Abstract
Nanotechnology has become a novel subject with impact in many research and technology areas. Nanoparticles (NPs), as a key component in nanotechnology, are widely used in many areas such as optical, magnetic, electrical, and mechanical engineering. The biomedical and pharmaceutical industries have embraced NPs as a viable drug delivery modality. As such, the potential for NP-induced cytotoxicity has emerged as a major concern for NP drug delivery systems. Thus, it is important to understand how NPs affect the innate immune system. As the most abundant myeloid cell type in innate immune responses, neutrophils are critical for concerns about potentially toxic side effects of NPs. When activated by innate immune stimuli, neutrophils may initiate NETosis to release neutrophil extracellular traps (NETs). Herein, we have reviewed the relationship between NPs and the induction of NETosis and release of NETs.
1. Introduction
Nanotechnology has emerged as one of the most exciting industrial innovations worldwide [1, 2] in diverse areas of structural and material design and device and systems engineering. Nanoparticles (NPs) are defined as structures whose sizes are within the range from 1 to 100 nm in one, two, or three dimensions while nanomaterials are a group of small-scale substances which are applied to carry out their distinct properties in many kinds of fields, including but not limited to optical, magnetic, mechanical, and electrical engineering [3–5]. NPs also have the unique biological characteristic of high surface-to-volume ratio and small size. Due to their unique structural and size properties, they can easily penetrate molecular, cellular, and extracellular matrix barriers to reach most body systems. While NPs can be easily taken up by cells, they may also bind to cell surface proteins, initiate signaling, activate or inactivate the relevant cells, and in some cases cause unexpected cellular interactions [6, 7]. At present, environmental exposure and deliberate administration are two approaches by which NPs may be introduced. As the potential for NP exposure from inhalation, ingestion, and direct skin contact has increased [8, 9], nanotoxicology has emerged as a new type of toxicology to evaluate the safety of nanostructures and nanodevices [10].
The innate immune system is the first line of immune defense for mammalian and other eukaryotic hosts including mice and humans. Innate immunity includes both soluble proteins such as secreted cytokines and acute-phase and complement system proteins [11–14] and cells from the myeloid, lymphoid, and mast cell lineages [13–21]. The myeloid cells include granulocytes (neutrophils, basophils, and eosinophils), monocytes, macrophages, and dendritic cells [16–18]. Innate lymphoid cells (ILC) [19], natural killer cells (NK) [20], and to some extent γδ T cells [21] are the lymphoid representatives to innate immunity. Mast cells, although similar in many respects to granulocytes, are a distinct lineage of innate immune cells [15]. Cells from all of these cell lineages are the main effector cells in innate immune responses [22] to both pathogenic and nonpathogenic challenges through pattern recognition receptor (PRR) recognition of pathogen-associated molecular patterns (PAMPs) to initiate an inflammatory response [23]. Polymorphonuclear leukocytes and neutrophils (PMNs) are not only the most abundant leukocytes in the blood, up to 65% of white blood cells in humans, but also short-lived. PMNs are derived from a granulocyte-monocyte precursor in adult bone marrow [24] and account for more than fifty percent of hematopoietic activity. Each day, there are about 5 × 1010 PMNs released from bone marrow into the peripheral circulation [25, 26]. Due to the PMN's short lifespan, close to 24 hours, homeostatic control is essential to maintain relatively stable cell numbers in the circulation. Acute bacterial or fungal infection, for example, stimulates an immediate inflammatory response by the vascular endothelium and the migration of PMNs to the site of infection in response to local chemokines and local changes in endothelial integrins [27]. The recruited PMNs phagocytose and kill the potential pathogens. Upon phagocytosis of potential pathogens, PMNs initiate a respiratory burst to generate reactive oxygen species (ROS) that are bactericidal [28].
2. Critical Role of Nanoparticles in Immune Response and Inflammation
The effects of NPs on the immune system, especially the innate immune system, are critical to a thorough understanding of the physiological and pathophysiological consequences of NP exposure. Intentional or unintentional NP exposure will initiate engagement of cellular and soluble protein components of the innate immune system to activate intracellular and extracellular signaling cascades [9, 29, 30] in response to the NPs. Both extracellular and intracellular innate immune receptors, pattern recognition receptors (PRR), may be engaged and stimulated by NPs [31–33]. Likewise, proteins in serum, particularly those in the complement [34, 35] and kallikrein [36] systems, may be engaged by NPs. Whether the NP interaction gradually leads to stimulation or inhibition of innate immunity and or inflammation is determined by the physicochemical properties of the relevant NPs [37–41]. NPs such as sand, dust particles, or pollen are generally ignored by the immune system. On the other hand, when NPs engage PRR, the NPs may mimic pathogen-associated molecular patterns (PAMPs) and initiate an innate immune reaction [42–44].
Upon exposure to NPs, neutrophils may initiate an inflammatory response, secreting signaling chemokines and evoking downstream reactions [14]. The physical and chemical properties of NPs are major factors that may affect the innate immune response. Differences in size, size distribution, charge, surface area, reactivity, crystallinity, aggregation in relevant medium, composition, surface coating, method of synthesis, and impurities not only affect biodistribution and cellular uptake of NPs but also affect innate immune responses [9, 45–48]. It still remains controversial whether the toxicity of NPs originates from the NPs themselves, metal ions released by dissolution of the NPs, or a combination of both. Several studies demonstrate that the released metal ions are the major or even the only cause of their toxicity. Soluble NPs, such as ZnO and FeO that have higher surface ion dissolution, were reported to be more toxic than NPs with less surface ion dissolution, such as CeO2 and TiO2 [49–52]. Some studies have also indicated that size is an important determinant for toxicity and the inflammatory potential of NPs. Larger-size NPs with a smaller surface-to-volume ratio have higher dissolution of toxic ions and induce more inflammatory ROS production [53]. Shape and composition are also critical determinants for NPs' toxicity and inflammatory potential [54]. The NPs' surface composition influences NPs' interactions with cell membranes and surface receptors. More positively charged NPs have higher potential to induce inflammatory reactions [55]. Thus, if aggregated NPs are dissociated through sonication, the cytotoxicity and ROS production may increase on account of the increased solubility and ion dissolution [56]. However, several studies report that the major source of toxicity of NPs is derived from their particulate characteristics [57, 58]. Wang et al. [59] reported that ZnO NP toxicity was due solely to the released Zn ions, and CuO NP toxicity originated from both the released Cu ions and the CuO particles. Toxicities of Fe2O3, Co3O4, Cr2O3, and NiO were caused by the particulate characteristics of the NPs. In consideration of the above, medical use of NPs must consider how NPs' physical properties, especially solubility, affect toxicity.
