Abstract
Dietary, fructose-containing sugars have been strongly associated with the development of nonalcoholic fatty liver disease (NAFLD). Recent studies suggest that fructose also can be produced via the polyol pathway in the liver, where it may induce hepatic fat accumulation. Moreover, fructose metabolism yields uric acid, which is highly associated with NAFLD. Here, using biochemical assays, reporter gene expression, and confocal fluorescence microscopy, we investigated whether uric acid regulates aldose reductase, a key enzyme in the polyol pathway. We evaluated whether soluble uric acid regulates aldose reductase expression both in cultured hepatocytes (HepG2 cells) and in the liver of hyperuricemic rats and whether this stimulation is associated with endogenous fructose production and fat accumulation. Uric acid dose-dependently stimulated aldose reductase expression in the HepG2 cells, and this stimulation was associated with endogenous fructose production and triglyceride accumulation. This stimulatory mechanism was mediated by uric acid–induced oxidative stress and stimulation of the transcription factor nuclear factor of activated T cells 5 (NFAT5). Uric acid also amplified the effects of elevated glucose levels to stimulate hepatocyte triglyceride accumulation. Hyperuricemic rats exhibited elevated hepatic aldose reductase expression, endogenous fructose accumulation, and fat buildup that was significantly reduced by co-administration of the xanthine oxidase inhibitor allopurinol. These results suggest that uric acid generated during fructose metabolism may act as a positive feedback mechanism that stimulates endogenous fructose production by stimulating aldose reductase in the polyol pathway. Our findings suggest an amplifying mechanism whereby soft drinks rich in glucose and fructose can induce NAFLD.
Keywords: fructose, uric acid, metabolic syndrome, liver metabolism, fatty acid, aldose reductase, polyol pathway, sorbitol
Introduction
Nonalcoholic fatty liver disease (NAFLD)2 has become one of the most important causes of chronic liver disease and cirrhosis. Strong evidence has linked the development of NAFLD with intake of sugary beverages (1–5), and experimental studies have shown that sugary beverages can induce fatty liver in humans (6). Sugar (sucrose) and high-fructose corn syrup are the two primary sweeteners that have been implicated in causing NAFLD, and both contain fructose, which is known to induce fatty liver in animals (7, 8). Our group has shown that the mechanism by which fructose induces fatty liver is due to the generation of uric acid during fructose metabolism that results in mitochondrial oxidative stress and an impairment in ATP production (9, 10). Indeed, hyperuricemia itself is also both highly associated with hypertriglyceridemia (11) and NAFLD and also predicts the development of NAFLD (1, 3, 4, 12–14).
Recently we have reported that fructose may not simply come from the diet but may also be generated in the liver because of activation of the polyol pathway, which results in the conversion of glucose to sorbitol by aldose reductase (AR), followed by the conversion of sorbitol to fructose by sorbitol dehydrogenase (SDH) (15, 16). Normally aldose reductase is minimally expressed in the human liver (17, 18). This has also been shown experimentally in WT mice in which hepatic production of fructose is minimal (15, 16). However, high-glycemic diets (glucose-enriched), as well as high-salt diets, can induce the expression of AR in the liver of mice, resulting in endogenous fructose production and fructose-dependent development of NAFLD (15, 16). The importance of this finding is supported by studies showing that inhibition of AR protects type 2 (db db) diabetic mice from developing fatty liver (19).
The discovery that the induction of AR by the liver may have a role in NAFLD emphasizes the need to understand what regulates AR. One of the key regulators of AR is increased osmolality (20), which is likely involved in how high-glycemic and high-salt diets act (15, 16). However, aldose reductase in endothelial cells is regulated by uric acid (21). Uric acid is also known to regulate fructokinase, the key enzyme that metabolizes fructose in the liver (22).
Here we tested the hypothesis that uric acid might regulate AR in the liver, and, in so doing, also regulate fructose generation and hepatic fat accumulation. We also investigated whether this involved nuclear factor of activated T cells 5 (NFAT5), a transcription factor well-known to regulate AR expression and synthesis (23, 24). If uric acid can be shown to regulate fructose production, then it provides another key link in understanding why hyperuricemia and sugar intake both appear to be major risk factors for NAFLD.
Results
Uric acid up-regulates aldose reductase expression and the polyol pathway in human hepatocytes
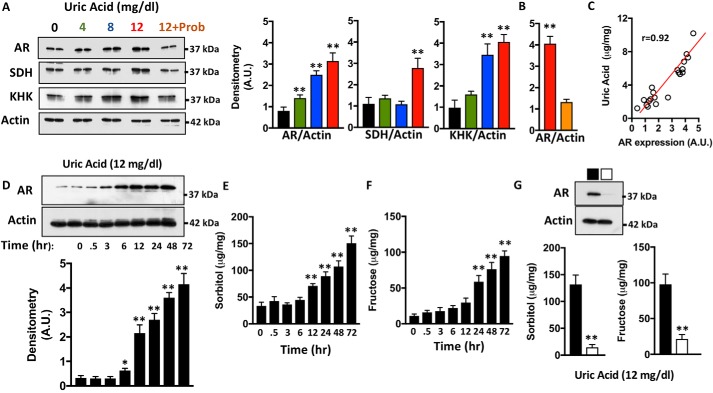
Human HepG2 cells were exposed to varying concentrations of uric acid including 4 (considered normouricemia), 8, and 12 mg/dl (hyperuricemia) for 72 h, and the expression of AR, SDH, and fructokinase/ketohexokinase (KHK) was analyzed. As shown in Fig. 1A, the expression of AR is up-regulated by uric acid in a dose-dependent manner. A small although significant difference in AR expression was already observed between unexposed and normouricemic levels (4 mg/dl). AR up-regulation was markedly increased with concentrations greater than 8 mg/dl (3.1- and 3.9-fold increase with 8 and 12 mg/dl, respectively; p < 0.01). Consistent with previous reports (22), high uric acid levels also resulted in the significant up-regulation of SDH (2.52-fold) and KHK (4.14-fold). Of interest, co-exposure of cells with uric acid and probenecid, a uric acid transporter inhibitor, resulted in the blockade of AR up-regulation, indicating that intracellular uric acid and not extracellular is driving AR expression in human hepatocytes (Fig. 1B). Consistently, AR expression correlated with intracellular uric acid levels as shown in Fig. 1C. We and others have previously shown that hypertonicity is an important stimulator of aldose reductase expression and activation of the polyol pathway (16, 25, 27). In this regard, the addition of uric acid from 4 to 12 mg/dl did not substantially raise the osmolality of the medium (289 ± 5 mOsm/KgH2O at baseline versus 284 ± 4 mOsm/KgH2O (4 mg/dl), 286 ± 6 mOsm/KgH2O (8 mg/dl), and 286 ± 7 mOsm/KgH2O (12 mg/dl)), indicating that the effects of uric acid on aldose reductase are independent of higher osmolality. To better characterize whether AR acts as an early or late response protein to uric acid, we then determined its expression in a time-dependent manner. As shown in Fig. 1D, AR expression starts to increase significantly at 6 h after exposure to uric acid, suggesting that the activation of the polyol pathway is an early response to the presence of high uric acid levels. Consistent with this observation, cellular sorbitol and fructose, intermediate products of the polyol pathway, are significantly up-regulated by uric acid at 12 and 24 h postexposure, respectively (Fig. 1, E and F). As expected, increased sorbitol and fructose levels are mediated by the up-regulation of AR because the levels of these metabolites were not up-regulated in AR-deficient cells (Fig. 1G).
Figure 1.
