Abstract
Objective:
Adenosine monophosphate–activated protein kinase (AMPK) is a cellular energy sensor whose phosphorylation increases energy production. We sought to evaluate the placenta-specific effect of AMPK activation on the handling of nutrients required for fetal development.
Methods:
Explants were isolated from term placenta of 29 women (pregravid body mass index: 29.1 ± 9.9 kg/m2) and incubated for 24 hours with 0 to 100 µmol/L resveratrol or 0 to 1 mmol/L of 5-aminoimidazole-4-carboxyamide ribonucleoside (AICAR). Following treatment, uptake and metabolism of radiolabeled fatty acids and glucose were measured. Phosphorylation of AMPK was measured by Western blotting. Adenosine diphosphate (ATP) production was assessed using the mitochondrial ToxGlo assay kit. P < .05 was considered statistically significant.
Results:
Resveratrol and AICAR increased AMPK phosphorylation in human placental explants. Exposure to resveratrol decreased the uptake of polyunsaturated fatty acids, arachidonic acid, and docosahexaenoic acid at 100 µmol/L (P < .0001). Fatty acid oxidation was decreased by 100 µmol/L (P < .05) resveratrol, while esterification was unchanged. Resveratrol decreased glucose uptake at the 50 and 100 µmol/L doses (P < .05). Glycolysis was not significantly affected. AICAR had similar effects, decreasing fatty acid uptake and glycolysis (P < .05). Production of ATP declined at doses found to decrease nutrient metabolism (P < .05).
Conclusions:
Activation of AMPK in the human placenta leads to global downregulation of metabolism, with mitotoxicity induced at the doses of resveratrol and AICAR used to activate AMPK. Although activation of this pathway has positive metabolic effects on other tissues, in the placenta there is potential for harm, as inadequate placental delivery of critical nutrients may compromise fetal development.
Keywords: AMP kinase, trophoblasts, resveratrol, AICAR, metabolism
Introduction
Changes in placental metabolism associated with maternal obesity and diabetes may affect fetal nutrient delivery and growth.1–3 The mechanism behind these changes is poorly understood. Adenosine monophosphate–activated protein kinase (AMPK) is a cellular energy sensor, which when phosphorylated in adenosine diphosphate (ATP)-poor conditions stimulates numerous metabolic processes within the cell with the goal of increasing energy production.4,5 The end result of AMPK phosphorylation is upregulation of vital nutrient metabolism and uptake pathways such as glycolysis and fatty acid (FA) oxidation. The role of AMPK in the placenta is understudied. Saben et al found that placentas of obese women have decreased levels of AMPK,6 which may account for our previous findings that these placentas have impaired mitochondrial function, changes in FA metabolism, and increased lipid storage.1
Despite the demonstrated beneficial effects of AMPK activation on metabolism in several tissues,7,8 its effects in the placenta have been more controversial. Resveratrol (trans-3,4′,5-trihydroxystilbene) is one of the active constituents of red wine, considered responsible for the “French paradox,” the finding that individuals from France, despite their diet high in saturated fat, have some of the lowest mortality from heart disease.9 The caloric restriction mimetic effects of resveratrol may rely on its ability to activate AMPK,9–15 which it has been shown to do in both in vivo and in vitro placental studies. Studies in Japanese macaque model of maternal obesity have shown resveratrol supplementation improves maternal fasting insulin levels with resultant decreased fat mass16 and stimulates placental AMPK phosphorylation and FA uptake.17 In murine models, resveratrol supplementation prevented embryonic oxidative stress and apoptosis and improved the glucose and lipid profile of diabetic mothers.18,19 Another compound shown to selectively activate AMPK is 5-aminoimidazole-4-carboxyamide ribonucleoside (AICAR), an adenosine analog.20 AICAR has been shown to activate AMPK in adipose tissue and skeletal muscle of pregnant women, thereby improving inflammation and insulin resistance.21 AICAR has also been shown to decrease infection-induced, proinflammatory cytokines in primary amnion cells in the setting of preterm premature rupture of membranes22 via the same AMPK pathway.
Although these data show some promising results regarding beneficial effects of upregulation of AMPK in pregnancy, the specific effect of AMPK activation on metabolism in the placenta is unclear. We sought to evaluate the effects of AMPK activation by both AICAR and resveratrol on the placental handling of the critical nutrients required for fetal development.
Methods
Study Design
This study was approved by the Institutional Review Board of MetroHealth Medical Center, Case Western Reserve University (IRB#13-00650). All participants provided written informed consent prior to collection of placental tissue. Twenty-nine healthy women (mean pregravid body mass index [BMI]: 29.1 ± 9.9 kg/m2) were recruited at term (37-40 weeks), and placental tissue was collected prior to scheduled cesarean delivery. Exclusion criteria were gestational diabetes, hypertension, drug use, and congenital anomalies. After chorionic membranes and maternal decidua were removed, placental tissue was collected from the maternal face of the placenta, avoiding calcified or underperfused cotyledons. Several full-depth samples were collected across the surface of the placenta from several cotyledons. Large samples were further dissected into small pieces that were blotted for the removal of blood. Explants were isolated as described previously.17,23 Briefly, placental explants were dissected in warm phosphate-buffered saline (PBS) and then placed into media (Dulbecco modified Eagle medium: nutrient mixture F-12 [DMEM:F12], 10% fetal bovine serum, 1% penicillin/streptomycin, and 0.25 μg/mL ascorbic acid) and incubated at 37°C for 30 minutes before addition of either resveratrol (0-100 μmol/L in ethanol) or the cell-permeable AMPK activator AICAR (0.1-1 mmol/L in water). Following incubation with drugs or their respective vehicles for 24 hours, explants underwent metabolic testing or were flash frozen in liquid nitrogen and stored at −80°C for protein analysis.
