Abstract
Applied Genetic Technologies Corporation (AGTC) is developing a recombinant adeno-associated virus (rAAV) vector AGTC-501, also designated AAV2tYF-GRK1-RPGRco, to treat retinitis pigmentosa (RP) in patients with mutations in the retinitis pigmentosa GTPase regulator (RPGR) gene. The vector contains a codon-optimized human RPGR cDNA (RPGRco) driven by a photoreceptor-specific promoter (G protein–coupled receptor kinase 1, GRK1) and is packaged in an AAV2 capsid with three surface tyrosine residues changed to phenylalanine (AAV2tYF). We conducted a safety and potency study of this vector administered by subretinal a injection in the naturally occurring RPGR-deficient Rd9 mouse model. Sixty Rd9 mice (20 per group) received a subretinal injection in the right eye of vehicle (control) or AAV2tYF-GRK1-RPGRco at one of two dose levels (4 × 108 or 4 × 109 vg/eye) and were followed for 12 weeks after injection. Vector injections were well tolerated, with no systemic toxicity. There was a trend towards reduced electroretinography b-wave amplitudes in the high vector dose group that was not statistically significant. There were no clinically important changes in hematology or clinical chemistry parameters and no vector-related ocular changes in life or by histological examination. Dose-dependent RPGR protein expression, mainly in the inner segment of photoreceptors and the adjacent connecting cilium region, was observed in all vector-treated eyes examined. Sequence integrity of the codon-optimized RPGR was confirmed by sequencing of PCR-amplified DNA, or cDNA reverse transcribed from total RNA extracted from vector-treated retinal tissues, and by sequencing of RPGR protein obtained from transfected HEK 293 cells. These results support the use of rAAV2tYF-GRK1-RPGRco in clinical studies in patients with XLRP caused by RPGR mutations.
Keywords: retinitis pigmentosa, XLRP, RPGR, gene therapy, AAV
Introduction
X-linked retinitis pigmentosa (XLRP) accounts for approximately 10% of all RP cases (range 1.5% to 17%), and approximately 80% of XLRP cases are caused by mutations in the retinitis pigmentosa GTPase regulator (RPGR) gene.1–3 Analysis of disease progression in patients with XLRP caused by RPGR gene mutations shows a steady deterioration of visual field extent, development of night blindness, reduced electroretinography (ERG) function at early ages, progressive loss of ERG amplitudes with aging and decline in visual acuity.4–6 A study of 113 males with RPGR mutations reported the mean annual exponential rate of decline was 4.0% for visual acuity, 4.7% for visual field area, and 7.1% for ERG amplitude, and the median age at which patients reached legally blind status, based on loss of acuity and/or visual field, was 45 years.6
There are multiple alternatively spliced transcripts of the RPGR gene, two of which have been extensively studied. The constitutive transcript, RPGR1–19, is widely expressed in many cell types, including within the retina.7 An alternatively spliced transcript containing exons 1–15 and a large part of intron 15, RPGR-ORF15 is localized in the connecting cilia of rod and cone photoreceptors in the retina of all species examined.8 Some RPGR-ORF15 can also be detected in the outer and inner segments.9,10 The highly repetitive purine-rich ORF15 region encodes glutamate and glycine repeats and is a mutation hotspot, accounting for up to 80% of all reported RPGR mutations.8,11 The physiological role of the RPGR-ORF15 protein is not fully elucidated, although it is likely involved in regulating transport through the photoreceptor cilia.3,12,13 Proof of concept studies in XLRP mouse and dog models have shown that subretinal delivery of recombinant adeno-associated virus (rAAV) vectors expressing a RPGR-ORF15 transgene can maintain photoreceptor structure and function.14–16
To treat patients with retinitis pigmentosa caused by mutations in RPGR, Applied Genetic Technologies Corporation (AGTC) is developing a rAAV viral vector (AAV2tYF-GRK1-RPGRco, or AGTC-501), which consists of an AAV2 capsid with three tyrosine to phenylalanine mutations on the capsid surface and a codon-optimized human RPGR-ORF15 gene driven by photoreceptor-specific promoter (G protein–coupled receptor kinase 1, GRK1). As part of our efforts to develop this vector for use in patients, we conducted a study in the naturally occurring RPGR-deficient Rd9 mouse model to evaluate vector safety by standard good laboratory-compliant toxicology methods, vector potency by determining dose-related protein expression and localization in vivo, and vector stability by DNA, RNA, and protein sequence in retinal tissues or transfected cells.
Results and Discussion
Objective and study design
The study was designed to evaluate the safety and pharmacology of rAAV2tYF-GRK1-RPGRco administered by subretinal injection in RPGR-deficient Rd9 mice, a naturally occurring model of XLRP caused by mutations in RPGR-ORF15. The retinal phenotype of Rd9 mice is relatively mild when compared with that of RPGR-XLRP patients, yet it shares important features with the human disease, including similar retinal pathology and reduction of ERG function at an early age.17 All procedures in the protocol were in compliance with applicable animal welfare acts and were approved by the local institutional animal care and use committee.