Uric acid stimulates aldose reductase and the polyol pathway in human hepatocytes. A, representative Western blotting and densitometry for AR, SDH, KHK, and actin in HepG2 cells exposed to increasing concentrations of uric acid for 72 h. A subset of cells exposed to 12 mg/dl uric acid was treated with the uric acid transporter URAT1 inhibitor probenecid (Prob). B, densitometry for AR expression in 12 mg/dl exposed cells in the absence (red) or presence (orange) of probenecid. C, correlation analysis between intracellular uric acid levels and AR expression in HepG2 cells. D, representative Western blotting and densitometry for AR and actin in HepG2 cells exposed to 12 mg/dl uric acid for different time points. E, intracellular sorbitol levels in the same groups as in D. F, intracellular fructose levels in the same groups as in D. G, representative Western blotting for AR in control (black) and silenced (white) HepG2 cells. Intracellular sorbitol and fructose levels in the same cells exposed to 12 mg/dl uric acid for 72 h. The data represent the results of three independent experiments. *, p < 0.05; **, p < 0.01 versus control; one-way ANOVA, Tukey post hoc analysis.
Regulation of aldose reductase by uric acid-dependent oxidative stress and NFAT5 activation
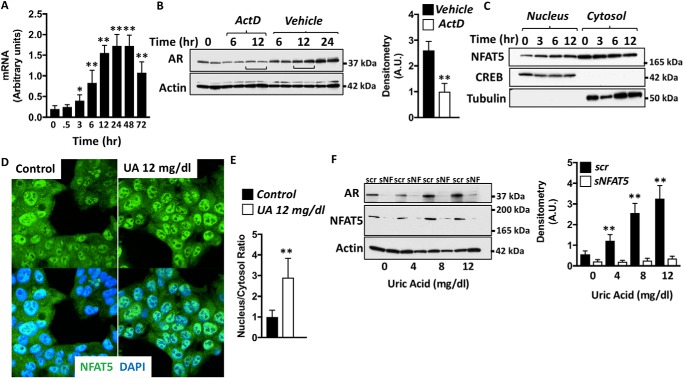
As shown in Figs. 2 and 3, increased AR protein expression could be secondary to either increased transcriptional/translational activity and/or increased half-life (reduced degradation). As shown in Fig. 2A, the elevation of AR mRNA by uric acid 3 h postexposure precedes the up-regulation of the protein, suggesting that uric acid-dependent AR expression is mediated by increased transcriptional activity. Consistently, the blockade of all transcriptional activity with actinomycin D markedly blunted the up-regulation of AR by uric acid (Fig. 2B). We and others have previously shown that AR is a target gene of the transcription factor NFAT5 (24, 25). In this regard, increased NFAT5 nuclear retention and transcriptional activity results in the up-regulation of AR and other osmolyte-dependent target genes (28–30). Of interest, we found a significant enrichment of NFAT5 in pure nuclear fractions of HepG2 cells exposed to uric acid (Fig. 2, C–E). Increased nuclear expression of NFAT5 mediated by uric acid occurs as early as 3 h after exposure, coinciding with the onset of expression of AR mRNA presented in Fig. 2A. Furthermore, and consistent with a key role of NFAT5 in regulating AR expression, AR up-regulation by uric acid is markedly reduced in NFAT5-deficient cells as shown in Fig. 2F.
Figure 2.
Aldose reductase expression in hepatocytes is mediated by the transcription factor NFAT5. A, AR mRNA expression in 12 mg/dl uric acid–exposed HepG2 cells for different time points. B, representative Western blotting and densitometry for AR and actin in HepG2 cells exposed to 12 mg/dl uric acid for different time points in the presence or absence of the transcription inhibitor actinomycin D (ActD). C, representative Western blotting and densitometry for NFAT5, CREB (nuclear marker), and tubulin (cytosol marker) in HepG2 cells exposed to 12 mg/dl uric acid for different time points. D, representative confocal images for NFAT5 (green) and the nucleus marker, 4′,6′-diamino-2-phenylindole (DAPI, blue) in HepG2 cells control and exposed to 12 mg/dl uric acid for 6 h. E, quantification of NFAT5 nucleus/cytosol ratio signal in control and exposed to 12 mg/dl uric acid for 6 h. F, representative Western blotting and densitometry for AR, NFAT5, and actin in control (scramble, scr) and NFAT5-silenced (sNF or sNFAT5) HepG2 cells exposed to increasing concentrations of uric acid for 72 h. The data represent the results of three independent experiments. *, p < 0.05; **, p < 0.01 versus control; one-way ANOVA, Tukey post hoc analysis.
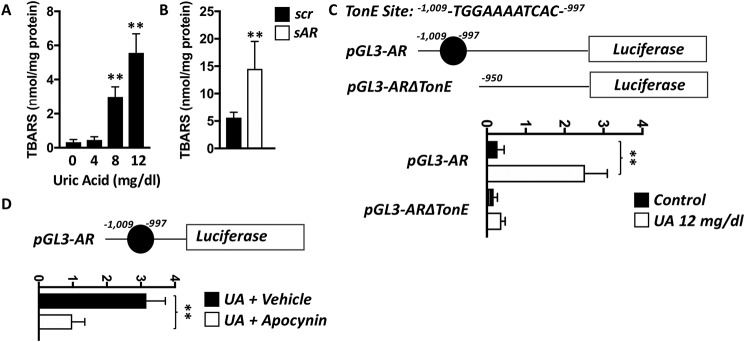
Figure 3.
Uric acid-dependent up-regulation of aldose reductase by NFAT5 is mediated by oxidative stress. A, intracellular TBARS (lipid peroxides) in HepG2 cells exposed to increasing levels of uric acid. B, intracellular TBARS in HepG2 cells control (scr) or deficient for AR expression (sAR) exposed to 12 mg/dl of uric acid. C, luciferase signal in HepG2 cells transfected with a luciferase reporter cassette containing a full AR promoter or a truncated version without the NFAT5 TonE site and exposed to 0 (control) or 12 mg/dl of uric acid for 3 h. D, luciferase signal in same experiment as in C in the presence or absence of the NADPH oxidase inhibitor apocynin. The data represent the results of three independent experiments. **, p < 0.01 versus control; one-way ANOVA, Tukey post hoc analysis.
Previous reports demonstrate a critical role for oxidants in stimulating NFAT5 transcriptional activity (31, 32). In this regard, uric acid, despite being an important antioxidant in the systemic circulation, can also act as a prooxidant molecule intracellularly (9, 33). Consistently, and as shown in Fig. 3A, uric acid markedly increased intracellular lipid peroxides determined as TBARS in HepG2 cells in a dose-dependent manner. Of interest, TBARS were significantly elevated in AR-deficient cells (Fig. 3B), suggesting that uric acid-mediated AR up-regulation would be an initial response to detoxify lipid peroxides as suggested by Rittner et al. (34). Therefore, to better determine whether oxidative stress drives AR promotion by NFAT5, we developed a luciferase-based system by cloning the human AR promoter upstream a luciferase expressing cassette. As shown in Fig. 3C, cells containing the full AR promoter expression cassette demonstrated a 9.1-fold (pGL3-AR, p < 0.01) increase in luciferase signal when exposed to uric acid. This signal was mediated by NFAT5 as deletion of the binding site in the AR promoter (pGL3-ARΔTonE) markedly blunted the luciferase signal (2.1-fold increase). Of interest, luciferase expression signal induced by uric acid was markedly blocked by the antioxidant molecule, apocynin (Fig. 3D), thus supporting that NFAT5-dependent activation of AR is mediated by oxidative stress.
