Abstract
Ovarian cancer as the most fatal gynecological malignancy is often manifested by excessive fluid accumulation known as ascites or effusion. Ascites-derived microRNAs (miRNAs) may be closely associated with ovarian cancer progression. However, our knowledge of their roles, altered expression, and clinical outcomes remained limited. In this study, large-scale expression profiling of 754 human miRNAs was performed using real-time quantitative polymerase chain reaction and 384-well TaqMan array human miRNA A and B cards to identify differentially expressed miRNAs between extracellular fraction of the ascitic fluid associated with high-grade serous ovarian carcinomas and control plasma. Of the 754 miRNAs, 153 were significantly differentially expressed relative to the controls. Expression of 7 individual miRNAs (miR-200a, miR-200b, miR-200c, miR-141, miR-429, miR-1290, and miR-30a-5p) was further validated in extended sample sets, including serous, endometrioid, and mucinous subtypes. All miR-200 family members and miR-1290 were conspicuously overexpressed, while miR-30a-5p was only weakly overexpressed. The ability of miRNAs expression to discriminate the pathological samples from the controls was strong. Receiver operating characteristic curve analyses found area under the curve (AUC) values of 1.000 for miR-200a, miR-200c, miR-141, miR-429, and miR-1290 and of AUC 0.996 and 0.885 for miR-200b and miR-30a-5p, respectively. Preliminary survival analyses indicated low expression level of miR-200b as significantly related to longer overall survival (hazard ratio [HR]: 0.25, mean survival 44 months), while high expression level was related to poor overall survival (HR: 4.04, mean survival 24 months). Our findings suggested that ascites-derived miRNAs should be further explored and evaluated as potential diagnostic and prognostic biomarkers for ovarian cancer.
Keywords: ovarian cancer; ascites; microRNA; diagnostic markers; prognostic markers; miR-200a,b,c, miR-141, miR-429, miR-1290; effusion
Introduction
Ovarian cancer is the most fatal gynecological malignancy with high metastatic potential and chemotherapeutic resistance. The disease is usually diagnosed in the advanced stages, leading to high levels of recurrence and substantially reduced survival relative to early diagnosis.1 Advanced stages are often manifested by ascites or effusion, the excessive accumulation of fluid within the peritoneal, and/or pleural cavity, but this fluid may also occur in early stages. The presence of ascites is a poor prognostic indicator, even in women with stage I/II disease,2 but dramatically decreases survival rates in advanced stages.3
Lymphocytes, mesothelial cells, macrophages, and malignant tumor cells (including cancer stem cells) occur in ascitic fluid in an environment of soluble growth factors, cytokines, chemokines, and extracellular matrix fragments.4,5 All these biologically active components may be linked to carcinogenesis, invasion, metastatic spread, chemoresistance, and the recurrence of ovarian cancer. Within this environment, the associated regulatory mechanisms may involve microRNAs (miRNAs) functioning at both cellular and extracellular levels, respectively.
MiRNAs are posttranscriptional regulators of gene expression with promising potential in cancer diagnostics, various therapeutic applications, and as novel biomarkers for monitoring the disease progression, predicting the response to treatment or estimating clinical outcomes.6–9 Expression and potential functional roles of ascites-derived miRNAs have been investigated only in a handful of recent pilot studies focused on cancer cells,10,11 extracellular miRNAs,12 or exosomal miRNAs.13,14
However, among the only available studies published up to now and focused on ascitic fluid-derived miRNAs in ovarian cancer, there is no study performing comprehensive large-scale profiling and validation of miRNA expression using entire cell-free fractions of ascitic fluid. Our knowledge of crucial regulatory processes and the roles of miRNAs in pathological fluids associated with ovarian cancer is limited. The aim of the present study was to explore and evaluate the expression of ascites-derived extracellular miRNAs and their possible associations with disease characteristics, cancer progression, and patient outcomes in ovarian cancer.
Material and Methods
Patients
This study was carried out in compliance with the Helsinki Declaration and was approved by a multicentric ethics committee of the General University Hospital in Prague (VFN Praha). All patients provided written informed consents. Patients initially diagnosed with primary ovarian cancer or pelvic mass and treated at the Faculty Hospital in Brno (FN Brno) were enrolled in this study and provided intraoperatively collected samples of ascitic fluid or peritoneal lavages. According to the histopathological examination, the analyzed samples included mostly (∼85%) primary ovarian carcinomas (OCs) and partially the indistinguishable samples of either primary ovarian/fallopian tube/peritoneal origin. Patients with mixed-histology ovarian tumors, Brenner tumor, granulosa tumor, recurrent OC, or who had received neoadjuvant chemotherapy treatment before the sampling were suspended from the study due to their limited numbers for relevant conclusions. In total, 18 ascitic fluid samples and 8 peritoneal lavage samples associated with 16 high-grade serous OCs, 1 low-grade serous OC, 1 high-grade fallopian tube/OC (primary site of origin possible either in fallopian tube or in ovary; the sample was analyzed with OCs according to standard clinical practice), 3 high-grade mucinous OCs, 2 high-grade endometrioid OCs, 1 high-grade serous peritoneal carcinoma, and 2 high-grade serous OC/peritoneal carcinoma were analyzed in this study. As a negative control, postmenopausal healthy women (n = 34) provided control blood samples at the Faculty Transfusion Center (VFN Praha). All patients were Caucasians. Clinicopathological characteristics are summarized in Table 1. Sample combinations used in the analyses (validation experiment) are listed in Supplemental Table S1.
Table 1.
Clinicopathological Characteristics of Patients.
| Parameters (Samples) | Patients (%) |
|---|---|
| Pelvic carcinoma (ovarian carcinoma, fallopian tube carcinoma, peritoneal carcinoma) | 26 (100) |
| Primary ovarian serous carcinoma | 17 (65) |
| High-grade (A2, A3, A6-9, A11-12, A14, L1, L3-4, L6-9) | 16 (62) |
| Low-grade (L5) | 1 (4) |
| Primary fallopian tube/ovarian high-grade serous carcinoma (A13) | 1 (4) |
| Primary ovarian high-grade mucinous carcinoma (A15-17) | 3 (12) |
| Primary ovarian high-grade endometrioid carcinoma (A18-19) | 2 (8) |
| Primary peritoneal high-grade serous carcinoma (A5) | 1 (4) |
| Primary ovarian/peritoneal high-grade serous carcinoma (A1, A4) | 2 (8) |
| Ascites | 18 (69) |
| Malignant (A1-6, A8-9, A11-19) | 17 (65) |
| Nonmalignant (A7) | 1 (4) |
| Lavage | 8 (31) |
| Malignant (L3-6) | 4 (15) |
| Nonmalignant (L1, L7-9) | 4 (15) |
| Median age | Years (range) |
| Ascites (A1-9, A11-19) | 60 (46-84) |
| Lavages (L1, L3-9) | 62 (31-82) |
| Lymph node metastasis | |
| N0 (A5-6, A8, A11, A13, A16, L3-6, L8-9) | 12 (46) |
| N1 (A1-4, A7, A9, A12, A14, A18-19, L1, L7) | 12 (46) |
| Unknown (A15, A17) | 2 (8) |
| Residual tumor | |
| R0 (no residual tumor; A1, A5-7, A9, A12, A15-16, A19, L3-6, L8) | 14 (54) |
| R1 (≤1 cm; L7, L9) | 2 (8) |
| R2 (>1 cm; A2-4, A8, A11, A13, A17) | 7 (27) |
| Unknown (A14, A18, L1) | 3 (12) |
| FIGO stage | |
| FIGO I/II (Ib: A7; Ic: A17, L5; IIb: L7; IIc: A13, A14) | 6 (23) |
| FIGO III/IV (IIIa: A12, A16, L8; IIIb: A8, A11, L4, L9; IIIc: A1-3, A5-6, A9, A15, A18-19, L1, L3, L6) | 19 (73) |
| Unknown (A4) | 1 (4) |
| Healthy postmenopausal women | 34 (100) |
| Control plasma (RP1-34, n = 34) | Years (range) |
| Median age | 56 (47-77) |
Abbreviations: A, ascites; FIGO, International Federation of Gynecology and Obstetrics; L, peritoneal lavages; RP, control plasma.
Clinical Samples
All samples were collected in special stabilization tubes ensuring inactivation of RNases. Ascitic fluid and ascites-derived lavages (effusions collected with saline solution) were collected in urine preservation tubes (Norgen Biotek, Canada, cat no 18122). Blood samples were collected in cell-free RNA BCT tubes (Streck, Omaha, United States, cat no 218975), and isolated plasma was used as the preferred standard control. Effusions (ascites and lavages) were considered “malignant” in cytologically positive samples (malignant cells present in the fluid) and “nonmalignant” in cytologically negative samples (no malignant cells present in the fluid).
