Abstract
Background:
Neoadjuvant chemotherapy (NAC) is increasingly used to treat patients with breast cancer, but the reliability of sentinel lymph node biopsy (SLNB) following chemotherapy is in doubt. In this meta-analysis, we aimed to evaluate studies that examine the results of SLNB after NAC to assess identification rate (IR) and false-negative rate (FNR).
Materials and Methods:
Systemic searches were performed in the PubMed, ISI Web of Sciences, Scopus, and Cochrane databases from January 1, 2000, to November 30, 2016, for studies of SLNB after NAC for breast cancer and followed by axillary lymph node (LN) dissection in two subgroups: initially node negative and node positive converted to node negative. Two reviewers independently review quality of included studies. A random-effects model was used to pool IR and FNR with 95% confidence intervals (CI), and heterogeneity among studies was assessed by I2 and Q-test.
Results:
A total of 23 studies with 1521 patients in the initially node-negative subgroup and 13 studies with 1088 patients in the node-positive converted to node-negative subgroup, were included in this meta-analysis with IR and FNR of 94% (95% CI: 92–96) and 7% (95% CI: 5–9) in the initially node-negative subgroup and 89% (95% CI: 85–94) and 13% (95% CI: 7–18) in the node-positive converted to node-negative subgroup, respectively.
Conclusion:
Our meta-analysis showed acceptable IR and FNR in initially node-negative group and it seems feasible in these patients, but these parameters did not reach to predefined value in node-positive converted to node-negative group, and thus, it is not recommended in these patients.
Keywords: Breast cancer, meta-analysis, neoadjuvant systemic therapy, sentinel lymph node biopsy
INTRODUCTION
Axillary lymph node (LN) evaluation plays the most important role in staging, treatment, and prognosis of breast cancer. Therefore, axillary LN dissection (ALND) is traditionally an important part of the breast cancer therapy.[1] However, this procedure has several complications, such as numbness, pain, restriction of shoulder range of motion, and upper limb lymphedema that leads to low quality of life. Hence, for decreasing complication risk, sentinel LN (SLN) – The first LN or group of LNs encountered in the lymphatic drainage – biopsy is recommended.[2,3,4] SLN biopsy (SLNB) is a minimally invasive technique with high accuracy in determining the status of axilla and led to less morbidity compared with ALND. This procedure is appropriate in early-stage clinically node-negative breast cancer. Approximately, half of the patients with a positive SLN are known to have additional axillary nodal involvement, and even in case of omitting an ALND, the risk of developing an axillary recurrence in the presence of a positive SLN is <1%. With the emergence of new medicines that have remarkable effect on breast cancer treatment, adjuvant chemotherapy has gained an integral part of the therapy. In recent years, a positive effect of neoadjuvant chemotherapy (NAC) on tumor and LN downstaging as well as overall prognosis has been identified. Studies have shown axillary pathological complete response (pCR) in 20%–40% of initially node-positive patients. Thus, NAC is widely used in breast cancer therapy. SLNB could be performed before NAC and that women with involved nodes could have ALND after the completion of chemotherapy. Avoiding the possible negative effects of lymphatic scarring or uneven nodal tumor response is the advantage of this strategy. The disadvantage of this approach is that these women would need to undergo two surgeries. Nevertheless, SLNB following NAC is a contraindication as NAC can distort lymphatic drainage and reduce SLN detection rate. However, this issue is recently in doubt, and many clinical researches have been conducted in this field.[3,5,6,7] The patient selection criteria, technique of mapping, type of tracer, pathologic staining, detection of involved SLN, and definition of positive SLN vary across individual literature. Thus, it is difficult to determine individual patient approach in clinical practice. There are three conditions in this subject: (1) node-negative breast cancer before and after NAC, (2) node positive before NAC that converted to node negative after NAC, and (3) node positive that does not respond to NAC and remains positive. At the present time, SLNB is a contraindication in the latest setting. It is important that the feasibility and reliability of SLNB is determined in two early items.
The aim of this study was to identify all of the clinical studies that have separately examined the results of SLNB after NAC in two subgroups, initially node negative and node positive converted to node negative, to evaluate identification rate (IR) and false-negative rate (FNR) and timing of SLNB in the context of NAC in these two subgroups.
METHODS
Literature search strategy
In this study, PubMed, ISI Web of Sciences, Scopus, and Cochrane databases were searched from January 1, 2000, to November 30, 2016. The following free text terms and Medical Subject Headings (Mesh) terms were used: “breast cancer” OR “breast carcinoma” OR “breast neoplasm” AND “sentinel lymph node biopsy” OR “sentinel lymph node dissection” OR “sentinel lymph node mapping” OR “SLNB” AND “preoperative chemotherapy” OR “neoadjuvant chemotherapy.” Only articles written in English were selected. The search strategy is depicted in Figure 1.
Figure 1.
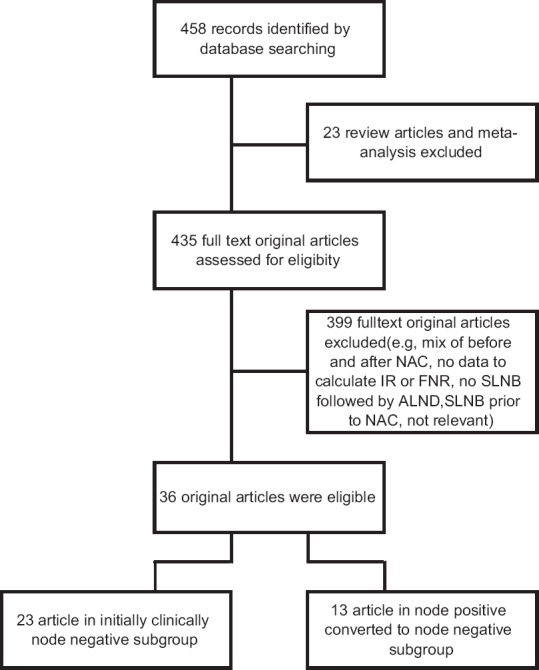
Flow diagram of literature search
Study inclusion and exclusion criteria
The inclusions criteria were as follows: (1) patients with breast cancer who received NAC, (2) patients who underwent SLNB after NAC, (3) patients who underwent ALND regardless of SLNB pathology, and (4) literatures that have clearly stated the status of LNs, either positive or negative, before and after NAC, or those from which we could accurately extract these information. Axillary LN-positive status was verified by clinically (physical examination or ultrasonic image), with or without histologic examination. The patients with inflammatory breast cancer, prior axillary surgery, and radiotherapy were excluded from the study.
