Abstract
Backgrounds
Since Mesenchymal epithelial transition (MET) amplification has been regarded as a potential treatment target, the knowledge of its prevalence and prognostic importance is crucial. However, its clinical pathologic characteristics are not well known in esophageal squamous cell carcinoma (ESCC).
Methods
We investigated MET gene status with fluorescence in situ hybridization (FISH) assay in 495 ESCC cases using tissue microarrays. Prognostic significance as well as correlations with various clinicopathological parameters was evaluated.
Results
Among 495 patients, 28 (5.7%) cases were MET FISH positive, including 5 cases (1%) with true gene amplification. There were no statistically significant associations between MET FISH-positivity and clinicopathologic characteristics. A significantly poorer prognosis was observed in 28 patients with MET FISH-positivity (disease free survival/DFS, P < 0.001 and overall survival/OS, P = 0.001). Multivariate analysis revealed MET FISH-positivity was an independent prognostic factor for DFS (hazard ratio/HR, 1.953; 95% confidence interval/CI, 1.271–2.999; P = 0.002) and OS (HR, 1.926; 95% CI, 1.243–2.983; P = 0.003). MET FISH-positivity was associated with DFS (P = 0.022 and 0.020) and OS (P = 0.046 and 0.024) both in stage I-II ESCC and in stage III-IVa ESCC. No statistical significance (DFS, P = 0.492 and OS, P = 0.344) was detected between stage I-II ESCC with MET FISH-positivity and stage III-IVa ESCC with FISH-negativity.
Conclusions
Increased MET gene copy number is an independent prognostic factor in ESCC, and ESCC might have potentially been up-staged by increased MET gene copy number. The results indicate that increased MET gene copy number is a very promising parameter, in clinical therapy and follow-up plans.
Keywords: Increased MET gene copy number, Esophageal squamous cell carcinoma (ESCC), Prognosis, Clinical stage, Fluorescence in situ hybridization (FISH)
Background
Esophageal cancer (EC) is the ninth most common cancer and the sixth leading causes of cancer death globally [1]. In China, there were about 477,900 newly diagnosed EC (the third most commonly cancers), and about 375,000 cases dead of EC (the fourth leading causes of cancer death) in 2015 [2]. Esophageal squamous cell carcinoma (ESCC) is the most common histological subtype of EC. In China, approximately 90% of EC are ESCC [3]. Despite the improvement in the traditionally therapeutic management for ESCC, the prognosis of some patients remains dismal [4]. Therefore, the identification of prognostic factors in these patients may be of great importance. Despite Tumor-node-metastasis (TNM) stage is the most important conventional prognostic factor in tumors, evidence is increasing that patients’ prognosis depends not only on tumor stage, but also on the tumor-specific molecular alteration [1]. Recent advancements in molecular biology have made it possible to detect molecular alteration in human tumors, and molecular prognostic markers are subjects of intense research [5–7].
Mesenchymal epithelial transition (MET) gene was first identified in 1984 in an osteosarcoma immortalized cell line [8]. As a proto-oncogene located on chromosome 7q31.2, it encodes a heterodimeric transmembrane receptor with tyrosine kinase activity (RTK) for the hepatocyte growth factor (HGF). MET activation triggers a variety of downstream signaling pathways, such as the PI3K/AKT/mTOR and RAS/ERK/MAPK pathways [9]. Normal MET activation is required for embryogenesis, cell growth, cell differentiation and angiogenesis. Aberrant MET activation has been reported in various types of cancer, and promotes tumor cell proliferation, motility, invasion and metastasis. The abnormally activating mechanism typically involves MET gene amplification, Met and/or HGF protein overexpression, or, rarely, domain-specific sequence mutations [10, 11].
Recent studies found different tumors with MET amplification were extraordinarily susceptible to the selective MET tyrosine kinase inhibitor (TKI) [12–14], and MET amplification was responsible for approximately 20% of the acquired resistance to epidermal growth factor receptor (EGFR) TKI treatment in lung adenocarcinomas [15, 16]. The inspiring findings trigger investigators to explore the prevalence and clinical relevance of MET gene amplification in different tumors. MET gene amplification is identified in 2–5% of gastric cancers [17, 18], 2–4% of esophageal adenocarcinoma (EAC) [5, 12], 1–8% of non-small cell lung cancer (NSCLC) [10, 13, 19], and 2–10% of colorectal cancers [13, 20]. And MET amplification is thought to be associated with metastasis and poorer outcome in gastric [21], lung [22] and colorectal cancers [23]. Despite the great interest on MET amplification, only few small studies evaluated its gene status in ESCC [24].
Therefore, in this study, we aimed to evaluate MET gene copy status in a large cohort of ESCC. In addition, we sought to analyze its clinicopathological features and prognostic value.
