Abstract
Chronic cerebral circulation insufficiency (CCCI) is viewed as an alarming state induced by long-term reduction in cerebral perfusion, which is associated with neurological deficits and high risk of stroke occurrence or recurrence. CCCI accounts for a large proportion of both outpatients and inpatients with cerebrovascular diseases, while management of CCCI remains a formidable challenge to clinicians. Normobaric oxygen (NBO) is an adjuvant hyperoxygenation intervention supplied with one atmosphere pressure (1 ATA =101.325 kPa). A plethora of studies have demonstrated the efficacy of NBO on the penumbra in acute stroke. NBO has been shown to increase the oxygen pressure, raise the intracranial blood flow, protect blood–brain barrier and enhance neuroprotective effects. As similar underlying mechanisms are shared by the penumbra in stroke and the ischemic–hypoxic brain tissues in CCCI, we speculate that NBO may serve as a promising therapeutic strategy for attenuating short-term symptoms or improving long-term clinical outcomes among patients with CCCI. Due to the scant research exploring the efficacy and safety of NBO for treating CCCI so far, both experimental and clinical studies are warranted to verify our hypothesis in the future.
Keywords: chronic cerebral circulation insufficiency, normobaric oxygen, therapeutic strategy, stroke, clinical outcome
Introduction
Chronic cerebral circulation insufficiency (CCCI), which was first proposed by Japanese scholars in 1990s, is considered as a pathological status induced by persistent reduction of cerebral blood volume and flow (CBV and CBF, respectively), leading to ischemia and hypoxia in the brain tissue.1 Long-time ischemic–hypoxic injury can cause various atypical brain dysfunctions such as headache, dizziness, cognitive decline and emotional abnormalities.2–6 Ischemic events, mainly referring to ischemic stroke or transient ischemic attack, are the most severe complications of CCCI. Under the low-perfusion background, the brain tissue is more vulnerable to ischemic–hypoxic insult; thus, the incidence of ischemic events among individuals with CCCI is substantially higher than in those without CCCI.2,3,5 It has been reported that intracranial atherosclerotic stenosis (ICAS) is the most common pathogenesis of CCCI, particularly in China, with the hypoperfusion being a vital mechanism accounting for these clinical presentations.1,5
Normobaric oxygen (NBO) is a routine adjuvant oxygenation intervention supplied by nasal cannula or facemask (such as Venturi mask) with one atmosphere pressure (1 ATA =101.325 kPa).7 Evidence available shows that NBO may be a safe, convenient and promising therapeutic strategy for multiorgan protection, which has garnered increasing attention of researchers over the past years.8 However, some studies have failed to support the favorable efficacy of NBO. For instance, a large meta-analysis conducted by Chu et al revealed that in acutely ill adult patients, oxygen supplementation might increase the mortality risk without improving patient-important outcomes.9 The negative outcomes may be secondary to acute critical conditions and some serious complications such as infection, arrhythmia and dyspnea. In contrast to previous experimental data confirming the neuroprotection afforded by NBO on acute stroke, a recent multicenter, randomized clinical trial concluded that this oxygen supplement does not reduce the rate of death or disability.10 The incongruent conclusions between clinical and animal studies may be attributable to the protective mechanisms of NBO behind cerebral ischemia: the protective effect afforded by NBO is based on freezing ischemic penumbra and extending the time window for reperfusion, meaning that NBO may be not applicable for patients with permanent vessel occlusion.11,12 Animal research has corroborated that NBO can reduce the infarct size and improve post-stroke outcomes after thrombolysis in ischemic stroke, and a large multicenter, randomized, prospective trial is ongoing.13,14
Theoretically, low cerebral blood perfusion in CCCI patients exposes the brain tissue to an ischemic–hypoxic condition, which is similar to that in the ischemic penumbra in stroke.1 Therefore, given the prominent effectiveness in the ischemic penumbra, NBO, which can supply abundant oxygen, may yield extra benefits to the ischemic–hypoxic brain tissue in CCCI patients. As there is a lack of studies designed to identify the efficacy and safety of NBO on CCCI, we proposed to conduct relevant animal experiments and clinical trials to validate the hypothesis.
