Abstract
Aims:
To estimate the level of alcohol consumption behaviors in adult survivors of childhood cancer and to test associations between alcohol consumption behaviors and symptoms of neurocognitive impairment and emotional distress.
Design:
Retrospective cohort study with longitudinal follow-up of self-reported health outcomes.
Setting:
Childhood Cancer Survivor Study (CCSS), a 26-center study of ≥5 year survivors of childhood cancer diagnosed ≤21 years of age between 1970 and 1986 in the United States and Canada.
Participants:
4,484 adult survivors of childhood cancer (mean [SD] age at evaluation = 34.8 [6.1] years; time from diagnosis = 24.8 [4.4] years) and 1,706 sibling controls who completed surveys reporting on alcohol use, neurocognitive impairment, and emotional distress.
Measurements:
Survivor report of alcohol use included age at drinking initiation and quantity and frequency of alcohol consumption. Neurocognition was assessed using the CCSS Neurocognitive Questionnaire. Emotional distress symptoms were measured using the Brief Symptoms Inventory – 18 and the Posttraumatic Stress Diagnostic Scale.
Findings:
After adjustment for childhood cancer treatment exposures, including cranial radiation therapy, drinking initiation prior to 18 years of age was associated with 30% increased risk of subsequent memory problems (Risk Ratio (RR) = 1.3; 95% Confidence Interval (CI), 1.1 – 1.5). Younger age at drinking initiation was associated with future risk of depression (RR = 1.3; 95% CI, 1.1 – 1.5), anxiety (RR = 1.6; 95% CI, 1.3 – 2.1), and somatization (RR = 1.2; 95% CI, 1.1 – 1.4). Persistent heavy/risky drinking was associated with 80% increased risk of persistent psychological distress (RR = 1.8, 95% CI, 1.4 – 2.3).
Conclusions:
Drinking initiation during adolescence is associated with modest increased risk for memory impairment and emotional distress in adult survivors of childhood cancer.
Introduction
It is well-established that survivors of childhood cancer are at-risk of developing neurocognitive morbidities that persist into adulthood.(1–6) Cancer-specific risk factors include disease involvement of the central nervous system (CNS) and/or treatment with neurotoxic agents (i.e., cranial radiation, antimetabolite chemotherapy) or neurosurgical procedures, particularly when these treatments occur at a young age.(7) Importantly, cancer survivors also are susceptible to factors that affect cognition in the general population, including aging,(8, 9) chronic health conditions,(10–12) and lifestyle factors such as physical activity,(13, 14) sleep,(15) and alcohol use.(16) It remains to be determined whether these lifestyle factors have a differential impact on survivor cognitive functioning given their potential vulnerability following exposure to neurotoxic cancer-directed therapies.
In the Childhood Cancer Survivor Study (CCSS) cohort, 16% of adult survivors reported risky drinking and eight percent reported heavy drinking at their baseline evaluation.(17) While the longitudinal trajectory of these drinking patterns has not yet been reported, heavy drinking and chronic alcohol use are strongly associated with neurocognitive impairment in non-cancer adult populations.(18) These impairments include difficulties with memory, attention, processing speed, executive functions, and visuospatial abilities.(19–21) In addition to the observed effects on performance-based neurocognitive tasks, patterns of brain activation as well as reduced gray and white matter volumes have been associated with heavy alcohol use.(22, 23) Moreover, individuals who have sustained traumatic brain injuries appear to have increased sensitivity to the effects of alcohol.(24, 25) As childhood cancer survivors who received neurotoxic cancer treatments such as cranial radiation may have sustained diffuse cerebral injury, they also may have increased sensitivity to the effects of alcohol on cognitive processes. However, to date, the impact of chronic, ongoing risky or heavy drinking on neurocognitive function has not been evaluated in aging adult survivors of childhood cancer.
Beyond potential adverse effects on cognition, alcohol consumption has been implicated in the emergence, persistence, and worsening of mental health conditions such as depression and anxiety.(26) Up to 40% of adults in the general population who seek treatment for alcohol use disorders have at least one independent mood disorder(27) and roughly 20% have an alcohol-induced mood or anxiety disorder. Results from a 25-year longitudinal study suggest a causal pathway from alcohol abuse or dependence to major depression,(28) though other studies have suggested reciprocal causation.(26)
Given survivors’ risk for treatment-induced neurocognitive impairment, the identification of modifiable lifestyle factors that may exacerbate or mitigate such deficits is important for informing the selection and/or development of cognitive intervention strategies. Moreover, understanding associations between health behaviors, such as alcohol consumption and psychological health has the potential to similarly inform mental health interventions for this population. Therefore, the aims of the current study were to (1) estimate the level of alcohol consumption behaviors in adult survivors of childhood cancer, (2) compare alcohol consumption behaviors between survivors and a randomly selected sample of sibling controls, (3) test associations between alcohol consumption behaviors and neurocognitive impairment in survivors, and (4) test associations between alcohol consumption behaviors and symptoms of emotional distress in survivors.