Methods
Patients
This retrospective study was conducted in a cohort of 495 treatment-naive ESCC patients who underwent esophagectomy at Zhongshan Hospital between January 2007 and December 2010. Patients were included in the study if the following criterias were met: (1) underwent primary resection, (2) with no prior treatment, and (3) with available complete medical records. Patients were excluded from the study if they had disease progression within three months after surgery. Clinical and histopathological data, including sex, age, smoking status, tumor size, tumor location, differentiation, vessel or nerve invasion, pT stage, and pN stage, was obtained from the patients’ medical and pathological records. The pathologic tumor-node-metastasis (pTNM) stage was performed according to the 8th edition of the American Joint Committee on Cancer (AJCC) staging system. All patients were followed up every 3–6 months after tumor resection, and patients underwent follow-up examinations to identify possible tumor recurrence. Exam methods included endoscopy, computed tomography, magnetic resonance imaging, abdominal ultrasonography, and measurement of serum tumor marker levels.
Written informed consent was obtained from all patients, and the study was approved by the ethical committee of the Zhongshan Hospital, in accordance with the ethical standards of the World Medical Association Declaration of Helsinki.
Tissue microarrays (TMAs)
TMA construction was performed as previously described [25]. Briefly, histological sections were examined by a pathologist, and representative tumor areas free from necrosis or hemorrhage were pre-marked in formalin-fixed paraffin-embedded (FFPE) donor blocks. Two or three core tissues (2 mm in width and 6 mm in length) from different representative areas per case were taken from the donor blocks and arranged in recipient blocks (tissue array blocks). Our TMAs contained the tumor samples, several normal esophagus and other control tissues.
Fluorescence in situ hybridization (FISH)
MET gene status was evaluated using a commercially available FISH assay [26], with Vysis MET Spectrum Red FISH Probe (Abbott Molecular, Chicago, IL, USA) and control Vysis CEP7 Centromere Spectrum Green Probe (Abbott Molecular) on 4 μm-thick TMA sections. The signals of each sample were counted in at least 50 well-defined nuclei using a fluorescence microscope (BX43, Olympus, Tokyo, Japan) equipped with a Microscope Digital Camera (DP73, Olympus, Tokyo, Japan). An average MET gene copy number ≥ 5 and a MET/CEP7 ratio ≥ 2 (true MET amplification) were regarded as MET FISH positive [22].
Statistical analysis
The Chi square and Fisher’s exact tests were used to evaluate the association between MET status and clinicopathological characteristics. The primary and secondary endpoints were cancer-related death and recurrence/metastasis. Disease free survival (DFS) and overall survival (OS) were defined as periods from the date of surgical treatment until the date of disease progression (event: recurrence, metastasis, deaths) and the date of cancer-specific survival (event: cancer-related death), respectively. The Kaplan–Meier analysis with the log-rank test was performed to determine the prognostic significance for DFS and OS. The univariate and multivariate Cox proportional hazard regression analysis was used to identify the independent prognostic factors. The hazard ratio (HR) and its 95% confidence interval (CI) were assessed for each factor.
Statistical analysis was carried out using SPSS 21.0 statistical software (SPSS, Chicago, IL, USA). All tests were two sided, and P-values < 0.05 were considered to be statistically significant.
Results
Clinical data
The patients’ clinicopathological characteristics are summarized in Table 1. The patient group consisted of 408 men (82.4%) and 87 women (17.6%) with a median age of 61 years (range, 34–83 years). One hundred ninety-nine subjects (40.2%) were ever-smokers or smokers, whereas 296 (59.8%) were nonsmokers. The mean tumor size was 3.4 cm. By anatomic site, 47.9% of tumors were located in the lower esophagus, 47.0% in the middle esophagus, and 5.1% in the upper esophagus. The tumors were poorly differentiated in 40.2%, moderately differentiated in 56.0%, and well differentiated in 3.8%. Vessel and nerve invasion were identified in 110 (22.2%) and 178 (36.0%) tumors, respectively. There were 9.3% patients at pathologic stage T1, with 22.2, 68.3, and 0.2% at stages T2, T3, and T4, respectively. About pathologic N stages, there were 53.3, 25.9, 15.8, 5.1% patients at N0, N1, N2, and N3 stages respectively. According to the 8th edition of TNM staging, 38 patients (7.7%) were classified as having stage I disease, 234 patients (47.3%) as stage II, 193 patients (39.0%) as stage III, and 30 patients (6.1%) as stage IVa.
Table 1.
