Abstract
Exposure to welding fumes may result in disorders of the pulmonary, cardiovascular, and reproductive systems. Welders are also at a greater risk of developing symptoms similar to those seen in individuals with idiopathic Parkinson’s disease. In welders, there are studies that suggest that alterations in circulating prolactin concentrations may be indicative of injury to the dopamine (DA) neurons in the substantia nigra. The goal of these studies was to use an established model of welding particulate exposure to mimic the effects of welding fume inhalation on reproductive functions. Since previous investigators suggested that changes in circulating prolactin may be an early marker of DA neuron injury, movement disorders, and reproductive dysfunction, prolactin, hypothalamic tyrosine hydroxylase (TH) levels (a marker of DA synthesis), and other measures of hypothalamic-pituitary-gonadal (HPG) function were measured after repetitive instillation of welding fume particulates generated by flux core arc-hard surfacing (FCA-HS), manual metal arc-hard surfacing (MMA-HS) or gas metal arc-mild steel (GMA-MS) welding, or manganese chloride (MnCl2). Exposure to welding fume particulate resulted in the accumulation of various metals in the pituitary and testes of rats, along with changes in hypothalamic TH and serum prolactin levels. Exposure to particulates with high concentrations of soluble manganese (Mn) appeared to exert the greatest influence on TH activity levels and serum prolactin concentrations. Thus, circulating prolactin levels may serve as a biomarker for welding fume/Mn-induced neurotoxicity. Other reproductive measures were collected, and these data were consistent with epidemiological findings that prolactin and testosterone may serve as biomarkers of welding particulate induced DA neuron and reproductive dysfunction.
Introduction
Exposure to welding fumes has been associated with a number of adverse health effects including development of allergies, acute and chronic respiratory problems, cardiovascular diseases (Antonini et al., 2012), Parkinson-like symptoms (Ellingsen et al., 2007a), and reproductive dysfunction (Bonde, 1990; Bonde & Ernst, 1992; Ellingsen et al., 2007b; Jensen et al., 2006). According to the US Bureau of Labor Statistics, in 2014, there were approximately 397,900 workers employed as welders, cutters, solders, and brazers, with the majority of workers (approximately 60%) being employed in the manufacturing sector (Bureau of Labor Statistics, 2016–17). However, welders are also employed in the shipbuilding industry, on pipelines, and in the construction industry. Thus, workers in a number of different occupational sectors are at risk of being exposed to fumes generated by the welding process, and these fumes may potentially exert detrimental health effects.
Depending on the surfaces and types of welding processes being performed, a number of different fume profiles might be generated. These fumes contain mixtures of various metal particulate matter (PM) including, but not limited to, iron (Fe), manganese, chromium (Cr), and nickel (Ni) (Antonini et al., 2009, 2011; Sriram et al., 2010). The concentrations of various metals are different in each welding fume, and thus the disorders associated with specific fumes may vary, or the rate at which a worker develops an illness from inhaling welding fumes may differ.
Exposure to welding fumes may affect the health of welders via a number of different underlying mechanisms. First, exposure to welding fumes might induce immune responses in the lungs (Zeidler-Erdely et al., 2012) and pulmonary-produced cytokines may affect other tissues in the body such as the cardiovascular (Zheng et al., 2015), central nervous (Sriram et al., 2010), and reproductive systems in male and female welders (Jensen et al., 2006; Niu et al., 2004; Smargiassi & Mutti, 1999). Solubilized metals and possibly intact welding particulates may also translocate from lung tissues into the blood or lymphatic system (Hubbs et al., 2013), and directly affect the functioning of other tissues. Sriram et al. (2010) found that after 7 weeks of intratracheal (IT) exposure to gas metal arc-mild steel (GMA-MS) welding particulate, there was an increase in Fe, Mn, and copper (Cu) concentrations in the lung, and a rise in Mn concentrations in various brain regions. A number of investigations examining the influence of particulate on welders noted that there are changes in circulating prolactin concentrations (Mutti et al., 1996; Niu et al., 2004). Prolactin synthesis and release from the anterior pituitary are regulated by dopamine (DA) released from the tuberoinfundibular dopamine (TIDA) neurons in the arcuate nucleus of the hypothalamus (Moore, 1987). Because TIDA neurons are located in a region of the hypothalamus with poorly developed blood brain barrier (BBB), these neurons may be affected by solubilized welding fume metal more rapidly than dopaminergic neurons in other regions of the brain where the BBB is well formed. Alterations in TIDA neuron function may serve as an early indicator of general dopaminergic dysfunction. Because DA released from TIDA neurons is a major factor regulating prolactin release, circulating prolactin concentrations, and possibly other reproductive measures, may be employed as a peripheral markers of TIDA neuron function (Moore, 1987). To determine if welding particulate exposure alters prolactin levels in our rat model, animals were initially exposed for 7 weeks to manual metal arc-hard surfacing (MMA-HS) and flux-core (FCA-HS), where particulate is more soluble, or gas metal arc-mild steel (GMA-MS) welding, where the particulate is less soluble, and the effects of these exposures examined on serum prolactin concentrations and tyrosine hydroxylase (TH) levels in the hypothalamus (a marker of DA synthesis). In a follow-up experiment, a longer exposure (28 weeks) was utilized to determine if welding fume (MMA-HS or GMA-MS) or manganese (Mn; such as MnCl2) exposure affected other measures of reproductive function, such as testosterone production and spermatogenesis, which may both be affected by a number of physiological factors including circulating prolactin concentrations (Krajnak et al., 1994, 1995).
Methods
Animals
Male Sprague–Dawley rats (H1a (SD) CVF) weighing approximately 250 g were purchased from Hilltop lab animals (Scottsdale, PA) at 6 weeks of age. Rats were maintained in an AAALAC accredited housing facility in a room with a 12:12 hr light/dark cycle with lights on at 0600 hr with food (Teklad 2918) and tap water available ad libitum. At the end of each experiment, rats were euthanized between 0900 and 1000 hr to reduce variability in measures of levels in prolactin and testosterone. All procedures were approved by the NIOSH Animal Care and Use Committee and were in compliance with NIH Guide for the Care and Use of Animals in Research.
Exposures
Welding particulate samples were collected by an experienced welder at Lincoln Electric Co. (Cleveland OH). Fume dusts were collected onto a 0.2 μm Nuclepore filter (Nuclepore Co., Pleasanton, CA) using a cubical open front fume chamber (volume = 1 m3). Gas metal arc fumes were generated using a mild steel E70S-3 electrode (GMA-MS; L-50 carbon steel electrode, Lincoln Electric Co, Cleveland OH). Manual metal arc-hard surfacing fumes were generated using Wearshield 15 CrMn electrode (MMA-HS; Lincoln Electric Co.). Flux core arc-hard surfacing fumes were produced using Lincore M electrode (FCA-HS; Lincoln Electric Co.). The particle size of the collected fume samples was determined using scanning electron microscopy and found to be in the respirable size range with a count mean diameter for each fume of <1 mm. Fumes produced by MMA-HS and FCA-HS electrodes contained higher levels of Mn than GMA-MS welding electrodes, and this Mn is in a more soluble form (Antonini et al., 2009). The concentrations of the primary metals or ions in each particulate are presented in Table 1. Metal composition of the welding fume samples was determined by inductively-coupled plasma atomic emission spectroscopy (ICP-AES) following the NIOSH Method 7300. A total of 31 elements were analyzed and Table 1 depicts some of the major elements in the respective aerosol particulates (National Institute for Occupational Safety and Health 1994). Values are expressed as weight % relative to all metals analyzed in the welding fume aerosol. Welding fume samples were analyzed in triplicate. Welding particulates were suspended in sterile phosphate buffered saline (PBS), pH 7.4 and sonicated using a Sonifier 450 Cell Disruptor (Branson Ultrasonics, Danbury, CT).
