Abstract
Background
The aim of this study was to determine if components of the endocannabinoid system are modulated in uterine leiomyomas (fibroids). Components studied included cannabinoid receptors 1 (CB1) and 2 (CB2); the G protein-coupled receptor GPR55; transient potential vanilloid receptor 1 (TRPV1) and the endocannabinoid modulating enzymes N-acylphosphatidylethanolamine-specific phospholipase D (NAPE-PLD) and fatty acid amide hydrolase (FAAH), and their N-acylethanolamine (NAE) ligands: N-arachidonylethanolamine (AEA), N-oleoylethanolamine (OEA), and N-palmityolethanaolamine (PEA).
Material/Methods
Transcript levels of CB1, CB2, TRPV1, GPR55, NAPE-PLD, and FAAH were measured using RT-PCR and correlated with the tissue levels of the 3 NAEs in myometrial tissues. The tissues studied were: 1) fibroids, 2) myometrium adjacent/juxtaposed to the fibroid lesions, and 3) normal myometrium. Thirty-seven samples were processed for NAE measurements and 28 samples were used for RT-PCR analyses.
Results
FAAH expression was significantly lower in fibroids, resulting in a NAPE-PLD: FAAH ratio that favors higher AEA levels in pre-menopausal tissues, whilst PEA levels were significantly lower, particularly in post-menopausal women, suggesting PEA protects against fibroid pathogenesis. The CB1: CB2 ratio was lower in fibroids, suggesting that loss of CB1 expression affects the fibroid cell phenotype. Significant correlations between reduced FAAH, CB1, and GPR55 expression and PEA in fibroids indicate that the loss of these endocannabinoid system components are biomarkers of leiomyomata.
Conclusions
Loss of expression of CB1, FAAH, GPR55, and PEA production are linked to the pathogenesis of uterine fibroids and further understanding of this might eventually lead to better disease indicators or the development of therapeutic potentials that might eventually be used in the management of uterine fibroids.
MeSH Keywords: Biological Markers, Endocannabinoids, Leiomyoma, Therapeutics, Uterus
Background
Uterine leiomyomas (fibroids) are the most common non-cancerous pelvic tumors found in women [1] with prevalence rates of up to 70% in those of reproductive age [2] and in the post-reproductive age uterus, these reduce in size with sarcomatous changes being more likely to occur within the fibroids of women in this age group [3]. Both of these pathologies are characterized by an increase in smooth muscle cell (SMC) proliferation [4,5] and can cause symptoms including abnormal uterine bleeding, pressure effects, pelvic pain, and reproductive dysfunction. The growth of fibroids is primarily mediated by the ovarian sex steroid hormones [6,7] in both pre- and post-menopausal women, although other more recognized growth factors have been reported to be associated with their growth and survival [8–10].
Fibroids present a major health [11] and economic [12] burden; and robot-assisted laparoscopic myomectomy rather than conventional surgery is becoming the treatment of choice [13,14] where it is available. There is currently a drive to identify possible mechanistic factor(s) that may be involved in fibroid pathogenesis (for review see [15]); manipulation of these factors might lead to cheaper pharmacological management of the disease/symptoms.
One such mechanistic factor might be the endocannabinoid system. This hypothesis comes from a single microarray study of fibroid gene expression in comparison to that of normal myometrium that showed that CB1 receptor transcript levels were decreased by 72% in fibroid tissue [16], as well as a pilot study performed by our group [17] where we found that tissue levels of N-arachidonylethanolamine (AEA) were increased relative to non-adjacent myometrial tissue, suggesting a possible role for the endocannabinoid system in the pathogenesis of fibroids.
The “endocannabinoid system” (ECS) consists of 2 major isoforms of cannabinoid receptor (CB1 and CB2) that bind a number of endogenous ligands collectively known as endocannabinoids. The ligands that belong to the N-acylethanolamines (NAE) group, namely N-arachidonylethanolamine (AEA), N-oleoylethanolamine (OEA), and N-palmitoylethanolamine (PEA), have been extensively studied in normal endometrium [18–21] and other reproductive tissues [22–25], but not in the myometrium [17]. The tissue and plasma concentrations of these ligands are primarily regulated by the expression and activities of the main enzymes that regulate ligand synthesis, N-acylphosphatidylethanolamine-specific phospholipase D (NAPE-PLD) and degradation, fatty acid amide hydrolase (FAAH) [26]. AEA, OEA, and PEA also partially activate other putative cannabinoid receptors such as the transient potential vanilloid receptor 1 (TRPV1) and the orphan G protein-coupled receptor 55 (GPR55) [15,27–30], although this is thought to not happen in the non-pregnant human myometrium [31–33]. Nevertheless, CB1, CB2, and FAAH have been demonstrated in the human term myometrium [34], and CB1, CB2, and the TRPV1 receptors are expressed in a non-pregnant human myometrial cell line, which has the capacity to synthesize, respond to, and degrade endocannabinoids [35]. In addition, it also responds when exposed to AEA by increasing ERK1/2 expression through a mechanism that involved CB1, Gαi/o, Src, and PI3K, but not phospholipase C, protein kinase C, Ca2+/calmodulin-dependent protein kinase, Ca2+ ions, CB2, or the TRPV1 receptor [36]. The cells also autoregulated CB1 receptor expression, through a desensitization pathway that causes reduced receptor expression. However, a targeted examination of the major endocannabinoid components in fibroids to the best of our knowledge is yet to be undertaken.
The aim of this study was therefore to compare the expression of the NAE-binding receptors, their modulating enzymes, and the levels of the NAE ligands in fibroids, tissue adjacent to the fibroid lesions, and in normal myometrial tissue, to further clarify the possible role that the endocannabinoid system might play in fibroid growth and development.
Material and Methods
Tissue collection and ethics statement
Thirty-eight women aged 37 to 77 years were recruited for this study at Leicester Royal Infirmary and participants gave written informed consent. None had received hormonal therapy for at least 3 months prior to surgery. Tissue samples were taken from the women undergoing hysterectomy for non-malignant indications, such as dysfunctional uterine bleeding or uterine prolapse. Fresh uteri specimens were immediately transported on ice to the Histopathology Department where biopsies were obtained by a consultant gynecological histopathologist who divided them into 2 samples; 1 sample for this study and the other sample for histological confirmation of diagnosis. Three types of biopsy were obtained: 1) normal myometrium, where no pathology could be identified; 2) normal myometrium adjacent to fibroid tissue; and 3) confirmed fibroid tissues. These samples were immediately frozen in liquid nitrogen and stored at −80°C for later analyses. All protocols for human tissue collection and experimental use were approved by the University Hospitals of Leicester R&D, and Leicestershire and Rutland Research Ethics Committees.
N-acylethanolamine measurements
N-acylethanolamine (NAE) concentrations were determined in normal myometrium and fibroids as previously described [19]. Briefly, frozen tissues were first pulverized under liquid nitrogen in a pestle and mortar. Approximately 100 mg of pulverized tissue was mixed with deuterated internal standards (12.5 pmol/g AEA-d8 and OEA-d2, and 25 pmol/g PEA-d4, Cayman Chemical, Ann Arbor, MI, USA) and 1 mL phosphoric acid (5%), and thoroughly mixed on a benchtop vortex mixer. The mixture was then diluted with 1 mL deionized water and homogenized for 40 seconds using TissueRuptor (QIAGEN) on ice. The samples were then subjected to centrifugation at 1500 g for 30 minutes at 4°C. The supernatant was carefully transferred to a fresh tube and NAEs extracted by solid phase extraction as described previously [17]. The extract was dried under a constant stream of nitrogen before being reconstituted in 80 μL of acetonitrile [19]. The NAEs were separated and quantified using a previously validated UHPLC-MS/MS method [17,37].