Sample Preparation and Isolation of Total RNA
Pure cell-free fractions were obtained via 2 consecutive centrifugations of the fluids, at 1300g for 15 minutes at room temperature and at 2500g for 10 minutes at 4°C. Total RNA (including exosomal RNA) was isolated using a plasma/serum circulating and exosomal RNA purification maxi kit (slurry format; Norgen Biotek, cat no 50900).
Large-Scale Screening of MiRNA Expression
The screening phase of the study involved large-scale real-time polymerase chain reaction (RT-PCR) analyses using 384-well TaqMan array human microRNA A and B cards (Applied Biosystems/Thermo Fisher Scientific, Foster City, United States, cat no 4444913) containing assays for 754 unique human miRNAs. A total of 11 samples (5 ascitic fluid samples, A2, A3, A6, A8, and A11 and 6 plasma samples, RP1-6) were initially analyzed using the type A cards. A total of 8 samples (4 ascitic fluid samples, A6, A7, A9, and A11, and 4 plasma samples, RP4-7) were analyzed using type B cards. We analyzed 1 sample per a card (no sample pooling). All pathological samples analyzed in the screening experiment were associated with primary high-grade serous OC.
Validation of Differential MiRNA Expression
With respect to results of the screening phase, 7 single miRNA assays (miR-200a, miR-200b, miR-200c, miR-141, miR-429, miR-1290, and miR-30a-5p) and 3 endogenous controls (miR-17, miR-93-5p, and miR-425; Applied Biosystems/Thermo Fisher Scientific, Foster City, United States) were used for validating the differential expression in the ascitic fluid and lavage samples relative to the plasma samples. Different combinations of samples were analyzed to increase the reliability of the results and to limit potential bias (Supplemental Table S1).
Reverse Transcription
Total RNA was reverse transcribed into complementary DNA (cDNA) in the screening phase using a TaqMan MicroRNA Reverse Transcription kit and Megaplex RT primers (Human Pool A v2.1 and Human Pool B v3.0; Applied Biosystems/Thermo Fisher Scientific, Foster City, United States). Reverse transcription in the validation phase used the TaqMan MicroRNA Reverse Transcription Kit and miRNA-specific RT primers following modified manufacturer’s instructions, that is, a scaled-down format (1/2 volume).
Real-Time PCR Amplification
The cDNA samples were preamplified in the screening phase using Megaplex PreAmp primers (Human Pool A v2.1 and Human Pool B v3.0; Applied Biosystems/Thermo Fisher Scientific, Foster City, United States). The reactions contained less nuclease-free water than that in the manufacturer’s protocol (2.5 µL instead 7.5 µL), and the difference was replaced with cDNA. The reaction mix for the RT-PCR amplifications consisted of TaqMan universal PCR master mix, no AmpErase UNG (450 μL), nuclease-free water (430 μL instead of 441 μL in the protocol), and preamplified cDNA (not diluted in Tris-EDTA buffer, a volume of 20 μL instead of 9 μL in the protocol). The RT-PCR amplifications in the validation phase were performed in scaled-down reactions (1/2, total volume of 10 µL) in triplicate in a 96-well MicroAmp optical 96-well reaction plates using Xceed qPCR Probe 2× Mix HI-ROX buffer as a master mix (IAB, Czech Republic, cat no HPCR10502L). The RT-PCR reactions were run using an Applied Biosystems 7900HT fast RT-PCR system thermocycler (Applied Biosystems/Thermo Fisher Scientific, Foster City, United States).
Normalization of miRNA Expression Data
Global means were used in the screening phase to normalize the expression data for the TaqMan array miRNA type A cards. Candidate endogenous controls for the validation phase in this experiment were identified using the geNorm module15 of qbase+ (Biogazelle, Belgium). Geometric means of miR-378, miR-30e-3p, miR-432, miR-320b, miR-1244, miR-151-3p, and miR-409-3p identified by geNorm were used to normalize the data for the TaqMan array miRNA type B cards because global means did not yield significant results. Three miRNAs suggested by geNorm (miR-17, miR-93-5p, and miR-425) in the screening experiment were further evaluated in the validation phase using geNorm and BestKeeper16 algorithms, and the geometric means of their expression were used for normalization.
Statistical Analyses
Expression data (log-transformed) were analyzed using qbase+17 and MedCalc statistical software, version 18.2.1, (bvba, Ostend, Belgium). Data processed in qbase+ were corrected for multiple testing using the Benjamini-Hochberg procedure. Mann-Whitney U tests, logistic regression, and receiver operating characteristic (ROC) curve analyses were used to find out fold differences (FD) in expression between pathological and control samples and to evaluate the area under the curve (AUC) values along with percentage of correct classification. Clinicopathological parameters examined were grouped in relation to histological subtype, age, grade, stage, residual tumor, and lymph node metastasis. Kaplan-Meier plots and log-rank tests were used to estimate overall- and progression-free survival in preliminary analyses. Progression-free survival was defined as time from primary surgery/ascitic fluid/lavage sampling until disease progression or death. Overall survival was defined as time from primary surgery/ascitic fluid/lavage sampling until death from any cause or end of follow-up. In the survival analyses, miRNA expression was divided into 2 groups (low expression and high expression) based on median miRNA expression in the representative sample set validation set (VS) VII. P values <.05 were considered statistically significant in all statistical tests.
Results
Screening Phase
Using TaqMan array miRNA type A cards, 2 analytical approaches were applied using global mean normalization, that is, analyses including (group 1) or not including (group 2) data points with Ct >35 (the cutoff value Ct <35), and miRNA expression was compared between the ascitic fluid and plasma samples.
We identified 134 miRNAs in group 1 that were significantly differentially expressed, with 82 miRNAs underexpressed and 52 miRNAs overexpressed. Twenty-three of the overexpressed miRNAs (Table 2) and 51 of the underexpressed miRNAs (Table 3) were consistently expressed in both groups 1 and 2. Among these miRNAs, the 4 miR-200 family members were notably overexpressed, that is, miR-200a (783-fold), miR-200b (584-fold), miR-200c (288-fold), and miR-141 (117-fold). miR-451 was the most significantly underexpressed miRNA (−1082-fold). Twenty-nine of the overexpressed miRNAs and 31 of the underexpressed miRNAs were significantly differentially expressed only in group 1 (Supplemental Table S2). The most conspicuously differentially expressed miRNA specific to group 1 were the overexpressed miR-135b (31 358-fold) and the underexpressed miR-18a (−813-fold). Group 2 had 2 overexpressed miRNAs (miR-708, 19-fold; miR-125b, 15-fold) and 7 underexpressed miRNAs (miR-486-3p was underexpressed the most, −15-fold), specifically in this group (Supplemental Table S3).
Table 2.
Fold Differences Between Ascitic Fluid and Control Plasma in TaqMan Array MiRNA Type A Cards and Consistently Overexpressed MiRNAs.a
| MiRNA | FD (Ct ≤40) | P | FD (Ct <35) | 95% CI Low | 95% CI High | P |
|---|---|---|---|---|---|---|
| miR-200a | 2 036.958 | .011 | 783.107 | 157.691 | 3 888.974 | .031 |
| miR-200b | 1 155.152 | .011 | 584.179 | 204.353 | 1 669.975 | .031 |
| miR-200c | 520.841 | .011 | 288.326 | 56.956 | 1 459.572 | .018 |
| miR-204 | 236.075 | .011 | 130.686 | 21.831 | 782.327 | .018 |
| miR-141 | 210.382 | .011 | 116.463 | 32.515 | 417.153 | .018 |
| miR-203 | 189.848 | .011 | 105.095 | 23.807 | 463.936 | .018 |
| miR-193b | 151.818 | .011 | 84.043 | 33.815 | 208.881 | .018 |
| miR-10b | 119.503 | .011 | 66.154 | 5.323 | 822.118 | .031 |
| miR-886-3p | 80.169 | .011 | 44.380 | 7.864 | 250.449 | .018 |
| miR-31 | 76.314 | .011 | 42.245 | 18.108 | 98.560 | .018 |
| miR-10a | 70.742 | .011 | 39.161 | 8.569 | 178.968 | .018 |
| miR-452 | 69.267 | .011 | 38.345 | 14.573 | 100.891 | .018 |
| miR-99a | 59.031 | .011 | 32.678 | 5.528 | 193.188 | .018 |
| miR-224 | 37.903 | .011 | 20.982 | 5.349 | 82.310 | .018 |
| miR-886-5p | 25.354 | .011 | 14.035 | 4.640 | 42.458 | .018 |
| miR-95 | 24.157 | .011 | 13.373 | 4.785 | 37.372 | .018 |
| miR-100 | 20.470 | .011 | 11.332 | 2.621 | 48.993 | .018 |
| miR-99b | 19.011 | .011 | 10.524 | 5.589 | 19.815 | .018 |
| miR-483-5p | 13.933 | .011 | 7.713 | 2.095 | 28.403 | .018 |
| miR-574-3p | 8.514 | .011 | 4.713 | 3.046 | 7.293 | .018 |
| miR-342-3p | 7.054 | .011 | 3.905 | 2.192 | 6.957 | .018 |
| miR-146b-5p | 6.386 | .011 | 3.535 | 1.993 | 6.269 | .018 |
| miR-155 | 5.554 | .011 | 3.074 | 1.773 | 5.332 | .018 |
Abbreviations: CI, confidence interval; FD, fold difference; miRNA, microRNA.
a Only significant differences (P < .05) are noted and include miRNAs overexpressed using both Ct cutoffs.
