Abstract
Intrauterine adhesion (IUA) is now recognized as one of the most common diseases in reproductive-age women. Metformin, a well-known frontline oral antidiabetic drug, has been found effective in numerous different diseases. The aim of this study was to determine the effect of metformin on reducing adhesions in an animal model of IUA. Sprague-Dawley rats were randomized into 4 groups: sham operation, control, metformin-treated for 7 days, and metformin-treated for 14 days. To establish the IUA model, mechanical injury to the endometria of rats was induced with a mini curette. Metformin was injected intraperitoneally after surgery. A significant amelioration in both the number of glands and the fibrotic area, compared to those of the control group, was detected 14 days after metformin intervention. The expression levels of antigen KI-67 and vascular endothelial growth factor were increased at 7 and 14 days after treatment. However, the transforming growth factor-β expression was decreased at 14 days after treatment. Endoplasmic reticulum stress-related apoptosis proteins (glucose-regulated protein 78, caspase-12, and CCAAT/enhancer binding protein (EBP) homologous protein) were downregulated after metformin treatment. Moreover, we determined that the effect of metformin was related to the inhibition of endoplasmic reticulum stress-induced apoptosis via the Phosphatidylinositol 3 kinase (PI3K)/Protein kinase B (AKT) and Extracellular regulated protein kinases1/2 pathways. In conclusion, metformin can attenuate the adhesion and promote the regeneration of the endometrium of the IUA rat, and metformin may serve as a novel therapeutic strategy for IUA patients.
Keywords: intrauterine adhesions, metformin, endometrial regeneration, endoplasmic reticulum stress, apoptosis
Introduction
Asherman syndrome, commonly known as intrauterine adhesion (IUA), is a consequence of mechanical or infectious injury to the basal layer of the endometrium, which causes the formation of fibrosis or adhesions within the uterine cavity.1Recent reports showed that 7% of secondary amenorrhea cases among Chinese women were caused by IUA and IUA is the root cause of more than one-third of female infertility cases.2 Clinical therapies for IUA are aimed at the prevention of readhesion by hysteroscopic adhesiolysis, the administration of hormones after surgery, and the use of intrauterine devices.3 Although multiple strategies may help relieve some symptoms, side effects and recurrence remain tough problems.4 Determining innovative therapies to repair and regenerate the endometria of IUA patients is urgent.
Abnormal protein accumulation in the endoplasmic reticulum (ER) can trigger the unfolded protein response (UPR). However, persistent UPR is destructive and can lead to the irregular expression of glucose-regulated protein 78 (GPR78) and CCAAT/enhancer binding protein (EBP) homologous protein (CHOP) and to the upregulation of caspase-12, which contributes to cell apoptosis.5 Observers have already drawn attention to the relationship between fibrosis and apoptosis, which are induced by ER stress, under conditions such as cardiac fibrosis,6 liver fibrosis,7 and pulmonary fibrosis.8 Recently, ER stress-induced apoptosis has been identified as a potential contributing factor to the formation of fibrosis in an IUA model.9 To some extent, ER stress-induced apoptosis is a potent therapeutic target for IUA.
Metformin, an effective drug in reducing the hepatic production of glucose, is widely used among patients with type 2 diabetes, especially in overweight patients. In addition to the glucose-lowering effects in the type 2 diabetes, numerous different functions for metformin have been confirmed in the past few years, which provides many benefits for patients.10,11 Recently, researchers have drawn attention to the relationship between metformin and ER stress.12,13 Various studies have shown that metformin alleviates disease progression by reducing abnormal ER stress and UPR-related apoptosis.14,15 No previous report, however, has discussed the role of metformin in IUA. Therefore, we hypothesized that metformin may have positive influences on the injured endometria of patients with IUA.
The present work was designed to determine the effect of metformin on healing the endometrium of an IUA rat model after mechanical damage and then to further determine the possible molecular mechanism underlying the phenomenon.
Materials and Methods
Animal Models and Experimental Groups
Female Sprague-Dawley rats (230-250 g, n = 32) aged 8 weeks were purchased from the Animal Center of Chinese Academy of Sciences, Shanghai, China. The experiments were carried out according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. This study was approved by the Animal Care and Use Committee of Wenzhou Medical University. Rats were housed under standard conditions for at least 7 days before the commencement of experiments. Vaginal smears were performed at 08:00 in the morning to determine the estrous stage on the basis of the amount of nucleated epithelial cells, leukocytes, and cornified epithelial cells under a light microscope. The IUA models were induced during the diestrus stage of the cycle. The animals (n = 32) were divided into 2 groups: the sham operation group (n = 8) and the mechanical injury group (n = 24). All animals were anaesthetized with 1% pentobarbital sodium (0.5 mL/kg, intraperitoneal). Afterward, a vertical incision was made in the abdominal midline of each animal, and a horizontal incision (0.2 cm) was made on the uterus 0.5 cm above the bifurcation. A mini curette was used to scrape the endometrium to induce mechanical injury. Rats in the mechanical injury group were randomly divided into 3 subgroups: control, metformin-treated for 7 days, and metformin-treated for 14 days. After surgery, the metformin-treated groups were intraperitoneally injected with 0.2 mL of metformin at a dose of 1 mg/mL. Additionally, the animals in the control group were intraperitoneally injected with 0.2 mL of saline. In the sham operation group, the animals only underwent abdominal incision without any uterine treatment. The metformin-treated rats were sacrificed at 7 and 14 days, whereas the rats in the sham group and control group were sacrificed at 14 days after surgery.
