Abstract
Since I started doing scientific research, I've been fascinated by the interplay of protein structure and dynamics and how they together mediate protein function. A particular area of interest has been in understanding the mechanistic basis of how lipid-signaling enzymes function on membrane surfaces. In this award lecture article, I will describe my laboratory's studies on the structure and dynamics of lipid-signaling enzymes on membrane surfaces. This is important, as many lipid-signaling enzymes are regulated through dynamic regulatory mechanisms that control their enzymatic activity. This article will discuss my continued enthusiasm in using a synergistic application of hydrogen–deuterium exchange MS (HDX–MS) with other structural biology techniques to probe the mechanistic basis for how membrane-localized signaling enzymes are regulated and how these approaches can be used to understand how they are misregulated in disease. I will discuss specific examples of how we have used HDX–MS to study phosphoinositide kinases and the protein kinase Akt. An important focus will be on a description of how HDX–MS can be used as a powerful tool to optimize the design of constructs for X-ray crystallography and EM. The use of a diverse toolbox of biophysical methods has revealed novel insight into the complex and varied regulatory networks that control the function of lipid-signaling enzymes and enabled unique insight into the mechanics of membrane recruitment.
Keywords: phosphoinositide, hydrogen-deuterium exchange mass spectrometry, HDX–MS, phosphatidylinositide 3-kinase (PI3K), phosphatidylinositol kinase, Akt PKB, protein dynamics, PI3K, PI4K, PI3K/Akt, Akt, lipid signaling
Introduction
Essential to many signaling processes is the recruitment of protein-signaling machinery to lipid membranes. This plays critical roles in regulating membrane trafficking, cellular signaling, and metabolism. My interests throughout my entire scientific career have been focused on understanding the mechanisms by which this recruitment is regulated and utilizing and developing biophysical tools that allow us to study this process. The fundamental driver for this research is that many of these lipid-signaling systems are dysregulated in disease. My laboratory's recent efforts have particularly focused on defining the regulation of protein and lipid kinases that work on cellular membranes, as well as the enzymes that regulate the Ras superfamily of GTPases. Our interest has been in defining the biophysical mechanisms that regulate these enzymes and how they can be dysregulated through mutations that mediate human disease.
Eukaryotic cells are compartmentalized into numerous intracellular membranous organelles, with a highly developed system that controls membrane trafficking between organelles. Signaling lipids, including sphingholipids and phosphoinositides, play a key role in mediating how signals are transduced from these organelles as well as how membranes traffic throughout the cell. The Ras superfamily of GTPases, which are lipidated membrane proteins, also participate in mediating signal transduction and membrane trafficking. The enzymes that regulate the formation of lipid signals and control the activation state of monomeric GTPases function primarily on membrane surfaces, and they must be properly recruited and activated in the correct membranous compartments. Dysregulation of lipid-signaling enzymes through either increased or decreased enzymatic activity is a frequent driver of human diseases, including cancer, immune diseases, inflammation, developmental disorders, and pathogen infection (1–3). Many lipid-signaling enzymes can be controlled by multiple levels of regulation, including post-translational modifications, protein-binding partners, and allosteric conformational changes. This is true for many lipid kinases, lipid-regulated protein kinases, and regulatory enzymes of Ras superfamily GTPases.
The goal of this article is to discuss a snapshot of my laboratory's studies on the molecular basis of regulation of lipid-signaling enzymes using a combination of both HDX2–MS and X-ray crystallography. The focus is on examining how the study of both protein structure and dynamics has allowed fundamental insight into the regulation of lipid signaling and the molecular basis for how disease-linked mutations cause disease. This work has revealed novel unexpected mechanisms of activation by disease-linked mutations in the phosphoinositide 3-kinase (PI3K) pathway and defined the molecular basis for how viral pathogens can subvert phosphoinositide signaling. The enzyme classes that will be highlighted in this article are the phosphoinositide kinases and the protein kinase Akt.
How to study protein dynamics on membranes?
Probing the structure and dynamics of peripheral membrane proteins and their interaction with membranes remains an extremely challenging biophysical problem. The study of integral membrane proteins using X-ray crystallography and cryo-EM using a combination of lipid cubic phase crystallography (4) and nanodisc-mediated biochemical reconstitution (5) has provided fundamental molecular insight into a number of membrane embedded signaling systems. However, structural studies of membrane interaction for peripheral membrane proteins that only transiently associate with membranes provide additional technical challenges. The proper recruitment to specific membrane compartments underlies the regulation and activation of many peripheral membrane proteins. These include enzymes that directly act on lipid substrate embedded in membranes (i.e. lipid kinases/phosphatases, phospholipases, etc.) or proteins that are regulated through interactions with lipid signals that mediate localization and activation (i.e. protein kinases (Akt, BTK, PKC), and Ras superfamily GTPase regulatory GEF and GAP proteins, etc.). Many of these enzymes are major players in human disease, exemplified by the class I PI3Ks, with activating mutations in the PIK3CA gene encoding the class I PI3K p110α catalytic subunit being one of the most frequently mutated genes in all of human cancer. There are also numerous mutations in class I PI3Ks that cause immune deficiencies and developmental disorders (6–11). Many of these mutations alter the association of this protein with lipid membranes, and therefore understanding the molecular mechanism of how membrane binding regulates the activity of lipid-signaling enzymes can have direct implications for many diseases.
Upon entering my Ph.D. studies with Dr. Edward Dennis at the University of California San Diego, my main challenge was how to examine at a molecular level the interaction of lipid-signaling proteins with membranes. The approach that we decided to use was the application of hydrogen–deuterium exchange MS (HDX–MS), which probes the exchange of amide hydrogens with solvent. As amide hydrogens are involved in hydrogen bonds in secondary structure elements, the exchange of amides can give a readout of protein dynamics. Our hope was that any conformational changes that occurred upon membrane binding would be detectable using this approach, and it would be able to define the membrane-binding interface as well as any allosteric conformational changes. The enzymes we chose to study were the phospholipase A2 (PLA2) family of enzymes, which is a large family of enzymes that catalyze the hydrolysis of the acyl bond at the sn-2-position of phospholipids. The PLA2 enzymes contain numerous isoforms, with diverse regulatory mechanisms, and provided an exciting testing group for our initial HDX–MS experiments. This research on the sPLA2, iPLA2, and cPLA2 isoforms of PLA2 (12–20), carried out in collaboration with a postdoctoral colleague, Dr. Howard Hsu, enabled novel insight into how these enzymes interact with membranes. This work gave me the first insight into the potential application of this approach to study the interaction of lipid-signaling enzymes with membranes.
HDX–MS
One of the main focuses of this article is on the use of HDX–MS as a tool to probe protein dynamics and its powerful use as a method to optimize and enhance other structural biology approaches. However, due to space limitations, there will not be a detailed overview of this technique, as numerous reviews have recently carefully described the fundamentals underlying the technique, and readers are advised to consult these for more details (21–26). As this is an award lecture, I feel it is critical that I acknowledge the training and guidance that I received from the late Dr. Virgil Woods during my Ph.D. career in this technique, as this was essential to my development as a scientist. Dr. Woods played a fundamental role in the development of tools and methodology to increase the throughput and accessibility of HDX, and his contributions made a sizable impact on the use of this technology (27).
