Abstract
Purpose
The aims of this study were to determine the effect of curcumin on osteosarcoma (OS) cells due to inactivation of the p-JAK2/p-STAT3 pathway and evaluate the prognostic value of this pathway in OS.
Materials and methods
We exposed a human OS cell line (MG-63) to different concentrations of curcumin. Then, we characterized the effects on MG-63 cells using assays (cell viability, colony formation, cell cycle, wound healing, invasion), flow cytometry, Western blot, immunohistochemical analyses, and tumor xenograft.
Results
The half-maximal inhibitory of curcumin for MG-63 cells at 24 hours was 27.6 µM. The number of colonies of MG-63 cells was decreased obviously upon curcumin (10 and 20 µM) treatment. We also found increased accumulation of MG-63 cells in the G2/M phase upon curcumin (10 and 20 µM) treatment. Apoptosis was increased in 10 and 20 µM curcumin-treated MG-63 cells. After incubation of physically wounded cells for 24 hours, the percentage wound width increased upon curcumin exposure. Curcumin obviously decreased the expression of pJAK-2 and pSTAT-3 in MG-63 cells in a dose-dependent manner. Curcumin dose-dependently inhibited the proliferation, migration, and invasion of MG-63 cells and induced arrest of the G0/G1 phase and apoptosis by inhibiting the p-JAK2/p-STAT3 pathway. The linear correlativity between expression of p-JAK2 and STAT3 was very prominent, and both were closely associated with lung metastasis. In vivo study suggested that curcumin suppressed tumor growth through JAK2/STAT3 signaling.
Conclusion
Curcumin-mediated inhibition of the proliferation and migration of MG-63 cells was associated with inactivation of JAK/STAT signaling.
Keywords: osteosarcoma, curcumin, multiplication, invasion
Introduction
Osteosarcoma (OS) is the most prevalent primary cancer of the bones. Standard treatment comprises multiagent neoadjuvant chemotherapy (eg, doxorubicin, cisplatin, high dose of methotrexate or ifosfamide) followed by surgery and adjuvant chemotherapy with the same agents. This widely applied treatment has improved 5-year survival from 25% in the early 1970s to ~70% in the last decade.1,2 However, outcomes for OS remain unsatisfactory for patients with metastasis.3 Moreover, high-dose chemotherapy also induces multidrug resistance and cachexia.4,5 Meanwhile, a high dose of currently used drugs is limited by their side effects: nephrotoxicity, cardiomyopathy, hemorrhagic cystitis, and nephrotoxicity.6,7 Therefore, development of novel, safe, efficacious therapeutic agents for late-stage OS is especially urgent.
Curcumin is a phenolic, yellow compound found in Curcuma longa. It has been reported to have a wide range of biologic and pharmacologic activities: anti-inflammatory, antidiabetes mellitus, and antioxidant.8 Recently, the anticancer effect of curcumin has garnered considerable attention. Unlike cytotoxic drugs, curcumin has shown minimal toxicity and high safety at high doses in clinical trials.9,10 Studies have shown curcumin’s activities against cancer of the breast,11,12 pancreas,13 colon,14 prostate gland,12 as well as melanoma15,16 and OS.17–20 Lee et al17 reported that curcumin caused the death of OS cells by blocking cells successively in G(1)/S and G(2)/M phases and activating the caspase-3 pathway. Leow et al18 found that curcumin exhibited anti-invasive and anti-metastatic effects in OS cells though activation of the Wnt/β-catenin pathway. Moreover, curcumin has been reported to inhibit the proliferation and invasion of OS cells by regulating miRNA-125a and miRNA-138.19,20 However, how curcumin works against OS is not known. We explored a pathway to explain the inhibitory property of curcumin on OS cells.
Materials and methods
Cell culture and reagents
A human OS cell line (MG-63) was obtained from the Shanghai Cell Bank of the Chinese Academy of Science (Shanghai, People’s Republic of China). Cells were grown in DMEM (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% FBS (Thermo Fisher Scientific) and 1% penicillin and streptomycin (100 mg/mL of each) in a humidified atmosphere of 5% CO2 at 37°C. Curcumin (99% purity) was purchased from Sigma-Aldrich Co. (St Louis, MO, USA), and 100 mM of it was stored in 99.9% dimethyl sulfoxide (Sangon Biotech, Shanghai, People’s Republic of China). Curcumin at 5, 10, 15, 20, 25, 30, 35, 40, and 80 µM was used to treat MG-63 cells.
Cell-viability assay
MG-63 cells (5×104/plate) were seeded in 96-well plates overnight and then treated with curcumin (0, 5, 10, 15, 20, 25, 30, 35, 40, and 80 µM) for 24 hours. A total of 10 µL of Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Dojindo, Japan) was added to each well for 3 hours. The OD was detected at 450 nm by an ELISA reader (Multiskan™ MK3; Thermo Fisher Scientific). The cell-viability assay was repeated at least thrice in each group with triplicate wells.
Colony-formation assay
MG-63 cells (5×104/dish) were seeded in 100 mm dishes with curcumin (0, 10, and 20 µM). Two weeks later, cells were washed twice with PBS, fixed with 10% formaldehyde for 5 minutes, and then stained with 1% crystal violet for 30 seconds. Each clone with >30 cells was counted using a dissection microscope.
Cell-cycle assay
MG-63 cells treated with 0, 10, or 20 µM of curcumin for 48 hours were harvested through trypsinization. Then, they were fixed in 70% (v/v) ethanol at −20°C for 24 hours. Before detection, cells were resuspended in a solution of RNase (0.2 mg) and 1 mL of propidium iodide (PI)/Triton X-100 (20 µg [PI]/0.1% Triton X-100) in the dark for 15 minutes at room temperature. After washing twice, cell-cycle distribution was analyzed using a FACSCalibur™ flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA).
