Abstract
Postsynaptic AMPA/glutamate receptors, essential for neuronal excitability, are important targets for anticonvulsant therapy. This single channel study of the selective noncompetitive AMPA receptor antagonist, perampanel, was performed on homotetrameric GluA3 receptor-channels that open in a stepwise manner to four distinct conductance levels through independent subunit activation. Previous structural studies show that perampanel binds to four sites located within the extracellular/transmembrane boundary of closed AMPA receptor-channel subunits. We found that channels exposed to 1 or 2 μM perampanel opened mainly to the two lower conductance levels in a dose-dependent manner. Comparison of the single channel results in the structures of the full length AMPA receptor in the closed state bound to perampanel, and the open state provide insights into the mechanism of allosteric reduction of AMPA-receptor-mediated excitation in epilepsy.
Keywords: AMPA receptor, glutamate receptor, epilepsy, single channel recording, allosteric modulation
Disruption of synaptic signaling underlies many forms of epilepsy, through either increased activity of glutamate receptors or through reduced inhibition when γ-amino butyric acid (GABA) receptors are primarily affected.1,2 In the presence of these alterations, glutamatergic synaptic transmission is largely responsible for generating seizure activity.3 Excitation spreads within the central nervous system (CNS) network by stimulated release of presynaptic glutamate followed by glutamate binding to and activation of postsynaptic ionotropic glutamate receptors (iGluRs). Among the potential glutamate targets, the AMPA and kainate receptors responsible for fast transient CNS signaling activate rapidly and desensitize rapidly, while N-methyl-d-aspartate (NMDA) receptors activate slowly and desensitize moderately in the continuous presence of glutamate. AMPA receptors are proving to be a particularly useful molecular target for a new class of noncompetitive antagonist anticonvulsants. Efforts to discover new anticonvulsants have led to the development of a highly selective negative allosteric modulator of AMPA receptors, perampanel (Fycompa: [2-(2-oxo-1-phenyl-5-pyridin-2-yl-1,2-dihydro-pyridin-3-yl)benzonitrile]).4−8 The binding sites for perampanel have been identified in the closed conformation of the AMPA receptor channel.9 Here, we have investigated how AMPA receptor gating machinery is modulated using single channel recordings in maximally activated AMPA receptor channels.
The amino acid sequences of GluA1–4 AMPA receptor subunits are nearly identical in their extracellular and pore domains and all share a common set of structural motifs.10 Each of the four subunits in the holoreceptor contributes an extracellular amino-terminal domain (ATD), a ligand-binding domain (LBD), a transmembrane/ion channel domain (TMD), and an intracellular C-terminal domain. Agonist molecules, including the neurotransmitter glutamate, bind between lobes 1 and 2 (L1, L2) of each of the bilobate LBDs, leading to a closed-cleft conformation, subunit activation and receptor desensitization.11−13 Asymmetry at the LBD level is manifested by the back-to-back orientation of adjacent L1/L1 subunits, which form a dimer interface in closed receptors. This interface breaks apart in desensitized receptors as closure of the cleft around the agonist places strain on the hydrogen bonds between subunits.13 The potent positive modulator cyclothiazide (CTZ), which binds to and stabilizes the dimer interface, suppresses desensitization, and increases open channel lifetime,14 was used here as described previously.15
Crystal structures of three negative modulators, including perampanel, bound to GluA2 show four binding sites, one per AMPA receptor subunit.9 Perampanel makes direct contacts to the preM1 linker and the M1, M3, and M4 helices within each subunit, with a possible contact to M3 of an adjacent subunit (S615). AMPA receptors open stepwise to four conductance levels in the absence of perampanel. In the single channel studies presented here, perampanel increased the proportion of lower conductance events in maximally activated AMPA receptors in a concentration-dependent manner, consistent with binding to and stabilization of individual subunits in a closed conformation. The mechanism of action of perampanel on single channel gating is discussed in the context of recently published structures of AMPA receptors in open and closed channel conformations.