Quantitative RT-PCR analysis of the endocannabinoid system
Biopsy fragments (100 mg) were pulverized under liquid nitrogen and homogenized in 1 mL of TRIzol reagent solution (Applied Biosystems) using an Ultra Turrax homogenizer on full power for 40 seconds on ice. RNA was extracted using 1-bromo-3-choro-propane (Sigma, Gillingham, Dorset, UK) as previously described [38] and RNA yield determined using a NanoDrop ND1000 spectrometer (ThermoFisher Scientific, Loughborough, Leics, UK). Samples with a 260 nm/280 nm ratio between 1.8 and 2.1, were reverse transcribed for PCR; any samples outside of this range were rejected. Total cellular RNA (500 ng) was reverse transcribed (High Capacity RNA-to-cDNA kit, Applied Biosystems) and the resultant cDNA stored at −20°C. Primers for the genes of interest (CB1, CB2, FAAH, NAPE-PLD, GPR55, and TRPV1; Table 1) were designed using Primer Express software (Applied Biosystems, Warrington, UK), whilst the endogenous control genes (housekeeping genes) [β-actin (BACT), tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein ζ (YWHAZ) and splicing factor 3a, subunit 1 (SF3A1)] were purchased as SYBR green-specific oligonucleotides as part of a geNorm endogenous control gene kit (PrimerDesign Ltd., Southampton, Hamp., UK). Quantitative real-time PCR reactions were carried out in a final reaction volume of 20 μL containing 10 μL of 2×SYBR Green PCR Universal Master Mix (Applied Biosystems), 300 nM of re-suspended endogenous control gene primer mix or 900 nM of target gene primers, 5 μL of diluted cDNA (diluted 1: 10 with RNase-free water) and 4 μL of RNAse/DNAse free water. The thermal cycler conditions for qRT-PCR were 95°C for 10 minutes followed by 40 cycles at 95°C for 15 seconds and 60°C for 1 minute and an extension at 72°C for 2 minutes. In all cases, a final extension incubation for 10 minutes at 72°C was performed. All reactions were performed in triplicate using an ABI PRISM® 7000 real-time PCR system (Applied Biosystems, Warrington, UK).
Table 1.
Primer sequences used for amplification of the indicated components of the endocannabinoid system within qRT-PCR.
| Gene | Primer sequences* | |
|---|---|---|
| NAPE-PLD | Forward | 5′-AAGAGATAGGAAAAAGATTTGGACCTT-3′ |
| Reverse | 5′-CTGGGTCTACATGCTGGTATTTCA-3′ | |
| FAAH | Forward | 5′-GGAGACCAAACAGAGCCTTGAG-3′ |
| Reverse | 5′-CTGAAGAGCCCACCTGTTGAC-3′ | |
| CB1 | Forward | 5′-TGCTGAACTCCACCGTGAAC-3′ |
| Reverse | 5′-TCCCCCATGCTGTTATCCA-3′ | |
| CB2 | Forward | 5′-GCCCAGCCACCCACAAC-3′ |
| Reverse | 5′-GCTATCTCTGTCACCCAGCATTC-3′ | |
| GPR55 | Forward | 5′-GGAAAGTGGAAAAATACATGTGCTT-3′ |
| Reverse | 5′-AACACCTCCAGCGGGAAGA-3′ | |
| TRPV1 | Forward | 5′-GAAGCCGTTGCTCAGAATAACTG-3′ |
| Reverse | 5′-AGCATGGCTTTCAGCAGACA-3′ |
All the primer sequences listed were designed using Primer Express software (Applied Biosystems, Warrington, UK) and purchased as HPLC-purified versions from Sigma-Aldrich (Poole, Dorset, UK).
The primers for the housekeeping genes (β-actin (BACT), tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein ζ (YWHAZ) and splicing factor 3a, subunit 1 (SF3A1), were designed and purchased as part of a SYBR green geNorm kit (Primer Design Ltd., Chandler’s Ford, Southampton, Hampshire, UK).
Statistical analysis
Results were analyzed using Graph-Pad Prism version 7.0 for Windows (Graph-Pad Software. San Diego, CA, USA; www.graphpad.com). Data are presented as mean ± standard deviation (SD) and statistical analysis performed using 2-tailed Student’s unpaired t-test for non-paired data and Student’s paired t-test for pairwise data. Correlation analysis was performed using Pearson correlation on non-logarithmic transformed data. P<0.05 was considered statistically significant.
Results
Patient samples
Although a total of 38 patient samples for NAE measurements were collected, 1 sample was lost due to patient withdrawal from the study. Of the remaining 37 samples, 16 samples were obtained from pre-menopausal women and 21 samples were obtained from post-menopausal women. All 37 samples were processed for NAE measurement. For PCR analyses, one sample was lost during processing and 8 had low quality RNA and were discarded. These 28 RNA samples comprised 8 normal myometrial samples and 20 fibroid tissues. Two extra normal myometrial samples were prepared and used in the PCR analyses, but were not used in the measurement of NAEs.
PEA tissue levels are increased in fibroid
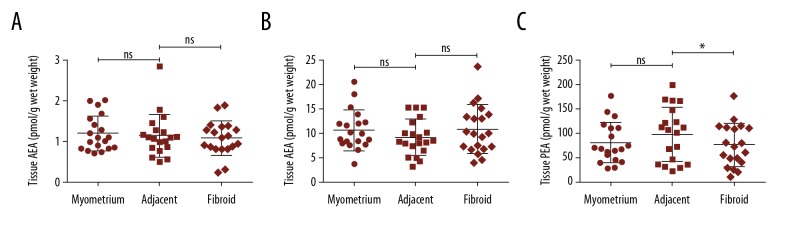
Figure 1 shows the levels of the NAEs (AEA, OEA, and PEA) in normal myometrium, myometrium adjacent to the fibroid and in fibroid for the entire cohort. AEA, OEA, or PEA levels were not significantly different between normal and adjacent myometrium, even though the mean (±SD) in PEA levels did increase to 80.0±42.0 pmol/g wet weight and 97.7±56.3 pmol/g wet weight in normal myometria and adjacent myometria, respectively. Similarly, there were no differences in AEA or OEA tissue levels between adjacent myometria and fibroids. Although OEA levels were higher in fibroid compared to adjacent myometrium (10.7±5.0 pmol/g wet weight versus 8.9±3.6 pmol/g wet weight); this difference was not statistically significant (P=0.77; Student’s paired t-test). By contrast, the levels of PEA (76.0±44.5 pmol/g wet weight) in fibroids were significantly lower than in adjacent myometrium (97.8±56.3 pmol/g wet weight; P=0.013; Student’s paired t-test).
Figure 1.
(A–C) Measurement of tissue NAE levels. The levels of AEA, OEA, and PEA in normal myometrium (myometrium; n=19), normal myometrium adjacent to fibroid (adjacent; n=19) and within fibroid tissue (n=19) were evaluated by ultra-high-performance liquid chromatography-tandem mass spectrometry. The data for each patient biopsy is presented together with the mean ± standard deviation for the measurement. Statistical differences in NAE levels between normal and adjacent myometrium were determined by unpaired Student’s t-test and by paired Student’s test between adjacent myometrium and fibroid: * P<0.05; n.s. – not significantly different.