Usually, neutrophils take up NPs through pinocytosis, macropinocytosis, clathrin/caveolar-mediated endocytosis, or phagocytosis. Both micropinocytosis and pinocytosis are nonspecific and related to immune response. When neutrophil PRR engage NP PAMPs, that engagement may initiate inflammasome-dependent neutrophil activation [31, 60]. Recently, NETosis, a new cell death specific to neutrophils, has become another significant way by which NPs may stimulate immune and inflammation response [61, 62]. Herein, we focus on the correlation and interaction between NPs and NETs in innate immune responses.
2.1. NP-Induced NET Formation in Inflammatory Response and Inflammation Resolution
Neutrophil extracellular traps (NETs), a network structure released during NETosis, consist of 15-17 nm chromatin strands decorated with as many as 20 different antimicrobial proteins and peptides including myeloperoxidase (MPO), neutrophil elastase (NE), proteinase 3 (PR3), cathepsin G, LL37, and histones 1, 2A, 2B, 3, and 4 [62]. Conventional suicidal NETosis is usually initiated by several stimuli (bacteria, viruses, and fungi) binding to neutrophil toll-like receptors (TLRs) [62, 63], which activate the endoplasmic reticulum to release stored calcium ions. Elevated calcium levels increase protein kinase C (PKC) activity, inducing NADPH oxidase to assemble into the functional phagocytic oxidase (PHOX) complex [64–66]. PHOX generates ROS that initiates nuclear and granular membrane rupture with subsequent chromatin decondensation and diffusion into the cytoplasm [64, 65]. The aforementioned neutrophil granular proteins and peptides attach to the cytoplasmic chromatin, and the complexes break through the plasma membrane and diffuse into the extracellular space as NETs [61, 67]. Vital NETosis is another pathway to release NETs induced by Staphylococcus aureus [68] and Candida albicans [69] via blebbing of the nuclear envelope and vesicular exportation. Consequently, this pathway preserves the integrity of the neutrophil plasma membrane [66, 68, 70]. Meanwhile, it still remains controversial whether and how suicidal NETosis and vital NETosis coexist. Recent data suggest that other immune cells such as mast cells [71], eosinophils [72], and macrophages [73] can also release extracellular traps. When NPs stimulate NETs, the NPs may be captured within the NETs in a phagocytosis-independent process [70]. Recent results indicate that NETs may function in the setting of noninfectious disease and its regulation [70]. Several kinds of NPs such as gold, silver, cationic lipid, polystyrene, nanodiamonds, and graphene oxide (GO) were found to trigger NETosis [37, 55, 74–78] (Table 1).
Table 1.
Nanoparticles (NPs) that induce NETs.
2.2. Gold Nanoparticles (AuNPs)
Gold nanoparticles (AuNPs) have great potential in diagnostics and therapeutic nanomedicine [79]. AuNPs are recognized as nonbiodegradable and mostly insoluble in biological media, and they cause activation of neutrophils by altering the surface charge density on neutrophil membranes [80, 81]. AuNPs function as excellent nanocarriers not only because of their small size, which is similar to cellular components, but also because of their biocompatibility. AuNPs larger than 10 nm in size have less cytotoxicity and are more biocompatible [82, 83]. Bartneck et al. [77] explored the interaction between gold NPs with diameters of 15–50 nm and neutrophils. They built a successful model library of AuNPs with different surface chemistries or different shapes and studied their effect on human primary peripheral PMNs. Accordingly, they use transmission electron microscopy (TEM) or electroless deposition to observe that neutrophils trapped AuNPs mostly within extracellular networks. NETosis was detected 15 minutes after AuNPs come in contact with neutrophils and progressively trapped more NPs with time. AuNPs in different shapes and modified surface properties such as cetyltrimethyl ammonium bromide (CTAB) and polyethylene oxide (PEO) were compared to determine how size and surface properties affect NET formation. From that research, they concluded that NP's surface chemical characteristic had only a slight effect on NET formation but had significantly more impact on the range and ratio of gold PMN aggregates. The positively charged CTAB- and PEO-NH2-coated AuNPs were more frequently located internally in the NETs than PEO-OH- or PEO-COOH-modified NPs. In this study, we also found that unless we use DNase-pretreated neutrophils before staining, gold PMN aggregates could be detected. Meanwhile, gold NPs remain in the structure. It proves that there are some proteins in the gold PMN aggregate structure that may not be influenced by DNase and still play a role in trapping NPs. Since DNA structure is the main component part and carries a net negative charge, positively charged particles of AuNPs trapped by NETs can be explained by electrostatic forces [77]. Ali et al. [84] did researches on gold nanorods (AuNRs). In this study, AuNRs showed ability to treat cancer [84]. Another study has investigated how AuNPs with a size of 60 nm induce the generation of free radicals that may be involved in NET formation [81].
