Abstract
Background: The objective of this study is to assess group differences in symptom reduction between individuals receiving group cognitive behavioral therapy (G-CBT) and attention bias modification (ABM) compared to their respective control interventions, control therapy (CT), and attention control training (ACT), in a 2 × 2 factorial design.
Methods: A total of 310 treatment-naive children (7–11 years of age) were assessed for eligibility and 79 children with generalized, separation or social anxiety disorder were randomized and received G-CBT (n = 42) or CT (n = 37). Within each psychotherapy group, participants were again randomized to ABM (n = 38) or ACT (n = 41) in a 2 × 2 factorial design resulting in four groups: G-CBT + ABM (n = 21), G-CBT + ACT (n = 21), CT + ABM (n = 17), and CT + ACT (n = 20). Primary outcomes were responder designation as defined by Clinical Global Impression-Improvement (CGI-I) scale (≤2) and change on the Pediatric Anxiety Rating Scale (PARS).
Results: There were significant improvements of symptoms in all groups. No differences in response rates or mean differences in PARS scores were found among groups: G-CBT + ABM group (23.8% response; 3.9 points, 95% confidence interval [CI] −0.3 to 8.1), G-CBT + ACT (42.9% response; 5.6 points, 95% CI 2.2–9.0), CT + ABM (47.1% response; 4.8 points 95% CI 1.08–8.57), and CT + ACT (30% response; 0.8 points, 95% CI −3.0 to 4.7). No evidence or synergic or antagonistic effects were found, but the combination of G-CBT and ABM was found to increase dropout rate.
Conclusions: We found no effect of G-CBT or ABM beyond the effects of comparison groups. Results reveal no benefit from combining G-CBT and ABM for anxiety disorders in children and suggest potential deleterious effects of the combination on treatment acceptability.
Keywords: : attention training, attention retraining, attention bias modification treatment, phobias, cognitive behavioral therapy, factorial
Introduction
Pediatric anxiety disorders are prevalent and debilitating conditions (Baxter et al. 2011) that lead to later adult psychopathology (Gregory et al. 2007) and other negative outcomes (Woodward and Fergusson 2001; Beesdo-Baum and Knappe 2012; Salum et al. 2013a). Selective serotonin reuptake inhibitors and cognitive behavioral therapies (CBTs) are first-line treatments (Ipser et al. 2009; James et al. 2013). Nevertheless, many individuals fail to fully respond (Ipser et al. 2009; James et al. 2013) or cannot tolerate these treatments (Ipser et al. 2009; de Souza et al. 2013), creating a need for novel effective, safe, and easy-to-disseminate treatments.
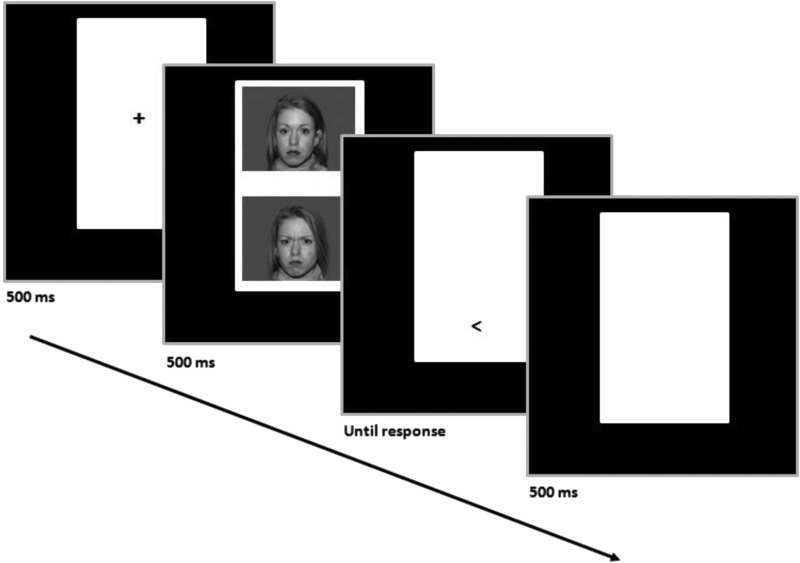
Attention bias modification (ABM) has emerged as one such possible treatment (MacLeod et al. 2002). ABM originated from the observation that anxious individuals show vigilance toward minor threats (Bar-Haim et al. 2007), using tasks such as the dot-probe paradigm (Fig. 1). In this task, threat and neutral stimuli are paired, competing for attention in different spatial locations. Each trial is followed by a probe at the spatial location of one of the two stimuli. Biases in attention allocation are measured as the mean difference in reaction times, in trials in which the probe replaces the neutral stimuli versus those in which the probe replaces the threatening stimuli. ABM alters the dot-probe paradigm to train attention by consistently pairing the location of the probe with the neutral stimuli, leading to reductions in threat bias through implicit learning (Abend et al. 2014; Shechner et al. 2014). Initial data suggest the treatment is effective in adults (Hakamata et al. 2010; Hallion and Ruscio 2011; Beard et al. 2012; Mogoase et al. 2014).
FIG. 1.
Dot-probe schematic representation.
Biases in threat-related attention also occur in pediatric anxiety disorders (Salum et al. 2013b; Shechner et al. 2012). Preliminary evidence also suggests that ABM may be effective treatment for pediatric anxiety (Bar-Haim et al. 2011; Eldar et al. 2012; Waters et al. 2013; Bechor et al. 2014; De Voogd et al. 2014), but efficacy of ABM as a standalone treatment in children is still controversial (Cristea et al. 2015; Heeren et al. 2015). Since ABM and CBT may target distinct mechanisms, synergistic effects between these two treatments may occur (Bar-Haim 2010). Randomized controlled trials integrating ABM into standard protocols indicate mixed results (Britton et al. 2013; Rapee et al. 2013; Riemann et al. 2013; Boettcher et al. 2014; Shechner et al. 2014), highlighting the need for more efficacy research on such combination treatments. In addition, only three randomized controlled trials targeting threat bias were conducted in children and adolescents. Two studies showed adding ABM to CBT, if compared to adding attention control conditions, resulted in a reduced number of anxiety symptoms at the end of treatment (Riemann et al. 2013; White et al. 2017), whereas one study showed adding both ABM and control conditions to CBT if compared to CBT alone resulted in benefits for anxiety symptoms, but failed to find differences between ABM and active control conditions (Shechner et al. 2014).
Previous evidence is limited in two important ways. First, the potential synergistic and antagonistic effects between CBT and ABM have not been examined previously using a factorial design, whereby both CBT and ABM are contrasted with control conditions, such as control therapy (CT) and attention control training (ACT). Second, because all available studies were conducted in high-income countries, no research examines efficacy in low- or middle-income countries where there is a great need for mental services (Kieling et al. 2011; de Souza et al. 2013). In this study, we address these limitations through a randomized controlled trial comparing efficacy of group CBT (G-CBT) and ABM in 7–11 year olds in a factorial design.
Methods
The methods are reported per CONSORT guidelines with the suggested amendments for factorial trials (McAlister et al. 2003).
