Abstract
Objectives
Emergence delirium (ED) is a common neurologic complication that can not only distress children and their families in the early postanesthetic period, but can also have adverse effects on children in the long-term. This study aimed to investigate the effects of single-dose dexmedetomidine on ED in children with sevoflurane anesthesia and to observe postoperative behavioral changes through long-term follow-up.
Methods
Patients aged 2–7 years, American Society of Anesthesiologists class (ASA) I or II, scheduled for tonsillectomy with and without adenoidectomy were randomized to receive dexmedetomidine 0.5 μg/kg (Group D) or volume-matched normal saline (Group C) over 10 minutes after induction of anesthesia. The primary outcome was the incidence of ED within 30 minutes after extubation. Other outcomes were the incidence of pain, extubation time, post-anesthesia care unit (PACU) length of stay after extubation, adverse events, and the incidence of negative postoperative behavioral changes (NPOBCs).
Results
Ninety children completed the study. Compared with the control group (Group C), dexmedetomidine decreased the incidence of ED (31.1% vs 53.3%; P=0.033) and pain (28.9% vs 57.8%; P=0.006), but it prolonged extubation time (P⩽0.001). PACU length of stay after extubation and the percentage of adverse events were similar between groups. The incidence of NPOBCs in Group D was significantly lower at 1 and 7 days after discharge (33.3% vs 60.0%; P=0.011% and 24.4% vs 46.7%; P=0.028, respectively) than it was in Group C, but no significant difference was found at the 30th day.
Conclusion
Dexmedetomidine 0.5 μg/kg reduced the incidence of ED after sevoflurane anesthesia and might be used to prevent NPOBCs.
Clinical trials registration
ChiCTR1800016828.
Keywords: dexmedetomidine, emergence delirium, postoperative behavioral changes, tonsillectomy
Introduction
Emergence delirium (ED) is a behavioral disturbance in children, which is characterized by crying, thrashing, inconsolability, and disorientation in the early postanesthetic period. It is reported that the incidence of ED in children anesthetized with sevoflu-rane is up to 80%, much higher than that in adults.1 Furthermore, it can increase the risks of self-injury, surgical dehiscence, accidental dislodgement of IV catheters, and negative postoperative behavior, and finally, it can pose threats to patients, parents, and primary caregivers.2,3
Currently, numerous interventions for managing ED have been studied, including medications and behavioral interventions.4 Among them, dexmedetomidine, a highly selective α2 adrenergic receptor agonist, has been suggested as a potential prophylactic intervention against sevoflurane-associated ED.5,6 It provides sedation and anxiolysis by acting on these receptors in the locus coeruleous of the pons. It also exerts dose-dependent analgesic effects through binding to α2-receptors in the dorsal horn and the supra-spinal sites.7 Unfortunately, there has been controversy in the published work about the effectiveness of single-dose dexmedetomidine in reducing ED following sevoflurane anesthesia in children.8 Furthermore, no studies reported any data for long-term negative behavioral changes after discharge.
To our best knowledge, higher doses of dexmedetomidine are often associated with delayed extubation, residual sedation, and prolonged post-anesthesia care unit (PACU) stay,9 and these factors may account for the fact that we chose intravenous dexmedetomidine 0.5 μg/kg administered after induction of anesthesia. In the present study, we hypothesized that dexmedetomidine could reduce the incidence of ED in children undergoing tonsillectomy following sevoflurane-based anesthesia. In addition, pain relief, the incidence of requiring rescue analgesic, hemodynamic parameters, adverse events, extubation time, PACU length of stay, and postoperative behavioral changes were assessed.
Materials and methods
This was a prospective, double-blind, randomized controlled study conducted between June and November 2018 in accordance with the principles of the Declaration of Helsinki. The study was registered in the Chinese Clinical Trial Registry (ChiCTR1800016828) and was approved by the Ethics Committee of The Affiliated Hospital of Xuzhou Medical University (Jiangsu, China; XYFY2018-KL036-01). Written informed consent was obtained from the parents or guardians before surgery.
Participants
We included pediatric patients aged 2–7 years with ASA physical status I or II who were scheduled for tonsillectomy with and without adenoidectomy under general anesthesia with sevoflurane. Children were excluded for the following reasons: emergency surgery, intellectual disability, neurological diseases with agitation-like symptoms, weight of more than 40 kg, renal or hepatic disease, cardiac or respiratory disease, allergy to dexmedetomidine, or major life changes 1 month before the operation, such as divorce of parents, death of parents, moving to a new home, changing to a new kindergarten, etc.
Randomization and masking
Subjects were randomly assigned to receive dexmedetomidine (Group D) or saline (Group C) in a 1:1 ratio. A random sequence was generated using a computer-generated randomization program and kept within sealed opaque envelopes by an assistant not involved in data collection. On the morning of the surgery, the assistant opened a sealed envelope and prepared the drugs in identical syringes according to the group allocation. The anesthesiologist who administered the injections, the outcome assessor, the parents, and the children were blinded to the allocation and study drugs.