Study quality assessment
The Quality Assessment of Diagnostic Accuracy Studies-2 (QUADAS-2)[8] tool was used to perform quality assessment of articles. This tool consists of four domains: patients’ selection, index test, references standard, and flow and timing. The risk of bias categorized as “low,” “high,” and “unclear.” If the answer to all the questions for domain is yes, the risk is low; if the answer to all the questions is no, the risk is high; and if there are insufficient data, the risk is unclear [Table 1].
Table 1a.
Results of quality assessment according to the Quality Assessment of Diagnostic Accuracy Studies-2 for the initially clinically node negative
| Study | Risk of bias | Applicability concerns | |||||
|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference Standard | |
| Nason et al. | 2 | 1 | 1 | 1 | 1 | 1 | 1 |
| Tafra et al. | 2 | 1 | 1 | 1 | 1 | 1 | 1 |
| Miller et al. | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Vigario et al. | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Piato et al. | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Shimazu et al. | 2 | 1 | 1 | 1 | 2 | 1 | 1 |
| Lang et al. | 2 | 1 | 1 | 1 | 2 | 1 | 1 |
| Tanaka et al. | 2 | 1 | 1 | 1 | 1 | 2 | 1 |
| Jones et al. | 2 | 1 | 1 | 1 | 2 | 1 | 1 |
| Mamounas et al. | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Yu et al. | 2 | 1 | 1 | 1 | 2 | 1 | 1 |
| Kinoshita et al. | 2 | 1 | 1 | 1 | 1 | 2 | 1 |
| Gimbergues et al. | 2 | 1 | 1 | 1 | 1 | 2 | 1 |
| Papa et al. | 2 | 1 | 1 | 1 | 1 | 2 | 1 |
| Classe et al. | 2 | 1 | 1 | 1 | 1 | 1 | 1 |
| Hunt et al. | 2 | 1 | 1 | 1 | 1 | 2 | 1 |
| Cheuny et al. | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Pecha et al. | 2 | 1 | 1 | 1 | 1 | 2 | 1 |
| Takashia et al. | 2 | 1 | 1 | 1 | 1 | 1 | 1 |
| Robollo-Aguirre et al. | 2 | 1 | 1 | 1 | 1 | 2 | 1 |
| Shigekawa et al. | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Piñero-Madrona et al. | 2 | 1 | 1 | 1 | 1 | 2 | 1 |
| Kida et al. | 2 | 1 | 1 | 1 | 1 | 2 | 1 |
1=Low risk; 2=High risk; ?=Unclear risk
Table 1b.
Results of quality assessment according to the Quality Assessment of Diagnostic Accuracy Studies-2 for the node positive converted to node negative
| Study | Risk of bias | Applicability concerns | |||||
|---|---|---|---|---|---|---|---|
| Patient selection | Index test | Reference standard | Flow and timing | Patient selection | Index test | Reference Standard | |
| Kinoshita et al. | 2 | 1 | 1 | 1 | 1 | 2 | 1 |
| Ozmen et al. | 2 | 1 | 1 | 1 | 1 | 2 | 1 |
| Thomas et al. | 2 | 1 | 1 | 2 | 1 | 2 | 1 |
| Chintamani et al. | 2 | 1 | 1 | 1 | 2 | 1 | 1 |
| RobolloAguirre et al. | 2 | 1 | 1 | 1 | 1 | 2 | 1 |
| Shigekawa et al. | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Takashia et al. | 2 | 1 | 1 | 1 | 1 | 1 | 1 |
| RobolloAguirre et al. | 2 | 1 | 1 | 1 | 1 | 2 | 1 |
| Kuehn et al. | 2 | 1 | 1 | 2 | 2 | 1 | 1 |
| Lee et al. | 1 | 2 | 1 | 1 | 1 | 1 | 1 |
| Yu et al. | 2 | 1 | 1 | 1 | 2 | 1 | 1 |
| Carrera et al. | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Cao et al. | 2 | 1 | 1 | 1 | 2 | 1 | 1 |
1=Low risk; 2=High risk; ?=Unclear risk
Data extraction and definitions
All studies were independently evaluated by two reviewers and checked by other reviewers for accuracy. Discrepancies were resolved by consensus after discussion. The first and second authors were separately extracted the following predefine items: first author, year of publication, study design, sample size, country of study, clinical tumor and nodal stage, the use immunohistochemistry (IHC) on axillary nodes, mapping method of SLN, definition of pathologic complete axillary response, IR, and FNR.
The results from each successfully identification SLN was categorized as true positive, true negative or false positive, taking the outcome of the complete ALND as reference standard. A true-negative SLN was define as a negative SLN and a negative ALND, a false negative as negative SLN with a positive LN in the ALND, and true positive as a positive SLN with or without a positive ALND. Based on these definitions, it was assumed that there was no false-positive case. The IR was defined as the number of patients with successful identification of SLNs was divided by the total number of patients. FNR was defined as the false negatives divided by the sum of false negatives and true positives. Axillary pCR was defined as the absence of cancer according to histological diagnosis after ALND.
Statistical analysis
Statistical analyses were performed using STATA Corp. 2011 (Stata Statistical Software, Release 11, Stata Corp LP, Package, College Station, TX, USA). The analysis heterogeneity was evaluated by a Chi-square test and was quantified by I2 statistic. The I2 statistic values of ≤30%, 30%–70%, and ≥70% were considered as mild, moderate, and severe heterogeneity, respectively.[9] P values of the Chi-square test of heterogeneity were considered as statistically significant at 0.1. Due to heterogeneity between studies, random-effects models (using the DerSimonian and Laird methods) for meta-analysis were used to calculate pooled estimates of IR and FNR with 95% confidence intervals (CIs). Potential publication bias was assessed using Egger's weighted regression tests, and the results of Egger's tests were statistically significant at P < 0.1. If there was publication bias, “trim-and-fill” method was used to adjust and correct the publication bias.[10] Subgroup analysis was performed according to the type of tracers (single/dual). Sensitivity analysis was used to assess the effect of excluding any study on the overall effect.
RESULTS
Study selection process
The articles search founded 458 studies. After removing 23 review articles and meta-analyses, 435 literature remained, and then, 399 full-text original papers were excluded (e.g., mix of before and after NAC, no data to calculate IR or FNR, no SLNB followed by ALND, SLNB before NAC, and not relevant) that resulted in 36 eligible papers. Twenty-three studies included initially node-negative cases, and 13 studies included cases of node-positive breast cancers converted to node negative after NAC. Overall, 2609 patients were enrolled: 1521 patients in the initially node-negative subgroup with a mean of 66.1 patients per studies (range: 9–320) and 1088 patients in the node positive converted to node negative after NAC subgroup with a mean of 83.6 patients per studies (range: 15–529). The studies are summarized in Table 2.