Histological Examination and Masson Trichrome Staining
The rats were anaesthetized with 1% pentobarbital sodium (0.5 mL/kg, intraperitoneal), and the uterine tissue was collected. One side of the uterus was fixed in cold 4% paraformaldehyde overnight and embedded in paraffin. Uterine sections (5 μm) were stained with hematoxylin and eosin (Beyotime Institute of Biotechnology, Shanghai, China) and with Masson trichrome staining (Beyotime Institute of Biotechnology). The histology was viewed and images were captured under a Nikon Eclipse 80i (Nikon, Japan).
Immunohistochemical Analysis
Immunostaining for antigen KI-67 (Ki-67), transforming growth factor-β (TGF-β), vascular endothelial growth factor (VEGF), CHOP, GRP78, and caspase-12 was performed in each experimental group. After dewaxing and rehydration, the sections were heated in sodium citrate buffer for antigen retrieval in a microwave oven and then rinsed in phosphate-buffered saline (PBS). The slides were blocked with 3% H2O2 for 10 minutes to inhibit the endogenous peroxidase activity, and then nonspecific antibody binding was blocked with 5% bovine serum albumin (BSA; Beyotime, Shanghai, China) for 30 minutes at 37°C. Thereafter, primary antibodies, including Ki-67 (1:100, Abcam, CB, UK), VEGF (1:100, Abcam, CB, UK), TGF-β (1:100, Abcam, CB, UK), CHOP (1:200, Santa Cruz, CA, USA), GRP78 (1:200, Santa Cruz, CA, USA), and caspase-12 (1:400, Abcam, CB, UK), were incubated with the sections at 4°C overnight. After thoroughly washing with PBS, the sections were incubated with the biotinylated secondary antibodies (goat anti-mouse or goat anti-rabbit) for 2 hours at 37°C. The sections were stained with diaminobezidin (DAB; ZSGB-BIO, Beijing, China) and counterstained with hematoxylin, followed by dehydration and cover slipping. The images were acquired on a light microscope (Olympus, Japan). Semiquantitative evaluation of the immunostaining intensity was conducted using Image-Pro plus 6.0 system. Negative controls were similarly stained, but the primary antibodies were replaced with PBS.
Western Blot
The other side of the uterus was stored at −80°C for later protein analysis. To extract protein, the uterine tissue was homogenized in modified RIPA buffer (Solarbio, Beijing, China) with 1 mmol/L phenyl methane sulfonyl fluoride (Solarbio). The complex was then centrifuged at 12 000 rpm at 4°C for 10 minutes, and then, the supernatant was obtained and quantified with bicinchoninic acid reagents (Thermo, Rockford, Illinois). Proteins (20 μg) were resolved on 12% sodium dodecyl sulfate–polyacrylamide gels and transferred onto a polyvinylidene difluoride membrane (Bio-Rad, Hercules, California). After blocking with 5% skim milk for 120 minutes at room temperature, the bands were incubated with primary antibodies, namely, CHOP (1:1000, Santa Cruz, CA, USA), GRP78 (1:1000, Santa Cruz, CA, USA), caspase-12 (1:1000, Abcam, CB, UK), phosphorylated-protein kinase B (p-AKT, 1:1000, Abcam, CB, UK), protein kinase B (AKT, 1:1000, Abcam, CB, UK), phosphorylated-extracellular regulated protein kinases (p-ERK, 1:1000, Abcam, CB, UK), and extracellular regulated protein kinases (ERK, 1:1000, Abcam, CB, UK), overnight at 4°C. The next day, the bands were treated with horseradish peroxidase-conjugated secondary antibodies (1:5000, Shanghai Boyun Biotech Co., Ltd., China). The bands were visualized and quantified with Image Lab 3.0 software (Bio-Rad).
Statistical Analysis
All data are expressed as the mean ± standard deviation (SD), and the analyses were performed using the GraphPad Prism 7.0 software. Two sample t tests were used to determine the significance of the differences between 2 groups. One-way analysis of variance was utilized to determine significant differences between multiple groups. P Values <0.05 were considered to indicate statistical significance.