Aldose reductase-mediated endogenous fructose production induces hepatic fat accumulation
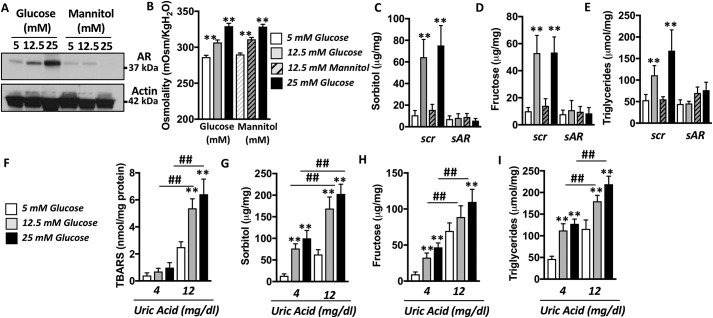
High glucose is known to stimulate AR in the kidney (35–37). As shown in Fig. 4A, glucose also stimulates AR in human hepatocytes in a dose-dependent manner from 5 mm (normoglycemia) to 25 mm (hyperglycemia). Of interest, equiosmolar levels of mannitol failed to up-regulate AR expression, indicating that glucose-dependent up-regulation of AR is independent of its osmolar content (Fig. 4, A and B). Consistent with increased AR expression, intracellular sorbitol and fructose levels are significantly elevated in cells exposed to high glucose (and not mannitol) levels (5.4- and 4.8-fold increase, respectively; p < 0.01; Fig. 4, C and D). Importantly, no significant increases in sorbitol or fructose in high glucose–exposed cells were observed in AR-deficient cells (Fig. 4, C and D; representative Western blotting for efficient silencing for AR is shown in Fig. 1G). We previously reported that increased endogenous fructose production and metabolism are elements of an important deleterious step in the development of fatty liver (15). Consistent with this observation, intracellular triglycerides were significantly elevated (3.04-fold increase, p < 0.01) in control but not AR knockout cells exposed to high glucose levels (Fig. 4E). Furthermore, and as shown in Fig. 4 (F–I), intracellular oxidative stress as well as products from the polyol pathway (sorbitol and fructose) and triglycerides were significantly greater in HepG2 cells exposed to both high glucose (12.5 and 25 mm) and high uric acid (12 mg/dl) compared with control or cells just exposed to one of these stimulants (either glucose or uric acid), documenting an exacerbation of this effect induced by the combination of two AR activators.
Figure 4.
Up-regulation of AR activates the polyol pathway and induces fat accumulation in HepG2 cells. A, representative Western blotting and densitometry for AR and actin in HepG2 cells exposed to increased glucose or mannitol (equiosmolar control) concentrations for 72 h. B, medium osmolality of cells exposed to 5, 12.5, or 25 mm glucose or mannitol. C, intracellular sorbitol levels in HepG2 cells exposed to normal (5 mm) or high (25 mm) glucose levels in control (scr) and AR-deficient (sAR) cells. D, intracellular fructose levels in the same groups as in B. E, intracellular triglyceride levels in the same groups as in B. F, intracellular TBARS (lipid peroxides) in HepG2 cells exposed to normal (5 mm, gray) or high (25 mm, black) glucose in the presence of low (4 mg/dl) or high (12 mg/dl) uric acid. G, intracellular sorbitol levels in the same groups as in F. H, intracellular fructose levels in the same groups as in F. I, intracellular triglyceride levels in the same groups as in F. **, p < 0.01 versus control within the same uric acid; ##, p < 0.01 versus control within the same glucose; one-way ANOVA, Tukey post hoc analysis.
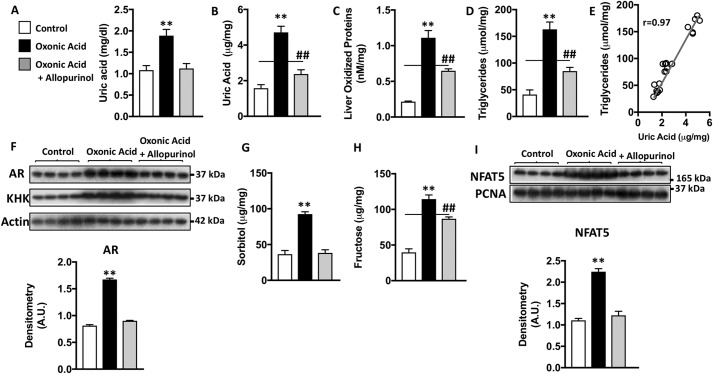
Most mammals, including rats and unlike humans, have low uric acid levels because of the presence of uricase in the liver. Therefore, to characterize the role of uric acid in rats, we inhibited uricase with oxonic acid as previously described (38). Because uricase blockade would not only raise uric acid but also other upstream purines like xanthine, hypoxanthine and inosine, a subset of animals were treated with allopurinol to inhibit xanthine oxidase and thus the production of uric acid. As shown in Fig. 5 (A and B), administration of oxonic acid resulted in a significant increase in both plasma and liver uric acid levels. Uric acid up-regulation was markedly reduced by co-administration of allopurinol. In the liver, uric acid levels were associated and correlated with oxidative stress (lipid peroxides) and intrahepatic triglyceride concentrations (Fig. 5, C–E). Furthermore, and as shown in Fig. 5 (F–H), elevated hepatic uric acid induced the up-regulation of AR and KHK in rats as well as the increase in intrahepatic sorbitol and fructose levels, thus documenting the activation of the polyol pathway in vivo. Consistently, we found increased NFAT5 expression in the nucleus of hyperuricemic rats compared with control or allopurinol treated animals (Fig. 5I).
Figure 5.
Activation of the polyol pathway by uric acid induces hepatic fat accumulation in rats. A, plasma uric acid levels in control rats (white) and rats exposed to the uricase inhibitor, oxonic acid, in the absence (black) and presence of the xanthine oxidase inhibitor, allopurinol (gray). B, intrahepatic levels of uric acid in the same groups as in A. C, intrahepatic liver oxidized protein levels of in the same groups as in A. D, intrahepatic triglycerides in the same groups as in A. E, correlation between intracellular uric acid and triglyceride levels in all rats. F, representative Western blotting and densitometry for AR, fructokinase/KHK, and actin in the same groups as in A. G, intrahepatic sorbitol levels in the same groups as in A. H, intrahepatic fructose levels in the same groups as in A. I, representative Western blotting and densitometry for NFAT5 and the nuclear marker proliferating cell nuclear antigen (PCNA) in the same groups as in A; n = 4–7 rats/group. **, p < 0.01 versus the rest of groups; ##, p < 0.01 versus control one-way ANOVA, Tukey post hoc analysis.