Table 2a.
Characteristics of the individual studies in initially node-negative subgroup
| Author | Year | Country | Center | Design | Sample size | Tracer | Pathology | Mean SLN | Percentage IR | Percentage FNR |
|---|---|---|---|---|---|---|---|---|---|---|
| Nason et al.[11] | 2000 | USA | Single | Prospective | 9 | D, I, LS | H & E, IHC | 88.8 | 22.2 | |
| Tafra et al.[12] | 2001 | USA | Multiple | Prospective | 29 | D, I | H & E, IHC | 2.5 | 93 | 0 |
| Miller et al.[13] | 2002 | USA | Single | Retrospective | 35 | D, I | H & E, IHC | 2.1 | 85.7 | 11.4 |
| Vigario et al.[14] | 2003 | Brazil | Single | Prospective | 37 | I, LS | H & E, IHC | 1.7 | 97 | 19.4 |
| Piato et al.[15] | 2003 | Brazil | Single | Retrospective | 42 | I, LS | H & E | 97.5 | 16.7 | |
| Shimazu et al.[3] | 2004 | Japan | Single | Retrospective | 25 | D, I, LS | H & E, IHC | 2.1 | 96 | 7.1 |
| Lang et al.[16] | 2004 | USA | Single | Retrospective | 30 | D, I, LS | H & E | - | 96.7 | 0 |
| Tanaka et al.[17] | 2005 | Japan | Single | Retrospective | 17 | D | H & E | 1.9 | 100 | 0 |
| Jones et al.[18] | 2005 | USA | Single | Retrospective | 17 | - | H & E, IHC | - | 94.1 | 10 |
| Mamounas et al.[19] | 2005 | USA | Multiple | Prospective | 326 | D, I | H & E, IHC | 84.4 | 12.4 | |
| Yu et al.[20] | 2007 | Taiwan | Single | Retrospective | 127 | D | H & E, IHC | - | 91.3 | 9.6 |
| Kinoshita[21] | 2007 | Japan | Single | Prospective | 54 | D, I, LS | H & E | - | 96.9 | 14.3 |
| Gimbergues et al.[22] | 2008 | France | Single | Prospective | 82 | I, LS | H & E, IHC | 1.7 | 93.9 | 0 |
| Papa et al.[23] | 2008 | Israel | Single | Prospective | 31 | D, I | H & E | - | 87 | 15.8 |
| Classe et al.[24] | 2009 | France | Multiple | Prospective | 130 | D, I | H & E, IHC | 1.9 | 94.6 | 9.4 |
| Hunt et al.[25] | 2009 | USA | Single | Retrospective | 84 | D, I | H & E, IHC | 2.7 | 97.4 | 5.9 |
| Cheung et al.[26] | 2009 | China | Single | Prospective | 78 | D, I | H & E, IHC | - | 88.3 | 10.3 |
| Pecha et al.[27] | 2011 | Czech | Multiple | Retrospective | 172 | D, I, LS | H & E, IHC | 1.3 | 89.5 | 16.3 |
| Takahashi et al.[28] | 2012 | Japan | Single | Prospective | 41 | D, I, LS | H & E, IHC | 3 | 87.8 | 5.6 |
| Rebollo-Aguirre et al.[29] | 2012 | Spain | Single | Prospective | 51 | I, LS | H & E, IHC, OSNA | 1.7 | 98 | 9.5 |
| Shigekawa et al.[30] | 2012 | Japan | Single | Retrospective | 21 | D, I, LS | H & E, IHC | - | 81 | 0 |
| Piñero-Madrona et al.[31] | 2015 | Spain | Multiple | Prospective | 49 | D, I | - | - | 90 | 18 |
| Kida et al.[32] | 2015 | Japan | Single | Prospective | 34 | D | H & E | 2.5 | 97.1 | 0 |
D=Dye; I=Radioisotope; LS=Lymphoscintigraphy; H & E=Hematoxylin-eosin; IHC=Immunohistochemistry; OSNA=One-step nucleic acid amplification; IR=Identification rate; FNR=False-negative rate; SLN=Sentinel lymph node
Table 2b.
Characteristics of the individual studies in node-positive converted to node-negative subgroup
| Author | Year | Country | Center | Design | Sample size | Tracer | pathology | Mean SLN | Percentage IR | Percentage FNR |
|---|---|---|---|---|---|---|---|---|---|---|
| Kinoshita[21] | 2007 | Japan | Single | Prospective | 50 | D, I, LS | H & E | - | 90 | 7 |
| Ozmen et al.[33] | 2010 | Turkey | Single | Retrospective | 77 | D, I | H & E, IHC | 2.1 | 92 | 13.7 |
| Thomas et al.[34] | 2011 | India | Single | Prospective | 30 | D | H & E, IHC | 1.5 | 86.6 | 20 |
| Chintamani et al.[35] | 2011 | India | Single | Retrospective | 15 | D | - | - | 100 | 0 |
| Rebollo-Aguirre et al.[29] | 2012 | Spain | Single | Prospective | 37 | I, LS | H & E, IHC, OSNA | 1.7 | 88.7 | 6.7 |
| Shigekawa et al.[30] | 2012 | Japan | Single | Retrospective | 47 | D, I, LS | H & E | - | 83 | 29.2 |
| Takahashi et al.[28] | 2012 | Japan | Single | Prospective | 46 | D, I, LS | H & E, IHC | 3 | 87 | 27.3 |
| Rebollo-Aguirre et al.[36] | 2013 | Spain | Single | Prospective | 53 | I, LS | H & E, IHC, OSNA | 1.9 | 84.9 | 8.3 |
| Kuehn et al.[37] | 2013 | Germany | Multiple | Prospective | 592 | D, I, LS | H & E | 2.7 | 80.1 | 13.5 |
| Lee et al.[38] | 2015 | Korea | Single | Prospective | 55 | I | H & E, IHC | 2 | 87.3 | 6.7 |
| Yu et al.[39] | 2016 | China | Single | Retrospective | 48 | D | H & E, IHC | 1.4 | 95 | 36 |
| Carrera et al.[40] | 2016 | Spain | Multiple | Prospective | 53 | I, LS | H & E, IHC | 2.2 | 90.5 | 9.7 |
| Cao et al.[6] | 2016 | China | Single | Prospective | 48 | D, I, LS | H & E | 2 | 100 | 17.2 |
D=Dye; I=Radioisotope; LS=Lymphoscintigraphy; H & E=Hematoxylin-Eosin; IHC=Immunohistochemistry; OSNA=One-step nucleic acid amplification; IR=Identification rate; FNR=False-negative rate; SLN=Sentinel lymph node
Measures of test performance of initially node negative
Characteristics of included studies