Sixty Rd9 mice, including hemizygous males and homozygous females, 6–8 weeks of age, in three groups of 20 animals each, were used in the study (Table 1). Animals in group 1 received a subretinal injection in the right eye of vehicle control (Alcon BSS with 0.014% Tween 20). Animals in group 2 and group 3 received a subretinal injection of vector in the right eye at 4 × 108 vector genomes (vg)/eye (low dose) or 4 × 109 vg/eye (high dose). All contralateral left eyes remained untreated. Dose analysis of residual formulated vector confirmed the vector concentration in this study was consistent with planned dose levels. Half of the study animals were euthanized at week 4 and the remaining animals at week 12.
Table 1.
Study design
| Group | Number of Animals (Sex) | Number of Animals (Sex) at Termination | |||||
|---|---|---|---|---|---|---|---|
| Day 28 (Week 4) | Day 84 (Week 12) | Vector | Concentration (vg/mL) | Volume (μL) | Total Dose (vg/eye) | ||
| 1 | 20 (11M/9F) | 10 (6M/4F) | 10 (5M/5F) | Vehicle (Alcon balanced salt solution with 0.014% Tween 20) | 0 | 1 | 0 |
| 2 | 20 (10M/10F) | 10 (5M/5F) | 10 (5M/5F) | rAAV2tYF-GRK1-RPGRco | 4.0 × 1011 | 1 | 4.0 × 108 |
| 3 | 20 (10M/10F) | 10 (5M/5F) | 10 (5M/5F) | rAAV2tYF-GRK1-RPGRco | 4.0 × 1012 | 1 | 4.0 × 109 |
F, female; M, male.
The primary safety endpoint of this study was the histopathology examination of tissues. Clinical observations, including body weights, ocular exams, assessments of hematology, clinical chemistry parameters, and ERGs, were also performed. Additional evaluation included immunohistochemical staining for RPGR, measurement of serum antibodies to RPGR in specimens collected at euthanasia, and the sequence integrity of the codon-optimized RPGR at both the mRNA and protein level.
Summary of data
Ophthalmic examination
A summary of the ophthalmic findings for each group is presented in Supplementary Table S1 (Supplementary Data are available online at www.liebertpub.com/humc). No vector-related ophthalmic findings were observed at weeks 4 or 12. All abnormal findings were considered procedure-related or background findings.
At both the week 4 and week 12 time points, procedure-related microscopic findings were characterized by the presence of pigmented cells in the subretinal space and within the photoreceptor cell layer, and/or degeneration of photoreceptor and inner and/or outer nuclear layer (thinning or absence of these layers). In addition, swollen lens fibers and/or lens fibrosis were present in a few animals (week 4: vehicle 1 of 10, high dose 2 of 10; week 12: vehicle 1 of 10, low dose 1 of 10). The findings were present across all groups, including vehicle controls, and/or the findings lacked a dose response and were therefore considered to be subretinal injection procedure–related instead of vector-related.
ERG
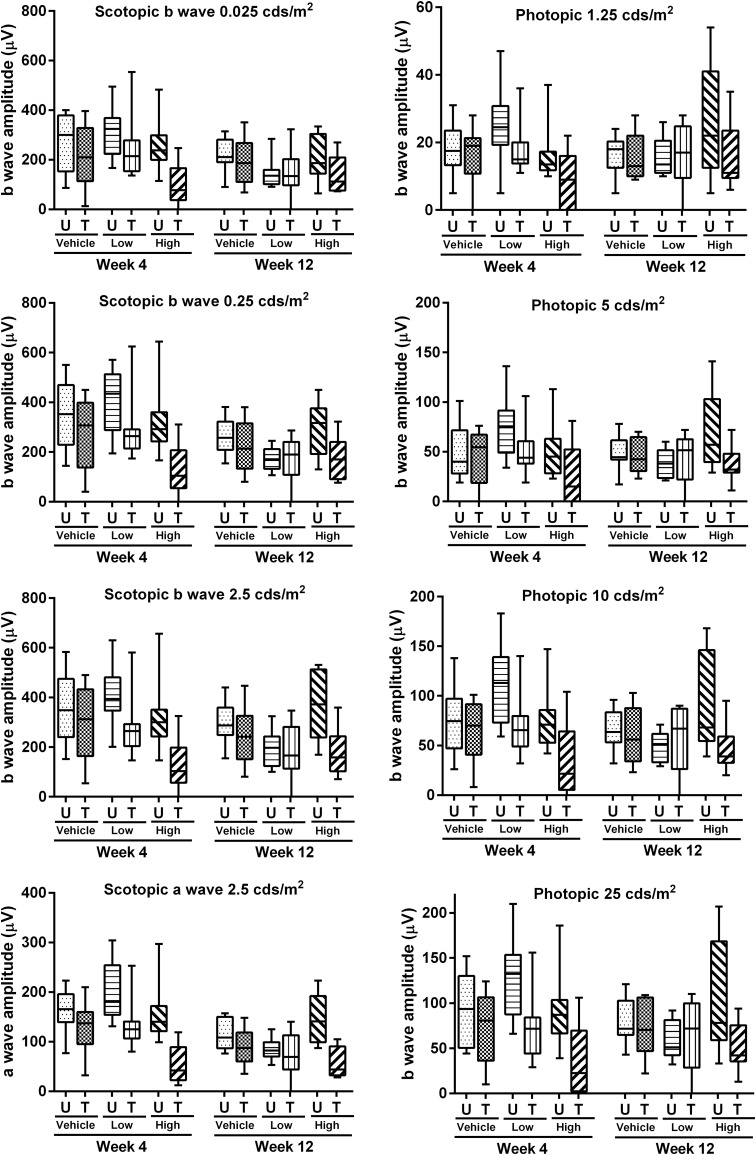
ERG responses were measured prior to sacrifice at 4 or 12 weeks after vector administration under dark-adapted (scotopic) conditions with stimulus intensities of 0.025, 0.25, and 2.5 cd·s/m2 and then under light-adapted (photopic) conditions with stimulus intensities of 1.25, 5, 10, and 25 cd·s/m2. It has been reported that under scotopic condition, the dimmer flashes generate an ERG b-wave driven by rod postreceptoral activity, whereas the brighter flash produces an a-wave dominated by rod photoreceptor activity and a b-wave that is driven by both rod and cone postreceptoral neurons.18 For scotopic ERG, we plotted b-wave amplitudes at all three light intensities and a-wave amplitudes at 2.5 cd·s/m2. There was a trend toward ERG amplitude reduction (both a-wave and b-wave) in animals administered with the higher vector dose. There was a large animal-to-animal variation, however, no statistically significant differences between the treated and untreated eyes were found (p > 0.05) (Fig. 1). It has been reported that administration of a rAAV-RPGR vector at high doses can cause photoreceptor degeneration,15,19 and the possibility of toxicity from the high dose of the AAV2tYF-GRK1-RPGRco vector will be considered during the design of clinical studies.
Figure 1.