Apoptosis detection by flow cytometry
Cells treated as stated earlier were trypsinized and harvested after washing with ice-cold PBS. Cells (5×105) were stained with Annexin V-fluorescein isothiocyanate/PI (Thermo Fisher Scientific) according to manufacturer’s instructions and resuspended in 500 µL of binding buffer. Flow cytometry was undertaken using a flow cytometer (FACSCalibur).
Wound-healing assay
Cell mobility was assessed by a wound-healing assay. MG-63 cells (1×106) were seeded in six-well plates until 80% confluence had been reached. A sterile yellow 100 µL pipette tip was used to make a straight scratch. Detached cells were removed by washing with PBS. Then, cells were treated with curcumin (0, 10, or 20 µM) in the serum-free medium. Phase-contrast images were taken in the same field at 0 and 24 hours. Experiments were carried out in triplicate.
Invasion assay in vitro
For the cell-invasion test, 24-well transwells (pore size =8 mm; Corning Incorporated, Corning, NY, USA) were coated with Matrigel (1 mg/mL) according to the manufacturer’s (Becton Dickinson) instructions. Briefly, 1×104 cells were seeded into the upper chamber containing 0, 10, or 20 µM of curcumin. Then, 24 hours later, noninvasive cells in the upper chamber were removed with a cotton swab. After fixation with 4% paraformaldehyde for 20 minutes, the upper chamber was stained with Giemsa solution for 5 minutes. Cells in five random fields were counted at a high power to obtain the mean value. Each experiment was carried out in triplicate on separate occasions.
Western blot
Cells were lysed by ice-cold RIPA buffer (Beyotime Biotechnology, Jiangsu, People’s Republic of China) with freshly added 0.01% protease inhibitor (Sigma-Aldrich Co.) according to manufacturer’s instructions. Total protein (50 µg) was run on 10% SDS-PAGE gel and transferred by electrophoretic means onto nitrocellulose membranes (EMD Millipore, Billerica, MA, USA). Then, 5% skimmed milk (Becton Dickinson) was used to block nonspecific combinations. Cells were incubated with primary antibodies against phosphorylated STAT3 (p-STAT3) (Cell Signaling Technology, Danvers, MA, USA; catalog number 9139), phospho-STAT3 (Cell Signaling Technology; catalog number 9145), p-JAK2 (Cell Signaling Technology; catalog number 3230), or glyceraldehyde 3-phosphate dehydrogenase (Fermentas, Burlington, ON, Canada; catalog number 2251–1) overnight. Blots were recognized using goat anti-mouse secondary antibody (Beyotime Biotechnology; catalog number A0208) or goat anti-rabbit secondary antibody (Beyotime Biotechnology; catalog number A0216). Images were visualized using chemiluminescence detection reagents (EMD Millipore).
Immunohistochemical (IH) analysis
OS tissue samples in paraffin from 80 patients who had been followed up for >3 years were collected. The baseline characteristics of patients are given in Table 1. Expression of p-JAK2 (BM4839; Boster Biological Technology, Wuhan, People’s Republic of China) and STAT3 (BM3922; Boster Biological Technology) was measured using a SV0004 two-step immunohistochemistry kit according to manufacturer’s instructions. The ethics committee of Shanghai Sixth People’s Hospital approved the research protocol on human tissues. All patients provided written informed consent.
Table 1.
Baseline characteristics of patients
| Characteristic | OS tissue with lung metastasis | OS tissue without lung metastasis | P-value |
|---|---|---|---|
|
| |||
| Age (years), n | |||
| <18 | 23 | 31 | 0.797 |
| ≥18 | 12 | 14 | |
| Sex, n | |||
| Male | 19 | 20 | 0.843 |
| Female | 16 | 25 | |
| Pathogenic site, n | |||
| All four | 22 | 27 | 0.858 |
| Other parts | 13 | 18 | |
| Necrosis, n | |||
| <90 | 10 | 22 | 0.081 |
| ≥90 | 25 | 23 | |
| ki67, n | |||
| <14 | 14 | 15 | 0.026 |
| 14–30 | 18 | 16 | |
| >30 | 3 | 14 | |
| p-JAK2, mean ± SD | 2.05±1.246 | 4.86±0.765 | 0.000 |
| STAT3, mean ± SD | 3.43±0.731 | 8.07±1.859 | 0.000 |
Abbreviation: OS, osteosarcoma.
Tumor xenograft model
A total of six BALB/c nude mice were divided into two groups (three per group). The animal experimental operations were approved by the Animal Care and Use Committee of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital. MG-63 cells (1×106) were injected subcutaneously into the right thigh of each mouse. As soon as the xenografts were 0.5 cm in diameter, curcumin (Sigma-Aldrich Co.) was administered orally to them at a dose of 50 mg/kg QD for four consecutive weeks. The tumor, pJAK2, and STAT3 levels were evaluated. All of the in vivo experimental protocols were approved by the Animal Care and Use Committee of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital. The procedures were performed according to the guidelines and regulations of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital (Shanghai, People’s Republic of China).
Statistical analyses
Data are the mean ± SD of triplicates. Statistical analyses of data were undertaken using Prism 5.0 (GraphPad Software, Inc., La Jolla, CA, USA). Statistical significance was determined by one-way or two-way ANOVA. P<0.05 was considered as significant.