Anesthesia and intervention
All patients fasted for at least 6 hours and received no pre-medication before procedure. After a patient entered the operating room, standard monitoring (non-invasive blood pressure, electrocardiogram, heart rate, and pulse oxygen saturation) and monitoring with a bispectral index (BIS; VISTA™ monitoring system; Aspect Medical Systems Inc., Norwood, MA, USA) were performed. Anesthesia was induced via a face-mask with 5%–8% sevoflurane in oxygen. Once consciousness was lost, intravenous access was established, and endotracheal intubation was then facilitated with IV propofol 2 mg/kg, fentanyl 2 μg/kg, and cisatracurium 0.1–0.2 mg/kg. After the induction of anesthesia, Group D received a 20 mL normal saline infusion containing dexmedetomidine 0.5 μg/kg slowly over 10 minutes, whereas patients in Group C were administered a volume matched normal saline infusion. All patients received antiemetics with dexamethasone 0.1 mg/kg and dolasetron 0.3 mg/kg for the prevention of postoperative nausea and vomiting (PONV). Postoperative analgesia was achieved with paracetamol 15 mg/kg IV over 15 minutes before the end of surgery.
After intubation, children were mechanically ventilated with volume-controlled ventilation mode aiming at an end-tidal carbon dioxide value of 35–45 mmHg. Anesthesia was maintained with remifentanil 0.2–0.3 μg/kg/min and sevoflurane 3%–5% with a BIS target range of 40–60. Hemodynamic changes were monitored at 5-minute intervals throughout the procedure. If systolic blood pressure values decreased 20% below the preoperative baseline value or decreased to 90 mmHg, 10 mL/kg Ringer’s solution and 0.1 mg/kg ephedrine were administered. A decrease in heart rate to 60 beats/min was considered bradycardia, and atropine 0.01 mg/kg was administered.
At the end of surgery, all patients were immediately transported to the PACU and continued to receive respiratory support. Patients were extubated when a normal respiratory pattern was observed, and neostigmine 0.02 mg/kg and atropine 0.01 mg/kg were given promptly to antagonize residual muscle relaxant. All children were observed for at least 30 minutes after extubation and were transferred to the ward when they became calm and met a modified Aldrete score ≥9.10
Observed parameters
An independent trained anesthesiologist who was blinded to the assigned group checked all measurements. Preoperative anxiety was evaluated using the modified Yale Preoperative Anxiety Scale (m-YPAS) after patients arrived to the preoperative holding room.11 Hemodynamic parameters such as heart rate (HR), systolic blood pressure (SBP), and diastolic blood pressure (DBP) were checked at five time points: immediately before administration of the study drug (baseline, T0); 5 minutes (T1), 10 minutes (T2), and 15 minutes (T3) after administration; and at the time of extubation (T4).
ED and pain scores were measured upon extubation and every 5 minutes thereafter until 30 minutes after extubation. ED was assessed with the Pediatric Anesthesia Emergence Delirium (PAED) scale12, which consists of 5 psychometric items describing emergence behavior, scores ranging from 0 to 20. Pain was measured by Face, Legs, Activity, Cry, and Consolability (FLACC) scale13, scores ranging from 0 to 10. The severity of ED increases with a higher score. Because of differences in the literature,14 two different cutoff points were used in our current study. Patients with maximum PAED scores of ≥10 or ≥12 were diagnosed as having ED, and PAED scores ≥15 were considered to be severe ED. Maximum FLACC scores of ≥4 meant that pain was present. For patients considered to be delirious by the attending anesthesiologist, the first measure taken was to facilitate oral comfort, and, when this failed, a 0.5 μg/kg fentanyl dose was treated as rescue medication if pain was present. Otherwise, patients would be treated with 1 mg/kg propofol (Figure 1).
Figure 1.
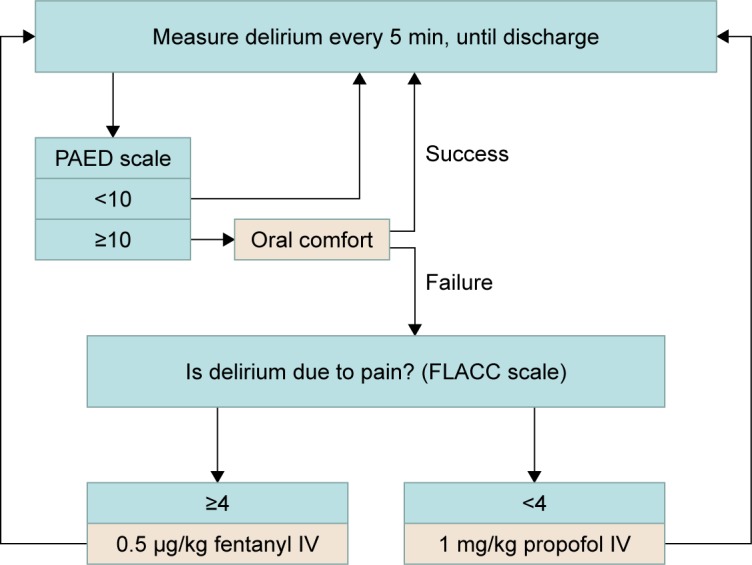
Delirium, pain and treatment flowchart during PACU, until discharge.