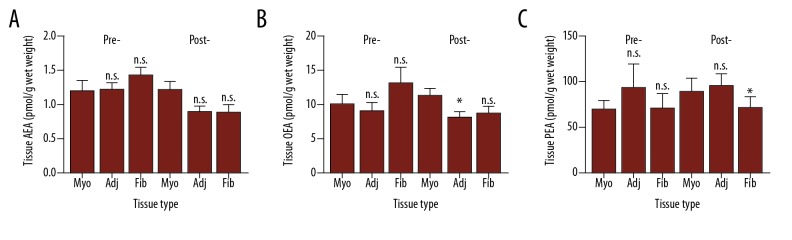
A sub-analysis of the tissue NAE levels using pre-and post-menopausal status as the discriminator is shown in Figure 2. In this analysis, the samples were divided into groups based on whether the patients were pre-menopausal (41.1±3.15 years) or post-menopausal (63.15±8.95 years). The age difference between pre- and post-menopausal women was significantly different (P<0.0001 for the myometrial samples and P<0.0001 for adjacent myometria/fibroid pair samples, respectively; Student’s unpaired t-test). The ages of the women (mean ±SD) providing the different tissue types were not statistically significantly different within the pre-menopausal group (normal myometrium=40.78±3.86 years [n=9] versus adjacent/fibroid pair 41.43±2.44 [n=7]; P=0.997 Student’s unpaired t-test) or within the post-menopausal group (normal myometrium=66.4±9.57 years [n=10] versus adjacent/fibroid pair 59.91±7.34 years [n=11]; P=0.147 Student’s unpaired t-test).
Figure 2.
(A–C) Tissue levels of the NAEs in pre- and post-menopausal myometrium and fibroids. Lipids extracted from normal myometrium (Myo), tissue adjacent (adj) to the fibroid and the fibroid (Fib) itself were measured by UHPLC-MS/MS as described in the Materials and Methods section and corrected for the amount of starting material. The numbers of patient samples analyzed were: n=9 for normal pre-menopausal myometrium and n=10 for normal post-menopausal myometrium; n=7 for pre-menopausal fibroids and their adjacent myometrium; n=11 for the post-menopausal fibroid and adjacent myometrium. The data are presented as mean ± standard deviation; * P<0.05 Student’s unpaired t-test compared to normal myometrium; n.s. – not significantly different.
There were no significant differences in the AEA, OEA, or PEA levels found between the pre-menopausal tissues (Figure 2). In the post-menopausal tissues, AEA levels at 1.21±0.40 pmol/g wet weight (mean ±SD) in the normal myometrium were higher than either the adjacent tissue (0.89±0.29 pmol/g wet weight) or in the fibroid (0.88±0.39 pmol/g wet weight), but neither of these differences were significant (P=0.080 and P=0.975, respectively).
OEA levels in the post-menopausal normal myometrium at 11.16±3.67 pmol/g wet weight were significantly (P=0.013) higher than that found in the tissue adjacent to the fibroid (7.96±3.19 pmol/g wet weight) but they were not significantly higher than that found in the fibroid (8.46±3.47 pmol/g wet weight); OEA levels in the pre-menopausal fibroid (12.92±6.10 pmol/g wet weight) were significantly (P=0.046) different to adjacent myometrium (8.85±3.29 pmol/g wet weight) but not to normal myometrium at a distant site (9.82±4.63 pmol/g wet weight).
The levels of PEA in both the pre-menopausal and post-menopausal tissues were more variable than those found for either AEA or OEA. In the pre-menopausal tissues, PEA levels were not significantly different to those found in the normal myometrium. In post-menopausal samples the levels of PEA in the myometrium adjacent to the fibroid (95.34±47.67 pmol/g wet weight) though similar to that of the normal myometrium (89.50±48.77 pmol/g wet weight; P=0.785), were significantly higher than that found in the fibroid (72.02±38.96 pmol/g wet weight; P=0.013).
The expression of NAE binding receptors in fibroid tissue
The expression of CB1, CB2, TRPV1, and GPR55 was not significantly different between fibroid tissues and normal myometrium (Table 2). The mean (SEM) levels of CB1 were 0.6±0.2 in the fibroid and 2.0±1.1 in myometrium, whilst that of CB2 were 2.2±0.7 in the fibroid and 0.9±0.5 in the myometrium. TRPV1 levels were very similar between myometrium and fibroid (3.7±0.8 and 4.6±2.1, respectively), whilst mean GPR55 transcript levels were 5 times higher in the myometrium (5.6±3.3) than in fibroid tissues (1.1±0.3). This was due to 2 samples of myometrium having levels of over 30 and 16. Because of the inverse expression patterns of CB1 and CB2; i.e., CB1 is higher in myometrium than fibroid and CB2 is higher in the fibroid than myometrium), the CB1: CB2 ratio was also examined. The data showed that the mean CB1: CB2 ratio was significantly (P=0.031) 10 times higher in myometrium (2.22±1.22) compared to that of the fibroid (0.27±0.09; Table 2).
Table 2.
The expression of components of the endocannabinoid system in the normal myometrium and fibroid tissue.
| CB1 | CB2 | CB1: CB2 | TRPV1 | GPR55 | NAPE-PLD | FAAH | NAPE-PLD: FAAH | |
|---|---|---|---|---|---|---|---|---|
| Myometrium | 2.0±1.1 | 0.9±0.5 | 2.22±1.22 | 3.7±0.8 | 5.6±3.3 | 14.1±2.0 | 1.3±0.5 | 10.8±1.54 |
| Fibroid | 0.6±0.2ns | 2.2±0.7ns | 0.27±0.09* | 4.6±2.1ns | 1.1±0.3ns | 12.5±3.8ns | 0.5±0.1* | 25.0±4.6* |
The data are presented as mean ±SEM from 10 normal myometrial and 20 fibroid tissue samples. In each case, the levels of the indicated transcripts were normalized to the geometric mean of the amount of transcript for the β-actin (ACTB), tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein ζ (YWHAZ) and splicing factor 3a, subunit 1 (SF3A1) housekeeping genes: (see Material and Methods section).
P<0.05;
ns – not significantly different, fibroid versus normal myometrium; Student’s unpaired t-test.
The ratio of NAPE-PLD: FAAH transcript levels in fibroids
Analysis of the expression of the NAE modulating enzymes showed that the levels of NAPE-PLD expression in fibroids (12.5±3.8) was similar to that found in myometrium (14.1±2.0; Table 2). By contrast, the levels of FAAH in fibroids at 0.5±0.1 were significantly lower (P=0.042) than in myometrium (1.3±0.5). Furthermore, the NAPE-PLD: FAAH ratio was also significantly (P=0.042) higher in fibroid tissue at (25.0±4.6) when compared with that in myometrium (10.8±1.5).
