Abstract
Background:
Decidual γδ T cells are known to regulate the function of trophoblasts at the maternal–fetal interface; however, little is known about the molecular mechanisms of cross talk between trophoblast cells and decidual γδ T cells.
Methods:
Expression of chemokine C-X-C motif ligand 6 (CXCL16) and its receptor CXCR6 was evaluated in first-trimester human villus and decidual tissues by immunohistochemistry. γδ T cells were isolated from first-trimester human deciduae and cocultured with JEG3 trophoblast cells. Cell proliferation and apoptosis-related molecules, together with cytotoxicity factor and cytokine production, were measured by flow cytometry analysis.
Results:
Expression of CXCL16 and CXCR6 was reduced at the maternal–fetal interface in patients who experienced unexplained recurrent spontaneous abortion as compared to healthy pregnancy women. With the administration of pregnancy-related hormones or coculture with JEG3 cells, CXCR6 expression was upregulated on decidual γδ T cells. CXCL16 derived from JEG3 cells caused a decrease in granzyme B production of decidual γδ T cells. In addition, decidual γδ T cells educated by JEG3-derived CXCL16 upregulated the expression of Bcl-xL in JEG3 cells.
Conclusion:
This study suggested that the CXCL16/CXCR6 axis may contribute to maintaining normal pregnancy by reducing the secretion of cytotoxic factor granzyme B of decidual γδ T cells and promoting the expression of antiapoptotic marker Bcl-xL of trophoblasts.
Keywords: decidual γδ T cells, trophoblasts, CXCL16, CXCR6, unexplained recurrent spontaneous abortion
Introduction
Blastocyst implantation is a complex process that requires not only the growth and differentiation of villi but also trophoblasts invading the maternal uterine arteries so as to establish sufficient blood supply for the conceptus.1 Trophoblasts are formed during the first stage of pregnancy and are the first cells differentiating from the fertilized egg.2 Trophoblasts could differentiate along either the villous or the extravillous cytotrophoblast (EVCT) pathway.3 At the tip of the anchoring villi, trophoblasts proliferate and differentiate into EVCTs, which invade into decidua to form giant cells with 2 or 3 nuclei or replace the uterine spiral arterial endothelial cells. In contrast, the cytotrophoblasts on the border layer of the floating villi differentiate by cell–cell fusion into multinucleate syncytiotrophoblasts that cover floating villi, provide substance exchange between fetus and mother, and execute endocrine functions of placenta.4,5 All processes are precisely regulated by modulators at the maternal–fetal interface. However, failure to regulate any of the processes may result in abnormalities of blastocyst implantation and placentation, which have been mentioned as main mechanisms that contributed to pregnancy complications, such as early pregnancy loss.6
The mechanisms by which the human semiallogeneic fetoplacental unit is not rejected by the maternal immune system have received intense attention. It has now become clear that a large specific population of leukocytes have special features in local cytokine production, cytotoxicity regulation, and placental development so as to keep pregnancy going smoothly.7–9 During decidualization, the uterine leukocytes appear to increase dramatically in number and account for at least 15% of all decidual cells from early pregnancy through term. Decidual leukocytes have an unusual composition: ∼70% natural killer (NK) cells, ∼15% macrophages, and ∼15% T cells.10,11 About 90% of circulating T cells use the αβ T-cell receptor (TCR), while 5% to 10% of circulating T cells use the alternate heterodimeric TCR composed of γ and δ chains.12,13 Although the γδ T cells represent a small subset of T cells, sufficient evidence showed that γδ T cells perform important protective roles for the host, such as maintaining homeostasis of the tissues where they reside and responding to tissue damage, infection, inflammation, and malignancy.14–16 Our previous study found decidual γδ T cells promoted trophoblast proliferation and invasion and suppressed trophoblast apoptosis through interleukin (IL)-10 secretion.17 However, little is known about the molecular mechanisms of cross talk between trophoblast cells and decidual γδ T cells.
Chemokines are a large family of chemotactic cytokines, whose principal role is orchestrating immune response in the body.18 Because the interactions between chemokines and chemokine receptors dominate the traffic of decidual leukocytes, it is most likely that the expression and secretion of chemokines at the maternofetal interface are involved in recruitment and maintenance of the decidual leukocytes. To date, it has been found that several chemokines are expressed in and are even secreted by cytotrophoblasts, and the corresponding receptors are expressed in the decidual immune cells.19,20 For example, CXCL12 expressed by cytotrophoblasts plays a fundamental role in attracting decidual NK cells.21,22 Our previous work provided evidence that CXCR6 has high expression levels in first-trimester human decidual γδ T cells and CXCL16 from first-trimester human cytotrophoblasts could recruit and maintain γδ T cells residing in human decidua.4 However, whether trophoblasts have regulation roles on the biological functions of decidual γδ T cells through CXCL16/CXCR6 axis is still largely unknown.
The aim of the current study was to investigate the effects of CXCL16/CXCR6 axis on the biological behaviors of trophoblasts and decidual γδ T cells. Our current results suggest that CXCL16/CXCR6 axis plays an important role in cross talk between decidual γδ T cells and trophoblasts and may contribute to maintaining normal pregnancy by reducing the secretion of cytotoxic factor granzyme B of decidual γδ T cells and promoting the expression of antiapoptotic markers of trophoblast cells.
Materials and Methods
Sample Collection
First-trimester human villus and decidual tissues were collected from clinically normal pregnant women and unexplained recurrent spontaneous abortion (URSA) pregnant women undergoing abortion in Obstetrics and Gynecology Hospital of Fudan University between October 2014 and October 2015. The URSA group comprised 10 women with a history of 2 to 6 spontaneous miscarriages at early pregnancy (7-10 weeks of gestation). The mean age was 30.2 ± 1.1 years. The URSA was diagnosed after excluding any verifiable causes, including infection, endocrine or metabolic disease, chromosomal abnormality, anatomic deformation, and autoimmune response. All patients had a single male partner for the pregnancies under investigation. All patients and their male partners had normal karyotypes, and all male partners had a normal semen status according to criteria from the World Health Organization. The control group comprised 30 randomly selected women who underwent a legal termination for nonmedical reasons (7-10 weeks of gestation) at the same facility during the same period. The mean age of the control group was 28.2 ± 3.4 years. All control patients had 1 or 2 living children and no history of spontaneous abortion, ectopic pregnancy, preterm delivery, or stillbirth. In all cases, fetal heart activity was verified and the embryonic karyotype was normal. There was no significant difference in age or gestation between the URSA and control groups. Written informed consent was obtained from all patients before sampling. The study protocol was approved by the research ethics committee of the Obstetrics and Gynecology Hospital, Fudan University.
