Abstract
Purpose
Marrow failure in some patients with myelodysplastic syndrome (MDS) responds to immunosuppressive treatment (IST), but long-term outcome after IST has not been described. We evaluated patients with MDS treated with IST at our institution to determine their clinical course compared with a comparable supportive care only group.
Patients and Methods
One hundred twenty-nine patients with MDS received IST with a median follow-up of 3.0 years (range, 0.03 to 11.3 years), using antithymocyte globulin (ATG) or cyclosporine (CsA) in combination or singly. Variables affecting response and survival were studied and outcomes were compared with those of 816 patients with MDS reported to the International Myelodysplasia Risk Analysis Workshop (IMRAW) who received only supportive care.
Results
Thirty-nine (30%) of 129 patients receiving IST responded either completely or partially: 18 (24%) of 74 patients responded to ATG, 20 (48%) of 42 patients responded to ATG plus CsA, and one (8%) of 13 patients responded to CsA. Thirty-one percent (12 of 39) of the responses were complete, resulting in transfusion independence and near-normal blood counts. In multivariate analysis, younger age was the most significant factor favoring response to therapy. Other favorable factors affecting response were HLA-DR15 positivity and combination ATG plus CsA treatment (P = .001 and P = .048, respectively). In multivariate analysis of the combined IMRAW and IST cohorts, younger age, treatment with IST, and intermediate or low International Prognostic Scoring System score significantly favored survival.
Conclusion
IST produced significant improvement in the pancytopenia of a substantial proportion of patients with MDS and was associated with improved overall and progression-free survival, especially in younger individuals with lower-risk disease.
INTRODUCTION
Myelodysplastic syndromes (MDS) include a group of disorders that vary widely in clinical presentation and severity.1,2 Typically, patients with MDS are older adults with comorbidities.3 Death from MDS is due to progression to acute leukemia or to the consequences of cytopenias.4 The International Prognostic Scoring System (IPSS) can predict survival based on clinical, hematologic, and karyotypic features.5 No treatment other than allogeneic stem-cell transplantation6 has yet been shown to prolong survival, although for some patients with MDS, hematopoietic growth factors,7 decitabine,8 and lenalidomide9 may improve cytopenias, and 5-azacytidine may reduce transfusion requirements, delay the time to leukemic transformation, and improve quality of life when compared with supportive care.10,11 Chemotherapy has a limited role in the management of leukemic progression,12 and autologous stem-cell transplantation does not prolong relapse-free survival.13
The concept that an immune-mediated response directed against hematopoietic cells can cause failure of the bone marrow leading to pancytopenia arose from early experience with bone marrow transplantation to treat severe aplastic anemia, in which some nonengrafting patients developed autologous hematologic recovery. Several investigators subsequently used antithymocyte globulin (ATG) to treat the bone marrow failure accompanying hypoplastic MDS with some success.14,15 Immunosuppressive therapy (IST) can benefit patients with MDS regardless of cellularity.16–23 We previously described hematologic responses, including transfusion independence, in 21 of 61 patients with MDS given ATG.23 We subsequently developed a scoring system to predict response to IST based on the patient’s age, duration of RBC transfusion dependence, and presence of an HLA-DR15 allele24 (designated the IST response probability score [ISTRPS]). Although IST can improve cytopenias in MDS, the impact of IST on survival and leukemic progression in both responders and nonresponders has not been studied. Here we examine the response rates and long-term survival of patients treated with IST and compare the outcome of IST responders and nonresponders with that of a control population of 816 patients drawn from the International Myelodysplasia Risk Analysis Workshop (IMRAW) database who received neither IST nor cytotoxic drugs.5
PATIENTS AND METHODS
Patients
Patients with MDS, classified according to French-American-British criteria25 as refractory anemia, refractory anemia with ringed sideroblasts, or refractory anemia with excess blasts, were enrolled to receive equine ATG (Pharmacia, Kalamazoo, MI), ATG plus cyclosporine (CsA), or CsA alone, in sequential protocols 00-H-0169, 04-H-0026, and 95-H-0189 approved by the institutional review board of the National Heart, Lung and Blood Institute. Patients included in the trials were diagnosed with MDS between 1971 and 2003. The first trial treated 62 consecutive patients with ATG alone. The second trial randomly assigned 23 patients to receive either ATG or CsA, and the third trial treated consecutive patients with ATG plus CsA. Patients who received IST in this study comprised 69 patients previously reported24 and an additional 60 patients. We compared survival with a group of 816 patients with MDS reported by the IMRAW5 diagnosed between 1973 and 1994 analyzed for survival (816 patients) and freedom from acute myeloid leukemia (AML) evolution (759 patients).
Eligibility for IST
Consecutive patients older than 18 years with MDS refractory anemia, refractory anemia with ringed sideroblasts, or refractory anemia with excess blasts25 were evaluated. The diagnosis of MDS was established by bone marrow morphology and cytogenetics. For study inclusion one or more of the following criteria was necessary: transfusion dependence (at least two units of RBCs or five units of platelets per month for a period of 2 months before enrollment), thrombocytopenia (platelet count ≤ 50,000/μL), or neutropenia (neutrophil count ≤ 500/μL), based on the mean of three blood counts within 2 weeks of enrollment. Granulocyte colony-stimulating factor (G-CSF) to treat severe neutropenia, but no other hematopoietic growth factors, was permitted. Patients with more than 20% blasts and those with secondary MDS were excluded from the study. Nonresponders were not treated with a second course of IST, although responders who experienced relapse were eligible for an additional course of IST based on the clinical judgment of the investigator.