Note: Delirium and pain were measured as scores on the PAED scale and FLACC scale, respectively.
Abbreviations: FLACC, Face, Legs, Activity, Cry, Consolability; IV, intravenous; PACU, post-anesthesia care unit; PAED, Pediatric Anesthesia Emergence Delirium.
Extubation time (defined as the interval from discontinuation of the anesthetic to extubation), PACU length of stay after extubation (defined as the interval from extubation to discharge from the PACU), length of hospital stay, adverse events such as PONV, laryngospasm, severe coughing (defined as ≥4 coughs in a short period of time with SpO2 ⩽95%), oxygen desaturation (defined as SpO2 ⩽90%), and bradycardia (defined as heart rate ⩽60 beats/min) were also noted.
Negative postoperative behavioral changes (NPOBCs) were observed at 1, 7, and 30 days after discharge via parent phone surveys. Parents identified NPOBCs using the Post-Hospital Behavior Questionnaire (PHBQ),15 a 27-item questionnaire having questions in six subcategories, to score changes in behavior compared to baseline. NPOBCs were defined by PHBQ scores ⩾0.
Statistical analysis
For the sample size calculation, we used a proportion of ED of 60% reported in previously published studies. Therefore, 41 subjects per group would have a significance level of 5% and a power of 80% in detecting a 30% reduction in ED incidence between groups. Anticipating a 15% dropout rate, we estimated that 48 patients were needed in each group.
Normally distributed variables were reported as the mean±standard deviation (mean±SD) and were analyzed with independent sample t-tests. Nonnormally distributed data were reported as median (interquartile range) and were analyzed with the Mann–Whitney U-test. Categorical data were reported as frequency (%) and were analyzed using the chi-squared test or Fisher’s exact test.
SPSS software (version 24.0; SPSS Inc., IBM, Chicago, IL, USA) was used to conduct all statistical analyses. All tests were two-sided, and P⩽0.05 was considered to be statistically significant.
Results
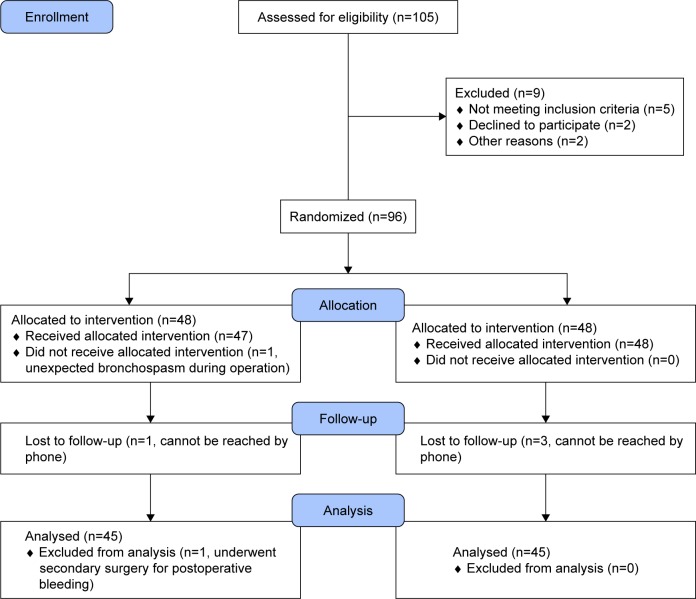
A total of 96 patients were initially enrolled, of which six were subsequently excluded; one developed bronchospasm during their operation, one underwent a secondary surgery for postoperative bleeding, and four were lost during follow-up. Thus, 90 patients were included in the final analysis for the study (Figure 2). The demographic and perioperative characteristics of the two groups were similar, as shown in Table 1 (P⩾0.05).
Figure 2.
CONSORT flowchart showing the number of patients at each phase of the study.
Table 1.
Demographic and perioperative characteristics by group
| Variables | Group C (n=45) | Group D (n=45) |
|---|---|---|
|
| ||
| Gender, M/F | 33 (73.3)/12 (26.7) | 34 (75.6)/11 (24.4) |
| Age (years) | 5.0 (4.0–6.0) | 5.0 (4.0–6.0) |
| Weight (kg) | 20.0 (16.0–23.0) | 20.0 (17.0–25.0) |
| m-YPAS score | 29.1 (22.9–45.8) | 33.3 (29.1–50.0) |
| History of surgery | 5 (11.1) | 6 (13.3) |
| Age | ||
| ≤3 years | 9 (18.9) | 8 (17.8) |
| >3 years | 36 (81.1) | 37 (82.2) |
| Type of surgery | ||
| Tonsillectomy | 19 (42.2) | 16 (35.6) |
| T&A | 26 (57.8) | 29 (64.4) |
| Duration of surgery (min) | 36.5±17.9 | 32.2±15.7 |
| Duration of anesthesia (min) | 45.5±21.5 | 40.8±16.2 |
Notes: Data are presented as median (interquartile range) for age, weight, m-YPAS score; mean±SD for duration of surgery, duration of anesthesia; and number of patients (%) for gender, history of surgery, age stratification, type of surgery. There were no significant differences among the two groups (P⩾0.05). Group C=control group, Group D=dexmedetomidine group.