Study overview
This study was conducted at the Hospital de Clinicas de Porto Alegre, Federal University of Rio Grande do Sul, Brazil, with a period of active recruitment from July 2011 and September 2012. Local institutional review board approval was obtained, and all parents of participants signed informed consent forms. The trial was registered at ClinicalTrials.gov (NCT01687764, “Combination of Active or Placebo Attentional Bias Modification Treatment [ABMT] to Either Cognitive Behavioral Group Therapy [CBGT] or Psychoeducational Control Intervention [PCI] for Anxiety Disorders in Children” https://clinicaltrials.gov/show/NCT01687764). Our registered protocol originally included a 6-month follow-up that was not performed due to logistic reasons.
Design
This is a unicenter factorial double-blind (child, caregivers and investigators), parallel-group randomized controlled trial in which participants were randomized using a 2 × 2 design to G-CBT or CT and subsequently to ABM or ACT.
Randomization
All subjects meeting study criteria were included in a list of eligible participants. After a sufficient number of participants were included in the list, randomization was performed for all participants at once in two moments as part of two separated lists (October 2011 and September 2012). This strategy was used to allow G-CBT and ACT therapy groups to be conducted in parallel and according to stratified randomization. We used computerized generated numbers used to allocate subjects to each group. A researcher not directly involved in treatment performed a stratified 1:1 randomization, stratified by age (7–9 vs. 10–11) and school shift (morning vs. afternoon). Stratification by age was performed to facilitate interactions among children in each group. Stratification by school shift was performed due to study logistics, since group were conducted in the shift in which the child was not at school.
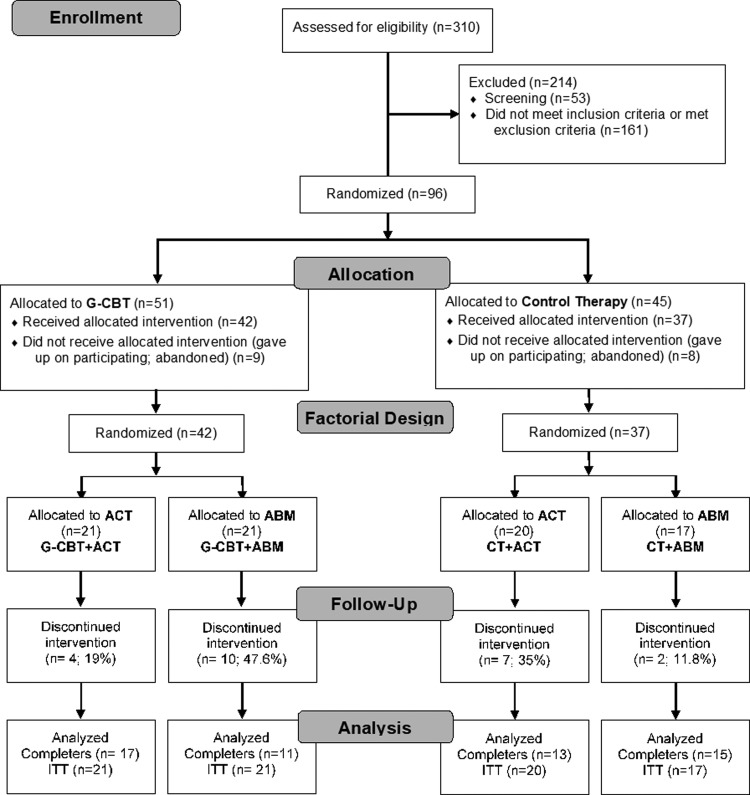
Participants were allocated in seven G-CBT groups and seven CT groups, each with five to seven children in a parallel group comparison. After that, participants within each group were again randomized 1:1 to receive ABM or ACT. A total of 96 participants who fulfilled inclusion criteria after the diagnostic assessment visit (see below) were randomized to CT or G-CBT and subsequently to ACT or ABM, resulting in four groups: G-CBT + ABM, G-CBT + ACT, CT + ABM, and CT + ACT (Fig. 2). Eight participants in CT and nine participants in G-CBT dropped out before receiving treatment and baseline symptom assessment, resulting in 79 who were randomized and received treatment in one of four groups: G-CBT + ABM, G-CBT + ACT, CT + ABM, and CT + ACT (Fig. 2).
FIG. 2.
CONSORT flow diagram.
Procedures
Participants were recruited by media advertisements and were prescreened by a brief telephone interview. Potentially eligible children and their parents were invited for a full diagnostic interview with a team of trained clinicians (diagnostic assessment visit). Children who fulfilled the inclusion criteria were randomized to each study intervention. Allocation to groups was concealed, so therapists did not know the participant's group allocation until the delivery of the first therapy session. Participants were not directly informed to which type of intervention group they were assigned. Participants and their parents were assessed for baseline symptoms of psychiatric disorders (primary and secondary outcomes) and threat bias before treatment started within the week of the first therapy session (baseline symptomatic assessment visit). Children were included in the trial irrespective of their initial threat bias status, as performed in other studies (Shechner et al. 2012). CT/G-CBT was delivered in groups and at the end of each therapy session, children performed the ABM/ACT training on individual laptops. One week after treatment ended, psychiatric symptoms and threat bias were reassessed (endpoint symptomatic assessment visit).
Participants
We included participants with the following characteristics: (i) primary diagnosis of generalized anxiety disorder (GAD), separation anxiety disorder (SeAD), or social anxiety disorder (SoAD) according to standard diagnostic procedures described below. Exclusion criteria were as follows: (i) other psychiatric disorder judged by the clinician to cause more impairment or distress than GAD, SeAD, or SoAD; (ii) any history of mental health treatment; and (iii) Intelligence Quotient lower than 70 (Raven).
Assessments
The Dot-probe task
The dot-probe task was used to measure biases in attention orienting to threatening stimuli before and after treatment. Tasks were presented by testers blinded to all clinical data as part of the Tel Aviv University - National Institute of Mental Health (TAU-NIMH) ABM Treatment Initiative, which provides standardized methods to measure and deliver ABM procedures (Bar-Haim et al. 2010). A full description of the dot-probe task and data preparation can be found in Supplementary Data (Supplementary Data are available online at www.liebertpub.com/cap).
Diagnostic assessment
All children underwent a comprehensive psychiatric evaluation with the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime (K-SADS-PL) (Kaufman et al. 1997; Brasil 2003) at the baseline assessment. Primary diagnoses were based on the Clinical Global Impression-Severity scale that was rated independently for each psychiatric disorder. Interviews were performed by doctoral or master degree-level clinicians (L.N.B, D.S., R.B.J., and R.T.) and weekly supervised by a senior clinician (G.G.M.). Before the trial, all interviewers received formal K-SADS-PL training, which included lectures on the instrument, in vivo interviews with more experienced clinicians, and the rating of video-taped interviews.
Primary outcomes
Clinician-based ratings from CGI-I and Pediatric Anxiety Rating Scale (PARS) were administered by the team that performed the diagnostic assessment and by physicians with experience in psychiatric research (G.A.S. and R.B.J.). No researchers involved in the delivery of interventions took part in any diagnostic or symptomatic assessment.
CGI-I scale
The primary dichotomous outcome was the percentage of participants rated as “much improved” (score of 2) or “very much improved” (score of 1) on the CGI-I scale in week 10. For the purposes of this analysis, we present statistics on both completers and intention to treat. For intention-to-treat analysis on this dichotomous measure, all dropouts are considered nonrespondents.
