Abstract
Background
Although dysmenorrhea is not a life-threatening condition, it can cause a substantial burden on individuals and communities. There is no data on the prevalence of dysmenorrhea in Kuwait. This study aimed to estimate the prevalence of dysmenorrhea among female public high-school students in Kuwait and investigate factors associated with dysmenorrhea.
Methods
A cross-sectional study using multistage cluster sampling with probability proportional to size method was conducted on 763 twelfth grade female public high-school students (aged 16–21 years). We used face-to-face interview with a structured questionnaire to collect data on dysmenorrhea and presumed risk factors. Weight and height of the students were measured using appropriate weight and height scales in a standardized manner. The association between dysmenorrhea and potential risk factors was assessed using multiple logistic regression.
Results
The one-year prevalence of dysmenorrhea was found to be 85.6% (95%CI: 83.1–88.1%). Of the participants with dysmenorrhea, 26% visited a public or a private clinic for their pain and 4.1% were hospitalized for their menstrual pain. Furthermore, 58.2% of students with dysmenorrhea missed at least one school day and 13.9% missed at least one exam. Age of menarche (p-value = 0.005), regularity and flow of the menstrual period (p-value = 0.025, p-value = 0.009; respectively), and drinking coffee (p-value = 0.004) were significantly associated with dysmenorrhea in multivariable analysis.
Conclusion
Dysmenorrhea seems to be highly prevalent among female high-school students in Kuwait, resembling that of high-income countries. Because of the scale of the problem, utilizing school nurses to reassure and manage students with primary dysmenorrhea and referring suspected cases of secondary dysmenorrhea is recommended.
Keywords: Dysmenorrhea, School girls, Kuwait, Menstrual pain
Background
Menstrual period is a cyclic physiological phenomenon; in which several problems can arise including irregular cycles, excessive bleeding, and dysmenorrhea. Dysmenorrhea is commonly described as a severe, painful, cramping sensation in the lower abdomen that is often associated with other symptoms, such as sweating, headaches, nausea, vomiting, and diarrhea [1]. These symptoms can occur during or a few days before menstruation. Dysmenorrhea can be primary, which is defined as pain without any clear pathological gynecological origin. Hypothesized pathways include endometrial release of large and imbalanced amounts of prostanoids and possibly eicosanoids during the menstrual cycle, which causes the uterus to contract frequently and dysrhythmically ultimately causing pain during menstruation [2]. On the other hand, secondary dysmenorrhea is caused by obvious underlying pelvic pathology and could occur years after menarche. It is associated with a variety of conditions including endometriosis, pelvic inflammatory disease, ovarian cysts, adenomyosis, and uterine myomas [3].
Although primary dysmenorrhea is not a life-threatening condition, it can cause a substantial burden on the quality of life of women or female adolescents [4–6]. A literature review of previous studies showed that dysmenorrhea negatively impacts the quality of life of affected women including their relationships with family members and friends, school or work performance in addition to social and recreational activities [7]. It has been also reported that women with dysmenorrhea tend to have higher sensitivity to pain in general even at the time when they have no menstrual pain [7]. Furthermore, dysmenorrhea is deemed to have significant economic consequences [8]. In the United States, the economic burden of dysmenorrhea has been estimated to be 600 million work hours or 2 billion dollars [9]. In Japan, it has been reported that the total healthcare cost for patients with primary dysmenorrhea is 2.2 times higher than the healthcare cost for females with no dysmenorrhea after adjusting for baseline characteristics [10].
There is large variation in the reported prevalence of primary dysmenorrhea between different countries and sometimes within the same country, which could be due to the use of different definitions of the condition [7]. Literature reviews of previous studies [5, 7] suggested that the prevalence of primary dysmenorrhea ranged from 34% or 45 to 95% among menstruating women. Dysmenorrhea tends to affect adolescent females more frequently than older women; therefore, results from studies reporting prevalence among adolescent girls cannot be generalized to older women [11]. As mentioned above, the lack of a uniform operational definition for dysmenorrhea to be used in epidemiological studies could be the underlying reason for the differences in the prevalence of primary dysmenorrhea between different settings.
Epidemiological studies have identified a number of factors associated with primary dysmenorrhea but the association between dysmenorrhea and many of these risk factors is still uncertain. In a review article, Ju et al. reported smoking, high body mass index (BMI), early age of menarche, longer and heavier menstrual flow, and family history of dysmenorrhea as predisposing factors for dysmenorrhea [12]. Although dysmenorrhea is deemed to be a sensitive issue in the Arab states in the Gulf region and Middle East, there have been some studies that explored the prevalence of dysmenorrhea [13–19] but none of these studies were in Kuwait. This study aimed to estimate the prevalence of dysmenorrhea among high-school female students and investigate factors associated with dysmenorrhea.
Methods
Study population and study participants
The total population of Kuwait is 4.2 million with 24.8% of the population below 20 years of age. The literacy in females aged between 15 to 24 years in Kuwait is 98.7% [20]. There are 77 female public high-schools in Kuwait with 40,095 students typically between the age 14 and 19 years. The study population was female students at the twelfth grade (typically aged 16–19) from public high-schools from all governorates in Kuwait. It was thought that this group would be easily accessible and would allow for a valid inference to be made on the prevalence of dysmenorrhea and its associated factors. The inclusion criteria were female students who attended their schools on the day of data collection and were willing to participate in the study.
Study design and sampling methods
A cross-sectional study was conducted on a representative sample of female high-school students at twelfth grade (aged 16–21 years) that were selected using probability proportional to size sampling after stratification by governorate. Using the list of all female public high-schools (with the number of students in each school), multistage random cluster sampling was used to select the participants at public high-schools in all governorates of Kuwait. The relative size of each governorate, which was judged by the number of female students in high-schools, was taken into account to set the number of participants required.
Data collection
Data on dysmenorrhea were gathered by face-to-face interviews conducted by six female senior medical students. The interview was based on a structured questionnaire that was developed after extensive review of the literature. The questionnaire comprised four major parts, which in addition to socio-demographic factors focused on the presence of dysmenorrhea, its associated symptoms (i.e. fatigue, headaches, breast tenderness … etc.), and impact on academic life. The presence of dysmenorrhea was assessed by asking the students if they had pain during their menstrual period in the past year. Participants who answered “yes, always”, “yes, often”, or “yes, sometimes” were considered to have dysmenorrhea; while those who answered “yes, rarely”, or “never” were considered to have no dysmenorrhea. A similar approach was used in a previous study in Canada [21]. The questionnaire was developed in English and was translated into Arabic and then back-translated to English by an independent person who was not part of this study. The original English questionnaire and the back-translated were then compared. The final Arabic version of the questionnaire was pretested on 20 students from the same age group, who were not included in the study.