Responses to perampanel were measured in whole cell recordings (Figure 1A). Each cell was exposed to 5 mM l-glutamate and CTZ in the presence and absence of perampanel. Perampanel blocked whole cell currents from GluA3 with relatively slow kinetics (Figure 1A), as has been described previously.8 The concentration–effect curve for perampanel-meditated inhibition is shown in Figure 1B. Assuming simple inhibition, the IC50 for perampanel is 2.1 μM. At perampanel concentrations that produced less than 50% inhibition at equilibrium, sufficient initial single channel activity was observed before steady-state inhibition was reached, allowing identification of patches containing only one channel.
Figure 1.
Concentration-dependent inhibition by perampanel in whole cell recordings. (A) Examples of responses to 1, 3, and 10 μM perampanel recorded from different cells (Vh = −60 mV) demonstrate the slow onset of inhibition. (B) Whole cell steady-state responses to inhibitors were normalized to currents in 5 mM glutamate in each of 19 cells, and concentration–effect data are shown (3–10 cells per point) along with the structure of perampanel (CAS 380917–97–5). Fifty percent inhibition was obtained at 2.1 μM.
Single homomeric GluA3i-G (i = flip,16 G in R/G editing site17) AMPA receptor-channels were recorded using patch-clamp in the cell-attached configuration (10–45 min) in the absence and presence of inhibitors using a high concentration of l-glutamate (5 mM) and saturating concentrations of cyclothiazide (150–200 μM CTZ) to inhibit desensitization. The low expression level of homomeric GluA3i-G AMPA receptor channels typically results in one channel per patch. In the absence of inhibitors, an individual AMPA receptor channel exposed to 5 mM l-glutamate can open to four distinct conductance levels (O1–O4) of approximately 10, 23, 33, and 47 pS.18
In the absence of desensitization, the population of each of these conductance states is dependent, in part, on the glutamate concentration, with the higher conductance levels more likely to be observed as the concentration of glutamate increases.15 This has been used, along with other evidence, to suggest that individual subunits can gate independently,19 with the highest conductance level (O4) associated with glutamate bound to all four subunits and all four subunits in the open conformation. The lowest conductance level (O1) would arise from the gating of a single subunit. Figure 2 shows a control high activity cell-attached patch and patches in the presence of perampanel (1 or 2 μM). The control patch (Figure 2A) retained high activity throughout the recording, while recordings with coapplication of perampanel and glutamate initially showed higher activity before the inhibitor reached its steady-state effects (Figure 2B,C). When steady-state was attained at 1 μM perampanel, channels opened to all four levels, but openings to O3 were infrequent and openings to O4 were rare. In 2 μM perampanel, openings to levels O3 were rare and to O4 were not detected.
Figure 2.
Perampanel raw data acquired initially upon patch formation and subsequently in steady-state conditions. A1, B1, and C1 show initial segments of recordings from cell-attached recordings for control and with 1 and 2 μM perampanel, respectively. Openings to O3 and O4 were relatively frequent before the effect of perampanel reached equilibrium (B2 and C2). Steady-state channel behavior with perampanel present occurred within 25–50 s (1 μM) and 10–15 s (2 μM) of patch formation.
Figure 3 compares the results of single channel analyses carried out on a high activity channel recorded in the absence of inhibitor under steady-state conditions (Figure 3A), to another high activity channel exposed to 1 μM perampanel (Figure 3B), and yet another channel exposed to 2 μM perampanel (Figure 3C). The channel in Figure 3B is defined as “high activity” based on its presteady state activity (see Figure 2B2). A reaction scheme with five conductance levels (one closed, C, and four open, O1, O2, O3, and O4) was used to idealize (SKM, segmental k-means algorithm20) the multilevel openings in the control (Figure 3A), generating a table of events and an amplitude histogram. For high activity control records, the relative occupancies of each conductance level were: O3 > O2 > O1> O4 > C. A linear-branched model was used for kinetic modeling.15,21 Transitions between adjacent conductance levels are connected in a line (each component assigned a numerical subscript (O1, O2, etc.)) and kinetic components within a given conductance level are connected to the trunk of the model by linear branches (subscripts denoting different kinetic components within a conductance level are letters, O1a, O1b, etc.).