Electroretinography (ERG) a-wave and b-wave responses after subretinal administration of rAAV2tYF-GRK1-RPGRco. ERG amplitudes were recorded from treated (T) and untreated (U) eyes from different groups (vehicle, low, and high doses) at scotopic (a-wave, 2.5 cds/m2; b-wave, 0.025, 0.25, and 2.5 cds/m2) and photopic (b-wave, 1.25, 5, 10, and 25 cds/m2) light intensities. The amplitudes from treated and untreated eyes in each group was plotted with box and whisker. In each diagram the box indicates the median and interquartile range, and bars indicate the minimum and maximum. Statistical analysis was performed to compare the ERG responses between treated and untreated eyes of each group at week 4 or week 12, and there is no statistically significant reduction of ERG response after adeno-associated virus (AAV) treatment comparing low- or high-dose group with vehicle-treated animals.
All eyes were considered for statistical analysis, including the five injected eyes (one vehicle control, one low dose, and three high dose injected eyes) that showed a nearly flat ERG under scotopic and photopic conditions. The near to total loss of ERG signal in these animals was considered likely related to injection-related damage.
Because of the expected slow progression of retinal degeneration in Rd9 mice, there was no obvious therapeutic effect on ERG responses at 3 months after vector administration. This is consistent with published results obtained in RPGR-KO mice that demonstrated no obvious therapeutic effect at 4 months after vector treatment and only a slightly better ERG response in the AAV-GRK1-hRPGR vector-treated eyes at 12 months after treatment.15,17 In our study, the average b-wave amplitudes of the noninjected eyes were reduced in animals sacrificed at week 12 compared with animals sacrificed at week 4, by 28.95 ± 9.39% with the scotopic low intensity stimulus and 23.95 ± 11.24% with the photopic high intensity stimulus (Supplementary Fig. S1). Because there was large animal-to-animal variability, however, the differences between week 4 and week 12 were not statistically significant.
In a previous study conducted in a more rapidly progressive animal model of XLRP (XLPRA2 dogs), we demonstrated that subretinal injection of rAAV2tYF-GRK1-RPGR was effective in reducing retinal degeneration and providing functional rescue of ERG scotopic and photopic responses.20
Hematology and clinical chemistry
No vector-related changes in hematology or clinical chemistry parameters were seen. Minimal changes in hematology parameters of red blood cell count, hemoglobin concentration, hematocrit, and monocyte, lymphocyte, and neutrophil counts as compared with the range of the concurrent vehicle controls were identified in individual animals in the low dose group but not in the high dose group. These minimal changes were considered consistent with individual animal variability and unrelated to administration of vector. Minimal to slight increases in the activities of aspartate aminotransferase (AST) and/or alanine aminotransferase (ALT), as compared with the range of concurrent vehicle controls, in individual males in either the low dose or high dose group and individual females in the low dose group, were considered not vector-related due to the marked variability within and among groups, including controls, and across time points. These increases were most likely artifacts of handling rather than effects of the test article.21
Postmortem findings
No vector-related changes in absolute or relative organ weight parameters and no vector-related macroscopic findings were observed at either week 4 or week 12.
Ocular histopathology
No vector-related histopathology findings were observed at week 4 or week 12. All findings were considered to be procedure related or incidental findings.
The subretinal injection site was observed microscopically in the injected eye of 53% and 59% of animals at the week 4 and week 12 sacrifice time points, respectively (Supplementary Tables S2 and S3). The most common abnormal retinal findings in the injected eyes were the presence of pigmented cells in the subretinal space and within the photoreceptor cell layer in about half of animals at week 4 and week 12, and degeneration of photoreceptor and inner and/or outer nuclear layer (thinning or absence of these layers) in about one-third of animals at week 4 and one-half of animals at week 12. Additionally, swollen lens fibers and/or lens fibrosis were present in <10% of injected eyes. Because each of these findings were present in all groups, including the vehicle control group, and lacked a dose response–relationship, they were considered related to the subretinal injection procedure and not related to the test article.
