Abstract
Background
Histidine-rich glycoprotein (HRG) displays anticoagulant and antifibrinolytic properties in animal models, but its effects in humans are unclear. We investigated serum HRG levels and their associations with the disease stage and prothrombotic alterations in lung cancer (LC) patients.
Methods
In 148 patients with advanced LC prior to anticancer therapy (87 non-small-cell LC and 61 small-cell LC) versus 100 well-matched controls, we measured HRG levels in association with clot permeability (Ks), clot turbidimetry (lag phase and maximum absorbance), and clot lysis time (CLT).
Results
Compared to controls, LC patients had 45.9% lower HRG levels with no associations with demographics and comorbidities. Decreased HRG, defined as the 90th percentile of control values (<52.7 μg/ml), was 16 times more common in subjects with than without LC (OR = 16.4, 95% CI 9.2-23.5, p < 0.01). HRG < 38 μg/ml discriminated stage IIIAB/limited disease from IV/extensive disease (ED) LC. In LC patients, HRG correlated inversely with CLT (r = −0.41, p < 0.001), but not with other fibrin variables. Among stage IV/ED LC, HRG correlated significantly with Ks and lag phase (r = 0.28 and r = 0.33, respectively, both p < 0.001). LC patients with low Ks (10th percentile of control values) combined with prolonged CLT (90th percentile of control values) had reduced HRG levels compared to the remainder (p = 0.003). No such observations were noted in controls.
Conclusions
Our study is the first to show that decreased HRG levels occur in advanced LC and are associated with the disease stage and hypofibrinolysis.
1. Introduction
Lung cancer incidence has raised from an infrequent entity in the year 1912 to one of the most commonly occurring cancers over the past century [1]. In 2012, almost 1.82 million new cases have been diagnosed, representing approximately 12.9% of all cancers worldwide [2]. It is estimated to account for almost every fifth death (1.59 million deaths per year, 19.4% of the total) [2]. Despite the introduction of new therapies such as chemotherapy and immunotherapy that prolong the lives of patients and in some cases make cancer a chronic disease, lung cancer remains the most prevalent fatal tumor [2, 3].
Venous thromboembolic disease (VTE) is common in subjects with active cancer with the prevalence of 20% in this disease [4]. Approximately 3% of lung cancer patients experience a VTE episode within two years after diagnosis, which is associated with a higher mortality for both non-small-cell lung carcinoma (NSCLC) and small-cell lung carcinoma (SCLC) [5]. Prothrombotic mechanisms resulting in VTE occurrence among lung cancer patients include enhanced expression and release of tissue factor, higher levels of thrombin-antithrombin complexes and plasmin-alpha 1-antiplasmin complexes, increase in platelet count, and elevated levels of procoagulant factors such as lupus anticoagulant, anticardiolipin antibodies to factor VIII, interleukin-6, and tumor necrosis factors [6].
Plasma fibrin clots are formed as ultimate products of thrombin-mediated conversion of fibrinogen into fibrin. Genetic and environmental factors affect the formation of compact, highly branched networks with thin fibers resistant to fibrinolysis [7, 8]. Fibrin clot properties are unfavorably altered by cigarette smoking [9], a well-established risk factor for nearly 90% cases of lung cancer [10]. Among patients with malignancy, subjects with digestive tract cancer [11] and multiple myeloma [12] were characterized by a prothrombotic clot phenotype. There is evidence for pathological clot microstructure with changes of physical properties, including higher fractal dimension in patients with extensive than those with localized lung cancer [13].
Since its discovery in 1972 [14], histidine-rich glycoprotein (HRG), an abundant plasma protein produced in the liver and circulating at a concentration of 100–150 μg/ml [15], remains poorly characterized in terms of its role in human pathophysiology. Plasma HRG concentrations increase with age but decrease in acute inflammation, chronic autoimmune diseases, advanced liver cirrhosis, after renal transplantation, in asthma and chronic obstructive pulmonary disease treated with corticosteroids, and in acquired immune deficiency syndrome [15, 16]. Reduced HRG levels have been found in patients with ovarian cancer and hepatocellular carcinoma [17, 18]. HRG binds to heparin, which prevents the formation of heparin-antithrombin complexes and results in inhibition of the antithrombin activity [19]. It is estimated that nearly 50% of plasminogen circulates bound to HRG, which reduces plasminogen available to bind fibrin [19, 20]. Moreover, HRG can be incorporated to fibrin clots and its presence causes the formation of thinner fibrils as demonstrated in a purified system [20]. HRG-deficient mice, compared to control, showed higher spontaneous fibrinolytic activity but shorter prothrombin and bleeding time, indicating both prothrombotic and antifibrinolytic properties [21]. A role of HRG in human hemostasis is unclear. There is evidence for an active involvement of HRG in cancer largely by displaying both pro- and antiangiogenic properties [22–25]. Moreover, HRG by promoting tumor-associated macrophage polarization into an antitumor phenotype [24, 25] may result in tumor growth inhibition and it is a possible mechanism of cancer progression. Available data on HRG in cancer patients is inconsistent [26–28]. To our knowledge, no reports on HRG in lung cancer and its role in prothrombotic alterations have been published until now. Based on the available data, we hypothesized that HRG levels are reduced in advanced lung cancer in association with the disease severity and they contribute to unfavorable fibrin clot properties.
