PubMed] [Google Scholar]
- King AN, Beer DG, Christensen PJ, Simpson RU, Ramnath N. 2010. The vitamin D/CYP24A1 story in cancer. Anticancer Agents Med Chem 10:213–224. [DOI] [PubMed] [Google Scholar]
- Kósa JP, Horváth P, Wölfling J, Kovács D, Balla B, Mátyus P, Horváth E, Speer G, Takács I, Nagy Z et al. 2013. CYP24A1 inhibition facilitates the anti-tumor effect of vitamin D3 on colorectal cancer cells. World J Gastroenterol 19:2621–2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusudo T, Sakaki T, Abe D, Fujishima T, Kittaka A, Takayama H, Hatakeyama S, Ohta M, Inouye K. 2004. Metabolism of A-ring diastereomers of 1α,25-dihydroxyvitamin D3 by CYP24A1. Biochem Biophys Res Commun 321:774–782. [DOI] [PubMed] [Google Scholar]
- Lechner D, Cross HS. 2003. Phytoestrogens and 17α-estradiol influence vitamin D metabolism and receptor expression-relevance for colon cancer prevention. Recent Results Cancer Res 164:379–391. [PubMed] [Google Scholar]
- Lou YR, Miettinen S, Kagechika H, Gronemeyer H, Tuohimaa P. 2005. Retinoic acid via RARα inhibits the expression of 24-hydroxylase in human prostate stromal cells. Biochem Biophys Res Commun 338:1973–1981. [DOI] [PubMed] [Google Scholar]
- Lou YR, Nazarova N, Talonpoika R, Tuohimaa P. 2005. 5α-Dihydrotestosterone inhibits 1α,25-dihydroxyvitamin D3-induced expression of CYP24 in human prostate cancer cells. Prostate 63:222–230. [DOI] [PubMed] [Google Scholar]
- Luo W, Hershberger PA, Trump DL, Johnson CS. 2013. 24-Hydroxylase in cancer: Impact on vitamin D-based anticancer therapeutics. J Steroid Biochem Mol Biol 136:252–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makin G, Lohnes D, Byford V, Ray R, Jones G. 1989. Target cell metabolism of 1,25-dihydroxyvitamin D3 to calcitroic acid. Evidence for a pathway in kidney and bone involving 24-oxidation. Biochem J 262:173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunawa M, Amano Y, Endo K, Uno S, Sakaki T, Yamada S, Makishima M. 2009. The aryl hydrocarbon receptor activator benzo[a]pyrene enhances vitamin D3 catabolism in macrophages. Toxicol Sci 109:50–58. [DOI] [PubMed] [Google Scholar]
- Muindi JR, Yu WD, Ma Y, Engler KL, Kong RX, Trump DL, Johnson CS. 2010. CYP24A1 inhibition enhances the antitumor activity of calcitriol. Endocrinology 151:4301–4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano H, Matsunawa M, Yasui A, Adachi R, Kawana K, Shimomura I, Makishima M. 2005. Enhancement of ligand-dependent vitamin D receptor transactivation by the cardiotonic steroid bufalin. Biochem Pharmacol 70:1479–1486. [DOI] [PubMed] [Google Scholar]
- Norlin M, Lundqvist J, Ellfolk M, Hellstrom Pigg M, Gustafsson J, Wikvall K. 2017. Drug-mediated gene regulation of vitamin D3 metabolism in primary human dermal fibroblasts. Basic Clin Pharmacol Toxicol 120:59–63. [DOI] [PubMed] [Google Scholar]
- Pan ST, Xue D, Li ZL, Zhou ZW, He ZX, Yang Y, Yang T, Qiu JX, Zhou SF. 2016. Computational identification of the paralogs and orthologs of human cytochrome P450 superfamily and the implication in drug discovery. Int J Mol Sci June 28;17(7). pii: ijms17071020. doi: 10.3390/ijms17071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascussi JM, Robert A, Nguyen M, Walrant-Debray O, Garabedian M, Martin P, Pineau T, Saric J, Navarro F, Maurel P et al. 2005. Possible involvement of pregnane X receptor-enhanced CYP24 expression in drug-induced osteomalacia. J Clin Invest 115:177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen JI, Hagenfeldt Y, Björkhem I. 1988. Assay and properties of 25-hydroxyvitamin D3 23-hydroxylase. Evidence that 23,25-dihydroxyvitamin D3 is a major metabolite in 1,25-dihydroxyvitamin D3-treated or fasted guinea pigs. Biochem J 250:527–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkovich M, Jones G. 2011. CYP24A1 and kidney disease. Curr Opin Nephrol Hypertens 20:337–344. [DOI] [PubMed] [Google Scholar]
- Posner GH, Crawford KR, Yang HW, Kahraman M, Jeon HB, Li H, Lee JK, Suh BC, Hatcher MA, Labonte T et al. 2004. Potent, low-calcemic, selective inhibitors of CYP24 hydroxylase: 24-Sulfone analogs of the hormone 1α,25-dihydroxyvitamin D3. J Steroid Biochem Mol Biol 89–90:5–12. eng. [DOI] [PubMed] [Google Scholar]
- Posner GH, Helvig C, Cuerrier D, Collop D, Kharebov A, Ryder K, Epps T, Petkovich M. 2010. Vitamin D analogues targeting CYP24 in chronic kidney disease. J Steroid Biochem Mol Biol 121:13–19. [DOI] [PubMed] [Google Scholar]
- Prosser DE, Jones G. 2004. Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem Sci 29:664–673. [DOI] [PubMed] [Google Scholar]
- Prosser DE, Kaufmann M, O’Leary B, Byford V, Jones G. 2007. Single A326G mutation converts human CYP24A1 from 25-OH-D3-24-hydroxylase into −23-hydroxylase, generating 1α,25-(OH)2D3-26,23-lactone. Proc Natl Acad Sci USA 104:12673–12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purnapatre K, Khattar SK, Saini KS. 2008. Cytochrome P450s in the development of target-based anticancer drugs. Cancer Lett 259:1–15. [DOI] [PubMed] [Google Scholar]
- Reddy GS, Tserng KY. 1989. Calcitroic acid, end product of renal metabolism of 1,25-dihydroxyvitamin D3 through C-24 oxidation pathway. Biochemistry 28:1763–1769. [DOI] [PubMed] [Google Scholar]
- Saito N, Suhara Y, Abe D, Kusudo T, Ohta M, Yasuda K, Sakaki T, Honzawa S, Fujishima T, Kittaka A. 2009. Synthesis of 2α-propoxy-1α,25-dihydroxyvitamin D3 and comparison of its metabolism by human CYP24A1 and rat CYP24A1. Bioorg Med Chem 17:4296–4301. [DOI] [PubMed] [Google Scholar]
- Sakaki T, Kagawa N, Yamamoto K, Inouye K. 2005. Metabolism of vitamin D3 by cytochromes P450. Frontiers Biosci 10:119–134. [DOI] [PubMed] [Google Scholar]
- Sakaki T, Sawada N, Komai K, Shiozawa S, Yamada S, Yamamoto K, Ohyama Y, Inouye K. 2000. Dual metabolic pathway of 25-hydroxyvitamin D3 catalyzed by human CYP24. Eur J Biochem 267:6158–6165. [DOI] [PubMed] [Google Scholar]
- Sakaki T, Sawada N, Nonaka Y, Ohyama Y, Inouye K. 1999. Metabolic studies using recombinant Escherichia coli cells producing rat mitochondrial CYP24. CYP24 can convert 1a,25-dihydroxyvitamin D3 to calcitroic acid. Eur J Biochem 262:43–48. [DOI] [PubMed] [Google Scholar]
- Schuster I, Bernhardt R. 2007. Inhibition of cytochromes P450: existing and new promising therapeutic targets. Drug Metab Rev 39:481–499. [DOI] [PubMed] [Google Scholar]
- Schuster I, Egger H, Astecker N, Herzig G, Schussler M, Vorisek G. 2001. Selective inhibitors of CYP24: Mechanistic tools to explore vitamin D metabolism in human keratinocytes. Steroids 66:451–462. [DOI] [PubMed] [Google Scholar]
- Schuster I, Egger H, Bikle D, Herzig G, Reddy GS, Stuetz A, Stuetz P, Vorisek G. 2001. Selective inhibition of vitamin D hydroxylases in human keratinocytes. Steroids 66:409–422. [DOI] [PubMed] [Google Scholar]
- Schuster I, Egger H, Herzig G, Reddy GS, Schmid JA, Schussler M, Vorisek G. 2006. Selective inhibitors of vitamin D metabolism--new concepts and perspectives. Anticancer Res 26:2653–2668. [PubMed] [Google Scholar]
- Schuster I, Egger H, Nussbaumer P, Kroemer RT. 2003. Inhibitors of vitamin D hydroxylases: Structure-activity relationships. J Cell Biochem 88:372–380. [DOI] [PubMed] [Google Scholar]
- Schuster I, Egger H, Reddy GS, Vorisek G. 2003. Combination of vitamin D metabolites with selective inhibitors of vitamin D metabolism. Recent Results Cancer Res 164:169–188. [DOI] [PubMed] [Google Scholar]
- Segersten U, Holm PK, Bjorklund P, Hessman O, Nordgren H, Binderup L, Akerstrom G, Hellman P, Westin G. 2005. 25-Hydroxyvitamin D3 1α-hydroxylase expression in breast cancer and use of non-1α-hydroxylated vitamin D analogue. Breast Cancer Res 7:R980–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon JD, Heitzer MD, Liu TT, Beumer JH, Parise RA, Normolle DP, Leach DA, Buchanan G, DeFranco DB. 2014. VDR activity is differentially affected by Hic-5 in prostate cancer and stromal cells. Mol Cancer Res 12:1166–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumantran VN, Mishra P, Bera R, Sudhakar N. 2016. Microarray Analysis of Differentially-Expressed Genes Encoding CYP450 and Phase II Drug Metabolizing Enzymes in Psoriasis and Melanoma. Pharmaceutics 8(1). February 17; pii: E4. doi: 10.3390/pharmaceutics8010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swami S, Krishnan AV, Peehl DM, Feldman D. 2005. Genistein potentiates the growth inhibitory effects of 1,25-dihydroxyvitamin D3 in DU145 human prostate cancer cells: Role of the direct inhibition of CYP24 enzyme activity. Mol Cell Endocrinol 241:49–61. [DOI] [PubMed] [Google Scholar]
- Taban IM, Zhu J, DeLuca HF, Simons C. 2017a. Analysis of the binding sites of vitamin D 1α-hydroxylase (CYP27B1) and vitamin D 24-hydroxylase (CYP24A1) for the design of selective CYP24A1 inhibitors: Homology modelling, molecular dynamics simulations and identification of key binding requirements. Bioorg Med Chem 25:5629–5636. [DOI] [PubMed] [Google Scholar]
- Taban IM, Zhu J, DeLuca HF, Simons C. 2017b. Synthesis, molecular modelling and CYP24A1 inhibitory activity of novel of (E)-N-(2-(1H-imidazol-1-yl)-2-(phenylethyl)-3/4-styrylbenzamides. Bioorg Med Chem 25:4076–4087. [DOI] [PubMed] [Google Scholar]
- Tashiro K, Abe T, Oue N, Yasui W, Ryoji M. 2004. Characterization of vitamin D-mediated induction of the CYP 24 transcription. Mol Cell Endocrinol 226:27–32. [DOI] [PubMed] [Google Scholar]
- Tieu EW, Li W, Chen J, Kim T-K, Ma D, Slominski AT, Tuckey RC. 2015. Metabolism of 20-hydroxyvitamin D3 and 20,23-dihydroxyvitamin D3 by rat and human CYP24A1. J Steroid Biochem Mol Biol 149:153–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuckey RC, Janjetovic Z, Li W, Nguyen MN, Zmijewski MA, Zjawiony J, Slominski A. 2008. Metabolism of 1α-hydroxyvitamin D3 by cytochrome P450scc to biologically active 1α,20-dihydroxyvitamin D3. J Steroid Biochem Mol Biol 112:213–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vantieghem K, Kissmeyer AM, De Haes P, Bouillon R, Segaert S. 2006. UVB-induced production of 1,25-dihydroxyvitamin D3 and vitamin D activity in human keratinocytes pretreated with a sterol △7-reductase inhibitor. J Cell Biochem 98:81–92. [DOI] [PubMed] [Google Scholar]
- Vrzal R, Doricakova A, Novotna A, Bachleda P, Bitman M, Pavek P, Dvorak Z. 2011. Valproic acid augments vitamin D receptor-mediated induction of CYP24 by vitamin D3: A possible cause of valproic acid-induced osteomalacia? Toxicol Lett 200:146–153. [DOI] [PubMed] [Google Scholar]
- Wegler C, Wikvall K, Norlin M. 2016. Effects of osteoporosis-inducing drugs on vitamin D-related gene transcription and mineralization in MG-63 and Saos-2 cells. Basic Clin Pharmacol Toxicol 119:436–442. [DOI] [PubMed] [Google Scholar]
- Wietrzyk J 2007. [The influence of isoflavonoids on the antitumor activity of vitamin D3]. Postepy higieny i medycyny doswiadczalnej 61:253–260. pol. [PubMed] [Google Scholar]
- Yee SW, Simons C. 2004. Synthesis and CYP24 inhibitory activity of 2-substituted-3,4-dihydro-2H-naphthalen-1-one (tetralone) derivatives. Bioorg Med Chem Lett 14:5651–5654. [DOI] [PubMed] [Google Scholar]
- Zhou YK, Liang Z, Guo Y, Zhang HT, Wang KH. 2015. High glucose upregulates CYP24A1 expression which attenuates the ability of 1,25(OH)2D3 to increase NGF secretion in a rat Schwann cell line RSC96. Mol Cell Endocrinol 404:75–81. [DOI] [PubMed] [Google Scholar]
- Zou A, Elgort MG, Allegretto EA. 1997. Retinoid X receptor (RXR) ligands activate the human 25-hydroxyvitamin D3-24-hydroxylase promoter via RXR heterodimer binding to two vitamin D-responsive elements and elicit additive effects with 1,25-dihydroxyvitamin D3. J Biol Chem 272:19027–19034. [DOI] [PubMed] [Google Scholar]
P450 27B1
P450 27B1, a kidney mitochondrial enzyme, is the vitamin D 1α-hydroxylase, acting on various derivatives of 25-hydroxyvitamin D. This is an activated form of vitamin D, and accordingly the enzyme has a very complex regulatory system (Guengerich 2015). Further, P450 27B1 deficiency is associated with type I vitamin D-dependent rickets, and ≥ 20 variants are known (Kitanaka et al. 1998; Wang et al. 1998; Smith et al. 1999; Portale and Miller 2000; Kim et al. 2007; Wu et al. 2007).
No crystal structures have been reported, although roles of several amino acids have been identified through the work with the clinical variants.
References
- Alesutan I, Feger M, Pakladok T, Mia S, Ahmed MS, Voelkl J, Lang F. 2013. 25-Hydroxyvitamin D3 1-α-hydroxylase-dependent stimulation of renal klotho expression by spironolactone. Kidney Blood Pressure Res 37:475–487. [DOI] [PubMed] [Google Scholar]
- Avila E, Diaz L, Barrera D, Halhali A, Mendez I, Gonzalez L, Zuegel U, Steinmeyer A, Larrea F. 2007. Regulation of Vitamin D hydroxylases gene expression by 1,25-dihydroxyvitamin D3 and cyclic AMP in cultured human syncytiotrophoblasts. J Steroid Biochem Mol Biol 103:90–96. [DOI] [PubMed] [Google Scholar]
- Avila E, Diaz L, Halhali A, Larrea F. 2004. Regulation of 25-hydroxyvitamin D3 1α-hydroxylase, 1,25-dihydroxyvitamin D3 24-hydroxylase and vitamin D receptor gene expression by 8-bromo cyclic AMP in cultured human syncytiotrophoblast cells. J Steroid Biochem Mol Biol 89–90:115–119. [DOI] [PubMed] [Google Scholar]
- Bland R, Walker EA, Hughes SV, Stewart PM, Hewison M. 1999. Constitutive expression of 25-hydroxyvitamin D3-1α-hydroxylase in a transformed human proximal tubule cell line: evidence for direct regulation of vitamin D metabolism by calcium. Endocrinology 140:2027–2034. [DOI] [PubMed] [Google Scholar]
- Bruno RD, Njar VC. 2007. Targeting cytochrome P450 enzymes: A new approach in anti-cancer drug development. Bioorg Med Chem 15:5047–5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzolino M, Vidal M, Arcidiacono MV, Tebas P, Yarasheski KE, Dusso AS. 2003. HIV-protease inhibitors impair vitamin D bioactivation to 1,25-dihydroxyvitamin D. AIDS (London) 17:513–520. [DOI] [PubMed] [Google Scholar]
- Du J, Wei X, Ge X, Chen Y, Li YC. 2017. Microbiota-dependent induction of colonic Cyp27b1 is associated with colonic inflammation: Implications of locally produced 1,25-dihydroxyvitamin D3 in inflammatory regulation in the colon. Endocrinology 158:4064–4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellfolk M, Norlin M, Gyllensten K, Wikvall K. 2009. Regulation of human vitamin D3 25-hydroxylases in dermal fibroblasts and prostate cancer LNCaP cells. Mol Pharmacol 75:1392–1399. [DOI] [PubMed] [Google Scholar]
- Farhan H, Cross HS. 2002. Transcriptional inhibition of CYP24 by genistein. Ann New York Acad Sci 973:459–462. [DOI] [PubMed] [Google Scholar]
- Farhan H, Wähälä K, Cross HS. 2003. Genistein inhibits vitamin D hydroxylases CYP24 and CYP27B1 expression in prostate cells. J Steroid Biochem Mol Biol 84:423–429. [DOI] [PubMed] [Google Scholar]
- Frizen BN, Zhegin VA. 1976. [Clinico-functional characteristics of the esophagus in rheumatoid arthritis and systemic lupus erythematosus in comparison with systemic scleroderma]. Terapevticheskii arkhiv 48:57–64. rus. [PubMed] [Google Scholar]
- Ge N, Chu XM, Xuan YP, Ren DQ, Wang Y, Ma K, Gao HJ, Jiao WJ. 2017. Associations between abnormal vitamin D metabolism pathway function and non-small cell lung cancer. Oncol Lett 14:7538–7544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guengerich FP. 2015. Chapter 9, Human cytochrome P450 enzymes. In: Ortiz de Montellano PR, editor. Cytochrome P450: Structure, Mechanism, and Biochemistry 4th ed. New York: Springer; p. 523–785. [Google Scholar]
- Inouye K, Sakaki T. 2001. Enzymatic studies on the key enzymes of vitamin D metabolism; 1α-hydroxylase (CYP27B1) and 24-hydroxylase (CYP24). Biotechnol Annu Rev 7:179–194. [DOI] [PubMed] [Google Scholar]
- Kim CJ, Kaplan LE, Perwad F, Huang N, Sharma A, Choi Y, Miller WL, Portale AA. 2007. Vitamin D 1α-hydroxylase gene mutations in patients with 1α-hydroxylase deficiency. J Clin Endocrinol Metab 92:3177–3182. [DOI] [PubMed] [Google Scholar]
- Kitanaka S, Takeyama K, Murayama A, Sato T, Okumura K, Nogami M, Hasegawa Y, Niimi H, Yanagisawa J, Tanaka T et al. 1998. Inactivating mutations in the 25-hydroxyvitamin D3 1α-hydroxylase gene in patients with pseudovitamin D-deficiency rickets. New Engl J Med 338:653–661. [DOI] [PubMed] [Google Scholar]
- Kozai M, Yamamoto H, Ishiguro M, Harada N, Masuda M, Kagawa T, Takei Y, Otani A, Nakahashi O, Ikeda S et al. 2013. Thyroid hormones decrease plasma 1α,25-dihydroxyvitamin D levels through transcriptional repression of the renal 25-hydroxyvitamin D3 1α-hydroxylase gene (CYP27B1). Endocrinology 154:609–622. [DOI] [PubMed] [Google Scholar]
- Lechner D, Bajna E, Adlercreutz H, Cross HS. 2006. Genistein and 17α-estradiol, but not equol, regulate vitamin D synthesis in human colon and breast cancer cells. Anticancer Res 26:2597–2603. [PubMed] [Google Scholar]
- Lechner D, Kallay E, Cross HS. 2007. 1α,25-Dihydroxyvitamin D3 downregulates CYP27B1 and induces CYP24A1 in colon cells. Mol Cell Endocrinol 263:55–64. [DOI] [PubMed] [Google Scholar]
- Maas RM, Reus K, Diesel B, Steudel WI, Feiden W, Fischer U, Meese E. 2001. Amplification and expression of splice variants of the gene encoding the P450 cytochrome 25-hydroxyvitamin D3 1,α-hydroxylase (CYP 27B1) in human malignant glioma. Clin Cancer Res 7:868–875. [PubMed] [Google Scholar]
- Pan ST, Xue D, Li ZL, Zhou ZW, He ZX, Yang Y, Yang T, Qiu JX, Zhou SF. 2016. Computational identification of the paralogs and orthologs of human cytochrome P450 superfamily and the implication in drug discovery. Int J Mol Sci June 28;17(7). pii: ijms17071020. doi: 10.3390/ijms17071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascussi JM, Robert A, Nguyen M, Walrant-Debray O, Garabedian M, Martin P, Pineau T, Saric J, Navarro F, Maurel P et al. 2005. Possible involvement of pregnane X receptor-enhanced CYP24 expression in drug-induced osteomalacia. J Clin Invest 115:177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portale AA, Miller WL. 2000. Human 25-hydroxyvitamin D-1a-hydroxylase: cloning, mutations, and gene expression. Pediatr Nephrol 14:620–625. [DOI] [PubMed] [Google Scholar]
- Sakaki T, Kagawa N, Yamamoto K, Inouye K. 2005. Metabolism of vitamin D3 by cytochromes P450. Frontiers Biosci 10:119–134. [DOI] [PubMed] [Google Scholar]
- Schuster I, Egger H, Bikle D, Herzig G, Reddy GS, Stuetz A, Stuetz P, Vorisek G. 2001. Selective inhibition of vitamin D hydroxylases in human keratinocytes. Steroids 66:409–422. [DOI] [PubMed] [Google Scholar]
- Schuster I, Egger H, Herzig G, Reddy GS, Schmid JA, Schussler M, Vorisek G. 2006. Selective inhibitors of vitamin D metabolism–new concepts and perspectives. AntiCancer Res 26:2653–2668. [PubMed] [Google Scholar]
- Schuster I, Egger H, Nussbaumer P, Kroemer RT. 2003. Inhibitors of vitamin D hydroxylases: Structure-activity relationships. J Cell Biochem 88:372–380. [DOI] [PubMed] [Google Scholar]
- Schuster I, Egger H, Reddy GS, Vorisek G. 2003. Combination of vitamin D metabolites with selective inhibitors of vitamin D metabolism. Recent Results Cancer Res 164:169–188. [DOI] [PubMed] [Google Scholar]
- Smith SJ, Rucka AK, Berry JL, Davies M, Mylchreest S, Paterson CR, Heath DA, Tassabehji M, Read AP, Mee AP et al. 1999. Novel mutations in the 1α-hydroxylase (P450c1) gene in three families with pseudovitamin D-deficiency rickets resulting in loss of functional enzyme activity in blood-derived macrophages. J Bone Mineral Res 14:730–739. [DOI] [PubMed] [Google Scholar]
- Somjen D, Katzburg S, Stern N, Kohen F, Sharon O, Limor R, Jaccard N, Hendel D, Weisman Y. 2007. 25-Hydroxy vitamin D3–1α hydroxylase expression and activity in cultured human osteoblasts and their modulation by parathyroid hormone, estrogenic compounds and dihydrotestosterone. J Steroid Biochem Mol Biol 107:238–244. [DOI] [PubMed] [Google Scholar]
- Somjen D, Knoll E, Sharon O, Many A, Stern N. 2017. Interaction between the effects of the selective estrogen modulator femarelle and a vitamin D analog in human umbilical artery vascular smooth muscle cells. J Steroid Biochem Mol Biol 174:9–13. [DOI] [PubMed] [Google Scholar]
- Somjen D, Weisman Y, Kohen F, Gayer B, Limor R, Sharon O, Jaccard N, Knoll E, Stern N. 2005. 25-Hydroxyvitamin D3-1α-hydroxylase is expressed in human vascular smooth muscle cells and is upregulated by parathyroid hormone and estrogenic compounds. Circulation 111:1666–1671. [DOI] [PubMed] [Google Scholar]
- Sumantran VN, Mishra P, Bera R, Sudhakar N. 2016. Microarray analysis of differentially-expressed genes encoding CYP450 and Phase II drug metabolizing enzymes in psoriasis and melanoma. Pharmaceutics 8(1) February 17;8(1). pii: E4. doi: 10.3390/pharmaceutics8010004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang EKY, Chen JJ, Janjetovic Z, Tieu EW, Slominski AT, Li W, Tuckey RC. 2013. Hydroxylation of CYP11A1-derived products of vitamin D3 metabolism by human and mouse CYP27B1. Drug Metab Dispos 41:1112–1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JT, Lin CJ, Burridge SM, Fu GK, Labuda M, Portale AA, Miller WL. 1998. Genetics of vitamin D 1α-hydroxylase deficiency in 17 families. Am J Hum Genet 63:1694–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wegler C, Wikvall K, Norlin M. 2016. Effects of osteoporosis-inducing drugs on vitamin D-related gene transcription and mineralization in MG-63 and Saos-2 cells. Basic Clin Pharmacol Toxicol 119:436–442. [DOI] [PubMed] [Google Scholar]
- Wietrzyk J 2007. [The influence of isoflavonoids on the antitumor activity of vitamin D3]. Postepy higieny i medycyny doswiadczalnej (Online) 61:253–260. pol. [PubMed] [Google Scholar]
- Wu S, Ren S, Nguyen L, Adams JS, Hewison M. 2007. Splice variants of the CYP27b1 gene and the regulation of 1,25-dihydroxyvitamin D3 production. Endocrinology 148(7):3410–3418. [DOI] [PubMed] [Google Scholar]
- Xie Z, Munson SJ, Huang N, Portale AA, Miller WL, Bikle DD. 2002. The mechanism of 1,25-dihydroxyvitamin D3 autoregulation in keratinocytes. J Biol Chem 277:36987–36990. [DOI] [PubMed] [Google Scholar]
- Zhalehjoo N, Shakiba Y, Panjehpour M. 2017. Gene expression profiles of CYP24A1 and CYP27B1 in malignant and normal breast tissues. Mol Med Rep 15:467–473. [DOI] [PubMed] [Google Scholar]
Retinoid metabolism
Several P450 enzymes are involved in oxidations of retinoids (Fig. 21), the regulation of which is very important, as in the case of vitamin D. The 4- and 18-oxidations are considered to be deactivating. The 3,4-desaturation by P450 27C1 in human skin is an efficient reaction but the biological significance has only been established in fish and amphibians.
