Abstract
Chronic traumatic encephalopathy (CTE) is associated with pathological changes, yet detecting these changes during life has proven elusive. Positron emission tomography (PET) offers the potential for identifying such pathology. Few studies have been completed to date and their approaches and results have been diverse. It was the objective of this review to systematically examine relevant research using ligands for PET that bind to identified pathology in CTE. We focused on identification of patterns of binding and addressing gaps in knowledge of PET imaging for CTE. A comprehensive literature search was conducted. Data used were published on or before May 22, 2017. As the extant literature is limited, any peer-reviewed article assessing military, contact sports athletes, or professional fighters was considered for inclusion. The main outcomes were regional binding to brain regions identified through control comparisons or through clinical metrics (e.g., standardized uptake volume ratios). A total of 1207 papers were identified for review, of which six met inclusion criteria. Meta-analyses were planned but were deemed inappropriate given the small number of studies identified. Methodological concerns in these initial papers included small sample sizes, lack of a control comparison, use of nonstandard statistical procedures to quantify data, and interpretation of potentially off-target binding areas. Across studies, the hippocampi, amygdalae, and midbrain had reasonably consistent increased uptake. Evidence for increased uptake in cortical regions was less consistent. The evidence suggests that the field of PET imaging in those at risk for CTE remains nascent. As the field evolves to include more stringent studies, ligands for PET may prove an important tool in identifying CTE in vivo.
Keywords: : adult brain injury, beta amyloid, head trauma, inflammation, PET scanning
Introduction
In recent years, chronic traumatic encephalopathy (CTE) has been the subject of much public and media interest as it has important potential ramifications, especially in sports and the military. There has been an upswing of published articles, news reports, books, and even a movie on the subject. Yet CTE remains a complex issue. CTE is a pathological diagnosis, defined at autopsy and retrospectively found to be a progressive, neurodegenerative condition brought about by a history of repetitive concussive or subconcussive injury.1 Pathologically, this disease is characterized as a tauopathy. This tauopathy manifests as neurofibrillary tangles composed of hyperphosphorylated tau protein in addition to astrocytic tangles occurring early on in superficial cortical laminae, perivascular regions, the base of cortical sulci, limbic regions, the brainstem, cerebellum, and in the basal ganglia.2,3
In as many as 47% of cases, however, the co-occurring presence of other pathological hallmarks, such as amyloid deposition, Lewy bodies, and TDP-43 proteins, are found.3 Indeed, many individuals across pathological studies of CTE also meet diagnostic criteria for another neurodegenerative condition (see Iverson and colleagues4 for review). Given the frequency with which extracellular amyloid beta (β-amyloid) deposition has been found, this evidence may suggest its role in the disease pathogenesis.5 Some debate continues about β-amyloid in CTE given its role in inflammatory processes6 and high prevalence in the brains of autopsy-confirmed CTE cases3 in juxtaposition to its high correlation with age in clinically healthy older adults. As β-amyloid's precise relationship to CTE pathogenesis remains elusive, it is an important pathological hallmark to research. Other research suggests that inflammatory processes may be linked to the pathology of CTE given its known role in the pathogenesis of traumatic brain injury and findings in CTE autopsy research.6–9
The advent of positron emission tomography (PET) imaging ligands that target hyperphosphorylated tau protein (e.g., AV145),10,11 β-amyloid (e.g. florbetapir, florbetaben, flutemetamol, Pittsburgh compound B)12–17 and neuroinflammatory processes (e.g., PBR28),18 may afford the potential to assess concerns voiced by critical reviewers of the pathology literature. Notably, PET imaging provides an empirically validated means of assessing pathology in vivo, which in turn allows researchers and clinicians to better assess the potential causal implications of β-amyloid pathology in the clinical presentation of the disorder. Studies using PET in those at risk for CTE are critical in characterizing CTE in vivo; however, little consensus about the findings of this research has been published. Therefore, a systematic review and synthesis of this evidence base is undertaken here. Specifically, imaging evidence for β-amyloid deposition, hyperphosphorylated tau protein as neurofibrillary tangles, and inflammatory processes are highlighted as used in populations at risk for CTE.
To date, a majority of CTE research has focused on detailed and thorough pathological studies2,3,19–21 that have characterized CTE postmortem. At the first consensus meeting of the National Institute of Neurological Disorders and Stroke/National Institute of Biomedical Imaging and Bioengineering,22 selected neuropathologists blindly evaluated several tauopathies, and identified CTE with up to 90% agreement.23 Of these 142 patients, 51 (∼36%) had one or more comorbid pathological diagnoses. These findings suggest acceptable efficacy of pathological CTE diagnoses. However, postmortem studies are obviously not able to assess for changes in the pathological hallmarks of the disease over time, and are also limited by the lack of ability to draw causal inferences in the relationship between repetitive head injury and pathology, the availability of only a small number of well-characterized individuals, and the lack of suitable controls. Consistent with this, in the 2015 consensus meeting noted above,22 the authors warned that a causal relationship between observed neuropathology and clinical symptoms remains tenuous and current neuropathological criteria are preliminary.22,23 These limitations are a major barrier to understanding CTE, which can potentially be addressed with PET studies.
Researchers have grappled with pathological (and in some instances phenotypical) similarities between CTE and other tauopathies such as Alzheimer's disease (AD), progressive supranuclear palsy (PSP), Pick's disease, and corticobasal degeneration, all of which involve varying degrees of tauopathy or amyloidopathy. Disease-specific isoform compositions of tau have been identified in these neurodegenerative conditions that may allow for targeted radioimaging (see Villemagne and Okamura24 for a recent review of tau imaging in neurodegenerative disease). Overall, the intraneuronal composition of neurofibrillary tangles found in CTE is not distinct to the form seen in Alzheimer's disease.3 CTE differs in that it involves more prominent astrocytic tangles than are reported in aging-related tau astrogliopathy (ARTAG).25 Paired helical filaments, which are the principal component of neurofibrillary tangles, are comprised of both 3- and 4-repeat isoforms of tau in both CTE and Alzheimer's disease. While the intraneuronal isoform and aggregates are not distinct from Alzheimer's, the topographic distribution, especially early in the disease process, is described as distinct from the predictable spread of Alzheimer's disease noted by Braak and Braak and more recent researchers.2,3,26–28 Similarly, the astrocytic tangles are not distinguishable from those found in ARTAG, although they are found in a distinct location, specifically in clusters at the depths of the cortical sulci and in a perivascular distribution in early stages.3 Thus, ligands sensitive to the tauopathy of AD should also be useful in identifying early CTE due to their affinity for neurofibrillary tangles, although only the spatial pattern would distinguish between the two disorders.