2. Materials and Methods
2.1. Patients
From May to September 2014, 148 consecutive adult patients with advanced lung cancer prior to anticancer treatment were recruited at the John Paul II Hospital in Cracow, Poland. This cohort was part of the study population reported previously in detail [29]. Briefly, lung cancer was confirmed and classified with the applicable histological classification of lung cancer according to the World Health Organization with the International Association for the Study of Lung Cancer modification for adenocarcinoma. Cancer stage was determined according to the American Joint Committee on Cancer 7th edition staging scheme. Controls (n = 100) matched for age, sex, and cardiovascular risk were recruited using the same screening protocol and included members of the hospital personnel and their relatives. The exclusion criteria for both groups were as follows: any infections, glomerular filtration rate < 60 ml/min, hypo- or hyperthyroidism, any acute vascular events, acute venous thromboembolism, and current anticoagulant therapy except for prophylactic doses of low-molecular-weight heparin administered for the last time 12 h or more prior to the sample collection.
The research has been complied with all the relevant national regulations and institutional policies and in accordance with the tenets of the Helsinki Declaration and has been approved by the local ethical committee. A written informed consent was obtained from each participant.
2.2. Laboratory Investigations
Fasting blood samples were drawn from an antecubital vein with minimal stasis between 8:00 and 11:00 AM. Routine laboratory techniques were applied to determine blood cell count, creatinine, and D-dimer. Fibrinogen was determined using the Clauss method. Plasminogen was measured by a chromogenic assay (STA Stachrom; Diagnostica Stago, Asnieres, France). HRG was measured using an ELISA according to the manufacturer's instruction (Cusabio Technologies, US).
2.3. Plasma Fibrin Clot Analysis
In citrated plasma (vol/vol, 9 : 1 of 3.2% sodium citrate), the following variables describing a plasma clot formation, structure, and lysability were determined in duplicate by technicians blinded to the origin of the samples (intra-assay and interassay coefficients of variation, 5% to 7%). Details of the methodology used were presented previously [30].
2.3.1. Clot Permeability
Permeation of plasma fibrin clots was determined as described [31]. Briefly, 20 mM calcium chloride and 1 U/ml human thrombin (Sigma-Aldrich, St. Louis, MO, USA) were added to citrated plasma. Tubes containing the clots were connected to a reservoir of a Tris-buffered saline (TBS; 0.01 M Tris, 0.1 M NaCl, pH 7.4), and its volume flowing through the gels was measured. A permeation coefficient (Ks), which indicates the pore size, was calculated from the following equation: Ks = Q × L × η/t × A × Δp, where Q is the flow rate in time t, L is the length of a fibrin gel, η is the viscosity of liquid (in poise), t is percolating time, A is the cross-sectional area (in cm2), and Δp is a differential pressure (in dyne/cm2).
2.3.2. Turbidity Measurements
Plasma citrated samples were mixed at a ratio of 2 : 1 with a TBS containing 0.6 U/ml human thrombin (Sigma-Aldrich) and 50 mM CaCl2 to initiate polymerization. Absorbance was read at 405 nm with a Perkin-Elmer Lambda 4B spectrophotometer (Molecular Devices). The lag phase of the turbidity curve, which reflects the time required for initial protofibril formation, and maximum absorbance at the plateau phase (ΔAbs), indicating the number of protofibrils per fiber, were recorded [31].