Fig. 21.
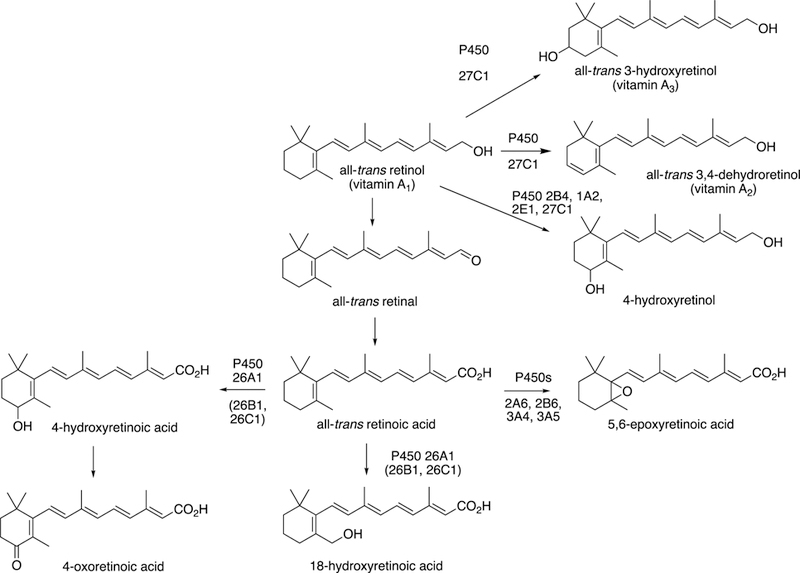
Some P450s involved in the metabolism of retinoids.
P450 26A1
Retinoic acid is the endogenous active metabolite of vitamin A1 (retinol) and acts as an essential regulator of cell growth, cell cycle, and differentiation (Gudas 2012; Napoli 2012). all-trans-Retinoic acid is believed to be the main biologically active form, binding predominantly to nuclear retinoic acid receptors (RARs), and plays a critical role in many biological processes including reproduction, maintenance of skin and epithelia, regulation of the immune system and T- and B-cell function, and in fetal development (Maden 2007; Ross 2012; Hogarth and Griswold 2013).
The production of the enzyme P450 26A1 is regulated by its substrate, retinoic acid, via the RXR receptor.
No crystal structures are available for the enzyme.
P450 26A1 has relevance in developmental biology, in that Cyp26a1−/− mice show distinct malformations and lethality (Tay et al. 2010). Gudas has demonstrated a role of (mouse) P450 26a1 in stem cell differentiation, and inhibiting P450 26A1 has been considered as an approach in cell/differentiation therapy for neurodegenerative diseases (Ricard and Gudas 2013).
Imbalance in vitamin A and retinoic acid homeostasis have been implicated in development and progression of several human diseases, e.g., acne, psoriasis and ichthyosis, type II diabetes, neurodegenerative diseases, and some cancers (Gudas 2012). The use and long term efficacy of all-trans retinoic acid has been limited by its high metabolic clearance, which limits oral administration of this agent, and the resistance that develops towards all-trans retinoic acid due to autoinduction of its metabolism (Nelson et al. 2013).
The clearance of all-trans retinoic acid is predominantly mediated by P450 Family 26 enzymes (Thatcher and Isoherranen 2009; Ross and Zolfaghari 2011), particularly P450 26A1 in human liver, considered to be responsible for majority of the hepatic clearance (Thatcher et al. 2010). It has been proposed that inhibition of the P450 Family 26 enzymes will increase all-trans retinoic acid concentrations in target tissues, and inhibition of P450 26 enzymes during could be used productively (Njar et al. 2006). Many of the existing inhibitors of P450 26 enzymes have not been evaluated for specificity (among the P450 Family 26 enzymes) and therefore the benefits and shortcomings of selective or broad P450 26 inhibition have not been established.
P450 Family 26 enzymes have cell type and tissue specific expression (Topletz et al. 2012) and therefore specific P450 26A1 (or P450 26B1) inhibitors may be beneficial for increasing retinoic acid concentrations in selected target tissues without causing broad side effects. P450 26A1 is the predominant P450 Family 26 enzyme in the liver, and therefore selective inhibition of P450 26A1 could decrease systemic clearance of retinoic acid and be useful in therapy resistance with individuals being treated with all-trans retinoic acid or 13-cis-retinoic acid. A P450 26A1 selective inhibitor would avoid side effects of P450 26B1 inhibitors, which have potential reproductive and teratogenic pissues, and be more likely to be beneficial than when systemic retinoic acid concentrations are targeted (Diaz et al. 2016).
References
- Armstrong JL, Ruiz M, Boddy AV, Redfern CP, Pearson AD, Veal GJ. 2005. Increasing the intracellular availability of all-trans retinoic acid in neuroblastoma cells. Brit J Cancer 92:696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GT, Cash BG, Blihoghe D, Johansson P, Alnabulsi A, Murray GI. 2014. The expression and prognostic significance of retinoic acid metabolising enzymes in colorectal cancer. PloS One 9:e90776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CL, Hong E, Lao-Sirieix P, Fitzgerald RC. 2008. A novel role for the retinoic acid-catabolizing enzyme CYP26A1 in Barrett’s associated adenocarcinoma. Oncogene 27:2951–2960. [DOI] [PubMed] [Google Scholar]
- Diaz P, Huang W, Keyari CM, Buttrick B, Price L, Guilloteau N, Tripathy S, Sperandio VG, Fronczek FR, Astruc-Diaz F et al. 2016. Development and characterization of novel and selective inhibitors of cytochrome P450 CYP26A1, the human liver retinoic acid hydroxylase. J Med Chem 59:2579–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomaa MS, Bridgens CE, Aboraia AS, Veal GJ, Redfern CP, Brancale A, Armstrong JL, Simons C. 2011. Small molecule inhibitors of retinoic acid 4-hydroxylase (CYP26): Synthesis and biological evaluation of imidazole methyl 3-(4-(aryl-2-ylamino)phenyl)propanoates. J Med Chem 54:2778–2791. [DOI] [PubMed] [Google Scholar]
- Gomaa MS, Bridgens CE, Veal GJ, Redfern CP, Brancale A, Armstrong JL, Simons C. 2011. Synthesis and biological evaluation of 3-(1H-imidazol- and triazol-1-yl)-2,2-dimethyl-3-[4-(naphthalen-2-ylamino)phenyl]propyl derivatives as small molecule inhibitors of retinoic acid 4-hydroxylase (CYP26). J Med Chem 54:6803–6811. [DOI] [PubMed] [Google Scholar]
- Gudas LJ. 2012. Emerging roles for retinoids in regeneration and differentiation in normal and disease states. Biochim Biophys Acta 1821:213–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helvig C, Taimi M, Cameron D, Jones G, Petkovich M. 2011. Functional properties and substrate characterization of human CYP26A1, CYP26B1, and CYP26C1 expressed by recombinant baculovirus in insect cells. J Pharmacol Toxicol Methods 64:258–263. [DOI] [PubMed] [Google Scholar]
- Hogarth CA, Griswold MD. 2013. Retinoic acid regulation of male meiosis. Curr Opin Endocrinol Diabetes Obesity 20:217–223. [DOI] [PubMed] [Google Scholar]
- Lampen A, Meyer S, Arnhold T, Nau H. 2000. Metabolism of vitamin A and its active metabolite all-trans-retinoic acid in small intestinal enterocytes. J Pharmacol Exp Ther 295:979–985. [PubMed] [Google Scholar]
- Lutz JD, Dixit V, Yeung CK, Dickmann LJ, Zelter A, Thatcher JE, Nelson WL, Isoherranen N. 2009. Expression and functional characterization of cytochrome P450 26A1, a retinoic acid hydroxylase. Biochem Pharmacol 77:258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maden M 2007. Retinoic acid in the development, regeneration and maintenance of the nervous system. Nat Rev Neurosci 8:755–765. [DOI] [PubMed] [Google Scholar]
- Napoli JL. 2012. Physiological insights into all-trans-retinoic acid biosynthesis. Biochim Biophys Acta 1821:152–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CH, Buttrick BR, Isoherranen N. 2013. Therapeutic potential of the inhibition of the retinoic acid hydroxylases CYP26A1 and CYP26B1 by xenobiotics. Curr Topics Med Chem 13:1402–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njar VC. 2002. Cytochrome P450 retinoic acid 4-hydroxylase inhibitors: Potential agents for cancer therapy. MiniRev Med Chem 2:261–269. [DOI] [PubMed] [Google Scholar]
- Njar VC, Gediya L, Purushottamachar P, Chopra P, Vasaitis TS, Khandelwal A, Mehta J, Huynh C, Belosay A, Patel J. 2006. Retinoic acid metabolism blocking agents (RAMBAs) for treatment of cancer and dermatological diseases. Bioorg Med Chem 14:4323–4340. [DOI] [PubMed] [Google Scholar]
- Ocaya P, Gidlöf AC, Olofsson PS, Törmä H, Sirsjö A. 2007. CYP26 inhibitor R115866 increases retinoid signaling in intimal smooth muscle cells. Arteriosclerosis Thrombosis Vasc Biol 27:1542–1548. [DOI] [PubMed] [Google Scholar]
- Osanai M, Lee GH. 2011a. Enhanced expression of retinoic acid-metabolizing enzyme CYP26A1 in sunlight-damaged human skin. Med Mol Morphol 44:200–206. [DOI] [PubMed] [Google Scholar]
- Osanai M, Lee GH. 2011b. Nicotine-mediated suppression of the retinoic acid metabolizing enzyme CYP26A1 limits the oncogenic potential of breast cancer. Cancer Sci 102:1158–1163. [DOI] [PubMed] [Google Scholar]
- Osanai M, Lee GH. 2014. Increased expression of the retinoic acid-metabolizing enzyme CYP26A1 during the progression of cervical squamous neoplasia and head and neck cancer. BMC Res Notes 7:697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osanai M, Lee GH. 2015. The retinoic acid-metabolizing enzyme CYP26A1 upregulates fascin and promotes the malignant behavior of breast carcinoma cells. Oncol Rep 34:850–858. [DOI] [PubMed] [Google Scholar]
- Ozpolat B, Mehta K, Lopez-Berestein G. 2005. Regulation of a highly specific retinoic acid-4-hydroxylase (CYP26A1) enzyme and all-trans-retinoic acid metabolism in human intestinal, liver, endothelial, and acute promyelocytic leukemia cells. Leukemia Lymphoma 46:1497–1506. [DOI] [PubMed] [Google Scholar]
- Ozpolat B, Mehta K, Tari AM, Lopez-Berestein G. 2002. all-trans-Retinoic acid-induced expression and regulation of retinoic acid 4-hydroxylase (CYP26) in human promyelocytic leukemia. Am J Hematol 70:39–47. [DOI] [PubMed] [Google Scholar]
- Ozpolat B, Tari AM, Mehta K, Lopez-Berestein G. 2004. Nuclear retinoid receptors are involved in N-(4-hydroxyphenyl) retinamide (Fenretinide)-induced gene expression and growth inhibition in HL-60 acute myeloid leukemia cells. Leukemia Lymphoma 45:979–985. [DOI] [PubMed] [Google Scholar]
- Pan ST, Xue D, Li ZL, Zhou ZW, He ZX, Yang Y, Yang T, Qiu JX, Zhou SF. 2016. Computational identification of the paralogs and orthologs of human cytochrome P450 superfamily and the implication in drug discovery. Int J Mol Sci June 28;17(7). pii: ijms17071020. doi: 10.3390/ijms17071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavez Lorie E, Chamcheu JC, Vahlquist A, Torma H. 2009. Both all-trans retinoic acid and cytochrome P450 (CYP26) inhibitors affect the expression of vitamin A metabolizing enzymes and retinoid biomarkers in organotypic epidermis. Arch Dermatol Res 301:475–485. [DOI] [PubMed] [Google Scholar]
- Pavez Lorie E, Cools M, Borgers M, Wouters L, Shroot B, Hagforsen E, Torma H, Vahlquist A. 2009. Topical treatment with CYP26 inhibitor talarozole (R115866) dose dependently alters the expression of retinoid-regulated genes in normal human epidermis. Brit J Dermatol 160:26–36. [DOI] [PubMed] [Google Scholar]
- Pavez Lorie E, Li H, Vahlquist A, Torma H. 2009. The involvement of cytochrome p450 (CYP) 26 in the retinoic acid metabolism of human epidermal keratinocytes. Biochim Biophys Acta 1791:220–228. [DOI] [PubMed] [Google Scholar]
- Purushottamachar P, Patel JB, Gediya LK, Clement OO, Njar VC. 2012. First chemical feature-based pharmacophore modeling of potent retinoidal retinoic acid metabolism blocking agents (RAMBAs): identification of novel RAMBA scaffolds. Eur J Med Chem 47:412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quere R, Baudet A, Cassinat B, Bertrand G, Marti J, Manchon L, Piquemal D, Chomienne C, Commes T. 2007. Pharmacogenomic analysis of acute promyelocytic leukemia cells highlights CYP26 cytochrome metabolism in differential all-trans retinoic acid sensitivity. Blood 109:4450–4460. [DOI] [PubMed] [Google Scholar]
- Ricard MJ, Gudas LJ. 2013. Cytochrome P450 cyp26a1 alters spinal motor neuron subtype identity in differentiating embryonic stem cells. J Biol Chem 288:28801–28813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AC. 2012. Vitamin A and retinoic acid in T cell-related immunity. Am J Clin Nutr 96:1166s–1172s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AC, Zolfaghari R. 2011. Cytochrome P450s in the regulation of cellular retinoic acid metabolism. Annu Rev Nutr 31:65–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster I, Bernhardt R. 2007. Inhibition of cytochromes P450: existing and new promising therapeutic targets. Drug Metab Rev 39:481–499. [DOI] [PubMed] [Google Scholar]
- Shimshoni JA, Roberts AG, Scian M, Topletz AR, Blankert SA, Halpert JR, Nelson WL, Isoherranen N. 2012. Stereoselective formation and metabolism of 4-hydroxy-retinoic acid enantiomers by cytochrome P450 enzymes. J Biol Chem 287:42223–42232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun B, Liu K, Han J, Zhao LY, Su X, Lin B, Zhao DM, Cheng MS. 2015. Design, synthesis, and biological evaluation of amide imidazole derivatives as novel metabolic enzyme CYP26A1 inhibitors. Bioorg Med Chem 23:6763–6773. [DOI] [PubMed] [Google Scholar]
- Sun B, Song S, Hao CZ, Huang WX, Liu CC, Xie HL, Lin B, Cheng MS, Zhao DM. 2015. Molecular recognition of CYP26A1 binding pockets and structure-activity relationship studies for design of potent and selective retinoic acid metabolism blocking agents. J Mol Graph Model 56:10–19. [DOI] [PubMed] [Google Scholar]
- Tay S, Dickmann L, Dixit V, Isoherranen N. 2010. A comparison of the roles of peroxisome proliferator-activated receptor and retinoic acid receptor on CYP26 regulation. Mol Pharmacol 77:218–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher JE, Buttrick B, Shaffer SA, Shimshoni JA, Goodlett DR, Nelson WL, Isoherranen N. 2011. Substrate specificity and ligand interactions of CYP26A1, the human liver retinoic acid hydroxylase. Mol Pharmacol 80:228–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher JE, Isoherranen N. 2009. The role of CYP26 enzymes in retinoic acid clearance. Expert Opin Drug Metab Toxicol 5:875–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thatcher JE, Zelter A, Isoherranen N. 2010. The relative importance of CYP26A1 in hepatic clearance of all-trans retinoic acid. Biochem Pharmacol 80:903–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topletz AR, Thatcher JE, Zelter A, Lutz JD, Tay S, Nelson WL, Isoherranen N. 2012. Comparison of the function and expression of CYP26A1 and CYP26B1, the two retinoic acid hydroxylases. Biochem Pharmacol 83:149–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topletz AR, Tripathy S, Foti RS, Shimshoni JA, Nelson WL, Isoherranen N. 2015. Induction of CYP26A1 by metabolites of retinoic acid: evidence that CYP26A1 is an important enzyme in the elimination of active retinoids. Mol Pharmacol 87:430–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villani MG, Appierto V, Cavadini E, Valsecchi M, Sonnino S, Curley RW, Formelli F. 2004. Identification of the fenretinide metabolite 4-oxo-fenretinide present in human plasma and formed in human ovarian carcinoma cells through induction of cytochrome P450 26A1. Clin Cancer Res 10:6265–6275. [DOI] [PubMed] [Google Scholar]
- Wang F, Kwak HS, Elbuluk N, Kaczmarek AL, Hamilton T, Voorhees JJ, Fisher GJ, Kang S. 2009. Retinoic acid 4-hydroxylase inducibility and clinical response to isotretinoin in patients with acne. J Am Acad Dermatol 61:252–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee SW, Jarno L, Gomaa MS, Elford C, Ooi LL, Coogan MP, McClelland R, Nicholson RI, Evans BA, Brancale A et al. 2005. Novel tetralone-derived retinoic acid metabolism blocking agents: synthesis and in vitro evaluation with liver microsomal and MCF-7 CYP26A1 cell assays. J Med Chem 48:7123–7131. [DOI] [PubMed] [Google Scholar]
P450 26B1
P450 26B1 is expressed in the brain and also in a number of other tissues but not liver (Topletz et al. 2012). It is induced by all-trans-retinoic acid and also regulated by certain transcription factors (Guengerich 2015).