RPGR expression
Vector-induced RPGR expression occurred in a time- and dose-dependent manner. At week 4, slight immunolabeling was observed in the retinas of animals in the low dose group and moderate staining was observed in the retinas of animals in the high dose group. At week 12, slight to marked RPGR immunolabeling in the retinas of animals in the low dose group and marked RPGR expression was observed in the retinas of animals in the high dose group (Supplementary Table S4).
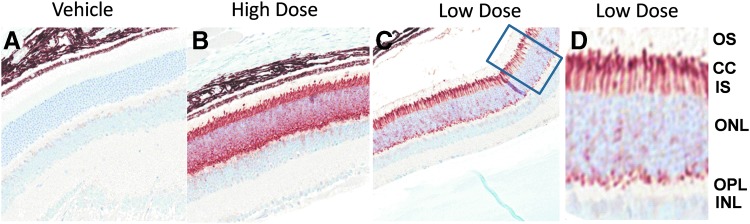
Although the function of RPGR-ORF15 in the retina is not fully understood, studies suggest that RPGR-ORF15 may act as a ciliary gate or participate in cargo trafficking and sorting in the transition zone of photoreceptor cells.3 To function correctly, RPGR-ORF15 must be localized at the correct subcellular compartment, predominantly in the connecting cilia of human and mouse photoreceptors. Detectable RPGR-ORF15 can also be found in the outer and inner segments.9,10 We demonstrated substantial, dose-dependent immunolabeling of vector-expressed RPGR mainly in the inner segment (IS) of photoreceptors (Fig. 2B and C) and adjacent connecting cilium (CC) region of the retina (Fig. 2D). Substantial RPGR labeling in the outer nuclear layer and outer plexiform was also detected in eyes of animals in the high dose group (Fig. 2B). No RPGR protein labeling was detected in the vehicle control-treated eyes (Fig. 2A), which is consistent with a previous report.17
Figure 2.
Immunohistochemical staining of retinitis pigmentosa GTPase regulator (RPGR) in retinas of Rd9 mice after subretinal injection of rAAV2tYF-GRK1-RPGRco. Retinal sections of eyes injected with vehicle control (A) or rAAV2tYF-GRK1-hRPGRco at high dose (B) or low dose (C) was stained with an antibody specific for human RPGR (brown staining). The magnified images of the marked areas are shown in (D). Vector-expressed RPGR protein was mainly detected in the inner segment of photoreceptors and localizes to the connecting cilia region of the retina. RPGR staining is shown in brown, and nuclei are stained blue by hematoxylin. OS, outer segments; CC, connecting cilia; IS, inner segments; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer.
Antibodies to vector-expressed RPGR protein
Sera collected at the week 4 and week 12 sacrifice time points from all animals were tested for antibodies to human RPGR protein by ELISA, and all samples tested were negative.
Stability of RPGRco cDNA and mRNA
Due to the highly repetitive nucleotide sequence composition of the RPGR-ORF15 region, unusually high rates of mutation and complex splicing patterns have been extensively reported.1,19,22 To overcome this challenge for developing a rAAV vector, the 3,459-bp coding sequence of RPGR-ORF15 was codon optimized based on human codon usage and further modified to reduce tandem repeats, increase the frequency of human codon usage and adjust G/C content to prolong mRNA half-life, as described in our previous publication.22 We previously verified the stability and fidelity of the RPGRco cDNA sequence by DNA sequencing at multiple steps including the large-scale AAV production.22 In the current study, we further confirmed the sequence integrity of the codon-optimized RPGR cDNA and mRNA by sequencing PCR-amplified DNA, or cDNA reverse transcribed from total mRNA extracted from HEK 293 cells (in vitro) and retinal tissue from animals (in vivo) treated with rAAV2tYF-GRK1-RPGRco respectively. The assembled overlapping sequences were 100% identical to the reference human RPGRco sequence (Supplementary Fig. S2)
Fidelity of vector-expressed RPGR protein
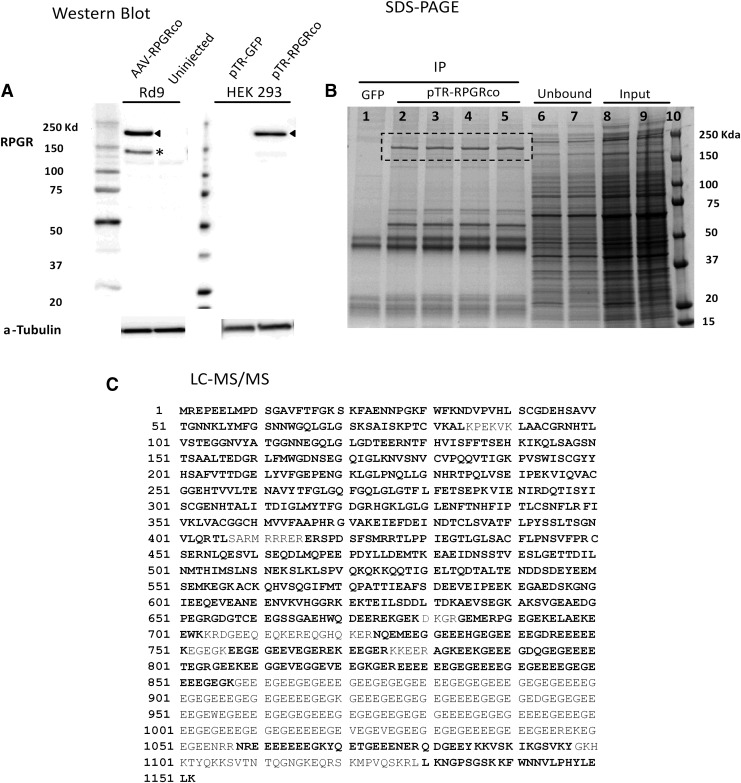
