Abstract
Objective
To analyze the etiology of nasopharyngeal hemorrhage after radiotherapy for nasopharyngeal carcinoma (NPC) and evaluate the relevant management and rescue approaches.
Methods
Seventeen cases of nasopharyngeal hemorrhage caused by radiotherapy of NPC, treated between January 2015 and March 2018, were retrospectively analyzed to study the etiology of nasopharyngeal hemorrhage. The management and rescue strategies, including anterior and posterior nostril packing, endoscopic nasopharynx electrocoagulation, and digital subtraction angiography embolization, were assessed for their effectiveness.
Results
Nasopharynx hemorrhage after radiotherapy of NPC was mainly associated with erosion of the internal carotid artery or maxillary artery by the tumor. Among the 17 cases, 11 patients were treated by digital subtraction arterial angiography embolization, and 3 were treated by endoscopic nasopharynx electrocoagulation. Overall, 13 patients survived, while 4 died.
Conclusion
Anterior and posterior nostril packing, endoscopic nasopharynx electrocoagulation, and digital subtraction angiography embolization are suitable for treating nasopharyngeal hemorrhage. However, effective hemostasis depends on early identification of the bleeding vessels.
Keywords: nasopharyngeal carcinoma (NPC), nasopharyngeal hemorrhage, radiotherapy, prognosis
Introduction
Nasopharyngeal carcinoma (NPC) is the most common head and neck cancer in southern China, affecting 30 out of 100,000 people. It is usually diagnosed with a high degree of malignancy and consequently leads to >34,000 deaths annually.1 Currently, the primary treatment modality for NPC is radical radiation therapy, with chemotherapy as an auxiliary option. Although radiotherapy has evolved from two-dimensional radiotherapy to intensity-modulated radiation therapy (IMRT), which has improved local control rates and minimized adverse effects on surrounding healthy tissue, radiation complications are frequently observed. These include xerostomia, sinusitis, temporal lobe necrosis, cranial nerve palsy, and brainstem damage.2 In addition, radiation arteritis with carotid stenosis, especially nasopharyngeal hemorrhage, also occurs often.3
The vascular injury resulting from radiation is believed to be a significant cause of bleeding after radiation treatment for NPC.4 Multiple factors may be involved, including local hypoxia, vascular remodeling, inflammatory infection, nasopharyngeal and nasal dysfunction, dry mucous membranes, tumor recurrence, and malnutrition.5 Nasopharyngeal hemorrhage represents a particular subtype of epistaxis after radiotherapy for NPC, described as a sudden hemorrhage of >100 mL or continuous bleeding of more than 300 mL in the nasopharyngeal area within a short period.6 The condition is usu ally dangerous and critical, with many complications, and the reported mortality rate has varied from 35.7% to 100%.7
There are no standard treatment methods for significant bleeding after radiotherapy, and bleeding-related factors should be avoided. Various hemostasis strategies are available, including packing of the anterior and posterior nostrils, applying the nasal cavity balloon, carotid artery ligation, and vascular interventional embolization. In the past five years, >800 new cases of NPC have been treated in our clinic every year. In the present study, we retrospectively analyzed the etiological factors of nasopharyngeal hemorrhage after radiotherapy for NPC and evaluated the management protocols to provide insight into improving the effectiveness of the treatments.
Materials and methods
Ethical statement and patient selection
This study was approved by the Ethics Committee of Hainan General Hospital, Haikou, China, and conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from each patient. The 17 patients included in this study were treated for nasopharyngeal hemorrhage after radiation treatment for NPC in Hainan General Hospital, China, from January 2015 to March 2018.
Management of nasopharyngeal hemorrhage
Upon the emergence of nasopharyngeal hemorrhage, the upper airway patency was ensured. The blood and blood clots in the throat were timely removed with a bedside mucus suction device to prevent tracheal obstruction. Meanwhile, single- or dual-chamber balloons, which were inflated with 10–15 mL of air or water, were used to fill the nasopharynx to control the bleeding. If the bleeding continued, nasal packing with Vaseline gauze was performed to fill the anterior nares. In cases with a large amount of blood loss, intravenous access was established to meet the potential needs for intravenous fluids and blood transfusion.