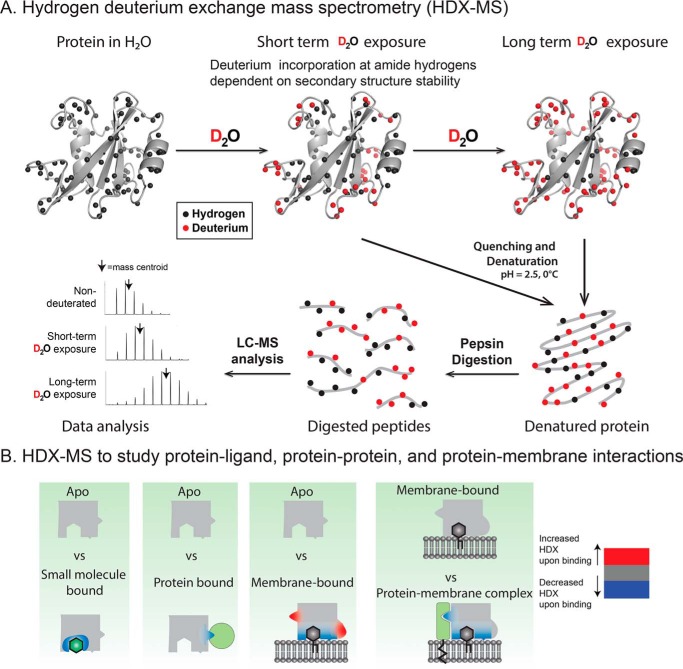
The basis of HDX–MS experiments is that amide hydrogens exchange with solvent at rates that are exquisitely sensitive to the presence of secondary structure. A basic schematic of the HDX reaction is shown in Fig. 1. There are four primary determinants of amide exchange: pH, temperature, primary sequence effects, and influence from secondary structure/solvent accessibility. The main application for HDX–MS experiments is comparison between different conditions (apo versus binding partner; i.e. membranes, proteins, ligands, etc.), and so pH, temperature and primary sequence can be controlled for, and differences reveal differences in secondary structure stability. Amide hydrogens are involved in hydrogen bonds in both α-helices and β-sheets and can only exchange when these bonds are transiently broken through protein motion. Therefore, amide hydrogen exchange provides a readout of the secondary structure dynamics. An additional bonus from an HDX experiment that is extremely useful to structural biologists is the determination of disordered regions lacking secondary structure, as this information can be used in the design of truncated constructs for other high-resolution structural approaches (28–30).
Figure 1.
Overview of HDX–MS to study lipid-signaling systems. A, schematic representation of the methodological steps in an HDX–MS experiment. Protein is exposed to deuterated solvent for a variety of different time periods, leading to exchange of solvent-accessible hydrogens. The exchange rate of amide hydrogens is determined by the involvement in secondary structure. To localize the exchange information, the protein sample is shifted to a denaturing condition that greatly decreases the exchange rate (pH ∼2.5, 0 °C), followed by proteolysis using immobilized pepsin and separation of the peptides on a reverse-phase column. The masses of the peptides are measured using a mass spectrometer. B, schematic of different conditions that can be studied using HDX–MS for lipid-signaling enzymes. This figure was adapted from Ref. 24. This research was originally published in Biochemical Society Transactions. Vadas, O., and Burke, J. E. Probing the dynamic regulation of peripheral membrane proteins using hydrogen deuterium exchange-MS (HDX-MS). Biochem. Soc. Trans. 2015; 43:773–786. ©Portland Press (United Kingdom).
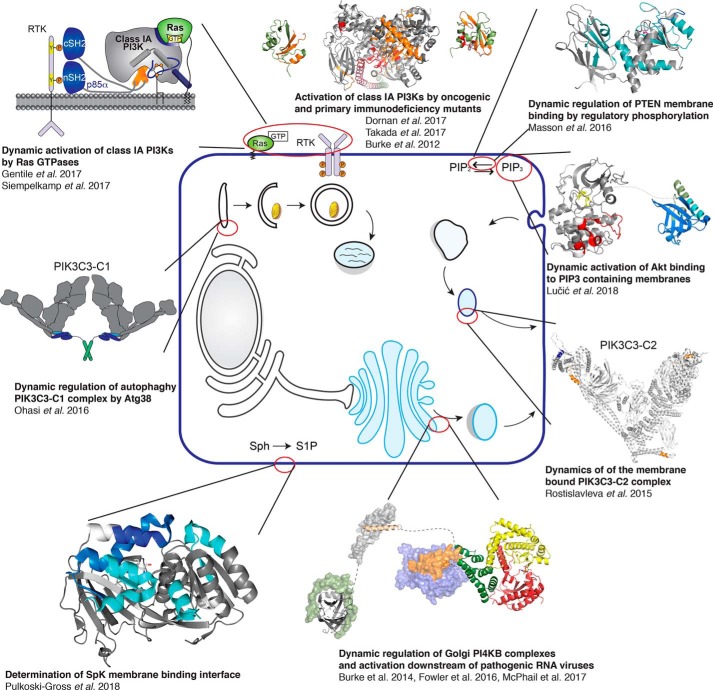
An overall schematic describing some of my laboratory's application of HDX–MS to study a variety of membrane-associated lipid-signaling enzymes is shown in Fig. 2. HDX–MS has been exceptionally useful to probe the dynamics of membrane binding (12, 17, 18, 31–39); examine how disease-linked mutations activate membrane signaling enzymes (31, 34, 38, 40, 41); and define protein–protein (33, 36, 37, 42–48), protein–ligand (49, 50), and protein–inhibitor (13, 21, 51–55) complexes. There is not sufficient room to fully describe all of these studies, with the focus of this article describing specific case studies from our work on lipid-signaling enzymes.
Figure 2.
Applications of HDX–MS to study lipid-signaling systems. Shown is a schematic of the cell with different lipid-signaling systems studied by HDX–MS highlighted. The different studies, which focus on the use of HDX–MS to study protein–protein, protein–ligand, and protein–membrane interactions, as well as looking at how disease-linked mutations cause allosteric activating conformational changes. Full details on these specific studies can be found in the References.
Case studies in the study of protein dynamics on membranes
One of the best-studied groups of lipid-signaling enzymes is the phosphoinositide kinases, comprising 19 different human genes that can generate seven distinct phosphoinositide species (3). Phosphoinositides play key roles in defining organelle identity, in mediating membrane traffic between organelles, and as lipid signals that can activate downstream signaling processes. My postdoctoral research with Dr. Roger Williams at the MRC Laboratory of Molecular Biology was focused on examining the regulation of class IA phosphoinositide 3-kinases and their interaction with lipid membranes. Class IA PI3Ks generate the lipid-signaling molecule PIP3 from the lipid substrate PIP2, and the generation of PIP3 downstream of phosphorylated receptors, G-protein–coupled receptors, and Ras superfamily GTPases plays key roles in growth, differentiation, and survival (2, 56). PI3Ks are large complicated macromolecular assemblies composed of a p110 catalytic and a p85 regulatory subunit, and they are intricately regulated through both intra- and intersubunit inhibitory interactions that control its association with lipid membranes. Studying the protein dynamics of PI3Ks on membranes using HDX–MS provided unique insight into their activation, with the details described below.
