Abstract
Background/Aims
Spexin is a novel peptide which has a potential role as a biomarker of insulin resistance, diabetes, and obesity. Our aim was to measure spexin levels in lean type 1 diabetic patients and its relevance to glycemic parameters without the presence of obesity or insulin resistance.
Subjects and Methods
This cross-sectional study included 29 type 1 and 30 type 2 diabetic patients and a control group of 23 healthy subjects with adjusted age, sex, and body mass index (BMI). Height and weight were measured using standard techniques. Glucose levels, triglycerides, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, serum cortisol levels, and spexin levels were measured in each patient.
Results
The median fasting serum spexin levels were significantly lower in patients with type 1 and type 2 diabetes than in control subjects (p = 0.008 and p = 0.041, respectively). Spexin levels were not correlated with glycemic parameters, lipids, BMI, cortisol levels, and thyroid-stimulating hormone (p > 0.05). Only age turned out to be correlated with spexin levels in patients with type 1 diabetes when we analyzed the groups separately. Regression models, including age and diabetes duration, revealed no association between age and spexin levels. Regression models, including cortisol, BMI, and HbA1c, revealed no association with spexin levels within each group.
Conclusion
The presence of type 1 diabetes is associated with lower spexin levels, independent of glucose, lipid parameters, and BMI. The expression of spexin in the pancreas apart from the current glycemic control of the patients may be the main determinant of spexin levels in type 1 diabetic patients.
Key Words: Spexin, Type 1 diabetes, Biomarker
Significance of the Study
Spexin is a novel peptide which has a potential role as a biomarker of insulin resistance, diabetes, and obesity. Our study revealed that the low levels of spexin in type 1 diabetes are not related with glycemia, lipid parameters, and body mass index. The expression of spexin in the pancreas may be the main determinant of circulatory levels in type 1 diabetes.
Introduction
Spexin is a novel peptide expressed in human endocrine and epithelial tissues. Porzionato et al. [1] showed the expression of spexin in various rat tissues such as the hypothalamus, pons, cerebral cortex, kidney, ovary, adrenal gland, thyroid, anterior pituitary, and stomach. The expression of spexin in endocrine cells suggested its possible role as an endocrine factor [1]. Hence, Gu et al. [2] examined the localization of spexin in a number of human endocrine tissues and found spexin expression in the adrenal glands, pancreas, visceral fat, and thyroid, with the highest expression in the adrenal glands followed by the pancreas.
Researchers identified spexin along with a second spexin gene in vertebrate genomes and showed that spexin, galanin, and kisspeptide genes are closely localized on the same ancestral chromosome. Alignment of mature peptide sequences showed some extent of sequence similarity among the three peptide groups. Gene structure analysis indicated that spexin is more closely related to galanin than kisspeptin. A ligand-receptor interaction study showed that spexins activate human, xenopus, and zebrafish GALR2/3 family receptors but not GALR1, suggesting that spexins are natural ligands for GALR2/3 [3]. Galanin accelerates the translocation of glucose transporter-4 to the plasma membrane of various insulin-sensitive cells, regulates glucose homeostasis, and reduces insulin resistance in peripheral tissues [4].
Spexin inhibits the binding of galanin to GALR2, mediates multiple biologic processes, improves glucose handling in obese mice with type 2 diabetes, and significantly depletes hepatic triglyceride both biochemically and histologically in mice with hepatic steatosis. Preliminary studies in mice with diet-induced obesity, type 2 diabetes, and hepatic steatosis suggest that the administration of spexin may be an effective treatment for these three conditions [5].