Table 1.
Elemental Analysis of the Different Aerosols. Values are Expressed as Weight % Relative to All Metals Analyzed in the Welding Fume Aerosol. Welding Fume Samples Were Analyzed in Triplicates
| Metal | GMA-MS (weight %) |
MMA-HS (weight %) |
FCA-HS (weight %) |
|---|---|---|---|
| Fe | 90.4 | 30.4 | 37.1 |
| Mn | 6.88 | 43.7 | 36.6 |
| Ca | 0.00 | 0.65 | 6.52 |
| Cr | 0.04 | 8.25 | 2.11 |
| Cu | 0.88 | 0.03 | 0.10 |
| K | 0.13 | 13.4 | 10.0 |
| Mg | 0.02 | 0.78 | 2.21 |
| Ni | 0.00 | 0.31 | 0.42 |
| Ti | 0.26 | 1.29 | 1.10 |
| Zn | 1.25 | 0.73 | 0.10 |
To prepare the particulate, suspensions were vortexed immediately prior to instillation to ensure a homogenous suspension. For treatment, rats were anesthetized by an intraperitoneal (ip) injection of 0.6 ml 1% solution of sodium methohexital (Brevital; Eli Lilly, Indianapolis, IN) and rats were then intratracheally instilled (IT) with 2 mg/rat of welding particulate matter (PM; 300μl instillation volume). In Experiment 1, animals were instilled weekly with FCA-HS, MMA-HS or GMA-MS particulate suspensions for 7 consecutive weeks (exposure occurred once each week), and euthanized 35 days following the last exposure. The length of this exposure was based on other studies that previously examined the effects of 7 weeks of particulate exposure on accumulation of metals in lungs and nervous system (Antonini et al., 2010; Sriram et al., 2010). A 35 day recovery period was used to determine if the effects of PM exposure were maintained over time. In Experiment 2, animals were instilled with 2 mg/animal PM from MMA-HS, GMA-MS, or MnCl2 for 28 consecutive weeks (exposure once per week) and euthanized 1 week following the last exposure. Rats in this second study were tested for behavioral effects of particulate exposure using a reaction time test. Thus, the exposure period was extended to 28 weeks to determine if welding fume particulate or MnCl2 exerted any behavioral effects. These data are not reported in this paper because no significant effects of exposure on reaction time were found. Reaction time was also measured after the last exposure. That is the basis for rats being euthanized one week after the last exposure.
The exposure dose was based upon a number of different factors. In certain work environments welders are exposed to concentrations of fumes that are higher than the threshold limit value (TLV), and consequently are at enhanced risk of adverse health effects. Indeed, the total welding fume levels measured in various industries (Korczynski, 2000; Susi et al., 2000), especially in confined spaces (Harris et al., 2005), were observed to often exceed the TLV. To mimic such adverse exposure scenarios, a high dose of the welding fume was selected. To relate pulmonary (IT) dosing paradigm employed in this study to workplace exposures of welders, a mathematical calculation was utilized to determine the daily lung burden of a welder on an 8hr work schedule. Incorporating factors such as fume concentration (5 mg/m3, previous TLV for welding fumes), human (worker) minute ventilation volume (20,000 ml/min × 10−6 m3/ml), exposure duration (8h/day × 60 min/hr) and a predicted deposition efficiency of approximately 15%, it was determined that the daily lung burden of a welder is about 7.2 mg. Using surface area of alveolar epithelium (rat = 0.4 m2; human =102 m2) as the dose metric, the daily lung burden for a similar exposure in the rat amounts to 0.0282 mg. Factoring the cumulative dosing paradigms used in this study (2 mg × 7 instillations =14 mg or 2 mg × 28 instillations = 56 mg) and the estimated daily lung burden for rat (0.0282 mg), the number of welder exposure days was derived for the two exposure paradigms as, 14mg/0.0282 mg = 496.5 days, and 56 mg/0.0282 mg = 1985.8 days. Thus, our short- and long-term pulmonary exposures in the rodent mimic worker exposures of approximately 1.4 and 5.4 years, respectively.
Rats were injected with sodium pentobarbital (100 mg/kg body weight, i.p.; Sleepaway, Fort Dodge Animal Health, Wyeth, Madison, NJ, USA) and then exsanguinated by severing the abdominal aorta. Bronchoalveolar lavage (BAL) was performed to assess parameters of lung injury and inflammation. Lungs were first repeatedly lavaged with a 1 ml/100 g body weight aliquot of calcium- and magnesium-free PBS, pH 7.4. The first fraction of recovered BAL fluid (BALF) was centrifuged at 500 × g for 10 min, and resultant cell-free supernatant analyzed for various biochemical parameters of injury. The lungs were further lavaged with 6 ml aliquots of PBS until 30 ml were collected. These samples also were centrifuged for 10 min at 500 × g and cell-free BALF discarded. Cell pellets from all washes for each rat were combined, washed, and re-suspended in 1 ml PBS buffer. The total cell numbers recovered by BALF were determined using a Coulter Multisizer II and AccuComp software (Coulter Electronics, Hialeah, FL, USA). Cells were differentiated using a Cytospin 3 centrifuge (Shandon Life Sciences International, Cheshire, England). Cell suspensions (5 × 104 cells) were spun for 5 min at 800 rpm and pelleted onto a slide. Cells (200/rat) were identified and differentiated after labeling with Leukostat stain (Fisher Scientific, Pittsburgh, PA, USA).
Using the acellular first fraction of BALF, albumin content, an index to quantify increased permeability of the bronchoalveolar-capillary barrier, and lactate dehydrogenase (LDH) activity, an indicator of general cell damage and toxicity, were measured. Albumin content was determined colorimetrically at 628 nm based on albumin binding to bromcresol green using an albumin BCG diagnostic kit (Sigma Chemical Co., St. Louis, MO, USA). LDH activity was determined by measuring the oxidation of lactate to pyruvate coupled with the formation of NADH at 340 nm. Measurements were performed with a COBAS MIRA auto-analyzer (Roche Diagnostic Systems, Montclair, NJ, USA).
Tissue collection
Thirty-five days following the short-term (7 week) exposure, and 1-week following the long-term (28 week exposure), blood was collected via cardiac puncture into vacutainer tubes (BD; Franklin Lakes, New Jersey), serum was isolated, and stored at −80°C, in 100 μl aliquots until use for assays. The median eminence/arcuate nucleus of the hypothalamus was dissected on a cold-plate, weighed and stored at −80°C until processed. In long-term studies, the pituitary, testes and epididymis were also collected in addition to serum and hypothalamus, to assess reproductive effects. The epididymis was dissected, cleaned, weighed, placed into a vial containing 1 ml 0.1 M PBS and vortexed for 1 min. Two 10 μl aliquots were placed on a grid micrometer and number of sperm in a 100 μm2 region of the micrometer counted using the 40X objective. An average of the counts was calculated and utilized for analyses. The testes and pituitary were also dissected, weighed and stored in cryotubes at −80°C until processed.
Prolactin and testosterone assays
Prolactin and testosterone levels were measured by enzyme immunosorbent assays (EIA). Serum prolactin concentrations were measured in duplicate using the manufacturer’s protocol (ALPCO diagnostics, Salem, NH 03079, cat #: 12-MKVEP1). The lower limit of detection (LOD) of the assay was 0.6 ng/ml and the coefficient of variation (CoV) between duplicate samples was 6.67% (±0.16). Serum testosterone was determined in duplicate as per the manufacturer’s protocol (ALPCO diagnostics, Cat# 11-TESHU-E01). The lower LOD for the assay was 0.002 ng/ml and CoV between duplicate samples was 5% (±0.05).