Response Criteria
Complete hematologic remission was defined as normalization of all affected hematopoietic lineages with less than 5% blasts in the marrow. Partial response was defined as improvement of blood counts, measured at 3 months after the last dose of ATG, sustained on at least two serial measurements performed 1 month apart. The parameters for response were previously described.18 The neutrophil response to IST in patients who had received G-CSF support was evaluated after a minimum 1-month G-CSF–free interval. In addition, we retrospectively graded response using the International Working Group criteria.26,27
Blood counts were obtained weekly during the study. Response was assessed by at least three serial measurements obtained 1 month apart. Transfusion independence was defined as no transfusion requirement for a 3-month period. Improvement in the transfusion requirement was a secondary end point, defined as a halving of the number of transfusions received in a 2-month period assessed 6 months after completion of treatment. Patients were followed up yearly to assess the durability of response, disease progression, and survival. For comparability with patients in the IMRAW database,5 leukemia was defined as more than 30% blasts in the bone marrow. Patients not surviving or those receiving alternative treatment, such as stem-cell transplantation for disease progression, were classified as nonresponders.
Statistical Methods
Results were analyzed on an intention-to-treat basis. Thus four patients unable to receive the full dose of ATG because of intolerance or comorbidity were included. Summary statistics, such as proportions, means, medians, and ranges, were used to describe patient characteristics, baseline variables, and treatment responses. Multivariate logistic regression was used to evaluate the treatment and other covariates on the response rates among the patients evaluated in the IST group. Cumulative incidence curves for mortality and leukemia and the multivariate Cox proportional hazard models were used to analyze the distributions of time to overall mortality and time to leukemia. In particular, the cumulative events of mortality and leukemia and their 95% CIs were estimated among subgroups of patients, and the multivariate Cox proportional hazard models were used to evaluate the effects of covariates, such as age, treatment received, IPSS categories, and other risk factors on time to death and time to leukemia. For the IMRAW data, the survival time and leukemia time were defined to be the time from enrollment to death or the development of leukemia. For the IST data, the survival time and leukemia time were defined to be the time from receiving the first dose of IST (enrollment) to death or the development of leukemia. Our multivariate Cox proportional hazards models evaluated the difference between the IMRAW and IST patients in overall survival time and time to the development of leukemia from enrollment after adjusting the other covariates and/or risk factors. Because the patients receiving IST did not immediately receive their treatments after diagnosis, the survival time and leukemia time based on this definition were shorter and hence more conservative than the time from diagnosis to death or the development of leukemia. Although it is possible to analyze the time to death or leukemia from diagnosis using the Cox model with IST as a time-dependent covariate,28,29 such models may not have a clear clinical interpretation because of the potential inaccuracy of the diagnosis time and the various reasons prompting a patient to receive IST treatment after diagnosis. Statistical tests based on t tests, likelihood ratio tests, and χ2 tests were used to compare the response rates, overall survival, and leukemia evaluation between subgroups. Data analysis was performed using the S-Plus 8 statistical package (Insightful Corp, Seattle, WA).
RESULTS
Patient Selection
All 129 patients enrolled had de novo MDS without preceding aplastic anemia or prior chemotherapy. By IPSS criteria,5 16 patients were considered low risk, 94 patients were considered intermediate-1 (int-1), 13 patients were considered int-2, and six patients were considered high risk. Median follow-up was 2.9 years (range, 0.03 to 11.3 years). Patient characteristics are listed in Table 1. Time from diagnosis to treatment ranged from 0 to 197 months (median, 19 months). Seventy four patients received equine ATG, 42 patients received a combination of ATG and CsA (maintaining CsA levels>100 ng/Ml for up to 6 months), and 13 patients received CsA alone on the same schedule.
Table 1.
