Abstract
Objective
Candida albicans and Candida dubliniensis are germ tube-positive pathogenic yeast species. Accurate identification of these two species is warranted since C. albicans is a highly pathogenic species while C. dubliniensis exhibits increased adherence to buccal epithelial cells, reduced susceptibility to azoles and resistance to flucytosine. We have developed a duplex real-time PCR assay for rapid detection and differentiation between clinical C. albicans and C. dubliniensis isolates.
Materials and Methods
A duplex real-time PCR assay was developed by using two species-specific primer pairs and SYBR Green dye to differentiate C. albicans and C. dubliniensis isolates via melting curve analysis of real-time PCR amplicons. Amplification products were also analyzed by agarose gel electrophoresis to confirm real-time PCR results.
Results
Melting temperatures (Tm) for reference strains of C. albicans and C. dubliniensis were 86.55 and 82.75°C, respectively. No amplicon was obtained with DNA from reference strains of 8 other common Candida spp. When real-time PCR was applied on 226 clinical isolates previously identified by the Vitek 2 system and/or PCR sequencing of rDNA, Tm values for C. albicans (n = 113) and C. dubliniensis (n = 98) were 86.68 ± 0.529 and 82.616 ± 0.535°C, respectively. The results were confirmed by agarose gel electrophoresis. No amplicon was obtained from 15 isolates belonging to 9 other Candida spp.
Conclusions
The real-time PCR assay described here does not require prior identification of clinical yeast isolates as C. albicans/C. dubliniensis by germ tube formation and accurately reports results within 2 h. Detection of amplicons by agarose gel electrophoresis is also suitable for resource-poor settings devoid of real-time PCR facilities.
Key Words: Candida albicans, Candida dubliniensis, Real-time polymerase chain reaction, Melting curve analysis
Significance of the Study
Accurate identification of Candida albicans and Candida dubliniensis is required as the two species vary in their pathogenicity and susceptibility to antifungal drugs. We have developed and evaluated a simple and rapid SYBR Green dye-based real-time PCR assay to differentiate these two species among clinical Candida spp. isolates. The method will help in the proper management of patients with invasive Candida infections.
Introduction
The predominant pathogenic Candida species in susceptible human hosts is Candida albicans [1, 2, 3]. However, recent years have also witnessed an increasing incidence of episodes of candidemia and invasive candidiasis by non-albicans Candida spp. including Candida dubliniensis [1, 2, 3]. C. dubliniensis shares many phenotypic characteristics such as the ability to form chlamydospores on cornmeal agar and germ tubes in serum with C. albicans [4, 5]. As the germ tube test is mainly used for the differentiation of C. albicans from other Candida species, routine identification of C. albicans based solely on this test leads to misidentification of some C. albicans isolates in routine mycology laboratories [6]. Accurate identification is desirable since C. albicans is the most prevalent and most pathogenic species while C. dubliniensis exhibits increased adherence to buccal epithelial cells, reduced susceptibility to azoles and genotype-specific resistance to flucytosine [4, 5, 6, 7]. The role of C. dubliniensis as a bloodstream pathogen has been increasingly recognized in recent years as it was recorded as the third or fourth most common Candida spp. causing candidemia, usually surpassing Candida tropicalis and even Candida parapsilosis [8, 9, 10, 11, 12, 13]. The isolation frequency of C. dubliniensis from the oral cavity is high, particularly from cancer patients, and it has been isolated in Kuwait from a variety of clinical specimens including blood, thus highlighting the increasing role of this non-albicans Candida species in human infections [9, 13, 14, 15].
