Abstract
Purpose
Bladder cancer is one of the top five cancers diagnosed in the U.S. with a high recurrence rate, and also one of the most expensive cancers to treat over the life-course. However, there are few observational, prospective studies of bladder cancer survivors.
Methods
The Bladder Cancer Epidemiology, Wellness, and Lifestyle Study (Be-Well Study) is a National Cancer Institute-funded, multi-center prospective cohort study of non-muscle invasive bladder cancer (NMIBC) patients (Stage Ta, T1, Tis) enrolled from the Kaiser Permanente Northern California (KPNC) and Southern California (KPSC) health care systems, with genotyping and biomarker assays performed at Roswell Park Comprehensive Cancer Center. The goal is to investigate diet and lifestyle factors in recurrence and progression of NMIBC, with genetic profiles considered, and to build a resource for future NMIBC studies.
Results
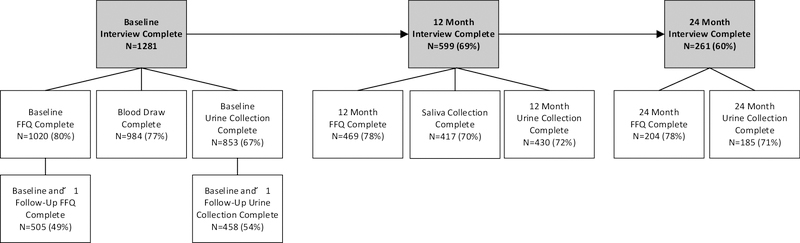
Recruitment began in February 2015. As of June 30, 2018, 1,281 patients completed the baseline interview (774 KPNC, 511 KPSC) with a recruitment rate of 54%, of whom 77% were male and 23% female, and 80% White, 6% Black, 8% Hispanic, 5% Asian, and 2% other race/ethnicity. Most patients were diagnosed with Ta (69%) or T1 (27%) tumors. Urine and blood specimens were collected from 67% and 73% of consented patients at baseline, respectively. To date, 599 and 261 patients have completed the 12- and 24-month follow-up questionnaires, respectively, with additional urine and saliva collection.
Conclusions
The Be-Well Study will be able to answer novel questions related to diet, other lifestyle, and genetic factors and their relationship to recurrence and progression among early-stage bladder cancer patients.
Keywords: non-muscle invasive bladder cancer, urothelial carcinoma, lifestyle and genetic factors, recurrence, prospective cohort study
Introduction
In the U.S., bladder cancer is among the top five most common cancers, with an expected 81,190 newly diagnosed cases in 2018 and 17,240 deaths (1, 2). It is also the most expensive cancer, per patient, from diagnosis to death (3), due in part to its high rate of recurrence (4, 5). Most cases (75%) are diagnosed as non-muscle invasive bladder cancer (NMIBC), yet 70% of NMIBC typically recurs and 25% progresses to muscle-invasive disease (6, 7). Established risk factors for bladder cancer encompass three general areas: genetic and molecular abnormalities (specific oncogenes and tumor suppressor genes), chemical or environmental exposures (primarily cigarette smoke), and chronic irritation (such as pelvic irradiation or indwelling catheters) (6, 7).
However, research on risk factors that might influence bladder cancer prognosis and survival of NMIBC patients has been limited, especially in the context of a prospective study with sufficient sample size and follow-up time. Importantly, there is a lack of studies examining the impact on prognosis of patient-specific factors including lifestyle, genetics, and quality of life, and clinical care-related factors including treatment, surveillance, and cost (8).
In 2015, the Bladder Cancer Epidemiology, Wellness, and Lifestyle Study (Be-Well Study) of newly diagnosed NMIBC patients was launched at Kaiser Permanente Northern California (KPNC) and Southern California (KPSC), two of the largest integrated health care systems in the U.S. Funded by the National Cancer Institute, the Be-Well Study is a prospective cohort study with 1,281 patients enrolled to date (projected 1,600 total patients), with a primary focus to examine diet and lifestyle factors and prognosis. Of particular interest is the consumption of cruciferous vegetables (CV) with their unique phytochemical isothiocyanate (ITC) exposure in relation to bladder cancer prognosis, and also considering the modifying effect of polymorphisms of ITC-metabolizing genes and interactions with treatment. Previously, in a small retrospective study of 239 NMIBC patients, we found that consumption of raw broccoli was associated with improved survival (9). Based on our strong preclinical data, the hypothesis of Be-Well is that high intake of dietary ITCs may prevent disease recurrence and progression in NMIBC patients (10–14).
