Abstract
Quinoline and quinazoline alkaloids, two important classes of N-based heterocyclic compounds, have attracted tremendous attention from researchers worldwide since the 19th century. Over the past 200 years, many compounds from these two classes were isolated from natural sources, and most of them and their modified analogs possess significant bioactivities. Quinine and camptothecin are two of the most famous and important quinoline alkaloids, and their discoveries opened new areas in antimalarial and anticancer drug development, respectively. In this review, we survey the literature on bioactive alkaloids from these two classes and highlight research achievements prior to the year 2008 (Part I). Over 200 molecules with a broad range of bioactivities, including antitumor, antimalarial, antibacterial and antifungal, antiparasitic and insecticidal, antiviral, antiplatelet, anti-inflammatory, herbicidal, antioxidant and other activities, were reviewed. This survey should provide new clues or possibilities for the discovery of new and better drugs from the original naturally occurring quinoline and quinazoline alkaloids.
Keywords: bioactivities, camptothecin, quinazoline alkaloids, quinine, quinoline alkaloids
1 |. INTRODUCTION
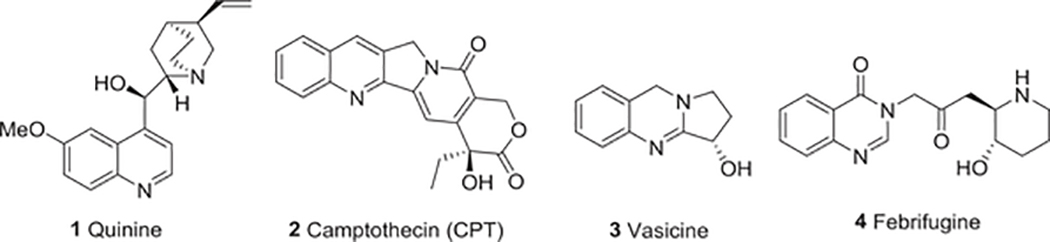
Quinoline alkaloids are important N-based heterocyclic aromatic compounds with a broad range of bioactivities. They have attracted significant attention from researchers over the past 200 years.1 After the quinoline alkaloid quinine (1) (Fig. 1) was isolated from the bark of the Cinchona tree in 1820, it replaced the crude bark in the treatment of malaria.2,3 Although 1 has relatively low efficacy and tolerability, it played a historical role in the development of quinoline alkaloids, and still plays an important role in the treatment of multi-resistant malaria.2,4 Camptothecin (CPT, 2) (Fig. 1), isolated from the Chinese tree Camptotheca acuminata in the early 1960s, is the most important and famous quinoline alkaloid from an anticancer aspect.5,6 Ever since mechanistic studies determined that CPT specifically targets DNA topoisomerase (topo) I, modified CPT analogs have been at the frontline of anticancer drug development. In addition, numerous quinoline alkaloids have been isolated and identified from natural sources, and many studies have documented their antitumor, antimalarial, antibacterial, antifungal, antiparasitic and insecticidal, antiviral, antiinflammatory, antiplatelet and other activities (Table 1).1,7 Now, quinoline alkaloids and their derivatives have extensive medical and agricultural applications.
FIGURE 1:
Chemical structures of historically important quinoline and quinazoline alkaloids 1–4
Table 1.
The active quinolone and quinazoline alkaloidsa
| No. | Compound | Activity | Reference |
|---|---|---|---|
| 1 | Quinine | Anti-tumor activity | 93 |
| Antimalarial activity | 2,3,166,167 | ||
| Trypanocidal activity | 108 | ||
| 2 | Camptothecin | Anti-tumor activity | 6,60–64 |
| 3 | Vasicine | Antifeedant activity | 215 |
| Cardiovascular protective activity | 281 | ||
| Bronchodilator activity | 332 | ||
| Oxytocic activity | 340 | ||
| 4 | Febrifugine | Antimalarial activity | 176–186 |
| Trypanocidal activity | 219 | ||
| 28 | Quinidine | Anti-tumor activity | 91,92 |
| Antiarrhythmic activity | 256–266 | ||
| Effect on CYP3A4 | 323 | ||
| 29 | Zanthosimuline | Anti-tumor activity | 94 |
| 30 | Huajiaosimuline | Anti-tumor activity | 94,95 |
| Antiplatelet activity | 95 | ||
| 31 | Flindersine | Anti-tumor activity | 96,97 |
| Anti-bacterial activity | 228 | ||
| Anti-fungal activity | 244 | ||
| SRS-A antagonist | 333 | ||
| 32 | Haplamine | Anti-tumor activity | 97 |
| Anti-fungal activity | 244 | ||
| 33 | γ-Fagarine | Anti-tumor activity | 100,121 |
| Antiplatelet activity | 95 | ||
| Cardiovascular protective activity | 275 | ||
| Anti-HIV activity | 284 | ||
| Mutagenicity | 336 | ||
| Estrogenic activity | 341 | ||
| 34 | Skimmianine | Anti-tumor activity | 98,100 |
| Antimalarial activity | 173 | ||
| Anti-leishmania activity | 199 | ||
| Trypanocidal activity | 200 | ||
| Anti-bacterial activity | 228 | ||
| Anti-fungal activity | 245 | ||
| Antiplatelet activity | 95,269–272 | ||
| Cardiovascular protective activity | 273,275 | ||
| Antagonists at the 5-HT2 receptor site | 307 | ||
| Mutagenicity | 335 | ||
| Estrogenic activity | 341 | ||
| 35 | Haplopine | Anti-tumor activity | 97 |
| Antimalarial activity | 173 | ||
| Anti-bacterial activity | 228 | ||
| Antiplatelet activity | 95 | ||
| Cardiovascular protective activity | 275 | ||
| Anti-HIV activity | 284 | ||
| Estrogenic activity | 341 | ||
| 36 | Evolitrine | Anti-tumor activity | 98 |
| Antifeedant activity | 201 | ||
| Antiplatelet activity | 269–272 | ||
| Cardiovascular protective activity | 275 | ||
| Antiviral activity | 292 | ||
| Anti-inflammatory activity | 293 | ||
| 37 | Kokusaginine | Anti-tumor activity | 98 |
| Antimalarial activity | 173 | ||
| Trypanocidal activity | 200 | ||
| Insecticidal activity | 203,204 | ||
| Anti-bacterial activity | 228 | ||
| Anti-fungal activity | 245 | ||
| Antiplatelet activity | 269–272 | ||
| Cardiovascular protective activity | 275 | ||
| Antagonists at the 5-HT2 receptor site | 307 | ||
| 38 | Maculosidine | Anti-tumor activity | 98 |
| 39 | 2,3-Methylenedioxy-4,7-dimethoxyquinoline | Anti-tumor activity | 98 |
| 40 | Dictamnine | Anti-tumor activity | 99,100 |
| Antifeedant activity | 201,202 | ||
| Anti-fungal activity | 245,246 | ||
| Antiplatelet activity | 267,269–272 | ||
| Cardiovascular protective activity | 275 | ||
| Antiviral activity | 292 | ||
| Mutagenicity | 335 | ||
| 41 | Graveoline | Anti-tumor activity | 99 |
| Herbicidal activity | 314 | ||
| 42 | Maculine | Anti-tumor activity | 101 |
| Anti-fungal activity | 245 | ||
| 43 | 5-Methoxymaculine | Anti-tumor activity | 101 |
| 44 | 5,8-Dimethoxymaculine | Anti-tumor activity | 101 |
| 45 | 4,5,6,7,8-Pentamethoxyfuroquinoline | Anti-tumor activity | 101 |
| 46 | Flindersiamine | Anti-tumor activity | 101 |
| Anti-bacterial activity | 227 | ||
| Anti-fungal activity | 245 | ||
| 47 | 7-(2′-Hydroxy-3′chloroprenyloxy)-4-methoxy-furoquinoline | Anti-tumor activity | 102 |
| 48 | 7-(2′,3′-Epoxyprenyloxy)-4-methoxyfuroquinoline | Anti-tumor activity | 102 |
| 49 | Pteleine | Anti-tumor activity | 103 |
| Antiplatelet activity | 269–272 | ||
| 50 | (+)-7,8-Dimethoxymyrtopsine | Anti-tumor activity | 102,103 |
| 51 | Medicosmine | Anti-tumor activity | 104 |
| 52 | Jineol | Anti-tumor activity | 105 |
| Anti-oxidant activity | 329 | ||
| 53 | 3,8-Dimethoxyquinoline | Anti-tumor activity | 105 |
| 54 | 3,8-Diacetoxyquinoline | Anti-tumor activity | 105 |
| 55 | Senepodine A | Anti-tumor activity | 106 |
| 56 | 7-Hydroxy-4-[5′-hydroxymethylfuran-2′-yl]-2-quinolone | Anti-tumor activity | 107 |
| 57 | Acetylcupreine | Anti-tumor activity | 108 |
| Insecticidal activity | 108 | ||
| 58 | 3,3-Diisopentenyl-N-methyl2,4-quinoldione | Anti-tumor activity | 109 |
| 59 | Cuspareine | Anti-tumor activity | 110 |
| Antimalarial activity | 110 | ||
| 60 | Galipeine | Anti-tumor activity | 110 |
| Antimalarial activity | 110 | ||
| 61 | Galipinine | Anti-tumor activity | 110 |
| Antimalarial activity | 110 | ||
| 62 | Angustureine | Anti-tumor activity | 110 |
| Antimalarial activity | 110 | ||
| 63 | Asimicilone | Anti-tumor activity | 111 |
| 64 | Lepadin A | Anti-tumor activity | 112,113 |
| 65 | Lepadin B | Anti-tumor activity | 112,113 |
| 66 | Lepadin C | Anti-tumor activity | 112,113 |
| 67 | Lepadin D | Antimalarial activity | 112,113 |
| Trypanocidal activity | 112,113 | ||
| Anti-fungal activity | 112,113 | ||
| 68 | Lepadin E | Anti-tumor activity | 112,113 |
| Antimalarial activity | 112,113 | ||
| Trypanocidal activity | 112,113 | ||
| Anti-fungal activity | 112,113 | ||
| 69 | Lepadin F | Anti-tumor activity | 112,113 |
| Antimalarial activity | 112,113 | ||
| Trypanocidal activity | 112,113 | ||
| Anti-fungal activity | 112,113 | ||
| 70 | Lepadin G | Anti-tumor activity | 112,113 |
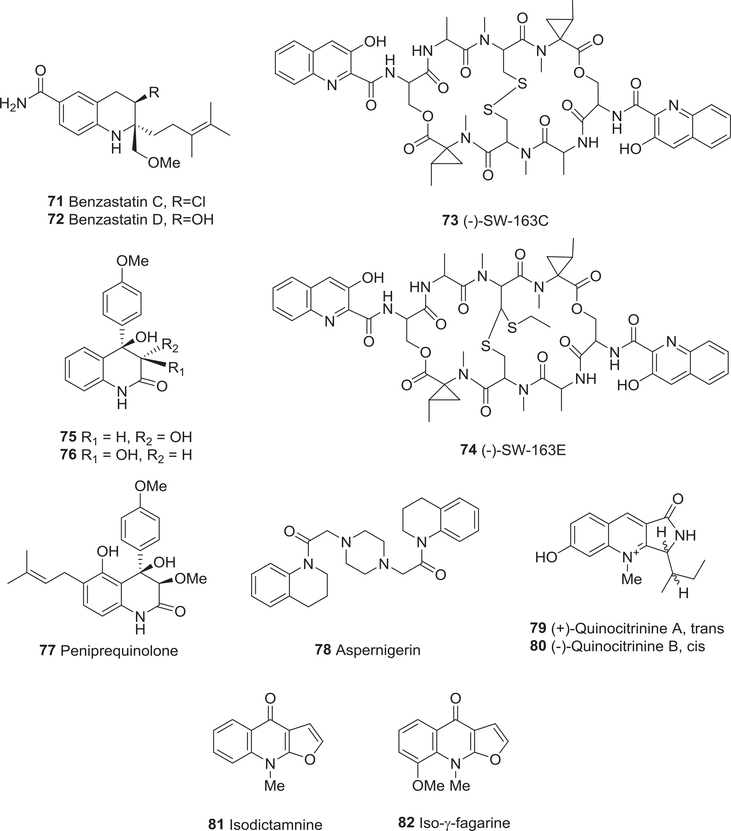
| 71 | Benzastatin C | Neuroprotective activity | 114,115 |
| Anti-oxidant activity | 114,115 | ||
| 72 | Benzastatin D | Neuroprotective activity | 114,115 |
| Anti-oxidant activity | 114,115 | ||
| 73 | (−)-SW-163C | Anti-tumor activity | 116,117 |
| 74 | (−)-SW-163E | Anti-tumor activity | 116,117 |
| 75 | 3S*,4R*-Dihydroxy-4-(4′methoxyphenyl)-3,4-dihydro-2(1H)-quinolinone | Anti-tumor activity | 118 |
| 76 | 3R*,4R*-Dihydroxy-4-(4′methoxyphenyl)-3,4-dihydro-2(1H)-quinolinone | Anti-tumor activity | 118 |
| 77 | Peniprequinolone | Anti-tumor activity | 118 |
| Nematicidal activity | 210 | ||
| 78 | Aspernigerin | Anti-tumor activity | 119 |
| 79 | (+)-Quinocitrinine A | Anti-tumor activity | 120 |
| Anti-bacterial activity | 120 | ||
| 80 | (−)-Quinocitrinine B | Anti-tumor activity | 120 |
| Anti-bacterial activity | 120 | ||
| 81 | Isodictamnine | Anti-tumor activity | 121 |
| 82 | Iso-γ-fagarine | Anti-tumor activity | 121 |
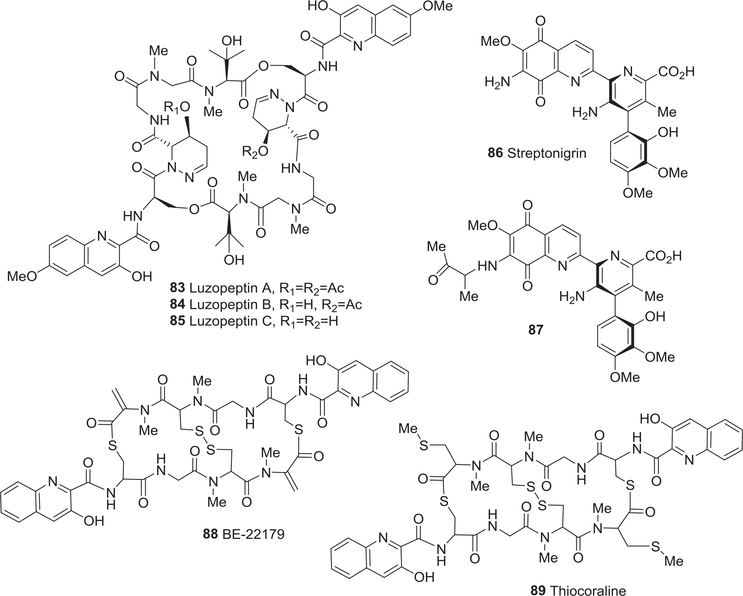
| 83 | Luzopeptin A | Anti-tumor activity | 121–126 |
| Anti-HIV activity | 286 | ||
| 84 | Luzopeptin B | Anti-tumor activity | 121–126 |
| Anti-HIV activity | 286 | ||
| 85 | Luzopeptin C | Anti-tumor activity | 121–126 |
| Anti-HIV activity | 286 | ||
| 86 | Streptonigrin | Anti-tumor activity | 127 |
| 87 | 7-(1-Methyl-2-oxopropyl)streptonigrin | Anti-tumor activity | 127 |
| 88 | BE-22179 | Anti-tumor activity | 128,129,132,133 |
| 89 | Thiocoraline | Anti-tumor activity | 130–133 |
| Anti-bacterial activity | 240 | ||
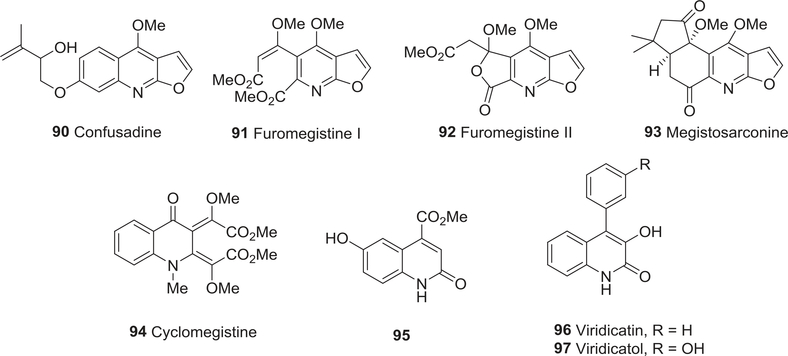
| 90 | Confusadine | Anti-tumor activity | 134 |
| Antiplatelet activity | 274 | ||
| 91 | Furomegistine I | Anti-tumor activity | 135 |
| 92 | Furomegistine II | Anti-tumor activity | 135 |
| 93 | Megistosarconine | Anti-tumor activity | 136 |
| 94 | Cyclomegistine | Anti-tumor activity | 137 |
| 95 | 4-Carbomethoxy-6-hydroxy2-quinolone | Anti-tumor activity | 138 |
| Anti-oxidant activity | 330 | ||
| 96 | Viridicatin | Anti-tumor activity | 139 |
| 97 | Viridicatol | Anti-tumor activity | 139,140 |
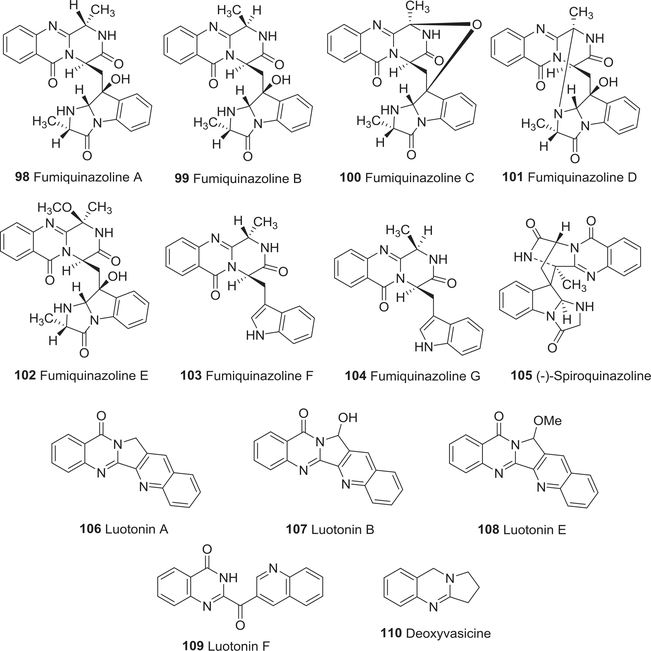
| 98 | Fumiquinazoline A | Anti-tumor activity | 141,142 |
| 99 | Fumiquinazoline B | Anti-tumor activity | 141,142 |
| 100 | Fumiquinazoline C | Anti-tumor activity | 141,142 |
| 101 | Fumiquinazoline D | Anti-tumor activity | 141,142 |
| 102 | Fumiquinazoline E | Anti-tumor activity | 141,142 |
| 103 | Fumiquinazoline F | Anti-tumor activity | 141,142 |
| 104 | Fumiquinazoline G | Anti-tumor activity | 141,142 |
| 105 | (−)-Spiroquinazoline | Anti-tumor activity | 143 |
| 106 | Luotonin A | Anti-tumor activity | 144–146 |
| 107 | Luotonin B | Anti-tumor activity | 144–146 |
| 108 | Luotonin E | Anti-tumor activity | 144–146 |
| 109 | Luotonin F | Anti-tumor activity | 144–146 |
| 110 | Deoxyvasicine | Anti-tumor activity | 148 |
| Anticholinesterase activity | 309 | ||
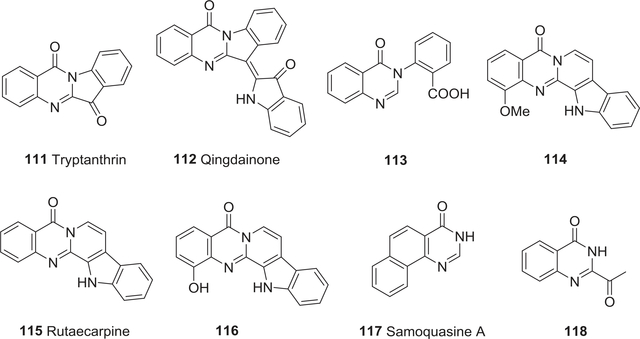
| 111 | Tryptanthrin | Anti-tumor activity | 152–155 |
| Antimalarial activity | 187,188 | ||
| Trypanocidal activity | 217 | ||
| Antifeedant activity | 216 | ||
| Anti-bacterial activity | 253,254 | ||
| Anti-inflammatory activity | 298,299 | ||
| 112 | Qingdainone | Anti-tumor activity | 152 |
| 113 | 3-(2-Carboxyphenyl)-4(3H)-quinazolinone | Anti-tumor activity | 156 |
| 114 | 1-Methoxy-7,8-dehydrorutaecarpine | Anti-tumor activity | 157 |
| 115 | Rutaecarpine | Anti-tumor activity | 157 |
| Antiplatelet activity | 277 | ||
| Anti-inflammatory activity | 306 | ||
| Effect on CYP1A1, CYP1A2 and CYP1B1 | 318–322 | ||
| 116 | 1-Hydroxyrutaecarpine | Anti-tumor activity | 157 |
| Antiplatelet activity | 277 | ||
| 117 | Samoquasine A | Anti-tumor activity | 158–161 |
| 118 | 2-Acetyl-4(3H)-quinazolinone | Anti-tumor activity | 162,163 |
| Anti-HIV activity | 162,163 | ||
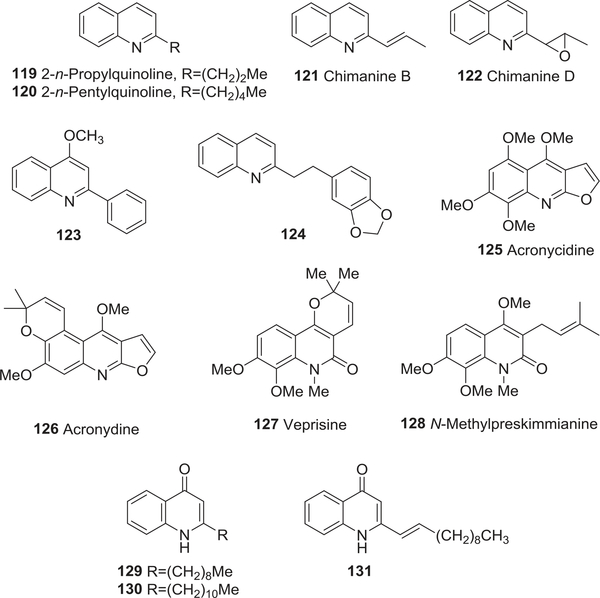
| 119 | 2-n-Propylquinoline | Antimalarial activity | 172 |
| Anti-leishmania activity | 192–196 | ||
| Trypanocidal activity | 197 | ||
| Molluscicidal activity | 198 | ||
| Antiviral activity | 289–291 | ||
| 120 | 2-Pentylquinoline | Antimalarial activity | 172 |
| Anti-leishmania activity | 192–196 | ||
| Trypanocidal activity | 197 | ||
| Molluscicidal activity | 198 | ||
| Antiviral activity | 289–291 | ||
| 121 | Chimanine B | Antimalarial activity | 172 |
| Anti-leishmania activity | 192–195 | ||
| 122 | Chimanine D | Antimalarial activity | 172 |
| Anti-leishmania activity | 192–195 | ||
| Antiviral activity | 289–291 | ||
| 123 | 4-Methoxy-2phenylquinoline | Antimalarial activity | 172 |
| 124 | 2-(3,4- Methylenedioxyphenylethyl)quinoline | Antimalarial activity | 172 |
| Anti-leishmania activity | 192–195 | ||
| Molluscicidal activity | 198 | ||
| Antiviral activity | 289–291 | ||
| 125 | Acronycidine | Antimalarial activity | 173 |
| 126 | Acronydine | Antimalarial activity | 173 |
| 127 | Veprisine | Antimalarial activity | 174 |
| SRS-A antagonists | 333 | ||
| 128 | N-Methylpreskimmianine | Antimalarial activity | 174 |
| 129 | 2-Nonyl-4-(1H)-quinolone | Antimalarial activity | 175 |
| Immunomodulatory activity | 297 | ||
| 130 | 2-Undecyl-4-(1H)-quinolone | Antimalarial activity | 175 |
| Immunomodulatory activity | 297 | ||
| 131 | 2-(Undec-1-enyl)quinolin4-(1H)-one | Antimalarial activity | 175 |
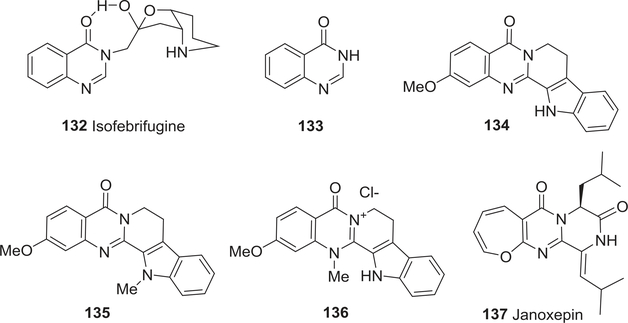
| 132 | Isofebrifugine | Antimalarial activity | 176–186 |
| 133 | Quinazolin-4-(3H)-one | Antimalarial activity | 181 |