Cell Culture
JEG3 cell lines were obtained from the Cell Bank of Chinese Academy of Sciences (Shanghai, China). JEG3 cells were cultured in α-minimum essential medium (Gibco, Life Technologies, Grand Island, New York) supplemented with 10% fetal bovine serum (Gibco) at 37°C under 5% CO2 humidified air.
Immunohistochemistry
For immunohistochemistry, paraffin sections of human decidua and villi from the early pregnancy were dehydrated in Tris-buffered saline (TBS) and incubated with 3% hydrogen peroxide and 1% bovine serum albumin (BSA)/TBS to block endogenous peroxidase. The samples were then incubated with mouse antihuman CXCL16 antibody (1:100; Abcam, Cambridge, MA, USA), mouse antihuman CXCR6 antibody (1:250; R&D Systems, Abingdon, United Kingdom), mouse immunoglobulin G (IgG) isotype (for CXCL16 and CXCR6 groups; Sino-America Biotechnology Co. Ltd, Qingdao, China), at 4°C overnight in a humidity chamber. After washing 3 times with TBS, the sections were over laid with peroxidase-conjugated anti-mouse IgG (SP-9002; Zhongshan Golden Bridge International, Inc., Beijing, China). The reaction was developed with 3,3-diaminobenzidine and counterstained with hematoxylin. The experiments were repeated in 10 different patients from each group.
Enzyme-Linked Immunosorbent Assay
JEG3 cells in different concentration of 2 × 104 cells/well, 5 × 104 cells/well, and 1 × 105 cells/well were cultured for 48 hours. The culture supernatants were collected and centrifuged to remove cellular debris and stored at −80°C for CXCL16 measurements. The secretion of CXCL16 in the cultured supernatant samples was determined by enzyme-linked immunosorbent assay (ELISA) using commercially available kits (R&D) according to the manufacturer’s instructions. The JEG3 cells were homogenized, and the total protein levels were measured using a bicinchoninic acid protein assay kit (Pierce). The ELISA data were standardized to the total protein levels of the cell lysates.
Decidual γδ T-Cell Enrichment
Decidual mononuclear cells were obtained from the decidual tissue of normal pregnancies by collagenase type IV (1.0 mg/mL, CLS-1; Worthington Biomedical, Lakewood, New Jersey) and DNase I (150 U/mL; AppliChem, Darmstadt, Germany) as described previously.23 The γδ T lymphocytes were enriched from the isolated decidual mononuclear cells using magnetic isolation kit (positive selection; MiltenyiBiotec, Bergisch Gladbach, Germany). Briefly, the isolated decidual mononuclear cells were suspended in phosphate-buffered saline (PBS)/EDTA/BSA buffer containing 0.5% BSA and 2 mM EDTA. The cells were first labeled with hapten-conjugated monoclonal antibodies directed against TCR γ/δ, followed by conjugate binding with antihapten microbeads. After incubation for 15 minutes at 4°C, the cells were resuspended in PBS/EDTA/BSA buffer and washed twice and passed through a magnetic separation column. After the column was removed from the magnetic field, the magnetically retained TCR γ/δ cells were eluted as the positively selected cell fraction, representing the enriched T-cell fraction. The purity of the enriched γδ T cells was evaluated by flow cytometry (FCM; Becton Dickinson, Palo Alto, California). The purity of the isolated cells was above 95%.
CXCR 6 Expression-Level Detection
Decidual mononuclear cells were plated at the density of 1 × 106 cells/well in 24-well flat-bottom plates and were cocultured with 1 × 105 JEG3 for 48 hours or treated by 10−7 M estrogen, 10−7 M progesterone, and 10 KU/L human chorionic gonadotropin, respectively, for 72 hours. The decidual mononuclear cells were collected for CXCR6 expression detection by FCM.
Cell Viability and Proliferation Assay
Decidual γδ T cells were isolated according to the protocols described in the previous section. An amount of 1 × 104 decidual γδ T cells were seeded in triplicate in 96-well plates and treated with 100 ng/mL recombination human CXCL16 (rhCXCL16), 1 μg/mL anti-CXCL16 antibody, 1 × 104 JEG3, and 1 × 104 JEG3 together with 1 μg/mL anti-CXCL16 antibody for 48 hours, respectively. Treated γδ T cells were collected for cell viability and proliferation assay. The cell viability was analyzed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The proliferation was assessed by Ki-67 expression by FCM.
Interferon γ/Perforin/Granzyme B Expression Analysis
To study the effects of JEG3 coculture on the cytotoxicity of decidual γδ T cells, the expression levels of interferon γ (IFN-γ), perforin, and granzyme B were examined. An amount of 1 × 105 decidual γδ T cells were seeded in 24-well plates and treated with 100 ng/mL rhCXCL16, 1 μg/mL anti-CXCL16 antibody, 1 × 104 JEG3, and 1 × 104 JEG3 together with 1 μg/mL anti-CXCL16 antibody for 48 hours, respectively. Then, the γδ T cells were collected and FCM was performed to evaluate the expression levels of IFN-γ, perforin, and granzyme B.
Cytokine Production Detection
To test the effects of JEG3 on the cytokine production in decidual γδ T cells, 1 × 105 decidual γδ T cells were seeded in 24-well plates and treated with 100 ng/mL rhCXCL16, 1 μg/mL anti-CXCL16 antibody, 1 × 104 JEG3, and 1 × 104 JEG3 together with 1 μg/mL anti-CXCL16 antibody for 48 hours, respectively. Then, the γδ T cells were collected and used for expression level evaluation of a panel of 6 cytokines (IL-4, IL-5, IL-10, IL-17A, transforming growth factor β [TGF-β], and tumor necrosis factor α [TNF-α]) by FCM.
Expression Analysis of Proliferation and Apoptosis-Related Markers of JEG3 Cells
To investigate the modulatory role that decidual γδ T cells may exert on the biological functions of human trophoblasts, we evaluated the effects of γδ T cells on the expression of cell proliferation and apoptosis-related markers in JEG3 cells. An amount of 5 × 105 γδ T cells were seeded in 24-well plates and treated with 5 × 105 JEG3 cells alone or combined with 1 μg/mL anti-CXCL16 antibody for 48 hours, respectively. Then, the γδ T cells were collected and cocultured with fresh JEG3 for another 48 hours. Thereafter, JEG3 was collected and used for ki67, Bcl-xL, FasL, and Fas expression level evaluation by FCM.