Fatty Acid Uptake in Human Placental Explants
Placental FA uptake studies using 14C-labeled palmitic acid (PA), oleic acid (OA), arachidonic acid (AA), and docosahexaenoic acid (DHA) were performed in placental explants as described previously17,23 with the following modifications: Briefly, following incubation for 24 hours with 100 µmol/L resveratrol or 1 mmol/L AICAR, media were removed and fragments equilibrated in uptake buffer (Hank balanced salt solution supplemented with 10 mM HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), pH 7.4) for 20 minutes before addition of albumin-bound FAs (1:1 ratio of FA and bovine serum albumin [BSA]). Radio-labeled [9,10-3H]-PA and [1-14C]-OA or [5,6,8,9,11,12,14,15-3H(N)]-AA and [1-14C]-DHA (Moravek Biochemicals, Brea, California) were added in pairs along with a mixture of nonlabeled FAs in a physiological ratio (110 µmol/L PA, 50 µmol/L OA, 30 µmol/L AA, and 10 µmol/L DHA; Sigma, St Louis, Missouri) in uptake buffer. Explants were incubated with the radiolabeled FAs for 45 minutes at 37°C. Reactions were stopped by addition of ice-cold “stop buffer” (uptake buffer with 0.1% BSA and 200 µmol/L of the nonspecific transporter inhibitor, phloretin; MP Biomedicals, Solon, Ohio). Explants were washed 3 times in cold stop buffer and finally in cold PBS. Explants were dissolved in 500 µL BioSol overnight at 37°C and were counted on a Beckman LS3801 liquid scintillation counter (Beckman Coulter, Brea, California). Total protein concentration was determined by bicinchoninic acid (BCA) assay (Sigma) following the manufacturer’s instructions. Uptake was calculated as nmol FA/mg/min and normalized to uptake in the presence of the drug vehicle alone (100%).
Immunoblotting
Tissue explant samples were homogenized in RIPA (Radioimmunoprecipitation assay) buffer (Cell Signaling Technology, Danvers, Massachusetts) supplemented with protease and phosphatase inhibitor cocktail (Pierce, Rockford, Illinois) on ice followed with agitation for 30 minutes at 4°C and then centrifuged at 16 000×g for 10 minutes at 4°C. The protein concentration of collected supernatants was determined using BCA protein assay (Pierce). Lysates containing equal amounts (20 µg) protein were diluted in sodium dodecyl sulfate sample loading buffer (Bio-Rad, Hercules, California) supplemented with β-mercaptoethanol and heated for 5 minutes at 95°C. Twenty micrograms of total protein were separated on 10% TGX™ gels (Bio-Rad). Resolved protein was electrophoretically transferred to a nitrocellulose membrane (Bio-Rad) using a Trans-Blot Turbo transfer system (Bio-Rad) according to the manufacturer’s protocols. After transfer, membranes were rinsed with water and incubated for 5 minutes in REVERT total protein stain (P/N 926-11011; Li-Cor, Lincoln, Nebraska) with agitation followed by 2× 30 second washes and water rinse according to manufacturer’s protocol. Membranes were immediately imaged in the 700 channel with an Odyssey infrared imaging system (Li-Cor). Detected REVERT total protein stain signal (data not shown) was used for the normalization. For imumunodetection, membranes were first incubated in Odyssey blocking buffer (Tris-buffered saline [TBS]; P/N 927-50100; Li-Cor) at room temperature for 1 hour and then with primary antibodies (each 1:1000) overnight at 4°C with agitation. The primary antibodies were p-AMPKα (#2535; Cell Signaling Technology), AMPKα (#2603; Cell Signaling Technology), and β-actin (sc-47778; Santa Cruz Biotechnology, Santa-Cruz, California). The following day membranes were washed 3 times with Tris-buffered saline–Tween 20 (TBST) and incubated at room temperature for 1 hour with infrared-labeled secondary antibody (antimouse immunoglobulin G [IgG] [H+L] DyLight 680 conjugate, #5470; Cell Signaling Technology) and anti-rabbit IgG (H+L) DyLight 800 conjugate (#5151; Cell Signaling Technology) at 1:5000 dilution. After 3 washes with TBST followed by 10 minutes wash in TBS, band intensities were determined by a sensitive near-infrared fluorescence scanner (Odyssey infrared imaging system). Fluorescence channel intensities ranged from 6.0 to 7.5 for AMPKα detection and 2.5 to 3.0 for β-actin detection.
Fatty Acid Esterification in Human Placental Explants
The FA esterification in placental explants was determined, as described previously,1,24 with modifications for explants. Briefly, fresh media were applied in the presence of 30% BSA, 0.1 mmol/L unlabeled palmitate, and 100 μmol/L [3H]-palmitate for 18 hours at 37°C. At the end of the treatment period, explants were washed with ice-cold PBS and then minced, homogenized, and sonicated in 400 μL of high-performance liquid chromatography–grade acetone on ice. Following lipid extraction, the supernatant was collected, and radioactivity (representing esterified palmitate) was counted in a 100-μL aliquot (Beckman LS3801 liquid scintillation counter). The dry protein pellet was resuspended in 200 µL of RIPA lysis buffer, and the total protein was determined using the BCA assay (Sigma). Esterification was determined by total 3H-lipid content and calculated as nmol palmitate/mg protein/h and expressed as a percentage of control. Each assay was performed in triplicate. Both placental weights (in mg) and protein content were used to normalize. Only the protein normalization is presented here.
Fatty Acid Oxidation Assay in Human Placental Explants
The FA oxidation assays were performed on placental explants, as described previously.1,24 Briefly, after 18 hours incubation with [3H]-palmitate, as described above, the media were collected, spun at 13K for 5 minutes, and placed in 200 μL aliquots submerged in 500 μL water in scintillation vials. These were incubated overnight at 50°C. At the end of the treatment period, water [3H]-H2O, representing the oxidized palmitate, was quantified in a scintillation counter. Data were calculated as nmol palmitate/mg protein/h and expressed as a percentage of control.