Fidelity of vector-expressed RPGR-ORF15 protein was evaluated by immunoblot analysis of protein extracted from plasmid-transfected HEK 293 cells and retinal tissue from animals administered rAAV2tYF-GRK1-RPGRco by subretinal injection. Full-length RPGR-ORF15 has 1,152 amino acids with a predicted relative molecular mass (Mr) of 127 kDa.3 However, the C-terminal glutamic acid-rich region results in aberrant migration on SDS-PAGE and an apparent Mr of approximately 200 kDa14 for RPGR-immunoreactive band expressed in plasmid-transfected HEK 293 cells and vector-treated Rd9 mouse retina (Fig. 3A, arrowheads). We also observed an RPGR-immunoreactive band at approximately 140 kDa (Fig. 3A, asterisk).
Figure 3.
Stability of vector-expressed RPGR protein. The stability of AAV vector-expressed RPGR protein was analyzed by western blot or immunoprecipitation followed by liquid chromatography and mass spectrometry (LC-MS/MS). (A) RPGR protein expression in AAV-transduced Rd9 retinal tissue or pTR-RPGRco-transfected HEK293 cells was examined by western blot using anti-RPGR antibody. Alpha-tubulin was used as the loading control. Arrow heads indicate the full length of RPGR protein and the asterisk denotes the shorter form of the RPGR. (B) Whole lysate from pTR-RPGRco-transfected HEK 293 cells was immunoprecipitated by anti-RPGR antibody and separated on SDS-PAGE. After Coomassie brilliant blue R-250 staining, the bands of full-length RPGR, as shown in the dashed rectangle, were excised and pooled for LC/MS/MS analysis. (C) Amino acids in bold are the ones that have been identified by LC-MS/MS and the amino acids non-bolded were the ones that have not been covered. Although the LC-MS/MS results did not achieve 100% coverage, the intact C-terminal sequence (LKNGPSGSKKFWNNVLPHYLELK) plus the correct molecular weight assured that an intact, full-length RPGR-ORF15, without open reading frame shift, was expressed.
To confirm that codon-optimized RPGR-ORF15 cDNA did not result in changes to the amino acid sequence, immunoprecipitated RPGR-ORF15 protein from HEK293 cells transfected with AAV plasmid pTR-CB-RPGRco was separated by SDS-PAGE (Fig. 3B), and the protein bands were excised and subjected to enzymatic cleavage for subsequent peptide sequence analysis by LC-MS/MS. Using this methodology, 76% of human RPGR-ORF15 protein sequence was confirmed to be 100% identical to the reference sequence, including the C-terminal sequence (Fig. 3C). For the other 24% of the protein, the special arrangement of limited proteolytic sites in the highly repetitive glutamate-glycine C-terminal region resulted in peptide fragments either too large or too small to be detected by LC-MS/MS. Endoproteinase Glu-C partial digestion was also attempted to produce appropriately sized oligopeptides through partial digestion. Due to the highly repetitive glutamate–glycine sequence of RPGR-ORF15; however, it was impossible to determine their precise localizations even when some fragments were identified.
In addition to the full-length RPGR-ORF15, Western blot detected a shorter and weaker RPGR band (∼140 kDa) in Rd9 retinas injected with AAV-RPGRco (Fig. 3, arrowhead). After immunoprecipitation and proteomic analysis by LC-MS/MS, the shorter band was determined to contain RPGR exons 1–14 (68% coverage, data not shown) and the C-terminal end of ORF15 (LKNGPSGSKKFWNNVLPHYLELK), indicating an in-frame deletion in ORF15. The 140 kDa protein is one of the multiple RPGR-ORF15 protein isoforms detected in human retina.7 Apparently, a protein band of similar size to the 140 kDa protein was also detected by immunoblot in the lysates of HEK293 cells transfected with codon-optimized or wild-type RPGR-ORF15 plasmid.23 The details of how this isoform is generated are not clear currently due to the lack of the complete amino acid sequence information. However, in-frame deletions or insertions in RPGR-ORF15 that retain the C-terminal end have been reported to be well tolerated,14 and we speculate that both of the full-length and shorter RPGR species expressed in Rd9 mouse from the AAV vector may be functional.