2.3. Silver Nanoparticles (AgNPs)
Silver nanoparticles (AgNPs) are widely used in many fields such as electronics, biosensing, and food adjuvants. AgNPs may also be used in medical applications such as drug delivery because of their size and antimicrobial properties [85]; however, AgNPs do have significant dose-dependent cytotoxicity. Meanwhile, it still remains controversial whether AgNPs or silver ions (Ag+) have been attributed to the cytotoxicity, because the majority of cell culture studies are done in suspension that makes it difficult to differentiate between particle and soluble Ag+ effects [86, 87]. A study in which AgNP particle dissolution (and aggregation) in cell culture media was prevented by using an air-liquid exposure cell system did not cause cytotoxicity or induce the release of proinflammatory markers [88]. However, more experiments are needed to clarify the fate of intracellular AgNPs and Ag+. Several studies have evaluated the effects of AgNPs on neutrophils including NET formation in vivo and in vitro. Liz et al. [74] reported that 15 nm AgNPs (AgNP15) induce atypical cell death in neutrophils in a caspase-1- and caspase-4-dependent process. AgNPs also induced ROS and IL1β [89]. The atypical cell death was also inhibited by the antioxidant n-acetylcysteine indicating ROS dependency on the AgNP-induced atypical cell death. AgNPs also induced NETosis in adherent neutrophils that could not be inhibited by caspase-1 and caspase-4 inhibitors [74]. During AgNP-induced activation, the volume of neutrophils increased when the expression of the neutrophil surface marker CD16 remained the same unlike apoptotic neutrophils where the CD16 expression decreased [90, 91]. These changes were related to oxidative stress.
2.4. Cationic Lipid Nanoparticles
Solid lipid nanoparticles (SLNs), which are made up of solid crystalline lipids at room and body temperature, are among the colloidal nanosystems [92]. Nowadays, SLNs are commonly used in nanomedicine as drug carriers for a variety of medical treatments including cancer therapy, medical diagnosis, and tissue impairing [93]. Cationic SLNs (cSLNs) have been useful as carriers for DNA and RNA in promoting gene transfection and expression, respectively [94, 95]. Investigation into the possible roles for cSLNs in inflammation is still lacking. Hwang et al. designed a study examining the effect of cSLNs on human primary neutrophils and whether cSLNs can induce NETosis. As noted above, NETosis is initiated when the nuclear and granular membranes rupture with subsequent chromatin decondensation and diffusion into the cytoplasm [61, 67]. Their results indicated that oxidative stress, Ca2+ influx, and MAPK pathway signaling were essential to cSLN-induced NET formation. All these findings indicate the significance of cSLNs in the activation of neutrophils [75].
2.5. Carbon and Polystyrene Nanopowders
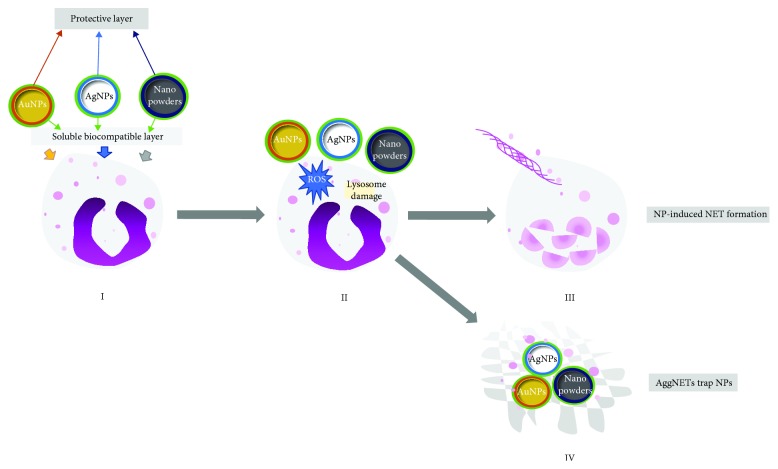
Muñoz et al. [76]. recently studied the interaction between neutrophils and carbon and polystyrene NPs. Environmental exposure to carbon NPs, including nanodiamonds, is unavoidable. Carbon NPs are a ubiquitous, necessary by-product of common procedures used in manufacturing and business including abrasive grinding and laser printing to carbon combustion that generates smoke. While NETs are induced by inflammation, aggregated NETs (aggNETs), which are generated under high neutrophil densities, may restrict and promote the absorption of inflammation [96]. Neutrophil NETs and aggNETs can capture and “neutralize” NPs in a size-dependent mechanism. When small NPs such as nanodiamonds (d) with a size of 10 nm (d10) and polystyrene beads (b) with a size of 40 nm (b40) were used to stimulate neutrophils, classical NET-like structures appear similar to those induced with PMA, whereas larger NPs (d1000 or b1000) did not induce NETs. Thus, there is a conclusion that both unipolar diamonds and polystyrene beads may induce NETs in a size-dependent way in vivo. This process activates a short-term inflammatory response and limits inflammation by immobilizing and entrapping NPs. They also got a conclusion that small-sized NPs may damage the cell molecular barrier and the function of cell membrane ion selectively. Oxidative stress and lysosomal damage are vital in NP-induced NETosis. The membranes were damaged by NPs and used for recycling in body systems firstly, then fused with primary lysosomes to form into phagolysosome. When lysosomes ruptured, the oxidative stress is being activated and the production of ROS is increasing beyond intracellular pathways. In order to prevent further tissue damage, neutrophils formed aggNETs to restrict and immobilize NPs that lead to an endpoint of inflammation [76] (Figure 1).
Figure 1.
Several kinds of nanoparticles (gold nanoparticles, silver nanoparticles, nanopowders, etc.) can induce NET formation (I). Once the NPs contact the cellular membrane, cellular activation and/or cellular membrane damage can lead to lysosomal damage and ROS production (II). NP stimulation may induce histone deimination and chromatin decondensation resulting in the formation of NETs (III). Neutrophils may form aggNETs and trap NPs in order to eliminate and immobilize inflammatory particles (IV).
