Abstract
During differentiation in the human, the gametocytes of the malaria parasite Plasmodium falciparum display a remarkable number of adhesive proteins on their plasma membrane. These include the PfCCp family of six secreted proteins that assemble to multimeric protein complexes (MPCs) within the gametocyte parasitophorous vacuole. We now show that the PfCCp-based MPCs are linked to the gametocyte plasma membrane via interactions with Pfs230, a binding-partner of the GPI-anchored Pfs48/45. Upon onset of gametogenesis, which takes place after gametocyte uptake by bloodfeeding mosquitoes, GPI-anchored Pfs25 joins the MPC, providing an additional link of its components to the plasma membrane. Gametogenesis also initiates cleavage of Pfs230 at its Nterminal site, resulting in its increased interaction with the MPC. Either lack of Pfs230 or impaired Pfs230 processing causes proteolysis of the PfCCp proteins and release from the MPC. Our data point to MPC reassembly as a crucial step for sexual reproduction.
Keywords: multi-protein complex, Plasmodium falciparum, PfCCp protein, Pfs230, gametogenesis, transmission
Multi-protein complexes (MPCs) are groups of two or more polypeptide chains linked by noncovalent protein-protein interactions. MPCs play important roles in regulating the activity and stability of polypeptides and are crucial for most cell biological processes. Moreover, protein complexes function in establishing cell-cell contacts and thus are of importance for interactions between pathogens and host cells. For intracellular pathogens such as malaria parasites, recognition, adhesion and invasion of host cells are essential steps during infection and are often mediated by protein-protein interactions. MPCs can also be found in the non-invasive, amotile gametocyte stages of Plasmodium falciparum (reviewed in [1]).
Gametocytes are dormant sexual precursor cells of the malaria pathogen, which differentiate within human red blood cells. Once taken up by a mosquito during the blood meal, the gametocytes become activated in the mosquito midgut by environmental stimuli. Within minutes upon activation, they exit the enveloping erythrocyte and transform into male and female gametes to initiate sexual reproduction and to continue the parasite’s life-cycle in the insect vector (reviewed in [2]).
A remarkable feature of sexual reproduction of P. falciparum is the co-ordinated expression of numerous sexual stage proteins with adhesive properties. One subset of these proteins includes the cysteine motif-rich proteins Pfs230 and Pfs48/45 and the six PfCCp proteins, all of which are expressed during gametocyte maturation and which associate with the plasma membrane of the parasite within the parasitophorous vacuole. Some of these proteins are subsequently present on the gamete surface, but its expression usually ceases latest during zygote formation.
The PfCCp proteins are a family of six secreted proteins with multiple adhesive modules, including a Limulus Coagulation factor C-like (LCCL) adhesion domain shared by five of the six proteins (thus also named LCCL-domain proteins). Orthologs of the PfCCp proteins were identified in other Plasmodium species as well as other Apicomplexan parasites, indicating an evolutionary conserved function across the apicomplexan clade (e.g. [3–6]). Previous studies demonstrated that the PfCCp proteins are expressed during gametocytogenesis and assemble to MPCs, which are localized in the lumen of the parasitophorous vacuole associated with the parasite plasma membrane (PPM) [7–9]. The MPCs remain associated with the surface of the developing macrogamete for a few hours following gametogenesis. The detailed mechanisms by which the secreted PfCCp proteins are linked to the PPM, however, remained unclear.
It was previously shown that Pfs230 is linked to the PPM of gametocytes and gametes via a molecular interaction with the GPI-anchored Pfs48/45 [10, 11]. Further, we previously identified an interaction of PfCCp4 with Pfs230 [8]. Based on these data, we aimed to investigate the role of Pfs230 as molecular coupler between the PfCCp-based MPCs and the gametocyte PPM to determine the fate of the MPCs following gametogenesis.