In the initially node-negative subgroup, 3 studies used blue dye alone,[17,20,32] 4 studies used radioisotope alone,[14,15,22,29] 15 studies used both blue dye and radioisotope,[3,11,12,13,16,19,21,23,24,25,26,27,28,30,31] and 1 study did not state the type of the tracer used.[18] One study considered abnormal palpable LNs as SLNs.[32] Concerning pathologic assessment, 6 articles used hematoxylin and eosin (H & E) only,[15,16,17,21,23,32] 10 articles used IHC for negative H & E samples,[11,12,13,14,18,20,22,24,25,26] 5 articles used both H & E and IHC,[3,19,27,28,30] 1 study used H & E, IHC, and one-step nucleic acid amplification (OSNA),[29] and 1 study did not state the type of the tracer used.[31] Importantly, 1 study considered micrometastasis (yp mi) as involved SLN[20] and 3 studies considered micrometastasis (yp mi) and isolated tumor cell (ITC) (yp i+) as positive SLN.[27,28,29,30]
Meta-analysis
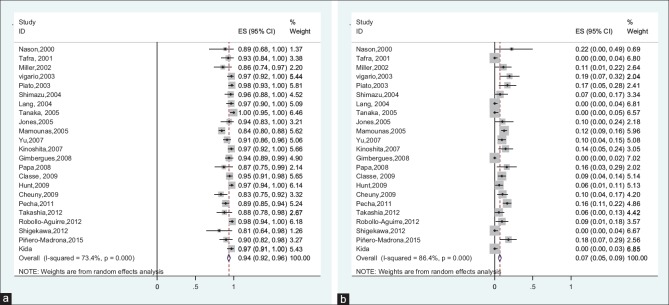
The reported IR between studies ranged from 81% to 100%. Between-study heterogeneity was high and statistically significant (I2 = 73.4%, Q-test: 82.68, P < 0.001). Due to severe heterogeneity, using random-effects meta-analysis model, the pooled IR estimated 94% (95% CI: 92%–96%).
The FNR ranged from 0% to 22%. Due to high and significant heterogeneity between studies (I2 = 86.4%, Q-test: 161.84, P < 0.001), random-effects meta-analysis estimated the pooled FNR 7% (95% CI: 5%–9%) [Figure 2].
Figure 2.
(a) Forest plot of the identification rate in initially node-negative patients, (b) forest plot of the false-negative rate in initially node-negative patients
Publication bias
Results of the Egger's test showed that there was no publication bias for IR. Results of the Egger's test showed that publication bias was existed between studies for FNR. Therefore, the missing data were imputed using “trim-and-fill” method to reduce the publication bias in pooled estimates of FNR. The results of this method showed that there were six missing studies, which after imputing these studies, the corrected overall FNR was estimated 5.5% (95% CI: 2.9%–8.1%).
Subgroup analysis
Subgroup analysis according to the type of tracer showed that the pooled IR for single and dual tracers was 97% (95% CI: 95%–99%) and 91% (95% CI: 86%–94%), respectively. Moreover, the pooled FNR for single and dual tracers was 4% (95% CI: 1%–7%) and 8% (95% CI: 5%–11%), respectively.
Sensitivity analysis
Results of sensitivity analysis showed that excluding none of the studies could not change the overall FNR and IR significantly.
Measures of test performance of node-positive converted to node-negative subgroup
Characteristics of included studies
In the node-positive converted to node-negative subgroup, 3 studies used blue dye alone,[34,35,39] 4 studies used radioisotope alone,[29,36,38,40] 6 studies used both blue dye and radioisotope,[6,14,33,30,28,37] and 1 study also included abnormal palpable LNs as SLN.[39] Regarding pathologic assessment, 4 studies used only H & E,[6,14,30,37] 2 studies performed IHC when H & E was negative,[33,38] 4 studies used both IHE and H & E,[34,28,39,40] 2 studies used OSNA,[29,36] and 1 study did not state the type of the pathologic staining.[35] One article considered (yp mi) as involved SLN[36] and 3 articles included (yp mi) and (yp i+) as positive SLN.[6,29,30]
Meta-analysis
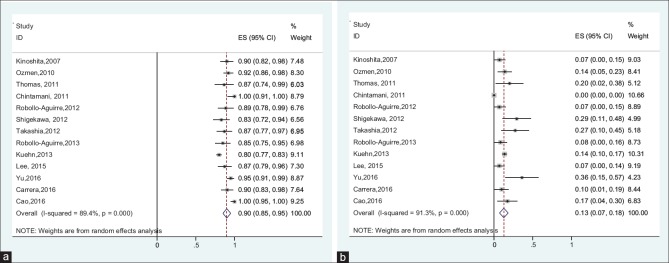
The reported IR between studies ranged from 80.1% to 100%. Between-study heterogeneity was high and statistically significant (I2 = 80.5%, Q-test: 71.18, P < 0.001). Due to sever heterogeneity, using random-effects meta-analysis model, the pooled IR estimated 89% (95% CI: 85%–94%).
The FNR ranged from 6.7% to 36%. Due to high and significant heterogeneity between studies (I2 = 91.3%, Q-test: 138.54, P < 0.001), random-effects meta-analysis estimated the pooled FNR 13% (95% CI: 7%–18%) [Figure 3].
Figure 3.
(a) Forest plot of the identification rate in node-positive converted to node-negative patients, (b) forest plot of the false-negative rate in node-positive converted to node-negative patients
Publication bias
Results of the Egger's test showed that there was no publication bias for IR. Results of the Egger's test showed that publication bias was existed between studies for FNR. Therefore, the missing data were imputed using “trim-and-fill” method to reduce the publication bias in pooled estimates of FNR. The results of this method showed that there were two missing studies, which after imputing these studies, the corrected overall FNR was estimated 10.5% (95% CI: 5.1%–15.9%).
Subgroup analysis
Subgroup analysis according to the type of tracer showed that the pooled IR for single and dual tracers was 92% (95% CI: 87%–96%) and 89% (95% CI: 80%–98%), respectively. Moreover, the pooled FNR for single and dual tracers was 9% (95% CI: 3%–15%) and 14% (95% CI: 10%–19%), respectively.