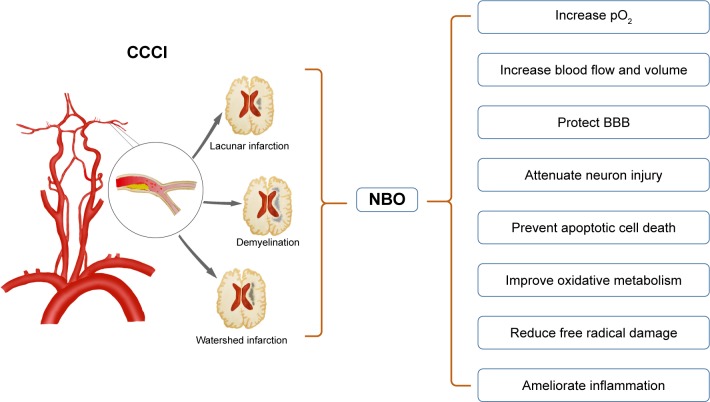
Theory of the hypothesis
Our hypothesis is that NBO can enhance the oxygen content in the ischemic–hypoxic brain tissue in CCCI patients, and subsequently improve both short-term symptoms and long-term clinical outcomes. The rationale of the hypothesis is inferred by several convincing theories presented below and in Figure 1.
Figure 1.
The potential protective mechanisms of NBO on CCCI.
Notes: CCCI can induce lacunar infarction, demyelination and watershed infarction. After the use of NBO, these lesions may be improved significantly. Although the protective mechanisms presented in the figure are derived from NBO in acute stroke, the penumbra in this setting is similar to the alarming condition in CCCI. Given that, we assume that CCCI patients may benefit from NBO intervention as well.
Abbreviations: BBB, blood–brain barrier; CCCI, chronic cerebral circulation insufficiency; NBO, normobaric oxygen.
NBO is capable of increasing the arterial partial pressure of oxygen (pO2) and raising the dissolved oxygen fraction in the aorta and the smallest arterioles.15 In this regard, Liu et al reported that after NBO treatment, the penumbral interstitial pO2 could be maintained close to preischemic normal value.16 Although the cerebral capillaries are expected to be disrupted in the penumbra, it has been verified that there is still a reduced blood supply to the region.17 This can explain why the tissue pO2 levels are restored upon reperfusion.16 Therefore, the oxygen supplied by NBO can be delivered to the penumbra region. Given the fact that both the penumbra in acute stroke and abnormal brain tissues in CCCI are caused by ischemia and hypoxia, NBO may be conceived as an effective adjuvant therapy for CCCI as well.
NBO can increase CBF/CBV in the penumbra in acute stroke.18 During NBO treatment, vasodilation occurs in the penumbra without any changes in the infarct core, while vasoconstriction occurs in the nonischemic regions.19 Unlike acute stroke, the ischemic–hypoxic tissues in CCCI are based on arterial stenosis instead of occlusion, whereby the ischemic–hypoxic tissues would not die as quickly as that in stroke, thus providing NBO enough time to elevate the pO2 and blood flow. The raised pO2 and increased blood flow in the penumbra are involved in downregulating zinc levels, which may be attributed to the neuroprotective effect afforded by NBO.20 Improving perfusion in the ischemic–hypoxic brain tissue gives relief from associated clinical symptoms.
NBO is able to attenuate blood–brain barrier (BBB) disruption in cerebral ischemia, possibly through inhibiting MMP-9-mediated degradation of tight junction proteins.13 The integrity of BBB is influenced by various pathological processes, such as invasion of inflammatory mediators, edema formation and hemorrhagic transformation. Similarly, BBB protection provided by NBO may also be available to CCCI patients, so that the impaired brain function may be at least partially restored.
