Abstract
In our continuing search for camptothecin (CPT)-derived antitumor drugs, novel 7-substituted CPT derivatives incorporating piperazinyl-sulfonylamidine moieties were designed, synthesized and evaluated for cytotoxicity against five tumor cell lines (A-549, MDA-MB-231, MCF-7, KB, and KB-VIN). All of the derivatives showed promising in vitro cytotoxic activity against the tested tumor cell lines, and were more potent than irinotecan. Remarkably, most of the compounds exhibited comparable cytotoxicity against the multidrug-resistant (MDR) KB-VIN and parental KB tumor cell lines, while irinotecan lost activity completely against KB-VIN. Especially, compounds 13r and 13p (IC50 0.38 and 0.85 μM, respectively) displayed the greatest cytotoxicity against the MDR KB-VIN cell line and merit further development into preclinical and clinical drug candidates for treating cancer, including MDR phenotype.
Keywords: Camptothecin, Cytotoxic activity, Piperazine, Sulfonylamidine, Synthesis
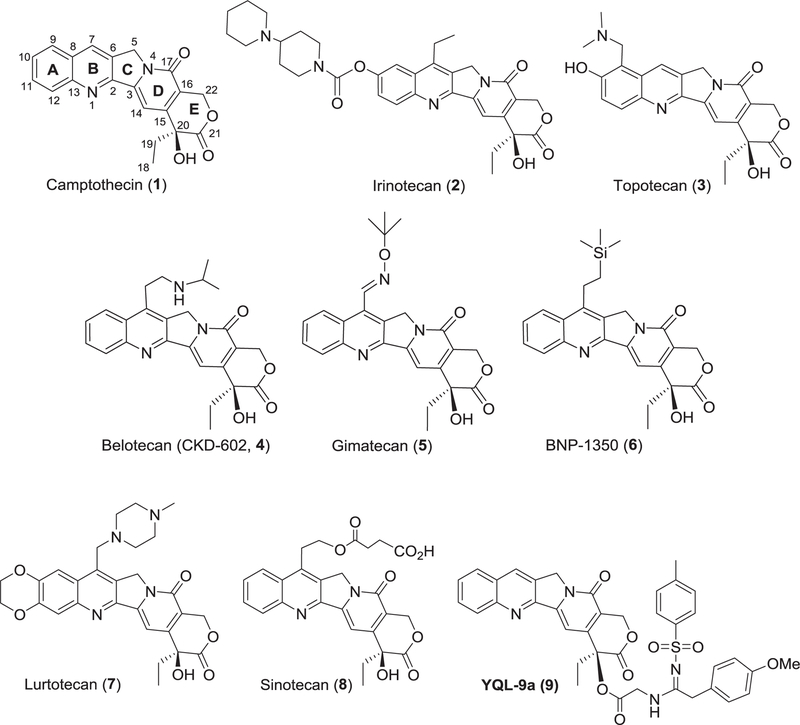
Camptothecin (CPT, 1) (Fig. 1) is a potent cytotoxic natural alkaloid, which acts against a broad spectrum of cancers via inhibition of DNA enzyme topoisomerase I (topo I).1,2 Its three semisynthetic analogues, irinotecan (CPT-11, 2), topotecan (TPT, 3), and belotecan (CKD-602, 4) (Fig. 1), received US government approval for the clinical treatment of ovarian, small-cell lung, and refractory colorectal cancers, while several other analogues are the subjects of ongoing preclinical or clinical evaluation.3–5
Fig. 1.
Structures of camptothecin derivatives.
From published accumulated structure-activity relationship (SAR) studies, modifications at the 7- or 10-position or both appear to be the most efficient approach to improve the antitumor potency, as well as increase E-ring stability.6–9 Furthermore, several reports documented that the introduction of lipophilic sub-stituents at the 7-position provides favorable molecular interactions and improved pharmacological features that could have potential therapeutic advantages.10–15 A binding model of CPT with biological macromolecules also indicated that the C-7 molecular area could accommodate considerable structural diversity.16 Accordingly, various substituents, such as ethyl,17 arylimi-nomethyl,18 alkylsilyl,19,20 and cycloalkyl,21 were introduced at the 7-position of CPT leading to either enhanced or comparable activity. Some C-7 modified analogues exhibited superior pharma-cological properties compared with 1, and several clinical trial drug candidates, including gimatecan (5), BNP-1350 (6), lurtotecan (7) and sinotecan (8) (Fig. 1) emerged as alternatives to overcome the drawbacks of 1.