Each tau ligand for PET has binding affinities for certain types of tauopathies that allow for selection of those best suited to CTE research. Marquie and colleagues29 found that F18-AV-1451 binds strongly to tau lesions made of paired helical filaments in Alzheimer's disease brains. A clinical comparison study found that AV1451 binding was consistent with the predicted pattern in Alzheimer's disease and PSP, and demonstrated elevated binding in an at-risk CTE case (although not entirely consistent with the pathognomonic distribution).30,31 Other studies suggest that AV1451 may have weak binding for straight tau filaments in non-Alzheimer tauopathy brains such as PSP.32 Ultimately, this ligand may be a good candidate agent for CTE imaging because it is tau-specific and may bind strongly to the tau pathology seen in Alzheimer's disease and CTE. Another tau ligand, 18F-FDDNP, binds to intracellular neurofibrillary tangles33 and may similarly be useful in CTE research; however, it is considered “non-selective” because it also binds to extracellular β-amyloid, a component of Alzheimer's disease, not considered necessary for CTE.3,23 Due to the lack of reproduction of FDDNP findings in other research labs, debate continues about the efficacy of FDDNP as a radiotracer.34 11C-PBB3 images a wide range of tau deposits (including Pick's bodies, tufted astrocytes, oligodendroglial coiled bodies, and astrocytic plaques, all typical of primary tauopathies). Ono and colleagues35 suggest that compared with AV1451, 11C-PBB3 may be less effective at identifying paired helical filaments-tau in CTE; however, no consensus exists that 11C-PBB3 may be better equipped to identify astrocytic tangles in CTE. Overall, the results of those studies of CTE using ligands such as AV1451 (which have higher specificity for neurofibrillary tangles) have been reported more commonly.
Neuroinflammatory ligands for PET target Translocator Protein 18 kDa as a marker for neuroinflammatory processes, since it is reliably elevated along with activated microglia in the central nervous system. Ligands that target Translocator Protein 18 kDa (TSPO) have been termed first-generation (e.g., 11C-PK11195) and second-generation (e.g., PBR111, DPA713, PBR06, DAA1106). 36 In this review, Turkheimer and colleagues36 explain that second-generation TSPO ligands aimed to address signal-to-noise ratio problems with first-generation ligands due to binding sites in blood and plasma proteins, as well binding in the normal brain to the blood–brain barrier and the brain itself. Unfortunately, second-generation ligands encounter challenges since genetics determine high-binders from low-binders more generally, and issues involving microglial presence in normal brain tissue, binding to the blood–brain barrier, and plasma binding have not been fully addressed. However, such ligands are our best tool to address neuroinflammatory processes and greater weight should be given to studies that address these methodological concerns on a genetic level. In addition to these methodological considerations for TSPO ligands, the picture of neuroinflammation in both traumatic brain injury (TBI) and CTE is murky. In cases of isolated TBI, there is some evidence to suggest that chronic neuroinflammatory changes are seen in subcortical structures (i.e., the thalamus and putamen), and cerebrospinal fluid cytokine studies have predicted unfavorable outcomes after TBI.6 The literature to date does not adequately addresses whether additive, multiplicative, or minimal neuroinflammatory damage may occur in the presence of repeated mechanical insult to the brain, although the presence of neuroinflammation in CTE is commonly touted. 6–9
This systematic review will target PET imaging of contact sports athletes and military personnel with repetitive head trauma (RHT) and healthy control comparisons where available to examine differences in PET tracer uptake. Additionally, given the evidence for a predictable pattern of neurofibrillary tangles (NFTs) in CTE, greater emphasis will be put on tau ligands such as AV1451 and that have demonstrated good affinity for NFT pathology. Our specific focus on PET tracer uptake will hopefully enable future research to precisely characterize CTE in vivo and develop targeted strategies for diagnosis.
Methods
Protocol and registration
No existing review protocol structured the present paper. We completed this systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 37 criteria.
Eligibility criteria
Given the above, our review relies on peer-reviewed research that fits two defined criteria: CTE and radioimaging of CTE pathology. For the purpose of this systematic review, we will consider CTE to be a degenerative neuropathological condition brought on by exposure to RHT. This follows from the pathological definition of McKee and colleagues.7 This definition is deliberately broad to allow for examination of literature addressing imaging of tau, β-amyloid, and neuroinflammation. The terms “head injury” and “concussion” are loosely defined constructs in the literature and are included in our definition to allow for inclusion of a larger subset of potentially relevant research. Head injury does not necessarily entail concussion, and evidence suggests that CTE antecedents can include subconcussive impacts38; a 2015 review found that 16% of CTE subjects had no previous concussion history.39 As such, “concussion” or “brain injury” were not required terms for inclusion and for the purposes of this review, RHT is used in favor of these terms. For this review, radioimaging of CTE pathology is considered to be PET imaging aimed at identifying tau pathology, β-amyloid pathology, or neuroinflammatory pathology. Given our terms, we excluded studies that a) did not include a population with exposure to RHT, and b) did not use radioisotopes shown to bind to tau, beta amyloid, or neuroinflammatory processes.
We identified imaging tracers a priori for inclusion in this review based on established evidence for their efficacy. The recent reviews by Okamura and colleagues39 and Villemagne and Okamura24 served as a scaffold for selection of tau-selective radioligands for this review, and identification of 18F-FDDNP as a dual tracer of tau and β-amyloid. Radioisotopes identified by these reviews as being tau selective are as follows: 18F-THK-5105, 18F-THK-5117, 18F-THK-5351, 18F-AV-1451 (18F-T807), 11C-PBB3, and 18F-RO6958948. Studies using these isotopes were therefore interpreted as tau imaging studies. In another review, Rowe and Villemagne40 identified 18F-florbetapir, 11C-PIB, 18F-florbetaben, and 18F-flutemetamol as selective radioisotopes for the imaging of β-amyloid. Studies using these isotopes were interpreted as β-amyloid imaging studies. For interpretation of studies as in vivo characterization of neuroinflammation, the radioisotopes 11C-PBR2818 and 11C-DPA-71341 were chosen since they have been shown to demonstrate glial activation characteristic of neuroinflammatory processes.
Information sources and search procedure
We searched computerized databases (PubMed, Ovid, and Google Scholar), citations in reviewed articles, and references provided by colleagues. In order to conduct a comprehensive review of the literature, a list of search terms encompassing general phrases and specific radioligands pertinent to our definitions was created. Our searches encompassed all possible permutations of nine CTE terms and 16 radioimaging search terms (Table 1) encompassing 144 individual search queries. These search queries were collapsed into a single-string Boolean search phrase (Appendix 1; see online supplementary material at http://www.liebertpub.com) for use in PubMed and Ovid, without the application of filters. Authors known to conduct research in this domain were used as search terms in PubMed, Ovid, and Google Scholar. Additional searches were conducted in Google Scholar using the search terms identified in Table 1. Only articles published or known to us before May 22, 2017, were included.
Table 1.
Search Terms
| Imaging terms | CTE terms |
|---|---|
| THK-5105 | CTE |
| THK-5117 | Chronic traumatic encephalopathy |
| THK-5351 | Head injury |
| AV-1451 | Concussion |
| T807 | Boxing |
| PBB3 | Football |
| RO6958948 | Rugby |
| PET | Contact sports |
| Positron emission Tomography | Military |
| Florbetapir | |
| PIB | |
| Florbetaben | |
| flutemetamol | |
| PBR28 | |
| Neuroinflammation |
CTE, chronic traumatic encephalopathy.
