Abstract
An increase in parasympathetic tone may be the main cause of some transient or permanent atrioventricular block cases. Some of these patients, defined as vagally mediated functional atrioventricular block, may be severely symptomatic and refractory to conventional therapies and may necessitate cardiac pacing. Cardioneuroablation is a relatively new strategy for management of patients with excessive vagal activation based on radiofrequency catheter ablation of main ganglionated plexi around the heart. Present review was dedicated to discuss potential usage of cardioneuroablation in patients with vagally mediated functional atrioventricular block.
Keywords: Cardiovascular, syncope, parasympathetic, bradycardia, ablation
Introduction
Syncope due to atrioventricular block (AVB) accounts for more than half of arrhythmic syncope.1 Paroxysmal AVB is a relatively little known type of AVB and may cause syncope because of a sudden change from apparently physiological atrioventricular conduction to transient second- or third-degree AVB, which leads to ventricular asystole. Despite this theoretical knowledge, determination of the underlying mechanism of AVB is usually difficult.2 Vagally mediated functional AVB is a condition in which a paroxysmal AVB occurs with a slowing of sinus rate and named as functional because there is no intrinsic abnormality in the atrioventricular conduction system.3 Although the great majority of patients may be followed without any special treatment, cardiac pacing may be necessary in patients with severe forms such as very frequent syncope altering quality of life, recurrent syncope without prodromal symptoms which exposes the patient to a risk of trauma, and syncope occurs during a high-risk activity. Furthermore, there is no a rationale recommendation for cardiac pacing due to the lack of sufficient evidence from studies.1 As a new treatment strategy, radiofrequency catheter ablation ablation (RFCA) of ganglionated plexi (GPs) located close to sinus and atrioventricular nodes was reported to abolish the vagal efferent output during vasovagal syncope, functional AVB, and sinus node dysfunction in some observational studies and case reports.4–7 The technique was named as cardioneuroablation (CNA) by operators who made the first application.4
This review aimed to discuss potential role of CNA as a new strategy to treat vagally mediated functional AVB.
Vagally mediated functional atrioventricular block as a clinical entity
Vagally mediated functional AVB is one of the paroxysmal AVB types which is characterized by a sudden change from apparently physiological atrioventricular conduction to transient second- or third-degree heart block due to parasympathetic influence on cardiac conduction. The other types of paroxysmal AVB are intrinsic paroxysmal AVB which is due to an intrinsic disease of the atrioventricular conduction system and extrinsic idiopathic paroxysmal AVB which is associated with low values of endogenous adenosine.8 Real incidence of vagally mediated functional AVB is not well defined. In the previous studies, epidemiology of paroxysmal AVB was researched on the basis of syncope coexistence. Zyśko et al.9 studied ECG characteristics of AVB induced by neurocardiogenic reflex provoked by tilt testing. Of 786 patients who underwent tilt testing, 31 (3.9%), developed AVB, mean age 45 ± 20 years. In another study, AVB was seen in 3.4% of patients with a neurally mediated response to tilt testing, mean age 21 ± 12 years.10 A female predominance (74% and 100%, respectively) was detected in both studies.9,10 Considering that up to 40% of the general population has experienced at least one episode of syncope in their lifetime and about 20% of all syncopes are reflex syncope, it may be speculated that vagally mediated functional AVB is not an uncommon but is an under-diagnosed clinical entity.1,11 Intrinsic and extrinsic idiopathic forms are usually characterized by recurrent syncope episodes with a duration of prodromal symptoms of ⩽5 s.12,13 However, vagally mediated form is associated with well characterized prodromal symptoms such as nausea, feeling warm, a cold-clammy sweat, blurred vision, or lightheadedness prior to syncope which last longer than 5 s.12,14 In reflex syncope, a potential trigger such as emotional stress, painful stimuli, or standing for long periods can be identified in the great majority of cases.