| Bronchodilator activity | 331,332 | ||
| 134 | 2-Methoxyrutaecarpine | Effect on CYP1A1, CYP1A2 and CYP1B1 | 318 |
| 136 | 5,8,13,14-Tetrahydro-2methoxy-14-methyl-5oxo-7H-indolo[2′,3′:3,4]-pyrido[2,1b] quinazolin-6-ium chloride | Antimalarial activity | 174 |
| 137 | (−)-Janoxepin | Antimalarial activity | 189 |
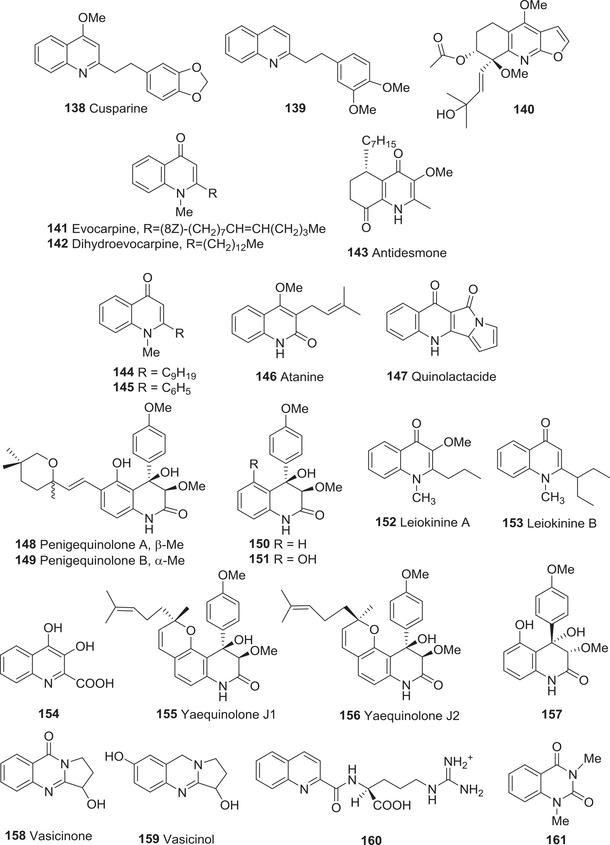
| 138 | Cusparine | Anti-leishmania activity | 199 |
| 140 | rel-(7R,8R)-8-[(E)-3-hydroxy3-methyl-1-butenyl]-4,8-dimethoxy-5,6,7,8-tetrahydrofuro[2,3-b]quinoline-7-yl acetate | Trypanocidal activity | 200 |
| 141 | Evocarpine | Insecticidal activity | 204 |
| Anti-bacterial activity | 222,223,225 | ||
| Cardiovascular protective activity | 276 | ||
| Immunomodulatory activity | 297 | ||
| Hypolipidaemic activity | 328 | ||
| 142 | Dihydroevocarpine | Insecticidal activity | 204 |
| Anti-bacterial activity | 222 | ||
| Immunomodulatory activity | 297 | ||
| Hypolipidaemic activity | 328 | ||
| 143 | Antidesmone | Trypanocidal activity | 205 |
| 144 | N-Methyl-2-nonylquinolin-4(1H)-one | Trypanocidal activity | 206 |
| 145 | N-Methyl-2-hexylquinolin-4(1H)-on | Trypanocidal activity | 206 |
| 146 | Atanine | Antiparasitic and anthelmintic activity | 207 |
| 147 | Quinolactacide | Insecticidal activity | 208,209 |
| 148 | Penigequinolone A | Nematicidal activity | 210 |
| Herbicidal activity | 315 | ||
| 149 | Penigequinolone B | Nematicidal activity | 210 |
| Herbicidal activity | 315 | ||
| 150 | 3-Methoxy-4-hydroxy-4-(4′-methoxyphenyl)quinolinone | Nematicidal activity | 210 |
| 151 | 3-Methoxy-4,6-dihydroxy-4- (4′-methoxyphenyl)quinolinone | Nematicidal activity | 210 |
| 152 | Leiokinine A | Antifeedant activity | 211 |
| 153 | Leiokinine A | Antifeedant activity | 211 |
| 154 | 3,4-Dihydroxyquinoline-2-carboxylic acid | Antifeedant activity | 212 |
| 155 | (−)-Yaequinolone J1 | Insecticidal activity | 213 |
| 156 | (−)-Yaequinolone J2 | Insecticidal activity | 213 |
| 157 | 3-Methoxy-4,5-dihydroxy-4- (4′-methoxyphenyl)quinolinone | Insecticidal activity | 214 |
| 158 | Vasicinone | Antifeedant activity | 215 |
| Bronchodilator activity | 331 | ||
| 159 | Vasicinol | Antifeedant activity | 215 |
| 160 | (+)-Nα-Quinaldyl-L-arginine | Antifeedant activity | 218 |
| 161 | 1,3-Dimethylquinazoline2,4-dione | Insecticidal activity | 220,221 |
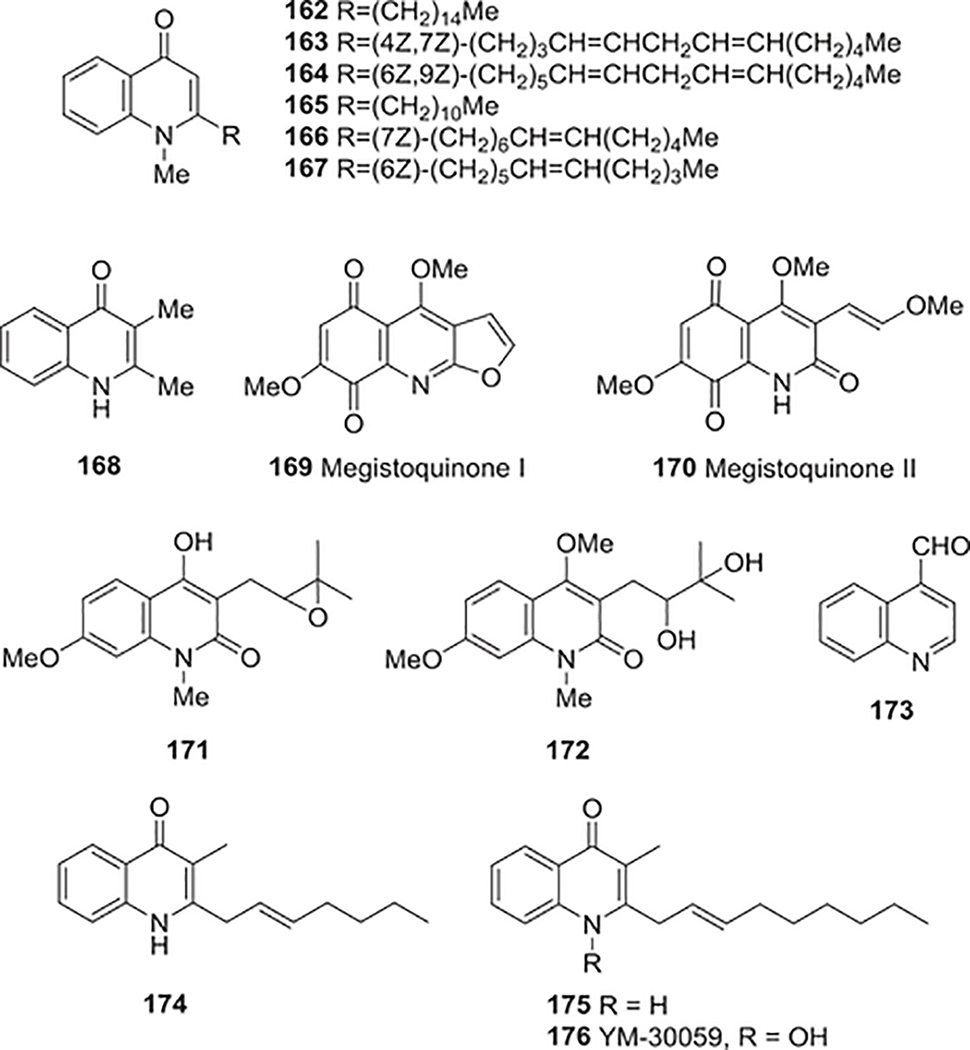
| 162 | 1-Methyl-2-pentadecyl4(1H)-quinolone | Anti-bacterial activity | 222 |
| Immunomodulatory activity | 297 | ||
| 163 | 1-Methyl-2-[(4Z,7Z)-4,7-tridecadienyl]-4(1H)-quinolone | Anti-bacterial activity | 222,225 |
| Cardiovascular protective activity | 276 | ||
| Immunomodulatory activity | 297 | ||
| Hypolipidaemic activity | 328 | ||
| 164 | 1-Methyl-2-[(6Z,9Z)-6,9-pentadecadienyl]-4(1H)-quinolone | Anti-bacterial activity | 222,225 |
| Cardiovascular protective activity | 276 | ||
| Immunomodulatory activity | 297 | ||
| Hypolipidaemic activity | 328 | ||
| 165 | 1-Methyl-2-undecyl-4(1H)-quinoline | Anti-bacterial activity | 222,225 |
| Immunomodulatory activity | 297 | ||
| 166 | 1-Methyl-2-[(Z)-7tridecadienyl]-4(1H)-quinolone | Anti-bacterial activity | 223 |
| 167 | 1-Methyl-2-(6Z)-6undecenyl-quinolone | Anti-bacterial activity | 225 |
| 168 | 2,3-Dimethyl-4-quinolone | Anti-bacterial activity | 226 |
| 169 | Megistoquinone I | Anti-bacterial activity | 230 |
| 170 | Megistoquinone II | Anti-bacterial activity | 230 |
| 172 | 3-(2,3-Dihydroxy-3-methylbutyl)-4,7-dimethoxy-1-methyl-1H-quinolin-2-one | Anti-bacterial activity | 231 |
| 173 | Quinoline-4-carbaldehyde | Anti-bacterial activity | 232,233 |
| Anti-fungal activity | 249 | ||
| 174 | 3-Methyl-2-(non-2enyl)quinolin-4(1H)-one | Anti-bacterial activity | 234,235 |
| 175 | 2-(2-Heptenyl)-3-methyl-4(lH)-quinolone | Anti-bacterial activity | 234,235 |
| Anti-fungal activity | 249 | ||
| 176 | YM-30059 | Anti-bacterial activity | 236 |
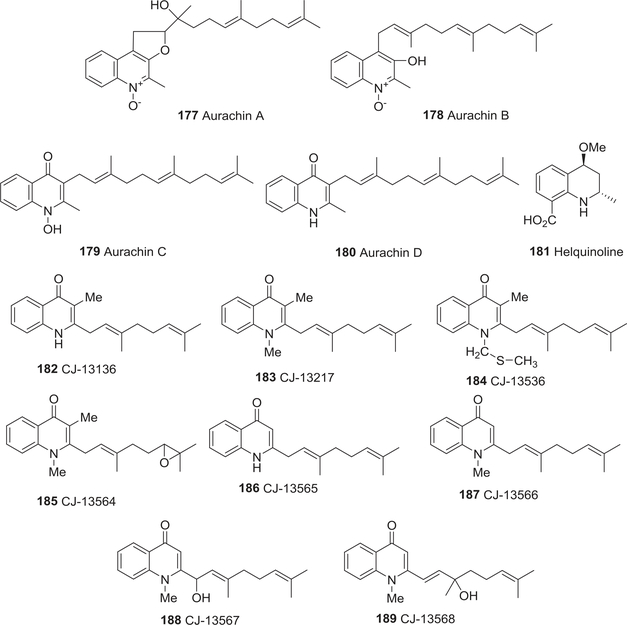
| 177 | Aurachin A | Anti-bacterial activity | 237 |
| Effect on cytochrome | 316,317 | ||
| 178 | Aurachin B | Anti-bacterial activity | 237 |
| Effect on cytochrome | 316,317 | ||
| 179 | Aurachin C | Anti-bacterial activity | 237 |
| Effect on cytochrome | 316,317 | ||
| 180 | Aurachin D | Anti-bacterial activity | 237 |
| Effect on cytochrome | 316,317 | ||
| 181 | Helquinoline | Anti-bacterial activity | 238 |
| 182 | CJ-13136 | Anti-bacterial activity | 239 |
| 183 | CJ-13217 | Anti-bacterial activity | 239 |
| 184 | CJ-13536 | Anti-bacterial activity | 239 |
| 185 | (−)-CJ-13564 | Anti-bacterial activity | 239 |
| 186 | CJ-13565 | Anti-bacterial activity | 239 |
| 187 | CJ-13566 | Anti-bacterial activity | 239 |
| 188 | (+)-CJ-13567 | Anti-bacterial activity | 239 |
| 189 | (−)-CJ-13568 | Anti-bacterial activity | 239 |
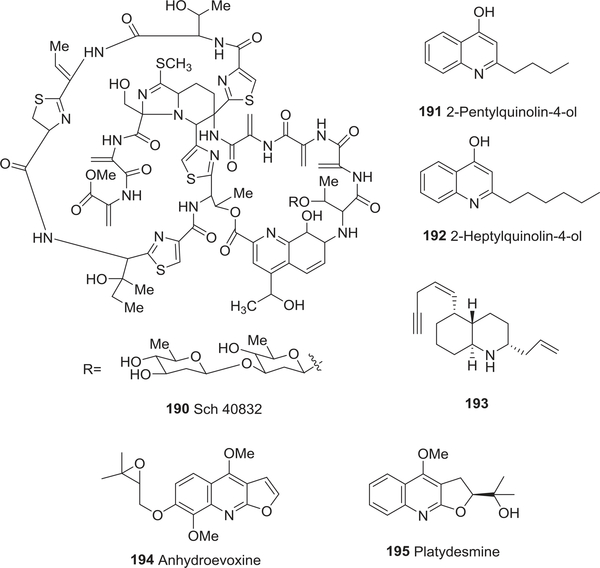
| 190 | Sch 40832 | Anti-bacterial activity | 241 |
| 191 | 2-Heptylquinolin-4-ol | Anti-bacterial activity | 242 |
| 192 | 2-Pentylquinolin-4-ol | Anti-bacterial activity | 242 |
| 193 | trans-Decahydroquinoline 243A | Anti-bacterial activity | 243 |
| 194 | Anhydroevoxine | Anti-fungal activity | 244 |
| 195 | Platydesmine | Anti-fungal activity | 245 |
| Anti-HIV activity | 284 | ||
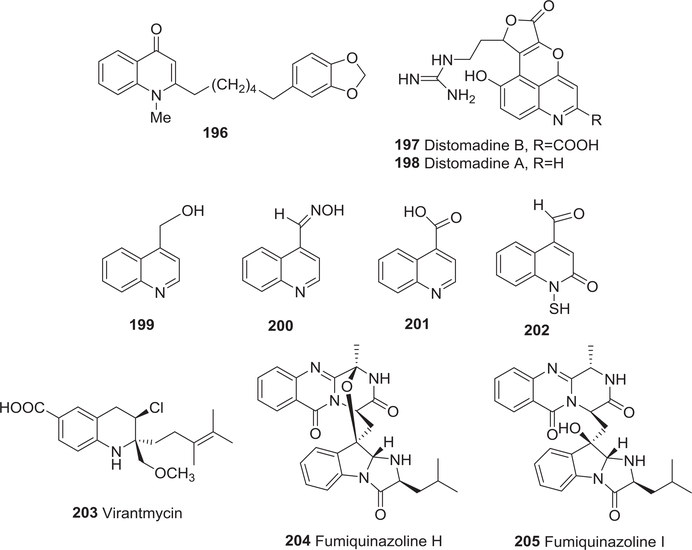
| 196 | 1-Methyl-2-[6′-(3′′,4′′-methylenedioxyphenyl)hexyl]-4-quinolone | Anti-fungal activity | 247 |
| 197 | Distomadine B | Anti-fungal activity | 248 |
| 198 | Distomadine A | Anti-fungal activity | 248 |
| 199 | 4-Hydroxymethylquinoline | Anti-fungal activity | 249 |
| 200 | Quinoline-4-carbaldoxime | Anti-fungal activity | 249 |
| 201 | Quinoline-4-carboxylic acid | Anti-fungal activity | 249 |
| 202 | N-Mercapto-4-formylcarbostyril | Anti-fungal activity | 250 |
| 203 | Virantmycin | Anti-fungal activity | 251,252 |
| Antiviral activity | 251,252 | ||
| 204 | Fumiquinazoline H | Anti-bacterial activity | 255 |
| 205 | Fumiquinazoline I | Anti-bacterial activity | 255 |
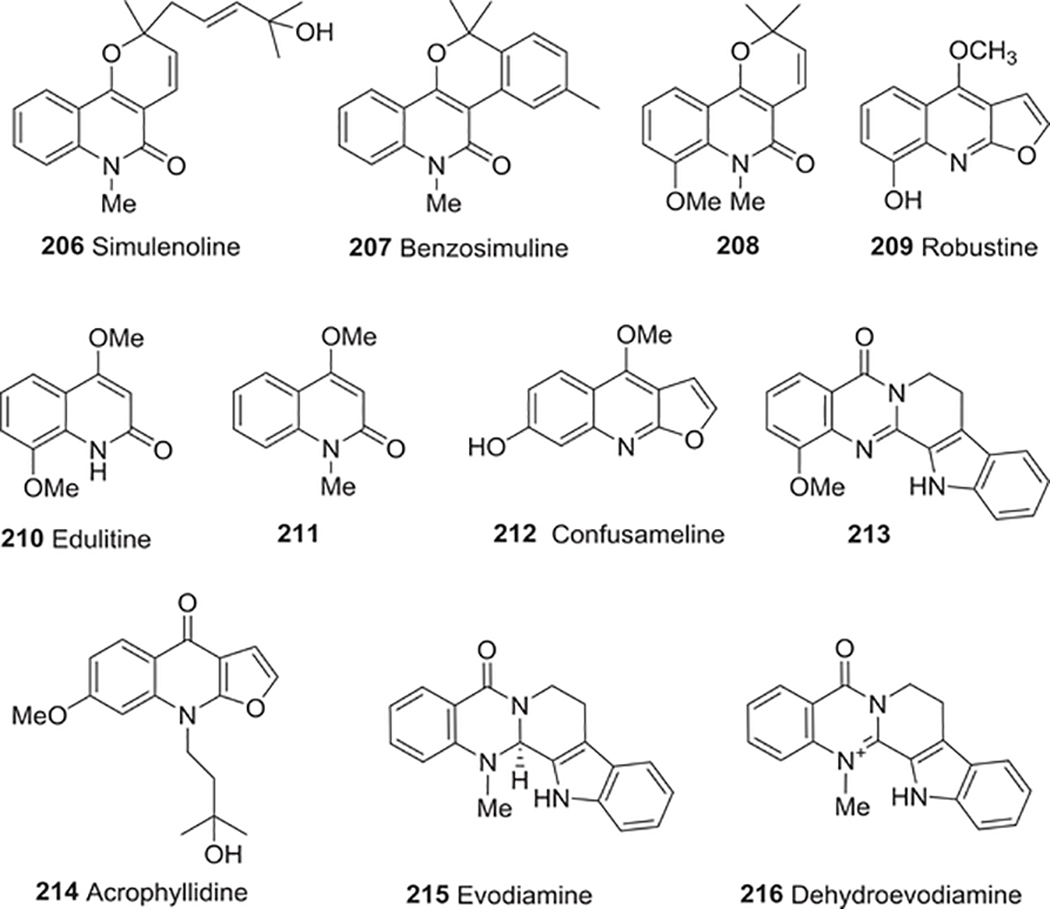
| 206 | Simulenoline | Antiplatelet activity | 95 |
| 207 | Benzosimuline | Antiplatelet activity | 95 |
| 208 | Zanthobungeanine | Antiplatelet activity | 95 |
| 209 | Robustine | Antiplatelet activity | 95 |
| Cardiovascular protective activity | 275 | ||
| 210 | Edulitine | Antiplatelet activity | 95 |
| 211 | 4-Methoxy-1-methylquinolin-2-one | Antiplatelet activity | 268 |
| Anti-HIV activity | 284 | ||
| 212 | Confusameline | Antiplatelet activity | 269–272 |
| Cardiovascular protective activity | 275 | ||
| Antagonists at the 5-HT2 receptor site | 307 | ||
| 213 | 1-Methoxyrutaecarpine | Antiplatelet activity | 277 |
| 214 | Acrophyllidine | Cardiovascular protective activity | 278 |
| 215 | Evodiamine | Anti-inflammatory activity | 297 |
| Vasodilatory effect | 279 | ||
| 216 | Dehydroevodiamine | Vasodilatory effect | 279 |
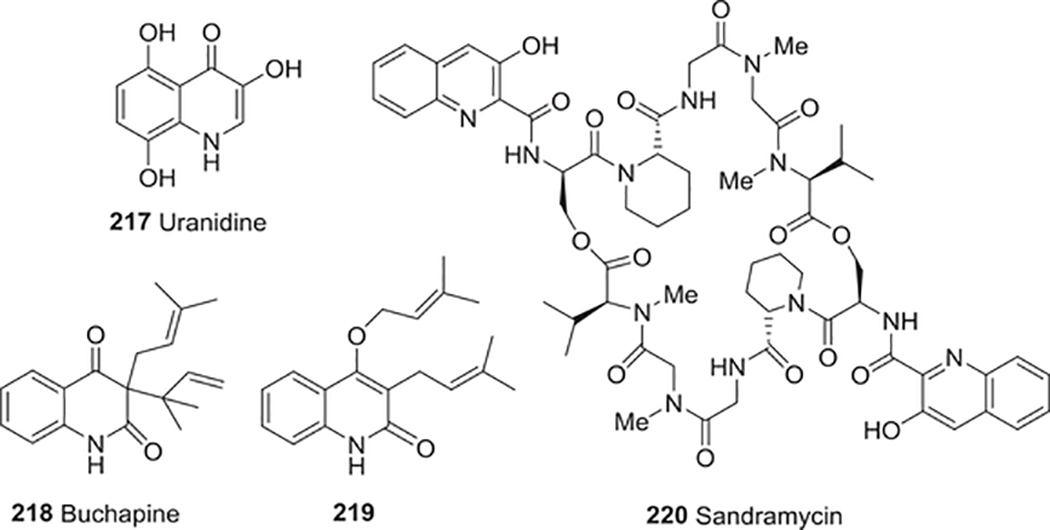
| 217 | Uranidine | Anti-HIV activity | 282 |
| 218 | Buchapine | Anti-HIV activity | 283 |
| 219 | 3-Prenyl-4-prenyloxyquinolin-2-one | Anti-HIV activity | 283 |
| 220 | Sandramycin | Anti-HIV activity | 285 |
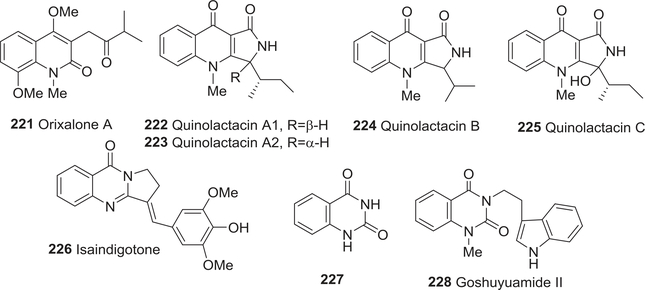
| 221 | Orixalone A | Anti-inflammatory activity | 294 |
| 222 | Quinolactacin A1 | Anti-inflammatory activity | 295,296 |
| Anticholinesterase activity | 310 | ||
| 223 | Quinolactacin A1 | Anti-inflammatory activity | 295,296 |
| Anticholinesterase activity | 310 | ||
| 224 | Quinolactacin B | Anti-inflammatory activity | 295,296 |
| 225 | Quinolactacin C | Anti-inflammatory activity | 295,296 |
| 226 | Isaindigotone | Anti-inflammatory activity | 304 |
| 227 | Quinazoline-2,4-dione | Anti-inflammatory activity | 305 |
| Antihypertensive activity | 305 | ||
| 228 | Goshuyuamide II | Anti-inflammatory activity | 306 |
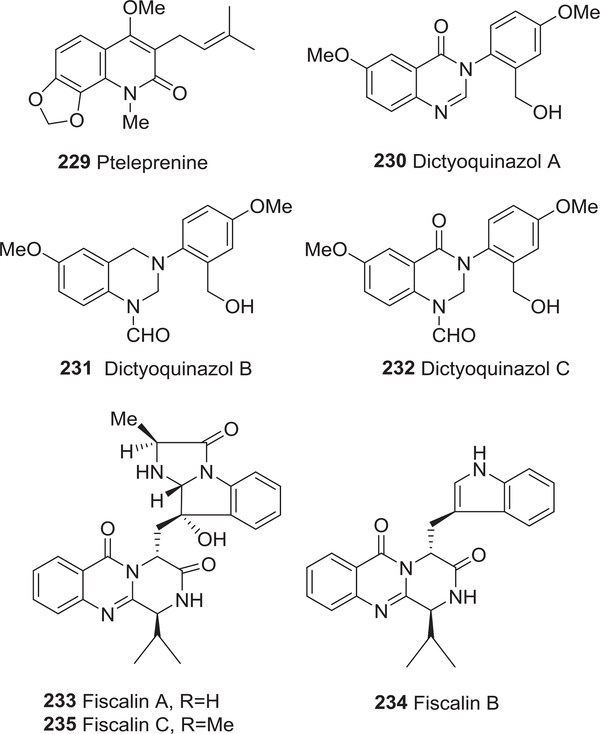
| 229 | Pteleprenine | Agonist of nicotinic acetylcholine receptors | 308 |
| 230 | Dictyoquinazol A | Cortical neurons protective activity | 312 |
| 231 | Dictyoquinazol B | Cortical neurons protective activity | 312 |
| 232 | Dictyoquinazol C | Cortical neurons protective activity | 312 |
| 233 | Fiscalin A | Inhibition of the binding of substance P | 313 |
| 234 | Fiscalin B | Inhibition of the binding of substance P | 313 |
| 235 | Fiscalin C | Inhibition of the binding of substance P | 313 |
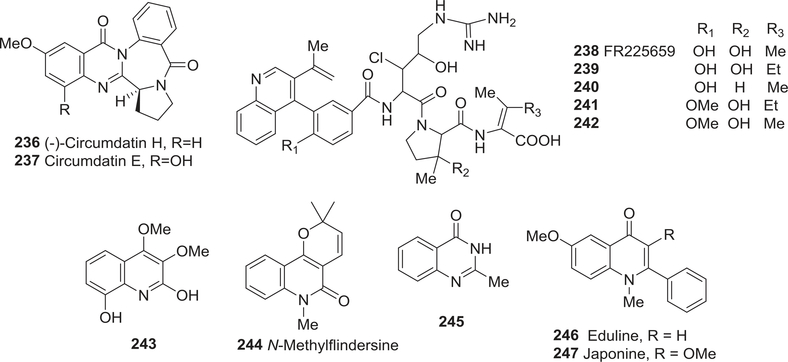
| 236 | Circumdatin H | Effect on the respiratory chain | 324 |
| 237 | Circumdatin E | Effect on the respiratory chain | 324 |
| 238 | FR225659 | Hypoglycemic activity | 325–327 |
| 239 | 239 | Hypoglycemic activity | 325–327 |
| 240 | 240 | Hypoglycemic activity | 325–327 |
| 241 | 241 | Hypoglycemic activity | 325–327 |
| 242 | 242 | Hypoglycemic activity | 325–327 |
| 243 | 2,8-Dihydroxy-3,4-dimethoxyquinoline | Anti-oxidant activity | 329 |
| 244 | N-Methylflindersine | SRS-A antagonists | 333 |
| 245 | 2-Methyl-4(3H)-quinazolinone | Inhibitor of poly(ADP-ribose) synthetase | 338 |
| 246 | Eduline | Effect on muscle | 339 |
| 247 | Japonine | Effect on muscle | 339 |
Compounds 5–27 are CPT analogs and are not specifically listed in this table.
Quinazoline alkaloids are another class of N-based heterocyclic compounds. To date, approximately 150 naturally occurring quinazoline alkaloids have been isolated from several families of the plant kingdom, as well as from animals and microorganisms; many are derived biogenetically from anthranilic acid.8,9 In 1888, the first quinazoline alkaloid, vasicine (3) (Fig. 1), was isolated from Adhatoda vasica and later from other species.10,11 Our group optimized the extraction technology of this compound from Peganum harmala and recently reported its acaricidal activity.12 In the 1950s, more comprehensive study of quinazoline alkaloids began after a new quinzolinone alkaloid, 3-[β-keto-γ-(3-hydroxy-2-piperidyl)-propyl]-4-quinazolone [febrifugine,2 4] (Fig. 1), with antimalarial effects was isolated from the Asian plant Dichroa febrifuga.13 Since then, many more quinazoline alkaloids and their derivatives were isolated, synthesized, and found to exhibit diverse pharmacological activities with broad agricultural and medical uses (Table 1).14–21
Several thousands of publications (journal articles, books, and patents) on quinoline and quinazoline alkaloids have been recorded through 2016. The topics include the extraction, synthesis, pharmacology, and other aspects of these compounds. The increasing numbers of publications reflect the importance and research intensity in this field, as well as the bright prospect for drug development of these compounds. Furthermore, some excellent reviews on quinoline and quinazoline alkaloids from a historical point of view are available.1,6,8,9,12,22–57 These publications focused mainly on the chemical structures of isolated compounds, the synthetic methods and approaches to new derivatives, and the derivatives’ biological properties. They have contributed significantly to the general scientific understanding of quinoline and quinazoline alkaloids. However, from 2008 to date, additional significant studies have been published, and a more comprehensive and up-to-date review is merited. Therefore, this review combines newer literature reports with the authors’ research as well as presents the developments in this field more from the perspective of biological activities. It covers quinoline and quinazoline alkaloids related not only to anticancer and antimalarial effects, but also other biological activities. We hope that this review will provide new clues or possibilities for the development of these compounds. Due to the vast amount of literature, we will split the material into two review papers. This review will cover the literature up to 2008 (Part I, all active quinoline and quinazoline alkaloids isolated are listed in Table 1), and the forthcoming review (Part II) will summarize the literature from 2009 to 2016.
2 |. BIOACTIVITIES OF QUINOLINE AND QUINAZOLINE ALKALOIDS
2.1 |. Antitumor activity
2.1.1 |. Quinoline alkaloids
Cancer is known medically as a malignant neoplasm, which includes over 200 human diseases, all involving unregulated cell growth.58 Many new natural products with anticancer activities have been isolated and could possibly The active quinoline and quinazoline alkaloidsa be used in the treatment of cancer. Among such potential anticancer compounds or agents, some quinoline and quinazoline alkaloids fused with various heterocycles have displayed potent anticancer activity. CPT (2) is one of the most important and famous.59 It is a specific and strong inhibitor of the DNA-replicating enzyme topo I.59,60 In the presence of CPT, cells either undergo cell cycle arrest in S-phase or continue progression with subsequent accumulation of DNA damage, ultimately resulting in cell death.61–63 Because of this distinct cytotoxic mechanism, CPT exhibits significant activity against established cell lines from leukemias and various solid cancers, such as colon, lung, breast, ovarian, and melanoma, in experimental systems. However, CPT is water insoluble and results in severe and unpredictable side effects. These shortcomings hampered the development of CPT in the 1970s. Meanwhile, these problems also stimulated interest in the synthesis of CPT analogs to find active and clinically useful anticancer drugs with the same mechanism of action.6 More than 5000 publications on CPT were recorded between 1966 and 2012. This dramatic number of publications not only reflects the research intensity, but also the importance and bright prospect of CPT derivatives in cancer treatment.
