Abstract
RARβ plays a critical role in cancer progression and is associated with several types of human cancer. It remains unclear, however, whether it is linked to the clinicopathological parameters of colorectal cancer (CRC). We therefore determined the expression of RARβ protein in patients with primary CRC and examined its relationship with clinical outcomes. RARβ expression in 234 samples of CRC patients and matched benign noncancerous tumors was detected by immunohistochemistry. RARβ mRNA expression was confirmed using the TCGA and Oncomine databases. COX regression analysis and Kaplan–Meier survival analysis were performed to determine the relationship between RARβ expression and CRC prognosis. Our results show that high expression of RARβ correlated with better prognosis in CRC patients. RARβ expression in CRC specimens was clearly lower than in peritumoral specimens (30.8% vs 58.8%, p < 0.001) and significantly correlated with gender (χ2 = 3.926, p = 0.048), tumor differentiation (χ2 = 5.978, p = 0.014), and tumor stage (χ2 = 6.642, p = 0.036). Multivariate analyses further revealed that low RARβ expression (p = 0.001), distant metastasis (p = 0.001), tissue differentiation (p = 0.006), and tumor stage (p = 0.002) were associated with overall survival in CRC patients. In addition, Kaplan–Meier analysis indicated that increased RARβ expression in cytoplasm (p = 0.001) and early tumor TNM stage (p = 0.030) was associated with a more favorable outcome in patients with CRC. In conclusion, RARβ expression was strongly correlated with several clinicopathological factors of CRC and may represent a favorable prognostic marker in patients with CRC.
1. Introduction
Colorectal cancer (CRC) is the third most prevalent cancer globally after lung and breast cancer [1, 2], and its incidence has increased in recent years along with an increasing number of old people [3]. According to the literature, 45.5% of the incidence and mortality of CRC in China is attributable to unhealthy lifestyles and environmental risk factors including obesity, alcohol consumption, smoking, physical inactivity, and dietary factors [4]. The treatment and prognosis of approximately half of CRC patients with late-stage disease are unsatisfactory owing to resistance to treatment and distant metastasis [2]. Among CRC patients with liver metastases, less than 20% are suitable candidates for surgical intervention [5]. The inhibition of apoptosis in the epithelium of the CRC may contribute to malignancy [6], although the precise molecular mechanisms responsible for metastasis in CRC remain unclear [7]. The identification of novel diagnostic markers and risk factors for CRC is therefore required.
Retinoic acid (RA), a metabolite or analog of vitamin A, is the active form of retinoid with biological functions in processes including cellular proliferation and differentiation, exerted through its receptors, A receptor (RAR) and X receptor (RXR). These receptors each include an α, β, and γ component. Several studies have indicated that RA might be effective in the treatment of malignancies. All-trans RA-based chemotherapy has been used as standard therapy for promyelocytic leukemia, with remission rates of more than 90% [8, 9]. Previous researchers have also reported that RARβ protein is epigenetically silenced with tumor progression, indicating that RARβ might be a tumor-suppressor gene [10–12].
Some studies have shown that decreased or absent RARβ expression correlates with an increase in metastatic processes in various tumor types, including breast cancer and cancers of the digestive tract. However, little has been reported on its role in CRC, particularly its tumor suppressor function [10–12].
In this study, we evaluated the level of RARβ expression in human CRC tissues compared with corresponding tumor-adjacent tissues from the same patients and assessed the relationship between RARβ expression and clinical prognosis.
2. Materials and Methods
2.1. Patients and Tissue Microarray Analysis
Formalin-fixed and paraffin-embedded tumor samples and corresponding normal adjacent tissues were obtained from CRC patients treated at the Affiliated Hospital of Nantong University between January 2008 and December 2008. The included patients did not receive neoadjuvant radiation, neoadjuvant chemotherapy, or immunotherapy. The work described has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki), and the study protocol was approved by the ethics committee of the Affiliated Hospital of Nantong University.
Immunohistochemistry (IHC) with tissue microarrays (TMAs) was used to assess RARβ expression in 234 patients with CRC. The average patient age was 60 years, (range: 34–92 years). Clinicopathological parameters including sex, age, tumor stage, tumor diameter, lymph node metastasis, and differentiation were obtained from patient medical records. Overall survival was evaluated from the date of surgical resection until death or the end of the follow-up period. Tumor stage was evaluated according to CRC TNM staging guidelines, version 7 of the World Health Organization classification.