2.6. Oxidative Stress Is the Major Mechanism of NP-Induced NET Formation
A number of studies indicate that oxidative stress is a major pathway in NP-induced NET formation by nanoparticles such as AgNPs, cSLNs, and nanopowders [76, 77, 85, 95]. In classical PMA-induced NETosis, reactive oxygen species is a vital factor. Thus, there was a hypothesis that ROS is the major pathway in NP-induced NET formation. Research with AgNPs revealed that AgNP-induced NETosis could not be reversed by the inhibitors of caspase-1 and caspase-4 [74, 85]. IL-1β, an inflammatory cytokine, is also measured, and it was found that its expression is decreased due to the function of caspase-1 and caspase-4 inhibitors. ROS was assayed by flow cytometry and found to be increased by AgNPs. Therefore, it was concluded that AgNPs rapidly induced an atypical cell death in neutrophils by a mechanism involving caspase-1, caspase-4, and ROS [74]. In the research of cSLNs, Hwang et al. [97] found that cSLNs can activate neutrophils through respiratory and degranulation pathways. cSLNs induce a dose-dependent increase in superoxide anion production. Uptake of cSLNs activated Ca2+ channels and increased Ca2+ influx. Pretreatment with the Ca2+ influx inhibitor BAPTA-AM inhibited increases in Ca2+ influx and ROS induced by cSLNs [75]. Muñoz et al. concluded that both carbon and polystyrene nanopowders induced NETs by an oxidative stress-dependent mechanism. The NPs damaged neutrophil cell membranes and caused lysosome to rupture to activate the production of ROS and induce NETosis [76].
3. Conclusion and Perspective
Nowadays, nanoparticles have become widely used in engineering, vaccine carrying, and drug delivery due to their biochemistry and biocompatibility. The interaction between NPs and the innate immune system, especially neutrophils, is a vital area of research to be further pursued. Currently, neutrophils release NETs and trap sterile NPs and nonsterile pathogens as soon as they can, and NPs can be trapped due to their different biochemical properties. Further studies are needed to understand the interaction between NPs and NETs. Meanwhile, it is important to know the most vital properties of NPs in NETosis. Thus, NP-induced NET formation needs to be further investigated to figure out their physiological roles to utilize NPs well in nanomedicine.
Acknowledgments
The present work was supported by the research project of the Health and Family Planning Commission of Sichuan Province (18PJ354 to HY), the Science and Technology Innovation and Entrepreneurship Seedling Project of Sichuan Province (key project) (2019JDRC0101 to HY), the China International Medical Foundation Simcere clinical research fund (Z-2014-06-2-1855 to HY), the National Natural Science Foundation of China (81771742 to YZ), and the international collaborative project of the Science and Technology Department of Sichuan Province (2015HH0050 to YZ and MH and 2014HH0027 to YL and MH).
Conflicts of Interest
The authors declare that they have no conflicts of interest.
Authors' Contributions
HY and YZ wrote the first draft of this article. HY, YL, LZ, XC, and YZ designed the figures. YZ, MH, and TM critically revised the manuscript for important intellectual content. All authors approved the final version.
References
- 1.Hartung T. Food for thought ... on alternative methods for nanoparticle safety testing. ALTEX. 2010;27(2):87–95. doi: 10.14573/altex.2010.2.87. [DOI] [PubMed] [Google Scholar]
- 2.Horie M., Kato H., Fujita K., Endoh S., Iwahashi H. In vitro evaluation of cellular response induced by manufactured nanoparticles. Chemical Research in Toxicology. 2012;25(3):605–619. doi: 10.1021/tx200470e. [DOI] [PubMed] [Google Scholar]
- 3.Goncalves D. M., De Liz R., Girard D. Activation of neutrophils by nanoparticles. Scientific World Journal. 2011;11(12):1877–1885. doi: 10.1100/2011/768350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Byrne H. J. Thomas J. Webster (Ed.): safety of nanoparticles. From manufacturing to medical applications. Analytical and Bioanalytical Chemistry. 2009;395:p. 1199. doi: 10.1007/s00216-009-3094-0. [DOI] [Google Scholar]
- 5.Tsuji J. S., Maynard A. D., Howard P. C., et al. Research strategies for safety evaluation of nanomaterials, part IV: risk assessment of nanoparticles. Toxicological Sciences. 2006;89(1):42–50. doi: 10.1093/toxsci/kfi339. [DOI] [PubMed] [Google Scholar]
- 6.Nogueira D. R., Tavano L., Mitjans M., Pérez L., Infante M. R., Vinardell M. P. In vitro antitumor activity of methotrexate via pH-sensitive chitosan nanoparticles. Biomaterials. 2013;34(11):2758–2772. doi: 10.1016/j.biomaterials.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 7.Robbens J., Vanparys C., Nobels I., et al. Eco-, geno- and human toxicology of bio-active nanoparticles for biomedical applications. Toxicology. 2010;269(2-3):170–181. doi: 10.1016/j.tox.2009.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Kreitinger J. M., Beamer C. A., Shepherd D. M. Environmental immunology: lessons learned from exposure to a select panel of immunotoxicants. Journal of Immunology. 2016;196(8):3217–3225. doi: 10.4049/jimmunol.1502149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petrarca C., Clemente E., Amato V., et al. Engineered metal based nanoparticles and innate immunity. Clinical and Molecular Allergy. 2015;13(1):p. 13. doi: 10.1186/s12948-015-0020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oberdörster G., Oberdörster E., Oberdörster J. Nanotoxicology: an emerging discipline evolving from studies of ultrafine particles. Environmental Health Perspectives. 2005;113(7):823–839. doi: 10.1289/ehp.7339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gouwy M., Struyf S., Proost P., van Damme J. Synergy in cytokine and chemokine networks amplifies the inflammatory response. Cytokine & Growth Factor Reviews. 2005;16(6):561–580. doi: 10.1016/j.cytogfr.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 12.Scott M. G., Hancock R. E. Cationic antimicrobial peptides and their multifunctional role in the immune system. Critical Reviews in Immunology. 2000;20(5):407–431. [PubMed] [Google Scholar]