2.3.3. Clot Lysis Assay
Clot lysis time (CLT) was measured as described previously [31]. Briefly, to 75 μl of citrated plasma we added TF (dilution 105 times; Innovin, Dade Behring, Deerfield, IL, USA), CaCl2 (final concentration, 17 mM), tissue plasminogen activator (tPA, final concentration, 30 U/ml; Boehringer Ingelheim, Ingelheim, Germany), and phospholipid vesicles [32] (final concentration, 10 mM). HEPES buffer was added to make a total volume of 150 μl.
2.4. Statistical Analysis
Data management was performed with STATISTICA 10.0 software (StatSoft Inc. 2011, Tulsa, OK, USA). Normal distribution for continuous variables was checked using the Shapiro-Wilk statistics. Continuous variables were presented as mean ± SD for normally distributed variables and as a median and interquartile range for nonnormally distributed variables. Proportional variables were compared by χ2 test and Student's t-test while continuous variables by Mann-Whitney U-test (Kruskal-Wallis and multiple repetition tests), as appropriate. Pearson's rank correlation coefficient was used to evaluate an association between two variables. To test the relationship between continuous variables, a univariate linear regression model was used. Because HRG had a skewed normal distribution, a 10 log-transformed data was entered for a better fit the model. Other variables were calculated on the original scale. Independent determinants of HRG were established in a multiple regression model, built by a forward stepwise selection procedure, verified by Snedecore's F statistics, with F > 1. The R2 was used as a measure of the variance. Log-linear analysis and unconditional multivariate logistic regression were performed to make adjustments for categorical variables (age, body mass index, and gender) [30]. To calculate odds ratios (ORs) with 95% CIs, Ks, CLT, and HRG values were divided using the value of 90th percentile in controls as a cut-off point. The best cut-off value that maximizes sensitivity and specificity and differentiates lung cancer patients from control subjects was calculated by using the receiver operating characteristic (ROC) curve. The significance level was set at p < 0.05.
3. Results
3.1. Patient Characteristics
A final analysis included 148 lung cancer patients and 100 matched control subjects (Table 1). There were 58 (39.19%) subjects diagnosed with SCLC, including 33 patients with limited disease and 25 patients with extensive disease, and 90 (60.81%) subjects with NSCLC (among them 24 with IIIAB stage/locally advanced inoperative neoplasm and 66 with IV stage/metastatic disease). As expected the lung cancer patients had lower BMI and were more often smokers (79 vs. 10; Table 1). They also had higher white blood cells (WBC), lower hemoglobin, and higher platelet count (Table 1). The cancer group had elevated D-dimer and fibrinogen. Among fibrin clot variables, lowered Ks (-26.52%), prolonged lag phase (+8.24%), increased ΔAbs (+6.25%), and longer CLT (+31.13%) were detected in lung cancer patients. The study groups did not differ in plasminogen levels.
Table 1.
Demographic, clinical, and laboratory characteristics of lung cancer patients and control subjects.
| Variable | Lung cancer patients (n = 148) | Control subjects (n = 100) | p value |
|---|---|---|---|
| Age (years) | 65 (46-82) | 63 (43-81) | 0.49 |
| Male, n (%) | 30 (20.3) | 20 (20.0) | 0.08 |
| Body mass index (kg/m2) | 25.0 (14.6-40.6) | 26.9 (19.9-36.2) | <0.0001 |
| Clinical features | |||
| Current smoking, n (%) | 53 (35.8) | 10 (10.0) | <0.0001 |
| Arterial hypertension, n (%) | 48 (32.4) | 55 (55.0) | 0.3 |
| Diabetes mellitus, n (%) | 12 (8.1) | 7 (7.0) | 0.19 |
| Previous stroke, n (%) | 4 (2.7) | 8 (8.0) | 0.09 |
| Medications | |||
| Statin, n (%) | 26 (17.6) | 42 (42.0) | 0.004 |
| Aspirin, n (%) | 25 (16.9) | 38 (38.0) | 0.005 |
| Laboratory investigations | |||
| WBC (103/μl) | 9.2 (8.4-10.0) | 6.0 (5.8-6.2) | <0.0001 |
| Hemoglobin (g/dl) | 12.8 (12.6-13.0) | 13.9 (13.7-14.1) | <0.0001 |
| Hematocrit (%) | 39.2 (38.7-39.7) | 41.3 (40.9-41.8) | <0.0001 |
| Platelets (103/μl) | 309 (298-320) | 237 (229-245) | <0.0001 |
| Creatinine (μM) | 74 (72-76) | 68 (66-69) | 0.028 |
| D-dimer (ng/ml) | 477 (402-552) | 225 (217-233) | <0.0001 |
| Fibrinogen (g/l) | 3.16 (3.10-3.25) | 2.47 (2.24-3.10) | <0.0001 |
| Plasminogen (%) | 102.8 (101.0-104.6) | 104.8 (102.9-106.6) | 0.79 |
| HRG (μg/ml) | 37.0 (36.2-37.9) | 68.4 (66.5-70.2) | <0.0001 |
| Fibrin clot characteristics | |||
| K s (10−9 cm2) | 6.65 (6.54-6.76) | 9.05 (8.84-9.26) | <0.0001 |
| Lag phase (s) | 39.0 (38.6-39.4) | 42.5 (40-46) | <0.0001 |
| ΔAbs (405 nm) | 0.85 (0.84-0.86) | 0.80 (0.79-0.81) | <0.0001 |
| Clot lysis time (min) | 99.0 (97.2-100.8) | 75.5 (73.7-77.3) | <0.0001 |
Data are given as number (percentage), mean ± SD, or median (interquartile range). Abbreviations: WBC: white blood cells; HRG: histidine-rich glycoprotein; Ks: fibrin clot permeability coefficient.