Glutamylation of vector-expressed RPGR protein
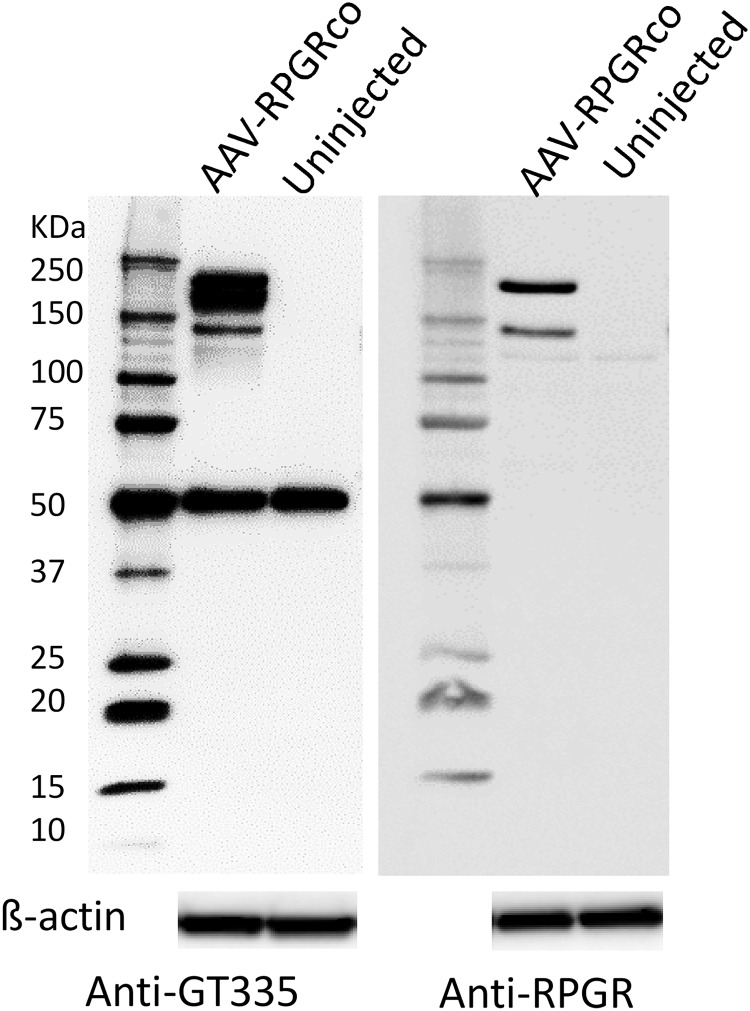
Glutamylation, a novel posttranslational modification of proteins, was initially observed on structural proteins such as α- and β-tubulins.24,25. RPGR-ORF15 was recently identified as a substrate for glutamylation and was shown to be important for its function.26. We used GT-335, a monoclonal antibody specific for glutamylation, to demonstrate that AAV-RPGRco vector-expressed RPGR-ORF15 was glutamylated in vivo. Western blot analysis of retinal tissue from Rd9 mice demonstrated the expected 50 kDa GT335-reactive tubulin band (Fig. 4, left). Subretinal administration of rAAV2tYF-GRK1-RPGRco led to the detection of additional GT335-reactive bands that co-migrated with RPGR-ORF15 immune reactive bands (Fig. 4, right), suggesting that vector-expressed RPGR-ORF15, both full length (∼200 kDa) and truncated (∼140 kDa), can be glutamylated.
Figure 4.
Glutamylation of vector-expressed RPGR protein. Western blots of whole lysates extracted from Rd9 mouse retina injected or uninjected with rAAV2tYF-GRK1-RPGRco were probed with anti-glutamylation antibody (anti-GT335) (left) or RPGR-specific monoclonal antibody (anti-RPGR) (right). β-Actin was used as a loading control. The bands detected by anti-glutamylation antibody co-migrates with the bands detected with anti-RPGR antibody.
Interaction of vector-expressed RPGR and RPGR-interacting protein 1
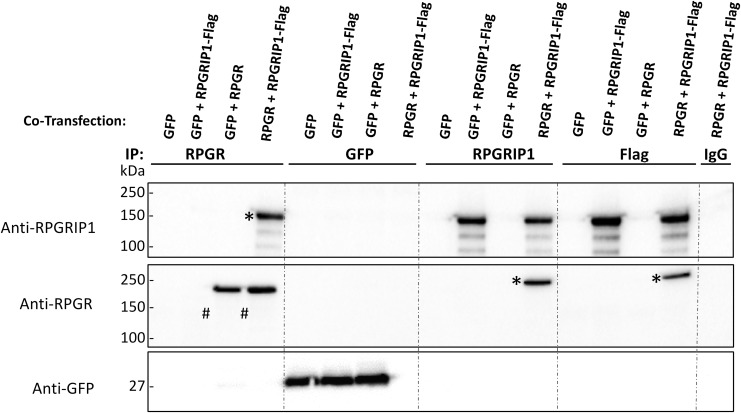
RPGR-interacting protein 1 (RPGRIP1) is one of the well-known proteins that binds to RPGR through the C-terminal RPGR-interacting domain.3 Moreover, the co-localization of RPGR and RPGRIP1 in the connecting cilia of photoreceptors also suggests the importance of their interaction in maintaining proper RPGR function. It has been reported that RPGR mutations impair the interaction between RPGR and RPGRIP1 in vivo,27 and this interaction is considered to be required for normal RPGR function in photoreceptor cells. We assessed the interaction of vector-expressed RPGR-ORF15 and RPGRIP1 in HEK 293 cells transfected with the respective expression constructs, pTR-SmCBA-RPGRco and/or pCMV-RPGRIP1-Flag (a C-terminal Flag-tagged RPGRIP1 plasmid). Co-immunoprecipitation of whole lysates of transfected HEK293 cells demonstrated that full length RPGR interacts with RPGRIP1 as expected (Fig. 5). Since the polyclonal anti-RPGR antibody that we used for Western blots can also weakly interact with RPGRIP1 nonspecifically when used in co-immunoprecipitation experiments, we then switched to a new anti-RPGR antibody which is monoclonal and specifically binds to RPGR only. This new antibody, however, could not efficiently pull down the short band of RPGR (∼140kDa) in co-immunoprecipitation, thus we could not conclude whether the short species can also bind to RPGRIP1.