Abbreviations: F, female; M, male; m-YPAS, modified Yale Preoperative Anxiety Scale; T&A, Tonsillectomy with Adenoidectomy.
Postoperative data and adverse events are presented in Figure 3 and Table 2. The incidence of ED (PAED≥10) in Group D was significantly lower than that in Group C [14 (31.1%) vs 24 (53.3%); P=0.033; Figure 3]. Similarly, the percentage of severe ED (PAED≥15) in Group D was significantly lower in comparison to Group C [4 (8.9%) vs 11 (24.4%); P=0.048; Figure 3]. However, when we performed a sensitivity analysis of ED (PAED≥12), there was no significant difference in the number of patients with ED between the two study groups [11 (24.4%) vs 19 (42.2%); P=0.074; Figure 3]. The maximal PAED score in Group D (7.1±4.8) was also dramatically decreased compared with Group C (9.8±5.8) (P=0.017; Table 2). Meanwhile, the maximal FLACC score was significantly lower in Group D [2.0 (0.0–4.0)] in comparison with the score in Group C [4.0 (2.0–6.0)] (P=0.002; Table 2). The incidence of pain was lower in Group D in comparison to Group C [13 (28.9%) vs 26 (57.8%); P=0.006; Table 2]. Likewise, the number of patients requiring additional fentanyl treatment to control ED in Group D was lower than the number in Group C, while there was no significant difference between the two groups [9 (20.0%) vs 17 (37.8%); P=0.063; Table 2]. Extubation time was slightly but significantly longer in Group D (31.7±9.8) than in Group C (23.7±8.8) (P⩽0.001; Table 2). Interestingly, there was no difference between the two groups in PACU length of stay after extubation [35.0 (32.5–46.0) vs 42.0 (32.0–50.0); P=0.108; Table 2] and length of hospital stay [7.0 (7.0–8.0) vs 7.0 (6.0–9.0); P=0.696; Table 2]. Of note, six patients in Group C suffered PONV, severe coughing, oxygen desaturation, or bradycardia, while four in Group D suffered oxygen desaturation or bradycardia, and all recovered uneventfully with proper treatment. None of the patients suffered laryngospasm in either group (Table 2).
Figure 3.
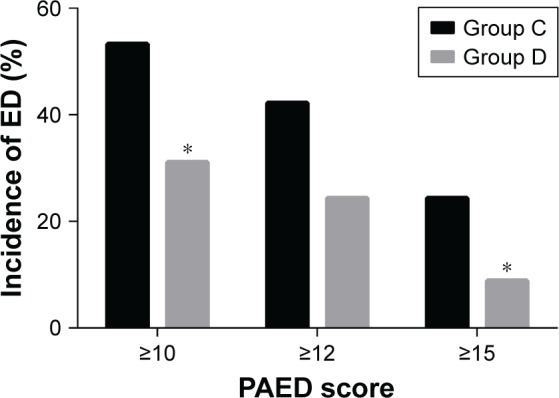
Incidence and severity of ED by study group.
Notes: *P⩽0.05 vs Group C. Group C=control group, Group D=dexmedetomidine group.
Abbreviations: ED, emergence delirium; PAED, Pediatric Anesthesia Emergence Delirium.
Table 2.
Postoperative outcomes and complications of children
| Variables | Group C (n=45) | Group D (n=45) | P-value |
|---|---|---|---|
|
| |||
| Maximal PAED score | 9.8±5.8 | 7.1±4.8 | 0.017 |
| Incidence of pain | 26 (57.8) | 13 (28.9) | 0.006 |
| Maximal FLACC score | 4.0 (2.0–6.0) | 2.0 (0.0–4.0) | 0.002 |
| Incidence of fentanyl rescue | 17 (37.8) | 9 (20.0) | 0.063 |
| Extubation time (min) | 23.7±8.8 | 31.7±9.8 | ⩽0.001 |
| PACU length of stay after extubation (min) | 42.0 (32.0–50.0) | 35.0 (32.5–46.0) | 0.108 |
| Length of hospital stay (days) | 7.0 (6.0–9.0) | 7.0 (7.0–8.0) | 0.696 |
| Adverse events | |||
| PONV | 2 (4.4) | 0 (0.0) | |
| Severe coughing | 2 (4.4) | 0 (0.0) | |
| Laryngospasm | 0 (0.0) | 0 (0.0) | |
| Desaturation | 1 (2.2) | 2 (4.4) | |
| Bradycardia | 1 (2.2) | 2 (4.4) | |
| Total | 6 (13.3) | 4 (8.9) | 0.502 |
Notes: Data are presented as median (interquartile range) for maximal FLACC score, PACU length of stay after extubation, length of hospital stay; mean±SD for maximal PAED score, extubation time; and number of patients (%) for incidence of pain, incidence of fentanyl rescue, adverse events. Group C=control group, Group D=dexmedetomidine group.