The reactions are similar to those catalyzed by P450 26A1, i.e. 4- and 18-hydroxylation of all-trans-retinoic acid and further oxidation of the 4-hydroxy product to 4-oxoretinoic acid.
References
- Bechor S, Zolberg Relevy N, Harari A, Almog T, Kamari Y, Ben-Amotz A, Harats D, Shaish A. 2016. 9-cis β-Carotene increased cholesterol efflux to HDL in macrophages. Nutrients 8: pii: E435. doi: 10.3390/nu8070435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GT, Cash BG, Blihoghe D, Johansson P, Alnabulsi A, Murray GI. 2014. The expression and prognostic significance of retinoic acid metabolising enzymes in colorectal cancer. PloS One 9:e90776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz P, Huang W, Keyari CM, Buttrick B, Price L, Guilloteau N, Tripathy S, Sperandio VG, Fronczek FR, Astruc-Diaz F et al. 2016. Development and characterization of novel and selective inhibitors of cytochrome P450 CYP26A1, the human liver retinoic acid hydroxylase. J Med Chem 59:2579–2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guengerich FP. 2015. Chapter 9, Human cytochrome P450 enzymes. In: Ortiz de Montellano PR, editor. Cytochrome P450: Structure, Mechanism, and Biochemistry 4th ed. New York: Springer; p. 523–785. [Google Scholar]
- Helvig C, Taimi M, Cameron D, Jones G, Petkovich M. 2011. Functional properties and substrate characterization of human CYP26A1, CYP26B1, and CYP26C1 expressed by recombinant baculovirus in insect cells. J Pharmacol Toxicol Methods 64:258–263. [DOI] [PubMed] [Google Scholar]
- Kumar S, Chatzi C, Brade T, Cunningham TJ, Zhao X, Duester G. 2011. Sex-specific timing of meiotic initiation is regulated by Cyp26b1 independent of retinoic acid signalling. Nat Commun 2:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Njar VC. 2002. Cytochrome P450 retinoic acid 4-hydroxylase inhibitors: Potential agents for cancer therapy. MiniRev Med Chem 2:261–269. [DOI] [PubMed] [Google Scholar]
- Njar VC, Gediya L, Purushottamachar P, Chopra P, Vasaitis TS, Khandelwal A, Mehta J, Huynh C, Belosay A, Patel J. 2006. Retinoic acid metabolism blocking agents (RAMBAs) for treatment of cancer and dermatological diseases. Bioorg Med Chem 14:4323–4340. [DOI] [PubMed] [Google Scholar]
- Ocaya P, Gidlöf AC, Olofsson PS, Törmä H, Sirsjö A. 2007. CYP26 inhibitor R115866 increases retinoid signaling in intimal smooth muscle cells. Arteriosclerosis Thrombosis Vasc Biol 27:1542–1548. [DOI] [PubMed] [Google Scholar]
- Ocaya PA, Elmabsout AA, Olofsson PS, Torma H, Gidlof AC, Sirsjo A. 2011. CYP26B1 plays a major role in the regulation of all-trans-retinoic acid metabolism and signaling in human aortic smooth muscle cells. J Vasc Res 48:23–30. [DOI] [PubMed] [Google Scholar]
- Pan ST, Xue D, Li ZL, Zhou ZW, He ZX, Yang Y, Yang T, Qiu JX, Zhou SF. 2016. Computational identification of the paralogs and orthologs of human cytochrome P450 superfamily and the implication in drug discovery. Int J Mol Sci June 28;17(7). pii: ijms17071020. doi: 10.3390/ijms17071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavez Lorie E, Chamcheu JC, Vahlquist A, Torma H. 2009. Both all-trans retinoic acid and cytochrome P450 (CYP26) inhibitors affect the expression of vitamin A metabolizing enzymes and retinoid biomarkers in organotypic epidermis. Arch Dermatol Res 301:475–485. [DOI] [PubMed] [Google Scholar]
- Pavez Lorie E, Cools M, Borgers M, Wouters L, Shroot B, Hagforsen E, Torma H, Vahlquist A. 2009. Topical treatment with CYP26 inhibitor talarozole (R115866) dose dependently alters the expression of retinoid-regulated genes in normal human epidermis. Brit J Dermatol 160:26–36. [DOI] [PubMed] [Google Scholar]
- Pavez Lorie E, Li H, Vahlquist A, Torma H. 2009. The involvement of cytochrome P450 (CYP) 26 in the retinoic acid metabolism of human epidermal keratinocytes. Biochim Biophys Acta 1791:220–228. [DOI] [PubMed] [Google Scholar]
- Ross AC, Zolfaghari R. 2011. Cytochrome P450s in the regulation of cellular retinoic acid metabolism. Annu Rev Nutr 31:65–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taimi M, Helvig C, Wisniewski J, Ramshaw H, White J, Amad M, Korczak B, Petkovich M. 2004. A novel human cytochrome P450, CYP26C1, involved in metabolism of 9-cis and all-trans isomers of retinoic acid. J Biol Chem 279:77–85. [DOI] [PubMed] [Google Scholar]
- Topletz AR, Thatcher JE, Zelter A, Lutz JD, Tay S, Nelson WL, Isoherranen N. 2012. Comparison of the function and expression of CYP26A1 and CYP26B1, the two retinoic acid hydroxylases. Biochem Pharmacol 83:149–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
P450 26C1
In contrast with the other Family 26 P450 enzymes, P450 26C1 is expressed mainly during development, although this conclusion is based largely on animal models (Ross and Zolfaghari 2011). However, P450 26C1 mRNA has been detected not only in human fetal liver and braion but also in adult human brain, lung, liver, spleen, and testis, which are retinoid target tissues (Xi and Yang 2008).
The reactions catalyzed (Fig. 23) are similar as for P450 26B1, although recent work by Zhong et al. (2018) has defined some important differences in both the substrate and product profiles. Zhong et al. (2018) reported that P450 26C1 has a specificty constant (kcat/Km) up to 10-fold higher than P450 26A1 or 26B1 in oxidizing all-trans-4-oxo retinoic acid. There are issues in defining the roles of the three different Family 26 P450s in retinoic acid metabolism, in light of the differneces in intrinssic catalytic acticity, cellular localizaiton, the concentration of each P450 protein in each tissue, and the potentialy different interactions of the P450s with cellular retinoic acid binding proteins (Zhong et al. 2018).
Fig. 23.
C4- and C18-oxidations of all-trans-retinol catalyzed by P450s 26B1 and 26C1.
No information about the structure of this protein is available yet, although at least one homology model has been presented (Zhong et al. 2018).
References
- Helvig C, Taimi M, Cameron D, Jones G, Petkovich M. 2011. Functional properties and substrate characterization of human CYP26A1, CYP26B1, and CYP26C1 expressed by recombinant baculovirus in insect cells. J Pharmacol Toxicol Methods 64:258–263. [DOI] [PubMed] [Google Scholar]
- Osanai M, Lee GH. 2016. Elevated expression of the retinoic acid-metabolizing enzyme CYP26C1 in primary breast carcinomas. Med Mol Morphol 49:22–27. [DOI] [PubMed] [Google Scholar]
- Pan ST, Xue D, Li ZL, Zhou ZW, He ZX, Yang Y, Yang T, Qiu JX, Zhou SF. 2016. Computational identification of the paralogs and orthologs of human cytochrome P450 superfamily and the implication in drug discovery. Int J Mol Sci June 28;17(7). pii: ijms17071020. doi: 10.3390/ijms17071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross AC, Zolfaghari R. 2011. Cytochrome P450s in the regulation of cellular retinoic acid metabolism. Annu Rev Nutr 31:65–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taimi M, Helvig C, Wisniewski J, Ramshaw H, White J, Amad M, Korczak B, Petkovich M. 2004. A novel human cytochrome P450, CYP26C1, involved in metabolism of 9-cis and all-trans isomers of retinoic acid. J Biol Chem 279:77–85. [DOI] [PubMed] [Google Scholar]
- Xi J, Yang Z. 2008. Expressioh of RALDHs (ALDH1As) and CYP26s in human tissues and druing the neural differentiation of P19 embryonal carcinoma stem cell. Gene Expr Patterns 8:438–442. [DOI] [PubMed] [Google Scholar]
- Zhong G, Ortiz D, Zelter A, Nath A, and Isoherranen N (2018) CYP26C1 is a hydroxylase of multiple active retinoids and interacts with cellular retinoic acid binding proteins. Mol Pharmacol., mol 117111039; DOI: 10.1124/mol.117.111039, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
P450 27C1
Desaturation of retinol to 3,4-dehydroretinol (vitamin A2) (Figs. 21, 24) has been reported in preparations of human breast skin (Törmä and Vahlquist 1985) and keratinocytes in culture (Rollman et al. 1993; Andersson et al. 1994). UV light exposure was reported to increase the formation of 3,4-dehydroretinol in cultured human keratinocytes (Tafrova et al. 2012). Zebrafish P450 27C1 is an efficient retinol 3,4-desaturase and that this enzyme is expressed in the eyes of fish and amphibians where it acts to red-shift the visual chromophore (Enright et al. 2015). No bird or mammal has been shown to express P450 27C1 in the eye, but the human CYP27C1 gene encodes a retinoid 3,4-desaturase, P450 27C1, with selectivity for all-trans retinol. This enzyme catalyzes the production of 3,4-dehydroretinoids in human skin (Kramlinger et al. 2016; Johnson et al. 2017).
Fig. 24.
3,4-Desaturation of all-trans-retinol by P450 27C1.
The specificity constant for human P450 27C1 is relatively high, at least for mammalian P450 enzymes (kcat/Km 7.7 × 105 M-1 s-1 for all-trans-retinol), and clearly indicates that retinoids should be the natural substrates (Kramlinger et al. 2016; Johnson et al. 2017). The rate-limiting steps in the catalytic cycle are the first-electron reduction and C-H bond-breaking, the latter revealed by kinetic deuterium isotope effects (Johnson et al. 2017).
In fish and some amphibians, the enzyme is localized in the retinal epithelium, and synthesis of P450 27C1 allows these animals to change their visual spectra (Enright et al. 2015). However, in humans this (mitochondrial) enzyme is clearly localized in the skin, as shown by extensive proteomic analysis (Johnson et al. 2017).
Although there is little question that P450 27C1 is a skin retinoid desaturase (Kramlinger et al. 2016; Johnson et al. 2017), what is not clear is why such a large fraction of the retinoids in human skin (1/4) is desaturated (Vahlquist 1980, 1982; Vahlquist et al. 1982). Rats and mice are devoid of the 27C1 gene and thus provide no insight into the function.
References
- Andersson E, Bjorklind C, Torma H, Vahlquist A. 1994. The metabolism of vitamin A to 3,4-didehydroretinol can be demonstrated in human keratinocytes, melanoma cells and HeLa cells, and is correlated to cellular retinoid-binding protein expression. Biochim Biophys Acta 1224:349–354. [DOI] [PubMed] [Google Scholar]
- Enright JM, Toomey MB, Sato SY, Temple SE, Allen JR, Fujiwara R, Kramlinger VM, Nagy LD, Johnson KM, Xiao Y et al. 2015. Cyp27c1 red-shifts the spectral sensitivity of photoreceptors by converting vitamin A1 into A2. Curr Biol 25:3048–3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KM, Phan TTN, Albertolle ME, Guengerich FP. 2017. Human mitochondrial cytochrome P450 27C1 is localized in skin and preferentially desaturates trans-retinol to 3,4-dehydroretinol. J Biol Chem 292:13672–13687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramlinger VM, Nagy LD, Fujiwara R, Johnson KM, Phan TT, Xiao Y, Enright JM, Toomey MB, Corbo JC, Guengerich FP. 2016. Human cytochrome P450 27C1 catalyzes 3,4-desaturation of retinoids. FEBS Lett 590:1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan ST, Xue D, Li ZL, Zhou ZW, He ZX, Yang Y, Yang T, Qiu JX, Zhou SF. 2016. Computational identification of the paralogs and orthologs of human cytochrome P450 superfamily and the implication in drug discovery. Int J Mol Sci June 28;17(7). pii: ijms17071020. doi: 10.3390/ijms17071020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollman O, Wood EJ, Olsson MJ, Cunliffe WJ. 1993. Biosynthesis of 3,4-didehydroretinol from retinol by human skin keratinocytes in culture. Biochem J 293:675–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tafrova JI, Pinkas-Sarafova A, Stolarzewicz E, Parker KA, Simon M. 2012. UVA/B exposure promotes the biosynthesis of dehydroretinol in cultured human keratinocytes. Mol Cell Biochem 364:351–361. [DOI] [PubMed] [Google Scholar]
- Törmä H, Stenström E, Andersson E, Vahlquist A. 1991. Synthetic retinoids affect differently the epidermal synthesis of 3,4-didehydroretinol. Skin Pharmacol 4:246–253. [DOI] [PubMed] [Google Scholar]
- Törmä H, Vahlquist A. 1985. Biosynthesis of 3-dehydroretinol (vitamin A2) from all-trans-retinol (vitamin A1) in human epidermis. J Invest Dermatol 85:498–500. [DOI] [PubMed] [Google Scholar]
- Vahlquist A. 1980. The identification of dehydroretinol (vitamin A2) in human skin. Experientia 36:317–318. [DOI] [PubMed] [Google Scholar]
- Vahlquist A. 1982. Vitamin A in human skin: I. Detection and identification of retinoids in normal epidermis. J Invest Dermatol 79:89–93. [DOI] [PubMed] [Google Scholar]
- Vahlquist A, Andersson E, Coble BI, Rollman O, Törmä H. 1996. Increased concentrations of 3,4-didehydroretinol and retinoic acid-binding protein (CRABPII) in human squamous cell carcinoma and keratoacanthoma but not in basal cell carcinoma of the skin. J Invest Dermatol 106:1070–1074. [DOI] [PubMed] [Google Scholar]
- Vahlquist A, Lee JB, Michaelsson G, Rollman O. 1982. Vitamin A in human skin: II. Concentrations of carotene, retinol and dehydroretinol in various components of normal skin. J Invest Dermatol 79:94–97. [DOI] [PubMed] [Google Scholar]
An Orphan P450
P450 20A1
The function of P450 20A1 remains unknown; however, the sites of mRNA expression in human brain and the conservation among species may suggest possible neurophysiological function (Stark et al. 2008). P450 20A1 appears to be the only human P450 Family 20 member. P450 20A1 EST sequences have recently been reported from kidney, brain, placenta, and urogenital tissues, according to the NCBI UniGene database (http://www.ncbi.nlm.nih.gov/UniGene/). Orthologous nucleotide sequences have been found in a number of mammals, including rat (84% identity), mouse (85%), chimpanzee (99.6%), and chicken (72%) (http://drnelson.utmem.edu/CytochromeP450.html). None of these (putative) proteins has been studied to date.
In contrast to our report in Stark et al. (Stark et al. 2008), the Protein Atlas Database (www.proteinatlas.org) indicates widespread mRNA expression and possibly higher levels in endocrine tissues. Although expression in E. coli has been reported (Stark et al. 2008), the levels were low and in our own hands this has not been easy to repeat. Although there is still no information about a substrate, deletion of the CYP20A1 gene in zebrafish has been reported to cause a hyperactivity disorder (Lemaire et al. 2016). At this time P450 20A1 remains a true orphan P450 (Guengerich and Cheng 2011) in the sense of not having any identified function.
It has been noted that this protein lacks one amino acid of the conserved heme binding site. It also lacks the conserved I-helix motif AGX(D,E)T, and it has been suggested that its substrate may carry its own oxygen (www.proteinatlas.org). However, there is no actual evidence that this is the case.
References
- Guengerich FP, Cheng Q 2011. Orphans in the human cytochrome P450 superfamily: approaches to discovering functions and relevance in pharmacology. Pharmacol. Rev 63:684–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire B, Kubota A, O’Meara CM, Lamb DC, Tanguay RL, Goldstone JV, Stegeman JJ 2016. Cytochrome P450 20A1 in zebrafish: Cloning, regulation and potential involvement in hyperactivity disorders. Tox Appl Pharmacol 296:73–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marek CJ, Tucker, Koruth M, Wallace K, Wright MC 2007. Expression of CYP2S1 in human hepatic stellate cells. FEBS Lett 581:781–786. [DOI] [PubMed] [Google Scholar]
- Stark K, Guengerich FP 2007. Characterization of orphan human cytochromes P450. Drug Metab Rev 39:627–637. [DOI] [PubMed] [Google Scholar]
- Stark K, Wu ZL, Bartleson CJ, Guengerich FP 2008. mRNA distribution and heterologous expression of orphan cytochrome P450 20A1. Drug Metab Dispos 36:1930–1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
CONCLUSIONS AND OUTLOOK
The human P450 family, which contains 57 members, can be divided into two major groups. Members of the first group are involved largely in the metabolism of xenobiotic compounds such as drugs and environmental chemicals (Families 1–4 with 35 enzymes). Members of the second group are enzymes that predominately catalyze biotransformation and metabolism of endobiotic compounds (Families 5–51 with 22 enzymes). However, there is overlap in these groups, i.e. the Family 5–51 enzymes can oxidize some non-physiological compounds.
The enzymes from the first group have been extensively investigated ever since they were discovered. These enzymes have been the subject of a great number of research publications and review papers. Scientific interest in the enzymes belonging the gene Families CYP1, CYP2, and CYP3) was strengthened by the fact that these enzymes were found to be involved in most of the metabolism of clinically relevant drugs and toxicological responses to xenobiotic compounds, with some exceptions (e.g., Family 4 which in addition to some drugs catalyzes reactions in the metabolism of fatty acids and arachidonic acid). The enzymes belonging to the second group (P450s 5–51), reviewed in the present publication, preferentially catalyze biotransformations and metabolism of compounds that are of major importance in the physiological status of humans and consequently for human health. These enzymes catalyze the biosynthesis and metabolism of eicosanoids (P450s 5A1 and 8A1; Figs. 1–3), steroids (P450s 7A1, 11A1, 11B1, 17A1, 19A11, 21A1, 21A2, 27A1; Figs. 4A, 4B, 8, 9, 10, 11, 12 ), bile acids (P450s 7A1, 7B1, 8B1, 27A1, 39A1, 46A1; Figs 4A, 14A ), vitamin D3 (P450 24A1, 27A1, 27B1; Figs. 4A, 14B ) and retinoids (26A1, 26B1, 26C1, 27C1; Fig. 21) and also the formation of cholesterol, the starting material for the biosynthesis of steroids, bile acids, and vitamin D) from lanosterol (Figs. 4A and 17). Also in this group there are exceptions regarding drugs as substrates of the enzymes: P450 11B1, 11B2, 19A1, 21A2, and 46A1. P450 20A1 is considered as orphan enzyme in that its function still remains unknown.
Fig. 3.
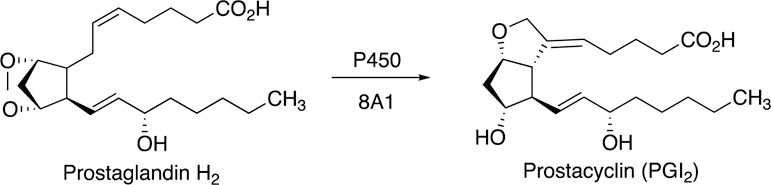
Conversion of prostaglandin H2 to prostacyclin (PGI2) by P450 8A1.
Fig. 8.
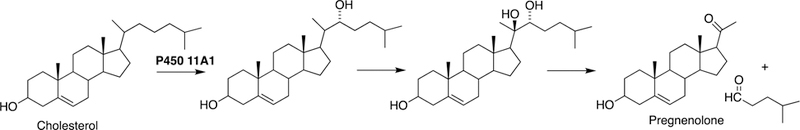
Three-step oxidation of cholesterol to pregnenolone by P450 11A1.
Fig. 10.
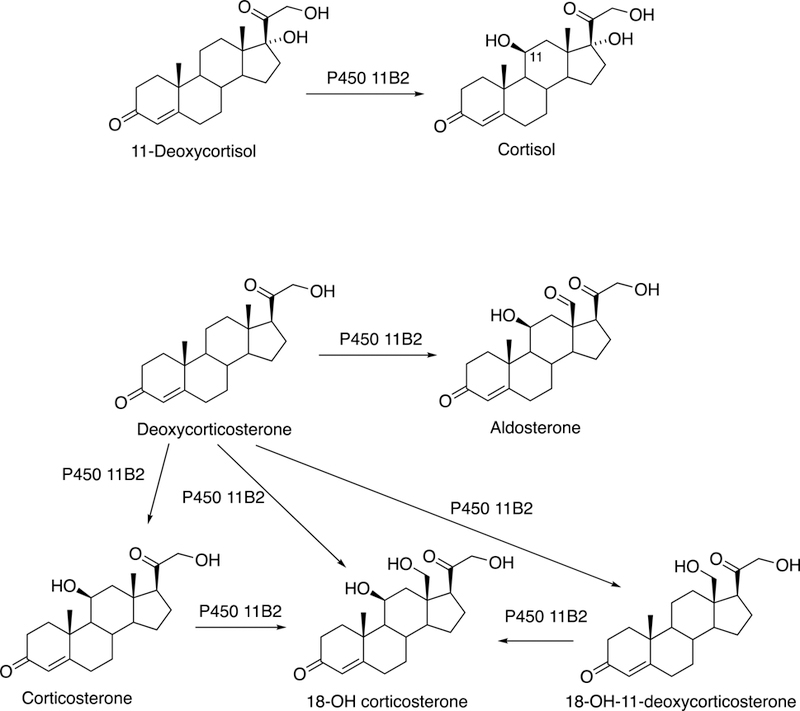
Steroid oxidations catalyzed by (human) P450 11B2.
