Abstract
This case study describes a 21-year-old male with congenital myotonic dystrophy referred to respiratory physiotherapy with a weak cough and upper respiratory tract secretions. Mechanical insufflation–exsufflation (MI–E) was prescribed. Post initiation, the patient described a worsening of secretions and increased attendances to hospital with suspected chest infection. He also described difficulties with speaking after use of MI–E. Multidisciplinary assessment of cough as well as bulbar and swallow function resulted in a primary diagnosis of oro-pharyngeal dysphagia as well as weak cough. An alternative prophylactic therapy programme including active cycle of breathing, chest wall percussions, and manually assisted cough, was prescribed to facilitate clearance of upper airway secretions and patient comfort. The case highlights some of the risks associated with cough augmentation techniques derived from single-discipline intervention in the neuromuscular patient population. Comprehensive multidisciplinary assessment and management were key to redefining this patient’s diagnosis, allowing effective and individualised treatment.
Key Words: Neuromuscular, Cough, Mechanical insufflation–exsufflation, Bulbar, Dysphagia, Multidisciplinary team
INTRODUCTION
Myotonic dystrophy type 1 (DM1) is the most prevalent form of neuromuscular disease in adults, affecting 1 in every 8000 people worldwide [1]. It is a progressive multisystem genetic disorder caused by unstable trinucleotide (CTG) repeated expansions in untranslated DNA. There is a moderate correlation between longer CTG repeat expansions, an earlier age of onset, and more severe disease [2].
Congenital DM1 is a distinct clinical phenotype of DM1, which includes the clinical features of severe generalised weakness, hypotonia, and respiratory compromise. Weakness progresses slowly in early adulthood with severe cardiopulmonery complications manifesting into the third and fourth decade of life. Adults also present with bulbar weakness and oro-pharyngeal dysphagia (henceforth referred to as dysphagia) [3]. The average age of death is 45 years [2, 4].
Respiratory insufficiency is the leading cause of morbidity and mortality in the neuromuscular disease population [5]. Decline in respiratory muscle function results in ventilatory failure and impaired cough effectiveness leading to secretion accumulation, recurrent chest infections, and possible death [6]. Bulbar impairment (most commonly in the form of weakness) also predisposes individuals to weak cough, aspiration, and difficulty clearing oral secretions [5].
Peak cough flow (PCF) is a measure of cough strength. A PCF above 250–260 L/min may be sufficient to prevent pneumonia in people with neuromuscular disease [7, 8]. Physiotherapists assess PCF and tailor treatment to the component(s) of the cough cycle affected: inspiratory muscle strength, glottic closure, and expiratory muscle strength [9]. Bulbar impairment results in altered range, strength, or sequencing of glottic movement, which reduces PCF even in the absence of respiratory muscle weakness [10, 11]. Speech and Language Therapists (SLT) assess bulbar function via direct testing of muscle strength and range and rate of movement, plus they assess voice, speech, and swallowing. Bulbar impairment also causes dysphagia resulting in poor clearance of food, fluids, and/or saliva from the mouth and pharynx with risk of material being aspirated into the airway.
Cough augmentation is helpful for clearing pulmonary secretions and is useful in the prevention and treatment of pneumonias [5, 6, 8]. Mechanical insufflation–exsufflation (MI–E), a form of cough augmentation, is used to maintain respiratory health in advanced neuromuscular disease [10]. Positive and negative pressure is sequentially applied to the airways to facilitate upward clearance of pulmonary secretions [12]. For those with bulbar impairment, the suitability of MI–E is currently unclear. MI–E can provoke significant laryngeal adduction during inspiratory and expiratory stages in patients with bulbar impairment secondary to amyotrophic lateral sclerosis [11, 13]. Whilst pressures should be titrated to suit the needs of the individual [11–14]; those with severe bulbar involvement are often excluded from studies evaluating the efficacy of MI–E [10, 15]. This lack of consensus leaves clinicians to assess the risk versus benefit of MI–E on an individual patient basis. This case study considers the relevance of comprehensive bulbar assessment to inform prophylactic cough augmentation in neuromuscular disease and suitability of MI–E in the presence of severe bulbar impairment.
CASE PRESENTATION
A 21-year-old male with a diagnosis of genetically confirmed congenital DM1 was electively admitted to adult neuromuscular services in October 2016 for review of his MI–E settings. MI–E had previously been provided by the respiratory physiotherapist upon request of the treating medical team because of weak cough and difficulty clearing secretions. Following initiation of MI–E, the patient had three hospital admissions, all for suspected chest infections. The patient’s mother also described an accumulation of secretions and difficulty speaking for up to an hour after use of MI–E. A referral was made to physiotherapy to adjust MI–E settings.