Dynamic activation of PI3Ks on membranes
PI3Ks are composed of three distinct classes (I, II, and III), with the class I PI3Ks generating the lipid signal PIP3. The class I are split into two subclasses (IA and IB) dependent on the presence of different regulatory subunits. The class IA PI3Ks are composed of three catalytic subunits (p110α, p110β, and p110δ) and five possible p85 regulatory subunits. The p85 regulatory subunits of class IA PI3Ks play three key roles in regulating PI3Ks; they stabilize the p110 catalytic subunit, they inhibit basal lipid kinase activity, and they allow for activation downstream of phosphorylated tyrosine residues present on receptor tyrosine kinases and their adaptor proteins (57). The class IA PI3Ks are master regulators of growth and metabolism, and disease-linked alterations that either increase or decrease kinase activity lead to cancer, immunodeficiencies, and developmental disorders. Extensive biophysical characterization revealed the structure of the p110 and p85 subunits; however, it did not fully explain their interaction with membranes and how activating and inactivating mutations can alter enzyme activity (57). Also, there was limited information on the molecular basis for how p110 isoforms can be differentially controlled by p85 regulatory subunits.
Intriguingly, HDX–MS studies on the different p110 catalytic isoforms revealed unexpected differences in how p85 regulatory subunits interact with and inhibit each p110 isoform. All p85 regulatory subunits contain an nSH2 and cSH2 domain that can bind phosphorylated tyrosine residues and an interspersing iSH2 coiled coil domain. Using a variety of domain deletions of p85 with each p110 catalytic subunit revealed that all p110 subunits are inhibited by the nSH2 and iSH2 domains present in p85, with the cSH2 domain only inhibiting p110β and p110δ (38, 39, 50), revealing a completely unique inhibition of p110α compared with other class IA isoforms. As only the p110α isoform frequently contains activating mutations in solid tumors, this isoform-specific difference in inhibition may explain why p110β and p110δ are not frequently mutated in cancer due to this additional inhibitory input.
The next thing we wanted to establish was the conformational changes that mediated/accompanied membrane binding in class I PI3Ks. Membrane binding of the PI3Ks was concordant with large-scale conformational changes in both the p110 and p85 regulatory subunits (38, 39). Many of these changes occurred at regions distant from both the active site and membrane-binding surface, specifically located at inter- and intrasubunit interfaces of domains within the p110 and p85 subunits. Intriguingly, HDX–MS studies of the tetrameric class III PI3Ks revealed large-scale conformational changes at intersubunit interactions upon membrane binding (36), suggesting that large scale allosteric conformational rearrangements may be involved in many membrane binding events. The oncogenic and overgrowth mutations in p110α as well as the primary immunodeficiency-causing mutations in p110δ cluster at these interfaces. HDX–MS studies of the p110α and p110δ mutations revealed that both led to conformational changes that mimicked and enhanced conformational changes associated with membrane binding, revealing the molecular mechanism of activation for the disease-linked mutants (34, 38, 40, 41). Different mutations/deletions in the p85α regulatory subunit can be both oncogenic or promote immune disorders. HDX–MS revealed that an N-terminal deletion of the iSH2 domain preferentially activates p110δ over p110α, revealing why this mutant primarily results in a disease that is most similar to activating mutations in p110δ in immune deficiencies (34). Together, the HDX–MS analysis combined with detailed biophysical and biochemical analysis has provided insight into how disease-linked mutations in class I PI3Ks cause disease through enhanced membrane binding.
The class IA PI3Ks are activated downstream of a number of membrane-localized signaling proteins, including phosphorylated receptors, G-protein–coupled receptors (GPCRs), and Ras superfamily GTPases. HDX–MS has been useful to study the molecular basis for how these complexes, many of which only form on membrane surfaces, activate PI3Ks. HDX–MS showed how a phosphopeptide derived from a receptor tyrosine kinase activated class IA PI3Ks through binding to the nSH2 and cSH2 domains of p85, causing disruption of a subset of the p85-inhibitory contacts with the p110 catalytic subunit (50). Intriguingly, this also revealed a number of large-scale allosteric conformational changes that occur distant from the direct p85-inhibitory interface, similar to the changes seen upon membrane binding. Class IA PI3Ks can be activated downstream of Ras GTPases, and this requires the direct interaction with membrane-localized Ras (for p110α and p110δ) or Rho (p110β) GTPases. HDX–MS experiments carried out with lipid-conjugated Ras allowed for the determination of conformational changes that occur upon Ras binding and revealed that Ras activation is mainly driven through enhanced membrane binding (33), with covalent inhibitors of active Ras preventing PI3K activation (52). The p110β isoform is the only class IA PI3K that is able to be activated by Gβγ subunits downstream of GPCRs. The Gβγ subunit binds to the helical-C2 linker of p110β, and this region undergoes conformational changes upon membrane binding (58), suggesting that part of the reason the complex formation occurs only on membrane surfaces might be through membrane-induced conformational changes. The Gβγ-binding site is conserved and is also found in the divergent p110γ class IB PI3Ks (37). Overall, HDX–MS has allowed for the biophysical analysis of membrane complexes of PI3Ks with their activators, allowing for the design of targeted mutations to disrupt specific inputs in biological models of PI3K function, and has revealed unexpected isoform-specific regulatory mechanisms. Together, the HDX–MS studies described here have provided unique molecular insight into PI3K activation and provided some of the first insight into the many mechanisms that control their proper recruitment and regulation on membrane surfaces.
Dynamics of PIP3-mediated activation of Akt/protein kinase B
The protein kinase Akt (also known as protein kinase B, or PKB) is activated downstream of the lipid signal PIP3 (59). Akt is directly activated by PIP3, through an interaction of its PH domain with PIP3, leading to disruption of an inhibitory interaction between the PH and kinase domains of Akt. It can also be indirectly activated by PIP3 through the PIP3-mediated activation of the protein kinase phosphoinositide-dependent kinase 1 (PDK1), which phosphorylates Akt. Akt is further phosphorylated by the kinase mTOR complex 2 (mTORC2), leading to its full activation. The inhibited state of Akt is maintained through an inhibitory interaction between the activation loop of the kinase domain and the PH domain. Critical aspects of Akt activation that have remained elusive are the molecular basis for activating conformational changes that occur upon recruitment to PIP3-containing membranes, as well as whether phosphorylation of the kinase domain of Akt leads to disruption of the inhibitory PH-kinase domain interface. HDX–MS revealed conformational changes that occur both upon PIP3 binding and upon phosphorylation of Thr-308 and Ser-473 (31). Through the use of an oncogenic mutation in Akt in the activation loop that disrupts the interface of the PH and kinase domains (60), the conformational differences between direct membrane binding and PH domain disruption were able to be determined. Importantly, this study revealed that phosphorylated Akt in the absence of PIP3 membranes still has an intact PH-kinase domain–inhibitory interface. These results suggest that Akt activity is tightly restricted, with Akt signaling being constrained to membrane locations containing its activating lipids PI(3,4)P2 and PIP3.