Rescue and management approaches were chosen appropriately to the clinical conditions and the bleeding rate. Patients with a small amount or continuous bleeding and bleeding point identified by nasal endoscopy were treated in the emergency with endoscopic nasopharynx electrocoagulation under general anesthesia. Patients with an unidentifiable bleeding point, due to an urgent medical condition or limited diagnostic options, underwent selective digital subtraction angiography (DSA) to identify the bleeding vessel. Hemorrhage in the external carotid artery was controlled by selective vascular embolization using a stainless steel coil, while bleeding from the internal carotid artery was managed using a stent. Endotracheal intubation was performed in an emergency that the patients presented respiratory abnormality, which might be caused by insufficient blood clot removal under conditions of restricted jaw opening and poor throat emission. A tracheotomy was performed when endotracheal intubation was infeasible. After the airway patency was ensured and bleeding controlled by using a single or double-lumen balloon tamponade, the patients were sent to the interventional room for DSA to further clarify the bleeding site and bleeding artery, which were eventually treated with selective vascular embolization. Endoscopic nasopharynx electrocoagulation hemostasis was generally not recommended for patients with nasopharyngeal hemorrhage after radiotherapy for NPC.
The treatment was considered effective if the active bleeding decreased or stopped, and hemorrhage symptoms disappeared within 72 hours.
Results
Physical and clinical characteristics of the patients
A total of 17 patients with nasopharyngeal hemorrhage after radiation treatment of NPC were included in this study, and their clinical characteristics are summarized in Table 1. The majority of these patients had stage III or IV NPC, which was classified according to the seventh edition of American Joint Committee on Cancer TNM classification. In the 17 patients, 10 cases received 1 course of radiotherapy, 7 cases received two courses of radiotherapy, and all patients had not undergone surgical resection. Among them, 3 cases were treated by two-dimensional radiotherapy and 14 cases were treated by IMRT. The nasopharynx dose was 68–72 Gy, the cervical metastatic lymph node was 64–70 Gy, the high-risk area was 60–62 Gy, and the low-risk area was 54–56 Gy. 13 (76.5%) presented with extensive erosion and necrosis in the nasopharyngeal tissue, with necrotic tissue located mostly adjacent to the posterior wall. These patients also exhibited extensive skull base destruction and sinusitis according to computed tomography (CT) scan performed at admission (Table 2). Fourteen patients had varying degrees of difficulty in opening their mouths, with an average gap of 2.35 cm and a minimum gap of 0.5 cm. Nasal discharge accompanied with oral or nasal odor was noted in 14 cases about two weeks before the occurrence of nasopharyngeal hemorrhage. Ten patients (58.8%) complained of recurrent headaches, including six cases with occipital pain, 2 cases with frontal pain, and two cases with palatal pain. Also, 6 patients (35.3%) suffered repeated nasal bleeding before nasopharyngeal hemorrhage. Of the 17 patients, 15 (88.2%) had unilateral illness, whereas the other 2 had bilateral hemorrhage (Table 3). The cumulative total blood loss ranged between 300 and 4,000 mL. All the clinical data and results are summarized in Tables 4 and 5.
Table 1.
Physico-pathological characteristics of NPC patients
| Characteristic | Category | Patients (n) |
|---|---|---|
|
| ||
| Age (years) | Median | 62 |
| Range | 46–75 | |
| Bleeding time (months) | Median | 31 |
| Range | 6–96 | |
| Bleeding time (hours) | Median | 1.5 |
| Range | 1–3 | |
| Sex, n (%) | Male | 12 (70.6) |
| Female | 5 (29.4) | |
| Radiotherapy courses, n (%) | 1 | 10 (58.8) |
| 2 | 7 (41.2) | |
| Clinical stage, n (%) | II | 2 (11.8) |
| III | 5 (29.4) | |
| IV | 10 (58.8) | |
| Anemia, n (%) | <120 g/l | 12 (70.6) |
| <60 g/L | 3 (17.6) | |
| Mouth opening, n (%) | Limited | 14 (82.4) |
| Normal | 3 (17.6) | |
| Distant metastases, n (%) | Yes | 3 (17.6) |
| No | 14 (82.4) | |
Abbreviation: NPC, nasopharyngeal carcinoma.
Table 2.
Imaging of skull base bone destruction
| Number | Bone destruction in the sphenoid bone slope area | Bone destruction in the basal part of sphenoid bone | Bone destruction in the petrous part of temporal bone | Bone destruction of occipital bone |
|---|---|---|---|---|
|
| ||||
| 1 | Yes | No | No | No |
| 2 | Yes | No | No | No |
| 3 | Yes | Yes | No | No |
| 4 | No | No | Yes | Yes |
| 5 | Yes | No | No | No |
| 6 | No | No | No | Yes |
| 7 | No | Yes | No | No |
| 8 | No | No | Yes | Yes |
| 9 | No | No | No | No |
| 10 | No | Yes | No | No |
| 11 | Yes | No | No | No |
| 12 | No | No | Yes | No |
| 13 | Yes | No | No | No |
| 14 | No | Yes | No | No |
| 15 | No | No | Yes | No |
| 16 | Yes | No | No | No |
| 17 | No | No | Yes | No |
Table 3.