TMAs were constructed as described previously [13]. Following hematoxylin and eosin staining, representative tumor areas were labeled in paraffin blocks. Specimen samples (2 mm in diameter) were prepared and sequentially aligned into fixed paraffin blocks, from which 4 μm optimum sections were prepared [13].
2.2. Immunohistochemical Analysis
We performed IHC on CRC tissues using a primary antibody against RARβ. In brief, tissue sections were deparaffinized and antigen retrieval was performed by boiling under high pressure in 0.01 M citrate buffer (pH 7.0) for 5 minutes. Next, sections were incubated with goat serum reagent in phosphate buffered saline (PBS) for 10 minutes to block nonspecific binding. Tissues were then incubated with primary rabbit anti-RARβ antibody (1 : 300, ab124701; Abcam, Cambridge, MA, this rabbit monoclonal antibody is against human RARβ amino acids 400 to the C-terminus following the instruction of the manufacturer) overnight at 4°C and subsequently incubated with goat anti-rabbit HRP (Dako, Carpinteria, CA) as secondary antibody for 30 minutes, followed by three washes with PBS. Immunostained CRC sections were evaluated by two experienced pathologists under blinded conditions.
2.3. Statistical Methods
The percentage of RARβ-positive cells was scored from 0%–100%. The staining intensity of RARβ-positive cells was defined as follows: 0, 1, 2, or 3 for negative, weak, moderate, or strong intensity, respectively. To evaluate statistical significance, an appropriate cut point was established based on RARβ expression score and overall survival using the X-tile software program (the Rimm Lab, Yale University, New Haven, CT). The final sum of percentage of RARβ-positive cells and intensity score represented the RARβ immunostaining score.
A chi-square test was used to compare protein expression of RARβ in CRC tissues with corresponding tumor-adjacent tissues and to evaluate the association between RARβ protein expression and clinicopathological variables. Kaplan–Meier survival curves and the log-rank test were used to calculate the overall survival rate. The prognostic significance of univariate data models was examined using a multivariate Cox regression analysis. We did univariate analysis using clinical parameters like gender, age, RARβ expression, and others, respectively. Then, all clinical parameters that meet pvalue < 0.05 in univariate analysis were recruited into multivariate analysis. pvalue < 0.05 was accepted as statistically significant. All analyses were performed using IBM SPSS software version 22 (SPSS Inc., Chicago, IL).
2.4. Validation of RARβ Expression in CRC
To validate RARβ expression and its relationship with clinical parameters in CRC, we explored expression of RARβ mRNA in online database Oncomine (https://www.oncomine.org). Two CRC datasets were used (Bittner Colon dataset: 373 samples and TCGA dataset: 237 samples).
3. Results
3.1. RARβ Protein Expression in CRC Tissues
As shown in Figure 1, RARβ expression was primarily observed in the cytoplasm of CRC cells. In CRC samples from 236 patients, RARβ expression was detected in 30.8% of samples compared with 58.8% of corresponding peritumoral tissue samples. RARβ protein expression was significantly lower in CRC samples compared with adjacent matched tumor tissues (χ2 = 18.767, p < 0.001) (Table 1).
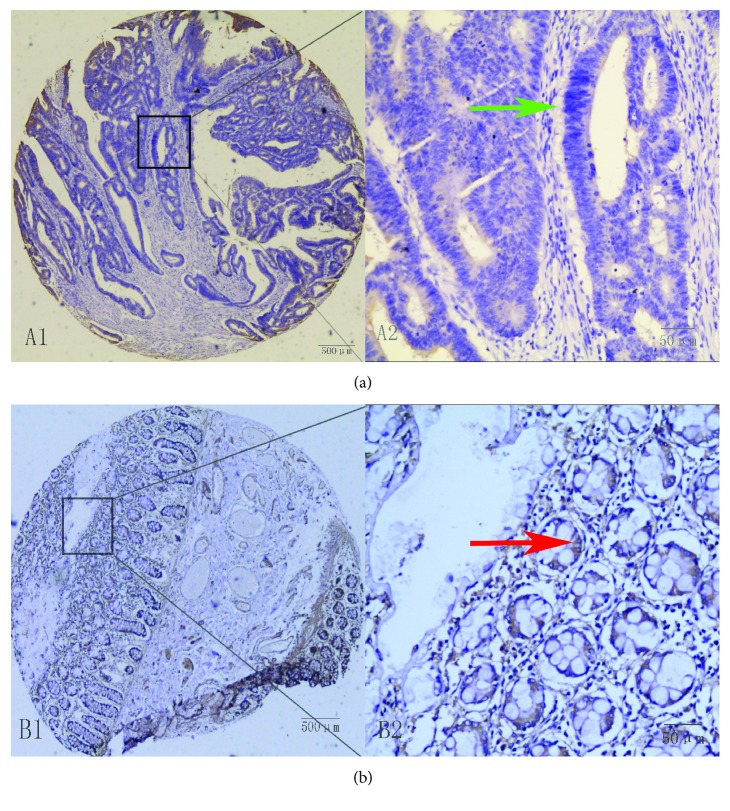
Figure 1.