Results
Metformin Treatment Ameliorates the Morphology of the Uterus and Increases the Number of Glands in the IUA Model
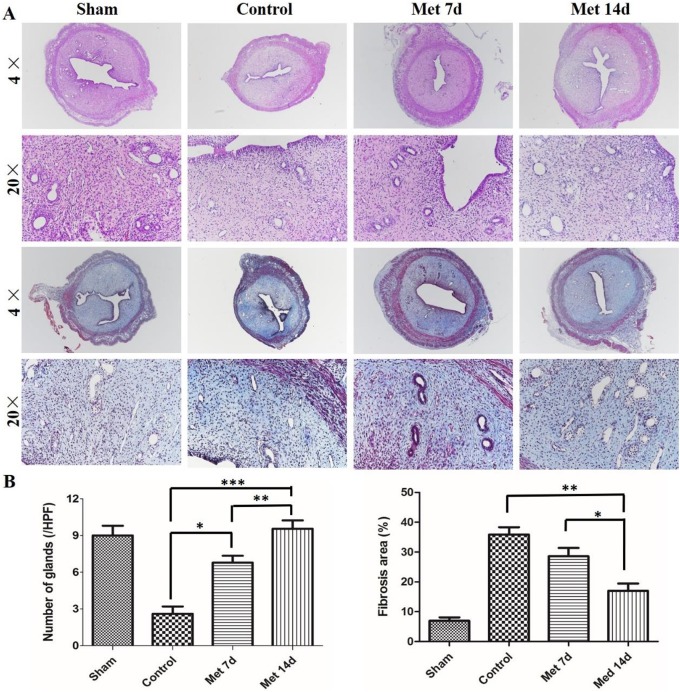
Hematoxylin and eosin staining was carried out to examine the tissue histology of the different groups and the results are shown in Figure 1. There was a simple layer of high columnar epithelial cells on the surface of the endometrium in the sham operation group. Round and oviform glands were also noted in the submucosa and basal layer (Figure 1A). In contrast, in the control group, the surface of the endometrium was largely covered by horizontal columnar epithelial cells, and there was only a small number of glands in the epithelial tissue. Moreover, some uterine adhesions were observed in some rats (Figure 1A). The metformin-treated group showed less damage. In contrast to the control group, the number of glands increased slightly after metformin treatment for 7 days (Figure 1A). The quantitative results indicated that the number of glands was significantly increased after metformin treatment for 14 days, and the number of glands was almost the same as that of the sham operation group and better than that of the rats treated for 7 days (Figure 1B). Collectively, these data suggest that metformin helped repair the endometrial injury, and the effect appeared to increase with time.
Figure 1.
Metformin treatment ameliorates the morphology of the uterus, increases the number of glands, and decreases the fibrosis in the intrauterine adhesion (IUA) model. (A) Representative images of hematoxylin and eosin (H&E) staining and Masson trichrome staining of the uterus in different groups (×40 and ×200). (B) Quantification of the number of the glands and fibrosis area percentage. The data in the figure are presented as the mean ± standard deviation (SD); n = 8; *P < 0.05, **P < 0.01, ***P < 0.001. Met indicates metformin.
Metformin Treatment Decreases the Fibrosis of the IUA Model
To characterize fibrosis after injury, the ratio of the area with endometrial fibrosis to the total endometrial area was measured by Masson trichrome staining. It was difficult to find any fibrosis in the endometrium of the sham operation group (Figure 1A). However, the distribution of endometrial fibrosis in the control group was apparently increased. In contrast to the control group, the administration of metformin for 7 days did not significantly decrease the degree of fibrosis in the endometrium (Figure 1B). However, at 14 days after metformin treatment, the ratio of the endometrial fibrosis area was dramatically reduced compared to those of the other groups. Taken together, these data suggest that endometrial fibrosis was reduced by metformin and that the degree of fibrosis had a negative relationship with the number of the glands.