In continuation of these efforts, we recently found that a series of 20-sulfonylamidine-CPT derivatives displayed potent antitumor activity with significantly different drug-resistance profiles from those of 1.22,23Among these derivatives, compound 9 is attractive as a potential candidate for anticancer chemotherapy and the modification with sulfonylamidine-substituted side chains may over-come some limitations of 1. These encouraging results prompted us to further extend our investigation by synthesizing a novel series of 7-piperazinyl-sulfonylamidine-CPT derivatives. A piperazine group is commonly found in various drugs24–27 and its introduction can contribute to improved drug-like properties, such as bioavailability and metabolism. Therefore, in the present study, we proposed to incorporate the two privileged fragments, sulfony-lamidine and piperazine, into the structure of 1 to design new derivatives.
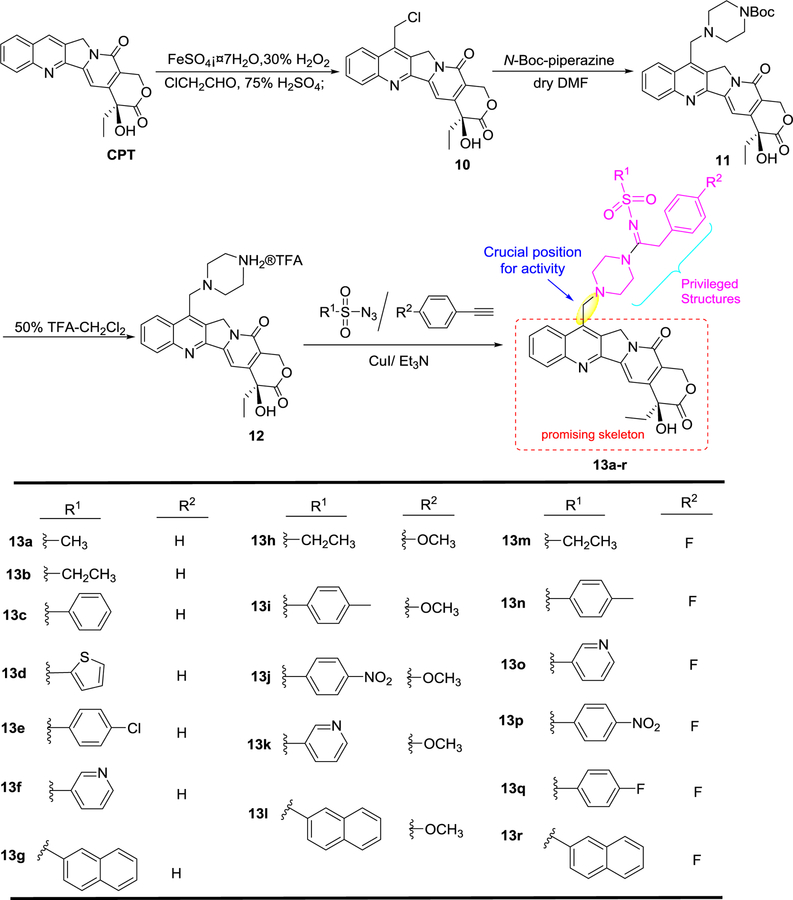
The synthetic routes to target compounds 13a–r are outlined in Scheme 1. Briefly, CPT was initially converted to 7-chloro-methyl-CPT (10) by using the Sawada method.17 Then, treatment of 10 with N-Boc protected piperazine in dry DMF solution furnished precursor 11 in 80% yield. Removal of the N-Boc group of 11 with TFA in CH2Cl2 (1:1) formed the TFA salts 12. Subsequently, the key intermediate 12 was reacted with various sulfonyl azides and alkynes in a Cu-catalyzed three-component reaction28 to produce the corresponding target compounds 13a– r in 50–72% yields.
Scheme 1.
General synthetic procedure for target compounds 13a–r.
The newly synthesized compounds were evaluated for in vitro cytotoxic activity against five tumor cell lines, KB (nasopharyngeal carcinoma), A-549 (lung carcinoma), MDA-MB-231 (breast carcinoma), KB-VIN (MDR KB subline) and MCF-7 (breast adenocarcinoma) by using a sulforhodamine B colorimetric assay29 with triplicate experiments. Irinotecan (2) was used as the positive control. The screening results are shown in Table 1.