Patient Characteristics
| Total Patients |
Patients With AML |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IMRAW |
IST |
IMRAW |
IST |
|||||||||
| Characteristic | No. | % | No. | % | P | AML (n) | No. Evaluated | % | AML (n) | No. Evaluated | % | P |
| Total | 816 | — | 129 | — | — | 168 | 759 | 22.1 | 21 | 129 | 16.3 | .103 |
| Sex | ||||||||||||
| Male | 491 | 60.2 | 86 | 66.7 | .152 | 101 | 459 | 22.0 | 14 | 86 | 16.3 | .197 |
| Female | 325 | 39.8 | 43 | 33.3 | .152 | 67 | 300 | 22.3 | 7 | 43 | 16.3 | .332 |
| PSS status | ||||||||||||
| Low | 262 | 32.1 | 16 | 12.4 | .000 | 22 | 231 | 9.5 | 2 | 16 | 12.5 | .742 |
| Int-1 | 318 | 39.0 | 94 | 72.9 | .000 | 69 | 313 | 22.0 | 12 | 94 | 12.8 | .028 |
| Int-2 | 180 | 22.1 | 13 | 10.1 | .000 | 45 | 141 | 31.9 | 2 | 13 | 15.4 | .158 |
| High | 56 | 6.9 | 6 | 4.7 | .285 | 32 | 74 | 43.2 | 5 | 6 | 83.3 | .062 |
| Age ≤ 60 years | ||||||||||||
| Total | 205 | 25.1 | 69 | 53.5 | .000 | 52 | 187 | 27.8 | 5 | 69 | 7.2 | .000 |
| IPSS status | ||||||||||||
| Low | 58 | 7.1 | 8 | 6.2 | .696 | 5 | 49 | 10.2 | 1 | 8 | 12.5 | .879 |
| Int-1 | 89 | 10.9 | 55 | 42.6 | .000 | 20 | 84 | 23.8 | 3 | 55 | 5.5 | .001 |
| Int-2 | 49 | 6.0 | 5 | 3.9 | .263 | 19 | 41 | 46.3 | 0 | 5 | 0.0 | .000 |
| High | 9 | 1.1 | 1 | 0.8 | .703 | 8 | 13 | 61.5 | 1 | 1 | 100.0 | — |
| Age > 60 years | ||||||||||||
| Total | 611 | 74.9 | 60 | 46.5 | .000 | 116 | 572 | 20.3 | 16 | 60 | 26.7 | .291 |
| IPSS status | ||||||||||||
| Low | 204 | 25.0 | 8 | 6.2 | .000 | 17 | 182 | 9.3 | 1 | 8 | 12.5 | .810 |
| Int-1 | 229 | 28.1 | 39 | 30.2 | .619 | 49 | 229 | 21.4 | 9 | 39 | 23.1 | .820 |
| Int-2 | 131 | 16.1 | 8 | 6.2 | .000 | 26 | 100 | 26.0 | 2 | 8 | 25.0 | .954 |
| High | 47 | 5.8 | 5 | 3.9 | .321 | 24 | 61 | 39.3 | 4 | 5 | 80.0 | .112 |
| IST | ||||||||||||
| ATG | — | — | 74 | 57.4 | — | — | — | — | 17 | 74 | 23.0 | — |
| ATG + CsA | — | — | 42 | 32.6 | — | — | — | — | 1 | 42 | 2.4 | — |
| CsA | — | — | 13 | 10.1 | — | — | — | — | 3 | 13 | 23.1 | — |
| Marrow | ||||||||||||
| Hypercellular | — | — | 47 | 36.4 | — | — | — | 8 | 47 | 17.0 | — | |
| Normal or variable | — | — | 39 | 30.2 | — | — | — | — | 9 | 39 | 23.1 | — |
| Hypocellular | — | — | 43 | 33.3 | — | — | — | — | 4 | 43 | 9.3 | — |
| PNH clone | — | — | 15 | 11.6 | — | _ | — | — | 1 | 15 | 6.7 | — |
Abbreviations: AML, acute myeloid leukemia; IMRAW, International Myelodysplasia Risk AnalysisWorkshop; IST, immunosuppressivetherapy; IPSS, International Prognostic Scoring System; Int, intermediate; ATG, antithymocyte globulin; CsA, cyclosporine; PNH, paroxysmal nocturnal hemoglobinuria.
Response to IST
Of the 129 patients receiving a single course of IST, 39 patients (30%) experienced either complete or partial response; 18 (24%) of 74 patients (95% CI, 14% to 34%) responded to ATG, 20 (45%) of 42 patients (95% CI, 32% to 63%) responded to ATG plus CsA (P=.01), and one (8%) of 13 patients responded to CsA. Thirty-one percent (12 of 39 patients; 95% CI, 16% to 46%) of the responses were complete, resulting in near-normal blood counts and transfusion independence; 32 (82%) of 39 (95% CI, 70% to 94%) of the responders had either a bi-lineage or trilineage responses. Of the partial responders, all but one became transfusion independent; this patient had paroxysmal nocturnal hemoglobinuria (PNH) with hemolysis and required eventual transplantation. One hundred twenty-two patients were RBC transfusion dependent before IST. Of these, 31% (95% CI, 23% to 39%) responded to IST at a median time of 4 months. Five responders but no nonresponders were re-treated with immunosuppression. Responses were the same when the response classification of the International Working Group criteria was applied (data not shown).26 Twenty-four (62%) of the 39 (95% CI, 46% to 77%) responders had neutrophil responses; 34 (87%) of the 39 responders (95% CI, 76% to 98%) had RBC responses, and 24 (62%) of the 39 responders (95% CI, 46% to 77%) had platelet responses. For int-1 IPSS patients, the response rate to ATG plus CsA was superior to that of ATG alone: 54% versus 29% (P = .004). The difference between treatments was also significant in multivariate analysis (P = .048; Table 2). The total number of int-1 responders for patients≤60 years was 28 (51%) of 54 versus six (15%) of 39 in patients older than 60 years (P<.001).Three of thirteen int-2 patients responded to IST, including three of five patients ≤ 60 years versus 0 of eight patients older than 60 years. Univariate analysis showed a small contribution of RBC transfusion duration on response rate and that the relationship between age was a continuous variable affecting probability of response. In multivariate analysis, age as a continuous variable and the presence of the DR15 allele were the most significant factors affecting response (P<.001 and P = .002, respectively; Table 2). There was no association of cellularity, PNH clone or absolute neutrophil count, sex, or duration of transfusion dependence with response (P=.543, .833, .978, and .116, respectively).
Table 2.
Coefficients Obtained From the Multivariate Stepwise Logistic Regression Models of Response Versus Study Medicine,* Age, Sex, and DR15 Allele
| Risk Factor | Coefficient (β) | 95% CI for β | P for β = 0 |
|---|---|---|---|
| Study medicine | |||
| ATG + CsA | — | — | — |
| ATG | −1.042 | −2.075 to −0.009 | .048 |
| Age | −0.097 | −0.144 to −0.050 | < .001 |
| Sex | |||
| Female | — | — | — |
| Male | 0.443 | −0.686 to 1.572 | .442 |
| DR15 allele | 1.690 | 0.643 to 2.737 | .002 |
Abbreviations: ATG, antithymocyte globulin; CsA, cyclosporine.