3.2. HRG Serum Level Associations
Lung cancer patients had 45.87% lower HRG level compared to control subjects (Figure 1). Serum HRG showed no associations with demographic variables. Comparing histological subtypes of LC in the studied cohort, we observed that patients diagnosed with adenocarcinoma (n = 41) had lowered HRG (median, 32 (28-40) μg/ml vs. 39 (32-44) μg/ml, p = 0.002). Reduced HRG levels were detected in patients with metastases (n = 101, 33.8 ± 7.6 vs. 41.4 ± 7.8 μg/ml, p = 0.044). Metastatic cancer was also associated with prolonged CLT (>105 min, OR = 2.09, 95% CI (1.18-3.73), p = 0.01), but not with other fibrin variables.
Figure 1.

Distribution of histidine-rich glycoprotein (HRG) serum levels in controls and lung cancer patients with stage IV/extensive disease and locally advanced inoperative stage IIIAB/limited disease. Data are presented as median, interquartile range, and maximum and minimum values. Numbers on the graph represent p values in comparison to control and between lung cancer patient groups. LD: limited disease; ED: extensive disease.
In the lung cancer group, HRG was inversely correlated with D-dimer (r = −0.37, p < 0.001) and fibrinogen (r = −0.18, p < 0.001), while it was positively correlated with lag phase (r = 0.20, p < 0.001). Ks and ΔAbs showed no associations with HRG in cancer patients, however CLT was inversely correlated with HRG (r = −0.38, p < 0.001). In the stage IIIAB/LD lung cancer patients, lowered HRG was associated with longer CLT (r = −0.41, p < 0.001), while in more advanced stage IV/ED, reduced HRG was associated with higher D-dimer (r = −0.35, p < 0.001), shorter lag phase (r = 0.33, p < 0.001), decreased Ks (r = 0.28, p < 0.001), and prolonged CLT (r = −0.5, p < 0.001). No such correlations were detected for HRG and fibrinogen or plasminogen.
Low HRG levels defined using the 90th percentile of control values (i.e., 57.2 μg/ml) observed in 143 (94.08%) lung cancer patients were detected in all patients diagnosed with stage IV/ED lung cancer. Metastatic stage IV/ED lung cancer patients had significantly decreased HRG compared to locally advanced lung cancer (stage IIIAB/LD) subjects (p < 0.0001, Figure 1). HRG cut-off levels which discriminate lung cancer patients from healthy subjects and stage IIIAB/LD from stage IV/ED lung cancer were below 49.2 μg/ml (sensitivity, 98% and specificity, 90%) and below 38 μg/ml (sensitivity, 80% and specificity, 70%) (Figure 2), respectively. HRG below 38 μg/ml was associated with stage IV/ED lung cancer (OR = 3.07, 95% CI 2.08-4.54, p < 0.01).
Figure 2.
Receiver operating characteristic (ROC) curve for serum levels of histidine-rich glycoprotein (HRG) to discriminate between lung cancer patients and healthy subjects (a) and between IIIAB/limited disease and IV/extensive disease lung cancer (b). The blue line represents serum HRG level and the red line represents the reference line. The best cut-off point of HRG at 49.2 μg/ml (a) and 38 μg/ml (b) is marked, P < 0.0001.