Methods
Design
The CCSS is a multi-institutional retrospective cohort study with longitudinal follow-up of survivors of childhood cancer recruited from 26 institutions across North America, including the United States and Canada. The institutional review board at each participating institution approved the CCSS protocol, and all participants provided informed consent. Medical record abstraction is performed for primary diagnosis, chemotherapy, radiation therapy, and surgical procedures. Survivors complete comprehensive surveys reporting on demographics, health care utilization, health outcomes, health behaviors, and psychosocial outcomes. A detailed description of the cohort methodology and study design has been reported previously.(29, 30)
Participants
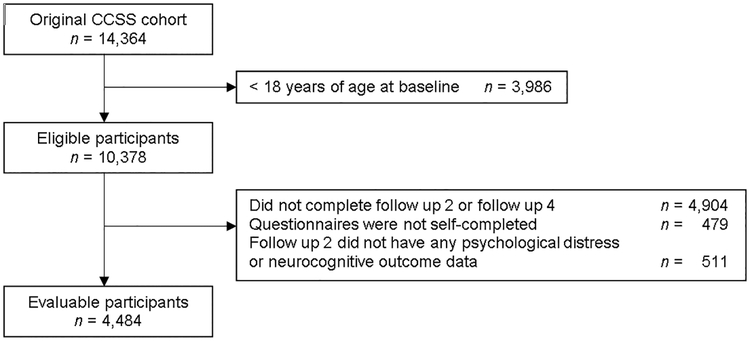
The CCSS cohort consists of survivors diagnosed with childhood cancer before age 21 between January 1, 1970 and December 31, 1986 who survived at least five years after their primary cancer diagnosis. The sibling comparison cohort was recruited from among a randomly selected subset of survivors. Survivors were eligible for the current analysis if they were at least 18 years of age at baseline and completed the Baseline, Follow-up 2, and Follow-up 4 surveys, which included questions related to alcohol consumption (see CONSORT diagram). The final study sample included 4,484 survivors and 1,651 siblings; however, the analysis samples for multivariable models varied slightly based on the missing data for specific predictors and outcomes. Outcomes
Neurocognitive functioning was measured using the CCSS Neurocognitive Questionnaire (CCSS-NCQ),(31) a 25-item questionnaire that provides a 3-point Likert scale (0=never a problem to 2=often a problem) for self-rating of neurocognitive problems. The CCSS-NCQ is comprised of four primary factors: task efficiency, emotion regulation, organization, and memory. The CCSS-NCQ was only administered at Follow-up 2. Consistent with previous CCSS reports, impairment was defined as a score falling above the 90th percentile based on values obtained in the sibling cohort.
Emotional distress was measured by the Brief Symptom Inventory-18 (BSI)(32) and the Posttraumatic Stress Diagnostic Scale (PDS).(33) The BSI includes subscales of anxiety, depression, and somatization. Using sex-specific normative data, distress on the BSI was defined as a T-score of 63 or greater (equivalent to distress in the highest 10% of the normative sample). Persistent/increasing emotional distress was defined as previously reported,(34) and longitudinally persisting distress (T-score ≥ 63 at Baseline, Follow-up 2, and Follow-up 4), and/or increasing emotional distress (non-significant distress at Baseline [T-score < 63] that increased at Follow-up 2 or Follow-up 4) for any of the three BSI subscales (depression, anxiety, somatization). The PDS is a 17-item measure of diagnostic symptom criteria listed in the Diagnostic and Statistical Manual for Psychiatric Disorders, 4th Edition (DSM-IV).(35) Consistent with prior CCSS reports, a positive endorsement of PTSS was defined by the report of at least one re-experiencing symptom, at least three avoidance symptoms and at least two arousal symptoms, with or without functional impairment.(36, 37)
Predictors
Consistent with past CCSS reports(17, 38) and the National Institute for Alcohol Abuse and Alcoholism (NIAAA) risky drinking was defined as >4 drinks per day or 14 drinks per week for men and >3 drinks per day or 7 drinks per week for women.(39) Heavy drinking was defined as ≥6 drinks per day for men and ≥5 drinks per day for women, at least once per month. Persistent heavy/risky drinking was defined as heavy and/or risky drinking at Baseline and at the Follow-up 4. Age at drinking initiation referred to the year that the participant reported having their first drink on the Baseline survey, and was dichotomized as <18 years of age or ≥18 years of age.
Covariates
For models including neurocognitive outcomes, covariates included age at Follow-up 2 (NCQ data completion), sex, age at diagnosis, physical health (poor/fair vs. good/very good/excellent), and cancer-related pain (none, small amount vs. medium amount, a lot, very bad/excruciating), which were ascertained at Follow-up 2. Neurocognitive models were stratified by cranial radiation therapy (CRT) exposure (yes vs. no). In the CRT model, radiation dose was included per 10Gy. In the non-CRT model, intravenous methotrexate dose per 100g/m2 and number of intrathecal injections were considered. For emotional distress models, covariates included age at Follow-up 2, sex, race, age at diagnosis, educational attainment (≤ high school; post-high school training; ≥college graduate), employment (unemployed; caring for family; part-time; full-time), cancer-related pain, and physical health status. Emotional distress models were adjusted for radiation exposure (none, non-cranial, and CRT [≤20Gy, >20Gy]).
Statistical Analysis
Demographic, diagnosis, treatment and health-related characteristics of participants and non-evaluable participants were compared using t-tests and chi-square tests, and descriptive statistics were calculated for all exposure, covariate and outcome variables. Associations between alcohol consumption and neurocognitive function and relative risks (RR) and corresponding 95% confidence intervals (CI) were estimated using Poisson regression modeling with robust error variance to account for potentially correlated observations.(40, 41) Because CRT and sex have been associated with both alcohol use and neurocognitive impairment in adult survivors of childhood cancer, we examined potential interaction effects. To examine the associations between alcohol consumption and emotional distress symptoms, RRs and corresponding 95% CIs were estimated using the same modified Poisson models as described above. All multivariable models were adjusted for a minimally sufficient set of covariates to reduce confounding bias. A Bonferroni correction was applied to multivariable models. For neurocognitive models with four primary outcomes, P≤0.0125 was considered statistically significant. For emotional distress models with five primary outcomes P≤0.01 was considered statistically significant. We did not adjust the significance threshold for number of covariates because these were select a priori and not tested in an exploratory manner.(42) Separate multivariable models were conducted with terms for primary cancer diagnosis included and terms for treatment exposures omitted. Analyses were performed using SAS version 9.4.
Results
Survivors (N=4,484) were a mean [standard deviation] of 10.5 [5.6] years of age at diagnosis, 27.2 [6.2] years of age at baseline, and 34.8 [6.1] years of age at follow-up 2 (Table 1). Thirty percent of survivors were diagnosed with leukemia, 28% with lymphoma, and 10% with a central nervous system tumor. One-third of survivors were treated with cranial irradiation (22% with exposure >20Gy).
Table 1.