Study selection
For each search engine queried, article titles were reviewed and irrelevant results were removed (i.e., review articles, animal models, or articles not addressing either of our searched criteria). After the initial database search, authors BL and ML independently reviewed the ensuing data and came to consensus on which articles to include for each step in the selection process. Articles were culled after abstract review if they did not use methods relevant to our research question (e.g., the use of FDG PET only or use of a sample with a single head injury only). We reviewed in full those articles that included people with a history of repetitive head trauma (RHT) and PET imaging using one of our preselected radioisotopes. When papers were read in full, they were assessed in terms of their content and methodological rigor. For articles read in full, authors BL, ML, and SB arrived at consensus regarding the interpretation of these results for discussion in this article.
Statistical analysis
The number of unique research articles using PET imaging to investigate CTE was not sufficient to conduct a meta-analysis. The present review included any studies that investigated our constructs adequately and met our inclusion and exclusion criteria. No additional statistical analyses were performed. Risks of bias for the studies included are addressed below.
As the number of participants in a given study was highly variable, we opted against a strict study-to-study comparison in our synthesis of the retrieved data. In our summary of the literature, the proportion of individuals with identified pathology in a given region will be reported along with the number of individuals with RHT on whom these observations were made. Note that these figures only include those with exposure to RHT. The number of control participants when included in studies are reflected in Table 2.
Table 2.
General Sample Information
| Article | Reference method | n = RHT/CN | Age (CN) | Education (CN) |
|---|---|---|---|---|
| Small and colleagues48 | Cerebellar gray matter | 5 (5) | 59 (60) | 17 (15) |
| Barrio and colleagues43 | Cerebellar gray matter | 14 (28) | 57.2 (64.3) | 16.2 (N.R.) |
| Jordan and colleagues46 | Whole cerebellum | 1 | 39 | 16 |
| Mitsis and colleagues47 | Whole cerebellum | 1 | 71 | 16 |
| Coughlin and colleagues45 | Regional DVR | 11 (9) | 64.8 (58.3) | 16.7 (16.0) |
| Coughlin and colleagues44 | Regional DVR | 12 (11) | 31.3 (27.6) | 16.8 (16.9) |
RHT, repeated head trauma; CN, control; N.R., not reported; DVR, distribution volume ratio.
Results
Study selection
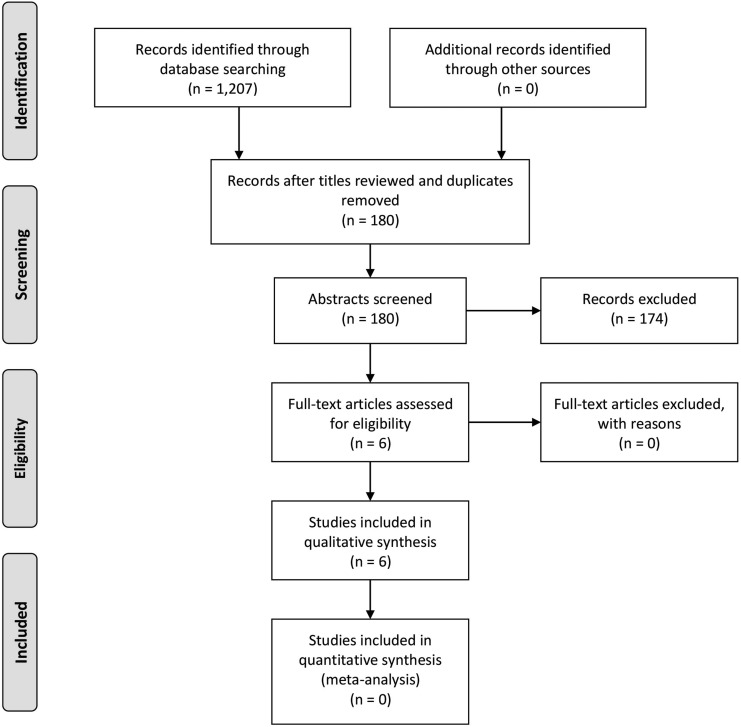
Our initial searches led to a total article sample of 1207. Initial review of article titles to exclude those that were clearly irrelevant reduced our sample to 180. After reading article abstracts, six articles42–47 were deemed to be appropriate for full review for inclusion in the study. Detailed data on the number of papers excluded between phases 1 and 3 as a function of criterion can be found in Figure 1. The total RHT sample across these articles was 39 individuals, accounting for studies that used the same participants across publications. General demographic information, a list of the included studies, and their PET reference method can be found in Table 2.
FIG. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)37 flow diagram.
Discussion
Evidence for regional uptake
The literature on CTE suggests that pathological hallmarks such as neurofibrillary tangles should be present in the bases of cortical sulci and in perivascular regions most prominently in the frontal lobe, then extending to other regions, such as midbrain and thalamic structures, and the basal ganglia.3,23,48–50 One of the articles included in this review42 employed a priori regions that align well with this conceptualization, and helped formulate our a priori target brain regions within which research observations were presented.
All studies assessed uptake in limbic regions, including the amygdala, hippocampal formation, and temporal pole to some extent. Table 3. Of the RHT participants in these studies, when compared with controls, all had evidence of increased uptake in the hippocampus (suggesting increased tau as NFTs and/ or amyloidopathy; see FDDNP caveats above); neuroinflammatory studies identified significantly more binding to Translocator Protein (TSPO) only in younger RHT samples. Interestingly, Barrio and colleagues42 found no significant difference between RHT samples and Alzheimer's samples in FDDNP uptake in the hippocampus. All studies that assessed amygdala uptake found that RHT participants had significantly greater binding when compared with controls, although neuroinflammatory evidence for increased uptake in this region was limited to the right amygdala in a sample of older RHT patients. Evidence for uptake in the temporal pole was less consistent, with two of three studies noting significant differences compared with controls: One imaging tau pathology45 found bilateral uptake increases in a single patient, and one study imaging neuroinflammation43 found left-sided increased uptake in their sample of 12 patients with RHT.
Table 3.
Findings in Limbic Regions
| Limbic | |||||
|---|---|---|---|---|---|
| Imaged pathology | Tracer(s) used | Article | Amygdala | Medial temporal lobe/ hippocampal formation | Temporal pole |
| Tau/amyloid imaging | Barrio and colleagues43 RHT (n = 14) > CN (n = 28) | x | x | – | |
| [F-18] FDDNP | Barrio and colleagues43 RHT (n = 14) > AD (n = 24) | x | NS | – | |
| [F-18] FDDNP | Small and colleagues48* RHT (5) > CN (5) | x | NS | – | |
| Tau only imaging | [18F] T807, AV1451 | Jordan and colleagues46 (n = 1) | – | x | x |
| 18F-Florbetapir, T807 | Mitsis and colleagues47 (n = 1) | – | x | – | |
| Neuroinflammation imaging | 18 kda TSPO | Coughlin and colleagues44 RHT (n = 12) > CN (n = 11) | x | x | x (L) |
| [11C] DPA-713 TSPO | Coughlin and colleagues45 RHT (n = 11) > CN (n = 9) | x (R) | NS | NS | |
| Proportion of patients with significant changes vs. CN; Small and colleagues48 excluded (Patient n) | 1 (47) | 0.72 (37) | 0.54 (24) | ||
RHT, repetitive head trauma; CN, control; x, significant finding; –, comparison was not identified a priori for the specific article; AD, Alzheimer's disease; NS, not significant; TSPO, Translocator Protein 18 kDa; L, left; R, right.