Results
Curcumin inhibited the survival of MG-63 cells
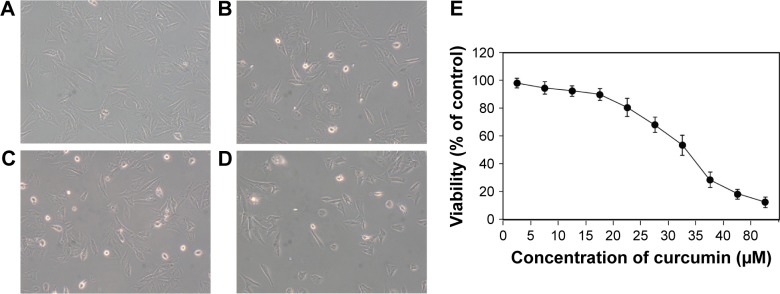
To explore the toxic effect of curcumin on MG-63 cells, a cell-viability assay was carried out. MG-63 cells were treated with various concentrations of curcumin for 24 hours. Curcumin clearly reduced the growth viability of MG-63 cells in a dose-dependent manner (Figure 1). The half-maximal inhibitory concentration of curcumin for MG-63 cells at 24 hours was 27.6 µM.
Figure 1.
Curcumin-induced toxic effects in MG-63 cells.
Note: (A) Untreated cells, magnification ×100, (B) cells treated with 5 µM of curcumin, (C) cells treated with 10 µM of curcumin, (D) cells treated with 20 µM of curcumin, and (E) concentration–response curves in MG-63 cells treated with different concentrations of curcumin.
Curcumin inhibits the proliferation of MG-63 cells
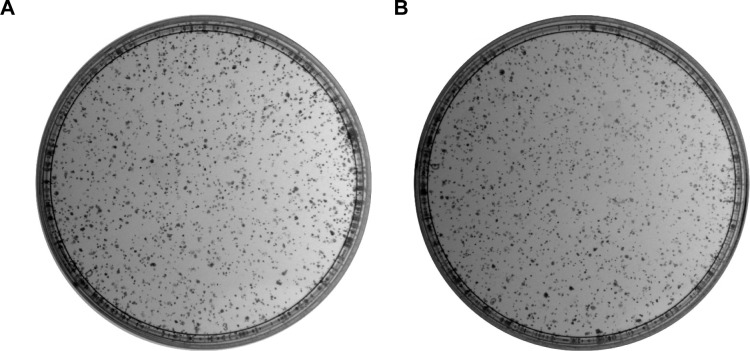
The number of colonies of MG-63 cells was decreased obviously from 1,182.33±102.42 in the control group to 983.33±87.31 and 812.00±53.74 at 10 and 20 µM of curcumin, respectively. These data demonstrated that the decrease in MG-63 growth was coincident with an increase in curcumin concentration in vitro (Figure 2).
Figure 2.
Clone formation of human osteosarcoma cell line (MG-63) treated with various concentrations of curcumin.
Notes: (A) Untreated cells, (B) cells treated with 10 µM of curcumin, and (C) cells treated with 20 µM of curcumin. (D) The colony numbers of 0, 10, and 20 µM were 1,182.3±102.4, 983.3±87.3, and 812.0±53.7, respectively. *P<0.05 and **P<0.01.
Abbreviation: OS, osteosarcoma.
Curcumin blocks progression of the G2/M phase in MG-63 cells
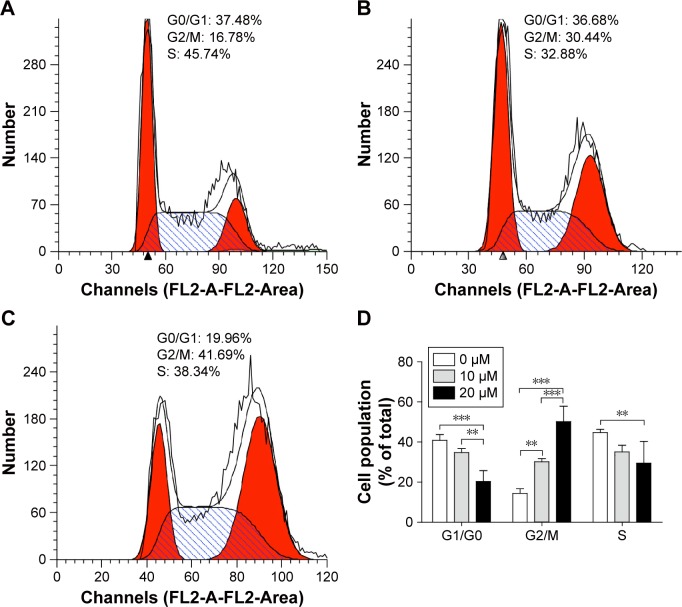
To ascertain if curcumin modulates the cycle in MG-63 cells, we treated MG-63 cells with 10 and 20 µM of curcumin for 24 hours and measured the cell-cycle distribution. We noted increased accumulation of the MG-63 cell population in the G2/M phase from 14.43%±1.92% in the control group to 30.17%±1.33% and 50.16%±6.28% upon treatment with 10 and 20 µM curcumin, respectively (Figure 3). These findings suggested that curcumin treatment could lead to arrest of the G2/M phase in MG-63 cells in a dose-dependent manner.
Figure 3.
Curcumin induces arrest the G2/M phase in MG-63 cells.
