Abstract
Two new sources of ECs were generated from human induced pluripotent stem cells (hiPSCs), and by direct reprogramming of somatic cells without undergoing the stages of stem or progenitor cell. These two types of ECs will advance our understanding of EC biology and can become a novel therapeutic option for treating ischemic cardiovascular diseases.
Keywords: endothelial cells, reprogramming, human pluripotent stem cells
Ischemic cardiovascular disease is the most common health burden worldwide. The discovery of stem or progenitor cells has provided new hope for many patients with advanced diseases, because cell therapy could alleviate ischemia by forming new vessels. To date, earlier studies using endothelial progenitor cells, bone marrow mononuclear cells, or mesenchymal stem cells fell short of generating durable vessels. However, two new developments have emerged for cell-based therapy for cardiovascular disease: human pluripotent stem cell-derived endothelial cells and directly reprogrammed or induced endothelial cells (Figure 1).
Figure 1.
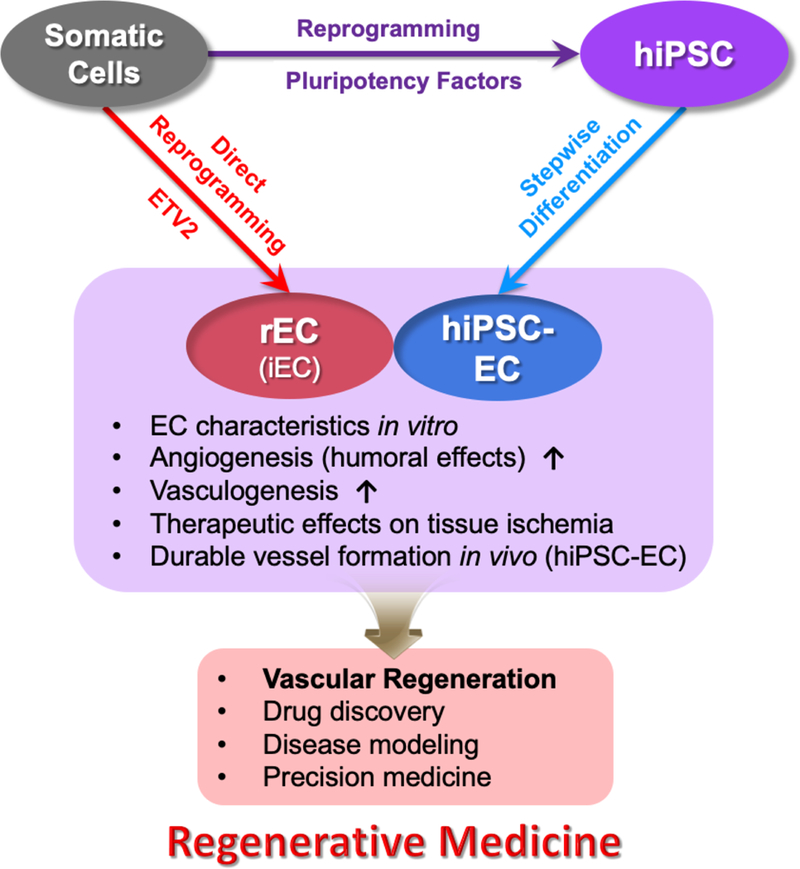
Schematic of two emerging methods for generating ECs from somatic cells and their applications for regenerative medicine
Endothelial Cells Generated from Human Pluripotent Stem Cells (hPSC-ECs)
To generate ECs, hPSCs, which include both embryonic stem cells (hESCs) and induced pluripotent stem cells (iPSCs), should be differentiated into ECs. Two EC differentiation systems have been developed: three-dimensional (3D) embryoid body (EB)-mediated differentiation and two-dimensional (2D) monolayer-directed differentiation. In EB-mediated differentiation methods, ESCs are permitted to differentiate spontaneously into a mass (EBs) of multiple lineage cells by suspension culture, and thus the yield of ECs is generally low and inconsistent1. 2D monolayer systems allow more homogeneous exposure of cells to the differentiation medium, generating higher and more consistent yields of ECs. 2D systems also employ stepwise differentiation methods following the embryonic stages of vascular development, enabling modular control of the yield at each stage and enhancing the final efficiency of EC generation2–4. In 2D systems, PSCs are first differentiated into the mesodermal lineage. Combinations of BMP4, FGF2, and small molecule inhibitors of GSK-3β are generally used2–4. In the next stage, mesodermal cells are differentiated into vascular progenitor cells or endothelial cells by adding angiogenic factors and small molecules2,3. Studies demonstrated that in combinations with VEGFA, treatment with SB431542, a TGF-β receptor I inhibitor, or forskolin showed higher expression of CDH5 (VE-Cadherin) in hPSC-ECs2. Even higher expression of CDH5 was achieved when DLL4, a Notch ligand, was combined3. The final stage is to select EC lineage cells via sorting with antibodies against EC surface markers. KDR or CD34 was used for selecting progenitor stage ECs4, and PECAM11, CDH52,3 or VWF5 was used for isolating more mature or committed ECs. The criteria determining the identity of hPSC-ECs include EC-specific gene and protein expression, cell biological characteristics, and EC generation in vivo. Among them, EC generation in vivo is the ultimate assay to prove the identity and function of hPSC-ECs. However, only a few studies performed in vivo experiments together with histological studies to confirm the genuine incorporation of transplanted hPSC-ECs into vascular walls3,5. Short-term cell survival after in vivo transplantation was also a problem for hPSC-ECs. Recently, transplantation of hPSC-ECs encapsulated with biomaterials was shown to prolong cell survival and to induce sustained incorporation of hPSC-ECs within the vessel wall3. Surprisingly, this study uncovered that many hPSC-ECs directly contributed to vessel formation after 6 months, suggesting the importance of long-term survival of the transplanted cells for vasculogenesis. Given the ethical concerns and the possibility of an exclusive allogeneic approach, hESC-EC is not a practical option for cell-based therapy. The studies have revealed that hiPSC-ECs are equally potent in their therapeutic potency to hESC-ECs.