Abbreviations: FLACC, Face, Legs, Activity, Cry, Consolability; PACU, post-anesthesia care unit; PAED, Pediatric Anesthesia Emergence Delirium; PONV, postoperative nausea and vomiting.
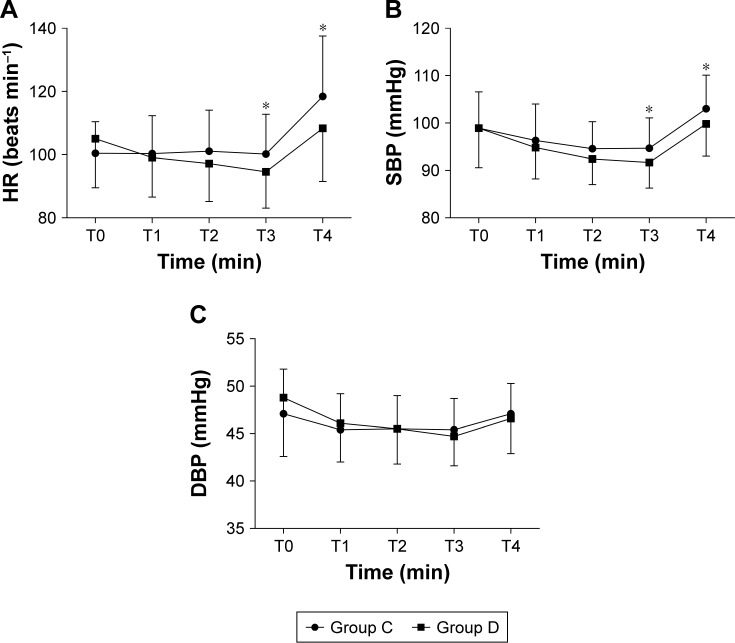
Hemodynamic changes of pediatric patients in the two groups are shown in Figure 4. Both groups were comparable in values of baseline SBP, DBP, and HR. Compared with Group C, Group D displayed a significant decrease in HR for 15 minutes (T3) after study drug administration and at the time of extubation (T4) [(94.6±11.5) vs (100.2±12.6); P=0.032 and (108.3±16.8) vs (118.4±19.1); P=0.009, respectively; Figure 4]. However, none of the patients needed treatment for heart rate falls. Likewise, SBP was lower in group D than in group C at the same two time points [(91.7±5.4) vs (94.7±6.4); P=0.017 and (99.8±6.8) vs (103.0±7.1); P=0.033, respectively; Figure 4]. There was no significant difference between the two groups in DBP at any time.
Figure 4.
Hemodynamic changes of pediatric patients in the two groups. (A) HR; (B) SBP; (C) DBP.
Notes: *P⩽0.05 vs Group C. Group C=control group, Group D=dexmedetomidine group.
Abbreviations: DBP, diastolic blood pressure; HR, heart rate; SBP, systolic blood pressure.
Figure 5 shows the prevalence of any NPOBCs at different follow-up times after discharge. The incidence of NPOBCs in both groups decreased over time after discharge. Compared with Group C, the number of patients with NPOBCs in Group D was significantly lower at 1 and 7 days after discharge [15 (33.3%) vs 27 (60.0%); P=0.011 and 11 (24.4%) vs 21 (46.7%); P=0.028, respectively; Figure 5], but there was no significant difference on the 30th day.
Figure 5.
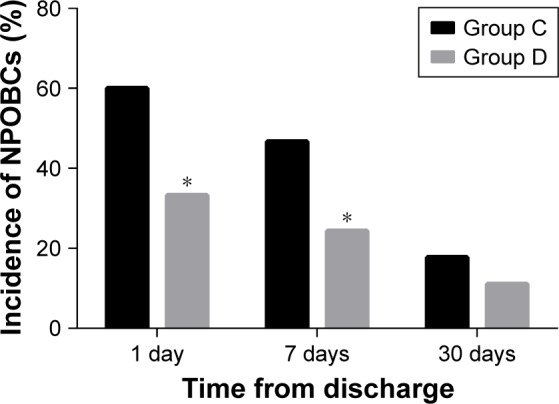
The incidence of any NPOBCs at different follow-up times after discharge.
Notes: *P⩽0.05 vs Group C. Group C=control group, Group D=dexmedetomidine group.
Abbreviation: NPOBCs, negative postoperative behavioral changes.
Discussion
The findings of this trial support the beneficial effect of intravenous dexmedetomidine 0.5 μg/kg administered after induction of anesthesia for prevention of ED in pediatric patients anesthetized with sevoflurane, and dexmedetomidine might be used to safely prevent postoperative behavior changes.