Phenotypic tests such as the formation of fringed/rough colonies on various seed agar-based media [16, 17] and tobacco agar [18], production of dark green colonies on CHROMagar Candida [19] and lack of growth in hypertonic Sabouraud dextrose broth [20] have been used to differentiate C. dubliniensis from C. albicans. However, these tests can only be applied on clinical isolates presumptively identified as C. albicans/C. dubliniensis by another test (such as germ tube formation), requiring a further 1–2 days and are not 100% specific [6]. Variations in growth conditions (incubation temperature, repeated subculturing and storage) may also impede accurate identification of these two species by phenotypic tests. Commercially available yeast identification systems (Vitek 2 ID-YST, API 20C and ID32C) based on assimilation of carbon/other compounds are useful for differentiating various Candida spp.; however, these methods are expensive and also take at least 1–2 days for results to be reported. The matrix-assisted laser desorption/ionization time-of-flight mass spectrometry has also been used for rapid discrimination between C. albicans and C. dubliniensis isolates. However, the method requires fresh cultures, and the Bruker Biotyper yields low scores and unacceptable identification for some C. dubliniensis isolates [21]. Molecular techniques offer unambiguous differentiation, and PCR-based methods are mostly employed. However, some PCR-based methods are complex and time-consuming while others are based on intronic sequences that may lead to erroneous results due to variations in intronic sequences among different strains [22, 23, 24]. In this study, we describe a duplex real-time PCR assay using primers derived from highly conserved rDNA sequences and used melting point analysis with SYBR Green dye for rapid detection and differentiation of strains of C. albicans and C. dubliniensis.
Material and Methods
Reference Strains and Clinical Isolates
Reference strains of C. albicans (ATCC 90029), C. dubliniensis (CD36), C. parapsilosis (ATCC 22019), Candida orthopsilosis (ATCC 96139), Candida glabrata (ATCC 15545), Candida nivariensis (CBS 9983), C. tropicalis (ATCC 750), Candida lusitaniae (CBS 1944), Candida guilliermondii (ATCC 6021) and Candida krusei (CBS 6258) were used. Clinical specimens including blood were collected from patients at various hospitals across Kuwait as part of routine patient care. Specimens were collected after obtaining consent from patients as part of the routine diagnostic workup. The isolates were sent to the Mycology Reference Laboratory, Department of Microbiology, Faculty of Medicine, Kuwait University, for identification and antifungal susceptibility testing. Clinical Candida isolates (n = 226) including C. albicans (n = 113), C. dubliniensis (n = 98) and other clinical Candida spp. (n = 15) were tested for evaluation of real-time PCR. All 226 clinical Candida spp. isolates were previously identified by the Vitek 2 yeast identification system and tested for germ tube formation in horse serum. All germ tube positive isolates were presumptively identified as C. albicans/C. dubliniensis.
Template DNA Preparation and Real-Time PCR Assay
A loopful of yeast colony was suspended in 1 mL of sterile water in a microcentrifuge tube containing 50 mg Chelex-100 (Sigma-Aldrich Co., St. Louis, MO, USA); the contents were heated at 95°C for 20 min and then centrifuged. The supernatant was transferred to a new tube and then used as source of genomic DNA in real-time PCR. Two species-specific primer pairs derived from the internally transcribed spacer (ITS) region (comprising ITS-1, 5.8S rRNA and ITS-2) of ribosomal DNA (rDNA) were designed for differentiation of C. albicans and C. dubliniensis strains by real-time PCR. For this purpose, sequences of the ITS region of rDNA from different Candida species, Aspergillus species and other phylogenetically related fungi were downloaded from the GenBank database and aligned using clustal omega (https://www.ebi.ac.uk/Tools/msa/clustalo/). Although we did not use any specific software for designing primers, species-specific sequences for primers SACALF and SACALR for C. albicans and SACDUF and SACDUR for C. dubliniensis were designed based on multiple sequence alignment (Table 1). The sequences of the primers, particularly at the 3′ end, were also inspected carefully to ensure that they will not form primer dimers. Species specificity of SACALF + SACALR primers for C. albicans and SACDUF + SACDUR primers for C. dubliniensis was indicated by BLAST searches (http://www/ncbi.nlm.nih.gov/BLAST/blast.cgi?) as they showed complete identity with the corresponding sequences only from these two species and not with other Candida species, Aspergillus species or other fungi. The LightCycler FastStart DNA Master SYBR Green kit and instrument (Roche Diagnostics, Mannheim, Germany) were used for amplification and analysis as directed by the manufacturer. Reaction mixture (in 20 μL) contained LightCycler FastStart DNA Master SYBR Green, 5 pmol of each (SACALF, SACALR, SACDUF and SACDUR) primer, 3 mM MgCl2 and 1 μL of template DNA. Cycling conditions included an initial denaturation at 95°C for 2 min followed by 30 cycles of 95°C for 10 s, 58°C for 10 s and 72°C for 15 s. The amplified DNA was detected by fluorescence quantification of the double-stranded DNA binding dye SYBR Green by melting curve analysis. The melting curve analysis consisted of 1 cycle at 72°C for 15 s and then an increase in temperature to 95°C at 0.1°C/s. Amplification products (10 μL) were also analyzed by agarose gel electrophoresis, performed as described previously [25], to confirm the results of real-time PCR.