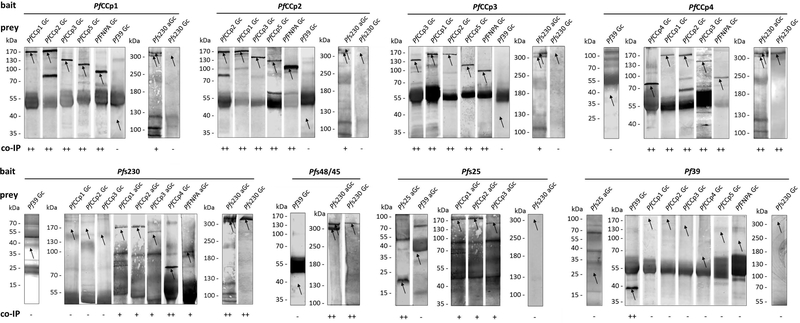
We performed co-immunoprecipitation assays on lysates of mature non-activated gametocytes and of gametocytes at 30 min post-activation in vitro to detect potential MPC interaction partners by Western blotting (for domain structures of MPC proteins see Fig. S1). When we used antisera directed against any of the respective PfCCp proteins as bait, we were able to precipitate the other PfCCp proteins from gametocyte lysate (Figs. 1 and S2), confirming the previously reported molecular interactions between the PfCCp proteins [9]. For PfCCp1-PfCCp3, PfCCp5 and PfFNPA the full length proteins were immunoprecipitated, for PfCCp4 a previously described processed form running at ~75 kDa was pulled down. Also a previously described additional processed PfCCp2 protein of ~80 kDa was detectable [8, 9]. While it was not possible to precipitate Pfs230 from lysate of non-activated gametocytes when using the anti-PfCCp1-PfCCp3 or anti-PfFNPA antisera, we detected Pfs230 via Western blotting when lysates of activated gametocytes were used in the assays (Figs. 1 and S2) (for experimental details, see the materials and method section of the supplemental data). Noteworthy, Pfs230 is proteolytically processed upon gametocyte activation, resulting in a protein of ~300 kDa bound to the gamete surface [12]. The PfCCp-specific precipitates contained both the full-length and the processed forms of Pfs230 (Fig. 1). When antisera directed against PfCCp4 were used as bait, it was possible to precipitate full-length Pfs230 from the non-activated gametocyte lysate, while full-length and processed Pfs230 were present in the precipitates from lysates of activated gametocytes (Fig. 1). Vice versa, we detected the PfCCp proteins in lysates of activated gametocytes, when antisera against Pfs230 were used as bait in the assays. With the exception of PfCCp4, however, the PfCCp proteins were not detected in Pfs230specific precipitates of lysates of non-activated gametocytes (Fig. 1). We confirmed that Pfs230 binds to Pfs48/45. When antibodies against Pfs48/45 were used as bait in the assays, full-length Pfs230 was detected in the precipitate of lysate from non-activated gametocytes and both Pfs230 forms were detected in the precipitate from lysate of activated gametocytes (Fig. 1).
Figure 1: Protein-protein interactions between MPC components.
Lysates of WT strain NF54 mature non-activated gametocytes (Gc) and activated (aGc) gametocytes (30 minpost activation) were subjected to co-immunoprecipitation assay, using mouse or rabbit antisera against PfCCp1-PfCCp4, Pfs230, Pfs48/45 and Pfs25 (bait), followed by Western blot analysis using the same antisera as well as mouse antisera against PfCCp5 or PfFNPA to detect the precipitated proteins (prey). Bands of immunoprecipitated proteins migrated at the expected molecular weights of 185 kDa (PfCCp1, PfCCp2), 143 kDa (PfCCp3), 180 kDa (PfCCp4), 119 kDa (PfCCp5), 100 kDa (PfFNPA), 360 and 300 kDa (Pfs230 unprocessed versus processed). PfCCp4 migrated in a processed form of 75 kDa and an additional processed form of PfCCp2 migrated at 80 kDa. Mouse antisera directed against Pf39 (39 kDa) were used as a negative control for bait and prey in the assays. The intensities of the precipitated protein bands are indicated (++, strong; +, regular; −, negative). Smeared protein bands, migrating at ~ 55 and 20 kDa, resemble the heavy and light chains of the antibody used for precipitation.
Female gametocytes are known to express the GPI-anchored protein Pfs25 in vesicular structures, from which the protein relocates to the PPM during gametogenesis [8, 9]. After demonstrating that the PfCCp-based MPC components interact with Pfs230 upon gametocyte activation, we aimed to evaluate if these components might also interact with Pfs25. When antiserum against Pfs25 was applied in the immunoprecipitation assays on activated gametocyte lysates, it was possible to detect PfCCp1-PfCCp3 in subsequent Western blot analyses (Fig. 1). In all assays, antisera against the endoplasmic reticulum-associated protein Pf39 [13] were used for negative control and Pf39 was not detected in any of the precipitates (Fig 1, S2). Similarly, when antiPf39 antisera were used as bait, neither the PfCCp proteins nor Pfs230 and Pfs25 were detected in the pull-downs (Fig. 1).