Relevant medical history included right hemiplegic cerebral palsy due to a neonatal intracerebral haemorrhage, adenotonsillectomy, sialorrhea, and tongue reduction surgery. A diagnosis of respiratory failure was made at 18 years of age when non-invasive ventilation was prescribed.
Informed consent was obtained from the patient after the nature of the procedure had been fully explained.
EXAMINATION
The individual had marked scoliosis (cobb angle > 90° ) with an asymmetrical posture in his powered wheelchair. Weight was reported to be stable at 51.2 kg. Peak cough flow was 140 L/min as measured by Wrights Mini Peak Flow Meter with a facemask. Maximum insufflation capacity was not completed owing to an inability to breath stack, suggestive of dysfunction at the level of the glottis. Clinical signs of pooled oral and pharyngeal secretions were evident despite active prescription of hyoscine. The physiotherapist initiated a referral to SLT to establish the influence of bulbar muscle weakness on the individual’s sialorrhea, weak cough, and poor outcomes of MI–E.
SLT assessment identified: macroglossia (large tongue), mandibular prognathism (protrusion of the mandible beyond the top teeth), bilateral weakness of the face and tongue, pooled saliva in the oral cavity, moderate–severe dysarthria, and intermittent wet vocal quality suggestive of a severe underlying dysphagia secondary to weakness.
We hypothesised that bulbar weakness was the primary cause of the patient’s weak cough. This same impairment was likely to be affecting swallowing function resulting in unmanaged saliva and pooled food and fluids in the pharynx.
INVESTIGATIONS
Investigations aimed to (i) examine the bulbar impairment and its impact on cough and swallowing and (ii) understand the influence of the bulbar impairment on the suitability of use of MI–E.
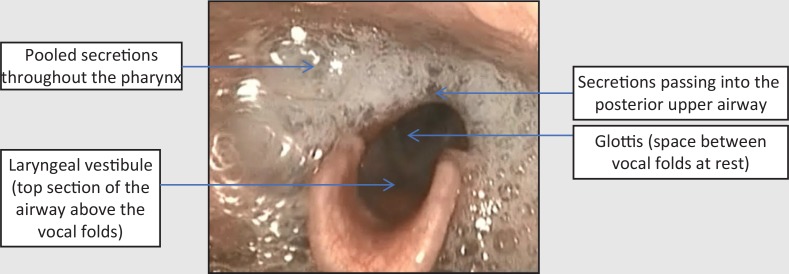
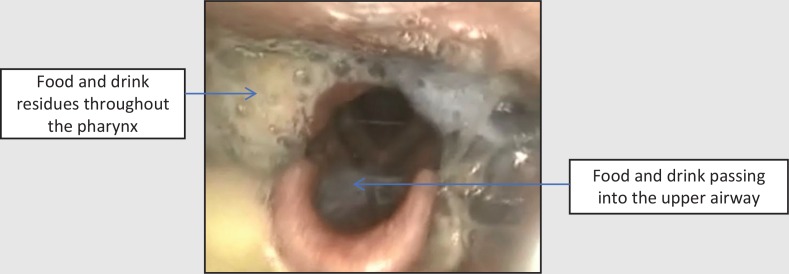
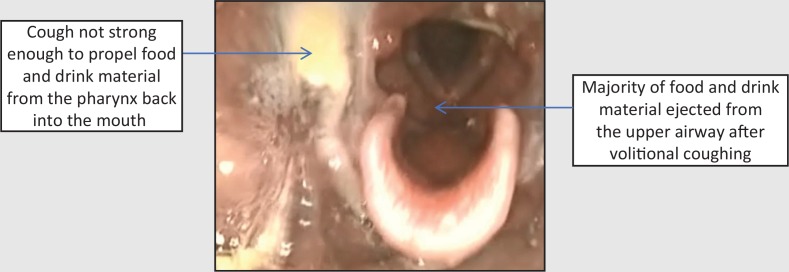
Assessment via fibreoptic endoscopic evaluation of swallowing (FEES) revealed gross pooling of secretions throughout the pharynx at rest which were breathed into and out of the airway. This indicated a diffuse pharyngeal motor and sensory impairment (Figure 1). On provision of food and drink, material remained in the pharynx entering the laryngeal vestibule with eventual aspiration (Figure 2). Volitional coughing was strong enough to eject some material from the laryngeal vestibule but not strong enough to propel material from the pharynx into the mouth (Figure 3). Secretions, food, and fluid continued to spill into the laryngeal vestibule without clearance with more to eat and drink.