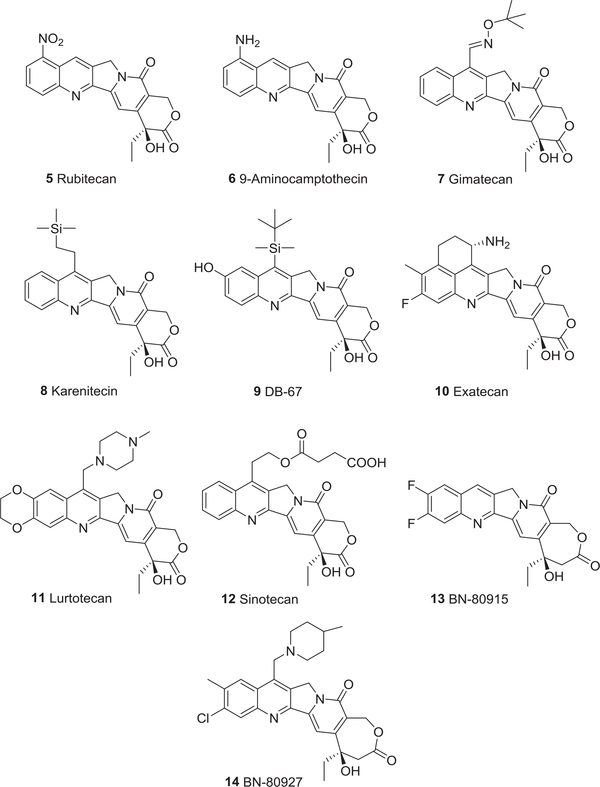
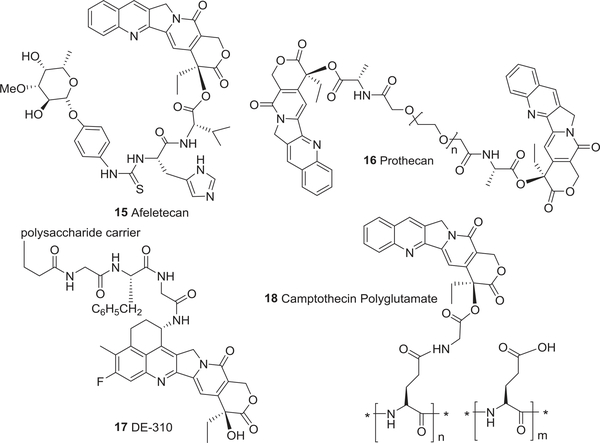
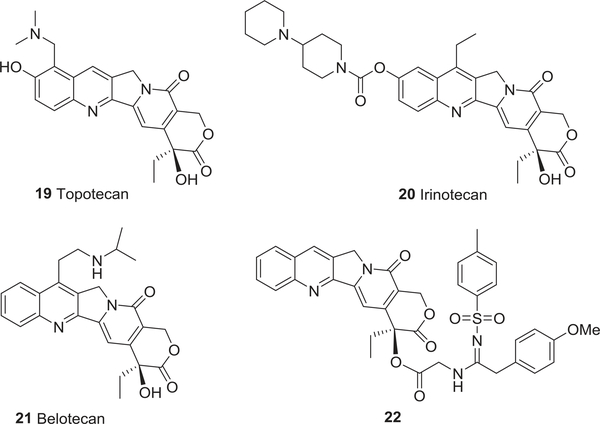
To date, five non-water-soluble CPT analogs, rubitecan (5),64,65 9-aminocamptothecin (6),66 gimatecan (7),67 karenitecin (8),68 DB-67 (9),69 and three water-soluble analogs, exatecan (10)70–72, lurtotecan (11),73,74 and sinotecan (12)75,76 (Fig. 2), are in preclinical and clinical studies. Newly emerging homocamptothecin (hCPT) derivatives, BN80915 (13) and BN-80927 (14)77,78 (Fig. 2) with a stabilized seven membered hydroxylactone ring, the CPT prodrug afeletecan (15),79,80 and different delivery systems (16–18)81–84 (Fig. 3) are also currently undergoing clinical trials. More importantly, three CPT analogs, topotecan (19),85 irinotecan (20),86 and belotecan (approved only in South Korea) (21),87 have received governmental approval for the clinical treatment of ovarian, small-cell lung, and refractory colorectal cancers.
FIGURE 2:
The chemical structures of camptothecin analogs 5–14
FIGURE 3:
The chemical structures of camptothecin analogs 15–18
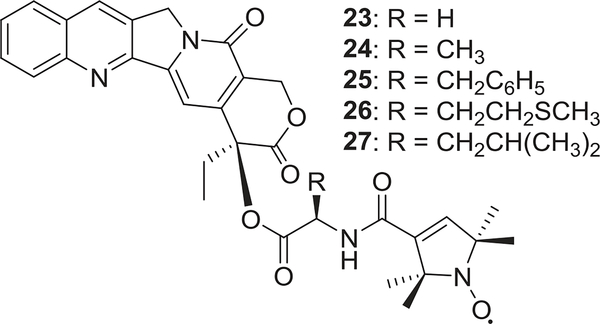
In recent years, the authors’ laboratories designed and synthesized several series of CPT derivatives. In 2008, a nitroxylradicalmoiety(1-oxyl-2,2,5,5-tetramethylpyrroline-3-carboxylicacid)waslinkedatthe20-hydroxylofCPTvia different hydrophilic amino acid spacers to generate a series of novel spin-labeled CPT derivatives (23–27) (Fig. 5).88 The new compounds showed similar or better in vitro cytotoxic activity than the parent drug CPT and the clinically available drug 20 against human bladder cancer T-24. In 2012, a series of 7-acyl CPT derivatives showed significant inhibition of A-549, DU-145, KB, and KBvin cell growth with IC50 values ranging from 0.0154 to 13.3 μM.89 In continued efforts, 20-sulfonylamidine CPT derivatives with potent antitumor activity were also synthesized.90 Among them, compound 22 (Fig. 4) showed the best potency against the growth of A549, DU-145, KB, and KBvin with IC50 values of 0.031, 0.050, 0.14, and 0.026 μM, respectively. It induced significant DNA damage by selectively inhibiting topo I and activating the ATM/Chk-related DNA damage-response pathway. Furthermore, compound 22 at 300 mg/kg (i.p.) showed no overt acute toxicity in contrast to CPT in vivo (LD50 56.2 mg/kg, i.p.). Thus, 22 is attractive as a potential candidate for anticancer chemotherapy, and the modification with sulfonylamidine-substituted side chains may overcome some limitations of CPT.
FIGURE 5:
The chemical structures of camptothecin analogs 23–27
FIGURE 4:
The chemical structures of camptothecin analogs 19–22
The antitumor activity of quinidine (28) (Fig. 6), another major quinoline alkaloid from the Cinchona tree, was observed in 1989.91 This compound effectively modulates resistance, increasing the sensitivity of the multidrug resistant breast cancer cell line MCF-7 to adriamycin by eight-fold. In other studies, a combination of 28 and epirubicin was not more toxic than epirubicin alone and, at a dose of 250 mg b.d., levels of 28 equivalent to those active in vitro were achieved in patients.92 Thus, the treatment of advanced breast cancer with a combination of 28 and epirubicin appears feasible. In addition, quinine (1) (Fig. 1) increased the cellular accumulation of anthracycline in resistant cells and enhanced the in vitro cytotoxic activity of epidoxorubicin in resistant DHD/K12 rat colon cancer cells, and also circumvented anthracycine resistance in clinical practice.93
FIGURE 6:
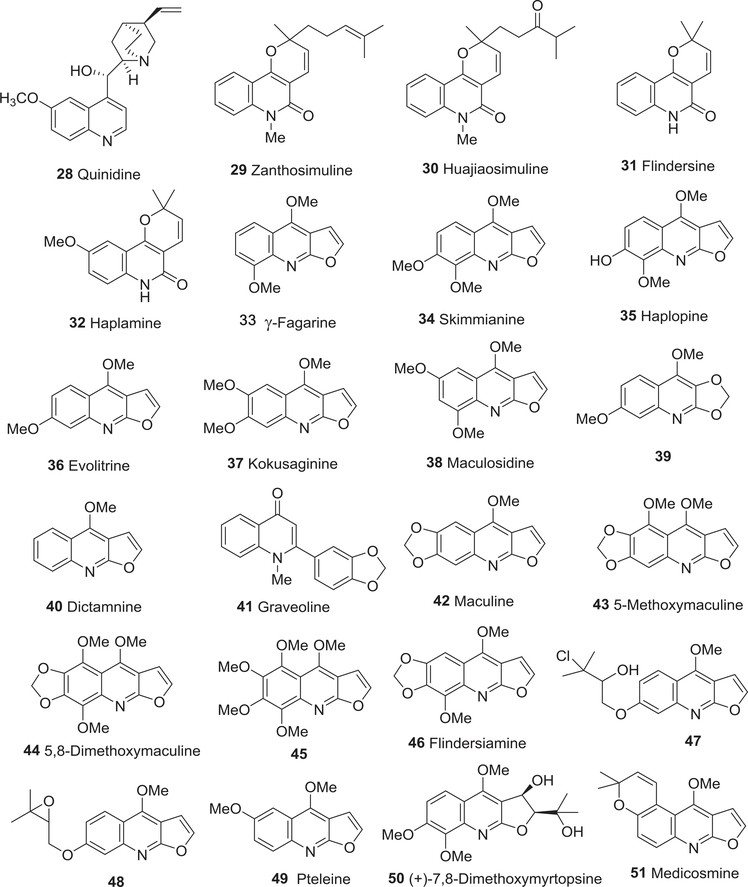
Chemical structures of compounds 28–51
Subsequently, more quinoline alkaloids were isolated and evaluated for cytotoxic activity. In 1994, Chen and coworkers isolated two pyranoquinoline alkaloids, zanthosimuline (29) and huajiaosimuline (30) (Fig. 6), from the root bark of Zanthoxylum simulans.94 In cytotoxicity testing, 29 exhibited a general cytotoxic response to various cultured human cancer cell lines, especially P-388 cells (EC50 5.20 μM). However, 30 produced a more selective cytotoxic activity profile and was especially effective against estrogen receptor-positive breast cancer ZR-75–1 (EC50 11.1 μM) and P-388 (EC50 9.80 μM) cells. The two compounds also induced the expression of cellular markers associated with cell differentiation in cultured HL-60 cells.94 In later studies, the same authors again verified the cytotoxic activity of 30.95
Two additional pyranoquinoline alkaloids, flindersine (31), and haplamine (32), as well as three furoquinoline alkaloids, γ-fagarine (33), skimmianine (34), and haplopine (35), (Fig. 6) from the genus Haplophyllum,96,97 showed cytotoxic activity against the HeLa cell line (IC50 < 50.0 μM), while only 32 was active against the HCT 116 cell line (IC50 64.5 μM). A structure–activity relationship (SAR) analysis showed that the aliphatic side chains at the 2′-position of the pyrano group of the pyranoquinoline alkaloids may increase the cytotoxic activity against human cancer cell lines. However, colchicine (positive drug) was much more potent with IC50 values of 1.10 and 1.30 μM against HeLa and HCT 116 cell lines, respectively.97
As indicated above, furoquinoline alkaloids, which are derived biogenetically from 2-substituted oxygenated 4-quinolones after a prenylation at C-3, can exhibit cytotoxic activity. In 1999, several furoquinoline alkaloids, including γ-fagarine (33), skimmianine (34), evolitrine (36), kokusaginine (37), and maculosidine (38), along with 2,3-methylenedioxy-4,7-dimethoxyquinoline (39) (Fig. 6), were isolated from the root bark of Acronychia laurifolia.98 Compounds 34 and 36–38 exhibited varying potencies of cytotoxic activity against specific human cancer cell lines, BC1 (EC50 15.4, 25.3, > 70, and > 70 μM, respectively), KB-V1+ (17.0, 12.7, 17.0, and 17.4 μM, respectively) and KB-V1− cell line (10.8, 16.6, 55.6, and 39.4 μM, respectively), but were inactive against Lu1, Col2, KB, and LNCaP cells.98
The furoquinoline dictamnine (40) and the 2-phenylquinolinone graveoline (41) from Ruta graveolens demonstrated greater cytotoxic activity against HeLa (EC50 12.6, 14 μM) compared with KB (EC50 103, 26.8 μM) cancer cell lines.99 In another study, 40, 33, and 34 were identified as moderate cytotoxic constituents from Z. pistaciiflorum against murine leukemia P-388, A549, and HT-29 cell lines.100
Five additional furoquinoline alkaloids, maculine (42); 5-methoxymaculine (43); 5,8-dimethoxymaculine (44); 4,5,6,7,8-pentamethoxyfuroquinoline (45); and flindersiamine (46) (Fig. 6), from Vepris punctate, showed modest cytotoxic activity toward the A2780 cell line (IC50 < 20 μM).101 In 2005 and 2006, 7-(2′-hydroxy-3′-chloroprenyloxy)4-methoxyfuroquinoline (47), 7-(2′,3′-epoxyprenyloxy)-4-methoxyfuroquinoline (48), pteleine (49), and (+)-7,8-dimethoxymyrtopsine (50) (Fig. 6) were isolated from two Melicope species, the former two compounds from M. bonwickii and the latter two from M. semecarpifolia.102,103 Compounds 47 and 48 showed cytotoxic activity when tested against the HeLa cell line (IC50 34 and 20.1 μM, respectively).102 Compound 49 showed similar potency toward the P-388 cell line (EC50 39.0 μM), but both 49 and 50 were less potent against the HT-29 cell line (EC50 66.4 and 124 μM, respectively).103 The rare furanoquinoline alkaloid medicosmine (51) (Fig. 6) has a fused 2,2-dimethyl2H-pyran ring rather than the simple methoxy group found in 49. It was isolated from the aerial parts of Boronella koniambiensis and was slightly cytotoxic against the murine L1210 leukemia cell line (IC50 48.0 μM).104
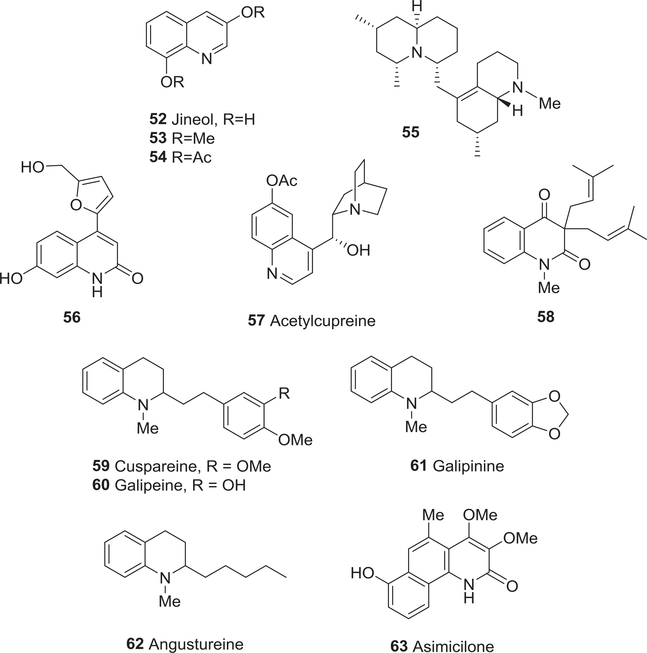
Jineol (52), a simple quinoline alkaloid from an animal rather than plant source, was isolated from the centipede Scolopendra subspinipes mutilans in 1996, together with 3,8-dimethoxyquinoline (53) and 3,8-diacetoxyquinoline (54) (Fig. 7).105 Compared with 53 and 54, compound 52 exhibited greater cytotoxic activity in vitro against five human tumor cell lines, A-549 (EC50 36.0 μM), SKOV-3 (EC50 27.9 μM), SK-Mel-2 (EC50 34.7 μM), XF-498 (EC50 62.1 μM) and HCT-15 (EC50 11.8 μM). It was less effective than cisplatin, but more effective than carboplatin.105 Senepodine A (55) (Fig. 7), a novel C22N2 alkaloid isolated from Lycopodium chinense, was significantly cytotoxic toward murine lymphoma L1210 cells (IC50 0.290 μM).106 7-Hydroxy-4-[5′-hydroxymethylfuran-2′-yl]-2-quinolone (56) (Fig. 7) from Aquilegia ecalcarata was moderately cytotoxic toward GLC-82 and HCT cells (IC50 8.80–10.1 μM) in vitro.107
FIGURE 7:
Chemical structures of compounds 52–63
Other studies found cytotoxic activity with acetylcupreine108 (57) (Fig. 7) from Remijia peruviana against mammalian CHO cells (ED50 43.8 μM) and with 3,3-diisopentenyl-N-methyl-2,4-quinoldione109 (58) (Fig. 7) from Esenbeckia almawillia against HL-60, CEM, B-16, HCT-8, and MCF-7 cancer cells (IC50 29.5– > 80.3 μM). The simple tetrahydroquinoline alkaloids cuspareine (59), galipeine (60), galipinine (61), and angustureine (62) (Fig. 7) were cytotoxic toward HeLa cells (IC50 18.6–161 μM), with 59 showing the highest potency (IC50 18.6 μM).110
In 1992, the new 2-quinolone alkaloid asimicilone (63) (Fig. 7) was isolated from Asimina parviflora.111 It showed cytotoxic activity against A-549, HT-29, and MCF-7 (IC50 7.47, 11.4, and 25.3 μM, respectively). The IC50 values of adriamycin (positive control) against the same three human tumor cell lines were 0.001, 0.008, and 0.425 μM respectively.
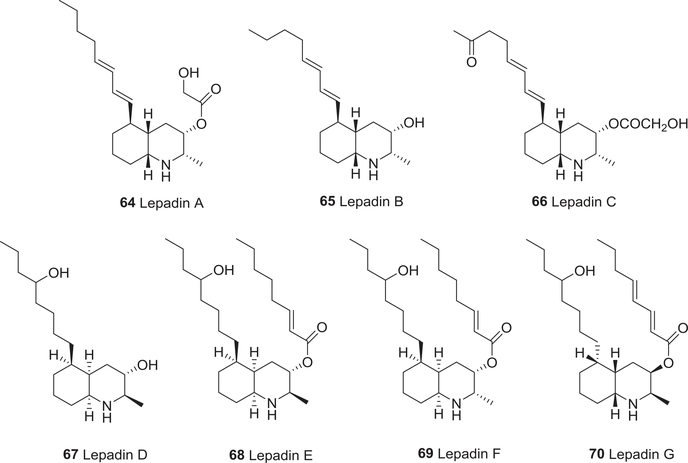
Then, in 1995 and 2002, seven novel decahydroquinoline alkaloids, lepadins A–G (64–70) (Fig. 8), were isolated.112,113 Compounds 65 and 66 showed significant in vitro cytotoxic activity toward various murine and human cancer cell lines, 69 and 70 showed mild activity, and 67 was inactive.112,113 The biological activity was postulated to be dependent on the configuration at C-2 and the nature of the functionality at C-3 in the decahydroquinoline.
FIGURE 8:
Chemical structures of compounds 64–70
In 1996, two tetrahydroquinoline alkaloids, benzastatins C (71) and D (72) (Fig. 9) were isolated by Kim et al. from the bacterium Streptomyces nitrosporeus 30643.114,115 The former chlorinated compound was cytotoxic against N18RE-105 cells with an IC50 value of 38.1 μM, but its hydroxylated congener 72 was inactive even at 100 μM.114,115 In addition, two new quinoline-containing octadepsipeptides, (−)-SW-163C (73) and E (74) (Fig. 9) were isolated from culture broth of the Streptomyces strain SNA15896.116,117 SW-163E (74) demonstrated better antitumor activity than SW-163C (73) in in vitro tests against various murine and human tumor cell lines (IC50 0.200–1.60 vs. 17.0–140 nM, respectively). When in vivo activity was assessed in mice implanted with P388 leukemia, 74 prolonged life span at a dose of 0.010 mg/kg, but was acutely toxic at higher doses (LD50 0.600 mg/kg for 74 vs. > 100 mg/kg for 73).
FIGURE 9 :
Chemical structures of compounds 71–82
In 2006, two new diastereomeric alkaloids 3S*,4R*-dihydroxy-4-(4′- methoxyphenyl)-3,4-dihydro-2(1H)-quinolinone (75) and 3R*,4R*-dihydroxy-4-(4′- methoxyphenyl)-3,4-dihydro-2(1H)-quinolinone (76), together with the prenyl-substituted peniprequinolone (77) (Fig. 9), were isolated from cultures of the marine fungus Penicillium janczewskii strain H-TW5/869.118 They showed moderate cytotoxic activity toward eight human tumor cell lines (MDA-MB 231, DU-145, SKOV-3, HT-29, A549, CAKI-1, SK-MEL 2, K562 cells). Among these compounds, 76 was markedly active against the SKOV-3 cell line. Furthermore, a novel cytotoxic alkaloid aspernigerin (78) (Fig. 9) from a culture of Aspergillus niger strain IFBE003 showed cytotoxic activity when tested against KB, HeLa, and SW1116 cell lines with IC50 values of 22.0, 46.0, and 35.0 μM, respectively.119 (+)-Quinocitrinine A (79) and (−)-quinocitrinine B (80) (Fig. 9) with a rare pyrrolo[3,4-b]quinoline ring system were isolated from cultures of P. citrinum Thom 1910 in 2003.120 Both compounds showed antiproliferative activity toward L-929, K-562, and HeLa cells.
Two naturally occurring isoalkaloids, isodictamnine (81), and iso-γ-fagarine (82) (Fig. 9), as well as γ-fagarine (33), were found in Glycosmis arborea.121 They showed inhibitory effects toward the tumor promoter 12-Otetradecanoylphorbol 13-acetate induced Epstein-Barr virus early antigen.
Luzopeptins A–C (83–85) (Fig. 10), quinoline-substituted cyclic decadepsipeptides from Actinomadura luzonensis, showed potent cytotoxic and antitumor activity.122–126 Compound 83, with two acetylated sites in its peptide ring, was active against several experimental animal tumor systems. Compound 84 (one acetylated site) was less active, and compound 85 (no acetylation) was inactive. However, compound 85 was slightly more effective than 83 and 84 in assays to evaluate bifunctional DNA intercalation and drug-induced DNA-DNA intermolecular cross-linking. The peptidic cyclic structure of luzopeptins is essential for the bifunctional intercalation of the twin chromophores, probably by providing proper conformational orientations of the chromophores.122–126
FIGURE 10:
Chemical structures of compounds 83–89
In 2002, streptonigrin (86) and its N-(1-methyl-2-oxopropyl) derivative, 7-(1-methyl-2-oxopropyl)-streptonigrin (87) (Fig. 10), were isolated from the fermentation broth of the actinomycete strain Micromonospora sp. IM 2670.127 They induced apoptosis through a p53-dependent pathway in human neuroblastoma SH-SY5Y cells. Compound 86 also caused nuclear accumulation of p53 and induced DNA ladders in SH-SY5Y cells as well as mediated p53-dependent apoptosis. Compound 86 was more cytotoxic than 87 (IC50 0.050 vs. 0.900 μM) toward SH-SY5Y cells.127
Furthermore, two quinoline-containing octadepsipeptides, BE-22179 (88) and thiocoraline (89) (Fig. 10), were isolated from the culture broths of Streptomyces strain A22179128,129 and Micromonospora sp. L-13-ACM2–092,130,131 respectively. BE-22179 (88) exhibited potent inhibition of topo П and significant in vitro cytotoxic activity against various murine leukemia and human stomach adenocarcinoma cell lines, as well as in vivo activity in mice transplanted with L1210 leukemic cells.128,129 More specifically, it inhibited the DNA-relaxing activity of L1210 topo П and prevented both DNA and RNA synthesis as well as the growth of L1210 mouse leukemic cells.128,129 Compound 89 also displayed significant cytotoxic effects against P-388, A-549, and MEL-28 cell lines (IC50 0.002 μM). It also inhibited RNA synthesis more specifically than DNA synthesis, bound to supercoiled DNA, but, unlike 88, did not inhibit topo II.130,131 Boger and co-workers reported the first total syntheses of both macrocyclic compounds and noted the exceptional IC50 values of 88 and 89 (200 and 400 pM, respectively) against the L1210 cell line.132,133
Of course, some isolated natural alkaloids exhibit weak or no cytotoxic activity in various studies against specific tumor cell lines. Confusadine (90) (Fig. 11) from the plant Melicope semecarpifolia showed poor cytotoxic activity toward P-388, A549, and HT-29 human cancer cell lines, and was substantially less potent than the related confusameline with a simple hydroxyl group and dutadrupine with a fused 2,2-dimethyl-2H-pyran ring rather than the 2-hydroxy-3-methylbut-3-enyloxy side chain.134 Furomegistines I (91) and II (92) (Fig. 11) were isolated from bark extracts of Sarcomelicope megistophylla;135 both alkaloids showed weak to no cytotoxic activity toward A549 and HT29 cells (IC50 90 and 100 μM, respectively). Megistosarconine (93, IC50 70 μM)136 and cyclomegistine (94, IC50 80 μM)137 (Fig. 11) from S. megistophylla also exhibited poor cytotoxic activity towards L1210 leukemia cells. 4-Carbomethoxy-6-hydroxy2-quinolone (95) (Fig. 11), a new alkaloid isolated from Oryza sativa cv. Mihyangbyo, did not exhibit antiproliferative activity toward the U937 cell line (IC50 539 μM).138
FIGURE 11:
Chemical structures of compounds 90–97
The fungal metabolites viridicatin (96) and viridicatol (97) (Fig. 11) were isolated from cultures of P. crustosum and P. discolor, respectively, grown on cheese agar.139 The compounds exhibited weak to no cytotoxic activity in an MTT assay; the IC50 values of 97 toward KB, KBv200, A549, HepG2, MCF7, K562, SMMC7221, and SGC 7901 tumor cell lines were 98.8, 65.2, 237, 336, 178, 98.8, 317, and 316 μM, respectively.140
2.1.2 |. Quinazoline alkaloids
In 1992 and 1995, fumiquinazolines A–C (98–100) and D–G (101–104) (Fig. 12) were isolated from the fungus A. fumigatus.141,142 All seven fumiquinazolines were moderately cytotoxic in a P388 lymphocytic leukemia test system. Meanwhile, (−)-spiroquinazoline (105) (Fig. 12) from cultures of the fungus A. flavipe inhibited the binding of substance P to human astrocytoma cells.143
FIGURE 12:
Chemical structures of compounds 98–110
Four important quinazoline alkaloids, luotonins A, B, E, and F (106–109) (Fig. 12), from the aerial parts of P. nigellastrum have two major skeleton types, pyrroloquinazolinoquinoline (106–108) and 4(3H)-quinazolinone (109).144,145 All four compounds exhibited promising cytotoxic activity toward P388 murine leukemia as well as potent topo II inhibition, but 106 was the most cytotoxic (IC50 6.32 μM)146 with the added ability to stimulate topo I-mediated cleavage of DNA.147 It stabilized the covalent binary complex formed between DNA and human topo I during DNA relaxation and mediated topo I-dependent activity in yeast Saccharomyces cerevisiae lacking the yeast topo but containing a plasmid with the human topo I gene. Due to its outstanding cytotoxic activity toward murine leukemia P-388 cells at low concentrations and the ability to inhibit topos I and II, 106 has been studied extensively.148 Alkaloid 106 and its derivatives were cytotoxic against a human lung large cell carcinoma cell line H460, but were less potent than a CPT-related control.149 To improve the biological as well as pharmacokinetic properties of 106 as an anticancer drug lead compound, systematic syntheses of derivatives have been performed.149–151 Another metabolite of this plant, deoxyvasicine (110) (Fig. 12), exhibited good cytotoxic activity toward mouse leukemia P-388 cells.148
Tryptanthrin (111, indolo[2,1-b]quinazolin-6,12-dione) and qingdainone (112) (Fig. 13) were first isolated from the traditional Chinese medicine Qingdai in 1985, and both compounds showed cytotoxic activity against melanoma B16 cells in vitro.152 Compound 111 also affected cell differentiation and apoptosis of U-937 and HL-60 leukemia cells.153 Low concentrations of 111 induced differentiation of leukemia cells but higher concentrations killed leukemia cells through apoptosis, possibly through a caspase-3/Fas antigen pathway. Meanwhile, 111 suppressed the growth of azoxymethane-induced intestinal tumors in F344 rats,154 and strongly inhibited the induction of hepatocyte growth factor in human dermal fibroblasts.155 3-(2-Carboxyphenyl)-4(3H)-quinazolinone (113) (Fig. 13) from Isatis indigotica, an open-ring analog of 111, showed endotoxic activity in vitro in the limulus amoebocyte lysate test.156
FIGURE 13:
Chemical structures of compounds 111–118
In 2005, Chen and co-workers isolated three new quinazoline alkaloids, 1-methoxy-7,8-dehydrorutaecarpine (114), rutaecarpine (115), and 1-hydroxyrutaecarpine (116) (Fig. 13), from the root bark of Z. integrifoliolum.157 In in vitro tests, all three alkaloids were cytotoxic toward murine P-388 (EC50 12.3, 36.8, and 12.4 μM, respectively) and human HT-29 (EC50 27.1, 118, and 24.7 μM, respectively) cells. Samoquasine A (117) (Fig. 13) with a benzo[h]quinazoline ring system was isolated from seeds of the custard apple Annona squamosa.158–161 It showed significant cytotoxic activity against murine lymphoma L1210 cells (IC50 1.94 μM).158 However, the original published structure was reinvestigated and revised.159–161 The simple quinazoline alkaloid 2-acetyl-4(3H)-quinazolinone (118) (Fig. 13) showed cytotoxic activity only at high concentrations.162,163
2.2 |. Antimalarial activity
2.2.1 |. Quinoline alkaloids
Malaria is the most lethal human parasitic infection. According to the WHO World Malaria Report 2015, an estimated 292,000 African children under five died from malaria, and the disease caused an estimated 306,000 deaths worldwide in the same age group.164 Malaria is caused by five species of protozoan parasites of the genus Plasmodium, including P. falciparum, P. vivax, P. ovale, P. malariae, and P. knowlesi. Of these, P. falciparum and P. vivax account for more than 95% of malaria cases in the world.165 The bark of the Cinchona tree was utilized in early clinical history to treat human malaria. With the development of natural product technology, quinine (1), a quinoline alkaloid, was isolated from the bark of the Cinchona tree in 1820. Due to its low price, parenteral administration, and high efficacy against P. falciparum, it was widely used to treat malaria worldwide.166,167 To meet the needs of this compound in southeast Asia during World War II, the synthesis of 1 was promoted and completed, and some derivatives were developed with better potency and lower toxicity.168–171 In 2006, WHO stopped recommending 1 as a first-line treatment for malaria, because of its high toxicity and the developing resistance of Plasmodium sp. However, it has still been used when artemisinins are not available.171 To date, 1 and its analogs have saved thousands of people’s lives worldwide and made an enormous contribution to human health.