To differentiate vagally mediated form, the ECG characteristics of AVB before, during, and after the episode should be carefully examined on continuous Holter tracings.8 In vagally mediated type, AVB is usually preceded by sinus node slowing, and sinus rhythm during AVB is slow and instable.9 A progressive PR prolongation before AVB may be noticeable. Mobitz type I, 2:1 second-degree AVB, advanced second-degree AVB, and third-degree AVB can be recognized during episode. However, PR remains unchanged and sinus rate increases or does not change in most cases with other types of paroxysmal AVB. Symptom–rhythm correlation cannot be established by Holter in some cases. A negative Holter cannot rule out the presence of paroxysmal AVB in patients demonstrating symptoms compatible symptoms; further investigations, that is, implantable loop recorder, may be warranted to establish a symptom–rhythm correlation. However, this strategy necessitates the implantation of a diagnostic device and waiting until a new episode occurs. As an alternative strategy, ECG characteristics of AVB provoked by tilt table testing seem to be sufficiently sensitive to identify patients with functional AVB in the presence of characteristic triggering factors and prodromal symptoms. However, the technique had some limitations such that different responses to the test may be seen within the same patients and the optimal protocol is still unknown.9 To exclude the diagnosis of intrinsic AVB and extrinsic idiopathic AVB, electrophysiological study and adenosine test may be used, respectively.
Treatment options in patients with paroxysmal atrioventricular block
Irrespective of paroxysmal or persistent nature of block, cardiac pacing is optimal treatment strategy in patients with intrinsic AVB (11). Aste et al.15 studied the efficacy of pacing in preventing syncope recurrences in a population suffering from syncope due to severe AVB. At long term, recurrence rate was only 1% in patients with documented AVB plus syncope, whereas it was 14% in patients with undocumented AVB plus syncope after permanent pacemaker implantation.
There is no clinical study investigating the potential role of cardiac pacing in patients with extrinsic idiopathic AVB. In a recently published study assessing the mechanism of syncope, 58 patients presenting with unexplained syncope, no prodromal episodes, and a normal heart received an implantable loop recorder and were followed up until a diagnosis was established.16 Their outcomes were compared with those of 389 patients affected by reflex syncope with prodromal episodes who received an implantable loop recorder. A 17% and a 13% of cases underwent cardiac pacing implantation and oral theophylline treatment, respectively. During subsequent 17 ± 12 months of follow-up, syncope recurred in one patient on theophylline and presyncope occurred in one patient with pacemaker.
Considering the fact that extrinsic idiopathic AVB is associated with low values of endogenous adenosine, the effect of treatment with theophylline, a non-selective adenosine receptor antagonist, in the prevention of syncope recurrences has been investigated. It saturates adenosine receptors which are known to be located in the atrioventricular node and, to a lesser extent, in the sinus node, and therefore prevents their activation which may cause syncope as a result of prolonged asystolic pauses due to AVB or sinus arrest. In a well-selected case series, theophylline was found to be effective in five of six patients.17
There is no clinical study specifically investigating the effect of permanent pacemaker in patients with functional AVB. Furthermore, Mobitz type I and type II second-degree AVB, and third-degree AVB were exclusion criteria in SUP2, ISSUE 2 and 3 studies.18–20 However, it should be kept in mind that excessive vagal tone may be the only underlying cause not only in intermittent but also in some permanent AVB cases.6,21
Atropine usage to differentiate the type of atrioventricular block
Atropine sulfate as a vagolytic is a competitive antagonist of actions of acetylcholine and other muscarinic agonists that accelerates both sinus node and atrial myocyte automaticity and increases the speed of atrioventricular conduction. According to Advanced Cardiac Life Support guidelines of the American Heart Association, if bradycardia produces signs and symptoms of instability, atropine is suggested as an initial treatment.22 Although atropine is usually ineffective in patients with primary conduction system disease, it is an effective treatment strategy in patients experiencing AVB due to heightened parasympathetic tone.23–26
Although effects of atropine on the cardiac rhythm have been known since 1912, there is scarce data for following items: (1) possible role of atropine response to reveal the etiology of AVB; (2) potential importance of the extent of response to atropine administration; (3) the dosage of atropine required to achieve required response. To demonstrate the functional nature of transient or permanent AVB and to select suitable candidates for CNA, atropine response was successfully used by different groups.4,6,21 In our study protocol, the dosing for atropine was 0.04 mg/k was used. The dosage was not indicated in other studies. However, according to the latest guideline, atropine should be used in bradycardia with 1 mg initial dose and repeated until desired heart rate is obtained in every 3 to 5 min with a maximum dosage of up to 3 mg.6,22 The next uncertainty is related to potential importance of the extent of response to atropine administration. Contrary to the general opinion, AVB does not need to be intermittent to be functional nature. In a recently published study, we used atropine to define functional nature of AVB in three persistent AVB cases. In two of cases, 2:1 AVB completely resolved after atropine administration whereas the response was partial with first-degree AVB in other case.6 Therefore, we can speculate that resolution of AVB with atropine may be accepted as good indicator to exclude functional AVB from intrinsic AVB whether it is intermittent or persistent.