Glucose Uptake in Human Placental Explants
Uptake of 2-deoxy-d-glucose (2-DOG, a nonmetabolizable form of glucose) was performed in placental explants as described previously by Visiedo et al with some modifications.2 These experiments were performed in a glucose-free HEPES-buffered Dulbecco’s phosphate-buffered saline (DPBS) solution with the following composition: 10 μmol/L 2-DOG (Sigma) and 0.05 μL/mL3 H-2-DOG (Moravek Biochemicals). Placental explants had been incubated with the drugs for 24 hours in media. Excess media were removed, and explants were rinsed with warm DPBS. The buffer solution with radioactive tracer was added. Uptake was terminated at 20 minutes by rapid addition of 2 mL cold DPBS. The explants were washed 3 times in cold DPBS and lysed in 500 μL of BioSol overnight at 37°C. After vortexing, 200 μL of sample was counted on a Beckman LS3801 liquid scintillation counter.
Glycolysis in Human Placental Explants
Placental explants were incubated as described above, with the addition of 5-[3H]-glucose (0.65 μL/1 mL medium; Moravek Biochemicals) for 18 hours at 37°C. Glycolysis was assessed as described previously.2 Glucose utilization was determined as the production of tritiated water (as for fatty acid oxidation experiments).
Production of ATP
Primary trophoblast ATP concentration was evaluated using the Promega mitochondrial ToxGlo assay kit (Madison, Wisconsin) per manufacturer’s specifications. Primary trophoblasts were isolated and cultured as described previously24 from a subset of 6 women recruited for metabolism studies (mean pregravid BMI: 23.5 ± 3.9 kg/m2). Trophoblasts were cultured in 96-well plates at a density of 150 000 per well. Following 24 hours treatment with resveratrol or AICAR utilizing the same concentrations as the metabolism experiments, ATP detection reagent, ATPase inhibitors, and thermostable Ultra-Glo luciferase were added to each well in DMEM media supplemented with 1.8 g/L galactose (as opposed to glucose which would serve as a nonmitochondrial ATP source). Cells were lysed, and luminescence was generated proportional to the amount of ATP produced. The plate was placed on an orbital shaker for 5 minutes and ATP concentration was determined by measuring luminescence with a Perkin Elmer EnSpire 2300 multilabel reader (Perkin Elmer, Shelton, Connecticut).
Data Analysis
All data are presented as means ± standard error of the mean unless noted otherwise. All data were analyzed as percentage control, as we sought to look at the effect of the drugs at varying doses, rather than the interplacental variation. Differences between drug and control in the FA uptake experiments were analyzed with a Wilcoxon signed rank test. All other metabolic data were analyzed with a nonparametric, repeated-measures 1-way analysis of variance (Friedman test), followed by Dunn multiple comparison test. Statistical analysis was performed with use of GraphPad Prism version 6. P < .05 was considered statistically significant.
Results
Maternal and neonatal characteristics are summarized in Table 1. Maternal demographic data included mean maternal age, prepregnancy BMI, gestational weight gain, and gestational age at delivery. The neonatal data included birth weight, body fat percentage, and gender. We did not detect a differential effect of maternal BMI on any of the treatment effects, so they were grouped together in the analysis. We have included Supplemental figures where results have been separated for lean and obese placentas.
Table 1.
Maternal and Neonatal Characteristics.
| All | FA Uptake | FA Oxidation and Esterification | Glucose Uptake | Glycolysis | |
|---|---|---|---|---|---|
| N | 29 | 18 | 8 | 6 | 7 |
| Maternal age | 30 ± 6 | 30 ± 6 | 31 ± 7 | 31 ± 4 | 31 ± 4 |
| Prepregnancy BMI, kg/m2 | 29.1 ± 9.9 | 30.0 ± 11 | 28.5 ± 7.6 | 29.0 ± 10 | 30.4 ± 9.7 |
| Gestational weight gain, kg | 14.8 ± 8.6 | 13.9 ± 9.9 | 11.8 ± 4.1 | 13.3 ± 4.5 | 13.8 ± 5.8 |
| Gestational age, weeks | 39 ± 1 | 39 ± 1 | 39 ± 1 | 39 ± 1 | 39 ± 1 |
| Birth weight, kg | 3.16 ± 0.35 | 3.16 ± 0.37 | 3.03 ± 0.26 | 2.88 ± 0.35 | 2.86 ± 0.32 |
| Body fat, % | 10.8 ± 3.2 | 11.9 ± 2.6 | 9.2 ± 3.1 | 10.1 ± 3.5 | 9.2 ± 3.4 |
| Female/male | 17/12 | 7/11 | 4/4 | 3/3 | 4/3 |
Abbreviations: FA, fatty acid; BMI, body mass index.
Resveratrol and AICAR Increased AMPK Phosphorylation in Human Placental Explants
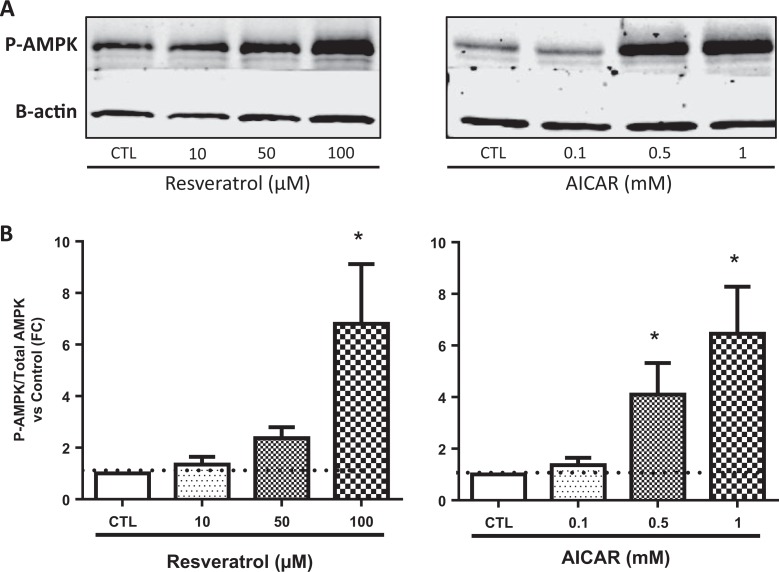
Both resveratrol and AICAR increased the phosphorylation of AMPK in a dose–response manner (Figure 1). The increase in phosphorylation of AMPK was detected in placentas of both lean and obese women.