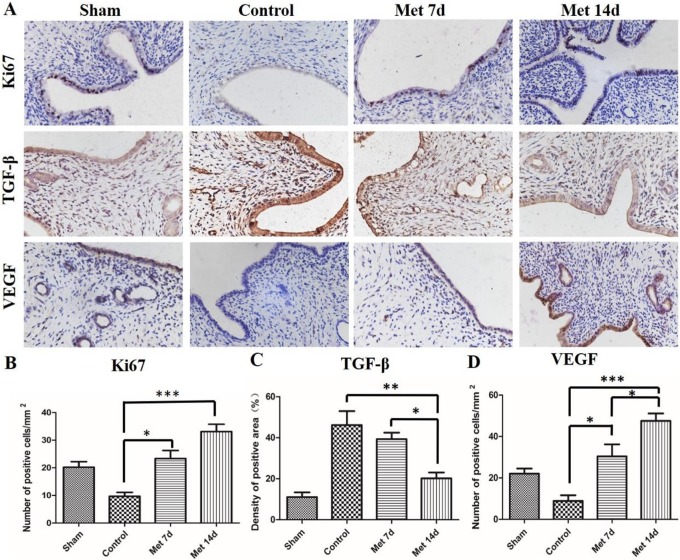
Metformin Treatment Promotes Cell Proliferation and Vascularization in the Endometrium of the Rat Model
The immunohistochemical staining results for Ki-67, a nuclear antigen for cell proliferation, are presented in Figure 2. The number of positive cells was decreased in the control group compared to the sham operation group (Figure 2A and B). After the administration of metformin for 7 days and 14 days, the expression of Ki-67 was increased obviously in the endometria of both groups compared to the control group. However, no significant difference was detected between the 7-day group and 14-day group. Overall, the administration of metformin promoted the proliferation of the endometrium of the injured uterus. Transforming growth factor-β is an important growth factor regulating cell growth and differentiation and is expressed in the nucleus and cytoplasm of the endometrial cell. The results showed that the density of the TGF-β-positive area in the control group was much higher than that in the sham group (Figure 2C). There was no obvious disparity between the control group and metformin 7-day group. However, when we extended the time of the metformin treatment to 14 days, TGF-β expression was noticeably reduced. To explore the vascularization of each group, VEGF was used to measure the vascular remodeling of the endometrium. In contrast to the sham group, the number of VEGF-positive cells in the control group was slightly decreased (Figure 2D). After 7 days of metformin injections, the expression level of VEGF was appreciably upregulated. Strikingly, the number of VEGF-positive cells in the metformin 14-day group was even higher than that in the metformin 7-day group. As shown in Figure 3, these findings showed that metformin is critical in enhancing the vascularization of the injured endometrium.
Figure 2.
Immunohistochemical staining for antigen KI-67 (Ki-67), transforming growth factor-β (TGF-β), and vascular endothelial growth factor (VEGF). A, Representative images of immunohistochemical staining for K-67, TGF-β, and VEGF in the endometrium (×400) in different groups. B, Quantification of Ki-67 positive cells. C, Quantification of TGF-β positive cells. D, Quantification of VEGF positive cells. The data in this figure represent the mean ± standard deviation (SD); n = 8; *P < 0.05, **P < 0.01, ***P < 0.001. Met indicates metformin.
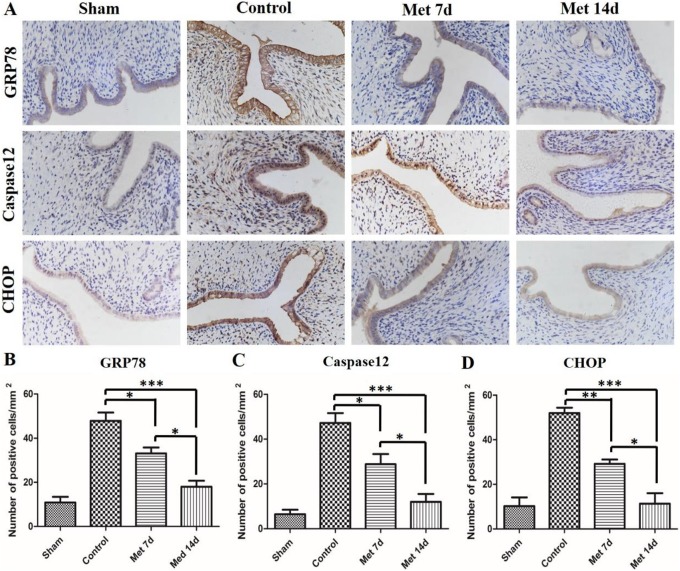
Figure 3.
The endoplasmic reticulum (ER) stress signaling pathway is inhibited by metformin in the intrauterine adhesion (IUA) model. A, Representative images of immunohistochemical staining of glucose-regulated protein 78 (GRP78), caspase12, and CCAAT/EBP homologous protein (CHOP) in the endometrium (×400) in different groups. B, Quantification of GRP78-positive cells. C, Quantification of caspase12-positive cells. D, Quantification of CHOP-positive cells. The data in the figure represent the mean ± standard deviation (SD); n = 8; *P < 0.05, **P < 0.01, ***P < 0.001. Met indicates metformin.