Figure 3.

Perampanel decreased openings to higher conductance levels. (A) Control data from a 10 min record of a high activity channel in a cell-attached patch recording. Single channel openings are mainly to level O3, and the amplitude histogram produced by SKM idealization shows five conductance levels: C, O1, O2, O3, and O4, with amplitudes of 0.14 ± 0.92, 1.7 ± 0.98, 3.6 ± 1.3, 5.0 ± 1.3, and 6.0 ± 1.3 pA and occupancies of 0.043, 0.11, 0.22, 0.51, and 0.12, respectively. Subjecting the 14 state linear-branched model to global fitting resulted in a LL/event of 4.16. The energy landscape shows the O3a,b and O2b,c were the most stable states with time constant (τ, ms) and area (in parentheses) values of 1.4 (0.68) and 4.9 (0.29) for O2b,c and 2.8 (0.61) and 11 (0.38) for O3a,b, respectively. Analyses of single channels at equilibrium with 1 μM and 2 μM perampanel are shown: (B) a channel in the presence of 1 μM perampanel was fit by amplitudes of 0.05 ± 1.2, 2.0 ± 1.3, 3.0 ± 1.3, 4.5 ± 1.3, and 6.4 ± 1.3 pA with fractional occupancies of 0.13, 0.33, 0.55, 0.023, and 0.001 for C, O1, O2, O3, and O4 levels, respectively. A linear-branched 11 state-model fit the events with a LL/event of 3.50; the preferred lower energy states were O1a,b,c and O2a,b with time constant (τ, ms) and area (in parentheses) values of 0.56 (0.44), 4.1 (0.34) and 26 (0.22) for O1a,b,c and 7.4 (0.53) and 26 (0.46) for O2a,b respectively. (C) A channel exposed to 2 μM perampanel opened mainly to O1 and the amplitude histogram showed O1 and O2 levels occupancies of 0.62 and 0.32 with amplitudes of 2.2 ± 1.2 and 3.3 ± 1.3, respectively. Channel activity was fit with a linear-branched model with seven states and a LL/event of 4.07, and despite the presence of the inhibitor, open states were more stable than closed ones. Time constant (τ, ms) and area (in parentheses) values were 1.3 (0.79), 5.3 (0.20) and 39 (0.003) for C, 4.1 (0.80) and 17 (0.20) for O1a,b, and 1.9 (0.68) and 10 (0.32) for O2a,b, respectively.
Similar models were applied to the recording in the presence of 1 and 2 μM perampanel (Figure 3B,C). However, in 1 μM perampanel, the relative occupancy patterns were O2 > O1 > O3 > C > O4 (Table 1), with each of the four levels having amplitudes similar to those observed in the absence of perampanel. In 2 μM perampanel, most events were in O1 > O2 levels. O3 was only 1–2% populated, and openings to O4 were not detected. The basic reaction scheme that fit all of the data was a linear-branched model with fewer states than in the control data. Inspection of the transition rates within the reaction scheme reveals that the forward rates from O2a to O3a and from O3a to O4a, were markedly decreased in 1 μM perampanel compared to control rates (Figure 3, B vs A). As expected channel activity was reduced more profoundly in 2 μM perampanel (Figure 3C). These results are consistent with a model in which perampanel inhibits the gating of individual subunits.