Remaining Questions and Challenges for hiiPSC-ECs
When using hiPSC-ECs for clinical cell-based therapy, several hurdles remain to be addressed. First, a guideline should be established to verify the characteristics of hiPSC-ECs. Many protocols to generate hiPSC-ECs were developed, but there is no consensus on the criteria to define the EC characteristics. Second, scalability of hiPSC-ECs is still a concern. For clinical application, a large number of cells are required. To date, no studies clearly addressed this point. More studies are needed to develop methods to proliferate hiPSC-ECs in culture while maintaining a reasonable EC phenotype. Moreover, the genomic stability of hiPSC-ECs during this expansion should be addressed. Third, long-term safety of transplanted hiPSC-ECs must be guaranteed using animal models because hiPSCs have the innate capacity for tumorigenicity and multi-lineage differentiation. Fourth, to maximize the vasculogenic effects of hiPSC-ECs, optimization of biomaterial-mediated cell delivery and tissue engineering would be necessary3. Finally, when considering autologous cell therapy, the effects of cardiovascular risk factors on hiPSCs and hiPSC-EC should be considered. In addition to these clinical concerns, many biological questions need further investigation. For example, the maturity of hiPSC-ECs needs to be addressed together with their biological or therapeutic potency. Type-specific EC generation toward arterial and venous specification was not well addressed. Studies only demonstrated that arterial and venous ECs are mixed in the differentiated hPSC-EC cultures but each type was not isolated and characterized6. However, lymphatic EC differentiation, their isolation by specific surface markers, and their therapeutic effects were reported7. There are still gaps between the therapeutic effects and mechanisms, particularly direct vessel-forming or vasculogenic effects of hiPSC-ECs. Unlike other hiPSC-derived cells, disease modeling and drug testing with hiPSC-ECs were hardly explored.
Direct Reprogramming of Somatic Cells into Endothelial Cells
Due to potential concerns of tumorigenicity and aberrant tissue generation associated with hPSC-derived ECs, a new approach in which somatic cells are directly converted to ECs via overexpression of lineage specific transcription factors has been developed. Another practical concern was difficulties in expanding hPSC-EC. Dr. Rafii’s group pioneered this approach, demonstrating that overexpression of ETS transcription factors, ETV2, FLI1, and ERG1 into human amniotic cells (ACs) generated expandable ECs called reprogrammed AC vascular endothelial cells (rAC-VECs)8. The successful reprogramming into ECs required transient expression of ETV2 along with treatment with SB431542 in the early phase of the reprogramming process and constitutive ectopic expression of FLI1 and ERG1. This study, while opening a new avenue for generating ECs, raised questions on the practicality of this method due to the failure to generate ECs from human postnatal fibroblasts. Later, Han et al generated ECs from adult skin fibroblasts using EC/hematopoietic lineage transcription factors (Etv2, Foxo1, Klf2, Lmo2, and Tal1) and named them induced ECs (iECs)9; however, only mouse cells were used in this study.