Flow Cytometry Analysis
The expression of cell-surface markers and the production of intracellular cytokine were evaluated by FCM. Briefly, the cells were resuspended in PBS at a density of 5 × 106/mL and added to Falcon 2054 polystyrene round-bottom tubes (Becton Dickinson) in 100-μL aliquots to each tube for immunolabeling. The cells were immediately stained by a standard immunofluorescence assay. Following incubation at 4°C for 30 minutes, the cells were washed twice and resuspended in PBS for surface marker analysis by FCM. For intracellular cytokine analysis, cells (1 × 106/well) were cocultured with phorbol myristate acetate, ionomycin, and brefeldin A for 4 hours in 24-well flat-bottom plates (Nunc, Roskilde, Denmark). Thereafter, the cells were harvested and resuspended in PBS at a density of 5 × 106/mL and distributed in 100-μL aliquots to Falcon 2054 polystyrene round-bottom tubes. The cells were then fixed, permeabilized, stained, washed twice, and resuspended in PBS for FCM analysis. In parallel, isotypic IgG antibodies were used as controls. Samples were analyzed in a FACS Calibur flow cytometer (Becton Dickinson) using Cellquest software (Becton Dickinson). Statistical analysis was conducted by using isotype-matched controls as references. Typically, fewer than 1% positive cells were allowed beyond the statistical marker in the appropriate controls. Fluorescein-isothiocyanate–conjugated anti-γδ TCR (mouse IgG1) monoclonal antibody (mAb); phycoerythrin (PE)-conjugated CXCL16 (mouse IgG2b); CXCR6 (mouse IgG2b); Ki67 (mouse IgG1); and IFN-γ, IL-4, IL-10, IL-5, TNF-α, and TGF-β, IL-17A (mouse IgG2b) mAbs; allophycocyanin-conjugated anti-perforin (mouse IgG1) mAb; PE-Cy5-conjugated anti-granzyme B (mouse IgG1) mAb; and corresponding isotype controls were purchased from Caltag (Burlingame, California).
Statistical Analysis
Data were analyzed with SPSS (version 16, IBM, USA) software, and statistical significance was determined using Student t test and 1-way analysis of variance with P values <.05 being considered statistically significant.
Results
CXCL16/CXCR6 Expression Levels Were Reduced in Villi of URSA Patients
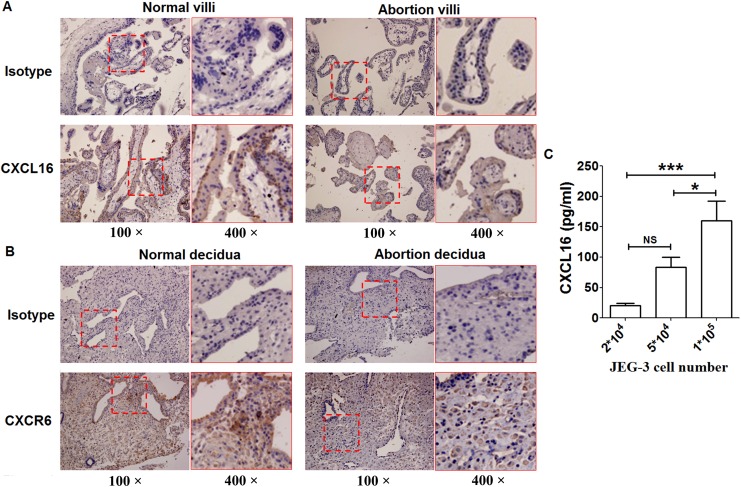
The localization and protein expression levels of CXCL16 and CXCR6 at the maternal–fetal interface were evaluated in pregnant individuals with a normal first trimester and compared to URSA patients by immunohistochemistry. It was observed that CXCL16 was localized in both the syncytiotrophoblast and cytotrophoblast layers of first-trimester villi (Figure 1A). Compared to villi from normal pregnant women, CXCL16 was weakly positive staining in villi from women experiencing URSA (each group included 10 different patients). CXCR6 was localized in stroma cells of decidua (Figure 1B) and was weakly positive staining in decidua from URSA patients as compared to normal decidua. CXCL16 protein expression was also analyzed in the culture medium of JEG3 cells by ELISA. As the cell number of JEG3 rises, CXCL16 protein levels increased (Figure 1C).
Figure 1.
Reduced expression of CXCL16 and CXCR6 was found in villi and decidua of URSA patients. Immunohistochemistry analysis of the expression and localization of CXCL16 (A) and CXCR6 (B) was performed in villi from 10 women in the first trimester of pregnancy and 10 URSA patients. Representative images (100× and 400×) were shown. C, CXCL16 protein expression in the culture of JEG3 cells was analyzed by ELISA. Data were mean ± SEM from 5 independent experiments. *P < .05; ***P < .001. ELISA indicates enzyme-linked immunosorbent assay; NS, nonsignificant; SEM, standard error of the mean; URSA, unexplained recurrent spontaneous abortion.
Trophoblast Cells or Pregnant-Related Hormones Upregulated CXCR6 Expression on Decidual γδ T Cells
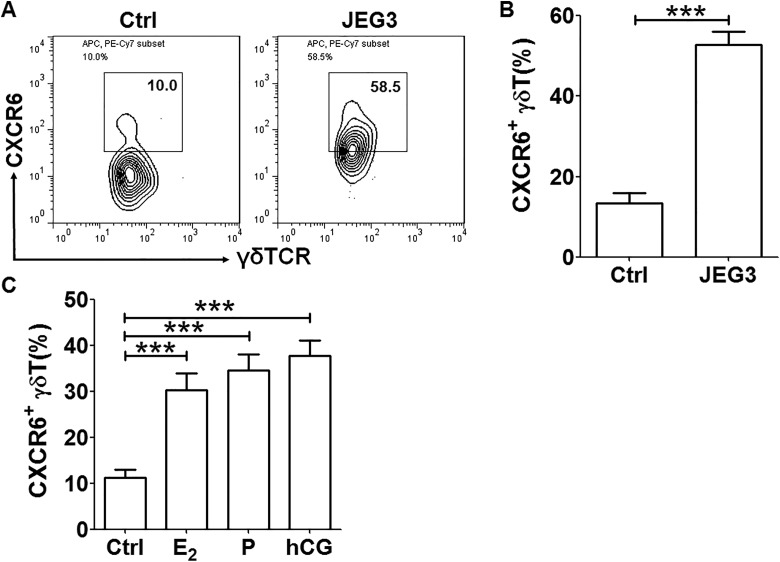
Decidual immune cells that were isolated from decidual tissues of women in the early stages of normal pregnancies were cocultured with JEG3 cells. The expression of CXCR6 on decidual γδ T cells was detected by FCM. When the decidual immune cells were cocultured with JEG3 trophoblast cells, the percentages of CXCR6+ cells in the γδ TCR+ cell population were induced about 3-fold (Figure 2A and B). When the decidual immune cells were treated by estrogen, progesterone, or human chorionic gonadotropin, the expression of CXCR6 on decidual γδ T cells was also significantly upregulated by more than 2 times (Figure 2C). Our results indicated that JEG3 cells and pregnant-related hormones could increase CXCR6 expression on decidual γδ T cells.