- 13.Colonna M., Krug A., Cella M. Interferon-producing cells: on the front line in immune responses against pathogens. Current Opinion in Immunology. 2002;14(3):373–379. doi: 10.1016/S0952-7915(02)00349-7. [DOI] [PubMed] [Google Scholar]
- 14.Scapini P., Lapinet-Vera J. A., Gasperini S., Calzetti F., Bazzoni F., Cassatella M. A. The neutrophil as a cellular source of chemokines. Immunological Reviews. 2000;177(1):195–203. doi: 10.1034/j.1600-065X.2000.17706.x. [DOI] [PubMed] [Google Scholar]
- 15.Galli S. J., Maurer M., Lantz C. S. Mast cells as sentinels of innate immunity. Current Opinion in Immunology. 1999;11(1):53–59. doi: 10.1016/S0952-7915(99)80010-7. [DOI] [PubMed] [Google Scholar]
- 16.Geissmann F., Manz M. G., Jung S., Sieweke M. H., Merad M., Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327(5966):656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gordon S., Plüddemann A. Tissue macrophages: heterogeneity and functions. BMC Biology. 2017;15(1):p. 53. doi: 10.1186/s12915-017-0392-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kolaczkowska E., Kubes P. Neutrophil recruitment and function in health and inflammation. Nature Reviews Immunology. 2013;13(3):159–175. doi: 10.1038/nri3399. [DOI] [PubMed] [Google Scholar]
- 19.Artis D., Spits H. The biology of innate lymphoid cells. Nature. 2015;517(7534):293–301. doi: 10.1038/nature14189. [DOI] [PubMed] [Google Scholar]
- 20.Marcenaro E., Dondero A., Moretta A. Multi-directional cross-regulation of NK cell function during innate immune responses. Transplant Immunology. 2006;17(1):16–19. doi: 10.1016/j.trim.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 21.Vantourout P., Hayday A. Six-of-the-best: unique contributions of γδ T cells to immunology. Nature Reviews Immunology. 2013;13(2):88–100. doi: 10.1038/nri3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beutler B. Microbe sensing, positive feedback loops, and the pathogenesis of inflammatory diseases. Immunological Reviews. 2010;227(1):248–263. doi: 10.1111/j.1600-065x.2008.00733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawai T., Akira S. The roles of TLRs, RLRs and NLRs in pathogen recognition. International Immunology. 2009;21(4):317–337. doi: 10.1093/intimm/dxp017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedman A. D. Transcriptional control of granulocyte and monocyte development. Oncogene. 2007;26(47):6816–6828. doi: 10.1038/sj.onc.1210764. [DOI] [PubMed] [Google Scholar]
- 25.Flannagan R. S., Jaumouillé V., Grinstein S. The cell biology of phagocytosis. Annual Review of Pathology. 2012;7(1):61–98. doi: 10.1146/annurev-pathol-011811-132445. [DOI] [PubMed] [Google Scholar]
- 26.Ward C., Dransfield I., Chilvers E. R., Haslett C., Rossi A. G. Pharmacological manipulation of granulocyte apoptosis: potential therapeutic targets. Trends in Pharmacological Sciences. 1999;20(12):503–509. doi: 10.1016/S0165-6147(99)01391-7. [DOI] [PubMed] [Google Scholar]
- 27.Ley K., Laudanna C., Cybulsky M. I., Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nature Reviews Immunology. 2007;7(9):678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 28.Bogdan C., Röllinghoff M., Diefenbach A. Reactive oxygen and reactive nitrogen intermediates in innate and specific immunity. Current Opinion in Immunology. 2000;12(1):64–76. doi: 10.1016/S0952-7915(99)00052-7. [DOI] [PubMed] [Google Scholar]
- 29.Dobrovolskaia M. A., Shurin M., Shvedova A. A. Current understanding of interactions between nanoparticles and the immune system. Toxicology and Applied Pharmacology. 2016;299:78–89. doi: 10.1016/j.taap.2015.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kononenko V., Narat M., Drobne D. Nanoparticle interaction with the immune system. Arhiv Za Higijenu Rada I Toksikologiju. 2015;66(2):97–108. doi: 10.1515/aiht-2015-66-2582. [DOI] [PubMed] [Google Scholar]
- 31.Demento S. L., Eisenbarth S. C., Foellmer H. G., et al. Inflammasome-activating nanoparticles as modular systems for optimizing vaccine efficacy. Vaccine. 2009;27(23):3013–3021. doi: 10.1016/j.vaccine.2009.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hafner A. M., Corthésy B., Merkle H. P. Particulate formulations for the delivery of poly(I:C) as vaccine adjuvant. Advanced Drug Delivery Reviews. 2013;65(10):1386–1399. doi: 10.1016/j.addr.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 33.Kato T., Suzuki F., Park E. Y. Purification of functional baculovirus particles from silkworm larval hemolymph and their use as nanoparticles for the detection of human prorenin receptor (PRR) binding. BMC Biotechnology. 2011;11(1):p. 60. doi: 10.1186/1472-6750-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reddy S. T., van der Vlies A. J., Simeoni E., et al. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nature Biotechnology. 2007;25(10):1159–1164. doi: 10.1038/nbt1332. [DOI] [PubMed] [Google Scholar]