Correlation between MET FISH-positivity and ESCC clinicopathological parameters
| MET FISH-positivity | ||||
|---|---|---|---|---|
| Number | No | Yes | P value | |
| Sex | 0.638 | |||
| Female | 87 | 83 | 4 | |
| Male | 408 | 384 | 24 | |
| Age | 0.932 | |||
| < 60 | 216 | 204 | 12 | |
| ≥60 | 279 | 263 | 16 | |
| Smoking | 0.919 | |||
| No | 296 | 279 | 17 | |
| Yes | 199 | 188 | 11 | |
| Tumor Size | 0.434 | |||
| < 3.4 | 283 | 265 | 18 | |
| ≥3.4 | 212 | 202 | 10 | |
| Tumor Location | 0.941 | |||
| Upper | 24 | 23 | 1 | |
| Middle | 220 | 207 | 13 | |
| Lower | 224 | 211 | 13 | |
| Differentiation | 0.957 | |||
| Well | 19 | 18 | 1 | |
| Middle | 277 | 262 | 15 | |
| Poor | 199 | 187 | 12 | |
| Vessel invasion | 0.194 | |||
| No | 385 | 366 | 19 | |
| Yes | 110 | 101 | 9 | |
| Nerve invasion | 0.706 | |||
| No | 317 | 300 | 17 | |
| Yes | 178 | 167 | 11 | |
| pT | 0.883 | |||
| T1 | 46 | 44 | 2 | |
| T2 | 110 | 105 | 5 | |
| T3 | 338 | 317 | 21 | |
| T4 | 1 | 1 | 0 | |
| pN | 0.088 | |||
| N0 | 264 | 252 | 12 | |
| N1 | 128 | 119 | 9 | |
| N2 | 78 | 75 | 3 | |
| N3 | 25 | 21 | 4 | |
| Clinical stage | 0.351 | |||
| I-II | 272 | 259 | 13 | |
| III-IVa | 223 | 208 | 15 | |
| Disease progression | 0.002 | |||
| No | 226 | 221 | 5 | |
| Yes | 269 | 246 | 23 | |
| Cancer-related death | 0.005 | |||
| No | 234 | 228 | 6 | |
| Yes | 261 | 239 | 22 | |
Increased MET gene copy number
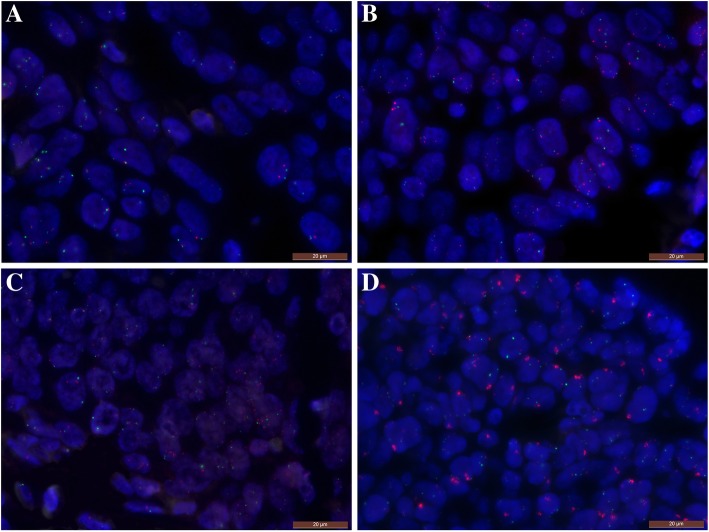
Among 495 patients, 28 (5.7%) cases were MET FISH positive (an average number of MET signals per nucleus ≥5.0), including 5 cases (1%) with true gene amplification (5 cases with MET: CEP7 ratio of ≥2.0) (Fig. 1c and d). Other specimens showed disomy or low polysomy (94.3%) (Fig. 1a and b).
Fig. 1.
Representative microscopic images of MET (red) and CEP7 (green) fluorescence in situ hybridization. A, Normal gene status; B, MET low polysomy; C, MET FISH-positivity (an average number of MET signal per nucleus ≥5.0) and D, MET gene amplification (MET: CEP7 ratio of ≥2.0)
The correlations between MET FISH-positivity and clinical pathologic characteristics are listed in Table 1. MET FISH-positivity was significantly associated with DFS (2.2% in patients without disease progression vs. 8.6% in patients with disease progression, P = 0.002) and OS (2.6% vs. 8.4%, P = 0.005). However, there were no statistically significant difference in sex (P = 0.638), age (P = 0.932), smoking (P = 0.919), tumor size (P = 0.434), tumor location (P = 0.941), differentiation (P = 0.957), vessel invasion (P = 0.194) and nerve invasion (P = 0.706), pT stage (P = 0.883), pN stage (P = 0.088), and clinical stage (P = 0.351).
Survival analysis
The median follow-up time was 35.0 months (range 3–102 months). Two hundred sixty-nine patients (54.3%) had disease progression and two hundred sixty-one patients (52.7%) had died from esophageal cancer during the follow-up. The 5-year DFS and disease-specific OS rates for all patients were 44.1 and 44.4%, respectively.