Figure 5.
Interaction of vector-expressed RPGR and RPGR-interacting protein 1 (RPGRIP1). Whole cell lysates of HEK 293 cells transfected with pTR-GRK1-GFP (GFP), pTR-SmCBA-RPGRco (RPGR), or both pTR-SmCBA-RPGRco (RPGR) and pCMV-RPGRIP1-Flag (RPGRIP1-Flag) were immunoprecipitated with anti-RPGR, anti-GFP, anti- RPGRIP1, or anti-Flag antibody or immunoglobulin-g and probed with anti-RPGRIP1, anti-RPGR, or anti-GFP antibody to assess the association of RPGR and RPGRIP1. pTR-GRK1-GFP transfection was used as a negative control to exclude the possibility of non-specific binding after protein overexpression. Both RPGR (denoted by #) and RPGRIP1-Flag (denoted by *) proteins were pulled down by either anti-RPGR, anti-RPGRIP1, or anti-Flag antibody from lysate of HEK293 cells expressing both RPGR and RPGRIP1-Flag, indicating that vector-expressed full-length RPGR protein interacts with RPGRIP1 as expected. The anti-RPGR monoclonal antibody used in immunoprecipitation could not efficiently pull down the short band of RPGR (∼140kDa), as evidenced by the absence of the 140 kDa protein band (denoted by arrow) in the immunoblot with anti-RPGR antibody.
Conclusions
The objectives of this study were to evaluate, in a naturally occurring mouse model of XLRP, the safety and pharmacology of a viral vector designed to treat XLRP patients by gene augmentation therapy. The viral vector used in this study, designated AGTC-501 or rAAV2tYF-GRK1-RPGRco, contains a codon-optimized human RPGR-ORF15 cDNA driven by a photoreceptor-specific GRK1 promoter, packaged in an AAV2tYF capsid, and manufactured using an HSV-based AAV production system.
Subretinal injection of AAV2tYF-GRK1-RPGRco at two dose levels (4 × 108 and 4 × 109 vg/eye) in RPGR-deficient Rd9 mice was well tolerated with no vector-related findings in ophthalmic exams, clinical pathology, or gross and microscopic pathology. There was a trend towards reduced ERG b-wave amplitudes in the high vector dose group that was not statistically significant. RPGR protein, mainly in the connecting cilia region and the inner segment of photoreceptors, was detected by immunolabeling in a dose-dependent manner in all vector-treated eyes examined. In addition, we confirmed the integrity of the RPGR-ORF15 sequence at both the mRNA and protein level and demonstrated the vector-expressed RPGR-ORF15 was glutamylated and appropriately interacted with RPGRIP1.
These results support the use of rAAV2tYF-GRK1-RPGRco in clinical studies in patients with XLRP caused by RPGR mutations.
Supplementary Material
Acknowledgments
We thank Jie Li for technical assistance with ERG. The funding for the Mass Spectrometry Center at the University of Florida was from the U.S. National Institutes of Health (S10 OD021758-01A1).
Author Disclosure
C.S., S.M., M.V.H., P.R., A.T., D.R.K., M.S., J.D.C., and G.Y. are employees and shareholders of AGTC and have a conflict of interest to the extent that this work potentially increases their financial interests. W.W.H. and the University of Florida have a financial interest in the use of AAV therapies and are shareholders of AGTC. Covance is the drug development business of Laboratory Corporation of America Holdings (LabCorp). Content was developed by a scientist (A.S.) who at the time was affiliated with the LabCorp Clinical Trials or Tandem Labs brands, now part of Covance. None of the other authors has a competing financial interest.