Structure and dynamics of phosphatidylinositol 4-kinases
The phosphatidylinositol 4-kinases (PI4Ks) generate the lipid phosphoinositide phosphatidylinositol 4-phosphate (PI4P), with four distinct PI4K isoforms that generate PI4K (61). Two of these PI4Ks are soluble proteins (PI4KIIIα and PI4KIIIβ, referred to as PI4KA and PI4KB in the rest of this work) and transiently associate with membrane surfaces. The generation of PI4P at the Golgi and the plasma membrane by PI4KB and PI4KA, respectively, play essential roles in mediating membrane trafficking, in lipid transport, and in the generation of substrate for the production of the lipid signals PIP2 and PIP3 (62). Also, both PI4KA and PI4KB are frequently hijacked by viruses to generate PI4P-enriched replication organelles that are essential in viral replication (63, 64). These viral replication organelles are used by the viruses to localize their viral replication machinery in the cell, with the PI4P produced also playing key roles in manipulating lipid transport. The PI4KB isoform is also a major anti-parasitic target, as small-molecule inhibitors directed at the Plasmodium and Cryptosporidium variants of PI4KB are able to kill all life cycles of the causative parasites of malaria and cryptosporidiosis and are extremely promising anti-parasitic therapeutics (65–69). Therefore, understanding the regulatory mechanisms that control PI4K activation has direct therapeutic implications for both viral and parasitic infections.
The critical role of PI4Ks in regulating essential membrane trafficking and their participation in disease progression for both viral and parasitic pathogens made them an extremely interesting target to study. Our initial studies focused on PI4KB, due to the intense interest in drug development for both antiviral and antiparasitic therapeutics. Numerous questions existed regarding the regulation of PI4KB, as it was known to play both catalytic and noncatalytic roles in regulating membrane trafficking, with limited information existing on the protein complexes that mediated these roles. Limiting the initial ability to study PI4KB was the lack of any high-resolution structural information. A powerful application of the HDX–MS approach is that it can be used to identify disordered regions that prevent crystallization of complex protein assemblies. Through an HDX–MS–optimized approach, we were able to make a number of N-terminal, C-terminal, and internal deletions in PI4KB that allowed for the determination of the structure of PI4KB bound to the small GTPase Rab11 (30, 46). The structure of PI4KB revealed that it shared a similar kinase and helical domain architecture compared with the PI3Ks. This allowed for a collaboration with the Shokat laboratory in the structure-guided drug design of novel potent and selective PI4KB inhibitors (70). PI4KB was known to play a key noncatalytic role in binding to Rab11, with PI4KB being required for the recruitment of a subpopulation of Rab11 to the Golgi and trans-Golgi network (71, 72). Intriguingly, the complex with Rab11 revealed the molecular basis of this recruitment, with the helical domain of PI4KB forming a unique Rab-binding site over the Rab11 nucleotide-binding pocket. This binding site on Rab11 still allowed for the binding of Rab11 effectors, which permitted for the formation of tertiary complexes of PI4KB–Rab11 and Rab11 effectors. This formation of a tertiary Rab complex is unique, and it potentially reveals a novel mechanism for Rab regulation by binding partners and reveals how PI4KB mediates Rab11 recruitment. Intriguingly, HDX–MS studies revealed that when Rab11 binds PI4KB, there is a substantial allosteric conformational change in the switch regions of Rab11 that bind effectors, possibly providing a mechanism for PI4KB to alter Rab11 effector specificity. This same Rab11-binding interface is also found in the Rab8 GEF Rabin8 (73), with this complex also allowing for formation of Rab11 tertiary complexes. Overall, this work revealed a platform for design of PI4KB inhibitors and provided novel insight into the role of PI4KB in mediating membrane trafficking.
Although the PI4KB–Rab11 structure provided insight into how PI4KB can play noncatalytic roles in regulating membrane transport, it did not explain the mechanism by which PI4KB is recruited to the Golgi and trans-Golgi network. It also did not provide insight into how pathogenic single-stranded RNA viruses are able to activate PI4KB downstream of the lipidated viral effector protein 3A. The 3A protein does not form a direct complex with PI4KB, which implied the possible involvement of a PI4KB-binding partner. The acyl-CoA–binding domain 3 protein (ACBD3) had been proposed as the main binding partner for recruiting PI4KB to the Golgi (74) and had emerged as a binding partner of 3A proteins from numerous different viruses (44, 74–79). HDX–MS revealed that the GOLD domain of ACBD3 binds directly to the Aichi virus 3A protein, with the Q domain forming a direct interaction with the disordered N terminus of PI4KB (44, 76). ACBD3 is located to the Golgi through a direct interaction with the Golgi protein giantin, and this shows how PI4KB can be recruited to the Golgi membrane through formation of a complex with ACBD3. The PI4KB–ACBD3 complex is formed through a disorder–order transition in the PI4KB N terminus upon binding ACBD3, with mutations in this region leading to disruption of complex formation. Defining the molecular chain of events that mediate how viruses hijack PI4KB through a 3A–ACBD3 tertiary complex provides a novel mechanism to develop novel antiviral therapeutic strategies. Much is still unknown about other protein-binding partners of the PI4Ks and how they regulate its role in membrane trafficking and viral infection, with further biophysical studies being essential to decipher the regulation of these enzymes.
Conclusions
This award lecture article has given a brief overview of how my laboratory has approached the study of lipid-signaling systems using a synergy of biophysical techniques. Phosphoinositide kinases are central signaling hubs that control many aspects of how cells respond to external stimuli and how membranes traffic between organelles. There are still many unexplored aspects of how these enzymes are regulated, specifically how protein-binding partners and post-translational modifications regulate their association with membranes. Due to their frequent involvement in disease, defining these regulatory mechanisms may provide novel approaches for therapeutic intervention. The use of HDX–MS has proven to be a powerful analytical tool in the study of these complicated systems. An exciting development in the study of lipid-signaling research is the potential to start combining the medium-resolution HDX–MS approach with high-resolution cryo-EM studies of lipid-signaling proteins bound to their native membrane substrates. This is still an ambitious objective, as this will require conditions where sufficient occupancy of membrane binding is maintained at the concentrations used in EM, but this has the potential to transform our molecular understanding of signaling at membrane surfaces. Also, the continued use of HDX–MS in a synergistic manner with X-ray crystallography and NMR for construct design is useful to generate high-resolution snapshots of the proteins involved, with this approach still underutilized. It is an exciting time to be a structural biologist interested in lipid signaling, as continued technological developments in EM and MS have the potential to revolutionize our ability to study membrane recruitment in unprecedented molecular detail. On a personal note, it is a great pleasure to win this prestigious Young Investigator Award and have the article published in JBC, as I began my academic career in lipid research with three published JBC articles in my Ph.D. I greatly appreciate the Journal's support of molecular studies into lipid signaling.
Acknowledgments
The work underlying this article and the accompanying award was only possible through the long-term support of many mentors, collaborators, and trainees. Primary acknowledgment has to go to Dr. Edward Dennis, Dr. Virgil Woods, Dr. Roger Williams, and Dr. Olga Perisic, who were fundamental to my training as a scientist. I have been lucky to be involved in a field that supports and mentors new faculty, and I am grateful for the guidance of Dr. Kevan Shokat, Dr. Tamas Balla, Dr. Robin Irvine, Dr. Julie Brill, Dr. Emilio Hirsch, Dr. Len Stephens, Dr. Phill Hawkins, Dr. Klaus Okkenhaug, Dr. Oscar Vadas, Dr. Carrie Lucas, and many others. Much of the work this award recognizes was only possible through the dedicated efforts of trainees in my laboratory, with specific thanks to Melissa Fowler, Jacob McPhail, Gillian Dornan, Meredith Jenkins, Manoj Rathinaswamy, Braden Siempelkamp, Reece Hoffmann, David Hamelin, and Jordan Stariha. The support of my institute and colleagues at the University of Victoria was essential in the establishment of my laboratory, with Dr. Martin Boulanger, Dr. Caroline Cameron, Dr. Alisdair Boraston, Dr. Perry Howard, and Dr. Chris Nelson playing a significant role.