Cardioneuroablation as an alternative strategy
Showing the increase in heart rate following different RFCA procedures caused the idea that elimination of parasympathetic innervation might be achieved by endocardial RFCA of GPs and may be used in the treatment of conditions associated with excessive vagal activation.27,28 Taking into account the results of animal studies and human autopsy specimens that the postganglionic parasympathetic cells are located either in the atrial wall or in the distinct GP sites close to the pericardium and great vessels, it might be presumed that properly selected methods may identify the parts of the atrial wall encompassing autonomic GP clusters.29–32 However, the main challenge is clear demonstration of those GP localizations during an electrophysiological study. Four strategies have been used to map GPs and identify the ablation targets: atrial electrogram characteristics, high-frequency stimulation, purely anatomical localization, and different combinations of these strategies.33
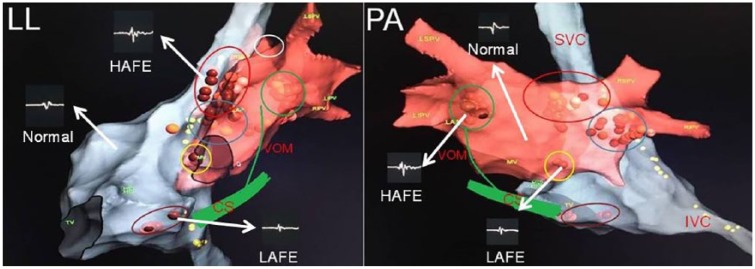
In the first human experience, Pachon et al.4 used Fast-Fourier Transformation analysis to differentiate GP sites that demonstrate fragmented and heterogeneous potentials than normal atrial myocardium differentiating with homogeneous and fast conduction pattern. In the second technique, the response of high-frequency stimulation is used for differentiation.33 While a vagal response, which is defined as a significant prolongation of the PR or RR intervals, demonstrates GP sites, the absence of any effect or non-significant changes on the PR or RR intervals is accepted as the clue of normal atrial myocardium. Empirical anatomic ablation in the presumed areas of usual GP localization sites was also used as stand-alone or adjunctive strategy (Figure 1). While atrial electrogram-based strategies require additional equipment, the others have low specificity to detect GPs.34
Figure 1.
A modified schematic view of GPs according to animal and human studies. The figure shows the commonly used location sites for GPs and different electrogram patterns seen in atria. Brown dots demonstrate ablation points. Ablation points were selected in the sites revealing high-amplitude or low-amplitude fractionated electrograms. Yellow dots demonstrate phrenic nerve trace by high-amplitude stimulation. Red ellipse demonstrates superior (anterior) right ganglionated plexus. Blue ellipse shows inferior (posterior) right ganglionated plexus. Brown ellipse reveals posterior right atrial ganglionated plexus. Green circle demonstrates posterolateral left ganglionated plexus. Yellow circle reveals posteromedial left ganglionated plexus. Since the vein of Marshall is also rich in parasympathetic neurons, it is added to the figure.
CS, the coronary sinus; HAFE, high-amplitude fractionated electrogram; IVC, the inferior vena cava; LAFE, low-amplitude fractionated electrogram; LIPV, the left inferior pulmonary vein; LL, left lateral view; LSPV, the left superior pulmonary vein; Normal, atrial electrogram demonstarting non-GP sites; PA, postero-anterior view; RIPV, the right inferior pulmonary vein; RSPV, the right superior pulmonary vein; SVC, the superior vena cava; VOM, the vein of Marshall.
In our recently published study, we used the electroanatomic mapping–guided CNA strategy and compared this technique our previous approach consisting of a special combination of high-frequency stimulation, spectral analysis, and additional anatomical ablation.7 Twenty vasovagal syncope cases with recurrent syncope (aged 36.0 ± 12.8 years; 50.0% female) were enrolled in the study. Twelve of these patients underwent the electroanatomic mapping–guided CAN, while 8 patients underwent combined CNA. During three-dimensional electroanatomic mapping of both atria, bipolar endocardial electrograms were recorded and divided into three subgroups at a special filter setting (300–500 Hz): normal electrogram, low-amplitude fractionated electrogram (LAFE), and high-amplitude fractionated electrogram (HAFE) (Figure 1). The sites demonstrating HAFE or LAFE pattern in a region that is compatible with the probable location of the vagal ganglia, which were discussed in Figure 1, were tagged as ablation targets. Almost complete elimination of atrial electrograms (<0.1 mV) in those sites was accepted as the ablation endpoint. In both groups, a similar improvement was detected in the heart rate variability parameters, demonstrating parasympathetic activity. There were no new syncopal episodes in any patients at the end of an at least six-month follow-up period.7 Prodromal symptoms demonstrated a significant and comparable decrease after CNA.