Tyrosine hydroxylase activity westerns
The hypothalamus was homogenized in 500 μl buffer (0.1 M PBS, 0.2% Tween 20 and protease inhibitors) using a sonic dismembrator. Twenty-five μl samples were assayed for the total protein concentrations using the BCA total protein assay (ThermoFisher, Life Technologies Corporation, Grand Island, NY). A protein standard (Pre-stained SDS PAGE Broad Range Standard, ThermoFisher), and each sample (15 μg total protein with 3 μl Laemmli’s sample buffer) were loaded onto 12% SDS page mini gels and proteins electrophoretically separated. Separated proteins were transferred onto nitrocellulose membranes and bands positive for TH activity levels were immunohistochemically identified using a rabbit anti-TH antibody (Chemicon International, Cat#AB152) at a final dilution of 1:500 in 0.1M TBS plus Odyssey Blocking Buffer. To visualize TH-labeled bands, an IRDye 800 conjugate affinity purified goat anti-rabbit IgG (Rockland; Cat#611-132-122) in 0.1 M Tris buffered saline (TBS) and (10%) Odyssey Blocking Buffer. Blots were air dried and bands were imaged using the Odyssey Infrared Imaging system with an 800 filter channel with a brightness and contrast setting of 50. Black and white images of the blots were saved and imported into SCION image (now ImageJ; NIH Bethesda, MD) to obtain band densities. Intra- and inter-blot variability was accounted for by analyzing the differences in densities of the standard ladder and β-actin (1:1000; anti-rabbit, cat #4967, Cell Signaling Inc, Mountainview, CA).
Metal analyses in tissues
Elemental analysis of pituitary and testicular tissues was performed using inductive coupled plasma atomic emission spectroscopy (ICP-AES) as previously described (Sriram et al., 2010). Briefly, all tissues were weighed and elemental content calculated based upon the dry tissue weights. To digest the tissues, 1 ml 3N hydrochloric acid/10% trichloroacetic acid solutions was added to pre-weighed tissues and heated to 70°C for 18 hr. The digested samples were then centrifuged at 600 g for 10 min and concentrations of the elements measured in the supernatants. The elements measured included aluminum (Al), chromium (Cr), copper, (Cu), iron (Fe), manganese (Mn), nickel (Ni), titanium (Ti), and zinc (Zn).
Statistical analyses
All data were analyzed using one-way ANOVA. Pairwise comparisons were performed using Dunnett’s tests where all measurements were compared to those of the saline controls. Differences with p < 0.05 were considered significant.
Results
Short-term (7 weeks) exposure, 35 days recovery
Pulmonary inflammation
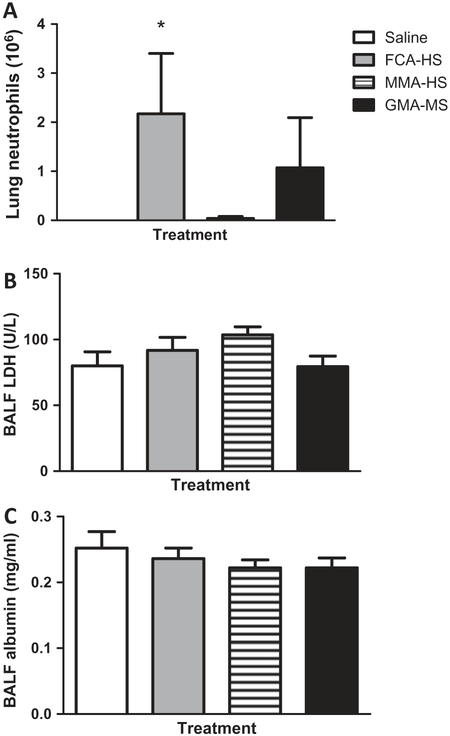
Analysis of BALF showed that 35 days following the short-term (7 week) exposure, there was an increase in lung neutrophils in the FCA-HS treated rats as compared to controls (Figure 1A). A numerical rise in neutrophil levels was also seen following GMA-MS treatment. LDH and albumin levels in the BALF of rats treated with welding fume particulate were not significantly affected (Figure 1B and C).
Figure 1.
Neutrophil number (A), LDH concentrations (B), and albumin concentrations (C) in BAL fluid collected 35 days after short-term (7 week) exposure to welding particulates.
* Significant from controls p < 0.05. T
Metal analyses in the pituitary and testes
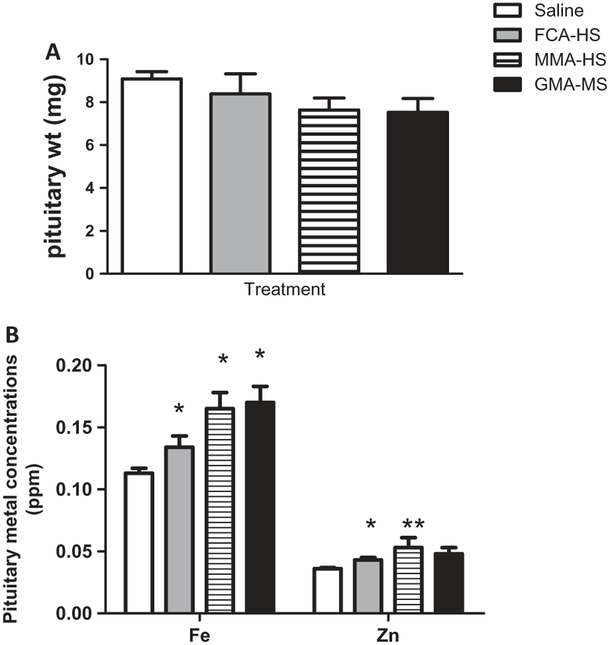
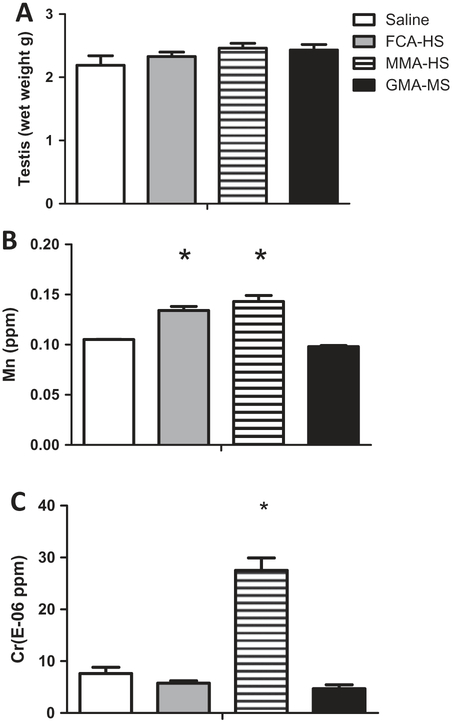
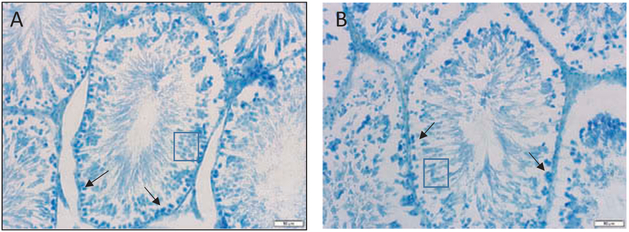
Pituitary weights were not significantly altered by exposure to welding particulates (Figure 2A). However, following short-term treatment, Fe and Zn concentrations were significantly elevated in pituitaries of exposed rats (Figure 2B). Concentrations of other metals that were measureable in the pituitary are presented in Table 2. Exposure to welding particulates did not markedly alter testicular weights (Figure 3A). However, exposure to FCA-HS and MMA-HS produced an increase in Mn in the testes (Figure 3B). MMA-HS exposure was also associated with a significant rise Cr in testes of treated rats (Figure 3C and D). Concentrations of other metals measured in the testes are presented in Table 3. Photomicrographs of toluidine blue stained sections of the testes (12 μM thick) were visualized using bright and dark field microscopy (Figure 4). A representative photomicrograph from a saline treated rat is presented in Figure 4A. The arrows show the spermatogonium within Sertoli cells and the box outlines the mature sperm cells. Although counts were not performed, examination of slides from a number of animals in control and particulate treated groups suggests that exposure to welding fume particulate results in a reduction in sperm production.