To confirm the direct protein interaction between Pfs230 and selected PfCCps, we then performed co-elution binding assays. The recombinantly expressed MBP-tagged region C of Pfs230 (see Fig. S1) was bound to a maltose column. When the recombinantly expressed GST-tagged region of recombinant protein (rp) rp6 of PfCCp1 or rp3 of PfCCp3 were added to the columns, the proteins bound to the recombinant region C of Pfs230 and were subsequently co-eluted with the Pfs230 fragment, as detected by Western blotting using anti-GST antibodies (Fig. S3. As negative controls recombinantly expressed, GST-tagged rp1 of Pf39 or buffer alone were added to the columns and no co-eluted proteins were detected via Western blotting using anti-GST antibodies. Immunoblotting with anti-MBP antibodies confirmed that the recombinantly expressed region C of Pfs230 was eluted from the column (Fig. S3). In conclusion, the protein-protein interaction data indicated that the PfCCp-based MPC is linked to the gametocyte PPM via binding to Pfs230 and that the interaction intensifies upon gametocyte activation.
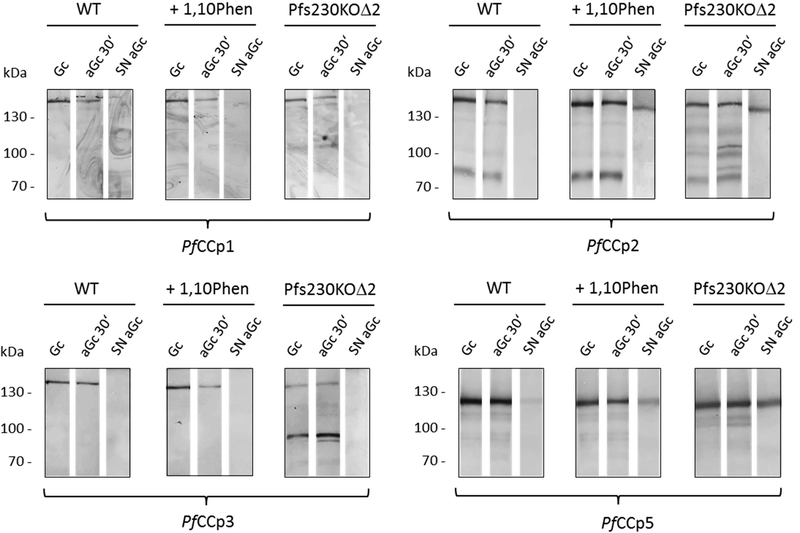
We then wished to know the fate of the PfCCp proteins in gametocytes which lack the PPMbound Pfs230 or which are chemically impaired in Pfs230 processing. For our studies, we used the Pfs230 gene-disruptant parasite line Pfs230-delta2 expressing an N-terminal 100 kDa fragment of the 360 kDa protein which is sequestered in a tubular compartment in the erythrocyte cytoplasm [14, 15], as well as NF54 wildtype (WT) parasites chemically impaired in Pfs230 processing. We investigated the presence of the PfCCp proteins in lysates of non-activated gametocytes and gametocytes at 30 min post activation as well as in the supernatants of the activated gametocyte cultures (for experimental details, see the materials and method section of the supplemental data). Samples of WT gametocytes, Pfs230-delta2 gametocytes and WT gametocytes treated with the metalloprotease inhibitor 1,10-phenanthroline, known to inhibit Pfs230 processing [12], were compared. Western blotting using antisera against PfCCp1-PfCCp3 and PfCCp5 demonstrated the presence of the full-length proteins as well as the PfCCp2-positive band of the ~80 kDa isoform of PfCCp2 in lysates of non-activated and activated gametocytes (Fig. 2A). In Pfs230-delta2 gametocyte lysate, additional protein bands of lower molecular weights were found for PfCCp1-PfCCp3 and PfCCp5, indicating proteolysis of these proteins. Furthermore, PfCCp2 and PfCCp5 were found in the supernatant of activated gametocytes of the Pfs230-delta2 line, and in the supernatant of WT gametocytes chemically impaired in Pfs230 processing. Noteworthy, the protein band of PfCCp2 detected in the supernatant was running at a slightly lower molecular weight than the one of the fulllength protein detected in gametocyte lysate (Fig. 2A). The data demonstrate that the correctly processed 300-kDa form of Pfs230 is required to retain the PfCCp-based MPC at the gamete surface and that incorrect processing or lack of WT Pfs230 results in partial proteolysis and/or release of the PfCCp proteins.