As humans are exposed to a great number of chemicals through food, environmental contamination, and medicines, there may be results with a significant positive or negative effect on catalytic activityies of the enzymes or expression of mRNAs or proteins. The data presented in the tables provide information to serve as a guide when considering possible drug-drug, drug-environmental chemicals, or chemical-physiological compounds interactions in clinic or in every day life. The positive effect of inhibition of P450 enzyme activity on human health is examplified by inhibition of P450 19A1, i.e. lowering estrogen levels by chemicals. Different chemicals can exert such an effect on the enzyme such as drugs (e.g., imidazoles, antiepileptic drugs, cyclooxygenase-selective inhibitors), natural compounds (e.g., flavonoids, polyphenols, and chalcones), physiological compounds (4-hydroxyandrostendione), and environmental chemicals, as well as number of developed drug candidates of different types explored for treatment of breast cancer (Table 10). The following examples illustrate effects provoked by the action of drugs or other chemicals on the enzymes activity relating to human health: P450 8A1—elevated risk of hypertension and infarction when inhibited, chemo-preventive activity when induced (Table 2); P450 5A1—anti-platelet aggregation and anti-tumor activity when inhibited, pro-carcinogenic role when induced (Table 1); P450 11A1 and 17A1—prostate cancer treatment when inhibited (Tables 6 and 9, respectivaly). Analysis of the data from clinical samples showed that increases/or down-regulation of mRNA and/or protein expression of P450s 5A1, 7B1, 19A1, 26A1, 26B1, 26C1, 27A1, and 27B1 might be used as markers of the aggressive biological potential of tumors and association with poor patient survival. It has been suggested that up-regulation of these enzymes might be useful as tumor markers in the diagnosis and prognosis of different malignancies.
Table 10.
P450 19A1
| Properties | References |
|---|---|
| Physiological substrates: Testosterone, androstendione, 16α-androstendione, 16β-OH-androstenedione, 17β-estradiol, dihydrotestosterone | (Zharikova et al. 2007; Pan et al. 2016;Ghosh et al. 2018;Magistrato et al. 2017;Neunzig et al. 2017) |
|
Other substrates: | |
|
Drugs: L-α-Acetylmethadol, methadone, buprenorphine, tiboline, nortestosterone (norethisterone) | |
|
Sex hormone derivatives: 7α-Methyl-19-nortestosterone (trestolone), androst-5-ene-4,17-dione, androstadiene-3,17-dione, androst-4,6-diene and −1–4-diene-3,17-diones, 6-alkyl- and 6-ether analogs |
|
|
Other compounds: Dibenzylfluorescein, 7-methoxy-4-trifluoromethylcoumarin, 6α-methoxyandrost-1,4-diene-3,17-dione dervatives |
|
|
Inhibition: Lowered estrogen levels in breast and endometrial cancers, decreased estradiol secretion, anti-androgen activity (Vanden Bossche et al. 1994; Lønning and Eikesdal 2013; Ahmad and Shagufta 2015; Linardi et al. 2017; Kang et al. 2018) | |
|
Inhibitors: (Yumoto et al. 1976; Zhou et al. 1990; Kelloff et al. 1998; Ahmed and Amanuel 2000; Seralini and Moslemi 2001; Conley et al. 2002; Lønning and Eikesdal 2013; Bois et al. 2017;Sgrignani et al. 2014;Punetha et al. 2011;Caporuscio et al. 2011;Hirani et al. 2004; Hadizadeh et al. 2018) | |
|
Drugs : | |
|
Aminoglutethimide a (Ayub and Levell 1988; Kitawaki et al. 1990; Zhou et al. 1990; Campbell and Kurzer 1993; Wang C et al. 1994; White et al. 1999) | |
| Antiepileptic drugs b (lamotrigine, tiagabine, oxcarbazepine, phenobarbital, phenytion, ethosuximide, valproic acid) b (Jacobsen et al. 2008; Glister et al. 2012; Chen Y et al. 2015) | |
| Cyclooxygenase-1 selective inhibitors c (SC-560) (Brueggemeier et al. 2005; Diaz-Cruz et al. 2005) | |
| Cyclooxygenase-2 selective inhibitors c (celecoxib, niflumic acid, nimesulide, NS-398 and N-methyl anolog, SC-58125) (Brueggemeier et al. 2005; Diaz-Cruz et al. 2005; Su et al. 2008) | |
| 15-Deoxy-prostaglandin J2 (15-dPGJ2) b (Andrews et al. 2005) | |
| Exemstane d and derivatives (Görlitzer et al. 2006; Hong et al. 2007; Lønning and Eikesdal 2013; Kümler et al. 2016; Chottanapund et al. 2017) | |
| Fenretinide b (Andrews et al. 2005) | |
| Formestane b (Pokhrel and Ma 2011) | |
| Fulvestrant (Kümler et al. 2016) | |
| Fungicides, imidazoles e (e.g., bifonazole, fadrozole, tioconazole, clotrimazole; anastrozole, econazole, miconazole, isoconazole, ketonazole, letrozole, tinidazole (weak inhibitor), carbimazole (weak inhibitor), metronideazole (weak inhibitor), econazole, miconazole, mebendazole, thiabendazole (weak inhibitor), nimorazole (weak inhibitor), astemizole (weak inhibitor), mebendazole (weak inhibitor), propiconazole, (Ayub and Levell 1988, 1990; Ahmed et al. 1995; Sanderson JT et al. 2002; Zarn et al. 2003; Sanderson JT et al. 2004; Trösken et al. 2004; Trösken, Fischer, Volkel, et al. 2006; Kjeldsen et al. 2013; Lønning and Eikesdal 2013; Linardi et al. 2017;Di Nardo et al. 2018;Magistrato et al. 2017) | |
| Metformin c (Takemura et al. 2007; Rice et al. 2009) | |
| Non-steroidal anti-inflammatory drugs c (ibuprofen, piroxicam, indomethacin, nimesulide) (Brueggemeier et al. 2005) | |
| Norendoxifen (tamoxifen metabolite) b (Lu WJ, Desta Z, et al. 2012; Lu WJ, Xu C, et al. 2012) | |
| Opiates a (sufentanil, L-acetylmethadol, methadone, EDDP, (nor)buprenorphine, Oxycodone (weak inhibitor), codeine (very weak inhibitor) | |
| Pentazocine (very weak inhibitor) (Zharikova et al. 2006; Zharikova et al. 2007) | |
| Plomestane d (19-acetylenic androstenedione) (White et al. 1999) | |
| Rifampicin a (Kim et al. 2013) | |
| Rogletimide b (Vanden Bossche et al. 1994) | |
| Tamoxifen, raloxifene b (Fiorelli et al. 1999) | |
| Testolactone b (low potency inhibitor) (Dunkel 2006) | |
| Tiboline, ∆4,7α-methyl norethisterone metabolite a (Raobaikady et al. 2007) | |
| Tibolone and its ∆4, 7α-methyl norethisterone metabolite b (Raobaikady et al. 2007) | |
| Troglitazone plus LG100268 c (Mu, Yanase, Nishi, Waseda, et al. 2000; Yanase et al. 2001) | |
|
Natural compounds; | |
|
Alkylresorcinols c (Oskarsson and Ohlsson Andersson 2016) | |
| Cree plant extracts b (Tam et al. 2009) | |
| Cicer arietinum seeds extracts b (Zhang et al. 2018) | |
| Depsidones b (unguinol and aspergillusidone A) (Sureram et al. 2012; Chottanapund et al. 2017) | |
| Flavonoids, polyphenols, chalcones c (e.g., biochanin (potent inhibitor), butein, 4-hydroxychalcone, 8-prenylnaringenin, apigenin (at high doses), quercetin (at high doses), genistein (at high doses—or no inhibition), baicalein, biochanin A (at high doses), daidzein (at high doses), naringenin (potent inhibitor), chrysin (potent inhibitor), coumestrol, rotenone (potent inhibitor), luteolin, kaempferol, 7,8-benzoflavone, 7-hydroxyflavones, 7-hydroxyflavanone, 4´-hydroxyflavanone, 7,4´-dihydroxyflavone, flavone (weak inhibitor), flavanone (weak or no inhibition), quercetin (weak inhibitor), genistein 4´-methyl ether (weak inhibitor), formononetin (also no inhibition) (Kellis J.T., Jr. and Vickery 1984; Ibrahim and Abul-Hajj 1990; Campbell and Kurzer 1993; Wang C et al. 1994; Le Bail et al. 1998; Fiorelli et al. 1999; Jeong et al. 1999; Le Bail et al. 2000; Le Bail et al. 2001; Saarinen et al. 2001; Vinh et al. 2001; Almstrup et al. 2002; Sanderson JT et al. 2004; Lacey et al. 2005; Wang Y et al. 2005; Rice et al. 2006; van Meeuwen et al. 2007; van Meeuwen et al. 2008; McNulty et al. 2009; Lu DF et al. 2012) | |
| (−) Gossypol b (Zhong et al. 2010; Dong et al. 2016) | |
| Green tea extract catechins b (Satoh et al. 2002) | |
| Isodon excisus var. Coreanus b (Jeong et al. 2000) | |
| Isoflavone derivatives b (Su et al. 2005) | |
| Lignans b (nectandrin-B) (Filleur et al. 2001) | |
| Lignans b (lignan, O-demethylsecoisolariciresinol, demethoxysecoisolariciresinol, didemethylsecoisolariciresinol) (Adlercreutz et al. 1993; Wang C et al. 1994) | |
| Melatonin, resveratrol b (Chottanapund et al. 2014) | |
| Phytoestrogens c (Le Bail et al. 2000; Whitehead and Lacey 2003; Lacey et al. 2005) | |
| Phytoestrogens b (chrysin, biochanin A, naringenin, formononetin; at low concentrations) (Almstrup et al. 2002) | |
| trans-Phytol (SA-20), (22E)-ergosta-6,9,22-triene-3β,5α,8α-triol (SA-48) c (Guo et al. 2014) | |
| Red wine exctract b (procyanidin B dimers, resveratrol) (Eng et al. 2001, 2002; Eng et al. 2003; Wang Y et al. 2006; Wang Y and Leung 2007; Linardi et al. 2017) | |
| Sesquiterpene lactones a (guaianolides 10-epi-8-deoxycumambrin B, dehydroleucodin, ludartin (Blanco et al. 1997) | |
| UVA-ursi herbal products b (Chauhan et al. 2007) | |
| White button mushroom phytochemicals b (Grube et al. 2001; Chen S et al. 2006) | |
| Yikun neiyi wan c (Wang Q et al. 2009) | |
| Zaraleone b (Lacey et al. 2005) | |
|
Physiological compounds: | |
|
17β-Estradiol c (in women) (Dieudonné et al. 2006) | |
| 4-Hydroxyandrostendione f (Abul-Hajj 1983; Ayub and Levell 1988, 1990; Zhou et al. 1990; White et al. 1999; Vinggaard et al. 2000; Almstrup et al. 2002; Numazawa et al. 2002) | |
| Leptin c (in women) (Dieudonné et al. 2006) | |
|
Physiological condition: | |
|
Hypoxia c (Jiang et al. 2000; Jiang and Mendelson 2005) | |
|
Other compounds, including drug candidates: | |
|
(±)-Abyssinone II and analogues b (Maiti et al. 2007) | |
| 7-(α-Azolylbenzyl)-1H-indoles and indolines b (Marchand et al. 2003) | |
| 4-(Aryl/heteroaryl)-2-(pyrimidin-2-yl)thiazole derivatives b (Ertas et al. 2018) | |
| 3–(1H-imidazol-1-yl)-2H-chromen-2-one a (Niinivehmas et al. 2018) | |
| Atrazine c (Pogrmic-Majkic et al. 2018) | |
| 1-[(Benzofuran-2-yl)(phenylmethyl)pyridine, -imidazole, and -triazole inhibitors b (Saberi et al. 2006) | |
| Bisphenol A, lindane b (longer incubation time, up to 18 h) (Almstrup et al. 2002; Nativelle-Serpentini et al. 2003; Benachour et al. 2007; Chu et al. 2018) | |
| 4´-tert-Butyl-quinolin-4-one b (Sanderson JT et al. 2004) | |
| C19 steroidal 17-oxime analogs b (Pokhrel and Ma 2011) | |
| Carbon black nanoparticles b (Simon et al. 2017) | |
| Chlordecone b (Benachour et al. 2007) | |
| Cinnamyl triazoles b (McNulty et al. 2014) | |
| Dibutyl phthalate c (Mauger 1989) | |
| Diethylstilbestrol b (Benachour et al. 2007) | |
| N,N-Disubstituted-5-aminopyrimidine derivatives b (Okada et al. 1997) | |
| Estrone and estradiol derivatives (2-, 4-, or 6-substituted) b (Numazawa et al. 2005) | |
| 19-(Ethyldithio)-androst-4-ene-3,17-dione (OrG 30958) b (Geelen et al. 1991) | |
| Fungicides b (prochloraz, imazalil, flusilazole, fenbuconazole, propioconazole, difenoconazole, penconazole, fenarimol, triadimenol, triadimefon, toxaphen, heptachlor, dicofol, ziram, vinclozolin, tributyltin oxide, myclobutanil) (Vinggaard et al. 2000; Heneweer et al. 2004; Trösken et al. 2004; Laville et al. 2006; Trösken, Fischer, Völkel, et al. 2006; Benachour et al. 2007; Chen L et al. 2017) | |
| Herbicides b (atrazine) (Benachour et al. 2007) | |
| ICI 182,780 c (Almstrup et al. 2002) | |
| 5-Hydroxy-BDE47, 6-hydroxy-BDE47, 6-hydroxy-BDE99) b (Cantón et al. 2005; Cantón et al. 2008) | |
| Imidazole,triazole and pyridine based compounds b (Ahmed et al. 1995; Saberi et al. 2006) | |
| 1-(1H-Imidazol-1-yl)methyl-6-chloro-9H-xanthen-9-one, lactones (CRI-7, 8, and 9) | |
| Insecticides b (dieldrin, toxaphenem)(Almstrup et al. 2002; Laville et al. 2006) | |
| Lactone derivatives b (Sanderson T et al. 2008) | |
| MDL 18,962 d (19-acetylenic androstenedione, and analogs) (Johnston et al. 1990) | |
| 4-Methoxy-androst-4-ene-3,17-dione (White et al. 1999) | |
| 2-Methoxyestrone-3-O-sulfamate b (Purohit et al. 2005) | |
| Mono-(2-ethylhexyl) phthalate f (Noda et al. 2007) | |
| Nonylphenol b (Almstrup et al. 2002; Benachour et al. 2007) | |
| α-Naphthoflavone and derivatives b (Kellis J. T., Jr. et al. 1986; Campbell and Kurzer 1993; Sanderson JT et al. 2004) | |
| Organochlorines c (e.g. TCDD, PCB126) (Drenth et al. 1998; Letcher et al. 1999) | |
| Pesticides b (prochloraz (IC(50) <1 μM), fenbuconazole (IC50 1.1 μM), propiconazole (IC50 1.5 μM) fenarimol (IC50 3.3 μM); toxaphen (10 μM) and heptachlor (10 μM) only after 24 h exposure), epoxyconazole, endosulfan (weak inhibitor) (Andersen et al. 2002; Heneweer et al. 2004; Laville et al. 2006) | |
| Pesticides j (i-butyl-, tributyl-, and triphenyltin chloride) (Sanderson JT et al. 2002; Benachour et al. 2007) | |
| Pesticides j (p,p´-DDT, o,p´-DDT, p,p´-DDE, o,p-DDE) (Sanderson JT et al. 2002; Wójtowicz et al. 2007) | |
| Polybrominated diphenyl ethers, brominated flame retardants b, (e.g., 2-hydroxy-BDE, 2,4,6-tribromophenol, 5-hydroxy-BDE47, 6-hydroxy-BDE47, and 6-hydroxy-BDE9) (Cantón et al. 2005; Cantón et al. 2008) | |
| Polychlorinated biphenyls c (Aroclor 1254, PCB39) (Benachour et al. 2007; Li 2007) | |
| Protein kinase inhibitors PD98059, KN-93m H-89 b (Watanabe et al. 2006) | |
| 2-Pyridinyl-substituted γ-naphthoflavones (at concentrations >30 μM) b (Sanderson JT et al. 2004) | |
| Pyridine-substituted thiazolylphenol derivatives (non-steroidal triazole bioisosteres) b (Ertas et al. 2018) | |
| Pyridoglutethimide a (Kitawaki et al. 1990) | |
| Pyridyl-substituted indanones, indans, and tetralins b (Hartmann et al. 1994) | |
| Pyridyl-substituted tetrahydrocyclopropa[a]naphthalenes b (Hartmann et al. 1995) | |
| Ro41–5253 f (RARα-selective antagonist) (Hartmann et al. 1995) | |
| RU486 c (antiglucocorticoid and antiprogestin) (Schmidt and Löffler 1997) | |
| Steroidal derivatives d (6-oxoandrostenedione and its 19-hydroxy analog, 10α-acetoxyestr-5-ene-7,17-dione, androst-5-ene-4,7,17-trione, and 17α-ethynyl-19-nortestosterone) (Hartmann et al. 1995; Numazawa and Yamaguchi 1998; Numazawa et al. 1998; Numazawa and Yamada 1999; Numazawa and Yamaguchi 1999; Numazawa et al. 2000; Numazawa et al. 2002; Numazawa et al. 2003) | |
| Sulfonanilide analogues c (Su et al. 2006) | |
| Synthetic lactones (TM-7, TM-8, TM-9 b (Cantón et al. 2008) | |
| Thioglutethimide derivatives a (Bednarski and Hartmann 1993) | |
| Triazole derivatives e (CGS 20267, CGS 47645, R 76 713, ICI D1033) (Vanden Bossche et al. 1994) | |
| Triazolylflavans b (Yahiaoui et al. 2004) | |
|
Induction: Over-expression associated with increased risk of developing estrogen-dependent mammary tumors | |
|
Inducers: | |
|
Drugs: | |
|
Dexamethasone g (Zhao et al. 1996; Heneweer et al. 2004; Enjuanes et al. 2005; Watanabe et al. 2005) | |
| Insulin h (Rice et al. 2006) | |
| Mibolerone i (Stillman et al. 1991) | |
| Opiates g (morphine, heroin, hydromorphone, oxymorphone, hydrocodone, propoxyphene, meperidine, levorphanol, dextrorphan, (−)-pentazocine, naloxone, naltrexone, sufentanil) (Zharikova et al. 2007; Cui et al. 2011) | |
| Pacitaxel (taxol) i (Morinaga et al. 2004) | |
|
Natural compounds: | |
|
1α,25-Dihydroxyvitamin D3 (calcitriol) i (Yanase et al. 2003; Enjuanes et al. 2005; Lou et al. 2005; Pino et al. 2006; Barrera et al. 2007) | |
| All-trans-Retinoic acid, 9-cis-retinoic acid h (Zhu et al. 2002) | |
| Cordyceps sinensis mycelium h (Huang et al. 2004) | |
| Egonol gentiobioside, egonol gentiotrioside i (Lu D et al. 2012) | |
| Epidermal growth factor h (Watanabe et al. 2006) | |
| Flavonoids (quercetin, genistein at 10 μM) i (Sanderson JT et al. 2004) | |
| Forskolin g (Watanabe et al. 2005) | |
| Buthus martensi Karsch (BMK) extract i (Jin et al. 2006) | |
| Zeranol g (Zhong et al. 2010) | |
|
Physiological compounds: | |
|
Ceramide h (Zhao et al. 1996) | |
| Collagen h (Tan et al. 2015) | |
| Cortisol platelet-derived growth factor BB h (Schmidt and Löffler 1997) | |
| Epidermal growth factor i (Jin et al. 2006) | |
| 17β-Estradiol g (in men) (Dieudonné et al. 2006) | |
| Interleukin 6 i (Lou et al. 2005) | |
| Leptin g (in men) (Dieudonné et al. 2006; Pino et al. 2006); | |
| Progesterone h (Purohit et al. 2005) | |
| Prostaglandins (PG) A1, PGB2, PGD2, PGE1, PGE2 h (Heneweer et al. 2004; Watanabe et al. 2006; Chen D et al. 2007; Tan et al. 2015) | |
| Protein kinase h (Watanabe et al. 2006) | |
| Redox-regulated transcription factor, NRF2h (Muralimanoharan et al. 2018) | |
| Retinoic acid receptor system RAR-RXR heterodimer ligands combined h (TTNPB and LG100268) (Mu, Yanase, Nishi, Hirase, et al. 2000) | |
| Testosterone, 5α-dihydrotestosterone i (Stillman et al. 1991) | |
| Transforming growth factor-β1 i (Stillman et al. 1991) | |
| Tumor necrosis factor-α h (Zhao et al. 1996) | |
|
Physiological conditions and illnesses: | |
|
Bladder cancer (tumor related stroma) g (Nguyen et al. 2017; Wu et al. 2018) | |
|
Other compounds, including drug candidates: | |
|
2-Phenylbenzo[b]furans i (Pu et al. 2016) | |
| Bisphenol A, lindane b (shorter time pre-incubation) (Nativelle-Serpentini et al. 2003) | |
| Brominated flame retardants i (2,4,6-tribromophenol) (Cantón et al. 2005) | |
| 8-Bromo-cyclic adenosine monophosphate (Heneweer et al. 2004) | |
| Ethanol i (Eng et al. 2002; Linardi et al. 2017) | |
| Fungicides—benzimidazole i (benomyl, and metabolite, carbendazim) (Morinaga et al. 2004) | |
| Fungicides h (vinclozolin, prochloraz) (Sanderson JT et al. 2002; Rieke et al. 2014) | |
| Herbicides, triazines h (atrazine, simazine, propazine, and metabolites-atrazine-desethyl, atrazine-desisopropyl) (Sanderson JT et al. 2000; Sanderson JT et al. 2001) | |
| 2-Hydroxy-BDE-123, 2-MeO-BDE-123 i (Song et al. 2008) | |
| Lactones (CRI-1, CRI-4 or Vioxx, CRI-11 and CRI-13) b (Sanderson T et al. 2008) | |
| Lindane, bisphenol A i (shorter incubation time, up to 6 h) (Nativelle-Serpentini et al. 2003) | |
| 3-Methylcholanthrene g (weak inducer) (Ning et al. 2008) | |
| 1-Methyl-3-isobutylxanthine h (Sanderson JT et al. 2002) | |
| Pesticides h (aldrin, chlordane, cypermethrin, parathion-methyl, endosulfan, methoxychlor, oxadiazon, metolachlor and atrazine after 24 h of exposure; tributyltin at 1 nM and 3 nM after 2 h and 24 h of exposure) (Laville et al. 2006) | |
| Pesticides i (terbuthylazine, propiconazolem prothioconazole-weak inducers) and pesticide mixtures i (Kjeldsen et al. 2013) | |
| Pesticides, herbicides h (atrazine, aldrin, chlordane, cypermethrin, parathion-methyl, endosulfan, methoxychlor, oxadiazon, metolachlor, terbuthylazine, propiconazole, prothioconazole, methomyl, pirimicarb, propamocarb, iprodion) (Andersen et al. 2002; Laville et al. 2006; Kjeldsen et al. 2013) | |
| Phorbol 12-myristate 13-acetate i (Heneweer et al. 2004; Jin et al. 2006) | |
| Polybrominated diphenyl ethers h (e.g. 2-hydroxy-BDE-123, 2-MeO-BDE-123 ) (Song et al. 2008) | |
| Polychlorinated biphenyls h (PCB126, at high concentrations)(Li 2007) | |
| 2-Pyridinyl-substituted γ-naphthoflavones (at concentrations <1 μM) (Sanderson JT et al. 2004) | |
| Ro40–6055 h (retinoic acid receptor α (RARα)-selective activator) (Zhu et al. 2002) | |
| Steroidal derivatives (6-oxoandrostenedione) | |
| 2,4,6-Tribromophenol, BDE38 i (Cantón et al. 2005) | |
Footnotes
Competitive inhibition, binding to enzyme active site
Decreased/suppressed/inhibited activity/product formation
Reduced/suppressed mRNA and/or protein level/expression and activity
Suicide inactivator(s)
Competitive inhibition, ligand binding
Gene downregulated/suppressed
Up-regulation of biosynthesis, increased expression of protein
Increased transcription/mRNA/protein expression/levels /and/or catalytic activity
Increased activity, and/or increased product formation
At cytotoxic concentrations
Table 2.