Table 1. Fractional Occupancy (± SD) for Each Conductance Level in Control and 1 and 2 μM Perampanel-Exposed Patches.
| group | C | O1 | O2 | O3 | O4 | total # events |
|---|---|---|---|---|---|---|
| control n = 3 | 0.037 ± 0.010 | 0.16 ± 0.059 | 0.23 ± 0.051 | 0.44 ± 0.14 | 0.14 ± 0.050 | 396,416 |
| 1 μM n = 2 | 0.078 ± 0.061 | 0.30 ± 0.059 | 0.53 ± 0.021 | 0.13 ± 0.16 | 0.001 | 112,096 |
| 2 μM n = 3 | 0.23 ± 0.15 | 0.49 ± 0.22 | 0.25 ± 0.16 | 0.015 ± 0.027 | 51,421 |
As shown in Figure 1, perampanel clearly inhibits whole cell AMPA receptor activity with relatively slow kinetics when glutamate is applied before coapplication of perampanel and glutamate. On the single channel level, inhibition increases with time and reaches its equilibrium level after the formation of a patch when 1 or 2 μM perampanel is coapplied with 5 mM glutamate (Figure 2B,C), and inhibition develops with the apparent blockade of transitions to higher conductance (O3 and O4) levels (Figure 3). Because previous work has shown that the kinetic behavior of AMPA receptors is complicated by the channel shifting between different kinetic modes in individual recordings,15,21−23 we entertained the hypothesis that perampanel may interact with complex modal gating. The different kinetic behaviors present in control recordings translate into the observation that, at high glutamate concentrations, a channel opening mainly to the O3 or O4 conductance level is in one of the higher kinetic modes, and when it is mainly to O1 and O2 conductance levels, it is in one of the lower modes.15,21 Here we sought to understand if perampanel changed the gating mode/occupancy pattern, or if inhibition more likely stems from perampanel binding to and stabilizing the closed conformation of individual independently gating subunits.
Figure 4A shows an example of the highest activity mode from a control channel. A control recording from another channel that was mainly in one of the lower modes is shown for comparison (Figure 4B). The high activity mode exhibits mainly occupancy in the O3 and O4 levels, and the low activity mode favors the O2 level. Comparing raw data in Figure 2, even initially highly active patches in the presence of 1 and 2 μM perampanel used in this study showed a predominance of O1 and O2 levels at equilibrium (Figures 3B,C). In 1 μM perampanel, the channel did open to O3 and O4, albeit rarely. By contrast, during the low activity pattern in the control, a significant number of openings to the O3 and O4 levels is observed. This is in stark contrast to the perampanel records, particularly at 2 μM, where essentially no transitions to the higher conductance levels are observed.
Figure 4.

Amplitude histograms, kinetic models, and energy landscapes from high (A) and low (B) activity control records.
Occupancy differences stem from kinetic differences, which also support the interaction of perampanel with individual subunits opening independently. In channels manifesting mainly higher activity patterns, the forward rates are faster than in channels with lower activity patterns, and there is greater fractional occupancy in the higher conductance levels (Figure 4). In the presence of 1 μM perampanel, the early forward rates (closed to O1 and O1 to O2) are faster than the later ones in the reaction scheme (O2 to O3 and O3 to O4). These later rates are much slower than those observed in the low activity control pattern. Taken together, these kinetic differences between lower modes in control and the more rapid rates for individual subunit opening in perampanel suggest that perampanel can bind to and inhibit the gating of individual subunits. Those subunits that are not bound by inhibitor appear to open and close independently.
Assuming that perampanel has only inhibitory action, two models could potentially explain its action: (1) the binding of one molecule of perampanel to a site on an AMPA receptor could completely inhibit its activity or (2) the binding to an individual subunit could inhibit the contribution of that subunit, while leaving unbound subunits unimpaired. An example of the first alternative is an open channel blocker. With rapid kinetics of binding (relative to the channel lifetime), a flickering block can be observed, which if sufficiently rapid can manifest as a decrease in channel conductance. This is clearly not the case with perampanel action of GluA3 channels. If, however, the block is slow, then depending upon the kinetics, one might observe only a decrease in channel lifetime or no channel activity at all. If the concentration of the blocker is sufficiently low, then unbound channels would be observed preferentially.24 Neither of these possibilities seems to apply here since perampanel decreases the population of the higher conductance states but seems to leave the lower conductance states intact. Given this and previous work showing that individual subunits gate independently, the second model seems more plausible.