Figure 2.
Pituitary weights (A), and metal concentrations in the pituitaries (B) of rats 35 days following short-term (7 week) exposure to welding particulates.
*Significant from control p < 0.05.
Table 2.
Metal Concentrations (μg/g wet wt) in the Pituitaries of Rats 35 days after a 7 week Exposure to Welding Particulate (IT Exposure 1/week for 7 weeks)
| Saline | FCA-HS | MMA-HS | GMA-MS | |
|---|---|---|---|---|
| Al | 0.134 ± 0.009 | 0.134 ± 0.009 | 0.018 ± 0.003 | 0.014 ± 0.003 |
| Cr | 0.0037 ± 0.0005 | 0.003 ± 0.0003 | 0.0041 ± 0.0006 | 0.0041 ± 0.0006 |
| Cu | 0.0054 ± 0.0003 | 0.006 ± 0.0003 | 0.0086 ± 0.0025 | 0.0005 ± 0.0045 |
| Fe | 0.113 ± 0.004 | 0.134 ± 0.009 | 0.17 ± 0.013* | 0.165 ± 0.013* |
| Ni | 0.0037 ± 0.007 | 0.004 ± 0.007 | 0.0036 ± 0.005 | 0.008 ± 0.003 |
| Zn | 0.036 ± 0.001 | 0.043 ± 0.002* | 0.048 ± 0.005* | 0.053 ± 0.008* |
| Mn | 0.0042 ± 0.0002 | 0.0035 ± 0.0005 | 0.0045 ± 0.0004 | 0.005 ± 0.018 |
Different than Saline Controls, p ≤ 0.05.
Figure 3.
Testicular weights (A), testicular concentrations of Mn (B) and Cr (C) 35 days following short-term (7 week) exposure to welding particulates.
*Significant from control p < 0.05.
Table 3.
Metal Concentrations (μg/g wt) in the Testes of rats 35 days after Exposure to Welding Particulate (IT Exposure 1/week for 7 weeks)
| Element | Saline | FCA-HS | MMA-HS | GMA-MS |
|---|---|---|---|---|
| Cr | 7.653E-05 ± 1.204E-05 | 5.761E-05 ± 4.633E-06 | 2.749E-04 ± 2.425E-05* | 4.700E-05 ± 7.537E-06 |
| Cu | 0.050 ± 0.001 | 0.052 ± 0.002 | 0.053 ± 0.001* | 0.051 ± 0.001 |
| Fe | 0.703 ± 0.045 | 0.749 ± 0.031 | 0.754 ± 0.02 | 0.723 ± 0.22 |
| Ni | 9.759E-05 ± 1.897E-05 | 2.007E-04 6.754E-05 | 6.312E-04 ±3.79E-04 | 8.240E-05 ± 2.040E-06 |
| Zn | 0.603 ± 0.028 | 0.635 ± 0.033 | 0.642 ± 0.012 | 1.884E-04 ± 6.971E-06 |
| Mn | 0.105 ± 0.002 | 0.134 ± 0.004* | 0.143 ± 0.006* | 0.098 ± 0.001 |
Different than Saline Controls, p ≤ 0.05.
Figure 4.
Bright field (A and B) photomicrographs taken from rats exposed to saline or GMA-MS particulate. Based on visual inspection, there appears to be a reduction in the number of both spermatogonium (arrows) and mature sperm (box) in welding fume treated rats. The magnification bars on the lower right designate 20 μM.
Prolactin and TH concentrations
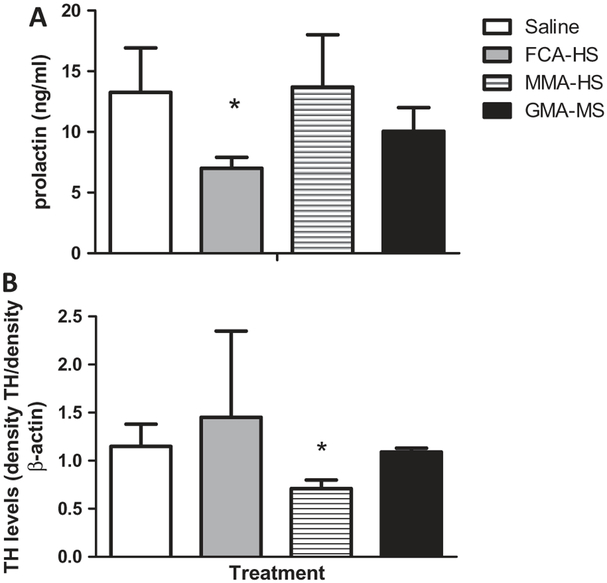
To determine if prolactin and TH activity levels are altered due to the direct effects of particulate metals in welding fumes, serum concentrations of prolactin and hypothalamic TH immunoreactivity were measured (Figure 5). After 7 weeks of exposure to welding particulates, prolactin was markedly reduced in animals treated with FCA-HS (Figure 5A). TH levels were not affected by exposure in FCA-HS treated rats, but they were significantly decreased in rats treated with MMA-HS (Figure 5B). Table 4 shows % change in specific elements after exposure to each welding fume particulate, and associated alterations in hormone and TH levels.
Figure 5.
Circulating prolactin concentrations (A) and hypothalamic TH concentrations (B) 35 days following short-term (7 week) exposure to welding particulates.
*Significant from control p < 0.05.
Table 4.
Changes in Metal Concentrations, Circulating Prolactin Concentrations and Hypothalamic TH Levels after 7 weeks of Exposure To Different Welding Fume Particulate.
| Cr | Fe | Mn | Zn | Prl | TH | ||
|---|---|---|---|---|---|---|---|
| FCA-HS | Pituitary | – | – | – | 16% ↑ | 53% ↓ | – |
| Testes | – | ||||||
| MMA-HS | Pituitary | – | 21% ↑ | – | 21% ↑ | – | 32% ↓ |
| Testes | 74% ↑ | – | 31% | – | |||
| GMA-MS | Pituitary | – | 26% ↑ | – | 26% ↑ | – | – |
| Testes | – | – | – | – |
All changes listed are significantly different and expressed as a percent change from saline control. Prl: prolactin, TH: tyrosine hydroxylase.