Characteristics of evaluable study participants (n=4,484)
| Mean | SD | |
|---|---|---|
| Age at Baseline | 27.2 | 6.2 |
| Age at Diagnosis | 10.5 | 5.6 |
| Age at Follow-up 2 | 34.8 | 6.1 |
| Age at Follow-up 4 | 39.5 | 6.0 |
| Frequency | % | |
| Sex | ||
| Female | 2120 | 47.3 |
| Male | 2364 | 52.7 |
| Race/Ethnicity | ||
| White/non-Hispanic | 3931 | 88.0 |
| Other | 536 | 12.0 |
| Diagnosis | ||
| Leukemia | 1353 | 30.2 |
| CNS Tumor | 453 | 10.1 |
| Hodgkin Lymphoma | 874 | 19.5 |
| Non-Hodgkin Lymphoma | 382 | 8.5 |
| Wilms Tumor | 290 | 6.5 |
| Neuroblastoma | 165 | 3.7 |
| Soft tissue sarcoma | 456 | 10.2 |
| Bone tumors | 511 | 11.4 |
| Radiation | ||
| None | 1240 | 29.9 |
| Non-cranial | 1435 | 34.6 |
| ≤20Gy Cranial | 523 | 12.6 |
| >20Gy Cranial | 946 | 22.8 |
| Intravenous Methotrexate | ||
| No | 3359 | 80.9 |
| Yes | 791 | 19.1 |
| No. Intrathecal Injections | ||
| None | 2819 | 66.7 |
| 1 | 1052 | 24.9 |
| ≥2 | 355 | 8.4 |
| Educational Attainment | ||
| ≤High School | 509 | 11.4 |
| Post-high school training | 1377 | 30.9 |
| ≥College graduate | 2565 | 57.6 |
| Employment | ||
| Unemployed | 425 | 9.6 |
| Caring for family | 301 | 6.8 |
| Part-time | 393 | 8.9 |
| Full-time | 3318 | 74.8 |
| Cancer-related pain | ||
| None, small amount | 3992 | 89.7 |
| Medium amount, a lot, very bad | 460 | 10.3 |
| Physical health status | ||
| Poor, fair | 599 | 13.4 |
| Good, very good, excellent | 3872 | 86.6 |
Alcohol consumption patterns
Compared with siblings, survivors were significantly less likely to report heavy drinking (P = 0.002), risky drinking (P < 0.001), persistent heavy/risky drinking (P < 0.001), and consuming their first drink before 18 years of age (P = 0.002; Table 2). Survivors treated with CRT were significantly less likely to report drinking before 18 years of age (P <0.001), heavy drinking (P <0.001), risky drinking (P <0.001), and persistent heavy/risky drinking (P <0.001) compared to survivors who did not receive CRT (Table S1). Females were significantly less likely to reported early drinking initiation (P <0.001), heavy/risky drinking at baseline (P <0.001), or persistent heavy/risky drinking (P = 0.001; Table S2) compared with males.
Table 2.
Prevalence of alcohol consumption behaviors and neurocognitive impairment and emotional distress among survivors and siblings
| Survivors | Siblings | ||||
|---|---|---|---|---|---|
| n | % | n | % | P-value | |
| Age at Drinking Initiation | 0.002 | ||||
| <18 years | 2077 | 48.9 | 881 | 53.4 | |
| ≥18 years | 2174 | 51.1 | 770 | 46.6 | |
| Heavy Drinking (baseline) | 0.002 | ||||
| Yes | 304 | 7.1 | 156 | 9.5 | |
| No | 3962 | 92.9 | 1485 | 90.5 | |
| Risky Drinking (baseline) | 0.001 | ||||
| Yes | 647 | 14.8 | 305 | 18.2 | |
| No | 3711 | 85.2 | 1367 | 81.8 | |
| Persistent heavy/risky drinking | <0.001 | ||||
| Yes | 274 | 6.2 | 147 | 8.7 | |
| No | 4134 | 93.8 | 1534 | 91.3 | |
| Neurocognitive impairmenta | n/a | ||||
| Task efficiency | 890 | 20.7 | --- | --- | |
| Emotion regulation | 475 | 11.0 | --- | --- | |
| Organization skills | 508 | 11.8 | --- | --- | |
| Memory | 579 | 13.5 | --- | --- | |
| Emotional distress | |||||
| Depressiona | 474 | 10.6 | 106 | 6.2 | <0.001 |
| Anxietya | 296 | 6.6 | 83 | 4.9 | 0.010 |
| Somatizationa | 602 | 13.5 | 109 | 6.4 | <0.001 |
| Posttraumatic stressb,c | 696 | 16.8 | 10 | 4.0 | <0.001 |
| Persistent/increasing emotional distress | |||||
| Depression | 257 | 6.0 | 61 | 3.7 | <0.001 |
| Anxiety | 160 | 3.7 | 43 | 2.6 | 0.031 |
| Somatization | 306 | 7.1 | 54 | 3.3 | <0.001 |
| Depression, anxiety, and/or somatization | 538 | 12.5 | 112 | 6.8 | <0.001 |
Impairment defined as level of symptoms above the 90th percentile of the reference group.
Impairment defined as ≥1 re-experiencing symptom, ≥3 avoidance symptoms, and ≥2 arousal symptoms.
Data only available for 248 siblings.
Neurocognitive and emotional distress outcomes
Twenty-one percent of survivors reported impairment in task efficiency, 11% in emotion regulation, 12% in organization skills, and 14% in memory (Table 2). Compared with siblings survivors reported a higher prevalence of depressive (P < 0.001, somatic (P < 0.001), and posttraumatic stress symptoms (P < 0.001). Thirteen percent of survivors experienced persistent or increasing distress symptoms characterized by elevated symptoms of depression, anxiety, and/or somatization compared to 7% of siblings (P < 0.001). Table S2 shows neurocognitive and emotional distress symptoms by sex. Tables S3 & S4 show the proportion of survivors with impaired neurocognitive function and emotional distress for each alcohol consumption behavior by CRT exposure, respectively.