Figure 2.
Expression of CXCR6 was induced in decidual γδ T cells cocultured with JEG3 or treated by pregnancy-related hormones. A, Coculture with JEG3 trophoblast cells for 48 hours, CXCR6+ decidual γδ T cells were determined by flow cytometric analysis. B, A t test was performed for the statistical significance of percentage of CXCR6+ decidual γδ T cells between control cells and treated cells. C, After treatment with estrogen, progesterone, and human chorionic gonadotropin for 72 hours, respectively, CXCR6+ γδ T cells were determined by FCM. A t test was performed for significance testing. Data were mean ± SEM from 3 independent experiments. ***P < .001. FCM indicates flow cytometry; SEM, standard error of the mean.
The Stimulatory Effects of Trophoblast Cells on Viability and Proliferation of Decidual γδ T Cells Were Independent on CXCL16
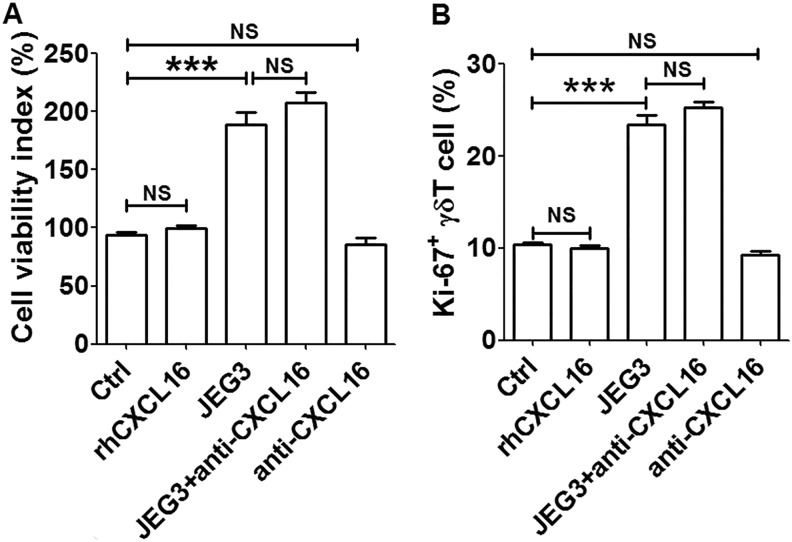
To investigate the modulatory roles those JEG3 cells may exert on the biological functions of decidual γδ T cells, decidual γδ T cells were enriched from isolated decidual immune cells using magnetic isolation kit, and the coculture system with JEG3 was established. We first evaluated the effects of JEG3 on the cell viability and proliferation of decidual γδ T cells. As shown in Figure 3, the cell viability index and proliferation of decidual γδ T cells were increased approximately 2 times after coculture with JEG3. When CXCL16 neutralizing antibody was added to the coculture system, the cell viability and proliferation of decidual γδ T cells maintained at high levels. When decidual γδ T cells were treated with rhCXCL16 or CXCL16 neutralizing antibody alone, the cell viability and proliferation were unchanged. Our results indicated that JEG3 cells could promote the viability and proliferation of decidual γδ T cells probably independent of CXC16.
Figure 3.
JEG3 trophoblast cells promoted the cell viability and proliferation of decidual γδ T cells. After coculture with JEG3 and/or treatment with rhCXCL16 or CXCL16 neutralizing antibody for 48 hours, respectively, the cell viability (A) and proliferation (B) of decidual γδ T cells were detected. A t test was performed for significance testing. Data were mean ± SEM from 3 independent experiments. ***P < .001. NS indicates nonsignificant; SEM, standard error of the mean.
Trophoblast Cells Regulated the Secretion of Cytotoxic Factors of Decidual γδ T Cells
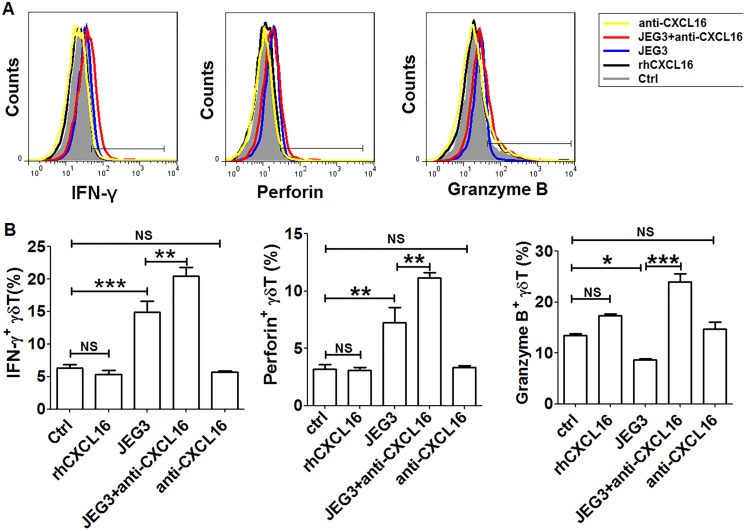
To study the effects of JEG3 cells on the cytotoxicity of decidual γδ T cells, the expression levels of IFN-γ, perforin, and granzyme B were examined by FCM. As shown in Figure 4, the expression levels of IFN-γ and perforin of decidual γδ T cells were upregulated after coculture with JEG3 cells. The CXCL16 neutralizing antibody further amplified the inducing effects of JEG3 on the expression of IFN-γ and perforin. After coculture with JEG3 cells, the secretion of granzyme B of decidual γδ T cells was downregulated. The CXCL16 neutralizing antibody could significantly reverse the inhibitory effects of JEG3 coculture on the secretion of granzyme B. When decidual γδ T cells were treated with rhCXCL16 or CXCL16 neutralizing antibody alone, neither IFN-γ, perforin, nor granzyme B had expression changes. The results indicated that JEG3 might reduce the secretion of cytotoxic factor granzyme B of decidual γδ T cells dependent of CXCL16.