In 1996, Gantier and co-workers isolated six quinoline alkaloids, 2-n-propylquinoline (119), 2-pentylquinoline (120), chimanines B (121) and D (122), 4-methoxy-2-phenylquinoline (123), and 2-(3,4-methylenedioxyphenylethyl)quinoline (124) (Fig. 14) from the bark of Galipea longiflora, which is used to treat recurrent fevers, such as malaria, in Bolivia.172 All six compounds showed the same approximate level of activity as the well-known antimalarial compound chloroquine against P. vinckei petteri infected mice. Four G. officinalis tetrahydroquinolines, cuspareine (59), galipeine (60), galipinine (61), and angustureine (62) (Fig. 7) exhibited antimalarial activity against one chloroquine-sensitive and two chloroquine-resistant strains of the malaria parasite P. falciparum; 61 was the most active compound (IC50 0.276–2.76 μM for the resistant strains at 24 and 72 h).110 Three novel decahydroquinoline alkaloids lepadins D–F (67–69) (Fig. 8) from the genus Didemnum also showed significant antiplasmodial activity; the most potent compound was 69.112,113
FIGURE 14:
Chemical structures of compounds 119–131
Certain furoquinoline alkaloids also demonstrated antimalarial activity. In in vitro tests, kokusaginine (37), skim-mianine (34), haplopine (35) (Fig. 6), acronycidine (125), and acronydine (126) (Fig. 14) were active against HB3 (chloroquine-sensitive) and W2 (chloroquine-resistant) clones of P. falciparum.173 The most active compound, 126, was at least fourfold more potent against the resistant clone (IC50 22.6 and 4.63 μM, respectively), although it was less potent than chloroquine (IC50 0.032 and 0.466 μM, respectively). The pyranoquinolone veprisine (127) and its prenylated congener N-methylpreskimmianine (128) (Fig. 14) also exhibited antimalarial activity against P. falciparum D6 (IC50 6.65 and > 14.8 μM, respectively) and W2 (IC50 6.98 and 5.68 μM, respectively) clones.174
In 1999, three quinolone alkaloids were isolated from a new gram-negative marine bacterial strain of Pseudomonas sp.175 Compounds 129–131 (Fig. 14) showed activity against the malaria parasite P. falciparum (ID50 3.51–16.8 μM).
2.2.2 |. Quinazoline alkaloids
Febrifugine (4) (Fig. 1) and isofebrifugine (132) (Fig. 15) were first isolated as active components of the traditional Chinese medicine Chan Shan (roots of D. febrifuga Lour.), which has marked antimalarial effects. Both compounds were named by Koepfli and co-workers in the 1940s.176–178 They found that 4 was 100 times more active against P. lophurae in ducks than quinine (1), while 132 possessed only modest activity against the same malaria strain.176–178
FIGURE 15:
Chemical structures of compounds 132–137
Additional antimalarial testing showed that 4 (EC50 0.910 nM) was almost 100 times more potent toward P. falciparum compared with chloroquine (EC50 18.0 nM), twice as potent as its hydrochloride salt (EC50 1.8 nM) and about ten times as potent as 132 (EC50 9.00 nM).179 Takaya and co-workers verified that compounds 4 and 132 exert powerful antimalarial activity in vitro, with similar potencies against chloroquine-sensitive P. falciparum FCR-3 (EC50 0.700 and 3.40 nM, respectively), as well as against chloroquine-resistant P. falciparum K1 (EC50 1.20 and 1.80 nM, respectively).180 In in vivo assays, the acetone adduct of 4 displayed better activity than the acetone adduct of 132 against mouse malaria P. berghei. In 2003, Murata et al. investigated the mechanisms of 4, 132, and quinazolin-4(3H)-one (133) (Fig. 15).181 The results indicated that 4 may act differently from other antimalarial drugs, and could be used as a novel lead compound for antiplasmodial chemotherapy. The basicity of both the 1- and the 1′′-nitrogen atoms of 4 is crucial in conferring powerful antimalarial activity.
To possibly decrease unacceptable emetic properties and other side effects, a combination of 4 and 132 was studied against a blood-induced infection with chloroquine-resistant P. berghei NK65 in ICR mice.180,182 A four-day dosage of 1 mg/kg of the 4/132 mixture alone showed slight antimalarial activity, but all mice died during days 19 to 27 with increasing parasitemia. However, mice treated with chloroquine (20 mg/kg) plus the two alkaloids survived the entire experiment. In addition, malaria parasites in the mice given chloroquine plus alkaloids decreased on day 6 and then were undetectable by microscopic examination during the remaining observation period. Several analogs, including halofuginone, a chloro-bromo substituted derivative of 4, were also synthesized to produce better efficacy and lower toxicity.183–186
Three new quinazoline alkaloids, 2-methoxyrutaecarpine (134), 2-methoxy-13-methylrutaecarpine (135), and the cationic variant 5,8,13,14-tetrahydro-2-methoxy-14-methyl-5-oxo-7H-indolo[2′,3′:3,4]pyrido[2,1-b]quinazolin-6-ium chloride (136) (Fig. 15), were isolated from stem bark of Araliopsis tabouensis.174 The two latter compounds showed promising antimalarial activity against P. falciparum D6 (IC50 5.44 and 5.99 μM, respectively) and W2 (IC50 > 14.2 μM) clones, but were less potent than the positive drug artemisinin (IC50 < 0.92 μM against both clones).
Furthermore, the indoloquinazolinedione tryptanthrin (111) (Fig. 13) showed significant in vitro antimalarial activity against P. falciparum, both sensitive and multidrug-resistant strains,187,188 and exhibited remarkable in vitro activity (below 100 ng/mL) against sensitive and multidrug-resistant P. falciparum malaria. The pharmacophore containing two hydrogen bond acceptors (lipid) and two hydrophobic (aromatic) features mapped well onto many well-known antimalarial drug classes, including quinolines, chalcones, rhodamine dyes, Pfmrk cyclin dependent kinase inhibitors, malarial FabH inhibitors, and plasmepsin inhibitors. Compound 111 and its analogs are also highly potent against strains of P. falciparum that are up to 5000-fold resistant to atovoquone, 50-fold resistant to chloroquine, and 20-fold resistant to mefloquine. This novel class of compounds has opened a new chapter for study in the chemotherapy of malaria (−)-Janoxepin (137) (Fig. 15), an interesting oxepine-pyrimidinone natural product, was isolated from a culture of the fungus A. janus.189 However, it did not⋅show antiplasmodial activity against P. falciparum.
2.3 |. Antiparasitic and insecticidal activities
2.3.1 |. Quinoline alkaloids
Leishmaniasis (kala-azar) is a major public health problem in Africa, Asia, and Latin America,190 causing significant morbidity and mortality. To date, more than 70 isolated natural alkaloids have been used to treat this disease. Some of these alkaloids are quinoline or quinazoline type.191 In the 1990s, Fournet et al. studied the antiprotozoal activity of several 2-substituted quinoline alkaloids isolated from G. longiflora.192–195 After administrating chimanine D (122) subcutaneously and 2-n-propylquinoline (119) orally (0.540 mmol/kg per day) to mice for 10 days, the liver parasites were suppressed by 86.6% and 99.9%, respectively. The reference drug resulted in 97.4% parasite suppression in the liver. The alkaloids did not cause any apparent toxicity during the experiment. Additional studies indicated that chimanine B (121) reduced lesion weight and parasite loads substantially after oral administration or intralesion injection, and showed improved performance compared with the positive drug glucantime in BALB/c mice infected with Leishmania amazonensis and L. venezuelensis. Compound 121 may be chosen as a lead molecule in the development of oral therapy against leishmaniasis. Compounds 119 and 122 were also more potent than glucantime against L. amazonensis PH8. After a single treatment with proximate injection, 119 reduced the lesion severity; however, it was less active than glucantime.
2-Propyl- and 2-pentyl-quinoline (119 and 120) were again investigated by Belliard and co-workers in 2003.196 The compounds exhibited significant activity against the virulent strain L. venezuelensis, and 119 decreased intestinal P-glycoprotein activity in mice infected with L. donovani. Based on the P-gp inhibition, 119 could be valuable as an oral drug to restrict leishmanial multi-drug-resistance in humans with kala-azar.
Besides its antiprotozoal activity, 119 was as clinically effective as the known trypanocidal agent benznidazole in mice chronically infected with Trypanosoma cruzi, the pathogenic parasite of Chagas disease.197 Benznidazole and 119 were administered orally at 25 mg/kg for 30 days starting at 60 days post-infection. At day 35 post-treatment, the 119-treated mice had a significantly different serological value from those of the control and the benznidazole-treated mice; however, at day 85 post-treatment, the difference was not statistically different. These results indicate that 119 and its analogs should be further investigated for potent trypanocidal activity and control of chronic Chagas’ disease. In addition, compounds 119 and 120, as well as 2-(3,4-methylenedioxyphenylethyl)quinoline (124), exhibited molluscicidal activity against the freshwater snail Biomphalaria glabrata.198
Four quinoline alkaloids 121, 124, cusparine (138) (Fig. 16), and 2-(3,4-dimethoxyphenylethyl)quinoline (139) (Fig. 16) as well as the furanoquinoline alkaloid skimmianine (34) were as effective as the positive control drug against the Leishmania parasite.199 In addition, 34 inhibited the parasite enzyme adenine phosphoribosyltransferase. Other furoquinoline alkaloids also exhibit antiparasitic and insecticidal activities. Kokusaginine (37) (IC50 0.560 mM), 34 (IC50 1.46 mM), and rel-(7R,8R)-8-[(E)-3-hydroxy-3-methyl-1-butenyl]-4,8-dimethoxy-5,6,7,8-tetrahydrofuro [2,3-b]quinolin-7-yl acetate (140) (Fig. 16) (IC50 0.977 mM) from Almeidea rubra exhibited moderate in vitro trypanocidal activity against the trypomastigote forms of T. cruzi.200 Dictamnine (40) and evolitrine (36, 8-methoxydictamnine) exhibited antifeedant activity against fourth instar larvae of the tobacco caterpillar Spodoptera litura.201 Compound 40 was also deterrent against two insect pests [Sitophilus zeamays (maize weevil) and Trilobium castaneum (red flour beetle)] responsible for spoilage of stored products.202 However, the furoquinoline alkaloid 37 (LC50 1420 μM) was extremely less potent than the quinolinone alkaloids evocarpine (141) and dihydroevocarpine (142) (Fig. 16) in a brine shrimp toxicity assay (LC50 2.27 and 62.6 μM, respectively).203,204
FIGURE 16:
Chemical structures of compounds 138–161
The Cinchona alkaloid quinine (1)108 and lepadins D–F (67–69) showed significant antitrypanosomal activity; the most potent compound was 69.112,113 Antidesmone (143) (Fig. 16), a tetrahydroquinolinedione alkaloid from Antidesma membranaceum, also displayed potent and selective antitrypanosomal activity (IC50 0.066 μM) against T. cruzi, but only weak antimalarial activity against P. falciparum K1 and NF254 and anti-leishmanial activity against L. donovani.205 In contrast, 2-nonylquinolin-4(1H)-one (129), N-methyl-2-nonylquinolin-4-one (144), and N-methyl-2-phenylquinolin-4-one (145) (Fig. 16) from Raulinoa echinata did not show activity against the trypomastigote forms of T. cruzi (IC50 > 300 μM), but compound 129 was weakly fungicidal toward Leucoagaricus gongylophorus.206
In 1995, Perrett and Whitfield reported that atanine (146) (Fig. 16), a quinolin-2-one alkaloid from Evodia rutaecarpa, showed antiparasitic and anthelmintic activity against larvae of the human parasite Schistosoma mansoni and the soil nematode Caenorhabditis elegans.207 The novel tetracyclic quinolin-4-one quinolactacide (147) (Fig. 16) from the fermentation broth of P. citrinum Thom F 1539 also showed excellent insecticidal activity against green peach aphids (Myzus persicae) (88% and 100% mortality at 250 and 500 ppm, respectively) and diamondback moth (Plutella xylostella) (42% at 500 ppm).208,209
Subsequently, peniprequinolone (77), penigequinolones A (148) and B (149), 3-methoxy-4-hydroxy-4-(4′-methoxyphenyl)quinolinone (150), and 3-methoxy-4,6-dihydroxy-4-(4′-methoxyphenyl)quinolinone (151) (Fig. 16) were isolated from Penicillium cf. simplicissimum in 2000.210 Compounds 148 and 149 showed potent nematicidal activity (LD50 100 mg/L) toward Pratylenchus penetrans. Thus, the penigequinolones may be useful for controlling parasitic nematodes.
Nakatsu and co-workers studied the anti-feedant activity of two unusual quinolin-4-ones, leiokinines A (152) and B (153) (Fig. 16), from E. leiocarpa.211 The compounds showed weak effects against the pink bollworm Pectinophora gossypiella. 3,4-Dihydroxyquinoline-2-carboxylic acid (154) (Fig. 16) from the sponge Aplysina cavernicola acted as a powerful feeding deterrent of the fish species Blennius sphynx,212 and acetylcupreine (57) affected the feeding behavior of the potato beetle Leptinotarsa decemlineata.108
(−)-Yaequinolone J1 (155) and (+)-yaequinolone J2 (156) (Fig. 16), two new alkaloids related to the abovementioned penigequinolones, were isolated from a Japanese soil sample of Penicillium sp. FKI-2140 in 2005.213 Both compounds showed activity in a brine shrimp assay with a minimum inhibitory concentration (MIC) of 13.9 μM. 3-Methoxy-4,5-dihydroxy-4-(4′-methoxyphenyl)-quinolinone (157) (Fig. 16), without the side chain at C-6, was also toxic to brine shrimp with an IC50 value of 63.5 μM.214
2.3.2 |. Quinazoline alkaloids
Among vasicine alkaloids found in A. vasica, vasicine (3), vasicinone (158), and vasicinol (159) (Fig. 16) showed feeding deterrence at concentrations of 0.05 and 0.1% against two beetle species Aulacophora foveicollis and Epilachna vigintioctopunctata.215 The latter compound blocked oocytes in the oviduct and exhibited severe antifertility effects against T. castaneum and the cotton pest Dysdercus koenigii.
The well-known alkaloid tryptanthrin (111) showed insecticidal activity against larvae of the house longhorn beetle Hylotrupes bajulus and the termite Reticulitermis santonensis. Moreover, the compound also displayed antifeedant activity, as termites avoided the treated pine samples.216 In addition, compound 111 showed antitrypanosomal activity against T. brucei with an EC50 value of 23.0 μM.217 Furthermore, (+)-Nα-quinaldyl-L-arginine (160) (Fig. 16) found in the exudates of the ladybird beetle Subcoccinella 24-punctata proved to be a highly effective feeding deterrent to the ant species Myrmica rubra.218
A mixture of the cis and trans isomers of febrifugine (4) was isolated from Hydrangea macrophylla.219 Trans-4 showed anticoccidial activity against Eimeria parasites in chickens, whereas cis-4 was inactive even at much higher dosages.
1,3-Dimethylquinazoline-2,4-dione (161) (Fig. 16) was identified as a sex pheromone of Phyllopertha diversa or chafer beetle.220 Female beetles release the compound in only picogram quantities. As many as 153 male beetles per trap per hour were successfully lured to field traps baited with 161, while the control captures were extremely low (0.4). The compound was catabolized by an antennal cytochrome P450 system, which was highly specific to male insects.221
2.4 |. Antibacterial and antifungal activities
2.4.1 |. Quinoline alkaloids
E. rutaecarpa extracts display antibacterial activity against Helicobacter pylori, which is implicated in the pathogenesis of chronic gastritis, peptic ulcers, and gastric cancers. Consequently, many compounds have been isolated and identified from this plant. In 1999, Rho and co-workers isolated six quinolone alkaloids, evocarpine (141), dihydroevocarpine (142), 1-methyl-2-pentadecyl-4(1H)-quinolone (162), 1-methyl-2-[(4Z,7Z)-4,7-tridecadienyl]-4(1H)-quinolone (163), 1-methyl-2-[(6Z,9Z)-6,9-pentadecadienyl]-4(1H)-quinolone (164), and 1-methyl-2-undecyl-4(1H)-quinolone (165), (Fig. 17), which showed potent anti-H. pylori activity with MIC values of 10–20 μg/mL.222
FIGURE 17:
Chemical structures of compounds 162–176
The following year, Hamasaki et al. explored the in vitro anti-H. pylori activity of an extract from the fruits of E. rutae-carpa (Gosyuyu), one part of the Chinese herbal medicine Gosyuyu-to (Wu-Chu-Yu).223 Two 1-methyl-2-tridecenyl-4(1H)-quinolones [141 (8Z) and 166 (7Z)] (Fig. 17) were identified as the strongest antibacterial principles. Their MIC values were less than 0.147 μM against clinically isolated and reference H. pylori strains and similar to the values of the antibiotics amoxicillin and clarithromycin.223 Additional studies indicated that these alkaloids were highly selective against H. pylori and almost inactive against other intestinal pathogens. They inhibited the bacterial respiration and reduced the bacterial growth in vivo, but not DNA synthesis.224 In addition, these compounds significantly decreased the number of viable H. pylori in the stomachs of infected Mongolian gerbils and reduced neutrophil infiltration without causing harmful adverse effects, including animal mortality.224 The above results indicated that these alkyl methyl quinolone alkaloids have a unique antimicrobial mechanism(s) different from those of other antibiotics such as amoxicillin and clarithromycin. They may be beneficial in the treatment of H. pylori-associated gastroduodenal diseases, whether used alone or together with the above-mentioned antibiotics or proton pump inhibitors.223
Five quinolone alkaloids, 141, 163–165, and 1-methyl-2-(6Z)-6-undecenyl-quinolone (167) (Fig. 17), from E. rutaecarpa also displayed promising antimycobacterial activities in in vitro tests with Mycobacterium fortuitum, M. smegmatis, and M. phlei (MICs 12.5–200 μM).225 Among these compounds, 141 was the most active (MIC 12.5 μM). Quinolone alkaloid 129, its N-methyl congener (144), and 2,3-dimethyl-4-quinolone (168) (Fig. 17) from Boronia bowmanii exhibited moderate antibacterial activity against Bacillus subtilis, Staphylocccus aureus, Sarcina lutea, exterotoxigenic E. coli, Salmonella typhi, and Klebsiella sp.226
The furoquinoline alkaloid flindersiamine (46) from E. yaaxhokob exhibited moderate antimicrobial activity against S. aureus and S. faecalis.227 In other studies, the furoquinolines kokusaginine (37), skimmianine (34) and haplopine (35), as well as the pyranoquinoline flindersine (31), exhibited photo-activated antimicrobial activity against S. aureus.228 Compounds 37, 34, and 35 displayed photo-activated DNA binding activity in the presence of several restriction enzymes and likely target DNA. However, the pyranoquinoline alkaloid 31 did not show photo-activated DNA binding activity and must act on other cellular target components to exert its photo-toxic activity.228 The furoquinoline pteleine (49) showed moderate antimicrobial activity against M. smegmatis, B. subtilis, S. aureus, and Candida albicans (MIC 4.39– 87.8 μM), while 34 and dictamnine (40) were less potent against the two former microbes and inactive against the latter two microbes.229
Megistoquinones I (169) and II (170) (Fig. 17), probable oxidation products of a furo[2,3-b]quinoline precursor, were isolated from the bark of S. megistophylla.230 Both alkaloids showed antibacterial properties against two gram-positive, S. aureus (MIC 9.073, 2.577 mM) and S. epidermidis (MIC 10.7, 2.51 mM), and four gram-negative, Pseudomonas aeruginosa (MIC 12.5, 3.33 mM), E. coli (MIC 18.3, 3.51 mM), Enterobacter cloacae (MIC, 12.0, 3.06 mM), and Klebsiella pneumoniae (MIC 20.3, 4.23 mM), bacteria.
Two new functionalized 3-prenylquinolinones, N-methyl-4-hydroxy-7-methoxy-3-(2,3-epoxy-3-methylbutyl)-1Hquinolin-2-one (171) and 3-(2,3-dihydroxy-3-methylbutyl)-4,7-dimethoxy-1-methyl-1H-quinolin-2-one (172) (Fig. 17) were isolated from Toddalia aculeata.231 Both compounds strongly inhibited the growth of the bacteria E. coli, B. cereus, and Lactobacillus lactis at millimolar concentrations.
A special carbaldehyde substituted compound, quinoline-4-carbaldehyde (173) (Fig. 17), was isolated from the herb R. chalepensis.232,233 It significantly inhibited the growth of Clostridium perfringens. This result may verify the phytoprotective effects of the herbal remedy. However, the compound’s effect on E. coli was weak, and effects on the beneficial gastrointestinal bacteria Bifidobacterium bifidum, B. longum, and L. acidophilus were slight or absent.
During a research escalation on the antibacterial activity of microorganism metabolites, two 2-alkyl-4(1H)-quinolinone alkaloids (174, 175) (Fig. 17) were isolated from P. cepacia strain RB425 collected from lettuce root234 and strain LT4–12-W,235 respectively. Both alkaloids exhibited antibiotic activity against fungal and bacterial plant pathogens. Meanwhile, YM-30059 (a structurally related N-hydroxyquinolin-4-one) (176) (Fig. 17) was isolated from Arthrobacter sp. YL-02729S as an antibacterial and cytotoxic compound.236 It displayed moderate activity against gram-positive bacteria, including B. subtilis and multiple-drug resistant S. aureus and S. epidermidis.
Four sesquiterpenoid quinoline antibiotics, aurachins A–D (177–180) (Fig. 18) from the myxobacterium, Stigmatella aurantiaca, were active against gram-positive bacteria and weakly active against some fungi.237 Against B. subtilis, S. aureus, Arthrobacter aurescens, Brevibacterium ammoniagenes, and Corynebacterium fascians, the four compounds showed the following MIC values, 177: 12.658, 6.329, 0.481, 0.987, 3.949; 178: 6.849, 3.425, 2.137, 3.425, 4.273; 179: 0.396, 1.029, 0.501, 0.132, 2.058; 180: 0.413, 1.074, 0.523, 0.138, 2.149 μM, respectively. Meanwhile, one of the simplest quinolines, helquinoline (181) (Fig. 18), from the fermentation broth of Janibacter limosus strain Hel-1, showed moderate activity toward B. subtilis, S. viridochromogenes Tü57, and S. aureus.238
FIGURE 18:
Chemical structures of compounds 177–189
In 1998, Dekker and co-workers isolated eight new quinolin-4-ones from the fermentation broth of the actinomycete Pseudonocardia sp. CL38489.239 These compounds were given the code numbers CJ-13136 (182), CJ-13217 (183), CJ-13536 (184), (–)-CJ-13564 (185), CJ-13565 (186), CJ-13566 (187), (+)-CJ-13567 (188), and (–)-CJ-13568 (189) (Fig. 18). All eight compounds inhibited the growth of H. pylori; the most potent compound was the epoxide CJ13564 (185) with minimum bacterial concentration (MBC) 30.769 nM and MIC 0.308 nM. Moreover, the antibacterial activity of these compounds was highly selective and specific. Thus, because they are less likely to disturb the normal gastro-intestinal microbial flora, they could be used as antiulcer agents.
In addition to promising antitumor activity with potential clinical value,240 the octadepsipeptide (−)-thiocoraline (89) exhibited potent antibiotic activity against S. aureus (MIC 0.05 μg/mL), B. subtilis (MIC 0.05 μg/mL), and Micrococcus luteus (MIC 0.03 μg/mL).130,131 Sch 40832 (190) (Fig. 19), a minor metabolite from the fermentation broth of M. carbonacea var. africana, also exhibited potent activity less than 0.504 μM against gram-positive bacteria.241
FIGURE 19:
Chemical structures of compounds 190–195
Two bacterial alkaloids 2-heptylquinolin-4-ol (191) and 2-pentylquinolin-4-ol (192) (Fig. 19) were isolated from Alteromonas sp.242 The latter compound inhibited respiration in other bacteria at a low concentration (75.0 nM) and DNA and protein synthesis, as well as bacterial motility, at micromolar concentrations. It also inhibited the growth of phytoplankton and diatoms, and altered the composition of bacterial communities growing on particles suspended in sea water.
Quinoline-related animal metabolites also show antibacterial activity. trans-Decahydroquinoline 243A (193) (Fig. 19) was isolated from amphibian (frog) skin in 2005.243 It inhibited the growth of the gram-positive bacterium B. subtilis, gram-negative bacterium E. coli, and the fungus C. albicans. The two novel pyrrolo[3,4-b]quinoline alkaloids quinocitrinine A (79) and (−)-quinocitrinine B (80) showed moderate antimicrobial activity toward a range of bacteria and fungi.120
As indicated above, quinoline alkaloids have also been investigated for antifungal activities. Decahydroquinoline alkaloids lepadins D–F (67–69) showed weak antifungal effects.112,113 The decahydroquinolone alkaloid anhydroevoxine (194) (Fig. 19), as well as two pyranoquinolone alkaloids flindersine (31), and haplamine (32) from Haplophyllum sieversii showed growth-inhibitory antifungal activity against Colletotrichum fragariae, C. gloeosporioides, C. acutatum, Botrytis cinerea, Fusarium oxysporum, and Phomopsis obscurans in a dose-response manner at 100, 50, and 150 μM.244 Among these compounds, 31 presented the highest antifungal activity. In addition, 32 was selectively more toxic toward freshwater phytoplanktons such as Pseudanabaena sp. LW397 and the odor-producing cyanobacterium Oscillatoria perornata. The furoquinoline alkaloid flindersiamine (46) and its congeners kokusaginine (37), skimmianine (34), dictamnine (40), maculine (42), and platydesmine (195) (Fig. 19) inhibited the growth of the fungus L. gongylophorus, a symbiotic fungus of the insect pest Atta sexdens rubropilosa.245 Dictamnine (40) also was a weak inhibitor of the pathogenic fungus Cladosporium cucumerinum (MIC 125.628 μM), while haplopine (35) exhibited relatively low activity.246
1-Methyl-2-[6′-(3′′,4′′-methylenedioxyphenyl)hexyl]-4-quinolone (196) (Fig. 20) from R. graveolens was highly active against the necrotrophic fungus B. cinerea.247 Distomadine B (197) and its analog (+)-distomadine A (198) (Fig. 20) with furo[3′,4′:5,6]pyrano[2,3,4-de]quinoline skeletons were isolated from Pseudodistoma aureum.248 Compound 198 showed moderate antifungal activity toward C. albicans, but was inactive in various antitumor, antiviral, anti-inflammatory, and antimycobacterial assays.
FIGURE 20:
Chemical structures of compounds 196–205
One quinolone [2-(hept-2-enyl)-3-methylquinolin-4-one (175)] and four quinoline [quinoline-4-carbaldehyde (173), 4-hydroxymethylquinoline (199), quinoline-4-carbaldoxime (200), and quinoline-4-carboxylic acid (201) (Fig. 20)] alkaloids were isolated from cultures of the soil myxobacterium Archangium gephyra (strain Ar T205) in 1996.249 Among these five alkaloids, compound 176 proved to be the most active against Phytophthora capsici and other fungal plant pathogens. In 2001, the simple antibiotic N-mercapto-4-formylcarbostyril (202) (Fig. 20) from P. fluorescens (strain G308) showed good activity against a range of plant pathogenic fungi, including F. oxysporum, F. culmorum, C. cucumerinum, and C. lagenarium.250 Moreover, a new antiviral antibiotic, virantmycin (203) (Fig. 20), was isolated from the culture broth of strain AM-2722 in 1980.251,252 It exhibited weak antifungal activity with MICs from 12.5 to 50 μg/mL against S. sake, Piricularia oryzae, Trichophyton interdigitale, A. niger, Alternaria kikuchiana, Mucor racemosus, and C. albicans.