Alcohol consumption and neurocognitive impairment
Assessment of interaction terms revealed no significant interactions between CRT exposure and alcohol consumption patterns or sex and alcohol consumptions patterns with neurocognitive impairment; therefore interaction terms were not included in our final models nor were analyses stratified by CRT or sex. In multivariable models adjusted for treatment exposures, associations between alcohol consumption behaviors and neurocognition largely failed to achieve statistical significance (Table 3). However, drinking initiation at younger than 18 years of age was associated with a 30% increased risk of memory impairment (P=0.003). Heavy/risky drinking at baseline and persistent heavy/risky drinking did not confer increased risk of neurocognitive impairment.
Table 3.
Associations between alcohol consumption behaviors and neurocognitive function among survivors of childhood cancer
| Task Completion |
Emotional Regulation |
Organization Skills |
Memory | |
|---|---|---|---|---|
| RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | |
| Model 1: Age at drinking initiation (<18 years) | ||||
| <18 years | 1.0 (0.9–1.1) | 1.2 (1.0–1.4) | 1.1 (1.0–1.3) | 1.3 (1.1–1.5) |
| ≥18 years | 1.0 | 1.0 | 1.0 | 1.0 |
| Sex | ||||
| Male | 1.0 | 1.0 | 1.0 | 1.0 |
| Female | 1.3 (1.2–1.5) | 1.80 (1.5–2.2) | 1.2 (1.0–1.4) | 1.5 (1.1–1.7) |
| Physical health | ||||
| Good, very good, excellent | 1.0 | 1.0 | 1.0 | 1.0 |
| Poor, fair | 2.2 (1.9–2.5) | 2.3 (1.8–2.8) | 2.0 (1.7–2.7) | 2.2 (1.8–2.7) |
| Cancer-related pain | ||||
| None, small amount | 1.0 | 1.0 | 1.0 | 1.0 |
| Medium amount, a lot, very bad | 1.5 (1.3–1.8) | 1.7 (1.3–2.1) | 1.2 (0.9–1.5) | 1.5 (1.2–1.9) |
| CRT does per 10gy | 1.2 (1.1–1.2) | 1.0 (1.0–1.1) | 1.0 (1.0–1.1) | 1.2 (1.1–1.3) |
| IV MTX does per 100g/m2 | 1.0 (0.8–1.3) | 0.9 (0.7–1.2) | 1.1 (0.9–1.4) | 1.0 (0.7–1.4) |
| No. IT injections | 1.0 (0.9–1.1) | 1.2 (1.0–1.3) | 1.0 (0.9–1.2) | 1.2 (1.0–1.3) |
| Model 2: Heavy/risky drinking (baseline) | ||||
| No | 1.0 | 1.0 | 1.0 | 1.0 |
| Yes | 0.9 (0.8–1.1) | 1.1 (0.9–1.4) | 0.8 (0.6–1.1) | 0.9 (0.7–1.2) |
| Sex | ||||
| Male | 1.0 | 1.0 | 1.0 | 1.0 |
| Female | 1.3 (1.1–1.5) | 1.8 (1.5–2.1) | 1.1 (0.9–1.3) | 1.4 (1.2–1.7) |
| Physical health | ||||
| Good, very good, excellent | 1.0 | 1.0 | 1.0 | 1.0 |
| Poor, fair | 2.1 (1.9–2.5) | 2.3 (1.9–2.9) | 2.1 (1.7–2.6) | 2.2 (1.8–2.6) |
| Cancer-related pain | ||||
| None, small amount | 1.0 | 1.0 | 1.0 | 1.0 |
| Medium amount, a lot, very bad | 1.5 (1.3–1.8) | 1.6 (1.3–2.1) | 1.2 (0.9, 1.5) | 1.7 (1.3–2.0) |
| CRT does per 10gy | 1.2 (1.1–1.2) | 1.0 (1.0–1.1) | 1.0 (1.0–1.1) | 1.2 (1.1–1.2) |
| IV MTX does per 100g/m2 | 1.0 (0.8–1.3) | 0.9 (0.7–1.2) | 1.1 (0.9–1.4) | 1.0 (0.7–1.4) |
| No. IT injections | 1.0 (1.0–1.1) | 1.2 (1.0–1.3) | 1.0 (0.9–1.2) | 1.1 (1.0–1.3) |
| Model 3: Persistent heavy/risky drinking | ||||
| No | 1.0 | 1.0 | 1.0 | 1.0 |
| Yes | 0.9 (0.7–1.2) | 1.2 (0.9–1.7) | 1.1 (0.8–1.6) | 1.1 (0.8–1.6) |
| Sex | ||||
| Male | 1.0 | 1.0 | 1.0 | 1.0 |
| Female | 1.3 (1.2–1.5) | 1.8 (1.5–2.2) | 1.2 (1.0–1.4) | 1.4 (1.2–1.7) |
| Physical health | ||||
| Good, very good, excellent | 1.0 | 1.0 | 1.0 | 1.0 |
| Poor, fair | 2.1 (1.8–2.4) | 2.3 (1.8–2.8) | 2.0 (1.6–2.5) | 2.1 (1.8–2.6) |
| Cancer-related pain | ||||
| None, small amount | 1.0 | 1.0 | 1.0 | 1.0 |
| Medium amount, a lot, very bad | 1.5 (1.3–1.8) | 1.6 (1.3–2.1) | 1.2 (0.9–1.5) | 1.6 (1.3–2.0) |
| CRT does per 10gy | 1.2 (1.1–1.2) | 1.0 (1.0–1.1) | 1.0 (1.0–1.1) | 1.2 (1.1–1.2) |
| IV MTX does per 100g/m2 | 1.0 (0.8–1.2) | 0.9 (0.6–1.2) | 1.1 (0.9–1.4) | 1.0 (0.7–1.4) |
| No. IT injections | 1.1 (1.0–1.1) | 1.2 (1.0–1.3) | 1.0 (0.9–1.2) | 1.1 (1.0–1.3) |
Abbreviations: RR=relative risk; CI=confidence interval, CRT=cranial radiation therapy, IV = intravenous, MTX = methotrexate, IT = intrathecal. Separate models presented for age at drinking initiation, heavy/risky drinking, persistent heavy/risk drinking. All models adjusted for age at diagnosis and age at follow-up. Bold font denotes statistical significance at P ≤ 0.0125.