Notes: (A) Untreated cells, (B) cells treated with 10 µM of curcumin, and (C) cells treated with 20 µM of curcumin. Treatment with curcumin resulted in an increase in the number of cells in the G2/M phase and a decrease in the number of cells in the G0/G1 phase, indicating a curcumin-induced arrest of the G2/M phase. Results are plotted as the percentage of cells in each phase. (D) Cell population of the G0/G1 phase in 0, 10, and 20 µM was 40.9%±2.4%, 34.8%±1.6%, and 20.4%±4.4%, respectively. Cell population of the G2/M phase in 0, 10, and 20 µM was 14.4%±1.9%, 30.2%±1.3%, and 50.2%±6.3%, respectively. Cell population of the S phase in 0, 10, and 20 was 44.7%±1.3%, 35.1%±2.7%, and 29.5%±8.9%, respectively. **P<0.01 and ***P<0.001.
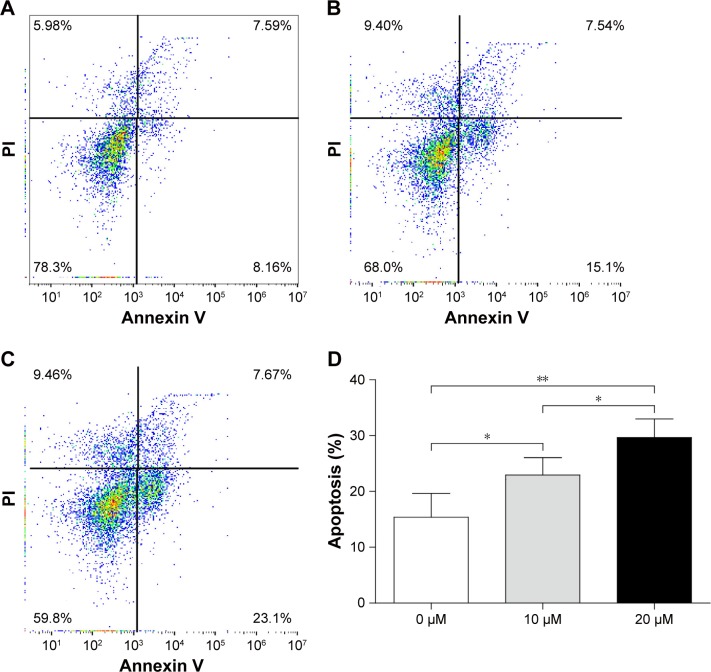
Curcumin induces the apoptosis of OS cells
To ascertain if curcumin induced the apoptosis of MG-63 cells, the latter were treated with different concentrations of curcumin for 48 hours. Apoptosis was increased from 15.40±3.47% in the control group to 22.95±2.53% and 29.65±2.71% upon treatment with 10 and 20 µM curcumin groups, respectively (Figure 4). These results suggested that curcumin triggered significant apoptosis of MG-63 cells in a dose-dependent manner.
Figure 4.
Curcumin induces the apoptosis of MG-63 cells.
Notes: (A) Untreated cells, (B) cells treated with 10 µM of curcumin, (C) cells treated with 20 µM of curcumin, and (D) treatment of MG-63 cells with curcumin resulted in a dramatic increase in the percentage of apoptotic and necrotic cells (including early apoptosis and late apoptosis). Flow cytometry was applied to analyze annexin V/PI double-stained MG-63 cells. The upper left quadrant of the histograms denotes necrotic cells, and the upper right quadrant denotes late apoptotic and necrotic cells. The lower right quadrant denotes the early apoptotic cells. Experiments were repeated thrice. The percentage of apoptotic cells of 0, 10, and 20 µM were 15.4%±3.5%, 23.0%±2.5%, and 29.6%±2.7%, respectively. *P<0.05 and **P<0.01.
Abbreviation: PI, propidium iodide.
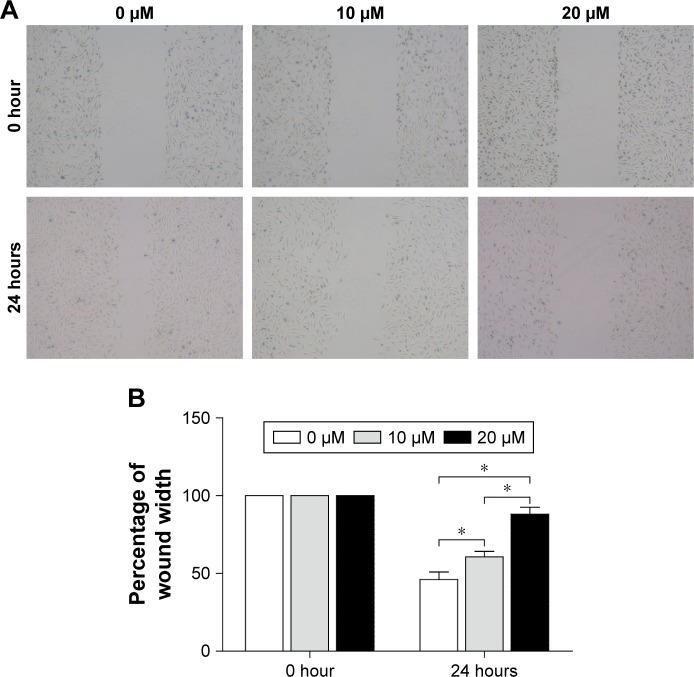
Curcumin inhibits the migration of OScells
The ability of MG-63 cells to migrate was examined by a wound-healing assay (Figure 5). After incubation of physically wounded cells for 24 hours, the percentage wound width increased from 46.00%±4.08% in the control group to 60.67%±2.87% and 88.00%±3.74% upon treatment with 10 and 20 µM curcumin, respectively (Figure 5). These results suggested that curcumin inhibited the migration of MG-63 cells significantly in a dose-dependent manner.