Pediatric Anxiety Rating Scale
The primary continuous outcome was the change on the PARS from baseline to week 10, computed by the sum of five items assessing anxiety severity, frequency, distress, avoidance, and interference at the end of treatment, in accordance to standardized methods (RUPP 2002). Total scores on this scale range from 0 to 25 (RUPP 2002), with scores from 8 to 10 suggesting strong treatment response (Caporino et al. 2013). We also present scores on PARS symptom count on the 50 symptoms rated as present/absent varying from 0 to 50. The Cronbach's alpha for PARS and PARS symptom count in the baseline of this sample was 0.818 and 0.795, respectively.
Secondary outcomes
Secondary measures included measures of anxiety symptoms (Screen for Child Anxiety Related Emotional Disorders [SCARED] and Spence Children's Anxiety Scale [SCAS]), depression (Children's Depression Inventory [CDI]), and attention-deficit hyperactivity (Swanson, Nolan, and Pelham Questionnaire [SNAP-IV]), and measures of overall level of symptoms and impairment (Strengths and Difficulties Questionnaire [SDQ]). Secondary outcome description, reliability, and references can be found in Supplementary Data.
Interventions
Interventions took place in a local clinic (“Projecto” clinic), comprising four specific interventions: CT + ACT, CT + ABM, G-CBT + ACT, and G-CBT + ABM. Both ACT and ABM were administered at the end of each CT or G-CBT group session.
ACT and ABM
The ABM consists of 160 trials (120 angry-neutral and 40 neutral-neutral presentations). In the ABM condition, the target appeared at the neutral-face location in all angry-neutral trials, creating a contingency between neutral face and target location. The ACT protocol consists of the same 160 trials as in the ABM protocol. However, threat face location, probe location, and actor were fully counterbalanced in presentation, thus implicitly teaching patients that there is no contingency between face emotion and target location. The set of faces used in bias assessment was different from the set used in the training protocols and the faces set used for measurement and training was counterbalanced across participants.
Cognitive behavioral therapy
The Friends for Life program follows the principles of G-CBT. It was developed with the aim of increasing social and emotional skills, promoting resilience and decreasing anxiety and depression. Studies have shown that the program helped children by reducing their anxiety symptoms and low mood (Barrett et al. 2001; Kosters et al. 2015). The protocol consists of 10 weekly 90-minute sessions and two booster sessions. A detailed description can be found in Supplementary Data. The “Friends for Life” protocol was delivered by trained psychologists who were supervised by a senior therapist, who has certificated rights to use the protocol in Brazil and coordinated the transcultural adaptation protocol (C.S.P.). Each group was delivered by a main therapist and an auxiliary therapist. A total of six trained psychologists participated in Friends for Life groups according to standardized procedures. Monitoring and fidelity of the treatment manual were assured by regular supervision with the senior therapist. In addition, the senior therapist participated in at least three sessions from each group and performed all meeting with parents.
Control therapy
The intervention manual control follows all the formal aspects of the Friends for Life program in relationship to the number and duration of sessions. However, cognitive and behavioral strategies used in the G-CBT protocol were replaced by recreational and educational tasks, through games, storytelling, and psychopedagogical activities in a structured and uniform script for all groups. In addition, an active psychoeducation program for anxiety was provided for children and parents in the first treatment session. Six undergraduates in psychology students delivered the control intervention. Those delivering the CT were required not to have formal G-CBT training and were formally instructed to avoid psychotherapeutic interventions, other than psychoeducation. Fidelity to the CT protocol was assured by regular supervision with a senior therapist (C.S.P.).
Statistical analysis
Sample size was estimated using data from previous ABM (Bar-Haim et al. 2011; Eldar et al. 2012) and G-CBT studies in children (James et al. 2005). With these data, we hypothesized a four-point between-group difference in PARS for both G-CBT and ABM (effect size of Cohen d = 0.8; assuming a standard deviation of 6) and a combined additive effect in the combined treatment group (i.e., eight-point difference, with an effect size of Cohen d = 1.3). Considering probabilities of 5% for type I error and 20% for type II error, this effect size resulted in an estimated sample size of a minimum of 74 participants.
To analyze the primary and secondary continuous outcomes, we performed only Intention-to-Treat (ITT) by using linear mixed models effects (implemented with the use of PROC MIXED), which uses all available information to estimate treatment effects. In all models, random effects included intercept and linear slope terms, and an unstructured covariance was used to account for within-subject correlation over time. Fixed effects tested included for each outcome the effects of time (two levels, baseline and endpoint), ABMT (two levels, active and control), and G-CBT (two levels, active or control) nested within 14 therapy groups (seven levels for each G-CBT level), as well as two-way and three-way interactions of G-CBT and ABMT with time.
This factorial model allowed us to perform three comparisons: (i) the effects of each group inside the cell (G-CBT + ABM vs. G-CBT + ACT vs. CT + ABM vs. CT + ACT); (ii) the effects of each factor at the margins (G-CBT vs. CT and ABM vs. ACT); and (iii) the conditional, higher-order interaction of G-CBT and ABM (G-CBT*ABM). We therefore determined whether treatments were additive or nonadditive according to one of two possible scenarios: (i) if the interaction is not significant, the factors are independent of each other; and (ii) if the interaction is significant (i.e., the effect of one factor is conditioned to the level of the other factor), then the combined effects of the two treatments are nonadditive. All analyses were performed using SAS University Edition, with two-sided significance tests at the 5% significance level. Missing data were considered to be at random.
Results
Participants
Of 310 children screened, a total of 96 fulfilled inclusion and exclusion criteria. After these children were randomized into the G-CBT and CT conditions, 17 declined further participation before the first study session (Fig. 2), generating a total of 79 children who started the interventions. Subjects who declined participation were not aware of the group allocations; therefore, nonparticipation could not be related to the forthcoming allocation. Children were also randomized into ABM and ACT within the G-CBT and the CT conditions. The four groups were similar in clinical characteristics, in demographics, and in attention bias measures at baseline (Table 1). Baseline scores are in the expected range of clinical populations.
Table 1.
Sample Description According to Each Treatment Group
| Estimated means | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CT + ACT (n = 10) | CT + ABM (n = 7) | G-CBT + ACT (n = 9) | G-CBT + AB M (n = 7) | Mixed model effects | |||||||
| Bas M (SE) | End M (SE) | Bas M (SE) | End M (SE) | Bas M (SE) | End M (SE) | Bas M (SE) | End M (SE) | Group | Time | Group × time | |
| PARS | 21.36 (1.09) | 22.68 (2.84) | 20.25 (1.28) | 17.51 (3.45) | 23.16 (1.16) | 17.73 (2.69) | 18.60 (1.31) | 15.62 (3.46) | F(3,9) = 1.23; p = 0.355 | F(1,9) = 2.93; p = 0.121 | F(3,9) = 1.22; p = 0.359 |
| PARS-S | 21.69 (2.26) | 19.64 (3.24) | 17.11 (2.64) | 16.31 (3.90) | 23.34 (2.38) | 15.56 (3.18) | 19.62 (2.70) | 12.97 (3.97) | F(3,9) = 0.60; p = 0.631 | F(3,9) = 9.34;p = 0.014 | F(3,9) = 1.56; p = 0.266 |
| Threat bias | 60.77 (15.96) | 12.56 (29.40) | 63.91 (18.72) | 23.12 (37.26) | 45.21 (16.83) | 7.53 (28.03) | 37.53 (19.15) | −59.47 (43.28) | F(3,9) = 1.11; p = 0.397 | F(3,9) = 8.68;p = 0.016 | F(3,9) = 0.740; p = 0.785 |
Bold indicates statistically significant results.