Alcohol consumption and emotional distress
In multivariable models adjusted for CRT dose (Table 4), younger age at drinking initiation (<18 years) was associated with a 30% increased risk of depression (P=0.007), 60% anxiety (P<0.001), and 20% somatization (P=0.007). Heavy/risky drinking at baseline was associated with a 40% increased risk of persistent/increasing emotional distress (P=0.008). Persistent heavy/risky drinking was associated with a nearly 2-fold increased risk of persistent/increasing emotional distress (P<0.001).
Table 4.
Associations between alcohol consumption behaviors and emotional distress symptoms among survivors
| Depression | Anxiety | Somatization | Posttraumatic Stress | Persistent/Increasing | |
|---|---|---|---|---|---|
| RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | |
| Model 1: Age at drinking initiation (<18 years) | |||||
| <18 years | 1.3 (1.1–1.5) | 1.6 (1.3–2.1) | 1.2 (1.1–1.4) | 1.1 (1.0–1.3) | 1.2 (1.0–1.4) |
| ≥18 years | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Sex | |||||
| Male | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Female | 0.8 (0.7–1.0) | 0.9 (0.7–1.1) | 1.4 (1.2–1.7) | 1.2 (1.1–1.4) | 1.1 (0.9–1.3) |
| Physical health | |||||
| Good, very good, excellent | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Poor, fair | 2.6 (2.1–3.2) | 3.0 (2.3–4.0) | 3.5 (2.9–4.2) | 2.0 (1.7–2.4) | 2.2 (1.8–2.8) |
| Cancer-related pain | |||||
| None, small amount | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Medium amount, a lot, very bad | 1.4 (1.1–1.8) | 1.8 (1.3–2.4) | 2.3 (1.9–2.8) | 1.9 (1.6–2.3) | 1.7 (1.4–2.2) |
| Radiation | |||||
| None | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Non-cranial | 0.8 (0.7–1.0) | 0.8 (0.6–1.1) | 1.2 (1.0–1.4) | 1.0 (0.9–1.3) | 1.1 (0.9–1.4) |
| CRT ≤ 20Gy | 0.9 (0.7–1.2) | 0.8 (0.5–1.2) | 1.6 (1.2–2.0) | 1.2 (1.0–1.5) | 1.1 (0.8–1.4) |
| CRT > 20Gy | 1.1 (0.8–1.3) | 0.8 (0.6–1.1) | 1.2 (0.9–1.5) | 1.1 (0.9–1.3) | 1.2 (1.0–1.5) |
| Model 2: Heavy/risky drinking (baseline) | |||||
| No | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Yes | 1.2 (1.0–1.5) | 1.3 (1.0–1.7) | 1.2 (1.0–1.4) | 1.2 (1.0–1.4) | 1.4 (1.1–1.6) |
| Sex | |||||
| Male | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Female | 0.8 (0.6–0.9) | 0.8 (0.6–1.0) | 1.4 (1.2–1.6) | 1.2 (1.1–1.4) | 1.1 (0.9–1.3) |
| Physical health | |||||
| Good, very good, excellent | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Poor, fair | 2.6 (2.1–3.2) | 3.2 (3.4–4.3) | 3.5 (3.0–4.3) | 1.9 (1.6–2.2) | 2.3 (1.8–2.8) |
| Cancer-related pain | |||||
| None, small amount | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Medium amount, a lot, very bad | 1.5 (1.2–1.8) | 1.8 (1.3–2.4) | 2.3 (1.9–2.7) | 2.0 (1.6–2.3) | 1.8 (1.4–2.2) |
| Radiation | |||||
| None | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Non-cranial | 0.8 (0.6–1.0) | 0.8 (0.6–1.1) | 1.1 (0.9–1.4) | 1.1 (0.9–1.3) | 1.1 (0.9–1.4) |
| CRT ≤ 20Gy | 0.9 (0.7–1.2) | 0.7 (0.5–1.1) | 1.5 (1.2–2.0) | 1.2 (1.0–1.6) | 1.0 (0.8–1.4) |
| CRT > 20Gy | 1.0 (0.8–1.3) | 0.7 (0.5–1.0) | 1.1 (0.9–1.4) | 1.1 (0.9–1.3) | 1.1 (0.9–1.4) |
| Model 3: Persistent heavy/risky drinking | |||||
| No | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Yes | 1.2 (0.9–1.7) | 1.5 (1.0–2.1) | 1.0 (0.8–1.4) | 1.2 (1.0–1.6) | 1.8 (1.4–2.3) |
| Sex | |||||
| Male | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Female | 0.8 (0.6–0.9) | 0.8 (0.6–1.0) | 1.4 (1.2–1.6) | 1.2 (1.1–1.4) | 1.1 (0.9–1.3) |
| Physical health | |||||
| Good, very good, excellent | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Poor, fair | 2.6 (2.1–3.2) | 3.2 (2.4–4.3) | 3.6 (3.0–4.3) | 1.9 (1.6–2.2) | 2.2 (1.8–2.8) |
| Cancer-related pain | |||||
| None, small amount | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Medium amount, a lot, very bad | 1.4 (1.2–1.8) | 1.7 (1.3–2.3) | 2.2 (1.9–2.7) | 2.0 (1.7–2.3) | 1.8 (1.5–2.2) |
| Radiation | |||||
| None | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Non-cranial | 0.9 (0.7–1.2) | 0.8 (0.6–1.1) | 1.1 (0.9–1.4) | 1.0 (0.9–1.2) | 1.1 (0.9–1.4) |
| CRT ≤ 20Gy | 0.8 (0.6–1.2) | 0.7 (0.5–1.1) | 1.5 (1.2–1.9) | 1.2 (1.0–1.5) | 1.1 (0.8–1.5) |
| CRT > 20Gy | 1.0 (0.8–1.3) | 0.8 (0.6–1.0) | 1.1 (0.9–1.3) | 1.1 (0.9–1.3) | 1.1 (0.9–1.4) |
Abbreviations: RR=relative risk; CI=confidence interval; CRT=cranial radiation therapy. Separate models presented for age at drinking initiation, heavy/risky drinking, persistent heavy/risk drinking. All models adjusted for race, age at diagnosis, age at follow-up, educational attainment, and employment status. Bold font denotes statistical significance at P ≤ 0.0
Discussion
The current study examined the association of alcohol consumption behaviors with neurocognitive and emotional functioning in adult survivors of childhood cancer. Our findings suggest that associations between alcohol consumption and neurocognition are relatively modest. In contrast, alcohol consumption was consistently associated with increased risk of emotional distress symptoms in long-term survivors.