A few studies investigated the potential role of spexin as a biomarker of insulin resistance and obesity. In particular, in obese human fat, the expression of spexin was found to be down-regulated and the administration of spexin to rats with diet-induced obesity resulted in weight loss. It has been found that spexin levels in type 2 diabetic patients were significantly lower than in healthy controls and spexin levels were correlated with glycemic parameters and lipid parameters. Furthermore, Gu et al. [2] evaluated spexin levels during an oral glucose tolerance test (OGTT) in healthy participants and found a negative correlation with blood glucose values. Their promising results on the possible role of spexin in diabetes and lipid metabolism led to new studies on this issue. Kumar et al. [6] found decreased levels of spexin in obese children compared to normal counterparts, suggesting a potential role of spexin in childhood obesity. However, recently, Hodges et al. [5] found neither an association between spexin levels and obesity as well as glucose parameters in adolescent patients nor a change in spexin levels during an OGTT. Results on the role of spexin in diabetes, obesity, and insulin resistance are controversial.
Therefore, in order to evaluate the effect of glycemic status and diabetes on spexin levels apart from obesity and insulin resistance, we investigated serum spexin levels in type 1 diabetic patients with normal weight compared to age-matched and body mass index (BMI)-matched controls.
Subjects and Methods
Patients and Study Protocol
This cross-sectional study was approved by the institutional ethics committee and included 29 patients (24 females, 5 males), 30 obese type 2 diabetic patients (24 females, 6 males), and 23 control patients (20 females, 3 males) diagnosed with type 1 diabetes according to American Diabetes Association (ADA) guidelines [7]. The patients were recruited in the Endocrinology Unit of the Ankara Training Hospital, Ankara, Turkey, between September 2015 and January 2017. Table 1 shows the characteristics of the patients included in the study. At recruitment, the patients had an average age of 30.6 ± 6. 4 years and type 1 diabetes duration of 10.55 ± 5.9 years. All patients received insulin with a multiple daily injection regimen. A control group of 23 healthy subjects with adjusted age, sex, and BMI distribution matching the group of type 1 diabetic subjects were enrolled. Those with acute or chronic infections, autoimmune disease, heart failure, hepatic disease, or renal disease were excluded. Thirty patients with type 2 diabetes were further analyzed in terms of spexin levels as well as glycemic and obesity parameters. A written informed consent was obtained from all participants. All procedures used were in accordance with the guidelines of the Helsinki Declaration on Human Experimentation (Fig. 1).
Table 1.
Background characteristics of the three groups
| Type 1 DM | Type 2 DM | Control | |
|---|---|---|---|
| Age, years | 30±6# | 51±7** | 33±9 |
| BMI | 24±5# | 36±6** | 24±5 |
| FPG, mg/dL | 209#, * | 150** | 90 |
| PPG, mg/dL | 234±9#, * | 217** | 89±9 |
| HbA1c | 9±2.2#, * | 7±1** | 5±0.2 |
| TSH | 2.48±1.93 | 2.72±3.35 | 2.65±2.04 |
| Cortisol | 10.27±4.34 | 11.08±4.65 | 11.17±4.65 |
FPG, fasting plasma glucose; PPG, postprandial plasma glucose; BMI, body mass index. Student t test:
p < 0.05, type 1 DM vs. controls;
p < 0.05, type 1 DM vs. type 2 DM;
p < 0.05, type 2 DM vs. controls.
Fig. 1.
CONSORT flow diagram of the study.
The patients' height and weight were measured using standard techniques. BMI was calculated as weight/height2. Glucose levels were measured by a colorimetric method with Beckman Coulter AU 860 (Beckman Coulter Inc., Brea, CA, USA). Insulin levels were measured by a chemiluminescent method with Beckman Coulter dxi 800 immunoassay analyzer (Beckman Coulter Inc.). Triglycerides, total cholesterol, and HDL cholesterol were measured by spectrophotometer with Beckman Coulter AU 5800 (Beckman Coulter Inc.). LDL cholesterol was calculated by the Friedewald equation method. Serum cortisol levels were detected by Human Cortisol Elisa Kit (Cusabio Biotech. Co. Ltd., USA). The analytical sensitivity was less than 0.049 ng/mL. The calculated overall intra-assay and inter-assay coefficients of variation (CV) were < 8 and < 10%, respectively. Human cortisol levels are expressed as ng/mL. The quantitative determination of human spexin was performed using commercially available ELISA according to the manufacturer's instructions (SunRed Biotechnology Co., Shanghai, China). Results are expressed as pg/mL. The calculated intra-assay CV were < 10%, and the inter-assay CV were < 12%. The functional sensitivity was 18,452 pg/mL, and the detection range of the kit was 20–6,000 pg/mL (Fig. 2).