Correlation analyses
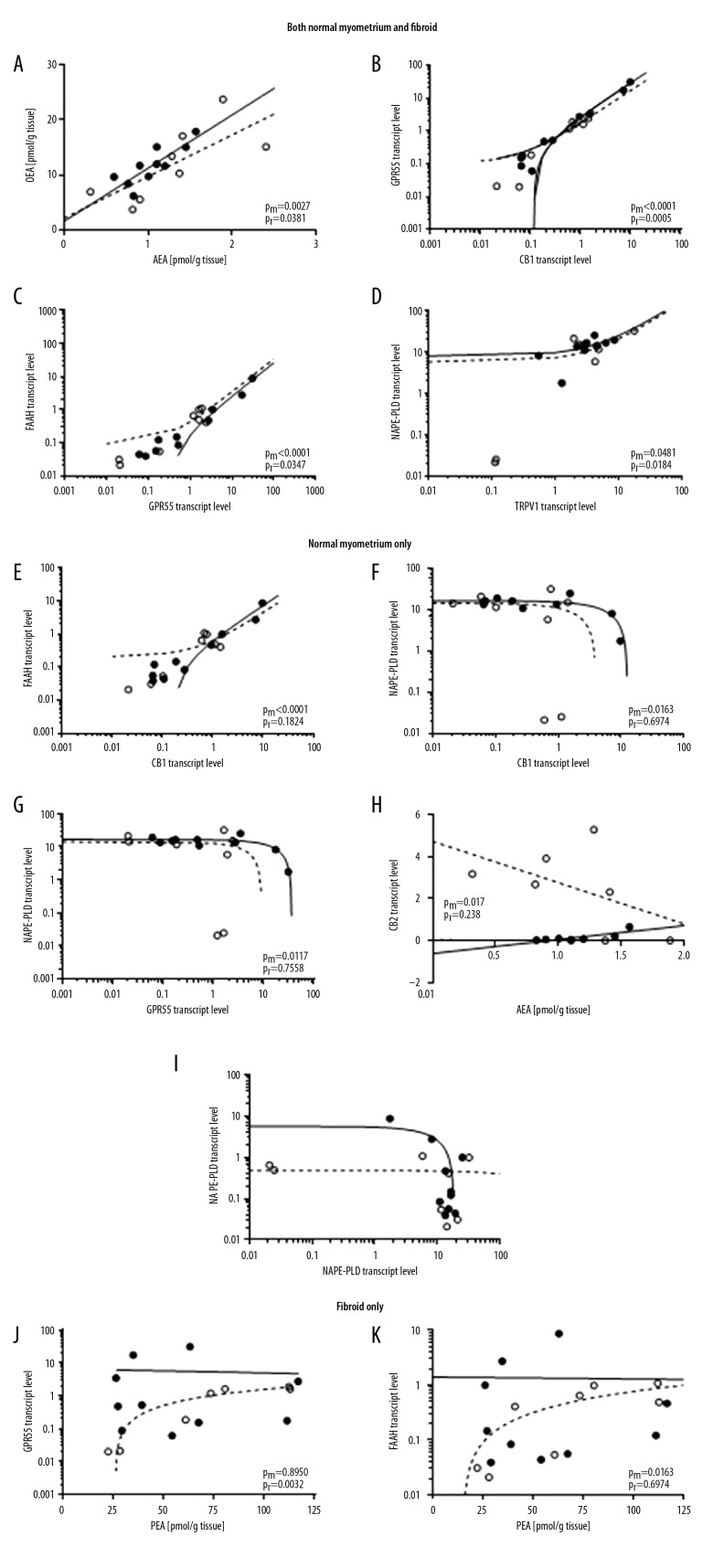
Table 3 shows the relationships between tissue NAE levels, and receptor and enzyme expression levels, for normal myometrium and fibroids. The data show that the levels of AEA and OEA were positively correlated both within normal myometrium and in fibroids. At the same time, there were positive correlates between GPR55 and CB1 (myometrium: R2=0.9802, P<0.0001; fibroids: R2=0.8872, P=0.0005), GPR55 and FAAH (myometrium: R2=0.9479, P<0.0001; fibroids: R2=0.5521, P=0.0347) and NAPE-PLD and TRPV1 (myometrium: R2=0.4043, P=0.0481; fibroids: R2=0.6320, P=0.0184) in both tissue types (Table 3, Figure 3).
Table 3.
Correlation analyses of the interactions between components of the endocannabinoid system in normal myometrium and fibroid tissue.
| Fibroid | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Normal | AEA | OEA | PEA | CB1 | CB2 | GPR55 | TRPV1 | NAPE-PLD | FAAH | |
| AEA | 0.5390 | −0.3058 | −4 x×10−7 | −0.2640 | −0.0160 | −0.1270 | −0.0172 | −0.1463 | ||
| OEA | 0.6946 | −0.4577 | 0.0024 | −0.1891 | −0.0003 | −0.0954 | −6.8×10−5 | −0.2029 | ||
| PEA | 0.0018 | −0.0689 | 0.2110 | 0.2579 | 0.8485 | 0.0056 | −0.2046 | 0.5678 | ||
| CB1 | −0.0730 | 0.0270 | −0.0085 | −0.0614 | 0.8872 | −0.0013 | −0.0304 | 0.2747 | ||
| CB2 | 0.6396 | −0.1086 | −0.1826 | 0.0086 | −0.0179 | 0.2000 | −0.0069 | 0.0632 | ||
| GPR55 | −0.0436 | 0.0414 | −0.0023 | 0.9802 | 0.0011 | 0.0098 | −0.0173 | 0.5521 | ||
| TRPV1 | 0.2309 | 0.0291 | 0.1505 | −0.3385 | −0.0582 | −0.3073 | 0.6320 | 0.1722 | ||
| NAPE-PLD | 0.0585 | −0.0096 | −0.0098 | −0.5345 | −0.0006 | −0.5690 | 0.4043 | −0.0004 | ||
| FAAH | −0.0094 | 0.0714 | −0.0001 | 0.8712 | −0.0049 | 0.9479 | −0.2294 | −0.5186 | ||
The data are presented as the Pearson correlation squared (R2); inverse correlations are indicated with a minus sign placed before each number. Correlations that are significantly different to zero are shown in a bold font and those relationships that are similar in both tissue types are shaded. The significant correlations (with p-values) are shown graphically in Figure 3.
Figure 3.
Correlation analysis of the endocannabinoid system in normal myometrium and fibroid. Pearson correlation analysis is shown for the expression of the indicated components of the endocannabinoid system in normal myometrium (solid line) and in fibroid tissue (dashed line). Factors that are either significantly positively or negatively correlated in both normal myometrium and fibroid tissue are shown in the upper panel (A–D) (previous page), whilst those correlated only in the normal myometrium are shown in the middle panel (E–I) (this page), and those only correlated in the fibroid tissue are shown in the lower panel (J, K) (next page). Statistically insignificant correlations are presented in Table 3. The P-values for the normal myometrium (Pm) and for fibroid (Pf) are shown on the individual graphs. (○ – fibroid; ● – normal myometrium). Transcript levels were corrected against the geometric mean of 3 housekeeping genes as described in the Material and Methods section.
By contrast, in the normal myometrium there was a positive correlation between FAAH and CB1 (myometrium: R2=0.8712, P<0.0001; fibroids: R2=0.2747, P=0.1824), a negative correlation between NAPE-PLD and GPR55 (myometrium: R2=0.5690, P=0.0117; fibroids: R2=0.0174, P=0.7558) and between NAPE-PLD and CB1 (myometrium: R2=0.5345, P=0.0163; fibroids: R2=0.0304, P=0.6794) that was not present in the corresponding fibroids (Table 3, Figure 3). Interestingly, there was also an inverse correlation between CB2 and AEA levels in the normal myometrium that was absent from the fibroid (myometrium: R2=0.6396, P=0.0172; fibroids: R2=0.2640, P=0.2381). Taken together, these observations suggest a dysregulation of the endocannabinoid system in fibroid tissue.