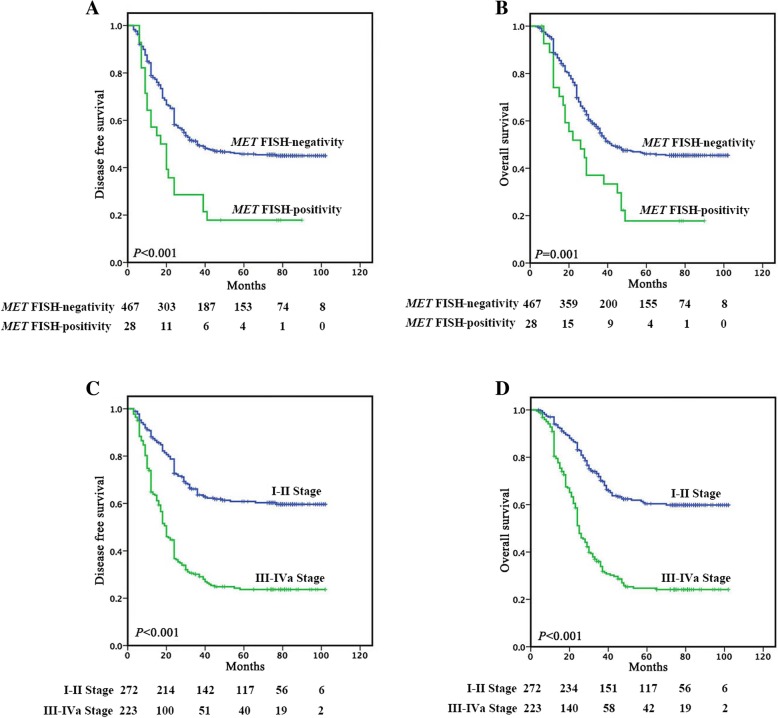
Figure 2a and b reveals that a significantly poorer prognosis was observed in 28 patients with MET FISH-positivity, showing a median DFS or OS of 17.0 or 26.0 months, respectively, compared with 36.0 or 42.0 months in the group with MET FISH-negativity (P < 0.001 or P = 0.001). The 5-year DFS (17.9%) and OS (17.8%) rates for patients with MET FISH-positivity were significantly lower than the corresponding rates (45.7 and 46.0%) for patients with MET FISH-negativity. Univariate analysis indicated that MET FISH-positivity, differentiation, vessel invasion, nerve invasion and clinical stage had significant impacts on DFS, and MET FISH positive, vessel invasion, nerve invasion and clinical stage had significant impacts on OS (both P < 0.05). Multivariate analysis revealed MET FISH-positivity was an independent prognostic factor for DFS (HR, 1.953; 95% CI, 1.271–2.999; P = 0.002) and OS (HR, 1.926; 95% CI, 1.243–2.983; P = 0.003). Clinical stage was also found to be an independent prognostic factor for DFS and OS (Table 2).
Fig. 2.
Kaplan-Meier Survival Analysis for DFS and OS in ESCC according to (A, B) MET FISH-positivity and (C, D) clinical stage, respectively
Table 2.
Univariate and Multivariate Analysis for DFS and OS in ESCC Patients
| DFS | OS | |||
|---|---|---|---|---|
| P value | HR (95% CI) | P value | HR(95% CI) | |
| Univariate analysis | ||||
| Sex | 0.251 | 1.204 (0.877–1.655) | 0.125 | 1.295 (0.931–1.802) |
| Age | 0.994 | 1.001 (0.787–1.273) | 0.982 | 0.997 (0.781–1.273) |
| Smoking | 0.538 | 1.079 (0.846–1.377) | 0.345 | 1.126 (0.880–1.440) |
| Tumor Size | 0.166 | 1.185 (0.932–1.509) | 0.113 | 1.218 (0.954–1.555) |
| Tumor Location | 0.879 | 0.984 (0.799–1.212) | 0.793 | 1.029 (0.831–1.274) |
| Differentiation | 0.047 | 1.246 (1.003–1.549) | 0.080 | 1.217 (0.977–1.518) |
| Vessel invasion | < 0.001 | 1.597 (1.228–2.076) | 0.001 | 1.576 (1.205 2.061) |
| Nerve invasion | 0.02 | 1.335 (1.046–1.703) | 0.008 | 1.401 (1.094–1.793) |
| Clinical stage | < 0.001 | 2.856 (2.230–3.659) | < 0.001 | 2.899 (2.255–3.727) |
| MET FISH-positivity | 0.001 | 2.114 (1.378–3.245) | 0.002 | 2.002 (1.293–3.099) |
| Mutivariate analysis | ||||
| Differentiation | 0.376 | 1.106 (0.885–1.381) | – | – |
| Vessel invasion | 0.425 | 1.119 (0.849–1.474) | 0.455 | 1.113 (0.841–1.472) |
| Nerve invasion | 0.506 | 1.089 (0.848–1.398) | 0.269 | 1.153 (0.896–1.485) |
| Clinical stage | < 0.001 | 2.672 (2.061–3.465) | < 0.001 | 2.745 (2.111–3.569) |
| MET FISH-positivity | 0.002 | 1.953 (1.271–2.999) | 0.003 | 1.926 (1.243–2.983) |
Survival analyses based on clinical stage
In stage I-II patients, one hundred four patients (38.2%) had disease progression and one hundred one patients (37.1%) had died from esophageal cancer during the follow-up. In stage III-IVa patients, one hundred sixty-five patients (74.0%) had disease progression and one hundred sixty patients (71.7%) had died from esophageal cancer during the follow-up.
Figure 2c and d reveals that a significantly poorer prognosis was observed in 223 stage III-IVa patients, showing a median DFS of 20.0 months or OS of 25.0 months, respectively, compared with not-reached median survival in 272 stage I-II patients (P < 0.001). The 5-year DFS (23.7%) and OS (24.7%) rates for stage III-IVa patients, were significantly lower than the corresponding rates (60.8 and 60.4%) for stage I-II patients.