Figure 4.
JEG3 trophoblast cells downregulated the secretion of granzyme B from decidual γδ T cells dependent of CXCL16. A, Coculture with JEG3 and/or treatment with rhCXCL16 or CXCL16 neutralizing antibody for 48 hours, respectively, the secretion of granzyme B, IFN-γ, and perforin of γδ T cells was analyzed by FCM. B, A t test was performed for significance testing. Data were mean ± SEM from 3 independent experiments. *P < .05; **P < .01; ***P < .001. FCM indicates flow cytometry; IFN-γ, interferon γ; NS, nonsignificant; SEM, standard error of the mean.
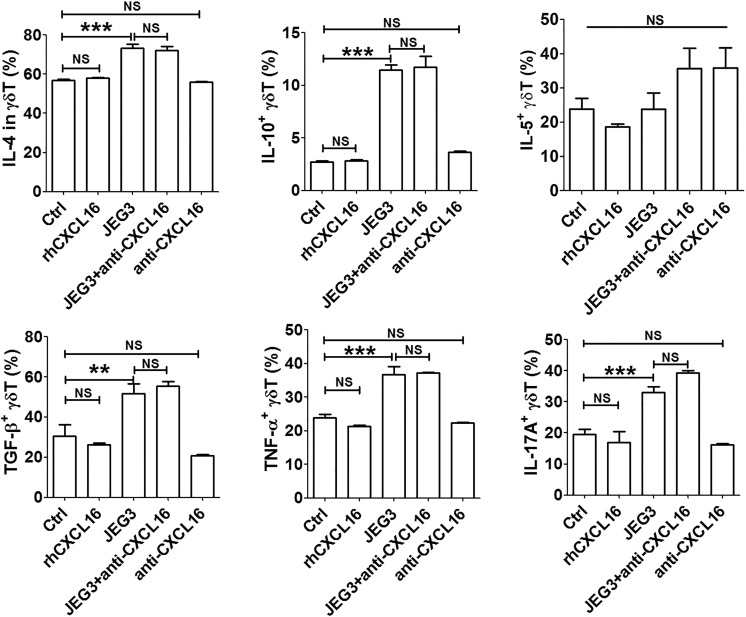
Trophoblast Cells Induced the Cytokine Production in Decidual γδ T Cells
To test the effects of JEG3 on the cytokine production in decidual γδ T cells, a panel of 6 cytokines (IL-4, IL-5, IL-10, IL-17A, TGF-β, and TNF-α) were evaluated by FCM. Our results showed that JEG3 could upregulate the secretion of IL-4, IL-10, TGF-β, TNF-α, and IL-17A (Figure 5). When neutralizing antibody against CXCL16 was added into the coculture system, the secretion of IL-4, IL-10, TGF-β, TNF-α, and IL-17A kept at high levels. Treated with rhCXCL16 or neutralizing antibody against CXCL16 alone, the secretion of all tested cytokines in decidual γδ T cells had no changes. Our results demonstrated that JEG3 trophoblast cells induced the cytokine production in decidual γδ T cells independent of CXCL16.
Figure 5.
JEG3 trophoblast cells induced the cytokine production in decidual γδ T cells. Coculture with JEG3 and/or treatment with rhCXCL16 or CXCL16 neutralizing antibody for 48 hours, respectively, the expression of cytokines (IL-4, IL-5, IL-10, TGF-β, TNF-α, and IL-17A) was determined by FCM. A t test and 1-way analysis of variance were performed for significance testing. Data were mean ± SEM from 12 independent experiments. **P < .01; ***P < .001. FCM indicates flow cytometry; IL, interleukin; NS, nonsignificant; SEM, standard error of the mean; TGF-β, transforming growth factor β; TNF-α, tumor necrosis factor α.
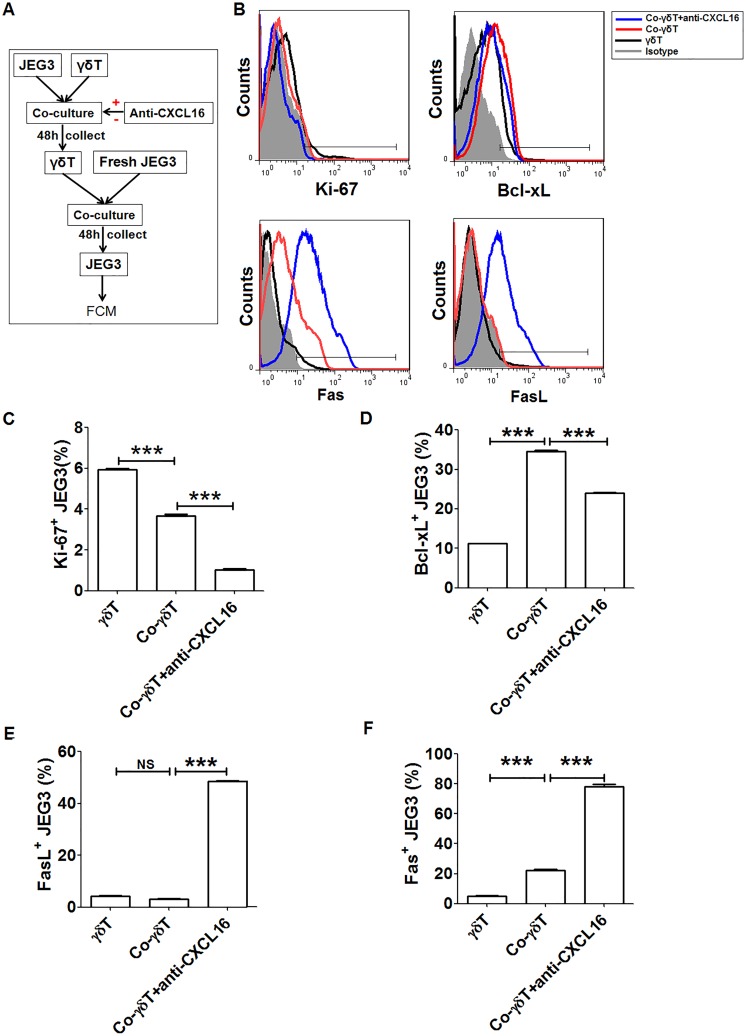
Decidual γδ T Cells Educated by JEG3-Derived CXCL16 Upregulated Bcl-xL
To investigate the roles the decidual γδ T cells may play on the biological functions of human trophoblasts, we evaluated the expression changes of cell proliferation and apoptosis-related molecules of JEG3 cells cocultured with decidual γδ T cells. As shown in Figure 6, decidual γδ T cells educated by excreted CXCL16 from JEG3 cells could promote the expression of Bcl-xL. Neutralizing antibody against CXCL16 significantly reversed the inhibitory effects of JEG3-derived CXCL16 on Bcl-xL expression of JEG3 cells. Our results demonstrated that decidual γδ T cells educated by trophoblast-derived CXCL16 might inhibit apoptosis of trophoblasts by upregulating Bcl-xL.