Usage of cardioneuroablation in patients with functional atrioventricular block
The procedure was first attempted in a mixed population consisting of vasovagal syncope, sinus node dysfunction, and functional AVB. The diagnosis was intermittent high-degree AVB in a total of 7 of 22 cases. In three of the patients, the block was only detected during sleep. In other four cases, there were mild to moderate abnormalities of atrioventricular conduction such as prolonged AH interval and high atrioventricular Wenckebach point during pre-procedural electrophysiological study. However it should be indicated that exact values of those pre-procedural parameters did not presented in the study. The procedure was found effective in six of seven cases. In one case demonstrating partial response which was revealed by nocturnal Mobitz I AVB episodes during follow-up Holter recordings, the procedure was accomplished only via the right atrium contrary to the usual approach composing of ablation of all the regions demonstrating features of vagal innervation in the regions of GP, one between the aorta and the superior vena cava (treated through the superior vena cava), the second between the right pulmonary veins and the right atrium (treated through the left atrium), and the last in the right posterior interatrial septum near the inferior vena cava (treated through the inferior vena cava and through the coronary sinus). Insufficient ablation has been claimed by authors as the cause of failure.
Same group tried to technique in a case with recurrent syncope due to high-degree AVB during high-vagal tone periods such as at rest, after meals, and during sleep.35 In baseline EPS, atrioventricular Wenckebach point was detected as 500 ms with normal His-Purkinje conduction which was totally corrected by atropine. Ablation was performed again only via the right atrium in the regions demonstrating segmented spectrum between the aorta and the superior vena cava (treated through the superior vena cava) and inside the coronary sinus ostium, and in the right posterior interatrial septum. At the end of the procedure, all the electrophysiological parameters studied were corrected. The patient was asymptomatic during the 21-month follow-up.
We performed the procedure in a 54-year-old woman who had undergone slow pathway ablation for atrioventricular nodal reentrant tachycardia 3 years ago and presented with recurrent dizziness and syncope due to 2:1 AVB.21 Unlike previous cases, repeated Holter recordings and rest ECGs showed 2:1 AVB that was ongoing throughout the day. Complete resolution of atrioventricular conduction abnormality was demonstrated with atropine administration and treadmill exercise test which suggested functional nature of AVB. Pre-procedural EPS revealed a suprahisian 2:1 AVB. Right atrial sites showing a parasympathetic response with high-frequency stimulation and fibrillar atrial myocardium pattern were assigned as targets and ablated until amplitude of atrial electrical potential lowered below 0.1 mV. The ablation points were basically similar to those previously described by Pachon et al.35 Atrioventricular conduction resolved completely and the patient was still asymptomatic with 1:1 atrioventricular conduction, while this article was written.
Rivarola et al.36 reported a 38-year-old male patient presenting no previous clinical record and using no medication, with asymptomatic frequent nocturnal ventricular pauses of >3 s length, the longest being of 11 s. Normal sinus function and atrioventricular conduction were detected while awake, which was characteristic of a vagally mediated AVB. By using spectral mapping, sites demonstrating segmented spectrum in both sides of interatrial septum were targeted. AH interval and atrial Wenckebach point decreased from 115 ms to 74 ms and 820 ms to 570 ms, respectively. Holter recordings after 3, 6, 9, 12, and 15 months demonstrated partial vagal denervation with continuing sinus pauses during sleep. In that case, authors claimed that targeting selective vagal innervations is likely to promote a parasympathetic attenuation instead of a total vagal blockade, and this could be seen as a technique limitation. When we consider anatomical distribution of GPs, insufficient ablation seems as the most plausible cause of failure rather than a limitation of ablation technique in our opinion. As supporting data of our argument, although authors detected a decrease on atrial Wenckebach point from 820 ms to 570 ms, the final value was still above the normal references, suggesting that a fair amount vagal effect on atrioventricular conduction continues. Also, the authors demonstrated that heart rate did not increase after atropine administration; however, it is unclear whether there was any change on atrial Wenckebach point with atropine.