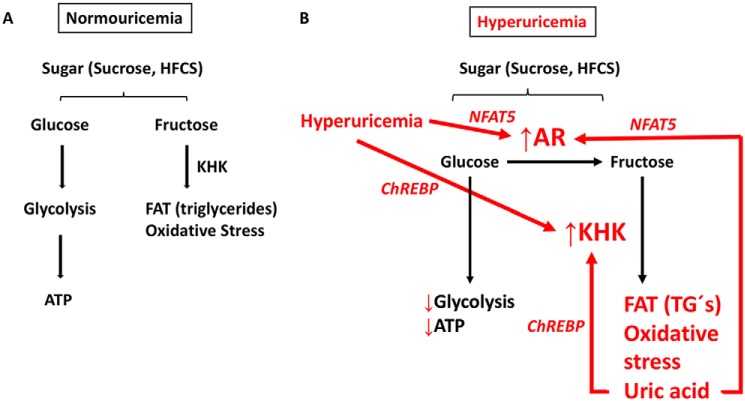
In summary, our data support the hypothesis presented in Fig. 6. Sugar, either as sucrose or high-fructose corn syrup, consists of a combination of glucose and fructose. Under normouricemic conditions (Fig. 6A), glucose is metabolized primarily through glycolysis for ATP production. Fructose is metabolized via the fructokinase/KHK for energy storage as fat while producing low amounts of oxidative stress. However, under high–uric acid conditions (Fig. 6B), AR expression is up-regulated by NFAT5. thus shifting glucose fate from glycolysis to endogenous fructose production and metabolism. Reduced glycolysis results in impaired ATP homeostasis, lower ATP levels, and uric acid/oxidative stress production. Uric acid also up-regulates KHK and fructose metabolism through the activation of the transcription factor ChREBP (22). In turn, increased fructolysis from uric acid-dependent endogenous fructose production results in marked fat accumulation, greater oxidative stress, and more uric acid accumulation, thus amplifying this vicious cycle.
Figure 6.
Schematic representing the effects of uric acid in the polyol pathway, oxidative stress, and fat accumulation. A, regular metabolism of glucose and fructose in normouricemic conditions. B, glucose is favored toward endogenous fructose production and metabolism, thus reducing glycolytic flux and promoting fat accumulation and oxidative stress.
Discussion
NAFLD is an important cause of liver disease and is strongly associated both with intake of sugary beverages (1–4, 7, 39–44) and with hyperuricemia (1, 2, 12–14, 45–51). Several studies suggest that the fructose component of added sugars may have an important role in mediating fatty liver (4, 42) and that this is mediated in part because of the generation of uric acid that occurs during fructose metabolism (9, 10, 22). Although dietary fructose constitutes a major source of fructose in the diet, recently the role of endogenously produced fructose has been shown to have a role in the fatty liver induced by high-glycemic and high-salt diets (15, 16). In humans, fructose can only be produced via the polyol (AR–SDH) pathway. Here we investigated whether uric acid might influence endogenous fructose generation via the stimulation of the AR–SDH pathway. Our primary finding was that uric acid potently increases AR expression (mRNA and protein), resulting in endogenous fructose generation and triglyceride accumulation. We further show that the mechanism involves uric acid induced intracellular oxidative stress and the induction of NFAT5 (also known as TonEBP) and that the presence of uric acid could enhance the production of fructose in response to glucose. In summary, we identify a key role for uric acid in amplifying the effects of added sugars to cause fatty liver.
The first major finding was that uric acid could stimulate AR expression in cultured HepG2 cells (a hepatocyte line), and similar documentation was shown in the liver of hyperuricemic rats (induced with the uricase inhibitor, oxonic acid) (Figs. 1, 2, and 4). This was associated with increased levels of sorbitol and fructose, documenting activation of the polyol pathway, and with triglyceride accumulation. Importantly, the activation of aldose reductase and the polyol pathway seem to be independent of any potential hyperosmolar effect because 1 mg/dl of uric acid equals 59 μmol/liter, and thus 12 mg/dl is only 0.7 mmol/KgH2O, which should translate into 0.7 mOsm/KgH2O g.
The important role of uric acid in this pathway was shown in the HepG2 cells by including probenecid in the medium, which is known to block urate uptake (52–55), and by the inclusion of a xanthine oxidase inhibitor (allopurinol) in the in vivo studies, which would reduce serum and intrahepatic uric acid levels. In both situations, the induction of AR expression, fructose generation, and triglyceride accumulation was prevented.
The second major finding was that the mechanism for the induction of AR was shown to be mediated by uric acid–induced oxidative stress, with the induction of NFAT5, a known transcription factor that drives AR expression (23, 24). Although uric acid functions as an anti-oxidant extracellularly (56), multiple studies show uric acid can induce both cytosolic and mitochondrial oxidative stress via the induction of NADPH oxidase (9, 45, 55, 57–59) and possibly by direct effects (60, 61). Oxidative stress also activates NFAT5 and AR expression (31, 32). Hence, these studies show that uric acid can accelerate fructose generation, similar to the way it also stimulates fructose metabolism by inducing the expression of fructokinase (22). The latter stimulation of fructokinase is via the induction of a different transcription factor, ChREBP (22).
Thus, these studies are important because they suggest that uric acid may contribute to the development of fatty liver in part by its ability to stimulate fructose generation and metabolism. This leads to the final key observation, which is that an elevated uric acid could potentiate the effects of high glucose to stimulate the AR pathway, resulting in augmented triglyceride accumulation. Thus, uric acid may be viewed as an amplifying pathway that may increase the biologic effects of glucose, high-salt diet, and fructose to stimulate fatty liver.
A limitation of our study is that the studies only involved cell culture and experimental animals. It will be important to determine whether lowering uric acid in humans can either prevent or improve fatty liver. Indeed, there is some tantalizing evidence that reducing either fructose intake (44) or lowering uric acid (62, 63) may have potential benefits on fatty liver. Moreover, both uric acid concentrations and fructose intake were independently associated with NASH in obese children and adolescents with NAFLD, suggesting that hyperuricemia is an aggravating factor for chronic hepatic disease (5).
In summary, we demonstrate that the relationship between fructose and uric acid is complex; although fructose metabolism generates uric acid, in turn uric acid can act as an amplifying pathway to stimulate upstream enzymes such as AR to generate more fructose and fructokinase to metabolize it. Hence, uric acid likely has an important role in driving NAFLD. We recommend placebo-controlled clinical trials to determine whether lowering uric acid can help reduce the development of NAFLD in high-risk individuals.
Experimental procedures
Materials
Cell culture medium (RPMI 1640), fetal calf serum, and antibiotics were from Gibco. Rabbit polyclonal antibody to KHK (HPA00740) was purchased from Sigma, and antibodies to Actin (4970) and CREB (9197) were from Cell Signaling Technologies, whereas the antibody to SDH (NB100-1486) was from Novus. Tubulin antibody was obtained from Santa Cruz Biotechnologies (sc-5274). Antibody to AR was a kind gift from Dr. Mark Petrash (University of Colorado), whereas antibody against NFAT5 was obtained from Dr. Moo Kwon (University of Maryland). Secondary antibodies conjugated with horseradish peroxidase were from Cell Signaling, and those conjugated with Alexa dyes were purchased from Molecular Probes (Eugene, OR). Glucose, uric acid, actinomycin D, and allopurinol were purchased from Sigma, whereas probenecid was obtained from Molecular Probes. Before using, uric acid solutions were ensured to be free of endotoxins. Also, no crystal formation in the medium was ensured by observing the solution microscopically under polarized light.
Determination of intracellular, intrahepatic, and serum uric acid and triglycerides
Cell lysates obtained with mitogen-activated protein kinase lysis buffer as well as serum collected from 8-h fasting rats were analyzed using a VetAce autoanalyzer (Alfa Wassermann, West Caldwell, NJ) as previously described (26). For triglyceride determination in cells, fat was solubilized by homogenization in 1 of ml solution containing 5% Nonidet P-40 in water, and slowly samples were exposed to 80–100 °C in a water bath for 2–5 min until the Nonidet P-40 became cloudy and then cooled down to room temperature. The samples were then centrifuged for 2 min to remove any insoluble material. Triglyceride determination with the VetAce autoanalyzer consisted in their initial breakdown into fatty acids and glycerol. Glycerol is then oxidized to generate a product that reacts with the probe to generate color at 570 nm. Similarly, uric acid determination is based in the conversion of uric acid to allantoin, H2O2, and carbon dioxide by uricase. The H2O2 is then determined by its reaction with the probe to generate color at ∼571 nm. Values obtained were normalized per mg of soluble protein in the lysates. Because rat livers contained endogenous uricase, intrahepatic uric acid in liver samples were analyzed employing the quantichrom uric acid determination kit (Bioassay Systems, Hayward, CA), which is a non–uricase-based system.