- 35.Andersen A. J., Hashemi S. H., Andresen T. L., Hunter A. C., Moghimi S. M. Complement: alive and kicking nanomedicines. Journal of Biomedical Nanotechnology. 2009;5(4):364–372. doi: 10.1166/jbn.2009.1045. [DOI] [PubMed] [Google Scholar]
- 36.Ekstrand-Hammarström B., Hong J., Davoodpour P., et al. TiO2 nanoparticles tested in a novel screening whole human blood model of toxicity trigger adverse activation of the kallikrein system at low concentrations. Biomaterials. 2015;51:58–68. doi: 10.1016/j.biomaterials.2015.01.031. [DOI] [PubMed] [Google Scholar]
- 37.Zahr A. S., Davis C. A., Pishko M. V. Macrophage uptake of core−shell nanoparticles surface modified with poly(ethylene glycol) Langmuir. 2006;22(19):8178–8185. doi: 10.1021/la060951b. [DOI] [PubMed] [Google Scholar]
- 38.Dobrovolskaia M. A., Mcneil S. E. Immunological properties of engineered nanomaterials. Nature Nanotechnology. 2007;2(8):469–478. doi: 10.1038/nnano.2007.223. [DOI] [PubMed] [Google Scholar]
- 39.Dobrovolskaia M. A., Aggarwal P., Hall J. B., Mcneil S. E. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Molecular Pharmaceutics. 2008;5(4):487–495. doi: 10.1021/mp800032f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Aggarwal P., Hall J. B., McLeland C. B., Dobrovolskaia M. A., McNeil S. E. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Advanced Drug Delivery Reviews. 2009;61(6):428–437. doi: 10.1016/j.addr.2009.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fang C., Shi B., Pei Y. Y., Hong M. H., Wu J., Chen H. Z. In vivo tumor targeting of tumor necrosis factor-α-loaded stealth nanoparticles: effect of MePEG molecular weight and particle size. European Journal of Pharmaceutical Sciences. 2006;27(1):27–36. doi: 10.1016/j.ejps.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 42.Fadeel B. Clear and present danger? Engineered nanoparticles and the immune system. Swiss Medical Weekly. 2012;142, article w13609:p. 24. doi: 10.4414/smw.2012.13609. [DOI] [PubMed] [Google Scholar]
- 43.Siefert A. L., Caplan M. J., Fahmy T. M. Artificial bacterial biomimetic nanoparticles synergize pathogen-associated molecular patterns for vaccine efficacy. Biomaterials. 2016;97:85–96. doi: 10.1016/j.biomaterials.2016.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dostert C., Petrilli V., van Bruggen R., Steele C., Mossman B. T., Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science. 2008;320(5876):674–677. doi: 10.1126/science.1156995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vivier E., Malissen B. Innate and adaptive immunity: specificities and signaling hierarchies revisited. Nature Immunology. 2004;6(1):17–21. doi: 10.1038/ni1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moyano D. F., Liu Y., Peer D., Rotello V. M. Modulation of immune response using engineered nanoparticle surfaces. Small. 2016;12(1):76–82. doi: 10.1002/smll.201502273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Getts D. R., Shea L. D., Miller S. D., King N. J. C. Harnessing nanoparticles for immune modulation. Trends in Immunology. 2015;36(7):419–427. doi: 10.1016/j.it.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Warheit D. B. How meaningful are the results of nanotoxicity studies in the absence of adequate material characterization? Toxicological Sciences. 2008;101(2):183–185. doi: 10.1093/toxsci/kfm279. [DOI] [PubMed] [Google Scholar]
- 49.Brunner T. J., Wick P., Manser P., et al. In vitro cytotoxicity of oxide nanoparticles: comparison to asbestos, silica, and the effect of particle solubility. Environmental Science & Technology. 2006;40(14):4374–4381. doi: 10.1021/es052069i. [DOI] [PubMed] [Google Scholar]
- 50.Brun N. R., Lenz M., Wehrli B., Fent K. Comparative effects of zinc oxide nanoparticles and dissolved zinc on zebrafish embryos and eleuthero-embryos: importance of zinc ions. Science of the Total Environment. 2014;476-477:657–666. doi: 10.1016/j.scitotenv.2014.01.053. [DOI] [PubMed] [Google Scholar]
- 51.Wehmas L. C., Anders C., Chess J., et al. Comparative metal oxide nanoparticle toxicity using embryonic zebrafish. Toxicology Reports. 2015;2(C):702–715. doi: 10.1016/j.toxrep.2015.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pandurangan M., Kim D. H. In vitro toxicity of zinc oxide nanoparticles: a review. Journal of Nanoparticle Research. 2015;17(3):p. 159. doi: 10.1007/s11051-015-2958-9. [DOI] [Google Scholar]
- 53.Magdolenova Z., Collins A., Kumar A., Dhawan A., Stone V., Dusinska M. Mechanisms of genotoxicity. A review of in vitro and in vivo studies with engineered nanoparticles. Nanotoxicology. 2014;8(3):233–278. doi: 10.3109/17435390.2013.773464. [DOI] [PubMed] [Google Scholar]
- 54.Yang H., Liu C., Yang D., Zhang H., Xi Z. Comparative study of cytotoxicity, oxidative stress and genotoxicity induced by four typical nanomaterials: the role of particle size, shape and composition. Journal of Applied Toxicology. 2009;29(1):69–78. doi: 10.1002/jat.1385. [DOI] [PubMed] [Google Scholar]