The menstrual pain was assessed using a horizontal visual analog scale (VAS) with a 100-mm line; one end of the line represents “no pain” and the other end represents “worst possible pain”. The participants were asked to rate the degree of their pain by making a mark on the line. The data collectors then measured the answers marked by the students with a ruler. The scores received from the scale were classified into mild (> 5 to ≤44 mm), moderate (> 44 to ≤74 mm), and severe (> 74 mm) [22]. The question regarding the location of the menstrual pain was illustrated using photo cards and the students were allowed to choose more than one site. Data on regularity of menstrual period were gauged using the question “Do you describe your period as being regular or irregular?” with the answers “regular or irregular”. Data on the management of pain were gathered using the question “What do you do to relieve your pain?” followed by a list of options (bed rest, heating pad, medication prescribed by a doctor, medication taken by myself, tea/herbs and others) with the student allowed to selected more than one option. Similarly data on symptoms were collected by the question “Do you experience any of the following symptoms during your period?” followed by a list of symptoms with the student allowed to select more than one option.
We collected data on socio-demographic factors in addition to hypothesized risk factors for dysmenorrhea such as characteristics of the menstrual period, smoking, second-hand smoking, consumption of specific dietary items and drinks. We also collected data on presence of any disease condition that was diagnosed by a medical doctors. Furthermore, we collected data on physical activity using a self-administered questionnaire, which has already been translated to Arabic and validated in our setting using accelerometers (Spearman correlation 0.92; p < 0.001 for total steps count) (not published). This questionnaire contained 14 questions, asking about different physical activities that participants did in the previous 7 days with their frequency and duration. We measured height using a portable stable stadiometer (SECATMR) to the nearest 0.1 cm, and weight using digital weight scale (BeurerR) to the nearest 0.1 kg.
Data analysis
Data were entered and analyzed using Statistical Package for Social Sciences (SPSS). Using the measured weight and height, BMI was calculated (weight (Kg)/height(m2)) and categorized into underweight, normal weight, overweight and obese for students below the age of 18 years according to WHO’s growth charts, and for those ≥18 years as per WHO’s classification (underweight< 18, normal 18.5 to 24.9, overweight 25 to 29.9, obese ≥30). The 95% CI for the one-year prevalence of dysmenorrhea was calculated using the exact binominal distribution. We used Chi-square test to investigate the association between dysmenorrhea and categorical variables. The adjusted association between dysmenorrhea and multiple factors was assessed using multiple unconditional logistic regression. The binary outcome in this analysis was created by categorizing participants who reported always, often, or sometimes having pain with their menstrual period during the past year in one group (dysmenorrhea group), while those who reported having pain with their menstrual period rarely or never in another group (no dysmenorrhea group).
Ethical approval
The study was approved by The Health Sciences Center Ethics Committee at Kuwait University (Ref: 3660–16/10/2017). We also obtained the permission from The Ministry of Education in Kuwait. Each participant completed a written informed consent, which outlined the objectives of the study before the interview was initiated.
Result
Of 787 students who were approached, 766 (97.3%) agreed to participate. Three participants have not reached menarche, and therefore, were excluded from the study. Thus, the analysis below comprised 763 participants. Table 1 shows the socio-demographic characteristics of the study participants. The mean (SD) age of the participants was 17.4 (0.7) years. Table 2 shows the description of the menstrual period of 763 female high-school students. The mean (SD) age of menarche was 12.1 (1.3) years; and about half of the study group described their menstrual period as irregular; while 56.1% reported having their period lasting between 6 and 8 days.
Table 1.
Socio-demographic characteristics of 763 female public high-school students in Kuwait, 2017
| Number | (Percent) | |
|---|---|---|
| Age in years, mean (SD) | 17.4 (0.7) | |
| Nationality | ||
| Kuwaiti | 655 | (85.8) |
| Non-Kuwaiti | 108 | (14.2) |
| Father’s educationa | ||
| Intermediate/below | 98 | (12.9) |
| Secondary (high school) | 230 | (30.2) |
| Diploma | 64 | (8.4) |
| University and above | 369 | (48.5) |
| Mother’s education | ||
| Intermediate/below | 132 | (17.3) |
| Secondary (high school) | 196 | (25.7) |
| Diploma | 76 | (10.0) |
| University and above | 359 | (47.0) |
| Father’s income per monthb | ||
| Less than 500 KD | 25 | (3.3) |
| 500–1000 KD | 93 | (12.2) |
| 1001–1500 KD | 140 | (18.4) |
| 1501–2000 KD | 99 | (13.0) |
| More than 2000 KD | 77 | (10.1) |
| Do not wish to tell | 328 | (43.0) |
| Mother’s income per month | ||
| Less than 500 KD | 102 | (13.4) |
| 500–1000 KD | 174 | (22.8) |
| 1001–1500 KD | 88 | (11.5) |
| 1501–2000 KD | 73 | (9.6) |
| More than 2000 KD | 33 | (4.3) |
| Do not wish to tell | 293 | (38.4) |
| Currently lives with: | ||
| Both parents | 671 | (87.9) |
| Mother without the father | 73 | (9.6) |
| Father without the mother | 15 | (2.0) |
| Other relatives | 4 | (0.5) |
| Number of sisters | ||
| ≤ 2 | 448 | (58.7) |
| 3–4 | 214 | (28.1) |
| ≥ 5 | 101 | (13.2) |
| Number of brothers | ||
| ≤ 2 | 421 | (55.2) |
| 3–4 | 272 | (35.6) |
| ≥ 5 | 70 | (9.2) |
a Missing for two participants; b Missing for one participant
Table 2.