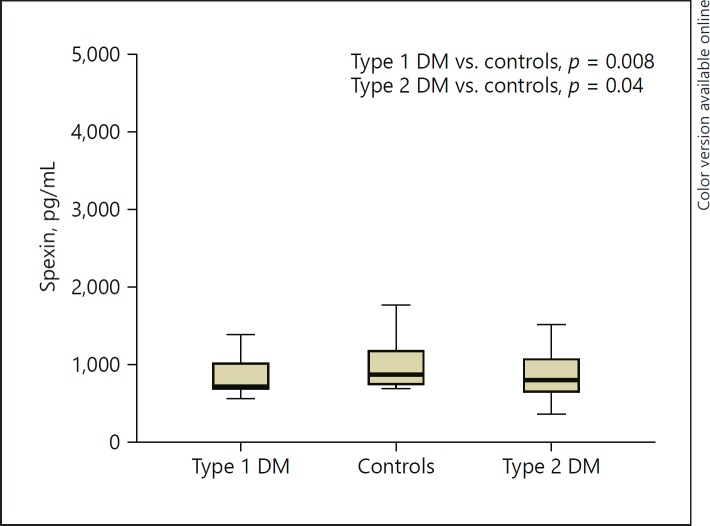
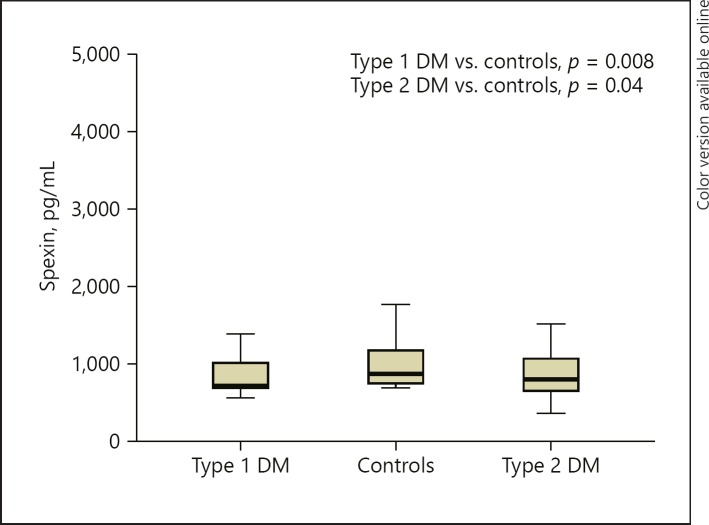
Fig. 2.
Spexin levels in all 3 patient groups. DM, diabetes mellitus.
Statistical Analysis
For statistical analysis, the SPSS for Windows version 20.0 (SPSS Inc., Chicago, IL, USA) was used. Results are presented as means ± standard deviation. Categorical variables were summarized by number and percentage of the subjects. The normality for continuous variables was assessed by the Kolmogorov-Smirnov test. In parameters with normal distribution, mean values were compared using a two-sample t test, whereas linear correlations were calculated with the Pearson correlation coefficient. In parameters with skewed distribution, significance was assessed with the Mann-Whitney test and the Spearman correlation coefficient, respectively. Comparisons among groups were performed by ANOVA. Multiple regression analyses were performed to identify the relative strength of each biochemical and clinical variable in predicting spexin levels. The limit of statistical significance was set at 0.05.