Immunohistochemical staining was carried out on paraffin-embedded 4 μm sections, and representative patterns of RARβ protein expression in colorectal cancer (a) and peritumoral tissue (b) are shown. In colorectal cancer tissues, no expression was observed (A1 and A2). In adjacent normal colorectal tissues, high RARβ expression was observed (B1 and B2). A1 and B1: original magnification ×40 (bar = 500 μm): A2 and B2: ×400 (bar = 50 μm).
Table 1.
Correlation of RARβ expression with clinicopathological characteristics in patients with colorectal cancer.
| RARβ | n | Low or no expression | High expression | Pearson χ2 | p |
|---|---|---|---|---|---|
| Total | 117 | 81 (69.20) | 36 (30.80) | ||
| Gender | 3.926 | 0.048 ∗ | |||
| Male | 74 | 56 (75.70) | 18 (24.30) | ||
| Female | 43 | 25 (58.10) | 18 (41.90) | ||
| Age at diagnosis (years) | 0.202 | 0.653 | |||
| ≤60 | 42 | 28 (66.70) | 14 (33.30) | ||
| >60 | 75 | 53 (70.70) | 22 (29.30) | ||
| Tumor diameter (cm) | 0.396 | 0.529 | |||
| ≤2 | 9 | 7 (77.80) | 2 (22.20) | ||
| >2 | 105 | 71 (67.60) | 34 (32.40) | ||
| 3a | |||||
| Differentiation | 5.978 | 0.014 ∗ | |||
| Well | 95 | 61 (64.20) | 34 (35.80) | ||
| Poor | 22 | 20 (90.90) | 2 (9.10) | ||
| Lymph node metastasis | 3.772 | 0.052 | |||
| No metastasis | 69 | 43 (62.30) | 26 (37.70) | ||
| Metastasis | 48 | 38 (79.20) | 10 (20.80) | ||
| Distant metastasis (M) | 0.590 | 0.442 | |||
| No metastasis | 111 | 76 (68.50) | 35 (31.50) | ||
| Metastasis | 6 | 5 (83.30) | 1 (16.70) | ||
| Stage grouping with TNM | 6.642 | 0.036 ∗ | |||
| Stage I | 29 | 15 | 14 | ||
| Stage II | 39 | 27 | 12 | ||
| Stage III-IV | 49 | 39 | 10 |
aImmeasurable tumor diameter, 3 cases; ∗p < 0.05.
3.2. Relationship between RARβ Expression and Clinicopathological Characteristics of CRC Patients
We examined the correlation between clinicopathological parameters and RARβ immunoreactivity (Table 1). Our data indicate that RARβ immunoreactivity was significantly correlated with gender (p = 0.048), tumor differentiation (p = 0.014), and tumor TNM stage (p = 0.036). However, no association was observed between RARβ expression and clinicopathological parameters including age, tumor diameter, lymph node metastasis, and distant metastasis (Table 1).
3.3. Univariate and Multivariate Analysis of Prognostic Factors
Univariate Cox regression tests revealed that low RARβ protein expression (p = 0.001), tumor TNM stage (p = 0.002), distant metastasis (p = 0.001), and differentiation (p = 0.006) were inferior prognostic factors for overall survival (Table 2). A multivariate Cox proportional hazards regression model demonstrated that negative or low RARβ expression (p = 0.001) and TNM stage (p = 0.030) were strong predictors of overall survival (Table 2).
Table 2.