Description of menstrual period of 763 female high-school students in Kuwait, 2017
| Number | Percent | |
|---|---|---|
| Age of menarche, mean (SD) years | 12.1 (1.3) | |
| Regularity of menstrual perioda | ||
| Regular | 367 | (48.1) |
| Irregular | 396 | (51.9) |
| Duration of menstrual period (days) | ||
| ≤ 3 | 31 | (4.1) |
| 4–5 | 268 | (35.1) |
| 6–8 | 428 | (56.1) |
| > 8 | 36 | (4.7) |
| Number of pads changed in the first three days of period | ||
| ≤ 3 | 240 | (31.5) |
| 4–5 | 351 | (46.0) |
| ≥ 6 | 172 | (22.5) |
| Flow of menstrual period (as described by the participants)b | ||
| Mild | 59 | (7.7) |
| Moderate | 467 | (61.3) |
| Heavy | 115 | (15.1) |
| Heavy with clots | 121 | (15.9) |
a As described by the study participants. b Missing for one participant
Prevalence of dysmenorrhea
The prevalence of dysmenorrhea in addition to the description of the pain intensity and its duration are shown in Table 3. Of 763 participants, 653 (85.6%; 95%CI: 83.1–88.1%) had dysmenorrhea according to the definition used in our study (those who reported pain with their menstrual period always, often, or sometimes during last year). This was 560 (85.5%) and 93 (86.1%) among Kuwaitis and non-Kuwaitis, respectively (p-value = 0.866). There was no significant difference in the prevalence of dysmenorrhea between different governorates (p-value = 0.137). If we include those who reported having the menstrual pain rarely, the prevalence becomes 702 (92.0%; 95%CI: 90.0–93.9%). In other words, only around 8.0% reported that they have never had pain with their menstrual period.
Table 3.
Description of dysmenorrhea and the characteristics of pain experienced by 653 female students from Kuwait public high-schools, 2017
| Questions | Number | % | n(%) |
|---|---|---|---|
| During the last year: | |||
| Did you have pain with your menstrual period? (N = 763) | |||
| Yes, always | 353 | (46.2) | 653 (85.6) |
| Yes, often | 131 | (17.1) | |
| Yes, sometimes | 169 | (22.1) | |
| Yes, rarely | 50 | (6.5) | 110 (14.4) |
| Never | 60 | (7.9) | |
| How long does the pain usually last? (N = 653) | |||
| Less than one day | 102 | (15.6) | |
| 1–2 days | 364 | (55.7) | |
| 3–4 days | 163 | (25.0) | |
| More than 4 days | 24 | (3.7) | |
| Where do you experience the pain?a (N = 653) | |||
| Lower abdomen | 640 | (98.0) | |
| Back | 455 | (69.7) | |
| Thighs | 189 | (28.9) | |
| Legs | 84 | (12.9) | |
| Have you visited a public/private clinic for this pain? (yes)(N = 653) | 170 | (26.0) | |
| Have you been hospitalized for the pain? (yes)(N = 653) | 27 | (4.1) | |
| Pain severity (N = 653) | |||
| Mild (5–44) | 119 | (18.2) | |
| Moderate (45–74) | 333 | (51.0) | |
| Severe (75–100) | 201 | (30.8) | |
| Have you ever missed a school day because of your menstrual pain? (yes)(N = 653) | 380 | (58.2) | |
| Have you ever missed an exam because of the menstrual pain? (yes)(N = 653) | 91 | (13.9) | |
| Does your pain severity change throughout the year depending on the weather? (yes))N = 653) | 305 | (46.7) | |
| Is it worse in cold or hot seasons? (N = 653) | |||
| Hot seasons | 39 | (6.0) | |
| Cold seasons | 266 | (40.7) | |
a Participants can select more than one option
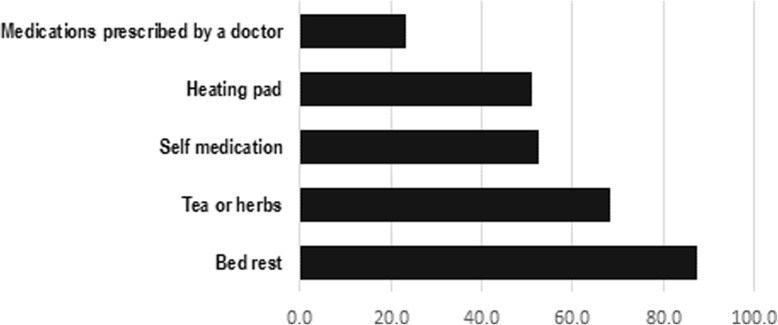
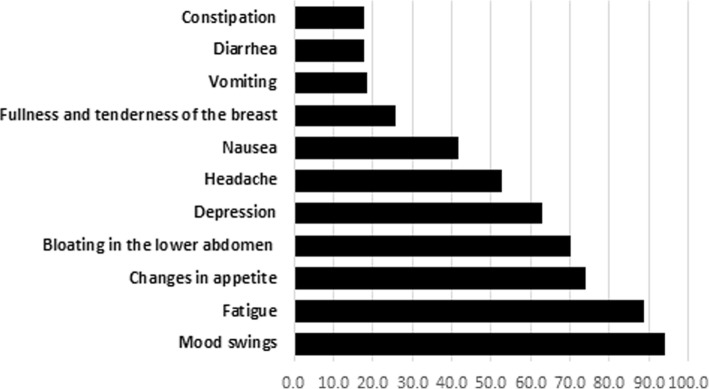
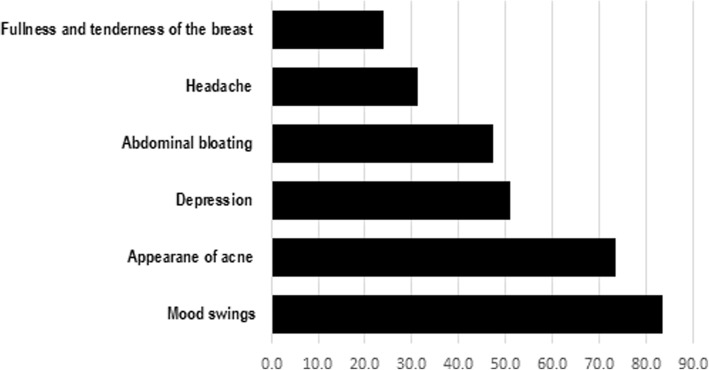
More than half of the participants with dysmenorrhea had pain for 1 to 2 days; and the most common site for pain was the lower abdomen. It is worth noting that 26% of the participants with dysmenorrhea (170 out of 653) have visited a public or private clinic because of their pain. Out of students with dysmenorrhea (N = 653), 27 (4.1%) were hospitalized for the management of their menstrual pain. Methods the participants used to relieve their pain are shown in Fig. 1, while the most common symptoms during and before the menstrual period are shown in Figs. 2 and 3. More than half of the participants with dysmenorrhea have missed at least one school day during the last academic year. Also, 91 (13.9%) of the participants with dysmenorrhea missed at least one school exam.