A summary of the evidence for uptake in subcortical structures can be found in Table 4. One study42 found increased uptake of FDDNP in the hypothalamus, thalamus, pons, and striatum; one study found increased uptake in the striatal substructures, globus pallidus, and putamen,46 and another found increased uptake in the globus pallidus only45 but did not identify the putamen a priori. Small and colleagues'47 finding of increased uptake in the caudate and putamen are not interpreted as all of these participants are included in Barrio and colleagues'42 larger sample; however, their unique findings of increased uptake in cerebral white matter and subthalamic nucleus are of note, as no other studies identified these comparisons a priori. Both Barrio and colleagues42 and Jordan and colleagues45 found increased uptake in the midbrain; however, no other studies identified the midbrain as a region of interest. Only one study accounting for a single subject found increased uptake in the substantia nigra.47 Whether this represents off-target binding of AV1451 to neuromelanin52 or a unique clinical phenotype cannot be determined due to the study's methods.
Table 4.
Findings in Subcortical Regions
| Imaged pathology | Tracer(s) used | Article | Substantia nigra | Subcortical hypothalamus | Thalamus | Subthalamic nucleus | Pons | Striatum | Globus pallidus | Caudate | Putamen | Cerebral white matter |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tau/amyloid Iimaging | Barrio and colleagues43 RHT (n = 14) > CN (n = 28) | – | x | x | – | x | x | – | – | – | – | |
| [F-18] FDDNP | Barrio and colleagues43 RHT (n = 14) > AD (n = 24) | – | x | NS | – | x | x | – | – | – | – | |
| [F-18] FDDNP | Small and colleagues48* RHT (5) >CN (5) | – | – | x | x | – | – | – | x | x | x | |
| Tau only imaging | [18F] T807, AV1451 | Jordan and colleagues46 (n = 1) | – | – | – | – | – | – | x | – | – | – |
| 18F-Florbetapir, T807 | Mitsis and colleagues47 (n = 1) | x | – | – | – | – | – | x | – | x | – | |
| Neuroinflammation imaging | 18 kda TSPO | Coughlin and colleagues44 (n = 12) > CN (n = 11) | – | – | – | – | – | – | – | – | – | – |
| [11C] DPA-713 TSPO | Coughlin and colleagues45 (n = 11) > CN (n = 9) | – | – | – | – | – | – | – | – | – | – | |
| Proportion of patients with significant changes vs. CN; Small and colleagues48 excluded (Patient n) | 1 (1) | 1 (14) | 1 (14) | 1 (5) | 1 (14) | 1 (14) | 1 (2) | 1 (5) | 1 (6) | 1 (5) | ||
The evidence for cortical regions (Table 5) is more varied. Two studies42,45 reported increased uptake of FDDNP and AV1451, respectively, in the frontal lobe, compared with controls, with Barrio and colleagues42 also reporting no significant difference between RHT and AD samples in frontal regional uptake. These same studies also identified the anterior cingulate cortex as a region with increased uptake for RHT patients. Evidence for the posterior cingulate cortex, however, is not at all consistent with only one study45 finding significant uptake in this region, and another finding no difference between controls and RHT using a larger sample.42 Both neuroinflammatory studies found evidence for increased uptake in the supramarginal gyrus; unfortunately, no other studies discussed analyses for this region specifically. Barrio and colleagues42 found increased FDDNP uptake for the broadly defined parietal lobe. Only Jordan and colleagues45 reported increased uptake in the retrosplenial cortex in a younger retired National Football League (NFL) player. Two studies found evidence for increased uptake for lateral temporal lobe regions including the narrower-defined primary auditory cortex.42,45
Table 5.
Findings in Cortical Regions
| Cortical | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Imaged pathology | Tracer(s) used | Article | Frontal lobe | Anterior cingulate | Posterior cingulate | Retrosplenial cortex | Parietal lobe | Supramarginal gyrus | Lateral temporal lobe | Occipital |
| Tau/amyloid imaging | Barrio and colleagues43 RHT (n = 14) > CN (n = 28) | x | x | NS | – | x | – | x | x | |
| [F-18] FDDNP | Barrio and colleagues43 RHT (n = 14) > AD (n = 24) | NS | NS | NS | – | NS | – | NS | NS | |
| [F-18] FDDNP | Small and colleagues48* RHT (5) > CN (5) | NS | – | NS | – | NS | – | NS | – | |
| Tau only imaging | [18F] T807, AV1451 | Jordan and colleagues46 (n = 1) | x | x | x | x | – | – | x (Primary A. Ctx) | x |
| 18F-Florbetapir, T807 | Mitsis and colleagues47 (n = 1) | – | – | – | – | – | – | – | – | |
| Neuroinflammation imaging | 18 kda TSPO | Coughlin and colleagues44 RHT (n = 12) > CN (n = 11) | – | – | – | – | – | x | – | – |
| [11C] DPA-713 TSPO | Coughlin and colleagues45 RHT (n = 11) > CN (n = 9) | – | – | – | – | – | x | – | – | |
| Proportion of patients with significant changes vs. CN; Small and colleagues48 excluded (Patient n) | 1(15) | 1 (15) | 0.07 (15) | 1 (1) | 1 (14) | 1 (23) | 1 (15) | 1 (15) | ||
RHT, repetitive head trauma; CN, control; x, significant finding; NS, not significant; –, comparison was not identified a priori for the specific article; AD, Alzheimer's disease; Primary A. Ctx, primary auditory cortex; TSPO, Translocator Protein 18 kDa.
Overall, the evidence for regional uptake across tracer type is strongest for limbic structures except the temporal pole and for midbrain structures when assessed. With regard to cortical regions, findings are harder to assess given the varied nature in which regions are parcellated for analysis across studies. There is some agreement that there is increased uptake in frontal, temporal, occipital, and parietal regions more broadly, although there were no comparisons made among neuroinflammatory studies for occipital, frontal cingulate, or lateral temporal regions. Hence, there is evidence for both neuroinflammatory processes and tau deposition in the parietal lobe only at this time. Reasonable evidence exists for increased tau or combined tau/ β-amyloid deposition in the anterior cingulate cortex but not the posterior cingulate.
Tau and CTE
As can be seen from the narrative summary in Tables 3 to 5, for studies using the tau-specific tracer 18F-T807/AV1451, there is a consistent pattern or uptake in the globus pallidus, hippocampus, and putamen for young and old former NFL athletes with a history of RHT and CTE symptomatology; however, sample size limitations make it difficult to assume the generalizability of these results. Additionally, Jordan and colleagues45 used a threshold for determining increased binding developed for florbetapir but applied it to AV14151. Since these two ligands bind to completely different targets, the results should be interpreted with caution.