CT, control therapy; ACT, attention control therapy; ABM, attentional bias modification; G-CBT, group cognitive behavior therapy; Bas, baseline; End, endpoint; M, mean; SE, standard error; PARS, Pediatric Anxiety Rating Scale score; PARS-S, number of symptoms in the PARS.
Response rates
Response rates for inside the cell analysis were comparable among groups for both intention-to-treat analysis (χ2 = 3.008, df = 3, p = 0.390) and completers (χ2 = 0.293, df = 3, p = 0.961): G-CBT + ABM (23.8% for ITT; 45.5% for completers), G-CBT + ACT (42.9% for ITT; 52.9% for completers), CT + ABM (47.1% for ITT; 53.3% for completers), and CT + ACT (30% for ITT; 46.2% for completers). Analysis at the margins for ITT response rates for the main effects of G-CBT versus CT (33.3% vs. 37.8%; Wald χ2 = 0.253, df = 1, p = 0.615), ABM versus ACT (34.2% vs. 36.6%; Wald χ2 = 0.023, df = 1, p = 0.880), and interaction term (Wald χ2 = 2.766, df = 1, p = 0.096) did not reveal any significant results. Same results were also obtained for response rates among completers: G-CBT versus CT (50% vs. 50% response; Wald χ2 = 0.002, df = 1, p = 0.968), ABM versus ACT (50% vs. 50% response; Wald χ2 < 0.001, df = 1, p = 0.991), and interaction term (Wald χ2 = 0.293, df = 1, p = 0.588).
Dropout rates
Twenty-three children dropped out. For analysis inside the cell, despite not being statistically significant (χ2 = 7.33, df = 3, p = 0.062), there were nominally different dropout rate among groups: 47.6% for G-CBT + ABM, 19% for G-CBT + ACT, 11.8% for CT + ABM, and 35% for CT + ACT group. Analyses at the margins revealed an ABM by G-CBT interaction on dropout rates (χ2 = 5.87, df = 1, p = 0.015).
To decompose this interaction, we investigate main effects of G-CBT and ABM within groups stratified by each 2 × 2 design allocation. This analysis revealed that among those allocated to CT, the addition of ABM if compared to ACT presented a tendency toward decreasing dropout rates (odds ratio [OR] = 0.248; χ2 = 2.478, df = 1, p = 0.115), whereas in those allocated to G-CBT, the addition of ABM if compared to ACT presented a tendency toward increasing dropout rates (OR = 3.86, χ2 = 3.66, df = 1, p = 0.056). Among those allocated to ACT, the addition of G-CBT did not change dropout rates (OR = 0.437, χ2 = 1.29, df = 1, p = 0.255), whereas in those allocated to ABM, the addition of G-CBT significantly increased the dropout rate (OR = 6.82, χ2 = 4.86, df = 1, p = 0.027).
Primary continuous outcome
The primary continuous outcome was change from baseline to week 10 in PARS scores. We found a significant time effect on PARS total score [F(1,50) = 16.03, p < 0.001]. However, no time-by-group interactions were found for neither inside the cell (Table 2; all p-values >0.05) nor at the margins analysis (Table 3; all p-values >0.05). Estimated mean differences from baseline to endpoint in PARS scores were as follows: G-CBT + ABM (mean difference = 3.9 points, 95% confidence interval [CI] −0.3 to 8.1, p = 0.066), G-CBT + ACT (mean difference = 5.6, 95% CI 2.2–9.0, p = 0.002), CT + ABM (mean difference = 4.8, 95% CI 1.08–8.57, p = 0.013), and CT + ACT (mean difference = 0.8, 95% CI −3.0 to 4.7, p = 0.666). In addition, no three-way interactions were detected.
Table 2.
Estimated Means and Mixed Model Effects in “Inside the Cell” Analysis for Symptomatic Outcomes and Biases in Attention Orienting Toward Threats
| Estimated means | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| G-CBT+ABM (n = 21) | G-CBT+ACT (n = 21) | CT+ABM (n = 17) | CT+ACT (n = 20) | Mixed model effects | |||||||
| Bas M (SE) | End M (SE) | Bas M (SE) | End M (SE) | Bas M (SE) | End M (SE) | Bas M (SE) | End M (SE) | Group | Time | Group × time | |
| PARS | 20.77 (0.95) | 16.83 (2.09) | 21.47 (0.98) | 15.86 (1.69) | 21.60 (1.06) | 16.78 (1.87) | 19.94 (0.97) | 19.10 (1.92) | F(3,50) = 0.11; p = 0.953 | F(1,50) = 16.03;p < 0.001 | F(3,50) = 1.26; p = 0.298 |
| PARS-S | 19.78 (1.54) | 10.37 (1.85) | 22.27 (1.58) | 14.43 (1.41) | 20.57 (1.70) | 14.62 (1.56) | 19.89 (1.56) | 15.07 (1.66) | F(3,50) = 1.19; p = 0.321 | F(1,50) = 50.30;p < 0.001 | F(3,50) = 1.00; p = 0.402 |
| SCARED-P | 37.79 (2.72) | 31.11 (3.84) | 42.26 (2.77) | 37.31 (3.30) | 36.83 (3.03) | 32.16 (3.51) | 41.19 (2.86) | 37.61 (3.63) | F(3,49) = 1.06; p = 0.374 | F(1,49) = 11.47;p = 0.001 | F(3,49) = 0.16; p = 0.920 |
| SCARED-C | 30.94 (3.14) | 27.14 (3.08) | 33.28 (2.77) | 28.18 (2.18) | 34.10 (3.21) | 24.58 (2.58) | 33.26 (2.92) | 23.01 (2.50) | F(3,47) = 0.21; p = 0.890 | F(1,47) = 36.85;p < 0.001 | F(3,47) = 1.81; p = 0.158 |
| SCAS-P | 37.73 (3.25) | 27.81 (4.36) | 47.95 (3.31) | 40.79 (3.78) | 41.37 (3.62) | 32.17 (4.03) | 41.09 (3.42) | 39.16 (4.15) | F(3,49) = 2.20; p = 0.100 | F(1,49) = 18.98;p < 0.001 | F(3,49) = 1.17; p = 0.333 |
| SCAS-C | 42.69 (4.00) | 36.58 (5.15) | 43.68 (3.60) | 39.60 (3.92) | 48.24 (4.35) | 37.21 (4.53) | 45.81 (3.72) | 39.79 (4.41) | F(3,45) = 0.15; p = 0.932 | F(1,45) = 10.00;p = 0.003 | F(3,45) = 0.46; p = 0.710 |
| CDI | 7.94 (1.27) | 5.99 (1.66) | 8.57 (1.14) | 6.35 (1.30) | 6.82 (1.34) | 5.16 (1.49) | 6.95 (1.18) | 4.65 (1.45) | F(3,46) = 0.48; p = 0.700 | F(1,46) = 10.03;p = 0.003 | F(3,46) = 0.05; p = 0.987 |
| SNAP-P | 27.20 (3.47) | 24.00 (4.45) | 29.18 (3.53) | 29.70 (4.11) | 24.20 (3.86) | 24.02 (4.42) | 30.42 (3.64) | 30.13 (4.39) | F(3,49) = 0.61; p = 0.614 | F(1,49) = 0.35; p = 0.557 | F(3,49) = 0.33; p = 0.800 |
| SDQ-total | 17.09 (1.52) | 15.63 (1.83) | 17.38 (1.55) | 16.99 (1.59) | 17.91 (1.69) | 15.63 (1.70) | 16.88 (1.64) | 14.69 (1.76) | F(3,48) = 0.16; p = 0.925 | F(1,48) = 5.34;p = 0.025 | F(3,48) = 0.48; p = 0.700 |
| SDQ-impact | 3.92 (0.61) | 3.65 (0.95) | 4.04 (0.62) | 3.48 (0.71) | 4.26 (0.67) | 3.13 (0.75) | 3.30 (0.64) | 3.99 (0.83) | F(3,49) = 0.01; p = 0.998 | F(1,49) = 0.47; p = 0.497 | F(3,49) = 0.69; p = 0.565 |
| Threat bias | −12.70 (13.92) | −23.10 (25.47) | 1.46 (13.49) | 21.78 (18.12) | 9.50 (16.23) | 1.73 (23.09) | 13.61 (13.74) | 9.25 (19.82) | F(3,50) = 0.98; p = 0.411 | F(1,50) < 0.01; p = 0.965 | F(3,50) = 0.38; p = 0.771 |
Bold indicates statistically significant results.