ATG plus CsA versus AtG.
Treatment Toxicity
Twelve patients required temporary admission to the intensive care unit during ATG treatment. Six patients did not complete the 4 days of ATG treatment: three developed shaking chills, two had hypotension associated with shaking chills, and one died from alveolar hemorrhage associated with leukemic pulmonary infiltrates.
Outcome After IST
Fifty-nine patients died: 20 patients (33%) died from leukemia and 36 patients (61%) died from marrow failure (bleeding or infection); three patients died from other causes (asthma, lung cancer, and pulmonary embolus). One patient died shortly after receiving ATG from alveolar hemorrhage. Death from leukemia occurred predominantly in older patients (five of 71 patients ≤ 60 years v 16 of 60 patients older than 60 years). Five patients including one IST responder underwent allogeneic stem-cell transplantation to treat leukemic progression (censored at transplantation for survival estimates). Patients were seen at yearly follow-up from the start of IST. Seventy patients survive with a median follow-up of 3 years.
Given differences in follow-up care and variation in the frequency of blood count monitoring, it was not possible to assign exact dates of relapse. Relapse after IST was therefore defined as the date of reinitiation of therapy (transfusions, cytokines, IST [ including CsA alone for patients treated with ATG/CsA], transplantation, or other drugs used to treat MDS). Four relapses occurred in the ATG plus CsA group (after discontinuation of CsA), whereas nine relapses occurred in the ATG-only group. The median duration of responses for the responding individuals was 3 years (range, 3 months to 10 years). Among the 12 patients with complete responses, four patients experienced relapse within the first year, but all responded to reinitiation of immunosuppression; of these, only two patients required reinitiation of CsA. Three of these patients remain in remission without further treatment at a median follow-up of 6.2 years. Two patients (both with trisomy 8) require continued low-dose CsA. Of the 27 partial responders, nine experienced relapse; three patients underwent transplantation and three patients responded to an additional course of IST. Three patients were treated long-term with CsA and remain in remission. Median relapse-free survival was greater than 10.5 years. Results of long-term follow-up of the IST cohort are listed in Table 3.
Table 3.
Numbers of Patients, Overall Mortality, and Mortality With AML Evolution of Patients With MDS Stratified by Pretreatment Disease Characteristics and Study Medications
| All Patients |
ATG |
ATG + CsA |
CsA |
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Died |
AML Death |
Died |
AML Death |
Died |
AML Death |
Died |
AML Death |
Median Survival (years) | 25% AML Evolution (years) | |||||||||||||
| Characteristic | N | No. | % | No. | % | N | No. | % | No. | % | N | No. | % | No. | % | N | No. | % | No. | % | ||
| Total | 129 | 59 | 45.7 | 20 | 15.5 | 74 | 37 | 50.0 | 16 | 21.6 | 42 | 15 | 35.7 | 1 | 2.4 | 13 | 7 | 53.8 | 3 | 23.1 | 10.5 | > 8.2 |
| Sex | ||||||||||||||||||||||
| Male | 86 | 46 | 53.5 | 14 | 16.3 | 43 | 27 | 62.8 | 11 | 25.6 | 32 | 13 | 40.6 | 1 | 3.1 | 11 | 6 | 54.5 | 2 | 18.2 | 5.4 | > 2.7 |
| Female | 43 | 13 | 30.2 | 6 | 14.0 | 31 | 10 | 32.3 | 5 | 16.1 | 10 | 2 | 20.0 | 0 | 0.0 | 2 | 1 | 50.0 | 1 | 50.0 | > 10 | > 8.2 |
| IPSS | ||||||||||||||||||||||
| Low | 16 | 8 | 50.0 | 2 | 12.5 | 14 | 7 | 50.0 | 2 | 14.3 | 2 | 1 | 50.0 | 0 | 0.0 | 0 | 0 | — | 0 | — | 8.1 | > 10.0 |
| Int-1 | 94 | 37 | 39.4 | 11 | 11.7 | 51 | 22 | 43.1 | 9 | 17.6 | 33 | 11 | 33.3 | 1 | 3.0 | 10 | 4 | 40.0 | 1 | 10.0 | 11.3 | > 8.2 |