2.4.2 |. Quinazoline alkaloids
The quinazoline alkaloid tryptanthrin (111) showed exciting potential as an antimycobacterial agent against a multiple drug-resistant strain of M. tuberculosis.253 It also exhibited good antibacterial activity against H. pylori in both in vitro and in vivo studies.254
Fumiquinazolines H and I (204, 205) (Fig. 19) were isolated from the culture broth and mycelia of an Acremonium sp. in 2000.255 Both compounds showed weak antifungal activity toward C. albicans in a broth microdilution assay, but no activity in antimicrobial assays or toward various cancer cell lines.
2.5 |. Cardiovascular protective and antiplatelet activities
2.5.1 |. Quinoline alkaloids
Although Pasteur first isolated quinidine (28) from the bark of the Cinchona tree in 1853, the compound’s possible use in arrhythmias was not noted until 1912 after patient observed that quinine, another Cinchona alkaloid and a stereoisomer of quinindine, had a beneficial effect on his own heart arrhythmia. Compound 28 was later noted to be the most effective Cinchona alkaloid on the heart. In 1920, Lewis proposed that 28 restores normal cardiac rhythm by closing the gap between the crest and wake of the circus wave generated in arrhythmia.256 Since then, alkaloid 28 has been widely investigated for its antiarrhythmic activity and was acknowledged as the most potent of the antiarrhythmic compounds in the early 20th century.257 In studies on the effect of reserpine pretreatment on the action of 28 in isolated cat hearts with complete heart blocks, exogenous catecholamines were demonstrated to antagonize the cardiac actions of 28, and cardiac catecholamines to antagonize the depressant action of 28.258–261 Alkaloid 28 slows amphibian heart rate with its foremost effects attributed to a rise in the threshold for electrical stimuli and its consequences.262 Further studies indicated that 28 interferes selectively with vasoconstrictor stimuli, which activate alpha adrenergic receptors, and this mechanism as well as a direct vasodilator effect may contribute to vasodilatation and hypotension.263 Therapeutic doses (10–20 μM) of 28 strongly inhibit fast inward current INa in isolated ventricular cells,264 affect the spontaneous contractions of rabbit atria,265 and depress the active transport of serotonin by platelets.266
Other quinoline and quinazoline alkaloids also have cardiovascular effects. At a concentration of 100 μg/mL, the furoquinoline alkaloid dictamnine (40), isolated from Zanthoxylum species in 1994,267,268 completely inhibited the platelet aggregation induced by arachidonic acid, and was also markedly effective in inhibiting platelet aggregation induced by collagen and PAF. Pyranoquinolone [huajiaosimuline (30), simulenoline (206), benzosimuline (207), zanthobungeanine (208)], furoquinoline [γ-fagarine (33), skimmianine (34), haplopine (35), robustine (209)], and quinolone [edulitine (210)] alkaloids (Fig. 21) also inhibited the aggregation induced by thrombin, arachidonic acid, collagen, and PAF in washed rabbit platelets.95 Likewise, 4-methoxy-1-methylquinolin-2-one (211) (Fig. 21) completely inhibited arachidonic acid-induced platelet aggregation in vitro at a concentration of 100 μg/mL.268
FIGURE 21:
Chemical structures of compounds 206–216
In other related studies on furoquinoline alkaloids from Zanthoxylum and Melicope species, confusameline (212) (Fig. 21), skimmianine (34), evolitrine (36), kokusaginine (37), dictamnine (40), and pteleine (49) showed significant antiplatelet aggregation activity.269–272 Compound 34 affected the cardiovascular function and vasopressor responses in rats,273 and confusadine (90) inhibited the platelet aggregation triggered by various inducers.274
Moreover, furoquinoline alkaloids also show cardiovascular protective activity. Robustine (209) and confusameline (212) (Fig. 21), as well as γ-fagarine (33), skimmianine (34), haplopine (35), evolitrine (36), kokusaginine (37), dictamnine (40), inhibited human phosphodiesterase 5, which regulates the intracellular levels of cGMP and influences vascular smooth muscle tone.275 Three quinolone alkaloids, evocarpine (141), 1-methyl-2-[(4Z,7Z)-4,7-tridecadienyl]-4(1H)-quinolone (163), and 1-methyl-2-[(6Z,9Z)-6,9-pentadecadienyl]-4(1H)-quinolone (164) from E. rutaecarpa blocked the angiotensin II receptor and inhibited angiotensin II binding to rat liver receptor (IC50 43.4, 34.1, and 48.2 μM, respectively).276
2.5.2 |. Quinazoline alkaloids
The antiplatelet activity of the quinazoline alkaloids rutaecarpine (115), 1-hydroxyrutaecarpine (116), and 1-methoxyrutaecarpine (213) (Fig. 21) from Z. integrifolium was investigated.277 In in vitro tests, 116 was the strongest inhibitor of arachidonic acid-induced platelet aggregation, with an IC50 values of 3.32–6.65 μM.
In 2000, studies showed that acrophyllidine (214) (Fig. 21) from A. haplophylla has antiarrhythmic activity.278 It suppressed ischemia/reperfusion-induced polymorphic ventricular tachyarrhythmias with an EC50 value of 4.40 μM in isolated rat heart, increased the atrioventricular and His-Purkinje system conduction intervals, ventricular repolarization time, and basic cycle length, and prolonged the refractory periods of the AV node, His-Purkinje system, and ventricle in a perfused whole-heart model. Moreover, this furoquinoline alkaloid prolonged the action potential duration and decreased both the maximal upstroke velocity of depolarization and action potential amplitude in a concentration-dependent manner in isolated rat ventricular myocytes.278 These changes alter the electrophysiological properties of the conduction system and may be responsible for the compound’s termination of ischaemia/reperfusion induced ventricular arrhythmias.
The quinazoline alkaloids rutaecarpine (115), evodiamine (215), and dehydroevodiamine (216) (Fig. 21) produced a vasodilatory effect on endothelium-intact rat aorta with equal potency in smooth muscle from rat thoracic aortas.279 Compound 115 produced a full nitric oxide (NO)-dependent vasodilation, whereas 216 and 217 exhibited partial endothelium-dependent effects, 50% and 10%, respectively. Another quinazoline alkaloid vasicine (3) also showed hypotensive and cardiac depressant properties.280,281
2.6 |. Antiviral activity
Uranidine (217) (Fig. 22), a quinolone alkaloid and well-known yellow pigment, inhibits the RNA-directed DNA synthesis of the reverse transcriptases (RTs) of human immunodeficiency viruses HIV-1 and HIV-2, with the 3-hydroxy-4-oxo system likely being a key structural element for the inhibitory activity.282 Furthermore, 2-undecyl-4(1H)-quinolone (130) from the gram-negative marine bacterial strain of Pseudomonas sp. showed activity against HIV-1.175
FIGURE 22:
Chemical structures of compounds 217–220
Buchapine [218, 3-(1,1-dimethylallyl)-3-(3-methylbut-2-enyl)-1H-quinoline-2,4-dione] and 3-prenyl-4-prenyloxy1H-quinolin-2-one (219) (Fig. 22) from E. roxburghiana also showed anti-HIV-1 activity.283 Both compounds were active against infectious HIV-1 (EC50 0.940 and 1.64 μM, respectively) in human lymphoblastoid host cells (cell growth IC50 29 and 26.9 μM, respectively). They also showed inhibitory activity in an HIV-1 RT assay (IC50 12 and 8 μM, respectively).
Three furoquinoline alkaloids, γ-fagarine (33), haplopine (35), and (+)-platydesmine (196), as well as 4-methoxy1-methylquinolin-2-one (212) also inhibited HIV-1 replication in H9 lymphocyte cells at low concentrations (EC50 < 5.85 μM) without significantly affecting the growth of uninfected H9 cells.284 Compound 33 showed the best therapeutic index, while 35, 196, and 212 were less effective. 2-Acetyl-4(3H)-quinazolinone (118) also inhibited HIV replication.162,163
Moreover, quinoline-containing decadepsipeptides can significantly inhibit HIV-1 RT, but also display notable cytotoxicity against tumor cell lines. Various modified derivatives of sandramycin (220) (Fig. 22) (HIV RT IC50 0.13 nM) retained its HIV potency, but exhibited 150- to 1000-fold less cytotoxic activity.285 Thus, promising candidates could be further developed as HIV-1 chemotherapeutic agents. Three other decadepsipeptides luzopeptins A–C (83–85) were identified as potent inhibitors of the HIV RT responsible for the emerging clinical resistance to recently introduced RT inhibitors.286 Moreover, the rank orders of cytotoxic potency (A > B > > C) and antiviral potency/HIV RT inhibition (C > B > A) were reversed, and 85 suppressed HIV replication in infected MT-4 cells at noncytotoxic concentrations.123–126,287
At very low concentrations, virantmycin (203) inhibited various RNA and DNA viruses, including the Indiana strain of vesicular stomatitis virus, Egypt Ar 339 strain of Sindbis virus, MCMILLAN strain of Western equine encephalitis virus, MIYADERA strain of Newcastle disease virus, DIE strain of vaccinia virus, IHD strain of vaccinia virus, HF strain of herpes simplex virus type 1, and UW strain of herpes simplex virus type 2.251,252 The compound affected the cell membranes, including specific virus receptor sites, and suppressed viral replication at a very early stage. In addition, compound 203 showed excellent growth inhibition of influenza virus.288
2-(3,4-Methylenedioxyphenethyl)quinoline (124), chimanine D (122), 2-pentylquinoline (120), and 2-nproplyquinoline (119) from G. longiflora inhibited the growth of cells infected with human T-lymphotropic virus type 1 (HLTV-1).289–291 Certain quinolines also showed antiproliferative activity against HTLV-1 infected HUT-102 cells. Evolitrine (36) and dictamnine (40) inhibited activation of Epstein-Barr virus early antigen in Raji cells.292
2.7 |. Anti-inflammatory and immunomodulatory activities
2.7.1 |. Quinoline alkaloids
In 2005, Lal and co-workers studied the anti-inflammatory activity of the furoquinoline alkaloid evolitrine (36) and its analogs.293 The results showed that 36 effectively inhibited the formation of edema resulting from sub-plantar injection of carrageena in rats (57% inhibition at a dosage of 20 mg/kg), but did not produce toxic symptoms, cardiovascular effects, or weight loss.293 Also, the quinolone alkaloid orixalone A (221) (Fig. 23) from Orixa japonica strongly inhibited NO production in murine macrophage RAW 264.7 cells stimulated with interferon-γ and LPS at micromolar concentrations and, thus, might be used as an anti-inflammatory or cancer-preventive agent to suppress excessive synthesis of NO.294
FIGURE 23:
Chemical structures of compounds 221–228
Quinolactacins A1 and A2 (222, 223), B (224), and C (225) (Fig. 23) were isolated from culture broth of the entomopathogenic fungus Penicillium sp. EPF-6.295,296 This rare compound class contains an N-methyl quinolone fused to a lactam ring. Only compound 223 inhibited the production of tumor necrosis factor (TNF) induced by LPS in murine peritoneal macrophages (IC50 12.2 μg/mL) and in macrophage-like J774.1 cells.
In 2003, nine 2-alkyl-4(1H)-quinolone alkaloids, 129, 130, 141, 142, 144, and 162–165 from the fruits of E. rutaecarpa were evaluated for immunomodulatory effects.297 With IC50 values between 0.910 and 15.9 μM, these alkaloids inhibited the activity of nuclear factor of activated T cells (NFAT), without affecting cell viability. Among the N-methylated quinolones, compounds with longer aliphatic side chains on the quinolone ring showed stronger inhibition of NFAT activity and comparable inhibitory effects against NF-κB activity. These results indicated that these quinolones could be used as lead compounds for treating diseases of the immune system.297
2.7.2 |. Quinazoline alkaloids
In 2000, the anti-inflammatory activity of tryptanthrin (111) was first reported.298 This alkaloid significantly inhibited the production of both NO and prostaglandin E2 (PGE2) in murine macrophage RAW 264.7 cells activated by interferon-γ and LPS inadose-dependent manner.This potential new anti-inflammatory agent was subsequently investigated for other anti-inflammatory effects and its mechanism of action. In pharmacological studies, 111 ameliorated artificially induced colitis in mice, as well as suppressed weight loss, tissue damage, and subsequent mortality.299 Meanwhile, it showed 100-fold greater selectivity toward COX-2 than COX-1 in the biosynthesis of eicosanoids, as well as inhibition of 5-lipoxygenase.300 Moreover, it inhibited the production of interferon-γ and interleukin-2 by lymphocytes in response to staphylococcal enterotoxin B.301 These results indicated that 111 not only has potent dual effects on prostaglandin and leukotriene synthesis for the treatment of inflammatory diseases, but also can potentially be used to control food-borne intestinal diseases. Finally, Oberthür et al.302 and Heinemann et al.303 postulated that 111 could be more easily absorbed through the skin than other alkaloids, because of its lower bioavailability resulting from ready crystallization from solution.
Subsequently, the natural vasicinone analog isaindigotone (226) (Fig. 23), isolated from I. tinctoria, was found to be a superior scavenger of superoxide generated in the hypoxanthine/xanthine oxidase system (IC50 42.2 nM).304 The compound inhibited PGE2 and NO generation in RAW 264.7 macrophages stimulated by LPS. Its free phenolic group wasimportanttotheanti-inflammatoryactivity.Thesimplealkaloidquinazoline-2,4-dione(227)(Fig. 23)alsoexhibited anti-inflammatory and antihypertensive properties.305
In 2006, two indolopyridoquinazolinone alkaloids rutaecarpine (115) and evodiamine (215), as well as the structurally related quinazoline-2,4-dione goshuyuamide II (228) (Fig. 23) were evaluated for anti-inflammatory activity.306 Compounds 115 and 215 strongly inhibited PGE2 synthesis in LPS-treated RAW 264.7 cells at 1 to 10 μM, and 215 also inhibited COX-2 induction and NF-κB activation. Compound 228 inhibited 5-lipoxygenase from RBL-1 cells (IC50, 6.60 μM), resulting in reduced synthesis of leukotrienes. However, these three compounds did not inhibit inducible NO synthase-mediated NO production.306
2.8 |. Anti-Alzheimer’s disease and other neurological disorders
2.8.1 |. Quinoline alkaloids
The furoquinoline alkaloids skimmianine (34), kokusaginine (37), and confusameline (212) inhibited 5-HT-induced contraction mediated by 5-HT2 receptors in the presence of methiothepin in rat isolated aorta.307 These three compounds may act on 5-HT receptors in animals, more selectively to the 5-HT2 subtype, in the rank order of 34 > 37 > 212. The quinoline alkaloids benzastatins C (71) and D (72) inhibited glutamate toxicity in N18-RE-105 cells with EC50 values of 2 and 5.40 μM, respectively.114,115
The quinolone alkaloid pteleprenine (229) (Fig. 24) from O. japonica significantly inhibited acetylcholine- and nicotine-induced contraction of guinea pig ileum.308 Thus, this natural product might be a novel lead compound as an agonist of nicotinic acetylcholine receptors.
FIGURE 24:
Chemical structures of compounds 229–235
2.8.2 |. Quinazoline alkaloids
In 1996, the anticholinergic natural product deoxyvasicine (110) was identified in a search for a new compound for the treatment of Alzheimer’s disease, and a 3-chloro derivative of the parent hexahydroazepino[2,1-b]quinazoline structure was found to be about eight-fold more potent as an acetylcholinesterase (AChE) inhibitor than the unsubstituted compound.309 In addition, both quinolactacin A2 (223) and quinolactacin A1 (222) inhibited AChE, but 223 was more potent (IC50 19.8 vs 280 μM).310 1-Methyl-2-undecylquinolin-4(1H)-one (165), an Evodia alkaloid, acted as an irreversible and selective inhibitor of type B monoamine oxidase.311
Dictyoquinazols A–C (230–232) (Fig. 24), from the mushroom Dictyophora indusiata, showed protective effects in primary cultured mouse cortical neurons against the excitotoxicity induced by glutamate and N-methyl-D-aspartate in a dose-dependent manner.312 These results indicated that the above compounds have potential value in the treatment of neurological disorders or neurodegenerative diseases of the brain, such as Parkinson’s and Alzheimer’s diseases and Huntington’s chorea. Fiscalins A (233), B (234), and C (235) (Fig. 24), from a fungal culture of Neosartorya fischeri, inhibited the binding of substance P, an undecapeptide neurotransmitter, to human neurokinin-I receptors with Ki values of 57, 174, and 68 μM, respectively.313
2.9 |. Herbicidal activity
In 2004, Hale and co-workers found that the 2-phenylquinolinone alkaloid graveoline (41) has marked herbicidal activity and may be useful as a biodegradable, environmentally friendly herbicide. It inhibited the germination of representative monocot and dicot seeds, impeded the growth of aquatic duckweed, and reduced cell division in onion.314 A mixture of two highly substituted 4-phenyl 2-quinolone alkaloids penigequinolones A (148) and B (149) inhibited the growth of tea pollen tubes by 40% at 10 mg/L and 100% at 100 mg/L.315 Compared with other natural pollen inhibitors, the mixture’s effects were stronger than those of emeniveol, but weaker than those of hericerin and isofunicone.315
2.10 |. Effects on CYP450 family and cytochromes
In 1990, Oettmeier et al. reported that aurachins A–D (177–180) inhibit photosynthetic electron transport.316 Aurachin C (179) was an extremely potent inhibitor of the quinol oxidation sites of two different cytochrome enzymes and competed for the binding sites normally occupied by quinones of the electron transport chain. Aurchin D (180) was a highly effective inhibitor of cytochrome bd. Both 179 and 180 were active on the cytochrome b6/f-complex, the latter showing the most pronounced inhibition to date.317
In 2003, Don et al. studied the indolopyridoquinazolinone alkaloid rutaecarpine (115) and its analogs for their effects on CYP450.318 2-Methoxyrutaecarpine (134) and 115 inhibited all three cytochromes (CYP1A1, CYP1A2, and CYP1B1) of the human cytochrome P450 family without particular selectivity. Alkaloid 115 also modulated the effects of CYP1A1 and 1A2 in human or mouse liver and kidney and CYP2B in rat liver.319–322 and the Cinchona alkaloid quinidine (28) also modified CYP3A4 activity.323
Finally, the quinazoline-benzodiazepine alkaloids, (−)-circumdatins H (236) and E (237) (Fig. 25), isolated from fungal sources, inhibited the mitochondrial respiratory chain in submitochondrial particles from beef heart.324 Their effects were presumably due to interference with NADH oxidase activity (IC50 1.50 and 2.50 μM, respectively).
FIGURE 25:
Chemical structures of compounds 236–247
2.11 |. Hypolipidemic and anti-hyperglycemic activities
FR225659 (238) and four related compounds (239–242) (Fig. 25) were isolated from the culture broth of Helicomyces sp. No. 19353 as novel gluconeogenesis inhibitors in 2003.325,326 Despite high hypoglycemic activity in vitro, 241 and 242 exhibited weak or no activity in vivo, while 240 showed weak activity in vitro and in vivo. Compounds 238 and 239 showed significant activity, and furthermore, orally administered 238 suppressed glucagon-induced hyperglycemia in mice. The peripheral blood glucose levels of db/db mice, an animal model of spontaneous type 2 diabetes, were significantly decreased in a dose-dependent manner by the administration of 238. Thus, this compound could be used as a novel lead to develop new hypoglycemic agents.325–327
Activity-guided fractionation based on inhibition of diacylglycerol acyltransferase led to the isolation of evocarpine (141), dihydroevocarpine (142), 1-methyl-2-[(4Z,7Z)-4,7-decadienyl]-4(1H)-quinolone (163), and 1-methyl-2-[(6Z,9Z)-6,9-pentadecadienyl]-4(1H)-quinolone (164) from the fruits of E. rutaecarpa.328 The four compounds displayed moderate activity (IC50 23.8, 69.5, 20.1, and 13.5 μM, respectively) suggesting they could be used in the design of hypolipidemic and antiobesity agents.
2.12 |. Anti-oxidant activity
In 2006, jineol (52) and 2,8-dihydroxy-3,4-dimethoxyquinoline (243) (Fig. 25) were isolated from the centipede S. subspinipes.329 Both compounds exhibited antioxidant activities on copper-mediated (IC50 2.60 and 63.0 μM), AAPHmediated oxidation (IC50 3.90 and 71.8 μM), and SIN-1-mediated oxidation (70% and 29% at 5 μM) in thiobarbituric acid reactive substances (TBARS) assay. Both compounds also showed 1,1-diphenyl-2-picrylhydrasyl radical scavenging activity and 52 exhibited metal chelating activity.329
Moreover, 4-carbomethoxy-6-hydroxy-2-quinolone (95), from the aleuronic layer of the dark purple anthocyaninpigmented rice cultivar O. sativa cv. Heugjinmi, exhibited moderate anti-oxidative activity in a radical-scavenging assay (IC50 166 μM).330 The quinoline alkaloids benzastatins C (71) and D (72) were also identified as free-radical scavengers, inhibiting lipid peroxidation in rat liver microsomes with EC50 values of 3.30 and 4.20 μM, but they were less effective than vitamin E (0.400 μM).114,115
2.13 |. Bronchodilator activity
In 1959, Amin and Merta identified the effect of quinazolin-4(3H)-one (133) and vasicinone (158) on bronchial musculature.331 In in vitro tests on guinea pig tracheal rings, these two compounds produced relaxation at 495 μM, about 1/2000 the activity of adrenaline, whereas vasicine (3) caused slight relaxation at 53.2 μM, but contraction at higher concentrations.332
In 1996, Kamikawa et al. reported that three pyranoquinolone alkaloids flindersine (31), veprisine (127), and N-methylflindersine (244) (Fig. 25) from Fagara chalybea showed good bronchodilator activity on both perfused guinea pig lungs and isolated tracheal preparations.333 Compounds 31 and 244 were slightly less potent than 127. Compound 127 also exhibited moderate positive inotropic activity on guinea pig left atria, which was inhibited by propranolol, indicating the presence of a β2-agonist action. In addition, all three compounds were antagonists of slow reacting substance-A (SRS-A), with 127 active at a concentration as low as 1 μg/mL without showing antihistaminic and anti-serotonin properties.333
2.14 |. Mutagenicity
In 1987, the mutagenic activity of extracts of R. graveolens was attributed in part to well-known furoquinoline alkaloids.334 Schimmer and co-workers reported further study results in 1988 and 1989.335,336 γ-Fagarine (33), skimmianine (34), and dictamnine (40) exhibited strong mutagenicity in S. typhimurium strains TA98 and TA100, but had comparatively little or no activity in the corresponding non-R-factor strains TA1538 and TA1535.335 The metabolic capacity of the corresponding liver microsome preparations was increased by pretreatment of rats with phenobarbital (Pb) and to a lesser amount with 3-methylkholanthrene. The results suggested that furoquinolines are activated to mutagenic metabolites by cytochrome P450 and cytochrome P448, and feasibly the flavin-containing monooxygenase.335 Alkaloid 33 induced sister chromatid exchange in human lymphocyte culture.336 Akaloid 40 showed photo-induced genotoxicity toward an E. coli lysogen, as determined by prophage induction.337
2.15 |. Other activities
2-Methyl-4(3H)-quinazolinone (245) (Fig. 25) from the culture broth of the micro-organism B. cereus BMH225-mF1 strongly inhibited poly(ADP-ribose) synthetase (IC50 1.10 μM) and was competitive with the substrate.338 It also had low acute toxicity, and the mice tolerated i.p. treatment with 250 mg/kg of compound.
Eduline (246) and japonine (247) (Fig. 25), two well-known 2-phenyl 4-quinolone alkaloids from O. japonica, showed strong relaxant activity on small intestine muscle, with equal potency (relaxative tension 0.17 ± 0.05 g at 10 μM for 245, 0.12 ± 0.03 g at 5 μM for 246) to that of papaverine (10 μM, 0.16 ± 0.03 g).339 The quinazoline alkaloid vasicine (3) potentiated the effect of oxytocin on rat mammary gland and stimulated muscular contraction in guinea pig ileum and uterus.340
The furoquinoline alkaloids μ-fagarine (33), skimmianine (34), and haplopine (35) showed pronounced estrogenic activity.341 When these three compounds (10 mg/kg) were administered to immature rats, the uterine mass increased by 193.9%, 22.6%, and 74.4% without liquid. The compounds differed structurally only in the C-7 substituent (H, OMe, OH, respectively). Results with other compounds also showed that the basicity/electronic state of the N atom influences the level of estrogenic activity.341
3 |. CONCLUSIONS AND OUTLOOK
Since the first quinoline alkaloid (quinine) and quinazoline alkaloid (vasicine) were identified in 1820 and 1888, respectively, quinoline and quinazoline alkaloids have attracted significant attention from researchers worldwide, and represent a promising and expanding platform for active natural compounds.
Among these compounds, CPT is the most famous and important as a DNA topo I inhibitor. Its discovery opened a new area for anticancer drug development. Subsequently, many alkaloids containing a quinoline ring, such as luzopeptin C, streptonigrin, BE-22179 and thiocoraline, with significant inhibitory effects on DNA and RNA synthesis and the topo II enzyme have been identified. The molecular structures have provided valuable clues for antitumor drug design. Besides quinine, the discoveries of febrifugine, tryptanthrin, and their analogs with significant antimalarial activity and different mechanism of action have provided additional modalities for treating malarial disease. These novel classes of compounds have opened a new chapter for study in the chemotherapy of malaria. Alkyl methyl quinolone alkaloids have a highly selective and unique antimicrobial mechanism different from that of other antibiotics, and thus, may be beneficial in the treatment of H. pylori-associated gastroduodenal diseases. In addition to the aforementioned activities, quinoline and quinazoline alkaloids exhibit other important bioactivities, such as antifungal, antiparasitic, insecticidal, anti-inflammatory, antiplatelet, and other effects. We hope that such compounds will provide more avenues for the development of new drugs in the future, particularly as improved methods of isolation and identification of quinoline and quinazoline alkaloids open the way to targeted pharmacological modeling and resulting synthetic modification.
Undoubtedly, these two alkaloid classes will attract tremendous continued attention and long-lasting interest from boththeacademiccommunityandthepharmaceuticalindustrytoadvancethediscoveryofnewandbetterdrugsbased on the original effects of the naturally occurring quinoline and quinazoline alkaloids.
ACKNOWLEDGMENTS
This work was supported financially by the National Natural Science Foundation of China (31371975, 21672092, 31302136) with partial support also supplied by the Fundamental Research Funds for the Central Universities (lzujbky-2016-147) and Youth Science Foundation of Gansu Province (1506RJYA144). Additional support was supplied by NIH grant CA177584 from the National Cancer Institute awarded to K.H. Lee.
Biography
Xiaofei Shang, M.S., received his B.S. in bioscience from Longdong University in 2007 and his M.S. in biochemistry from Lanzhou University in 2010, and he is now a Ph.D. candidate in medicinal chemistry at Lanzhou University. He is currently an Assistant Professor of the Key Laboratory of Veterinary Pharmaceutical Development of Ministry of Agriculture, Lanzhou Institute of Husbandry and Pharmaceutical Sciences, Chinese Academy of Agricultural Science (CAAS). His research interests include the isolation, structural elucidation, and structural modification of bioactive compounds from natural products. He has published about 20 articles, and received several projects.
Susan L. Morris-Natschke, Ph.D., received her B.S. in chemistry from the University of Maryland-College Park in 1975 and her Ph.D. in organic chemistry from the University of North Carolina-Chapel Hill (UNC-CH) in 1982. She is currently a Research Professor in the Division of Chemical Biology and Medicinal Chemistry, UNC Eshelman School of Pharmacy, UNC-CH, where she has been on the faculty since 1983. Her interests include scientific writing/editing, as well as the synthesis and SARs of bioactive natural products.
Ying-Qian Liu, Ph.D., received his B.S. in chemistry from Hebei Normal University in 2002 and his Ph.D. in bioorganic chemistry from Lanzhou University in 2007. He worked as a postdoctoral scholar in the School of Life Sciences at Lanzhou University from 2008 to 2013 and then as a visiting scholar at the University of North Carolina at Chapel Hill from 2011 to 2012. He is currently a Professor in the School of Pharmacy at Lanzhou University. His research interests include the design and synthesis of bioactive compounds as potential anticancer or pesticide agents, development of novel methodologies in organic chemistry, as well as drug mechanism and pharmacokinetics evaluation. He has published more than 50 research articles, applied for more than 28 patents, and directed several projects.