The features of 17 NPC patients with nasopharyngeal hemorrhage
| Number | Difficulty in opening mouth | Pre-bleeding symptoms
|
Hemoglobin level on hospitalization (g/L) | Nasal hemorrhage
|
|||
|---|---|---|---|---|---|---|---|
| Stench from mouth and nose | Recurrent headache | A few nosebleeds one week before | Unilateral | Bilateral | |||
|
| |||||||
| 1 | No | Yes | Yes | Yes | 83 | Yes | No |
| 2 | No | Yes | No | No | 125 | Yes | No |
| 3 | Yes | Yes | No | No | 105 | Yes | No |
| 4 | Yes | Yes | Yes | Yes | 74 | No | Yes |
| 5 | Yes | No | Yes | No | 112 | Yes | No |
| 6 | Yes | No | Yes | No | 43 | Yes | No |
| 7 | Yes | Yes | Yes | No | 133 | Yes | No |
| 8 | Yes | Yes | No | Yes | 89 | Yes | No |
| 9 | Yes | No | Yes | No | 97 | Yes | No |
| 10 | Yes | Yes | No | No | 101 | Yes | No |
| 11 | Yes | Yes | Yes | Yes | 79 | Yes | No |
| 12 | Yes | No | No | Yes | 56 | No | Yes |
| 13 | Yes | Yes | Yes | No | 67 | Yes | No |
| 14 | Yes | Yes | No | No | 110 | Yes | No |
| 15 | No | Yes | Yes | No | 104 | Yes | No |
| 16 | Yes | No | No | No | 112 | Yes | No |
| 17 | Yes | Yes | Yes | Yes | 51 | Yes | No |
Abbreviation: NPC, nasopharyngeal carcinoma.
Table 4.
Demographics and outcomes of 17 NPC patients with nasopharyngeal hemorrhage
| Number | Sex | Age (years) | Nasopharyngeal features under endoscope | The time of massive hemorrhage after radiotherapy (years) | Clinical stage | RNPC for two-stage radiotherapy | Therapy | Affected artery | Survival |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| 1 | F | 46 | OR | 0.5 | IVa | Yes | NP + EE | SA | Alive |
| 2 | M | 49 | OR | 2 | III | Yes | NP | BOECA | Alive |
| 3 | M | 63 | OR | 3 | II | No | NP | ROICA | Alive |
| 4 | M | 75 | OR | 0.75 | IVa | Yes | NP | undefined | DLBS |
| 5 | F | 58 | MN | 3 | IVa | Yes | NP | BOECA | Alive |
| 6 | M | 59 | OR | 1 | IVa | Yes | NP + TR | undefined | DLB |
| 7 | F | 66 | No | 8 | III | No | NP | POICA | Alive |
| 8 | M | 57 | OR | 4 | III | No | NP + TR | undefined | DLB |
| 9 | M | 64 | No | 3 | III | No | NP | BOECA | Alive |
| 10 | M | 52 | OR | 6 | IVa | No | NP + EE | PNSA | Alive |
| 11 | M | 65 | MN | 2 | III | No | NP | BOECA | Alive |
| 12 | M | 69 | OR | 4 | Iva | No | NP | POICA | MODS |
| 13 | F | 62 | No | 2 | IVa | No | NP | BOECA | Alive |
| 14 | M | 73 | OR | 2 | IVa | No | NP + EE | SA | Alive |
| 15 | M | 56 | MN | 3 | II | No | NP | BOECA | Alive |
| 16 | M | 74 | No | 0.5 | IVa | Yes | NP | ROICA | Alive |
| 17 | F | 61 | OR | 1 | IVa | Yes | NP | BOECA | Alive |
Abbreviations: DLB, died of loss of blood; DLBS, died of loss of blood and suffocation; IMA, internal maxillary artery; NP, nasal packing; NPC, nasopharyngeal carcinoma; TR, tracheostomy; APS, the arteriae pharyngea ascendens of the external carotid artery; EE, endoscopic electrocoagulation; POICA, pseudoaneurysm of internal carotid artery; OR, osteoradionecrosis; MN, member necrosis; RNPC, recurrent nasopharyngeal carcinoma; SA, sphenopalatine artery; DSA, digital subtraction angiography; PNSA, posterior nasal septum artery; BOECA, branch of external carotid artery; ROICA, rupture of internal carotid artery; MODS, multiple organ dysfunction.
Table 5.