Table 3.
FDs Between Ascitic Fluid and Control Plasma in TaqMan Array MiRNA Type A Cards and Consistently Underexpressed MiRNAs.a
| MiRNA | Minus FD (Ct ≤40) | P | Minus FD (Ct <35) | 95% CI Low (minus) | 95% CI High (minus) | P |
|---|---|---|---|---|---|---|
| miR-451 | 598.843 | .011 | 1 081.769 | 3 019.676 | 387.533 | .018 |
| miR-185 | 310.425 | .011 | 260.640 | 575.569 | 118.028 | .031 |
| miR-15b | 138.183 | .011 | 249.618 | 456.172 | 136.591 | .018 |
| miR-223 | 131.143 | .011 | 236.902 | 755.220 | 74.313 | .018 |
| miR-142-3p | 106.116 | .011 | 191.692 | 665.192 | 55.241 | .018 |
| miR-16 | 101.453 | .011 | 183.268 | 400.573 | 83.848 | .018 |
| miR-652 | 86.518 | .011 | 66.579 | 209.182 | 21.191 | .031 |
| miR-126 | 76.626 | .011 | 138.420 | 322.409 | 59.428 | .018 |
| miR-26b | 69.606 | .011 | 40.518 | 75.762 | 21.669 | .031 |
| miR-19a | 69.221 | .011 | 80.826 | 139.057 | 46.980 | .031 |
| miR-20a | 45.640 | .011 | 82.446 | 173.103 | 39.268 | .018 |
| miR-19b | 27.853 | .011 | 50.315 | 87.871 | 28.810 | .018 |
| miR-301a | 43.401 | .011 | 34.675 | 66.850 | 17.986 | .031 |
| miR-20b | 26.450 | .011 | 47.780 | 124.771 | 18.297 | .018 |
| miR-140-5p | 25.665 | .011 | 46.362 | 76.233 | 28.196 | .018 |
| miR-140-3p | 25.047 | .011 | 41.931 | 92.403 | 19.028 | .031 |
| miR-106b | 24.881 | .011 | 44.946 | 81.356 | 24.831 | .018 |
| miR-25 | 23.222 | .011 | 41.948 | 74.975 | 23.470 | .018 |
| miR-374a | 21.698 | .011 | 39.195 | 169.944 | 9.040 | .018 |
| miR-93 | 20.514 | .011 | 37.057 | 65.974 | 20.815 | .018 |
| miR-192 | 19.671 | .011 | 35.534 | 70.411 | 17.932 | .018 |
| miR-29c | 18.436 | .011 | 27.388 | 53.460 | 14.032 | .031 |
| miR-199a-3p | 17.559 | .036 | 17.245 | 72.266 | 4.115 | .031 |
| let-7b | 17.498 | .011 | 31.609 | 105.259 | 9.492 | .018 |
| miR-302b | 17.217 | .011 | 31.101 | 96.755 | 9.997 | .018 |
| miR-17 | 16.745 | .011 | 30.249 | 61.102 | 14.975 | .018 |
| miR-532-3p | 15.367 | .011 | 8.257 | 19.703 | 3.460 | .031 |
| miR-590-5p | 14.579 | .011 | 26.336 | 44.049 | 15.745 | .018 |
| miR-208 | 14.354 | .011 | 25.930 | 62.696 | 10.724 | .018 |
| miR-486-5p | 12.497 | .011 | 22.575 | 39.935 | 12.762 | .018 |
| miR-425 | 12.339 | .011 | 22.289 | 27.030 | 18.379 | .018 |
| miR-145 | 13.099 | .011 | 16.141 | 84.504 | 3.083 | .031 |
| miR-195 | 11.888 | .011 | 21.474 | 42.774 | 10.781 | .018 |
| miR-324-3p | 10.140 | .011 | 18.318 | 45.336 | 7.401 | .018 |
| miR-454 | 9.217 | .011 | 16.650 | 31.566 | 8.782 | .018 |
| miR-30c | 9.048 | .011 | 16.345 | 45.589 | 5.860 | .018 |
| miR-191 | 7.550 | .011 | 13.639 | 22.260 | 8.356 | .018 |
| miR-618 | 6.556 | .036 | 11.843 | 52.234 | 2.685 | .031 |
| let-7a | 6.384 | .011 | 11.532 | 22.961 | 5.792 | .018 |
| let-7d | 6.345 | .011 | 11.462 | 18.574 | 7.074 | .018 |
| miR-30b | 6.009 | .036 | 10.855 | 30.621 | 3.848 | .018 |
| miR-26a | 4.688 | .011 | 8.468 | 11.945 | 6.003 | .018 |
| miR-221 | 4.473 | .011 | 8.081 | 20.841 | 3.133 | .018 |
| miR-194 | 4.473 | .019 | 8.081 | 24.977 | 2.614 | .018 |
| miR-181a | 4.344 | .011 | 7.847 | 10.328 | 5.962 | .018 |
| miR-92a | 3.762 | .036 | 6.796 | 14.268 | 3.237 | .018 |
| miR-186 | 3.518 | .011 | 6.354 | 9.149 | 4.413 | .018 |
| miR-532-5p | 3.303 | .011 | 5.966 | 8.479 | 4.198 | .018 |
| miR-501-5p | 2.886 | .011 | 5.213 | 7.073 | 3.842 | .018 |
| let-7g | 2.643 | .036 | 4.774 | 10.731 | 2.123 | .018 |
| miR-28-5p | 2.328 | .011 | 4.205 | 7.908 | 2.236 | .018 |
Abbreviations: CI, confidence interval; FD, fold difference; miRNA, microRNA.
a Only significant differences (P < .05) are noted and include miRNAs underexpressed using both Ct cutoffs.
Using TaqMan array microRNA type B cards, the analysis of miRNA expression using the cutoff Ct <35 (only this procedure yielded significant results) identified 10 significantly overexpressed miRNAs, with miR-1290 overexpressed the most (89-fold). Nine significantly underexpressed miRNAs were also found, with miR-766 underexpressed the most (−139-fold; for details, see Supplemental Table S4).
Validation Phase
The expression of 7 miRNAs (miR-200a, miR-200b, miR-200c, miR-141, miR-429, miR-1290, and miR-30a-5p) identified in the screening phase as potential biomarkers was evaluated in the validation phase analyzing different sample combinations (VS I-VIII; see Supplemental Table S1). In these combinations, ascitic fluid and lavages were analyzed either separately or altogether along with various combinations of input samples with respect to histological subtype (high-grade serous carcinomas, endometrioid, mucinous carcinomas), malignancy of ascites (nonmalignant samples in some analyses were excluded), and primarily in comparison with plasma control samples. Alternatively, malignant samples were compared with nonmalignant samples.
Remarkably, the similar expression pattern, that is, all miRNAs of the miR-200 family and miR-1290 with strongly increased expression, and miR-30a-5p with weakly increased expression, was observed across all sample combinations, independently on the fluid type (ascitic fluid vs lavage) or histological subtype (serous, mucinous, and endometrioid carcinomas). The results obtained in the validation phase were consistent with the screening phase except for miR-30a-5p. This miRNA was underexpressed in cancer samples in comparison with control samples in the screening phase but was only weakly overexpressed in the validation phase.
Validation Set I
In this sample combination, primary high-grade serous OCs (malignant ascites) were compared with postmenopausal control plasma samples.
The expression of miR-200 family ranged from 1698-fold (miR-200c) to 3657-fold (miR-200a) when comparing malignant ascitic fluid-derived miRNAs of high-grade serous OCs to control plasma-derived miRNAs (Supplemental Table S1). In this comparison, increased expression of miR-1290 was also notable (1535-fold). On the contrary, the lowest fold-difference was observed for miR-30a-5p (4.7-fold; for details, see Table 4).
Table 4.