Fibroids also showed 2 correlates that were absent in normal myometrium; these were between GPR55 and PEA (myometrium: R2=0.0023, P=0.8950; fibroids: R2=0.8485, P=0.0032, and FAAH and PEA (myometrium: R2=0.0001, P=0.9734; fibroids: R2=0.5678, P=0.0309). In each case, the relationship was positive (i.e., as one parameter increases so does the other). The strongest relationship was between GPR55 and CB1 expression in fibroids (R2-value=0.887; Table 3).
Discussion
In this study, we have shown that the 3 N-acylethanolamines, AEA, OEA, and PEA are present in the normal myometrium and in fibroid tissue. PEA levels were significantly lower in fibroids compared to adjacent normal myometrial tissue, particularly in the post-menopausal group, suggesting that the lower levels or indeed a loss of this NAE might be associated with disease regression. Conversely, the slightly lower AEA levels and slightly higher OEA levels in the fibroid in the post-menopausal group, points to possible alterations in these 2 NAEs as disease progresses. As far as we are aware, the actions of PEA in the human myometrium have not been studied previously, but in the mouse prostaglandin-stimulated model, PEA inhibits myometrial contractility [39], suggesting that it might play a similar role in the human myometrium. One hypothesis related to fibroid development is that during the normal menstrual cycle and with postmenopausal bleeding, the clonal expansion of damaged smooth muscle cells, especially close to the stratum basalis of the endometrium (subserosal fibroids) are normally shed by a myometrial contractile mechanism [40,41], but in the presence of multiple fibroids the normal contractile force involved is impaired [15]. This results in fibroid smooth muscle cell retention, growth and, in some cases, infertility [42]. If PEA acts to prevent human myometrial contractility, as it does in the mouse [39], then PEA could thus be the missing link in this hypothesis.
We also observed that mean transcript levels for CB1 decreased from 2.0 to 0.6 while that for CB2 increased from 0.9 to 2.2 resulting in an almost 10-fold change in the CB1 to CB2 transcript ratio. Although none of the receptor expression level changes reached significance, the significant reduction in CB1 transcript levels in relation to other genes (CB2) is in keeping with the observations of Lee et al. [16] who reported a similar 72% reduction in CB1 transcript levels in their microarray study. Unfortunately, they did not report any changes in CB2, NAPE-PLD, or FAAH transcript levels. The reason for this was unavailability of cDNA targets for these 3 genes (and TRPV1 and GPR55) on their microarray at the time of their study. We are unaware of any other studies that have evaluated the levels of NAPE-PLD, FAAH, TRPV1, or GPR55 transcript levels in fibroid tissue or normal myometrium, and definitely none that have reported a comparative study of these endocannabinoid system components in myometrial disease.
There is ample support in the literature for the loss of CB1 receptor expression coinciding with a loss of apoptosis or cellular autophagy (see [43] and references therein), which has led to the suggestion that cannabinoids (both natural and synthetic) might be useful in the prevention of both benign [31,44,45] and malignant [46–48] tumor growth. The observations that the transcript level for the GPR55 receptor was 5-fold lower in fibroids, whilst the level for TRPV1 remained constant probably indicate that the loss of GPR55 might also be involved the pathogenesis of fibroid formation, whilst TRPV1 is not. The available evidence suggests that the expression of GPR55 is closely linked to malignant transformation in many tumor types [49] and its reduction in fibroids might be involved in preventing the transformation of the fibroid into its malignant form, the leiomyosarcoma, even though such transformations are incredibly rare.
We also observed changes in FAAH expression, but not in NAPE-PLD. There was a decrease in FAAH expression from 1.3 to 0.5 resulting in a significant 2.5-fold increase in the NAPE-PLD: FAAH ratio. These data suggest that the NAPE-PLD: FAAH expression ratio might be involved in the regulation of tissue NAE levels in fibroids. The increase in the NAPE-PLD: FAAH ratio should have resulted in an increased production of at least one of the NAEs, as it does in other reproductive tissues [50], and although this was originally observed in our pilot study [19], but this was not observed when a larger patient cohort was examined. It is possible, of course, that under these circumstances, mRNA levels are not indicative of functional protein levels; i.e., regulation is not at the transcription level but at the translation stage of protein synthesis or that other enzymes are involved in the NAE catalysis in these tissues, however, these studies are beyond the scope of the present study. What was observed was a positive correlation between OEA and AEA in both normal myometrium and fibroids (Table 3, Figure 3) suggesting that the increased NAPE-PLD: FAAH ratio is biased towards these 2 NAEs, resulting in a preference for PEA degradation and thus the significantly decreased PEA levels observed in the fibroids. There was also a strong inverse correlation between NAPE-PLD and FAAH expression, but only in the fibroid (Table 3, Figure 3) suggesting that reciprocal regulation of these 2 enzymes occurs in this tissue. This observation is supported by the differential regulation of these 2 enzymes in other reproductive tissues [24,50,51]. An interesting observation from the correlation analyses, is that GPR55 expression correlated positively with FAAH expression in both tissue types, and that PEA correlated positively with GPR55 and FAAH expression, but only in the fibroid tissue. Since PEA binds to and activates the GPR55 receptor [52] then in the normal myometrial smooth muscle it could be postulated that PEA binds to its receptor (GPR55) resulting in an intracellular cascade that alters gene expression including increased FAAH expression. In the fibroids, this mechanism does not take place because there is a decreased amount of PEA, and there would thus be reduced GPR55 and FAAH expression relative to that in normal myometrium, which was observed (Table 2).
Changes in the expression of the endocannabinoid system in the uterus have been attributed to changes in estrogen/progesterone homeostasis. In rodents, estradiol (E2) appears to upregulate endocannabinoid biosynthesis and directly correlates with uterine and endocannabinoid plasma concentrations in women [18,53]. On the other hand, progesterone increases the expression of FAAH [54] suggesting that menstrual cycle-dependent dysregulation of estrogen and progesterone homeostasis might cause increased mitotic activity and enhanced DNA replication in myometrial smooth muscle cells leading to fibroid growth. Indeed, there is evidence that tissue aromatase expression and activity is increased in fibroid tissue, which results in increased local E2 tissue levels [55,56]. The large age range of the volunteers (37 to 77 years) and mixture of pre- and post-menopausal status suggests that across the group there were marked differences in estrogen and progesterone concentrations and their ratio, which may have contributed to the variability in the data and the consequential lack of statistical significance. To overcome this limitation, we reclassified our data into pre-menopausal and post-menopausal samples and revealed that the effect of disease on the levels of 2 of the NAEs, namely OEA and PEA, was aged-related. This effect has led us to postulate a new working hypothesis for the pathogenesis of leiomyomata (Figure 4), in which the actions of estrogen and progesterone might, through the endocannabinoid system, attenuate disease progression. In this scenario, we predict that loss of estrogen and progesterone action results in the observed imbalance of enzyme expression and activity, thus allowing the selective destruction of OEA and PEA in and around the myometrial cells that are destined to become the fibroid lesion. More work in this area to test this hypothesis is needed, but we would predict that women on hormone replacement therapy (HRT) (not examined in this study) might be provided with some protection against the development of some forms of leiomyomata.