Other underlying neuroprotective mechanisms, including reducing peri-infarct depolarization, improving aerobic metabolism, preventing apoptotic cell death and ameliorating inflammation, can offer extra benefits to patients with cerebral ischemia.21–24 On the other hand, NBO is safe enough, as it does not augment the formation of ROS, nitrogen species and some other mediators implicated in the exacerbation of oxidative stress injury.25
Currently, there are very few reports in literature regarding the application of NBO in CCCI, and this is undoubtedly a brand new field that deserves more attention. Unlike the beneficial effects observed in experimental stroke models, most of the clinical trials failed to reach favorable results.8 As we discussed above, the ischemic penumbra is a vital target for NBO and a relatively low rate of revascularization is responsible for poor outcomes in acute stroke patients.8 CCCI refers to a state of long-term reduction in cerebral perfusion secondary to ICAS or other pathogenesis. The afflicted brain tissues in CCCI are in ischemic-hypoxic conditions, which is similar to the penumbra in acute stroke.1 This phenomenon suggests that supplying enough oxygen may hold the potential of enhancing the resistance of brain tissues to hypoxic insults, slowing down the deterioration and preventing secondary ischemic stroke in CCCI patients. Meanwhile, rapid enhancement of oxygen content enables immediate improvement in ischemic–hypoxic conditions, allowing to get relief from clinical symptoms in a short period of time. Nagatomo et al indicated that exposure to oxygen concentrations higher than 40% for 24 hours could induce excessive levels of oxidative stress in rats.26 However, Liu et al found that 95% NBO for 90 minutes reduced production of ROS, MMP-9 and caspase-8.18 Permanent hyperoxia may lead to harmful or even detrimental consequences, such as ROS formation, perfusion reduction and severe pulmonary congestion; thus, intermittent hyperoxia is recommended.27
Evaluation of the hypothesis
During the CCCI process, a plethora of remarkable pathological changes, such as white matter lesion (WML) formation, neuronal degeneration, cortical atrophy, overexpression of inflammatory factors and glial cell proliferation, may be associated with neurological deficits, which in turn contribute to the relevant clinical presentations.1 As there is a lack of studies exploring the efficacy and safety of NBO for treating CCCI, both experimental and clinical studies are warranted to test our hypothesis in the future.
Animal studies
The CCCI model used in our study will adopt adult male Sprague Dawley rats with the two-vessel occlusion (2VO) as described previously, and the sham-operated model will receive similar surgery but without vessel occlusion.28,29 These rats will be randomly assigned to three groups: sham-operated group, 2VO group and 2VO + NBO group, and they will be maintained on a 12-hour light/dark cycle with unlimited access to food and water. The specific intervention (NBO or room air) will be administered 3 days after surgery.30 Rats in the 2VO + NBO group will be placed in an environmental chamber and exposed to a normobaric hyperoxic atmosphere (90% oxygen, 1 ATA) intermittently (4 hours/day for 28 consecutive days). Similarly, rats in the sham-operated group and 2VO group will be placed in the environmental chamber, but exposed to room air (21% oxygen, 1 ATA) for the same period of time. The main measurements include spatial learning memory (evaluated by the Morris water maze), the level of ROS, BBB disruption (evaluated by MMP-9 and occludin), apoptosis (evaluated by caspase-8 cleavage) and so on. In order to further assess the dose–response effect of NBO on CCCI, the efficacy and safety of different concentrations and treatment durations in NBO will be conducted as well and the dose–response curves will be depicted. All animal-use procedures have been examined by the Institutional Ethics Committee (Xuanwu Hospital, Capital Medical University) in accordance with the Guide for the Care and Use of Laboratory Animals (China).
Clinical trials
Patients who are diagnosed with ICAS-induced CCCI will be recruited in this study. The involved patients are screened with the following inclusion and exclusion criteria.
Inclusion criteria: 1) age ranging from 45 to 80 years; 2) identified ICAS of anterior circulation (including the internal carotid artery and middle cerebral artery, with ≥50% stenosis); 3) presence of chronic cerebral hypoperfusion symptoms such as headache, vertigo, insomnia and paresthesia; 4) the duration between enrollment and prior ischemic events longer than 2 months and 5) modified Rankin scale (mRS) ≤4 and ABCD2 ≥4 on admission.
Patients will be excluded if they meet any of the following criteria: 1) presence of severe life-threatening diseases, 2) pregnancy, 3) severe respiratory diseases, 4) intracranial malignancy, 5) hematological and blood coagulation disturbances, 6) intracranial hemorrhage within 3 months and 7) no informed consent.