Univariate and multivariate analysis of prognostic factors for overall survival in CRC patients.
| Characteristic | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| HR | p > |Z| | 95% CI | HR | p > |Z| | 95% CI | |
| RARβ expression | 0.032 | 0.001 ∗ | 0.004 0.232 | 0.038 | 0.001 ∗ | 0.005 0.278 |
| Tumor diameter (cm) ≤2 vs >2 |
4.596 | 0.132 | ||||
| Lymph node metastasis No metastasis vs metastasis |
1.379 | 0.268 | 0.781 2.434 | |||
| Distant metastasis (M) No metastasis vs metastasis |
5.189 | 0.001 ∗ | 2.028 13.272 | |||
| Stage grouping with TNM Stage I vs II vs III-IV |
1.758 | 0.002 ∗ | 1.235 2.503 | 1.523 | 0.030 ∗ | 1.043 2.225 |
| Gender Male vs female |
1.584 | 0.148 | 0.849 2.953 | |||
| Age at diagnosis (years) ≤60 vs >60 |
1.121 | 0.708 | 0.615 2.044 | |||
| Differentiation Well vs moderate vs poorly |
0.418 | 0.006 ∗ | 0.224 0.780 | 0.687 | 0.243 | 0.366 1.289 |
∗ p < 0.05.
Following surgical resection for CRC, Kaplan–Meier survival curves showed that RARβ-positive tumors and early-stage cancer (TNM stage I) were associated with longer overall survival compared with RARβ-negative tumors and advanced cancer (TNM stages II–IV) in CRC patients (Figures 2 and 3).
Figure 2.
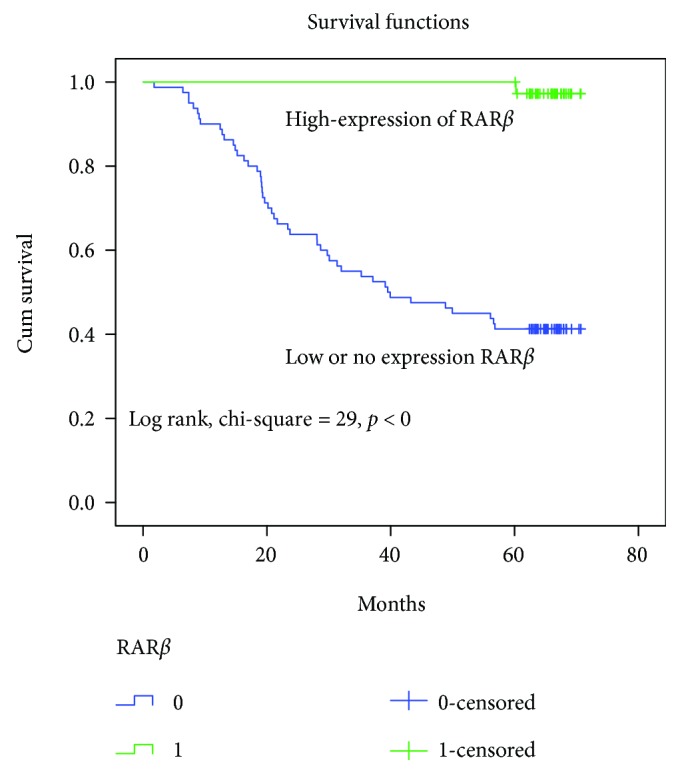
Patients with low or no RARβ expression (blue line) exhibited significantly poorer survival compared with the high-expression group (green line).
Figure 3.
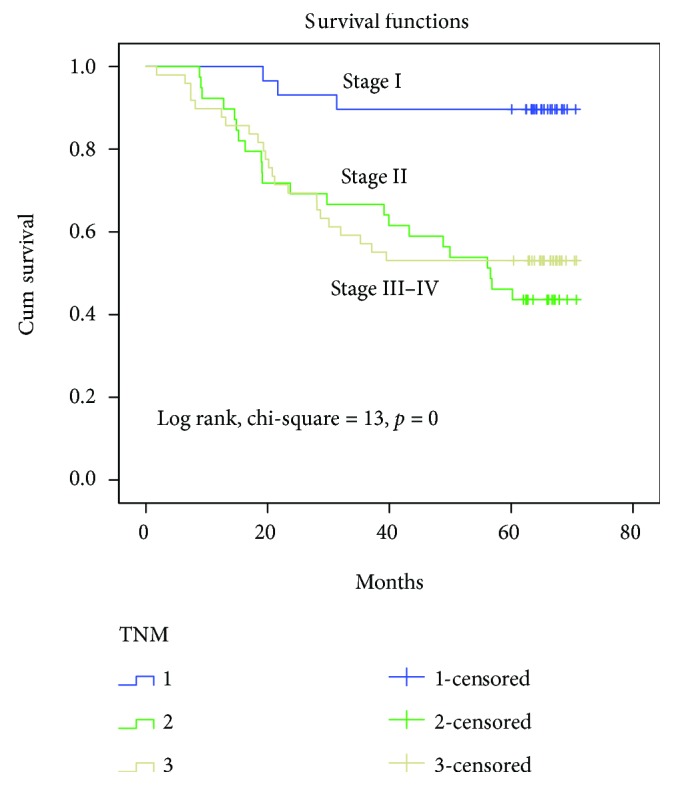
Kaplan–Meier survival curves demonstrate that survival of patients with stage II and stage III–IV CRC tumors is significantly reduced with patients with stage I disease.