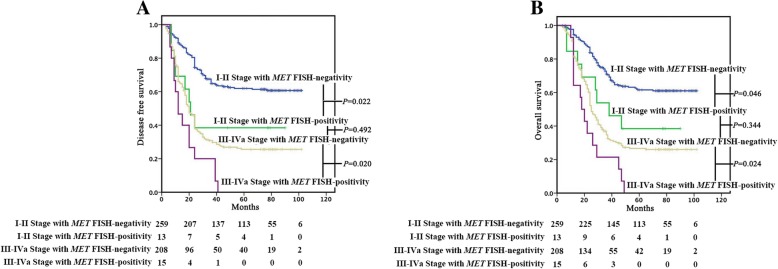
MET FISH-positivity was associated with DFS (P = 0.022) and OS (P = 0.046) in patients with stage I-II ESCC (Fig. 3a and b). In detail, a poorer prognosis was observed in 13 patients with MET FISH-positivity, with a median DFS or OS of 21.0 or 38.0 months, respectively, while those with MET FISH-negativity (n = 259) did not reach the median survival. MET FISH-positivity was also associated with DFS (P = 0.020) and OS (P = 0.024) in patients with stage III-IVa ESCC (n = 223) (Fig. 3a and b). In detail, a poorer prognosis was observed in 15 patients with MET FISH-positivity, with a median DFS or OS of 12.0 or 18.0 months, respectively, while those with MET FISH-negativity (n = 208), with a median DFS or OS of 20.0 or 25.0 months, respectively. What’s more, no statistical significance (DFS, P = 0.492 and OS, P = 0.344) was detected between stage I-II ESCC with MET FISH-positivity and stage III-IVa ESCC with FISH-negativity.
Fig. 3.
Kaplan-Meier Survival Analysis for DFS (A) and OS (B) in ESCC based on clinical stage and MET FISH-positivity
Discussion
In our study, MET gene status was detected in 495 ESCC patients by FISH method. FISH analysis is a semiquantitative method that can be performed with two probes for determination of the number of signals for a target gene and for the centromere of the corresponding chromosome [27]. Comparing with southern blot and PCR-based methods, FISH has several advantages over other methods. It can be applied to FFPE tumor tissues for routine pathologic diagnosis, and is now widely used in clinical practice for the detection of gene amplification [28–30].
Our findings showed MET FISH positive rate was 5.7% and gene amplification rate was 1% using Cappuzzo criteria, which was consistent with the somatic copy number alteration data generated by The Cancer Genome Atlas Research Network [5]. As has been published previously in other tumors [31–33], the rate of MET amplification is relatively low. MET genetic alterations were detected using increasing gene copy number. The increasing gene copy number can result from mainly two genetic mechanisms [34]: 1) polysomy, a copy number gain, due to extra copies of the entire chromosome; and 2) gene amplification, the amplification of specific gene or a group of genes in a given chromosome. In 2009, Cappuzzo et al. found the survival outcome of patients with a mean MET gene copy number per cell higher than 5 and higher than 6 was similar, and worse than the other four groups with a mean copy number lower than 5 in NSCLC [22]. Gradually, the Cappuzzo criteria (MET /CEP7 ratio ≥ 2.0 and/or MET ≥ 5.0 copies) has been widely accepted and used in other tumors, such as NSCLC [10, 35], gastric cancer [21, 36], gastroesophageal adenocarcinoma [17], tonsillar squamous cell carcinoma [37], and mesothelioma [38].
Since Lennerz etal has demonstrated that 2% of patients (10/489) with esophagogastric adenocarcinoma, who harbored MET amplification and were treated with a MET inhibitor, experienced tumor shrinkage in 2011 [12], MET gene status has gained considerable interest in solid tumors [13, 14]. Increased MET gene copy number has an established prognostic role in NSCLC, gastric cancer and gastroesophageal adenocarcinoma patients [17, 21, 39, 40]. However, its clinical pathologic characteristics are not well known in ESCC [24, 41], and to our knowledge, no previous study with a large number of ESCC has been reported. Our data demonstrated that 28 patients with MET FISH-positivity had a significantly worse DFS and OS than 467 individuals with FISH-negativity. Moreover, MET FISH-positivity was an independent prognostic factor for both DFS and OS, further indicating increased MET gene copy number is a negative prognostic factor in ESCC.
Subgroup analyses according to the disease stage were also conducted in our study. Lee et al. reported in gastric cancer, MET amplification did not have an impact on prognosis in early TNM stage (stage I or II), unlike in advanced TNM stage (stage III or IV) [21]. Our results demonstrated MET FISH-positivity has an impact on prognosis both in early TNM stage (stage I-II) and in advanced TNM stage (stage III-IVa). And there was no prognostic difference between stage I-II ESCC with MET FISH-positivity and stage III-IVa ESCC with MET-negativity. The findings indicate that MET gene alteration could be acquired during the early phase of ESCC development, and exaggerated the cancer progression [41].
Conclusions
We investigated MET gene copy status using FISH, in a large series of ESCC. Our data show that increased MET gene copy number is an independent prognostic factor in surgically ESCC, and we firstly find that ESCC might have potentially been up-staged by increased MET gene copy number, which indicates increased MET gene copy number is a very promising parameter, in clinical therapy and follow-up plans.
Acknowledgements
Not applicable.