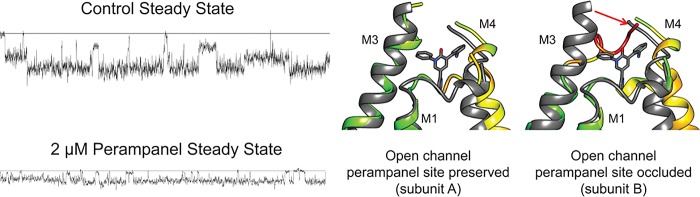
The crystal and cryo-EM structures of the perampanel-bound9 and the partially25 and fully open states26 of GluA2 can provide insight into the mechanism. In the closed state, perampanel binds to each of the four subunits at a site where the linkers between the ligand binding domain and the extracellular part of the ion channel meet (Figures 5A,B).9 This is the point at which the protein transitions from the 2-fold symmetry of the binding domains to the 4-fold symmetry of the ion channel. One might envision that inhibitory action would involve preventing the movement of helices required for transitioning to the open state. The number of perampanel molecules bound would determine the number of inoperable subunits within the tetramer. However, the fully open-state structures provide an additional view that is further consistent with the single channel data. In the fully open state, the binding sites for perampanel are no longer identical (4-fold symmetry) but now show a 2-fold symmetry, with two of the four subunits available and two occluded by rearrangement of linker domains (Figure 5C). Although the structure of a channel in a lower conductance state has not been determined, an open channel of any conductance may have at least one binding site occluded and perhaps two. This leaves two or three perampanel sites available that could then limit the opening to only the second conductance level as is seen in Figure 2. It is likely that this phenomenon would only be seen at concentrations of perampanel near its IC50, as is used in this study. At much higher concentrations, the probability of maintaining binding to all four subunits is increased, completely preventing channel opening. This would not be observed in the single channel experiments (only open channels are observed) but is the explanation for the complete inhibition in whole cell studies. At concentrations much lower than the IC50, channels without perampanel bound would be preferentially observed.
Figure 5.

(A,B) Structures showing the binding site for perampanel on GluA2 (PDBid: 5L1F(9)). (C) Individual subunits of the open structure (PDBid: 5WEO(26)) of the AMPA receptor ion channel domain were aligned with the best fit perampanel-bound closed structure (gray, PDBid: 5L1F(9)) using UCSF-Chimera.27 Open-state channel subunit A (left) maintains a perampanel binding site, while in channel subunit B (right), the perampanel binding site is occluded by the shift in M3 linker (red arrow).
The combination of single channel and structural information provide new insights into the subtleties of perampanel action. It is not a simple noncompetitive inhibitor of AMPA receptor channels, in that the channel can function when a subset of binding sites on the protein is occupied by perampanel. This shifts the distribution of open states to lower conductances, producing a more graded response characteristic. This tuning of the response may have beneficial therapeutic effects in that it permits channel activity but with lower amplitude perhaps allowing affected neurons to function normally in the face of an epileptic challenge.
Acknowledgments
The authors would like to thank Michael A. Rogawski (U.C. Davis) for supplying perampanel, and Matthew T. Sipple and Gregory A. Weiland for reading and making valuable comments on the manuscript. We are indebted to the late Ms. Estefania Negrete-Alvarado whose technical assistance and infectious enthusiasm we valued greatly.