| Int-2 | 13 | 8 | 61.5 | 2 | 15.4 | 3 | 2 | 66.7 | 0 | 0.0 | 7 | 3 | 42.9 | 0 | 0.0 | 3 | 3 | 100 | 2 | 66.7 | 1.9 | 1.1 |
| High | 6 | 6 | 100 | 5 | 83.3 | 6 | 6 | 100 | 5 | 83.3 | 0 | 0 | — | 0 | — | 0 | 0 | — | 0 | — | 0.4 | 0.2 |
| Age ≤ 60 years | ||||||||||||||||||||||
| Total | 69 | 17 | 24.6 | 4 | 5.8 | 37 | 11 | 29.7 | 3 | 8.1 | 28 | 6 | 21.4 | 1 | 3.6 | 4 | 0 | 0.00 | 0 | 0.0 | ||
| IPSS | ||||||||||||||||||||||
| Low | 8 | 3 | 37.5 | 1 | 12.5 | 7 | 3 | 42.9 | 1 | 14.3 | 1 | 0 | 0.0 | 0 | 0.0 | 0 | 0 | — | 0 | — | > 9.8 | > 9.8 |
| Int-1 | 55 | 12 | 21.8 | 2 | 3.6 | 27 | 6 | 22.2 | 1 | 3.7 | 24 | 6 | 25.0 | 1 | 4.2 | 4 | 0 | 0.0 | 0 | 0.0 | > 10.5 | > 8.2 |
| Int-2 | 5 | 1 | 20.0 | 0 | 0.0 | 2 | 1 | 50.0 | 0 | 0.0 | 3 | 0 | 0.0 | 0 | 0.0 | 0 | 0 | — | 0 | — | > 9.3 | > 9.3 |
| High | 1 | 1 | 100 | 1 | 100 | 1 | 1 | 100 | 1 | 100 | 0 | 0 | — | 0 | — | 0 | 0 | — | 0 | — | — | — |
| Age > 60 years | ||||||||||||||||||||||
| Total | 60 | 42 | 70.0 | 16 | 26.7 | 37 | 26 | 70.3 | 13 | 35.1 | 14 | 9 | 64.3 | 0 | 0.00 | 9 | 7 | 77.8 | 3 | 33.3 | ||
| IPSS | ||||||||||||||||||||||
| Low | 8 | 5 | 62.5 | 1 | 12.5 | 7 | 4 | 57.1 | 1 | 14.3 | 1 | 1 | 100 | 0 | 0.0 | 0 | 0 | — | 0 | — | 6.5 | > 9.5 |
| Int-1 | 39 | 25 | 64.1 | 9 | 23.1 | 24 | 16 | 66.7 | 8 | 33.3 | 9 | 5 | 55.6 | 0 | 0.0 | 6 | 4 | 66.7 | 1 | 16.7 | 2.3 | 2.5 |
| Int-2 | 8 | 7 | 87.5 | 2 | 25.0 | 1 | 1 | 100 | 0 | 0.0 | 4 | 3 | 75.0 | 0 | — | 0 | 0 | — | 0 | — | 0.6 | |
| High | 5 | 5 | 100 | 4 | 80.0 | 5 | 5 | 100 | 4 | 80.0 | 0 | — | 0 | 0 | — | 0 | 0 | — | 0 | — | 0.6 | 0.2 |
Abbreviations: AML, acute myeloid leukemia; MDS, myelodysplastic syndrome; ATG, antithymocyte globulin; CsA, cyclosporine; IPSS, International Prognostic Scoring System; Int, intermediate.
Comparison of Outcome in IST and IMRAW Cohorts
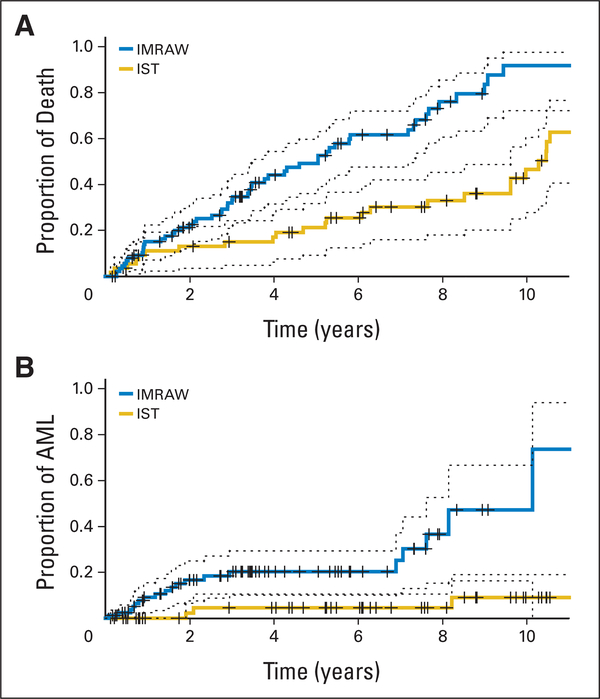
Multivariate Cox regression models were used to analyze the combined IMRAW-IST data for survival from enrollment into both study and time to develop leukemia. Continuous measurements were used for age, absolute neutrophil count, and platelet counts. Discrete covariates were sex, study cohort (IMRAW or IST), IPSS categories (low, int-1, int-2, and high), and marrow blast categories (0% to 5%,>5% to 10%, and>10%). Cytogenetics were grouped as follows: 1=normal and 20q-; 2 = 5q-; 3 = chromosome 7 abnormalities and complex chromosome abnormalities; 4 = other cytogenetic abnormalities; 5 = trisomy 8. Variables affecting survival were age, number of blasts, female sex, cytopenias, cytogenetics, and treatment with IST. The negative regression coefficient and less than 1.00 relative risk for IST indicated that IST was associated with better survival after adjusting these covariates (Table 4). Benefit from IST became more significant in multivariate analysis of patients≤60 years of age; conversely, there was no significant survival benefit for IST patients older than 60 years of age when compared with the IMRAW cohort (P=.10). IPSS score and age emerged as major independent factors affecting survival for both the IST and the IMRAW cohorts. We compared outcomes for the two cohorts in the subset of int-1 patients aged ≤ 60 years and older than 60 years. For the int-1 patients ≤ 60 years, survival of the 55 IST patients was longer than for the 89 IMRAW patients (median > 8.1 v 5.2 years; P = .001; Fig 1). Similarly, the proportion of int-1 patients ≤ 60 years developing AML was lower in the IST versus IMRAW patient cohort (time for 25% of cohort to develop AML was 6.9 years for IMRAW v>8.2 years of the IST group; P=.002). When used as a continuous variable, age was shown to be highly predictive for response to IST (Fig 2; P < .001). In contrast, duration of RBC transfusion need was not.