In this paper, we describe the design of the Be-Well Study and characteristics of the cohort enrolled to date, as well as discuss possible future studies that can build upon this cohort.
Materials and Methods
Study Design
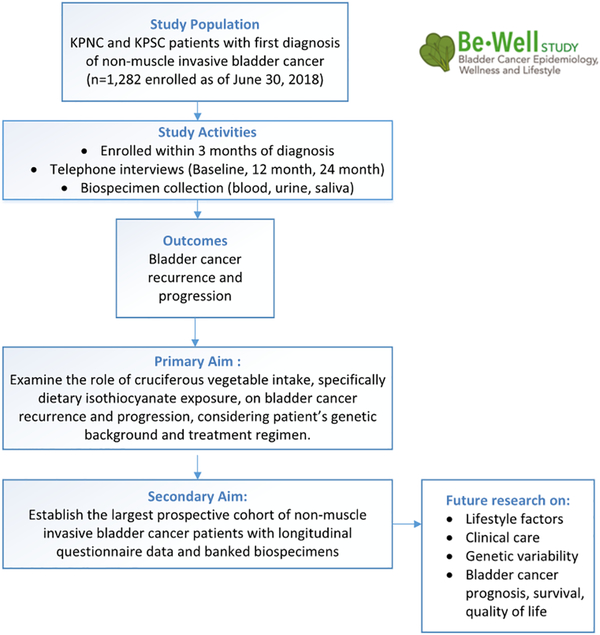
The Be-Well Study is a prospective cohort study of NMIBC patients, examining the role of nutritional, lifestyle, and genetic factors in bladder cancer treatment and outcomes (R01CA172855; Figure 1) (15). Patients are recruited from KPNC and KPSC, and data are collected at three timepoints post-diagnosis: baseline (mean time 2.9 months), 12 months, and 24 months. This includes lifestyle and nutritional factors via patient telephone interview, along with confirmed recurrence and progression status via medical record abstraction at 24 months post-diagnosis, given that the majority of recurrences occur within 24 months of diagnosis (6, 7). In addition, blood, urine, and saliva are collected to provide accompanying genetic and biomarker data and are stored and analyzed at Roswell Park Comprehensive Cancer Center (Roswell Park). Human subjects approval was obtained from KPNC, KPSC, and Roswell Park Institutional Review Boards, and all enrolled patients provided informed consent for biospecimen collection.
Figure 1.
Overview of Bladder Cancer Epidemiology, Wellness, and Lifestyle Study (Be-Well Study), funded by the National Cancer Institute, R01CA172855
Clinical data on patients including diagnosis, treatment, and follow-up surveillance are available from KPNC and KPSC electronic data sources connected to the electronic health record (EHR) at each site. In addition, both sites have Surveillance, Epidemiology, and End Results-affiliated cancer registries with information on tumor characteristics at diagnosis.
Patient Identification and Enrollment
The eligibility criteria at the time of patient identification are: at least 21 years of age, first diagnosis of NMIBC (stage Ta, Tis, T1), current KP member, alive, and not in hospice care. Exclusion criteria include: previous bladder cancer, other cancers not including non-melanoma skin cancer diagnosed within one year of bladder cancer, and concurrent cancer diagnosis.
At KPNC, newly-diagnosed NMIBC patients are first identified from automated daily scans of electronic pathology records using pre-selected Systematized Nomenclature of Medicine--Clinical Terms (SNOMED codes) indicative of malignant bladder cancer. At KPSC, newly-diagnosed patients are first identified from electronic extraction of pathology reports using text string searches for “bladder” or “urothelial” on a semi-monthly schedule. After this initial identification of patients at both sites, study eligibility and evidence that patients have been notified of his/her diagnosis are assessed from medical record review. Next the physician of record (the urologist) for each patient is contacted to ensure that there is no reason to not contact a patient for study recruitment. Recruitment materials are then mailed to the patient, followed by the first recruitment call to confirm eligibility and schedule the baseline telephone interview. The consent form for biospecimen collection is mailed to the patient once the baseline telephone interview is scheduled. Enrollment into the study is signified by completion of the baseline interview.