Table 1.
Specific features and sequences of oligonucleotide primers used in this study and the melting temperature values of amplicons from C. albicans and C. dubliniensis
| Primer | Target gene | Purpose | DNA sequence | Amplicon size, bp | Melting temperature Tm, °C |
|---|---|---|---|---|---|
| SACALF | ITS-1 | Forward primer for C. albicans | 5′-TTTATCAACTTGTCACACCAGA-3′ | ~354 | 86.55 |
| SACALR | ITS-2 | Reverse primer for C. albicans | 5′-GGTCAAAGTTTGAAGATATACGT-3′ | ||
| SACDUF | ITS-2 | Forward primer for C. dubliniensis | 5′-TTGGGTTTGCTTGAAAGATGAT-3′ | ~119 | 82.75 |
| SACDUR | ITS-2 | Reverse primer for C. dubliniensis | 5′-AAAGTTTGAAGAATAAAATGGC-3′ | ||
| ITS-1 | 18S rRNA | Panfungal forward primer for ITS region | 5′-TCCGTAGGTGAACCTGCGG-3′ | ~400–900 | not done |
| ITS-4 | 28S rRNA | Panfungal reverse primer for ITS region | 5′-TCTTTTCCTCCGCTTATTGATATG-3′ |
The Tm values are shown for amplicons obtained from C. albicans ATCC 90029 and C. dubliniensis CD36 reference strains.
PCR Sequencing of ITS Region of rDNA
The ITS region of rDNA from selected isolates was amplified by using panfungal ITS1 and ITS4 primers and the amplicons were sequenced by using internal (ITS1FS, ITS2, ITS3 and ITS4RS) primers, as described in detail previously [14].
Results
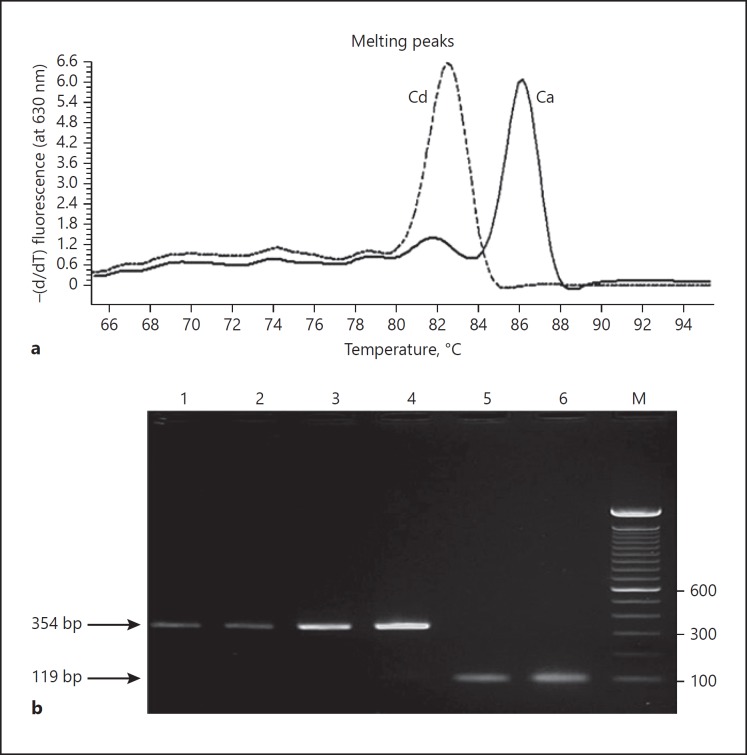
Melting temperatures (Tm) of amplicons were measured from melting curves derived by continuous monitoring of fluorescence during the temperature ramp. Recorded Tm values for C. albicans ATCC 90029 (86.55°C) and C. dubliniensis CD36 (82.75°C) showed a difference of nearly 4°C which is sufficient for their accurate differentiation by real-time PCR (Fig. 1). Reference strains of other Candida species or human DNA extracted from the serum of healthy subjects or controls lacking genomic DNA yielded negative (no amplification) results. Although DNA samples from Aspergillus and other molds were not tested, based on the results of BLAST searches of primer sequences, they are not expected to yield amplicons either. Agarose gels of real-time PCR products confirmed the amplification of DNA fragments of expected size