Tau/β-amyloid and CTE
A larger number of studies employ 18F-FDDNP, a radiotracer shown to bind to both tau as neurofibrillary tangles and to β-amyloid52; however, there is difficulty in knowing to what protein the ligand is bound, and all studies have been completed by the same academic group. Two studies used 18F-FDDNP across 14 patients with suspected CTE. It should be noted that a subset of five patients from the more recent study43 were the same sample used for the earlier47 investigation, and interpretations are herein drawn from the larger total sample, and clarified by observations from this smaller subset. Barrio and colleagues'42 study design is unique because it offers comparisons between healthy controls, those with RHT, and an Alzheimer's dementia population. They found that NFL players with repetitive head injuries showed increased binding for limbic and subcortical regions and for the frontal and anterior cingulate gyrus, while compared with patients with Alzheimer's disease, RHT participants showed significantly greater binding primarily for subcortical and non-hippocampal limbic structures including the midbrain, hypothalamus, pons, striatum, and amygdala (accounting for multiple comparisons), but not for cortical regions. Small and colleagues48 similarly found that compared with controls, NFL football players with RHT had increased uptake in the amygdala, caudate, putamen, thalamus, subthalamic nucleus, midbrain, and cerebellar white matter. With the larger sample included in Barrio and colleagues'42 paper, all investigated subcortical brain regions and the anterior cingulate and frontal lobes demonstrated increased uptake when compared with healthy controls, accounting for multiple comparisons. As 18F-FDDNP is not specific to tau protein, it is difficult to know whether this binding represents tauopathy, amyloidopathy, or a combination of both in these NFL players with RHT.
β-Amyloid and CTE
No studies in the reviewed literature employed sufficient β-amyloid imaging regional uptake values to assess evidence for regional uptake of β-amyloid pathology. Those that employed amyloid imaging used this as a rule-out criterion for Alzheimer's disease45,46 and as such did not report regional uptake. This is surprising since pathological evidence suggests that β-amyloid pathology as diffuse plaques, neuritic plaques, or vascular amyloid was found in the brains of 44.1% of brains with CTE, including 27.4% of those brains identified as having “pure” CTE pathology in McKee and colleagues'3 sample. Although 18F-FDDNP imaging captures some β-amyloid pathology in addition to pTau, it is impossible to determine the extent to which the pathology imaged represents the inclusion of β-amyloid pathology. One study that did not meet our inclusion criteria53 reported a case series of NFL players, two of whom had identified β-amyloid pathology (in one case diffusely and in another case in the left anterior superior temporal lobe). Given the clear evidence that β-amyloid increases with age in pathological samples assessing CTE3 and in the healthy ageing population more broadly,55–60 the presence of β-amyloid pathology may ultimately be a spurious finding in imaging studies of CTE; however, imaging research investigating this hypothesis may help build diagnostic specificity in vivo.
Neuroinflammation and CTE
The works by Coughlin and colleagues43,44 represent steps forward in examining potential neuroinflammatory biomarkers for CTE. The steps taken by their later work in controlling for genetic factors in Translocator Protein 18 kDa (TSPO) binding allow more specificity as some individuals are considered “non-binders.” Given the pathological findings by groups such as McKee and colleagues,3,23 Stern and colleagues,21 and the PET findings examining tau deposition in this population42,45–47 that report early and prominent tau deposition in midbrain and subcortical structures, it is interesting that these regions were not identified as a priori regions of interest for these investigations. Further studies may benefit from examining subcortical nuclei and midbrain structures for evidence of neuroinflammation in this population. Additionally, these studies suggest that the evidence for neuroinflammatory pathology is greater for younger RHT samples. This may be due to methodological differences or to TSPO evidence for neuroinflammatory damage subsiding over time post-injury. Further research may benefit from including an RHT sample with a wide age range and assessing for this uptake as a function of age, or utilizing serial imaging within participants over time post-exposure.
Technicalities in studying neurodegenerative disorders with PET
Several important issues in identifying reliable PET biomarkers in CTE should be addressed. Individuals with this disorder identified at autopsy often have significant atrophy. This can cause complications for PET analysis and interpretation due to partial volume effects, which can distort the amount of apparent uptake in a small region (either due to its initial size or later atrophy), and correcting for this can change results (e.g., Ossenkoppele and colleagues, 2016).60 The manner in which brain regions are parcellated will also impact results. In the current review, the studies employing FDDNP imaging42,47 use manually-drawn neuroanatomic regions. Jordan and colleagues45 defined anatomic regions by co-referencing to magnetic resonance imaging (MRI) images and employing an MNI-Brodmann area atlas for extraction of brain regions. Mitsis and colleagues46 spatially normalized their neuroimaging data to a template image and applied a modified Hammers volume template. Coughlin and colleagues' studies43,44 co-registered PET images to MRI and used anatomic subdomains automatically generated using Freesurfer. The reference region (e.g., whole cerebellum) used is also important and in the case of tau imaging, continues to evolve. As considerations related to parcellation, reference regions, and volume correction are continually evolving in the field, it is recommended that future studies follow up-to-date standards of practice in these matters. Studies examining neuroinflammation using TSPO ligands should consider the influence of genetics on binding.
Conclusions, Limitations, and Future Directions
Even though the use of radioimaging in CTE is a newer and costly avenue of research, the small number of participants included in these studies is surprising. It remains a limitation and more studies are needed to gain insight into the efficacy of these techniques for identification of CTE pathology in vivo. Patients included in the studies tend to be symptomatic, presenting a selection bias, and controls with similar symptoms without head injury exposure are rarely included. Studies that use larger cohorts are needed, since the small sample size that encompasses the extant published literature limits the degree to which results can be generalized. Although selectively-binding tau and amyloid ligands for PET may be particularly useful in the identification and monitoring of CTE pathology in vivo, no studies to date that report such ligands include adequate control comparisons. Ideally, since these ligands have been developed and validated for the identification of other neuropathological entities (commonly Alzheimer's disease), studies that include a population at risk for CTE versus matched healthy control and diseased comparisons would be particularly important in bridging this literature gap.
Additionally, the vast majority of RHT participants are professional football players. Further research may broaden our understanding by including other populations such as military and professional fighters who may be at enhanced risk for CTE given the frequency and intensity of head trauma they sustain. One such longitudinal study, the Professional Fighters Brain Health Study, is ongoing and may expand the current state of imaging research examining CTE pathology. Further, the recently initiated DIAGNOSE CTE study, a multi-site longitudinal study of professional and college football players and controls including AV1451 and β-amyloid imaging will be informative. (Disclosure: authors SJB and CB are investigators on both these studies).