Figure 1.
Representative Western blots showing phosphorylated AMPK and β-actin levels in placental explants incubated for 24 hours with 0 to 100 µmol/L resveratrol and 0 to 1 mmol/L AICAR (A). Quantification of fold change of phosphorylated to total AMPK versus control (B), N = 5. *P < .05 versus control by the Friedman test followed by Dunn multiple comparison test. AMPK indicates adenosine monophosphate–activated protein kinase; AICAR, 5-aminoimidazole-4-carboxyamide ribonucleoside.
Effects of Resveratrol and AICAR on Human Placental FA Uptake and Metabolism
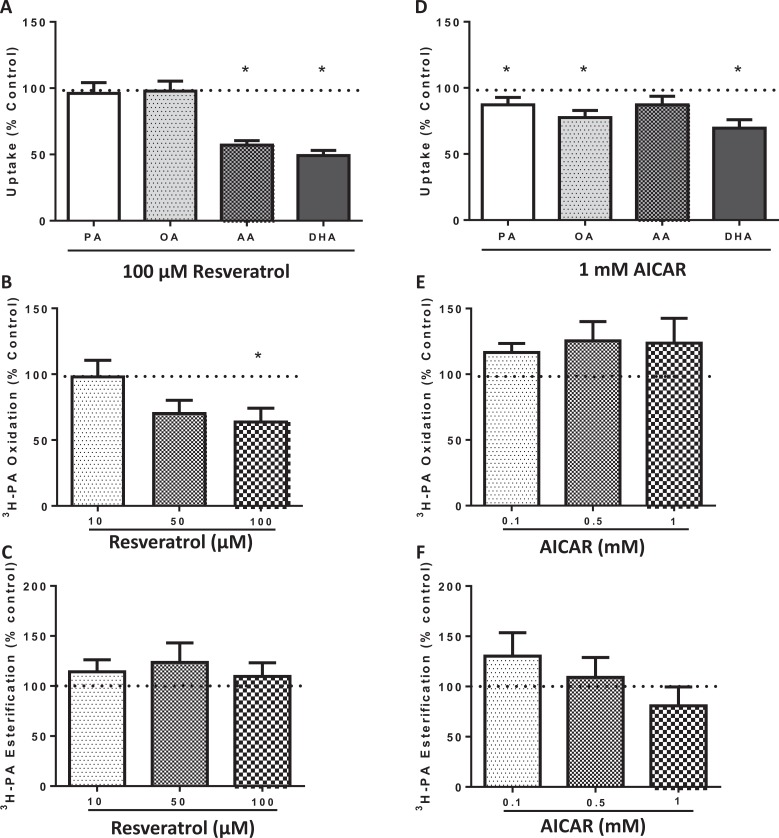
To assess the effect of AMPK phosphorylation on placental FA uptake, we incubated placental explants for 24 hours with 100 µmol/L resveratrol and 1 mmol/L AICAR. Unexpectedly, exposure to 100 µmol/L resveratrol decreased the uptake of polyunsaturated FAs, AA and DHA, when compared to control but did not affect uptake of the saturated PA and monounsaturated OA (P < .0001; N = 18; Figure 2A). Similarly, treatment with 1 mmol/L of AICAR (N = 13) decreased the uptake of the polyunsaturated FA DHA (P = .0012; Figure 2D), in addition to the saturated (PA; P = .04) and monounsaturated (OA; P = .0027) FAs. There was a trend for decreasing AA uptake with AICAR (P = .0708).
Figure 2.
Effects of resveratrol and AICAR on human placental fatty acid uptake and metabolism. Treatment with 100 µmol/L of resveratrol decreased the uptake of polyunsaturated fatty acids, AA and DHA, compared to control but did not affect uptake of the saturated PA and monounsaturated OA (A), N = 18. Fatty acid oxidation was decreased by 100 µmol/L resveratrol (B), N = 8; while esterification was unchanged (C), N = 8. Similarly, AICAR decreased uptake of fatty acids PA, OA, and DHA (D), N = 13. There was no effect on oxidation (E) or esterification (F), N = 8. *P < .05 versus control by the Wilcoxon signed rank test for fatty acid uptake, and the Friedman test followed by Dunn multiple comparison test for metabolism. AICAR indicates 5-aminoimidazole-4-carboxyamide ribonucleoside; AA, arachidonic acid; DHA, docosahexaenoic acid.
Palmitate, the most abundant FA in circulation, was chosen for measurement of FA metabolism. Additionally, lower doses of AMPK agonists were used to determine whether effects on cellular metabolism were dose dependent. The FA oxidation was decreased by 100 µmol/L resveratrol (P = .04; Figure 2B) while esterification was unchanged (N = 8; Figure 2C). AICAR had no effect on FA oxidation (N = 8; Figure 2E) or esterification (Figure 2F) at any dose.