for both, C. albicans and C. dubliniensis (Table 1), and no primer dimer artifacts were visible indicating that Tm values were derived from expected amplicons only (data not shown). The analytical sensitivity was determined by extracting DNA from serial dilutions of pure cell suspension of C. albicans culture, and at least 5 colony-forming units (CFUs) were needed for a positive result in real-time PCR. Similar results were obtained when the assay was repeated demonstrating the reproducibility of the test. The amplicons from reference strains always yielded Tm values characteristic for C. albicans (approx. 86.5°C) and C. dubliniensis (approx. 82.7°C). When real-time PCR was applied on template DNA from 226 clinical isolates speciated by the Vitek 2 yeast identification system, the measured Tm values for C. albicans (n = 113) and C. dubliniensis (n = 98) were 86.68 ± 0.529 and 82.616 ± 0.535°C, respectively (Fig. 1). Slight variations in Tm values are expected since the ITS region sequences vary slightly among different strains of both C. albicans and C. dubliniensis [5, 7]. Again, no amplification was obtained from other Candida species (Table 2). Identification of all 98 C. dubliniensis, 15 other Candida spp. and 73 selected C. albicans strains was confirmed by PCR sequencing of the ITS region of rDNA. With the rapid DNA extraction method from clinical isolates described here, the whole procedure could be completed within 2 h.
Fig. 1.
a Melting curve analysis of amplicons of real-time PCR assay with SYBR Green I dye. The solid line (Ca) is C. albicans ATCC 90029; the dashed line (Cd) is C. dubliniensis CD36. b An agarose gel of real-time PCR amplified product with DNA from 4 different clinical C. albicans isolates (lanes 1–4) and 2 different clinical C. dubliniensis isolates (lanes 5 and 6). Amplicons from C. albicans (354 bp) and C. dubliniensis (119 bp) are marked by arrows. DNA from reference/clinical isolates of other Candida species was not amplified in real-time PCR (data not shown). Lane M is a 100-bp DNA ladder, and positions of migration of 100-, 300- and 600-bp fragments are marked.
Table 2.