Clinical characteristics of 10 NPC patients with just 1 course of RT
| Number | Sex | Age (years) | Stage | Skull base erosion at diagnosis | GTV volume of the primary cancer (cm3) | RT dose | GTV Vol >105% (cm3) | Chemotherapy manner | Cycles of chemotherapy | Trismus before the incidence of bleeding | Hypertension | Diabetic | Use of TCM | Nasopharynx irrigation | Use of brachytherapy | Surgical intervention |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||||||||
| 1 | M | 63 | II | No | 50.6 | 70 Gy/30F | 20.1 | None | None | Yes | Yes | No | No | None | No | No |
| 2 | F | 66 | III | Yes | 88.9 | 70 Gy/32F | 29.8 | C+A | 4 | Yes | No | Yes | No | KDI | No | No |
| 3 | M | 57 | III | Yes | 135.6 | 70 Gy/32F | 91.5 | C+A | 4 | Yes | No | No | No | None | No | No |
| 4 | M | 64 | III | No | 87.4 | 70 Gy/31F | 72.5 | Cc | 2 | Yes | No | Yes | No | KDI | No | No |
| 5 | M | 52 | IVa | Yes | 107.9 | 70 Gy/32F | 67.2 | C+A | 5 | Yes | Yes | No | No | ODI | No | No |
| 6 | M | 65 | III | Yes | 62.7 | 70 Gy/31F | 28.1 | C+A | 4 | Yes | Yes | No | No | ODI | No | No |
| 7 | M | 69 | IVa | Yes | 165.8 | 70 Gy/35F | 94.2 | C+A | 6 | Yes | No | Yes | No | None | No | No |
| 8 | F | 62 | IVa | Yes | 145.6 | 70 Gy/32F | 99.2 | C+A | 6 | Yes | No | No | No | ODI | No | No |
| 9 | M | 73 | IVa | Yes | 107.2 | 70 Gy/32F | 71.1 | C+A | 6 | Yes | Yes | Yes | No | None | No | No |
| 10 | M | 56 | II | No | 49.2 | 69 Gy/30F | 39.6 | None | None | No | No | No | No | None | No | No |
Notes: Concurrent chemotherapy program consisted of cisplatin; the adjuvant chemotherapy program consisted of cisplatin and 5-fluorouracil.
Abbreviations: GTV, gross tumor volume; KDI, keep doing irrigation; NPC, nasopharyngeal carcinoma; ODI, occasionally doing irrigation; RT, radiotherapy; TCM, traditional Chinese medicine; C+A, concurrent + adjuvant; Cc, concurrent chemotherapy.
Origin and treatment of the nasopharyngeal hemorrhage
Among the 17 cases, sphenopalatine artery bleeding was found in two patients, and posterior nasal septal artery bleeding was identified in one patient. These three patients were treated by endoscopic nasopharyngeal electrocoagulation and hemostasis.
Eleven patients were treated by digital subtraction arterial angiography embolization. These included seven patients with bleeding sites on the external carotid artery branches, such as intra-maxillary arteries, facial arteries, and ascending pharyngeal artery (Figures 1–4), 2 patients with vascular hemorrhage caused by internal carotid artery stenosis, and two patients with hemorrhage resulting from the rupture of a pseudoaneurysm in the internal carotid artery (Figures 5 and 6). The bleeding sites were identified by the bilateral external carotid artery and internal carotid artery angiography in which the contrast-enhanced Seldinger technique was used to percutaneously puncture the right femoral artery with a 4F or 5F Cobra catheter or a multipurpose catheter. Embolization approaches were chosen based on the results of angiography. External carotid artery branches were embolized with a steel coil alone, a steel coil plus gelatin sponge, or a steel coil together with polyvinyl alcohol particles. Internal carotid artery embolization was achieved using a removable balloon with a closed-cell stent. Eight (72.7%) of these 11 patients underwent single intervention, 2 patients received a double intervention and one required triple intervention. After interventional treatment, 81.8% (9/11) of patients had bilateral maxillofacial pain, pharyngeal numbness, and difficulties in opening the mouth. The symptoms improved significantly after hormone-assisted treatment for half a month and had entirely resolved after one month.
Figure 1.
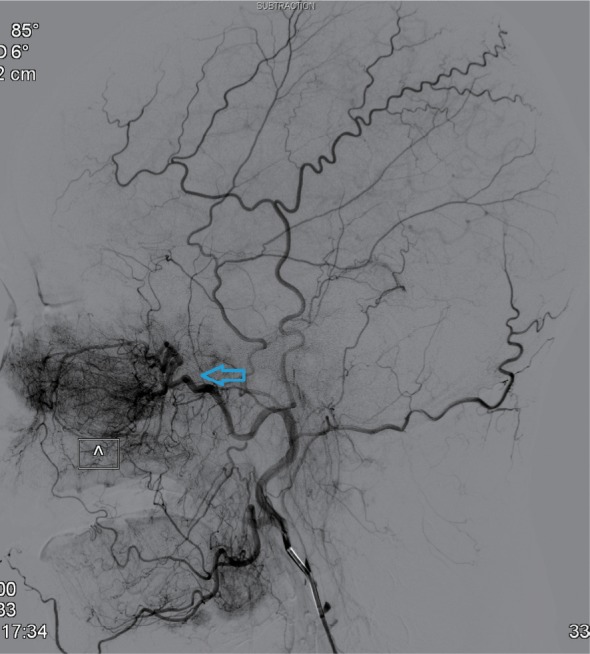
External carotid artery angiogram showing the bleeding sites on the internal maxillary artery (indicated by the arrow).