Determination of polyol pathway activity
Intracellular sorbitol, fructose, and TBARS levels were determined biochemically following manufacturer's protocol (Bioassay Systems ESBT-100, EFRU-100, and DTBA-100, respectively). For sorbitol determination, samples were deproteinized to remove endogenous SDH using 10K Spin columns (Biovision).
Silencing AR expression in HepG2 cells
AR expression was deleted by transducing HepG2 with lentiviral particles containing small hairpin RNA sequences against AR mRNA. Lentiviral particles were obtained from Santa Cruz Biotechnologies (sc-37119-v). After transduction, HepG2 cells were selected with puromycin (10 μg/ml) for generating stable clones. Data are presented from three different clones.
Luciferase reporter assay
Two different luciferase reporter constructs were engineered from the first 2 kb (pGL3-AR) and 950 bp (pGL3-ARΔTonE) 5′ upstream of the first exon of human akr1b3, which were cloned before the luciferase cassette of the pGL3-Luc vector (Promega). The luciferase activity was measured in HeLa cells using the Luciferase assay system (Promega, Madison, WI) according to the manufacturer's instructions. Briefly, cells in 6-well plates grown to 50% confluence were transiently transfected with each construct and a β-galactosidase reporter (used for normalizing the transfection efficiency). Replicates were incubated either with 4 or 12 mg/dl of uric acid for 24 h. The cells were then lysed directly in luciferase reporter lysis buffer (Promega). Supernatants from cell lysates were mixed with luciferase substrate and measured immediately with a Luminoskan Ascent DCReady luminometer (Labsystems). All transfection experiments were performed in triplicate. Luciferase activity was normalized to β-galactosidase expression.
Confocal fluorescence microscopy
HepG2 cells expressing were grown to confluency on 8-well glass slides (catalog no. 177402, NUNC, Thermo Fisher Scientific), fixed for 2 min at −20 °C with methanol, and permeabilized at room temperature with PBS containing 0.3% Triton X-100 before staining with an NFAT5 antibody (from Dr. Moo Kwon, University of Maryland). For nucleus identification, 4′,6′-diamino-2-phenylindole staining was used in combination with a fluorescence fading retardant (Vector Laboratories, Burmingdale, CA) before imaging by confocal microscopy. Immunostained preparations were imaged and analyzed using a laser-scanning confocal microscope (LSM510, Carl Zeiss, Thornwood, NY) with a 40× water immersion objective and the corresponding postacquisition software.
Protein extraction and Western blotting
Protein lysates were prepared from confluent cell cultures and rat tissue employing mitogen-activated protein kinase lysis buffer as previously described (26). Sample protein content was determined by the BCA protein assay (Pierce). 50 μg of total protein was loaded per lane for SDS-PAGE (10% w/v) analysis and then transferred to PVDF membranes. The membranes were incubated with primary antibodies and visualized using a horseradish peroxidase secondary antibody and the horseradish peroxidase Immunstar® detection kit (Bio-Rad). Chemiluminescence was recorded with an Image Station 440CF, and the results were analyzed with 1D Image software (Kodak Digital Science, Rochester, NY).
Nuclear isolation from HepG2 cells
The nuclei were purified with the NE-PER kit from Pierce following the manufacturer's protocol. Quantization of nuclear protein was performed by the BCA assay. CREB and proliferating cell nuclear antigen expression was employed for equally nuclear loading control by Western blotting analysis.
Animal model
All experiments were conducted with adherence to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. The animal protocol was approved by the Animal Care and Use Committee of the University of Colorado. Hyperuricemia was induced in male Wistar rats (230–250 g) by administering the uricase inhibitor oxonic acid (750 mg/kg/day, by gastric gavage, n = 6) during 2 weeks. Allopurinol was added to oxonic acid-receiving rats to prevent the rise in uric acid (150 mg/liter, oxonic acid + allopurinol, n = 7). Also, a Control group which received oxonic acid vehicle (1% hydroxyethyl cellulose by gavage n = 7) was also included.
At the end of the 2 weeks, the rats were euthanized by isoflurane anesthesia and abdominal aortae exsanguination. The liver was washed by perfusion with cold PBS, and the median liver lobe was excised and frozen in liquid nitrogen in fragments. Plasma aliquots and liver tissue were stored at −80 °C until further processing.
Author contributions
L. G. S.-L. and M. A. L. conceptualization; L. G. S.-L., A. A.-H., F. E. G.-A., C. C., N. L., M. K., C. A. R.-J., and M. A. L. data curation; L. G. S.-L., A. A.-H., F. E. G.-A., C. C., N. L., M. K., R. J. J., and M. A. L. formal analysis; L. G. S.-L. and M. A. L. supervision; L. G. S.-L., R. J. J., and M. A. L. funding acquisition; L. G. S.-L. and R. J. J. methodology; L. G. S.-L., R. J. J., and M. A. L. writing-original draft; A. A.-H., F. E. G.-A., C. C., N. L., and C. A. R.-J. investigation; M. K. validation; M. K. writing-review and editing.
This work was supported by National Institutes of Health Grants NIDDK 1R01DK109408-01A1 and NIDDK R01 DK108859-01. Some of the authors are members of Colorado Research Partners LLC, a start-up company developing inhibitors of fructose metabolism (M. A. L., A. A.-H., C. A. R.-J., R. J. J., and L. G. S.-L.). Dr. Johnson also has equity with a start-up company (XORT Therapeutics) developing novel xanthine oxidase inhibitors. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
- NAFLD
- nonalcoholic fatty liver disease
- AR
- aldose reductase
- SDH
- sorbitol dehydrogenase
- KHK
- ketohexokinase
- NFAT5
- nuclear factor of activated T cells 5
- CREB
- cAMP-responsive element-binding protein
- ANOVA
- analysis of variance
- TBARS
- thiobarbituric acid-reactive substances.