In a recently published article, we investigated the effectiveness of CNA in the patients with not only intermittent (4 cases) but also with persistent AVB (3 cases).6 In patients with paroxysmal AVB, AH interval and atrial Wenckebach point decreased from 149 ± 19 ms to 70 ± 7 ms and 471 ± 39 ms to 260 ± 12 ms, respectively. In the patients with persistent AVB, supra-His 2:1 atrioventricular conduction block, which was revealed in baseline EPS, was completely resolved in two of three cases with a mean of 81 ms of AH interval and 248 ms of atrial Wenckebach point after CNA. As an important point, the failed single case had some visible differences from the other six patients. First, this patient was the oldest patient in the study population. He was 69 years old. It should have been kept in mind that fibrosis and sclerosis of the conduction system account for about one-half of cases of AVB and advanced age is the most common cause of fibrosis. More importantly, the patient’s response to pre-procedural atropine test was partial with first-degree AVB, unlike other persistent AVB cases demonstrating complete resolution. Therefore, as it is mentioned under the “Atropine usage to differentiate the type of atrioventricular block” section, a complete response to the atropine test should be used as an absolute criterion in all persistent AVB cases.
In a prospective non-randomized study, Rivarola et al.37 investigated immediate end points and critical atrial regions responsible for vagal denervation. Primary diagnosis was paroxysmal AVB presenting with recurrence (⩾3 episodes) of syncope or poorly tolerated presyncope in 9 of 14 cases. Primary dysfunction of atrioventricular conduction system was excluded with conventional EPS. Specific atrium sites which were empirically identified as GP by presumed anatomic location were targeted in both left and right atria. Procedural endpoint was ablation of all prespecified anatomic sites. AH interval and atrial Wenckebach point decreased from 105 ± 41 ms to 72 ± 13 ms and from 454 ± 106 ms to 365 ± 37 ms, respectively. Again, although they revealed that heart rate did not increase after atropine administration, they did not check whether there is any change in AH interval or atrial Wenckebach point with atropine after the ablation procedure. As a response to the question of “what the study adds,” they concluded that the interatrial septum is responsible for most of the parasympathetic tone modification because targeting a single spot on the left side (64% of the patients) or right side (36%) of the interatrial septum was observed to be responsible for ⩾80% of the final RR and AH interval shortening during ablation. During a mean follow-up time of 22.5 ± 11.3 months, 10 of 14 cases demonstrated significant clinical improvement. However, 2 of 9 AVB cases demonstrated transient second-degree AVB, exclusively at night, during follow-up Holter recordings. The remaining 4 AVB cases, although presenting RR interval, WC length, and AH interval shortening similar to the rest of the cohort, had syncope recurrence or symptomatic bradycardia and underwent pacemaker implantation. Although the authors concluded that the interatrial septum is a critical area and responsible for most of the parasympathetic tone modification observed after GP ablation, it is well known that there is significant variation in the number and distribution of GP based on postmortem studies.38 Therefore, non-selective ablation may cause insufficient vagal denervation and may explain higher recurrence rate in the long term.
Our electroanatomic mapping–guided technique was used successfully in a case with symptomatic functional second-degree AVB and recurrent syncope.39 During left atrial ablation, the intrinsic basic cycle length of sinus node accelerated to 800 milliseconds despite AVB persistence. Subsequently, 1:1 atrioventricular conduction was achieved when ablation was applied around the coronary sinus ostium. The patient was completely asymptomatic, experiencing no episodes of dizziness or syncope and was taking no medications at the end of the 9-month follow-up. Whether cardiac innervation be sufficiently lateralized for selective vagal denervation is a critical question. In our case, although we performed biatrial ablation, improvement of AVB appeared during radiofrequency application around the coronary sinus. It may be further evidence of our earlier hypothesis that ablation in different parts of the atria may cause different effects on the sinus node or atrioventricular conduction system.6 The ablation of GPs around the coronary sinus and septal side of the superior vena cava is responsible for most of the parasympathetic tone modification of atrioventricular node and a right atrial approach might suffice in patients with AVB.