Long-term (28 weeks) exposure, 1 week recovery
Pulmonary inflammation
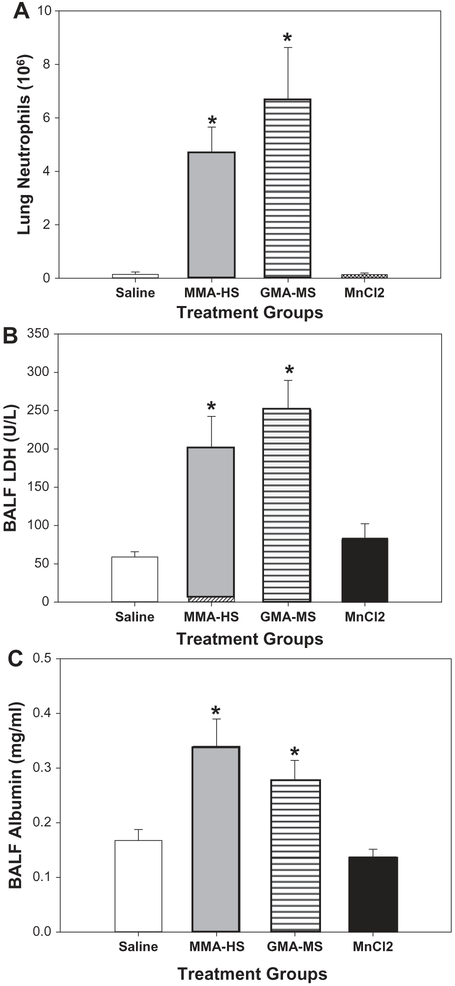
The goal of this study was to determine how Mn exposure may affect hypothalamic-pituitary-gonadal function. To reduce the number of animals used in the study rats were not exposed to FCA-HS. Instead animals were exposed to particulate from welding fumes with a low level of Mn (GMA-MS), a high level of Mn (MMA-HS) or MnCl2, which is a highly soluble form of Mn. This was undertaken to specifically examine the effects Mn on measures DA neurotoxicity and reproduction. Measures of pulmonary inflammation are presented in Figure 6. Neutrophils (Figure 6A), LDH (Figure 6B) and albumin (Figure 6C) levels were significantly increased in BALF after long-term exposure (28 weeks) to MMA-HS and GMA-MS but not MnCl2.
Figure 6.
Neutrophil number (A), LDH concentrations (B), and albumin concentrations (C) in BAL fluid collected 1 day after long-term (28 weeks) of exposure to welding particulates or MnCl2.
*Significant from control p < 0.05.
Metal analyses
Metal concentrations in the pituitary of rats exposed to welding fume particulates are presented in Table 5. Values designated by (*) are significantly different than those measured in saline controls. Highlighted values are different than those in MnCl2 treated rats. Fe and Zn levels were significantly reduced in animals expose to MMA-HS and GMA-MS. Mn concentrations were elevated after exposure to MMA-HS and MnCl2. Table 6 displays % changes in accumulation of metals in the pituitary and correlation to alterations in various physiological measures of reproduction and hypothalamic TH concentrations.
Table 5.
Metal Concentrations (μg/g wet tissue wt) in the Pituitary. Levels after 1 week Following a 28 week Exposure to Saline, MMA-HS, GMA-MS or MnCl2 Welding Particulate.
| Saline | MMA-HS | GMA-MS | MnCl2 | |
|---|---|---|---|---|
| Al | 5.736 ± 3.853 | 2.833 ± 0.316 | 0.744 ± 0.062* | 1.813 ± 0.116 |
| Cr | 1.287 ± 0.192 | 1.103 ± 0.089 | 1.218 ± 0.123 | 4.567 ± 2.755 |
| Cu | 2.468 ± 0.143 | 2.130 ± 0.330 | 2.282 ± 0.489 | 2.213 ± 0.181 |
| Fe | 54.015 ± 6.963 | 35.134 ± 1.039* | 45.549 ± 2.510* | 55.210 ± 4.337 |
| Ni | 0.903 ± 0.093 | 0.716 ± 0.078 | 0.504 ± 0.092* | 0.863 ± 0.073 |
| Zn | 13.922 ± 1.085 | 10.343 ± 0.773* | 13.653 ± 0.355 | 16.459 ± 2.019 |
| Mn | 0.919 ± 0.106 | 2.982 ± 0.250* | 1.214 ± 0.059 | 3.077 ± 0.425* |
Concentrations with (*) are different than saline controls (p < 0.05). Highlighted concentrations are different than MnCl2 (p < 0.05)
Table 6.
Changes in Metal Concentrations, Circulating Prolactin Concentrations and Hypothalamic TH Levels after 28 weeks of Exposure to Different Welding Fume Particulate.
| Tissue | Al | Fe | Mn | Zn | Prl | TH | Epi. | Pit wt | Sperm count | Testosterone | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MMA-HS | Pit | – | 36%↓ | 224%↑ | 16%↑ | 53%↓ | – | – | 23%↑ | 38%↓ | – |
| GMA-MS | Pit | 87%↓ | 16%↓ | – | 21%↑ | – | 32%↓ | – | – | 45%↓ | 348%↑ |
| MnCl2 | Pit | – | – | 242%↑ | 26%↑ | – | – | 38%↓ | 31%↓ | 77%↓ | – |
All changes listed are significantly different and expressed as a percent change from saline control. Prl: prolactin, Epi wt: epididymis wt, TH: tyrosine hydroxylase, Pit wt: pituitary wt
Effects of welding fumes on male reproductive measures
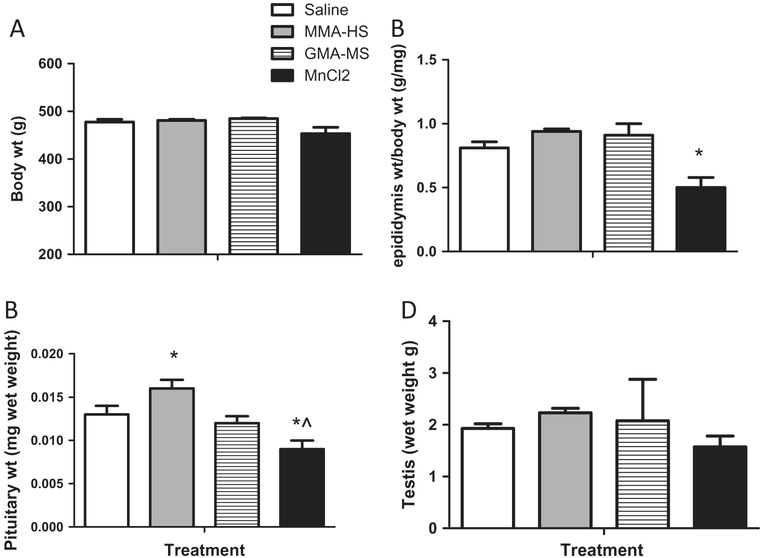
Body and organ weights are presented in Figure 7. Body (Figure 7A) and testicular weights (Figure 7D) were not significantly different from control after treatment with any of the welding samples. However, epididymis (Figure 7B) and pituitary weights were markedly lower in the MnCl2 treated group (Figure 7C, different than control and MMA-HS-treated). Interestingly, exposure to MMA-HS increased pituitary weights. These differences were also apparent when body weight was used as a control for organ weight.
Figure 7.
The effects of exposure on body weight (A), epididymis (B), pituitary (C) and testis weights (D) are presented. There were no treatment effects on body or testis weights.
However, * Significantly different than saline controls, p < 0.05, ^ Significantly different than MMA-HS treated rats, p < 0.05.
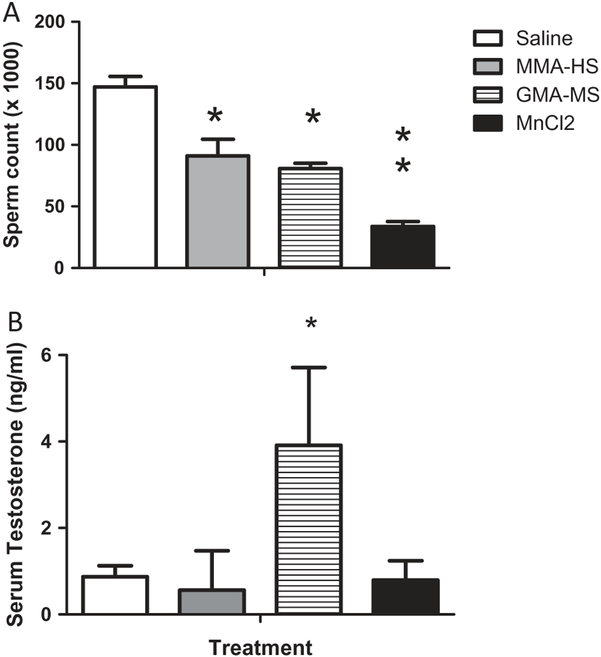
All rats exposed to welding particulates displayed a reduction in sperm count, regardless of the exposure (Figure 8A). However, sperm counts were significantly lower in rats treated with MnCl2 than those exposed with MMA-HS or GMA-MS. Rats treated with GMA-MS displayed higher circulating testosterone concentrations than in any other treatment groups (Figure 8B).