Figure 2:
A. Release of PfCCp proteins in gametocytes lacking processed Pfs230.
Lysates were produced from non-activated gametocytes (Gc) and activated (aGc) gametocytes (30 min post activation) and supernatant (SN) was collected from activated gametocytes of WT strain NF54, Pfs230-delta2 and of WT strain NF54 cultures treated with 1mM of the metalloprotease inhibitor 1,10-phenanthroline (1,10Phen) for 2 h prior to activation. Samples were subjected to Western blot analysis utilizing mouse antisera directed against PfCCp1-PfCCp3 and PfCCp5. Bands of PfCCp proteins migrated at the expected molecular weights of 185 kDa (PfCCp1, PfCCp2), 143 kDa (PfCCp3), and 119 kDa (PfCCp5); an additional processed form of PfCCp2 migrated at 80 kDa.
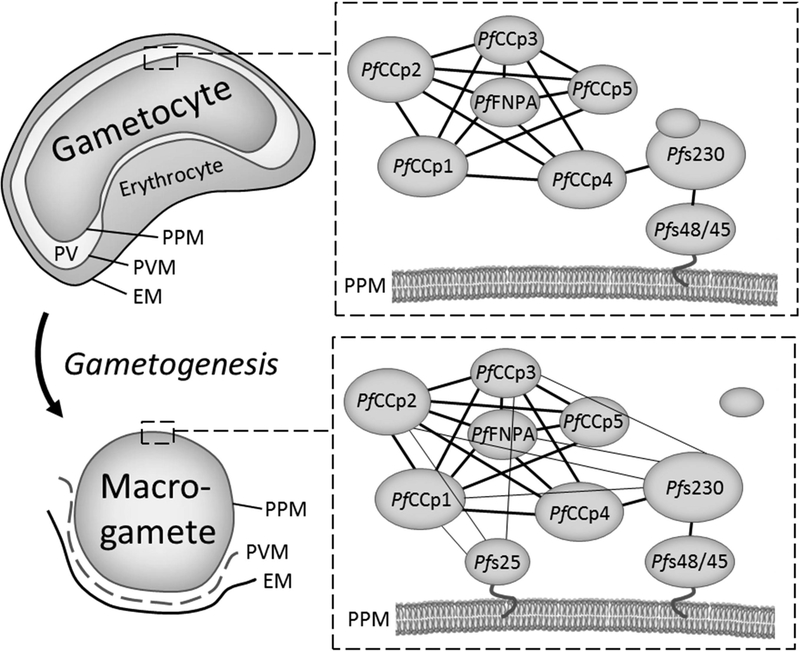
B. Schematic of MPC composition and reassembly during gametogenesis.
Thick lines represent interactions of MPC proteins in the parasitophorous vacuole of gametocytes associated to the parasite plasma membrane. Thin lines demonstrate additional protein-protein interactions of MPC components after onset of gametogenesis on macrogamete PPM. Processing of Pfs230 during gametogenesis is represented by the release of a protein fragment. EM, erythrocyte membrane; PPM, parasite plasma membrane; PV, parasitophorous vacuole; PVM, parasitophorous vacuole membrane.
In conclusion we propose that the PfCCp proteins form MPCs via intermolecular binding between the six family members, which are located in the parasitophorous vacuole lumen of gametocytes and which are linked to the gametocyte PPM via an interaction of PfCCp4 with Pfs230 that itself binds to PPM-integrated Pfs48/45. Following gametocyte activation Pfs230 becomes processed, while at the same time Pfs25 reaches the gamete surface, connected to the PPM via a GPI-anchor. These events result in an increased binding of the PfCCp-based MPC to the PPM of gametocytes, probably promoting its stability and its linkage to the parasite surface, once the parasitophorous vacuole dissolves and the gametes emerge from the enveloping red blood cell (Fig. 2B). All of the MPC components have previously been linked to parasite reproduction and further development in the mosquito midgut [4, 8, 9, 16, 17]. In view of the available data on the MPC components we hypothesize that these, due to their strong adhesive properties, might play a role during fertilization, when the mating partners have to adhere to each other prior to fusion.
Supplementary Material
Research Highlights:
Plasmodium falciparum gametocytes display multi-protein complexes (MPCs) on plasma membrane
MPCs are linked to gametocyte plasma membrane via protein Pfs230
Protein complex stability requires proteolysis of Pfs230
During gametogenesis Pfs25 integrates into MPC
Acknowledgments:
We thank Ludmilla Sologub for technical assistance. This research was funded by grants PR905/1–3, PR905/4–1 and SFB479/B8 from the Deutsche Forschungsgemeinschaft and additionally supported by MALSIG of the EU 7th framework program (to GP). NS received a fellowship from the program for female scientists of the University of Würzburg. AK was an associated member of the BioMedTec International Graduate School of Science “Lead structures of cell function” of the Elite Network Bavaria.