The points to be considered related to cardioneuroablation
To date, there are no clinical studies consisting only of cases with AVB. Distinction of whether an AVB is vagally mediated functional, idiopathic extrinsic, or intrinsic is crucial for the determination of suitable candidates for CNA. Once diagnosis of vagally mediated functional AVB is confirmed in a patient with recurrent syncopal episodes or significant complaints related with bradycardia, the patient should be accepted as a potential candidate for CNA whether it is intermittent or persistent. In patients with paroxysmal AVB, the following parameters can be used to differentiate the vagally mediated form from the other: no AVB or intraventricular conduction disease on baseline ECG, PR prolongation and decrease in sinus rate just before AVB, and resolving of AVB with an increase in sinus rate.40 In patients with persistent AVB, complete resolution of AVB with both atropine infusion and exercise test may be suggested to exclude functional AVB on the basis of our study results.6 The patients with structural cardiopathy were excluded in all studies. Therefore, it should be accepted as an exclusion criterion. Although clear data are not available, acceptance of exclusion criterion of age of >60 years seems reasonable considering age and fibrosis in the conduction system relationship.
Although the technique is still in its infancy, CNA may markedly improve health and quality of life and might be a potential alternative modality in patients with pronounced symptoms due to functional AVB when conventional modalities are inadequate or pacemaker implantation is declined. Although there is no reported major complication, which is defined as such an event that results in permanent injury or death, requires intervention for treatment, or prolongs or requires hospitalization for >48 h, CNA is a complex procedure linked to potentially life-threatening complications. Possible complications related to transseptal puncture or left atrial ablation may be overcome by unifocal right-sided ablation. Minor complications such as pseudoaneurysm or groin hematoma related to vascular intervention were reported regardless of the side of ablation.41,42 Also inappropriate sinus tachycardia was reported in up to 23% of cases, which were treated temporarily (1–3 months) with beta-blockers or ivabradine.7,41
However, the procedure carries some risk of serious complications as septal puncture. It should be kept in mind that only patients demonstrating all the features of functional AVB might be a candidate for this new modality. Also, the patients who are suitable candidates for treatment receive comprehensive and objective information concerning the risks and the expected benefits of the method.
Conclusion
Ablation of GPs may be a feasible and valuable adjunctive therapy in patients with functional AVB. Instead of complex spectral analysis or high-frequency stimulation application, analysis of electrogram characteristics in terms of the presence of fractionated patterns may be used to define parasympathetic innervation sites. Further larger and randomized controlled studies with a sham procedure are required to demonstrate the efficiency and reliability of CNA.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Tolga Aksu  https://orcid.org/0000-0001-8061-9660
https://orcid.org/0000-0001-8061-9660
References
- 1. Brignole M, Moya A, de Lange FJ, et al. 2018 ESC guidelines for the diagnosis and management of syncope. Eur Heart J 2018; 39: 1883–1948. [DOI] [PubMed] [Google Scholar]
- 2. Lee S, Wellens HJJ, Josephson ME. Paroxysmal atrioventricular block. Heart Rhythm 2009; 6: 1229–1234. [DOI] [PubMed] [Google Scholar]
- 3. Alboni P, Holz A, Brignole M. Vagally mediated atrioventricular block: pathophysiology and diagnosis. Heart 2013; 99(13): 904–908. [DOI] [PubMed] [Google Scholar]
- 4. Pachon JC, Pachon EI, Pachon JC, et al. “Cardioneuroablation”—new treatment for neurocardiogenic syncope, functional AV block and sinus dysfunction using catheter RF-ablation. Europace 2005; 7(1): 1–13. [DOI] [PubMed] [Google Scholar]