ED is among common troublesome complications in children involving sevoflurane anesthesia. The incidence of ED has varied greatly among different studies (10%–80%), attributed to the application of different rating scales, criteria, and evaluation time points. The PAED scale, an ideal, reliable, and valid rating tool, is widely accepted to evaluate ED in children after surgery or diagnostic procedures.16 It consists of five psychometric items describing emergence behavior, and the last two items, restlessness and inconsolability, are similar to the irritable behaviors caused by pain. Therefore, PAED score was employed in the present study. Although there is no consensus as to what score determines ED on this 20-point scale, studies have cited scores of 10 and 12.14
In our study, the incidence of ED was 53.3% (24/45) in the control group when we defined ED as PAED scores ≥10. Similar to our findings, Guler et al17 demonstrated that dexmedetomidine 0.5 μg/kg 5 minutes before the end of surgery reduced the incidence of ED from 57% to 17%. Shukry et al18 evaluated the effect of continuous infusions of dexmedetomidine 0.2 μg/kg/h during surgery, and determined that the incidence of ED decreased from 61% to 26%. However, when PAED scores greater than 12 were defined as agitation, the results of dexmedetomidine-associated reductions in ED were negated. This could have been due to the sensitivity to diagnosis of ED not remaining reliable higher than 10 points. Regardless, our study found that dexmedetomidine had a better preventive effect on severe ED, and the decline in maximal PAED score confirmed our views.
Postoperative pain, a potential confounding factor after tonsillectomy, must be taken into account when assessing ED. In this study, the commonly used FLACC scale was used to distinguish delirium from irritability caused by pain to the greatest extent. However, there was general overlap in the scales, making separation of the two difficult because a child who was restless or thrashing would score high on both scales. Our results showed that the incidence of pain and maximal FLACC score in Group D were significantly lower than Group C, which were consistent with the analgesic characteristics of dexmedetomidine. However, there was no significant difference between the two groups in the number of patients requiring additional fentanyl treatment, which was inconsistent with conclusions from other trials.14,19 The reason for this discrepancy, on the one hand, could be the setting of our research center where parents were not allowed to enter sterile areas, including the operating room and PACU. Although controversial,20 parental presence or a mother’s recorded voice during anesthetic emergence might play an important role in decreasing the incidence of ED.21,22 For children with a PAED score of 10 or higher, therefore, oral comfort was first given rather than fentanyl, and this may avoid unnecessary opioids use. On the other hand, the calculation of our sample size was based on the primary outcome, that is, the incidence of ED, but this sample size may not be sufficient for testing efficacy of the secondary outcomes.
The prolonged extubation time for the dexmedetomidine group is in agreement with previous studies.9,23 The study conducted by Bong et al8 concluded that recovery time is an important predictor of ED, with every minute increase in wake-up time reducing the odds of delirium by 7%. Therefore, we support the viewpoint that prolonging recovery time may be associated with improved quality of recovery. To our knowledge, ED is generally self-limiting and can be relieved after approximately 5–15 minutes in most cases. The results of the current study also showed that PACU length of stay after extubation was similar between the two groups. Furthermore, there was no difference in the length of hospital stay.
Administration of dexmedetomidine can be accompanied by a decrease in heart rate and blood pressure.24,25 In the present study, HR and SBP in Group D were significantly lower than that in Group C for 15 minutes after study drug administration, but neither required any treatment. Furthermore, no evidence was found for changes in the DBP during the study period. The decline in hemodynamic parameters at the time of extubation indicated that dexmedetomidine was able to effectively alleviate the stress response. In addition, patients in the two groups experienced comparable adverse events while in the PACU, such as PONV, severe coughing, oxygen desaturation or bradycardia, and all were effectively relieved after proper treatment.
Unlike dexmedetomidine-associated reductions in ED, data about the effects of dexmedetomidine on postoperative behavioral changes are limited. It has been demonstrated that negative postoperative behavioral changes (NPOBCs) occur in over 50% of children with general anesthesia and manifest as behavioral issues, such as excessive dependence on parents, sleep and eating disorders, nightmares, and temper tantrums.26 Consistent with earlier studies,27,28 we showed that 60.0% of children had NPOBCs on day 1, with 46.7% and 17.8% continuing to exhibit problems 1 week after discharge and 1 month post-procedure, respectively. Video distraction and parental presence in a study by Kim et al29 appeared to have an advantage in preventing NPOBCs in preschool children undergoing surgery. Intravenous infusion of dexmedetomidine may be another effective measure in reducing the incidence of NPOBCs, as was shown in the present study. We speculate the role of dexmedetomidine in preventing NPOBCs may be associated with reductions in delirium, rather than a direct effect of the intervention drug. However, the connection between the two negative behaviors remains to be further explored.
Several limitations related to this study must be addressed. First, the evaluation of the outcomes in this study mainly depended on subjective scales. However, the instruments utilized were validated ED, pain, and behavior scales that have been widely used clinically and provided standardization of the responses. Second, we did not evaluate the baseline temperament of children using a validated assessment tool, which has been suggested as an important contributor to ED and NPOBCs. Further studies should be conducted to focus on this issue. Third, our conclusions were based on a relatively small sample size, and, thus, the number of children recruited may have been insufficient to detect the effects of dexmedetomidine on postoperative pain and behavioral changes. Fourth, this was a single-center study, and whether single-dose dexmedetomidine can prevent ED and NPOBCs after sevoflurane anesthesia for populations in other areas remains uncertain. Therefore, more high-quality, multi-center, large-simple randomized trials are still needed to confirm the findings above.