Figure 6.
Decidual γδ T cells educated by JEG3-derived CXCL16 upregulated Bcl-xL expression. A, A diagram of cell treatment and sample collection progresses was given. B, The expression of cell proliferation and apoptosis-related markers (ki-67, Fas, FasL, and Bcl-xL) was analyzed by FCM for JEG3. C-F A t test was performed for significance testing. Data were mean ± SEM from 3 independent experiments. **P < .01; ***P < .001. FCM indicates flow cytometry; NS, nonsignificant; SEM, standard error of the mean.
Discussion
The implantation of a semiallogeneic fetus in the maternal uterus presents a major challenge to the maternal immune system. One of the ways in which the immune system deals with the challenge is the unique distribution of decidual immune cells. The decidual immune cells are recruited from the periphery rather than by self-renewal in the deciduae. Because chemokines have been implicated as pivotal players in trafficking of immune cells to decidua, it implies that some chemokines are expressed highly at the maternal–fetal interface and are required for effective homing of the leukocytes. It was reported that chemokine CXCL12 is expressed by invasive trophoblasts and induces the specific migration of human NK cells where CXCR4 is preferentially expressed.22 Chemokine CXCL16 is highly expressed in and secreted by the first-trimester human trophoblasts, and CXCR6 as the sole receptor of CXCL16 is preferentially expressed on decidual γδ T cells.4 The CXCL16/CXCR6 interaction is involved in the migration of the peripheral γδ T cells.4 The results suggested that fetus-derived trophoblasts can attract γδ T cells by producing CXCL16 and interacting with CXCR6 on these cells, leading to the formation of a specialized immune milieu at the maternofetal interface. However, whether there is a mutual biological regulation mechanism between trophoblasts and decidual γδ T cells mediated by CXCL16/CXCR6 axis is still largely unknown. In the current work, we established the coculture system for JEG3 trophoblast cells and decidual γδ T cell. The modulatory roles of JEG3 cells may exert on the biological functions of decidual γδ T cells, and the possible effects of educated decidual γδ T cells on JEG3 cells were investigated.
CXCL16 is one of the members of the plasma-membrane chemokines, consisting of a chemokine domain followed by a glycosylated mucin-like stalk, a single transmembrane helix and a short cytoplasmic tail.24 It has dual functions as a membrane-bound molecule and a soluble chemokine; the soluble CXCL16 induces homing of some leukocytes, whereas the transmembrane molecule functions as a scavenger receptor for oxidized low-density lipoprotein and an adhesion molecule to CXCR6-expressing cells.25 Our study showed that after treatment with estrogen, progesterone, or human chorionic gonadotropin or coculture with trophoblast cells, CXCR6 expression was upregulated on decidual γδ T cells. The chemokine receptor CXCR6 expression is finely controlled during cell activation and development.26,27 Cytokines IL-12, IL-4, IL-15, IL-5, and IFN-γ are important modulators of CXCR6 expression.28,29 Notably, previous studies reported that estrogen and progesterone stimulate the production of cytokines IL-10, IL-4, and TGF-β.30,31 Thus, our results indicated that pregnancy-related hormones and trophoblast cells upregulated CXCR6 expression probably by inducing the production of cytokines (IL-10, IL-4, IL-17A, TNF-α, and TGF-β) in decidual γδ T cells. The detailed intracellular regulating mechanisms need to be further studied.
γδ T cells play important roles in innate immunity against tumors and infections. The killing of tumor cells is associated with increased expression of perforin, granzyme B, and IFN-γ.32 In this study, first-trimester γδ T cells were cocultured with trophoblast cells and then the expression of perforin, granzyme B, and IFN-γ in γδ T cells was estimated. Only granzyme B expression was found to be inhibited by trophoblast cells and the inhibition could be reversed by anti-CXCL16 antibody. Our results suggest that the trophoblast cells might reduce cytotoxic factor granzyme B expression of decidual γδ T cells in the first-trimester by producing CXCL16 and interaction with CXCR6 on γδ T cells. Another interesting finding in this study is that decidual γδ T cells educated by trophoblast cells were found to upregulate the expression of antiapoptotic protein Bcl-xL of trophoblast cells; the increased Bcl-xL expression could be reversed by pretreatment of anti-CXCL16 antibody. CXCL16/CXCR6 signal seems to be activated by interaction between decidual γδ T cells and trophoblast cells to modulate apoptosis-related protein expression in trophoblast cells. There is evidence that CXCL16/CXCR6 could directly activate NF-κB signaling transduction pathway to induce cell proliferation and inhibit cell apoptosis.33,34 Future work would focus on investigating the intracellular mechanisms of the CXCL16/CXCR6 chemokine axis reducing the killing activity of decidual γδ T cells and inhibiting apoptosis of trophoblast cells. Although antiapoptosis protein Bcl-xL was increased, cell proliferation–related protein ki67 was not upregulated in trophoblast cells after coculture with decidual γδ T cells. It seems that other cytokines from the coculture system might downregulate the proliferation of trophoblast cells and neutralize the promoting effects of CXCL16 on proliferation. In this study, we also found that CXCL16 and CXCR6 expression levels were decreased at the maternal–fetal interface of URSA patients as compared to normal pregnant women, consistent with the hypothesis that insufficient expression of CXCL16/CXCR6 probably disturbs the blastocyst implantation and further increases the risk of abortion.