Glossary
ABBREVIATIONS
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid;
- ATD
amino terminal domain
- CNS
central nervous system
- CTZ
cyclothiazide
- GABA
γ-amino butyric acid
- LBD
ligand binding domain
- MIL
maximum interval likelihood analysis
- NMDA
N-methyl d-aspartic acid
- SD
standard deviation
- SKM
segmental k-means algorithm
- TMD
transmembrane domain.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsmedchemlett.8b00322.
Experimental details regarding cell culture, whole cell and single channel recording, and data analysis (PDF)
Author Present Address
† EYS SUNY Downstate Medical Center, 450 Clarkson Avenue, Brooklyn, New York 11203, United States.
Author Contributions
‡ These authors contributed equally. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
This work was supported by a grant from NINDS (R01 NS085239) to R.E.O.
The authors declare no competing financial interest.
Supplementary Material
References
- Macdonald R. L.; Kang J. Q.; Gallagher M. J. Mutations in GABAA receptor subunits associated with genetic epilepsies. J. Physiol. 2010, 588 (Pt 11), 1861–9. 10.1113/jphysiol.2010.186999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan H.; Low C. M.; Moody O. A.; Jenkins A.; Traynelis S. F. Ionotropic GABA and Glutamate Receptor Mutations and Human Neurologic Diseases. Mol. Pharmacol. 2015, 88 (1), 203–17. 10.1124/mol.115.097998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogawski M. A.; Donevan S. D. AMPA receptors in epilepsy and as targets for antiepileptic drugs. Adv. Neurol 1999, 79, 947–63. [PubMed] [Google Scholar]
- Rogawski M. A. Revisiting AMPA receptors as an antiepileptic drug target. Epilepsy Curr. 2011, 11 (2), 56–63. 10.5698/1535-7511-11.2.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogawski M. A. AMPA receptors as a molecular target in epilepsy therapy. Acta Neurol Scand Suppl 2013, 127 (197), 9–18. 10.1111/ane.12099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogawski M. A.; Hanada T. Preclinical pharmacology of perampanel, a selective non-competitive AMPA receptor antagonist. Acta Neurol Scand Suppl 2013, 127 (197), 19–24. 10.1111/ane.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBonaventura C.; Labate A.; Maschio M.; Meletti S.; Russo E. AMPA recepotors and perampanel behind selected epilepsies: current evidence and future perspectives. Expert Opin. Pharmacother. 2017, 18, 1751–1764. 10.1080/14656566.2017.1392509. [DOI] [PubMed] [Google Scholar]
- Chen C. Y.; Matt L.; Hell J. W.; Rogawski M. A. Perampanel inhibition of AMPA receptor currents in cultured hippocampal neurons. PLoS One 2014, 9 (9), e108021. 10.1371/journal.pone.0108021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelshanskaya M. V.; Singh A. K.; Sampson J. M.; Narangoda C.; Kurnikova M.; Sobolevsky A. I. Structural Bases of Noncompetitive Inhibition of AMPA-Subtype Ionotropic Glutamate Receptors by Antiepileptic Drugs. Neuron 2016, 91 (6), 1305–15. 10.1016/j.neuron.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galen Wo Z.; Oswald R. E. Unraveling the modular design of glutamate-gated ion channels. Trends Neurosci. 1995, 18, 161–168. 10.1016/0166-2236(95)93895-5. [DOI] [PubMed] [Google Scholar]
- Armstrong N.; Gouaux E. Mechanisms for activation and antagonism of an AMPA-sensitive glutamate receptor: crystal structures of the GluR2 ligand binding core. Neuron 2000, 28 (1), 165–81. 10.1016/S0896-6273(00)00094-5. [DOI] [PubMed] [Google Scholar]