Baseline and Follow-up Data Collection
The baseline interview consists of the main questionnaire and a food frequency questionnaire (FFQ), which is administered to the participant primarily via computer-assisted telephone interview (CATI). Web- and paper-versions of the FFQ are also available at the patient’s request. The questionnaire domains include: Bladder Cancer Diagnosis, Medical History, Current Bladder and Bowel Function (16), Current Quality of Life (PROMIS), Current Residence and Lifestyle Factors, Smoking History, Environmental and Occupational Exposure History, and Sociodemographic characteristics. The FFQ includes the Block Brief FFQ (70 items) (17) augmented with 22 items specific to cruciferous vegetables (18).
The 12-month and 24-month follow-up intervals consist of the main questionnaire and the FFQ. The main questionnaire includes updates on lifestyle factors, quality of life, cost burden, self-report of bladder cancer recurrence and progression status, and diagnosis of other conditions.
Biospecimen Collection, Processing, and Storage
After completing the baseline telephone interview and after the written consent form is received, the participant is instructed to go to a local KP lab to provide a research blood sample. We collect 1 or 2 serum separator tubes (SSTs) (6.0–8.5 ml) and 1 or 2 EDTA tubes (5.0–6.0 ml), enabling storage of serum, plasma, and buffy coat. The baseline blood is shipped, processed into aliquots, and stored at the national KP Biorepository in Berkeley, CA. The participant also receives a urine kit in the mail with instructions to collect three consecutive days of first void urine, for a total volume of 10 ml. The urine collection device is a specialized sponge to absorb the urine for ease of collection and shipment, along with an accompanying sponge holder for storage. The participant then ships the baseline urine to Roswell Park for processing and storage. Similarly, urine samples are also collected at the 12-month and 24-month follow-ups. Finally, a saliva specimen is requested (12 month follow-up only) as a back-up source of DNA for genetic analyses. The Oragene® saliva kit OG-250 (DNA Genotek Inc., Ontario, Canada) with instructions is included with the mailed urine kit to the participant.
Outcomes Ascertainment
All enrolled participants undergo chart review by a trained medical records abstractor (KPNC) or research associate (KPSC) at the 24-month follow-up. The EHR is searched to update the initial diagnosis and treatment details, as well as for ascertainment of study outcomes. The primary outcomes of interest are recurrence and progression of NMIBC. Recurrence is defined as a newly-detected bladder tumor at least 3 months after previous negative or ambiguous cystoscopy findings. Progression is defined as tumor progression by stage and/or grade into muscle-invasive disease (stage T2 or higher) or within non-muscle invasive categories. Outcomes are confirmed by review of pathology reports, and detailed information on the tumor characteristics and treatment are abstracted. Tumor information includes stage, concomitant carcinoma in situ, size, grade, multiplicity, and lymphovascular invasion. Treatment-related information includes a description of the initial treatment plan, treatment received (e.g., Bacillus Calmette-Guerin (BCG), interferon, chemotherapy such as mitomycin C, thiotepa, doxorubicin, Gemzar, Valstar, radiation therapy for other cancer diagnosed more than one year ago), start and end dates, and any reasons for treatment discontinuation. If BCG was administered, the number of cycles and dose received are also recorded. Chart review abstraction forms are entered directly in the study database.
The primary source of information on bladder cancer recurrence and progression is chart review, and the secondary source is patient self-report from the 12- and 24-month follow-up main questionnaire. Comparison of study outcomes across these two sources will be conducted to ensure complete capture of outcomes, and any positive self-report of recurrence or progression will be chart-reviewed for confirmation.
Results
Data are presented as frequency distributions for sociodemographic, clinical, and treatment variables.
Recruitment and Follow-up of Cohort
On January 21, 2015, patient recruitment officially began at both study sites. As of June 30, 2018, a total of 1,281 patients have been enrolled (770 KPNC, 511 KPSC). Figure 2 shows recruitment and enrollment numbers at baseline and 12- and 24- month follow-ups, respectively.
Figure 2.
Baseline Recruitment, Follow-up, and Data/Biospecimen Collection
Regarding non-participants, we have thus far confirmed non-participation status on 1,541 (96%) out of 1,602 non-participants where 1,103 (72%) refused, 153 (10%) were ineligible, and 285 (20%) were unreachable (Table 1). The overall Be-Well recruitment rate was 54% among those who were ultimately found to be eligible, contacted for study participation, and completed the baseline questionnaire. There were no noticeable age or gender differences between participants and non-participants at the study sites, yet at KPNC, less whites were enrolled compared to the unenrolled (74% vs. 80%, respectively), and at KPSC, more whites were enrolled compared to the unenrolled (81% vs. 64%, respectively).