Figure 2.
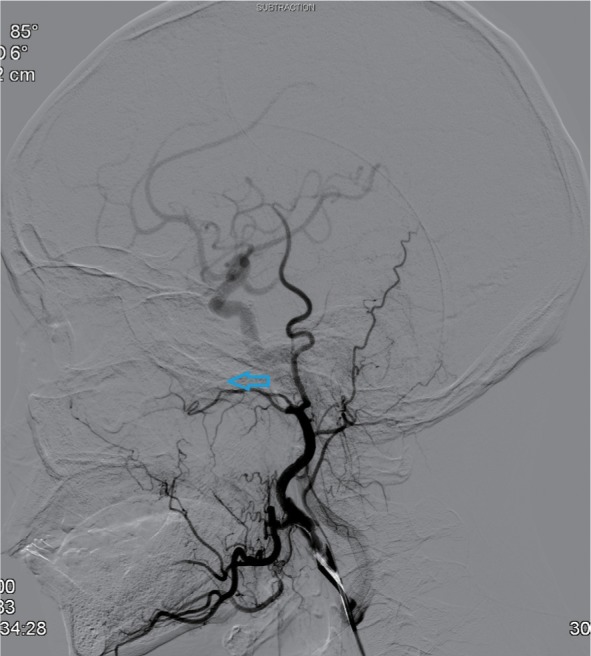
Post-embolization internal maxillary artery angiograms revealing total occlusion of the artery (indicated by the arrow).
Figure 3.
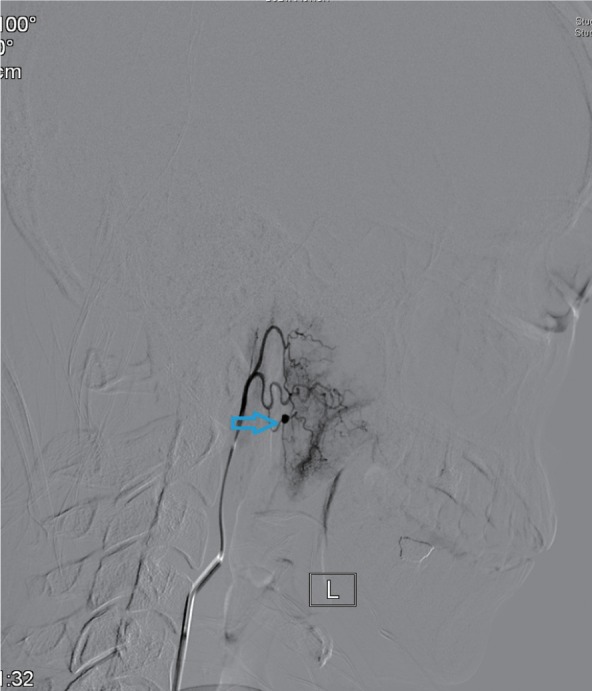
External carotid artery branch angiogram showing the bleeding sites on ascending pharyngeal artery (indicated by the arrow).
Note: L= blood vessel on the left.
Figure 4.
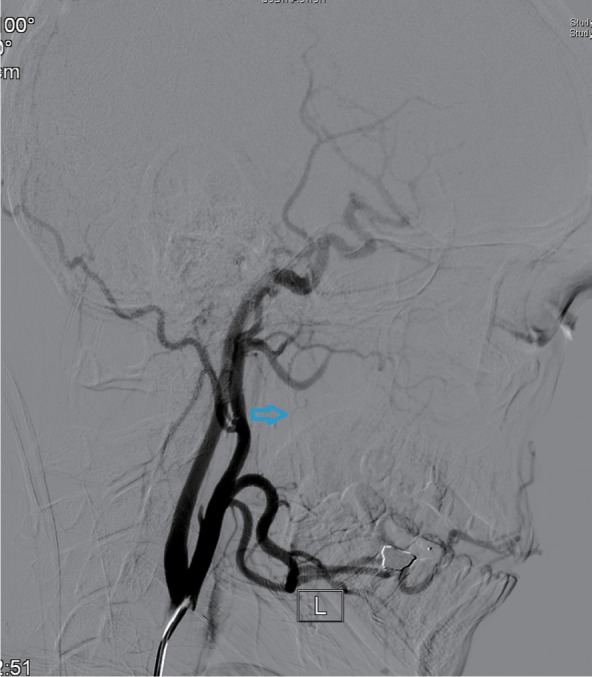
Post-embolization ascending pharyngeal artery angiogram revealing total occlusion of the artery (indicated by the arrow).