Fig. 1.
Management of menstrual pain by 653 female public high-school students with dysmenorrhea, Kuwait 2017
Fig. 2.
Reported symptoms of dysmenorrhea during the menstrual period by 763 female public high-school students, Kuwait 2017
Fig. 3.
Reported symptoms of dysmenorrhea before the menstrual period by 763 female public high-school students, Kuwait 2017
Risk factors for dysmenorrhea
Table 4 shows the association between dysmenorrhea and presumed risk factors in univariable analysis. Factors that showed significant association with dysmenorrhea in univariable analysis were age of menarche, the regularity of the menstrual period as reported by the participants, the flow of the menstrual period and the frequency of drinking coffee. Table 5 shows the association between dysmenorrhea and risk factors in multivariable logistic regression analysis. The age of menarche was found to be significantly associated with dysmenorrhea; adjusted odds ratio 0.80 (95%CI: 0.69–0.93), (p-value = 0.005). Similarly, having irregular menstrual period, as described by the participants, was negatively associated with dysmenorrhea; adjusted odds ratio 0.59 (95%CI: 0.38–0.91), (p-value = 0.018). The flow of menstrual period as reported by the participants was also positively associated with dysmenorrhea (p-value = 0.006). Furthermore, drinking coffee four or more times per week was positively associated with dysmenorrhea, adjusted odds ratio 2.19 (95%CI: 1.39–3.44), (p-value = 0.001). We also repeated the analysis after recoding those who rarely had menstrual pain as dysmenorrhea (i.e. never had menstrual pain vs. rarely, sometimes, often, and always had menstrual pain). In multivariable analysis, only age of menarche, regularity and flow of the menstrual period and weekly drinking of coffee were significantly associated with dysmenorrhea. These findings are identical to the results from the previous analysis.
Table 4.
Association between dysmenorrhea and potential risk factors among 763 female high-school students in Kuwait in univariable analysis
| ORa | [95% CI] | p-valueb | |
|---|---|---|---|
| Variables | |||
| Nationality | |||
| Non-Kuwaiti | 1.00 | [Reference] | 0.866 |
| Kuwaiti | 0.95 | [0.53–1.71] | |
| Father’s education | |||
| Intermediate school or belowc | 0.84 | [0.46–1.55] | 0.830 |
| Secondary (high-school) | 1.05 | [0.65–1.70] | |
| Diploma | 0.79 | [0.39–1.61] | |
| University and above | 1.00 | [Reference] | |
| Mother’s education | |||
| Intermediate school or belowc | 0.89 | [0.51–1.55] | 0.419 |
| Secondary (high-school) | 1.34 | [0.78–2.28] | |
| Diploma | 0.75 | [0.39–1.44] | |
| University and above | 1.00 | [Reference] | |
| Currently lives | |||
| With mother | 1.00 | [Reference] | 0.637 |
| Without the mother | 1.43 | [0.32–6.27] | |
| Number of sisters | |||
| ≤ 2 | 1.00 | [Reference] | 0.317 |
| 3–4 | 0.85 | [0.54–1.33] | |
| ≥ 5 | 1.52 | [0.75–3.06] | |
| Number of brothers | |||
| ≤ 2 | 1.00 | [Reference] | 0.183 |
| 3–4 | 1.32 | [0.84–2.08] | |
| ≥ 5 | 0.70 | [0.37–1.34] | |
| Passive smoking at household | |||
| Yes | 0.89 | [0.59–1.33] | 0.565 |
| No | 1.00 | [Reference] | |
| Sleep hours per night during week days | |||
| < 6 h | 1.64 | [0.95–2.82] | 0.201 |
| 6–7 h | 1.18 | [0.74–1.89] | |
| > 7 h | 1.00 | [Reference] | |
| Sleep hours per night during weekends | |||
| < 8 h | 1.25 | [0.74–2.133] | 0.647 |
| 8–10 h | 1.19 | [0.75–1.92] | |
| > 10 h | 1.00 | [Reference] | |
| Presence of disease condition diagnosed by doctor | |||
| No | 1.00 | [Reference] | 1.21 |
| Yes | 1.21 | [0.75–1.95] | |
| Taking supplements | |||
| No | 1.00 | [Reference] | 0.269 |
| Yes | 1.29 | [0.82–2.02] | |
| Taking medication | |||
| No | 1.00 | [Reference] | 0.077 |
| Yes | 2.06 | [0.92–4.58] | |
| History of surgeries | |||
| No | 1.00 | [Reference] | 0.143 |
| Yes | 1.49 | 0.87–2.55 | |
| Age of menarche | 0.81 | [0.70–0.94] | 0.005 |
| Regularity of menstrual periodd | |||
| Regular | 1.00 | [Reference] | 0.025 |
| Irregular | 0.62 | [0.41–0.94] | |
| Duration of menstrual period | |||
| ≤ 3 | 1.00 | [Reference] | 0.081 |
| 4–5 | 2.48 | [1.06–5.78] | |
| 6–8 | 2.77 | [1.21–6.33] | |
| > 8 | 1.69 | [0.55–5.26] | |
| Number of pads changed in the first three days of the period | |||
| 4–5 | 1.00 | [Reference] | 0.323 |
| ≤ 3 | 0.624 | [0.39–0.99] | |
| ≥ 6 | 0.743 | [0.44–1.26] | |
| Flow of menstrual periodd | |||
| Mild | 0.38 | [0.17–0.86] | 0.009 |
| Moderate | 0.70 | [0.38–1.30] | |
| Heavy | 1.75 | [0.70–4.34] | |
| Heavy with clots | 1.00 | [Reference] | |
| Family history of menstrual pain | |||
| No | 1.00 | [Reference] | 0.298 |
| Yes | 1.29 | [0.79–2.1] | |
| Diet description by the participant | |||
| I eat meat, fish, or both | 1.00 | [Reference] | 0.398 |
| I don’t eat meat or fish/I am vegetarian and eat eggs/I am vegetarian and don’t eat eggs | 1.57 | [0.55–4.51] | |
| Weekly consumption of fast food | |||
| ≤ 2 days | 1.00 | [Reference] | 0.337 |
| ≥ 3 days | 0.81 | [0.52–1.25] | |
| Weekly fried food consumption | |||
| ≤ 2 days | 1.00 | [Reference] | 0.344 |
| ≥ 3 days | 1.22 | [0.81–1.83] | |
| Energy drinks consumption | |||
| No | 1.00 | [Reference] | 0.592 |
| Yes | 0.88 | [0.56–1.39] | |
| Weekly drinking of coffee | |||
| ≤ 3 times per week | 1.00 | [Reference] | 0.004 |
| ≥ 4 times per week | 1.9 | [1.23–2.96] | |
| Weekly drinking of tea | |||
| ≤ 3 times per week | 1.00 | [Reference] | 0.497 |
| ≥ 4 times per week | 1.21 | [0.7–2.07] | |