Patients enrolled will be randomly divided into two groups: NBO group, in which the patients will receive NBO treatment (5–8 L/min, via nasal cannula or face mask) 1 hour per time, three times per day for 6 months, and other routine medical or surgical therapies; and the control group, in which patients will undergo similar routine therapy without NBO for the same period of time. Primary outcomes include change in WMLs and the incidence of ischemic events (including symptomatic ischemic stroke and transient ischemic attack) at 1 year. Therefore, the overall 5% type I error is divided into two parts, that is, a significance level of 4% will be adopted for the analysis of WMLs and a significance level of 1% will be adopted for the analysis of the incidence of ischemic events. Thus, the minimum number of subjects in this clinical trial is 30 in each group. Secondary outcomes include the overall treatment response (evaluated by scores of global impression of change), headache severity (evaluated by headache visual analog scale), vertigo severity (evaluated by European evaluation of vertigo scale), relevant cognitive behavior change (evaluated by Mini-Mental State Examination and Montreal Cognitive Assessment), disability (evaluated by mRS) and activities of daily living (evaluated by Barthel index) at 1, 3 and 6 months. NBO-related adverse events might involve respiratory infection, abnormal blood pressure or heart rate fluctuation and nasal or oral mucosal injury. Any potential adverse events should be recorded during the whole follow-up process.
The study protocol has been registered on Clinical Trial Registration (NCT03373292). The trial was performed in accordance with the guidelines of the 1964 Declaration of Helsinki and approved by the Institutional Ethics Committee (Xuanwu Hospital, Capital Medical University), and written informed consent was obtained from all patients before initiating any study-specific procedures.
Implications of the hypothesis
In real clinical practice, there are a large number of patients suffering from CCCI and current therapeutic strategies are far from satisfactory.1,5 Conservative therapies mainly involve anti-platelets, lipid-lowering agents and neuroprotective agents, but their effectiveness is still uncertain. The efficacy of endovascular treatment, such as endarterectomy and intravascular stenting, is still controversial and should not be considered superior to conservative treatment.1,5 Moreover, endovascular treatment may be not suitable for all patients with CCCI. Recently, remote ischemic conditioning (RIC) has emerged as an innovative and promising adjunctive approach for multiorgan protection.31–33 It has been demonstrated that daily RIC can reduce the rate of stroke recurrence and improve the long-term clinical outcomes in patients with ICAS.31,32 Nonetheless, it is reasonable to expect that RIC requires a longer time to show its effect, and therefore, patients cannot be relieved of their symptoms within a short period of time following initiation of treatment. Meanwhile, there is still a portion of patients who may not benefit from or be contraindicated to RIC. According to available evidence and our hypothesis, oxygen administration is able to enhance the oxygen content of ischemic regions, increase cerebral perfusion and prevent the brain tissues from secondary injury, all of which could help relieve CCCI-induced symptoms in a short period of time and improve the long-term clinical outcomes profoundly. Taken together, oxygen therapy may serve as a promising adjunctive alternative to current treatment strategies.
Accumulating evidence indicates that over-oxygenation during hyperbaric oxygen (HBO) therapy may result in oxidative stress and free radical damage.34,35 Oxidative stress can activate MMPs and caspases, which further promote BBB damage and neuronal demise.36,37 Moreover, the implementation requirements and adverse effects on the lung largely limit the use of HBO.38 In contrast, NBO raises the interstitial pO2 levels to a proper range and the ensuing mild oxygen radical accumulation can stimulate the activation of antioxidant enzymes such as superoxide dismutase, glutathione peroxidase, catalase and glutathione reductase, followed by reduction in ROS production and MMP levels.39,40 Given this, NBO may confer greater neuroprotection to CCCI patients than HBO.
Conclusion
As NBO may profoundly improve both short-term symptoms and long-term clinical outcomes in CCCI patients, it should be deemed as a brand new effective and convenient adjuvant treatment strategy if our hypothesis is validated. Well-designed animal experiments and clinical trials are urgently warranted in the next step to corroborate the effectiveness of NBO on brain protection in patients with CCCI.