Figure 4.
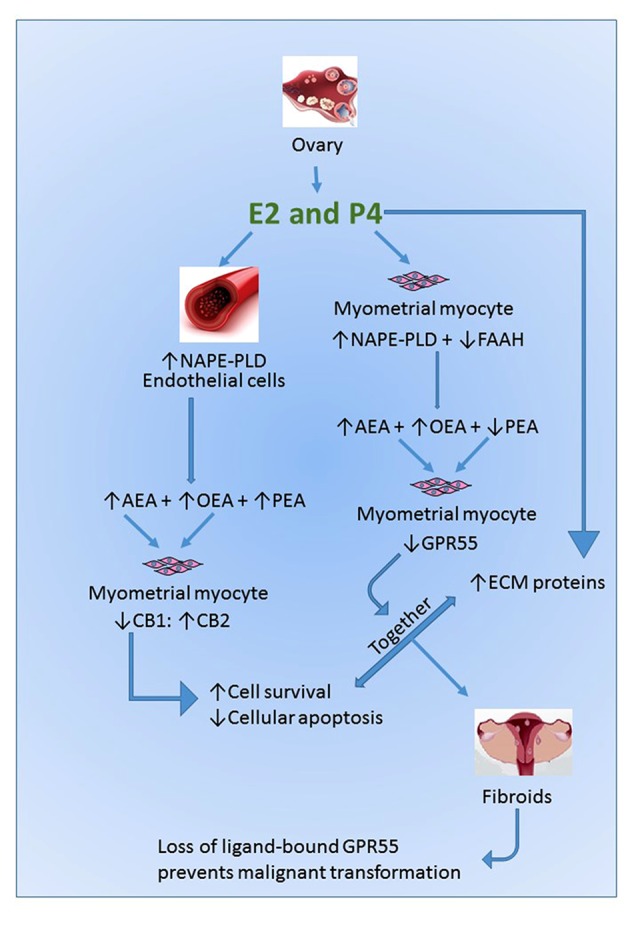
Working Hypothesis Model. Our working hypothesis is that gonadal sex steroid hormones act independently and synergistically to alter the phenotype of myometrial smooth muscle cells (SMCs). Estradiol (E2) acts directly on uterine blood vessel endothelial cells to increase the expression and activity of NAPE-PLD to increase systemic NAE concentrations. Paracrine activation of the cannabinoid receptors that have altered expression on the SMCs results in increased cell survival and proliferation. Simultaneously, progesterone (P4) acts directly on the SMCs to increase the expression and deposition of extracellular matrix proteins (ECM), a common feature of fibroids [67], and alter the ratio of NAPE-PLD: FAAH expression, resulting in increased degradation of PEA and reduced expression of GPR55 receptor protein. The combination of these factors moves the SMC towards a fibroid generating phenotype. The lack of an inducible GPR55 receptor in the resultant fibroid tissue prevents the formation of a malignant uterine leiomyosarcoma.
In summary, the data presented suggest a potential role for the loss of PEA and FAAH in the development of fibroids and suggest them as possible future targets for the development of novel therapies, even if that development might be a long-time coming. The suggestion that an imbalance in estrogen and progesterone at the tissue level is associated with fibroid development [27,30] is supported and it might be that subtle changes in the expression of some components of the endocannabinoid system are insufficient to prevent the increase in the mitotic activity of smooth myometrial cells and enhanced DNA replication that leads to the fibroid growth [15], and that the loss of GPR55 prevents malignant transformation. These findings, together with previous investigations providing evidence of the ECS and its roles in myometrial smooth muscle cell fates [57] suggest a key role for this system in normal and abnormal myometrial development and function.
Conclusions
In the past decade, interest in the impact of uterine fibroids on women’s health, its etiology and implications for future therapy has increased [15,58–66]. Although renewed interest in this area is gathering pace, and new therapeutic options are being developed [59], a clearer understanding of the factors that underpin the mechanism(s) causing this increasingly prevalent disease [15] is currently lacking. Any studies that improve our understanding behind the etiopathogenesis of fibroids are likely to provide the rationale for developing potential additional therapeutic options. In this regard, the endocannabinoid system may provide a new avenue of research.
Acknowledgements
The authors thank Dr. Lawrence Brown (Gynaecology Pathologist, Department of Pathology, University Hospitals Leicester NHS Trust, Leicester Royal Infirmary), for providing the dissected tissues samples.
Footnotes
Source of support: This work was funded by grants from the University Hospitals of Leicester NHS Trust and miscellaneous income from Postgraduate (MRCOG) courses run by Professor Konje at the University of Leicester
Conflicts of interest
None.
References
- 1.Cramer SF, Patel A. The frequency of uterine leiomyomas. Am J Clin Path. 1990;94:435–38. doi: 10.1093/ajcp/94.4.435. [DOI] [PubMed] [Google Scholar]
- 2.Tropeano G, Amoroso S, Scambia G. Non-surgical management of uterine fibroids. Hum Reprod Update. 2008;14:259–74. doi: 10.1093/humupd/dmn006. [DOI] [PubMed] [Google Scholar]
- 3.Hosh M, Antar S, Nazzal A, et al. Uterine sarcoma: analysis of 13,089 cases based on surveillance, epidemiology, and end results database. Intl J Gynecol Cancer. 2016;26:1098–104. doi: 10.1097/IGC.0000000000000720. [DOI] [PubMed] [Google Scholar]
- 4.Stewart EA, Friedman AJ, Peck K, Nowak RA. Relative overexpression of collagen type I and collagen type III messenger ribonucleic acids by uterine leiomyomas during the proliferative phase of the menstrual cycle. J Clin Endocrinol Metab. 1994;79:900–6. doi: 10.1210/jcem.79.3.8077380. [DOI] [PubMed] [Google Scholar]
- 5.Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science. 2005;308:1589–92. doi: 10.1126/science.1112063. [DOI] [PubMed] [Google Scholar]
- 6.Rein MS, Barbieri RL, Friedman AJ. Progesterone: A critical role in the pathogenesis of uterine myomas. Am J Obstet Gynecol. 1995;172:14–18. doi: 10.1016/0002-9378(95)90077-2. [DOI] [PubMed] [Google Scholar]
- 7.Sadovsky Y, Kushner PJ, Roberts JM, Riemer RK. Restoration of estrogen-dependent progesterone receptor expression in a uterine myocyte cell line. Endocrinology. 1993;132:1609–13. doi: 10.1210/endo.132.4.8462459. [DOI] [PubMed] [Google Scholar]
- 8.Shimomura Y, Matsuo H, Samoto T, Maruo T. Up-regulation by progesterone of proliferating cell nuclear antigen and epidermal growth factor expression in human uterine leiomyoma. J Clin Endocrinol Metab. 1998;83:2192–98. doi: 10.1210/jcem.83.6.4879. [DOI] [PubMed] [Google Scholar]
- 9.Strawn EY, Jr, Novy MJ, Burry KA, Bethea CL. Insulin-like growth factor I promotes leiomyoma cell growth in vitro. Am J Obstet Gynecol. 1995;172:1837–43. doi: 10.1016/0002-9378(95)91420-x. discussion 1843–44. [DOI] [PubMed] [Google Scholar]
- 10.Yu L, Saile K, Swartz CD, et al. Differential expression of receptor kinases (RTKs) and IGF-1 pathway activation in human uterine leiomyomas. Mol Med. 2008;14:264–75. doi: 10.2119/2007-00101.Yu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Catherino WH, Parrott E, Segars J. Proceedings from the National Institute of Child Health and Human Development conference on the Uterine Fibroid Research Update Workshop. Fertil Steril. 2011;95:9–12. doi: 10.1016/j.fertnstert.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cardozo ER, Clark AD, Banks NK, et al. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol. 2012;206:211e1–9. doi: 10.1016/j.ajog.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Advincula AP, Xu X, Goudeau St, Ransom SB. Robot-assisted laparoscopic myomectomy versus abdominal myomectomy: A comparison of short-term surgical outcomes and immediate costs. J Minim Invas Gyn. 2007;14:698–705. doi: 10.1016/j.jmig.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Subramanian S, Spies JB. Uterine artery embolization for leiomyomata: Resource use and cost estimation. J Vasc Interv Radiol. 2001;12:571–74. doi: 10.1016/s1051-0443(07)61477-8. [DOI] [PubMed] [Google Scholar]