Table 1.
Participation in the Be-Well Study, as of June 30, 2018
| Eligible Patientsa | 2,883 | (100%) |
| Participants | 1,281 | (44%) |
| Non-Participants | 1,602 | (56%) |
| Non-Participants | 1,602 | (100%) |
| Deceased | 7 | (<1%) |
| Doctor Refused | 32 | (2%) |
| Not Yet Contacted | 22 | (1%) |
| Status Confirmed | 1,541 | (96%) |
| Non-Participation Status Confirmed | 1,541 | (100%) |
| Unreachable | 285 | (18%) |
| Refused | 1,103 | (72%) |
| Ineligible | 153 | (10%) |
| Overall Recruitment Rateb | 54% |
After eligibility chart review
1,281 participants were enrolled out of a possible 2,384 potential participants (sum of 1,103 refusals and 1,281 enrolled) for an overall recruitment rate of 54%.
Demographic Characteristics of Cohort
Sociodemographic characteristics of the Be-Well cohort are presented in Table 2. As expected, 77% of the cohort are male and 80% are white, with an average age of bladder cancer diagnosis of 70 years. Nearly half of the cohort has a college degree or higher, and 33% has an annual household income of greater than/equal to $100,000. One-third reported ever being in an occupation involving exposure to chemical or environmental exposures, as well as two-thirds being ever or current smokers. Finally, 70% of the cohort reported being overweight or obese at diagnosis. Descriptively, characteristics are similar across KP regions, except that KPNC participants have a higher body mass index (BMI) and report higher education, higher household income, being currently employed, and being a non-smoker, compared to KPSC participants.
Table 2.
Sociodemographic characteristics of Be-Well cohort from baseline interview, as of June 30, 2018
| KPNC | KPSC | Totala | |
|---|---|---|---|
| N=770 N (%) | N=511 N (%) | N=1281 N (%) | |
| Gender | |||
| Male | 596 (77.4) | 387 (75.7) | 983 (76.7) |
| Female | 174 (22.6) | 124 (24.3) | 298 (23.3) |
| Race/Ethnicity | |||
| White | 619 (80.4) | 402 (78.7) | 1021 (79.7) |
| Black or African American | 38 (4.9) | 36 (7.1) | 74 (5.8) |
| Hispanic | 50 (6.5) | 48 (9.4) | 98 (7.6) |
| Asian | 49 (6.4) | 17 (3.3) | 66 (5.1) |
| Other | 14 (1.8) | 8 (1.6) | 22 (1.7) |
| Age Group (y) | |||
| <50 | 36 (4.7) | 22 (4.3) | 58 (4.5) |
| 50–64 | 199 (25.8) | 123 (24.1) | 322 (25.1) |
| 65–79 | 381 (49.5) | 282 (55.2) | 663 (51.8) |
| ≥80 | 154 (20.0) | 84 (16.4) | 238 (18.6) |
| Mean (SD) | 70.0 (11.2) | 69.4 (10.2) | 69.8 (10.8) |
| Marital Status | |||
| Never married | 42 (5.5) | 15 (2.9) | 57 (4.5) |
| Married or living as married | 534 (70.2) | 367 (72.0) | 901 (70.9) |
| Separated/Divorced | 105 (13.8) | 75 (14.7) | 180 (14.2) |
| Widowed | 80 (10.5) | 53 (10.4) | 133 (10.5) |
| Educational Attainment | |||
| High school or less | 127 (16.7) | 96 (19.0) | 223 (17.6) |
| Some college | 265 (34.8) | 171 (33.8) | 436 (34.4) |
| College graduate | 214 (28.1) | 129 (25.5) | 343 (27.1) |
| Post-graduate | 156 (20.5) | 110 (21.7) | 266 (21.0) |
| Household Income | |||
| <$60K | 227 (33.9) | 175 (38.5) | 402 (35.7) |
| $60K–99K | 172 (25.7) | 126 (27.7) | 298 (26.5) |
| ≥$100K | 271 (40.4) | 154 (33.8) | 425 (37.8) |
| Employment Status | |||
| Currently employed | 297 (38.6) | 183 (35.8) | 480 (37.5) |
| Overall Exposure to Chemical or Environmental Exposuresb | |||
| Ever | 523 (67.9) | 324 (63.4) | 847 (66.1) |
| Never | 247 (32.1) | 187 (36.6) | 434 (33.9) |
| Occupation Involving Chemical or Environmental Exposuresc | |||
| Ever | 265 (34.4) | 184 (36.0) | 449 (35.0) |
| Never | 505 (65.6) | 327 (64.0) | 832 (65.0) |
| Smoking History | |||
| Never smoker | 270 (35.2) | 159 (31.1) | 429 (33.5) |
| Former smoker | 446 (58.1) | 309 (60.5) | 755 (59.0) |