References
- 1. Abdelmalek M. F., Lazo M., Horska A., Bonekamp S., Lipkin E. W., Balasubramanyam A., Bantle J. P., Johnson R. J., Diehl A. M., Clark J. M., and Fatty Liver Subgroup of Look AHEAD Research Group (2012) Higher dietary fructose is associated with impaired hepatic adenosine triphosphate homeostasis in obese individuals with type 2 diabetes. Hepatology 56, 952–960 10.1016/j.jhep.2011.08.025,10.1002/hep.25741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Abdelmalek M. F., Suzuki A., Guy C., Unalp-Arida A., Colvin R., Johnson R. J., Diehl A. M., and Nonalcoholic Steatohepatitis Clinical Research Network (2010) Increased fructose consumption is associated with fibrosis severity in patients with nonalcoholic fatty liver disease. Hepatology 51, 1961–1971 10.1002/hep.23535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jensen T., Abdelmalek M. F., Sullivan S., Nadeau K. J., Green M., Roncal C., Nakagawa T., Kuwabara M., Sato Y., Kang D. H., Tolan D. R., Sanchez-Lozada L. G., Rosen H. R., Lanaspa M. A., Diehl A. M., et al. (2018) Fructose and sugar: A major mediator of non-alcoholic fatty liver disease. J. Hepatol. 68, 1063–1075 10.1016/j.jhep.2018.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ouyang X., Cirillo P., Sautin Y., McCall S., Bruchette J. L., Diehl A. M., Johnson R. J., and Abdelmalek M. F. (2008) Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J. Hepatol. 48, 993–999 10.1016/j.jhep.2008.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mosca A., Nobili V., De Vito R., Crudele A., Scorletti E., Villani A., Alisi A., and Byrne C. D. (2017) Serum uric acid concentrations and fructose consumption are independently associated with NASH in children and adolescents. J. Hepatol. 66, 1031–1036 10.1016/j.jhep.2016.12.025 [DOI] [PubMed] [Google Scholar]
- 6. Maersk M., Belza A., Stødkilde-Jorgensen H., Ringgaard S., Chabanova E., Thomsen H., Pedersen S. B., Astrup A., and Richelsen B. (2012) Sucrose-sweetened beverages increase fat storage in the liver, muscle, and visceral fat depot: a 6-mo randomized intervention study. Am. J. Clin. Nutr. 95, 283–289 10.3945/ajcn.111.022533 [DOI] [PubMed] [Google Scholar]
- 7. Ackerman Z., Oron-Herman M., Grozovski M., Rosenthal T., Pappo O., Link G., and Sela B. A. (2005) Fructose-induced fatty liver disease: hepatic effects of blood pressure and plasma triglyceride reduction. Hypertension 45, 1012–1018 10.1161/01.HYP.0000164570.20420.67 [DOI] [PubMed] [Google Scholar]
- 8. Ishimoto T., Lanaspa M. A., Le M. T., Garcia G. E., Diggle C. P., Maclean P. S., Jackman M. R., Asipu A., Roncal-Jimenez C. A., Kosugi T., Rivard C. J., Maruyama S., Rodriguez-Iturbe B., Sánchez-Lozada L. G., Bonthron D. T., et al. (2012) Opposing effects of fructokinase C and A isoforms on fructose-induced metabolic syndrome in mice. Proc. Natl. Acad. Sci. U.S.A. 109, 4320–4325 10.1073/pnas.1119908109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lanaspa M. A., Sanchez-Lozada L. G., Choi Y. J., Cicerchi C., Kanbay M., Roncal-Jimenez C. A., Ishimoto T., Li N., Marek G., Duranay M., Schreiner G., Rodriguez-Iturbe B., Nakagawa T., Kang D. H., Sautin Y. Y., et al. (2012) Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: potential role in fructose-dependent and -independent fatty liver. J. Biol. Chem. 287, 40732–40744 10.1074/jbc.M112.399899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lanaspa M. A., Cicerchi C., Garcia G., Li N., Roncal-Jimenez C. A., Rivard C. J., Hunter B., Andrés-Hernando A., Ishimoto T., Sánchez-Lozada L. G., Thomas J., Hodges R. S., Mant C. T., and Johnson R. J. (2012) Counteracting roles of AMP deaminase and AMP kinase in the development of fatty liver. PLoS One 7, e48801 10.1371/journal.pone.0048801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kuwabara M., Borghi C., Cicero A. F. G., Hisatome I., Niwa K., Ohno M., Johnson R. J., and Lanaspa M. A. (2018) Elevated serum uric acid increases risks for developing high LDL cholesterol and hypertriglyceridemia: a five-year cohort study in Japan. Int. J. Cardiol. 261, 183–188 10.1016/j.ijcard.2018.03.045 [DOI] [PubMed] [Google Scholar]
- 12. Sirota J. C., McFann K., Targher G., Johnson R. J., Chonchol M., and Jalal D. I. (2013) Elevated serum uric acid levels are associated with non-alcoholic fatty liver disease independently of metabolic syndrome features in the United States: liver ultrasound data from the National Health and Nutrition Examination Survey. Metabolism 62, 392–399 10.1016/j.metabol.2012.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xu C., Yu C., Xu L., Miao M., and Li Y. (2010) High serum uric acid increases the risk for nonalcoholic fatty liver disease: a prospective observational study. PLoS One 5, e11578 10.1371/journal.pone.0011578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Jensen T., Niwa K., Hisatome I., Kanbay M., Andres-Hernando A., Roncal-Jimenez C. A., Sato Y., Garcia G., Ohno M., Lanaspa M. A., Johnson R. J., and Kuwabara M. (2018) Increased serum uric acid over five years is a risk factor for developing fatty liver. Sci. Rep. 8, 11735 10.1038/s41598-018-30267-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lanaspa M. A., Ishimoto T., Li N., Cicerchi C., Orlicky D. J., Ruzycki P., Rivard C., Inaba S., Roncal-Jimenez C. A., Bales E. S., Diggle C. P., Asipu A., Petrash J. M., Kosugi T., Maruyama S., et al. (2013) Endogenous fructose production and metabolism in the liver contributes to the development of metabolic syndrome. Nat. Commun. 4, 2434 10.1038/ncomms3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lanaspa M. A., Kuwabara M., Andres-Hernando A., Li N., Cicerchi C., Jensen T., Orlicky D. J., Roncal-Jimenez C. A., Ishimoto T., Nakagawa T., Rodriguez-Iturbe B., MacLean P. S., and Johnson R. J. (2018) High salt intake causes leptin resistance and obesity in mice by stimulating endogenous fructose production and metabolism. Proc. Natl. Acad. Sci. U.S.A. 115, 3138–3143 10.1073/pnas.1713837115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. O'Connor T., Ireland L. S., Harrison D. J., and Hayes J. D. (1999) Major differences exist in the function and tissue-specific expression of human aflatoxin B1 aldehyde reductase and the principal human aldo-keto reductase AKR1 family members. Biochem. J. 343, 487–504 10.1042/bj3430487,10.1042/0264-6021:3430487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brown K. E., Broadhurst K. A., Mathahs M. M., Kladney R. D., Fimmel C. J., Srivastava S. K., and Brunt E. M. (2005) Immunodetection of aldose reductase in normal and diseased human liver. Histol. Histopathol. 20, 429–436 [DOI] [PubMed] [Google Scholar]