Endoplasmic Reticulum Stress Signaling Pathway Is Inhibited by Metformin in IUA Model
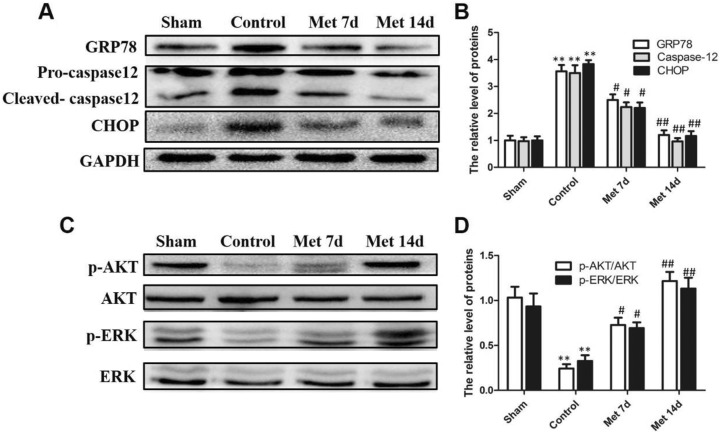
To investigate whether ER stress-related apoptosis was involved in the protective effect of metformin on the injured endometrium, ER stress-related apoptosis proteins (GRP78, caspase-12, and CHOP) were detected by immunohistochemical staining (Figure 3). The damage to the endometrium caused remarkable increases in the apoptosis proteins (GRP78, caspase-12, and CHOP) in the control group compared to the sham operation group. However, the administration of metformin significantly decreased the expression levels of the apoptosis proteins. Moreover, the number of positive cells in the metformin 14-day group was even lower than that in the metformin 7-day group. To further strengthen the finding, Western blot was used to evaluate the expression levels of GRP78, caspase-12, and CHOP (Figure 4). The results were approximate to the immunohistochemical staining. As shown in Figure 4, the expression of ER stress-related apoptosis proteins in the control group increased considerably compared to the sham group. Metformin injection helped to reduce the protein expression level, and 14-day injection of metformin had a better effect than injection for 7 days.
Figure 4.
The protective effect of metformin is related to the Phosphatidylinositol 3 kinase (PI3K)/Protein kinase B (AKT) and Extracellular regulated protein kinases1/2 pathways. A, Western blot results of glucose-regulated protein 78 (GRP78), pro-caspase-12, cleaved caspase-12, and C/EBP homologous protein (CHOP) in the different groups. Glyceraldehyde-3-phosphate dehydrogenase (GADPH) was used as the loading control and for band density normalization. B, Quantification of protein expression. **P < 0.01 versus sham group, # P < 0.05 versus control group, ## P < 0.01 versus control group. C, Western blot results of phosphorylated-protein kinase B (p-AKT), protein kinase B (AKT), phosphorylated-extracellular regulated protein kinases (p-ERK), and extracellular regulated protein kinases (ERK) in different groups. D, Quantification of protein expression. **P < 0.01 versus sham group, # P < 0.05 versus control group, ## P < 0.01 versus control group.
The Shielding Effect of Metformin Is Related to the PI3K/Akt and ERK1/2 Signal Pathways
The PI3K/Akt and ERK1/2 pathways are linked to cell survival, migration, and differentiation. We used Western blot to explore whether the PI3K/Akt and ERK1/2 pathways were involved in endometrial regeneration (Figure 4). The protein levels of p-ERK and p-AKT were reduced after endometrial injury, whereas metformin treatment for 7 days significantly increased the levels of these proteins. Moreover, the protein levels after 14 days of metformin treatment were obviously elevated compared to those of the control group. These results indicated that the function of metformin in protecting the endometrium was related to the downregulation of ER stress through the activation of the PI3K/Akt and ERK1/2 pathways.
Discussion
It is widely believed that curettage, postpartum hemorrhage, infection, and some surgical treatments can cause trauma to the endometrium with consequent IUA.16 There are avascular strands of fibrous tissue that bridge the anterior and posterior uterine walls in patients with IUA.17 As time passes, the thin endometrial bands become thicker bands with more severe fibrosis and less glandular proliferation, causing the obliteration of the uterine cavity.18 Eventually, IUA patients have various symptoms, such as menstrual aberrations, pelvic pain, secondary infertility, recurrent abortion, intrauterine growth restriction, and other complications during pregnancy, caused by these pathological processes.17
In this work, metformin showed a positive effect in the recovery of endometrium in IUA rats. The numbers of glands were significantly increased after the metformin treatment. Moreover, we revealed that the prolonged treatment of metformin in the IUA model exhibited more effective function in promoting endometrium regeneration. On the other hand, metformin reversed the decrease in Ki-67 expression that was observed in the injured endometrium. Recently, a research by Xu et al revealed that expression of Ki-67, a cell proliferation marker, was enhanced after keratinocyte growth factor treatment in IUA rats.19 Therefore, the elevated expression level of Ki-67 indicated the potential efficacy of metformin in promoting cell proliferation. This was in line with the histological results, which means that metformin can regenerate the endometrium by enhancing the proliferation of endometrium cell and increasing the number of glands. Many studies have revealed that endometrial stem cells contribute to the regeneration of endometrium after menstruation.20 A research published by Gargett et al revealed that stem cells derived from bone marrow played an important role in the remodeling and regeneration of the human uterus.21 Additionally, some studies have confirmed that metformin can enhance the recruitment of neural stem cells and promote its proliferation, self-renewal, and differentiation.22 However, there are few studies focusing on the role of metformin in the recruitment of stem cells in endometrium. Thus, more studies are urgently needed to explore its role in the recruitment of stem cells.