It is also worth noting that in early stages, the changes found at pathology can be small and distributed over a fairly wide area in the brain. Thus group-level differences in relatively young people who could have been exposed to RHT may be difficult to elucidate in analysis of PET data. In older patients, there may be concern with regard to off-target binding of AV1451 to neuromelanin,61 which increases with age,62 and is not a pathological hallmark of CTE. In order to avoid spurious findings, future studies will benefit from adopting a priori hypotheses about the neuroanatomic regions germane to CTE pathology demonstrated by empirical research (e.g. McKee and colleagues, 2016).7 Potential regions of off-target binding of AV1451 to neuromelanin include the choroid plexus and basal ganglia.62
Moving forward, future research endeavors may benefit from using selectively binding radiotracers in the examination of CTE. Only two studies included the use of tau-specific ligands, accounting for a total of two individuals likely reflecting the relative novelty of these ligands for use in CTE research and the academic publication delay. A third report, not included in the above review,31 found increased left anterior temporal lobe uptake of AV1451 in one of two former NFL players assessed, again suggesting the utility of such compounds in those at risk of CTE. Given that CTE is conceptualized as a primary tauopathy, results from studies currently using tau-specific ligands will be important; however, it is recognized that AV1451 has lower affinity for astrocytic tau than other isoforms and this may increase the false-negative rate of this compound in CTE. Those studies using isotopes such as FDDNP that examine the presence of pTau along with β-amyloid may address CTE; however, greater attention should be paid to the role age plays in the presence of amyloidopathy. Additionally, debate continues about the validity of FDDNP in the assessment of tauopathies, and caution is warranted in drawing conclusions based solely on this isotope.
Studies should statistically or experimentally control for age or risk significant threats to internal validity. It is noted that under-reporting of nonsignificant findings may bias the observations reported in this systematic review, leading to an over-representation of the consensus across authors for regional uptake in neuroanatomic subdomains. Future studies may address methodological flaws intrinsic to PET research in CTE by following results with pathological confirmation postmortem as has been done with these ligands in Alzheimer's research. Future studies that examine visually discernable patterns of tau deposition in PET imaging of those with RHT may be particularly useful as a diagnostic aid and in prediction of symptomatic decline.
Supplementary Material
Author Disclosure Statement
Funding for this study came from the Lincy Foundation, Belator, and Ultimate Fighting Championship.
References
- 1.Baugh C.M., Stamm J.M., Riley D.O., Gavett B.E., Shenton M.E., Lin A., Nowinski C.J., Cantu R.C., McKee A.C., and Stern R.A. (2012). Chronic traumatic encephalopathy: neurodegeneration following repetitive concussive and subconcussive brain trauma. Brain Imaging Behav. 6, 244–254 [DOI] [PubMed] [Google Scholar]
- 2.McKee A.C., Cantu R.C., Nowinski C.J., Hedley-Whyte E.T., Gavett B.E., Budson A.E., Santini V.E., Lee H.S., Kubilus C.A., and Stern R.A., (2009). Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J. Neuropathol. Exp. Neurol. 68, 709–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKee A.C., Stein T.D., Nowinski C.J., Stern R.A., Daneshvar D.H., Alvarez V.E., Lee H.S., Hall G., Wojtowicz S.M., Baugh C.M., and Riley D.O., (2013). The spectrum of disease in chronic traumatic encephalopathy. Brain 136, 43–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iverson G., Gardner A., McCrory P., Zafonte R., and Castellani R. (2015). A critical review of chronic traumatic encephalopathy. Neurosci. Biobehav. Rev. 56, 276–293 [DOI] [PubMed] [Google Scholar]
- 5.Catafau A. M. and Bullich S. (2015). Amyloid PET imaging: applications beyond Alzheimer's disease. Clin. Transl. Imaging 3, 39–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Faden A.I. and Joane D. J. (2015). Chronic neurodegeneration after traumatic brain injury: Alzheimer disease, chronic traumatic encephalopathy, or persistent neuroinflammation? Neurotherapeutics, 12, 143–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McKee A.C., Cairns N.J., Dickson D.W., Folkerth R.D., Keene C.D., Litvan I., Perl D.P., Stein T.D., Vonsattel J.P., Stewart W., and Tripodis Y. (2016). The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol. Commun. 131, 75–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mez J., Daneshvar D.H., Kiernan P.T., Abdolmohammadi B., Alvarez V.E., Huber B.R., Alosco M.L., Solomon T.M., Nowinski C.J., McHale L., and Cormier K.A. (2017). Clinicopathological evaluation of chronic traumatic encephalopathy in players of American football. JAMA 318, 360–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramlackhansingh A.F., Brooks D.J., Greenwood R.J., Bose S.K., Turkheimer F.E., Kinnunen K.M., Gentleman S., Heckemann R.A., Gunanayagam K., Gelosa G., and Sharp D.J. (2011). Inflammation after trauma: microglial activation and traumatic brain injury. Ann. Neurol. 70, 374–383 [DOI] [PubMed] [Google Scholar]
- 10.Chien D.T., Bahri S., Szardenings A.K., Walsh J.C., Mu F., Su M.Y., Shankle W.R., Elizarov A., and Kolb H.C. (2013). Early clinical PET imaging results with the novel PHF-tau radioligand [F-18]-T807. J. Alzheimers Dis. 34, 457–468 [DOI] [PubMed] [Google Scholar]
- 11.Xia C.F., Arteaga J., Chen G., Gangadharmath U., Gomez L.F., Kasi D., Lam C., Liang Q., Liu C., Mocharla V.P., and Mu F. (2013). [18 F] T807, a novel tau positron emission tomography imaging agent for Alzheimer's disease. Alzheimers Dement. 9, 666–676 [DOI] [PubMed] [Google Scholar]
- 12.Klunk W.E., Lopresti B.J., Ikonomovic M.D., Lefterov I.M., Koldamova R.P., Abrahamson E.E., Debnath M.L., Holt D.P., Huang G.F., Shao L., and DeKosky S.T. (2005). Binding of the positron emission tomography tracer Pittsburgh compound-B reflects the amount of amyloid-β in Alzheimer's disease brain but not in transgenic mouse brain. J. Neurosci. 25, 10598–10606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamin G. and Teplow B. D. (2017). Pittsburgh Compound-B (PiB) binds amyloid B-protein protofibrils. J. Neurochem. 140, 210–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dani M., Brooks D.J., and Edison P. (2016). Tau imaging in neurodegnerative diseases. Eur. J. Nucl. Med. Mol. Imaging 43, 1139–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clark C.M., Pontecorvo M.J., Beach T.G., Bedell B.J., Coleman R.E., Doraiswamy P.M., Fleisher A.S., Reiman E.M., Sabbagh M.N., Sadowsky C.H., and Schneider J.A. (2012). Cerebral PET with florbetapir compared with neuropathology at autopsy for detection of neuritic amyloid-β plaques: a prospective cohort study. Lancet Neurol. 11, 669–678 [DOI] [PubMed] [Google Scholar]