- Armstrong N.; Sun Y.; Chen G. Q.; Gouaux E. Structure of a glutamate-receptor ligand-binding core in complex with kainate. Nature 1998, 395, 913–917. 10.1038/27692. [DOI] [PubMed] [Google Scholar]
- Sun Y.; Olson R.; Horning M.; Armstrong N.; Mayer M.; Gouaux E. Mechanism of glutamate receptor desensitization. Nature 2002, 417 (6886), 245–53. 10.1038/417245a. [DOI] [PubMed] [Google Scholar]
- Mitchell N. A.; Fleck M. W. Targeting AMPA receptor gating processes with allosteric modulators and mutations. Biophys. J. 2007, 92 (7), 2392–402. 10.1529/biophysj.106.095091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon K.; Nowak L. M.; Oswald R. E. Characterizing single-channel behavior of GluA3 receptors. Biophys. J. 2010, 99 (5), 1437–46. 10.1016/j.bpj.2010.06.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer B.; Keinänen K.; Verdoorn T. A.; Wisden W.; Burnashev N.; Herb A.; Köhler M.; Takagi T.; Sakmann G.; Seeburg P. H. Flip and flop: A cell-specific functional switch in glutamate-operated channels of the CNS. Science 1990, 249, 1580–1584. 10.1126/science.1699275. [DOI] [PubMed] [Google Scholar]
- Lomeli H.; Mosbacher J.; Melcher T.; Hoger T.; Geiger J. R.; Kuner T.; Monyer H.; Higuchi M.; Bach A.; Seeburg P. H. Control of kinetic properties of AMPA receptor channels by nuclear RNA editing. Science 1994, 266, 1709–1713. 10.1126/science.7992055. [DOI] [PubMed] [Google Scholar]
- Shi E. Y.; Yuan C. L.; Sipple M. T.; Srinivasan J.; Ptak C. P.; Oswald R. E.; Nowak L. M.. Tuning AMPA receptor-channel gating with noncompetitive antagonists. In preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C.; Stern-Bach Y.; Stevens C. F. The tetrameric structure of a glutamate receptor channel. Science 1998, 280, 1596–1599. 10.1126/science.280.5369.1596. [DOI] [PubMed] [Google Scholar]
- Nicolai C.; Sachs F. Solving ion channel kinetics with the QuB software. Biophys. Rev. Lett. 2013, 8 (03n04), 191–211. 10.1142/S1793048013300053. [DOI] [Google Scholar]
- Poon K.; Oswald R. E.; Nowak L. M., Current Recording and Kinetic Analyses for Single AMPA Receptors. In Ionotropic Glutamate Receptor Technologies, Popescu K. G., Ed.; Springer: New York, 2016; pp 257–272. [Google Scholar]
- Poon K.; Ahmed A. H.; Nowak L. M.; Oswald R. E. Mechanisms of modal activation of GluA3 receptors. Mol. Pharmacol. 2011, 80 (1), 49–59. 10.1124/mol.111.071688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prieto M. L.; Wollmuth L. P. Gating modes in AMPA receptors. J. Neurosci. 2010, 30 (12), 4449–59. 10.1523/JNEUROSCI.5613-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald J. F.; Nowak L. M. Mechanisms of blockade of excitatory amino acid receptor channels. Trends Pharmacol. Sci. 1990, 11 (4), 167–72. 10.1016/0165-6147(90)90070-O. [DOI] [PubMed] [Google Scholar]
- Chen S.; Zhao Y.; Wang Y.; Shekhar M.; Tajkhorshid E.; Gouaux E. Activation and Desensitization Mechanism of AMPA Receptor-TARP Complex by Cryo-EM. Cell 2017, 170 (6), 1234. 10.1016/j.cell.2017.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twomey E. C.; Yelshanskaya M. V.; Grassucci R. A.; Frank J.; Sobolevsky A. I. Channel opening and gating mechanism in AMPA-subtype glutamate receptors. Nature 2017, 549 (7670), 60–65. 10.1038/nature23479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersen E. F.; Goddard T. D.; Huang C. C.; Couch G. S.; Greenblatt D. M.; Meng E. C.; Ferrin T. E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25 (13), 1605–12. 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.