Fold-Differences Between Ascitic Fluid/Lavages Versus Control Plasma in Validation of MiRNAs Expression.a,b
| FD | 95% CI Low | 95% CI High | P | |
|---|---|---|---|---|
| miR-200c | ||||
| VS I | 1697.61 | 1045.67 | 2756.03 | .00000000 |
| VS II | 469.82 | 302.57 | 729.54 | .00000002 |
| VS III | 2.34 | 0.42 | 13.02 | .60000000 |
| VS IV | 691.93 | 383.44 | 1248.64 | .00370370 |
| VS V | 1525.91 | 964.08 | 2415.16 | .00030030 |
| VS VI | 1398.10 | 883.85 | 2211.56 | .00000000 |
| VS VII | 930.21 | 610.78 | 1416.72 | .00000000 |
| VS VIII | 1480.83 | 761.33 | 2880.31 | .00036036 |
| miR-429 | ||||
| VS I | 2417.14 | 1250.19 | 4673.36 | .00000000 |
| VS II | 474.00 | 241.40 | 930.73 | .00000002 |
| VS III | 0.71 | 0.09 | 5.44 | .88571429 |
| VS IV | 770.22 | 243.74 | 2433.91 | .00370370 |
| VS V | 2584.94 | 1 004.34 | 6653.04 | .00030030 |
| VS VI | 1340.75 | 679.98 | 2643.59 | .00000000 |
| VS VII | 1188.40 | 694.39 | 2033.86 | .00000000 |
| VS VIII | 1008.73 | 390.88 | 2603.20 | .00036036 |
| miR-200b | ||||
| VS I | 1815.73 | 976.38 | 3376.65 | .00000000 |
| VS II | 288.84 | 114.60 | 728.00 | .00000014 |
| VS III | 7.78 | 0.22 | 281.35 | .60000000 |
| VS IV | 603.16 | 192.13 | 1893.52 | .00370370 |
| VS V | 2121.98 | 839.22 | 5365.48 | .00030030 |
| VS VI | 1411.73 | 799.93 | 2491.45 | .00000000 |
| VS VII | 814.76 | 422.52 | 1571.15 | .00000000 |
| VS VIII | 781.35 | 304.92 | 2002.23 | .00036036 |
| miR-141 | ||||
| VS I | 1734.82 | 824.83 | 3648.75 | .00000000 |
| VS II | 464.51 | 233.08 | 925.70 | .00000002 |
| VS III | 2.39 | 0.54 | 10.62 | .60000000 |
| VS IV | 270.13 | 75.63 | 964.83 | .00370370 |
| VS V | 648.83 | 232.76 | 1808.62 | .00030030 |
| VS VI | 1337.81 | 674.68 | 2652.71 | .00000000 |
| VS VII | 777.70 | 447.44 | 1351.74 | .00000000 |
| VS VIII | 733.95 | 255.50 | 2108.41 | .00036036 |
| miR-1290 | ||||
| VS I | 1534.58 | 432.08 | 5450.26 | .00000000 |
| VS II | 2917.32 | 945.20 | 9004.18 | .00000002 |
| VS III | 0.23 | 0.01 | 8.91 | .68000000 |
| VS IV | 635.74 | 95.61 | 4227.15 | .00370370 |
| VS V | 3857.60 | 895.22 | 16622.77 | .00030030 |
| VS VI | 1928.90 | 624.29 | 5959.87 | .00000000 |
| VS VII | 2194.90 | 869.27 | 5542.06 | .00000000 |
| VS VIII | 57.73 | 10.45 | 319.02 | .03063063 |
| miR-200a | ||||
| VS I | 3656.88 | 1732.58 | 7718.42 | .00000000 |
| VS II | 1905.00 | 899.37 | 4035.08 | .00000002 |
| VS III | 0.92 | 0.24 | 3.47 | .88571429 |
| VS IV | 1752.96 | 414.66 | 7410.53 | .00370370 |
| VS V | 3801.85 | 1175.54 | 12295.72 | .00030030 |
| VS VI | 2787.28 | 1419.00 | 5474.94 | .00000000 |
| VS VII | 2630.97 | 1597.79 | 4332.23 | .00000000 |
| VS VIII | 837.08 | 254.92 | 2748.77 | .00036036 |
| miR-30a-5p | ||||
| VS I | 4.72 | 2.77 | 8.06 | .00033592 |
| VS II | 2.26 | 1.28 | 3.96 | .00186986 |
| VS III | 0.23 | 0.03 | 1.91 | .60000000 |
| VS IV | 1.71 | 0.87 | 3.38 | .01904762 |
| VS V | 2.30 | 1.30 | 4.07 | .00283140 |
| VS VI | 3.19 | 1.79 | 5.71 | .00090527 |
| VS VII | 3.25 | 2.03 | 5.18 | .00000098 |
| VS VIII | 1.26 | 0.72 | 2.19 | .14800515 |
Abbreviations: CI, confidence interval; FD, fold difference; miRNA, microRNA; VS, validation set.
aSample combinations are listed in Supplemental Table S1.
b P values <.05 were considered statistically significant. Nonsignificant results are indicated in italics.
Validation Set II
In this sample combination, primary serous OCs (∼88% high-grade peritoneal lavages) were compared with postmenopausal control plasma samples.
Independent evaluation of ascitic fluid–derived miRNAs collected as peritoneal lavages (mostly high-grade, serous OCs) and compared with plasma controls (Supplemental table S1) revealed similar results and miR-200a exhibited the most increased expression (1905-fold). Interestingly, the fold-differences of other miR-200 family members were mostly lower, while fold-difference for miR-1290 was almost 2-fold higher in comparison with VS II to the VS I results (for details, see Table 4). The congruent results obtained for ascites and peritoneal lavages suggest that both types of samples are usable for this kind of research.
Validation Set III
In this sample combination, primary serous OCs (∼88% high-grade malignant peritoneal lavages) were compared with nonmalignant peritoneal lavages.
In an effort to further evaluate the potential differences between malignant and nonmalignant effusions (the latter used in some other investigations as controls), we compared the expression of 4 malignant lavages with 4 nonmalignant lavages (mostly high-grade serous OCs; Supplemental Table S1). There was limited number of nonmalignant effusions for definite conclusions. However, no significant differences were found for the validated miRNAs. Nonsignificantly decreased expression was found for the 4 investigated miRNAs (miR-429, miR-1290, miR-200a, and miR-30a5p), and nonsignificantly increased expression was noted for miR-200c, miR-200b, and miR-141, in comparison with malignant versus nonmalignant samples (for details, see Table 4). It could be assumed that the absence of malignant cells in the effusion itself is a poor indicator of nonmalignant status of the fluid particularly in otherwise malignant disease. This also limits the usability of such samples as controls.
Validation Sets IV and V
In these sample combinations, primary endometrioid OCs (ascitic fluid, VS IV) and primary mucinous OCs (ascitic fluid, VS V) were compared with postmenopausal control plasma samples.
Evaluation of miRNA expression showed that both endometrioid (VS IV) and mucinous (VS V) subtypes (Supplemental Table S1) have similar expression pattern, as it was observed in VS I in high-grade serous samples. Of note, the expression was higher in mucinous samples as compared to endometrioid samples (for details, see Table 4).
Validation Sets VI and VII
In these sample combinations, primary high-grade serous OCs (malignant ascites and malignant lavages, VS VI) and primary OCs of serous, endometrioid, and mucinous subtypes (VS VII), ascites and lavages (both malignant and nonmalignant), were compared with postmenopausal control plasma samples.
In both VS VI and VS VII (Supplemental Table S1), miR-200 family members and miR-1290 were highly expressed, and miR-30a-5p had significantly but weakly (∼3-fold) elevated expression in both sample combinations (for details, see Table 4).
Validation Set VIII
In this sample combination, primary OC/peritoneal carcinoma (ascitic fluid) were compared with postmenopausal control plasma samples.
In some cases, the primary origin of advanced serous pelvic carcinoma may be either in the ovary or in the peritoneum, and it is impossible to identify the exact primary site. Two such samples (A1 and A4—high-grade serous carcinomas) were combined with A5 (primary serous peritoneal carcinoma) in the VS VIII, and their expression was compared with plasma controls. Highest overexpression of all miR-200 family members (range 734-fold in miR-141 to 1481-fold in miR-200c) in pathological samples was accompanied with remarkably less elevated expression of miR-1290 (58-fold), and almost unchanged expression of miR-30a-5p (FD = 1.3; for details, see Table 4).
Logistic Regression and ROC Curve Analysis (Ovarian Cancer vs Control Plasma)
The ability of particular miRNAs to discriminate samples of all subtypes of OCs (VS VII, malignant and nonmalignant samples of ascitic fluid and lavages, n = 23) from control plasma samples (RP1-34, n = 34), was evaluated using logistic regression and ROC curve analyses. Expression of individual miR-141, miR-200a, miR-200c, miR-429, and miR-1290 was able to correctly classify 100% of the samples, with an AUC of 1.000. miR-200b (96.49% correct classification, AUC: 0.996) also performed well. The AUC for miR-30a-5p was 0.885, with 87.72% correct classification.