The data were obtained using the JEG-3 cell line that originated from choriocarcinoma explants. They are phenotypically similar to extravillous trophoblast and have been extensively used as an in vitro model to study the properties of trophoblasts.35–37 However, it should be understood that cell lines do not completely mimic the primary cells. Hence, further studies of the primary trophoblast cells are required to verify the findings.
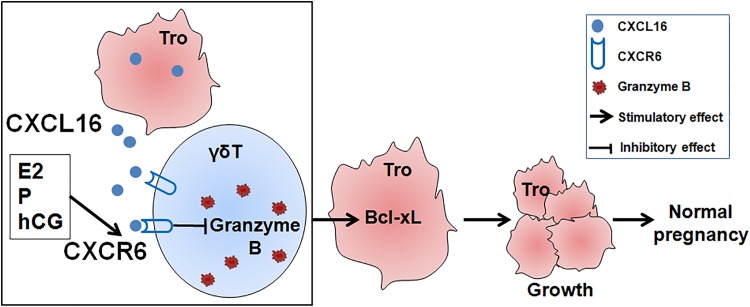
In summary, this study indicates that chemokine CXCL16 and its receptor CXCR6 were expressed in first-trimester villi and deciduae, respectively. The expression levels of CXCL16/CXCR6 were reduced at the maternal–fetal interface of URSA patients as compared to healthy pregnancy women. Moreover, CXCR6 expression levels were upregulated on decidual γδ T cells treated with pregnant-related hormones or cocultured with JEG3 trophoblast cells. In addition, CXCL16 derived from JEG3 cells caused a decrease in granzyme B production of decidual γδ T cells. Decidual γδ T cells educated by JEG3-derived CXCL16 further upregulated the expression of Bcl-xL of JEG3 cells. Collectively, our current study suggest that CXCL16/CXCR6 axis plays an important role in cross talk between decidual γδ T cells and trophoblasts and may contribute to maintaining normal pregnancy by reducing the secretion of cytotoxic factor granzyme B of decidual γδ T cells and promoting the expression of antiapoptotic marker Bcl-xL of trophoblasts (Figure 7). These findings contribute to a better understanding about the complex process of pregnancy and may provide us a clue for searching effective treatments to prevent failure of pregnancy.
Figure 7.
The proposed mechanism of cross talk between decidual γδ T cells and trophoblast cells mediated by CXCL16/CXCR6 during pregnancy. Treatment with pregnancy-related hormones or coculture with JEG3 trophoblast cells, CXCR6 expression was upregulated on decidual γδ T cells. CXCL16 derived from JEG3 trophoblast cells caused a decrease in granzyme B production of decidual γδ T cells. Decidual γδ T cells educated by JEG3-derived CXCL16 upregulated the expression of Bcl-xL in JEG3 trophoblast cells. Maintaining appropriate trophoblast growth is important for a normal healthy pregnancy.
Footnotes
Authors’ Note: Deng-Xuan Fan and Wen-Jie Zhou contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by National Natural Science Foundation of China (81300552, 31170870, 81501270), National 863 Program (2014AA020706), the Shanghai Outstanding Academic Leaders Program (15XD1500900), Program for Shanghai leaders, Training Program for excellent academic leaders of Shanghai Health System (XBR2013093), the Innovation Project of Shanghai Municipal Education Commission (14ZZ011), the Foundation of Shanghai Science and Technology Committee (134119a4300), the grant from National Key R&D Program of China (2016YFC1303100), and Shanghai Medical Center of Key Programs for Female Reproductive Diseases (2017ZZ01016).
References
- 1. Fujiwara H, Higuchi T, Yamada S, et al. Human extravillous trophoblasts express laeverin, a novel protein that belongs to membrane-bound gluzincin metallopeptidases. Biochem Biophys Res Commun. 2004;313(4):962–968. [DOI] [PubMed] [Google Scholar]
- 2. Ji L, Brkic J, Liu M, Fu G, Peng C, Wang YL. Placental trophoblast cell differentiation: physiological regulation and pathological relevance to preeclampsia. Mol Aspects Med. 2013;34(5):981–1023. [DOI] [PubMed] [Google Scholar]
- 3. Baczyk D, Dunk C, Huppertz B, et al. Bi-potential behaviour of cytotrophoblasts in first trimester chorionic villi. Placenta. 2006;27(4-5):367–374. [DOI] [PubMed] [Google Scholar]
- 4. Huang Y, Zhu XY, Du MR, Li DJ. Human trophoblasts recruited T lymphocytes and monocytes into decidua by secretion of chemokine CXCL16 and interaction with CXCR6 in the first-trimester pregnancy. J Immunol. 2008;180(4):2367–2375. [DOI] [PubMed] [Google Scholar]
- 5. Huang Y, Zhu XY, Du MR, Wu X, Wang MY, Li DJ. Chemokine CXCL16, a scavenger receptor, induces proliferation and invasion of first-trimester human trophoblast cells in an autocrine manner. Hum Reprod. 2006;21(4):1083–1091. [DOI] [PubMed] [Google Scholar]
- 6. Quaranta M, Erez O, Mastrolia SA, et al. The physiologic and therapeutic role of heparin in implantation and placentation. Peer J. 2015;3:e691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Boyson JE, Rybalov B, Koopman LA, et al. CD1d and invariant NKT cells at the human maternal–fetal interface. Proc Natl Acad Sci U S A. 2002;99(21):13741–13746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Croy BA, Chantakru S, Esadeg S, Ashkar AA, Wei Q. Decidual natural killer cells: key regulators of placental development (a review). J Reprod Immunol. 2002;57(1-2):151–168. [DOI] [PubMed] [Google Scholar]
- 9. Sun J, Yang M, Ban Y, et al. Tim-3 is upregulated in NK cells during early pregnancy and inhibits NK cytotoxicity toward trophoblast in galectin-9 dependent pathway. PLoS One. 2016;11(1):e0147186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Du MR, Wang SC, Li DJ. The integrative roles of chemokines at the maternal–fetal interface in early pregnancy. Cell Mol Immunol. 2014;11(5):438–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Red-Horse K, Drake PM, Gunn MD, Fisher SJ. Chemokine ligand and receptor expression in the pregnant uterus: reciprocal patterns in complementary cell subsets suggest functional roles. Am J Pathol. 2001;159(6):2199–2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mincheva-Nilsson L. Pregnancy and gamma/delta T cells: taking on the hard questions. Reprod Biol Endocrinol. 2003;1:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Glusman G, Rowen L, Lee I, et al. Comparative genomics of the human and mouse T cell receptor loci. Immunity. 2001;15(3):337–349. [DOI] [PubMed] [Google Scholar]