- 15.Bulun SE. Uterine fibroids. New Engl J Med. 2013;369:1344–55. doi: 10.1056/NEJMra1209993. [DOI] [PubMed] [Google Scholar]
- 16.Lee EJ, Kong G, Lee SH, et al. Profiling of differentially expressed genes in human uterine leiomyomas. Int J Gynecol Cancer. 2005;15:146–54. doi: 10.1111/j.1048-891x.2005.15016.x. [DOI] [PubMed] [Google Scholar]
- 17.Marczylo TH, Lam PM, Amoako AA, Konje JC. Anandamide levels in human female reproductive tissues: solid-phase extraction and measurement by ultraperformance liquid chromatography tandem mass spectrometry. Anal Biochem. 2010;400:155–62. doi: 10.1016/j.ab.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 18.El-Talatini MR, Taylor AH, Konje JC. The relationship between plasma levels of the endocannabinoid, anandamide, sex steroids, and gonadotrophins during the menstrual cycle. Fertil Steril. 2010;93:1989–96. doi: 10.1016/j.fertnstert.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 19.Marczylo TH, Lam PM, Konje JC. Measurement of anandamide in gynaecological tissue samples: Levels are elevated in endometrial cancer and other pathologies. Reprod Sci. 2009;16:358A. [Google Scholar]
- 20.Sanchez AM, Cioffi R, Vigano P, et al. Elevated systemic levels of endocannabinoids and related mediators across the menstrual cycle in women with endometriosis. Reprod Sci. 2016;23:1071–79. doi: 10.1177/1933719116630414. [DOI] [PubMed] [Google Scholar]
- 21.Taylor AH, Abbas MS, Habiba MA, Konje JC. Histomorphometric evaluation of cannabinoid receptor and anandamide modulating enzyme expression in the human endometrium through the menstrual cycle. Histochem Cell Biol. 2010;133:557–65. doi: 10.1007/s00418-010-0695-9. [DOI] [PubMed] [Google Scholar]
- 22.Di Blasio AM, Vignali M, Gentilini D. The endocannabinoid pathway and the female reproductive organs. J Mol Endocrinol. 2013;50:R1–9. doi: 10.1530/JME-12-0182. [DOI] [PubMed] [Google Scholar]
- 23.El-Talatini MR, Taylor AH, Elson JC, et al. Localisation and function of the endocannabinoid system in the human ovary. PLoS One. 2009;4:e4579. doi: 10.1371/journal.pone.0004579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gebeh AK, Willets JM, Bari M, et al. Elevated anandamide and related N-acylethanolamine levels occur in the peripheral blood of women with ectopic pregnancy and are mirrored by changes in peripheral fatty acid amide hydrolase activity. J Clin Endocrinol Metab. 2013;98:1226–34. doi: 10.1210/jc.2012-3390. [DOI] [PubMed] [Google Scholar]
- 25.Taylor AH, Finney M, Lam PM, Konje JC. Modulation of the endocannabinoid system in viable and non-viable first trimester pregnancies by pregnancy-related hormones. Reprod Biol Endocrinol. 2011;9:152. doi: 10.1186/1477-7827-9-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bambang K, Karisu T, Gebeh A, et al. From fertilisation to implantation in mammalian pregnancy – modulation of early human reproduction by the endocannabinoid system. Pharmaceuticals. 2010;3:2910–29. doi: 10.3390/ph3092910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.De Vivo A, Mancuso A, Giacobbe A, et al. Uterine myomas during pregnancy: A longitudinal sonographic study. Ultrasound Obstet Gynecol. 2011;37:361–65. doi: 10.1002/uog.8826. [DOI] [PubMed] [Google Scholar]
- 28.Dennedy MC, Friel AM, Houlihan DD, et al. Cannabinoids and the human uterus during pregnancy. Am J Obstet Gynecol. 2004;190:2–9. doi: 10.1016/j.ajog.2003.07.013. discussion 3A. [DOI] [PubMed] [Google Scholar]
- 29.Habayeb OM, Bell SC, Konje JC. Endogenous cannabinoids: Metabolism and their role in reproduction. Life Sci. 2002;70:1963–77. doi: 10.1016/s0024-3205(01)01539-9. [DOI] [PubMed] [Google Scholar]
- 30.Laughlin SK, Herring AH, Savitz DA, et al. Pregnancy-related fibroid reduction. Fertil Steril. 2010;94:2421–23. doi: 10.1016/j.fertnstert.2010.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dmitrieva N, Nagabukuro H, Resuehr D, et al. Endocannabinoid involvement in endometriosis. Pain. 2010;151:703–10. doi: 10.1016/j.pain.2010.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Resuehr D, Glore DR, Taylor HS, et al. Progesterone-dependent regulation of endometrial cannabinoid receptor type 1 (CB1-R) expression is disrupted in women with endometriosis and in isolated stromal cells exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) Fertil Steril. 2012;98:948–956e1. doi: 10.1016/j.fertnstert.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanchez AM, Vigano P, Mugione A, et al. The molecular connections between the cannabinoid system and endometriosis. Mol Hum Reprod. 2012;18:563–71. doi: 10.1093/molehr/gas037. [DOI] [PubMed] [Google Scholar]
- 34.Matsuda LA, Lolait SJ, Brownstein MJ, et al. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–64. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 35.Ledent C, Valverde O, Cossu G, et al. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–4. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- 36.Zimmer A, Zimmer AM, Hohmann AG, et al. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:5780–85. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lam PM, Marczylo TH, El-Talatini M, et al. Ultra performance liquid chromatography tandem mass spectrometry method for the measurement of anandamide in human plasma. Anal Biochem. 2008;380:195–201. doi: 10.1016/j.ab.2008.05.033. [DOI] [PubMed] [Google Scholar]
- 38.Chomczynski P, Mackey K. Substitution of chloroform by bromo-chloropropane in the single-step method of RNA isolation. Anal Biochem. 1995;225:163–64. doi: 10.1006/abio.1995.1126. [DOI] [PubMed] [Google Scholar]
- 39.Pagano E, Orlando P, Fonzie S, et al. Role of the endocannabinoid system in the control of mouse myometrium contractility during the menstrual cycle. Biochem Pharmacol. 2017;124:83–93. doi: 10.1016/j.bcp.2016.11.023. [DOI] [PubMed] [Google Scholar]
- 40.Pritts EA, Parker WH, Olive DL. Fibroids and infertility: An updated systematic review of the evidence. Fertil Steril. 2009;91:1215–23. doi: 10.1016/j.fertnstert.2008.01.051. [DOI] [PubMed] [Google Scholar]