Associations of MiRNA Expression With Clinicopathological Data
In representative sample set VS VII, miRNAs expression did not vary significantly with histological subtype, age, grade, stage, residual tumor, or lymph node metastasis.
Impact of MiRNA Expression Level on Survival
In the representative sample set VS VII (n = 22 with available survival data), the follow-up time (and overall survival in weeks) for the patients ranged between 1 and 226 weeks (mean 103 weeks, 95% confidence interval [CI]: 79-126; median 106 weeks, 95% CI: 70-132). Progression-free survival ranged between 1 and 178 weeks (mean 80 weeks, 95% CI: 60-99; median 70 weeks, 95% CI: 61-104). Of the 22 patients, 13 (59%) have died within the follow-up. Of the remaining 9 patients alive, 3 (33.3%) have been reported with the recurrence of the disease.
Kaplan-Meier survival estimates and log-rank models for both progression-free survival and overall survival, respectively, were performed to assess the predictive value of miRNA expression and clinical outcome in low and high expression groups for each miRNA. These groups were defined by median expression using log-transformed expression value (qbase+, calibrated normalized relative quantities [CNRQ] values) for the samples in VS VII group.
No significant association of miRNA expression was found for progression-free survival. On the contrary, miR-200b expression was significantly associated with overall survival (log-rank test, P = .019). Low expression level of miR-200b (<1.428 log-transformed CNRQ value) was associated with improved survival (hazard ratio [HR]: 0.25, 95% CI: 0.083-0.737, mean survival ± standard error [SE]: 44.2 ± 6.1 months), while high expression level was associated with worsened survival (HR: 4.043, 95% CI: 1.356-12.051, mean survival ± SE: 24.2 ± 3.6 months; Figure 1).
Figure 1.
Overall survival in relation to miR-200b expression levels. Univariate Kaplan-Meier survival curves related to low and high concentrations of ascites-derived miR-200b in ovarian cancer VS VII group (n = 22), including ascites and lavages of primary ovarian carcinomas (serous, endometrioid, and mucinous subtypes). For details on used samples, see Supplemental Table S1. VS indicates validation set.
Discussion
Extracellular MiRNAs as Novel Biomarkers
MiRNAs are small (∼22 nucleotides in length), endogenously expressed molecules of single-stranded noncoding RNA undergoing rapid turnover beginning with the transcription of their encoding genes in the nucleus, processing of the transcripts, their transport and localization, and functioning as the key posttranscriptional regulators of gene expression until their final degradation.7,18 However, in addition to cellular miRNAs, there is another remarkable fraction of miRNAs, occurring outside the cells. Several years ago, extracellular miRNAs have been detected in an extensive set of 12 body fluids (blood plasma, urine, breast milk, colostrum, saliva, seminal fluid, tears, amniotic fluid, cerebrospinal fluid, pleural fluid, and peritoneal fluid).19 Since then, extracellular miRNAs (generally known in a simplified way as circulating, c-miRNAs, or under somewhat confusing term cell-free miRNAs) have emerged as novel potential biomarkers for many diseases, including cancer.20,21 Extracellular miRNAs may exist attached to various forms of their carriers: encapsulated in extracellular vesicles including shedding vesicles and exosomes, associated with high-density lipoprotein particles or as a fraction bound in complex with AGO proteins.22,23 Different pathways of biogenesis, mechanisms of sorting and transport, and biological significance of extracellular miRNAs still remain to be determined in detail.18
Extracellular Ascites-Derived miRNAs in Ovarian Cancer
Similarly to other research, the vast majority of miRNA investigations of ovarian cancer have been focused on the tumor tissues or cell lines. However, even tumor tissue may be a very heterogeneous entity of various, not only, cancerous cells including tumor-infiltrating lymphocytes among the other.24,25 Representativeness of cell lines may also be questionable. But the most challenging issue is the limited capability of tumor tissues to be used for the early diagnosis of ovarian cancer. Therefore, the samples of liquid biopsies, such as blood plasma/serum or urine, have become the target of investigations in search for novel diagnostic, predictive, and prognostic biomarkers, including their miRNAs.26
Unfortunately, the research focused on ascites has been standing aside from the vast majority of body fluid-based investigations focused on ovarian cancer. It should be noted that ascites may have the advantage over other biofluids due its closer relationship with the tumor progression and metastatic spread within peritoneum and omentum, commonly found in affected patients. It is important because ovarian cancer metastasizes primarily via direct extension or detachment from the primary tumors and by passive carriage of tumor cells by the ascites fluid as cancer cell spheroids and dissemination within the peritoneal cavity.27
Our study is the first comprehensive and large-scale evaluation of extracellular ascites-derived miRNAs in ovarian cancer. There are several other recent studies reporting analyzes of ascitic fluid-derived miRNAs in ovarian cancer and differing in the analyzed material. Vaksman et al10 have explored the dynamics of miRNA regulation in ovarian cancer cells in tumors and effusions and found distinct expression pattern between these anatomical sites. Some miRNAs were highly expressed only in primary carcinomas (n = 6 miRNAs) or only in effusions (n = 12), while the third group of miRNAs consisted of miRNAs highly expressed in both groups (n = 16). However, many miRNAs showed inconclusive results differing between experiments. For example, miR-200c was overexpressed in effusions in sample set 2, but not in sample set 1.10 Congruently with10 and using the same TaqMan miRNA array type A, we found several miRNA overexpressed in the screening experiment (see Table 2): miR-99a among 6 miRNAs expressed only in primary OC cells, miR-200b and miR-200c among 16 miRNAs overexpressed both in effusions and in OC, and finally miR-224, miR-31, miR-342, and miR-99b among 12 miRNAs overexpressed only in effusions.10 All these miRNAs were found overexpressed in the screening experiment of the present study in comparison of ascitic fluid and control plasma and may be good candidates for further evaluation as ovarian oncomiRs. On the other hand, different results were obtained for miRNAs overexpressed in OC (miR-145 and miR-126) or overexpressed both in OC and effusions (let-7-a, let-b, miR-16, miR-17, miR-191, and miR-26a)10—we found all these miRNAs underexpressed in the screening phase (Table 3).
More recently, Vaksman et al14 have focused on exosomal fraction of OC effusions, pooled this fraction (n = 9), and compared the miRNA expression with pooled reactive mesothelial cells (RMC, n = 8) and pooled effusion-derived tumor cells (n = 13). They found high levels of miR-21, miR-23b, and miR-29a associated with poor progression-free survival, and high expression of miR-21 correlated with poor overall survival. The results also indicated that about 75% of the miRNAs in the effusion supernatant may have the origin in OC cells. Despite the loss of biological variation by pooling the samples and lacking statistical evaluation, the authors used an elimination method and identified “highly expressed” miRNAs (Ct <30) unique for each corresponding sample type and some overlapping miRNAs, 19 of them were specific for OC effusions. Among these miRNAs, miR-452 and miR-95 were overexpressed in the screening phase of our study (see Table 2) using the same TaqMan array miRNA platform, suggesting their function as ovarian oncomiRs. Within the group of 19 miRNAs specific to effusions, miR-21 has not been found specific for effusions though its high expression was associated with poor overall survival.14 Expression of this miRNA has also been elevated in OC cells and effusions previously.10
As known oncomiR, miR-21 was the subject of investigation in the study of Cappellesso et al.13 The authors demonstrated that miR-21 expression was elevated in OC cells and exosomes from peritoneal effusions associated with serous OCs as compared with nonneoplastic controls and found an associated inverse expression of its potential target tumor suppressor programmed cell death 4 (PDCD4).
Most recently, Nymoen et al11 evaluated expression and possible clinical roles of 9 miRNAs (miR-29a, miR-31, miR-99b, miR-182, miR-210, miR-221, miR-222, miR-224, and miR-342) previously shown to be overexpressed in cell pellets of ovarian cancer effusions.10 In the study based again on cell pellets of the effusions, Nymoen et al11 identified miR-29a as a candidate biomarker significantly related to longer overall survival in patients with metastatic high-grade serous carcinoma.
As regards the large-scale miRNA profiling of the entire extracellular fraction of the ascitic fluid, there has been only 1 study available so far.12 In this study, 5 miRNAs (miR-132, miR-26a, let-7b, miR-145, and miR-143) were consistently underexpressed in 3 different sample types (tumor tissues, serum, and ascites). Unfortunately, large-scale miRNA expression profiling was conducted on samples of only 2 patients with ovarian cancer and 1 control patient, and the results were validated assessing only serum samples,12 bringing down the informative value of the study with respect to ascitic fluid-derived miRNAs. Despite this, the abovementioned miRNAs (except for miR-132) were underexpressed in the screening phase of our study and their tumor suppressor roles in ovarian cancer may be tentatively assumed.