Sensitivity analysis
Sensitivity analysis showed that removing studies by Chintamani et al.,[35] Kuehn et al.,[37] and Cao et al.[6] can considerably change the effect of IR to 89% (95% CI: 83%–95%), 99% (95% CI: 97%–100%), and 89% (95% CI: 83%–95%), respectively. Moreover, excluding none of the studies could not change the overall FNR significantly.
DISCUSSION
The present study analyzed literature about the feasibility and accuracy of SLNB after NAC in two subgroups of breast cancer: the initially node negative and node positive converted to node negative after NAC. IRs and FNRs were evaluated because of the most important test clinically. The most important meta-analyses related to this issue were published without regarding the node status before and after NAC, and overall results were stated. We found only one meta-analysis that refers to the node status before and after NAC, specifically initially clinically node-negative cases, which was published by Geng et al.[41] The meta-analyses in this subject are summarized in Table 3. We think that the present meta-analysis is one of the most unique and comprehensive studies in this territory.
Table 3.
Systematic reviews and meta-anaiyses
| Study | Year of publication | Number of literatures | Number of patients |
|---|---|---|---|
| Geng et al[41] | 2016 | 16 | 1456 |
| Mocellin et al[42] | 2016 | 72 | 7451 |
| Van Nijnatten et al[43] | 2015 | 8 | 1395 |
| Fu et al[44] | 2014 | 15 | 2471 |
| Fontein et al[45] | 2013 | 40 | 3328 |
| Tan et al[46] | 2011 | 10 | 449 |
| Van Deurzen et al[47] | 2009 | 27 | 2148 |
| Xing et al[48] | 2006 | 21 | 1273 |
Based on studies of SLNB in node-negative patients with upfront surgery, IR >90% and FNR <10% have been accepted as oncologically aspect. In the patients who underwent NAC and then SLNB, these cutoff values were also considered; however, keep in mind that, these figures are arbitrary.[49,50] In the meta-analysis established by Geng et al.,[41] the pooled IR and FNR were 96% and 6%, respectively. The present study revealed pooled IR of 94% and FNR of 7% in the initially node-negative subgroup, while the pooled IR and FNR were 80.5% and 13%, respectively, in the node-positive converted to node-negative subgroup. Theoretically, chemotherapy leads to fibrosis and shrinkage and induces emboli and debris depositions in lymphatic routes that can alter lymphatic mapping and decrease IR. In addition, uneven disappear tumor burdens in LNs so as SLNs are sterilized, but non-SLNs remain involved, leading to high FNR.[29,41,42,51] However, this concept has not been proved. Van der Heiden-van der Loo et al.[52] showed that there was no statistical difference between IR of SLN before or after NAC. However, it seems that IR in upfront SLNB is higher than in IR of SLNB after NAC.[18,23,48,53] In a study established by Hunt et al.,[25] there were no differences in FNR between upfront SLN and SLN after NAC. Furthermore, some studies in clinically node negative have revealed identical accuracy in primary SLNB and SLNB after NAC.[53,54]
The SENTINA study has shown that dual tracer increases IR.[37] The GANEA series has stated an IR of 90% with dual tracer.[24] In the NASBP-B27 study, IR was 78.1%, 88.9%, and 87.6% in blue dye alone, radioisotope alone, and in combination, respectively.[19] In the SN-FNAC trial, the use of dual tracer was associated with lower FNR.[55] The Alliance research has revealed low FNR with dual tracer.[56] Hunt et al.[25] reported lower FNR with radioisotope alone or combination of two tracers. It seems that for increasing IR and decreasing FNR, dual-tracer mapping is required. However, Geng et al.[41] concluded that there were no differences between the type of tracer mapping agents. In particular, it has been suggested that this difference may be related to the fact that the initial axillary status varied among the patients included in their study. With respect to these studies, dual tracer deems better than one.
The SENTINA and Alliance trials have resulted that for decreasing FNR <10%, at least 3 SLNs should be harvested.[37,56] SN-FNAC study has recommended that at least 2 SLNs should be retrieved for this goal.[55] Furthermore, some studies had demonstrated a higher FNR when 1 SLN was biopsied, instead of 2 or more.[56,57] Wong et al.[58] stated higher FNR in initially node-negative patients in whom only 1 SLN was biopsied compared to 2 or more (14.3% vs. 4.3%). The average of SLNs excised in the SENTINA, SN-FNAC, and GANEA trials were 2, 2.7, and 1.9, respectively.[24,37,55] Our analysis showed an average SLN dissection of 2.09 and 2.05 in the initially node-negative and node-positive converted to node-negative subgroups, respectively. In the meta-analysis conducted by Fu et al.,[44] it was concluded that for decreasing FNR, both mapping and suspicious palpable LNs should be considered as SLNs. However, in our study, only 2 articles considered suspicious palpable LNs as SLNs.[33,39] It appears that to increase IR and decrease FNR, especially in the node-positive converted to node-negative subgroup, at least 2 SLNs should be harvested and the use of dual tracer is mandatory.
In the primary SLNB without NAC, evaluation of the ITC and micrometastasis were not recommended because there was no effect on survival. However, in SLNB after NAC, ITC and micrometastasis can result from partial response and downstage of macrometastasis before NAC.[41,43,59] The ACOSOG Z0010 trial has concluded that IHC-detected metastases in SLNs have no influence on overall survival.[60] The SN-FNAC study has recommended that ITC and micrometastasis should be considered positive in SLN to decrease FNR in the NAC setting.[55] Meta-analyses established by Tan et al.[46] and Geng et al.[41] have shown low FNR when IHC adds H and E stain. However, there was no consensus regarding the utility of IHC for evaluating SLN in the NAC setting.
CONCLUSION
There are great discrepancies between studies concerning SLNB after NAC in breast cancer. Therefore, this issue resulted in conflicting guideline recommendations. In initially node-negative patients, with regard to IR (94%) and FNR (7%) in our meta-analysis, it seems that SLNB after NAC in this group is feasible with acceptable accuracy.
In node-positive converted to node-negative patients, we did not found any meta-analysis to address this subject. Our results in this group were IR (89%) and FNR (13%) that did not reach to predefined value. Thus, SLNB after NAC in node-positive converted to node-negative patients is not recommended at this time, and novel techniques for increasing IR and decreasing FNR are required.
ASH contributed in the conception of the work, conducting the study, revising the draft, interpretation of data for the work, approval of the final version of the manuscript, and agreed for all aspects of the work.
HM contributed in the drafting and revising the draft, conducting the study, approval of the final version of the manuscript, and agreed for all aspects of the work.