Many previous studies have found that the levels of fibrosis markers were significantly increased in the endometrium with IUA compared to the control group, suggesting fibrosis formation may play an important role in the progression of IUA.23,24 Recently, several studies have shown that TGF-β, a central mediator of fibrogenesis, is upregulated in liver fibrosis, and metformin treatment can reverse the high expression level of TGF-β.25 In accord with previous studies,26 metformin showed a similar efficacy in this study. Our results revealed that the level of TGF-β was considerably reduced after the administration of metformin for 14 days. The density of TGF-β in the 7-day group was lower than that in the control group, yet a significant reduction was not achieved. However, there was a significant difference in the TGF-β levels between the 7- and 14-day groups. The findings by immunohistochemistry were in line with Masson trichrome staining. Whereas, a previous research suggested that, after the combination therapy of Interceed and estrogen, the fibrosis of endometrium in the rabbit IUA model resumed at 7 days after intervention.27 This may partly due to the different animal species and distinct therapeutic effects in these two studies. The results above indicated that metformin can suppress the fibrosis within the uterus. While it seems that achieving antifibrosis effect took more time than renewing the endometrium.
It is well known that the uterus is an organ with an abundant blood supply. Previous studies have reported that collagen-binding VEGF promotes remodeling of the uterine scar of the full-thickness injury and improves the function of the uterus28 and that angiogenesis plays a vital role in endometria regeneration.19 Moreover, the beneficial effect of metformin in angiogenesis has been reported in a number of studies.29 Coincidently, our data showed that metformin enhanced the expression of VEGF in the injured endometrium, and the drug effect became more pronounced with the extended time. The results above showed that metformin can improve the angiogenesis by upregulating the level of VEGF, which was in accord with previous study.30 Taken together, metformin has a positive effect on the healing of the injured endometrium.
An emerging body of evidence implicates that abnormal ER function is closely related to the pathogenesis of fibrosis. A recent research published by Luo and Chen demonstrated that the cardiac ER stress response is sustained during the procedure of myocardial fibrosis, whereas the attenuation of ER stress significantly reduces myocardial fibrosis.31 In our current study, we discovered that the expression of proteins related to ER stress-induced apoptosis, including GRP78, CHOP, and caspase-12, was remarkably enhanced in the injured endometria of IUA rats. In view of these findings, preventing ER stress-related apoptosis may be a novel target to attenuate fibrosis of the endometrium in IUA. Prior studies have noted the role of metformin in suppressing aberrant ER stress and UPR-related apoptosis. A study by Cai et al indicated that pretreatment with metformin reduced the overexpression of ER stress markers including cleaved caspase-12 and CHOP and attenuated the myocardial trauma and apoptosis induced by isoproterenol in a rat model.15 In our work, after the administration of metformin, ER stress associated-apoptosis proteins, including GRP78, CHOP, and caspase-12 were dramatically decreased. Moreover, there were obvious differences between the 2 metformin groups; the 14-day treatment was more effective in decreasing ER stress-induced apoptosis than 7-day treatment. Briefly, there was a clear benefit of metformin in attenuating ER stress-induced apoptosis, and therefore, curing the injured thin endometrium can be feasible.
In this work, we not only evaluated the effectiveness of metformin on IUA, but also explored the possible mechanisms underlying it. The PI3K/Akt and ERK1/2 pathways are vital regulators of cell survival, metabolism, and proliferation in many physiological and pathological processes. A recent study revealed that metformin can enhance the therapeutic effect of gall bladder cancer cells through the PI3K/Akt/ERK pathway.32 To explore whether the PI3K/AKT and ERK1/2 pathways were involved, we evaluated the phosphorylation of Akt and the phosphorylation of ERK in different groups. Our results indicated that the levels of p-Akt and p-ERK were significantly decreased in IUA rats and the declined protein levels of p-Akt and p-ERK were reversed after metformin administration. Moreover, the gap was considerably wider with the prolonged period of treatment. Furthermore, in hepatocellular carcinoma cells, ER stress-induced apoptosis has been reported to be mediated by crosstalk between the PI3K/Akt and ERK1/2 pathways.33 We tentatively put forward that the potential of metformin in the injured endometrium may due to or partially relied on the inhibition of the ER stress-induced apoptosis and activation of the PI3K/Akt and ERK1/2 cascades.