To sum up, the research on ascites-derived miRNAs is still in its infancy. However, the achieved results clearly indicate that all cellular, extracellular, and exosomal miRNA fractions of ascitic fluid may be closely related to ovarian carcinogenesis with potential impact on patient’s outcomes.
Expression of the miR-200 Family in Ovarian Cancer
All members of the miR-200 family (miR-200a, miR-200b, miR-200c, miR-141, and miR-429) were overexpressed in ascitic fluid relative to control plasma in the screening phase, and these findings were confirmed in the validation phase. Higher expression of miR-200b was related to poor overall survival (24 months) in contrast to low level of miR-200b expression associated with improved overall survival (44 months).
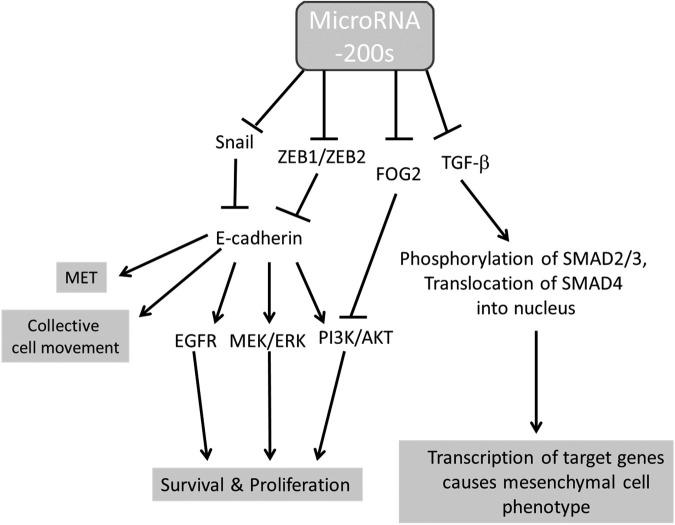
The role of the miR-200 family in ovarian cancer is not clear, suggesting that the spatial and temporal diversity of expression during carcinogenesis is associated with an epithelial to mesenchymal transition (EMT) and vice versa (mesen-chymal to epithelial transition, MET) linked to metastasis. Most previous profiling studies have demonstrated the increased expression of miR-200 family members in ovarian cancer tissues or cancer cell lines, implicating an oncogenic character of this miRNA family. Associations between the expression of the family members and an increased expression of epithelial markers, induction of MET, and limited migration and cell invasiveness, however, suggest biphasic expression patterns during ovarian carcinogenesis. Besides targeting ZEB1/ZEB2 resulting to upregulation of E-cadherin expression, miR-200s also target snail to increase E-cadherin expression in ovarian cancer.28,29 Choi and Ng30 reviewed the functions of miR-200s in ovarian cancer. They suggest that the common scenario noted in cancer research is that this miRNA family is active in suppressing EMT, leading to E-cadherin overexpression, epithelial cell identity, and cancer metastasis inhibition, appears to be different in ovarian cancer, as ovarian cancer cells are more epithelial in nature compared to their normal counterparts. Generally, miR-200s are highly conserved among vertebrate species and may possess important functions in a diversity of developmental processes, such as proliferation of neurons, podocyte differentiation, taste bud formation, insulin signaling pathway regulation in the control of fat body and body size, and for the hormonal regulation of endometrial stromal decidualization during embryo implantation. In ovarian cancer, however, clinical studies are not conclusive to relate the expression level of miR-200 family with disease stage.30
The spatial and temporal variation in expression of the miR-200 family members in various body fluids is not yet fully known. Kan et al31 found that serum miR-200a, miR-200b, and miR-200c were overexpressed in serous ovarian cancer samples relative to controls. Similarly, Gao and Wu32 reported that the levels of serum miR-200c and miR-141 were significantly higher in patients with ovarian cancer than healthy controls but also found that expression from early to advanced stages tended to decrease for miR-200c but increase for miR-141. Higher levels of miR-200c were associated with improved survival, and patients with low levels of miR-141 had significantly higher survival rates. Meng et al33 though reported that serum miR-429 continuously increased from healthy controls to International Federation of Gynecology and Obstetrics (FIGO) I-II patients to FIGO III-IV patients and that the levels of miR-429 were significantly correlated with lower overall survival (HR: 1.78).
In the present study, expression varied among the ascites-derived miR-200 family members, along with their possible effects associated with clinical outcomes (as found in miR-200b). This finding may also indicate their differential activity in ovarian carcinogenesis. The magnitude of the increased expression of the miR-200 family in the pathological samples of ascitic fluid versus the control plasma samples relative to other miRNAs nevertheless indicated the strong oncogenic character of the entire miR-200 family in ovarian cancer. Potential roles of miR-200s are depicted in Figure 2.
Figure 2.
Potential roles of miR-200 family in ovarian cancer. Adopted from Choi and Ng.30 MET indicates mesenchymal–epithelial transition.
Overexpressed Expression of miR-1290 in Ovarian Cancer
Ovarian cancer ascites-derived miR-1290 was conspicuously overexpressed relative to the control plasma. In contrast, Shapira et al34 did not find altered expression in plasma-derived miR-1290 between ovarian cancer and control samples. Interestingly, they found an association between increased expression of miR-1290 in plasma and higher overall survival. MiR-1290 has been shown to act as an oncomiR in another gynecological cancer, that is, endometrial cancer.35 Wu et al36 have identified miR-1290 upregulated in colon cancer tissues and found that upregulation of miR-1290 postponed cytokinesis and led to the formation of multinucleated cells. KIF13B, a target of miR-1290, was involved in aberrant cytokinesis.36 Similarly, it has been reported that miR-1290 may function as a tumor oncogene in the progression of esophageal squamous cell carcinoma by targeting nuclear factor I/X.37 Interestingly, inhibition of miR-1290 resulted in decreased stemness markers and EMT markers in non-small cell lung cancer (NSCLC), while anti-miR-1290 suppressed proliferation, sphere formation, colony formation, and invasion of NSCLC.38
Our findings also suggest an oncogenic character for miR-1290, mostly in advanced stages of ovarian cancer, which may provide further evidence for discrepancies between the expression levels of miRNAs in pathological plasma and ascitic fluid samples, indicating the complex nature of these body fluids. Ascites-derived miRNAs may also potentially be more advantageous than plasma/serum or tissue-derived miRNAs as novel biomarkers, because ascites may more closely represent the relationship with cancer progression in the abdominal cavity, overcoming the dilution of cancer-related miRNAs in blood and the vast heterogeneity of tumor tissue samples. Currently, there is limited knowledge on the particular functional roles of miR-1290 in ovarian cancer potentially resembling the roles observed in other cancers (see Figure 3). However, a specific nature of ovarian cancer deserves further research on this issue.
Figure 3.
Presumable roles of miR-1290 in ovarian cancer. This schematic diagram of assumed functions of miR-1290 is based on data reported for non-small cell lung cancer,38 esophageal squamous cell carcinoma,37 and colon cancer.36 However, these functions need to be evaluated for ovarian cancer.
Expression of miR-30a-5p in Ovarian Cancer
The function of miR-30a-5p is not fully understood, with both tumor suppressor and oncogenic roles in ovarian cancer.
Marchini et al39 found a slightly lower expression of miR-30a-5p (0.39-fold) in relapsers than nonrelapsers in ovarian cancer tissues, implicating possible tumor suppressor roles for this miRNA. Experimentally induced ectopic expression of miR-30a-5p may decrease cell proliferation and invasion and increase sensitivity to chemotherapeutic agents.40 In contrast, Chen et al41 reported opposite findings, where the increased expression of miR-30a-5p was associated with decreased paclitaxel sensitivity, supporting the view that miR-30a-5p acts as an oncogene. A low upregulation of miR-30a-5p (4.6-fold) in urine has recently been associated with serous ovarian cancer relative to controls. Surprisingly, expression was also higher in stage I-II than stage III-IV samples.42
As regards to other diagnoses and possible roles of miR-30a-5p, this miRNA was demonstrated to act as tumor suppressor in many studies. For example, miR-30a-5p inhibited proliferation, metastasis, and EMT and upregulated the expression of tight junction protein claudin-5 in upper tract urothelial carcinoma cells.43 Tumor suppressive roles of miR-30a-5p have also been shown in renal cell carcinoma.44 Similarly, miR-30a-5p inhibited the proliferation, invasion, and tumor growth of hepatocellular cancer cells by inhibition of forkhead box A1 (FOXA1).45 Moreover, miR-30-5p could inhibit muscle cell differentiation and regulate the alternative splicing of Trim55 and insulin receptor (INSR) by targeting Muscleblind-Like (MBNL).46
Expression of miR-30a-5p in our study was weakly (up to 4.7-fold in VS I) but significantly elevated in ascitic fluid versus controls in the validation phase. An oncogenic character of miR-30a-5p in OCs may be suggested but possibly to much lesser degree than in other validated miRNAs and should be further explored with respect to opposite functions found in other cancers.