Note: L= blood vessel on the left.
Figure 5.
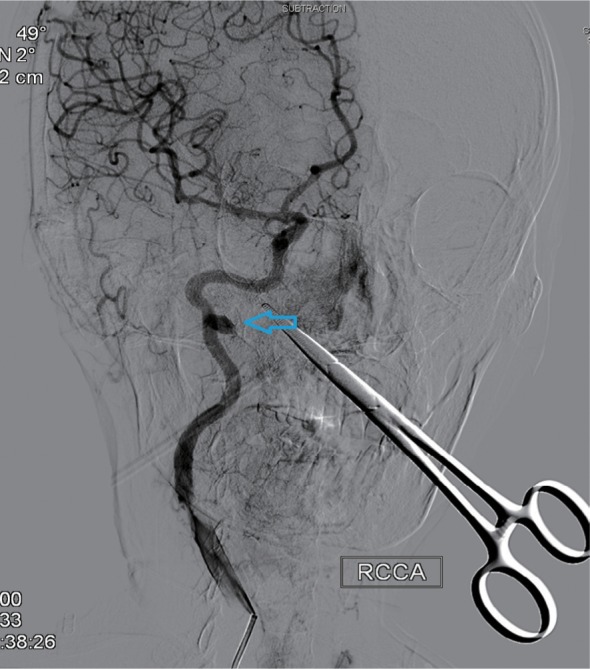
Right lateral internal carotid artery angiogram showing radiation arteritis with pseudoaneurysm (indicated by the arrow).
Note: RCCA=right common carotid artery.
Figure 6.
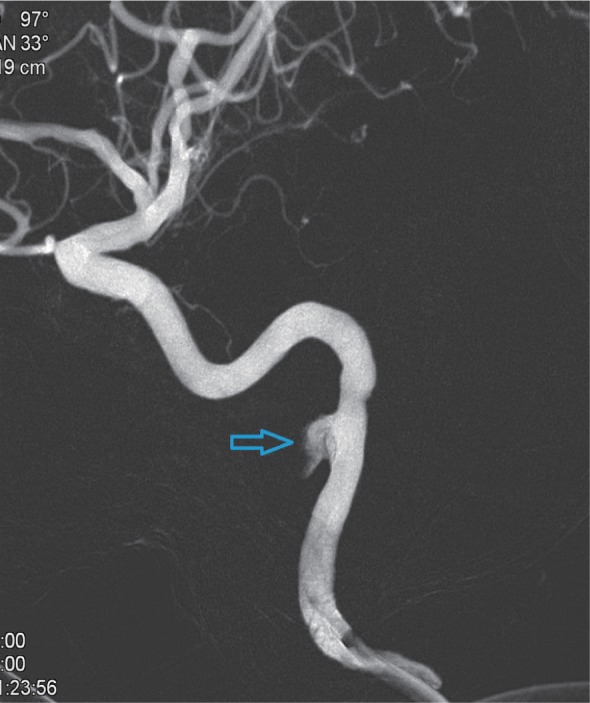
Vascular imaging of the pseudoaneurysm after 3D reconstruction (indicated by the arrow).
Patient outcomes
Thirteen of the 17 patients were successfully rescued, whereas four died. Six months of follow-up in the 13 surviving patients showed no recurrence of hemorrhage. Of the four patients who died, 2 patients died of hemorrhagic shock, and 1 died of suffocation resulting from the blocking of the airway by blood clots. The fourth patient had blood clots blocking the airway after the hemorrhage but was saved by an emergency tracheotomy. The patient was then sent to the interventional theater for emergency digital subtraction angiography embolization and transferred to the intensive care unit for postoperative treatment. The patient subsequently died due to systemic multiple organ failure. The clinical stages of all four deaths were relatively late, with one in stage III and the other three in stage IV.
Discussion
While radical radiotherapy represents the primary and most effective approach to treat NPC, the adverse effect of radiation on the adjacent healthy tissues remains to be resolved. Nasopharyngeal bleeding can be caused by tumor ulceration, necrosis, shedding, or mucosal hyperemia during radiotherapy.8 In the present study, 17 cases of nasopharyngeal hemorrhage after radiation treatment for NPC were collectively reviewed, and the rescue and management strategies were discussed.