In conclusion, we discovered the treatment effect of metformin on endometrium in an IUA model and demonstrated that metformin could protect the endometrium against mechanical injury in rats, at least partially, by alleviating aberrant ER stress-induced apoptosis and activating the PI3K/Akt and ERK1/2 pathways. However, the exact mechanisms of endometrium regeneration after the administration of metformin are still unclear. Therefore, the specific mechanism underlying this process should be further studied. Moreover, further experimental investigations are needed to confirm the ability of metformin to improve fertility in IUA rats and determine whether metformin administration will negatively influence the offspring.
Footnotes
Authors’ Note: Xin-Xin Xu and Si-Si Zhang contributed equally to this work. The experiments were carried out according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. This study was approved by the Animal Care and Use Committee of Wenzhou Medical University.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Natural Science Foundation of China (No.81873822), Shenzhen Science and Technology Innovation Committee Project (grant number JCYJ20170818100355168), Zhejiang Provincial Natural Science Foundation of China (grant number LY17H040009), High-level talent innovation and technology projects of Wenzhou (2017), the Science and Technology Project of Zhejiang Province (grant number 2016C37132).
References
- 1. Evans-Hoeker EA, Young SL. Endometrial receptivity and intrauterine adhesive disease. Semin Reprod Med. 2014;32(5):392–401. [DOI] [PubMed] [Google Scholar]
- 2. Johary J, Xue M, Zhu X, Xu D, Velu PP. Efficacy of estrogen therapy in patients with intrauterine adhesions: systematic review. J Minim Invasive Gynecol. 2014;21(1):44–54. [DOI] [PubMed] [Google Scholar]
- 3. March CM. Management of Asherman’s syndrome. Reprod Biomed Online. 2011;23(1):63–76. [DOI] [PubMed] [Google Scholar]
- 4. Yu D, Li TC, Xia E, Huang X, Liu Y, Peng X. Factors affecting reproductive outcome of hysteroscopic adhesiolysis for Asherman’s syndrome. Fertil Steril. 2008;89(3):715–722. [DOI] [PubMed] [Google Scholar]
- 5. Ohri SS, Maddie MA, Zhao Y, Qiu MS, Hetman M, Whittemore SR. Attenuating the endoplasmic reticulum stress response improves functional recovery after spinal cord injury. Glia. 2011;59(10):1489–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ayala P, Montenegro J, Vivar R, et al. Attenuation of endoplasmic reticulum stress using the chemical chaperone 4-phenylbutyric acid prevents cardiac fibrosis induced by isoproterenol. Exp Mol Pathol. 2012;92(1):97–104. [DOI] [PubMed] [Google Scholar]
- 7. Harris TR, Bettaieb A, Kodani S, et al. Inhibition of soluble epoxide hydrolase attenuates hepatic fibrosis and endoplasmic reticulum stress induced by carbon tetrachloride in mice. Toxicol Appl Pharmacol. 2015;286(2):102–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee MR, Lee GH, Lee HY, et al. BAX inhibitor-1-associated V-ATPase glycosylation enhances collagen degradation in pulmonary fibrosis. Cell Death Dis. 2014;5:e1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zhang SS, Xia WT, Xu J, et al. Three-dimensional structure micelles of heparin-poloxamer improve the therapeutic effect of 17beta-estradiol on endometrial regeneration for intrauterine adhesions in a rat model. Int J Nanomedicine. 2017;12:5643–5657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhao Y, Sun H, Feng M, et al. Metformin is associated with reduced cell proliferation in human endometrial cancer by inhibiting PI3K/AKT/mTOR signaling. Gynecol Endocrinol. 2017;34(5):428–432. [DOI] [PubMed] [Google Scholar]
- 11. Penzias A, Bendikson K, Butts S, et al. Role of metformin for ovulation induction in infertile patients with polycystic ovary syndrome (PCOS): a guideline. Fertil Steril. 2017;108(3):426–441. [DOI] [PubMed] [Google Scholar]
- 12. Diaz-Morales N, Iannantuoni F, Escribano-Lopez I, et al. Does metformin modulate endoplasmic reticulum stress and autophagy in type 2 diabetic peripheral blood mononuclear cells? Antioxid Redox Signal. 2017;28(17):1562–1569. [DOI] [PubMed] [Google Scholar]