Ascites-Derived Extracellular MiRNAs in Future Clinical Practice
As in other miRNA research, the problem of compatibility of results and their general acceptance is a function of their extensive and independent international validation. The methods of sampling (peritoneal/pleural effusions, or lavages), extracellular fraction separation, RNA isolation, and quantification analyses may differ among the investigations, similarly as normalization, use of control samples (RMCs vs plasma), or with respect to ethnogeographical and biological variation and often limited sets of samples. All these factors may potentially alter the results. Moreover, there is no generally accepted standard for the abovementioned issues and methods at this moment while each method may have its own advantages and drawbacks. Therefore, further validation of results will be necessary also with respect to serous peritoneal carcinomas and other less frequent subtypes of OCs.
Complexity of body fluids and their cellular and extracellular components raises other important questions. For example, what parts of the liquid biopsy are the true representatives of the disease? It has become evident that every fraction of particular body fluid may have its own but often overlapping miRNA expression pattern, informative value, and clinical potential. Focus on exosomes may identify differences attributed to secretory activity of the cells (note, however, that exosomal miRNAs account only for a few percent of the c-miRNA pool),18,22 while cellular miRNAs representing different cancer cells may show the oncogene- or tumor suppressor roles of miRNAs, but this is affected largely by the most abundant/active cells. Without cell sorting, the differences cannot be attributed to 1 type of the cells. On the other hand, analysis of the broad spectrum of extracellular miRNAs (in cell-free fraction including exosomes) may have the advantage to provide a global picture of the changes in miRNA expression in particular body fluid.
It should be emphasized that the levels of some ascites-derived miRNAs may be extensively altered relative to the control plasma samples. We identified 153 differentially expressed miRNAs, including many overexpressed (potential oncomiRs) and underexpressed miRNAs (potential tumor suppressors). High expressions suggesting oncogenic functions were most notable for all miR-200 family members and miR-1290. The function of the limited overexpression of miR-30a-5p remains elusive. Association between ascites-derived miRNA expression and clinical outcomes was found only in miR-200b, but it may suggest a promising prognostic potential within the group of extracellular miRNAs found in ascites. It might be expected that novel important parts of miRNA regulatory networks associated with ovarian carcinogenesis may be discovered in ascites. Meanwhile, novel diagnostic, predictive, and prognostic ascites-derived miRNA biomarkers should be evaluated in future studies for ovarian cancer. Such biomarkers may not only come from the entire extracellular fraction in ascitic fluids, but these may be accompanied with the novel miRNA biomarkers found in exosomes or cells in the pathological effusions associated with ovarian cancer. With this knowledge, novel targeted therapies and other clinical applications may also be developed for battling ovarian cancer.
Supplemental Material
Supplemental Material, Supplementary_Table_S1_RS_FINAL for Ascites-Derived Extracellular microRNAs as Potential Biomarkers for Ovarian Cancer by Luděk Záveský, Eva Jandáková, Vít Weinberger, Luboš Minář, Veronika Hanzíková, Daniela Dušková, Lenka Záveská Drábková, Iveta Svobodová, and Aleš Hořínek in Reproductive Sciences
Supplemental Material
Supplemental Material, Supplementary_Table_S2_RS_FINALafter_review for Ascites-Derived Extracellular microRNAs as Potential Biomarkers for Ovarian Cancer by Luděk Záveský, Eva Jandáková, Vít Weinberger, Luboš Minář, Veronika Hanzíková, Daniela Dušková, Lenka Záveská Drábková, Iveta Svobodová, and Aleš Hořínek in Reproductive Sciences
Supplemental Material
Supplemental Material, Supplementary_Table_S3_RS_FINALafter_review for Ascites-Derived Extracellular microRNAs as Potential Biomarkers for Ovarian Cancer by Luděk Záveský, Eva Jandáková, Vít Weinberger, Luboš Minář, Veronika Hanzíková, Daniela Dušková, Lenka Záveská Drábková, Iveta Svobodová, and Aleš Hořínek in Reproductive Sciences
Supplemental Material
Supplemental Material, Supplementary_Table_S4_RS_FINALafter_review for Ascites-Derived Extracellular microRNAs as Potential Biomarkers for Ovarian Cancer by Luděk Záveský, Eva Jandáková, Vít Weinberger, Luboš Minář, Veronika Hanzíková, Daniela Dušková, Lenka Záveská Drábková, Iveta Svobodová, and Aleš Hořínek in Reproductive Sciences
Footnotes
Authors’ Note: All patients provided an informed consent. The study was approved by a multicentric ethics committee of the General University Hospital in Prague.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Luděk Záveský received institutional funding from the Charles University in Prague (Progres Q28/LF1) and research support from the Avast Foundation (0F2016\2016020048), CEZ Foundation (STE EPP 126_16), and ČEPS a.s. (1410001544). Aleš Hořínek has received research support from the Ministry of Health of the Czech Republic (grant project RVO-VFN 64165). Vít Weinberger and Luboš Minář received research support from the Ministry of Health of the Czech Republic (CZ-DRO FNBr 65269705).
ORCID iD: Luděk Záveský, PhD  https://orcid.org/0000-0001-9592-7535
https://orcid.org/0000-0001-9592-7535
Supplemental Material: Supplementary material for this article is available online.
References
- 1. Matz M, Coleman MP, Carreira H, Salmeron D, Chirlaque MD, Allemani C. Worldwide comparison of ovarian cancer survival: histological group and stage at diagnosis (CONCORD-2). Gynecol Oncol. 2017;144(2):396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wei W, Li N, Sun Y, Li B, Xu L, Wu L. Clinical outcome and prognostic factors of patients with early stage epithelial ovarian cancer. Oncotarget. 2017;8(14):23862–23870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Puls LE, Duniho T, Hunter JE, Kryscio R, Blackhurst D, Gallion H. The prognostic implication of ascites in advanced-stage ovarian cancer. Gynecol Oncol. 1996;61(1):109–112. [DOI] [PubMed] [Google Scholar]
- 4. Ahmed N, Stenvers KL. Getting to know ovarian cancer ascites: opportunities for targeted therapy-based translational research. Front Oncol. 2013;3:256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shender VO, Pavlyukov MS, Ziganshin RH, et al. Proteome-metabolome profiling of ovarian cancer ascites reveals novel components involved in intercellular communication. Mol Cell Proteomics. 2014;13(12):3558–3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van Jaarsveld MT, Helleman J, Berns EM, Wiemer EA. MicroRNAs in ovarian cancer biology and therapy resistance. Int J Biochem Cell Biol. 2010;42(8):1282–1290. [DOI] [PubMed] [Google Scholar]
- 7. Iorio MV, Croce CM. MicroRNA dysregulation in cancer: diagnostics, monitoring and therapeutics. A comprehensive review. EMBO Mol Med. 2012;4(3):143–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zuberi M, Khan I, Gandhi G, Ray PC, Saxena A. The conglomeration of diagnostic, prognostic and therapeutic potential of serum miR-199a and its association with clinicopathological features in epithelial ovarian cancer. Tumor Biol. 2016;37(8):11259–11266. [DOI] [PubMed] [Google Scholar]
- 9. Rupaimoole R, Slack FJ. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discovery. 2017;16(3):203–221. [DOI] [PubMed] [Google Scholar]
- 10. Vaksman O, Stavnes HT, Kaern J, Trope CG, Davidson B, Reich R. . miRNA profiling along tumour progression in ovarian carcinoma. J Cell Mol Med. 2011;15(7):1593–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nymoen DA, Slipicevic A, Holth A, et al. MiR-29a is a candidate biomarker of better survival in metastatic high-grade serous carcinoma. Hum Pathol. 2016;54:74–81. [DOI] [PubMed] [Google Scholar]
- 12. Chung YW, Bae HS, Song JY, et al. Detection of microRNA as novel biomarkers of epithelial ovarian cancer from the serum of ovarian cancer patient. Int J Gynecol Cancer. 2013;23(4):673–679. [DOI] [PubMed] [Google Scholar]