Figure 1. Fibreoptic endoscopic evaluation of swallowing image at rest suggesting diffuse motor and sensory pharyngeal impairment.
Figure 2. Fibreoptic endoscopic evaluation of swallowing image after food and drink: diffuse residues with spillage into the upper airway.
Figure 3. Fibreoptic endoscopic evaluation of swallowing images after volitional coughing: clearance from the airway but not the pharynx.
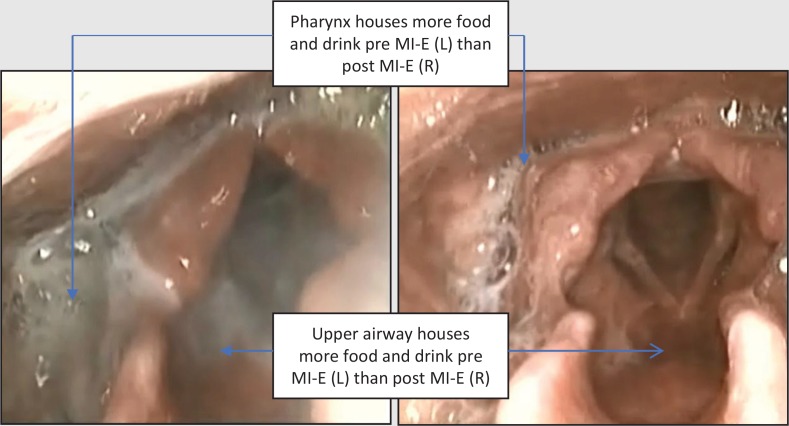
To support decisions regarding ongoing use of MI–E, nasendoscopic images were recorded before and after its use (insufflation pressure +10 cmH2O, exsufflation pressure –40 cmH2O manual). After use of MI–E, secretions were immediately expectorated from the oral cavity and the nasendoscopic view of the pharynx and upper airway was visually clearer (Figure 4).
Figure 4. Fibreoptic endoscopic evaluation of swallowing images pre- (left) and post- (right) mechanical insufflation–exsufflation.
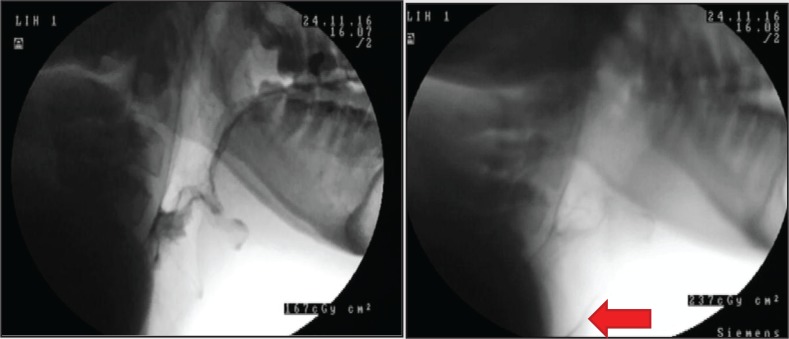
Videofluoroscopy (VFS) was conducted to examine the impact of MI–E on the lower airway, anatomy that cannot be visualised via FEES. Prior to MI–E, swallowing strategies were provided to clear the pharynx as much as possible and continued until residues failed to clear any further. Figure 5 shows pre- and post-MI–E VFS images. Material not visible prior to MI–E was subsequently seen in the lower airway suggesting that although MI–E is useful to propel upper airway material from the pharynx to mouth with low inspiratory pressures, it may have also propelled pooled material into the lower airway.
Figure 5. Videofluoroscopy images pre (left) and post (right) mechanical insufflation–exsufflation (MI–E). Arrow on right highlights material in trachea not evident pre MI-E.
A diagnosis of severe bulbar impairment affecting both cough and swallow was confirmed. Poor glottic function contributed to poor cough strength. Poor swallowing function resulted in pharyngeal residues of food, fluid, and pooled saliva. Using MI–E in the presence of pharyngeal residues is likely to have provoked aspiration.
MANAGEMENT
Given the severity of the dysphagia, a recommendation was made for enteral feeding to eliminate risk of aspiration from oral food and fluids. Pharmacological review was requested to optimise anticholinergic management of saliva. Given the likely provocation of aspiration from MI–E, the history of patient discomfort following its use, and the multiple hospital attendances since its prescription, a recommendation was made to cease MI–E until dysphagia and secretion management had been optimised.