This work was supported by a Canadian Institutes of Health Research New Investigator award and Open Operating Grant CRN-142393, Cancer Research Society Operating Grant CRS-22641, Natural Sciences and Engineering Research Council of Canada Discovery Grant NSERC-05218, and Michael Smith Foundation for Health Research Scholar Award 17686. The author declares that he has no conflicts of interest with the contents of this article.
- HDX
- hydrogen–deuterium exchange
- PI3K
- phosphoinositide 3-kinase
- ACBD3
- acyl-CoA–binding domain 3 protein
- GPCR
- G-protein–coupled receptor
- PI4K
- phosphatidylinositol 4-kinase
- PI4P
- phosphatidylinositol 4-phosphate
- PLA2
- phospholipase A2
- PIP3
- phosphatidylinositol (3,4,5)-trisphosphate
- PIP2
- phosphatidylinositol (4,5)-bisphosphate.
References
- 1. Wymann M. P., and Schneiter R. (2008) Lipid signalling in disease. Nat. Rev. Mol. Cell Biol. 9, 162–176 10.1038/nrm2335 [DOI] [PubMed] [Google Scholar]
- 2. Fruman D. A., Chiu H., Hopkins B. D., Bagrodia S., Cantley L. C., and Abraham R. T. (2017) The PI3K pathway in human disease. Cell 170, 605–635 10.1016/j.cell.2017.07.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Burke J. E. (2018) Structural basis for regulation of phosphoinositide kinases and their involvement in human disease. Mol. Cell 71, 653–673 10.1016/j.molcel.2018.08.005 [DOI] [PubMed] [Google Scholar]
- 4. Caffrey M., and Cherezov V. (2009) Crystallizing membrane proteins using lipidic mesophases. Nat. Protoc. 4, 706–731 10.1038/nprot.2009.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Efremov R. G., Gatsogiannis C., and Raunser S. (2017) Lipid nanodiscs as a tool for high-resolution structure determination of membrane proteins by single-particle cryo-EM. Methods Enzymol. 594, 1–30 10.1016/bs.mie.2017.05.007 [DOI] [PubMed] [Google Scholar]
- 6. Lucas C. L., Kuehn H. S., Zhao F., Niemela J. E., Deenick E. K., Palendira U., Avery D. T., Moens L., Cannons J. L., Biancalana M., Stoddard J., Ouyang W., Frucht D. M., Rao V. K., Atkinson T. P., et al. (2014) Dominant-activating germline mutations in the gene encoding the PI(3)K catalytic subunit p110δ result in T cell senescence and human immunodeficiency. Nat. Immunol. 15, 88–97 10.1038/ni.2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Angulo I., Vadas O., Garçon F., Banham-Hall E., Plagnol V., Leahy T. R., Baxendale H., Coulter T., Curtis J., Wu C., Blake-Palmer K., Perisic O., Smyth D., Maes M., Fiddler C., et al. (2013) Phosphoinositide 3-kinase δ gene mutation predisposes to respiratory infection and airway damage. Science 342, 866–871 10.1126/science.1243292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dyment D. A., Smith A. C., Alcantara D., Schwartzentruber J. A., Basel-Vanagaite L., Curry C. J., Temple I. K., Reardon W., Mansour S., Haq M. R., Gilbert R., Lehmann O. J., Vanstone M. R., Beaulieu C. L., FORGE Canada Consortium, et al. (2013) Mutations in PIK3R1 Cause SHORT Syndrome. Am. J. Hum. Genet. 93, 158–166 10.1016/j.ajhg.2013.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rivière J.-B., Mirzaa G. M., O'Roak B. J., Beddaoui M., Alcantara D., Conway R. L., St-Onge J., Schwartzentruber J. A., Gripp K. W., Nikkel S. M., Worthylake T., Sullivan C. T., Ward T. R., Butler H. E., Kramer N. A., et al. (2012) De novo germline and postzygotic mutations in AKT3, PIK3R2 and PIK3CA cause a spectrum of related megalencephaly syndromes. Nat. Genet. 44, 934–940 10.1038/ng.2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lindhurst M. J., Parker V. E. R., Payne F., Sapp J. C., Rudge S., Harris J., Witkowski A. M., Zhang Q., Groeneveld M. P., Scott C. E., Daly A., Huson S. M., Tosi L. L., Cunningham M. L., Darling T. N., et al. (2012) Mosaic overgrowth with fibroadipose hyperplasia is caused by somatic activating mutations in PIK3CA. Nat. Genet. 44, 928–933 10.1038/ng.2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Samuels Y., Wang Z., Bardelli A., Silliman N., Ptak J., Szabo S., Yan H., Gazdar A., Powell S. M., Riggins G. J., Willson J. K., Markowitz S., Kinzler K. W., Vogelstein B., and Velculescu V. E. (2004) High frequency of mutations of the PIK3CA gene in human cancers. Science 304, 554 10.1126/science.1096502 [DOI] [PubMed] [Google Scholar]
- 12. Hsu Y.-H., Burke J. E., Li S., Woods V. L. Jr., and Dennis E. A. (2009) Localizing the membrane binding region of Group VIA Ca2+-independent phospholipase A2 using peptide amide hydrogen/deuterium exchange mass spectrometry. J. Biol. Chem. 284, 23652–23661 10.1074/jbc.M109.021857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Burke J. E., Babakhani A., Gorfe A. A., Kokotos G., Li S., Woods V. L. Jr., McCammon J. A., and Dennis E. A. (2009) Location of inhibitors bound to group IVA phospholipase A2 determined by molecular dynamics and deuterium exchange mass spectrometry. J. Am. Chem. Soc. 131, 8083–8091 10.1021/ja900098y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Burke J. E., and Dennis E. A. (2009) Phospholipase A2 structure/function, mechanism, and signaling. J. Lipid Res. 50, S237–S242 10.1194/jlr.R800033-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burke J. E., and Dennis E. A. (2009) Phospholipase A2 biochemistry. Cardiovasc. Drugs Ther. 23, 49–59 10.1007/s10557-008-6132-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Prévost N., Mitsios J. V., Kato H., Burke J. E., Dennis E. A., Shimizu T., and Shattil S. J. (2009) Group IVA cytosolic phospholipase A2 (cPLA2α) and integrin αIIbβ3 reinforce each other's functions during αIIbβ3 signaling in platelets. Blood 113, 447–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burke J. E., Hsu Y.-H., Deems R. A., Li S., Woods V. L. Jr., and Dennis E. A. (2008) A phospholipid substrate molecule residing in the membrane surface mediates opening of the lid region in group IVA cytosolic phospholipase A2. J. Biol. Chem. 283, 31227–31236 10.1074/jbc.M804492200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Burke J. E., Karbarz M. J., Deems R. A., Li S., Woods V. L. Jr., and Dennis E. A. (2008) Interaction of group IA phospholipase A2 with metal ions and phospholipid vesicles probed with deuterium exchange mass spectrometry. Biochemistry 47, 6451–6459 10.1021/bi8000962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hsu Y.-H., Burke J. E., Stephens D. L., Deems R. A., Li S., Asmus K. M., Woods V. L. Jr., and Dennis E. A. (2008) Calcium binding rigidifies the C2 domain and the intradomain interaction of GIVA phospholipase A2 as revealed by hydrogen/deuterium exchange mass spectrometry. J. Biol. Chem. 283, 9820–9827 10.1074/jbc.M708143200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cao J., Burke J. E., and Dennis E. A. (2013) Using hydrogen/deuterium exchange mass spectrometry to define the specific interactions of the phospholipase A2 superfamily with lipid substrates, inhibitors, and membranes. J. Biol. Chem. 288, 1806–1813 10.1074/jbc.R112.421909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Masson G. R., Jenkins M. L., and Burke J. E. (2017) An overview of hydrogen deuterium exchange mass spectrometry (HDX-MS) in drug discovery. Expert Opin. Drug Discov. 12, 981–994 10.1080/17460441.2017.1363734 [DOI] [PubMed] [Google Scholar]