CT, control therapy; ACT, attention control therapy; ABM, attentional bias modification; G-CBT, group cognitive behavior therapy; Bas, baseline; End, endpoint; M, mean; SE, standard error; PARS, Pediatric Anxiety Rating Scale score; PARS-S, number of symptoms in the PARS; SCARED, Screen for Child Anxiety Related Emotional Disorders; SCAS, Spence Children's Anxiety Scale; CDI, Children's Depression Inventory; SNAP, Swanson, Nolan, and Pelham questionnaire; SDQ, Strengths and Difficulties Questionnaire; P, parent version; C, child version.
Table 3.
Estimated Means and Mixed Model Effects in “At the Margins” Analysis for Symptomatic Outcomes and Biases in Attention Orienting Toward Threats
| G-CBT vs. CT | ABM vs. ACT | Three-way interaction | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| G-CBT (n = 42) | CT (n = 37) | ABM (n = 38) | ACT (n = 41) | ||||||||
| Bas M (SE) | End M (SE) | Bas M (SE) | End M (SE) | CBT × time | Bas M (SE) | End M (SE) | Bas M (SE) | End M (SE) | ABM × time | G-CBT × ABM × time | |
| PARS | 21.12 (0.68) | 16.35 (1.35) | 20.77 (0.72) | 17.94 (1.34) | F(1,50) = 1.04; p = 0.312 | 21.19 (0.71) | 16.81 (1.40) | 20.70 (0.69) | 17.48 (1.28) | F(1,50) = 0.37; p = 0.545 | F(1,50) = 2.22; |
| p = 0.143 | |||||||||||
| PARS-S | 21.03 (1.10) | 12.40 (1.16) | 20.23 (1.16) | 14.85 (1.14) | F(1,50) = 2.69; p = 0.107 | 20.17 (1.15) | 12.50 (1.21) | 21.08 (1.11) | 14.75 (1.09) | F(1,50) = 0.47; p = 0.497 | F(1,50) = 0.01; |
| p = 0.910 | |||||||||||
| SCARED-P | 40.02 (1.94) | 34.21 (2.53) | 39.01 (2.08) | 34.88 (2.53) | F(1,49) = 0.33; | 37.31 (2.04) | 31.63 (2.60) | 41.72 (1.99) | 37.46 (2.46) | F(1,49) = 0.23; p = 0.633 | F(1,49) = 0.01; p = 0.913 |
| p = 0.568 | |||||||||||
| SCARED-C | 32.11 (2.10) | 27.66 (1.89) | 33.68 (2.17) | 23.79 (1.80) | F(1,47) = 5.31;p = 0.026 | 32.52 (2.25) | 25.86 (2.01) | 33.27 (2.01) | 25.60 (1.67) | F(1,47) = 0.19; p = 0.669 | F(1,47) = 0.01; p = 0.907 |
| SCAS-P | 42.84 (2.32) | 34.30 (2.89) | 41.23 (2.49) | 35.64 (2.89) | F(1,49) = 0.83; p = 0.367 | 39.55 (2.43) | 29.99 (2.97) | 44.52 (2.38) | 39.95 (2.81) | F(1,49) = 2.37; p = 0.130 | F(1,49) = 0.47; p = 0.495 |
| SCAS-C | 43.19 (2.69) | 38.09 (3.24) | 47.02 (2.86) | 38.50 (3.16) | F(1,45) = 0.63; p = 0.431 | 45.47 (2.96) | 36.89 (3.43) | 44.74 (2.59) | 39.69 (2.95) | F(1,45) = 0.67; p = 0.418 | F(1,45) = 0.12; p = 0.731 |
| CDI | 8.26 (0.86) | 6.17 (1.05) | 6.89 (0.89) | 4.89 (1.04) | F(1,46) < 0.01; p = 0.948 | 7.38 (0.92) | 5.56 (1.12) | 7.76 (0.82) | 5.50 (0.97) | F(1,46) = 0.12; p = 0.735 | F(1,46) = 0.02; p = 0.892 |
| SNAP-P | 28.2 (2.47) | 26.8 (3.02) | 27.3 (2.66) | 27.1 (3.11) | F(1,49) = 0.17; p = 0.681 | 25.7 (2.60) | 24.0 (3.13) | 29.8 (2.54) | 29.9 (3.00) | F(1,49) = 0.46; p = 0.502 | F(1,49) = 0.51; p = 0.476 |
| SDQ-total | 17.2 (1.08) | 16.3 (1.21) | 17.4 (1.18) | 15.2 (1.22) | F(1,48) = 0.93; p = 0.339 | 17.5 (1.14) | 15.6 (1.25) | 17.1 (1.13) | 15.8 (1.19) | F(1,48) = 0.18 p = 0.674 | F(1,48) = 0.13 p = 0.721 |
| SDQ-impact | 3.98 (0.43) | 3.57 (0.59) | 3.78 (0.46) | 3.56 (0.56) | F(1,49) = 0.04; p = 0.837 | 4.09 (0.45) | 3.39 (0.61) | 3.67 (0.44) | 3.74 (0.55) | F(1,49) = 0.68; p = 0.413 | F(1,49) = 1.27; p = 0.265 |
| Threat bias | −5.62 (9.69) | −66 (15.63) | 11.56 (10.63) | 5.49 (15.22) | F(1,50) = 0.20 p = 0.659 | −1.60 (10.69) | −10.68 (17.19) | 7.54 (9.63) | 15.52 (13.46) | F(1,50) = 0.47 p = 0.494 | F(1,50) = 0.30; p = 0.584 |
Bold indicates statistically significant results.
Statistics: The CBT, ABMT, and CBT × ABMT effects are not being presented for simplicity, but were included in the models.