Recently, two groups demonstrated direct reprogramming or conversion of human postnatal fibroblasts into ECs10,11. Both groups reported that the transduction of a single transcription factor, ETV2, in a doxycycline inducible lentiviral system was sufficient to directly convert fibroblasts into functional ECs. Morita et al demonstrated that PECAM1high cells sorted after 15 days of ETV2 transduction, called ETVECs, displayed EC characteristics in vitro. ETVECs improved blood flow in ischemic hindlimb of NOD-SCID mice when they were implanted with Matrigel, but their vascular incorporation was uncertain10. Another study more convincingly demonstrated direct reprogramming of human postnatal fibroblasts into functional ECs with ETV2 alone11. In this study, fibroblasts were converted to reprogrammed ECs (rECs) through two stages. KDR+ cells sorted after 7 days of ETV2 overexpression, referred to as early rECs, exhibited less mature but enriched EC characteristics as well as functional and therapeutic potential such as blood vessel incorporation, blood flow recovery, and ischemic tissue repair in an animal model. Early rECs, when further cultured for about three months with transient re-induction of ETV2, became late rECs, which showed a transcriptome profile similar to human postnatal ECs, more mature phenotypes, and were incorporated into functional vessels.
There was another approach of direct EC reprogramming, which did not involve overexpression of transcription factors12. The activation of Toll-like receptor (TLR3) by poly I:C (polyinosinic: polycytidylic acid) treatment to human fibroblasts followed by cultivation in EC differentiation medium with 8-Br-cAMP and SB431542 directly converted human fibroblasts into ECs. The resulting iECs improved blood flow recovery and increased capillary density through paracrine effects. While appealing for a transgene-free strategy, this approach suffers from a low reprogramming efficiency and lack of direct incorporation into vasculature.
Remaining Questions and Challenges for Directly Reprogrammed ECs
This direct conversion or reprogramming approach for EC generation is still at an early stage, facing many challenges. First, clinically compatible gene delivery methods need to be developed. To date, most studies used lentiviral8–11 vectors, which can cause insertional mutations. Therefore, non-integrating vectors need to be developed. A cytoplasmic RNA vector, Sendai viral vector, is a promising candidate and was shown to generate iPSCs and induced cardiomyocytes13. Non-integrating DNA viral vectors, such as adenovirus or adeno-associated virus (AAV) could be a practical option. Other attractive options include modified mRNAs, which showed effectiveness for direct reprogramming and iPSC generation. Second, reprogramming of human somatic cells into ECs is a low efficiency process (<20%)10 and the expansion of directly reprogrammed ECs generated from human postnatal cells were not demonstrated. Thus, improving efficiency and developing methods to proliferate reprogrammed ECs are clearly needed. To enhance the reprogramming efficiency, other transcription factors, epigenetic modulators, and non-coding RNAs can be explored. Third, while vascular incorporation is a final test for the cell’s identity as ECs, some studies lack this assay. A stricter definition of directly reprogrammed, or induced, ECs needs to be established. Fourth, studies are required to explore the effects of in vivo application of direct reprogramming factors as were shown in other cellular reprogramming approaches. Fifth, mechanisms of direct conversion into ECs are still elusive. Sixth, while the heterogeneity of the reprogrammed ECs was demonstrated including arterial, venous and lymphatic ECs10,12, no studies showed isolation of each type of ECs or subtype specific EC reprogramming. Addition of specific transcription factors such as HEY1/2 (artery) and NR2F2 (vein), and growth factors can potentially generate subtype-specific rECs. Seventh, biomaterials and tissue engineering technologies can be combined to enhance the survival of implanted cells and to increase neovascularizing effects.
Supplementary Material
Acknowledgments
Acknowledgment (Sources of Funding)
This work was supported by grants from NIDDK (DP3-DK108245) and NHLBI (R01HL127759, R01HL129511), and from Ministry of Food and Drug Safety in 2018 (18172MFDS182) and the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI) funded by the Ministry of Health & Welfare (HI15C2782, HI16C2211), Republic of Korea, the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIP) (No 2015M3A9C6031514).