Table 4.
Regression Coefficients (β) and Covariate Adjusted Relative Risks Obtained From the Multivariate Cox Proportional Hazards Models of Survival Time and Leukemia Time From Enrollment Versus Risk Factors and Treatments for the Combined IST and IMRAW Cohorts
| Survival |
Time to AML |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient |
Coefficient |
|||||||||
| Risk Factor | β | SD | RR | 95% CI | P for β = 0 | β | SD | RR | 95% CI | P for β = 0 |
| Age | 0.033 | 0.004 | 1.03 | 1.03 to 1.04 | < .001 | 0.009 | 0.006 | 1.01 | 0.998 to 1.02 | .120 |
| Sex | ||||||||||
| Female | — | — | 1.00 | — | — | — | — | 1.00 | — | — |
| Male | 0.400 | 0.092 | 1.49 | 1.25 to 1.79 | < .001 | 0.030 | 0.155 | 1.03 | 0.76 to 1.40 | .840 |
| ANC | 0.046 | 0.013 | 1.05 | 1.02 to 1.07 | < .001 | −0.003 | 0.031 | 0.997 | 0.94 to 1.06 | .930 |
| Platelets | −0.002 | 0.0003 | 0.998 | 0.998 to 0.999 | < .001 | −0.001 | 0.001 | 0.999 | 0.997 to 1.00 | .022 |
| Blasts* | ||||||||||
| >10% | — | — | 1.00 | — | — | — | — | 1.00 | — | — |
| 5% to 10% | −0.589 | 0.135 | 0.56 | 0.43 to 0.72 | < .001 | −0.758 | 0.205 | 0.47 | 0.31 to 0.70 | < .001 |
| ≤ 5% | −1.261 | 0.114 | 0.28 | 0.23 to 0.35 | < .001 | −1.845 | 0.187 | 0.16 | 0.11 to 0.23 | < .001 |
| Cyto† | ||||||||||
| Cyto 1 | ||||||||||
| Cyto 2 | — | — | 1.00 | — | — | — | −1.00 | — | ||
| Cyto 3 | 0.395 | 0.189 | 1.49 | 1.03 to 2.15 | 0.037 | 0.390 | 0.340 | 1.48 | 0.76 to 2.87 | .250 |
| Cyto 4 | 1.165 | 0.124 | 3.21 | 2.52 to 4.09 | < .001 | 1.443 | 0.202 | 4.23 | 2.85 to 6.29 | < .001 |
| Cyto 5 | 0.365 | 0.120 | 1.44 | 1.14 to 1.82 | .002 | 0.865 | 0.188 | 2.38 | 1.64 to 3.44 | < .001 |
| Cohort | 0.580 | 0.260 | 1.79 | 1.07 to 2.97 | .026 | 0.314 | 0.447 | 1.37 | 0.57 to 3.29 | .480 |
| IMRAW | — | — | 1.00 | — | — | — | — | 1.00 | — | — |
| IST | −0.506 | 0.162 | 0.60 | 0.44 to 0.83 | .002 | −0.452 | 0.268 | 0.64 | 0.38 to 1.08 | .092 |
Abbreviations: IST, immunosuppressive therapy; IMRAW, International Myelodysplasia Risk Analysis Workshop; AML, acute myeloid leukemia; RR, relative risk; ANC, absolute neutrophil count.
There were no patients with blasts > 20% in the IST group and were only two patients with blasts > 15%. Therefore, these variables were not used in the analysis.
Cytogenetics: 1, normal and 20q; 2, 5q-; 3, chromosome 7 abnormalities and complex chromosome abnormalities; 4, other; 5, trisomy 8.
Fig 1.
Clinical outcomes of 89 National Institutes of Health (NIH) immunosuppressive therapy (IST) and 55 International Myelodysplasia Risk Analysis Workshop (IMRAW) patients ≤ 60 years of age with intermediate-1 myelodysplastic syndrome. (A) Mortality rates and their 95% CIs from time in years of IST (NIH patients) and time in years from diagnosis (IMRAW patients). (B) Leukemia rates and their 95% CIs from time of IST (NIH patients) and from time of diagnosis (IMRAW patients). AML, acute myeloid leukemia.
Fig 2.
Role of age in predicting response to immunosuppressive therapy. Estimated probability of response versus age was determined using logistic regression analysis. Each “|” symbol represents a patient with his or her corresponding age. The vertical lines at the bottom represent nonresponders, whereas those at the top represent responders. As indicated, older patients are more likely to be nonresponders, and younger patients are more likely to be responders (P < .001).
DISCUSSION
Our data demonstrated clinical benefit of IST for a substantial proportion of patients with MDS. Of the criteria previously identified to predict IST response, age emerged as the strongest factor for predicting survival after IST: responders predominated in the group of 55 patients≤60 years of age. Improved responses and clinical outcomes also predominated in the IPSS int-1 patient subset. This translated into survival benefit for responders who also had a 96% leukemic progression-free survival at a median follow-up of 6 years. Patients treated with ATG plus CsA had superior response rates compared with patients treated with ATG alone, although there were no survival differences between these groups. Neither marrow cellularity30 nor the presence of a PNH clone31 influenced the probability of response to IST. A recent study of 96 patients with MDS indicated that hypocellularity and low IPSS score predicted response to immunosuppression.32 Others have shown that hypocellular marrow positively affects response33 as well. Differences in cohort composition as well as differences in pathologic discrimination of aplastic anemia and MDS may have accounted for these differing findings.