Figure 8.
The effects of treatment on sperm counts (A) and serum testosterone levels (B).
*Significantly different than saline controls, p < 0.05, ** Different than MMA-HS and GMA-HS treated rats, p < 0.05.
Prolactin and TH concentrations
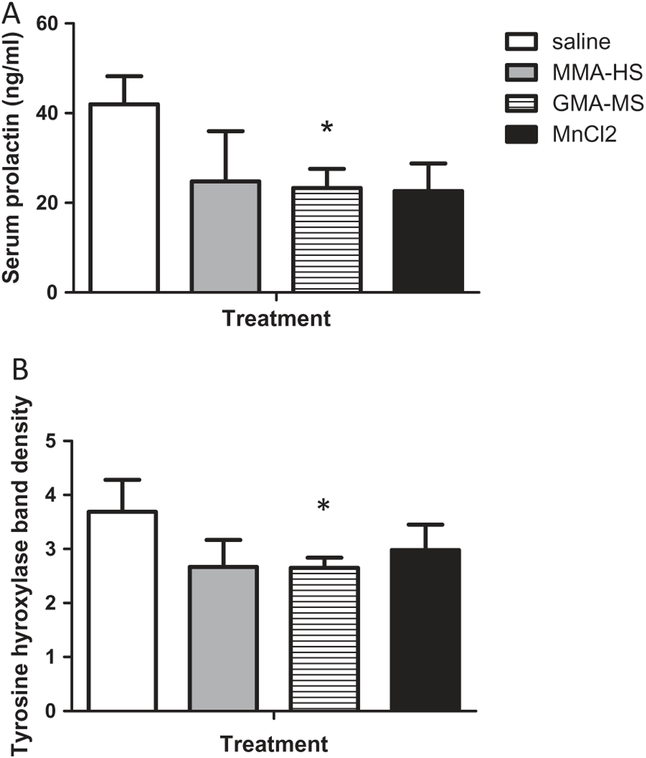
Circulating prolactin concentrations and TH levels in the hypothalamus are presented in Figure 9. Although prolactin appeared to be lower in all welding particulate-treated groups, concentrations were only significantly reduced in rats treated with GMA-MS (Figure 9A). Hypothalamic TH levels were only significantly lower in animals treated with GMA-MS (Figure 9B).
Figure 9.
Circulating prolactin (A) and hypothalamic median eminence/arcuate nucleus TH concentrations (B).
*Significant from control p < 0.05. P
Discussion
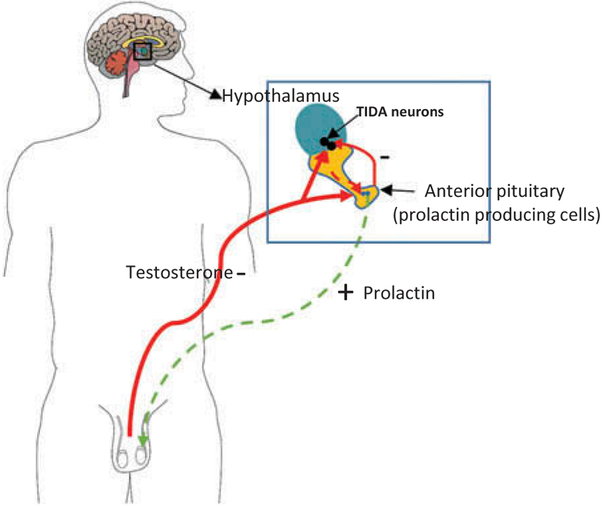
Epidemiological studies demonstrated that welders display increases in circulating prolactin concentrations (Niu et al., 2004; Smargiassi & Mutti, 1999), along with changes in other measures that are associated with reproductive dysfunction (Ellingsen et al., 2007b; Mutti et al., 1996; Niu et al., 2004; Smargiassi & Mutti, 1999). Investigators suggested that these alterations, particularly the elevation in circulating prolactin concentrations, may be an early biomarker of welding fume-induced neurotoxicity (Niu et al., 2004; Smargiassi & Mutti, 1999). Because many of these alterations have been attributed to Mn, the short-term effects of three welding fumes with different levels of Mn, or containing Mn with different solubility (FCA-HS, GMA-MS and MMA-HS) were examined. The results of the short-term study demonstrated that exposure to welding particulates affected the HPG axis with rats exposed to FCA-HS and MMA-HS displaying changes in circulating prolactin and hypothalamic TH concentrations. Although sperm were not counted after the shorter exposure, examination of photomicrographs suggests that sperm production was reduced in rats treated with welding particulate. With the long-term exposure MMA-HS, GMA-MS and MnCl2 all exerted effects on functioning of the HPG-axis that were consistent with the results of the short-term study. Figure 10 shows the relationship between various neuroendocrine and endocrine organs, and how exposure to welding fume particulate may have affected functioning of this system.
Figure 10.
This diagram illustrates the hypothalamic-pituitary gonadal (HPG) axis in the adult male (the hypothalamus and pituitary, are magnified in the box). DA from the TIDA neurons in the hypothalamus inhibits the release of prolactin from the anterior pituitary. Prolactin acts at the level of the gonad to stimulate sperm and testosterone production. In turn, testosterone feeds back onto TIDA neurons and the pituitary to inhibit the release of DA and prolactin. The green lines represents positive feedback, and the red lines are inhibitory feedback of hormone release and spermatogenesis. Dashed lines designate places where welding fume particulate and associated solubilized metals may interfere with reproductive function.
Changes in neuroendocrine function were associated with pulmonary inflammation. Following short-term exposures inflammation persisted in the lungs 35 days after exposure to FCA-HS; FCA-treated rats exhibited a higher number of neutrophils than controls. These findings are consistent with previous findings (Zeidler-Erdely et al., 2012) demonstrating that certain measures of pulmonary inflammation are maintained even after cessation of welding fume particulate exposure. After the 28 week long-term exposure, lung inflammation rose in rats exposed to GMA-MS and MMA-HS, but not MnCl2. These results are similar to Zeidler-Erdely et al. (2012) demonstrating that exposure to welding fumes produces an increase in pulmonary inflammation and development of pulmonary disorders following chronic IT exposure to welding fume particulate (Antonini et al., 2012). However, it should be noted that the effects of IT exposure and inhalation might be different. For example, inhalation of GMA-MS aerosol failed to initiate significant inflammation of the respiratory system (Antonini et al., 2011).
Pulmonary inflammation due to the inhalation of nanoparticles (NP) or other workplace generated fumes, or systemic inflammation associated with the inhalation of irritants, has been associated with disorders in a number of physiological systems, including cardiovascular, peripheral and central nervous system, renal and reproductive system (Antonini et al., 2010, 2011; Chakraborty et al., 2017; Kermanizadeh et al., 2016; Roberts et al., 2013; Zeidler-Erdely et al., 2012; Zheng et al., 2015). Inhalation of PM generated during the welding processes, in particular fumes with a high concentration of Mn, or those that have Mn with high solubility levels were found to induce manganism, a disorder with symptoms similar to those seen in idiopathic Parkinson’s disease (Sriram et al., 2010).
In the short-term study, accumulation of specific metals in the pituitary and testes was still measureable 35 days following the last exposure. Previous studies demonstrated that translocation of specific metals from lungs to peripheral organs occurs shortly after exposure (Antonini et al., 2010). Data suggest that these metals are still present and may induce changes in neuroendocrine function even after exposure was terminated. Elevated levels of Fe and Zn in the pituitary correlated with reductions in circulating (serum) prolactin concentrations and an increasing trend in TH activity levels in hypothalamus of FCA-HS exposed rats. There was also a decrease in hypothalamic TH levels in the MMA-HS treated rats.