References
- 1. Fishman GA. Retinitis pigmentosa. Genetic percentages. Arch Ophthalmol 1978;96:822–826 [DOI] [PubMed] [Google Scholar]
- 2. Testa F, Rossi S, Colucci R, et al. Macular abnormalities in Italian patients with retinitis pigmentosa. Br J Ophthalmol 2014;98:946–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Megaw RD, Soares DC, Wright AF. RPGR: its role in photoreceptor physiology, human disease, and future therapies. Exp Eye Res 2015;138:32–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bird AC. X-linked retinitis pigmentosa. Br J Ophthalmol 1975;59:177–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet 2006;368:1795–1809 [DOI] [PubMed] [Google Scholar]
- 6. Sandberg MA, Rosner B, Weigel-DiFranco C, Dryja TP, Berson EL. Disease course of patients with X-linked retinitis pigmentosa due to RPGR gene mutations. Invest Ophthalmol Vis Sci 2007;48:1298–1304 [DOI] [PubMed] [Google Scholar]
- 7. He S, Parapuram SK, Hurd TW, et al. Retinitis pigmentosa GTPase regulator (RPGR) protein isoforms in mammalian retina: insights into X-linked retinitis pigmentosa and associated ciliopathies. Vision Res 2008;48:366–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vervoort R, Lennon A, Bird AC, et al. Mutational hot spot within a new RPGR exon in X-linked retinitis pigmentosa. Nat Genet 2000;25:462–466 [DOI] [PubMed] [Google Scholar]
- 9. Khanna H, Hurd TW, Lillo C, et al. RPGR-ORF15, which is mutated in retinitis pigmentosa, associates with SMC1, SMC3, and microtubule transport proteins. J Biol Chem 2005;280:33580–33587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mavlyutov TA, Zhao H, Ferreira PA. Species-specific subcellular localization of RPGR and RPGRIP isoforms: implications for the phenotypic variability of congenital retinopathies among species. Hum Mol Genet 2002;11:1899–1907 [DOI] [PubMed] [Google Scholar]
- 11. Wright AF, Shu X. Focus on molecules: RPGR. Exp Eye Res 2007;85:1–2 [DOI] [PubMed] [Google Scholar]
- 12. Hosch J, Lorenz B, Stieger K. RPGR: role in the photoreceptor cilium, human retinal disease, and gene therapy. Ophthalmic Genet 2011;32:1–11 [DOI] [PubMed] [Google Scholar]
- 13. Khanna H. Photoreceptor Sensory Cilium: Traversing the ciliary gate. Cells 2015;4:674–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pawlyk BS, Bulgakov OV, Sun X, et al. Photoreceptor rescue by an abbreviated human RPGR gene in a murine model of X-linked retinitis pigmentosa. Gene Ther 2016;23:196–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu Z, Hiriyanna S, Qian H, et al. A long-term efficacy study of gene replacement therapy for RPGR-associated retinal degeneration. Hum Mol Genet 2015;24:3956–3970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Beltran WA, Cideciyan AV, Guziewicz KE, et al. Canine retina has a primate fovea-like bouquet of cone photoreceptors which is affected by inherited macular degenerations. PLoS One 2014;9:e90390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thompson DA, Khan NW, Othman MI, et al. Rd9 is a naturally occurring mouse model of a common form of retinitis pigmentosa caused by mutations in RPGR-ORF15. PLoS One 2012;7:e35865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weymouth AE, Vingrys AJ. Rodent electroretinography: methods for extraction and interpretation of rod and cone responses. Prog Retin Eye Res 2008;27:1–44 [DOI] [PubMed] [Google Scholar]
- 19. Deng WT, Dyka FM, Dinculescu A, et al. Stability and safety of an AAV vector for treating RPGR-ORF15 X-linked retinitis pigmentosa. Hum Gene Ther 2015;26:593–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ye GJ, Beltran WA, Dufour VL, Marinho FP, et al. Evaluation of AAV2tYF-GRK1-RPGR vectors in a canine model of RPGR-XLRP. The Association for Research and Ophthalmology (ARVO) Annual Meeting, Baltimore, MD, May2017, Abstract/Poster B0141 [Google Scholar]
- 21. Swaim LD, Taylor HW, Jersey GC. The effect of handling techniques on serum ALT activity in mice. J Appl Toxicol 1985;5:160–162 [DOI] [PubMed] [Google Scholar]
- 22. Beltran WA, Cideciyan AV, Boye SE, et al. Optimization of retinal gene therapy for X-linked retinitis pigmentosa due to RPGR mutations. Mol Ther 2017;25:1866–1880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fischer MD, McClements ME, Martinez-Fernandez de la Camara C, et al. Codon-optimized RPGR improves stability and efficacy of AAV8 gene therapy in two mouse models of X-linked retinitis pigmentosa. Mol Ther 2017;25:1854–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Edde B, Rossier J, Le Caer JP, Desbruyeres E, Gros F, Denoulet P. Posttranslational glutamylation of alpha-tubulin. Science 1990;247:83–85 [DOI] [PubMed] [Google Scholar]
- 25. Wolff A, de Nechaud B, Chillet D, et al. Distribution of glutamylated alpha and beta-tubulin in mouse tissues using a specific monoclonal antibody, GT335. Eur J Cell Biol 1992;59:425–432 [PubMed] [Google Scholar]
- 26. Sun X, Park JH, Gumerson J, et al. Loss of RPGR glutamylation underlies the pathogenic mechanism of retinal dystrophy caused by TTLL5 mutations. Proc Natl Acad Sci U S A 2016;113:E2925–2934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Roepman R, Bernoud-Hubac N, Schick DE, et al. The retinitis pigmentosa GTPase regulator (RPGR) interacts with novel transport-like proteins in the outer segments of rod photoreceptors. Hum Mol Genet 2000;9:2095–2105 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.