Drinking initiation prior to 18 years of age was associated with 30% increased risk of memory impairment. This risk was observed while accounting for the effects of known neurotoxic treatments (i.e. CRT, methotrexate). The effects of heavy drinking on cognition may be especially salient during adolescence given the continued maturation of the brain during this stage of development. Animal studies have shown decreased neurogenesis in the adolescent forebrain and hippocampus following ethanol exposure.(43) In human adolescents, the prefrontal cortex and hippocampal volumes appear reduced in heavy drinkers. Moreover, longitudinal studies have demonstrated that persistent heavy, chronic alcohol use from adolescence to young adulthood is associated with visuospatial and memory deterioration.(44) It has been previously established that survivors of childhood cancer who initiate alcohol use during adolescence have a two-fold increased risk for later heavy drinking compared to siblings who initiate drinking at the same age.(17) Although we did not observe an association between heavy/risky drinking and self-reported memory impairment in our study, we speculate that survivors of childhood cancer who initiate drinking in adolescence may be at increased risk for memory problems due to: 1) increased risk for later heavy drinking and associated neurocognitive morbidities and/or 2) the direct effects of alcohol on brain maturation in a potentially compromised nervous system following exposure to neurotoxic cancer treatments.
We observed consistent associations between alcohol consumption patterns (i.e. younger age at drinking initiation, heavy/risky drinking at baseline, persistent heavy/risky drinking) and increased risk of emotional distress symptoms. Similar associations have been reported in non-cancer populations,(45, 46) however, causal pathways are often difficult to discern and may be reciprocal. For example, symptoms of depression may lead to alcohol consumption through efforts to manage emotional symptoms (e.g. self-medication). Alternatively, the consumption of alcohol may result in neurochemical changes in the brain resulting in depressive symptoms (i.e. interference with serotonin uptake).(26, 47) Additional prospective longitudinal studies are necessary to further elucidate these associations in survivors of childhood cancer.
Independent of alcohol consumption patterns, survivors who reported cancer-related pain and poor physical health status were significantly more likely to report neurocognitive problems and emotional distress symptoms. For example, poor health status was associated with an approximately 2-fold increased likelihood of neurocognitive impairment for survivors. This is consistent with data from the general population indicating that health conditions, such as cardiopulmonary disease, are associated with deficits in memory, executive functions, and processing speed. Because survivors are at increased risk of developing chronic health conditions future efforts should aim to better understand the impact of these highly prevalent health morbidities on neurocognitive functioning.(48) Cancer-related pain and poor health also have been associated with distress symptoms in past studies,(49–51) however, similar to alcohol consumption patterns, the reciprocal associations between these constructs have not adequately been investigated in survivors.
The findings of the current analysis should be considered in the context of several limitations. First, we relied on self-report of neurocognitive problems, which makes our outcomes vulnerable to misclassification. In addition, recall bias may influence self-report of alcohol consumption behaviors, particularly among survivors with memory problems. Moreover, survivors may not accurately report their drinking behaviors, particularly in the context of a study designed to evaluate health outcomes in survivors of childhood cancer. For example, recent data demonstrate that rates of misclassification of self-reported smoking status among survivors range from 8% to 37% for past and current smokers, respectively.(52) Results of potential misclassification (i.e. underreporting) may have reduced our ability to detect associations between alcohol use patterns and our outcomes. Results also may be influenced by selection bias and loss to follow-up in our cohort. These factors may limit the external validity of our results. A comparison of evaluable study participants and non-evaluable participants indicated that participants in the current analysis were more likely to be female (52.7% vs. 41.3%), and less likely to have a CNS tumor diagnosis (10.1% vs. 14.7%) or treatment with cranial radiation (35.4% vs. 40.8%; Table S5). These differences may have resulted in an underestimation of alcohol consumption in survivors because women, in general, consume less alcohol and are more likely to abstain.(17) In fact, female survivors in our study were less likely to engage in alcohol consumption behaviors than were males survivors, yet they were more likely to report neurocognitive problems. In addition, past CCSS data suggest that survivors who received potentially neurotoxic treatments are less likely to engage in risky/heavy drinking; however, these same survivors are more likely to experience neurocognitive problems in adulthood.
Limitations notwithstanding, our results underscore the importance of considering modifiable lifestyle factors, such as alcohol consumption, when selecting or implementing interventions to treat psychological late effects. Beyond cognitive and emotional consequences, alcohol consumption also has been associated with oropharyngeal, esophageal, liver, and stomach cancers. Screening for engagement in risky health behaviors should begin early in survivorship and survivors should be proactively counseled on the risks of excessive alcohol consumption. Moreover, targeted efforts to prevent survivors from engaging in risky health behaviors, particularly beginning in adolescence, may modify the effects of childhood treatment exposures on neurocognitive and emotional functioning in aging adult survivors.
Supplementary Material
Figure 1.
Consort diagram of study participation.
Acknowledgements:
This work was supported by the National Cancer Institute (CA55727, G.T. Armstrong, Principal Investigator). Support to St. Jude Children’s Research Hospital also provided by the Cancer Center Support (CORE) grant (CA21765, C. Roberts, Principal Investigator) and the American Lebanese Syrian Associated Charities (ALSAC).
Footnotes
Disclosures: The authors have no conflicts of interest to disclose.