In summary, this randomized trial showed that dexmedetomidine administration seems rational and feasible to reduce the incidence of ED after tonsillectomy with sevoflurane anesthesia, despite the increased extubation time, and might be used to safely prevent postoperative behavioral changes.
Data sharing statement
We will share all of the individual deidentified participant data that underlie the results reported in this article. Other study-related documents, including study protocol and statistical analyse plan, will be made available. Data are available indefinitely at https://doi.org/10.6084/m9.figshare.7609766.v2. Anyone who wishes to access the data can acquire them immediately following publication with no end date.
Acknowledgments
The authors thank Dr Liu for his support in conducting this study and the colleagues and staff of the operating room and PACU for their cooperation in data collection. This work was supported by the Xuzhou Science and Technology Planning Project only [grant numbers KC17199]. No commercial funding was received.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Moore AD, Anghelescu DL. Emergence delirium in pediatric anesthesia. Pediatric Drugs. 2017;19(1):11–20. doi: 10.1007/s40272-016-0201-5. [DOI] [PubMed] [Google Scholar]
- 2.Mohkamkar M, Farhoudi F, Alam-Sahebpour A, et al. Postanesthetic emergence agitation in pediatric patients under general anesthesia. Iran J Pediatr. 2014;24(2):184–190. [PMC free article] [PubMed] [Google Scholar]
- 3.Kain ZN, Caldwell-Andrews AA, Maranets I, et al. Preoperative anxiety and emergence delirium and postoperative maladaptive behaviors. Anesth Analg. 2004;99:1648–1654. doi: 10.1213/01.ANE.0000136471.36680.97. [DOI] [PubMed] [Google Scholar]
- 4.Mason KP. Paediatric emergence delirium: a comprehensive review and interpretation of the literature. Br J Anaesth. 2017;118(3):335–343. doi: 10.1093/bja/aew477. [DOI] [PubMed] [Google Scholar]
- 5.Sun L, Guo R, Sun L. Dexmedetomidine for preventing sevoflurane-related emergence agitation in children: a meta-analysis of randomized controlled trials. Acta Anaesthesiol Scand. 2014;58(6):642–650. doi: 10.1111/aas.12292. [DOI] [PubMed] [Google Scholar]
- 6.Pickard A, Davies P, Birnie K, Beringer R. Systematic review and meta-analysis of the effect of intraoperative α2-adrenergic agonists on postoperative behaviour in children. Br J Anaesth. 2014;112(6):982–990. doi: 10.1093/bja/aeu093. [DOI] [PubMed] [Google Scholar]
- 7.Chrysostomou C, Schulman SR, Herrera Castellanos M, et al. A phase II/III, multicenter, safety, efficacy, and pharmacokinetic study of dexmedetomidine in preterm and term neonates. J Pediatr. 2014;164(2):276–282. doi: 10.1016/j.jpeds.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Bong CL, Lim E, Allen JC, et al. A comparison of single-dose dexmedetomidine or propofol on the incidence of emergence delirium in children undergoing general anaesthesia for magnetic resonance imaging. Anaesthesia. 2015;70(4):393–399. doi: 10.1111/anae.12867. [DOI] [PubMed] [Google Scholar]
- 9.Zhu M, Wang H, Zhu A, Niu K, Wang G. Meta-analysis of dexmedetomidine on emergence agitation and recovery profiles in children after sevoflurane anesthesia: different administration and different dosage. PLoS One. 2015;10(4):e0123728. doi: 10.1371/journal.pone.0123728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aldrete JA, Kroulik D. A postanesthetic recovery score. J Am Coll Surg. 2007;205(5):e3–e4. doi: 10.1016/j.jamcollsurg.2007.07.034. [DOI] [PubMed] [Google Scholar]
- 11.Kain ZN, Mayes LC, Cicchetti DV, et al. The Yale Preoperative Anxiety Scale: how does it compare with a “gold standard”? Anesth Analg. 1997;85(4):783–788. doi: 10.1097/00000539-199710000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Ringblom J, Wåhlin I, Proczkowska M. A psychometric evaluation of the pediatric anesthesia emergence delirium scale. Pediatr Anaesth. 2018;28(4):332–337. doi: 10.1111/pan.13348. [DOI] [PubMed] [Google Scholar]
- 13.Willis MH, Merkel SI, Voepel-Lewis T, Malviya S. FLACC behavioral pain assessment Scale: a comparison with the child’s self-report. Pediatr Nurs. 2003;29(3):195–198. [PubMed] [Google Scholar]
- 14.Hauber JA, Davis PJ, Bendel LP, et al. Dexmedetomidine as a rapid bolus for treatment and prophylactic prevention of emergence agitation in anesthetized children. Anesth Analg. 2015;121(5):1308–1315. doi: 10.1213/ANE.0000000000000931. [DOI] [PubMed] [Google Scholar]