Figure 5.
Effects of curcumin on the migration of MG-63 cells according to a wound-healing assay.
Notes: (A) The migration of MG-63 cells. (B) Migration of MG-63 cells was quantified by measuring wound-closure areas before and after injury. The percentages of wound width of 0, 10, and 20 μM were 46.0%±4.1%, 60.7%±2.9%, and 88.0%±3.7%, respectively. Experiments were repeated thrice. *P<0.05.
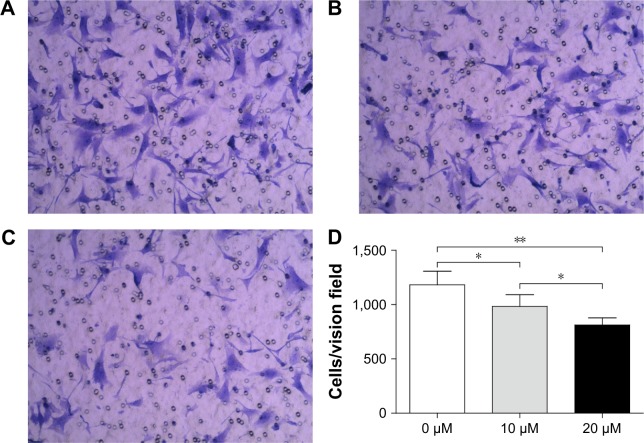
Curcumin inhibits invasion of OS cells
Matrigel-coated membranes were used to investigate the impact of curcumin on the invasion of OS cells. The number of cells penetrating into the lower chamber was decreased significantly from 64.00±2.45 in the control group to 47.33±8.34 and 29.33±4.99 upon treatment with 10 and 20 µM curcumin, respectively (Figure 6). These results suggested that curcumin inhibited the invasion of OS cells in a dose-dependent manner.
Figure 6.
Curcumin impaired the invasion of MG-63 cells as assessed by a transwell chamber assay.
Notes: (A) Untreated cells, (B) cells treated with 10 µM of curcumin, and (C) cells treated with 20 µM of curcumin. Magnification ×100. (D) Number of cells in each field was counted and averaged. The cell numbers per vision field of 0, 10, and 20 µM were 64.0±2.4, 647.3±8.3, and 29.3±5.0, respectively. Experiments were repeated thrice. *P<0.05 and **P<0.01.
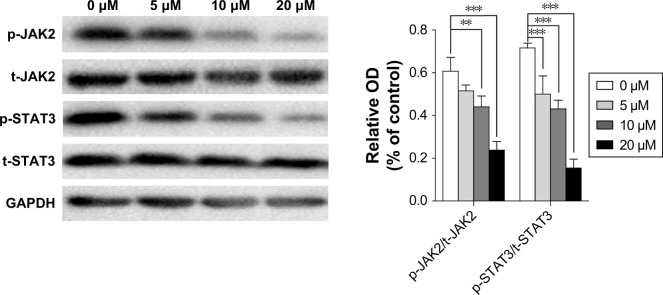
Curcumin inhibits the p-JAK2/p-STAT3 pathway
JAK/STAT signaling has been reported to play a central part in the proliferation and invasion of cancer cells. To ascertain if the p-JAK2/p-STAT3 pathway is involved in the antitumor effect of curcumin, the protein level of JAK2, p-JAK2, STAT3, and p-STAT3 in MG-63 cells was examined by Western blot. Curcumin treatment decreased expression of p-JAK2 and p-STAT3 in MG-63 cells obviously in a dose-dependent manner (Figure 7).
Figure 7.
Effect of curcumin on p-JAK2/p-STAT3 signaling in MG-63 cells.
Notes: MG-63 cells were treated with curcumin (0, 5, 10, or 20 µM) for 24 hours. Cell lysates were harvested, and expression of p-JAK2, JAK2, p-STAT3, and STAT3 was determined by Western blot. GAPDH was used as an internal control. **P<0.01 and ***P<0.001.
Abbreviation: p, phosphorylated.
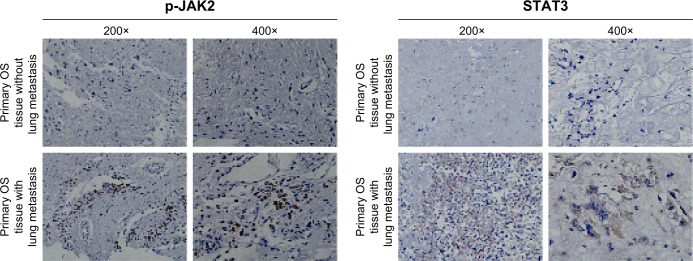
JAK2/STAT3 signaling is closely related to metastasis
The importance of JAK2 and STAT3 in OS is uncertain. We measured expression of p-JAK2 and STAT3 by the IH method in the tissue of OS patients with or without lung metastasis (Figure 8). For lung-metastasis samples, median expression of p-JAK2 and STAT3 was 9.23 and 14.82, respectively, which was much higher than that in patients without metastasis (2.43 and 5.06, respectively). There was a positive correlation between expression of p-JAK2 and STAT3 (r=0.715, P=0.002).
Figure 8.
Expression of p-JAK2 and STAT3 in OS tissue samples.
Notes: IH staining of p-JAK2 and STAT3 in osteosarcoma tumor tissues. Positive cells contain dark-brown particles.
Abbreviations: p, phosphorylated; OS, osteosarcoma; IH, immunohistochemical.