Effects of Resveratrol and AICAR on Human Placental Glucose Uptake and Metabolism
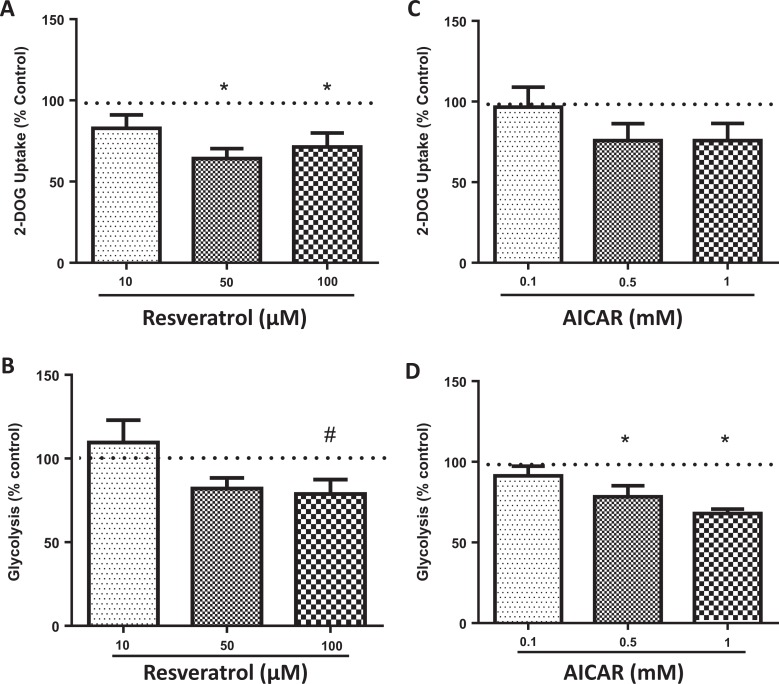
Resveratrol decreased 2-DOG (a nonmetabolizable form of glucose) uptake at both the 50 (P = .002) and 100 µmol/L (P = .04; Figure 3A) doses. AICAR had no effect on 2-DOG uptake at any dose (Figure 3C). Glycolysis was not significantly affected by resveratrol, although a trend was seen at the 100 µmol/L dose (P = .077; N = 7; Figure 3B). AICAR significantly decreased glycolysis at higher doses of 0.5 (P = .046) and 1 mmol/L (P = .0038; Figure 3D).
Figure 3.
Effects of resveratrol and AICAR on human placental glucose uptake and metabolism. Resveratrol decreased 2-deoxy-d-glucose (2-DOG, a nonmetabolizable form of glucose) uptake (A), N = 6. A trend was seen for glycolysis by 100 µmol/L resveratrol (B), N = 7. AICAR had no effect on 2-DOG uptake (C), N = 6. Glycolysis was decreased in the explants treated by both 0.5 and 1 mM AICAR (D), N = 7. *P < .05 versus control by the Friedman test followed by Dunn multiple comparison test. AICAR indicates 5-aminoimidazole-4-carboxyamide ribonucleoside.
Resveratrol and AICAR Induce Mitotoxicity in Human Trophoblasts
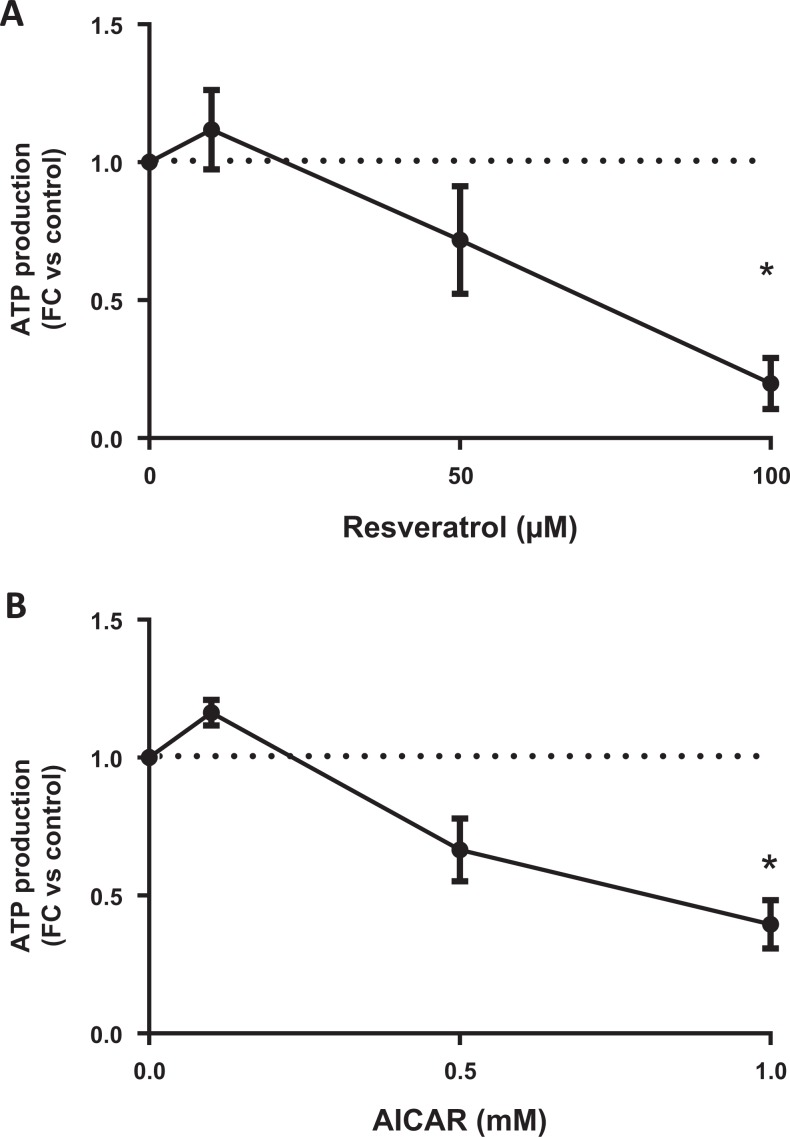
To evaluate potential mitotoxic effects of resveratrol and AICAR on human placental cells, we used MitoTox assay kit (Promega). We found that resveratrol transiently increased ATP production (N = 6; Figure 4A) at the 10-µmol/L dose but then rapidly declined at higher doses, reaching significance at 100 µmol/L (P = .0002). AICAR (N = 5; Figure 4B) was mitotoxic (P = .0066) at the highest dose (1 mmol/L).