Funding
This work was financially supported by Shanghai Natural Science Foundation of China (No. 18ZR1406800), National Natural Science Foundation of China (No. 81702372), and Shanghai Municipal Commission of Health and Family Planning, Key-developing disciplines (No. 2015ZB0201). The funding agencies were not involved in in the design of the study and collection, analysis, and interpretation of data and in writing the manuscript.
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AJCC
American Joint Committee on Cancer
- DFS
Disease free survival
- EAC
esophageal adenocarcinoma
- EC
Esophageal cancer
- EGFR
epidermal growth factor receptor
- ESCC
esophageal squamous cell carcinoma
- FFPE
formalin-fixed paraffin-embedded
- FISH
fluorescence in situ hybridization
- HGF
hepatocyte growth factor
- MET
Mesenchymal epithelial transition
- NSCLC
non-small cell lung cancer
- OS
overall survival
- RTK
tyrosine kinase activity
- TKI
tyrosine kinase inhibitor
- TMA
Tissue microarrays
Authors’ contributions
YW and ZJ analyzed and interpreted the patient data regarding the esophageal squamous cell carcinoma and the MET gene copy number. YH, QS, CX, HW, LT, SA, XW and DJ performed the histological examination of all esophageal cancer. YH and QS was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Written informed consent was obtained from all patients, and the study was approved by the ethical committee of the Zhongshan Hospital, in accordance with the ethical standards of the World Medical Association Declaration of Helsinki.
The authors declare no competing financial interests.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yanqiu Wang, Email: wang.yanqiu@zs-hospital.sh.cn.
Zhengzeng Jiang, Email: jiang.zhengzeng@zs-hospital.sh.cn.
Chen Xu, Email: xu.chen@zs-hospital.sh.cn.
Hao Wang, Email: wang.hao@zs-hospital.sh.cn.
Lijie Tan, Email: tan.lijie@zs-hospital.sh.cn.
Jieakesu Su, Email: su.jieakesu@zs-hospital.sh.cn.
Xin Wang, Email: 13211030056@fudan.edu.cn.
Dongxian Jiang, Email: jiangdongxian3@aliyun.com.
Yingyong Hou, Phone: +8602164041990, Email: houyingyong@aliyun.com.
Qi Song, Phone: +8602164041990, Email: qisong725@gmail.com.
References
- 1.Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet. 2017;390:2383–96. [DOI] [PubMed]
- 2.Zheng R, Zeng H, Zhang S, Chen W. Estimates of cancer incidence and mortality in China, 2013. Chin J Cancer. 2017;36:66. doi: 10.1186/s40880-017-0234-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abnet CC, Arnold M, Wei WQ. Epidemiology of Esophageal Squamous Cell Carcinoma. Gastroenterology. 2018;154:360–73. [DOI] [PMC free article] [PubMed]
- 4.Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med. 2014;371:2499–2509. doi: 10.1056/NEJMra1314530. [DOI] [PubMed] [Google Scholar]
- 5.Cancer Genome Atlas Research Network, Analysis Working Group: Asan University, BC Cancer Agency, Brigham and Women’s Hospital, Broad Institute, Brown University, Case Western Reserve University, Dana-Farber Cancer Institute, Duke University, Greater Poland Cancer Centre, et al: Integrated genomic characterization of oesophageal carcinoma. Nature. 2017;541:169–75. [DOI] [PMC free article] [PubMed]
- 6.Deng J, Chen H, Zhou D, Zhang J, Chen Y, Liu Q, Ai D, Zhu H, Chu L, Ren W, et al. Comparative genomic analysis of esophageal squamous cell carcinoma between Asian and Caucasian patient populations. Nat Commun. 2017;8:1533. doi: 10.1038/s41467-017-01730-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin DC, Hao JJ, Nagata Y, Xu L, Shang L, Meng X, Sato Y, Okuno Y, Varela AM, Ding LW, et al. Genomic and molecular characterization of esophageal squamous cell carcinoma. Nat Genet. 2014;46:467–473. doi: 10.1038/ng.2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper CS, Park M, Blair DG, Tainsky MA, Huebner K, Croce CM, Vande Woude GF. Molecular cloning of a new transforming gene from a chemically transformed human cell line. Nature. 1984;311:29–33. doi: 10.1038/311029a0. [DOI] [PubMed] [Google Scholar]
- 9.Perez-Ramirez C, Canadas-Garre M, Jimenez-Varo E, Faus-Dader MJ, Calleja-Hernandez MA. MET: a new promising biomarker in non-small-cell lung carcinoma. Pharmacogenomics. 2015;16:631–647. doi: 10.2217/pgs.15.11. [DOI] [PubMed] [Google Scholar]
- 10.Park S, Koh J, Kim DW, Kim M, Keam B, Kim TM, Jeon YK, Chung DH, Heo DS. MET amplification, protein expression, and mutations in pulmonary adenocarcinoma. Lung Cancer. 2015;90:381–387. doi: 10.1016/j.lungcan.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 11.Yan B, Lim M, Zhou L, Kuick CH, Leong MY, Yong KJ, Aung L, Salto-Tellez M, Chang KT. Identification of MET genomic amplification, protein expression and alternative splice isoforms in neuroblastomas. J Clin Pathol. 2013;66:985–991. doi: 10.1136/jclinpath-2012-201375. [DOI] [PubMed] [Google Scholar]