These results support findings in humans showing that exposure to welding fumes affects measures of reproductive and neuroendocrine function, and that these changes may be biomarkers of welding fume-induced toxicity of hypothalamic neurons or male reproductive function. However, the reduction in prolactin levels produced by welding fume exposure in rats is in contrast to the alterations (increased levels) seen in humans (Mutti et al., 1996; Smargiassi & Mutti, 1999). The differential effects of welding fume exposure on prolactin concentrations may be due to a number of factors. It is known that different fumes are composed of varying concentrations of different metals, and these may alter the release of DA from TIDA neurons, and act to inhibit prolactin release from the anterior pituitary (Freeman et al., 2000). Although DA concentrations and DA release were not determined directly, TH, which is the rate limiting enzyme for DA synthesis, was measured as an indicator of DA levels (Freeman et al., 2000; Krajnak et al., 1995; Moore, 1987). In the short-term study, hypothalamic TH concentrations were significantly lower in rats treated with MMA-HS. In addition, following long-term exposure, all treatments tended to reduce levels of hypothalamic TH. These findings suggest that the effects of welding particulates on the hypothalamic-pituitary system are not solely due to Mn exposure, but may also involve accumulation of a number of different particulates after exposure.
Reductions in TH levels measured by immunoblotting may be indicative of; (1) a decrease in DA due to inhibition in the rate limiting enzyme responsible for synthesis, and/or (2) diminished in synthesis of the enzyme. DA is a major inhibitor of prolactin, and prolactin concentrations were reduced in rats exposed to GMA-MS and MnCl2. A reduction in prolactin might be attributed to an increase in DA release and synthesis (13). However, there are situations where both DA and prolactin may be reduced. For example, in animals that display reproductive changes in response to differences in the length of day, both DA and prolactin are diminished at the same time (Krajnak et al., 1994). It is possible the adverse effects of welding particulate for 28 weeks may have resulted in a temporary loss of function in one of the endocrine organs, which in turn resulted in decreased function of other organs.
Following long-term exposures, sperm counts were reduced in animals exposed to all welding particulates. This decrease may be a consequence of changes in prolactin release from the pituitary (Freeman et al., 2000), or metal-induced toxicity in the testes. Analyses of metal concentrations in the pituitary showed that Mn concentrations were elevated in rats treated with MMA-HS and MnCl2. Although concentrations of metals in the testes of rats exposed to welding particulates were not determined after long-term exposure, observations from short-term exposure studies indicate that repetitive exposure to welding fumes, particularly MMA-HS, result in accumulation of Ni, Cr and Mn in the testes. Previous investigators demonstrated that metal accumulation, especially Cr accumulation, is toxic to the reproductive system (Bonde & Ernst, 1992; Danadevi et al., 2003). Specifically, exposure to welding fumes was found to damage the sperm germ line of workers that were exposed to the fumes, resulting in an enhanced risk of testicular cancer in male offspring (Danadevi et al., 2003). Similar effects on male reproduction were noted after exposure to other NP, such as crystalline cellulose (Farcas et al., 2016).
Although the changes in circulating prolactin concentrations in these studies were not similar to those detected in workers exposed to welding fumes, evidence indicates that changes in prolactin, or other circulating hormones, may be used as a marker of welding fume-induced toxicity. Alterations in circulating prolactin were associated with changes in hypothalamic TH concentrations, particularly following long-term exposure. Previously, Sriram et al. (2010) demonstrated that similar exposures produced changes in TH and DA concentrations in regions of the brain affected by Parkinson’s disease, and thus, it is possible that changes in hypothalamic DA concentrations and circulating prolactin concentrations may serve as a marker for welding fume and Mn-induced toxicity. Alterations in spermatogenesis occurred in all groups and may be attributed to welding fume-induced changes in the release of prolactin or gonadotropins from the pituitary (Freeman et al., 2000; McLachlan et al., 1996), or the direct effect of accumulated metals on the testes (Bonde, 1990; Jensen et al., 2006). However, it should be noted that welders are also exposed to a number of other factors that influence reproductive function, including excessive heat and physical stress due to working in confined areas (Ellingsen et al., 2007b). Although these other factors certainly might affect reproductive functions, this study specifically examined the influence of welding particulates, and thus, data presented here are consistent with epidemiological observations suggesting that inhalation of fumes alone may exert significant effects on reproduction (Bonde, 1990; Danadevi et al., 2003; Wirth et al., 2006).
Although it was beyond the scope of this study, it is possible that welding fume exposure might lead to neurobehavioral effects. In humans, exposure to welding fumes was associated with increased incidence of fatigue, insomnia, depression, anxiety, Parkinson’s like symptoms as well as pre-senile dementia, which might all, in part be related to changes in DA levels (Ellingsen et al., 2007a; Park, 2013). It is interesting to note however, that rats exposed to 11 weeks MMA-HS in our lab showed no robust behavioral changes on a choice reaction time task which assesses reaction speed, and other motivational, attentional, and motor processes. The effect of longer duration exposures on neurobehavioral effects of welding fumes are needed (Hayashi et al., 2010).
The results of this study are consistent with earlier findings showing that inhalation of welding fumes affects reproductive functions. Although changes in prolactin were contradictory to previous observations reported in welders, it is possible that human studies and current findings assessed the effects of fume inhalation at different time points during development of this disorder. However, it appears that exposure to welding fumes affected function of the neuroendocrine axis and that circulating prolactin concentrations might serve as a biomarker for welding fume-induced neurotoxicity. Future studies including additional time points, metal particulate, and doses, may provide more information regarding the reliability and validity of this measure.
Acknowledgments
We thank Kimberly Clough-Thomas for assistance with the diagram of the feedback loop. We also thank Patricia Zeidler-Erdley, Tina Sager, and Stacy Waugh for their critical review of the manuscript.
Footnotes
Disclaimer
The findings and conclusions in this report are those of the author and do not necessarily represent the views of the National Institute for Occupational Safety and Health.
References
- Antonini JM, Roberts JR, Chapman RS, Soukup JM, Ghio AJ, and Sriram K 2010. Pulmonary toxicity and extra-pulmonary tissue distribution of metals after repeated exposure to different welding fumes. Inhal. Toxicol 22: 805–816. [DOI] [PubMed] [Google Scholar]
- Antonini JM, Roberts JR, Stone S, Chen BT, Schwegler-Berry D, Chapman R, Andrews RN, and Frazer DG 2011. Persistence of deposited metals in the lungs after stainless steel mand mild steel welding fume inhalation in rats. Arch. Toxicol 85: 487–498. [DOI] [PubMed] [Google Scholar]
- Antonini JM, Sriram K, Benkovic SA, Roberts JR, Stone S, Chen BT, Schwegler-Berry D, Jefferson AM, Billig BK, Felton CM, Hammer MA, Ma F, and Frazer DG 2009. Mild steel welding fume causes manganese acumulation and subtle neuroinflammatory changes but not overt neuronal damage in discrete brain regions of rats after short-term inhalation exposure. Neurotoxicology 30: 915–925. [DOI] [PubMed] [Google Scholar]
- Antonini JM, Zeidler-Erdley PC, Young SH, Roberts JR, and Erdley A 2012. Systemic immune cell response in rats after pumonary exposure to manganese-containing particles collected from welding aerosols. J. Immunotoxicol 9:184–192. [DOI] [PubMed] [Google Scholar]
- Bonde JP 1990. Semen quality and sex hormones among mild steel and stainless steel welders: a cross sectional study. Br. J. Ind. Med 47: 508–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonde JP and Ernst E 1992. Sex hormones and semen quality in welders exposed to hexavalent chromium. Hum. Exp. Toxicol 11: 259–263. [DOI] [PubMed] [Google Scholar]
- Bureau of Labor Statistics. 2016–17. Welders, cutters, solderers, and brazers, edited by http://www.bls.gov.ooh/production/welders-cutters-solderers-and-brazers.htm.