Evaluation of real-time PCR for detection and differentiation of C. albicans and C. dubliniensis among 226 clinical Candida isolates speciated by Vitek 2 yeast identification system
| Candida species by Vitek 2 | Clinical isolates tested, n | Tm by melting curve analysis (mean + SD), ° C | C. albicans or C. dubliniensis isolates correctly detected by real-time PCR, n | Isolates sequenced, n |
|---|---|---|---|---|
| C. albicans | 113 | 86.680±0.529 | 113 | 73 |
| C. dubliniensis | 98 | 82.616±0.535 | 98 | 98 |
| C. glabrata | 2 | no amplification detected | not applicable | 2 |
| C. parapsilosis | 2 | no amplification detected | not applicable | 2 |
| C. orthopsilosis | 2 | no amplification detected | not applicable | 2 |
| C. tropicalis | 2 | no amplification detected | not applicable | 2 |
| C. lusitaniae | 2 | no amplification detected | not applicable | 2 |
| C. krusei | 2 | no amplification detected | not applicable | 2 |
| C. haemulonii | 1 | no amplification detected | not applicable | 1 |
| C. famata | 1 | no amplification detected | not applicable | 1 |
| C. kefyr | 1 | no amplification detected | not applicable | 1 |
Discussion
Although several end point PCR-based methods targeting rDNA have been described previously for the detection and differentiation of C. albicans and C. dubliniensis, they usually involve two separate PCR reactions for each strain [22, 26, 27] or further manipulations (such as restriction digestion of amplicons to generate restriction fragment length polymorphism or DNA sequencing) are needed for species-specific identification [22, 23, 26, 27, 28, 29]. These additional steps add to the cost of the test and/or consume additional time, thus delaying the results. In a previous study, we developed a thermal duplex PCR assay with two pairs of species-specific primers for detection and differentiation of strains of C. albicans and C. dubliniensis, and the amplicons were detected by agarose gel electrophoresis [30]. However, when the same primers were used in real-time PCR, the difference in Tm values between C. albicans and C. dubliniensis strains was small which was not suitable for routine use as the ITS region sequences vary among clinical C. albicans and C. dubliniensis strains [5, 7, 31]. Thus, other sets of primers were tested, and the primers used in this study yielded a Tm difference of nearly 4°C between reference strains of C. albicans and C. dubliniensis which was most suitable for accurate screening of a large number of samples. A novel real-time PCR assay was described previously for the detection of 6 Candida species including C. albicans and C. dubliniensis [32]. The assay involved amplification of the ITS region of rDNA with pan-Candida primers and detection of amplicons by the LightCycler with 6 specific probe primers corresponding to C. albicans, C. dubliniensis, C. tropicalis, C. parapsilosis, C. glabrata and C. krusei [32]. The DNA was extracted by the High Pure PCR template preparation kit, the probe primers were labeled with Cy5 fluorescent dye and the analytical sensitivity of the assay was reported as 1 CFU per PCR [32]. Although the analytical sensitivity of our assay was slightly lower (5 CFU per PCR in our assay), the DNA was extracted by a more rapid and inexpensive but less efficient boiling method, and the amplicons were detected directly with SYBR Green dye without reliance on expensive probe primers. Another real-time PCR assay using melting point analysis has also been described for detection and differentiation of C. albicans, C. dubliniensis, C. tropicalis and C. glabrata strains [33]. The target chosen in this study was 18S rRNA gene, the DNA was obtained from Candida isolates by using a commercial DNA extraction kit and PCR amplification was performed in LightCycler with pan-Candida primers followed by detection of amplicons by hybridization with 4 separate species-specific probe primers labeled with a fluorescent dye [33]. Three real-time PCR assays using SYBR Green dye have also been described [34, 35, 36]. Two of these real-time PCR assays used panfungal primers targeting the ITS region of rDNA [34, 35]. The panfungal nature of the primers makes prior identification of strains of C. albicans/C. dubliniensis by another rapid test (such as the germ tube test) absolutely essential to avoid misidentification of other Candida/yeast species (false-positive results) and the additional step is also time-consuming [34, 35]. Furthermore, the difference in Tm values for C. albicans and C. dubliniensis strains in these reports was either small (approx. 0.5°C) or negligible which may lead to misidentification of some isolates since ITS region sequences vary among clinical C. albicans and C. dubliniensis isolates [5, 7, 31]. The other method is a nested PCR involving a first round of amplification with pan-Candida primers followed by real-time PCR amplification with 8 different species-specific primer pairs for the identification of 8 different Candida species including C. albicans and C. dubliniensis [36]. The additional requirement of the nested step in this procedure [36] is time-consuming, increases cost and the possibility of false-positive results due to amplicon carry-over. Our real-time PCR assay involves only one round of amplification and the method can also be used as a thermal PCR assay with detection of amplicons by agarose gels (Fig. 1b) for detection and differentiation of C. albicans and C. dubliniensis for resource-limited mycology laboratories that do not have a real-time PCR machine.