- 19. Qiu L., Lin J., Xu F., Gao Y., Zhang C., Liu Y., Luo Y., and Yang J. Y. (2012) Inhibition of aldose reductase activates hepatic peroxisome proliferator-activated receptor-α and ameliorates hepatosteatosis in diabetic db/db mice. Exp. Diabetes Res. 2012, 789730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ruepp B., Bohren K. M., and Gabbay K. H. (1996) Characterization of the osmotic response element of the human aldose reductase gene promoter. Proc. Natl. Acad. Sci. U.S.A. 93, 8624–8629 10.1073/pnas.93.16.8624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huang Z., Hong Q., Zhang X., Xiao W., Wang L., Cui S., Feng Z., Lv Y., Cai G., Chen X., and Wu D. (2017) Aldose reductase mediates endothelial cell dysfunction induced by high uric acid concentrations. Cell Commun. Signal. 15, 3 10.1186/s12964-016-0158-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lanaspa M. A., Sanchez-Lozada L. G., Cicerchi C., Li N., Roncal-Jimenez C. A., Ishimoto T., Le M., Garcia G. E., Thomas J. B., Rivard C. J., Andres-Hernando A., Hunter B., Schreiner G., Rodriguez-Iturbe B., Sautin Y. Y., et al. (2012) Uric acid stimulates fructokinase and accelerates fructose metabolism in the development of fatty liver. PLoS One 7, e47948 10.1371/journal.pone.0047948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Na K. Y., Woo S. K., Lee S. D., and Kwon H. M. (2003) Silencing of TonEBP/NFAT5 transcriptional activator by RNA interference. J. Am. Soc. Nephrol. 14, 283–288 10.1097/01.ASN.0000045050.19544.B2 [DOI] [PubMed] [Google Scholar]
- 24. Woo S. K., Lee S. D., and Kwon H. M. (2002) TonEBP transcriptional activator in the cellular response to increased osmolality. Pflugers Arch. 444, 579–585 10.1007/s00424-002-0849-2 [DOI] [PubMed] [Google Scholar]
- 25. Lanaspa M. A., Andres-Hernando A., Li N., Rivard C. J., Cicerchi C., Roncal-Jimenez C., Schrier R. W., and Berl T. (2010) The expression of aquaporin-1 in the medulla of the kidney is dependent on the transcription factor associated with hypertonicity, TonEBP. J. Biol. Chem. 285, 31694–31703 10.1074/jbc.M109.093690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lanaspa M. A., Andres-Hernando A., Rivard C. J., Dai Y., and Berl T. (2008) Hypertonic stress increases claudin-4 expression and tight junction integrity in association with MUPP1 in IMCD3 cells. Proc. Natl. Acad. Sci. U.S.A. 105, 15797–15802 10.1073/pnas.0805761105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lin L. R., Carper D., Yokoyama T., and Reddy V. N. (1993) The effect of hypertonicity on aldose reductase, αB-crystallin, and organic osmolytes in the retinal pigment epithelium. Invest. Ophthalmol. Vis. Sci. 34, 2352–2359 [PubMed] [Google Scholar]
- 28. Andres-Hernando A., Lanaspa M. A., Rivard C. J., and Berl T. (2008) Nucleoporin 88 (Nup88) is regulated by hypertonic stress in kidney cells to retain the transcription factor tonicity enhancer-binding protein (TonEBP) in the nucleus. J. Biol. Chem. 283, 25082–25090 10.1074/jbc.M802381200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Al-Attar R., Zhang Y., and Storey K. B. (2017) Osmolyte regulation by TonEBP/NFAT5 during anoxia-recovery and dehydration-rehydration stresses in the freeze-tolerant wood frog (Rana sylvatica). PeerJ 5, e2797 10.7717/peerj.2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. López-Rodríguez C., Antos C. L., Shelton J. M., Richardson J. A., Lin F., Novobrantseva T. I., Bronson R. T., Igarashi P., Rao A., and Olson E. N. (2004) Loss of NFAT5 results in renal atrophy and lack of tonicity-responsive gene expression. Proc. Natl. Acad. Sci. U.S.A. 101, 2392–2397 10.1073/pnas.0308703100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhou X., Ferraris J. D., Cai Q., Agarwal A., and Burg M. B. (2005) Increased reactive oxygen species contribute to high NaCl-induced activation of the osmoregulatory transcription factor TonEBP/OREBP. Am. J. Physiol. Renal Physiol. 289, F377–F385 10.1152/ajprenal.00463.2004 [DOI] [PubMed] [Google Scholar]
- 32. Kim N. H., Choi S., Han E. J., Hong B. K., Choi S. Y., Kwon H. M., Hwang S. Y., Cho C. S., and Kim W. U. (2014) The xanthine oxidase-NFAT5 pathway regulates macrophage activation and TLR-induced inflammatory arthritis. Eur. J. Immunol. 44, 2721–2736 10.1002/eji.201343669 [DOI] [PubMed] [Google Scholar]
- 33. Mohandas R., Sautina L., Beem E., Schuler A., Chan W. Y., Domsic J., McKenna R., Johnson R. J., and Segal M. S. (2014) Uric acid inhibition of dipeptidyl peptidase IV in vitro is dependent on the intracellular formation of triuret. Exp. Cell Res. 326, 136–142 10.1016/j.yexcr.2014.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rittner H. L., Hafner V., Klimiuk P. A., Szweda L. I., Goronzy J. J., and Weyand C. M. (1999) Aldose reductase functions as a detoxification system for lipid peroxidation products in vasculitis. J. Clin. Invest. 103, 1007–1013 10.1172/JCI4711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Hodgkinson A. D., Søndergaard K. L., Yang B., Cross D. F., Millward B. A., and Demaine A. G. (2001) Aldose reductase expression is induced by hyperglycemia in diabetic nephropathy. Kidney Int. 60, 211–218 10.1046/j.1523-1755.2001.00788.x [DOI] [PubMed] [Google Scholar]
- 36. Shah V. O., Dorin R. I., Sun Y., Braun M., and Zager P. G. (1997) Aldose reductase gene expression is increased in diabetic nephropathy. J. Clin. Endocrinol. Metab. 82, 2294–2298 10.1210/jcem.82.7.4082,10.1210/jc.82.7.2294 [DOI] [PubMed] [Google Scholar]
- 37. Lanaspa M. A., Ishimoto T., Cicerchi C., Tamura Y., Roncal-Jimenez C. A., Chen W., Tanabe K., Andres-Hernando A., Orlicky D. J., Finol E., Inaba S., Li N., Rivard C. J., Kosugi T., Sanchez-Lozada L. G., et al. (2014) Endogenous fructose production and fructokinase activation mediate renal injury in diabetic nephropathy. J. Am. Soc. Nephrol. 25, 2526–2538 10.1681/ASN.2013080901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. García-Arroyo F. E., Gonzaga G., Muñoz-Jiménez I., Blas-Marron M. G., Silverio O., Tapia E., Soto V., Ranganathan N., Ranganathan P., Vyas U., Irvin A., Ir D., Robertson C. E., Frank D. N., Johnson R. J., et al. (2018) Probiotic supplements prevented oxonic acid-induced hyperuricemia and renal damage. PLoS One 13, e0202901 10.1371/journal.pone.0202901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Abid A., Taha O., Nseir W., Farah R., Grosovski M., and Assy N. (2009) Soft drink consumption is associated with fatty liver disease independent of metabolic syndrome. J. Hepatol. 51, 918–924 10.1016/j.jhep.2009.05.033 [DOI] [PubMed] [Google Scholar]
- 40. Collison K. S., Saleh S. M., Bakheet R. H., Al-Rabiah R. K., Inglis A. L., Makhoul N. J., Maqbool Z. M., Zaidi M. Z., Al-Johi M. A., and Al-Mohanna F. A. (2009) Diabetes of the liver: the link between nonalcoholic fatty liver disease and HFCS-55. Obesity (Silver Spring) 17, 2003–2013 10.1038/oby.2009.58 [DOI] [PubMed] [Google Scholar]
- 41. Jin R., Le N. A., Liu S., Farkas Epperson M., Ziegler T. R., Welsh J. A., Jones D. P., McClain C. J., and Vos M. B. (2012) Children with NAFLD are more sensitive to the adverse metabolic effects of fructose beverages than children without NAFLD. J. Clin. Endocrinol. Metab. 97, E1088–E1098 10.1210/jc.2012-1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lim J. S., Mietus-Snyder M., Valente A., Schwarz J. M., and Lustig R. H. (2010) The role of fructose in the pathogenesis of NAFLD and the metabolic syndrome. Nat. Rev. Gastroenterol. Hepatol. 7, 251–264 10.1038/nrgastro.2010.41 [DOI] [PubMed] [Google Scholar]