| Current smoker | 52 (6.8) | 43 (8.4) | 95 (7.4) |
| Smoking History in Pack- Years, Mean (SD) | |||
| Former smoker | 25.6 (23.5) | 26.4 (22.8) | 25.9 (23.2) |
| Current smoker | 29.9 (23.3) | 28.7 (20.5) | 29.4 (22.2) |
| Body Mass Index at Diagnosis | |||
| Normal weight (≤24.9) | 198 (25.7) | 136 (26.6) | 334 (26.1) |
| Overweight (25.0–29.9) | 321 (41.7) | 207 (40.5) | 528 (41.2) |
| Obese (≥30.0) | 251 (32.6) | 168 (32.9) | 419 (32.7) |
| Mean (SD) | 28.8 (5.9) | 27.8 (5.8) | 28.4 (5.8) |
Total unknown values: Marital Status n=10, Educational Attainment n=13, Household Income n=156, Smoking History n=2
Includes asbestos, chemicals/acids/solvents, coal/stone dusts, coal tar/pitch/asphalt, diesel engine exhaust, dyes, formaldehyde, gasoline exhaust, pesticides/herbicides, textile fibers/dusts, wood dust, x-rays/radioactive materials, smoke other than from cigarettes
Includes coal miner, furniture maker, hairdresser, nail salon worker, machinist, painter, printer, truck/taxi/bus driver, leather industry worker, rubber industry worker, paint industry worker, textile industry worker
Tumor Characteristics of Cohort
Clinical and treatment characteristics at initial bladder cancer diagnosis of the Be-Well cohort are presented in Table 3. Since there is a 6- to 12-month lag in data availability from KP cancer registries, we present the subset of 888 patients (69%) with complete cancer registry information. Most patients were diagnosed with Ta (69%) or T1 (27%) tumors and received an initial diagnostic transurethral resection of the bladder tumor (TURBT, 94%). About 43% were low grade Ta, followed by 26% high grade Ta, 24% high grade T1, and 3% low grade T1. Around one-third received perioperative chemotherapy (34%) and one-third received intravesical therapy (38%). About 1% of patients had a cystectomy within 6 months of the initial NMIBC diagnosis (data not shown).
Table 3.
Clinical and treatment characteristics at initial bladder cancer diagnosis of BeWell Cohort, as of June 30, 2018, among participants in the regional cancer registry
| KPNC | KPSC | Totala | |
|---|---|---|---|
| N=588 N (%) | N=300 N (%) | N=888 N (%) | |
| Stageb | |||
| Ta | 413 (70.2) | 197 (67.0) | 610 (69.2) |
| Tis | 18 (3.1) | 11 (3.7) | 29 (3.3) |
| T1 | 157 (26.7) | 86 (29.3) | 243 (27.5) |
| Gradec | |||
| Low (LG) | 265 (45.1) | 149 (50.3) | 414 (46.8) |
| High (HG) | 315 (53.6) | 137 (46.3) | 452 (51.1) |
| Other (CIS) | 8 (1.4) | 10 (3.4) | 18 (2.0) |
| Stage/Gradeb,c | |||
| LG Ta | 251 (42.7) | 127 (43.9) | 378 (43.1) |
| LG T1 | 10 (1.7) | 20 (6.9) | 30 (3.4) |
| HG Ta | 160 (27.2) | 67 (23.2) | 227 (25.9) |
| HG T1 | 146 (24.8) | 64 (22.1) | 210 (23.9) |
| Tis | 21 (3.6) | 11 (3.8) | 32 (3.6) |
| Receipt of Initial TURBTd | |||
| Yes | 583 (99.2) | 251 (83.7) | 834 (93.9) |
| No | 5 (0.8) | 49 (16.3) | 54 (6.1) |
| Receipt of Re-TURBT (within 2 months of initial diagnosis)d | |||
| Yes | 131 (22.3) | 43 (14.3) | 174 (19.6) |
| No | 457 (77.7) | 257 (85.7) | 714 (80.4) |
| Receipt of Perioperative Chemotherapyd | |||
| No | 348 (59.2) | 240 (80.0) | 588 (66.2) |
| Yes | 240 (40.8) | 60 (20.0) | 300 (33.8) |
| Receipt of Induction Intravesical Therapyd | |||
| No | 336 (57.1) | 211 (70.3) | 547 (61.6) |
| Yes | 252 (42.9) | 89 (29.7) | 341 (38.4) |
Total unknown values: Stage n=6, Grade n=4, Stage/Grade n=11
Limited to stage data from KPNC Cancer Registry for cases diagnosed as of June 30, 2017 and the KPSC Cancer Registry for cases diagnosed as of Dec 31, 2016. Missing data were supplemented with chart review. If multiple bladder tumors for an individual were in the registry, the record with diagnosis date closest to the qualifying pathology date was selected.