A limitation of our study is that the real-time PCR assay described here was only tested with culture isolates but was not tested for the detection of C. albicans and C. dubliniensis directly in clinical specimens obtained from patients in Kuwait.
In conclusion, the real-time PCR method based on melting point analysis with SYBR Green dye described here is a simple and rapid molecular tool for accurate detection and differentiation of strains of C. albicans and C. dubliniensis without relying on germ tube or other such tests. The method described here also does not require the use of expensive hybridization probes specific for C. albicans and C. dubliniensis.
Statement of Ethics
Specimens were collected after obtaining consent from patients as part of the routine diagnostic workup.
Disclosure Statement
No conflict of interest to declare.
Acknowledgments
We thank A. Theyyathel for technical assistance. The study was supported by College of Graduate Studies and Research Sector Grant YM10/11, Kuwait University.
References
- 1.Leroy O, Gangneux JP, Montravers P, et al. Epidemiology, management, and risk factors for death of invasive Candida infections in critical care: a multicenter, prospective, observational study in France (2005–2006) Crit Care Med. 2009;37:1612–1618. doi: 10.1097/CCM.0b013e31819efac0. [DOI] [PubMed] [Google Scholar]
- 2.Neofytos D, Fishman JA, Horn D, et al. Epidemiology and outcome of invasive fungal infections in solid organ transplant recipients. Transpl Infect Dis. 2010;12:220–229. doi: 10.1111/j.1399-3062.2010.00492.x. [DOI] [PubMed] [Google Scholar]
- 3.Pfaller MA, Moet GJ, Messer SA, et al. Geographic variations in species distribution and echinocandin and azole antifungal resistance rates among Candida bloodstream infection isolates: report from the SENTRY Antimicrobial Surveillance Program (2008 to 2009) J Clin Microbiol. 2011;49:396–399. doi: 10.1128/JCM.01398-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moran GP, Coleman DC, Sullivan DJ. Candida albicans versus Candida dubliniensis: why is C. albicans more pathogenic? Int J Microbiol. 2012;2012:205921. doi: 10.1155/2012/205921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McManus BA, Coleman DC. Molecular epidemiology, phylogeny and evolution of Candida albicans. Infect Genet Evol. 2014;21:166–178. doi: 10.1016/j.meegid.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 6.Ells R, Kock JLF, Pohl CH. Candida albicans or Candida dubliniensis. Mycoses. 2011;54:1–16. doi: 10.1111/j.1439-0507.2009.01759.x. [DOI] [PubMed] [Google Scholar]
- 7.Asadzadeh M, Ahmad S, Al-Sweih N, et al. Population structure and molecular genetic characterization of 5-flucytosine-susceptible and -resistant clinical Candida dubliniensis isolates from Kuwait. PLoS One. 2017;12:e0175269. doi: 10.1371/journal.pone.0175269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Odds FC, Hanson MF, Davidson AD, et al. One year prospective survey of Candida bloodstream infections in Scotland. J Med Microbiol. 2007;56:1066–1075. doi: 10.1099/jmm.0.47239-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khan Z, Ahmad S, Joseph L, et al. Candida dubliniensis: an appraisal of its clinical significance as a bloodstream pathogen. PLoS One. 2012;7:e32952. doi: 10.1371/journal.pone.0032952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Idelevich EA, Grunewald CM, Wüllenweber J, et al. Rapid identification and susceptibility testing of Candida spp. from positive blood cultures by combination of direct MALDI-TOF mass spectrometry and direct inoculation of Vitek 2. PLoS One. 2014;9:e114834. doi: 10.1371/journal.pone.0114834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pannanusorn S, Fernandez V, Römling U. Prevalence of biofilm formation in clinical isolates of Candida species causing bloodstream infection. Mycoses. 2013;56:264–272. doi: 10.1111/myc.12014. [DOI] [PubMed] [Google Scholar]