- 16.Barthel H., Gertz H.J., Dresel S., Peters O., Bartenstein P., Buerger K., Hiemeyer F., Wittemer-Rump S.M., Seibyl J., Reininger C., and Sabri O. (2011). Cerebral amyloid-β PET with florbetaben (18 F) in patients with Alzheimer's disease and healthy controls: a multicentre phase 2 diagnostic study. Lancet Neurol. 10, 424–435 [DOI] [PubMed] [Google Scholar]
- 17.Sabri O., Sabbagh M.N., Seibyl J., Barthel H., Akatsu H., Ouchi Y., Senda K., Murayama S., Ishii K., Takao M., and Beach T.G. (2015). Florbetaben PET imaging to detect amyloid beta plaques in Alzheimer's disease: phase 3 study. Alzheimers Dement 11, 964–974 [DOI] [PubMed] [Google Scholar]
- 18.Albrecht D. S., Granziera C., Hooker J. M., and Loggia M. L. (2016). In vivo imaging of human neuroinflammation. ACS Chem. Neurosci. 7, 470–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Omalu B.I., Dekosky S.T., Minster R.L., Kamboh M.I., Hamilton R.L., Wecht C.H. (2005). Chronic traumatic encephalopathy in a National Football League player. Neurosurgery, 57, 128–134 [DOI] [PubMed] [Google Scholar]
- 20.Omalu B.I., Fitzsimmons R.P., Hammers J., Bailes J. (2010). Chronic Traumatic Encephalopathy in a professional American wrestler. J. Forensic Nurs. 6, 130–136 [DOI] [PubMed] [Google Scholar]
- 21.Stern R.A., Daneshvar D.H., Baugh C.M., Seichepine D.R., Montenigro P.H., Riley D.O., Fritts N.G., Stamm J.M., Robbins C.A., McHale L., and Simkin I. (2013). Clinical presentation of chronic traumatic encephalopathy. Neurology 81, 1122–1129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Institute of Neurological Disorders and Stroke. (2015). NIH Chronic Traumatic Encephalopathy Diagnosis Conference, Boston [Google Scholar]
- 23.Mckee A.C., Stein T.D., Kieman P.T., and Alvarez V.E. (2015) The Neuropathology of Chronic Traumatic Encephalopathy, Brain Pathol. 25, 350–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villemagne V.L. and Okamura N. (2016). Tau imaging in the study of ageing, Alzheimer's disease, and other neurodegenerative conditions. Curr. Opin. Neurobiol, 36, 43–41 [DOI] [PubMed] [Google Scholar]
- 25.Kovacs G. G., Ferrer I., Grinberg L. T., Alafouzoff I., Attemus J., Budka H., and Halliday G. M. (2016). Aging-related tau astrogliopathy (ATAGS): Harmonized evaluation strategy. Acta Neuropathol. 131, 87–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Braak H. and Braak E. (1995). Staging of Alzheimer's disease-related neurofibrillary changes. Neurobiol. Aging 16, 271–278 [DOI] [PubMed] [Google Scholar]
- 27.Dubois B., Feldman H.H., Jacova C., Hampel H., Molinuevo J.L., Blennow K., DeKosky S.T., Gauthier S., Selkoe D., Bateman R., and Cappa S. (2014). Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. Lancet Neurol. 13, 614–629 [DOI] [PubMed] [Google Scholar]
- 28.Sperling R.A., Aisen P.S., Beckett L.A., Bennett D.A., Craft S., Fagan A.M., Iwatsubo T., Jack C.R., Kaye J., Montine T.J., and Park D.C. (2011). Toward defining the preclinical stages of Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 7, 280–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marquié M., Normandin M.D., Vanderburg C.R., Costantino I.M., Bien E.A., Rycyna L.G., Klunk W.E., Mathis C.A., Ikonomovic M.D., Debnath M.L., and Vasdev N. (2015). Validating novel tau positron emission tomography tracer [F-18]-AV-1451 (T807) on postmortem brain tissue. Ann. Neurol. 78, 787–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rabinovici G., Schonhaut D., Ossenkoppele R., Baker S., Lazaris A., Lockhart S., Schwimmer H., O'Neil J., Santos M., Miller Z., and Bettcher B. (2015). Initial experience with [18F]AV1451 PET in AD and non-AD tauopathies (P5.005). Neurology 84 (14 Supplement), P5.005 [Google Scholar]
- 31.Rabinovici G.D., Schonhaut D., Baker S., Lazaris A., Ossenkoppele R., Lockhart S., Schöll M., Schwimmer H., Vogel J.W., Ayakta N., and Rosen H. (2015). Tau PET with 18F- AV1451 in non-alzheimer's disease neurodegenerative syndromes. Alzheimers Dement. 11, 107–10925732924 [Google Scholar]
- 32.Passamonti L., Vázquez Rodríguez P., Hong Y.T., Allinson K.S., Williamson D., Borchert R.J., Sami S., Cope T.E., Bevan-Jones W.R., Jones P.S., and Arnold R. (2017). 18F-AV-1451 positron emission tomography in Alzheimer's disease and progressive supranuclear palsy. Brain 140, 781–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harada R., Okamura N., Furumoto S., Tago T., Yanai K., Arai H., and Kudo Y. (2016). Characteristics of Tau and its ligands in PET imaging. Biomolecules 6, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Underwood E. (2015). Seeking tests for a contested brain disease. Science 348, 378–379 [DOI] [PubMed] [Google Scholar]
- 35.Ono M., Sahara N., Kumata K., Ji B., Ni R., and Koga S. (2017) Distinct binding of PET ligands PBB3 and AV-1451 to tau fibril strains in neurodegnerative tauopathies. Brain 140, 764–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Turkheimer F. E., Rizzo G., Bloomfield P. S., Howes O., Zanotti-Fregonara P., Bertoldo A., and Veronese M. (2015). The methodology of TSPO imaging with positron emission tomography. Biochem. Soc. Trans. 43, 586–592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Moher D., Liberati A., Tetzlaff. J., and Altman D. G.; The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. http://prisma-statement.org [PMC free article] [PubMed]
- 38.Tagge C.A., Fisher A.M., Minaeva O.V., Gaudreau-Balderrama A., Moncaster J.A., Zhang X.L., Wojnarowicz M.W., Casey N., Lu H., Kokiko-Cochran O.N., Saman S., Ericsson. M., Onos K.D., Veksler R., Senatorov V.V., Jr., Kondo A., Zhou X.Z., Miry O., Vose L.R., Gopaul K.R., Upreti C., Nowinski C.J., Cantu R.C., Alvarez V.E., Hildebrandt A.M., Franz E.S., Konrad J., Hamilton J.A., Hua. N., Tripodis Y., Anderson A.T., Howell G.R., Kaufer D., Hall G.F., Lu K.P., Ransohoff R.M., Cleveland R.O., Kowall N.W., Stein T.D., Lamb B.T., Huber B.R., Moss W.C., Friedman A., Stanton P.K., McKee A.C., and Goldstein L.E. (2018) Concussion, microvascular injury, and early tauopathy in young athletes after impact head injury and an impact concussion mouse model. Brain 141, 422–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stein T.D., Alvarez V.E., and McKee A.C. (2015) Concussion in chronic traumatic encephalopathy. Curr. Pain Headache Rep. 19, 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okamura N., Harada R., Furukawa K., Furumoto S., Tago T., Yanai K., Arai H., and Kudo Y. (2016). Advances in the development of tau PET radiotracers and their clinical applications. Ageing Res. Rev. 30, 107–113 [DOI] [PubMed] [Google Scholar]