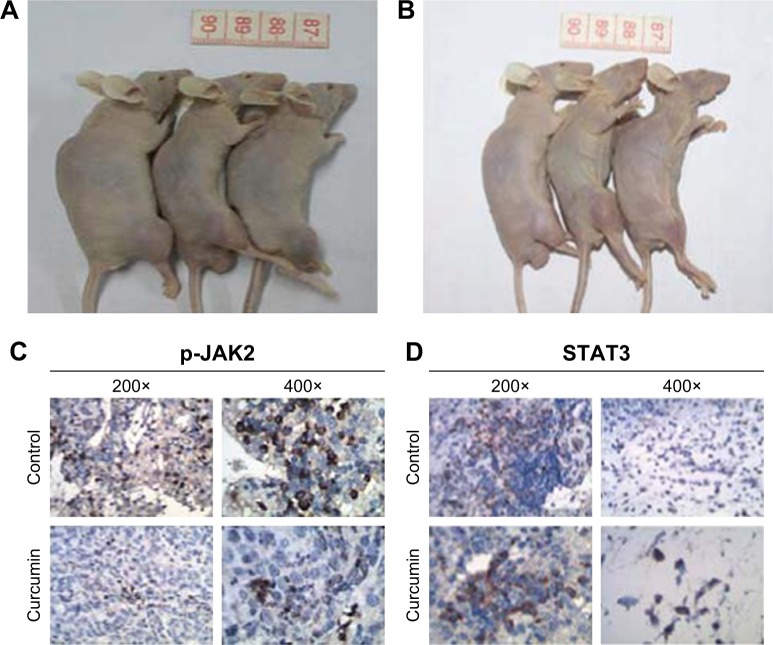
Curcumin inhibited OS cells in vivo
We established OS xenografts to evaluate the effect of curcumin on tumor growth in vivo. After curcumin treatment for 4 weeks, the tumor sizes were significantly smaller than those in the control group (Figure 9A and B). The IH analysis also demonstrated the expression of p-JAK2 and STAT3 (Figure 9C and D), confirming that curcumin suppressed OS tumor growth through JAK2/STAT3 signaling.
Figure 9.
In vivo effects of curcumin in nude mice.
Notes: (A) The tumor sizes in the control group after 4 weeks of placebo treatment. (B) The tumor sizes in the study group after 4 weeks of curcumin treatment. (C) Expression of p-JAK2 in the xenograft sample. (D) Expression of STAT3 in the xenograft sample. After curcumin treatment for 4 weeks, the tumor sizes were significantly smaller than those in the control group. The expression of p-JAK2 and STAT3 in the curcumin group was much lower than that in the control group.
Abbreviation: p, phosphorylated.
Discussion
Curcumin has been shown to possess potent activity in vitro and in vivo against cancer of the prostate gland,21 breast,22 colon,23 as well as OS.24 In the present study, curcumin elicited dramatic inhibition of proliferation and arrest of the G2⁄M cycle in MG-63 OS cells as demonstrated by the CCK-8 assay and flow cytometry, respectively. These results are consistent with data published by other research teams.25–28 Curcumin undertakes anticancer activities through nuclear factor-kappa B,29 AKT/mTOR,30 Wnt,31 and JAK/STAT32 signaling pathways. Whether curcumin exerts an antitumor effect in OS cells via JAK/STAT signaling has yet to be determined.
We found that the phosphorylation levels of STAT3 in curcumin-treated MG-63 cells were decreased. JAK/STAT signaling has a central role in tumorigenesis; p-STAT3 activation contributes to cell proliferation by regulating expression of various genes required for cell proliferation. After activation by cytokine-mediated transphosphorylation, JAK2 triggers the phosphorylation of STAT3 proteins. JAK2 is involved in epithelial–mesenchymal transition and promotes the metastasis of several types of cancer cell. JAK2 phosphorylation was decreased significantly in curcumin-treated groups, especially in the high-concentration groups (10 and 20 µM), similar to STAT3 phosphorylation. This observation suggests that curcumin exerts its effects by inhibiting JAK2 phosphorylation (and thus inhibiting STAT3 phosphorylation) without affecting expression of both proteins in MG-63 cells.
Aberrant activation of JAK/STAT signaling has been documented in cancers of the head and neck,33 breast,34 and prostate gland35 and in OS.36,37 Few reports have focused on the role of JAK/STAT signaling in OS. Gerland et al38 found that growth hormone induced expression of the JAK/STAT negative-regulatory proteins, which was inhibited by 1-alpha, 25-dihydroxyvitamin D. Sandoval-Usme et al reported that imvastatin decreases the proliferation, migration, and invasion of OS cells in a time- and dose-dependent manner through the JAK/STAT pathway.36 We found a positive correlation between expression of p-JAK2 and STAT3, and both were closely related to lung metastasis.
Conclusion
We showed curcumin to be an antitumor agent. Curcumin-mediated inhibition of the proliferation and migration of MG-63 cells was associated with inactivation of JAK/STAT signaling. Expression of JAK2 and STAT3 was much higher in OS patients with lung metastasis than in OS patients without metastasis. Downregulation of JAK/STAT signaling by curcumin could be a new strategy for OS treatment.