P450 8A1
| Properties | References |
|---|---|
| Physiological substrates: Prostaglandins (PG) ( H1, H2, H3, G2), 15-keto-prostaglandin H2, 6-keto-prostaglandin F1α, 15-hydroperoxyeicosatetraenoic acid, 10-hydroperoxyoctadeca-8,12-dienoic acid | (Hecker and Ullrich 1989; Ullrich and Hecker 1990; Yeh et al. 2005; Chiang et al. 2006; Yeh et al. 2007; Cathcart et al. 2010; Nakayama 2010; Pan et al. 2016) |
|
Function: Prostacyclin synthase, isomerization of prostaglandin H2 to prostacyclin (PGI2) (Fig. 3), inhibition of platelet activation and aggregation and induction of vasodilation. Believed to have anti-tumor effects as overexpression has been shown to be chemopreventive in a murine model of the disease, suggesting that the expression and activity of this enzyme may protect against tumor development. |
|
|
Inhibition: Risk of essential hypertension, myocardial infarction and cerebral infarction (Chiang et al. 2006) | |
|
Inhibitors: | |
|
Drugs: | |
|
Actinomycin D a (Camacho et al. 2008) | |
| Cycloheximide a (Camacho et al. 2008) | |
| Cyclosporine a (Sharanek et al. 2015) | |
| Miconazole, clotrimazole b (Yeh et al. 2005) | |
| Minoxidil b (Yeh et al. 2005; Li et al. 2008) | |
| Rofecoxib c (Griffoni et al. 2007) | |
| Simvastatin a (Skogastierna et al. 2013) | |
| Tranylcipromine b (Ding et al. 2002) | |
|
Other compounds: | |
|
Imidazole derivatives (4-phenylimidazole, 1-phenylimidazole, 1-benzylimidazole) b (Yeh et al. 2005) | |
| U46619, U51605, or U44069 (PGH2 analogues) b (Wada et al. 2004; Yeh et al. 2005; Li et al. 2008; Chao et al. 2011) | |
|
Induction: Enhanced activity in tumor cells, potential anti-neoplastic and chemopreventive activity | |
|
Inducers: | |
|
Natural compounds: | |
|
13-cis-Retinoic acid, 9-cis-retinoic acid, all-trans- retinoic acid d (Camacho et al. 2008) | |
| 13-cis-Retinoic acid, 9-cis-retinoic acid, all-trans- retinoic acid d (Camacho et al. 2008) | |
| Curcumin d (Tan et al. 2011) | |
| Grape seed procyanidin extract d (Mao et al. 2016) | |
| 13-cis-Retinoic acid, 9-cis-retinoic acid, all-trans- retinoic acid d (Camacho et al. 2008) | |
|
Physiological compounds: | |
|
17β-Estradiol d (Korita et al. 2004) | |
|
Physiological condition: | |
|
Hypoxia e (Camacho et al. 2011; Wang et al. 2013) | |
| Pregnancy d (Okahara et al. 1998) | |
| Shear stress e (Korita et al. 2002) | |
Footnotes:
Reduced/suppressed mRNA and/or protein level/expression and activity
Competitive inhibition, ligand binding
Decreased/suppressed/inhibited activity/product formation
Up-regulation of biosynthesis, increased expression of protein
Increased transcription/mRNA/protein expression/levels /and/or catalytic activity
Table 1.
P450 5A1
| Properties | References |
|---|---|
| Physiological substrates: Prostaglandin (PG) H2, PGH1, 8-iso-PGH2, 13(S)-hydroxy-PGH2, 15-keto-PGH2, PGG2, PGH3 15-hydroperoxyeicosatetraenoic acid, charged lipids (phosphatidylserine and phosphatidylethanolamine) | (Hecker and Ullrich 1989; Ullrich and Hecker 1990; Wang et al. 1996; Yeh et al. 2007; Cathcart et al. 2010; Pan et al. 2016) |
|
Function: Thromboxane A2 synthase, conversion of PGH2 to TXA2 (Figs. 1, 2), stimulates platelet secretion, platelet aggregation and vasoconstriction. Demonstrated to have a pro-carcinogenic role in a variety of cancers. |
|
|
Inhibition: Decrease of thromboxane A2 levels, antiplatelet aggregation, and thrombosis; increase of of PGI2 generation in vivo; antitumor activity by inhibition tumor cell growth, invasion, metastasis, and angiogenesis (de Leval et al. 2003; Dogne et al. 2004) | |
|
Inhibitors a | |
|
Drugs: | |
|
Cinnamophilin (Yu et al. 1994) | |
| Furegrelate, ozagrel (Moussa et al. 2008) | |
| Itraconazole, ketoconazole, terbinafine (Kanda and Watanabe 2006; Kanda et al. 2011) | |
| Miconazole (Steinhilber et al. 1990) | |
| Picotamide and derivatives (Dogne et al. 2004; Liu 2015) | |
| Picotamide, dazoxiben, dazmagrel, pirmagrel, ozagrel, isbogrel, ridogrel, terbogrel, camonagrel, samixogrel, uregrelate, furegrelate (Rowe et al. 2000; Schuster and Bernhardt 2007; Davi et al. 2012) | |
| Terbogrel (Muck et al. 1998; Soyka et al. 1999; Michaux et al. 2001; Michaux et al. 2003; Dogne et al. 2004) | |
| Torasemide and derivatives (de Leval et al. 2006) | |
|
Natural compounds: | |
|
Aloe vera glycoprotein fraction (Yagi et al. 2003) | |
| Cinnamophilin (Yu et al. 1994) | |
| Nicotine, cotinine and methylnicotine (Goerig and Habenicht 1988; Goerig et al. 1992; Saareks et al. 1998) | |
| Onion extract (Moon CH et al. 2000) | |
|
Other compounds, including drug candidates a (Dogne et al. 2000; Dogne et al. 2004; de Leval et al. 2006; Dogne et al. 2006; Davi et al. 2012) | |
|
(E)-3-(1,4-Benzoquinonyl)-2-[(3-pyridyl)-alkyl]-2-propenoic acid derivatives (Hibi et al. 1996) | |
| (E)-3-[4-(3-Pyridylmethyl) phenyl]-2-methylacrylate, OKY-1581 (Oketani et al. 2001) | |
| (E)-3-[p-(1H-Imidazol-1-ylmethyl)phenyl]-2-propenoic acid, OKY-046 (Hiraku et al. 1986) | |
| 1-Imidazolyl(alkyl)-substituted di- and tetrahydroquinolines and pyridines (Ackerley et al. 1995; Hartmann and Frotscher 1999; Jacobs et al. 2000) | |
| 4-(2-Phenyltetrahydrofuran-3-yl) benzene sulfonamide analogs (Sekhar et al. 2009) | |
| 6,6-Disubstituted hex-5-enoic acid derivatives (Soyka et al. 1994) | |
| Benzene sulfonylurea derivatives (Dogne, Rolin, et al. 2001; Rolin et al. 2001; de Leval et al. 2003; Michaux et al. 2003; Rolin et al. 2004; Ghuysen et al. 2005; Hanson et al. 2005; de Leval et al. 2006; Jarrar et al. 2013) | |
| Dioxane derivatives (Ackerley et al. 1995; Faull et al. 1995; Hartmann and Frotscher 1999; Jacobs et al. 2000) | |
| DP-1904, imidazolylmethyl-tetrahydronaphthalene derivative (Trochtenberg et al. 1992) | |
| E3040, benzothiazole (Oketani et al. 2001) | |
| FK070 (KDI-792) (pyrrolidine derivative) (Uematsu et al. 1996) | |
| Guanidine derivatives (Soyka et al. 1999) | |
| Imidazo[1,5-a]pyridines (Ford et al. 1985) | |
| Imidazolyl-substituted tetralones (Hartmann et al. 1996) | |
| MED 27, theophylline derivative (Tubaro et al. 1996) | |
| NV-52, a synthetic flavonoid derivative (Howes et al. 2007) | |
| Oxazolecarboxamide-substituted ω-phenyl-ω-(3-pyridyl)alkenoic acid derivatives (Takeuchi et al. 1998) | |
| Oxazolexarboxamide derivatives (Takeuchi et al. 1998) | |
| Ridogrel diazine derivatives (Heinisch et al. 1996) | |
| Sulfonylcyanoguanidine compounds (Dogne, Wouters, et al. 2001; Michaux et al. 2003) | |
| Tetrahydronaphthalene derivatives (Hartmann et al. 1996; Wachter et al. 1996; Cimetiere et al. 1998) | |
| Tetrahydroquinolines and analogues (Ackerley et al. 1995; Hartmann and Frotscher 1999; Jacobs et al. 2000) | |
| ω-Disubstituted alkenoic acid derivatives (Soyka et al. 1994) | |
|
Induction: Enhanced cholesterol biosynthesis | |
|
Inducers: | |
|
Natural compounds: | |
|
Biochanin A d (Moon et al. 2007) | |
| Phorbol b (Ihara et al. 1992) | |
|
Physiological compounds: | |
|
Cytotrophoblasts c (Ding et al. 2002) | |
|
Physiological conditions and illnesses: | |
|
Human colorectal carcinoma d (Sakai et al. 2006) | |
| Hypoxia c (Rowe et al. 2000) | |
| Papillary thyroid carcinoma b (Casey et al. 2004; Kajita et al. 2005) | |
| Pituitary adenomas and carcinoma d (Onguru et al. 2004) | |
| Prostate carcinoma b (Nie et al. 2004) | |
Footnotes:
Decreased/suppressed/inhibited activity/product formation
Increased transcription/mRNA/protein expression/levels /and/or catalytic activity
Increased activity, and/or increased product formation
Up-regulation of biosynthesis, increased expression of protein
Table 6.
P450 11A1
| Properties | References |
|---|---|
| Physiological substrate: Cholesterol, cholesterol 25- and 20ξ-hydroperoxides, cholesterol 25-hydroperoxide | (Tuckey and Cameron 1993b; Tuckey, Janjetovic, et al. 2008; Tuckey, Nguyen, et al. 2008; Mast et al. 2011; Pagotto et al. 2011; Slominski A. T. et al. 2014; Tuckey et al. 2014; Mosa et al. 2015; Slominski Andrzej T. et al. 2015; van Lier et al. 2015; Acimovic et al. 2016; Pan et al. 2016; Schiffer et al. 2016) |
|
Other substrates: Lathosterol, zymostenol, desmosterol, 7-dehydrocholesterol, plant sterols (including campesterol and β-sitosterol), 7-dehydrocholesterol, ergosterol, lumisterol 3, vitamins D3 and D2, 1α-hydroxyvitamin D3, turinabol, androstenedione, deoxycorticosterone, dehydroepiandosterone, testosterone (6β-hydroxylation) | |
|
Function: Conversion of cholesterol to pregnenolone (P450scc) (Figs. 4, 8) |
|
|
Inhibition: Block synthesis of androgens (e.g., possible application in prostate cancer therapy) | |
|
Inhibitor: | |
|
Drugs: | |
|
Aminoglutethimide a (Fassnacht et al. 2000; Johansson et al. 2002) | |
| Cisplatin a (Garcia et al. 2012) | |
| Cycloheximide b, puromycin b (Picado-Leonard et al. 1988) | |
| Etomidate a (Fassnacht et al. 2000) | |
| Ketoconazole c, posaconazole c, carbenoxolone c, selegiline c (Johansson et al. 2002; Mast et al. 2013) | |
| Metyrapone a (Fassnacht et al. 2000) | |
| Mitotane b (Schuster and Bernhardt 2007; Lin et al. 2012) | |
| Nitric oxide a (Drewett et al. 2002) | |
| Omeprazole a (Dowie et al. 1988) | |
| Turinabol b (Schiffer et al. 2016) | |
|
Natural compounds: | |
|
Digoxin, ouabain b (Kau et al. 2005) | |
| Gossypol a (Ye et al. 2011) | |
|
Physiological compounds: | |
|
22-Ketocholesterol d (Lambeth 1983) | |
| 24-Hydroxycholesterol (at high concentrations) d (Tuckey and Cameron 1993b) | |
| 25-Hydroxycholesterol (at high concentrations) d (Tuckey and Cameron 1993b, 1993a) | |
| Interferon-β b (van Koetsveld et al. 2013) | |
|
Other compounds: | |
|
2-(4-Pyridylmethyl)-1-indan 15 a, HB60 (Hartmann et al. 2003) | |
| 2,3,7,8-Tetrabromodibenzo-p-dioxin | |
| 2,6-Dibromophenol b (Ding et al. 2007) | |
| Dibutyl phthalate b (Mauger 1989) | |
| Lindane b (Ye et al. 2011) | |
| Methoxychlor and its metabolite 2,2-bis(p-hydroxyphenyl)-1,1,1-trichloroethane b (Wu et al. 2013) | |
| Paraquat a (Milczarek et al. 2016) | |
| Pentachlorobenzene b (Gregoraszczuk et al. 2014) | |
| Perfluorooctanoic acid e (Kraugerud et al. 2011) | |
|
Induction: Stimulation of androgen biosynthesis | |
|
Inducers: | |
|
Drugs: | |
| Clobenpropit f, dexmedetomidine f, desogestrel f, pemirolast f, tizanidine f (Mast et al. 2013) | |
| Valproate g (Nelson-DeGrave et al. 2004) | |
|
Natural compounds: | |
|
Alkylresorcinols h (Oskarsson and Ohlsson Andersson 2016) Cordyceps sinensis mycelium h (Huang et al. 2004) | |
| Forskolin h (Breckwoldt et al. 1996; Sawetawan et al. 1996; Nelson-DeGrave et al. 2004; Asif et al. 2006) | |
|
Physiological compounds: | |
|
17β-Estradiol h (Babischkin et al. 1997) | |
| 25-Hydroxycholesterol h (Babischkin et al. 1997) | |
| Adrenocorticotropic hormone (ACTH) h (Di Blasio et al. 1987; Ghayee and Auchus 2007) | |
| Cardiolipin f (Kisselev et al. 1999) Cyclic AMP g (Ghayee and Auchus 2007; Aumo et al. 2010) | |
| Cyclic AMP-inducible factors g (Aumo et al. 2010) | |
| Follicle-stimulating hormone, choriogonadotropin g (Breckwoldt et al. 1996) | |
| Insulin-like growth factors I and II h (Mesiano et al. 1997) | |
| Transcription factors g (SF-1, Sp1, AP-2, TReP-132, LBP-1b, LBP-9, AP-1, NF-1, EtsSF-1, c-Jun) (Guo et al. 2003; Guo et al. 2007; Shih et al. 2011) | |
|
Other compounds: | |
|
Bisphenol A h (Lan et al. 2015) | |
| Hexachlorobenzene h (Gregoraszczuk et al. 2014) | |
| Polybrominated biphenyls, brominated flame retardants c, polybrominated dibenzofurans g (Ding et al. 2007) | |
| Polybrominated diphenyl ethers (e.g., 2´-hydroxy-2,3´,4,5´-tetrabromodiphenyl ether, 2´-hydroxytetrabromodiphenyl ether-68) g (Song et al. 2008) | |
Footnotes:
Decreased/suppressed/inhibited activity/product formation
Reduced/suppressed mRNA and/or protein level/expression and activity
Competitive inhibition, ligand binding
Competitive inhibition, binding to enzyme active site
Gene downregulated/suppressed
Increased activity, and/or increased product formation
Up-regulation of biosynthesis, increased expression of protein
Increased transcription/mRNA/protein expression/levels /and/or catalytic activity
Table 9.
P450 17A1
| Properties | References |
|---|---|
| Physiological substrates; Progesterone, 17α-hydroxyprogesterone,17α-hydroxypregnenolone, 16α-hydroxyprogesterone | (Pikuleva et al. 2008; Petrunak et al. 2014; Pan et al. 2016; Gonzalez and Guengerich 2017; Yadav et al. 2017; Gonzalez et al. 2018) |
|
Function: Biosynthesis of androgens and estrogens (cortisol), 16α-hydroxylase, 17α-hydroxylase, 17,20-lyase (Figs. 4, 11); controls the levels of mineralocorticoids influencing blood pressure, glucocorticoids involved in immune and stress responses, as well as androgens and estrogens involved in development and homeostasis of reproductive tissues |
|
|
Inhibition: Lower androgen level and production in prostate cancer, treating diseases caused by cortisol overproduction; increases the pool of precursors for mineralocorticoid production and blocks P450 17A1-mediated production of glucocorticoids (Leroux 2005; Bird and Abbott 2016; Bonomo et al. 2016) | |
|
Inhibitors: (Ahmed 1999; Clement et al. 2003) | |
|
Drugs: | |
|
Abiraterone and analogsa (Pinto-Bazurco Mendieta et al. 2008; Ferraldeschi and de Bono 2013; Auchus R. J. et al. 2014; Petrunak et al. 2014; Yin and Hu 2014; Schroeder et al. 2016; Malikova et al. 2017) | |
| Abiraterone, galeterone, seviteronel, orteronela (Yamaoka et al. 2012; Stein et al. 2014; Bird and Abbott 2016; Petrunak et al. 2017) | |
| Azole drugsa (e.g., ketoconazole, liarozole—weak inhibitors) (Ayub and Levell 1989; Ideyama et al. 1998; Ahmed 1999; Clement et al. 2003; Bird and Abbott 2016) Ketoconazole (at high concentrations) (De Coster et al. 1986; Ayub and Levell 1989; Engelhardt and Weber 1994; Johansson et al. 2002) | |
| Rifampicinb (Kim et al. 2013) | |
| Valproic acidc (at high concentrations) (Glister et al. 2012) | |
|
Natural compounds: | |
|
1α,25-Dihydroxyvitamin D3 d (Lundqvist et al. 2010) | |
| Daidzein, hesperitin, resveratrol d (Lin et al. 2014) | |
| Polyphenols d (e.g., apigenin, aringenin, eriodictyol, genistein) d (Hasegawa et al. 2013) | |
|
Physiological compounds: | |
|
Bone morphogenetic protein 4 c (Rege et al. 2015) | |
| Epidermal growth factorc (Doi et al. 2001) | |
| Interferon-βc (van Koetsveld et al. 2013) | |
|
Other compounds, including drug candidates: | |
|
16- and 17-Azolyl steroids a (Njar et al. 1998) | |
| 5-(Phenoxymethyl)-1,3-dioxane analogs d (Schroeder et al. 2016) | |
| 6-Hydroxy-2,2´,4,4´-tetrabromodiphenyl ether d, bromodiphenyl ether-183 d (Canton et al. 2006; Song et al. 2008) | |
| Biphenylmethylene 4-pyridines d (Hu et al. 2010) | |
| Bisphenol A d (Niwa et al. 2001) | |
| Imidazole, pyridine, 1,2,4- and 1,2,3-triazole, pyrazine, pyrimidine, pyrazole, oxazole, thiazole, isoxazole, 1,2,3,4- and 1,2,3,5-tetrazole and isothiazole containing compounds d (Bonomo et al. 2016) | |
| Methoxy- and hydroxy-substituted methyleneimidazolyl biphenylsa (Hille et al. 2009) | |
| α-Naphthoflavonec (Lin et al. 2014) | |
| Nitrophenols d (Furuta et al. 2008) | |
| Prochloraz d (Ohlsson et al. 2009) | |
| Steroidal C-17 benzoazoles d (Handratta et al. 2005) | |
| Vioxx (rofecoxib)-related lactones d (van Duursen et al. 2010) | |
| YM116 d (Ideyama et al. 1998) | |
|
Induction: stimulation of androgen biosynthesis in man and risk of development of prostate cancer, or enhanced estrogen biosynthesis and risk of breast cancer | |
|
Inducers: | |
|
Drugs: | |
|
Cycloheximidee (Doi et al. 2001) | |
| Valproic acidf (Nelson-DeGrave et al. 2004) | |
|
Natural compounds: | |
|
Retinoids e (Wickenheisser et al. 2005) | |
| Forskolin e (Sawetawan et al. 1996; Nelson-DeGrave et al. 2004; Asif et al. 2006; Lin et al. 2014) | |
|
Physiological compounds: | |
|
Adrenocorticotropic hormone (ACTH) e (Di Blasio et al. 1987) | |
| Cardiolipin g (Kisselev et al. 1999) | |
| Cortisol e (Hsu et al. 2001; Auchus R. J. et al. 2014) | |
| Insulin-like growth factors I and II e (Mesiano et al. 1997) | |
|
Other compounds: | |
|
PD98059 (MAPK kinase (MEK) inhibitor) e (Doi et al. 2001) | |
| Polybrominated diphenyl ethers f (e.g. 2´-hydroxybromodiphenyl ether-68) (Song et al. 2008) | |
Footnotes:
Competitive inhibition, ligand binding
Competitive inhibition, binding to enzyme active site
Reduced/suppressed mRNA and/or protein level/expression and activity
Decreased/suppressed/inhibited activity/product formation
Increased transcription/mRNA/protein expression/levels /and/or catalytic activity
Up-regulation of biosynthesis, increased expression of protein
Increased activity, and/or increased product formation
In summary, the data presented show functional targets of P450 enzymes belonging to the Families 5–51. Of these enzymes, targets for drugs in clinical treatment are P450s 5A1, 11B1, 11B2, 17A1, and 19A1. The enzymes of this group synthesize important physiological molecules but over-production or down-regulation may be an issue in some diseases. The present review brings updated information and has focused on the properties of different chemical entieties (drugs, environmental, physiological, and natural compounds, as well as examples of drug candidates under investigation) as inhibitors and/or inducers of the enzymes activity and clinical implications.