From eligibility check chart review
From KP electronic data sources
Discussion
The Be-Well Study is one of the first prospective cohort studies of lifestyle factors, specifically diet, and outcomes among recently-diagnosed NMIBC patients. This study will provide contemporaneous data on the impact of lifestyle, genetics, and treatment on bladder cancer recurrence, progression, and other outcomes. The integrated health care settings of KPNC and KPSC provide an unparalleled resource for epidemiologic studies of this kind, enabling rapid case ascertainment through electronic pathology records, efficient follow-up through member identification systems, and linkage to other clinical data recorded in the EHR. Recruitment is expected to continue through at least spring 2019, and the final projected cohort size will be about 1,600 patients.
Strengths of the Be-Well Study include being one of the largest prospective cohort studies to date of NMIBC patients, and the only such study we know of to collect longitudinal questionnaire data on lifestyle, genetics, treatment, bladder function, and quality of life, as well as biospecimens of blood, urine, and saliva. Importantly, detailed clinical data on the patient and the treating providers are available from the EHR. Given this cohort is derived from the KPNC and KPSC integrated health care systems, it will most likely be representative of NMIBC patients being treated in community settings. While KP membership is limited to those with health insurance through KP, KP covers approximately 30% of the population in the KPNC service region and 25% in the KPSC service region, which represents about 23% of the total California population. Some potential constraints include lower participation rates at the 12-month (69%) and 24-month (60%) follow-ups out of the number of enrolled patients at baseline, and limited data on occupational exposures.
As we complete enrollment of cohort members and continue follow-up for updated information and documentation of outcomes, we will conduct descriptive and association analyses on cruciferous vegetable intake, urinary ITC levels, and ITC-metabolizing genes with risk of recurrence or progression. Findings from this study could be translated into behavioral strategies after diagnosis focused on diet if we demonstrate that cruciferous vegetable and ITC intake are associated with lower risk of recurrence and progression. Furthermore, given an ITC-rich cruciferous vegetable extract has been developed and is commercially available, next steps could include a cancer control clinical trial among high risk patients.
The Be-Well Study is not only an opportunity to examine provocative questions on the role of cruciferous vegetables and ITCs on bladder cancer prognosis, but also establishes a rich epidemiologic resource for future important studies of bladder cancer survivors. Potential topics include health-related quality of life, occupational exposures, smoking, and clinical surveillance and care, as well as biospecimen studies involving urine and blood metabolomics and biomarker discovery.
Conclusion and Future Steps
Bladder cancer is an understudied disease, disproportionate to its high incidence and recurrence, high cost of clinical management, and significant impact on quality of life. The Be-Well Study, built on two of the largest U.S. integrated health care systems with detailed information on clinical factors including treatments received, is the first and largest to investigate the role of diet in bladder cancer prognosis, including consideration of genetic profiles. This study is poised to inform new strategies for improvement of bladder cancer prognosis and contribute to future research to benefit NMIBC survivors.
Acknowledgements
We thank all Be-Well Study staff, and most importantly, the study participants. The Be-Well Study is supported by the National Cancer Institute (R01 CA172855). The contents are solely the responsibility of the authors and do not necessarily represent the official views of the funding agency.
Footnotes
Conflict of Interest Disclosure Statement: The authors declare no potential conflicts of interest.
References
- 1.American Cancer Society, Cancer Facts & Figures 2016. 2016, American Cancer Society: Atlanta. [Google Scholar]
- 2. https://seer.cancer.gov/statfacts/html/urinb.html.