Figure 4.
Measurement of mitotoxicity in trophoblasts incubated with either resveratrol or AICAR for 24 hours. An initial increase in ATP production at 10 µmol/L is followed by mitotoxicity at 100 µmol/L of resveratrol (A), N = 6. AICAR resulted in trophoblast mitotoxicity at 1 mmol/L (B), N = 5. *P < .05 versus control by the Friedman test followed by Dunn’s multiple comparison test. AICAR indicates 5-aminoimidazole-4-carboxyamide ribonucleoside; ATP, adenosine diphosphate.
Discussion
The primary finding in this study is that activation of AMPK in the human placenta leads to global downregulation of metabolism. Furthermore, mitochondrial toxicity was induced in the trophoblasts at the doses of resveratrol and AICAR used to activate AMPK. These findings suggest that although activation of this pathway has positive metabolic effects on other tissues, in the placenta, this may not be the case.
We have shown in human placental explants that both resveratrol and AICAR act as AMPK agonists and upregulate phosphorylation of this compound, in a dose-dependent manner. This is consistent with prior studies by Um et al which demonstrate that the metabolic effects of resveratrol depend on activation of AMPK.5 Although Saben et al demonstrated that placentas of obese women have decreased total and phosphorylated AMPK protein levels,6 we found that placental AMPK was activated by resveratrol and AICAR similarly in lean and obese women.
Phosphorylation of AMPK by resveratrol and AICAR was associated with lower FA uptake in human placental explants; however, the types of FAs affected varied by the drug used. Unexpectedly, resveratrol decreased the uptake of the polyunsaturated FAs, AA and DHA. Interestingly, uptake of saturated and monounsaturated FAs was not affected. AICAR decreased the uptake of both polyunsaturated and saturated FAs. We suspected the decrease in FA uptake was driven by changes in FA metabolism.2 Consistent with that hypothesis, we found that AMPK activation by resveratrol was associated with decreases in oxidation although this was not associated with increases in esterification. Interestingly, we found AICAR, unlike resveratrol, did not decrease FA oxidation; if anything, a nonsignificant trend toward increase was seen. We think this trend for increasing oxidation by AICAR combined with lower uptake rates resulted in unchanged if not lower rates of esterification at the highest dose (1 mmol/L).
We then hypothesized that reduction in FA uptake and metabolism by these compounds may be due to activation of alternate energy pathways by AMPK. Indeed, in experiments in placental explants performed by Visiedo et al, FA oxidation was reduced in a high glucose state, such as in gestational diabetes.25 We speculated that AMPK activation was stimulating glucose metabolism in placental explants, subsequently downregulating FA metabolism. Unexpectedly, resveratrol and AICAR decreased glucose uptake and metabolism in human placental explants at doses confirmed to stimulate phosphorylated AMPK. These findings are inconsistent with results from nonplacental tissues. Jimenez-Gomez et al showed resveratrol supplementation improved insulin sensitivity in visceral adipose tissue in monkeys.4 A double-blinded, placebo-controlled randomized controlled trial in obese men showed resveratrol had no effect on endogenous glucose production, turnover, or oxidation.26 The abovementioned findings drove us to evaluate the mitochondrial toxicity of these drug doses, despite being used consistently in the literature, at even higher doses.
We are the first to report the potential mitotoxic effects of resveratrol and AICAR in the placenta. The AMPK activation induced significant mitotoxicity in our explants at the highest doses of resveratrol and AICAR. Reviewing the literature, the range of doses of resveratrol and AICAR used in prior studies and in other tissues varied greatly. In human trials, doses of resveratrol ranged from 8 mg/d to 5 g/d as well as the duration of exposure—from a single 45-minute exposure to recurrent doses for as long as 12 months,27 Although the absorption, metabolism, and bioavailability may be different in vivo. In a study that looked at the effects of AICAR-induced activation of AMPK in rat adipocytes, the dose used was 2 mmol/L28—twice our highest dose. Since it has not been previously examined, it is difficult to ascertain whether these toxic effects are specific to human placental cells or would also be seen in other tissues.
A critical impact of this study is in the clinical application of resveratrol, given the toxicity demonstrated here. DSM, a nutritional company that manufactures and sells resveratrol supplements, has published the acceptable daily intake (ADI) recommendations for this compound, not to exceed 7.5 mg/kg body weight/d.29 We found mitotoxic effects at the 100 µmol/L dose of resveratrol in vitro, which corresponds to 10 times this recommended ADI but is comparable to the highest doses used in humans.27 However, the 10 µmol/L dose of resveratrol was within the recommended ADI, equivalent to 7 mg/kg/d. Interestingly, we found that although mitotoxicity was not statistically significant at 10 or 50 µmol/L of resveratrol, neither was phosphorylation of AMPK, suggesting a minimal effect on placental tissue at these doses.