MQ contributed in statistical calculations, interpretation of data for the work, approval of the final version of the manuscript, and agreed for all aspects of the work.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank the Imam Ali Hospital Clinical Research Development Unit for their assistance. Authors would like to acknowledge all those who cooperated to the research project. Thank you for your consideration. I look forward to hearing from you.
REFERENCES
- 1.Patten DK, Zacharioudakis KE, Chauhan H, Cleator SJ, Hadjiminas DJ. Sentinel lymph node biopsy after neo-adjuvant chemotherapy in patients with breast cancer: Are the current false negative rates acceptable? Breast. 2015;24:318–20. doi: 10.1016/j.breast.2015.02.026. [DOI] [PubMed] [Google Scholar]
- 2.Benson JR, Jatoi I. Sentinel lymph node biopsy and neoadjuvant chemotherapy in breast cancer patients. Future Oncol. 2014;10:577–86. doi: 10.2217/fon.13.231. [DOI] [PubMed] [Google Scholar]
- 3.Shimazu K, Noguchi S. Sentinel lymph node biopsy before versus after neoadjuvant chemotherapy for breast cancer. Surg Today. 2011;41:311–6. doi: 10.1007/s00595-010-4404-z. [DOI] [PubMed] [Google Scholar]
- 4.Kang SH, Kang JH, Choi EA, Lee ES. Sentinel lymph node biopsy after neoadjuvant chemotherapy. Breast Cancer. 2004;11:233–41. doi: 10.1007/BF02984543. [DOI] [PubMed] [Google Scholar]
- 5.Rubio IT. Sentinel lymph node biopsy after neoadjuvant treatment in breast cancer: Work in progress. Eur J Surg Oncol. 2016;42:326–32. doi: 10.1016/j.ejso.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 6.Cao XS, Li HJ, Cong BB, Sun X, Qiu PF, Liu YB, et al. Axillary and internal mammary sentinel lymph node biopsy in breast cancer after neoadjuvant chemotherapy. Oncotarget. 2016;7:74074–81. doi: 10.18632/oncotarget.12615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andreis D, Bonardi S, Allevi G, Aguggini S, Gussago F, Milani M, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with T2 to T4, N0 and N1 breast cancer. Breast. 2016;29:55–61. doi: 10.1016/j.breast.2016.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155:529–36. doi: 10.7326/0003-4819-155-8-201110180-00009. [DOI] [PubMed] [Google Scholar]
- 9.Fletcher J. What is heterogeneity and is it important? BMJ. 2007;334:94–6. doi: 10.1136/bmj.39057.406644.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talebi M. Study of publication bias in meta-analysis using trim and fill method. Int Res J Appl Basic Sci. 2013;4:31–6. [Google Scholar]
- 11.Nason KS, Anderson BO, Byrd DR, Dunnwald LK, Eary JF, Mankoff DA, et al. Increased false negative sentinel node biopsy rates after preoperative chemotherapy for invasive breast carcinoma. Cancer. 2000;89:2187–94. [PubMed] [Google Scholar]
- 12.Tafra L, Verbanac KM, Lannin DR. Preoperative chemotherapy and sentinel lymphadenectomy for breast cancer. Am J Surg. 2001;182:312–5. doi: 10.1016/s0002-9610(01)00718-8. [DOI] [PubMed] [Google Scholar]
- 13.Miller AR, Thomason VE, Yeh IT, Alrahwan A, Sharkey FE, Stauffer J, et al. Analysis of sentinel lymph node mapping with immediate pathologic review in patients receiving preoperative chemotherapy for breast carcinoma. Ann Surg Oncol. 2002;9:243–7. doi: 10.1007/BF02573061. [DOI] [PubMed] [Google Scholar]
- 14.Vigario A, Sapienza MT, Sampaio AP, Piato JR, Barros N, Barros A, et al. Primary chemotherapy effect in sentinel node detection in breast cancer. Clin Nucl Med. 2003;28:553–7. doi: 10.1097/00003072-200307000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Piato JR, Barros AC, Pincerato KM, Sampaio AP, Pinotti JA. Sentinel lymph node biopsy in breast cancer after neoadjuvant chemotherapy. A pilot study. Eur J Surg Oncol. 2003;29:118–20. doi: 10.1053/ejso.2002.1349. [DOI] [PubMed] [Google Scholar]
- 16.Lang JE, Esserman LJ, Ewing CA, Rugo HS, Lane KT, Leong SP, et al. Accuracy of selective sentinel lymphadenectomy after neoadjuvant chemotherapy: Effect of clinical node status at presentation. J Am Coll Surg. 2004;199:856–62. doi: 10.1016/j.jamcollsurg.2004.08.023. [DOI] [PubMed] [Google Scholar]
- 17.Tanaka Y, Maeda H, Ogawa Y, Nishioka A, Itoh S, Kubota K, et al. Sentinel node biopsy in breast cancer patients treated with neoadjuvant chemotherapy. Oncol Rep. 2005;15:927–31. [PubMed] [Google Scholar]
- 18.Jones JL, Zabicki K, Christian RL, Gadd MA, Hughes KS, Lesnikoski BA, et al. Acomparison of sentinel node biopsy before and after neoadjuvant chemotherapy: Timing is important. Am J Surg. 2005;190:517–20. doi: 10.1016/j.amjsurg.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 19.Mamounas EP, Brown A, Anderson S, Smith R, Julian T, Miller B, et al. Sentinel node biopsy after neoadjuvant chemotherapy in breast cancer: Results from national surgical adjuvant breast and bowel project protocol B-27. J Clin Oncol. 2005;23:2694–702. doi: 10.1200/JCO.2005.05.188. [DOI] [PubMed] [Google Scholar]
- 20.Yu JC, Hsu GC, Hsieh CB, Yu CP, Chao TY. Role of sentinel lymphadenectomy combined with intraoperative ultrasound in the assessment of locally advanced breast cancer after neoadjuvant chemotherapy. Ann Surg Oncol. 2007;14:174–80. doi: 10.1245/s10434-006-9132-7. [DOI] [PubMed] [Google Scholar]
- 21.Kinoshita T. Sentinel lymph node biopsy is feasible for breast cancer patients after neoadjuvant chemotherapy. Breast Cancer. 2007;14:10–5. doi: 10.2325/jbcs.14.10. [DOI] [PubMed] [Google Scholar]
- 22.Gimbergues P, Abrial C, Durando X, Le Bouedec G, Cachin F, Penault-Llorca F, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy is accurate in breast cancer patients with a clinically negative axillary nodal status at presentation. Ann Surg Oncol. 2008;15:1316–21. doi: 10.1245/s10434-007-9759-z. [DOI] [PubMed] [Google Scholar]