- 3.Botteman MF, Pashos CL, Redaelli A, Laskin B, and Hauser R, The health economics of bladder cancer: a comprehensive review of the published literature. Pharmacoeconomics, 2003. 21(18): p. 1315–30. [DOI] [PubMed] [Google Scholar]
- 4.Avritscher EB, Cooksley CD, Grossman HB, Sabichi AL, Hamblin L, Dinney CP, and Elting LS, Clinical model of lifetime cost of treating bladder cancer and associated complications. Urology, 2006. 68(3): p. 549–53. [DOI] [PubMed] [Google Scholar]
- 5.Barocas DA, Globe DR, Colayco DC, Onyenwenyi A, Bruno AS, Bramley TJ, and Spear RJ, Surveillance and treatment of non-muscle-invasive bladder cancer in the USA. Advances in urology, 2012. 2012: p. 421709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Josephson DY, Pasin E, and Stein JP, Superficial bladder cancer: part 1. Update on etiology, classification and natural history. Expert Rev Anticancer Ther, 2006. 6(12): p. 1723–34. [DOI] [PubMed] [Google Scholar]
- 7.Kaufman DS, Shipley WU, and Feldman AS, Bladder cancer. Lancet, 2009. 374(9685): p. 239–49. [DOI] [PubMed] [Google Scholar]
- 8.Lotan Y, Kamat AM, Porter MP, Robinson VL, Shore N, Jewett M, Schelhammer PF, deVere White R, Quale D, and Lee CT, Key concerns about the current state of bladder cancer: a position paper from the Bladder Cancer Think Tank, the Bladder Cancer Advocacy Network, and the Society of Urologic Oncology. Cancer, 2009. 115(18): p. 4096–103. [DOI] [PubMed] [Google Scholar]
- 9.Tang L, Zirpoli GR, Guru K, Moysich KB, Zhang Y, Ambrosone CB, and McCann SE, Intake of cruciferous vegetables modifies bladder cancer survival. Cancer Epidemiol Biomarkers Prev, 2010. 19(7): p. 1806–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang L and Zhang Y, Isothiocyanates in the chemoprevention of bladder cancer. Curr Drug Metab, 2004. 5(2): p. 193–201. [DOI] [PubMed] [Google Scholar]
- 11.Tang L, Zirpoli GR, Guru K, Moysich KB, Zhang Y, Ambrosone CB, and McCann SE, Consumption of raw cruciferous vegetables is inversely associated with bladder cancer risk. Cancer Epidemiol Biomarkers Prev, 2008. 17(4): p. 93844. [DOI] [PubMed] [Google Scholar]
- 12.Bhattacharya A, Tang L, Li Y, Geng F, Paonessa JD, Chen SC, Wong MK, and Zhang Y, Inhibition of bladder cancer development by allyl isothiocyanate. Carcinogenesis, 2010. 31(2): p. 281–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Munday R, Mhawech-Fauceglia P, Munday CM, Paonessa JD, Tang L, Munday JS, Lister C, Wilson P, Fahey JW, Davis W, and Zhang Y, Inhibition of urinary bladder carcinogenesis by broccoli sprouts. Cancer Res, 2008. 68(5): p. 1593–600. [DOI] [PubMed] [Google Scholar]
- 14.Tang L and Zhang Y, Dietary isothiocyanates inhibit the growth of human bladder carcinoma cells. J Nutr, 2004. 134(8): p. 2004–10. [DOI] [PubMed] [Google Scholar]
- 15.Kwan ML, Garren B, Nielsen ME, and Tang L, Lifestyle and nutritional modifiable factors in the prevention and treatment of bladder cancer. Urol Oncol, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilbert SM, Wood DP, Dunn RL, Weizer AZ, Lee CT, Montie JE, and Wei JT, Measuring health-related quality of life outcomes in bladder cancer patients using the Bladder Cancer Index (BCI). Cancer, 2007. 109(9): p. 1756–62. [DOI] [PubMed] [Google Scholar]
- 17. http://nutritionquest.com/.
- 18.Thomson CA, Newton TR, Graver EJ, Jackson KA, Reid PM, Hartz VL, Cussler EC, and Hakim IA, Cruciferous vegetable intake questionnaire improves cruciferous vegetable intake estimates. J Am Diet Assoc, 2007. 107(4): p. 631–43. [DOI] [PubMed] [Google Scholar]