| Weekly drinking of green tea | |||
| ≤ 3 times per week | 1.00 | [Reference] | 0.547 |
| ≥ 4 times per week | 1.28 | [0.57–2.9] | |
| Weekly drinking of carbonated drinks | |||
| ≤ 3 times per week | 1.00 | [Reference] | 0.371 |
| ≥ 4 times per week | 0.83 | [0.54–1.25] | |
| BMIe | |||
| Normal | 1.00 | [Reference] | 0.212 |
| Overweight | 0.82 | [0.49–1.35] | |
| Obese | 0.59 | [0.37–0.96] | |
| Physical activity in the previous weekf | |||
| < 3.67 h | 1.00 | [Reference] | 0.983 |
| 3.67–9.50 h | 0.95 | [0.57–1.58] | |
| > 9.50 h | 0.97 | [0.58–1.61] | |
a Crude unadjusted odds ratio; b P-values were generated using Chi-square test; c Intermediate school or below group included non-educated and primary level education; d as reported by the participants; e Weight and height were measured and for those who are below 18 years old, z-score was calculated using WHO’s growth chart, otherwise WHO’s BMI classification was used; f It includes only 737 participants
Table 5.
Association between dysmenorrhea and potential risk factors among 763 female high-school students in Kuwait in multivariable analysis
| Variables | ORa | [95% CI] | p-valueb |
|---|---|---|---|
| Age of menarche | 0.80 | [0.69–0.93] | 0.005 |
| Regularity of menstrual period | |||
| Regular | 1.00 | [Reference] | 0.018 |
| Irregular | 0.59 | [0.38–0.91] | |
| Flow of menstrual periodc | |||
| Mild | 0.44 | [0.19–1.01] | 0.006 |
| Moderate | 0.78 | [0.41–1.47] | |
| Heavy | 2.10 | [0.83–5.31] | |
| Heavy with clots | 1.00 | [Reference] | |
| Weekly drinking of coffee | |||
| ≤ 3 times per week | 1.00 | [Reference] | 0.001 |
| ≥ 4 times per week | 2.19 | [1.39–3.44] | |
a Adjusted odds ratio; b p-values were generated using likelihood ratio test; c as reported by the participants
Discussion
This study aimed to estimate the prevalence of dysmenorrhea among female public high-school students in Kuwait and to explore the relationship between dysmenorrhea and several presumed risk factors. There is a paucity of data on dysmenorrhea and its associated factors in Kuwait. We have demonstrated that the majority of female students in Kuwait had dysmenorrhea and that a large number had sought medical treatment from private or public healthcare services.
The one-year prevalence of dysmenorrhea was found to be 85.6%. Because pain is a highly subjective symptom and therefore difficult to quantify, there is a lack of consensus on an operational definition of dysmenorrhea in epidemiological studies. As a result, it is difficult to compare our findings with that of other studies at a regional or an international level. Unfortunately, in some studies, the definition of dysmenorrhea was not clearly stated [23, 24]; while in other studies dysmenorrhea was defined as pain with menstrual period without further specification of intensity and/or frequency [25, 26]. Developing a consensus on a standard definition of dysmenorrhea is an important step to study the geographical distribution of dysmenorrhea and its trends over time.
Overall, the one-year prevalence was very high in our setting similar to that reported in other studies in the region such as Saudi Arabia (60.9% among female medical students) [13], Oman (94% among high-school students) [14], and Iran (98.4% among female medical students) [27]. Our findings are also consistent with that reported from high-income countries such as Canada (60% in girls aged 18 and above) [21] and Australia (88% among females aged 16 to 25 years old) [28].
Dysmenorrhea can be a major cause for school absenteeism and missing exams. In Saudi Arabia, of the university students with dysmenorrhea, 28.3% had absenteeism [13], while in Turkey 32% of female high-school students reported school absenteeism due to dysmenorrhea [29]. In our study, 58.2% of students with dysmenorrhea missed at least one school day during the last academic year. Also, 13.9% of the participants with dysmenorrhea missed at least one exam in the past academic year. The difference between our findings and other studies could be due to an increased tendency to report dysmenorrhea as a reason for school absenteeism among female high-school students compared to students in other countries. Approximately, 26% of the participants with dysmenorrhea visited public or private clinic because of their menstrual pain, which is different from that reported amongst Hispanic adolescents in Texas (only 14% of school girls with dysmenorrhea had sought physician advice) [30] or Egyptian girls (the majority of students did not seek medical advice for dysmenorrhea) [18]. The easier access to healthcare services in Kuwait could explain the higher proportion of adolescents seeking medical care for dysmenorrhea compared to other settings.
Significant association between early age of menarche and dysmenorrhea was found in univariable and multivariable analysis (Tables 4 and 5), which could be due to the fact that early menarche reflects longer exposure to uterine prostaglandins that plays a major role in dysmenorrhea through increasing uterine contractility resulting in pain [31]. Another reason could be that dysmenorrhea typically occurs with ovulatory cycles, which are not established immediately after menarche [32]. Therefore, later onset of menarche means that females have unovulatory cycles and are less likely to report pain; although this does not mean that they will not experience dysmenorrhea later on in their life.