In the absence of a prospective study randomly assigning patients with MDS to receive IST or conventional support, it has not been possible to ascertain the overall impact of IST on survival and leukemia progression. Thus the IMRAW patient cohort was used as an untreated historical control group. Patients in the IMRAW group were studied from time of diagnosis, whereas patients in the National Institutes of Health (NIH) group were studied from time of treatment. This could have introduced a bias, as healthier patients may have survived longer to be entered into the clinical trial. However, the time from diagnosis to treatment averaged 1 year in the NIH study, and survivals between IST and conservatively treated patients were so great that it is unlikely to have accounted for the results. Therefore, we analyzed factors affecting outcome in the combined NIH and IMRAW cohorts. Age, cytopenias, number of blasts, and treatment with IST were major variables affecting survival. It was previously noted that age older than 60 years was a major discriminating factor for survival in the IMRAW cohort.5 In this study, IST patients ≤ 60 years had a median survival significantly longer than that of patients in the IMRAW cohort (> 8.1 years v 5.2 years). Multivariate analysis examining the factors affecting survival in both cohorts showed that younger age and IST were independent factors positively affecting both survival and leukemic progression.
Although the mechanism whereby younger patients with MDS benefit from IST is not clear, it is possible that powerful immunosuppressive effects causing myelosuppression dominate MDS occurring in younger patients, whereas MDS developing in older patients maybe predominantly a stem cell abnormality predisposing to cytopenias and leukemia without the autoimmune component. Older patients may also have a smaller marrow reserve, diminishing the chance of hematologic response to IST.
Many studies have confirmed a beneficial effect of IST on the cytopenias of patients with MDS.20–23,34,35 However, there are no prior reports on the durability of response and survival after IST. Our data indicate that improved survival in IST responders is associated with a sustained improvement in cytopenias and a lower risk of leukemic progression. Importantly, there was no evidence that nonresponders to IST were adversely affected by more rapid progression to leukemia, because outcomes for the older than 60 years age group (containing predominantly IST nonresponders) were comparable between IST and IMRAW patients.
Our findings are of clinical importance because they indicate that a portion (mainly younger int-1 patients) of individuals with MDS benefit from IST, with sustained improvement of cytopenias and improved survival and freedom from AML evolution. Prospective clinical trials are warranted to further clarify the role of IST in MDS.
Acknowledgment
We thank the International Myelodysplasia Risk Analysis Workshop for providing the database with clinical information on their patients.
Footnotes
AUTHORS’ DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The author(s) indicated no potential conflicts of interest.
REFERENCES
- 1.List AF, Vardiman J, Issa JP, et al. : Myelodysplastic syndromes. Hematology Am Soc Hematol Educ Program 297-317, 2004 [DOI] [PubMed] [Google Scholar]
- 2.Barrett J, Sloand EM, Young N: Immunologic mechanisms and gene expression patterns in myelodysplastic syndromes, in Greenberg PL (ed): Myelodysplastic Syndromes: Clinical and Biological Advances. Cambridge, England, Cambridge University Press, 2006, pp 147–171 [Google Scholar]
- 3.Toyama K, Ohyashiki K, Yoshida Y, et al. : Clinical implications of chromosomal abnormalities in 401 patients with myelodysplastic syndromes: A multicentric study in Japan. Leukemia 7:499–508, 1993 [PubMed] [Google Scholar]
- 4.Guralnik JM, Eisenstaedt RS, Ferrucci L, et al. : Prevalence of anemia in persons 65 years and older in the United States: Evidence for a high rate of unexplained anemia. Blood 104:2263–2268, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Greenberg P, Cox C, LeBeau MM, et al. : International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood 89:2079–2088, 1997 [PubMed] [Google Scholar]
- 6.Wong R, Shahjahan M, Wang X, et al. : Prognostic factors for outcomes of patients with refractory or relapsed acute myelogenous leukemia or myelodysplastic syndromes undergoing allogeneic progenitor cell transplantation. Biol Blood Marrow Transplant 11:108–114, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Jädersten M, Montgomery SM, Dybedal I, et al. : Long-term outcome of treatment of anemia in MDS with erythropoietin and G-CSF. Blood 106:803–811, 2005 [DOI] [PubMed] [Google Scholar]
- 8.van den Bosch J, Lübbert M, Verhoef G, et al. : The effects of 5-aza-2′-deoxycytidine (Decitabine) on the platelet count in patients with intermediate and high-risk myelodysplastic syndromes. Leuk Res 28:785–790, 2004 [DOI] [PubMed] [Google Scholar]