XiaoGuo,M.S., received her B.S. in bioscience from Northwest Normal University in 2010 and her M.S. in plant sciences from Northwest Normal University in 2013, and she now is a Ph.D. candidate in veterinary science from CAAS. Her research interests include the isolation, structural elucidation, and bioactivity of natural products.
Xiao-Shan Xu, B.S., received his B.S. in pharmacy from Chengdu University of Traditional Chinese Medicine in 2015, and he is now a Masters candidate in medicinal chemistry at Lanzhou University. His research interests include the design and synthesis of bioactive compounds as anticancer agents and the development of novel methodologies in medicinal chemistry.
Masuo Goto, Ph.D., received his B.S. degree in pharmacology in 1987, M.S. in pharmaceutical science in 1989, and Ph.D. in molecular biology in 1993 from Kanazawa University. He was a research fellow in cell and developmental biology at the National Institutes of Health from 1999 to 2003, followed by a postdoctoral fellow at the School of Medicine, UNC-CH from 2003–2009. Currently, he is Research Assistant Professor, UNC Eshelman School of Pharmacy, UNCCH. He is interested in elucidating the molecular mechanisms of action of antiproliferative small molecules mainly discovered and developed from natural products. His research goals are to develop novel strategies to prevent and overcome multidrug-resistant cancers.
Jun-Cai Li, B.S., received his B.S. in pharmaceutical engineering from School of Life Science and Engineering of South-west Jiaotong University in 2016, and he is currently a M.S. student in the School of Pharmacy at Lanzhou University. His research interests include the design and synthesis of bioactive compounds and natural products, as well as the development of novel methodologies in medicinal chemistry.
Guan-Zhou Yang, B.S., received his B.S. degree in pharmacy from Lanzhou University in 2016, and he is currently a M.S. student in the School of Pharmacy at Lanzhou University. His research interests include the design, synthesis and bioactivity of natural products and bioactive compounds, as well as development of novel methodologies in medicinal chemistry.
Kuo-Hsiung Lee, Ph.D., received his B.S. in pharmacy from Kaohsiung Medical University, Taiwan (1961), M.S. in pharmaceutical chemistry from Kyoto University, Japan (1965), and Ph.D. in medicinal chemistry from University of Minnesota, Minneapolis (1968). He joined the faculty of UNC Eshelman School of Pharmacy, University of North Carolina-Chapel Hill, in 1970 and is now Kenan Distinguished Professor of Medicinal Chemistry and Director of the Natural Products Research Laboratories. He has published more than 868 research articles, been granted more than 117 patents, and received numerous awards, including most recently, the Third Cheung On Tak International Award for Outstanding Achievement in Chinese Medicine from Hong Kong Baptist University in 2016.
REFERENCES
- 1.Prajapati SM, Patel KD, Vekariya RH, Panchal SN, Patel HD. Recent advances in the synthesis of quinolines: a review. RSC Adv. 2014;4:24463–24476. [Google Scholar]
- 2.Wiesner J, Ortmann R, Jomaa H, Schlitzer M. New antimalarial drugs. Angew Chem Int Ed. 2003;42:5274–5293. [DOI] [PubMed] [Google Scholar]
- 3.Manske RH. The chemistry of quinolines. Chem Rev. 1942;30:113–144. [Google Scholar]
- 4.Tumer RR, Woodward RR. The chemistry of the Cinchona alkaloids. In: Manske RHF, ed. The Alkaloids. New York: Academic Press; 1953:16. [Google Scholar]
- 5.Wall ME, Wani MC, Cook CE, Palmer KH, McPhail AT, Sim GA. Plant antitumor agents. I. The isolation and structure of camptothecin, a novel alkaloidal leukemia and tumor inhibitor from Camptotheca acuminate. J Am Chem Soc. 1966;88:3888–3890. [Google Scholar]
- 6.Liu YQ, Li WQ, Morris-Natschke SL, et al. Perspectives on biologically active camptothecin derivatives. Med Res Rev. 2015;35:753–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anjali PD, Singh D. Quinoline: a diverse therapeutic agent. Int J Pharm Sci Res. 2016;7:1–13. [Google Scholar]
- 8.Kshirsagar UA. Recent developments in the chemistry of quinazolinone alkaloids. Org Biomol Chem. 2015;13:9336–9352. [DOI] [PubMed] [Google Scholar]
- 9.Armarego WLF. Naturally occurring and biologically active quinazolines. Chemistry of Heterocyclic Compounds: Fused Pyrimidines, Part I, Quinazolines. 2008:490. [Google Scholar]
- 10.Khan I, Ibrar A, Abbas N, Saeed A. Recent advances in the structural library of functionalized quinazoline and quinazolinone scaffolds: synthetic approaches and multifarious applications. Eur J Med Chem. 2014;76:193–214. [DOI] [PubMed] [Google Scholar]
- 11.Johne SP. The quinazoline alkaloids. Prog Chem Org Nat Prod. 1984;46:159–229. [DOI] [PubMed] [Google Scholar]
- 12.Shang XF, Guo X, Li B, et al. Microwave-assisted extraction of three bioactive alkaloids from Peganum harmala L. and their acaricidal activity against Psoroptes cuniculi in vitro. J Ethnopharmacol. 2016;192:350–361. [DOI] [PubMed] [Google Scholar]
- 13.Mhaske SB, Argade NP. The chemistry of recently isolated naturally occurring quinazolinone alkaloids. Tetrahedron. 2006;62:9787–9826. [Google Scholar]
- 14.Witt A, Bergman J. Recent developments in the field of quinazoline chemistry. Curr Org Chem. 2003;7:659–677. [Google Scholar]
- 15.Armarego WLF. Quinazolines. Adv Heterocycl Chem. 1979;24:1. [DOI] [PubMed] [Google Scholar]
- 16.Johne S In: Ansell MF, ed. Rodd’s Chemistry of Carbon Compounds. Amsterdam: Elsevier; 1995:223–240. Supplements to the 2nd ed. [Google Scholar]
- 17.Brown DJ. Quinazolines. The Chemistry of Heterocyclic Compounds. New York: Wiley; 1996:55. Supplement I. [Google Scholar]
- 18.Ďyakonov AL, Telezhenetskaya MV. Quinazoline alkaloids in nature. Chem Nat Comp. 1997;33:221–267. [Google Scholar]
- 19.Johne S In: Sainsbury M, ed. Rodd’s Chemistry of Carbon Compounds. Amsterdam: Elsevier; 2000:203. Second Supplements to the 2nd ed. [Google Scholar]
- 20.Padala SR, Padi PR, Thipireddy V. A review on 2-heteryl- and heteroalkyl-4(3H)-quinazolinones. Heterocycles2003;60:183–226. [Google Scholar]
- 21.Ma Z, Hano Y, Nomura T. Luotonin A: a lead toward anti-cancer agent development. Heterocycles. 2005;65:2203–2219. [Google Scholar]
- 22.Khan I, Ibrar A, Ahmed W, Saeed A. Synthetic approaches, functionalization and therapeutic potential of quinazoline andquinazolinone skeletons: the advances continue. Eur J Med Chem. 2015;90:124–169. [DOI] [PubMed] [Google Scholar]
- 23.Madapa S, Tusi Z, Batra S. Advances in the syntheses of quinoline and quinoline-annulated ring systems. Curr Org Chem. 2008;12:1116–1183. [Google Scholar]
- 24.Kouznetsov VV, Méndez LYV, Gómez CMM. Recent progress in the synthesis of quinolines. Curr Org Chem. 2005;9:141–161. [Google Scholar]
- 25.Afzal O, Kumar S, Haider MR, et al. A review on anticancer potential of bioactive heterocycle quinoline. Eur J Med Chem. 2015;97:871–910. [DOI] [PubMed] [Google Scholar]
- 26.Kumar S, Bawa S, Gupta H. Biological activities of quinoline derivatives. Mini-Rev Med Chem. 2009;9:1468–1454. [DOI] [PubMed] [Google Scholar]
- 27.Chung PY, Bian ZX, Pun HY, et al. Recent advances in research of natural and synthetic bioactive quinolines. Future Med Chem. 2015;7:947–967. [DOI] [PubMed] [Google Scholar]
- 28.Witt A, Bergman J. Recent developments in the field of quinazoline chemistry. Cur Org Chem. 2003;7:659–677. [Google Scholar]
- 29.Connolly DJ, Cusack D, O’Sullivan TP, Guiry PJ. Synthesis of quinazolinones and quinazolines. Tetrahedron. 2005;61:10153–10202. [Google Scholar]
- 30.Khan I, Zaib S, Batool S, et al. Quinazolines and quinazolinones as ubiquitous structural fragments in medicinal chemistry: an update on the development of synthetic methods and pharmacological diversification. Bioorg Med Chem. 2016;24:2361–2381. [DOI] [PubMed] [Google Scholar]
- 31.Asif M Quinolene derivatives as anti-cancer agents: a review. J der Pharm Forschung. 2014;2:81–96. [Google Scholar]
- 32.Grundon MF. In: Grundon MF, ed. The Alkaloids London: The Royal Society of Chemistry; 1979:79. Specialist Periodical Reports. [Google Scholar]
- 33.Grundon MF. In: Grundon MF, ed. The Alkaloids London: The Royal Society of Chemistry; 1981:72. Specialist Periodical Reports. [Google Scholar]
- 34.Grundon MF. In: Grundon MF, ed. The Alkaloids London: The Royal Society of Chemistry; 1983:100–116. Specialist Periodical Reports. [Google Scholar]
- 35.Grundon MF. Quinoline, quinazoline and acridone alkaloids. Nat Prod Rep. 1984;1:195–200. [DOI] [PubMed] [Google Scholar]
- 36.Grundon MF. Quinoline, quinazoline and acridone alkaloids. Nat Prod Rep. 1985;2:393–400. [DOI] [PubMed] [Google Scholar]
- 37.Grundon MF. Quinoline, quinazoline and acridone alkaloids. Nat Prod Rep. 1987;4:225–36. [DOI] [PubMed] [Google Scholar]
- 38.Grundon MF. Quinoline, quinazoline and acridone alkaloids. Nat Prod Rep. 1988;5:293–307. [DOI] [PubMed] [Google Scholar]
- 39.Grundon MF. Quinoline, quinazoline and acridone alkaloids. Nat Prod Rep. 1990;7:131–138. [DOI] [PubMed] [Google Scholar]
- 40.Michael JP. Quinoline, quinazoline and acridone alkaloids. Nat Prod Rep. 1991;8:53–68. [Google Scholar]
- 41.Michael JP. Quinoline, quinazoline and acridone alkaloids. Nat Prod Rep. 1992;9:25–35. [DOI] [PubMed] [Google Scholar]
- 42.Michael JP. Quinoline, quinazoline and acridone alkaloids. Nat Prod Rep. 1993;10:99–108. [Google Scholar]
- 43.Michael JP. Quinoline, quinazoline and acridone alkaloids. Nat Prod Rep. 1994;11:163–172. [DOI] [PubMed] [Google Scholar]
- 44.Michael JP. Quinoline, quinazoline and acridone alkaloids. Nat Prod Rep. 1995;12:77–89. [Google Scholar]
- 45.Michael JP. Quinoline, quinazoline and acridone alkaloids. Nat Prod Rep. 1995;12:465–475. [Google Scholar]
- 46.Michael JP. Quinoline, quinazoline and acridone alkaloids. Nat Prod Rep. 1997;14:11–20. [DOI] [PubMed] [Google Scholar]
- 47.Michael JP. Quinoline, quinazoline and acridone alkaloids. Nat Prod Rep. 1997;14:605–618. [DOI] [PubMed] [Google Scholar]
- 48.Michael JP. Quinoline, quinazoline and acridone alkaloids. Nat Prod Rep. 1998;15:595–606. [Google Scholar]
- 49.Michael JP. Quinoline, quinazoline and acridone alkaloids. Nat Prod Rep. 1999;16:697–709. [DOI] [PubMed] [Google Scholar]
- 50.Michael JP. Quinoline, quinazoline and acridone alkaloids. Nat Prod Rep. 2000;17:603–620. [DOI] [PubMed] [Google Scholar]
- 51.Michael JP. Quinoline, quinazoline and acridone alkaloids. Nat Prod Rep. 2001;18:543–559. [DOI] [PubMed] [Google Scholar]
- 52.Michael JP. Quinoline, quinazoline and acridone alkaloids. Nat Prod Rep. 2002;19:742–760. [DOI] [PubMed] [Google Scholar]
- 53.Michael JP. Quinoline, quinazoline and acridone alkaloids. Nat Prod Rep. 2003;20:476–493. [DOI] [PubMed] [Google Scholar]
- 54.Michael JP. Quinoline, quinazoline and acridone alkaloids. Nat Prod Rep. 2004;21:650–668. [DOI] [PubMed] [Google Scholar]
- 55.Michael JP. Quinoline, quinazoline and acridone alkaloids. Nat Prod Rep. 2005;22:627–646. [DOI] [PubMed] [Google Scholar]
- 56.Michael JP. Quinoline, quinazoline and acridone alkaloids. Nat Prod Rep. 2007;24:223–246. [DOI] [PubMed] [Google Scholar]
- 57.Michael JP. Quinoline, quinazoline and acridone alkaloids. Nat Prod Rep. 2008;25:166–187. [DOI] [PubMed] [Google Scholar]
- 58.Anand P, Kunnumakkara AB, Sundaram C, et al. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res. 2008;25:2097–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gallo RC, Whang-Peng J, Adamson RH. Studies on the antitumor activity, mechanism of action, and cell cycle effects of camptothecin. Natl J Cancer Inst. 1971;46:789–795. [PubMed] [Google Scholar]
- 60.Herben VM, ten Bokkel Huinink WW, Beijnen JH. Clinical pharmacokinetics of topotecan. Clin Pharmacokinet. 1996;31:85–102. [DOI] [PubMed] [Google Scholar]
- 61.Jones CB, Clements MK, Wasi S, Daoud SS. Enhancement of camptothecin-induced cytotoxicity with UCN-01 in breast cancer cells: abrogation of S/G2 arrest. Cancer Chemother Pharmacol. 2000;45:252–258. [DOI] [PubMed] [Google Scholar]
- 62.Takahashi P, Polson A, Reisman D. Elevated transcription of the p53 gene in early S-phase leads to a rapid DNA-damageresponse during S-phase of the cell cycle. Apoptosis. 2011;16:950–958. [DOI] [PubMed] [Google Scholar]
- 63.Zhou Y, Gwadry FG, Reinhold WC, et al. Transcriptional regulation of mitotic genes by camptothecin-induced DNA damage. Cancer Res. 2002;62:1688–1695. [PubMed] [Google Scholar]
- 64.Koshkina NV, Kleinerman ES, Waidrep C, Jia SF, Worth LL, Gilbert BE. 9-Nitrocamptothecin liposome aerosol treatment of melanoma and osteosarcoma lung metastases in mice. Clin Cancer Res. 2000;6:2876–2880. [PubMed] [Google Scholar]
- 65.Verschraegen CF, Gilbert BE, Huaringa AJ, Newman R, Harris N, Leyva FJ. Feasibility, phase I, and pharmacological study of aerosolized liposomal 9-nitro-20(S)-camptothecin in patients with advanced malignancies in the lungs. Ann NY Acad Sci. 2000;922:352–354. [DOI] [PubMed] [Google Scholar]
- 66.Takimoto CH, Thomas R. The clinical development of 9-aminocamptothecin. Ann NY Acad Sci. 2000;922:224–236. [DOI] [PubMed] [Google Scholar]
- 67.Pratesi G, Beretta GL, Zunino F. Gimatecan, a novel camptothecin with a promising preclinical profile. Anticancer Drugs. 2004;15:545–552. [DOI] [PubMed] [Google Scholar]
- 68.Haridas K, Hausheer FH. Preparation of highly lipophilic camptothecin derivatives. US patent 6057303 2000. [Google Scholar]
- 69.Bom D, Curran DP, Zhang J, Zimmer SG, Bevins R, Kruszewski S. The highly lipophilic DNA topoisomerase Iinhibitor DB-67 displays elevated lactone levels in human blood and potent anticancer activity. J Control Rel. 2001;74:325–333. [DOI] [PubMed] [Google Scholar]
- 70.Wani MC, Nicholas AW, Wall ME. Plant antitumor agents. 23. Synthesis and antileukemic activity of camptothecinanalogs. J Med Chem. 1986;29:2358–2363. [DOI] [PubMed] [Google Scholar]
- 71.van Hattum AH, Pinedo HM, Schluper HMM, Erkelens CAM, Tohgo A, Boven E. The activity profile of the hexacyclic camptothecin derivative DX-8951f in experimental human colon cancer and ovarian cancer. Biochem Pharm.2002;64:1267–1277. [DOI] [PubMed] [Google Scholar]
- 72.Royce ME, Rowinsky EK, Hoff PM, Coyle J, DeJager R, Pazdur R. A phase II study of intravenous exatecan mesylate (DX-8951f) administered daily for five days every three weeks to patients with metastatic adenocarcinoma of the colon or rectum. Invest New Drugs. 2004;22:53–61. [DOI] [PubMed] [Google Scholar]
- 73.Stevenson JP, DeMaria D, Sludden J, Kaye SB, Paz-Ares L, Grochow LB. Phase I/pharmacokinetic study of the topoisomerase I inhibitor GG211 administered as a 21-day continuous infusion. Ann Oncol. 1999;10:339–344. [DOI] [PubMed] [Google Scholar]
- 74.Giles FJ, Tallman MS, Guillermo GM, Cortes JE, Thomas DA, Wierda WG. Phase I and pharmacokinetic study of alow clearance, unilamellar liposomal formulation of lurtotecan, a topoisomerase I inhibitor, in patients with advanced leukemia. Cancer. 2004;100:1449–1458. [DOI] [PubMed] [Google Scholar]
- 75.Dong P, Zuo C, Chen ZL, Gao Y, inventors: Jiangsu Chiatai Tianqing Pharmaceutical Co. Ltd, assignee. Pharmaceutical composition of camptothecin derivative and preparation method thereof. China Patent CN102764260.A. 2012. 11.7. [Google Scholar]
- 76.Yu Y, Zhan Y, Chen Z, Zhang Y, Zhong D. Development and validation of a sensitive LC-MS/MS method for simultaneous quantification of sinotecan and its active metabolite in human blood. J Chromatogr B. 2014;951–952:62–68. [DOI] [PubMed] [Google Scholar]
- 77.Demarquay D, Huchet M, Coulomb H. The homocamptothecin BN 80915 is a highly potent orally active topoisomeraseI poison. Anticancer Drugs. 2001;12:9–19. [DOI] [PubMed] [Google Scholar]
- 78.Lavergne O, Lesueur-Ginot L, Pla Rods M, et al. Homocamptothecins: synthesis and antitumor activity of novel E-ring-modified camptothecin analogs. J Med Chem. 1998;41:5410–5419. [DOI] [PubMed] [Google Scholar]
- 79.Bayés M, Rabasseda X, Prous JF. Gateways to clinical trials. Methods Find Exp Clin Pharmacol. 2003;25:747–771. [PubMed] [Google Scholar]
- 80.Bayés M, Rabasseda X, Prous JF. Gateways to clinical trials. March 2003 Methods Find Exp Clin Pharmacol. 2003;25:145–168. [PubMed] [Google Scholar]
- 81.Onishi H, Machida Y. Macromolecular and nanotechnological modification of camptothecin and its analogs to improve the efficacy. Curr Drug Discover Technol. 2005;2:169–183. [DOI] [PubMed] [Google Scholar]
- 82.Lerchen HG, Baumgarten J, von dem Bruch K, et al. Design and optimization of 20-O-linked camptothecin glycoconju-gates as anticancer agents. J Med Chem. 2001;44:4186–4195. [DOI] [PubMed] [Google Scholar]
- 83.Singer JW, Bhatt R, Tulinsky J, et al. Water-soluble poly-(l-glutamic acid)-Gly-camptothecin conjugates enhance camptothecin stability and efficacy in vivo. J Control Release. 2001;74:243–247. [DOI] [PubMed] [Google Scholar]
- 84.Greenwald RB, Pendri A, Conover C, Gilbert C, Yang R, Xia J. Drug delivery systems. 2. Camptothecin 20-O-polyethyleneglycol ester transport forms. J Med Chem. 1996;39:1938–1940. [DOI] [PubMed] [Google Scholar]
- 85.Houghton PJ, Cheshire PJ, Myers L, Stewart CF, Synold TW, Houghton JA. Evaluation of 9-dimethylaminomethyl-10hydroxycamptothecin against xenografts derived from adult and childhood solid tumors. Cancer Chemother Pharmacol. 1992;31:229–239. [DOI] [PubMed] [Google Scholar]
- 86.Kunimoto T, Nitta K, Tanaka T, et al. Antitumor activity of 7-ethyl-10-[4-(1-piperidino)-1-piperidino] carbonyloxycamptothecin, a novel water soluble derivative of camptothecin, against murine tumors. Cancer Res. 1987;47: 5944–5947. [PubMed] [Google Scholar]
- 87.Ahn SK, Choi NS, Jeong BS, Kim KK, Journ DJ, Kim JK. Practical synthesis of (S)-7-(2-isopropylamino)ethylcamptothecin hydrochloride, potent topoisomerase I inhibitor. J Heterocycl Chem. 2000;37:1141–1144. [Google Scholar]
- 88.Liu YQ, Tian X, Yang L, Zhan ZC. First synthesis of novel spin-labeled derivatives of camptothecin as potential antineoplastic agents. Eur J Med Chem. 2008;43:2610–2614. [DOI] [PubMed] [Google Scholar]
- 89.Liu YQ, Dai W, Wang CY, et al. Design and one-pot synthesis of new 7-acyl camptothecin derivatives as potent cytotoxic agents. Bioorg Med Chem Lett. 2012;22:7659–7661. [DOI] [PubMed] [Google Scholar]
- 90.Wang MJ, Liu YQ, Chang LC, et al. Design, synthesis, mechanisms of action, and toxicity of novel 20(S)-sulfonylamidine derivatives of camptothecin as potent antitumor agents. J Med Chem. 2014;57:6008–6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Stallard S, Kaye SB. Reversal of resistance in the breast cancer cell line MCF-7/AdrR was most effective with the modulating agent quinidine. Bri J Cancer. 1989;60:500–505. [Google Scholar]
- 92.Jones RD, Kerr DJ, Harnett AN, Rankin EM, Ray S, Kaye SB. A pilot study of quinidine and epirubicin in the treatment ofadvanced breast cancer. Brit J Cancer. 1990;62:133–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chauffert B, Pelletier H, Corda C, et al. Potential usefulness of quinine to circumvent the anthracycline resistance in clinical practice. Bri J Cancer. 1990;62:395–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen IS, Wu SJ, Tsai IL, et al. Chemical and bioactive constituents from Zanthoxylum simulans. J Nat Prod. 1994;57:1206–1211. [DOI] [PubMed] [Google Scholar]
- 95.Chen IS, Tsai IW, Teng CM, et al. Pyranoquinoline alkaloids from Zanthoxylum simulans. Phytochemistry. 1997;46:525–529. [Google Scholar]
- 96.Ulubelen A Alkaloids from Haplophyllum suaveolens. Phytochemistry. 1984;23:2123–2124. [Google Scholar]
- 97.Jansen O, Akhmedjanova V, Angenot L, et al. Screening of 14 alkaloids isolated from Haplophyllum A. Juss. for their cytotoxic properties. J Ethnopharmacol. 2006;105:241–245. [DOI] [PubMed] [Google Scholar]
- 98.Cui B, Chai H, Dong Y, et al. Quinoline alkaloids from Acronychia laurifolia. Phytochemistry. 1999;52:95–98. [DOI] [PubMed] [Google Scholar]
- 99.Wu TS, Shi LS, Wang JJ, et al. Cytotoxic and antiplatelet aggregation principles of Ruta graveolens. J Chin Chem Soc. 2003;50:171–178. [Google Scholar]
- 100.Chen JJ, Huang HY, Duh CY, Chen IS. Cytotoxic constituents from the stem bark of Zanthoxylum pistaciiflorum. J Chin Chem Soc. 2004;51:659–663. [Google Scholar]
- 101.Chaturvedula VSP, Schilling JK, Miller S, Andriantsiferana R, Rasamison VE, Kingston DGI. New cytotoxic alkaloids fromthe wood of Vepris punctate from the Madagascar rainforest. J Nat Prod. 2003;66:532–534. [DOI] [PubMed] [Google Scholar]
- 102.Komala I, Rahmani M, Sukari MA, Ismail HBM, Lian GEC, Rahmat A. Furoquinoline alkaloids from Melicope bonwickii (F. Muell.) T. Hartley. Nat Prod Res. 2006;20:355–360. [DOI] [PubMed] [Google Scholar]
- 103.Chou HC, Chen JJ, Duh CY, Huang TF, Chen IS. Cytotoxic and anti-platelet aggregation constituents from the root woodof Melicope semecarpifolia. Planta Med. 2005;71:1078–1081. [DOI] [PubMed] [Google Scholar]
- 104.Grougnet R, Magiatis P, Fokialakis N, et al. Koniamborine, the first pyrano[3,2-b]indole alkaloid and other secondary metabolites from Boronella koniambiensis. J Nat Prod. 2005;68:1083–1086. [DOI] [PubMed] [Google Scholar]
- 105.Moon SS, Cho N, Shin J, Seo Y, Lee CO, Choi SU. Jineol, a cytotoxic alkaloid from the centipede Scolopendra subspinipes. J Nat Prod. 1996;59:777–779. [Google Scholar]
- 106.Morita H, Hirasawa Y, Yoshida N, Kobayashi J. Senepodine A, a novel C22N2 alkaloid from Lycopodium chinense. Tetra-hedron Lett 2001;42:4199–4201. [Google Scholar]
- 107.Chen SB, Gao GY, Li YS, Yu SC, Xiao PG. Cytotoxic constituents from Aquilegia ecalcarata. Planta Med. 2002;68:554–556. [DOI] [PubMed] [Google Scholar]
- 108.Ruiz-Mesía L, Ruiz-Mesía W, Reina M, et al. Bioactive Cinchona alkaloids from Remijia peruviana. J Agric Food Chem. 2005;53:1921–1926. [DOI] [PubMed] [Google Scholar]
- 109.Nunes FM, Barros-Filho BA, de Oliveira MCF, et al. 3,3-Diisopentenyl-N-methyl-2,4-quinoldione from Esenbeckia almaw-illia: the antitumor activity of this alkaloid and its derivatives. Nat Prod Commun. 2006;1:313–318. [Google Scholar]
- 110.Jacquemond-Collet I, Benoit-Vical F, Valentin A, Stanislas E, Mallié M, Fourasté I. Antiplasmodial and cytotoxic activity of galipinine and other tetrahydroquinolines from Galipea officinalis. Planta Med. 2002;68:68–69. [DOI] [PubMed] [Google Scholar]
- 111.Ratnayake S, Fang XP, Anderson JE, McLaughlin JL, Evert DR. Bioactive constituents from the twigs of Asimina parviflora. J Nat Prod. 1992;55:1462–1467. [DOI] [PubMed] [Google Scholar]
- 112.Kubanek J, Williams DE, de Silva ED, Allen T, Anderse RJ. Cytotoxic alkaloids from the flatworm Prostheceraeus villatus and its tunicate prey Clavelina lepadiformis. Tetrahedron Lett. 1995;36:6189–6192. [Google Scholar]
- 113.Wright AD, Goclik E, König GM, Kaminsky R. Lepadins D-F: antiplasmodial and antitrypanosomal decahydroquinoline derivatives from the tropical marine tunicate Didemnum sp. J Med Chem. 2002;45:3067–3072. [DOI] [PubMed] [Google Scholar]
- 114.Kim WG, Kim JP, Kim CJ, Lee KH, Yoo ID. Benzastatins A, B, C, and D: new free radical scavengers from Streptomyces nitrosporeus 30643 I. Taxonomy, fermentation, isolation, physico-chemical properties and biological activities. J Antibiot. 1996;49:20. [DOI] [PubMed] [Google Scholar]
- 115.Kim WG, Kim JP, Yoo ID. Benzastatins A, B, C, and D: new free radical scavengers from Streptomyces nitrosporeus 30643 II. Structure determination. J Antibiot. 1996;49:26–30. [DOI] [PubMed] [Google Scholar]