Acknowledgments
The authors thank Dr Yang Su for her great help in immunohistochemistry experiments.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma Study Group protocols. J Clin Oncol. 2002;20(3):776–790. doi: 10.1200/JCO.2002.20.3.776. [DOI] [PubMed] [Google Scholar]
- 2.Longhi A, Errani C, De Paolis M, Mercuri M, Bacci G. Primary bone osteosarcoma in the pediatric age: state of the art. Cancer Treat Rev. 2006;32(6):423–436. doi: 10.1016/j.ctrv.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 3.Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the surveillance, epidemiology, and end results program. Cancer. 2009;115(7):1531–1543. doi: 10.1002/cncr.24121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whelan JS, Bielack SS, Marina N, et al. EURAMOS-1, an international randomised study for osteosarcoma: results from pre-randomisation treatment. Ann Oncol. 2015;26(2):407–414. doi: 10.1093/annonc/mdu526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bakhshi S, Radhakrishnan V. Prognostic markers in osteosarcoma. Expert Rev Anticancer Ther. 2010;10(2):271–287. doi: 10.1586/era.09.186. [DOI] [PubMed] [Google Scholar]
- 6.Marina N, Gebhardt M, Teot L, Gorlick R. Biology and therapeutic advances for pediatric osteosarcoma. Oncologist. 2004;9(4):422–441. doi: 10.1634/theoncologist.9-4-422. [DOI] [PubMed] [Google Scholar]
- 7.D’Adamo DR. Appraising the current role of chemotherapy for the treatment of sarcoma. Semin Oncol. 2011;38(Suppl 3):S19–S29. doi: 10.1053/j.seminoncol.2011.09.004. [DOI] [PubMed] [Google Scholar]
- 8.Lin JK, Lin-Shiau SY. Mechanisms of cancer chemoprevention by curcumin. Proc Natl Sci Counc Repub China B. 2001;25(2):59–66. [PubMed] [Google Scholar]
- 9.Gupta SC, Kismali G, Aggarwal BB. Curcumin, a component of turmeric: from farm to pharmacy. Biofactors. 2013;39(1):2–13. doi: 10.1002/biof.1079. [DOI] [PubMed] [Google Scholar]
- 10.Yang C, Su X, Liu A, et al. Advances in clinical study of curcumin. Curr Pharm Des. 2013;19(11):1966–1973. [PubMed] [Google Scholar]
- 11.Liu Y, Zhou J, Hu Y, Wang J, Yuan C. Curcumin inhibits growth of human breast cancer cells through demethylation of DLC1 promoter. Mol Cell Biochem. 2017;425(1–2):47–58. doi: 10.1007/s11010-016-2861-4. [DOI] [PubMed] [Google Scholar]
- 12.Lin L, Hutzen B, Ball S, et al. New curcumin analogues exhibit enhanced growth-suppressive activity and inhibit Akt and signal transducer and activator of transcription 3 phosphorylation in breast and prostate cancer cells. Cancer Sci. 2009;100(9):1719–1727. doi: 10.1111/j.1349-7006.2009.01220.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z, Zhang Y, Banerjee S, Li Y, Sarkar FH. Notch-1 down-regulation by curcumin is associated with the inhibition of cell growth and the induction of apoptosis in pancreatic cancer cells. Cancer. 2006;106(11):2503–2513. doi: 10.1002/cncr.21904. [DOI] [PubMed] [Google Scholar]
- 14.Tong W, Wang Q, Sun D, Suo J. Curcumin suppresses colon cancer cell invasion via AMPK-induced inhibition of NF-κB, uPA activator and MMP9. Oncol Lett. 2016;12(5):4139–4146. doi: 10.3892/ol.2016.5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faião-Flores F, Quincoces Suarez JA, Fruet AC, Maria-Engler SS, Pardi PC, Maria DA. Curcumin analog DM-1 in monotherapy or combinatory treatment with dacarbazine as a strategy to inhibit in vivo melanoma progression. PLoS One. 2015;10(3):e0118702. doi: 10.1371/journal.pone.0118702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao G, Han X, Zheng S, et al. Curcumin induces autophagy, inhibits proliferation and invasion by downregulating Akt/mTOR signaling pathway in human melanoma cells. Oncol Rep. 2016;35(2):1065–1074. doi: 10.3892/or.2015.4413. [DOI] [PubMed] [Google Scholar]
- 17.Lee DS, Lee MK, Kim JH. Curcumin induces cell cycle arrest and apoptosis in human osteosarcoma (HOS) cells. Anticancer Res. 2009;29(12):5039–5044. [PubMed] [Google Scholar]
- 18.Leow PC, Tian Q, Ong ZY, Yang Z, Ee PL. Antitumor activity of natural compounds, curcumin and PKF118-310, as Wnt/β-catenin antagonists against human osteosarcoma cells. Invest New Drugs. 2010;28(6):766–782. doi: 10.1007/s10637-009-9311-z. [DOI] [PubMed] [Google Scholar]
- 19.Chen P, Wang H, Yang F, Chen H, He W, Wang J. Curcumin promotes osteosarcoma cell death by activating miR-125a/ERRα signal pathway. J Cell Biochem. 2017;118(1):74–81. doi: 10.1002/jcb.25612. [DOI] [PubMed] [Google Scholar]
- 20.Yu D, An F, He X, Cao X. Curcumin inhibits the proliferation and invasion of human osteosarcoma cell line MG-63 by regulating miR-138. Int J Clin Exp Pathol. 2015;8(11):14946–14952. [PMC free article] [PubMed] [Google Scholar]