- 12.Lennerz JK, Kwak EL, Ackerman A, Michael M, Fox SB, Bergethon K, Lauwers GY, Christensen JG, Wilner KD, Haber DA, et al. MET amplification identifies a small and aggressive subgroup of esophagogastric adenocarcinoma with evidence of responsiveness to crizotinib. J Clin Oncol. 2011;29:4803–4810. doi: 10.1200/JCO.2011.35.4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jardim DL, Tang C, Gagliato Dde M, Falchook GS, Hess K, Janku F, Fu S, Wheler JJ, Zinner RG, Naing A, et al. Analysis of 1,115 patients tested for MET amplification and therapy response in the MD Anderson phase I clinic. Clin Cancer Res. 2014;20:6336–6345. doi: 10.1158/1078-0432.CCR-14-1293. [DOI] [PubMed] [Google Scholar]
- 14.Angevin E, Spitaleri G, Rodon J, Dotti K, Isambert N, Salvagni S, Moreno V, Assadourian S, Gomez C, Harnois M, et al. A first-in-human phase I study of SAR125844, a selective MET tyrosine kinase inhibitor, in patients with advanced solid tumours with MET amplification. Eur J Cancer. 2017;87:131–139. doi: 10.1016/j.ejca.2017.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Okuda K, Sasaki H, Yukiue H, Yano M, Fujii Y. Met gene copy number predicts the prognosis for completely resected non-small cell lung cancer. Cancer Sci. 2008;99:2280–2285. doi: 10.1111/j.1349-7006.2008.00916.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Engelman JA, Zejnullahu K, Mitsudomi T, Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen J, et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science. 2007;316:1039–1043. doi: 10.1126/science.1141478. [DOI] [PubMed] [Google Scholar]
- 17.Catenacci DV, Ang A, Liao WL, Shen J, O'Day E, Loberg RD, Cecchi F, Hembrough T, Ruzzo A, Graziano F. MET tyrosine kinase receptor expression and amplification as prognostic biomarkers of survival in gastroesophageal adenocarcinoma. Cancer. 2017;123:1061–1070. doi: 10.1002/cncr.30437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang Y, Wu N, Shen J, Teixido C, Sun X, Lin Z, Qian X, Zou Z, Guan W, Yu L, et al. MET overexpression and amplification define a distinct molecular subgroup for targeted therapies in gastric cancer. Gastric Cancer. 2016;19:778–788. doi: 10.1007/s10120-015-0545-5. [DOI] [PubMed] [Google Scholar]
- 19.Schildhaus HU, Schultheis AM, Ruschoff J, Binot E, Merkelbach-Bruse S, Fassunke J, Schulte W, Ko YD, Schlesinger A, Bos M, et al. MET amplification status in therapy-naive adeno- and squamous cell carcinomas of the lung. Clin Cancer Res. 2015;21:907–915. doi: 10.1158/1078-0432.CCR-14-0450. [DOI] [PubMed] [Google Scholar]
- 20.Di Renzo MF, Olivero M, Giacomini A, Porte H, Chastre E, Mirossay L, Nordlinger B, Bretti S, Bottardi S, Giordano S, et al. Overexpression and amplification of the met/HGF receptor gene during the progression of colorectal cancer. Clin Cancer Res. 1995;1:147–154. [PubMed] [Google Scholar]
- 21.Lee HE, Kim MA, Lee HS, Jung EJ, Yang HK, Lee BL, Bang YJ, Kim WH. MET in gastric carcinomas: comparison between protein expression and gene copy number and impact on clinical outcome. Br J Cancer. 2012;107:325–333. doi: 10.1038/bjc.2012.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cappuzzo F, Marchetti A, Skokan M, Rossi E, Gajapathy S, Felicioni L, Del Grammastro M, Sciarrotta MG, Buttitta F, Incarbone M, et al. Increased MET gene copy number negatively affects survival of surgically resected non-small-cell lung cancer patients. J Clin Oncol. 2009;27:1667–1674. doi: 10.1200/JCO.2008.19.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zeng ZS, Weiser MR, Kuntz E, Chen CT, Khan SA, Forslund A, Nash GM, Gimbel M, Yamaguchi Y, ATt C, et al. c-met gene amplification is associated with advanced stage colorectal cancer and liver metastases. Cancer Lett. 2008;265:258–269. doi: 10.1016/j.canlet.2008.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kato H, Arao T, Matsumoto K, Fujita Y, Kimura H, Hayashi H, Nishiki K, Iwama M, Shiraishi O, Yasuda A, et al. Gene amplification of EGFR, HER2, FGFR2 and MET in esophageal squamous cell carcinoma. Int J Oncol. 2013;42:1151–1158. doi: 10.3892/ijo.2013.1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi Y, He D, Hou Y, Hu Q, Xu C, Liu Y, Jiang D, Su J, Zeng H, Tan Y. An alternative high output tissue microarray technique. Diagn Pathol. 2013;8:9. doi: 10.1186/1746-1596-8-S1-S9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang D, Li X, Wang H, Shi Y, Xu C, Lu S, Huang J, Xu Y, Zeng H, Su J, et al. The prognostic value of EGFR overexpression and amplification in esophageal squamous cell carcinoma. BMC Cancer. 2015;15:377. doi: 10.1186/s12885-015-1393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seres-Santamaria A, Catala V, Cuatrecasas E, Villanueva R. Fluorescent in-situ hybridisation and Down's syndrome. Lancet. 1993;341:1544. doi: 10.1016/0140-6736(93)90690-I. [DOI] [PubMed] [Google Scholar]