Surprisingly, irregular menstrual period (as described by the participants) was found to be negatively associated with dysmenorrhea. We found no evidence in literature suggesting that having irregular periods is protective against dysmenorrhea. In fact, several studies have demonstrated a positive relationship between having irregular periods and dysmenorrhea [11, 33]; while other studies reported no association between dysmenorrhea and regularity of the menstrual period [14, 34]. In our study, it is possible that females with dysmenorrhea were more likely to report their menstrual period as being regular since they anticipate their period (hence their pain) each month. We also found that the flow of the menstrual period (the amount of blood lost during a menstrual period as reported by the participants) was significantly associated with dysmenorrhea, which is consistent with several studies [13, 35]. Both the flow of menstruation and dysmenorrhea are thought to be determined by prostaglandins. In case of increased blood flow, prostaglandins can disturb the homeostatic mechanism of the endometrium; hence, increasing the blood flow. Moreover, platelets aggregation and/or various coagulation factors are affected by prostaglandins leading to the increase of menstrual blood flow [36, 37]. It is also possible that the link between the flow of the menstrual period and dysmenorrhea is not genuine and that females who experienced pain were more likely to report their period as being heavy (recall bias).
Drinking coffee was the only modifiable risk factor that showed an association with dysmenorrhea in our analysis. Drinking coffee four or more times per week was positively associated with dysmenorrhea in univariable and multivariable analysis (Tables 4 and 5). Results from studies that assessed the association between coffee drinking and dysmenorrhea are controversial. Some studies reported positive association between coffee drinking and dysmenorrhea [38, 39]; while others showed no association between dysmenorrhea and daily caffeine intake [34, 40–42]. Caffeine, which is the main ingredient of coffee, is an adenosine analogue that inhibits adenosine (a potent vasodilator) receptors [43]. Blocking these receptors causes vasoconstriction that will decrease the blood flow to the uterus causing further increase in the degree of menstrual pain [44]. In our study, drinking coffee was common (more than 37% of the study group consumed coffee 6 or more times per week), but consumption of other caffeine drinks such as energy drinks was too low, and thus, we were unable to look for the association between other caffeinated drinks and dysmenorrhea.
Similar to other studies [45, 46], no significant association was found between physical activity and dysmenorrhea. Measuring physical activity among young adults is difficult and may result in a substantial non-differential misclassification, which can explain the lack of association between physical activity and dysmenorrhea in our study and other studies [45, 46]. In addition to the studies that showed no association between dysmenorrhea and physical activity, a study in Japan reported that physical activity is inversely associated with dysmenorrhea [47]. However, with the cross-sectional design, the relationship could be explained by the reverse-causality (girls with dysmenorrhea and heavy blood flow may refrain from exercise and other physical activities). Prospective studies are better to address this question and other questions related to the risk factors of dysmenorrhea.
This is the first study to explore the prevalence of dysmenorrhea and its related factors in Kuwait. We used a nationally representative sample; and only few students refused to participate. There are several limitations in the study, including the inherent weakness of the cross-sectional design, which does not allow for causal inferences. As an example, it is not clear if physical inactivity predisposes to dysmenorrhea or dysmenorrhea itself hinders physical activity (in our study we found no association between physical activity and dysmenorrhea). Finally, it is possible that students with severe menstrual pain (dysmenorrhea) were absent during the data collection, which would underestimate the prevalence of dysmenorrhea. However, the high prevalence we reported does not suggest that we missed a large number of students with dysmenorrhea.
Conclusion
Dysmenorrhea seems to be highly prevalent among female high-school students in Kuwait, resembling that of high-income countries. A substantial number of those with dysmenorrhea visited private or public healthcare services and also missed school days and school exams. Univariable and multivariable analysis have shown that age of menarche, regularity and flow of the menstrual period, and coffee drinking are significant independent predictors for dysmenorrhea. Although dysmenorrhea is not a life-threatening health condition, our data suggest dysmenorrhea as a major public health problem among female high-school students causing social burden on families and students alike in addition to affecting school attendance. In Kuwait, each school has a clinic with a nurse, which can be utilized to manage and reassure girls with dysmenorrhea. Training school nurses on management of primary dysmenorrhea and referral of suspected cases of secondary dysmenorrhea seems to be logical in the light of the size of the problem. Currently, the literature shows contradictory results with respect to the factors associated with dysmenorrhea which could be due to the lack of a uniform operational definition of dysmenorrhea in epidemiological studies. The only modifiable risk factor we found was drinking coffee. Although this is biologically plausible, the studies are inconclusive about this issue. Avoidance of heavy caffeine consumption is recommended for several other reasons and might be also useful for reducing dysmenorrhea.
Acknowledgements
We would like to thank Dr. Reem Al-Sabah for her comments on the first draft of the manuscript.
Funding
No funding was received for this study.
Availability of data and materials
The datasets used and analyzed during the current study are available from the corresponding author (Abdullah Al-Taiar) on reasonable request.
Abbreviations
- 95%CI
95% Confidence interval
- BMI
Body mass index
- SD
Standard deviation
- SPSS
Statistical Package for Social Sciences
- VAS
Visual analog scale
Authors’ contributions
SM: Help drafting the paper, collected the data, searched literature and revised the manuscript for significant intellectual input. HM: Help drafting the paper, collected the data, searched literature and revised the manuscript for significant intellectual input (SM and HM contributed equally to the paper). OM: Collected the data and revised the manuscript for significant intellectual input. FA: Collected the data and revised the manuscript for significant intellectual input. DB: Collected the data and revised the manuscript for significant intellectual input. ME: Collected the data and revised the manuscript for significant intellectual input AT: Drafted the paper, supervised data collection and conducted statistical analysis. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The study was approved by The Ethics Committee at Health Science Center, Kuwait University (Ref: 3660–16/10/2017). Written informed consent was taken from each participant. As per the waiver from The Ethics Committee, no consents were sought from the parents.
Consent for publication
Not applicable as no individual data are published.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sharefah Al-Matouq, Email: Sharefah.matouq@hsc.edu.kw.
Hessah Al-Mutairi, Email: Hessah.mutairi@hsc.edu.kw.
Ohood Al-Mutairi, Email: Ohood.mutairi@hsc.edu.kw.
Fatima Abdulaziz, Email: fatima.abdulaziz@hsc.edu.kw.
Dana Al-Basri, Email: Danah.basari@hsc.edu.kw.
Mona Al-Enzi, Email: mona.enzi@hsc.edu.kw.