- 55.Ahamed M., Karns M., Goodson M., et al. DNA damage response to different surface chemistry of silver nanoparticles in mammalian cells. Toxicology and Applied Pharmacology. 2008;233(3):404–410. doi: 10.1016/j.taap.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 56.Lee W. M., An Y. J., Yoon H., Kweon H. S. Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiatus) and wheat (Triticum aestivum): plant agar test for water-insoluble nanoparticles. Environmental Toxicology & Chemistry. 2008;27(9):1915–1921. doi: 10.1897/07-481.1. [DOI] [PubMed] [Google Scholar]
- 57.Applerot G., Lipovsky A., Dror R., et al. Enhanced antibacterial activity of nanocrystalline ZnO due to increased ROS-mediated cell injury. Advanced Functional Materials. 2009;19(6):842–852. doi: 10.1002/adfm.200801081. [DOI] [Google Scholar]
- 58.Xiao Y., Vijver M. G., Chen G., Peijnenburg W. J. G. M. Toxicity and accumulation of Cu and ZnO nanoparticles in Daphnia magna. Environmental Science & Technology. 2015;49(7):4657–4664. doi: 10.1021/acs.est.5b00538. [DOI] [PubMed] [Google Scholar]
- 59.Wang D., Lin Z., Wang T., et al. Where does the toxicity of metal oxide nanoparticles come from: the nanoparticles, the ions, or a combination of both? Journal of Hazardous Materials. 2016;308:328–334. doi: 10.1016/j.jhazmat.2016.01.066. [DOI] [PubMed] [Google Scholar]
- 60.Najafi-Hajivar S., Zakeri-Milani P., Mohammadi H., et al. Overview on experimental models of interactions between nanoparticles and the immune system. Biomedicine & Pharmacotherapy. 2016;83:1365–1378. doi: 10.1016/j.biopha.2016.08.060. [DOI] [PubMed] [Google Scholar]
- 61.Fuchs T. A., Abed U., Goosmann C., et al. Novel cell death program leads to neutrophil extracellular traps. Journal of Cell Biology. 2007;176(2):231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brinkmann V., Reichard U., Goosmann C., et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 63.Munks M. W., McKee A. S., MacLeod M. K., et al. Aluminum adjuvants elicit fibrin-dependent extracellular traps in vivo. Blood. 2010;116(24):5191–5199. doi: 10.1182/blood-2010-03-275529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaplan M. J., Radic M. Neutrophil extracellular traps: double-edged swords of innate immunity. Journal of Immunology. 2012;189(6):2689–2695. doi: 10.4049/jimmunol.1201719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Papayannopoulos V., Metzler K. D., Hakkim A., Zychlinsky A. Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. The Journal of Cell Biology. 2010;191(3):677–691. doi: 10.1083/jcb.201006052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang H., Biermann M. H., Brauner J. M., Liu Y., Zhao Y., Herrmann M. New insights into neutrophil extracellular traps: mechanisms of formation and role in inflammation. Frontiers in Immunology. 2016;7(5663):p. 302. doi: 10.3389/fimmu.2016.00302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Steinberg B. E., Grinstein S. Unconventional roles of the NADPH oxidase: signaling, ion homeostasis, and cell death. Sciences Stke Signal Transduction Knowledge Environment. 2007;2007(379, article pe11) doi: 10.1126/stke.3792007pe11. [DOI] [PubMed] [Google Scholar]
- 68.Pilsczek F. H., Salina D., Poon K. K. H., et al. A novel mechanism of rapid nuclear neutrophil extracellular trap formation in response to Staphylococcus aureus. Journal of Immunology. 2010;185(12):7413–7425. doi: 10.4049/jimmunol.1000675. [DOI] [PubMed] [Google Scholar]
- 69.Byrd A. S., O'Brien X. M., Johnson C. M., Lavigne L. M., Reichner J. S. An extracellular matrix-based mechanism of rapid neutrophil extracellular trap formation in response to Candida albicans. Journal of Immunology. 2013;190(8):4136–4148. doi: 10.4049/jimmunol.1202671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jorch S. K., Kubes P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nature Medicine. 2017;23(3):279–287. doi: 10.1038/nm.4294. [DOI] [PubMed] [Google Scholar]
- 71.von Kockritz-Blickwede M., Goldmann O., Thulin P., et al. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood. 2008;111(6):3070–3080. doi: 10.1182/blood-2007-07-104018. [DOI] [PubMed] [Google Scholar]
- 72.Yousefi S., Simon D., Simon H. U. Eosinophil extracellular DNA traps: molecular mechanisms and potential roles in disease. Current Opinion in Immunology. 2012;24(6):736–739. doi: 10.1016/j.coi.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 73.Chow O. A., von Köckritz-Blickwede M., Bright A. T., et al. Statins enhance formation of phagocyte extracellular traps. Cell Host & Microbe. 2010;8(5):445–454. doi: 10.1016/j.chom.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liz R., Simard J. C., Leonardi L. B. A., Girard D. Silver nanoparticles rapidly induce atypical human neutrophil cell death by a process involving inflammatory caspases and reactive oxygen species and induce neutrophil extracellular traps release upon cell adhesion. International Immunopharmacology. 2015;28(1):616–625. doi: 10.1016/j.intimp.2015.06.030. [DOI] [PubMed] [Google Scholar]
- 75.Hwang T. L., Aljuffali I. A., Hung C. F., Chen C. H., Fang J. Y. The impact of cationic solid lipid nanoparticles on human neutrophil activation and formation of neutrophil extracellular traps (NETs) Chemico-Biological Interactions. 2015;235:106–114. doi: 10.1016/j.cbi.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 76.Muñoz L. E., Bilyy R., Biermann M. H. C., et al. Nanoparticles size-dependently initiate self-limiting NETosis-driven inflammation. Proceedings of the National Academy of Sciences of the United States of America. 2016;113(40):E5856–E5865. doi: 10.1073/pnas.1602230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bartneck M., Keul H. A., Zwadlo-Klarwasser G., Groll J.¨. Phagocytosis independent extracellular nanoparticle clearance by human immune cells. Nano Letters. 2010;10(1):59–63. doi: 10.1021/nl902830x. [DOI] [PubMed] [Google Scholar]