References
- 1.Armstrong GT, Reddick WE, Petersen RC, Santucci A, Zhang N, Srivastava D, et al. Evaluation of memory impairment in aging adult survivors of childhood acute lymphoblastic leukemia treated with cranial radiotherapy. Journal of the National Cancer Institute. 2013;105(12):899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kadan-Lottick NS, Zeltzer LK, Liu Q, Yasui Y, Ellenberg L, Gioia G, et al. Neurocognitive functioning in adult survivors of childhood non-central nervous system cancers. Journal of the National Cancer Institute. 2010;102(12):881–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krull KR, Brinkman TM, Li C, Armstrong GT, Ness KK, Srivastava DK, et al. Neurocognitive outcomes decades after treatment for childhood acute lymphoblastic leukemia: a report from the St Jude lifetime cohort study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2013;31(35):4407–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krull KR, Sabin ND, Reddick WE, Zhu L, Armstrong GT, Green DM, et al. Neurocognitive function and CNS integrity in adult survivors of childhood hodgkin lymphoma. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2012;30(29):3618–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krull KR, Zhang N, Santucci A, Srivastava DK, Krasin MJ, Kun LE, et al. Long-term decline in intelligence among adult survivors of childhood acute lymphoblastic leukemia treated with cranial radiation. Blood. 2013;122(4):550–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brinkman TM, Krasin MJ, Liu W, Armstrong GT, Ojha RP, Sadighi ZS, et al. Long-Term Neurocognitive Functioning and Social Attainment in Adult Survivors of Pediatric CNS Tumors: Results From the St Jude Lifetime Cohort Study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2016;34(12):1358–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schreiber JE, Gurney JG, Palmer SL, Bass JK, Wang M, Chen S, et al. Examination of risk factors for intellectual and academic outcomes following treatment for pediatric medulloblastoma. Neuro Oncol. 2014;16(8):1129–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woods AJ, Cohen RA, Pahor M. Cognitive frailty: frontiers and challenges. J Nutr Health Aging. 2013;17(9):741–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schonknecht P, Pantel J, Kruse A, Schroder J. Prevalence and natural course of aging-associated cognitive decline in a population-based sample of young-old subjects. The American journal of psychiatry. 2005;162(11):2071–7. [DOI] [PubMed] [Google Scholar]
- 10.Debette S, Bauters C, Leys D, Lamblin N, Pasquier F, de GP. Prevalence and determinants of cognitive impairment in chronic heart failure patients. Congest Heart Fail. 2007;13(4):205–8. [DOI] [PubMed] [Google Scholar]
- 11.Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42(9):2672–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaffashian S, Dugravot A, Nabi H, Batty GD, Brunner E, Kivimaki M, et al. Predictive utility of the Framingham general cardiovascular disease risk profile for cognitive function: evidence from the Whitehall II study. Eur Heart J. 2011;32(18):2326–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Edwards MK, Loprinzi PD. Combined associations of sedentary behavior and cardiorespiratory fitness on cognitive function among older adults. Int J Cardiol. 2017;229:71–4. [DOI] [PubMed] [Google Scholar]
- 14.Edwards MK, Loprinzi PD. The Association Between Sedentary Behavior and Cognitive Function Among Older Adults May Be Attenuated With Adequate Physical Activity. J Phys Act Health. 2017;14(1):52–8. [DOI] [PubMed] [Google Scholar]
- 15.Fortier-Brochu E, Morin CM. Cognitive impairment in individuals with insomnia: clinical significance and correlates. Sleep. 2014;37(11):1787–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Friend KB, Malloy PF, Sindelar HA. The effects of chronic nicotine and alcohol use on neurocognitive function. Addict Behav. 2005;30(1):193–202. [DOI] [PubMed] [Google Scholar]
- 17.Lown EA, Goldsby R, Mertens AC, Greenfield T, Bond J, Whitton J, et al. Alcohol consumption patterns and risk factors among childhood cancer survivors compared to siblings and general population peers. Addiction. 2008;103(7):1139–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Woods AJ, Porges EC, Bryant VE, Seider T, Gongvatana A, Kahler CW, et al. Current Heavy Alcohol Consumption is Associated with Greater Cognitive Impairment in Older Adults. Alcoholism, clinical and experimental research. 2016;40(11):2435–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Houston RJ, Derrick JL, Leonard KE, Testa M, Quigley BM, Kubiak A. Effects of heavy drinking on executive cognitive functioning in a community sample. Addict Behav. 2014;39(1):345–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riege WH, Holloway JA, Kaplan DW. Specific memory deficits associated with prolonged alcohol abuse. Alcoholism, clinical and experimental research. 1981;5(3):378–85. [DOI] [PubMed] [Google Scholar]
- 21.Green A, Garrick T, Sheedy D, Blake H, Shores EA, Harper C. The effect of moderate to heavy alcohol consumption on neuropsychological performance as measured by the repeatable battery for the assessment of neuropsychological status. Alcoholism, clinical and experimental research. 2010;34(3):443–50. [DOI] [PubMed] [Google Scholar]
- 22.Sutherland GT, Sheedy D, Kril JJ. Using Autopsy Brain Tissue to Study Alcohol-Related Brain Damage in the Genomic Age. Alcohol Clin Exp Res. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Downer B, Jiang Y, Zanjani F, Fardo D. Effects of alcohol consumption on cognition and regional brain volumes among older adults. Am J Alzheimers Dis Other Demen. 2015;30(4):364–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jorge RE, Starkstein SE, Arndt S, Moser D, Crespo-Facorro B, Robinson RG. Alcohol misuse and mood disorders following traumatic brain injury. Arch Gen Psychiatry. 2005;62(7):742–9. [DOI] [PubMed] [Google Scholar]
- 25.Baguley IJ, Felmingham KL, Lahz S, Gordan E, Lazzaro I, Schotte DE. Alcohol abuse and traumatic brain injury: effect on event-related potentials. Arch Phys Med Rehabil. 1997;78(11):1248–53. [DOI] [PubMed] [Google Scholar]