Resveratrol has also been reported as a chemopreventive,30 antiinflammatory,31 as well as working in a neuro32,33 and cardioprotective capacity.34 It has been shown to improve insulin sensitivity and glycemic control in mice, monkeys, and humans.4,11,27,35 Several studies have focused on its beneficial effects in pregnancy. A study published in the American Journal of Obstetrics and Gynecology by Cudmore et al concluded that resveratrol may offer therapeutic potential in preeclampsia by inhibiting sFlt-1 release, a key causative factor in this disease.36 This study was performed in human umbilical vein endothelial cells, transformed human trophoblast-8 (HTR/SVneo)-8/SVneo trophoblast cells, and placental explants. This study used 100 µmol/L dose of resveratrol with the caveat that “resveratrol has been safely administered to healthy volunteers at very high doses and caused no serious adverse effects” but did not test for toxicity in the trophoblast cells. Bourque et al looked at treatment of severe hypoxia in pregnancy with 4 g resveratrol/kg diet per gestational day in a rat model.18 The primary finding in this study was that consumption of resveratrol by the dam improved fetal outcomes in hypoxic pregnancies. An interesting additional finding was that the fetuses in the hypoxic group that survived were 42% growth restricted compared to normoxic controls. In the normoxic pregnancies fed resveratrol, placental weight was 11% lower; the authors concluded that that “these data could indicate a maladaptation of the placenta.” Roberts et al studied the effects of resveratrol supplementation on a high-fat Western diet in Japanese macaques and found beneficial effects on maternal weight loss and glucose clearance.16 Interestingly, placental weight was significantly decreased in the cohort supplemented with resveratrol. Although fetal body weight was unchanged, a 42% increase in pancreatic mass was seen. Alterations in placental weight following resveratrol supplementation did not ultimately impact fetal growth in these animal models, although it was noted by Roberts et al that the “primate placenta does contain a significant reserve capacity to conserve fetal growth, thus a 20% to 25% reduction in placental weight can be well tolerated.” The impact of reduced placental size on placental function remains to be investigated.
Exploring the potential for mitotoxic effects of AMPK upregulation by resveratrol and AICAR is a strength of this study, as this information has not been reported previously. It remains to be established whether the placenta is more sensitive to AMPK agonists than other tissue types. The small number of placentas (N = 29) is a study limitation that may have left us underpowered to detect smaller effects of these drugs at lower doses. The nature of the in vitro model is a study limitation. As resveratrol continues to be purported as a popular supplement with potential for continued use in pregnancy, further studies should evaluate whether the metabolic effects of AMPK activation by this compound lead to an increased rate of fetal growth restriction.
In summary, we found that AMPK activation in human placental explants led to impaired FA uptake and oxidation in addition to a decrease in glucose uptake and metabolism, presumably secondary to the mitotoxic effects and that mitochondrial dysfunction was induced in trophoblasts at the drug doses used to activate AMPK. In obese women, total placental AMPK is reduced.6 Our in vitro experiments suggest a potential for harm in attempting to upregulate this compound, as inadequate placental delivery of critical nutrients may potentially compromise fetal development.
Supplemental Material
Supplemental_Figures for Activation of AMPK in Human Placental Explants Impairs Mitochondrial Function and Cellular Metabolism by Daphne Landau, Maricela Haghiac, Judi Minium, Yelenna Skomorovska-Prokvolit, Virtu Calabuig-Navarro, and Perrie O’Tierney-Ginn in Reproductive Sciences
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health ([R00HD062841]).
Supplemental Material: Supplementary material for this article is available online.
References
- 1. Calabuig-Navarro V, Haghiac M, Minium J, et al. Effect of maternal obesity on placental lipid metabolism. Endocrinology. 2017;158(8):2543–2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Visiedo F, Bugatto F, Quintero-Prado R, Cozar-Castellano I, Bartha JL, Perdomo G. Glucose and fatty acid metabolism in placental explants from pregnancies complicated with gestational diabetes mellitus. Reprod Sci. 2015;22(7):798–801. [DOI] [PubMed] [Google Scholar]
- 3. Perazzolo S, Hirschmugl B, Wadsack C, Desoye G, Lewis RM, Sengers BG. The influence of placental metabolism on fatty acid transfer to the fetus. J Lipid Res. 2017;58(2):443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jimenez-Gomez Y, Mattison JA, Pearson KJ, et al. Resveratrol improves adipose insulin signaling and reduces the inflammatory response in adipose tissue of rhesus monkeys on high-fat, high-sugar diet. Cell Metab. 2013;18(4):533–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Um JH, Park SJ, Kang H, et al. AMP-activated protein kinase–deficient mice are resistant to the metabolic effects of resveratrol. Diabetes. 2010;59(3):554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saben J, Lindsey F, Zhong Y, et al. Maternal obesity is associated with a lipotoxic placental environment. Placenta. 2014;35(3):171–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen KH, Cheng ML, Jing YH, Chiu DT, Shiao MS, Chen JK. Resveratrol ameliorates metabolic disorders and muscle wasting in streptozotocin-induced diabetic rats. Am J Physiol Endocrinol Metab. 2011;301(5):E853–E863. [DOI] [PubMed] [Google Scholar]
- 8. Zhang BB, Zhou G, Li C. AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 2009;9(5):407–416. [DOI] [PubMed] [Google Scholar]
- 9. Kulkarni SS, Canto C. The molecular targets of resveratrol. Biochim Biophys Acta. 2015;1852(6):1114–1123. [DOI] [PubMed] [Google Scholar]