3.4. Relationship between RARβ Protein Expression and Prognosis
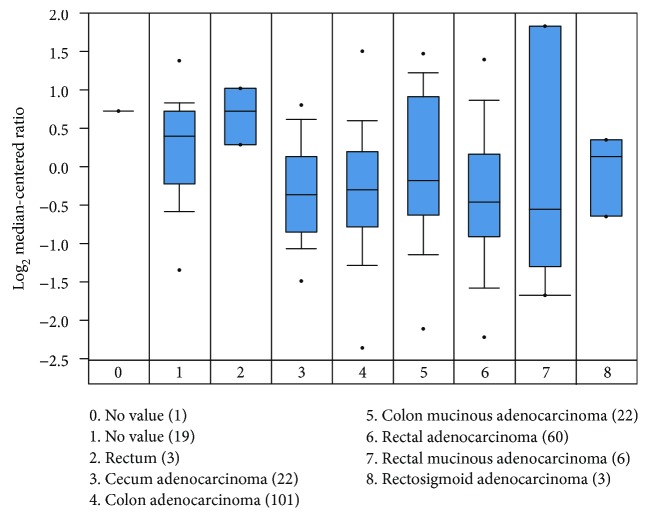
To assess the correlation between RARβ mRNA expression and its clinical parameters of CRC patients, we analyzed data in the Oncomine database and found that the expression of RARβ mRNA correlates negatively with CRC TNM stages (p < 0.05) (Figure 4). We also analyzed data in the TCGA database and found that low or no RARβ expression was shown in CRC tissues compared with high RARβ expression in benign noncancerous tissues (Figure 5). These results analyzed using the Oncomine and TCGA databases (Bittner Colon dataset: 373 samples and TCGA dataset: 237 samples) were consistent with our RARβ protein expression data in the previous research, which suggested that high RARβ expression was a favorable prognostic factor in CRC.
Figure 4.
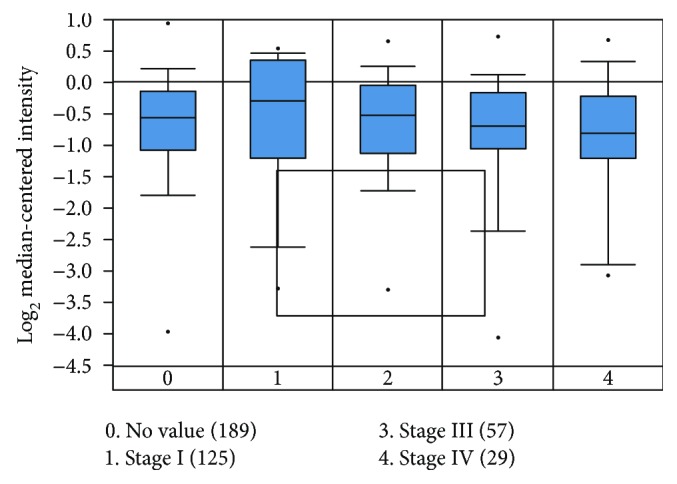
The expression of RARβ mRNA correlates negatively with the TNM stages (p < 0.05). Among 373 samples of Bittner Colon dataset, we utilized 300 samples of the colon adenocarcinoma which is the most common malignancy of the gastrointestinal tract.
Figure 5.
RARβ showed higher expression in benign noncancerous tissues compared with colorectal cancer in 237 samples of TCGA dataset.