CT, control therapy; ACT, attention control therapy; ABM, attention bias modification; G-CBT, group cognitive behavior therapy; Bas, baseline; End, endpoint; M, mean; SE, standard error; PARS, Pediatric Anxiety Rating Scale score; PARS-S, number of symptoms in the PARS; SCARED, Screen for Child Anxiety Related Emotional Disorders; SCAS, Spence Children's Anxiety Scale; CDI, Children's Depression Inventory; SNAP, Swanson, Nolan and Pelham questionnaire; SDQ, Strengths and Difficulties Questionnaire; P, parent version; C, child version; ABMT, attentional bias modification treatment.
Secondary outcomes
The same pattern of a significant time effect was found for all secondary anxiety measures used (i.e., SCARED and SCAS, in both parental and self-report forms). All groups showed lower anxiety levels by the end of the intervention as assessed by those measures, and there were no time-by-treatment condition effects on this response (Tables 2 and 3). There was also a time effect on depression symptomatology as measured by the CDI and general mental health symptoms measured by the SDQ showing all groups presented lower scores in the endpoint. For externalizing symptoms measured by the SNAP and impact measured by the SDQ, there were no significant time effects. Except for one significant time-by-treatment interaction for SCARED-C, which has a higher symptom decrease in CT if compared to G-CBT, no other significant time-by-treatment condition effects were found. In addition, no time nor time-by-group effects were found for threat bias.
Minimal detectable differences
Given our negative results, we conducted an analysis to investigate the minimal detectable differences on PARS scores among groups in this trial. With a standard deviation of 7 on PARS score change, a power of 80%, and a total of 79 randomized patients, between-group differences higher than 4.47 can be detected by our trial. A power analysis on unconditional effect sizes with this sample is also provided as Supplementary Data (Supplementary Fig. S1).
Supplemental analysis
Supplemental analysis was conducted investigating treatment effects on subjects with baseline levels of threat bias of eight or higher (n = 33), as performed in other studies (Eldar et al. 2012). This analysis showed similar results as the main analysis, with significant or trend-level effects for time, but no significant group or time-by-group interactions for primary outcome. Different from the main analysis, we found significant time effects for threat bias, but again, no group or time-by-group interactions. (Supplementary Table S1).
Discussion
This controlled clinical trial compared the main and interactive effects of a traditional group G-CBT protocol (“Friends for Life”) and ABM using a factorial design with two control treatments. All groups improved from anxiety symptoms over time and among completers, about half achieved response criteria at the study endpoint. There were no consistent differences in any clinical outcome among groups. No evidence emerged for changes in threat bias. No synergistic and antagonistic effects were detected for primary or secondary outcomes. However, dropout rates were increased with the combination of G-CBT and ABM.
The basic premise of factorial trials states that in the absence of interactions between groups, treatment effects can be interpreted as two independent trials (McAlister et al. 2003), investigating concomitantly the effects of ABM and G-CBT as standalone treatments. With respect to this trial, interactions from treatment effects on symptom measures were not detected, although we did find nonanticipated interactions between G-CBT and ABM on dropout rates. This interaction raises the question on whether G-CBT and ABM might have deleterious effects when delivered in combination, at least for measures of treatment acceptability. Our findings are discussed below in light of the current literature on ABM and G-CBT as standalone treatments. Finally, we focus our discussion on trials investigating the addition of ABM to G-CBT protocols.
Unlike the preliminary evidence from earlier studies investigating attention training (Bar-Haim et al. 2011; Eldar et al. 2012; Bechor et al. 2014), we were not able to demonstrate effectiveness of ABM for children and adolescents with anxiety disorders in our study, when delivered in combination with G-CBT and CT. Inconsistencies about the efficacy of ABM as a standalone trial do exist in the current literature (Hakamata et al. 2010; Mogoase, et al. 2014; Cristea et al. 2015a, b; Heeren et al. 2015; Linetzky et al. 2015). However, there are still only a handful of trials aiming to assess ABM for children and adolescents with anxiety disorders (Linetzky et al. 2015). The field is now focusing on potential treatment moderators that could explain inconsistent results. For example, one recent meta-analysis showed a small, but significant effect when the intervention was delivered at schools, showing potential settings in which this treatment strategy might be beneficial, which are different from the setting in which our study was performed (Cristea et al. 2015b). In addition, preliminary evidence shows that ABM efficacy might be affected by children's age, showing that the treatment might be particularly helpful for adolescents, but not for younger children, which is also the case for this specific study (Pergamin-Hight et al. 2016). Other avenues for research in ABM in children and adolescents include improving the training interface for this population (Lau 2015), advancing on more tailored interventions, such as targeting anxiety related to specific stimuli (Pergamin-Hight et al. 2015) or advancing in gamified ABM paradigms that are motivationally engaging (Notebaert et al. 2015) and provide feedback during training (Bernstein and Zvielli 2014).
We were also not able to show any additional improvement of G-CBT over an active CT. At least one recent meta-analysis suggests that limited and inconclusive evidence demonstrates G-CBT to be more effective than active control conditions or treatments as usual (James et al. 2015). CBT is indisputably a recommended treatment for anxiety disorders in children. However, our study raises questions about the degree to which nonspecific therapeutic effects from this treatment format contribute to clinical outcome. It is important to highlight that our trial used an unusually active comparator, which included psychoeducation and playful activities that facilitate group interactions. The comparable effects of this CT to the G-CBT protocol raise questions about the importance of investigating specific ingredients of G-CBT formats as a way to further improve rates of response with techniques that are known to reduce anxiety symptoms in children. Also, a higher proportion of children from low-income and low parent education families might have contributed to challenges to parents reinforcing G-CBT principals at home and decreases levels of treatment response to specialized treatment if compared to nonspecialized treatment.
Finally, we were not able to show any benefit of adding ABM to G-CBT protocols. This is the first trial to test synergic and antagonistic effects of ABM and G-CBT combinations in a fully factorial design, and these effects were not supported by this analysis. Previous randomized controlled trials mostly compared adding ABM or ACT to patients receiving G-CBT and while some reported some advantages for combining these treatment modalities (Riemann et al. 2013; Shechner et al. 2014), others also failed to report additional benefits (Rapee et al. 2013; Boettcher et al. 2014). The two trials conducted in anxious children and adolescents (Riemann et al. 2013; Shechner et al. 2014) have found benefits of combining training conditions to G-CBT. Riemann et al. (2013) showed benefits above and beyond the training control condition in patients receiving G-CBT and psychiatric medication. Shechner et al. (2014) showed some modest benefits of ABM and ACT compared to G-CBT alone. These effects were noted in some, but not other outcome measures, and CBT alone in this study produced minimal effects on symptoms. Unlike in Schechner et al. (2014), all our groups were receiving either ABM or ACT interventions. In addition, subjects in this study were younger than in Schechner et al. (2014), and Schechner et al. (2014) used individual CBT protocol, whereas our trial focused on group CBT—which might also account for discrepant results among trials. Thus, further and larger trials are needed to investigate effects of ABM delivered alone or with other active treatments (Waters et al. 2013; De Voogd et al. 2014).
This study has several strengths. First, this is the first study to investigate the main effects as well as potential interactions between ABM and G-CBT for anxiety disorders in children. Second, the study used 10 sessions of both G-CBT/CT and ABM/ACT training, which is a larger dose than most ABM trails and comparable to current CBT protocols. Third, the study was performed in a clinical treatment-seeking sample, reflecting the reality of the clinical practice for which most of the evidence is targeted.