- Chakraborty S, Castranova V, Perez MK, and Piedmont G 2017. Nanoparticles induced apoptosis of human airway epithelium is mediated by proNGF/p75NTR signaling. J. Toxicol. Environ. Health A 80: 53–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danadevi K, Rozati R, Reddy PP, and Grover P 2003. Semen quality of Indian welders occupationally exposed to nickel and chromium. Reprod. Toxicol 17: 451–456. [DOI] [PubMed] [Google Scholar]
- Ellingsen DG, Chashchin V, Haug E, Chashchin M, Tkachenko V, Lubnina N, Bast-Pettersen R, and Thomassen Y 2007a. An epidemiological study of reproductive function biomarkers in male welders. Biomarkers 12: 497–509. [DOI] [PubMed] [Google Scholar]
- Ellingsen DG, Konstantinov R, Bast-Pettersen R, Merkurjeva L, Chashchin M, Thomassen Y, and Chashchin V 2007b. A neurobehavioral study of current and former welders exposed to manganese. Neurotoxicology 29: 48–59. [DOI] [PubMed] [Google Scholar]
- Farcas MT, Kisin ER, Menas AL, Gutkin DW, Star A, Reiner RS, Yaqnamala N, Savolainen K, and Shvedova AA 2016. Pulmonary exposure to cellulose nanocrystals caused deleeterious effects to reproductive system in male mice. J. Toxicol. Environ. Health A 79: 984–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman ME, Kanyicska B, Lerant A, and Nagy G 2000. Prolactin: structure, function, and regulation of secretion. Physiol. Rev 80: 1523–1631. [DOI] [PubMed] [Google Scholar]
- Harris MK, Ewing WM, Longo W, DePasquale C, Mount MD, Hatfield R, and Stapleton R 2005. Manganese exposures during shielded metal arc welding (SMAW) in an enclosed space. J. Occup. Environ. Hyg 2: 375–382. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Antonini JM, and Wirth OA 2010. A choice reaction-time procedure for assessing the neurobehavioral effects of drugs and toxicants with rats, ed A.F.B. Analysis. Abstracts Association for Behavior Analysis: NIOSH. [Google Scholar]
- Hubbs AF, Sargent LM, Porter DW, Sager TM, Chen BT, Frazer DG, Castranova V, Sriram K, Nurkiewicz TR, Reynolds SH, Battelli LA, Schwegler-Berry D, McKinney W, Fluharty KL, and Mercer RR 2013. Nanotechnology: toxicologic pathology. Toxicol. Pathol 41: 395–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen TK, Bonde JP, and Joffe M 2006. The influence of occupational exposure on male reproductive function. Occup. Med 56: 544–553. [DOI] [PubMed] [Google Scholar]
- Kermanizadeh A, Gosens I, MacCalman L, Johnston H, Danielsen PE, Jacobsen NR, Lenz A-G, Fernandes T, Schins RPF, Cassee FR, Wallin H, Kreyling W, Stoeger T, Loft S, Moller P, Tran L, and Stone V 2016. A multilaboratory toxicological assessment of a panel of 10 engineered nanomaterials to human health-ENPRA project-the highlights, limitations, and current and future challenges. J. Toxicol. Environ. Health B 19: 1–28. [DOI] [PubMed] [Google Scholar]
- Korczynski RE 2000. Occupational health concerns in the welding industry. Appl. Occup. Environ. Hyg 15: 936–945. [DOI] [PubMed] [Google Scholar]
- Krajnak K, Lookingland KJ, and Nunez AA 1995. Seasonal changes in median eminence dopamine in male Syrian hamsters: role of the gonads and duration of exposure to short days. Brain Res. Bull 37: 617–622. [DOI] [PubMed] [Google Scholar]
- Krajnak K, Manzanares J, Lookingland KJ, and Nunez AA 1994. The effect of short-photoperiod exposure on tuberoinfundibular dopamine neurons in male and female Syrian hamsters. J. Biol. Rhythms 9: 125–135. [DOI] [PubMed] [Google Scholar]
- McLachlan RI, Wreford NG, O’Donnell LO, De Krester DM, and Robertson DM 1996. The endocrine regulation of spermatogenesis: independent roles for testosterone and FSH. J. Endocrinol 148: 1–9. [DOI] [PubMed] [Google Scholar]
- Moore KE 1987. Interactions between prolactin and dopaminergic neurons. Biol. Reprod. 36: 47–58. [DOI] [PubMed] [Google Scholar]
- Mutti A, Bergamaschi E, Alinovi R, Lucchini R, Vettori MV, and Franchini I 1996. Serum prolactin in subjects occupationally exposed to manganese. Ann. Clin. Lab. Sci 26: 10–17. [PubMed] [Google Scholar]
- National Insititute for Occupational Safety Elements (ICP): Method 7300. 1994. In NIOSH manual of analytical methods. Washington, DC: U.S. Department of Health and Human Services. [Google Scholar]
- Niu Q, Shuchang H, Sheng W, Di Gioacchino M, Verna N, Volpe AR, Di Giampaolo L, Carmignani M, and Boscolo P 2004. Neurobehavioral functions, serum prolactin and plasma renin activity of manganese-exposed workers. Int. J. Immunopathol. Pharmacol 17: 17–24. [DOI] [PubMed] [Google Scholar]
- Park RM 2013. Neurobehavioral deficits and Parkinsonism in occupations with manganese exposure: a review of methodological issues in the epidemiological literature. Saf. Health Work 4: 123–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JR, McKinney W, Kan H, Krajnak K, Frazer DF, Thomas TA, Waugh S, Kenyon A, MacCuspie RI, Hackley VA, and Castranova V 2013. Pulmonary and cardiovascular responses of rats to inhalation of silver nanoparticles. J. Toxicol. Environ. Health A 76: 651–668. [DOI] [PubMed] [Google Scholar]
- Smargiassi A and Mutti A 1999. Peripheral biomarkers and exposure to manganese. Neurotoxicology 20: 401–406. [PubMed] [Google Scholar]
- Sriram K, Lin GX, Jefferson AM, Roberts JR, Wirth O, Hayashi Y, Krajnak KM, Soukup JM, Ghio AJ, Reynolds SH, Castranova V, Munsone AE, and Antonini JM 2010. Dopaminergic neurotoxicity following pulmonary exposure to manganese-containing welding fumes. Arch. Toxicol 84: 521–540. [DOI] [PubMed] [Google Scholar]
- Susi P, Goldberg M, Barnes P, and Stafford E 2000. The use of a task-based exposure model (T-BEAM) for assessment of metal fume exposures during welding and thermal cutting. Appl. Occup. Environ. Hyg 15: 26–33. [DOI] [PubMed] [Google Scholar]
- Wirth JJ, Rossano MG, Daly DC, Paneth N, Puscheck E, Potter RC, and Diamond MP 2006. Ambient manganese exposure is negatively associated withn human sperm motility and concentration. Epidemiology 18: 270–273. [DOI] [PubMed] [Google Scholar]
- Zeidler-Erdely PC, Erdely A, and Antonini JM 2012. Immunotoxicology of arc welding fume: worker and experimental animal studies. J. Immunotoxicol 9: 411–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng W, Antonini JM, Lin YC, Roberts JR, Kashon ML, Castranova V, and Kan H 2015. Cardiovascular effects in rats after intratracheal installation of metal welding particles. Inhal. Toxicol 27: 45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]