Fig. 2.
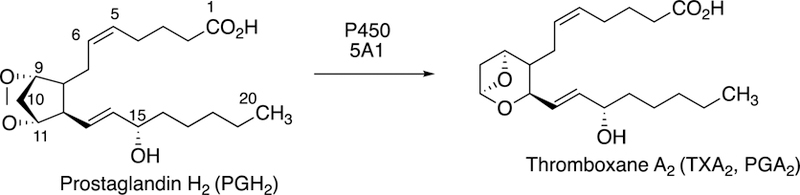
Conversion of prostaglandin H2 to thromboxane A2 by P450 5A1 (thromboxane synthase).
Fig. 6.
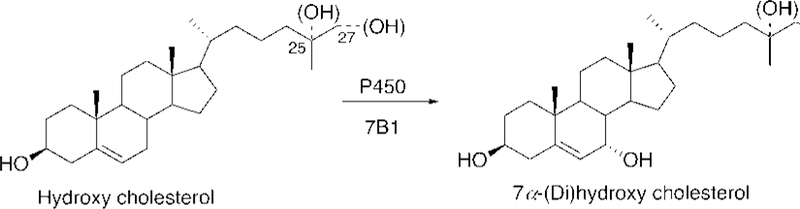
7α-Hydroxylation of 25- or 27-hydroxycholesterol by P450 7B1.
Fig. 16.
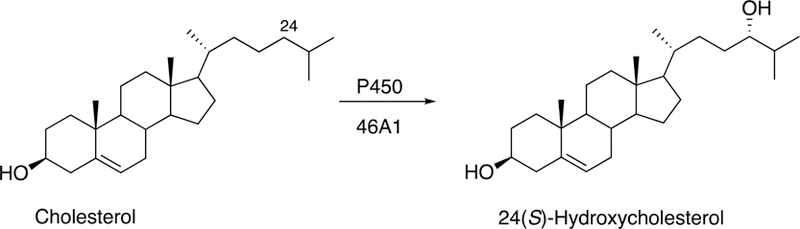
24(S)-Hydroxylation of cholesterol by P450 46A1.
Fig. 22.
C4-Oxidation of all-trans-retinoic acid catalyzed by P450 26A1.
Table 3.
P450 7A1
| Properties | References |
|---|---|
| Physiological substrates: Cholesterol, oxysterols, zimosterol, lathosterol, zymostenol, desmosterol, cholestanol, 24(S) and (R)-hydroxycholesterol, 25-hydroxycholesterol, 27-hydroxycholesterol, 20(S)-hydroxycholesterol, 7-dehydrocholesterol, cholest-4-en-3-one, bile acids (taurocholic acid, deoxycholic acid, lithocholic acid, ursodeoxycholic acid) | (Norlin, Andersson, et al. 2000; Norlin, Toll, et al. 2000; Smelt 2010; Shinkyo and Guengerich 2011; Shinkyo et al. 2011; Tempel et al. 2014; Acimovic et al. 2016; Pan et al. 2016) |
|
Function: Cholesterol 7α-hydroxylase (Figs. 4A, 5), first step in conversion of cholesterol to bile acids |
|
|
Inhibition: Causes increased cholesterol saturation of bile, enhanced liver and lower circulating LDL cholesterol content, lowering biosynthesis and excretion of bile acids | |
|
Inhibitors: | |
|
Drugs: | |
|
Cyclosporin A a (Sharanek et al. 2015) | |
| Fibrates a (clofibrate, bezafibrate, fenofibrate, gemfibrozil) (Marrapodi and Chiang 2000; Gbaguidi and Agellon 2004; Roglans et al. 2004; Zak et al. 2007; Honda et al. 2013) | |
| Obeticholic acid a (Zhang, Jackson, et al. 2017) | |
|
Natural compounds: | |
|
Guggulsterone b (Owsley and Chiang 2003) | |
| Phorbol 12-myristate-13-acetate a (Wang et al. 1996) | |
| Red grapefruit juice a (Davalos et al. 2006) | |
| α-Tocopherol a (Gonzalez, Cruz, Ferrin, Lopez-Cillero, Fernandez-Rodriguez, et al. 2011) | |
|
Physiological compounds: | |
|
25-Hydroxycholesterol a (Taniguchi et al. 1994; Gbaguidi and Agellon 2004) | |
| 7-Oxocholesterol a (Norlin, Toll, et al. 2000) | |
| Arachidonic acid a (Zhang, Wand, et al. 2017) | |
| Bile acids (free and glycine conjugated) a (chenodeoxycholic acid, deoxycholic acid, glycoursodeoxycholic acid, glycodeoxycholic acid, glycocholic acid) (Chiang et al. 2000; Chen et al. 2001; Ellis E et al. 2003; Li T, Jahan, et al. 2006; Smelt 2010; Gonzalez, Cruz, Ferrin, Lopez-Cillero, Briceno, et al. 2011; Gonzalez, Cruz, Ferrin, Lopez-Cillero, Fernandez-Rodriguez, et al. 2011; Liu J et al. 2014) | |
| cAMP a (Song KH and Chiang 2006) | |
| Glucagon a (Song KH and Chiang 2006) | |
| Insulin a (prolonged treatment) (Wang et al. 1996; Li T, Kong, et al. 2006) | |
| Proinflammatory cytokines (interleukin-1β) a(Jahan and Chiang 2005; Li T, Jahan, et al. 2006) | |
| Sitosterol c (Nguyen et al. 1998) | |
| Thyroid hormones (triiodothyronine, T3) a (Wang et al. 1996; Drover et al. 2002; Drover and Agellon 2004; Ellis EC 2006; Song Y et al. 2015) | |
| α1-Antitrypsin peptide a (Gerbod-Giannone et al. 2002) | |
|
Other compounds, including drug candidates: | |
|
WY-14643 (pirinixic acid) a (Marrapodi and Chiang 2000; Gbaguidi and Agellon 2004) | |
|
Induction: Causes enhanced biosynthesis of bile acids, cholesterol lowering effect, increased risk of gallstone formation | |
|
Inducers: Decrease of cholesterol levels, alteration of bile acid profiles | |
|
Drugs: | |
|
Dexamethasone d (Andreou and Prokipcak 1998) | |
| NO-1666 (Ibrolipim) d (Li Q et al. 2010) | |
| Rifampicin d (Gonzalez, Cruz, Ferrin, Lopez-Cillero, Briceno, et al. 2011) | |
| Statins e (atorvastatin, pitavastatin, pravastatin, simvastatin, lactostatin) (Fan et al. 2004; Morikawa et al. 2007; Parker et al. 2013) | |
|
Natural compounds: | |
|
Dipeptides d (Asp-Lys, Glu-Lys, and Trp-Lys) (Norlin and Wikvall 2007) | |
| Farnesylquinone and derivatives e (Liu D et al. 2014) | |
| Fish oil f (Jonkers et al. 2006; Smelt 2010) | |
| Crocin, chlorogenic acid, geniposide, and quercetin (in combination) d (Leng et al. 2018) | |
|
Physiological compounds: | |
|
Cholestyramine g (Norlin, Andersson, et al. 2000; Norlin and Wikvall 2007) | |
| Glucose g (Li T et al. 2010; Li T et al. 2012) | |
| Insulin d (short-term treatment) (Li T, Kong, et al. 2006; Li T et al. 2012) | |
| Taurine d (Guo et al. 2017) | |
| Thyroid hormones e (Lammel Lindemann et al. 2014) | |
|
Other compounds: | |
|
2,4,6-Trihydroxyacetophenone g (Charoenteeraboon et al. 2005) | |
| Oleic acid anilide d (An et al. 2008) | |
Footnotes:
Reduced/suppressed mRNA and/or protein level/expression and activity
Decreased/suppressed/inhibited activity/product formation
Competitive inhibition, binding to enzyme active site
Increased transcription/mRNA/protein expression/levels /and/or catalytic activity
Up-regulation of biosynthesis, increased expression of protein
Increased bile acid synthesis and mRNA levels
Increased activity, and/or increased product formation
Table 5.
P450 8B1
| Properties | References |
|---|---|
| Physiological substrates: 27-Hydroxycholesterol, 24-hydroxycholesterol, 7α-hydroxy-4-cholesten-3-one | Gafvels et al. 1999; Pan et al. 2016) |
|
Function: Sterol 12α-hydroxylase, production of cholic acids (bile acids) in the liver, conversion of 7α -hydroxy-4-cholesten-3-one into 7α,12α -dihydroxy-4-cholesten-3-one |
|
|
Inhibition: Lowered biosynthesis of bile acids | |
|
Inhibitors: | |
|
Drugs: | |
|
Chlorpromazine a (Antherieu et al. 2013) | |
| Cyclosporine A b (Sharanek et al. 2015) | |
|
Physiological compounds: | |
|
Bile acids b (chenodeoxycholic acid, glycochenodeoxycholic acid, glycodeoxycholic acid) (Zhang and Chiang 2001; Jahan and Chiang 2005) | |
| Cholesterol b (Zhang and Chiang 2001) | |
| Insulin b (Zhang and Chiang 2001) | |
| Pro-inflammatory cytokines b (e.g. interleukin-1β) (Jahan and Chiang 2005) | |
| Triiodothyronine (T3) b (Andersson et al. 1999; Ellis EC 2006) | |
|
Physiological condition: | |
|
Restricted feeding c (Pathak et al. 2013) | |
|
Induction: Enhanced biosynthesis of bile acids, cholesterol lowering effect | |
|
Inducers: | |
|
Drugs: | |
|
Atorvastatin d (Parker et al. 2013) | |
| Cycloheximide c (Andreou and Prokipcak 1998) | |
| Dexamethasone c (Andreou and Prokipcak 1998; Mörk et al. 2016) | |
|
Natural compounds: | |
|
Soy isoflavones e (genistein, daidzein, equol) (Li et al. 2007) | |
|
Physiological compounds: | |
|
Hepatocyte nuclear factor 4α (HNF4α) c (Zhang and Chiang 2001) | |
|
Physiological condition: | |
|
Fasting c (Pathak et al. 2013) | |
Footnotes:
Decreased/suppressed/inhibited activity/product formation
Reduced/suppressed mRNA and/or protein level/expression and activity
Increased transcription/mRNA/protein expression/levels /and/or catalytic activity
Up-regulation of biosynthesis, increased expression of protein
Increased bile acid synthesis and mRNA levels
Table 7.
P450 11B1
| Properties | References |
|---|---|
| Physiological substrate: 11β-Deoxycortisol | (Denner et al. 1996; Coulter and Jaffe 1998; Parr et al. 2012; Bernhardt 2016; Pan et al. 2016; Schiffer, Brixius-Anderko, et al. 2016; Schiffer, Müller, et al. 2016) |
|
Other substrates: Turinabol and metandienone (doping agents in sports), spironolactone, canrenone | |
|
Function: Steroid 11β- and 18-hydroxylation, cortisol formation (Figs. 4, 9) |
|
|
Inhibition: Block synthesis of cortisol in Cushing’s syndrome and in chronic skin wounds, treating diseases caused by cortisol overproduction, interfere with the biosynthesis of aldosterone, possible application in treatment of adrenocortical carcinoma | |
|
Inhibitors: (Ehmer et al. 2002; Hartmann et al. 2003; Bureik et al. 2004; Muller-Vieira et al. 2005; Papillon et al. 2015) | |
|
Drugs: | |
|
Aminoglutethimide b (at high concentrations) (Vermeulen et al. 1983) | |
| Canrenone, potassium canrenoate b (Cheng et al. 1976) | |
| Cimetidine a (Kenyon et al. 1986) | |
| Clotrimazole, miconazole, fluconazole a (Ayub and Levell 1989; Denner et al. 1995); | |
| Etomidate a (Engelhardt and Weber 1994; Fassnacht et al. 2000) | |
| Digoxin c (Kau et al. 2005) | |
| (R)-Etomidate a (Roumen et al. 2007) | |
| Fadrozole a (weak inhibitor) (Muller-Vieira et al. 2005) | |
| (R)- and (S)-Fadrozole a (Roumen et al. 2007) | |
| Iodometomidate, metomidate, fluoroetomidatec (Hahner et al. 2008) | |
| Ketoconazoled (Ayub and Levell 1989; Denner et al. 1995; Johansson MK et al. 2002) | |
| Metyrapone b (Fassnacht et al. 2000; Johansson MK et al. 2002; Roumen et al. 2007; Yin et al. 2012, Denner et al. 1995; Okahara et al. 1998) | |
| Mitotane c (Lin CW et al. 2012) | |
| Spironolactone b (Cheng et al. 1976; Denner et al. 1995) | |
| Trilostane b (Johansson MK et al. 2002) | |
| Turinabol c (Schiffer, Brixius-Anderko, et al. 2016) | |
|
Natural compounds: | |
|
Bufalin, cinobufagin b (Kau et al. 2012) | |
| Chelerythrine, rottlerin b (Bureik, Zeeh, et al. 2002) | |
| Ouabain c (Kau et al. 2005) | |
|
Physiological compounds: | |
|
Interferon-β c (van Koetsveld et al. 2013) | |
|
Other compounds, including drug candidates (only some examples are presented because of the large number of synthetic derivatives): | |
|
Abiraterone analogues a (Pinto-Bazurco Mendieta et al. 2008) | |
| 4,5-Dihydro-[1,2,4]triazolo[4,3-a]quinolines a (Hu et al. 2015) | |
| Arylsulfonyltetrahydroquinolines b (Zhu et al. 2014) | |
| Biphenylmethylene 4-pyridines b (Hu et al. 2010) | |
| Gö 6976 b (Bureik, Zeeh, et al. 2002) | |
| Heteroaryl-substituted dihydronaphthalenes and indenes b (Voets et al. 2006) | |
| Heteroaryl-substituted naphthalenes b (Voets et al. 2005) | |
| Imidazol-1-ylmethyl substituted 1,2,5,6-tetrahydropyrrolo[3,2,1-ij]quinolin-4-ones, pyridylmethyl pyridine compounds b (Yin et al. 2012) | |
| Imidazolylmethylenetetrahydronaphthalenes and imidazolylmethyleneindanes b (Ulmschneider et al. 2005) | |
| Imidazolylmethylxanthones b (Otton et al. 1993; Gobbi et al. 2016, 2017) | |
| 3-MeSO2-DDE d (Johansson MK et al. 2002) | |
| 3-Methylsulfonyl-DDE, MeSO2-PCBs (4-MeSO2-2,3,6,4´-tetrachlorobiphenyl, and 4-MeSO2-2,3,6,3´,4´-pentachlorobiphenyl b (Johansson M et al. 1998; Johansson MK et al. 2002); | |
| 5-(Phenoxymethyl)-1,3-dioxane analogs b (Schroeder et al. 2016) | |
| 1-Phenylsulfinyl-3-(pyridin-3-yl)naphthalen-2-ols b (Grombein et al. 2015) | |
| Pyridyl substituted arylsulfonyltetrahydroquinolines b (Zhu et al. 2014) | |
| Pyridylmethyl isoxazoles b (Emmerich et al. 2017) | |
| Pyridylmethyl pyridine compounds b (Emmerich et al. 2018) | |
| 2,3,7,8-Tetrabromodibenzo-p-dioxin, TBDD c (Ding et al. 2007) | |
| Tetralins b (Ehmer et al. 2002;Muller-Vieira et al. 2005) | |
|
Induction: Overproduction of cortisol is associated with Cushing’s syndrome and chronic wound diseases | |
|
Inducer: | |
|
Drugs: | |
|
Metyraponee (Coulter and Jaffe 1998) | |
| Mitotanef (Lin CW et al. 2012) | |
| Rifampicin f (Kim et al. 2013) | |
|
Natural compounds: | |
|
3´,4´-Dimethoxyflavone f (Lin TC et al. 2006) | |
| Forskolin e (Denner et al. 1996) | |
|
Physiological compounds: | |
|
Adrenocorticotropic hormone g (Vukelic et al. 2011) | |
| Angiotensin II e (Denner et al. 1996) | |
| Dibutyryl cAMP e (Denner et al. 1996) | |
|
Other compounds: | |
|
2,3,7,8-Tetrabromodibenzo-p-dioxin, TBDD e (Ding et al. 2007) | |
| 3´,4´-Dimethoxyflavone f (Lin TC et al. 2006) | |
| 3-Methylsulfonyl-DDE (o, p´-DDT metabolite, at lower concentrations) f (at lower concentrations) (Asp et al. 2010) | |
| Heteroaryl-substituted naphthalenes b (Voets et al. 2005) | |
| Polychlorinated biphenyls (PCBs) −39, −77, −126, −132, −156, and −169) f (Lin TC et al. 2006) | |
Footnotes:
competitive inhibition, ligand binding
decreased/suppressed/inhibited activity/product formation
reduced/suppressed mRNA and/or protein level/expression and activity
competitive inhibition, binding to enzyme active site
Increased transcription/mRNA/protein expression/levels /and/or catalytic activity
Up-regulation of biosynthesis, increased expression of protein
Increased activity, and/or increased product formation
Table 8.