- 22. Vadas O., Jenkins M. L., Dornan G. L., and Burke J. E. (2017) Using hydrogen-deuterium exchange mass spectrometry to examine protein-membrane interactions. Methods Enzymol. 583, 143–172 10.1016/bs.mie.2016.09.008 [DOI] [PubMed] [Google Scholar]
- 23. Guttman M., and Lee K. K. (2016) Isotope labeling of biomolecules: structural analysis of viruses by HDX-MS. Methods Enzymol. 566, 405–426 10.1016/bs.mie.2015.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vadas O., and Burke J. E. (2015) Probing the dynamic regulation of peripheral membrane proteins using hydrogen deuterium exchange-MS (HDX-MS). Biochem. Soc. Trans. 43, 773–786 10.1042/BST20150065 [DOI] [PubMed] [Google Scholar]
- 25. Engen J. R., and Wales T. E. (2015) Analytical aspects of hydrogen exchange mass spectrometry. Annu. Rev. Anal. Chem. (Palo Alto Calif) 8, 127–148 10.1146/annurev-anchem-062011-143113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Konermann L., Pan J., and Liu Y.-H. (2011) Hydrogen exchange mass spectrometry for studying protein structure and dynamics. Chem. Soc. Rev. 40, 1224–1234 10.1039/C0CS00113A [DOI] [PubMed] [Google Scholar]
- 27. Englander J. J., Del Mar C., Li W., Englander S. W., Kim J. S., Stranz D. D., Hamuro Y., and Woods V. L. (2003) Protein structure change studied by hydrogen-deuterium exchange, functional labeling, and mass spectrometry. Proc. Natl. Acad. Sci. U.S.A. 100, 7057–7062 10.1073/pnas.1232301100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Pantazatos D., Kim J. S., Klock H. E., Stevens R. C., Wilson I. A., Lesley S. A., and Woods V. L. (2004) Rapid refinement of crystallographic protein construct definition employing enhanced hydrogen/deuterium exchange MS. Proc. Natl. Acad. Sci. U.S.A. 101, 751–756 10.1073/pnas.0307204101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sharma S., Zheng H., Huang Y. J., Ertekin A., Hamuro Y., Rossi P., Tejero R., Acton T. B., Xiao R., Jiang M., Zhao L., Ma L.-C., Swapna G. V. T., Aramini J. M., and Montelione G. T. (2009) Construct optimization for protein NMR structure analysis using amide hydrogen/deuterium exchange mass spectrometry. Proteins. 76, 882–894 10.1002/prot.22394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fowler M. L., McPhail J. A., Jenkins M. L., Masson G. R., Rutaganira F. U., Shokat K. M., Williams R. L., and Burke J. E. (2016) Using hydrogen deuterium exchange mass spectrometry to engineer optimized constructs for crystallization of protein complexes: case study of PI4KIIIβ with Rab11. Protein Sci. 25, 826–839 10.1002/pro.2879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lučić I., Rathinaswamy M. K., Truebestein L., Hamelin D. J., Burke J. E., and Leonard T. A. (2018) Conformational sampling of membranes by Akt controls its activation and inactivation. Proc. Natl. Acad. Sci. U.S.A. 115, E3940–E3949 10.1073/pnas.1716109115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pulkoski-Gross M. J., Jenkins M. L., Truman J.-P., Salama M. F., Clarke C. J., Burke J. E., Hannun Y. A., and Obeid L. M. (2018) An intrinsic lipid-binding interface controls sphingosine kinase 1 function. J. Lipid Res. 59, 462–474 10.1194/jlr.M081307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Siempelkamp B. D., Rathinaswamy M. K., Jenkins M. L., and Burke J. E. (2017) Molecular mechanism of activation of class IA phosphoinositide 3-kinases (PI3Ks) by membrane-localized HRas. J. Biol. Chem. 292, 12256–12266 10.1074/jbc.M117.789263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dornan G. L., Siempelkamp B. D., Jenkins M. L., Vadas O., Lucas C. L., and Burke J. E. (2017) Conformational disruption of PI3Kδ regulation by immunodeficiency mutations in PIK3CD and PIK3R1. Proc. Natl. Acad. Sci. U.S.A. 114, 1982–1987 10.1073/pnas.1617244114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Masson G. R., Perisic O., Burke J. E., and Williams R. L. (2016) The intrinsically disordered tails of PTEN and PTEN-L have distinct roles in regulating substrate specificity and membrane activity. Biochem. J. 473, 135–144 10.1042/BJ20150931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rostislavleva K., Soler N., Ohashi Y., Zhang L., Pardon E., Burke J. E., Masson G. R., Johnson C., Steyaert J., Ktistakis N. T., and Williams R. L. (2015) Structure and flexibility of the endosomal Vps34 complex reveals the basis of its function on membranes. Science 350, aac7365 10.1126/science.aac7365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vadas O., Dbouk H. A., Shymanets A., Perisic O., Burke J. E., Abi Saab W. F., Khalil B. D., Harteneck C., Bresnick A. R., Nürnberg B., Backer J. M., and Williams R. L. (2013) Molecular determinants of PI3Kγ-mediated activation downstream of G-protein-coupled receptors (GPCRs). Proc. Natl. Acad. Sci. U.S.A. 110, 18862–18867 10.1073/pnas.1304801110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Burke J. E., Perisic O., Masson G. R., Vadas O., and Williams R. L. (2012) Oncogenic mutations mimic and enhance dynamic events in the natural activation of phosphoinositide 3-kinase p110α (PIK3CA). Proc. Natl. Acad. Sci. U.S.A. 109, 15259–15264 10.1073/pnas.1205508109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Burke J. E., Vadas O., Berndt A., Finegan T., Perisic O., and Williams R. L. (2011) Dynamics of the phosphoinositide 3-kinase p110δ interaction with p85α and membranes reveals aspects of regulation distinct from p110α. Structure 19, 1127–1137 10.1016/j.str.2011.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dornan G. L., and Burke J. E. (2018) Molecular mechanisms of human disease mediated by oncogenic and primary immunodeficiency mutations in class IA phosphoinositide 3-kinases. Front. Immunol. 