- 78.Mukherjee S. P., Gliga A. R., Lazzaretto B., et al. Graphene oxide is degraded by neutrophils and the degradation products are non-genotoxic. Nanoscale. 2018;10(3):1180–1188. doi: 10.1039/C7NR03552G. [DOI] [PubMed] [Google Scholar]
- 79.Almeida J. P. M., Figueroa E. R., Drezek R. A. Gold nanoparticle mediated cancer immunotherapy. Nanomedicine: Nanotechnology, Biology, and Medicine. 2014;10(3):503–514. doi: 10.1016/j.nano.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim K.-H., Kim J. U., Cha S. H., Lee J. C. Reversible formation and dissolution of gold nanoparticles through turning on and off sequences of UV light. Journal of the American Chemical Society. 2009;131(22):7482–7483. doi: 10.1021/ja8088394. [DOI] [PubMed] [Google Scholar]
- 81.Chekanov A. V., Baranova O. A., Levin A. D., Solov'eva ÉIu, Fedin A. I., Kazarinov K. D. Study of the influence of gold nanoparticles on activation of human blood neutrophils. Biofizika. 2013;58(3):495–500. [PubMed] [Google Scholar]
- 82.Chandolu V., Dass C. R. Treatment of lung cancer using nanoparticle drug delivery systems. Current Drug Discovery Technologies. 2013;10(2):170–176. doi: 10.2174/1570163811310020010. [DOI] [PubMed] [Google Scholar]
- 83.Coelho S. C., Rocha S., Juzenas P., et al. Gold nanoparticle delivery-enhanced proteasome inhibitor effect in adenocarcinoma cells. Expert Opinion on Drug Delivery. 2013;10(10):1345–1352. doi: 10.1517/17425247.2013.827659. [DOI] [PubMed] [Google Scholar]
- 84.Ali M. R. K., Rahman M. A., Wu Y., et al. Efficacy, long-term toxicity, and mechanistic studies of gold nanorods photothermal therapy of cancer in xenograft mice. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(15):E3110–E3118. doi: 10.1073/pnas.1619302114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ahamed M., Alsalhi M. S., Siddiqui M. K. J. Silver nanoparticle applications and human health. Clinica Chimica Acta. 2010;411(23-24):1841–1848. doi: 10.1016/j.cca.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 86.Behra R., Sigg L., Clift M. J. D., et al. Bioavailability of silver nanoparticles and ions: from a chemical and biochemical perspective. Journal of the Royal Society Interface. 2013;10(87, article 20130396) doi: 10.1098/rsif.2013.0396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kawata K., Osawa M., Okabe S. In vitro toxicity of silver nanoparticles at noncytotoxic doses to HepG2 human hepatoma cells. Environmental Science & Technology. 2009;43(15):6046–6051. doi: 10.1021/es900754q. [DOI] [PubMed] [Google Scholar]
- 88.Herzog F., Clift M. J. D., Piccapietra F., et al. Exposure of silver-nanoparticles and silver-ions to lung cells in vitro at the air-liquid interface. Particle and Fibre Toxicology. 2013;10(1):11–14. doi: 10.1186/1743-8977-10-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lamkanfi M., Dixit V. M. Mechanisms and functions of inflammasomes. Cell. 2014;157(5):1013–1022. doi: 10.1016/j.cell.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 90.Dransfield I., Buckle A. M., Savill J. S., McDowall A., Haslett C., Hogg N. Neutrophil apoptosis is associated with a reduction in CD16 (Fc gamma RIII) expression. The Journal of Immunology. 1994;153(3):1254–1263. [PubMed] [Google Scholar]
- 91.de Liz R., Horst H., Pizzolatti M. G., Fröde T. S., Girard D. Activation of human neutrophils by the anti-inflammatory mediator Esenbeckia leiocarpa leads to atypical apoptosis. Mediators of Inflammation. 2012;2012:10. doi: 10.1155/2012/198382.198382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rostami E., Kashanian S., Azandaryani A. H., Faramarzi H., Dolatabadi J. E. N., Omidfar K. Drug targeting using solid lipid nanoparticles. Chemistry and Physics of Lipids. 2014;181:56–61. doi: 10.1016/j.chemphyslip.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 93.Etheridge M. L., Campbell S. A., Erdman A. G., Haynes C. L., Wolf S. M., McCullough J. The big picture on nanomedicine: the state of investigational and approved nanomedicine products. Nanomedicine. 2013;9(1):1–14. doi: 10.1016/j.nano.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Vighi E., Leo E. Studying the in vitro behavior of cationic solid lipid nanoparticles as a nonviral vector. Nanomedicine. 2012;7(1):9–12. doi: 10.2217/nnm.11.168. [DOI] [PubMed] [Google Scholar]
- 95.Carrillo C., Sánchez-Hernández N., García-Montoya E., et al. DNA delivery via cationic solid lipid nanoparticles (SLNs) European Journal of Pharmaceutical Sciences. 2013;49(2):157–165. doi: 10.1016/j.ejps.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 96.Schauer C., Janko C., Munoz L. E., et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nature Medicine. 2014;20(5):511–517. doi: 10.1038/nm.3547. [DOI] [PubMed] [Google Scholar]
- 97.Hwang T.-L., Sung C.-T., Aljuffali I.-A., Chang Y.-T., Fang J.-Y. Cationic surfactants in the form of nanoparticles and micelles elicit different human neutrophil responses: a toxicological study. Colloids and Surfaces B: Biointerfaces. 2014;114(8):334–341. doi: 10.1016/j.colsurfb.2013.10.021. [DOI] [PubMed] [Google Scholar]