- 13. Cappellesso R, Tinazzi A, Giurici T, et al. Programmed cell death 4 and microRNA 21 inverse expression is maintained in cells and exosomes from ovarian serous carcinoma effusions. Cancer Cytopathol. 2014;122(9):685–693. [DOI] [PubMed] [Google Scholar]
- 14. Vaksman O, Tropę C, Davidson B, Reich R. Exosome-derived miRNAs and ovarian carcinoma progression. Carcinogenesis. 2014;35(9):2113–2120. [DOI] [PubMed] [Google Scholar]
- 15. Vandesompele J, De Preter K, Pattyn F, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):0034.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: Bestkeeper – Excel-based tool using pair-wise correlations. Biotechnol Lett. 2004;26(6):509–515. [DOI] [PubMed] [Google Scholar]
- 17. Hellemans J, Mortier G, De Paepe A, Speleman F, Vandesompele J. . qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007;8(2): R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Makarova JA, Shkurnikov MU, Wicklein D, et al. Intracellular and extracellular microRNA: an update on localization and biological role. Prog Histochem Cytochem. 2016;51(3-4):33–49. [DOI] [PubMed] [Google Scholar]
- 19. Weber JA, Baxter DH, Zhang SL, et al. The microRNA spectrum in 12 body fluids. Clin Chem. 2010;56(11):1733–1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kosaka N, Iguchi H, Ochiya T. Circulating microRNA in body fluid: a new potential biomarker for cancer diagnosis and prognosis. Cancer Sci. 2010;101(10):2087–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Witwer KW. Circulating microRNA biomarker studies: pitfalls and potential solutions. Clin Chem. 2015;61(1):56–63. [DOI] [PubMed] [Google Scholar]
- 22. Turchinovich A, Weiz L, Burwinkel B. Extracellular miRNAs: the mystery of their origin and function. Trends Biochem Sci. 2012;37(11):460–465. [DOI] [PubMed] [Google Scholar]
- 23. Witwer KW, Buzas EI, Bemis LT, et al. Standardization of sample collection, isolation and analysis methods in extracellular vesicle research. J Extracell Vesicles. 2013;2(1):20360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Blagden SP. Harnessing pandemonium: the clinical implications of tumor heterogeneity in ovarian cancer. Front Oncol. 2015;5:149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nelson BH. New insights into tumor immunity revealed by the unique genetic and genomic aspects of ovarian cancer. Curr Opin Immunol. 2015;33:93–100. [DOI] [PubMed] [Google Scholar]
- 26. Zavesky L, Jandakova E, Turyna R, et al. New perspectives in diagnosis of gynaecological cancers: emerging role of circulating microRNAs as novel biomarkers. Neoplasma. 2015;62(4):509–520. [DOI] [PubMed] [Google Scholar]
- 27. Lengyel E. Ovarian cancer development and metastasis. Am J Pathol. 2010;177:1053–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jabbari N, Reavis AN, McDonald JF. Sequence variation among members of the miR-200 microRNA family is correlated with variation in the ability to induce hallmarks of mesenchymal–epithelial transition in ovarian cancer cells. J Ovarian Res. 2014;7:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu S, Xu P, Wu W, et al. The biphasic expression pattern of miR-200a and E-cadherin in epithelial ovarian cancer and its correlation with clinicopathological features. Curr Pharm Des. 2014;20(11):1888–1895. [DOI] [PubMed] [Google Scholar]
- 30. Choi PW, Ng SW. The functions of microRNA-200 family in ovarian cancer: beyond epithelial–mesenchymal transition. Int J Mol Sci. 2017;18(6):1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kan CWS, Hahn MA, Gard GB, et al. Elevated levels of circulating microRNA-200 family members correlate with serous epithelial ovarian cancer. BMC Cancer. 2012;12:627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gao YC, Wu J. MicroRNA-200c and microRNA-141 as potential diagnostic and prognostic biomarkers for ovarian cancer. Tumor Biol. 2015;36(6):4843–4850. [DOI] [PubMed] [Google Scholar]
- 33. Meng X, Joosse SA, Muller V, et al. Diagnostic and prognostic potential of serum miR-7, miR-16, miR-25, miR-93, miR-182, miR-376a and miR-429 in ovarian cancer patients. Br J Cancer. 2015;113(9):1358–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shapira I, Oswald M, Lovecchio J, et al. Circulating biomarkers for detection of ovarian cancer and predicting cancer outcomes. Br J Cancer. 2014;110(4):976–983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Torres A, Torres K, Pesci A, et al. Diagnostic and prognostic significance of miRNA signatures in tissues and plasma of endometrioid endometrial carcinoma patients. Int J Cancer. 2013;132(7):1633–1645. [DOI] [PubMed] [Google Scholar]
- 36. Wu J, Ji X, Zhu L, et al. Up-regulation of microRNA-1290 impairs cytokinesis and affects the reprogramming of colon cancer cells. Cancer Lett. 2013;329(2):155–163. [DOI] [PubMed] [Google Scholar]
- 37. Mao Y, Liu J, Zhang D, Li B. MiR-1290 promotes cancer progression by targeting nuclear factor I/X (NFIX) in esophageal squamous cell carcinoma (ESCC). Biomed Pharmacother. 2015;76:82–93. [DOI] [PubMed] [Google Scholar]
- 38. Kim G, An HJ, Lee MJ, et al. Hsa-miR-1246 and hsa-miR-1290 are associated with stemness and invasiveness of non-small cell lung cancer. Lung Cancer. 2016;91:15–22. [DOI] [PubMed] [Google Scholar]
- 39. Marchini S, Cavalieri D, Fruscio R, et al. Association between miR-200c and the survival of patients with stage I epithelial ovarian cancer: a retrospective study of two independent tumour tissue collections. Lancet Oncol. 2011;12(3):273–285. [DOI] [PubMed] [Google Scholar]
- 40. Sestito R, Cianfrocca R, Rosano L, et al. miR-30a inhibits endothelin A receptor and chemoresistance in ovarian carcinoma. Oncotarget. 2016;7(4):4009–4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen N, Chon HS, Xiong Y, et al. Human cancer cell line microRNAs associated with in vitro sensitivity to paclitaxel. Oncol Rep. 2014;31(1):376–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhou J, Gong G, Tan H, et al. Urinary microRNA-30a-5p is a potential biomarker for ovarian serous adenocarcinoma. Oncol Rep. 2015;33(6):2915–2923. [DOI] [PubMed] [Google Scholar]
- 43. Chung YH, Li SC, Kao YH, et al. MiR-30a-5p inhibits epithelial-to-mesenchymal transition and upregulates expression of tight junction protein Claudin-5 in human upper tract urothelial carcinoma cells. Int J Mol Sci. 2017;18(8):1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Li Y, Li Y, Chen D, et al. MiR-30a-5p in the tumorigenesis of renal cell carcinoma: a tumor suppressive microRNA. Mol Med Rep. 2016;13(5):4085–4094. [DOI] [PubMed] [Google Scholar]
- 45. Zhang SL, Liu Q, Zhang Q, Liu L. MicroRNA-30a-5p suppresses proliferation, invasion and tumor growth of hepatocellular cancer cells via targeting FOXA1. Oncol Lett. 2017;14(4):5018–5026. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46. Zhang BW, Cai HF, Wei XF, et al. MiR-30-5p regulates muscle differentiation and alternative splicing of muscle-related genes by targeting MBNL. Int J Mol Sci. 2016;17(2):E182. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Supplementary_Table_S1_RS_FINAL for Ascites-Derived Extracellular microRNAs as Potential Biomarkers for Ovarian Cancer by Luděk Záveský, Eva Jandáková, Vít Weinberger, Luboš Minář, Veronika Hanzíková, Daniela Dušková, Lenka Záveská Drábková, Iveta Svobodová, and Aleš Hořínek in Reproductive Sciences
Supplemental Material, Supplementary_Table_S2_RS_FINALafter_review for Ascites-Derived Extracellular microRNAs as Potential Biomarkers for Ovarian Cancer by Luděk Záveský, Eva Jandáková, Vít Weinberger, Luboš Minář, Veronika Hanzíková, Daniela Dušková, Lenka Záveská Drábková, Iveta Svobodová, and Aleš Hořínek in Reproductive Sciences
Supplemental Material, Supplementary_Table_S3_RS_FINALafter_review for Ascites-Derived Extracellular microRNAs as Potential Biomarkers for Ovarian Cancer by Luděk Záveský, Eva Jandáková, Vít Weinberger, Luboš Minář, Veronika Hanzíková, Daniela Dušková, Lenka Záveská Drábková, Iveta Svobodová, and Aleš Hořínek in Reproductive Sciences
Supplemental Material, Supplementary_Table_S4_RS_FINALafter_review for Ascites-Derived Extracellular microRNAs as Potential Biomarkers for Ovarian Cancer by Luděk Záveský, Eva Jandáková, Vít Weinberger, Luboš Minář, Veronika Hanzíková, Daniela Dušková, Lenka Záveská Drábková, Iveta Svobodová, and Aleš Hořínek in Reproductive Sciences