- 5. Yao Y, Shi R, Wong T, et al. Endocardial autonomic denervation of the left atrium to treat vasovagal syncope: an early experience in humans. Circ Arrhythm Electrophysiol 2012; 5(2): 279–286. [DOI] [PubMed] [Google Scholar]
- 6. Aksu T, Golcuk E, Yalin K, et al. Simplified cardioneuroablation in the treatment of reflex syncope, functional AV block, and sinus node dysfunction. Pacing Clin Electrophysiol 2016; 39(1): 42–53. [DOI] [PubMed] [Google Scholar]
- 7. Aksu T, Guler TE, Mutluer FO, et al. Electroanatomic-mapping-guided cardioneuroablation versus combined approach for vasovagal syncope: a cross-sectional observational study. J Interv Card Electrophysiol. Epub ahead of print 28 July 2018. DOI: 10.1007/s10840-018-0421-4. [DOI] [PubMed] [Google Scholar]
- 8. Aste M, Brignole M. Syncope and paroxysmal atrioventricular block. J Arrhythm 2017; 33: 562–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zyśko D, Gajek J, Koźluk E, et al. Electrocardiographic characteristics of atrioventricular block induced by tilt testing. Europace 2009; 11(2): 225–230. [DOI] [PubMed] [Google Scholar]
- 10. Vanerio G, Vanerio de, León A, Vidal Amaral JL, et al. Atrioventricular block during upright tilt table test. Pacing Clin Electrophysiol 2004; 27(5): 632–638. [DOI] [PubMed] [Google Scholar]
- 11. Soteriades ES, Evans JC, Larson MG, et al. Incidence and prognosis of syncope. N Engl J Med 2002; 347(12): 878–885. [DOI] [PubMed] [Google Scholar]
- 12. Calkins H, Shyr Y, Frumin H, et al. The value of the clinical history in the differentiation of syncope due to ventricular tachycardia, atrioventricular block, and neurocardiogenic syncope. Am J Med 1995; 98(4): 365–373. [DOI] [PubMed] [Google Scholar]
- 13. Brignole M, Deharo JC, Guieu R. Syncope and idiopathic (paroxysmal) AV block. Cardiol Clin 2015; 33(3): 441–447. [DOI] [PubMed] [Google Scholar]
- 14. Brignole M, Auricchio A, Baron-Esquivias G, et al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy: the task force on cardiac pacing and resynchronization therapy of the European Society of Cardiology (ESC). Developed in collaboration with the European Heart Rhythm Association (EHRA). Eur Heart J 2013; 34: 2281–2329. [DOI] [PubMed] [Google Scholar]
- 15. Aste M, Oddone D, Donateo P, et al. Syncope in patients paced for atrioventricular block. Europace 2016; 18(11): 1735–1739. [DOI] [PubMed] [Google Scholar]
- 16. Brignole M, Guieu R, Tomaino M, et al. Mechanism of syncope without prodromes with normal heart and normal electrocardiogram. Heart Rhythm 2017; 14(2): 234–239. [DOI] [PubMed] [Google Scholar]
- 17. Brignole M, Solari D, Iori M, et al. Efficacy of theophylline in patients affected by low adenosine syncope. Heart Rhythm 2016; 13(5): 1151–1154. [DOI] [PubMed] [Google Scholar]
- 18. Brignole M, Ammirati F, Arabia F, et al. Assessment of a standardized algorithm for cardiac pacing in older patients affected by severe unpredictable reflex syncopes. Eur Heart J 2015; 36(24): 1529–1535. [DOI] [PubMed] [Google Scholar]
- 19. Brignole M, Sutton R, Menozzi C, et al. Early application of an implantable loop recorder allows effective specific therapy in patients with recurrent suspected neurally mediated syncope. Eur Heart J 2006; 27: 1085–1092. [DOI] [PubMed] [Google Scholar]
- 20. Brignole M, Donateo P, Tomaino M, et al. Benefit of pacemaker therapy in patients with presumed neurally mediated syncope and documented asystole is greater when tilt test is negative: an analysis from the third International Study on Syncope of Uncertain Etiology (ISSUE-3). Circ Arrhythm Electrophysiol 2014; 7(1): 10–16. [DOI] [PubMed] [Google Scholar]
- 21. Aksu T, Golcuk SE, Erdem Guler T, et al. Functional permanent 2:1 atrioventricular block treated with cardioneuroablation: case report. Heartrhythm Case Rep 2015; 1(2): 58–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hazinski MF, Nolan JP, Aickin R, et al. Part 1: executive summary: 2015 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Circulation 2015; 132(Suppl. 1): S2–S39. [DOI] [PubMed] [Google Scholar]