4. Discussion
As previously reported [14], RARβ is the most commonly expressed RAR subtype in CRC. The expression position of RARβ is primarily localized in the CRC cellular cytoplasm. RARβ has been shown to function as a tumor suppressor, representing an interesting target in cancer research [10–12]. Indeed, our data suggest that RA-mediated apoptosis in human CRC cells and the significant tumor suppressive effects of RARβ may contribute to the chemopreventive actions of retinoid. Recent reports have also indicated that RA, and RARβ agonists in particular, inhibits invasion and the migratory potential of breast cancer and endometrial cancer cells [15]. In parallel, the RARα-specific agonist BMS453 significantly reduced the viability of colorectal cells. These results indicate the potential relevance of RARβ expression kinetics to the antitumor effect of RARβ in CRC. Importantly, the correlation between RARβ expression and clinicopathological parameters or prognosis in CRC has been less reported. Given that several studies have shown that marker protein E-cadherin and RARβ expression were downregulated in breast cancer samples and that decreased E-cadherin expression is linked to distant metastasis in patients with CRC, we propose that RARβ expression may also be relevant in CRC.
An understanding of the multiple prognostic factors impacting survival is critical to the management of cancer after surgical resection [16, 17]. In the present study, we investigated the association between RARβ cellular expression and clinicopathological parameters in CRC patients utilizing TMA and appropriate statistical analyses, focusing on the level of RARβ expression in CRC specimens compared with adjacent tumor specimens. Our data showed that CRC patients expressing low levels of RARβ protein were more likely to have a poor prognosis compared with those expressing high levels of RARβ protein in tumors. Although RARβ also showed weak expression in tumor-adjacent tissues, high RARβ protein expression was associated with a higher overall survival rate, indicating that RARβ expression might play a critical role in tumorigenesis among CRC patients.
In consideration of these factors, we demonstrated that high RARβ expression correlated with increased overall survival of CRC patients. RARβ expression was also associated with gender in CRC patients, an observation that has been insufficiently examined in the previous research. This finding may be associated with the physiological features of patients with advanced CRC. The expression among males is gradually decreasing, but it continues to increase among females, with more proportion. Tumor TNM staging has also been shown to be a significant early prognostic factor in CRC in clinical settings (Table 1). Our findings demonstrated that increased RARβ expression and early tumor stage are independent prognostic biomarkers in patients with CRC.
Similarly, previous studies reported a significant correlation between RARβ expression and esophageal cancer [18], non-small-cell lung cancer [19, 20], endometrial cancer, and breast cancer [21, 22]. Furthermore, silencing of the RARβ gene by promoter hypermethylation is frequently observed in metastatic lung, brain, and bone lesions compared with primary breast cancer [23]. RARβ hypermethylation has been found in 92% of endometrial cancers and 75% of endometrial hyperplasias [24]. Previous studies have demonstrated various relationships between clinicopathological parameters and RARβ expression in different types of cancer. Taken together, the evidence suggests that RARβ expression is reduced in human tumor cells, likely because of the hypermethylation of its promoter [24, 25]. RARβ appears to be associated with multiple human regulatory pathways, further indicating its critical role in CRC.
RARβ comprises three major isoforms, β1, β2, and β4, with different biological functions. RAR-β2 is the most abundant and the major RA-inducible isoform, and thus the term RARβ in the literature usually refers to the RAR-β2 isoform [26]. Collectively, one of the most important functions of retinoids is their antitumor activity, including the inhibition of tumor growth and the promotion of apoptosis, which has led to their function as chemotherapeutic agents [11, 27]. Although the therapeutic efficacy of retinoids remains controversial based on previous studies, the future study interestingly raises various hypotheses [28].
In summary, previous studies have shown the potential of RA for the treatment of malignancies, given its biological characteristics. Our findings indicate that RARβ protein expression is decreased in CRC compared with normal colorectal tissues and is also decreased in tumor tissues compared with corresponding adjacent tissue, indicating that RARβ might have a major role in the suppression of tumor invasion and metastasis. Inactivation of the RARβ gene might therefore lead to carcinogenesis. It therefore represents a promising candidate for the development of molecular-targeted therapies to ensure a more favorable prognosis for patients with CRC.
As a result of limitations of the present study size and the restriction of the TMA observational findings, further in vivo and in vitro studies are therefore required to examine the anticancer effect of RARβ in CRC tumor biology, including the inhibition of cell proliferation, apoptosis, and migration and the significance of RARβ and its crosstalk with other markers to increasing the understanding of RARβ mechanisms in CRC.