Abdullah Al-Taiar, Phone: +965-24986553, Email: altaiar@hsc.edu.kw.
References
- 1.Lobo RA, Gershenson DM, Lentz GM, Valea FA. Comprehensive gynecology E-book: Elsevier Health Sciences; 2016.
- 2.Dawood M. Primary dysmenorrhea: advances in pathogenesis and management. Obstet Gynecol. 2006;108(2):428–441. doi: 10.1097/01.AOG.0000230214.26638.0c. [DOI] [PubMed] [Google Scholar]
- 3.Unsal A, Ayranci U, Tozun M, Arslan G, Calik E. Prevalence of dysmenorrhea and its effect on quality of life among a group of female university students. Ups J Med Sci. 2010;115(2):138–145. doi: 10.3109/03009730903457218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raine-Fenning N. Dysmenorrhoea. Curr Obstet Gynaecol. 2005;15(6):394–401. doi: 10.1016/j.curobgyn.2005.09.007. [DOI] [Google Scholar]
- 5.De Sanctis V, Soliman AT, Elsedfy H, Soliman NA, Soliman R, El Kholy M. Dysmenorrhea in adolescents and young adults: a review in different country. Acta Biomed. 2017;87(3):233–246. [PMC free article] [PubMed] [Google Scholar]
- 6.Wong CL. Health-related quality of life among Chinese adolescent girls with Dysmenorrhoea. Reprod Health. 2018;15(1):80. [DOI] [PMC free article] [PubMed]
- 7.Iacovides S, Avidon I, Baker FC. What we know about primary dysmenorrhea today: a critical review. Hum Reprod Update. 2015;21(6):762–778. doi: 10.1093/humupd/dmv039. [DOI] [PubMed] [Google Scholar]
- 8.Jones AE. Managing the pain of primary and secondary dysmenorrhoea. Nurs Times. 2004;100(10):40–43. [PubMed] [Google Scholar]
- 9.Dawood MY. Nonsteroidal anti-inflammatory drugs and changing attitudes toward dysmenorrhea. Am J Med. 1988;84(5A):23–29. doi: 10.1016/0002-9343(88)90473-1. [DOI] [PubMed] [Google Scholar]
- 10.Akiyama S, Tanaka E, Cristeau O, Onishi Y, Osuga Y. Evaluation of the treatment patterns and economic burden of dysmenorrhea in Japanese women, using a claims database. ClinicoEconomics Outcomes Res. 2017;9:295–306. doi: 10.2147/CEOR.S127760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Latthe P, Mignini L, Gray R, Hills R, Khalid K: Factors predisposing women to chronic pelvic pain: systematic review. 2006. [DOI] [PMC free article] [PubMed]
- 12.Ju H, Jones M, Mishra G. The prevalence and risk factors of dysmenorrhea. Epidemiol Rev. 2014;36(1):104–113. doi: 10.1093/epirev/mxt009. [DOI] [PubMed] [Google Scholar]
- 13.Ibrahim N, AlGhamdi M, Al-Shaibani A, AlAmri F, Alharbi H, Al-Jadani A, Alfaidi R. Dysmenorrhea among female medical students in king Abdulaziz University: prevalence, predictors and outcome. Pak J Med Sci. 2015;31(6):1312–1317. doi: 10.12669/pjms.316.8752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Al-Kindim R, Al-Bulushi A. Prevalence and impact of dysmenorrhea among Omani high school students. SQU MED J. 2011;11(4):485–491. [PMC free article] [PubMed] [Google Scholar]
- 15.Bata MS. Age at menarche, menstrual patterns, and menstrual characteristics in Jordanian adolescent girls. Int J Gynaecol Obstet. 2012;119(3):281–283. doi: 10.1016/j.ijgo.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 16.Karout N, Hawai SM, Altuwaijri S. Prevalence and pattern of menstrual disorders among Lebanese nursing students. East Mediterr Health J. 2012;18(4):346–352. doi: 10.26719/2012.18.4.346. [DOI] [PubMed] [Google Scholar]
- 17.Abd El-Mawgod MM, Alshaibany AS, Al-Anazi AM. Epidemiology of dysmenorrhea among secondary-school students in northern Saudi Arabia. J Egypt Public Health Assoc. 2016;91(3):115–119. doi: 10.1097/01.EPX.0000489884.20641.95. [DOI] [PubMed] [Google Scholar]
- 18.Kamel DM, Tantawy SA, Abdelsamea GA. Experience of dysmenorrhea among a group of physical therapy students from Cairo University: an exploratory study. J Pain Res. 2017;10:1079–1085. doi: 10.2147/JPR.S132544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rafique N, Al-Sheikh MH. Prevalence of primary dysmenorrhea and its relationship with body mass index. J Obstet Gynaecol Res. 2018;44(9):1773–1778. doi: 10.1111/jog.13697. [DOI] [PubMed] [Google Scholar]
- 20.At a glance: Kuwait [https://www.unicef.org/infobycountry/kuwait_statistics.html#120].