A multiple logistic regression analysis showed associations between HRG levels and Ks as well as CLT in lung cancer patients (Table 2), also after adjustment for aspirin use (data not shown). In the combined analysis, compact fibrin clots (1st quartile of control values of Ks) and hypofibrinolysis (4th quartile of control values of CLT) were more often found in subjects with lung cancer (OR = 3.08, 95% CI 2.29-4.14 and OR = 2.95, 95% CI 2.2-3.95, respectively). Among patients who had HRG below 52.7 μg/ml, 62.24% of them formed dense and poorly lysable fibrin clots (Figure 3).
Table 2.
Determinants of histidine-rich glycoprotein serum levels in lung cancer patients.
| Simple regression | Multiple regression | ||||
|---|---|---|---|---|---|
| β | p | β | p | R 2 | |
| Lung cancer patients | |||||
| K s | 0.09 | 0.27 | -0.13 | <0.001 | 0.19 |
| CLT | -0.41 | <0.001 | -0.42 | ||
| Fibrinogen | -0.18 | 0.03 | -0.15 | ||
| Stage IIIAB/LD lung cancer patients | |||||
| K s | -0.09 | 0.71 | -0.09 | 0.014 | 0.17 |
| CLT | -0.41 | 0.001 | -0.44 | ||
| Fibrinogen | -0.09 | 0.48 | -0.00 | ||
| Stage IV/ED lung cancer patients | |||||
| K s | 0.28 | 0.008 | 0.07 | <0.001 | 0.26 |
| CLT | -0.5 | <0.001 | -0.46 | ||
| Fibrinogen | -0.18 | 0.09 | -0.06 | ||
K s: fibrin clot permeability coefficient; CLT: clot lysis time; LD: limited disease; ED: extensive disease.
Figure 3.
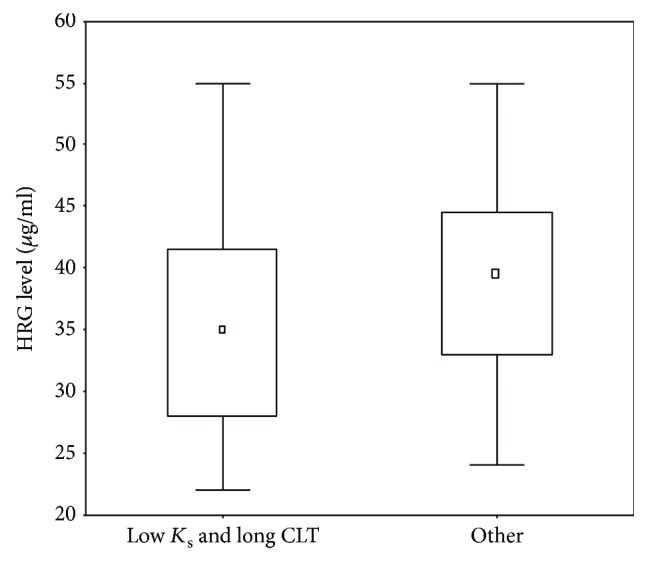
Distribution of histidine-rich glycoprotein (HRG) levels among lung cancer patients who formed compact fibrin clots (defined as the low value of fibrin clot permeability coefficient, 1st quartile in the control subjects) and clots resistant to lysis (defined as long clot lysis time, 4th quartile in the control group) compared to other subjects. Data are presented as median, interquartile range, and maximum/minimum values. p = 0.003. Ks: fibrin clot permeability coefficient; CLT: clot lysis time.