Table 1.
In vitro cytotoxicity data for 13a–r against five tumor cell lines.
| Entry |
IC50 (μM) |
||||
|---|---|---|---|---|---|
| A-549 | MDA-MB-231 | KB | MCF-7 | KB-VIN | |
| 13a | 0.65 ± 0.02 | 1.57 ± 0.46 | 0.13 ± 0.06 | 1.59 ± 0.47 | 3.82 ± 0.71 |
| 13b | 0.73 ± 0.005 | 3.50 ± 0.12 | 0.49 ± 0.09 | 1.9 ± 0.18 | 4.29 ± 0.44 |
| 13c | 0.65 ± 0.06 | 4.41 ± 0.11 | 0.63 ± 0.09 | 2.25 ± 0.64 | 1.77 ± 0.37 |
| 13d | 0.65 ± 0.01 | 3.99 ± 0.61 | 0.59 ± 0.01 | 1.25 ± 0.19 | 1.27 ± 0.36 |
| 13e | 0.64 ± 0.02 | 5.01 ± 1.36 | 0.74 ± 0.02 | 3.19 ± 0.31 | 1.55 ± 0.59 |
| 13f | 0.76 ± 0.02 | 3.84 ± 0.41 | 0.72 ± 0.06 | 3.40 ± 0.49 | 2.09 ± 0.54 |
| 13g | 3.15 ± 0.44 | 11.26 ± 1.54 | 2.11 ± 0.94 | 9.37 ± 1.04 | 0.89 ± 0.01 |
| 13h | 0.71 ± 0.06 | 3.81 ± 0.19 | 0.47 ± 0.06 | 2.90 ± 0.53 | 2.73 ± 1.00 |
| 13i | 0.74 ± 0.03 | 5.84 ± 0.66 | 0.83 ± 0.03 | 5.67 ± 0.43 | 3.08 ± 0.85 |
| 13j | 2.93 ± 0.60 | 9.75 ± 1.09 | 1.06 ± 0.16 | 8.36 ± 0.74 | 0.97 ± 0.03 |
| 13k | 0.80 ± 0.04 | 4.96 ± 0.27 | 0.88 ± 0.04 | 4.22 ± 1.04 | 2.38 ± 0.86 |
| 13l | 0.74 ± 0.04 | 9.36 ± 0.07 | 0.84 ± 0.07 | 6.56 ± 0.55 | 3.66 ± 0.38 |
| 13m | 0.50 ± 0.05 | 2.51 ± 0.47 | 0.33 ± 0.09 | 1.60 ± 0.28 | 0.97 ± 0.03 |
| 13n | 1.77 ± 0.07 | 6.01 ± 0.69 | 1.07 ± 0.25 | 7.04 ± 0.59 | 2.77 ± 0.28 |
| 13o | 2.59 ± 0.98 | 6.31 ± 1.42 | 6.76 ± 1.78 | 6.05 ± 0.95 | 5.82 ± 2.07 |
| 13p | 0.63 ± 0.02 | 3.54 ± 0.47 | 4.43 ± 2.74 | 2.18 ± 0.40 | 0.85 ± 0.11 |
| 13q | 4.31 ± 0.68 | 10.17 ± 0.81 | 10.12 ± 1.92 | 8.85 ± 0.05 | 1.81 ± 0.04 |
| 13r | 0.60 ± 0.03 | 5.41 ± 0.46 | 4.72 ± 3.72 | 4.74 ± 0.49 | 0.38 ± 0.08 |
| 2 | 8.31 ± 0.28 | 15.56 ± 0.30 | 7.99 ± 0.31 | 11.32 ± 0.25 | >20 |
| 3 | 0.11 ± 0.02 | 0.77 ± 0.03 | 0.101 ± 0.01 | 0.28 ± 0.02 | 0.66 ± 0.02 |
As shown in Table 1, all target compounds exhibited significant in vitro cytotoxic activity against the five tested tumor cell lines, with IC50 values ranging from 0.13 to 11.26 μM. All of the compounds were less potent than 3 in the assay, which is a common feature of prodrugs; whereas, except for 13q (against KB), all new compounds exhibited comparable or superior cytotoxic activity to 2. Significantly, all of the compounds were more potent than 2 (IC50 >20 μM) against the MDR KB-VIN cell line, with 13p (IC50 0.85 μM) and 13r (IC50 0.38 μM) showing the greatest cytotoxicity against this cell line. All of the tested compounds also showed increased cytotoxic potency against the triple-negative breast cancer (MDA-MB-231) cell line compared with 2. This result implied that the introduction of a sulfonylamidine group at C-7 might combat the tumor MDR phenotype caused by P-glycoprotein overexpression. The IC50 values in Table 1 also revealed that the A-549 cell line was more sensitive than the other four cell lines to these compounds, which is consistent with the clinical behavior of other CPT derivatives.30