- 14. Chen ZW. Comparative biology of gamma delta T cells. Sci Prog. 2002;85(Pt 4):347–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hayday AC. Gammadelta T cells and the lymphoid stress-surveillance response. Immunity. 2009;31(2):184–196. [DOI] [PubMed] [Google Scholar]
- 16. Rani M, Schwacha MG. The composition of T-cell subsets are altered in the burn wound early after injury. PLoS One. 2017;12(6):e0179015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fan DX, Duan J, Li MQ, Xu B, Li DJ, Jin LP. The decidual gamma-delta T cells up-regulate the biological functions of trophoblasts via IL-10 secretion in early human pregnancy. Clin Immunol. 2011;141(3):284–292. [DOI] [PubMed] [Google Scholar]
- 18. Deruaz M, Luster AD. Chemokine-mediated immune responses in the female genital tract mucosa. Immunol Cell Biol. 2015;93(4):347–354. [DOI] [PubMed] [Google Scholar]
- 19. Oghumu S, Varikuti S, Terrazas C, et al. CXCR3 deficiency enhances tumor progression by promoting macrophage M2 polarization in a murine breast cancer model. Immunology. 2014;143(1):109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ren L, Liu YQ, Zhou WH, Zhang YZ. Trophoblast-derived chemokine CXCL12 promotes CXCR4 expression and invasion of human first-trimester decidual stromal cells. Hum Reprod. 2012;27(2):366–374. [DOI] [PubMed] [Google Scholar]
- 21. Hanna J, Wald O, Goldman-Wohl D, et al. CXCL12 expression by invasive trophoblasts induces the specific migration of CD16-human natural killer cells. Blood. 2003;102(5):1569–1577. [DOI] [PubMed] [Google Scholar]
- 22. Wu X, Jin LP, Yuan MM, Zhu Y, Wang MY, Li DJ. Human first-trimester trophoblast cells recruit CD56brightCD16-NK cells into decidua by way of expressing and secreting of CXCL12/stromal cell-derived factor 1. J Immunol. 2005;175(1):61–68. [DOI] [PubMed] [Google Scholar]
- 23. Du MR, Guo PF, Piao HL, et al. Embryonic trophoblasts induce decidual regulatory T cell differentiation and maternal–fetal tolerance through thymic stromal lymphopoietin instructing dendritic cells. J Immunol. 2014;192(4):1502–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Abel S, Hundhausen C, Mentlein R, et al. The transmembrane CXC-chemokine ligand 16 is induced by IFN-gamma and TNF-alpha and shed by the activity of the disintegrin-like metalloproteinase ADAM10. J Immunol. 2004;172(10):6362–6372. [DOI] [PubMed] [Google Scholar]
- 25. Shimaoka T, Nakayama T, Fukumoto N, et al. Cell surface-anchored SR-PSOX/CXC chemokine ligand 16 mediates firm adhesion of CXC chemokine receptor 6-expressing cells. J Leukoc Biol. 2004;75(2):267–274. [DOI] [PubMed] [Google Scholar]
- 26. Heesch K, Raczkowski F, Schumacher V, Hünemörder S, Panzer U, Mittrücker HW. The function of the chemokine receptor CXCR6 in the T cell response of mice against Listeria monocytogenes. PLoS One. 2014;9(5):e97701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim CH, Kunkel EJ, Boisvert J, et al. Bonzo/CXCR6 expression defines type 1-polarized T-cell subsets with extralymphoid tissue homing potential. J Clin Invest. 2001;107(5):595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hydes T, Noll A, Salinas-Riester G, et al. IL-12 and IL-15 induce the expression of CXCR6 and CD49a on peripheral natural killer cells. Immun Inflamm Dis. 2018;6(1):34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Calabresi PA, Yun SH, Allie R, Whartenby KA. Chemokine receptor expression on MBP-reactive T cells: CXCR6 is a marker of IFNgamma-producing effector cells. J Neuroimmunol. 2002;127(1-2):96–105. [DOI] [PubMed] [Google Scholar]
- 30. Salem ML. Estrogen, a double-edged sword: modulation of TH1- and TH2-mediated inflammations by differential regulation of TH1/TH2 cytokine production. Curr Drug Targets Inflamm Allergy. 2004;3(1):97–104. [DOI] [PubMed] [Google Scholar]
- 31. Maccarrone M, Valensise H, Bari M, Lazzarin N, Romanini C, Finazzi-Agrò A. Progesterone up-regulates anandamide hydrolase in human lymphocytes: role of cytokines and implications for fertility. J Immunol. 2001;166(12):7183–7189. [DOI] [PubMed] [Google Scholar]
- 32. Zhou ZH, Chen FX, Xu WR, et al. Enhancement effect of dihydroartemisinin on human gammadelta T cell proliferation and killing pancreatic cancer cells. Int Immunopharmacol. 2013;17(3):850–857. [DOI] [PubMed] [Google Scholar]
- 33. Chandrasekar B, Bysani S, Mummidi S. CXCL16 signals via Gi, phosphatidylinositol 3-kinase, Akt, I kappa B kinase, and nuclear factor-kappa B and induces cell-cell adhesion and aortic smooth muscle cell proliferation. J Biol Chem. 2004;279(5):3188–3196. [DOI] [PubMed] [Google Scholar]
- 34. Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Genes Dev. 1999;13(22):2905–2927. [DOI] [PubMed] [Google Scholar]
- 35. Hannan NJ, Paiva P, Dimitriadis E, Salamonsen LA. Models for study of human embryo implantation: choice of cell lines? Biol Reprod. 2010;82(2):235–245. [DOI] [PubMed] [Google Scholar]
- 36. Gong L, Zhu L, Wang S, Zhang Z. Transthyretin regulates the migration and invasion of JEG-3 cells. Oncol Lett. 2017;13(3):1242–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Fu Y, Wei J, Dai X, Ye Y. Increased NDRG1 expression attenuate trophoblast invasion through ERK/MMP-9 pathway in preeclampsia. Placenta. 2017;51:76–81. [DOI] [PubMed] [Google Scholar]