- 26.Schuckit MA, Tipp JE, Bergman M, Reich W, Hesselbrock VM, Smith TL. Comparison of induced and independent major depressive disorders in 2,945 alcoholics. Am J Psychiatry. 1997;154(7):948–57. [DOI] [PubMed] [Google Scholar]
- 27.Grant BF, Stinson FS, Dawson DA, Chou SP, Dufour MC, Compton W, et al. Prevalence and co-occurrence of substance use disorders and independent mood and anxiety disorders: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Arch Gen Psychiatry. 2004;61(8):807–16. [DOI] [PubMed] [Google Scholar]
- 28.Fergusson DM, Boden JM, Horwood LJ. Tests of causal links between alcohol abuse or dependence and major depression. Arch Gen Psychiatry. 2009;66(3):260–6. [DOI] [PubMed] [Google Scholar]
- 29.Robison LL, Armstrong GT, Boice JD, Chow EJ, Davies SM, Donaldson SS, et al. The Childhood Cancer Survivor Study: a National Cancer Institute-supported resource for outcome and intervention research. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27(14):2308–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robison LL, Mertens AC, Boice JD, Breslow NE, Donaldson SS, Green DM, et al. Study design and cohort characteristics of the Childhood Cancer Survivor Study: a multi-institutional collaborative project. Medical and pediatric oncology. 2002;38(4):229–39. [DOI] [PubMed] [Google Scholar]
- 31.Krull KR, Gioia G, Ness KK, Ellenberg L, Recklitis C, Leisenring W, et al. Reliability and validity of the Childhood Cancer Survivor Study Neurocognitive Questionnaire. Cancer. 2008;113(8):2188–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Derogatis LR. Brief Symptom Inventory (BSI): Administration, scoring, and procedures manual. Minneapolis, MN: NCS Pearson; 2000. [Google Scholar]
- 33.Foa E Posttraumatic stress diagnostic scale manual. Minneapolis, MN: National Computer Systems; 1996. [Google Scholar]
- 34.Brinkman TM, Zhu L, Zeltzer LK, Recklitis CJ, Kimberg C, Zhang N, et al. Longitudinal patterns of psychological distress in adult survivors of childhood cancer. British journal of cancer. 2013;109(5):1373–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.American Psychiatric Association. DSM-IV-TR: Diagnostic and statistical manual of mental disorders, text revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 36.Stuber ML, Meeske KA, Krull KR, Leisenring W, Stratton K, Kazak AE, et al. Prevalence and predictors of posttraumatic stress disorder in adult survivors of childhood cancer. Pediatrics. 2010;125(5):e1124–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stuber ML, Meeske KA, Leisenring W, Stratton K, Zeltzer LK, Dawson K, et al. Defining medical posttraumatic stress among young adult survivors in the Childhood Cancer Survivor Study. Gen Hosp Psychiatry. 2011;33(4):347–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lown EA, Mertens AC, Korcha RA, Leisenring W, Hudson MM, Greenfield TK, et al. Prevalence and predictors of risky and heavy alcohol consumption among adult siblings of childhood cancer survivors. Psycho-oncology. 2013;22(5):1134–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Institute for Alcohol Abuse and Alcoholism. The Physician’s Guide to Helping Patients with Alcohol Problems. Rockville, MD: 1995. [Google Scholar]
- 40.McNutt LA, Wu C, Xue X, Hafner JP. Estimating the relative risk in cohort studies and clinical trials of common outcomes. American journal of epidemiology. 2003;157(10):940–3. [DOI] [PubMed] [Google Scholar]
- 41.Zou G A modified poisson regression approach to prospective studies with binary data. American journal of epidemiology. 2004;159(7):702–6. [DOI] [PubMed] [Google Scholar]
- 42.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–6. [PubMed] [Google Scholar]
- 43.Crews FT, Mdzinarishvili A, Kim D, He J, Nixon K. Neurogenesis in adolescent brain is potently inhibited by ethanol. Neuroscience. 2006;137(2):437–45. [DOI] [PubMed] [Google Scholar]
- 44.Hanson KL, Medina KL, Padula CB, Tapert SF, Brown SA. Impact of Adolescent Alcohol and Drug Use on Neuropsychological Functioning in Young Adulthood: 10-Year Outcomes. J Child Adolesc Subst Abuse. 2011;20(2):135–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rehm J, Room R, Graham K, Monteiro M, Gmel G, Sempos CT. The relationship of average volume of alcohol consumption and patterns of drinking to burden of disease: an overview. Addiction. 2003;98(9):1209–28. [DOI] [PubMed] [Google Scholar]
- 46.Nathan PC, Ford JS, Henderson TO, Hudson MM, Emmons KM, Casillas JN, et al. Health Behaviors, Medical Care, and Interventions to Promote Healthy Living in the Childhood Cancer Survivor Study Cohort. Journal of Clinical Oncology. 2009;27(14):2363–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.LeMarquand D, Pihl RO, Benkelfat C. Serotonin and alcohol intake, abuse, and dependence: Clinical evidence. Biological Psychiatry. 1994;36(5):326–37. [DOI] [PubMed] [Google Scholar]
- 48.Cheung YT, Brinkman TM, Li C, Mzayek Y, Srivastava D, Ness KK, et al. Chronic Health Conditions and Neurocognitive Function in Aging Survivors of Childhood Cancer: A Report from the Childhood Cancer Survivor Study. Journal of the National Cancer Institute. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zaza C, Baine N. Cancer pain and psychosocial factors: a critical review of the literature. Journal of pain and symptom management. 2002;24(5):526–42. [DOI] [PubMed] [Google Scholar]
- 50.D’Agostino NM, Edelstein K, Zhang N, Recklitis CJ, Brinkman TM, Srivastava D, et al. Comorbid symptoms of emotional distress in adult survivors of childhood cancer. Cancer. 2016;122(20):3215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zeltzer LK, Recklitis C, Buchbinder D, Zebrack B, Casillas J, Tsao JC, et al. Psychological status in childhood cancer survivors: a report from the Childhood Cancer Survivor Study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2009;27(14):2396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang I, Klosky JL, Young C, Murphy S, Srivastava DK, Hudson MM, Robison LL. Accuracy of self-reported smoking status in adult survivors of childhood cancer: A report from the St. Jude Lifetime Cohort Study. Journal of clnical oncology. 2017;35(15):10570-. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.