In conclusion, RARβ may be utilized as a prognostic factor in the management of CRC and represents a novel therapeutic target in CRC therapy.
Acknowledgments
This study was supported by grants from Nantong Science and Technology Program (MS22015055, MS12017010-2), general program from Jiansu Commission of Health, and the fifth batch 226 Talent Program from Nantong City (W.X.D.).
Data Availability
This publication is supported by multiple datasets, which are openly available at locations cited in the reference section.
Conflicts of Interest
These authors state that they have no conflict of interest.
Authors' Contributions
Wei Wang and Shuang Liu contributed equally to the manuscript.
References
- 1.Jemal A., Siegel R., Xu J., Ward E. Cancer statistics, 2010. CA: A Cancer Journal for Clinicians. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Ervik M., Lam F., Ferlay J., Mery L., Soerjomataram I., Bray F. Cancer today. International Agency for Research on Cancer; 2016. http://gco.iarc.fr/today. [Google Scholar]
- 3.Siegel R., DeSantis C., Virgo K., et al. Cancer treatment and survivorship statistics, 2012. CA: A Cancer Journal for Clinicians. 2012;62(4):220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 4.Gu M.-J., Huang Q.-C., Bao C.-Z., et al. Attributable causes of colorectal cancer in China. BMC Cancer. 2018;18(1):p. 38. doi: 10.1186/s12885-017-3968-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Power D. G., Kemeny N. E. Chemotherapy for the conversion of unresectable colorectal cancer liver metastases to resection. Critical Reviews in Oncology/Hematology. 2011;79(3):251–264. doi: 10.1016/j.critrevonc.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Kikuchi Y., Dinjens W. N. M., Bosman F. T. Proliferation and apoptosis in proliferative lesions of the colon and rectum. Virchows Archiv. 1997;431(2):111–117. doi: 10.1007/s004280050076. [DOI] [PubMed] [Google Scholar]
- 7.Enquist I. B., Good Z., Jubb A. M., et al. Lymph node-independent liver metastasis in a model of metastatic colorectal cancer. Nature Communications. 2014;5(1):p. 3530. doi: 10.1038/ncomms4530. [DOI] [PubMed] [Google Scholar]
- 8.Yoo E. S. Recent advances in the diagnosis and management of childhood acute promyelocytic leukemia. Korean Journal of Pediatrics. 2011;54(3):95–105. doi: 10.3345/kjp.2011.54.3.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Masetti R., Vendemini F., Zama D., Biagi C., Gasperini P., Pession A. All-trans retinoic acid in the treatment of pediatric acute promyelocytic leukemia. Expert Review of Anticancer Therapy. 2012;12(9):1191–1204. doi: 10.1586/era.12.101. [DOI] [PubMed] [Google Scholar]
- 10.Alvarez S., Germain P., Alvarez R., Rodríguez-Barrios F., Gronemeyer H., de Lera A. R. Structure, function and modulation of retinoic acid receptor beta, a tumor suppressor. The International Journal of Biochemistry & Cell Biology. 2007;39(7-8):1406–1415. doi: 10.1016/j.biocel.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 11.Mongan N. P., Gudas L. J. Diverse actions of retinoid receptors in cancer prevention and treatment. Differentiation. 2007;75(9):853–870. doi: 10.1111/j.1432-0436.2007.00206.x. [DOI] [PubMed] [Google Scholar]
- 12.Swift C. B., Hays J. L., Petty W. J. Distinct functions of retinoic acid receptor beta isoforms implications for targeted therapy. Endocrine, Metabolic & Immune Disorders - Drug Targets. 2008;8(1):47–50. doi: 10.2174/187153008783928389. [DOI] [PubMed] [Google Scholar]
- 13.Huang J., Mei H., Tang Z., et al. Triple-amiRNA VEGFRs inhibition in pancreatic cancer improves the efficacy of chemotherapy through EMT regulation. Journal of Controlled Release. 2017;245:1–14. doi: 10.1016/j.jconrel.2016.11.024. [DOI] [PubMed] [Google Scholar]
- 14.Lee M. O., Han S. Y., Jiang S., Han Park J., Kim S. J. Differential effects of retinoic acid on growth and apoptosis in human colon cancer cell lines associated with the induction of retinoic acid receptor β. Biochemical Pharmacology. 2000;59(5):485–496. doi: 10.1016/S0006-2952(99)00355-X. [DOI] [PubMed] [Google Scholar]