- 12.Orasch C, Marchetti O, Garbino J, et al. Candida species distribution and antifungal susceptibility testing according to European Committee on Antimicrobial Susceptibility Testing and new vs old Clinical and Laboratory Standards Institute clinical breakpoints: a 6-year prospective candidaemia survey from the fungal infection network of Switzerland. Clin Microbiol Infect. 2014;20:698–705. doi: 10.1111/1469-0691.12440. [DOI] [PubMed] [Google Scholar]
- 13.Aslani N, Janbabaei G, Abastabar M, et al. Identification of uncommon oral yeasts from cancer patients by MALDI-TOF mass spectrometry. BMC Infect Dis. 2018;18:24. doi: 10.1186/s12879-017-2916-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mokaddas E, Khan ZU, Ahmad S. Prevalence of Candida dubliniensis among cancer patients in Kuwait: a 5-year retrospective study. Mycoses. 2011;54:e29–e34. doi: 10.1111/j.1439-0507.2009.01822.x. [DOI] [PubMed] [Google Scholar]
- 15.Ellepola AN, Joseph BK, Altarakemah Y, et al. In vitro adhesion of oral Candida dubliniensis isolates to acrylic denture surfaces following brief exposure to sub-cidal concentrations of polyenes, azoles and chlorhexidine. Med Princ Pract. 2015;24:58–64. doi: 10.1159/000369019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lees S, Barton RC. The use of Niger seed agar to screen for Candida dubliniensis in the clinical microbiology laboratory. Diagn Microbiol Infect Dis. 2003;46:13–17. doi: 10.1016/s0732-8893(02)00551-5. [DOI] [PubMed] [Google Scholar]
- 17.Khan ZU, Ahmad S, Mokaddas E, et al. Simplified sunflower (Helianthus annuus) seed agar for differentiation of Candida dubliniensis from Candida albicans. Clin Microbiol Infect. 2004;10:574–592. doi: 10.1111/j.1469-0691.2004.00923.x. [DOI] [PubMed] [Google Scholar]
- 18.Khan ZU, Ahmad S, Mokaddas E, et al. Tobacco agar, a new medium for differentiating Candida dubliniensis from Candida albicans. J Clin Microbiol. 2004;42:4796–4798. doi: 10.1128/JCM.42.10.4796-4798.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Odds FC, Davidson A. “Room temperature” use of CHROMagar Candida. Diagn Microbiol Infect Dis. 2000;38:147–150. doi: 10.1016/s0732-8893(00)00197-8. [DOI] [PubMed] [Google Scholar]
- 20.Alves SH, Milan EP, de Laet Sant'Ana P, et al. Hypertonic Sabouraud broth as a simple and powerful test for Candida dubliniensis screening. Diagn Microbiol Infect Dis. 2002;43:85–86. doi: 10.1016/s0732-8893(02)00368-1. [DOI] [PubMed] [Google Scholar]
- 21.Jamal WY, Ahmad S, Khan ZU, et al. Comparative evaluation of two matrix-assisted laser desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) systems for the identification of clinically significant yeasts. Int J Infect Dis. 2014;26:167–170. doi: 10.1016/j.ijid.2014.05.031. [DOI] [PubMed] [Google Scholar]
- 22.Ahmad S, Khan Z, Mokaddas E, et al. Isolation and molecular identification of Candida dubliniensis from non-human immunodeficiency virus-infected patients in Kuwait. J Med Microbiol. 2004;56:633–637. doi: 10.1099/jmm.0.05315-0. [DOI] [PubMed] [Google Scholar]
- 23.Graf B, Trost A, Eucker J, et al. Rapid and simple differentiation of Candida dubliniensis from Candida albicans. Diagn Microbiol Infect Dis. 2004;48:149–151. doi: 10.1016/j.diagmicrobio.2003.09.016. [DOI] [PubMed] [Google Scholar]