4. Discussion
The present study is the first to show associations between unfavorably altered fibrin clot properties and serum HRG level in advanced lung cancer patients. Moreover, we observed lower HRG levels in this patient group in association with more advanced cancer stage. Lower HRG was linked to reduced clot permeability and impaired susceptibility to lysis, which might contribute to thromboembolic events in cancer patients [33]. The study increases the current knowledge on the role of HRG in lung cancer by showing that the lower the HRG, the more advanced the disease stage and the more altered the clot features. Existing evidence indicates that HRG represents an adaptor protein with the potential to modulate immune, vascular, and coagulation systems, which are involved in cancer development. In the tumor environment, HRG promotes tumor-associated macrophage polarization into an antitumor (M1) phenotype [24, 25]. The presence of HRG normalizes tumor vasculature, allowing cytotoxic T-lymphocytes, natural killers, and dendritic cells to infiltrate into its microenvironment [24, 25], resulting in tumor growth inhibition. HRG released from platelets have been shown to promote angiogenesis in cancer by interfering with thrombospondin 1 and thrombospondin receptor CD36-mediated antiangiogenic signaling [22, 23]. Other studies have postulated an antiangiogenic activity of HRG. In mice, HRG deficiency was associated with reduced expression of antiangiogenic chemokines such as chemokine C-X-C motif ligand 10 and chemokine C-X-C motif ligand 11 and also decreased vessel coverage and perfusions [24]. In HRG-treated mice, tumor vascularization was inhibited [25]. Proteomics study using isobaric tagging and LC-MS/MS analysis revealed a significant reduction in serum HRG associated with progression of hepatitis B virus-related hepatocellular carcinoma [18]. Wang et al. observed downregulation of serum HRG precursor by LC-MS/MS in patients with atypical endometrial hyperplasia compared to normal donor controls, indicating that lower HRG levels lead to endometrial cancer progression [27]. Wu et al. [17] assessed ovarian cancer patients and showed decreased HRG levels in patients with stage I/II as well as with stage III ovarian cancer compared to healthy controls [17]. HRG levels may also be increased in some malignancies as reported by Matboli et al. who observed higher HRG levels in breast cancer (median, 17.1 mg/dl) compared to benign breast lesions (median, 1.56 mg/dl) and the control (median, 1.1 mg/dl) group [26]. Ahmed et al. [28] have observed in acute lymphoblastic leukemia that both HRG serum levels and protein RNA expression decreased during the leukemia treatment. Taken together, the present study shows that advanced lung cancer is associated with decreased HRG levels, which appears to be a common feature in most advanced cancers. A novel observation is that prothrombotic alterations are related to this decrease.
This study has several limitations. First, the sample size was limited and HRG levels were measured at a single time point. Second, we did not measure several potential fibrin clot modifiers including homocysteine, which were beyond the scope of the study. Third, the correlations demonstrated in the present study may not necessarily mean cause-effect relationships. Fourth, the current data cannot be necessarily extrapolated to patients with stages I and II lung cancer. Finally, in vitro experiments to better characterize the function of HRG in lung cancer patients, including HRG binding to heparin and fibrin clots [34–36], should be performed in the future. It is also worth investigating whether HRG levels and fibrin properties in lung cancer patients are associated with inflammation and cellular senescence [37].
5. Conclusions
The current study shows that decreased HRG levels occur in advanced lung cancer and are associated with the disease stage and prothrombotic plasma clot characteristics, including faster formation of more compact clots and impaired susceptibility to lysis. Clinical relevance of these findings needs further investigation.
Acknowledgments
This work was supported by a grant from Jagiellonian University Medical College (K/ZDS/007717 to A.U.).