- 43. Nomura K., and Yamanouchi T. (2012) The role of fructose-enriched diets in mechanisms of nonalcoholic fatty liver disease. J. Nutr. Biochem. 23, 203–208 10.1016/j.jnutbio.2011.09.006 [DOI] [PubMed] [Google Scholar]
- 44. Schwarz J. M., Noworolski S. M., Erkin-Cakmak A., Korn N. J., Wen M. J., Tai V. W., Jones G. M., Palii S. P., Velasco-Alin M., Pan K., Patterson B. W., Gugliucci A., Lustig R. H., and Mulligan K. (2017) Effects of dietary fructose restriction on liver fat, de novo lipogenesis, and insulin kinetics in children with obesity. Gastroenterology 153, 743–752 10.1053/j.gastro.2017.05.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Choi Y. J., Shin H. S., Choi H. S., Park J. W., Jo I., Oh E. S., Lee K. Y., Lee B. H., Johnson R. J., and Kang D. H. (2014) Uric acid induces fat accumulation via generation of endoplasmic reticulum stress and SREBP-1c activation in hepatocytes. Lab. Invest. 94, 1114–1125 10.1038/labinvest.2014.98 [DOI] [PubMed] [Google Scholar]
- 46. Ferreira V. S., Pernambuco R. B., Lopes E. P., Morais C. N., Rodrigues M. C., Arruda M. J., Silva L. M., and Vilar L. (2010) Frequency and risk factors associated with non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus. Arq. Bras. Endocrinol. Metabol. 54, 362–368 10.1590/S0004-27302010000400004 [DOI] [PubMed] [Google Scholar]
- 47. Lee J. W., Cho Y. K., Ryan M., Kim H., Lee S. W., Chang E., Joo K. J., Kim J. T., Kim B. S., and Sung K. C. (2010) Serum uric acid as a predictor for the development of nonalcoholic Fatty liver disease in apparently healthy subjects: a 5-year retrospective cohort study. Gut Liver 4, 378–383 10.5009/gnl.2010.4.3.378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li Y., Xu C., Yu C., Xu L., and Miao M. (2009) Association of serum uric acid level with non-alcoholic fatty liver disease: a cross-sectional study. J. Hepatol. 50, 1029–1034 10.1016/j.jhep.2008.11.021 [DOI] [PubMed] [Google Scholar]
- 49. Nakatsu Y., Seno Y., Kushiyama A., Sakoda H., Fujishiro M., Katasako A., Mori K., Matsunaga Y., Fukushima T., Kanaoka R., Yamamotoya T., Kamata H., and Asano T. (2015) The xanthine oxidase inhibitor febuxostat suppresses development of nonalcoholic steatohepatitis in a rodent model. Am. J. Physiol. Gastrointest. Liver Physiol. 309, G42–G51 10.1152/ajpgi.00443.2014 [DOI] [PubMed] [Google Scholar]
- 50. Petta S., Cammà C., Cabibi D., Di Marco V., and Craxì A. (2011) Hyperuricemia is associated with histological liver damage in patients with non-alcoholic fatty liver disease. Aliment Pharmacol. Ther. 34, 757–766 10.1111/j.1365-2036.2011.04788.x [DOI] [PubMed] [Google Scholar]
- 51. Vos M. B., Colvin R., Belt P., Molleston J. P., Murray K. F., Rosenthal P., Schwimmer J. B., Tonascia J., Unalp A., Lavine J. E., and NASH CRN Research Group (2012) Correlation of vitamin E, uric acid, and diet composition with histologic features of pediatric NAFLD. J. Pediatr. Gastroenterol. Nutr. 54, 90–96 10.1097/MPG.0b013e318229da1a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kang D. H., Han L., Ouyang X., Kahn A. M., Kanellis J., Li P., Feng L., Nakagawa T., Watanabe S., Hosoyamada M., Endou H., Lipkowitz M., Abramson R., Mu W., and Johnson R. J. (2005) Uric acid causes vascular smooth muscle cell proliferation by entering cells via a functional urate transporter. Am. J. Nephrol. 25, 425–433 10.1159/000087713 [DOI] [PubMed] [Google Scholar]
- 53. Kang D. H., Nakagawa T., Feng L., Watanabe S., Han L., Mazzali M., Truong L., Harris R., and Johnson R. J. (2002) A role for uric acid in the progression of renal disease. J. Am. Soc. Nephrol. 13, 2888–2897 10.1097/01.ASN.0000034910.58454.FD [DOI] [PubMed] [Google Scholar]
- 54. Kang D. H., Park S. K., Lee I. K., and Johnson R. J. (2005) Uric acid-induced C-reactive protein expression: implication on cell proliferation and nitric oxide production of human vascular cells. J. Am. Soc. Nephrol. 16, 3553–3562 10.1681/ASN.2005050572 [DOI] [PubMed] [Google Scholar]
- 55. Sautin Y. Y., Nakagawa T., Zharikov S., and Johnson R. J. (2007) Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am. J. Physiol. Cell Physiol. 293, C584–C596 10.1152/ajpcell.00600.2006 [DOI] [PubMed] [Google Scholar]
- 56. Ames B. N., Cathcart R., Schwiers E., and Hochstein P. (1981) Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: a hypothesis. Proc. Natl. Acad. Sci. U.S.A. 78, 6858–6862 10.1073/pnas.78.11.6858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Yu M. A., Sánchez-Lozada L. G., Johnson R. J., and Kang D. H. (2010) Oxidative stress with an activation of the renin-angiotensin system in human vascular endothelial cells as a novel mechanism of uric acid-induced endothelial dysfunction. J. Hypertens. 28, 1234–1242 [PubMed] [Google Scholar]
- 58. Sánchez-Lozada L. G., Lanaspa M. A., Cristóbal-García M., García-Arroyo F., Soto V., Cruz-Robles D., Nakagawa T., Yu M. A., Kang D. H., and Johnson R. J. (2012) Uric acid-induced endothelial dysfunction is associated with mitochondrial alterations and decreased intracellular ATP concentrations. Nephron Exp. Nephrol. 121, e71–e78 10.1159/000345509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sánchez-Lozada L. G., Soto V., Tapia E., Avila-Casado C., Sautin Y. Y., Nakagawa T., Franco M., Rodríguez-Iturbe B., and Johnson R. J. (2008) Role of oxidative stress in the renal abnormalities induced by experimental hyperuricemia. Am. J. Physiol. Renal Physiol. 295, F1134–F1141 10.1152/ajprenal.00104.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gersch C., Palii S. P., Imaram W., Kim K. M., Karumanchi S. A., Angerhofer A., Johnson R. J., and Henderson G. N. (2009) Reactions of peroxynitrite with uric acid: formation of reactive intermediates, alkylated products and triuret, and in vivo production of triuret under conditions of oxidative stress. Nucleosides Nucleotides Nucleic Acids 28, 118–149 10.1080/15257770902736400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Imaram W., Gersch C., Kim K. M., Johnson R. J., Henderson G. N., and Angerhofer A. (2010) Radicals in the reaction between peroxynitrite and uric acid identified by electron spin resonance spectroscopy and liquid chromatography mass spectrometry. Free Radic. Biol. Med. 49, 275–281 10.1016/j.freeradbiomed.2010.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Paschos P., Athyros V. G., Tsimperidis A., Katsoula A., Grammatikos N., and Giouleme O. (2018) Can serum uric acid lowering therapy contribute to the prevention or treatment of nonalcoholic fatty liver disease? Curr. Vasc. Pharmacol. 16, 269–275 10.2174/1570161115666170621082237 [DOI] [PubMed] [Google Scholar]
- 63. Sylvia S., Sedhom O. A., Salwa H. S., and Mokhles M. A. (2016) Assessment of the therapeutic effect of allopurinol in patients with nonalcoholic fatty liver disease associated with hyperuricemia by cytokeratin 18, 9th Euro Global Gastroenterology Conference, October 24–25, 2016, Valencia, Spain [Google Scholar]