- 116.Kurosawa K, Takahashi K, Tsuda E. SW-163C and E, novel antitumor depsipeptides produced by Streptomyces sp. I. Taxonomy, fermentation, isolation and biological activities. J Antibiot. 2001;54:615–621. [DOI] [PubMed] [Google Scholar]
- 117.Takahashi K, Koshino H, Esumi Y, Tsuda E, Kurosawa K. SW-163C and E, novel antitumor depsipeptides produced byStreptomyces sp. II. Structure elucidation. J Antibiot. 2001;54:622–627. [DOI] [PubMed] [Google Scholar]
- 118.He J, Lion U, Sattler I, et al. Diastereomeric quinolinone alkaloids from the marine-derived fungus Penicillium janczewskii. J Nat Prod. 2005;68:1397–1399. [DOI] [PubMed] [Google Scholar]
- 119.Shen L, Ye YH, Wang XT, et al. Structure and total synthesis of aspernigerin: a novel cytotoxic endophyte metabolite. Chem Eur J. 2006;12:4393–4396. [DOI] [PubMed] [Google Scholar]
- 120.Kozlovsky AG, Zhelifonova VP, Antipova TV, et al. Quinocitrinines A and B, new quinoline alkaloids from Penicillium citrinum Thom 1910, a permafrost fungus. J Antibiot. 2003;56:488–491. [DOI] [PubMed] [Google Scholar]
- 121.Ito C, Itoigawa M, Sato A, et al. Chemical constituents of Glycosmis arborea: three new carbazole alkaloids and their biological activity. J Nat Prod. 2004;67:1488–1491. [DOI] [PubMed] [Google Scholar]
- 122.Konishi M, Ohkuma H, Sakai F, et al. BBM-928, a new antitumor antibiotic complex III. Structure determination of BBM928 A, B and C. J Antibiot. 1981;34:148–159. [DOI] [PubMed] [Google Scholar]
- 123.Huang CH, Mong S, Crooke ST. Interactions of a new antitumor antibiotic BBM-928A with deoxyribonucleic acid. Bifunctional intercalative binding studied by fluorometry and viscometry. Biochemistry. 1980;19:5537–5542. [DOI] [PubMed] [Google Scholar]
- 124.Huang CH, Prestayko AW, Crooke ST. Bifunctional intercalation of antitumor antibiotics BBM-928A and echinomycin with DNA. Effects of intercalation on DNA degradative activity of bleomycin and phleomycin. Biochemistry. 1982;21:3704–3710. [DOI] [PubMed] [Google Scholar]
- 125.Huang CH, Mirabelli CK, Mong S, Crooke ST. Intermolecular cross-linking of DNA through bifunctional intercalation of an antitumor antibiotic, luzopeptin A (BBM-928A). Cancer Res. 1983;43:2718–2724. [PubMed] [Google Scholar]
- 126.Huang CH, Crooke ST. Effects of structural modifications of antitumor antibiotics (luzopeptins) on the interactions with deoxyribonucleic acid. Cancer Res. 1985;45:3768–3773. [PubMed] [Google Scholar]
- 127.Wang H, Yeo SL, Xu J, et al. Isolation of streptonigrin and its novel derivative from Micromonospora as inducing agents of p53-dependent cell apoptosis. J Nat Prod. 2002;65:721–724. [DOI] [PubMed] [Google Scholar]
- 128.Okada H, Suzuki H, Yoshinari T, Arakawa H, Okura A, Suda H. A new topoisomerase II inhibitor, BE-22179, produced bya Streptomycete. I. Producing strain, fermentation, isolation and biological activity. J Antibiot. 1994;47:129–135. [DOI] [PubMed] [Google Scholar]
- 129.Yoshinar T, Okada H, Yamada A, et al. Inhibition of topoisomerase II by a novel antitumor cyclic depsipeptide, BE-22179. Jpn J Cancer Res. 1994;85:550–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Romero F, Espliego F, Baz JP, et al. Thiocoraline, a new depsipeptide with antitumor activity produced by a marine Micromonospora. I. Taxonomy, fermentation, isolation, and biological activities. J Antibiot. 1997;50:734–737. [DOI] [PubMed] [Google Scholar]
- 131.Perez Baz J, Cañedo LM, Fernández-Puentes JL, Silva Elipe MV. Thiocoraline, a novel depsipeptide with antitumor-activity produced by a marine Micromonospora. II. Physico-chemical properties and structure determination. J Antibiot. 1997;50:738–741. [DOI] [PubMed] [Google Scholar]
- 132.Boger DL, Ichikawa S. Total syntheses of thiocoraline and BE-22179: establishment of relative and absolute stereochemistry. J Am Chem Soc. 2000;122:2956–2957. [Google Scholar]
- 133.Boger DL, Ichikawa S, Tse WC, Hedrick MP, Jin Q. Total syntheses of thiocoraline and BE-22179 and assessment of their DNA binding and biological properties. J Am Chem Soc. 2001;123:561–568. [DOI] [PubMed] [Google Scholar]
- 134.Chen JJ, Duh CY, Huang HY, Chen IS. Furoquinoline alkaloids and cytotoxic constituents from the leaves of Melicope semecarpifolia. Planta Med. 2003;69:542–546. [DOI] [PubMed] [Google Scholar]
- 135.Fokialakis N, Magiatis P, Aligiannis N, Mitaku S, Tillequin F, Sévenet T. Furomegistines I and II, two furanopyridine alkaloids from the bark of Sarcomelicope megistophylla. Phytochemistry. 2001;57:593–596. [DOI] [PubMed] [Google Scholar]
- 136.Fokialakis N, Mitakua S, Mikros E, Skaltsounis AL, Tillequin F. Megistosarcimine and megistosarconine, two alka-loids from Sarcomelicope megistophylla. Phytochemistry. 1999;52:1745–1748. [DOI] [PubMed] [Google Scholar]
- 137.Fokialakis N, Magiatis P, Terzis A, Tillequin F, Skaltsounis AL. Cyclomegistine, the first alkaloid with the newcyclobuta[b]quinoline ring system from Sarcomelicope megistophylla. Tetrahedron Lett. 2001;42:5323–5325. [Google Scholar]
- 138.Chung HS. A quinolone alkaloid, from the aleurone layer of Oryzasativa cv. Mihyangbyo, inhibits growth of cultured human leukemia cell. Nutraceuticals Food. 2002;7:119–122. [Google Scholar]
- 139.Larsen TO, Gareis M, Frisvad JC. Cell cytotoxicity and mycotoxin and secondary metabolite production by common penicillia on cheese agar. J Agric Food Chem. 2002;50:6148–6152. [DOI] [PubMed] [Google Scholar]
- 140.Chen MY, Yang RY, Shao CL, et al. Isolation, structure elucidation, crystal structure, and biological activity of a marine natural alkaloid, viridicatol. Chem Nat Comp 2011;47:322–325. [Google Scholar]
- 141.Numata A, Takahashi C, Matsushita T, et al. Fumiquinazolines, novel metabolites of a fungus isolated from a saltfish. Tetrahedron Lett. 1992;33:1621–1624. [Google Scholar]
- 142.Takahashi C, Matsushita T, Doi M, et al. Fumiquinazolines A-G, novel metabolites of a fungus separated from a Pseudolabrus marine fish. J Chem Soc Perkin Trans 1. 1995:2345–2353. [Google Scholar]
- 143.Barrow CJ, Sun HH. Spiroquinazoline, a novel substance P inhibitor with a new carbon skeleton, isolated from Aspergillus flavipes. J Nat Prod. 1994;57:471–476. [DOI] [PubMed] [Google Scholar]
- 144.Ma ZZ, Hano Y, Nomura T, Chen YJ. Two new pyrroloquinazolinoquinoline alkaloids from Peganum nigellastrum Hetero-cycles 1997;46:541–546. [Google Scholar]
- 145.Ma ZZ, Hano Y, Nomura T, Chen YJ. Two new quinazoline-quinoline alkaloids from Pegnaum nigellastrum Heterocycles 1999;51:1883–1889. [Google Scholar]
- 146.Ma Z, Hano Y, Nomura T, Chen Y. Novel quinazoline-quinoline alkaloids with cytotoxic and DNA topoisomerase II inhibitory activities. Bioorg Med Chem Lett. 2004;14:1193–1196. [DOI] [PubMed] [Google Scholar]
- 147.Cagir A, Jones SH, Gao R, Eisenhauer BM, Hecht SM. Luotonin A. a naturally occurring human DNA topoisomerase Ipoison. J Am Chem Soc. 2003;125:13628–13629. [DOI] [PubMed] [Google Scholar]
- 148.Liang JL, Cha HC, Jahng Y. Recent advances in the studies on luotonins. Molecules 2011;16:4861–4863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Dallavalle S, Merlini L, Beretta GL, Tinelli S, Zunino F. Synthesis and cytotoxic activity of substituted luotonin A derivatives. Bioorg Med Chem Lett. 2004;14:5757–5761. [DOI] [PubMed] [Google Scholar]
- 150.Cagir A, Jones SH, Eisenhauer BM, Gao R, Hecht SM. Synthesis and biochemical properties of E-ring modified luotonin Aderivatives. Bioorg Med Chem Lett. 2004;14:2051–2054. [DOI] [PubMed] [Google Scholar]
- 151.Cagir A, Eisenhauer BM, Gao R, Thomas SJ, Hecht SM. Synthesis and topoisomerase I inhibitory properties of luotonin Aanalogues. Bioorg Med Chem Lett. 2004;12:6287–6299. [DOI] [PubMed] [Google Scholar]
- 152.Zou J, Huang L. Isolation, structure elucidation, and synthesis of tryptanthrin and qingdainone from Baphicacanthus cusia (Nees) Bremek. Yaoxue Xuebao 1985;20:45–48. [Google Scholar]
- 153.Kimoto T, Hino K, Koya-Miyata S, et al. Cell differentiation and apoptosis of monocytic and promyelocytic leukemia cells(U-937 and HL-60) by tryptanthrin, an active ingredient of Polygonum tinctorium Lour. Pathol Int. 2001;51:315–325. [DOI] [PubMed] [Google Scholar]
- 154.Koya-Miyata S, Kimoto T, Micallef MJ, et al. Prevention of azoxymethane-induced intestinal tumors by a crude ethylacetate-extract and tryptanthrin extracted from Polygonum tinctorium Lour. Anticancer Res. 2001;21:3295–3300. [PubMed] [Google Scholar]
- 155.Motoki T, Takami Y, Yagi Y, Tai A, Yamamoto I, Gohda E. Inhibition of hepatocyte growth factor induction in human dermalfibroblasts by tryptanthrin. Biol Pharm Bull. 2005;28:260–266. [DOI] [PubMed] [Google Scholar]
- 156.Wu X, Liu Y, Sheng W, Sun J, Qin G. Chemical constituents of Isatis indigotica. Planta Med. 1997;63:55–57. [DOI] [PubMed] [Google Scholar]
- 157.Chen JJ, Fang HY, Duh CY, Chen IS. New indolopyridoquinazoline, benzo[c] phenanthridines and cytotoxic constituentsfrom Zanthoxylum. Planta Med. 2005;71:470–475. [DOI] [PubMed] [Google Scholar]
- 158.Morita H, Sato Y, Chan KL, et al. Samoquasine A, a benzoquinazoline alkaloid from the seeds of Annona squamosa. J Nat Prod. 2000;63:1707–1708. [DOI] [PubMed] [Google Scholar]
- 159.Morita H, Sato Y, Chan KL, et al. Samoquasine A, a benzoquinazoline alkaloid from the seeds of Annona squamosa. J Nat Prod. 2002;65:1748. [DOI] [PubMed] [Google Scholar]
- 160.Monsieurs K, Tapolcsányi P, Loones KTJ, et al. Is samoquiasine A indeed benzo(f)phthalazin-4(3H)-one. Unambiguous, straightforward synthesis of benzo(f)phthalazin-4(3H)-one and its regioisomer benzo(f)phthalazin-1(2H)-one. Tetrahedron. 2007;63:3870–3881. [Google Scholar]
- 161.Timmons C, Wipf P. Densioty functional theory calculation of 13C NMR shifts of diazaphenanthrene alkaloids: reinvestigations of the structure of samoquasine A. J Org Chem. 2008;73:9168–9170. [DOI] [PubMed] [Google Scholar]
- 162.Tzantrizos YS, Xu XJ, Sauriol F, Hynes RC. Novel quinazolinones and enniatins from Fusarium lateritium Nees. Can J Chem. 1994;72:1415. [Google Scholar]
- 163.Tzantrizos YS, Xu XJ, Sauriol F, Hynes RC. Novel quinazolinones and enniatins from Fusarium lateritium Nees. Can J Chem. 1993;71:1362–1367. [Google Scholar]
- 164.World Malaria Report, 2015. http://www.who.int/malaria/publications/world-malaria-report-2015/report/en/.
- 165.Mishra M, Mishra VK, Kashaw V, Iyer AK, Kashaw SK. Comprehensive review on various strategies for antimalarial drugdiscovery. Eur J Med Chem. 2017;125:1300–1320. [DOI] [PubMed] [Google Scholar]
- 166.Quinine. Scientific American. 1848; 3: 326. doi: 10.1038/scientificamerican07011848-326d. [DOI] [Google Scholar]
- 167.White NJ, Looareesuwan S, Warrel DA, et al. Quinine loading dose in cerebral malaria. Am J Trop Med Hyg. 1983;32:1–5. [DOI] [PubMed] [Google Scholar]
- 168.Kelsey FE, Oldham FK, Cantrell W, Geiling EMK. Antimalarial activity and toxicity of a metabolic derivative of quinine. Nature. 1946;157:440. [DOI] [PubMed] [Google Scholar]
- 169.Ball P History of science: quinine steps back in time. Nature. 2008;28:451. [DOI] [PubMed] [Google Scholar]
- 170.Bateman DN, Dyson EH. Quinine toxicity. Adverse Drug React Poisoning Rev. 1986;4:215–233. [PubMed] [Google Scholar]
- 171.World Health Organization (WHO). Guidelines for the treatment of malaria, 1st ed. Geneva Switzerland. [Google Scholar]
- 172.Gantier JC, Fournet A, Munos MH, Hocquemiller R. The effect of some 2-substituted quinolines isolated from Galipea longiflora on Plasmodium vinckei petteri infected mice. Planta Med. 1996;62:285–286. [DOI] [PubMed] [Google Scholar]
- 173.Basco LK, Mitaku S, Skaltsounis AL, et al. In vitro activities of furoquinoline and acridone alkaloids against Plasmodium falciparum. Antimicrob Agents Chemother. 1994;38:1169–1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Christopher E, Bedir E, Dunbar C, et al. Indoloquinazoline alkaloids from Araliopsis tabouensis. Helv Chim Acta. 2003;86:2914–2918. [Google Scholar]
- 175.Bultel-Poncé V, Berge JP, Debitus C, Nicolas JL, Guyot M. Metabolites from the sponge-associated bacterium Pseudomonas species. Mar Biotechnol. 1999;1:384–390. [DOI] [PubMed] [Google Scholar]
- 176.Koepfli M, Mead JF, Brockmen JA Jr. An alkaloid with high antimalarial activity from Dichor febrifuga. J Am Chem Soc. 1947;69:1837. [DOI] [PubMed] [Google Scholar]
- 177.Koepfli M, Brockmen JA Jr. Alkaloids of Dichroa febrifuga. I. isolation and degradative studies. J Am Chem Soc. 1949;71:1048. [DOI] [PubMed] [Google Scholar]
- 178.Koepfli M, Brockmen JA Jr, Moffat J. The structure of febrifugine and isofebrifugine. J Am Chem Soc. 1950;72:3323. [Google Scholar]
- 179.Takeuchi Y, Azuma K, Takakura K, et al. Asymmetric synthesis of (+)-febrifugine and (+)-isofebrifugine using yeast reduction. Tetrahedron. 2001;57:1213–1218. [Google Scholar]
- 180.Takaya Y, Tasaka H, Chiba T, et al. New type of febrifugine analogues, bearing a quinolizidine moiety, show potent antimalarial activity against Plasmodium malaria parasite. J Med Chem. 1999;42:3163–3166. [DOI] [PubMed] [Google Scholar]
- 181.Murata K, Takano F, Fushiya S, Oshima Y. Enhancement of NO production in activated macrophages in vivo by an antimalarial crude drug, Dichroa febrifuga. J Nat Prod. 1998;61:729–733. [DOI] [PubMed] [Google Scholar]
- 182.Ishih A, Suzuki T, Watanabe M, Miyase T, Terada M. Combination effects of chloroquine with the febrifugine andisofebrifugine mixture against a blood-induced infection with chloroquine-resistant Plasmodium berghei NK65 in ICR mice. Phytother Res. 2003;17:1234–1236. [DOI] [PubMed] [Google Scholar]
- 183.Hirai S, Kikuchi H, Kim HS, et al. Metabolites of febrifugine and its synthetic analogue by mouse liver S9 and their anti-malarial activity against Plasmodium malaria parasite. J Med Chem. 2003;46:4351–4359. [DOI] [PubMed] [Google Scholar]
- 184.Jaidane M, Ali-El-Dein B, Ounaies A, Hafez AT, Mohsen T, Bazeed M. The use of halofuginone in limiting urethral strictureformation and recurrence: an experimental study in rabbits. J Urol. 2003;170:2049–2052. [DOI] [PubMed] [Google Scholar]
- 185.Özçlik MF, Pekmezci S, Saribeyoglu K, Ünal E, Gümüstas K, Doğusoy G. The effect of halofuginone, a specific inhibitor of collagen type 1 synthesis, in the prevention of esophageal strictures related to caustic injury. Am J Surg. 2004;187:257–260. [DOI] [PubMed] [Google Scholar]
- 186.Xavier S, Piek E, Fujii M, et al. Amelioration of radiation-induced fibrosis. Inhibition of transforming growth factor-β signaling by halofuginone. J Biol Chem. 2004;279:15167–15176. [DOI] [PubMed] [Google Scholar]
- 187.Pitzer KK, Kyle DE, Gerena L. Indolo[2,1-b]quinazole-6,12-dione antimalarial compounds and methods of treating malaria therewith. Patent 6. 2001;284:772. [Google Scholar]
- 188.Bhattacharjee AK, Hartell MG, Nichols DA, et al. Structure-activity relationship study of antimalarial indolo[2,1-b]quinazoline-6,12-diones (tryptanthrins). Three dimensional pharmacophore modeling and identification of new anti-malarial candidates. Eur J Med Chem. 2004;39:59–67. [DOI] [PubMed] [Google Scholar]
- 189.Sprogøe K, Manniche S, Larsen TO, Christophersen C. Janoxepin and brevicompanine B: antiplasmodial metabolites fromthe fungus Aspergillus janus. Tetrahedron. 2005;61:8718–8721. [Google Scholar]
- 190.World Health Organization (WHO). 2017. Leishmaniasis (http://www.who.int/mediacentre/factsheets/fs375/en/). Accessed 04/2017.
- 191.Rocha LG, Almeida JRGS, Macêdo RO, Barbosa-Filho JM. A review of natural products with antileishmanial activity. Phytomedicine 2005;12:514–535. [DOI] [PubMed] [Google Scholar]
- 192.Fournet A, Angelo Barrios A, Muiios V, et al. Antiprotozoal activity of quinoline alkaloids isolated from Galipea longiflora, a Bolivian plant used as a treatment for cutaneous leishmaniasis. Phytother Res. 1994;8:174–178. [Google Scholar]
- 193.Fournet A, Angelo Barrios A, Muiioz V, Hocquemiller R, Cave A, Bruneton J. 2-Substituted quinoline alkaloids as potential antileishmanial drugs. Antimicrob Agents Chemother. 1993;37:859–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Fournet A, Gantier JC, Gautheret A, et al. The activity of 2-substituted quinoline alkaloids in BALB/c mice infected withLeishmania donovani. J Antimicrob Chemother. 1994;33:537–544. [DOI] [PubMed] [Google Scholar]
- 195.Fournet A, Ferreira ME, de Arias AR, et al. In vivo efficacy of oral and intralesional administration of 2-substituted quinolines in experimental treatment of new world cutaneous leishmaniasis caused by Leishmania amazonensis. Antimicrob Agents Chemother. 1996;40:2447–2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Belliard AM, Leroy C, Banide H, Farinotti R, Lacour B. Decrease of intestinal P-glycoprotein activity by 2-npropylquinoline, a new oral treatment for visceral leishmaniasis. Exp Parasitol. 2003;103:51–56. [DOI] [PubMed] [Google Scholar]
- 197.Nakayama H, Ferreira ME, de Arias AR, et al. Experimental treatment of chronic Trypanosoma cruzi infection in mice with 2-n-propylquinoline. Phytother Res. 2001;15:630–632. [DOI] [PubMed] [Google Scholar]
- 198.Mester I The occurrence of the alkaloids in Rutaceae. Fitoterapia. 1973;44:123–152. [Google Scholar]
- 199.Napolitano HB, Silva M, Ellena J, et al. Redetermination of skimmianine: a new inhibitor against the Leishmania APRT enzyme. Acta Crystallogr Sect E Struct Rep Online. 2003;59:1503–1505. [Google Scholar]
- 200.Ambrozin ARP, Mafezoli J, Vieira PC, et al. New pyrone and quinoline alkaloid from Almeidea rubra and their trypanocidal activity. J Braz Chem Soc. 2005;16:434–439. [Google Scholar]
- 201.Jagadeesh SG, Krupadanam GLD, Srimannarayana G. Antifeedant activity of the constituents of Evodia lunu-ankenda. Indian J Chem Sect B: Org Chem Incl Med Chem. 2000;39:475–476. [Google Scholar]
- 202.Liu ZL, Xu YJ, Wu J, Goh SH, Ho SH. Feeding deterrents from Dictamnus dasycarpus Turcz against two stored-product insects. J Agri Food Chem. 2002;50:1447–1450. [DOI] [PubMed] [Google Scholar]
- 203.Rios MY, Delgado G. Terpenoids and alkaloids from Esenbeckia belizencis. Spontaneous oxidation of furoquinoline alka-loids. J Nat Prod. 1992;55:1307–1309. [Google Scholar]
- 204.Kim YC, Kim NY, Jeong SJ, Sohn DH, Miyamoto T, Higuchi R. Biologically active quinolone alkaloids from Evodia rutae-carpa on Artemia salina. Planta Med. 1998;64:490. [DOI] [PubMed] [Google Scholar]
- 205.Bringmann G, Schlauer S, Rischer H, et al. Antidesmone, a novel antitrypanosomal alkaloid. Pharm Pharmacol Lett. 2001;11:47–48. [Google Scholar]
- 206.Biavatti MW, Vieira PC, das GF da Silva MF, et al. Biological activity of quinoline alkaloids from Raulinoa echinata and X-ray structure of flindersiamine. J Braz Chem Soc. 2002;13:66–70. [Google Scholar]
- 207.Perrett IS, Whitfield PJ. Atanine (3-dimethylallyl-4-methoxy-2-quinolone), an alkaloid with anthelmintic activity fromthe Chinese medicinal plant, Evodia rutaecarpa. Planta Med. 1995;61:276–278. [DOI] [PubMed] [Google Scholar]
- 208.Abe M, Imai T, Ishii N, Usui M, Okuda T, Oki T. Quinolactacide, a new quinolone insecticide from Penicillium citrinum Thom F 1539. Biosci Biotechnol Biochem. 2005;69:1202–1205. [DOI] [PubMed] [Google Scholar]
- 209.Abe M, Imai T, Ishii N, Usui M. Synthesis of quinolactacide via an acyl migration reaction and dehydrogenation with manganese dioxide, and its insecticidal activities. Biosci Biotechnol Biochem. 2006;70:303–306. [DOI] [PubMed] [Google Scholar]
- 210.Kusano M, Koshino H, Uzawa J, Fujioka S, Kawano T, Kimura Y. Nematicidal alkaloids and related compounds producedby the fungus Penicillium cf. simplicissimum. Biosci Biotechnol Biochem. 2000;64:2559–2568. [DOI] [PubMed] [Google Scholar]
- 211.Nakatsu T, Johns T, Kubo I, et al. Isolation, structure, and synthesis of novel 4-quinolinone alkaloids from Esenbeckia leiocarpa. J Nat Prod. 1990;53:1508–1813. [DOI] [PubMed] [Google Scholar]
- 212.Thoms C, Wolff M, Padmakumar K, Ebel R, Proksch P. Chemical defense of Mediterranean sponges Aplysina cavernicola and Aplysina aerophoba. Z Naturforsch C. 2004;59:113–122. [DOI] [PubMed] [Google Scholar]
- 213.Uchida R, Imasato R, Shiomi K, Tomoda H, Omura S. Yaequinolones J1 and J2, novel insecticidal antibiotics from Penicillium sp. FKI-2140. Org Lett. 2005;7:5701–5704. [DOI] [PubMed] [Google Scholar]
- 214.Hayashi H, Nakatani T, Inoue Y, Nakayama M, Nozaki H. New dihydroquinolinone toxic to Artemia salina produced by Penicillium sp. NTC-47. Biosci Biotech Biochem. 1997;61:914–916. [DOI] [PubMed] [Google Scholar]
- 215.Saxena BP, Tikku K, Atal CK, Koul O. Insect antifertility and antifeedant allelochemics in Adhatoda vasica Insect Sci Its Appl. 1986;7:489–493. [Google Scholar]
- 216.Seifert K, Unger W. Insecticidal and fungicidal compounds from Isatis tinctoria. Z Naturforsch Teil C. 1994;49:44–48. [DOI] [PubMed] [Google Scholar]
- 217.Scovill J, Blank E, Konnick M, Nenortas E, Shapiro T. Antitrypanosomal activities of tryptanthrins. Antimicrob Agents Chemother. 2002;46:882–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Wang SF, Braekman JC, Daloze D, et al. Nα-Quinaldyl-L-arginine HCl, a new defensive alkaloid from Subcoccinella-24-punctata (Coleoptera, Coccinellidae). Experientia. 1996;52:628–630. [Google Scholar]
- 219.Kato M, Inaba M, Itahana H, et al. Studies on anticoccidial constituents of crude drugs and related plants (I). Isolation andbiological activities of cis- and trans-febrifugine from Hydrangea macrophylla Shoyakugaku Zasshi 1990;44:288–292. [Google Scholar]
- 220.Leal WS, Zarbin PHG, Wojtasek H, Kuwahara S, Hasegawa M, Ueda Y. Medicinal alkaloid as a sex pheromone. Nature. 1997;385:213. [DOI] [PubMed] [Google Scholar]
- 221.Wojtasek H, Leal WS. Degradation of an alkaloid pheromone from the pale-brown chafer, Phyllopertha diversa (Coleoptera: Scarabaeidae), by an insect olfactory cytochrome P450. FEBS Lett. 1999;458:333–336. [DOI] [PubMed] [Google Scholar]
- 222.Rho TC, Bae EA, Kim DH, et al. Anti-Helicobacter pylori activity of quinolone alkaloids from Evodiae fructus. Biol Pharm Bull. 1999;22:1141–1143. [DOI] [PubMed] [Google Scholar]
- 223.Hamasaki N, Ishii E, Tominaga K, et al. Highly selective antibacterial activity of novel alkyl quinolone alkaloids from a Chinese herbal medicine, Gosyuyu (Wu-Chu-Yu), against Helicobacter pylori in vitro. Microbiol Immunol. 2000;44: 9–15. [DOI] [PubMed] [Google Scholar]
- 224.Tominaga K, Higuchi K, Hamasaki N, et al. In vivo action of novel alkyl methyl quinolone alkaloids against Helicobacter pylori. J Antimicrob Chemother. 2002;50:547–552. [DOI] [PubMed] [Google Scholar]
- 225.Adams M, Wube AA, Bucar F, Bauer R, Kunert O, Haslinger E. Quinolone alkaloids from Evodia rutaecarpa: a potent new group of antimycobacterial compounds. Int J Antimicrob Agents. 2005;26:262–264. [DOI] [PubMed] [Google Scholar]
- 226.Islam SKN, Gray AI, Waterman PG, Ahasan M. Screening of eight alkaloids and ten flavonoids isolated from four species of the genus Boronia (Rutaceae) for antimicrobial activities against seventeen clinical microbial strains. Phytother Res 2002;16:672–674. [DOI] [PubMed] [Google Scholar]
- 227.Aguilar-Guadarrama AB, Rios MY. Geranyl N-dimethylallylanthranilate, a new compound from Esenbeckia yaaxhokob. Planta Med. 2004;70:85–86. [DOI] [PubMed] [Google Scholar]
- 228.Hanawa F, Fokialakis N, Skaltsounis AL. Photo-activated DNA binding and antimicrobial activities of furoquinoline and pyranoquinolone alkaloids from rutaceae. Planta Med. 2004;70:531–535. [DOI] [PubMed] [Google Scholar]
- 229.El Sayed K, Al-Said MA, El-Feraly FS, Ross SA. New quinoline alkaloids from Ruta chalepensis. J Nat Prod. 2000;63:995–997. [DOI] [PubMed] [Google Scholar]