- 23.Papa MZ, Zippel D, Kaufman B, Shimon-Paluch S, Yosepovich A, Oberman B, et al. Timing of sentinel lymph node biopsy in patients receiving neoadjuvant chemotherapy for breast cancer. J Surg Oncol. 2008;98:403–6. doi: 10.1002/jso.21128. [DOI] [PubMed] [Google Scholar]
- 24.Classe JM, Bordes V, Campion L, Mignotte H, Dravet F, Leveque J, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy for advanced breast cancer: Results of ganglion sentinelle et chimiotherapie neoadjuvante, a French prospective multicentric study. J Clin Oncol. 2009;27:726–32. doi: 10.1200/JCO.2008.18.3228. [DOI] [PubMed] [Google Scholar]
- 25.Hunt KK, Yi M, Mittendorf EA, Guerrero C, Babiera GV, Bedrosian I, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy is accurate and reduces the need for axillary dissection in breast cancer patients. Ann Surg. 2009;250:558–66. doi: 10.1097/SLA.0b013e3181b8fd5e. [DOI] [PubMed] [Google Scholar]
- 26.Cheung TT, Suen DT, Kwong A. Is sentinel lymph node biopsy after neoadjuvant chemotherapy feasible in Chinese patients with invasive breast cancers? Surg Oncol. 2009;79:719–23. doi: 10.1111/j.1445-2197.2009.05057.x. [DOI] [PubMed] [Google Scholar]
- 27.Pecha V, Kolarik D, Kozevnikova R, Hovorkova K, Hrabetova P, Halaska M, et al. Sentinel lymph node biopsy in breast cancer patients treated with neoadjuvant chemotherapy. Cancer. 2011;117:4606–16. doi: 10.1002/cncr.26102. [DOI] [PubMed] [Google Scholar]
- 28.Takahashi M, Jinno H, Hayashida T, Sakata M, Asakura K, Kitagawa Y, et al. Correlation between clinical nodal status and sentinel lymph node biopsy false negative rate after neoadjuvant chemotherapy. World J Surg. 2012;36:2847–52. doi: 10.1007/s00268-012-1704-z. [DOI] [PubMed] [Google Scholar]
- 29.Rebollo-Aguirre AC, Gallego-Peinado M, Menjón-Beltrán S, García-García J, Pastor-Pons E, Chamorro-Santos CE, et al. Sentinel lymph node biopsy in patients with operable breast cancer treated with neoadjuvant chemotherapy. Rev Esp Med Nucl Imagen Mol. 2012;31:117–23. doi: 10.1016/j.remn.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 30.Shigekawa T, Sugitani I, Takeuchi H, Misumi M, Nakamiya N, Sugiyama M, et al. Axillary ultrasound examination is useful for selecting patients optimally suited for sentinel lymph node biopsy after primary systemic chemotherapy. Am J Surg. 2012;204:487–93. doi: 10.1016/j.amjsurg.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 31.Piñero-Madrona A, Escudero-Barea MJ, Fernández-Robayna F, Alberro-Adúriz JA, García-Fernández A, Vicente-García F, et al. Selective sentinel lymph node biopsy after neoadjuvant chemotherapy in breast cancer: Results of the GEICAM 2005-07 study. Cir Esp. 2015;93:23–9. doi: 10.1016/j.ciresp.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 32.Kida K, Ishikawa T, Yamada A, Shimizu D, Tanabe M, Sasaki T, et al. Aprospective feasibility study of sentinel node biopsy by modified indigocarmine blue dye methods after neoadjuvant chemotherapy for breast cancer. Eur J Surg Oncol. 2015;41:566–70. doi: 10.1016/j.ejso.2014.10.066. [DOI] [PubMed] [Google Scholar]
- 33.Ozmen V, Unal ES, Muslumanoglu ME, Igci A, Canbay E, Ozcinar B, et al. Axillary sentinel node biopsy after neoadjuvant chemotherapy. Eur J Surg Oncol. 2010;36:23–9. doi: 10.1016/j.ejso.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 34.Thomas S, Prakash A, Goyal V, Popli MB, Agarwal S, Choudhury M, et al. Evaluation of sentinel node biopsy in locally advanced breast cancer patients who become clinically node-negative after neoadjuvant chemotherapy: A preliminary study. Int J Breast Cancer. 2011;2011:870263. doi: 10.4061/2011/870263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chintamani, Tandon M, Mishra A, Agarwal U, Saxena S. Sentinel lymph node biopsy using dye alone method is reliable and accurate even after neo-adjuvant chemotherapy in locally advanced breast cancer – A prospective study. World J Surg Oncol. 2011;9:19. doi: 10.1186/1477-7819-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rebollo-Aguirre AC, Gallego-Peinado M, Sánchez-Sánchez R, Pastor-Pons E, García-García J, Chamorro-Santos CE, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with operable breast cancer and positive axillary nodes at initial diagnosis. Rev Esp Med Nucl Imagen Mol. 2013;32:240–5. doi: 10.1016/j.remn.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Kuehn T, Bauerfeind I, Fehm T, Fleige B, Hausschild M, Helms G, et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): A prospective, multicentre cohort study. Lancet Oncol. 2013;14:609–18. doi: 10.1016/S1470-2045(13)70166-9. [DOI] [PubMed] [Google Scholar]
- 38.Lee HD, Ahn SG, Lee SA, Lee HM, Jeong J. Prospective evaluation of the feasibility of sentinel lymph node biopsy in breast cancer patients with negative axillary conversion after neoadjuvant chemotherapy. Cancer Res Treat. 2015;47:26–33. doi: 10.4143/crt.2013.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu Y, Cui N, Li HY, Wu YM, Xu L, Fang M, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy for breast cancer: Retrospective comparative evaluation of clinically axillary lymph node positive and negative patients, including those with axillary lymph node metastases confirmed by fine needle aspiration. BMC Cancer. 2016;16:808. doi: 10.1186/s12885-016-2829-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carrera D, de la Flor M, Galera J, Amillano K, Gomez M, Izquierdo V, et al. Validation of sentinel lymph node biopsy in breast cancer women N1-N2 with complete axillary response after neoadjuvant chemotherapy. Multicentre study in Tarragona. Rev Esp Med Nucl Imagen Mol. 2016;35:221–5. doi: 10.1016/j.remn.2015.12.007. [DOI] [PubMed] [Google Scholar]