9, 575 10.3389/fimmu.2018.00575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Takeda A. J., Zhang Y., Dornan G. L., Siempelkamp B. D., Jenkins M. L., Matthews H. F., McElwee J. J., Bi W., Seeborg F. O., Su H. C., Burke J. E., and Lucas C. L. (2017) Novel PIK3CD mutations affecting N-terminal residues of p110δ cause activated PI3Kδ syndrome (APDS) in humans. J. Allergy Clin. Immunol. 140, 1152–1156.e10 10.1016/j.jaci.2017.03.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Walser R., Burke J. E., Gogvadze E., Bohnacker T., Zhang X., Hess D., Küenzi P., Leitges M., Hirsch E., Williams R. L., Laffargue M., and Wymann M. P. (2013) PKCβ phosphorylates PI3Kγ to activate it and release it from GPCR control. PLoS Biol. 11, e1001587 10.1371/journal.pbio.1001587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Powell C. J., Jenkins M. L., Parker M. L., Ramaswamy R., Kelsen A., Warshaw D. M., Ward G. E., Burke J. E., and Boulanger M. J. (2017) Dissecting the molecular assembly of the Toxoplasma gondii MyoA motility complex. J. Biol. Chem. 292, 19469–19477 10.1074/jbc.M117.809632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. McPhail J. A., Ottosen E. H., Jenkins M. L., and Burke J. E. (2017) The molecular basis of Aichi virus 3A protein activation of phosphatidylinositol 4 kinase IIIβ, PI4KB, through ACBD3. Structure 25, 121–131 10.1016/j.str.2016.11.016 [DOI] [PubMed] [Google Scholar]
- 45. Ohashi Y., Soler N., García Ortegón M., Zhang L., Kirsten M. L., Perisic O., Masson G. R., Burke J. E., Jakobi A. J., Apostolakis A. A., Johnson C. M., Ohashi M., Ktistakis N. T., Sachse C., and Williams R. L. (2016) Characterization of Atg38 and NRBF2, a fifth subunit of the autophagic Vps34/PIK3C3 complex. Autophagy 12, 2129–2144 10.1080/15548627.2016.1226736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Burke J. E., Inglis A. J., Perisic O., Masson G. R., McLaughlin S. H., Rutaganira F., Shokat K. M., and Williams R. L. (2014) Structures of PI4KIIIβ complexes show simultaneous recruitment of Rab11 and its effectors. Science 344, 1035–1038 10.1126/science.1253397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Powell C. J., Ramaswamy R., Kelsen A., Hamelin D. J., Warshaw D. M., Bosch J., Burke J. E., Ward G. E., and Boulanger M. J. (2018) Structural and mechanistic insights into the function of the unconventional class XIV myosin MyoA from Toxoplasma gondii. Proc. Natl. Acad. Sci. U.S.A. 115, E10548–E10555 10.1073/pnas.1811167115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jenkins M. L., Margaria J. P., Stariha J. T. B., Hoffmann R. M., McPhail J. A., Hamelin D. J., Boulanger M. J., Hirsch E., and Burke J. E. (2018) Structural determinants of Rab11 activation by the guanine nucleotide exchange factor SH3BP5. Nat. Commun. 9, 3772 10.1038/s41467-018-06196-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. D'Angelo G., Uemura T., Chuang C.-C., Polishchuk E., Santoro M., Ohvo-Rekilä H., Sato T., Di Tullio G., Varriale A., D'Auria S., Daniele T., Capuani F., Johannes L., Mattjus P., Monti M., Pucci P., Williams R. L., Burke J. E., Platt F. M., Harada A., and De Matteis M. A. (2013) Vesicular and non-vesicular transport feed distinct glycosylation pathways in the Golgi. Nature 501, 116–120 10.1038/nature12423 [DOI] [PubMed] [Google Scholar]
- 50. Burke J. E., and Williams R. L. (2013) Dynamic steps in receptor tyrosine kinase mediated activation of class IA phosphoinositide 3-kinases (PI3K) captured by H/D exchange (HDX-MS). Adv. Biol. Regul. 53, 97–110 10.1016/j.jbior.2012.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Nnadi C. I., Jenkins M. L., Gentile D. R., Bateman L. A., Zaidman D., Balius T. E., Nomura D. K., Burke J. E., Shokat K. M., and London N. (2018) Novel K-Ras G12C Switch-II covalent binders destabilize Ras and accelerate nucleotide exchange. J. Chem. Inf. Model. 58, 464–471 10.1021/acs.jcim.7b00399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gentile D. R., Rathinaswamy M. K., Jenkins M. L., Moss S. M., Siempelkamp B. D., Renslo A. R., Burke J. E., and Shokat K. M. (2017) Ras binder induces a modified switch-II pocket in GTP and GDP states. Cell Chem. Biol. 24, 1455–1466.e14 10.1016/j.chembiol.2017.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. McGregor L. M., Jenkins M. L., Kerwin C., Burke J. E., and Shokat K. M. (2017) Expanding the scope of electrophiles capable of targeting K-Ras oncogenes. Biochemistry 56, 3178–3183 10.1021/acs.biochem.7b00271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Clarke J. H., Giudici M. L., Burke J. E., Williams R. L., Maloney D. J., Marugan J., and Irvine R. F. (2015) The function of phosphatidylinositol 5-phosphate 4-kinase γ (PI5P4Kγ) explored using a specific inhibitor that targets the PI5P-binding site. Biochem. J. 466, 359–367 10.1042/BJ20141333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Castro-Falcón G., Seiler G. S., Demir Ö., Rathinaswamy M. K., Hamelin D., Hoffmann R. M., Makowski S. L., Letzel A.-C., Field S. J., Burke J. E., Amaro R. E., and Hughes C. C. (2018) Neolymphostin A is a covalent phosphoinositide 3-kinase (PI3K)/mammalian target of rapamycin (mTOR) dual inhibitor that employs an unusual electrophilic vinylogous ester. J. Med. Chem. 61, 10463–10472 10.1021/acs.jmedchem.8b00975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Burke J. E., and Williams R. L. (2015) Synergy in activating class I PI3Ks. Trends Biochem. Sci. 40, 88–100 10.1016/j.tibs.2014.12.003 [DOI] [PubMed] [Google Scholar]
- 57. Vadas O., Burke J. E., Zhang X., Berndt A., and Williams R. L. (2011) Structural basis for activation and inhibition of class I phosphoinositide 3-kinases. Sci. Signal. 4, re2 10.1126/scisignal.2002165 [DOI] [PubMed] [Google Scholar]
- 58. Dbouk H. A., Vadas O., Shymanets A., Burke J. E., Salamon R. S., Khalil B. D., Barrett M. O., Waldo G. L., Surve C., Hsueh C., Perisic O., Harteneck C., Shepherd P. R., Harden T. K., Smrcka A. V., et al. (2012) G protein-coupled receptor-mediated activation of p110β by Gβγ is required for cellular transformation and invasiveness. Sci. Signal. 5, ra89 10.1126/scisignal.2003264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Manning B. D., and Toker A. (2017) AKT/PKB signaling: navigating the network. Cell 169, 381–405 10.1016/j.cell.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Ebner M., Lučić I., Leonard T. A., and Yudushkin I. (2017) PI(3,4,5)P3 engagement restricts Akt activity to cellular membranes. Mol. Cell 65, 416–431.e6 10.1016/j.molcel.2016.12.028 [DOI] [PubMed] [Google Scholar]