References:
- [1].Kuehn A, Simon N, Pradel G, 2010. Family members stick together: multi-protein complexes of malaria parasites. Med. Microbiol. Immunol 199, 209–26. [DOI] [PubMed] [Google Scholar]
- [2].Kuehn A, Pradel G, 2010. The coming-out of malaria gametocytes. J. Biomed. Biotechnol 2010, 976827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dessens JT, Sinden RE, Charles C, 2004. LCCL proteins of apicomplexan parasites. Trends Parasitol. 20, 99–102. [DOI] [PubMed] [Google Scholar]
- [4].Pradel G, Hayton K, Aravind L, Iyer LM, Abrahamsen MS, Bonawitz A, Mejia C, Templeton TJ, 2004. A multidomain adhesion protein family expressed in Plasmodium falciparum is essential for transmission to the mosquito. J. Exp. Med 199, 1533–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Templeton TJ, Iyer LM, Anantharaman V, Enomoto S, Abrahante JE, Subramanian GM, Hoffman SL, Abrahamsen MS, Aravind L, 2004. Comparative analysis of apicomplexa and genomic diversity in eukaryotes. Genome Res. 14, 1686–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bastos RG, Suarez CE, Laughery JM, Johnson WC, Ueti MW, Knowles DP, 2013. Differential expression of three members of the multidomain adhesion CCp family in Babesia bigemina, Babesia bovis and Theileria equi. PLoS One 8, e67765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Pradel G, Wagner C, Mejia C, Templeton TJ, 2006. Plasmodium falciparum: Co-dependent expression and co-localization of the PfCCp multi-adhesion domain proteins. Exp. Parasitol 112, 263–8. [DOI] [PubMed] [Google Scholar]
- [8].Scholz SM, Simon N, Lavazec C, Dude M-A, Templeton TJ, Pradel G, 2008. PfCCp proteins of Plasmodium falciparum: gametocyte-specific expression and role in complement-mediated inhibition of exflagellation. Int. J. Parasitol 38, 327–40. [DOI] [PubMed] [Google Scholar]
- [9].Simon N, Scholz SM, Moreira CK, Templeton TJ, Kuehn A, Dude M-A, Pradel G, 2009. Sexual stage adhesion proteins form multi-protein complexes in the malaria parasite Plasmodium falciparum. J. Biol. Chem 284, 14537–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kumar N, 1987. Target antigens of malaria transmission blocking immunity exist as a stable membrane bound complex. Parasite Immunol. 9, 321–35. [DOI] [PubMed] [Google Scholar]
- [11].Kumar N, Wizel B, 1992. Further characterization of interactions between gamete surface antigens of Plasmodium falciparum. Mol. Biochem. Parasitol 53, 113–20. [DOI] [PubMed] [Google Scholar]
- [12].Brooks SR, Williamson KC, 2000. Proteolysis of Plasmodium falciparum surface antigen, Pfs230, during gametogenesis. Mol. Biochem. Parasitol 106, 77–82. [DOI] [PubMed] [Google Scholar]
- [13].Templeton TJ, Fujioka H, Aikawa M, Parker KC, Kaslow DC, 1997. Plasmodium falciparum Pfs40, renamed Pf39, is localized to an intracellular membrane-bound compartment and is not sexual stage-specific. Mol. Biochem. Parasitol 90, 359–65. [DOI] [PubMed] [Google Scholar]
- [14].Eksi S, Stump A, Fanning SL, Shenouda MI, Fujioka H, Williamson KC, 2002. Targeting and sequestration of truncated Pfs230 in an intraerythrocytic compartment during Plasmodium falciparum gametocytogenesis. Mol. Microbiol 44, 1507–16. [DOI] [PubMed] [Google Scholar]
- [15].Eksi S, Czesny B, van Gemert G-J, Sauerwein RW, Eling W, Williamson KC, 2006. Malaria transmission-blocking antigen, Pfs230, mediates human red blood cell binding to exflagellating male parasites and oocyst production. Mol. Microbiol 61, 991–8. [DOI] [PubMed] [Google Scholar]
- [16].Van Dijk MR, Janse CJ, Thompson J, Waters a P., Braks J. a, Dodemont HJ, Stunnenberg HG, van Gemert GJ, Sauerwein RW, Eling W, 2001. A central role for P48/45 in malaria parasite male gamete fertility. Cell 104, 153–64. [DOI] [PubMed] [Google Scholar]
- [17].Tomas A, Margos G, 2001. P25 and P28 proteins of the malaria ookinete surface have multiple and partially redundant functions. EMBO J. 20, 3975–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.