Footnotes
Disclosures
Nothing to disclose
Contributor Information
Sangho Lee, Department of Medicine, Division of Cardiology, Emory University School of Medicine, Atlanta, GA.
Shin-Jeong Lee, Biomedical Science Institute, Yonsei University College of Medicine, Seoul, Korea.
Young-sup Yoon, Department of Medicine, Division of Cardiology, Emory University School of Medicine, Atlanta, GA; Biomedical Science Institute, Yonsei University College of Medicine, Seoul, Korea.
References
- 1.Levenberg S, Golub JS, Amit M, Itskovitz-Eldor J, Langer R. Endothelial cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2002;99:4391–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Patsch C, Challet-Meylan L, Thoma EC, et al. Generation of vascular endothelial and smooth muscle cells from human pluripotent stem cells. Nat Cell Biol. 2015;17:994–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee SJ, Sohn YD, Andukuri A, Kim S, Byun J, Han JW, Park IH, Jun HW, Yoon YS. Enhanced Therapeutic and Long-Term Dynamic Vascularization Effects of Human Pluripotent Stem Cell-Derived Endothelial Cells Encapsulated in a Nanomatrix Gel. Circulation. 2017;136:1939–1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park SW, Jun Koh Y, Jeon J, Cho YH, Jang MJ, Kang Y, Kim MJ, Choi C, Sook Cho Y, Chung HM, Young Koh G, Han YM. Efficient differentiation of human pluripotent stem cells into functional CD34+ progenitor cells by combined modulation of the MEK/ERK and BMP4 signaling pathways. Blood. 2010;116:5762–72. [DOI] [PubMed] [Google Scholar]
- 5.Cho SW, Moon SH, Lee SH, Kang SW, Kim J, Lim JM, Kim HS, Kim BS, Chung HM. Improvement of postnatal neovascularization by human embryonic stem cell derived endothelial-like cell transplantation in a mouse model of hindlimb ischemia. Circulation. 2007;116:2409–19. [DOI] [PubMed] [Google Scholar]
- 6.Rufaihah AJ, Huang NF, Kim J, Herold J, Volz KS, Park TS, Lee JC, Zambidis ET, Reijo-Pera R, Cooke JP. Human induced pluripotent stem cell-derived endothelial cells exhibit functional heterogeneity. Am J Transl Res. 2013;5:21–35. [PMC free article] [PubMed] [Google Scholar]
- 7.Lee SJ, Park C, Lee JY, Kim S, Kwon PJ, Kim W, Jeon YH, Lee E, Yoon YS. Generation of pure lymphatic endothelial cells from human pluripotent stem cells and their therapeutic effects on wound repair. Sci Rep. 2015;5:11019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ginsberg M, James D, Ding BS, et al. Efficient direct reprogramming of mature amniotic cells into endothelial cells by ETS factors and TGFbeta suppression. Cell. 2012;151:559–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Han JK, Chang SH, Cho HJ, et al. Direct conversion of adult skin fibroblasts to endothelial cells by defined factors. Circulation. 2014;130:1168–78. [DOI] [PubMed] [Google Scholar]
- 10.Morita R, Suzuki M, Kasahara H, Shimizu N, Shichita T, Sekiya T, Kimura A, Sasaki K, Yasukawa H, Yoshimura A. ETS transcription factor ETV2 directly converts human fibroblasts into functional endothelial cells. Proc Natl Acad Sci U S A. 2015;112:160–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee S, Park C, Han JW, et al. Direct Reprogramming of Human Dermal Fibroblasts Into Endothelial Cells Using ER71/ETV2. Circ Res. 2017;120:848–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sayed N, Wong WT, Ospino F, Meng S, Lee J, Jha A, Dexheimer P, Aronow BJ, Cooke JP. Transdifferentiation of Human Fibroblasts to Endothelial Cells: Role of Innate Immunity. Circulation. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miyamoto K, Akiyama M, Tamura F, et al. Direct In Vivo Reprogramming with Sendai Virus Vectors Improves Cardiac Function after Myocardial Infarction. Cell Stem Cell. 2018;22:91–103 e5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