Acknowledgments
We would like to thank all patients and doctors who participated in this study for their cooperation. This study was sponsored by the National Key R&D Program of China (2017YFC1308401), the National Natural Science Foundation of China (81371289), and the Project of Beijing Municipal Top Talent for Healthy Work of China (2014-2-015).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Zhou D, Meng R, Li SJ, et al. Advances in chronic cerebral circulation insufficiency. CNS Neurosci Ther. 2018;24(1):5–17. doi: 10.1111/cns.12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hirai S. MRI in patients with cerebral infarct and chronic cerebral circulation insufficiency. No To Shinkei. 1991;43(9):811–816. Japanese. [PubMed] [Google Scholar]
- 3.Wu C, Liao L, Yan X, et al. Effects of Yangxue Qingnao Granules on chronic cerebral circulation insufficiency: a randomized, double-blind, double-dummy, controlled multicentre trial. Psychogeriatrics. 2013;13(1):29–34. doi: 10.1111/j.1479-8301.2012.00423.x. [DOI] [PubMed] [Google Scholar]
- 4.Safouris A, Hambye AS, Sculier C, et al. Chronic brain hypoperfusion due to multi-vessel extracranial atherosclerotic disease: a potentially reversible cause of cognitive impairment. J Alzheimers Dis. 2015;43(1):23–27. doi: 10.3233/JAD-141203. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Zhao X, Liu L, et al. Prevalence and outcomes of symptomatic intracranial large artery stenoses and occlusions in China: the Chinese Intracranial Atherosclerosis (CICAS) study. Stroke. 2014;45(3):663–669. doi: 10.1161/STROKEAHA.113.003508. [DOI] [PubMed] [Google Scholar]
- 6.Calabrese V, Giordano J, Signorile A, et al. Major pathogenic mechanisms in vascular dementia: roles of cellular stress response and hormesis in neuroprotection. J Neurosci Res. 2016;94(12):1588–1603. doi: 10.1002/jnr.23925. [DOI] [PubMed] [Google Scholar]
- 7.Rusyniak DE, Kirk MA, May JD, et al. Hyperbaric oxygen therapy in acute ischemic stroke: results of the hyperbaric oxygen in acute ischemic stroke trial pilot study. Stroke. 2003;34(2):571–574. doi: 10.1161/01.str.0000050644.48393.d0. [DOI] [PubMed] [Google Scholar]
- 8.Ding J, Zhou D, Sui M, et al. The effect of normobaric oxygen in patients with acute stroke: a systematic review and meta-analysis. Neurol Res. 2018;40(6):433–444. doi: 10.1080/01616412.2018.1454091. [DOI] [PubMed] [Google Scholar]
- 9.Chu DK, Kim LH, Young PJ, et al. Mortality and morbidity in acutely ill adults treated with liberal versus conservative oxygen therapy (IOTA): a systematic review and meta-analysis. Lancet. 2018;391(10131):1693–1705. doi: 10.1016/S0140-6736(18)30479-3. [DOI] [PubMed] [Google Scholar]
- 10.Roffe C, Nevatte T, Sim J, et al. Effect of routine low-dose oxygen supplementation on death and disability in adults with acute stroke: the stroke oxygen study randomized clinical trial. JAMA. 2017;318(12):1125–1135. doi: 10.1001/jama.2017.11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang J, Qi Z, Liu W, et al. Normobaric hyperoxia slows blood–brain barrier damage and expands the therapeutic time window for tissue-type plasminogen activator treatment in cerebral ischemia. Stroke. 2015;46(5):1344–1351. doi: 10.1161/STROKEAHA.114.008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu J, Zhang Y, Liang Z, et al. Normobaric hyperoxia retards the evolution of ischemic brain tissue toward infarction in a rat model of transient focal cerebral ischemia. Neurol Res. 2016;38(1):75–79. doi: 10.1080/01616412.2015.1135558. [DOI] [PubMed] [Google Scholar]