Active cycle of breathing exercises and manual therapeutic techniques including chest wall percussions and manually assisted cough were prescribed as alternative chest clearance techniques. Continued use of regular oral suction to clear excess salvia was advised. Recommendations were made that MI–E should still be considered in the event of an acute chest infection where the necessity of airway clearance for optimal ventilation may likely outweigh chronic risks of infection secondary to provoked aspiration.
OUTCOMES
After balancing the risks and benefits of oral and enteral feeding, the patient elected to continue eating and drinking in the least restrictive manner unless he became critically unwell with aspiration pneumonia. In June 2017, eight months after an alternative chest clearance programme had been implemented, the individual reported a reduction in upper respiratory tract secretions and denied any further hospital admissions. He remains under the care of the local SLT team for monitoring of his symptoms and continues to have regular discussions regarding enteral feeding.
DISCUSSION
Respiratory insufficiency secondary to neuromuscular disease can lead to ventilatory and cough failure resulting in recurrent pneumonias [6]. Dysphagia and bulbar weakness provoke symptoms similar to those associated with respiratory weakness, namely upper respiratory secretions, chest infections, and weak cough. In this case, respiratory symptoms were indicative of both dysphagia and cough failure. We argue that implementation of MI–E without having explored the significance of the dysphagia and bulbar impairment may have resulted in recurrent aspiration and persistence of these respiratory symptoms. Consideration of dysphagia should therefore be integral to routine neuromuscular respiratory assessment.
There is a growing literature regarding implementation of MI–E in patients with neuromuscular disease and bulbar impairment [11, 13, 16, 17]. The need for individual titration of MI–E in patients with bulbar impairment to minimise laryngeal adduction during insufflation and exsufflation is well documented [11, 13]. Bulbar impairment can however lead to dysphagia. Studies have not explored the influence of this dysphagia, which may constitute a contraindication for MI–E.
Retained oral and pharyngeal residues are well documented in DM1 [18, 19] and are a symptom of other neuromuscular disease groups [20]. These residues are the primary concern for patients requiring cough augmentation, regardless of primary aetiology. Although the additional diagnoses of cerebral palsy and scoliosis may also have affected swallowing and respiratory function in this case, examining this influence is beyond the scope of this report.
MI–E settings of insufflation (+10 cmH2O) and exsufflation (−40 cmH2O) with a low flow were tailored to bias upward movement of secretions in this case. In the presence of pharyngeal secretions caused by dysphagia, positive pressure is likely to have provoked aspiration as well as support some upward clearance to the mouth. Daily provoked aspiration as a consequence of a chest clearance plan undermines the very purpose for which it has been implemented; hence, the decision to cease rather than titrate MI–E. Nasendoscopy was a useful tool in establishing the presence of pharyngeal secretions and the need for dysphagia management prior to provision of positive pressure adjuncts.
We advocate for collaborative working between physiotherapy and speech and language therapy in the context of patient-centred prophylactic neuromuscular respiratory management.
CONCLUSIONS
Careful evaluation of the physiological benefits and consequences of cough augmentation is required when assessing people with bulbar impairment. Clinicians need to challenge the habits of prophylactic MI-E provision without considering the potential impact of providing positive pressure adjuncts in the presence of pooled secretions secondary to an underlying dysphagia. Multidisciplinary collaboration is essential in providing individually tailored care in this complex patient group.
DISCLOSURES
All authors contributed to the conception or design of the work, the acquisition, analysis, or interpretation of the data. All authors were involved in drafting and commenting on the paper and have approved the final version. This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors declare no financial relationships with any organizations that might have an interest in the submitted work in the previous three years and no other relationships or activities that could appear to have influenced the submitted work.