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- 1.Spiro S. G., Silvestri G. A. One hundred years of lung cancer. American Journal of Respiratory and Critical Care Medicine. 2005;172(5):523–529. doi: 10.1164/rccm.200504-531OE. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J., Soerjomataram I., Dikshit R., et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. International Journal of Cancer. 2014;135(5):E359–E386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 3.Mao Y., Yang D., He J., Krasna M. J. Epidemiology of lung cancer. Surgical Oncology Clinics of North America. 2016;25(3):439–445. doi: 10.1016/j.soc.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Windyga J. Thromboembolic complications in neoplastic disease - management recommendations of the Institute of Hematology and Transfusion Medicine. Hematologia. 2013;4:56–64. [Google Scholar]
- 5.Chew H. K., Davies A. M., Wun T., Harvey D., Zhou H., White R. H. The incidence of venous thromboembolism among patients with primary lung cancer. Journal of Thrombosis and Haemostasis. 2008;6(4):601–608. doi: 10.1111/j.1538-7836.2008.02908.x. [DOI] [PubMed] [Google Scholar]
- 6.Vitale C., D’Amato M., Calabrò P., Stanziola A. A., Mormile M., Molino A. Venous thromboembolism and lung cancer: a review. Multidisciplinary Respiratory Medicine. 2015;10(1):p. 28. doi: 10.1186/s40248-015-0021-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Undas A., Ariens R. A. S. Fibrin clot structure and function: a role in the pathophysiology of arterial and venous thromboembolic diseases. Arteriosclerosis, Thrombosis, and Vascular Biology. 2011;31(12):e88–e99. doi: 10.1161/ATVBAHA.111.230631. [DOI] [PubMed] [Google Scholar]
- 8.Undas A. Fibrin clot properties and their modulation in thrombotic disorders. Thrombosis and Haemostasis. 2014;112(1):32–42. doi: 10.1160/TH14-01-0032. [DOI] [PubMed] [Google Scholar]
- 9.Barua R. S., Sy F., Srikanth S., et al. Acute cigarette smoke exposure reduces clot lysis – association between altered fibrin architecture and the response to t-PA. Thrombosis Research. 2010;126(5):426–430. doi: 10.1016/j.thromres.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 10.Biesalski H. K., de Mesquita B. B., Chesson A., et al. European consensus statement on lung cancer: risk factors and prevention. Lung cancer panel. CA: a Cancer Journal for Clinicians. 1998;48(3):167–176. doi: 10.3322/canjclin.48.3.167. [DOI] [PubMed] [Google Scholar]
- 11.Gronostaj K., Richter P., Nowak W., Undas A. Altered plasma fibrin clot properties in patients with digestive tract cancers: links with the increased thrombin generation. Thrombosis Research. 2013;131(3):262–267. doi: 10.1016/j.thromres.2012.11.033. [DOI] [PubMed] [Google Scholar]
- 12.Undas A., Zubkiewicz-Usnarska L., Helbig G., et al. Altered plasma fibrin clot properties and fibrinolysis in patients with multiple myeloma. European Journal of Clinical Investigation. 2014;44(6):557–566. doi: 10.1111/eci.12269. [DOI] [PubMed] [Google Scholar]
- 13.Davies N. A., Harrison N. K., Keith Morris R. H., et al. Fractal dimension (df) as a new structural biomarker of clot microstructure in different stages of lung cancer. Thrombosis and Haemostasis. 2017;114(12):1251–1259. doi: 10.1160/TH15-04-0357. [DOI] [PubMed] [Google Scholar]
- 14.Haupt H., Heimburger N., Kranz T., Baudner S. Humanserumproteine mit hoher affinität zu carboxymethyl- cellulose, III. Physikalisch-chemische und immunologische charakterisierung eines metallbindenden 9,5S-αi-glykoproteins (CM-protein III) Hoppe-Seyler's Zeitschrift für Physiologische Chemie. 1972;353(2):1841–1849. doi: 10.1515/bchm2.1972.353.2.1841. [DOI] [PubMed] [Google Scholar]
- 15.Poon I. K. H., Patel K. K., Davis D. S., Parish C. R., Hulett M. D. Histidine-rich glycoprotein: the Swiss Army knife of mammalian plasma. Blood. 2011;117(7):2093–2101. doi: 10.1182/blood-2010-09-303842. [DOI] [PubMed] [Google Scholar]
- 16.Morgan W. T. Serum histidine-rich glycoprotein levels are decreased in acquired immune deficiency syndrome and by steroid therapy. Biochemical Medicine and Metabolic Biology. 1986;36(2):210–213. doi: 10.1016/0885-4505(86)90127-1. [DOI] [PubMed] [Google Scholar]
- 17.Wu J., Xie X., Liu Y., et al. Identification and confirmation of differentially expressed fucosylated glycoproteins in the serum of ovarian cancer patients using a lectin array and LC-MS/MS. Journal of Proteome Research. 2012;11(9):4541–4552. doi: 10.1021/pr300330z. [DOI] [PubMed] [Google Scholar]
- 18.He X., Wang Y., Zhang W., et al. Screening differential expression of serum proteins in AFP-negative HBV-related hepatocellular carcinoma using iTRAQ -MALDI-MS/MS. Neoplasma. 2014;61(1):17–26. doi: 10.4149/neo_2014_001. [DOI] [PubMed] [Google Scholar]
- 19.Johnson L. D., Goubran H. A., Kotb R. R. Histidine rich glycoprotein and cancer: a multi-faceted relationship. Anticancer Research. 2014;34(2):593–603. [PubMed] [Google Scholar]