Multiple physico-pathological factors contribute to nasopharyngeal hemorrhage. Patients of older age, with advanced disease, or who receive higher irradiation doses are more likely to develop nasopharyngeal bleeding after radiotherapy.9 Among the 17 patients recruited in this study, only two had stage II NPC, whereas 5 and 10 patients had stage III and IV disease, respectively. Nasopharyngeal hemorrhage is also associated with repeated radiotherapy, which is the major treatment for recurrent NPC.10 Among the complications of radiotherapy for NPC, nasopharyngeal hemorrhage was reported to account for 77.8%.11 Spreading more extensively in the skull base than the primary tumor, recurrent NPC requires an increased irradiation area and dose, which cause more damage to the surrounding tissues and increase the chance of nasopharyngeal hemorrhage. Consistently, in this study, seven patients suffered from recurrent NPC and underwent a second course of radiotherapy. In addition, fibrosis of the nasopharyngeal-cranial base tissue after radiotherapy increases the brittleness of blood vessels and hampers tissue repair, triggering long-term ulceration and necrosis.12 Patients with NPC may have nasal and nasopharyngeal mucosal atrophy after radiotherapy, resulting in reduced mucus production and dryness of the mucous membranes. Furthermore, radiotherapy destroys the motor function of the nasal and nasopharyngeal cilia and weakens the self-cleaning of the nasal cavity and nasopharynx. This increases the occurrence of radiotherapy-induced rhinosinusitis and retention of the nasopharyngeal secretions, leading to infections and inflammation, which are associated with damage to the nasopharynx.13 Extensive tissue necrosis and erosion of the skull base bone result in the exposure and loss of major blood vessels in the nasopharynx, thereby causing potentially fatal hemorrhage. Moreover, the blood vessels may not be effectively repaired and occluded after the removal, by radiotherapy, of NPC cells that invade the blood vessels, causing spontaneous rupture and heavy bleeding.14
A shorter incubation period has been reported previously in patients of older age, with advanced disease, or repetitive irradiation.15 Among the 17 patients included in this study, nasopharyngeal hemorrhage occurred between 6 months and 8 years after radiotherapy, suggesting that NPC patients treated by radiotherapy need to remain alert to nasal bleeding over a long period. This is of importance despite advances in radiotherapy technologies that have reduced the local residual rate of NPC and the complications of radiotherapy and improved the efficacy of radiotherapy.16 Stereotactic radiosurgery and adaptive IMRT have been reported to increase the chance of nasopharyngeal hemorrhage, while both reduce the local residual rate of NPC and the occurrence of complications.17
The amount of hemorrhage after nasopharyngeal radiotherapy ranges from dozens to thousands of milliliters, and massive hemorrhage can be lethal within a short period. The likelihood of post-radiotherapy nasopharyngeal hemorrhage in patients with NPC can be assessed by the rate and frequency of symptomatic bleeding and the CT imaging results, which indicate the extent of damage to the nasopharynx and skull base. Patients with frequent or increased symptomatic nasal or oral bleeding, or CT images showing nasopharynx–skull base damage involving the adjacent blood vessels, require close observation and easy accessibility to rescue equipment. In the management of nasopharyngeal hemorrhage, maintaining upper airway patency is critical. Mucus suction, endotracheal intubation, and tracheotomy are approaches of choice depending on the upper airway patency. Once the bleeding sites are identified, anterior and posterior nostril packing, endoscopic nasopharynx electrocoagulation, and digital subtraction angiography embolization can be performed to stop the nasopharyngeal bleeding.
In summary, post-radiotherapy nasopharyngeal hemorrhage is often caused by erosion of the internal carotid artery and maxillary artery. Anterior and posterior nostril packing, endoscopic nasopharynx electrocoagulation, and digital subtraction angiography embolization are effective approaches for managing nasopharyngeal hemorrhage.