- 230.Fokialakis N, Magiatis P, Chinou I, Mitaku S, Tillequin F. Megistoquinones I and II, two quinoline alkaloids with antibacterial activity from the bark of Sarcomelicope megistophylla. Chem Pharm Bull. 2002;50:413–414. [DOI] [PubMed] [Google Scholar]
- 231.Jain SC, Pandey MK, Upadhyay RK, Kumar R, Hundal G, Hundal MS. Alkaloids from Toddalia aculeata. Phytochemistry. 2006;67:1005–1010. [DOI] [PubMed] [Google Scholar]
- 232.Cho JH, Lee CH, Lee HS. Antimicrobial activity of quinoline derivatives isolated from Ruta chalepensis toward human intestinal bacteria. J Microbiol Biotechnol. 2005;15:646–651. [Google Scholar]
- 233.Kumru M, Küçük V, Akyürek P. Vibrational spectra of quinoline-4-carbaldehyde: Combined experimental and theoreticalstudies. Spectrochimica Acta A: Mol Biomol Spect. 2013;113:72–79. [DOI] [PubMed] [Google Scholar]
- 234.Homma Y, Sato Z, Hirayama F, Konno K, Shirahama H, Suzui T. Production of antibiotics by Pseudomonas cepacia as an agent for biological control of soilborne plant pathogens. Soil Biol Biochem. 1989;21:723–728. [Google Scholar]
- 235.Roitman JN, Mahoney NE, Janisiewicz WJ, Benson M. A new chlorinated phenylpyrrole antibiotic produced by the anti-fungal bacterium Pseudomonas cepacia. J Agric Food Chem. 1990;38:538–541. [Google Scholar]
- 236.Kamigiri K, Tokunaga T, Shibazaki M, et al. YM-30059, a novel quinolone antibiotic produced by Arthrobactersp. J Antibiot. 1996;49:823–825. [DOI] [PubMed] [Google Scholar]
- 237.Kunze B, Hofle G, Reichenbach H. The aurachins, new quinoline antibiotics from myxobacteria: production, physicochemical and biological properties. J Antibiot. 1987;40:258–265. [DOI] [PubMed] [Google Scholar]
- 238.Asolkar RN, Schröder D, Heckmann R, Lang S, Wagner-Döbler I, Laatsch H. Helquinoline, a new tetrahydroquinoline antibiotic from Janibacter limosus Hel 1+. J Antibiot. 2004;57:17–23. [DOI] [PubMed] [Google Scholar]
- 239.Dekker KA, Inagaki T, Gootz TD, et al. New quinolone compounds from Pseudonocardia sp. with selective and potent anti-Helicobacter pylori activity: taxonomy of producing strain, fermentation, isolation, structural elucidation and biological activities. J Antibiot. 1998;51:145–152. [DOI] [PubMed] [Google Scholar]
- 240.Dawson S, Malkinson JP, Paumier D, Searcy M. Bisintercalator natural products with potential therapeutic applications: isolation, structure determination, synthetic and biological studies. Nat Prod Rep. 2007;24:109–126. [DOI] [PubMed] [Google Scholar]
- 241.Puar MS, Chan TM, Hegde V, et al. Sch 40832: a novel thiostrepton from Micromonospora carbonacea. J Antibiot. 1998;51:221–224. [DOI] [PubMed] [Google Scholar]
- 242.Long RA, Qureshi A, Faulkner DJ, Azam F. 2-n-Pentyl-4-quinolinol produced by a marine Alteromonas sp. and its potential ecological and biogeochemical roles. Appl Environ Microbiol. 2003;69:568–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Macfoy C, Danosus D, Sandit R, et al. Alkaloids of anuran skin: antimicrobial function. Z Naturforsch C. 2005;60c:932–937. [DOI] [PubMed] [Google Scholar]
- 244.Cantrell CL, Schrader KK, Mamonov LK, et al. Isolation and identification of antifungal and antialgal alkaloids from Haplophyllum sieversii. J Agri Food Chem. 2005;53:7741–7748. [DOI] [PubMed] [Google Scholar]
- 245.de Souza RC, Fernandes JB, Vieira PC, et al. A new imidazole alkaloid and other constituents from Pilocarpus grandifloras and their antifungal activity. Z Naturforsch B. 2005;60b:787–791. [Google Scholar]
- 246.Zhao W, Wolfender JL, Hostettmann K, Xu R, Qin G. Antifungal alkaloids and limonoid derivatives from Dictamnus dasycarpus. Phytochemistry. 1998;47:7–11. [DOI] [PubMed] [Google Scholar]
- 247.Oliva A, Meepagala KM, Wedge DE, et al. Natural fungicides from Ruta graveolens L. leaves, including a new quinolone alkaloid. J Agri Food Chem. 2003;51:890–896. [DOI] [PubMed] [Google Scholar]
- 248.Pearce AN, Appleton DR, Babcock RC, Copp BR. Distomadines A and B, novel 6-hydroxyquinoline alkaloids from theNew Zealand ascidian, Pseudodistoma aureum. Tetrahedron Lett. 2003;44:3897–3899. [Google Scholar]
- 249.Böhlendorf B, Forche E, Bedorf N, et al. Antibiotics from gliding bacteria, LXXIII† indole and quinoline derivatives as metabolites of tryptophan in myxobacteria. Liebigs Ann 1996:49–53. [Google Scholar]
- 250.Fakhouri W, Walker F, Vogler B, Armbruster W, Buchenauer H. Isolation and identification of N-mercapto-4-formylcarbostyril, an antibiotic produced by Pseudomonas fluorescens. Phytochemistry. 2001;58:1297–1303. [DOI] [PubMed] [Google Scholar]
- 251.Omura S, Nakagawa A, Hashimoto H, et al. Virantmycin, a potent antiviral antibiotic produced by a strain of Streptomyces. J Antibiot. 1980;33:1395–1396. [DOI] [PubMed] [Google Scholar]
- 252.Nakagawa A, Iwai Y, Hashimoto H, et al. Virantmycin, a new antiviral antibiotic produced by a strain of Streptomyces. J Antibiot. 1981;34:1408–1415. [DOI] [PubMed] [Google Scholar]
- 253.Mitscher LA, Baker WR. A search for novel chemotherapy against tuberculosis amongst natural products. Pure Appl Chem. 1998;70:365–371. [Google Scholar]
- 254.Kataoka M, Hirata K, Kunikata T, et al. Antibacterial action of tryptanthrin and kaempferol, isolated from the indigoplant (Polygonum tinctorium Lour.), against Helicobacter pylori-infected Mongolian gerbils. J Gastroenterol. 2001;36: 5–9. [DOI] [PubMed] [Google Scholar]
- 255.Belofsky GN, Anguera M, Jensen PR, Fenical W, Köck M. Oxepinamides A–C and fumiquinazolines H–I: bioactive metabolites from a marine isolate of a fungus of the genus Acremonium. Chem Eur J. 2000;6:1355–1360. [DOI] [PubMed] [Google Scholar]
- 256.Quinine Hollman A. and quinidine. Bri Heart J. 1991;66:301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Wenckebach KF. Cinchona derivatives in the treatment of heart disorders. J Am Med Assoc. 1923;81:472. [Google Scholar]
- 258.Mancini MA, Graubner W. Antagonism between quinine and adrenaline-like substances. Boll Soc Ital Biol. 1940;15:380. [Google Scholar]
- 259.Roberts J, Baer R. A method for evaluation of depressants of subatrial rhythmic function in the heart of the intact animal. J Pharm Exper Ther. 1960;129:36–41. [PubMed] [Google Scholar]
- 260.Dresel PE, Hart MC, Strümblad BCR. Cardiac arrhythmias induced by injection of isoproterenol into the coronary arteries [of dogs]. J Pharm Exper Ther. 1963;140:67–72. [Google Scholar]
- 261.Nye E, Roberts J. Effect of reserpine on the reactivity of atrial and ventricular pacemakers to quinidine. Nature. 1966;210:1376–1377. [DOI] [PubMed] [Google Scholar]
- 262.Wedd AM, Blair HA, Gosselin RE. The action of quinine on the cold blood heart. J Pharm Exper Ther. 1942;75:251. [Google Scholar]
- 263.Schmid PG, Nelson LD, Mark AL, Heistad DD, Abboud FM. Inhibition of adrenergic vasoconstriction by quinine. J Pharm Exper Ther. 1974;188:124–134. [PubMed] [Google Scholar]
- 264.Lee KS, Hume JR, Giles W, Brown AM. Sodium current depression by lidocaine and quinidine in isolated ventricular cells. Nature. 1981;291:325–327. [DOI] [PubMed] [Google Scholar]
- 265.Johnson EA, Robertson PA. Effect of acetylcholine and quinidine on atrial cellular potentials. Nature. 1957;180:1483–1484. [DOI] [PubMed] [Google Scholar]
- 266.Bridges JM, Baldini M. Effect of quinidine and related compounds on uptake and release of serotonin by blood platelets. Nature. 1966;210:1364–1365. [DOI] [PubMed] [Google Scholar]
- 267.Chen IS, Wu SJ, Lin YC, et al. Dimeric 2-quinolone alkaloid and antiplatelet aggregation constituents of Zanthoxylum simulans. Phytochemistry. 1994;36:237–239. [Google Scholar]
- 268.Sheen WS, Tsai IL, Teng CM, Chen IS. Nor-neolignan and phenyl propanoid from Zanthoxylum ailanthoides. Phytochemistry. 1994;36:213–216. [Google Scholar]
- 269.Chen KS, Chang YL, Teng CM, Chen CF, Wu YC. Furoquinolines with antiplatelet aggregation activity from leaves of Melicope confusa. Planta Med. 2000;66:80–81. [DOI] [PubMed] [Google Scholar]
- 270.Chen IS, Chen HF, Cheng MJ, et al. Quinoline alkaloids and other constituents of Melicope semecarpifolia with antiplatelet aggregation activity. J Nat Prod. 2001;64:1143–1147. [DOI] [PubMed] [Google Scholar]
- 271.Chen IS, Lin YC, Tsai IL, et al. Coumarins and anti-platelet aggregation constituents from Zanthoxylum schinifolium. Phytochemistry. 1995;39:1091–1097. [DOI] [PubMed] [Google Scholar]
- 272.Tsai IL, Lin WY, Teng CM, et al. Coumarins and antiplatelet constituents from the root bark of Zanthoxylum schinifolium. Planta Med. 2000;66:618–623. [DOI] [PubMed] [Google Scholar]
- 273.Cheng JT, Chang SS, Chen IS. Cardiovascular effect of skimmianine in rats. Arch Int Pharmacodyn Ther. 1990;306:65–74. [PubMed] [Google Scholar]
- 274.Chen JJ, Chang YL, Teng CM, Su CC, Chen IS. Quinoline alkaloids and anti-platelet aggregation constituents from theleaves of Melicope semecarpifolia. Planta Med. 2002;68:790–793. [DOI] [PubMed] [Google Scholar]
- 275.Nam KW, Je KH, Shin YJ, Kang SS, Mar W. Inhibitory effects of furoquinoline alkaloids from Melicope confusa and Dictamnus albus against human phosphodiesterase 5 (hPDE5A) in vitro. Arch Pharm Res. 2005;28:675–679. [DOI] [PubMed] [Google Scholar]
- 276.Lee HS, Oh WK, Choi HC, et al. Inhibition of angiotensin II receptor binding by quinolone alkaloids fromEvodia rutaecarpa. Phytother Res. 1998;12:212–214. [Google Scholar]
- 277.Sheen WS, Tsai IL, Teng CM, Ko FN, Chen IS. Indolopyridoquinazoline alkaloids with antiplatelet aggregation activity from Zanthoxylum integrifoliolum. Planta Med. 1996;62:175–176. [DOI] [PubMed] [Google Scholar]
- 278.Chang GJ, Wu MH, Chen WP, Kuo SC, Su MJ. Electrophysiological characteristics of antiarrhythmic potential of acro-phyllidine, a furoquinoline alkaloid isolated from Acronychia halophylla. Drug Dev Res. 2000;50:170–185. [Google Scholar]
- 279.Chiou WF, Liao JF, Chen CF. Comparative study on the vasodilatory effects of three quinazoline alkaloids isolated fromEvodia rutaecarpa. J Nat Prod. 1996;59:374–378. [DOI] [PubMed] [Google Scholar]
- 280.Gupta OP, Sharma ML, Ghatak BJR, Atal CK. Pharmacological investigations of vasicine and vasicinone–the alkaloids of Adhatoda vasica. Indian J Med Res. 1977;66:680–691. [PubMed] [Google Scholar]
- 281.Sharma RL, Prabhakar A. Synthesis of some new ring A subsituted vasicine analogues. Orient J Chem 1994;10:2–5. [Google Scholar]
- 282.Loya S, Rudi A, Tal R, Kashman Y, Loya Y, Hizi A. 3,5,8-Trihydroxy-4-quinolone, a novel natural inhibitor of thereverse transcriptases of human immunodeficiency viruses type 1 and type 2. Arch Biochem Biophys. 1994;309: 315–322. [DOI] [PubMed] [Google Scholar]
- 283.McCormick JL, McKee TC, Cardellina II JH, MR Boy. HIV inhibitory natural products. 26. Quinoline alkaloids from Euodia roxburghiana. J Nat Prod. 1996;59:469–471. [DOI] [PubMed] [Google Scholar]
- 284.Cheng MJ, Lee KH, Tsai IL, Chen IS. Two new sesquiterpenoids and anti-HIV principles from the root bark of Zanthoxylum ailanthoides. Bioorg Med Chem. 2005;13:5915–5920. [DOI] [PubMed] [Google Scholar]
- 285.Boger DL, Chen JH, Saionz KW, Jin Q. Synthesis of key sandramycin analogs: systematic examination of the intercalation chromophore. Bioorg Med Chem. 1998;6:85–102. [DOI] [PubMed] [Google Scholar]
- 286.Take Y, Inouye Y, Nakamura S, Allaudeen HS, Kubo A. Comparative studies of the inhibitory properties of antibiotics on human immunodeficiency virus and avian myeloblastosis virus reverse transcriptases and cellular DNA polymerases. J Antibiot. 1989;42:107–115. [DOI] [PubMed] [Google Scholar]
- 287.Boger DL, Ledeboer MW, Kume M. Total synthesis of luzopeptins A–C. J Am Chem Soc. 1999;121:1098–1099. [Google Scholar]
- 288.Morimoto Y, Shirahama H. Synthetic studies on virantmycin. 2. Total synthesis of unnatural (+)–virantmycin and determination of its absolute stereochemistry. Tetrahedron. 1996;52:10631–10652. [Google Scholar]
- 289.Fournet A, Hocquemiller R, Roblot F, et al. Les Chimanines, nouvelles quinoleines substituees en 2, isolees d’Une plante bolivienne antiparasitaire: Galipea longiflora. J Nat Prod. 1993;56:1547–1552. [Google Scholar]
- 290.Fournet A, Vagneur B, Richomme P, Bruneton J. Aryl-2 et alkyl-2 quinoléines nouvelles isolées d’une Rutacée bolivienne: Galipealongiflora. Can J Chem. 1989;67:2116–2118. [Google Scholar]
- 291.Fournet A, Mahieux R, Fakhfakh MA, Franck X, Hocquemiller R, Figadère B. Substituted quinolines induce inhibition ofproliferation of HTLV-1 infected cells. Bioorg Med Chem Lett. 2003;13:891–894. [DOI] [PubMed] [Google Scholar]
- 292.Ito C, Itoigawa M, Otsuka T, Tokuda H, Nishino H, Furukawa H. Constituents of Boronia pinnata. J Nat Prod. 2000;63:1344–1348. [DOI] [PubMed] [Google Scholar]
- 293.Lal B, Bhise NB, Gidwani RM, Lakdawala AD, Joshi K, Patvardhan S. Isolation, characterization, total synthesis, biologicalactivity of alkaloid evolitrine and its analogues. ARKIVOC 2005;2005:77–97. [Google Scholar]
- 294.Ito C, Itoigawa M, Furukawa A, et al. Quinolone alkaloids with nitric oxide production inhibitory activity from Orixa japonica. J Nat Prod. 2004;67:1800–1803. [DOI] [PubMed] [Google Scholar]
- 295.Kakinuma N, Iwai H, Takahashi S, et al. Quinolactacins A, B and C: novel quinolone compounds from Penicillium sp. EPF-6. I. Taxonomy, production, isolation and biological properties. J Antibiot. 2000;53:1247–1251. [DOI] [PubMed] [Google Scholar]
- 296.Takahashi S, Kakinuma N, Iwai H, et al. Quinolactacins A, B and C: novel quinolone compounds from Penicillium sp. EPF-6. II. Physico-chemical properties and structure elucidation. J Antibiot. 2000;53:1251–1256. [DOI] [PubMed] [Google Scholar]
- 297.Jin HZ, Lee JH, Lee D, et al. Quinolone alkaloids with inhibitory activity against nuclear factor of activated T cells from the fruits of Evodia rutaecarpa. Biol Pharm Bull. 2004;27:926–928. [DOI] [PubMed] [Google Scholar]
- 298.Ishihara T, Kohno K, Ushio S, Iwaki K, Ikeda M, Kurimoto M. Tryptanthrin inhibits nitric oxide and prostaglandin E2 synthesis by murine macrophages. Eur J Pharmacol. 2000;407:197–204. [DOI] [PubMed] [Google Scholar]
- 299.Micallef MJ, Iwaki K, Ishihara T, et al. The natural plant product tryptanthrin ameliorates dextran sodium sulfate-induced colitis in mice. Int Immunopharmacol. 2002;2:565–578. [DOI] [PubMed] [Google Scholar]
- 300.Danz H, Stoyanova S, Thomet OAR, et al. Inhibitory activity of tryptanthrin on prostaglandin and leukotriene synthesis. Planta Med. 2002;68:875–880. [DOI] [PubMed] [Google Scholar]
- 301.Takei Y, Kunikata T, Aga M, et al. Tryptanthrin inhibits interferon-gamma production by Peyer’s patch lymphocytes derived from mice that had been orally administered staphylococcal enterotoxin. Biol Pharm Bull. 2003;26:365–367. [DOI] [PubMed] [Google Scholar]
- 302.Oberthür C, Heinemann C, Elsner P, Benfeldt E, Hamburger M. A comparative study on the skin penetration of puretryptanthrin and tryptanthrin in Isatis tinctoria extract by dermal microdialysis coupled with isotope dilution ESI-LC-MS. Planta Med. 2003;69:385–389. [DOI] [PubMed] [Google Scholar]
- 303.Heinemann C, Schliemann-Willers S, Oberthür C, Hamburger M, Elsner P. Prevention of experimentally induced irritantcontact dermatitis by extracts of Isatis tinctoria compared to pure tryptanthrin and its impact on UVB-induced erythema. Planta Med. 2004;70:385–390. [DOI] [PubMed] [Google Scholar]
- 304.Molina P, Tárraga A, Gonzalez-Tejero A, et al. Inhibition of leukocyte functions by the alkaloid isaindigotone from Isatis indigotica and some new synthetic derivatives. J Nat Prod. 2001;64:1297–1300. [DOI] [PubMed] [Google Scholar]
- 305.Li L, Yang G, Dong T, Chen Z. Studies on the chemical constituents of dyers woad (Isatis tinctoria). Zhongcaoyao 1996;27:389–391. [Google Scholar]
- 306.Choi YH, Shin EM, Kim YS, Cai XF, Lee JJ, Kim HP. Anti-inflammatory principles from the fruits of Evodia rutaecarpa and their cellular action mechanisms. Arch Pharm Res. 2006;29:293–297. [DOI] [PubMed] [Google Scholar]
- 307.Cheng JT, Chang TK, Chen IS. Skimmianine and related furoquinolines function as antagonists of 5-hydroxytryptaminereceptors in animals. J Auton Pharmacol. 1994;14:365–374. [DOI] [PubMed] [Google Scholar]
- 308.Seya K, Miki I, Murata K, et al. Pharmacological properties of pteleprenine, a quinoline alkaloid extracted from Orixa japonica, on guinea-pig ileum and canine left atrium. J Pharm Pharmacol. 1998;50:803–807. [DOI] [PubMed] [Google Scholar]
- 309.Jaén JC, Gregor VE, Lee C, Davis R, Emmerling M. Acetylcholinesterase inhibition by fused dihydroquinazoline compounds. Bioorg Med Chem Lett. 1996;6:737–742. [Google Scholar]
- 310.Kim WG, Song NK, Yoo ID. Quinolactacins A1 and A2, new acetylcholinesterase inhibitors from Penicillium citrinum. J Antibiot. 2001;54:831–835. [DOI] [PubMed] [Google Scholar]
- 311.Lee MK, Hwang BY, Lee SA, et al. 1-Methyl-2-undecyl-4(1H)-quinolone as an irreversible and selective inhibitor of type B monoamine oxidase. Chem Pharm Bull. 2003;51:409–411. [DOI] [PubMed] [Google Scholar]
- 312.Lee IK, Yun BS, Han G, Cho DH, Kim YH, Yoo ID. Dictyoquinazols A, B, and C, new neuroprotective compounds from the mushroom Dictyophora indusiata. J Nat Prod. 2002;65:1769–1772. [DOI] [PubMed] [Google Scholar]
- 313.Wong SM, Musza LL, Kydd GC, Kullnig R, Gillum AM, Cooper R. Fiscalins: new substance P inhibitors produced by the fungus Neosartorya fischeri. Taxonomy, fermentation, structures, and biological properties. J Antibiot. 1993;46:545–553. [DOI] [PubMed] [Google Scholar]
- 314.Hale AL, Meepagala KM, Oliva A, Aliotta G, Duke SO. Phytotoxins from the leaves of Ruta graveolens. J Agri Food Chem. 2004;52:3345–3349. [DOI] [PubMed] [Google Scholar]
- 315.Kimura Y, Kusano M, Koshino H, Uzawa J, Fujioka S, Tani K. Penigequinolones A and B, pollen-growth inhibitors producedby Penicilium sp., No. 410. Tetrahedron Lett. 1996;37:4961–4964. [Google Scholar]
- 316.Oettmeier W, Dostatni R, Majewski C, et al. The aurachins, naturally occurring inhibitors of photosynthetic electron flow through photosystem II and the cytochrome b6/f-complex. Z Naturforsch Teil C. 1990;45:322–328. [Google Scholar]
- 317.Meunier B, Madgwick SA, Reil E, Oettmeier W, Rich PR. New inhibitors of the quinol oxidation sites of bacterial cytochromes bo and bd. Biochemistry. 1995;34:1076–1083. [DOI] [PubMed] [Google Scholar]
- 318.Don MJ, Lewis DFV, Wang SY, Tsai MW, Ueng YF. Effect of structural modification on the inhibitory selectivity of rutae-carpine derivatives on human CYP1A1, CYP1A2, and CYP1B1. Bioorg Med Chem Lett. 2003;13:2535–2538. [DOI] [PubMed] [Google Scholar]
- 319.Lee SK, Kim NH, Lee J, et al. Induction of cytochrome P450s by rutaecarpine and metabolism of rutaecarpine by cytochrome P450s. Planta Med. 2004;70:753–757. [DOI] [PubMed] [Google Scholar]
- 320.Ueng YF, Wang JJ, Lin LC, Park SS, Chen CF. Induction of cytochrome P450-dependent monooxygenase in mouse liver and kidney by rutaecarpine, an alkaloid of the herbal drug Evodia rutaecarpa. Life Sci. 2001;70:207–217. [DOI] [PubMed] [Google Scholar]
- 321.Ueng YF, Jan WC, Lin LC, Chen TL, Guengerich FP, Chen CF. The alkaloid rutaecarpine is a selective inhibitor of cytochrome P450 1A in mouse and human liver microsomes. Drug Metab Dispos. 2002;30:349–353. [DOI] [PubMed] [Google Scholar]
- 322.Ueng YF, Tsai TH, Don MJ, Chen RM, Chen TL. Alteration of the pharmacokinetics of theophylline by rutaecarpine, analkaloid of the medicinal herb Evodia rutaecarpa, in rats. J Pharm Phamacol 2005;57:227–232. [DOI] [PubMed] [Google Scholar]
- 323.Galetin A, Clarke SE, Houston JB. Quinidine and haloperidol as modifiers of CYP3A4 activity: multisite kinetic modelapproach. Drug Metab Dispos. 2002;30:1512–1522. [DOI] [PubMed] [Google Scholar]
- 324.López-Gresa MP, Gonz′alez MC, Primo J, Moya P, Romero V, Estornell E. Circumdatin H, a new inhibitor of mitochondrial NADH oxidase, from Aspergillus ochraceus. J Antibiot. 2005;58:416–419. [DOI] [PubMed] [Google Scholar]
- 325.Ohtsu Y, Sasamura H, Tsurumi Y, et al. The novel gluconeogenesis inhibitors FR225659 and related compounds that originate from Helicomyces sp. No. 19353. I. Taxonomy, fermentation, isolation and physico-chemical properties. J Antibiot. 2003;56:682–688. [DOI] [PubMed] [Google Scholar]
- 326.Ohtsu Y, Sasamura H, Shibata T, Nakajima H, Hino M, Fujii T. The novel gluconeogenesis inhibitors FR225659 and related compounds that originate from Helicomyces sp. No. 19353. II. Biological profiles. J Antibiot. 2003;56:689–693. [DOI] [PubMed] [Google Scholar]
- 327.Zenkoh T, Hatori H, Tanaka H, et al. Design and synthesis of a solid-supported FR225659 derivative for its receptor screening. Org Lett. 2004;6:2477–2480. [DOI] [PubMed] [Google Scholar]
- 328.Ko JS, Rho MC, Chung MY, et al. Quinolone alkaloids, diacylglycerol acyltransferase inhibitors from the fruits of Evodia rutaecarpa. Planta Med. 2002;68:1131–1133. [DOI] [PubMed] [Google Scholar]
- 329.Yoon MA, Jeong TS, Park DS, et al. Antioxidant effects of quinoline alkaloids and 2,4-di-tert-butylphenol isolated from Scolopendra subspinipes. Biol Pharm Bull. 2006;29:735–739. [DOI] [PubMed] [Google Scholar]
- 330.Chung HS, Woo WS. A quinolone alkaloid with antioxidant activity from the aleurone layer of anthocyanin-pigmented rice. J Nat Prod. 2001;64:1579–1580. [DOI] [PubMed] [Google Scholar]
- 331.Amin AH, Merta DR. A bronchodilator alkaloid (vasicinone) from Adhatoda vasica Nees. Nature. 1959;184:1317–1320. [DOI] [PubMed] [Google Scholar]
- 332.Cambridge GW, Jansen ABA, Jaeman DA. Bronchodilating action of vasicinone and related compounds. Nature. 1962;196:1217. [DOI] [PubMed] [Google Scholar]
- 333.Kamikawa T, Hanaoka Y, Fujie S, et al. SRS-A antagonist pyranoquinolone alkaloids from east African Fagara plants and their synthesis. Bioorg Med Chem. 1996;4:1317–1320. [DOI] [PubMed] [Google Scholar]
- 334.Paulini H, Eilert U, Schimmer O. Mutagenic compounds in an extract from rutae herba (Ruta graveolens L.). I. Mutagenicity is partially caused by furoquinoline alkaloids. Mutagenesis. 1987;2:271–273. [DOI] [PubMed] [Google Scholar]
- 335.Häfele F, Schimmer O. Mutagenicity of furoquinoline alkaloids in the Salmonella/microsome assay. Mutagenicity of dic-tamnine is modified by various enzyme inducers and inhibitors. Mutagenesis. 1988;3:349–353. [DOI] [PubMed] [Google Scholar]
- 336.Schimmer O, Leimeister U. The SCE-inducing potency of the furoquinoline alkaloid, gamma-fagarine, and a gamma-fagarine-containing tincture from Rutae Herba, in cultured human lymphocytes. Mutagenesis. 1989;4:467–470. [DOI] [PubMed] [Google Scholar]
- 337.Fujita H, Kakishima H. Further evidence for photoinduced genotoxicity of dictamnine as shown by prophage induction. Chem Biol Interactions. 1989;72:105–111. [DOI] [PubMed] [Google Scholar]
- 338.Yoshida S, Aoyagi T, Harada S, et al. Production of 2-methyl-4[3H]-quinazolinone, an inhibitor of poly(ADP-ribose) synthetase, by bacterium. J Antibiot. 1991;44:111–112. [DOI] [PubMed] [Google Scholar]
- 339.Funayama S, Tanaka R, Kumekawa Y, et al. Rat small intestine muscle relaxation alkaloids from Orixa japonica leaves. Biol Pharm Bull. 2001;24:100–102. [DOI] [PubMed] [Google Scholar]
- 340.Madappa C, Sankaranarayanan A, Sharma PL. A study on the selectivity of action of (+) INPEA and vasicine in different isolated tissue preparations. Indian J Pharmacol. 1989;21:144–152. [Google Scholar]
- 341.Nazrullaev SS, Bessonova IA, Akhmedkhodzhaeva KS. Estrogenic activity as a function of chemical structure in haplo-phyllum quinoline alkaloids. Chem Nat Comp. 2001;37:551–555. [Google Scholar]