We also have some important limitations. First, despite being one of the largest trials published in ABM literature, the sample size is still limited to investigate small to moderate effect sizes of intervention, which is particularly important for investigating interactions between ABM and G-CBT. Second, the absence of a follow-up period limits our ability to investigate the maintenance of treatment gains. This is particularly important for evaluating the efficacy of the G-CBT protocol, given that there is evidence that some differences from active treatments might only be evident in larger follow-up periods. Finally, as stated earlier, differences in dropout rates among groups might limit our ability to discriminate treatment gains from treatment acceptability. In addition, the interaction between ABM and G-CBT on dropout rates raises questions on whether interpreting treatment gains over symptom differences in each treatment condition is valid.
Conclusions
In conclusion, this trial has three important messages. First, it adds to a literature showing that benefits of ABM training in the context of G-CBT and CT for children with anxiety disorders are still inconsistent and more research is needed to clarify efficacy. Second, it adds to the body of literature showing the role of nonspecific factors on reduction of anxiety symptoms. Finally, it provides further evidence that despite theoretical propositions of synergistic effects, these findings suggest that, if any interactive effects, the combination of ABM and G-CBT might have deleterious effects on treatment acceptability, which have to be confirmed in further trials.
Future studies with larger sample sizes and longer follow-up periods are needed to fully elucidate the role of ABM training as a treatment option for anxiety disorders. Also, strategies such as gaze-contingent music reward therapy (Lazarov et al. 2017), which seems to be a more ecologically sound method to train attention control, and other strategies of gamification of the attention control as ways of making the ABM more acceptable to children are promising areas of future research.
Clinical Significance
We found no effect of G-CBT or ABM beyond the effects of active comparison groups. Also, results reveal no benefit from combining G-CBT and ABM for anxiety disorders in children and suggest potential deleterious effects of the combination on treatment acceptability.
Supplementary Material
Disclosures
No competing financial interests exist.
References
- Abend R, Pine D, Fox NA, Bar-Haim Y: Learning and memory consolidation processes of attention-bias modification in anxious and nonanxious individuals. Clin Psychol Sci 2:620–627, 2014 [Google Scholar]
- Bar-Haim Y: Research review: Attention bias modification (ABM): A novel treatment for anxiety disorders. J Child Psychol Psychiatry 51:859–870, 2010 [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Abend R, Pine D: Tel-Aviv University/National Institute of Mental Health Attention Bias Modification Treatment. 2010. http://people.socsci.tau.ac.il/mu/anxietytrauma/files/2013/08/TAU-NIMH-ABMT-Project-Overview.pdf (Accessed June26, 2018)
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IMH: Threat-related attentional bias in anxious and nonanxious individuals: A meta-analytic study. Psychol Bull 133:1–24, 2007 [DOI] [PubMed] [Google Scholar]
- Bar-Haim Y, Morag I, Glickman S: Training anxious children to disengage attention from threat: A randomized controlled trial. J Child Psychol Psychiatry 52:861–869, 2011 [DOI] [PubMed] [Google Scholar]
- Barrett PM, Duffy AL, Dadds MR, Rapee RM: Cognitive-behavioral treatment of anxiety disorders in children: Long-term (6-year) follow-up. J Consult Clin Psychol 69:135–141, 2001 [PubMed] [Google Scholar]
- Baxter AJ, Charlson FJ, Somerville AJ, Whiteford HA: Mental disorders as risk factors: Assessing the evidence for the Global Burden of Disease Study. BMC Med 9:134, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard C, Sawyer AT, Hofmann SG: Efficacy of attention bias modification using threat and appetitive stimuli: A meta-analytic review. Behav Ther 43:724–740, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechor M, Pettit JW, Silverman WK, Bar-Haim Y, Abend R, Pine DS, Vasey MW, Jaccard J: Attention bias modification treatment for children with anxiety disorders who do not respond to cognitive behavioral therapy: A case series. J Anxiety Disord 28:154–159, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesdo-Baum K, Knappe S: Developmental epidemiology of anxiety disorders. Child Adolesc Psychiatr Clin N Am 21:457–478, 2012 [DOI] [PubMed] [Google Scholar]
- Bernstein A, Zvielli A: Attention feedback awareness and control training (A-FACT): Experimental test of a novel intervention paradigm targeting attentional bias. Behav Res Ther 55:18–26, 2014 [DOI] [PubMed] [Google Scholar]
- Boettcher J, Hasselrot J, Sund E, Andersson G, Carlbring P: Combining attention training with internet-based cognitive-behavioural self-help for social anxiety: A randomised controlled trial. Cogn Behav Ther 43:34–48, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil HHA: Development of the Brazilian version of K-SADS-PL (Schedule for Affective Disorders and Schizophrenia for School Aged Children Present and Lifetime Version) and study of psychometric properties. São Paulo, Brazil, Department of Psychiatry, Universidade Federal de São Paulo, 2003 [Google Scholar]
- Britton JC, Bar-Haim Y, Clementi MA, Sankin LS, Chen G, Shechner T, Norcross MA, Spiro CN, Lindstrom KM, Pine DS: Training-associated changes and stability of attention bias in youth: Implications for attention bias modification treatment for pediatric anxiety. Dev Cogn Neurosci 4:52–64, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caporino NE, Brodman DM, Kendall PC, Albano AM, Sherrill J, Piacentini J, Sakolsky D, Birmaher B, Compton SN, Ginsburg G, Rynn M, McCracken J, Gosch E, Keeton C, March J, Walkup JT: Defining treatment response and remission in child anxiety: Signal detection analysis using the pediatric anxiety rating scale. J Am Acad Child Adolesc Psychiatry 52:57–67, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristea IA, Kok RN, Cuijpers P: Efficacy of cognitive bias modification interventions in anxiety and depression: Meta-analysis. Br J Psychiatry 206:7–16, 2015a [DOI] [PubMed] [Google Scholar]
- Cristea IA, Mogoase C, David D, Cuijpers P: Practitioner review: Cognitive bias modification for mental health problems in children and adolescents: A meta-analysis. J Child Psychol Psychiatry 56:723–734, 2015b [DOI] [PubMed] [Google Scholar]
- de Souza MA, Salum GA, Jarros RB, Isolan L, Davis R, Knijnik D, Manfro GG, Heldt E: Cognitive-behavioral group therapy for youths with anxiety disorders in the community: Effectiveness in low and middle income countries. Behav Cogn Psychother 41:255–264, 2013 [DOI] [PubMed] [Google Scholar]
- De Voogd EL, Wiers RW, Prins PJ, Salemink E: Visual search attentional bias modification reduced social phobia in adolescents. J Behav Ther Exp Psychiatry 45:252–259, 2014 [DOI] [PubMed] [Google Scholar]