- 20.Jones A. L., Hulett M. D., Parish C. R. Histidine-rich glycoprotein: a novel adaptor protein in plasma that modulates the immune, vascular and coagulation systems. Immunology and Cell Biology. 2005;83(2):106–118. doi: 10.1111/j.1440-1711.2005.01320.x. [DOI] [PubMed] [Google Scholar]
- 21.Tsuchida-Straeten N., Ensslen S., Schafer C., et al. Enhanced blood coagulation and fibrinolysis in mice lacking histidine-rich glycoprotein (HRG) Journal of Thrombosis and Haemostasis. 2005;3(5):865–872. doi: 10.1111/j.1538-7836.2005.01238.x. [DOI] [PubMed] [Google Scholar]
- 22.Hale J. S., Li M., Sinyuk M., Jahnen-Dechent W., Lathia J. D., Silverstein R. L. Context dependent role of the CD36 - thrombospondin - histidine-rich glycoprotein axis in tumor angiogenesis and growth. PLoS One. 2012;7(7, article e40033) doi: 10.1371/journal.pone.0040033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klenotic P. A., Huang P., Palomo J., et al. The American Journal of Pathology. 2010;176(4):2039–2050. doi: 10.2353/ajpath.2010.090782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tugues S., Honjo S., Konig C., et al. Genetic deficiency in plasma protein HRG enhances tumor growth and metastasis by exacerbating immune escape and vessel abnormalization. Cancer Research. 2012;72(8):1953–1963. doi: 10.1158/0008-5472.CAN-11-2194. [DOI] [PubMed] [Google Scholar]
- 25.Rolny C., Mazzone M., Tugues S., et al. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through down-regulation of PlGF. Cancer Cell. 2011;19(1):31–44. doi: 10.1016/j.ccr.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 26.Matboli M., Eissa S., Said H. Evaluation of histidine-rich glycoprotein tissue RNA and serum protein as novel markers for breast cancer. Medical Oncology. 2014;31(4):p. 897. doi: 10.1007/s12032-014-0897-4. [DOI] [PubMed] [Google Scholar]
- 27.Wang Y., Cao R., Jin H., et al. Altered protein expression in serum from endometrial hyperplasia and carcinoma patients. Journal of Hematology & Oncology. 2011;4(1):p. 15. doi: 10.1186/1756-8722-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahmed M. B., Almogbel E., Khirry I., Hassan S., Salem T., Saeed A. Diagnostic and prognostic significance of histidine-rich glycoprotein in acute lymphoblastic leukemia. Open Journal of Blood Diseases. 2017;7(1):16–28. doi: 10.4236/ojbd.2017.71002. [DOI] [Google Scholar]
- 29.Królczyk G., Ząbczyk M., Czyżewicz G., et al. Altered fibrin clot properties in advanced lung cancer: impact of chemotherapy. Journal of Thoracic Disease. 2018;10(12):6863–6872. doi: 10.21037/jtd.2018.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bazan-Socha S., Mastalerz L., Cybulska A., et al. Asthma is associated with enhanced thrombin formation and impaired fibrinolysis. Clinical and Experimental Allergy. 2016;46(7):932–944. doi: 10.1111/cea.12734. [DOI] [PubMed] [Google Scholar]
- 31.Undas A., Zawilska K., Ciesla-Dul M., et al. Altered fibrin clot structure/function in patients with idiopathic venous thromboembolism and in their relatives. Blood. 2009;114(19):4272–4278. doi: 10.1182/blood-2009-05-222380. [DOI] [PubMed] [Google Scholar]
- 32.Lisman T., de Groot P. G., Meijers J. C., Rosendaal F. R. Reduced plasma fibrinolytic potential is a risk factor for venous thrombosis. Blood. 2005;105(3):1102–1105. doi: 10.1182/blood-2004-08-3253. [DOI] [PubMed] [Google Scholar]
- 33.Ząbczyk M., Undas A. Plasma fibrin clot structure and thromboembolism: clinical implications. Polish Archives of Internal Medicine. 2017;127(12):873–881. doi: 10.20452/pamw.4165. [DOI] [PubMed] [Google Scholar]
- 34.Lijnen H. R., Hoylaerts M., Collen D. Heparin binding properties of human histidine-rich glycoprotein. Mechanism and role in the neutralization of heparin in plasma. The Journal of Biological Chemistry. 1983;258(6):3803–3808. [PubMed] [Google Scholar]
- 35.Leung L. L. Interaction of histidine-rich glycoprotein with fibrinogen and fibrin. The Journal of Clinical Investigation. 1986;77(4):1305–1311. doi: 10.1172/JCI112435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stachowicz A., Zabczyk M., Natorska J., et al. Differences in plasma fibrin clot composition in patients with thrombotic antiphospholipid syndrome compared with venous thromboembolism. Scientific Reports. 2018;8(1, article 17301) doi: 10.1038/s41598-018-35034-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kuźnar-Kamińska B., Mikuła-Pietrasik J., Książek K., Tykarski A., Batura-Gabryel H. Lung cancer in chronic obstructive pulmonary disease patients: importance of cellular senescence. Polish Archives of Internal Medicine. 2018;128(7-8):462–468. doi: 10.20452/pamw.4297. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data used to support the findings of this study are available from the corresponding author upon request.