- 21.Burnett MA, Antao V, Black A, Feldman K, Grenville A, Lea R, Lefebvre G, Pinsonneault O, Robert M. Prevalence of primary dysmenorrhea in Canada. J Obstet Gynaecol Can. 2005;27(8):765–770. doi: 10.1016/S1701-2163(16)30728-9. [DOI] [PubMed] [Google Scholar]
- 22.Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: visual analog scale for pain (VAS pain), numeric rating scale for pain (NRS pain), McGill pain questionnaire (MPQ), short-form McGill pain questionnaire (SF-MPQ), chronic pain grade scale (CPGS), short Form-36 bodily pain scale (SF-36 BPS), and measure of intermittent and constant osteoarthritis pain (ICOAP) Arthritis Care Res (Hoboken) 2011;63(S11):S240–S252. doi: 10.1002/acr.20543. [DOI] [PubMed] [Google Scholar]
- 23.Agarwal K, Agarwal A. A study of dysmenorrhea during menstruation in adolescent girls. Indian J Commun Med. 2010;35(1):159–164. doi: 10.4103/0970-0218.62586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Omidvar S, Bakouei F, Amiri F, Begum K. Primary dysmenorrhea and menstrual symptoms in Indian female students: prevalence, impact and management. Glob J Health Sci. 2016;8(8):135–144. doi: 10.5539/gjhs.v8n8p135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patel V, Tanksale V, Sahasrabhojanee M, Gupte S, Nevrekar P. The burden and determinants of dysmenorrhoea: a population-based survey of 2262 women in Goa, India. BJOG. 2006;113(4):453–463. doi: 10.1111/j.1471-0528.2006.00874.x. [DOI] [PubMed] [Google Scholar]
- 26.Zondervan T, Yudkin L, Vessey P, Jenkinson P, Dawes G, Barlow H, Kennedy H. The community prevalence of chronic pelvic pain in women and associated illness behaviour. Br J Gen Pract. 2001;51(468):541–547. [PMC free article] [PubMed] [Google Scholar]
- 27.Ghaderi F, Asghari Jafarabadi M, Mohseni Bandpei MA. Dysmenorrhea and self-care strategies in Iranian female students: a regression modeling of pain severity and underlying factors. Int J Adolesc Med Health. 2016;29(6). [DOI] [PubMed]
- 28.Subasinghe AK, Happo L, Jayasinghe YL, Garland SM, Gorelik A, Wark JD. Prevalence and severity of dysmenorrhoea, and management options reported by young Australian women. Aust Fam Physician. 2016;45(11):829–834. [PubMed] [Google Scholar]
- 29.Esen I, Oguz B, Serin HM. Menstrual characteristics of pubertal girls: a questionnaire-based study in Turkey. J Clin Res Pediatr Endocrinol. 2016;8(2):192–196. doi: 10.4274/jcrpe.2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banikarim C, Chacko MR, Kelder SH. Prevalence and impact of dysmenorrhea on Hispanic female adolescents. Arch Pediatr Adolesc Med. 2000;154(12):1226–1229. doi: 10.1001/archpedi.154.12.1226. [DOI] [PubMed] [Google Scholar]
- 31.French L. Dysmenorrhea. Am Fam Physician. 2005;71(2):285–91. [PubMed]
- 32.Harel Z. Dysmenorrhea in adolescents and young adults: etiology and management. J Pediatr Adolesc Gynecol. 2006;19(6):363–371. doi: 10.1016/j.jpag.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 33.DiCintio E, Parazzini F, Tozzi L. Dietary habits, reproductive and menstrual factors and risk of dysmenorrhoea. Eur J Epidemiol. 1997;13(8):925–930. doi: 10.1023/A:1007427928605. [DOI] [PubMed] [Google Scholar]
- 34.Alia S, Shamssain M, Shahwan M. Prevalence and impact of dysmenorrhea on health related quality of life in the United Arab Emirates. Eur J Pharm Med Res. 2016;3(2):77–86. [Google Scholar]
- 35.Habibi N, Huang MS, Gan WY, Zulida R, Safavi SM. Prevalence of primary dysmenorrhea and factors associated with its intensity among undergraduate students: a cross-sectional study. Pain Manag Nurs. 2015;16(6):855–861. doi: 10.1016/j.pmn.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Jensen D, Andersen K, Wagner G. Prostaglandins in the menstrual cycle of women. A review. Dan Med Bull. 1987;34(3):178–182. [PubMed] [Google Scholar]
- 37.Nygren K, Rybo G. Prostaglandins and menorrhagia. Acta Obstetrica Gynecologica Scandinavica. 1983;113:101–103. doi: 10.3109/00016348309155208. [DOI] [PubMed] [Google Scholar]
- 38.Ozerdogan N, Sayiner D, Ayranci U, Unsal A, Giray S. Prevalence and predictors of dysmenorrhea among students at a university in Turkey. Int J Gynecol Obstet. 2009;107(1):39–43. doi: 10.1016/j.ijgo.2009.05.010. [DOI] [PubMed] [Google Scholar]
- 39.Faramarzi M, Salmalian H. Association of Psychologic and Nonpsychologic Factors with Primary Dysmenorrhea. Iran Red Crescent. 2014;16(8):e16307. 10.5812/ircmj.16307. [DOI] [PMC free article] [PubMed]
- 40.Chung FF, Yao CC, Wan GH. The associations between menstrual function and life style/working conditions among nurses in Taiwan. J Occup Health. 2005;47(2):149–156. doi: 10.1539/joh.47.149. [DOI] [PubMed] [Google Scholar]
- 41.Unsal A, Tozun M, Aslan G, Ayranci U, Alkan G. Evaluation of dysmenorrhea among women and its impact on quality of life in a region of western Turkey. Pak J Med Sci. 2010;26(1):142–147. [Google Scholar]
- 42.Pejcic A, Jankovic S. Risk factors for dysmenorrhea among young adult female university students. Ann Ist Super Sanita. 2016;52(1):98–103. doi: 10.4415/ANN_16_01_16. [DOI] [PubMed] [Google Scholar]
- 43.Ribeiro JA, Sebastiao AM. Caffeine and adenosine. J Alzheimers Dis. 2010;20(Suppl 1):S3–15. doi: 10.3233/JAD-2010-1379. [DOI] [PubMed] [Google Scholar]
- 44.Åkerlund M. Pathophysiology of dysmenorrhea. Acta Obstet Gynecol Scand. 1979;58(sup87):27–32. doi: 10.3109/00016347909157786. [DOI] [PubMed] [Google Scholar]
- 45.Blakey H, Chisholm C, Dear F, Harris B, Hartwell R, Daley AJ, Jolly K. Is exercise associated with primary dysmenorrhoea in young women? BJOG Int J Obstet Gynaecol. 2010;117(2):222–224. doi: 10.1111/j.1471-0528.2009.02220.x. [DOI] [PubMed] [Google Scholar]
- 46.Wilson C, Emans J, Mansfield J, Podolsky C, Grace E. The relationships of calculated percent body fat, sports participation, age, and place of residence on menstrual patterns in healthy adolescent girls at an independent new England high school. J Adolesc Health Care. 1984;5(4):248–253. doi: 10.1016/S0197-0070(84)80126-6. [DOI] [PubMed] [Google Scholar]
- 47.Kazama M, Maruyama K, Nakamura K. Prevalence of dysmenorrhea and its correlating lifestyle factors in Japanese female junior high school students. Tohoku J Exp Med. 2015;236:107–113. doi: 10.1620/tjem.236.107. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analyzed during the current study are available from the corresponding author (Abdullah Al-Taiar) on reasonable request.