P450 11B2
| Properties | References |
|---|---|
| Physiological substrate: 11-Deoxycorticosterone | (Denner et al. 1996; Coulter and Jaffe 1998; Roumen et al. 2011; Parr et al. 2012; Bernhardt 2016; Pan et al. 2016; Schiffer, Brixius-Anderko, et al. 2016; Schiffer, Müller, et al. 2016) |
|
Other substrates: Turinabol and metandienone (doping agents misused in sports), spironolactone, canrenone | |
|
Function: Aldosterone synthase, conversion of 11-deoxycorticosterone to aldosterone via corticosterone and 18-hydroxycorticosterone (Figs. 4, 10) |
|
|
Inhibition: Lower level of aldosterone, hypertension and heart failure treatment (Azizi et al. 2013; Karns et al. 2013) | |
|
Inhibitors: (Ehmer et al. 2002; Hartmann et al. 2003; Bureik et al. 2004; Muller-Vieira et al. 2005; Lucas, Heim, Negri, et al. 2008; Lucas, Heim, Ries, et al. 2008; Hakki et al. 2011; Papillon et al. 2015) | |
|
Drugs: | |
|
(R)- and (S)-Fadrozole a (Roumen et al. 2007) | |
| (R)-Etomidate a (Roumen et al. 2007) Aminoglutethimide b (Schuster and Bernhardt 2007) | |
| Aminoglutethimide b (Schuster and Bernhardt 2007) | |
| Digoxin c (Kau et al. 2005) | |
| Eplerone b (Moore et al. 2003; White et al. 2003) | |
| Etomidate, iodometomidate, metomidate, fluoroetomidate c (Roumen et al. 2007; Hahner et al. 2008) | |
| Fadrozole b (Muller-Vieira et al. 2005) | |
| LCI699, silodrostat b (Amar et al. 2010; Karns et al. 2013)) | |
| Metyrapone (weak inhibitor) a (Roumen et al. 2007) | |
| Miconazole, ketoconazole, clotrimazole, isoconazole a (Bureik et al. 2004; Hakki et al. 2011); | |
| Mitotane b (Hakki et al. 2011) | |
| Nifedipine c (Denner et al. 1996) | |
| Phenelzineb (Hakki et al. 2011) | |
| Staurosporine b (Bureik et al. 2002; Bureik et al. 2005) | |
|
Natural compounds: | |
|
Bufalin, cinobufagin c (Kau et al. 2012) | |
| Chelerythrine, rottlerin b (Bureik et al. 2002) | |
| Ellipticine b (Hakki et al. 2011) | |
| Ouabain c (Kau et al. 2005) | |
|
Physiological compounds: | |
|
4-Androstene-3,17-dione b (Hakki et al. 2011) | |
|
Other compounds and drug candidates: | |
|
12-O-Tetradecanoyphorbol-13-acetate (TPA) c (LeHoux et al. 2001; LeHoux and Lefebvre 2004) | |
| Phenylsulfinyl-3-(pyridin-3-yl)naphthalen-2-ols b (Grombein et al. 2015) | |
| 20-Hydroxyiminopregna-5,14-diene-3β-ol b (Ehmer et al. 2002) | |
| 1–3-Methylsulfonyl-DDE (at high concentrations) | |
| 4,5-Dihydro-[1,2,4]triazolo[4,3-a]quinolines b (Hu et al. 2015) | |
| 4-Anilino-pyrimidines b (Meguro et al. 2017) | |
| Abiraterone analogues b (Pinto-Bazurco Mendieta et al. 2008) | |
| Biphenylmethylene 4-pyridines b (Hu et al. 2010) | |
| Calmidazolium b (Condon et al. 2002) | |
| D, L-p-Chlorophenylalanine methyl ester b (Hakki et al. 2011) | |
| FAD286 b (Brunssen et al. 2017) | |
| Gö 6976 b (Bureik et al. 2002) | |
| Heteroaryl-substituted dihydronaphthalenes and indenes b (Voets et al. 2006) | |
| Heteroaryl-substituted naphthalenes b (Voets et al. 2005; Lucas, Heim, Negri, et al. 2008) | |
| Imidazolylmethylenetetrahydronaphthalenes and imidazolylmethyleneindanes b (Ulmschneider et al. 2005) | |
| Imidazolylmethylxanthones b (Gobbi et al. 2016) | |
| Imidazopyridyl compounds (e.g. RO6836191) d (Bogman et al. 2017; Whitehead et al. 2017) | |
| Indazole compounds b (Hoyt et al. 2017) | |
| KN93 b (Condon et al. 2002) | |
| Naphthalene based phenyl or benzyl derivatives a (Lucas, Heim, Negri, et al. 2008) | |
| PA024 c (Suzuki et al. 2017) | |
| Pyridine substituted 3,4-dihydro-1H-quinolin-2-one derivatives b (Lucas, Heim, Negri, et al. 2008) | |
| Pyridyl- or isoquinolinyl-substituted indolines and indoles b (Yin et al. 2014) | |
| Pyridyl substituted 4,5-dihydro-[1,2,4]triazolo[4,3-a]quinolines b (Hu et al. 2015) | |
| Pyridyl substituted acenaphthene derivatives b (Ulmschneider et al. 2006) | |
| Tetralins a (Ehmer et al. 2002; Muller-Vieira et al. 2005) | |
| Triazoloquinolines b (Hu et al. 2015) | |
| Turinabol c (Schiffer, Brixius-Anderko, et al. 2016) | |
|
Induction: affects sex hormone production, overproduction of aldosterone leads to hypertension and end-organ damage such as cardiac and renal hypertrophy | |
|
Inducers: | |
|
Drug(s): | |
|
BAYK 8644 | |
| Rifampicin e (Kim et al. 2013) | |
| Telmisartan e (Matsuda et al. 2014) | |
|
Natural compounds: | |
|
3´,4´-Dimethoxyflavone e (Lin et al. 2006) | |
| Calneuron 1 e (Kobuke et al. 2018) | |
| Forskolin f (weak induction) (Denner et al. 1996) | |
| Glucose (high concentrations) e (Shimada et al. 2017) | |
|
Physiological compounds: | |
|
Angiotensin IIg (Denner et al. 1996; Gennari-Moser et al. 2013;Matsuda, 2014, 59503} | |
| Vascular endothelial growth factor (VEGF) g (Gennari-Moser et al. 2013) | |
| Very low-density lipoprotein f (Tsai et al. 2017) | |
|
Other compounds: | |
|
2,4-Dibromophenol e (Ding et al. 2007) | |
| 3 -Methylsulfonyl-DDE (o, p´-DDT metabolite, at lower concentrations) e (Asp et al. 2010) | |
| BAYK 8644 f (Denner et al. 1996) | |
| Benzo[a]pyrene e (Keshava et al. 2005) | |
| Brominated flame retardants e (e.g., 2,4-dibromophenol, pentabromophenol, 2,3,7,8-tetrabromodibenzofuran, TBDF) (Ding et al. 2007) | |
| Calcium e (Shimada et al. 2017; Kobuke et al. 2018) | |
| Organic sediment contaminants e (Blaha et al. 2006) | |
| PD98059 e (LeHoux and Lefebvre 2004) | |
| Polybrominated diphenyl ethers (e.g. 2´-OH-BDE-68) f (Song et al. 2008) | |
| Polychlorinated and polybrominated biphenyls e (PCB- and PBD-39, −77, −126, −132, −156, and −169) e (Lin et al. 2006) | |
| Potassiumf (Denner et al. 1996; Matsuda et al. 2014) | |
| Tetrabromodibenzo-p-dioxin e (Ding et al. 2007) | |
Footnotes:
Competitive inhibition, ligand binding
Decreased/suppressed/inhibited activity/product formation
Reduced/suppressed mRNA and/or protein level/expression and activity
Competitive inhibition, binding to enzyme active site
Increased transcription/mRNA/protein expression/levels /and/or catalytic activity
Up-regulation of biosynthesis, increased expression of protein
Increased activity, and/or increased product formation
Table 11.
P450 21A2
| Properties | References |
|---|---|
| Physiological substrates: Progesterone, 17α-hydroxyprogesterone | (Coulter and Jaffe 1998; Zöllner et al. 2010; Parr et al. 2012; Yoshimoto et al. 2012; Pallan, Wang, et al. 2015; Pan et al. 2016; Wang et al. 2017) |
|
Other substrate(s): Normetandienone, metandienone, 17-fluoroprogesterone |
|
|
Function: Steroid 21-hydroxylase, formation of 11-deoxycorticosterone from progesterone and 11-deoxycortisol from 17α-hydroxyprogesterone (Figs. 2, 13) |
|
|
Inhibition: May lead to congenital adrenal hyperplasia | |
|
Inhibitors: | |
|
Drugs: | |
|
Abiraterone a (Malikova et al. 2017) | |
| Imidazole drugs b (e.g. clotrimazole, bifonazole, isoconazole, miconazole, ketoconazole, tioconazole) (Ayub and Levell 1990; Johansson et al. 2002) | |
| Mitotane c (Lin CW et al. 2012) | |
| Omeprazole d (weak inhibitor) (Dowie et al. 1988) | |
|
Natural compounds: | |
|
1α,25-Dihydroxyvitamin D3 d (Lundqvist et al. 2010) | |
| Alkylresorcinols c (Ayub and Levell 1990; Oskarsson and Ohlsson Andersson 2016) | |
| Polyphenols d (e.g., apigenin, genistein, hesperitin, naringenin, apigenin, eriodictyol, daidzein, resveratrol (Hasegawa et al. 2013; Lin CJ et al. 2014) | |
|
Physiological compounds: | |
|
ent-Progesterone e (Auchus et al. 2003) | |
|
Other compounds: | |
|
2,3,7,8-Tetrabromodibenzo-p-dioxin c (Ding et al. 2007) | |
| 5-(Phenoxymethyl)-1,3-dioxane analogs d (Schroeder et al. 2016) | |
| Brominated flame retardants c (2,6-dibromophenol, 2,4,6-tribromophenol, 2,3,7,8-tetrabromodibenzofuran) (Ding et al. 2007) | |
| Crude sediment extracts f (containing high concentrations of polycyclic aromatic hydrocarbons (PAHs) and moderate amounts of polychlorinated biphenyls and organochlorine pesticides) (Blaha et al. 2006) | |
| Prochloraz d (Ohlsson et al. 2009) | |
| YZ5ay (imidazole biphenyl derivative b (Dragan et al. 2006) | |
|
Induction: causes cortisol overproduction | |
|
Inducers: | |
|
Natural compound: | |
|
Forskolin g (Asif et al. 2006; Lin CJ et al. 2014) | |
|
Physiological compound: | |
|
Orexin/hypocretin h (Wenzel et al. 2009) | |
|
Other compounds: | |
|
Brominated flame retardants g (weak inducer) (e.g., pentabromophenol) (Ding et al. 2007) | |
| Polybrominated diphenyl ethers g (e.g., 2´-hydroxy-BDE-68) h (Song et al. 2008) | |
| Polybrominated biphenyls g (e.g., 3,3´,4,4,5,5´´-tetrabromobiphenyl, 3,3´,4,4´-tetrabromobiphenyl) (Ding et al. 2007) | |
Footnotes:
Irreversible binding the iron heme complex, inactivator
Competitive inhibition, ligand binding
Reduced/suppressed mRNA and/or protein level/expression and activity
Decreased/suppressed/inhibited activity/product formation
Competitive inhibition, binding to enzyme active site
Gene downregulated/suppressed
Increased transcription/mRNA/protein expression/levels /and/or catalytic activity
Up-regulation of biosynthesis, increased expression of protein
Table 12.
27A1
| Properties | References |
|---|---|
| Physiological substrates: Cholesterol, 4-cholesten-3-one, 7α-ketocholesterol, 7-dehydrocholesterol, lathosterol, zymosterol, zymostenol, desmosterol, 27-hydroxycholesterol, 25-hydroxycholesterol, 5β-cholestane-3α,7α,12α-triol, 7α-hydroxy-4-cholesten-3-one, 4-cholesten-3-one, 24-hydroxy-4-cholesten-3-one, 25-hydroxy-4-cholesten-3-one, 24-hydroxycholesterol | (Norlin et al. 2003; Abe et al. 2005; Sakaki et al. 2005; Pettersson et al. 2008; Pikuleva et al. 2008; Tieu et al. 2012; van Lier et al. 2015; Acimovic et al. 2016; Pan et al. 2016) |
|
Natural substrates: Vitamin D3 (cholecalciferol), vitamin D2 (ergocalciferol), 20-hydroxyvitamin D3, 1α-hydroxyvitamin D3, 2α-propoxy-1α,25-dihydroxyvitamin D3, 2α-(3-hydroxypropoxy)-1α,25-dihydroxyvitamin D3 |
|
|
Other compounds: 3β-Hydroxy-24S-methyl-5α-cholesta-8(14),22-dien-15-one, cholesterol 25-hydroperoxide |
|
|
Function: Degradation of cholesterol to bile acids (Fig. 14A), vitamin D3 25-hydroxylase (Fig. 14B) |
|
|
Inhibition: May be effective as an adjuvant in the treatment of ER-positive breast cancer | |
|
Inhibitors: : (Gueguen et al. 2007; Taban et al. 2017) | |
|
Drugs: | |
|
Anastrozole, fadrozole, (R)-bicalutamide, dexmedetomidine, ravuconazole, posaconazole a (Mast et al. 2015) | |
| Bezafibrate b (Honda et al. 2013) | |
| Carfilzomib c (Federspiel et al. 2016) | |
| Clevidipine, delaviridne, etravirine, felodipine and analogs, nicardipine, sorafenib, abiratone, candesartan, celecoxib, dasatanib, nilvadipine, nimodipine, nilotinib, regorafenib b (Lam et al. 2018) | |
| Cyclosporine A, rapamycin d (Lyons and Brown 2001; Gueguen et al. 2007; Sharanek et al. 2015) | |
| SDZ-286907 d (Schuster et al. 2006) | |
| Statins (e.g., atorvastatin) d (Llaverias et al. 2006; Kimbung et al. 2017) | |
|
Natural compounds: | |
|
Phorbol 12-myristate 13-acetate d (Araya et al. 2003; Quinn et al. 2005) | |
|
Physiological compounds: | |
|
Bile acids d (glycochenodeoxycholic acid, glycodeoxycholic acid) (Chen and Chiang 2003; Ellis E et al. 2003) | |
| Estrogens d (17β-estradiol in HepG2 and RWPE-1 prostate cells) (Tang et al. 2007) | |
| Luteinizing hormone, human chorionic gonadotropin´´ (Xu et al. 2018) | |
| Sitosterol e (Nguyen et al. 1998) | |
| L-Thyroxine (T4), triiodothyronine (T3) d (Araya et al. 2003; Ellis EC 2006; Norlin and Wikvall 2007) | |
|
Physiological conditions and illnesses: | |
|
Prostate cancer d (Alfaqih et al. 2017; Lutz et al. 2018) | |
|
Other compounds: | |
|
GI268267X, GW273297X, GW9662 d (Lyons and Brown 2001; Quinn et al. 2005) | |
|
Induction: enhanced biosynthesis of bile acids, cholesterol lowering effect | |
|
Inducers: | |
|
Drugs: | |
|
Dexamethasone f (Araya et al. 2003) | |
| Pioglitazone g (Quinn et al. 2005) | |
| Rifampicin g (Li et al. 2007) | |
| Rosiglitazone g (Quinn et al. 2005; Llaverias et al. 2006) | |
|
Natural compounds: | |
|
1,25-Dihydroxyvitamin D3, vitamin D3 g (Tokar and Webber 2005; Diesing et al. 2006; Yin et al. 2015) | |
| Retinoid X receptor ligands (9-cis-retinoic acid) g (Szanto et al. 2004; Quinn et al. 2005) | |
|
Physiological compounds: | |
|
5α-Dihydrotestosterone g (Tang et al. 2007) | |
| Estrogens g (e.g., 17β-estradiol in LNCaP prostate cancer cells) (Tang et al. 2007) | |
| Growth hormone f (Araya et al. 2003) | |
| Insulin like growth factor-1, growth hormone f (Araya et al. 2003) | |
| Nuclear factor 4α g (Chen and Chiang 2003) | |
|
Physiological condition and illnesses: | |
|
Breast cancer g (Kimbung et al. 2017) | |
|
Other compounds: | |
|
Oleic acid anilide g (An et al. 2008) | |
| Peroxisome-proliferator-activated receptor-γ ligands g (e.g., GW1929) (Szanto et al. 2004; Quinn et al. 2005) | |
Footnotes:
Competitive inhibition, ligand binding
Decreased/suppressed/inhibited activity/product formation
Irreversible binding, inactivator
Reduced/suppressed mRNA and/or protein level/expression and activity
Competitive inhibition, binding to enzyme active site
Increased activity, and/or increased product formation
Increased transcription/mRNA/protein expression/levels /and/or catalytic activity
Table 13.
P450 39A1
| Properties | References |
|---|---|
| Physiological Substrates: 24(S)-Hydroxycholesterol, 25-hydroxycholesterol, and 27-hydroxycholesterol | (Li-Hawkins, Lund, Bronson, et al. 2000; Pan et al. 2016) |
|
Function: Oxysterol 7α-hydroxylase, C7-hydroxylation of 24-hydroxycholesterol, biosynthesis of bile acids (Figs. 4, 15), downregulated in melanoma |
|
|
Inhibition: No information available | |
|
Inhibitors: | |
|
Physiological condition and illness: | |
|
downregulation in melanoma a (Sumantran et al. 2016) | |
|
Induction: | |
|
Inducer: | |
|
Drugs: | |
|
carbamazepine b (Oscarson et al. 2006) | |
Footnotes:
Gene downregulated/suppressed
Increased transcription/mRNA/protein expression/levels /and/or catalytic activity
Table 14.
P450 46A1
| Properties | References |
|---|---|
| Physiological Substrates: Cholesterol, 4 βhydroxycholesterol, 24(S)-hydroxycholesterol, 7 αhydroxycholesterol, cortisol, 7-ketocholesterol, 7-dehydrocholesterol, cholestanol, desmosterol, zymostenol, zymosterol, progesterone, testosterone, lanosterol | (Mast N. et al. 2003; Mast N. et al. 2008; Liao et al. 2009; Mast N. et al. 2010; Goyal et al. 2014; Acimovic et al. 2016; Pan et al. 2016) |
|
Other substrates: |
|
|
Drugs: Diclofenac, bufuralol, phenacetin, dextromethorphan, clotrimazole |
|
|
Natural compounds: 25-Hydroxyvitamin D3 |
|
|
Functions: Cholesterol 24S-hydroxylase, 25- and 27-hydroxylase (minor extent) (Figs. 4, 16), eliminates cholesterol from brain, metabolizes neurosteroids and drugs that can cross the blood-brain barrier, 27-hydroxylation of 7-ketocholesterol (detoxication) |
|
|
Inhibition: Reduced brain cholesterol excretion | |
|
Inhibitors: (Mast N. et al. 2010) | |
|
Drugs: | |
|
Azole drugs a (ketoconazole, posaconazole, clotrimazole, voriconazole) (Mast N. et al. 2010; Shafaati et al. 2010; Mast N. et al. 2013) | |
| (E)-Fluvoxamine, bicalutamide, dexmedetomidine b (Mast N. et al. 2012) | |
| Tranylcypromine, thioperamide a (Mast N. et al. 2010) | |
|
Natural compounds: | |
|
Okadaic acid c (Nunes et al. 2012) | |
|
Stimulation: Increased activity potentially involved in the pathogenesis of Alzheimer’s disease (Testa et al. 2016) | |
|
Stimulators: (Mast N. et al. 2017) | |
|
Drugs: | |
|
Efavirenz (Anderson et al. 2016) Trichostatin A (Nunes et al. 2012) | |
|
Natural compounds: | |
|
L-Glutamate, L-aspartate, γ-aminobutyric acid, acetylcholine, 1α,25-dihydroxyvitamin D3d (Mast N. et al. 2017) | |
|
Other compounds: | |
|
-PD98059, U0126 e (MEK1 inhibitors) (Nunes et al. 2012) | |
Footnotes
Competitive inhibition, ligand binding
Competitive inhibition, binding to enzyme active site
Reduced/suppressed mRNA and/or protein level/expression and activity
Activation/stimulation
Increased transcription/mRNA/protein expression levels and/or catalytic activity
Table 15.
P450 51A1
| Properties | References | |
|---|---|---|
| Physiological substrate: Lanosterol | (Lepesheva and Waterman 2007; Hargrove et al. 2012; Pan et al. 2016; Guengerich 2017) | |
|
Other substrates: 24, 25-Dihydrolanosterol, 24-methylenedihydrolanosterol, obtusifoliol, 4β-desmethyllanosterol, eburicol | ||
|
Function: Sterol 14α-demethylase, 14α-lanosterol demethylase (Figs. 4, 17), a key step in the biosynthesis of membrane sterols and steroid hormones |
||
|
Inhibition: Block biosynthesis of cholesterol in tumors, used for treatment of hypercholesterolemia; fungal enzyme inhibited to treat fungal infections and tropical diseases in humans |
||
|
Inhibitors: | ||
|
Drugs: | ||
|
Antifungal drugs, imidazole and triazole drugs a, weak or non-inhibitors of human enzyme, potent inhibitors of protozoal and fungal enzyme, systemic fungicides, e.g. ketoconazole (Pont et al. 1982; Harwood et al. 2005; Trösken et al. 2006; Strushkevich et al. 2010) | ||
| Azalanstat (used for treatment of hyperlipidemia, benzenamine) (Swinney et al. 1994; Burton et al. 1995; Trzaskos 1995) | ||
| Clotrimazole, bifonazole (Trösken et al. 2006) | ||
| CP-320626 b (used of tratment of type 2 diabetes, indole-2-carboxamide) and analogs (Harwood et al. 2005) | ||
| Cytostatic drugs a: fadrozole, letrozole (Trösken et al. 2006) | ||
| Fluconazole (Harwood et al. 2005; Trösken et al. 2006; Arlt 2007; Krone et al. 2007) | ||
| Itraconazole ((Trösken et al. 2006; Schuster and Bernhardt 2007)) | ||
| Miconazole, econazole (Trösken et al. 2004; Trösken et al. 2006; Strushkevich et al. 2010) | ||
| Voriconazole (Trösken et al. 2006) | ||
|
Natural compounds: | ||
|
Betulafolientriol d, holothurin A d, teasaponin d, capsicoside A d (Kaluzhsiy et al. 2014) | ||
|
Physiological compounds: | ||
|
Oxysterols (e.g., 25-hydroxycholesterol, 15-ketolanosterol c ) (Swinney et al. 1994; Trzaskos 1995; Lepesheva and Waterman 2007) | ||
|
Other compounds and drug candidates: | ||
|
l5α-Fluorosterol (Trzaskos et al. 1993) | ||
| Lanosterol analogs c: 7-oxo-, 15-oxime, 15-keto, 15-hydroxy, 26-oxo, and 32-carboxylic acid derivatives of lanosterol c (Frye and Leonard 1999) | ||
| 2-Phenylimidazole a (Harwood et al. 2005) | ||
| 4-Phenylimidazole a (Matsuura et al. 2005) | ||
| SKF 104976, (32-carboxylic acid derivative of lanosterol) (Mayer et al. 1991) | ||
| Triazoles a (Lepesheva and Waterman 2007) | ||
| VFV a (azole derivative) (Hargrove et al. 2016) | ||
|
Induction: Enhanced cholesterol biosynthesis (possible) | ||
|
Inducers: | ||
|
Physiological compounds: | ||
|
Cholesterol deprivation e (Stromstedt et al. 1996) | ||
| Growth differentiation factor 9 and follicle-stimulating hormone combined treatment e (Nakamura et al. 2015) | ||
Footnotes:
Competitive inhibition, ligand binding, type II binding
Decrease (inhibition) of activity
Reduced/suppressed mRNA level
Competitive inhibition, binding to enzyme active site
Increased mRNA levels
Table 16.