- 9.List A, Kurtin S, Roe DJ, et al. : Efficacy of lenalidomide in myelodysplastic syndromes. N Engl J Med 352:549–557, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Silverman LR, Demakos EP, Peterson BL, et al. : Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: A study of the cancer and leukemia group B. J Clin Oncol 20:2429–2440, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Silverman LR: DNA methyltransferase inhibitors in myelodysplastic syndrome. Best Pract Res Clin Haematol 17:585–594, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Estey EH: Current challenges in therapy of myelodysplastic syndromes. Curr Opin Hematol 10: 60–67, 2003 [DOI] [PubMed] [Google Scholar]
- 13.de Witte T, Suciu S, Verhoef G, et al. : Intensive chemotherapy followed by allogeneic or autologous stem cell transplantation for patients with myelodysplastic syndromes (MDSs) and acute myeloid leukemia following MDS. Blood 98:2326–2331, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Biesma DH, van den Tweel JG, Verdonck LF: Immunosuppressive therapy for hypoplastic myelodysplastic syndrome. Cancer 79:1548–1551, 1997 [DOI] [PubMed] [Google Scholar]
- 15.Tichelli A, Gratwohl A, Wuersch A, et al. : Antilymphocyte globulin for myelodysplastic syndrome. Br J Haematol 68:139–140, 1988 [DOI] [PubMed] [Google Scholar]
- 16.Asano Y, Maeda M, Uchida N, et al. : Immunosuppressive therapy for patients with refractory anemia. Ann Hematol 80:634–638, 2001 [DOI] [PubMed] [Google Scholar]
- 17.Barrett AJ, Molldrem JJ, Saunthrajarian Y, et al. : Prolonged transfusion independence and disease stability in patients with myelodysplastic syndrome (MDS) responding to antithymocyte glogulin (ATG). Blood 10:713a, 1998. (suppl, abstr) [Google Scholar]
- 18.Molldrem J, Caples M, Mavroudis D, et al. : Antithymocyte globulin (ATG) abrogates cytopenias in patients with myelodysplastic syndrome. Br J Haematol 99:699–705, 1997 [DOI] [PubMed] [Google Scholar]
- 19.Steensma DP, Dispenzieri A, Moore SB, et al. : Antithymocyte globulin has limited efficacy and substantial toxicity in unselected anemic patients with myelodysplastic syndrome. Blood 101:2156–2158, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Killick SB, Mufti G, Cavenagh JD, et al. : A pilot study of antithymocyte globulin (ATG) in the treatment of patients with ‘low-risk’ myelodysplasia. Br J Haematol 120:679–684, 2003 [DOI] [PubMed] [Google Scholar]
- 21.Yazji S, Giles FJ, Tsimberidou AM, et al. : Antithymocyte globulin (ATG)-based therapy in patients with myelodysplastic syndromes. Leukemia 17:2101–2106, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Aivado M, Rong A, Stadler M, et al. : Favourable response to antithymocyte or antilymphocyte globulin in low-risk myelodysplastic syndrome patients with a ‘non-clonal’ pattern of X-chromosome inactivation in bone marrow cells. Eur J Haematol 68:210–216, 2002 [DOI] [PubMed] [Google Scholar]
- 23.Molldrem JJ, Leifer E, Bahceci E, et al. : Antithymocyte globulin for treatment of the bone marrow failure associated with myelodysplastic syndromes. Ann Intern Med 137:156–163, 2002 [DOI] [PubMed] [Google Scholar]
- 24.Saunthararajah Y, Nakamura R, Wesley R, et al. : A simple method to predict response to immunosuppressive therapy in patients with myelodysplastic syndrome. Blood 102:3025–3027, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Bennett JM, Catovsky D, Daniel MT, et al. : Proposals for the classification of chronic (mature) B and T lymphoid leukaemias: French-American-British (FAB) Cooperative Group. J Clin Pathol 42:567–584, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheson BD, Bennett JM, Kantarjian H, et al. : Report of an international working group to standardize response criteria for myelodysplastic syndromes. Blood 96:3671–3674, 2000 [PubMed] [Google Scholar]
- 27.Cheson BD, Greenberg PL, Bennett JM, et al. : Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood 108: 419–425, 2006 [DOI] [PubMed] [Google Scholar]
- 28.Therneau TM, Li H: Computing the Cox model for case cohort designs. Lifetime Data Anal 5:99–112, 1999 [DOI] [PubMed] [Google Scholar]
- 29.Fisher LD, Lin DY: Time-dependent covariates in the Cox proportional-hazards regression model. Annu Rev Public Health 20:145–157, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Cazzola M, Malcovati L: Myelodysplastic syndromes: Coping with ineffective hematopoiesis. N Engl J Med 352:536–538, 2005 [DOI] [PubMed] [Google Scholar]
- 31.Dunn DE, Tanawattanacharoen P, Boccuni P, et al. : Paroxysmal nocturnal hemoglobinuria cells in patients with bone marrow failure syndromes. Ann Intern Med 131:401–408, 1999 [DOI] [PubMed] [Google Scholar]
- 32.Lim ZY, Killick S, Germing U, et al. : Low IPSS score and bone marrow hypocellularity in MDS patients predict hematological responses to antithymocyte globulin. Leukemia 21:1436–1441, 2007 [DOI] [PubMed] [Google Scholar]
- 33.Yue G, Hao S, Fadare O, et al. : Hypocellularity in myelodysplastic syndrome is an independent factor which predicts a favorable outcome. Leuk Res 32:553–558, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Stadler M, Germing U, Kliche KO, et al. : A prospective, randomised, phase II study of horse antithymocyte globulin vs rabbit antithymocyte globulin as immune-modulating therapy in patients with low-risk myelodysplastic syndromes. Leukemia 18: 460–465, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Broliden PA, Dahl IM, Hast R, et al. : Antithymocyte globulin and cyclosporine A as combination therapy for low-risk non-sideroblastic myelodysplastic syndromes. Haematologica 91:667–670, 2006 [PubMed] [Google Scholar]