Furthermore, some preliminary SAR correlations were also observed for these derivatives. For compounds 13a–g, when the R2 group was fixed as hydrogen and the R1 group in the sulfony-lamidines was varied, methyl (13a) and ethyl (13b) gave the best results compared with the aromatic groups in compounds 13c–g, suggesting that small aliphatic chains appear to be the best R1 sub-stituents for greater cytotoxic potency. For example, against the KB cell line, the rank order of cytotoxic potency was 13a (methyl) > 13b (ethyl) > 13d (2-thienyl) > 13c (phenyl) > 13f (3-pyridinyl) 13e (chlorophenyl) ≥ 13g (2-naphthyl). In two other types of derivatives bearing a methoxy (13h–13l) or fluoro (13m–13r) R2 group, the compounds with an ethyl R1 group (13h and 13m) were generally more potent than the remaining compounds in each series with aromatic R1 groups. These results indicated that an aromatic group in R1 substituents is much less favorable than a short aliphatic group. The conjugation of the aromatic (R1) sub-stituents with the sulfonyl moiety might disturb the electronic properties of ring B to a greater extent than alkyl groups. Moreover, the potency of the compounds with the aromatic substituents (R1) depended significantly upon the nature of the R2 substituent on the aromatic ring; for example, compare 13p (4-fluorophenyl) with 13n (4-methylphenyl) or 13q (4-nitrophenyl).
In summary, novel series of 7-substituted-CPT derivatives incorporating piperazinyl-sulfonylamidine moieties were designed, synthesized and evaluated for cytotoxicity against five tumor cell lines (A-549, MDA-MB-231, MCF-7, KB and KB-VIN) by using a sulforhodamine B colorimetric assay. All synthesized compounds showed comparable or superior cytotoxicity activity compared with 2. Notably, all compounds were more potent than 2 against MDR KB-VIN cells. The SAR study found that the size, electron density, and distribution of the substituents within the sulfonylamidine side chain are critical to the derivatives’ activity. With a concise synthesis and potent cytotoxic profiles, the new CPT-derivatives, especially compounds 13r and 13p with the greatest cytotoxicity against the MDR KB-VIN cell line, merit further development into preclinical and clinical drug candidates for treating cancer, including MDR phenotype. Further optimization and mechanism of action studies on 13r and 13p are ongoing in our laboratory, and the results will be reported in due course. In this paper, two privileged fragments, sulfonylamidine and piperazine, were successfully introduced into our prior modified 1-analogue to produce a new derivative type with an improved cytotoxicity profile. We believe that such a strategy may be generally useful or, at least, shed light on other 1-derived antitumor drug discovery.
Supplementary Material
Acknowledgements
This work was supported financially by the National Natural Science Foundation of China (31371975, 21672092); Partial financial support was supplied by the Fundamental Research Funds for the Central Universities (lzujbky-2017-k23, lzujbky-2016-147). Support was also supplied by NIH grant CA177584 from the National Cancer Institute awarded to K.H. Lee. Thanks are also due to the support of Health and Welfare Surcharge of Tobacco Products, China Medical University Hospital Cancer Research Center of Excellence (MOHW103-TD-B-111-03, Taiwan).
Abbreviations:
- CKD-602
belotecan
- CPT
camptothecin
- CPT-11
irinotecan
- DMF
dimethylformamide
- MDR
multi-drug resistant
- SAR
structure-activity relationship
- TFA
trifluoroacetic acid
- topo
topoisomerase
- TPT
topotecan
Footnotes
A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bmcl.2017.07.078.