P450 24A1
| Properties | References |
|---|---|
| Natural substrates: Vitamin D3, 1,25-dihydroxy vitamin D3, 25-hydroxy vitamin D3, 1α-hydroxyvitamin D2, 1α,25-dihydroxy vitamin D3, 1α25-dihydroxyvitamin D3, 1α,25-dihydroxy vitamin D2, 1α,25-dihydroxy-20epi-vitamin D3, 1α,24(R)-dihydroxy vitamin D3, 1α,4α,25-trihydroxy vitamin D3, 25-hydroxy vitamin D3-26,23-lactol, 23(S),25,26-trihydroxy vitamin D3, 21α-hydroxy vitamin D2, 1α,25-dihydroxy-19-nor-vitamin D3, 1α,25-dihydroxy-3-epi-vitamin D3, 1β 25-dihydroxy-3-epi-vitamin D3, 1α,25-dihydroxycholecalciferol, 1α,24-dihydroxy vitamin D2, 1α,4β,25-trihydroxy vitamin D3, 20-hydroxy vitamin D3, 20,23-dihydroxy vitamin D3 | (Sakaki et al. 1999; Inouye and Sakaki 2001; Kusudo et al. 2004; Abe et al. 2005; Sakaki et al. 2005; Saito et al. 2009; Kaufmann et al. 2011; Jones et al. 2012, 2014; Tieu et al. 2015; Pan et al. 2016) |
|
Other substrates: 2α-Propoxy-1α,25-dihydroxy vitamin D3, 2α-(3-hydroxypropoxy)-1α,25-dihydroxy vitamin D3, 6,27-hexafluoro-1α,25-dihydroxy vitamin D3 |
|
|
Function: 1,25-Dihydroxyvitamin D3 24-hydroxylase (Figs. 3, 19), inactivates the active vitamin D3 metabolite 1,25-dihydroxyvitamin D3 |
|
|
Inhibition: Elevated level of 1,25-dihydroxyvitamin D3 active vitamin D metabolite, enhancement of the anti-tumor effect of 1α,25-dihydroxyvitamin D3 |
|
|
Inhibitors: (Schuster, Egger, Astecker, et al. 2001; Taban et al. 2017a) | |
|
Drugs: | |
|
Ketoconazole, liarozole a (Schuster, Egger, Bikle, et al. 2001; Vantieghem et al. 2006; Bruno and Njar 2007; Muindi et al. 2010; Luo et al. 2013) | |
| Ritonavir, indinavir, nelfinavir (mild inhibitors of activity) b (Cozzolino et al. 2003) | |
|
Natural compounds: | |
|
Isoflavonoids b (genistein, dihydroxygenistein, tetrahydroxygenistein) (Farhan and Cross 2002; Lechner and Cross 2003; Swami et al. 2005; Bruno and Njar 2007; Wietrzyk 2007) | |
| All trans-Retinoic acid b (Lou, Miettinen, et al. 2005) | |
|
Physiological compound: | |
|
5α-Dihydrotestosterone b (Lou, Nazarova, et al. 2005) | |
|
Physiological condition and illnesses: | |
|
Cancer b (e.g., breast tumor) (Anderson et al. 2006) | |
|
Other compounds: | |
|
Azoles c (e.g., VID400) (Schuster, Egger, Nussbaumer, et al. 2003; Schuster, Egger, Reddy, et al. 2003; Schuster et al. 2006; Bruno and Njar 2007) | |
| Azoles c (e.g., NFP-VAB636, SDZ-89789, SDZ-284814, SDZ-286907, (S)-SDZ 285428), (Schuster, Egger, Nussbaumer, et al. 2003; Schuster et al. 2006) | |
| 1,25-Dihydroxyvitamin D3 analogues d (Posner et al. 2010) | |
| N-(2-(1H-Imidazol-1-yl)-2-phenylethyl)arylamides a (Muindi et al. 2010) | |
| (E)-N-(2-(1H-Imidazol-1-yl)-2-(phenylethyl)-3/4-styrylbenzamides a (Taban et al. 2017b) | |
| MK-24(S)-S(O)(NH)Ph b (Bruno and Njar 2007) | |
| QW1624F2–2 b (Bruno and Njar 2007; Purnapatre et al. 2008) | |
| RC2204 d (Muindi et al. 2010) | |
| Styrylbenzamides d (Taban et al. 2017b) | |
| Styrylindoles a (Ferla et al. 2014) | |
| 24-Sulfone analogs of the hormone 1α,25-dihydroxyvitamin D3 d (Posner et al. 2004) | |
| Sulfone and sulfoximine derivatives (CTA018, CTA091) d (Schuster and Bernhardt 2007) | |
| Tetralones d (e.g., KD-35) (Yee and Simons 2004; Aboraia et al. 2010; Kósa et al. 2013; Luo et al. 2013) | |
|
Induction: enhanced steroid metabolism, proposed for treatment of congenital adrenal hyperplasia, may cause osteomalacia | |
|
Inducers: | |
|
Drugs: | |
|
Bufalin f (Nakano et al. 2005; Amano et al. 2009) | |
| Carbamezepine (very weak inducer), phenobarbital, phenytoin, rifampicin f (Pascussi et al. 2005; Schuster et al. 2006) | |
| Cycloheximide f (Pascussi et al. 2005) | |
| Efavirenz, stavudine, ritonavir f (Ikezoe et al. 2006; Norlin et al. 2017) | |
| Valproic acid f (Vrzal et al. 2011) | |
|
Natural compounds and metabolites: | |
|
1α,25-Dihydroxyvitamin D3 f (Tashiro et al. 2004; Lou, Nazarova, et al. 2005; Pascussi et al. 2005; Ikezoe et al. 2006; Kemmis et al. 2006; Tuckey et al. 2008; Muindi et al. 2010; Horvath et al. 2012; Kósa et al. 2013; Wegler et al. 2016) | |
| 24R,25-Dihydroxyvitamin D3 (Tashiro et al. 2004) | |
| High glucose levels f (Zhou et al. 2015) | |
| 25-Hydroxyvitamin D3 f (Kemmis et al. 2006) | |
| Hyperforin f (Pascussi et al. 2005) | |
|
Physiological compounds: | |
|
Calcitonin f (Gao et al. 2004) | |
| Lithocholic acid f (Ishizawa et al. 2008) | |
| PXR-ligands (Pascussi et al. 2005) | |
| Retinoids f (Zou et al. 1997) | |
| Transforming growth factor β f (Solomon et al. 2014) | |
|
Physiological conditions and illnesses: | |
|
Cancer f (e.g., colon, ovary, prostatic, lung tumors) (Anderson et al. 2006; King et al. 2010; Luo et al. 2013; Ge et al. 2017) | |
| Chronic kidney disease g (Petkovich and Jones 2011) | |
| Psoriasis h (Sumantran et al. 2016) | |
| UVB irradiation f (Vantieghem et al. 2006) | |
|
Other compounds: | |
|
(25R)-25-Adamantyl-1α,25-dihydroxy-2-methylene-22,23-didehydro-19,26,27-trinor-20-epivitamin D3 f (Choi et al. 2011) | |
| Benzo[a]pyrene f (Matsunawa et al. 2009) | |
| Non-1α-hydroxylated vitamin D analogues (Segersten et al. 2005) | |
| ZK 158222 (vitamin D receptor antagonist)´ f (Avila et al. 2007) | |
Footnotes:
Competitive inhibition, ligand binding
Reduced/suppressed mRNA and/or protein level/expression and activity
Competitive inhibition, binding to enzyme active site
Decreased/suppressed/inhibited activity/product formation
Gene downregulated/suppressed
Increased transcription/mRNA/protein expression/levels /and/or catalytic activity
Increased activity, and/or increased product formation
Up-regulation of biosynthesis, increased expression of protein
Table 17.
P450 27B1
| Properties | References |
|---|---|
| Natural substrates: 25-Hydroxyvitamin D3, 27-hydroxyvitamin D3, 24-oxo-,25-hydroxyvitamin D3, 25-hydroxy-3-epi-vitamin D3, 24(R),25-dihydroxyvitamin D3, 20,24-, 20,25-, and 20,26-dihydroxyvitamin D3 | (Inouye and Sakaki 2001; Maas et al. 2001; Sakaki et al. 2005; Tang et al. 2013; Pan et al. 2016) |
|
Function: 25-Hydroxyvitamin D3 1α-hydroxylase (Figs. 18, 20), synthesizes 1α,25-dihydroxyvitamin D3, the active form of vitamin D3, 24(R),25-dihydroxyvitamin D3 1α-hydroxylation |
|
|
Inhibition: Decreased plasma 1α,25-dihydroxyvitamin D levels | |
|
Inhibitors: (Schuster, Egger, Nussbaumer, et al. 2003) | |
|
Drugs: | |
|
Azole drugs a (ketoconazole, liarozole) (Frizen and Zhegin 1976; Schuster et al. 2001; Bruno and Njar 2007) | |
| Efavirenz b (Ellfolk et al. 2009; Wegler et al. 2016) | |
| Ritonavir, indinavir, nelfinavir c (Cozzolino et al. 2003) | |
|
Natural compounds: | |
|
17α,20-Dihydroxyvitamin D3 b (Tang et al. 2013) | |
| 1α,25-Dihydroxyvitamin D3 b (Bland et al. 1999; Xie et al. 2002; Pascussi et al. 2005; Avila et al. 2007; Lechner et al. 2007; Wietrzyk 2007; Wu et al. 2007; Ellfolk et al. 2009) | |
| Genistein c (Farhan and Cross 2002; Farhan et al. 2003) | |
|
Physiological compounds: | |
|
Short hairpin RNA c ((Wu et al. 2007) | |
| Thyroid hormones c (Kozai et al. 2013) | |
|
Physiological condition and illnesses: | |
|
Breast cancer d (Zhalehjoo et al. 2017) | |
|
Other compounds: | |
|
Azoles a ((R)-VID400 weak inhibitor) (Xie et al. 2002; Schuster, Egger, Reddy, et al. 2003; Bruno and Njar 2007) | |
| 8-Bromo cAMP c (Avila et al. 2004) | |
| Calcium b (high concentrations) (Bland et al. 1999) | |
| Imidazoles a (e.g. SDZ-88357, SDZ 89–443, SDZ 284971, SDZ 284814, (R)- SDZ 287871, (R)-SDZ 286907, SDZ 283251, (S)- SDZ 285428, (R)-VAB636) (Schuster et al. 2001; Schuster, Egger, Nussbaumer, et al. 2003; Schuster et al. 2006) | |
| Pyridine compounds a (Schuster, Egger, Nussbaumer, et al. 2003) | |
|
Induction: Increased production of 1,25-dihydroxyvitamin D3 | |
|
Inducers: | |
|
Drugs: | |
|
Raloxifene d (Somjen et al. 2005) | |
| Ritonavir, dexamethasone d (Wegler et al. 2016) | |
|
Natural compounds: | |
|
Carboxy biochainin A, genistein d (Somjen et al. 2005; Lechner et al. 2006; Somjen et al. 2007) | |
| 25-Hydroxyvitamin D3, femarelle, daidzein d (Somjen et al. 2017) | |
| Forskolin e (Bland et al. 1999) | |
|
Physiological compounds: | |
|
5α-Dihydrotestosterone d (Somjen et al. 2007) | |
| 17β-Estradiol d (Lechner et al. 2006) | |
| Parathyroid hormone d (Somjen et al. 2005; Somjen et al. 2007) | |
| Spironolactone d (Alesutan et al. 2013) | |
|
Physiological condition and illnesses: | |
|
Colonic inflammation e (Du et al. 2017) | |
| Psoriasis e (Sumantran et al. 2016) | |
| Non-small cell lung cancer d (Ge et al. 2017) | |
|
Other compounds: | |
|
Calcium e (low concentrations) (Bland et al. 1999) | |
Footnotes:
Competitive inhibition, ligand binding
Gene downregulated/suppressed
Reduced/suppressed mRNA and/or protein level/expression and activity
Increased transcription/mRNA/protein expression/levels /and/or catalytic activity
Up-regulation of biosynthesis, increased expression of protein
Table 18.
P450 26A1
| Properties | References |
|---|---|
| Natural substrates: all-trans-Retinoic acid, all-trans-dihydroretinoic acid, 9-cis-retinoic acid, 4-hydroxyretinoic acid, 18-hydroxyretinoic acid, 4-oxo-retinoic acid, retinal, and retinol | (Lutz et al. 2009; Thatcher et al. 2010; Helvig et al. 2011; Shimshoni et al. 2012; Topletz et al. 2012; Pan et al. 2016) |
|
Function: Retinoic acid-metabolizing enzyme, inactivation of all-trans-retinoic acid to hydroxylated forms 4-hydroxy- and 4-oxo- all-trans-retinoic acid and 18-hydroxy- all-trans-retinoic acid (Figs. 3, 22) |
|
|
Inhibition: Block degradation of endogenous retinoids, application in retinoid therapy, increase the levels of endogenous all-trans-retinoic acid | |
|
Inhibitors: (Njar et al. 2006; Osanai and Lee 2011b; Purushottamachar et al. 2012; Sun, Song, et al. 2015) | |
|
Drugs: | |
|
Azoles (ketoconazole, liarozole, talarozole) (Ocaya et al. 2007; Schuster and Bernhardt 2007; Chang et al. 2008; Pavez Lorie, Chamcheu, et al. 2009; Helvig et al. 2011; Thatcher et al. 2011; Diaz et al. 2016) | |
| Rosiglitazone, pioglitazone a (Thatcher et al. 2011) | |
|
Other compounds: | |
|
(Amide)imidazole derivatives c (Sun, Liu, et al. 2015) | |
| 4-Azoly retinoids (Njar 2002; Quere et al. 2007)CD2503 d (retinoic acid receptor-αspecific antagonist) (Ozpolat et al. 2002; Thatcher et al. 2011) | |
| 3-(1H-Imidazol- and triazol-1-yl)-2,2-dimethyl-3-(4-(naphthalen-2-ylamino)phenyl)propyl derivatives a (Gomaa, Bridgens, Veal, et al. 2011) | |
| Imidazole methyl 3-(4-(aryl-2-ylamino)phenyl)propanoates c (Gomaa, Bridgens, Aboraia, et al. 2011) | |
| Imidazole- and triazole-containing inhibitors a (Thatcher et al. 2011) | |
| R116010 a (benzoimidazolamine derivative) (Armstrong et al. 2005) | |
| Tetralones 4-hydroxyphenyl substituted b (e.g. KD-35) (Yee et al. 2005; Njar et al. 2006) | |
| 3-{4-[2-(5,5,8,8-Tetramethyl-5,6,7,8-tetrahydronaphthalen-2-yl)-1,3-dioxolan-2-yl] phenyl}4-propanoic acid a (Diaz et al. 2016) | |
|
Induction: increased catabolism of retinoids, diminishing the levels of active all-trans-retinoic acid in the course of therapy | |
|
Inducers: | |
|
Drugs: | |
| Fenretinide e (Ozpolat et al. 2004; Villani et al. 2004) | |
| Talarozole e (Pavez Lorie, Cools, et al. 2009; Pavez Lorie, Li, et al. 2009) | |
|
Natural compounds: | |
|
(4S)-Hydroxy all-trans-retinoic acid, (4R)-hydroxy-all-trans-retinoic acid, 4-oxo-all-trans-retinoic acid, 18-hydroxy-all-trans-retinoic acid e (Topletz et al. 2015) | |
| 13-cis-Retinoic acid, 9-cis-retinoic acid, all-trans-retinoic acid e (Lampen et al. 2000; Ozpolat et al. 2002; Armstrong et al. 2005; Ozpolat et al. 2005; Chang et al. 2008; Pavez Lorie, Chamcheu, et al. 2009; Pavez Lorie, Li, et al. 2009; Wang et al. 2009; Topletz et al. 2015) | |
| Lithocholic acid e (Chang et al. 2008) | |
| Nicotine d (Osanai and Lee 2011b) | |
|
Physiological conditions and illnesses: | |
|
Barrett’s associated adenocarcinoma e (Chang et al. 2008) | |
| Breast, colorectal, cervical squamous neoplasia, and head and neck cancers e (Brown et al. 2014; Osanai and Lee 2014, 2015) | |
| Skin from chronically sun-exposed body areas e (Osanai and Lee 2011a) | |
Footnotes:
Decreased/suppressed/inhibited activity/product formation
Competitive inhibition, binding to enzyme active site
Competitive inhibition, ligand binding
Reduced/suppressed mRNA and/or protein level/expression and activity
Increased transcription/mRNA/protein expression/levels /and/or catalytic activity
Table 19.
P450 26B1
| Properties | References |
|---|---|
| Natural substrates: all-trans-Retinoic acid, all-trans-dihydroretinoic acid, 9-cis-retinoic acid, 4-hydroxyretinoic acid, 18-hydroxyretinoic acid, 4-oxo retinoic acid, retinal, and retinol | (Helvig et al. 2011; Ross and Zolfaghari 2011; Topletz et al. 2012; Pan et al. 2016) |
|
Function: Retinoic acid-metabolizing enzyme, inactivation of all-trans-retinoic acid to hydroxylated forms 4-hydroxy- and 4-oxo-all-trans-retinoic acid and 18-hydroxy-all-trans-retinoic acid (Figs. 21, 23) |
|
|
Inhibition: Application in retinoid therapy, increase the levels of endogenous all-trans-retinoic acid | |
|
Inhibitors: (Njar 2002; Njar et al. 2006) | |
|
Drugs: | |
|
Azoles a (ketoconazole, liarozole, talarozole) (Ocaya P et al. 2007; Pavez Lorie, Chamcheu, et al. 2009; Helvig et al. 2011; Kumar et al. 2011; Diaz et al. 2016) | |
|
Natural compounds: | |
|
9-cis-Retinoic acid, 13-cis-retinoic acid, all-trans-retinoic acid b (Taimi et al. 2004; Helvig et al. 2011) | |
|
Other compounds: | |
|
4-Azoly retinoids (Njar 2002) | |
|
Induction: May increase the catabolism of retinoids, diminishing the levels of active all-trans-retinoic acid in the course of therapy | |
|
Inducers: | |
|
Drugs: | |
|
Talarozole c (Pavez Lorie, Cools, et al. 2009; Ocaya PA et al. 2011) | |
|
Natural compounds: | |
|
9-cis β-Carotene c (Bechor et al. 2016) | |
| all-trans-Retinoic acid c (Pavez Lorie, Chamcheu, et al. 2009; Pavez Lorie, Li, et al. 2009) | |
|
Physiological conditions and illnesses: | |
|
Colorectal cancer c (Brown et al. 2014) | |
| Ovarian cancer c (Downie et al. 2005) | |
Footnotes
Competitive inhibition, ligand binding
Competitive inhibition, binding to enzyme active site
Increased transcription/mRNA/protein expression/levels /and/or catalytic activity
Table 20.
P450 26C1
| Properties | References |
|---|---|
| Natural substrates: all-trans-Retinoic acid, 9-cis-retinoic acid, 13-cis-retinoic acid, all-trans-4-oxo retinoic acid, all-trans-dihydroretinoic acid | (Taimi et al. 2004; Helvig et al. 2011; Pan et al. 2016; Zhong et al. 2018) |
|
Function: Retinoic acid metabolism, conversion of all-trans-retinoic acid to hydroxylated forms (4-hydroxy- and 4-oxo- all-trans-retinoic acid and 18-hydroxy-all-trans-retinoic acid (Figs. 21, 23)). All-trans-4-oxo retinoic acid is oxidized to all-trans-4-oxo-16-hydroxy retinoic acid, and 13-cis-retinoic acid is hydroxylated at the 4 and 16 positions. |
|
|
Inhibition: Increases the levels of endogenous all-trans-retinoic acid | |
|
Inhibitors: | |
|
Drugs: | |
|
Ketoconazole a (Taimi et al. 2004; Zhong et al. 2018) | |
| Talarozole a (Zhong et al. 2018) | |
|
Induction: May contribute to increase the catabolism of retinoids, diminishing the levels of active all-trans-retinoic acid in the course of therapy | |
|
Inducers: | |
|
Natural compounds: | |
|
all-trans-Retinoic acid, 9-cis retinoic acid c (Taimi et al. 2004) | |
|
Physiological condition and illnesses: | |
|
Primary breast carcinoma c (Osanai and Lee 2016) | |
Footnotes:
Competitive inhibition, ligand binding
Competitive inhibition, binding to enzyme active site
Increased transcription/mRNA/protein expression/levels /and/or catalytic activity
Table 21.
P450 27C1
| Properties | References |
|---|---|
| Natural substrates: all-trans Retinol (vitamin A1), all-trans retinal and all-trans retinoic acid, 11-cis-retinal, all-trans retinol acetate | (Vahlquist 1980; Törmä and Vahlquist 1985; Kramlinger et al. 2016; Pan et al. 2016; Johnson et al. 2017) |
|
Function: Retinoid 3,4-desaturase, oxidation of all-trans retinol to 3,4-didehydroretinol (vitamin A2) (Figs. 21, 24) |
|
|
Inhibitorsa: | |
| Drugs: | |
|
Acitretin, arotenoid Ro 13–7410, 4-(N-hydroxyphenyl)retinamide, ketoconazole (Törmä et al. 1991) | |
|
Natural product: | |
|
13-cis-Retinoic acid (Rollman et al. 1993; Törmä et al. 1991) | |
| Citral (Törmä et al. 1991) | |
|
Inducersa: | |
|
Physiological compound: | |
|
Cellular retinoid-binding protein (Andersson et al. 1994) | |
|
Physiological condition amd illnesses: | |
|
Squamous cell carcinoma and keratoacanthoma (Vahlquist et al. 1996) | |
| UV light exposure (UVA, UVB) (Tafrova et al. 2012) | |
Footnote:
Mechanisms have not been investigated. All cited studies were only done at a cellular activity level before the assignment of P450 27C1.
Table 22.
P450 20A1
| Properties | References |
|---|---|
| Natural substrates: function unknown, still considered to be an “orphan” enzyme | (Marek et al. 2007; Stark and Guengerich 2007; Stark et al. 2008; Lemaire et al. 2016) |
|
Inhibition: | |
| No information available | |
|
Induction: | |
| No information available | |
Acknowledgments.
In memory of Vjekoslava (Vjeka) Rendic.
Funding details: This work was supported in part by the U. S. National Iinstitutes of Health under Grants R01 GM118122 and R01 GM103937. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Disclosure of Interest: The authors report no conflicts of interest with the contents of this article.