- 61. Dornan G. L., McPhail J. A., and Burke J. E. (2016) Type III phosphatidylinositol 4 kinases: structure, function, regulation, signalling and involvement in disease. Biochem. Soc. Trans. 44, 260–266 10.1042/BST20150219 [DOI] [PubMed] [Google Scholar]
- 62. Balla T. (2013) Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol. Rev. 93, 1019–1137 10.1152/physrev.00028.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. van der Schaar H. M., Dorobantu C. M., Albulescu L., Strating J. R. P. M., and van Kuppeveld F. J. M. (2016) Fat(al) attraction: picornaviruses usurp lipid transfer at membrane contact sites to create replication organelles. Trends Microbiol. 24, 535–546 10.1016/j.tim.2016.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Altan-Bonnet N., and Balla T. (2012) Phosphatidylinositol 4-kinases: hostages harnessed to build panviral replication platforms. Trends Biochem. Sci. 37, 293–302 10.1016/j.tibs.2012.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Brunschwig C., Lawrence N., Taylor D., Abay E., Njoroge M., Basarab G. S., Le Manach C., Paquet T., Cabrera D. G., Nchinda A. T., de Kock C., Wiesner L., Denti P., Waterson D., Blasco B., et al. (2018) UCT943, a next-generation Plasmodium falciparum PI4K inhibitor preclinical candidate for the treatment of malaria. Antimicrob. Agents Chemother. 62, e00012–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Manjunatha U. H., Vinayak S., Zambriski J. A., Chao A. T., Sy T., Noble C. G., Bonamy G. M. C., Kondreddi R. R., Zou B., Gedeck P., Brooks C. F., Herbert G. T., Sateriale A., Tandel J., Noh S., et al. (2017) A cryptosporidium PI(4)K inhibitor is a drug candidate for cryptosporidiosis. Nature 546, 376–380 10.1038/nature22337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Paquet T., Le Manach C., Cabrera D. G., Younis Y., Henrich P. P., Abraham T. S., Lee M. C. S., Basak R., Ghidelli-Disse S., Lafuente-Monasterio M. J., Bantscheff M., Ruecker A., Blagborough A. M., Zakutansky S. E., Zeeman A.-M., et al. (2017) Antimalarial efficacy of MMV390048, an inhibitor of Plasmodium phosphatidylinositol 4-kinase. Sci. Transl. Med. 9, eaad9735 10.1126/scitranslmed.aad9735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. McNamara C. W., Lee M. C. S., Lim C. S., Lim S. H., Roland J., Nagle A., Simon O., Yeung B. K. S., Chatterjee A. K., McCormack S. L., Manary M. J., Zeeman A.-M., Dechering K. J., Kumar T. R. S., Henrich P. P., et al. (2013) Targeting Plasmodium PI(4)K to eliminate malaria. Nature 504, 248–253 10.1038/nature12782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kato N., Comer E., Sakata-Kato T., Sharma A., Sharma M., Maetani M., Bastien J., Brancucci N. M., Bittker J. A., Corey V., Clarke D., Derbyshire E. R., Dornan G. L., Duffy S., Eckley S., et al. (2016) Diversity-oriented synthesis yields novel multistage antimalarial inhibitors. Nature 538, 344–349 10.1038/nature19804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Rutaganira F. U., Fowler M. L., McPhail J. A., Gelman M. A., Nguyen K., Xiong A., Dornan G. L., Tavshanjian B., Glenn J. S., Shokat K. M., and Burke J. E. (2016) Design and structural characterization of potent and selective inhibitors of phosphatidylinositol 4 kinase IIIβ. J. Med. Chem. 59, 1830–1839 10.1021/acs.jmedchem.5b01311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Polevoy G., Wei H.-C., Wong R., Szentpetery Z., Kim Y. J., Goldbach P., Steinbach S. K., Balla T., and Brill J. A. (2009) Dual roles for the Drosophila PI 4-kinase four wheel drive in localizing Rab11 during cytokinesis. J. Cell Biol. 187, 847–858 10.1083/jcb.200908107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. de Graaf P., Zwart W. T., van Dijken R. A. J., Deneka M., Schulz T. K. F., Geijsen N., Coffer P. J., Gadella B. M., Verkleij A. J., van der Sluijs P., and van Bergen en Henegouwen P. M. P. (2004) Phosphatidylinositol 4-kinasebeta is critical for functional association of rab11 with the Golgi complex. Mol. Biol. Cell 15, 2038–2047 10.1091/mbc.e03-12-0862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Vetter M., Stehle R., Basquin C., and Lorentzen E. (2015) Structure of Rab11-FIP3-Rabin8 reveals simultaneous binding of FIP3 and Rabin8 effectors to Rab11. Nat. Struct. Mol. Biol. 22, 695–702 10.1038/nsmb.3065 [DOI] [PubMed] [Google Scholar]
- 74. Sasaki J., Ishikawa K., Arita M., and Taniguchi K. (2012) ACBD3-mediated recruitment of PI4KB to picornavirus RNA replication sites. EMBO J. 31, 754–766 10.1038/emboj.2011.429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Klima M., Chalupská D., Różycki B., Humpolickova J., Rezabkova L., Silhan J., Baumlová A., Dubankova A., and Boura E. (2017) Kobuviral non-structural 3A proteins act as molecular harnesses to hijack the host ACBD3 protein. Structure 25, 219–230 10.1016/j.str.2016.11.021 [DOI] [PubMed] [Google Scholar]
- 76. Klima M., Tóth D. J., Hexnerova R., Baumlová A., Chalupská D., Tykvart J., Rezabkova L., Sengupta N., Man P., Dubankova A., Humpolickova J., Nencka R., Veverka V., Balla T., and Boura E. (2016) Structural insights and in vitro reconstitution of membrane targeting and activation of human PI4KB by the ACBD3 protein. Sci. Rep. 6, 23641 10.1038/srep23641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ishikawa-Sasaki K., Sasaki J., and Taniguchi K. (2014) A complex comprising phosphatidylinositol 4-kinase IIIβ, ACBD3, and Aichi virus proteins enhances phosphatidylinositol 4-phosphate synthesis and is critical for formation of the viral replication complex. J. Virol. 88, 6586–6598 10.1128/JVI.00208-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Téoulé F., Brisac C., Pelletier I., Vidalain P.-O., Jégouic S., Mirabelli C., Bessaud M., Combelas N., Autret A., Tangy F., Delpeyroux F., and Blondel B. (2013) The Golgi protein ACBD3, an interactor for poliovirus protein 3A, modulates poliovirus replication. 87, 11031–11046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Greninger A. L., Knudsen G. M., Betegon M., Burlingame A. L., and DeRisi J. L. (2012) The 3A protein from multiple picornaviruses utilizes the golgi adaptor protein ACBD3 to recruit PI4KIIIβ. J. Virol. 86, 3605–3616 10.1128/JVI.06778-11 [DOI] [PMC free article] [PubMed] [Google Scholar]