- 21.Mukhopadhyay A, Banerjee S, Stafford LJ, Xia C, Liu M, Aggarwal BB. Curcumin-induced suppression of cell proliferation correlates with down-regulation of cyclin D1 expression and CDK4-mediated retinoblastoma protein phosphorylation. Oncogene. 2002;21(57):8852–8861. doi: 10.1038/sj.onc.1206048. [DOI] [PubMed] [Google Scholar]
- 22.Moghtaderi H, Sepehri H, Attari F. Combination of arabinogalactan and curcumin induces apoptosis in breast cancer cells in vitro and inhibits tumor growth via overexpression of p53 level in vivo. Biomed Pharmacother. 2017;88:582–594. doi: 10.1016/j.biopha.2017.01.072. [DOI] [PubMed] [Google Scholar]
- 23.Zhang J, Feng Z, Wang C, et al. Curcumin derivative WZ35 efficiently suppresses colon cancer progression through inducing ROS production and ER stress-dependent apoptosis. Am J Cancer Res. 2017;7(2):275–288. [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z, Zhang K, Zhu Y, Wang D, Shao Y, Zhang J. Curcumin inhibits hypoxia-induced proliferation and invasion of MG-63 osteosarcoma cells via downregulating Notch1. Mol Med Rep. 2017;15(4):1747–1752. doi: 10.3892/mmr.2017.6159. [DOI] [PubMed] [Google Scholar]
- 25.Dasiram JD, Ganesan R, Kannan J, Kotteeswaran V, Sivalingam N. Curcumin inhibits growth potential by G1 cell cycle arrest and induces apoptosis in p53-mutated COLO 320DM human colon adenocarcinoma cells. Biomed Pharmacother. 2017;86:373–380. doi: 10.1016/j.biopha.2016.12.034. [DOI] [PubMed] [Google Scholar]
- 26.Su J, Zhou X, Wang L, Yin X, Wang Z. Curcumin inhibits cell growth and invasion and induces apoptosis through down-regulation of Skp2 in pancreatic cancer cells. Am J Cancer Res. 2016;6(9):1949–1962. [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang H, Xu W, Li B, et al. Curcumin promotes cell cycle arrest and inhibits survival of human renal cancer cells by negative modulation of the PI3K/Akt signaling pathway. Cell Biochem Biophys. 2015;73(3):681–686. doi: 10.1007/s12013-015-0694-5. [DOI] [PubMed] [Google Scholar]
- 28.Zhang X, Chen M, Zou P, et al. Curcumin analog WZ35 induced cell death via ROS-dependent ER stress and G2/M cell cycle arrest in human prostate cancer cells. BMC Cancer. 2015;15(1):866. doi: 10.1186/s12885-015-1851-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dai C, Li B, Zhou Y, et al. Curcumin attenuates quinocetone induced apoptosis and inflammation via the opposite modulation of Nrf2/HO-1 and NF-kB pathway in human hepatocyte L02 cells. Food Chem Toxicol. 2016;95:52–63. doi: 10.1016/j.fct.2016.06.025. [DOI] [PubMed] [Google Scholar]
- 30.Zhu FQ, Chen MJ, Zhu M, et al. Curcumin suppresses epithelial-mesenchymal transition of renal tubular epithelial cells through the inhibition of Akt/mTOR pathway. Biol Pharm Bull. 2017;40(1):17–24. doi: 10.1248/bpb.b16-00364. [DOI] [PubMed] [Google Scholar]
- 31.Zhu JY, Yang X, Chen Y, et al. Curcumin suppresses lung cancer stem cells via inhibiting Wnt/β-catenin and Sonic hedgehog pathways. Phytother Res. 2017;31(4):680–688. doi: 10.1002/ptr.5791. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, Wu J, Ye B, Wang Q, Xie X, Shen H. Protective effect of curcumin on TNBS-induced intestinal inflammation is mediated through the JAK/STAT pathway. BMC Complement Altern Med. 2016;16(1):299. doi: 10.1186/s12906-016-1273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lai SY, Johnson FM. Defining the role of the JAK-STAT pathway in head and neck and thoracic malignancies: implications for future therapeutic approaches. Drug Resist Updat. 2010;13(3):67–78. doi: 10.1016/j.drup.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 34.Hosford SR, Miller TW. Clinical potential of novel therapeutic targets in breast cancer: CDK4/6, Src, JAK/STAT, PARP, HDAC, and PI3K/Akt/mTOR pathways. Pharmgenomics Pers Med. 2014;7:203–215. doi: 10.2147/PGPM.S52762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duzagac F, Inan S, Ela Simsek F, et al. JAK/STAT pathway interacts with intercellular cell adhesion molecule (ICAM) and vascular cell adhesion molecule (VCAM) while prostate cancer stem cells form tumor spheroids. J BUON. 2015;20(5):1250–1257. [PubMed] [Google Scholar]
- 36.Sandoval-Usme MC, Umaña-Pérez A, Guerra B, et al. Simvastatin impairs growth hormone-activated signal transducer and activator of transcription (STAT) signaling pathway in UMR-106 osteosarcoma cells. PLoS One. 2014;9(1):e87769. doi: 10.1371/journal.pone.0087769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yan J, Wang Q, Zou K, et al. Inhibition of the JAK2/STAT3 signaling pathway exerts a therapeutic effect on osteosarcoma. Mol Med Rep. 2015;12(1):498–502. doi: 10.3892/mmr.2015.3439. [DOI] [PubMed] [Google Scholar]
- 38.Gerland K, Bataillé-Simoneau N, Baslé M, Fourcin M, Gascan H, Mercier L. Activation of the JAK/STAT signal transduction pathway in GH-treated rat osteoblast-like cells in culture. Mol Cell Endocrinol. 2000;168(1–2):1–9. doi: 10.1016/s0303-7207(00)00314-2. [DOI] [PubMed] [Google Scholar]