- 15.Flamini M. I., Gauna G. V., Sottile M. L., Nadin B. S., Sanchez A. M., Vargas-Roig L. M. Retinoic acid reduces migration of human breast cancer cells: role of retinoic acid receptor beta. Journal of Cellular and Molecular Medicine. 2014;18(6):1113–1123. doi: 10.1111/jcmm.12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rees M., Tekkis P. P., Welsh F. K. S., O’Rourke T., John T. G. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Annals of Surgery. 2008;247(1):125–135. doi: 10.1097/SLA.0b013e31815aa2c2. [DOI] [PubMed] [Google Scholar]
- 17.Fong Y., Fortner J., Sun R. L., Brennan M. F., Blumgart L. H. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Annals of Surgery. 1999;230(3):309–318. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiu H., Zhang W., el-Naggar A. K., et al. Loss of retinoic acid receptor-β expression is an early event during esophageal carcinogenesis. The American Journal of Pathology. 1999;155(5):1519–1523. doi: 10.1016/S0002-9440(10)65467-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu X. C., Sozzi G., Lee J. S., et al. Suppression of retinoic acid receptor β in non-small-cell lung cancer in vivo: implications for lung cancer development. Journal of the National Cancer Institute. 1997;89(9):624–629. doi: 10.1093/jnci/89.9.624. [DOI] [PubMed] [Google Scholar]
- 20.Picard E., Seguin C., Monhoven N., et al. Expression of retinoid receptor genes and proteins in non-small-cell lung cancer. Journal of the National Cancer Institute. 1999;91(12):1059–1066. doi: 10.1093/jnci/91.12.1059. [DOI] [PubMed] [Google Scholar]
- 21.Suzuki T., Moriya T., Sugawara A., Ariga N., Takabayashi H., Sasano H. Retinoid receptors in human breast carcinoma: possible modulators of in situ estrogen metabolism. Breast Cancer Research and Treatment. 2001;65(1):31–40. doi: 10.1023/A:1006433929792. [DOI] [PubMed] [Google Scholar]
- 22.Widschwendter M., Berger J., Daxenbichler G., et al. Loss of retinoic acid receptor β expression in breast cancer and morphologically normal adjacent tissue but not in the normal breast tissue distant from the cancer. Cancer Research. 1997;57(19):4158–4161. [PubMed] [Google Scholar]
- 23.Mehrotra J., Vali M., McVeigh M., et al. Very high frequency of hypermethylated genes in breast cancer metastasis to the bone, brain, and lung. Clinical Cancer Research. 2004;10(9):3104–3109. doi: 10.1158/1078-0432.CCR-03-0118. [DOI] [PubMed] [Google Scholar]
- 24.Li R., Saito T., Tanaka R., et al. Hypermethylation in promoter region of retinoic acid receptor-beta gene and immunohistochemical findings on retinoic acid receptors in carcinogenesis of endometrium. Cancer Letters. 2005;219(1):33–40. doi: 10.1016/j.canlet.2004.06.044. [DOI] [PubMed] [Google Scholar]
- 25.Sun S. Y. Retinoic acid receptor beta and colon cancer. Cancer Biology & Therapy. 2004;3(1):87–88. doi: 10.4161/cbt.3.1.686. [DOI] [PubMed] [Google Scholar]
- 26.Nagpal S., Zelent A., Chambon P. RAR-beta 4, a retinoic acid receptor isoform is generated from RAR-beta 2 by alternative splicing and usage of a CUG initiator codon. Proceedings of the National Academy of Sciences of the United States of America. 1992;89(7):2718–2722. doi: 10.1073/pnas.89.7.2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Altucci L., Gronemeyer H. The promise of retinoids to fight against cancer. Nature Reviews Cancer. 2001;1(3):181–193. doi: 10.1038/35106036. [DOI] [PubMed] [Google Scholar]
- 28.Perraud A., Nouaille M., Akil H., et al. Retinoid acid receptors in human colorectal cancer: an unexpected link with patient outcome. Experimental and Therapeutic Medicine. 2011;2(3):491–497. doi: 10.3892/etm.2011.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
This publication is supported by multiple datasets, which are openly available at locations cited in the reference section.