- 41.Taylor AH, Habiba MA. The myometrium in health and disease. In: Habiba MA, Benagiano G, editors. Uterine Adenomyosis. Springer; New York, USA: 2015. pp. 71–79. [Google Scholar]
- 42.Klatsky PC, Tran ND, Caughey AB, Fujimoto VY. Fibroids and reproductive outcomes: A systematic literature review from conception to delivery. Am J Obstet Gynecol. 2008;198:357–66. doi: 10.1016/j.ajog.2007.12.039. [DOI] [PubMed] [Google Scholar]
- 43.Costa L, Amaral C, Teixeira N, et al. Cannabinoid-induced autophagy: Protective or death role? Prost Other Lipid Med. 2016;122:54–63. doi: 10.1016/j.prostaglandins.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 44.Biglic E, Meydanli EG, Kose S, et al. Endocannabinoids modulate apoptosis in endometriosis and adenomyosis. Acta Histochem. 2017;119:523–32. doi: 10.1016/j.acthis.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 45.Leconte M, Nicco C, Ngo C, et al. Antiproliferative effects of cannabinoid agonists on deep infiltrating endometriosis. Am J Pathol. 2010;177:2963–70. doi: 10.2353/ajpath.2010.100375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ayakannu T, Taylor AH, Willets JM, Konje JC. The endocannabinoid system and sex steroid hormone-dependent cancers. Int J Endocrinol. 2013;2013 doi: 10.1155/2013/259676. 259676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Javid FA, Phillips RM, Afshinjavid S, et al. Cannabinoid pharmacology in cancer research: A new hope for cancer patients? Eur J Pharmacol. 2016;775:1–14. doi: 10.1016/j.ejphar.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 48.Velasco G, Hernandez-Tiedra S, Davila D, Lorente M. The use of cannabinoids as anticancer agents. Prog Neuropsychopharmacol Biol Psych. 2016;64:259–66. doi: 10.1016/j.pnpbp.2015.05.010. [DOI] [PubMed] [Google Scholar]
- 49.Andradas C, Caffarel MM, Perez-Gomez E, et al. The role of GPR55 in cancer. In: Abood ME, Sorensen RG, Nephi S, editors. endoCANNABINOIDS: Actions at non-CB1/CB2 cannabinoid receptors. Springer; New York, USA: 2013. pp. 115–33. [Google Scholar]
- 50.Karasu T, Marczylo TH, Marczylo EL, et al. The effect of mifepristone (RU486) on the endocannabinoid system in human plasma and first-trimester trophoblast of women undergoing termination of pregnancy. J Clin Endocrinol Metab. 2014;99:871–80. doi: 10.1210/jc.2013-2922. [DOI] [PubMed] [Google Scholar]
- 51.Gebeh AK, Willets JM, Marczylo EL, et al. Ectopic pregnancy is associated with high anandamide levels and aberrant expression of FAAH and CB1 in fallopian tubes. J Clin Endocrinol Metab. 2012;97:2827–35. doi: 10.1210/jc.2012-1780. [DOI] [PubMed] [Google Scholar]
- 52.Ryberg E, Larsson N, Sjogren S, et al. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152:1092–101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Scotchie JG, Savaris RF, Martin CE, Young SL. Endocannabinoid regulation in human endometrium across the menstrual cycle. Reprod Sci. 2015;22:113–23. doi: 10.1177/1933719114533730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gasperi V, Fezza F, Spagnuolo P, et al. Further insights into the regulation of human FAAH by progesterone and leptin implications for endogenous levels of anandamide and apoptosis of immune and neuronal cells. Neurotoxicology. 2005;26:811–17. doi: 10.1016/j.neuro.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 55.Bulun SE, Moravek MB, Yin P, et al. Uterine stem cells: Linking progesterone to growth. Semin Reprod Med. 2015;33:357–65. doi: 10.1055/s-0035-1558451. [DOI] [PubMed] [Google Scholar]
- 56.Sumitani H, Shozu M, Segawa T, et al. In situ estrogen synthesized by aromatase P450 in uterine leiomyoma cells promotes cell growth probably via an autocrine/intracrine mechanism. Endocrinology. 2000;141:3852–61. doi: 10.1210/endo.141.10.7719. [DOI] [PubMed] [Google Scholar]
- 57.Guzman M, Sanchez C, Galve-Roperh I. Cannabinoids and cell fate. Pharmacol Therapeut. 2002;95:175–84. doi: 10.1016/s0163-7258(02)00256-5. [DOI] [PubMed] [Google Scholar]
- 58.Agnihotri S. Assessment and treatment of uterine fibroids. Prescriber. 2016;27:28–33. [Google Scholar]
- 59.Donnez J, Dolmans M-M. Uterine fibroid management: from the present to the future. Hum Reprod Update. 2016;22:665–86. doi: 10.1093/humupd/dmw023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hou P, Zhao L, Li Y, et al. Comparative expression of thioredoxin-1 in uterine leiomyomas and myometrium. Mol Hum Reprod. 2014;20:148–54. doi: 10.1093/molehr/gat069. [DOI] [PubMed] [Google Scholar]
- 61.Islam MS, Protic O, Giannubilo SR, et al. Uterine leiomyoma: Available medical treatments and new possible therapeutic options. J Clin Endocrinol Metab. 2013;98:921–34. doi: 10.1210/jc.2012-3237. [DOI] [PubMed] [Google Scholar]
- 62.Karmon AE, Cardozo ER, Rueda BR, Styer AK. MicroRNAs in the development and pathobiology of uterine leiomyomata: Does evidence support future strategies for clinical intervention? Hum Reprod Update. 2014;20:670–87. doi: 10.1093/humupd/dmu017. [DOI] [PubMed] [Google Scholar]
- 63.Moravek MB, Yin P, Ono M, et al. Ovarian steroids, stem cells and uterine leiomyoma: therapeutic implications. Hum Reprod Update. 2015;21:1–12. doi: 10.1093/humupd/dmu048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rice KE, Secrist JR, Woodrow EL, et al. Etiology, diagnosis, and management of uterine leiomyomas. J Midwifery Wom Heal. 2012;57:241–47. doi: 10.1111/j.1542-2011.2012.00176.x. [DOI] [PubMed] [Google Scholar]
- 65.Soliman AM, Yang H, Du EX, et al. The direct and indirect costs of uterine fibroid tumors: A systematic review of the literature between 2000 and 2013. Am J Obstet Gynecol. 2015;213:141–60. doi: 10.1016/j.ajog.2015.03.019. [DOI] [PubMed] [Google Scholar]
- 66.Tal R, Segars JH. The role of angiogenic factors in fibroid pathogenesis: Potential implications for future therapy. Hum Reprod Update. 2014;20:194–216. doi: 10.1093/humupd/dmt042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bulun SE, Simpson ER, Word RA. Expression of the CYP19 gene and its product aromatase cytochrome P450 in human uterine leiomyoma tissues and cells in culture. J Clin Endocrinol Metab. 1994;78:736–43. doi: 10.1210/jcem.78.3.8126151. [DOI] [PubMed] [Google Scholar]