Acknowledgments
This study was supported by the Natural Science Foundation of Hainan Province, China (No. 818MS130). The authors thank all the patients and the research staff for their contributions to this project.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Zhang S, Zhou L, Huang X, Lin S. A retrospective study of concurrent chemoradiotherapy plus S-1 adjuvant chemotherapy on curative effect for treatment of patients with N3 stage nasopharyngeal carcinoma. Cancer Manag Res. 2018;10:1705–1711. doi: 10.2147/CMAR.S165804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang S, Lin S, Hu L. Lobaplatin combined with docetaxel neo-adjuvant chemotherapy followed by concurrent lobaplatin with intensity-modulated radiotherapy increases the survival of patients with high-risk lymph node positive nasopharyngeal carcinoma. J BUON. 2016;21(1):161–167. [PubMed] [Google Scholar]
- 3.Kane MF, Loda M, Gaida GM, et al. Methylation of the hMLH1 promoter correlates with lack of expression of hMLH1 in sporadic colon tumors and mismatch repair-defective human tumor cell lines. Cancer Res. 1997;57(5):808. [PubMed] [Google Scholar]
- 4.Cheng KM, Chan CM, Cheung YL, et al. Endovascular treatment of radiation-induced petrous internal carotid artery aneurysm presenting with acute haemorrhage. A report of two cases. Acta Neurochir. 2001;143(4):351–356. doi: 10.1007/s007010170089. [DOI] [PubMed] [Google Scholar]
- 5.Cheng K-Y, Lee K-W, Chiang F-Y, Ho K-Y, Kuo W-R. Rupture of radiation-induced internal carotid artery pseudoaneurysm in a patient with nasopharyngeal carcinoma--spontaneous occlusion of carotid artery due to long-term embolizing performance. Head Neck. 2008;30(8):1132–1135. doi: 10.1002/hed.20753. [DOI] [PubMed] [Google Scholar]
- 6.Chen H-Y, Ma X-M, Bai Y-R. Repeated massive epistaxis after re-irradiation in recurrent nasopharyngeal carcinoma. Contemp Oncol. 2014;18(5):371–376. doi: 10.5114/wo.2014.45290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Agulnik M, Epstein JB. Nasopharyngeal carcinoma: current management, future directions and dental implications. Oral Oncol. 2008;44(7):617–627. doi: 10.1016/j.oraloncology.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Mok JS, Marshall JN, Chan M, van Hasselt CA. Percutaneous embolization to control intractable epistaxis in nasopharyngeal carcinoma. Head Neck. 21(3):211–216. doi: 10.1002/(sici)1097-0347(199905)21:3<211::aid-hed5>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 9.Wang CC. Re-irradiation of recurrent nasopharyngeal carcinoma–treatment techniques and results. Int J Radiat Oncol Biol Phys. 1987;13(7):953–956. doi: 10.1016/0360-3016(87)90030-7. [DOI] [PubMed] [Google Scholar]
- 10.Han F, Zhao C, Huang S-M, et al. Long-term outcomes and prognostic factors of re-irradiation for locally recurrent nasopharyngeal carcinoma using intensity-modulated radiotherapy. Clin Oncol. 2012;24(8):569–576. doi: 10.1016/j.clon.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Tokuuye K, Akine Y, Sumi M, et al. Reirradiation of brain and skull base tumors with fractionated stereotactic radiotherapy. Int J Radiat Oncol Biol Phys. 1998;40(5):1151–1155. doi: 10.1016/s0360-3016(97)00954-1. [DOI] [PubMed] [Google Scholar]
- 12.Kodani N, Yamazaki H, Tsubokura T, et al. Stereotactic body radiation therapy for head and neck tumor: disease control and morbidity outcomes. J Radiat Res. 2011;52(1):24–31. doi: 10.1269/jrr.10086. [DOI] [PubMed] [Google Scholar]
- 13.Qiu S, Lu J, Zheng W, et al. Advantages of intensity modulated radiotherapy in recurrent T1-2 nasopharyngeal carcinoma: a retrospective study. BMC Cancer. 2014;14(1):797. doi: 10.1186/1471-2407-14-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wong GKC, Chan KK, Yu SCH, Tsang RKY, Poon WS. Treatment of profuse epistaxis in patients irradiated for nasopharyngeal carcinoma. ANZ J Surg. 2007;77(4):270–274. doi: 10.1111/j.1445-2197.2007.04032.x. [DOI] [PubMed] [Google Scholar]
- 15.Elden L, Montanera W, Terbrugge K, Willinsky R, Lasjaunias P, Charles D. Angiographic embolization for the treatment of epistaxis: a review of 108 cases. Otolaryngol Head Neck Surg. 1994;111(1):44–50. doi: 10.1177/019459989411100110. [DOI] [PubMed] [Google Scholar]
- 16.Low YM, Goh YH. Endovascular treatment of epistaxis in patients irradiated for nasopharyngeal carcinoma. Clin Otolaryngol Allied Sci. 2003;28(3):244–247. doi: 10.1046/j.1365-2273.2003.00699.x. [DOI] [PubMed] [Google Scholar]
- 17.Dhanachai M, Kraiphibul P, Dangprasert S, et al. Fractionated stereo-tactic radiotherapy in residual or recurrent nasopharyngeal carcinoma. Acta Oncol. 2007;46(6):828–833. doi: 10.1080/02841860601103050. [DOI] [PubMed] [Google Scholar]