- 41.Rowe C. C. and Villemagne V. L. (2013). Brain amyloid imaging. J. Nucl. Med. Technol. 41, 11–18 [DOI] [PubMed] [Google Scholar]
- 42.Boutin H., Chauveau F., Thominiaux C., Grégoire M.C., James M.L., Trebossen R., Hantraye P., Dollé F., Tavitian B., and Kassiou M. (2007). 11C-DPA-713: A novel peripheral benzodiazepine receptor PET ligand for in vivo imaging of neuroinflammation. J. Nucl. Med. 48, 573–581 [DOI] [PubMed] [Google Scholar]
- 43.Barrio J.R., Small G.W., Wong K., Huang S., Liu J., and Merrill D.A. (2015). In vivo characterization of chronic traumatic encephalopathy using [F-18]FDDNP PET brain imaging. Proc. Natl. Acad. Sci. U. S. A. 112, E2039–E2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coughlin J.M., Wang Y., Minn I., Bienko N., Ambinder E.B., Xu X., Peters M.E., Dougherty J.W., Vranesic M., Koo S.M., and Ahn H.H. (2017). Imaging of glial cell activation and white matter iIntegrity in brains of active and recently retired National Football League players. JAMA Neurol. 74, 67–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coughlin J.M., Wang Y., Munro C.A., Ma S., Yue C., Chen S., Airan R., Kim P.K., Adams A.V., Garcia C., and Higgs C. (2015). Neuroinflammation and brain atrophy in former NFL players: An in vivo multimodal imaging pilot study. Neurobiol. Dis. 74, 58–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jordan B.D., Delman B.N., Fernandez C., Tang C.Y., Dickstein D.L., Wong E., Short J.A., Stone J.R., Dams-O'Connor K., Knesaurek K., and Kostakoglu L. (2016). Cerebral 18F-T807/AV1451 retention pattern in clinically probable CTE resembles pathognomonic distribution of CTE tauopathy. Transl. Psychiatry 6, e900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mitsis E.M., Riggio S., Kostakoglu L., Dickstein D.L., Machac J., Delman B., Goldstein M., Jennings D., D'Antonio E., Martin J., and Naidich T.P. (2014). Tauopathy PET and amyloid PET in the diagnosis of chronic traumatic encephalopathies: studies of a retired NFL player and of a man with FTD and a severe head injury. Transl. Psychiatry 4, e441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Small G.W., Kepe V., Siddarth P., Ercoli L.M., Merrill D.A., Donoghue N., Bookheimer S.Y., Martinez J., Omalu B., Bailes J., and Barrio J.R. (2013). PET Scanning of brain tau in retired national football league players: preliminary findings. Am. J. Geriatr. Psychiatry 21, 138–144 [DOI] [PubMed] [Google Scholar]
- 49.Safinia C., Bershad E.M., Clark H.B., SantaCruz K., Alakbarova N., Suarez J.I., and Divani A.A. (2016). Chronic traumatic encephalopathy in athletes involved with high-impact sports. J. Vasc. Interv. Neurol. 9, 34–48 [PMC free article] [PubMed] [Google Scholar]
- 50.Kriegel J., Papadopoulos Z., and Mckee A.C. (2018). Chronic traumatic encephalopathy: is latency in symptom onset explained by Tau propagation? Cold Spring Harb. Perspect. Med. 8, pii: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Perrine K., Helcer J., Tsiouris A.J., Pisapia D.J., and Stieg P. (2017). The current status of research on chronic traumatic encephalopathy. World Neurosurg. 102, 533–544 [DOI] [PubMed] [Google Scholar]
- 52.Marquié M., Normandin M.D., Meltzer A.C., Siao Tick Chong M., Andrea N.V., Antón‐Fernández A., Klunk W.E., Mathis C.A., Ikonomovic M.D., Debnath M., and Bien E.A. (2017). Pathological correlations of [F-18]-AV-1451 in non-Alzheimer tauopathies. Ann. Neurol. 81, 117–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vallabhajosula S. (2011). Positron emission tomography radiopharmaceuticals for imaging brain Beta-amyloid. Semin. Nucl. Med. 41, 283–299 [DOI] [PubMed] [Google Scholar]
- 54.Gardner R.C., Possin K.L., Hess C.P., Huang E.J., Grinberg L.T., Nolan A.L., Cohn-Sheehy B.I., Ghosh P.M., Lanata S., Merrilees J., and Kramer J.H. (2015). Evaluating and treating neurobehavioral symptoms in professional American football players: lessons from a case series. Neurol. Clin. Pract. 5, 285–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Aizenstein H.J., Nebes R.D., Saxton J.A., Price J.C., Mathis C.A., Tsopelas N.D., Ziolko S.K., James J.A., Snitz B.E., Houck P.R., and Bi W. (2008). Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch. Neurol. 65, 1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bangen K.J., Clark A.L., Edmonds E.C., Evangelista N.D., Werhane M.L., Thomas K.R., Locano L.E., Zlatar Z.Z., Nation D.A., Bondi M.W., and Delano-Wood L. (2017). Cerebral blood flow and amyloid beta interact to affect memory performance in cognitively normal older Adults. Front. Aging Neurosci. 9, 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hatashita S. and Yamasaki H. (2010). Clinically different stages of Alzheimer's disease associated by amyloid deposition with 11C–PIB PET imaging. J. Alzheimers Dis. 21, 995–1003 [DOI] [PubMed] [Google Scholar]
- 58.Price J.L. and Morris J.C. (1999). Tangles and plaques in nondemented aging and “preclinical” Alzheimer's disease. Ann. Neurol. 45, 358–368 [DOI] [PubMed] [Google Scholar]
- 59.Villemagne V.L., and Rowe C.C. (2013). Long night's journey into the day: amyloid Beta imaging in Alzheimer's disease. J. Alzheimers Dis. 33, 349–359 [DOI] [PubMed] [Google Scholar]
- 60.Rodrigue K.M., Kennedy K.M., and Park D.C. (2009) Beta-amyloid deposition and the aging brain. Neuropsychol. Rev. 19, 436–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ossenkoppele R., Schonhaut D.R., Schöll M., Lockhart S.N., Ayakta N., Baker S.L., O'Neil J.P., Janabi M., Lazaris A., Cantwell A., and Vogel J. (2016). Tau PET patterns mirror clinical and neuroanatomical variability in Alzheimer's disease. Brain 139, 1551–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Amaral A.C., Meltzer A.C., Vanderburg C.R., Agüero C., Verwer E.E., Gonzalez J., Johnson K.A., Normandin M.D., Marquié M., Frosch M.P., and Chong M.S.T. (2017). Lessons learned about [F-18]-AV-1451 off-target binding from an autopsy-confirmed Parkinson's case. Acta Neuropathol. Commun. 5, 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zecca L., Tampellini D., Gerlach M., Riederer P., Fariello R. G., and Sulzer D. (2001). Substantia nigra neuromelanin: structure, synthesis, and molecular behaviour. Mol. Pathol. 54, 414. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.