- 13. Duan Q, Song P, Ding Y, Zou MH. Activation of AMP-activated protein kinase by metformin ablates angiotensin II-induced endoplasmic reticulum stress and hypertension in mice in vivo. Br J Pharmacol. 2017;174(13):2140–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kim H, Moon SY, Kim JS, et al. Activation of AMP-activated protein kinase inhibits ER stress and renal fibrosis. Am J Physiol Renal Physiol. 2015;308(3):F226–F236. [DOI] [PubMed] [Google Scholar]
- 15. Cai H, Zhang G, Chen W, et al. Metformin protects the myocardium against isoproterenol–induced injury in rats through alleviating endoplasmic reticulum stress. Pharmazie. 2014;69(1):64–69. [PubMed] [Google Scholar]
- 16. Deans R, Abbott J. Review of intrauterine adhesions. J Minim Invasive Gynecol. 2010;17(5):555–569. [DOI] [PubMed] [Google Scholar]
- 17. March CM. Asherman’s syndrome. Semin Reprod Med. 2011;29(2):83–94. [DOI] [PubMed] [Google Scholar]
- 18. Sabry D, Mostafa A, Marzouk S, et al. Neupogen and mesenchymal stem cells are the novel therapeutic agents in regeneration of induced endometrial fibrosis in experimental rats. Biosci Rep. 2017;37(5):BSR20170794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xu HL, Xu J, Zhang SS, et al. Temperature-sensitive heparin-modified poloxamer hydrogel with affinity to KGF facilitate the morphologic and functional recovery of the injured rat uterus. Drug Deliv. 2017;24(1):867–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hu J, Zeng B, Jiang X, et al. The expression of marker for endometrial stem cell and fibrosis was increased in intrauterine adhesious. Int J Clin Exp Pathol. 2015;8(2):1525–1534. [PMC free article] [PubMed] [Google Scholar]
- 21. Gargett CE, Chan RWS, Schwab KE. Hormone and growth factor signaling in endometrial renewal: role of stem/progenitor cells. Mol Cell Endocrinol. 2008;288(1-2):22–29. [DOI] [PubMed] [Google Scholar]
- 22. Fatt M, Hsu K, He L, et al. Metformin acts on two different molecular pathways to enhance adult neural precursor proliferation/self-renewal and differentiation. Stem Cell Reports. 2015;5(6):988–995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhu Y, Hu J, Yu T, Ren Y, Hu L. High molecular weight hyaluronic acid inhibits fibrosis of endometrium. Med Sci Monit. 2016;22:3438–3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Foix A, Bruno RO, Davison T, Lema B. The pathology of postcurettage intrauterine adhesions. Am J Obstet Gynecol. 1966;96(7):1027–1033. [DOI] [PubMed] [Google Scholar]
- 25. Fan K, Wu K, Lin L, et al. Metformin mitigates carbon tetrachloride-induced TGF-beta1/Smad3 signaling and liver fibrosis in mice. Biomed Pharmacother. 2017;90:421–426. [DOI] [PubMed] [Google Scholar]
- 26. Salma U, Xue M, Ali Sheikh MS, et al. Role of transforming growth factor-β1 and smads signaling pathway in intrauterine adhesion. Mediators Inflamm. 2016;2016:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cai H, Li H, He Y. Interceed and estrogen reduce uterine adhesions and fibrosis and improve endometrial receptivity in a rabbit model of intrauterine adhesions. Reprod Sci. 2016;23(9):1208–1216. [DOI] [PubMed] [Google Scholar]
- 28. Lin N, Li X, Song T, et al. The effect of collagen-binding vascular endothelial growth factor on the remodeling of scarred rat uterus following full-thickness injury. Biomaterials. 2012;33(6):1801–1807. [DOI] [PubMed] [Google Scholar]
- 29. Bakhashab S, Ahmed F, Schulten HJ, et al. Proangiogenic effect of metformin in endothelial cells is via upregulation of VEGFR1/2 and their signaling under hyperglycemia-hypoxia. Int J Mol Sci. 2018;19(1):293–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ding L, Li X, Sun H, et al. Transplantation of bone marrow mesenchymal stem cells on collagen scaffolds for the functional regeneration of injured rat uterus. Biomaterials. 2014;35(18):4888–4900. [DOI] [PubMed] [Google Scholar]
- 31. Luo T, Chen B, Wang X. 4-PBA prevents pressure overload-induced myocardial hypertrophy and interstitial fibrosis by attenuating endoplasmic reticulum stress. Chem Biol Interact. 2015;242:99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bi T, Zhu A, Yang X, et al. Metformin synergistically enhances antitumor activity of cisplatin in gallbladder cancer via the PI3K/AKT/ERK pathway. Cytotechnology. 2017;70(1):439–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dai R, Chen R, Li H. Cross-talk between PI3K/Akt and MEK/ERK pathways mediates endoplasmic reticulum stress-induced cell cycle progression and cell death in human hepatocellular carcinoma cells. Int J Oncol. 2009;34(6):1749–1757. [DOI] [PubMed] [Google Scholar]