- 28.Bhargava R, Dabbs DJ. Interpretation of human epidermal growth factor receptor 2 (HER2) in situ hybridization assays using 2013 update of American Society of Clinical Oncology/College of American Pathologists HER2 guidelines. J Clin Oncol. 2014;32:1855. doi: 10.1200/JCO.2013.53.9213. [DOI] [PubMed] [Google Scholar]
- 29.Donaldson AR, Shetty S, Wang Z, Rivera CL, Portier BP, Budd GT, Downs-Kelly E, Lanigan CP, Calhoun BC. Impact of an alternative chromosome 17 probe and the 2013 American Society of Clinical Oncology and College of American Pathologists guidelines on fluorescence in situ hybridization for the determination of HER2 gene amplification in breast cancer. Cancer. 2017;123:2230–2239. doi: 10.1002/cncr.30592. [DOI] [PubMed] [Google Scholar]
- 30.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 31.Kim WY, Shim SH, Jung HY, Dong M, Kim SN, Lee SJ. The gene copy number of c-MET has a significant impact on progression-free survival in Korean patients with ovarian carcinoma. Hum Pathol. 2017;64:98–105. doi: 10.1016/j.humpath.2017.04.002. [DOI] [PubMed] [Google Scholar]
- 32.Kwak Y, Yun S, Nam SK, Seo AN, Lee KS, Shin E, Oh HK, Kim DW, Kang SB, Kim WH, Lee HS. Comparative analysis of the EGFR, HER2, c-MYC, and MET variations in colorectal cancer determined by three different measures: gene copy number gain, amplification status and the 2013 ASCO/CAP guideline criterion for HER2 testing of breast cancer. J Transl Med. 2017;15:167. doi: 10.1186/s12967-017-1265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li Y, Li W, He Q, Xu Y, Ren X, Tang X, Wen X, Yang X, Sun Y, Zeng J, et al. Prognostic value of MET protein overexpression and gene amplification in locoregionally advanced nasopharyngeal carcinoma. Oncotarget. 2015;6:13309–13319. doi: 10.18632/oncotarget.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Albertson DG. Gene amplification in cancer. Trends Genet. 2006;22:447–455. doi: 10.1016/j.tig.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 35.Casadevall D, Gimeno J, Clave S, Taus A, Pijuan L, Arumi M, Lorenzo M, Menendez S, Canadas I, Albanell J, et al. MET expression and copy number heterogeneity in nonsquamous non-small cell lung cancer (nsNSCLC) Oncotarget. 2015;6:16215–16226. doi: 10.18632/oncotarget.3976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kawakami H, Okamoto I, Arao T, Okamoto W, Matsumoto K, Taniguchi H, Kuwata K, Yamaguchi H, Nishio K, Nakagawa K, Yamada Y. MET amplification as a potential therapeutic target in gastric cancer. Oncotarget. 2013;4:9–17. doi: 10.18632/oncotarget.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kwon MJ, Kim DH, Park HR, Shin HS, Kwon JH, Lee DJ, Kim JH, Cho SJ, Nam ES. Frequent hepatocyte growth factor overexpression and low frequency of c-met gene amplification in human papillomavirus-negative tonsillar squamous cell carcinoma and their prognostic significances. Hum Pathol. 2014;45:1327–1338. doi: 10.1016/j.humpath.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 38.Bois MC, Mansfield AS, Sukov WR, Jenkins SM, Moser JC, Sattler CA, Smith CY, Molina JR, Peikert T. Roden AC: c-met expression and MET amplification in malignant pleural mesothelioma. Ann Diagn Pathol. 2016;23:1–7. doi: 10.1016/j.anndiagpath.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 39.Sterlacci W, Fiegl M, Gugger M, Bubendorf L, Savic S, Tzankov A. MET overexpression and gene amplification: prevalence, clinico-pathological characteristics and prognostic significance in a large cohort of patients with surgically resected NSCLC. Virchows Arch. 2017;471:49–55. doi: 10.1007/s00428-017-2131-1. [DOI] [PubMed] [Google Scholar]
- 40.Landi L, Minuti G, D'Incecco A, Salvini J, Cappuzzo F. MET overexpression and gene amplification in NSCLC: a clinical perspective. Lung Cancer (Auckl) 2013;4:15–25. doi: 10.2147/LCTT.S35168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang H, Jiang D, Song Q, Xu C, Shi Y, Li X, Huang J, Xu Y, Sujie A, Zeng H, et al. Prognostic impact and potential interaction of EGFR and c-met in the progression of esophageal squamous cell carcinoma. Tumour Biol. 2016;37:9771–9779. doi: 10.1007/s13277-015-4692-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.