- 24.Romeo O, Criseo G. First molecular method for discriminating between Candida africana Candida albicans and Candida dubliniensis by using hwp1 gene. Diagn Microbiol Infect Dis. 2008;62:230–233. doi: 10.1016/j.diagmicrobio.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 25.Ahmad S, Mustafa AS, Khan Z, et al. PCR-enzyme immunoassay of rDNA in the diagnosis of candidemia and comparison with amplicon detection by agarose gel electrophoresis. Int J Med Microbiol. 2004;294:45–51. doi: 10.1016/j.ijmm.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Ahmad S, Mokaddas E, Al-Sweih N, et al. Phenotypic and molecular characterization of Candida dubliniensis isolates from clinical specimens in Kuwait. Med Princ Pract. 2005;14((suppl 1)):77–83. doi: 10.1159/000086188. [DOI] [PubMed] [Google Scholar]
- 27.Lau A, Halliday C, Chen SC, et al. Comparison of whole blood, serum and plasma for early detection of candidemia by multiplex-tandem PCR. J Clin Microbiol. 2010;48:811–816. doi: 10.1128/JCM.01650-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Than LT, Chong PP, Ng KP, et al. Detection of 10 medically important Candida species by seminested polymerase chain reaction. Diagn Microbiol Infect Dis. 2012;72:196–198. doi: 10.1016/j.diagmicrobio.2011.10.008. [DOI] [PubMed] [Google Scholar]
- 29.Rezazadeh E, Moazeni M, Sabokbar A. Use of cost effective and rapid molecular tools for identification of Candida species, opportunistic pathogens. Curr Med Mycol. 2016;2:1–4. doi: 10.18869/acadpub.cmm.2.3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ahmad S, Khan Z, Asadzadeh M, et al. Performance comparison of phenotypic and molecular methods for detection and differentiation of Candida albicans and Candida dubliniensis. BMC Infect Dis. 2012;12:230. doi: 10.1186/1471-2334-12-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ahmad S, Khan ZU, Joseph L, et al. Genotypic heterogeneity and molecular basis of 5-flucytosine resistance among Candida dubliniensis isolates recovered from clinical specimens in Kuwait. Med Mycol. 2012;50:244–251. doi: 10.3109/13693786.2011.597446. [DOI] [PubMed] [Google Scholar]
- 32.Schabereiter-Gurtner C, Selitsch B, Rotter ML, et al. Development of novel real-time PCR assays for detection and differentiation of eleven medically important Aspergillus and Candida species in clinical specimens. J Clin Microbiol. 2007;45:906–914. doi: 10.1128/JCM.01344-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fricke S, Fricke C, Schimmelpfennig C, et al. A real-time PCR assay for the differentiation of Candida species. J Appl Microbiol. 2010;109:1150–1158. doi: 10.1111/j.1365-2672.2010.04736.x. [DOI] [PubMed] [Google Scholar]
- 34.Somogyvari F, Doczi I, Serly J, et al. Rapid discrimination between Candida albicans and Candida dubliniensis by using real-time polymerase chain reaction. Diagn Microbiol Infect Dis. 2007;58:367–369. doi: 10.1016/j.diagmicrobio.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 35.Khan ZU, Mustafa AS, Alam FF. Real-time LightCycler polymerase chain reaction and melting temperature analysis for differentiation of clinically important Candida species. J Microbiol Immunol Infect. 2009;42:290–295. [PubMed] [Google Scholar]
- 36.Zhang J, Hung GC, Nagamine K, et al. Development of Candida-specific real-time PCR assays for the detection and identification of eight medically important Candida species. Microbiol Insights. 2016;9:21–28. doi: 10.4137/MBI.S38517. [DOI] [PMC free article] [PubMed] [Google Scholar]