- 23. Kim KO, Oh JS. Vagally mediated atrioventricular block with ventricular asystole immediately after assuming prone position under spinal anesthesia: a case report. Korean J Anesthesiol 2016; 69(1): 63–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kakuchi H, Sato N, Kawamura Y. Swallow syncope associated with complete atrioventricular block and vasovagal syncope. Heart 2000; 83(6): 702–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Strasberg B, Lam W, Swiryn S, et al. Symptomatic spontaneous paroxysmal AV nodal block due to localized hyperresponsiveness of the AV node to vagotonic reflexes. Am Heart J 1982; 103(5): 795–801. [DOI] [PubMed] [Google Scholar]
- 26. Talwar KK, Edvardsson N, Varnauskas E. Paroxysmal vagally mediated AV block with recurrent syncope. Clin Cardiol 1985; 8(6): 337–340. [DOI] [PubMed] [Google Scholar]
- 27. Soejima K, Akaishi M, Mitamura H, et al. Increase in heart rate after radiofrequency catheter ablation is mediated by parasympathetic nervous withdrawal and related to site of ablation. J Electrocardiol 1997; 30(3): 239–246. [DOI] [PubMed] [Google Scholar]
- 28. Pappone C, Stabile G, Oreto G, et al. Inappropriate sinus tachycardia after radiofrequency ablation of para-Hisian accessory pathways. J Cardiovasc Electrophysiol 1997; 8(12): 1357–1365. [DOI] [PubMed] [Google Scholar]
- 29. Chiou CW, Eble JN, Zipes DP. Efferent vagal innervation of the canine atria and sinus and atrioventricular nodes. The third fat pad. Circulation 1997; 95(11): 2573–2584. [DOI] [PubMed] [Google Scholar]
- 30. Armour JA, Murphy DA, Yuan BX, et al. Gross and microscopic anatomy of the human intrinsic cardiac nervous system. Anat Rec 1997; 247(2): 289–298. [DOI] [PubMed] [Google Scholar]
- 31. Yuan BX, Ardell JL, Hopkins DA, et al. Gross and microscopic anatomy of the canine intrinsic cardiac nervous system. Anat Rec 1994; 239(1): 75–87. [DOI] [PubMed] [Google Scholar]
- 32. Po SS, Nakagawa H, Jackman WM. Localization of left atrial ganglionated plexi in patients with atrial fibrillation. J Cardiovasc Electrophysiol 2009; 20(10): 1186–1189. [DOI] [PubMed] [Google Scholar]
- 33. Aksu T, Guler TE, Yalin K, et al. Catheter ablation of bradyarrhythmia: from the beginning to the future. Am J Med Sci 2018; 355(3): 252–265. [DOI] [PubMed] [Google Scholar]
- 34. Aksu T, Güler TE, Bozyel S, et al. Cardioneuroablation in the treatment of neurally mediated reflex syncope: a review of the current literature. Turk Kardiyol Dern Ars 2017; 45(1): 33–41. [DOI] [PubMed] [Google Scholar]
- 35. Pachon M JC, Pachon M EI, Lobo TJ, et al. Syncopal high-degree AV block treated with catheter RF ablation without pacemaker implantation. Pacing Clin Electrophysiol 2006; 29(3): 318–322. [DOI] [PubMed] [Google Scholar]
- 36. Rivarola E, Hardy C, Sosa E, et al. Selective atrial vagal denervation guided by spectral mapping to treat advanced atrioventricular block. Europace 2016; 18(3): 445–449. [DOI] [PubMed] [Google Scholar]
- 37. Rivarola EW, Hachul D, Wu T, et al. Targets and end points in cardiac autonomic denervation procedures. Circ Arrhythm Electrophysiol 2017; 10: e004638. [DOI] [PubMed] [Google Scholar]
- 38. Pauza DH, Skripka V, Pauziene N, et al. Morphology, distribution, and variability of the epicardiac neural ganglionated subplexuses in the human heart. Anat Rec 2000; 259(4): 353–382. [DOI] [PubMed] [Google Scholar]
- 39. Aksu T, Güler TE, Yalin K, et al. A new and simple technique for vagal ganglia ablation in a patient with functional atrioventricular block: electroanatomical approach. Turk Kardiyol Dern Ars 2018; 46(6): 494–500. [DOI] [PubMed] [Google Scholar]
- 40. Komatsu S, Sumiyoshi M, Miura S, et al. A proposal of clinical ECG index “vagal score” for determining the mechanism of paroxysmal atrioventricular block. J Arrhythm 2017; 33(3): 208–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pachon JC, Pachon EI, Cunha Pachon MZ, et al. Catheter ablation of severe neurally meditated reflex (neurocardiogenic or vasovagal) syncope: cardioneuroablation long-term results. Europace 2011; 13(9): 1231–1242. [DOI] [PubMed] [Google Scholar]
- 42. Debruyne P, Rossenbacker T, Collienne C. Unifocal right-sided ablation treatment for neurally mediated syncope and functional sinus node dysfunction under computed tomographic guidance. Circ Arrhythm Electrophysiol 2018; 11(9): e006604. [DOI] [PubMed] [Google Scholar]