- 10. Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci U S A. 2007;104(17):7217–7222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. de Ligt M, Timmers S, Schrauwen P. Resveratrol and obesity: can resveratrol relieve metabolic disturbances? Biochim Biophys Acta. 2015;1852(6):1137–1144. [DOI] [PubMed] [Google Scholar]
- 12. Dolinsky VW, Chakrabarti S, Pereira TJ, et al. Resveratrol prevents hypertension and cardiac hypertrophy in hypertensive rats and mice. Biochim Biophys Acta. 2013;1832(10):1723–1733. [DOI] [PubMed] [Google Scholar]
- 13. Price NL, Gomes AP, Ling AJY, et al. SIRT1 is required for AMPK activation and the beneficial effects of resveratrol on mitochondrial function. Cell Metab. 2012;15(5):675–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rivera L, Moron R, Zarzuelo A, Galisteo M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem Pharmacol. 2009;77(6):1053–1063. [DOI] [PubMed] [Google Scholar]
- 15. Timmers S, Konings E, Bilet L, et al. Calorie restriction-like effects of 30 days of resveratrol (resVida™) supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14(5): doi:10.1016/j.cmet.2011.1010.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Roberts VH, Pound LD, Thorn SR, et al. Beneficial and cautionary outcomes of resveratrol supplementation in pregnant nonhuman primates. FASEB J. 2014;28(6):2466–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O’Tierney-Ginn P, Roberts V, Gillingham M, et al. Influence of high fat diet and resveratrol supplementation on placental fatty acid uptake in the Japanese macaque . Placenta. 2015;36(8):903–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bourque SL, Dolinsky VW, Dyck JR, Davidge ST. Maternal resveratrol treatment during pregnancy improves adverse fetal outcomes in a rat model of severe hypoxia. Placenta. 2012;33(5):449–452. [DOI] [PubMed] [Google Scholar]
- 19. Singh CK, Ndiaye MA, Ahmad N. Resveratrol and cancer: challenges for clinical translation. Biochim Biophys Acta. 2015;1852(6):1178–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mullane K, Bullough D, Shapiro D. From academic vision to clinical reality: a case study of acadesine. Trends Cardiovasc Med. 1993;3(6):227–234. [DOI] [PubMed] [Google Scholar]
- 21. Liong S, Lappas M. Activation of AMPK improves inflammation and insulin resistance in adipose tissue and skeletal muscle from pregnant women. J Physiol Biochem. 2015;71(4):703–717. [DOI] [PubMed] [Google Scholar]
- 22. Lim R, Barker G, Lappas M. Activation of AMPK in human fetal membranes alleviates infection-induced expression of pro-inflammatory and pro-labour mediators. Placenta. 2015;36(4):454–462. [DOI] [PubMed] [Google Scholar]
- 23. Brass E, Hanson E, O’Tierney-Ginn PF. Placental oleic acid uptake is lower in male offspring of obese women. Placenta. 2013;34(6):503–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Calabuig-Navarro V, Puchowicz M, Glazebrook P, et al. Effect of omega-3 supplementation on placental lipid metabolism in overweight and obese women. Am J Clin Nutr. 2016;103(4):1064–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Visiedo F, Bugatto F, Sanchez V, Cozar-Castellano I, Bartha JL, Perdomo G. High glucose levels reduce fatty acid oxidation and increase triglyceride accumulation in human placenta. Am J Physiol Endocrinol Metab. 2013;305(2):E205–E212. [DOI] [PubMed] [Google Scholar]
- 26. Poulsen MM, Vestergaard PF, Clasen BF, et al. High-dose resveratrol supplementation in obese men. An investigator-initiated, randomized, placebo-controlled clinical trial of substrate metabolism, insulin sensitivity, and body composition. Diabetes. 2013;62(4):1186–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Park EJ, Pezzuto JM. The pharmacology of resveratrol in animals and humans. Biochim Biophys Acta. 2015;1852(6):1071–1113. [DOI] [PubMed] [Google Scholar]
- 28. Gaidhu MP, Fediuc S, Ceddia RB. . 5-Aminoimidazole-4-carboxamide-1-beta-d-ribofuranoside-induced AMP-activated protein kinase phosphorylation inhibits basal and insulin-stimulated glucose uptake, lipid synthesis, and fatty acid oxidation in isolated rat adipocytes. J Biol Chem. 2006;281(36):25956–25964. [DOI] [PubMed] [Google Scholar]
- 29. Edwards JA, Beck M, Riegger C, Bausch J. Safety of resveratrol with examples for high purity, trans-resveratrol, resVida™. Anna N Y Acad Sci. 2011;1215(1):131–137. [DOI] [PubMed] [Google Scholar]
- 30. Patel KR, Brown VA, Jones DJ, et al. Clinical pharmacology of resveratrol and its metabolites in colorectal cancer patients. Cancer Res. 2010;70(19):7392–7399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Poulsen MM, Fjeldborg K, Ornstrup MJ, Kjær TN, Nøhr MK, Pedersen SB. Resveratrol and inflammation: challenges in translating pre-clinical findings to improved patient outcomes. Biochim Biophys Acta. 2015;1852(6):1124–1136. [DOI] [PubMed] [Google Scholar]
- 32. Bastianetto S, Ménard C, Quirion R. Neuroprotective action of resveratrol. Biochim Biophys Acta. 2015;1852(6):1195–1201. [DOI] [PubMed] [Google Scholar]
- 33. Pasinetti GM, Wang J, Ho L, Zhao W, Dubner L. Roles of resveratrol and other grape-derived polyphenols in Alzheimer’s disease prevention and treatment. Biochim Biophys Acta. 2015;1852(6):1202–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zordoky BN, Robertson IM, Dyck JR. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim Biophys Acta. 2015;1852(6):1155–1177. [DOI] [PubMed] [Google Scholar]
- 35. Wang B, Sun J, Li L, Zheng J, Shi Y, Le G. Regulatory effects of resveratrol on glucose metabolism and T-lymphocyte subsets in the development of high-fat diet-induced obesity in C57BL/6 mice. Food Funct. 2014;5(7):1452–1463. [DOI] [PubMed] [Google Scholar]
- 36. Cudmore MJ, Ramma W, Cai M, et al. Resveratrol inhibits the release of soluble fms-like tyrosine kinase (sFlt-1) from human placenta. Am J Obstet Gynecol. 2012;206(3):253 e210–253 e255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental_Figures for Activation of AMPK in Human Placental Explants Impairs Mitochondrial Function and Cellular Metabolism by Daphne Landau, Maricela Haghiac, Judi Minium, Yelenna Skomorovska-Prokvolit, Virtu Calabuig-Navarro, and Perrie O’Tierney-Ginn in Reproductive Sciences