- 13.Shi S, Qi Z, Ma Q, et al. Normobaric hyperoxia reduces blood occludin fragments in rats and patients with acute ischemic stroke. Stroke. 2017;48(10):2848–2854. doi: 10.1161/STROKEAHA.117.017713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rodriguez P, Zhao J, Milman B, Tiwari YV, Duong TQ. Methylene blue and normobaric hyperoxia combination therapy in experimental ischemic stroke. Brain Behav. 2016;6(7):e00478. doi: 10.1002/brb3.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ivanov KP, Sokolova IB, Vovenko EP. Oxygen transport in the rat brain cortex at normobaric hyperoxia. Eur J Appl Physiol Occup Physiol. 1999;80(6):582–587. doi: 10.1007/s004210050637. [DOI] [PubMed] [Google Scholar]
- 16.Liu S, Shi H, Liu W, Furuichi T, Timmins GS, Liu KJ. Interstitial pO2 in ischemic penumbra and core are differentially affected following transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2004;24(3):343–349. doi: 10.1097/01.WCB.0000110047.43905.01. [DOI] [PubMed] [Google Scholar]
- 17.Dunn AK, Bolay H, Moskowitz MA, Boas DA. Dynamic imaging of cerebral blood flow using laser speckle. J Cereb Blood Flow Metab. 2001;21(3):195–201. doi: 10.1097/00004647-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 18.Liu S, Liu W, Ding W, Miyake M, Rosenberg GA, Liu KJ. Electron paramagnetic resonance-guided normobaric hyperoxia treatment protects the brain by maintaining penumbral oxygenation in a rat model of transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2006;26(10):1274–1284. doi: 10.1038/sj.jcbfm.9600277. [DOI] [PubMed] [Google Scholar]
- 19.Wu O, Lu J, Mandeville JB, et al. Dynamic functional cerebral blood volume responses to normobaric hyperoxia in acute ischemic stroke. J Cereb Blood Flow Metab. 2012;32(9):1800–1809. doi: 10.1038/jcbfm.2012.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dong W, Qi Z, Liang J, et al. Reduction of zinc accumulation in mitochondria contributes to decreased cerebral ischemic injury by normobaric hyperoxia treatment in an experimental stroke model. Exp Neurol. 2015;272:181–189. doi: 10.1016/j.expneurol.2015.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singhal AB, Ratai E, Benner T, et al. Magnetic resonance spectroscopy study of oxygen therapy in ischemic stroke. Stroke. 2007;38(10):2851–2854. doi: 10.1161/STROKEAHA.107.487280. [DOI] [PubMed] [Google Scholar]
- 22.Shin HK, Dunn AK, Jones PB, et al. Normobaric hyperoxia improves cerebral blood flow and oxygenation, and inhibits peri-infarct depolarizations in experimental focal ischaemia. Brain. 2007;130(Pt 6):1631–1642. doi: 10.1093/brain/awm071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ejaz S, Emmrich JV, Sitnikov SL, et al. Normobaric hyperoxia markedly reduces brain damage and sensorimotor deficits following brief focal ischaemia. Brain. 2016;139(Pt 3):751–764. doi: 10.1093/brain/awv391. [DOI] [PubMed] [Google Scholar]
- 24.Poli S, Veltkamp R. Oxygen therapy in acute ischemic stroke – experimental efficacy and molecular mechanisms. Curr Mol Med. 2009;9(2):227–241. doi: 10.2174/156652409787581619. [DOI] [PubMed] [Google Scholar]
- 25.Sun L, Wolferts G, Veltkamp R. Oxygen therapy does not increase production and damage induced by reactive oxygen species in focal cerebral ischemia. Neurosci Lett. 2014;577:1–5. doi: 10.1016/j.neulet.2014.05.060. [DOI] [PubMed] [Google Scholar]
- 26.Nagatomo F, Fujino H, Kondo H, Ishihara A. Oxygen concentration-dependent oxidative stress levels in rats. Oxid Med Cell Longev. 2012;2012:381763. doi: 10.1155/2012/381763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bigdeli MR, Asheghabadi M, Khalili A. Time course of neuroprotection induced by normobaric hyperoxia in focal cerebral ischemia. Neurol Res. 2012;34(5):439–446. doi: 10.1179/1743132812Y.0000000013. [DOI] [PubMed] [Google Scholar]