- 15.Cai Y, Lopata L, Dodhia S, et al. Differences in postoperative maladaptive behavioral changes between partial and total tonsillectomy patients. Int J Pediatr Otorhinolaryngol. 2018;106:55–58. doi: 10.1016/j.ijporl.2017.12.036. [DOI] [PubMed] [Google Scholar]
- 16.Sikich N, Lerman J. Development and psychometric evaluation of the pediatric anesthesia emergence delirium scale. Anesthesiology. 2004;100(5):1138–1145. doi: 10.1097/00000542-200405000-00015. [DOI] [PubMed] [Google Scholar]
- 17.Guler G, Akin A, Tosun Z, et al. Single-dose dexmedetomidine reduces agitation and provides smooth extubation after pediatric adenotonsillectomy. Paediatr Anaesth. 2005;15(9):762–766. doi: 10.1111/j.1460-9592.2004.01541.x. [DOI] [PubMed] [Google Scholar]
- 18.Shukry M, Clyde MC, Kalarickal PL, Ramadhyani U. Does dexmedetomidine prevent emergence delirium in children after sevoflurane-based general anesthesia? Paediatr Anaesth. 2010;15(12):1098–1104. doi: 10.1111/j.1460-9592.2005.01660.x. [DOI] [PubMed] [Google Scholar]
- 19.Patel A, Davidson M, Tran MCJ, et al. Dexmedetomidine infusion for analgesia and prevention of emergence agitation in children with obstructive sleep apnea syndrome undergoing tonsillectomy and adenoidectomy. Anesth Analg. 2010;111(4):1004–1010. doi: 10.1213/ANE.0b013e3181ee82fa. [DOI] [PubMed] [Google Scholar]
- 20.Costi D, Cyna AM, Ahmed S, et al. Effects of sevoflurane versus other general anaesthesia on emergence agitation in children. Cochrane Database Syst Rev. 2014;9:CD007084. doi: 10.1002/14651858.CD007084.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Byun S, Song S, Kim JH, et al. Mother’s recorded voice on emergence can decrease postoperative emergence delirium from general anaesthesia in paediatric patients: a prospective randomised controlled trial. Br J Anaesth. 2018;121(2):483–489. doi: 10.1016/j.bja.2018.01.042. [DOI] [PubMed] [Google Scholar]
- 22.In W, Kim YM, Kim HS, et al. The effect of a parental visitation program on emergence delirium among postoperative children in the PACU. J Perianesth Nurs. 2019;34(1):108–116. doi: 10.1016/j.jopan.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 23.Cao JL, Pei YP, Wei JQ, Zhang YY. Effects of intraoperative dexmedetomidine with intravenous anesthesia on postoperative emergence agitation/delirium in pediatric patients undergoing tonsillectomy with or without adenoidectomy: a CONSORT-prospective, randomized, controlled clinical trial. Medicine. 2016;95(49):e5566. doi: 10.1097/MD.0000000000005566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao Y, Qian B, Lin Y, et al. Intranasal dexmedetomidine premedication reduces minimum alveolar concentration of sevoflurane for laryngeal mask airway insertion and emergence delirium in children: a prospective, randomized, double-blind, placebo-controlled trial. Paediatr Anaesth. 2015;25(5):492–498. doi: 10.1111/pan.12574. [DOI] [PubMed] [Google Scholar]
- 25.Aouad MT, Zeeni C, Al Nawwar R, et al. Dexmedetomidine for improved quality of emergence from general anesthesia: a dose-finding study. Anesth Analg. 2017 Dec 29; doi: 10.1213/ANE.0000000000002763. Epub. [DOI] [PubMed] [Google Scholar]
- 26.Lee-Archer P, McBride C, Paterson R, et al. Does dexmedetomidine given as a premedication or intraoperatively reduce post-hospitalisation behaviour change in children? A study protocol for a randomised controlled trial in a tertiary paediatric hospital. BMJ Open. 2018;8(4):e019915. doi: 10.1136/bmjopen-2017-019915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kain ZN, Mayes LC, O’Connor TZ, Cicchetti DV. Preoperative anxiety in children. predictors and outcomes. Arch Pediatr Adolesc Med. 1996;150(12):1238. doi: 10.1001/archpedi.1996.02170370016002. [DOI] [PubMed] [Google Scholar]
- 28.Fortier MA, Kain ZN. Treating perioperative anxiety and pain in children: a tailored and innovative approach. Paediatr Anaesth. 2015;25(1):27–35. doi: 10.1111/pan.12546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim H, Jung SM, Yu H, et al. Video Distraction and Parental Presence for the Management of Preoperative Anxiety and Postoperative Behavioral Disturbance in Children: A Randomized Controlled Trial. Anesth Analg. 2015;121:778–784. doi: 10.1213/ANE.0000000000000839. [DOI] [PubMed] [Google Scholar]