References
- 1.Slichenmyer WJ, Rowinsky EK, Donehower RC, Kaufmann SH. J Natl Cancer Inst 1993;85:271. [DOI] [PubMed] [Google Scholar]
- 2.Liu YQ, Li WQ, Morris-Natschke SL, et al. Med Res Rev 2015;35:753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liew ST, Yang LX. Curr Pharm Des 2008;14:1078. [DOI] [PubMed] [Google Scholar]
- 4.Li QY, Zu YG, Shi RZ, Yao LP. Curr Med Chem 2006;13:2021. [DOI] [PubMed] [Google Scholar]
- 5.Zunino F, Dallavalle S, Laccabue D, Beretta G, Merlini L, Pratesi G. Curr Pharm Des 2002;8:2505. [DOI] [PubMed] [Google Scholar]
- 6.Burke TG. Ann N Y Acad Sci 1996;803:29. [DOI] [PubMed] [Google Scholar]
- 7.Dallavalle S, Delsoldato S, Ferrari A, et al. J Med Chem 2000;43:3963. [DOI] [PubMed] [Google Scholar]
- 8.Dallavalle S, Ferrari A, Merlini L, et al. Bioorg Med Chem Lett 2001;11:291. [DOI] [PubMed] [Google Scholar]
- 9.Dallavalle S, Ferrari A, Biasotti B, et al. J Med Chem 2000;43:3963. [DOI] [PubMed] [Google Scholar]
- 10.Wang L, Huang Y, Zhang J, et al. Bioorg Med Chem Lett 2014;24:1597. [DOI] [PubMed] [Google Scholar]
- 11.Xiao F, Xue YD, Luo Y, Zhang B, Lu W, Yang B. Chin Chem Lett 2009;20:566. [Google Scholar]
- 12.Bom D, Curran DP, Zhang J, et al. J Controlled Release 2001;74:325. [DOI] [PubMed] [Google Scholar]
- 13.Niizuma S, Tsukazaki M, Suda H, et al. Bioorg Med Chem Lett 2009;19:2018. [DOI] [PubMed] [Google Scholar]
- 14.Dallavalle S, Giannini G, Alloatti D, et al. J Med Chem 2006;49:5177. [DOI] [PubMed] [Google Scholar]
- 15.Zhao XB, Goto M, Song ZL, et al. Bioorg Med Chem Lett 2014;24:3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan J, Weinstein JN, Kohn KW, Shi LM, Pommier YM. J Med Chem 1998;41:2216. [DOI] [PubMed] [Google Scholar]
- 17.Sawada S, Nokata K, Furuta T, Yokokura T, Miyasaka T. Chem Pharm Bull 1991;39:2574. [DOI] [PubMed] [Google Scholar]
- 18.Dallavalle S, Merlini L, Morini G, et al. Eur J Med Chem 2004;39:507. [DOI] [PubMed] [Google Scholar]
- 19.Josien H, Bom D, Curran DP. Bioorg Med Chem Lett 1997;7:3189. [Google Scholar]
- 20.Du W, Kaskar B, Blumbergs P, Subramanian PK, Curran DP. Bioorg Med Chem 2003;11:451. [DOI] [PubMed] [Google Scholar]
- 21.Li MZ, Jin W, Jiang C, et al. Bioorg Med Chem Lett 2009;19:4107. [DOI] [PubMed] [Google Scholar]
- 22.Wang MJ, Liu YQ, Chang LC, et al. J Med Chem 2014;57:6008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song ZL, Wang MJ, Li LL, et al. Eur J Med Chem 2016;115:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaquiquzzaman M, Verma G, Marella A, et al. Eur J Med Chem 2015;102:487. [DOI] [PubMed] [Google Scholar]
- 25.Butini S, Budriesi R, Hamon M, et al. J Med Chem 2009;52:6946. [DOI] [PubMed] [Google Scholar]
- 26.Bourbeau MP, Siegmund A, Allen JG, et al. J Med Chem 2013;56:10132. [DOI] [PubMed] [Google Scholar]
- 27.Moussa IA, Banister SD, Beinat C, Giboureau N, Reynolds AJ, Kassiou M. J Med Chem 2010;53:6228. [DOI] [PubMed] [Google Scholar]
- 28.Yoo EJ, Ahlquist M, Bae I, Sharpless KB, Fokin VV, Chang S. J Org Chem 2008;73:5520. [DOI] [PubMed] [Google Scholar]
- 29.Skehan P, Storeng R, Scudiero D, et al. J Natl Cancer Inst 1990;82:1107. [DOI] [PubMed] [Google Scholar]
- 30.Cao Z, Harris N, Kozielski A, Vardeman D, Stehlin JS, Giovanella B. J Med Chem 1998;41:31. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.