- 28.Hess DC, Hoda MN, Khan MB. Humoral mediators of remote ischemic conditioning: important role of eNOS/NO/nitrite. Acta Neurochir Suppl. 2016;121:45–48. doi: 10.1007/978-3-319-18497-5_8. [DOI] [PubMed] [Google Scholar]
- 29.Li X, Ren C, Li S, et al. Limb remote ischemic conditioning promotes myelination by upregulating PTEN/AKT/mTOR signaling activities after chronic cerebral hypoperfusion. Aging Dis. 2017;8(4):392–401. doi: 10.14336/AD.2016.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ren C, Li N, Li S, et al. Limb ischemic conditioning improved cognitive deficits via eNOS-dependent augmentation of angiogenesis after chronic cerebral hypoperfusion in rats. Aging Dis. 2018;9(5):869–879. doi: 10.14336/AD.2017.1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meng R, Asmaro K, Meng L, et al. Upper limb ischemic preconditioning prevents recurrent stroke in intracranial arterial stenosis. Neurology. 2012;79(18):1853–1861. doi: 10.1212/WNL.0b013e318271f76a. [DOI] [PubMed] [Google Scholar]
- 32.Meng R, Ding Y, Asmaro K, et al. Ischemic conditioning is safe and effective for octo- and nonagenarians in stroke prevention and treatment. Neurotherapeutics. 2015;12(3):667–677. doi: 10.1007/s13311-015-0358-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou D, Ding J, Ya J, et al. Remote ischemic conditioning: a promising therapeutic intervention for multi-organ protection. Aging. 2018;10(8):1825–1855. doi: 10.18632/aging.101527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jamieson D, Chance B, Cadenas E, Boveris A. The relation of free radical production to hyperoxia. Annu Rev Physiol. 1986;48:703–719. doi: 10.1146/annurev.ph.48.030186.003415. [DOI] [PubMed] [Google Scholar]
- 35.Narkowicz CK, Vial JH, Mccartney PW. Hyperbaric oxygen therapy increases free radical levels in the blood of humans. Free Radic Res Commun. 1993;19(2):71–80. doi: 10.3109/10715769309056501. [DOI] [PubMed] [Google Scholar]
- 36.Sugawara T, Yu F, Ma L, Hsia CJ, Chan PH. Delayed treatment with polynitroxyl albumin reduces infarct size after stroke in rats. Neuroreport. 2001;12(16):3609–3612. doi: 10.1097/00001756-200111160-00047. [DOI] [PubMed] [Google Scholar]
- 37.Anantharam V, Kitazawa M, Wagner J, Kaul S, Kanthasamy AG. Caspase-3-dependent proteolytic cleavage of protein kinase Cdelta is essential for oxidative stress-mediated dopaminergic cell death after exposure to methylcyclopentadienyl manganese tricarbonyl. J Neurosci. 2002;22(5):1738–1751. doi: 10.1523/JNEUROSCI.22-05-01738.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mickel HS, Vaishnav YN, Kempski O, von Lubitz D, Weiss JF, Feuerstein G. Breathing 100% oxygen after global brain ischemia in Mongolian Gerbils results in increased lipid peroxidation and increased mortality. Stroke. 1987;18(2):426–430. doi: 10.1161/01.str.18.2.426. [DOI] [PubMed] [Google Scholar]
- 39.Bigdeli MR, Rasoulian B, Meratan AA. In vivo normobaric hyperoxia preconditioning induces different degrees of antioxidant enzymes activities in rat brain tissue. Eur J Pharmacol. 2009;611(1–3):22–29. doi: 10.1016/j.ejphar.2009.03.034. [DOI] [PubMed] [Google Scholar]
- 40.Bigdeli MR. Preconditioning with prolonged normobaric hyperoxia induces ischemic tolerance partly by upregulation of antioxidant enzymes in rat brain tissue. Brain Res. 2009;1260:47–54. doi: 10.1016/j.brainres.2008.12.065. [DOI] [PubMed] [Google Scholar]