- 41.Geng C, Chen X, Pan X, Li J. The feasibility and accuracy of sentinel lymph node biopsy in initially clinically node-negative breast cancer after neoadjuvant chemotherapy: A Systematic review and meta-analysis. PLoS One. 2016;11:e0162605. doi: 10.1371/journal.pone.0162605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mocellin S, Goldin E, Marchet A, Nitti D. Sentinel node biopsy performance after neoadjuvant chemotherapy in locally advanced breast cancer: A systematic review and meta-analysis. Int J Cancer. 2016;138:472–80. doi: 10.1002/ijc.29644. [DOI] [PubMed] [Google Scholar]
- 43.van Nijnatten TJ, Schipper RJ, Lobbes MB, Nelemans PJ, Beets-Tan RG, Smidt ML, et al. The diagnostic performance of sentinel lymph node biopsy in pathologically confirmed node positive breast cancer patients after neoadjuvant systemic therapy: A systematic review and meta-analysis. Eur J Surg Oncol. 2015;41:1278–87. doi: 10.1016/j.ejso.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 44.Fu JF, Chen HL, Yang J, Yi CH, Zheng S. Feasibility and accuracy of sentinel lymph node biopsy in clinically node-positive breast cancer after neoadjuvant chemotherapy: A meta-analysis. PLoS One. 2014;9:e105316. doi: 10.1371/journal.pone.0105316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fontein DB, van de Water W, Mieog JS, Liefers GJ, van de Velde CJ. Timing of the sentinel lymph node biopsy in breast cancer patients receiving neoadjuvant therapy – Recommendations for clinical guidance. Eur J Surg Oncol. 2013;39:417–24. doi: 10.1016/j.ejso.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 46.Tan VK, Goh BK, Fook-Chong S, Khin LW, Wong WK, Yong WS, et al. The feasibility and accuracy of sentinel lymph node biopsy in clinically node-negative patients after neoadjuvant chemotherapy for breast cancer – a systematic review and meta-analysis. J Surg Oncol. 2011;104:97–103. doi: 10.1002/jso.21911. [DOI] [PubMed] [Google Scholar]
- 47.van Deurzen CH, Vriens BE, Tjan-Heijnen VC, van der Wall E, Albregts M, van Hilligersberg R, et al. Accuracy of sentinel node biopsy after neoadjuvant chemotherapy in breast cancer patients: A systematic review. Eur J Cancer. 2009;45:3124–30. doi: 10.1016/j.ejca.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 48.Xing Y, Foy M, Cox DD, Kuerer HM, Hunt KK, Cormier JN, et al. Meta-analysis of sentinel lymph node biopsy after preoperative chemotherapy in patients with breast cancer. Br J Surg. 2006;93:539–46. doi: 10.1002/bjs.5209. [DOI] [PubMed] [Google Scholar]
- 49.Harlow SP, Krag DN, Julian TB, Ashikaga T, Weaver DL, Feldman SA, et al. Prerandomization surgical training for the national surgical adjuvant breast and bowel project (NSABP) B-32 trial: A randomized phase III clinical trial to compare sentinel node resection to conventional axillary dissection in clinically node-negative breast cancer. Ann Surg. 2005;241:48–54. doi: 10.1097/01.sla.0000149429.39656.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim T, Giuliano AE, Lyman GH. Lymphatic mapping and sentinel lymph node biopsy in early-stage breast carcinoma: A metaanalysis. Cancer. 2006;106:4–16. doi: 10.1002/cncr.21568. [DOI] [PubMed] [Google Scholar]
- 51.Han A, Moon HG, Kim J, Ahn SK, Park IA, Han W, et al. Reliability of sentinel lymph node biopsy after neoadjuvant chemotherapy in breast cancer patients. J Breast Cancer. 2013;16:378–85. doi: 10.4048/jbc.2013.16.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van der Heiden-van der Loo M, de Munck L, Sonke GS, van Dalen T, van Diest PJ, van den Bongard HJ, et al. Population based study on sentinel node biopsy before or after neoadjuvant chemotherapy in clinically node negative breast cancer patients: Identification rate and influence on axillary treatment. Eur J Cancer. 2015;51:915–21. doi: 10.1016/j.ejca.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 53.Lee S, Kim EY, Kang SH, Kim SW, Kim SK, Kang KW, et al. Sentinel node identification rate, but not accuracy, is significantly decreased after pre-operative chemotherapy in axillary node-positive breast cancer patients. Breast Cancer Res Treat. 2007;102:283–8. doi: 10.1007/s10549-006-9330-9. [DOI] [PubMed] [Google Scholar]
- 54.Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. The role of sentinel lymph node surgery in patients presenting with node positive breast cancer (T0-T4, N1-2) who receive neoadjuvant chemotherapy – Results from the ACOSOG Z1071 trial. Cancer Res Suppl. 2012;72:94s. [Google Scholar]
- 55.Boileau JF, Poirier B, Basik M, Holloway CM, Gaboury L, Sideris L, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: The SN FNAC study. J Clin Oncol. 2015;33:258–64. doi: 10.1200/JCO.2014.55.7827. [DOI] [PubMed] [Google Scholar]
- 56.Boughey JC, Suman VJ, Mittendorf EA, Ahrendt GM, Wilke LG, Taback B, et al. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: The ACOSOG Z1071 (Alliance) clinical trial. JAMA. 2013;310:1455–61. doi: 10.1001/jama.2013.278932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park S, Park JM, Cho JH, Park HS, Kim SI, Park BW, et al. Sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with cytologically proven node-positive breast cancer at diagnosis. Ann Surg Oncol. 2013;20:2858–65. doi: 10.1245/s10434-013-2992-8. [DOI] [PubMed] [Google Scholar]
- 58.Wong SL, Edwards MJ, Chao C, Tuttle TM, Noyes RD, Carlson DJ, et al. Sentinel lymph node biopsy for breast cancer: Impact of the number of sentinel nodes removed on the false-negative rate. J Am Coll Surg. 2001;192:684–9. doi: 10.1016/s1072-7515(01)00858-4. [DOI] [PubMed] [Google Scholar]
- 59.Lyman GH. Appropriate role for sentinel node biopsy after neoadjuvant chemotherapy in patients with early-stage breast cancer. J Clin Oncol. 2015;33:232–4. doi: 10.1200/JCO.2014.58.9838. [DOI] [PubMed] [Google Scholar]
- 60.Hunt KK, Ballman KV, McCall LM, Boughey JC, Mittendorf EA, Cox CE, et al. Factors associated with local-regional recurrence after a negative sentinel node dissection: Results of the ACOSOG Z0010 trial. Ann Surg. 2012;256:428–36. doi: 10.1097/SLA.0b013e3182654494. [DOI] [PMC free article] [PubMed] [Google Scholar]