REFERENCES
- 1.Emery AE. Population frequencies of inherited neuromuscular diseases – A world survey. Neuromuscul Disord 1991;1(1): 19–29. 10.1016/0960-8966(9190039-U [DOI] [PubMed] [Google Scholar]
- 2.Turner C, Hilton-Jones D. Myotonic dystrophy: Diagnosis, management and new therapies. Curr Opin Neurol 2014;27: 599–606. 10.1097/WCO.0000000000000128 [DOI] [PubMed] [Google Scholar]
- 3.LaDonna KA, Koopman WJ, Venance SL. Myotonic dystrophy (DM1) and dysphagia: The need for dysphagia management guidelines and an assessment tool. Can J Neurosci Nurs 2011;33(1): 42–6. [PubMed] [Google Scholar]
- 4.Turner C, Hilton-Jones D. The myotonic dystrophies: Diagnosis and management. J Neurol Neurosurg Psychiatr 2008;81(4): 358–67. 10.1136/jnnp.2008.158261 [DOI] [PubMed] [Google Scholar]
- 5.Boitano LJ. Management of airway clearance in neuromuscular disease. Respir Care 2006;51(8): 913–22. [PubMed] [Google Scholar]
- 6.Benditt JO, Boitano LJ. Pulmonary issues in patients with chronic neuromuscular disease. Am J Respir Crit Care Med 2013;187: 1046–55. 10.1164/rccm.201210-1804CI [DOI] [PubMed] [Google Scholar]
- 7.Dohna-Schwake C, Ragette R, Teschler H, et al. Predictors of severe chest infections in pediatric neuromuscular disorders. Neuromuscul Disord 2006;16: 325–8. 10.1016/j.nmd.2006.02.003 [DOI] [PubMed] [Google Scholar]
- 8.Kang SW, Bach JR. Maximum insufflation capacity: Vital capacity and cough flows in neuromuscular disease. Am J Phys Med Rehabil 2000;79: 222–7. 10.1097/00002060-200005000-00002 [DOI] [PubMed] [Google Scholar]
- 9.Toussaint M, Chatwin M, Gonzalez J, et al. ENMC respiratory therapy consortium. 228th ENMC International Workshop: Airway clearance techniques in neuromuscular disorders Naarden, The Netherlands, 3–5 March, 2017. Neuromuscul Disord 2018;28: 289–98. 10.1016/j.nmd.2017.10.008 [DOI] [PubMed] [Google Scholar]
- 10.Chatwin M, Simmonds A. The addition of mechanical insufflation/exsufflation shortens airway-clearance sessions in neuromuscular patients with chest infection. Respir Care 2009;54(11): 1473–9 [PubMed] [Google Scholar]
- 11.Anderson T, Sandnes A, Brekka AK, et al. Laryngeal response patterns influence the efficacy of mechanical assisted cough in amyotrophic lateral sclerosis. Thorax 2017;72: 221–9. 10.1136/thoraxjnl-2015-207555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bott J, Blumenthal S, Buxton M, et al. Physiotherapy management of the adult, medical spontaneously breathing patient. Thorax 2009;64(Suppl I): i1–i51. 10.1136/thx.2008.110726 [DOI] [PubMed] [Google Scholar]
- 13.Anderson TM, Sandnes A, Fondenes O, et al. Laryngeal responses to mechanically assisted cough in progressing amyotrophic lateral sclerosis. Respir Care 2018;63(5): 538–49. 10.4187/respcare.05924 [DOI] [PubMed] [Google Scholar]
- 14.Senet C, Golmard JL, Salachas F, et al. A comparison of assisted cough techniques in stable patients with severe respiratory insufficiency due to amyotrophic lateral sclerosis. Amyotroph Lateral Scler 2011;12: 26–32. 10.3109/17482968.2010.535541 [DOI] [PubMed] [Google Scholar]
- 15.Chatwin M, Ross E, Hart N, et al. Cough augmentation with mechanical insufflation/exsufflation in patients with neuromuscular weakness. European Respiratory Journal 2003;21(3): 502–8. [DOI] [PubMed] [Google Scholar]
- 16.Hadjikoutis S, Wiles CM. Respiratory complications related to bulbar dysfunction in motor neuron disease. Acta Neurol Scand 2001;103: 207–13. 10.1034/j.1600-0404.2001.103004207.x [DOI] [PubMed] [Google Scholar]
- 17.Simmonds AK. Progress in respiratory management of bulbar complications of motor neuron disease/amyotrophic lateral sclerosis? Thorax 2016;72(3): 199–201. 10.1136/thoraxjnl-2016-208919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pilz W, Baijens LWJ, Passos VL, et al. Swallowing assessment in myotonic dystrophy type 1 using fiberoptic endoscopic evaluation of swallowing (FEES). Neuromuscul Disord 2014;24(12): 1054–62. 10.1016/j.nmd.2014.06.002 [DOI] [PubMed] [Google Scholar]
- 19.Leonard RJ, Kendall KA, Johnson R, et al. Swallowing in myotonic muscular dystrophy: A videofluoroscopy study. Arch Phys Med Rehabil 2001;82(7): 979–85. 10.1053/apmr.2001.23962 [DOI] [PubMed] [Google Scholar]
- 20.Leighton SE, Burton MJ, Lumd WS, et al. Swallowing in motor neurone disease. J Roy Soc Med 1994;87(12): 801–5. [PMC free article] [PubMed] [Google Scholar]