- Eldar S, Apter A, Lotan D, Edgar KP, Naim R, Fox NA, Pine DS, Bar-Haim Y: Attention bias modification treatment for pediatric anxiety disorders: A randomized controlled trial. Am J Psychiatry 169:213–220, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory AM, Caspi A, Moffitt TE, Koenen K, Eley TC, Poulton R: Juvenile mental health histories of adults with anxiety disorders. Am J Psychiatry 164:301–308, 2007 [DOI] [PubMed] [Google Scholar]
- Hakamata Y, Lissek S, Bar-Haim Y, Britton JC, Fox NA, Leibenluft E, Ernst M, Pine DS: Attention bias modification treatment: A meta-analysis toward the establishment of novel treatment for anxiety. Biol Psychiatry 68:982–990, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallion LS, Ruscio AM: A meta-analysis of the effect of cognitive bias modification on anxiety and depression. Psychol Bull 137:940–958, 2011 [DOI] [PubMed] [Google Scholar]
- Heeren A, Mogoase C, Philippot P, McNally RJ: Attention bias modification for social anxiety: A systematic review and meta-analysis. Clin Psychol Rev 40:76–90, 2015 [DOI] [PubMed] [Google Scholar]
- Ipser JC, Stein DJ, Hawkridge S, Hoppe L: Pharmacotherapy for anxiety disorders in children and adolescents. Cochrane Database Syst Rev CD005170, 2009 [DOI] [PubMed]
- James A, Soler A, Weatherall R: Cognitive behavioural therapy for anxiety disorders in children and adolescents. Cochrane Database Syst Rev CD004690, 2005 [DOI] [PubMed]
- James AC, James G, Cowdrey FA, Soler A, Choke A: Cognitive behavioural therapy for anxiety disorders in children and adolescents. Cochrane Database Syst Rev CD004690, 2013 [DOI] [PubMed]
- James AC, James G, Cowdrey FA, Soler A, Choke A: Cognitive behavioural therapy for anxiety disorders in children and adolescents. Cochrane Database Syst Rev CD004690, 2015 [DOI] [PMC free article] [PubMed]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, Williamson D, Ryan N: Schedule for affective disorders and schizophrenia for school-age children-present and lifetime version (K-SADS-PL): Initial reliability and validity data. J Am Acad Child Adolesc Psychiatry 36:980–988, 1997 [DOI] [PubMed] [Google Scholar]
- Kieling C, Baker-Henningham H, Belfer M, Conti G, Ertem I, Omigbodun O, Rohde LA, Srinath S, Ulkuer N, Rahman A: Child and adolescent mental health worldwide: Evidence for action. Lancet 378:1515–1525, 2011 [DOI] [PubMed] [Google Scholar]
- Kosters MP, Chinapaw MJ, Zwaanswijk M, van der Wal MF, Koot HM: Indicated prevention of childhood anxiety and depression: Results from a practice-based study up to 12 months after intervention. Am J Public Health 105:2005–2013, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau JY: Commentary: A glass half full or half empty? Cognitive bias modification for mental health problems in children and adolescents - reflections on the meta-analysis by Cristea et al. (2015). J Child Psychol Psychiatry 56:735–737, 2015 [DOI] [PubMed] [Google Scholar]
- Lazarov A, Pine DS, Bar-Haim Y: Gaze-contingent music reard terap for social anxiety disorders: A randomized controlled trial. Am J Psychiatry 174:649–656, 2017 [DOI] [PubMed] [Google Scholar]
- Linetzky M, Pergamin-Hight L, Pine DS, Bar-Haim Y: Quantitative evaluation of the clinical efficacy of attention bias modification treatment for anxiety disorders. Depress Anxiety 32:383–391, 2015 [DOI] [PubMed] [Google Scholar]
- MacLeod C, Rutherford E, Campbell L, Ebsworthy G, Holker L: Selective attention and emotional vulnerability: Assessing the causal basis of their association through the experimental manipulation of attentional bias. J Abnorm Psychol 111:107–123, 2002 [PubMed] [Google Scholar]
- McAlister FA, Straus SE, Sackett DL, Altman DG: Analysis and reporting of factorial trials: A systematic review. JAMA 289:2545–2553, 2003 [DOI] [PubMed] [Google Scholar]
- Mogoase C, David D, Koster EH: Clinical efficacy of attentional bias modification procedures: An updated meta-analysis. J Clin Psychol 70:1133–1157, 2014 [DOI] [PubMed] [Google Scholar]
- Notebaert L, Clarke PJ, Grafton B, MacLeod C: Validation of a novel attentional bias modification task: The future may be in the cards. Behav Res Ther 65:93–100, 2015 [DOI] [PubMed] [Google Scholar]
- Pergamin-Hight L, Naim R, Bakermans-Kranenburg MJ, van IMH, Bar-Haim Y: Content specificity of attention bias to threat in anxiety disorders: A meta-analysis. Clin Psychol Rev 35:10–18, 2015 [DOI] [PubMed] [Google Scholar]
- Pergamin-Hight L, Pine DS, Fox NA, Bar-Haim Y: Attention bias modification for youth with social anxiety disorder: The moderating roles of age, attention control, and threat bias. J Child Psychol Psychiatry 57:1317–1325, 2016 [DOI] [PubMed] [Google Scholar]
- Rapee RM, MacLeod C, Carpenter L, Gaston JE, Frei J, Peters L, Baillie AJ: Integrating cognitive bias modification into a standard cognitive behavioural treatment package for social phobia: A randomized controlled trial. Behav Res Ther 51:207–215, 2013 [DOI] [PubMed] [Google Scholar]
- Riemann BC, Kuckertz JM, Rozenman M, Weersing VR, Amir N: Augmentation of youth cognitive behavioral and pharmacological interventions with attention modification: A preliminary investigation. Depress Anxiety 30:822–828, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUPP: The Pediatric Anxiety Rating Scale (PARS): Development and psychometric properties. J Am Acad Child Adolesc Psychiatry 41:1061–1069, 2002 [DOI] [PubMed] [Google Scholar]
- Salum GA, Desousa DA, do Rosario MC, Pine DS, Manfro GG: Pediatric anxiety disorders: From neuroscience to evidence-based clinical practice. Rev Bras Psiquiatr 35 Suppl 1:S03–S21, 2013a [DOI] [PubMed] [Google Scholar]
- Salum GA, Mogg K, Bradley BP, Gadelha A, Pan P, Tamanaha AC, Moriyama T, Graeff-Martins AS, Jarros RB, Polanczyk G, do Rosario MC, Leibenluft E, Rohde LA, Manfro GG, Pine DS: Threat bias in attention orienting: Evidence of specificity in a large community-based study. Psychol Med 43:733–745, 2013b [DOI] [PubMed] [Google Scholar]
- Shechner T, Britton JC, Perez-Edgar K, Bar-Haim Y, Ernst M, Fox NA, Leibenluft E, Pine DS: Attention biases, anxiety, and development: Toward or away from threats or rewards? Depress Anxiety 29:282–294, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechner T, Rimon-Chakir A, Britton JC, Lotan D, Apter A, Bliese PD, Pine DS, Bar-Haim Y: Attention bias modification treatment augmenting effects on cognitive behavioral therapy in children with anxiety: Randomized controlled trial. J Am Acad Child Adolesc Psychiatry 53:61–71, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waters AM, Pittaway M, Mogg K, Bradley BP, Pine DS: Attention training towards positive stimuli in clinically anxious children. Dev Cogn Neurosci 4:77–84, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White LK, Sequeira S, Britton JC, Brotman MA, Gold AL, Berman E, Towbin K, Abend R, Fox NA, Bar-Haim Y, Leibenluft E, Pine DS: Complementary features of attention bias modification therapy and cognitive-behavioral therapy in pediatric anxiety disorders. Am J Psychiatry 174:775–784, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward LJ, Fergusson DM: Life course outcomes of young people with anxiety disorders in adolescence. J Am Acad Child Adolesc Psychiatry 40:1086–1093, 2001 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.