Results
As expected, type 1 diabetic patients had higher FPG, PPG, and HbA1c levels than the controls. Uric acid and albumin levels were slightly higher in the control group than in type 1 diabetic patents although within the normal range in both groups, which seems to be an accidental finding (p < 0.047). Type 1 diabetic patients had lower levels of 25 OH vitamin D than control subjects; however, both groups had vitamin D deficiency. The cortisol and thyroid-stimulating hormone (TSH) levels were similar in both groups.
The median serum spexin levels were not normally distributed among the two groups. Serum levels of spexin were significantly lower in patients with type 1 diabetes than in control subjects (p = 0.008). Type 2 diabetic patients had lower levels of spexin than control subjects (p = 0.041) but similar levels of spexin as type 1 diabetic patients (p = 0.752) (Fig. 1). In the type 1 diabetes group, spexin levels were not correlated with glycemic parameters, lipids, BMI, cortisol levels, and TSH (p > 0.05). Only age turned out to be correlated with spexin levels in patients with type 1 diabetes when groups were analyzed separately. Diabetes duration was not correlated with spexin levels in type 1 diabetic patients (p = 0.070). Regression analyses including age and diabetes duration revealed no association between age and spexin levels. Regression analyses including cortisol, BMI, and HbA1c revealed no association with spexin levels within each group. On the other hand, in the type 2 diabetes group, correlations did not reveal any associations between spexin levels and glycemic parameters, lipids, BMI, cortisol levels, and TSH in type 2 diabetic patients. Age- and BMI-adjusted (age 51.7 ± 7.8 years and BMI 36.6 ± 6.5) correlations did not reveal any associations between spexin levels and glycemic parameters, lipids, and cortisol levels.
Discussion
In our study, we found lower levels of spexin in patients with type 1 diabetes compared to age- and BMI-matched controls. Similarly, Gu et al. [2] reported lower levels of spexin in type 2 diabetic patients with normal weight than in healthy controls. They found a correlation between spexin levels and glycemic parameters as well as lipid parameters. Furthermore, they evaluated spexin levels during an oral glucose tolerance test in healthy participants and found a negative correlation with blood glucose values. They suggested that spexin may play a role in glucose and lipid metabolism in type 2 diabetes. In our study, serum spexin levels were not correlated with glycemic parameters unlike in the study by Gu et al. which included older type 2 diabetic patients aged around 50 years. The discrepancy between our results and those of Gu et al. may be related to the age of the cohort and the associated insulin resistance with type 2 diabetes. Hodges et al. [5] investigated spexin levels in normal weight, obese with normal glucose tolerance, and obese with type 2 diabetes adolescents. They could not find any difference in spexin levels between the three groups suggesting that the presence of diabetes did not affect the serum levels of spexin. Furthermore, they measured spexin levels in normal weight adolescents during an oral glucose tolerance test but contrary to Gu et al. they did not report any change in spexin levels. Spexin was not correlated with glycemic parameters, BMI, fat mass, lean mass, insulin, lipid parameters, C-reactive protein, and homeostatic model assessment (HOMA). Our patients were type 1 diabetic patients who were younger than Gu et al.'s cohort and older than Hodges et al.'s cohort. Age could be a determinant for spexin levels, and we found a correlation with age and spexin level in type 1 diabetic patients. However, regression analyses including age and diabetes duration could not confirm this correlation.
Another possible factor may be the absence of obese patients in our cohort. Unlike in the study by Hodges et al., our study included type 1 diabetic patients and control subjects without obesity. Expression of spexin was reported to be low in obese human fat, and daily administration of intraperitoneal spexin to diet-induced obese rats resulted in a reduction in food consumption and body weight. Additionally, expression of the spexin gene was observed to be markedly downregulated in obese human fat and serum levels of spexin were found to be lower than in patients with normal weight. Moreover, spexin produces weight loss in diet-induced obese rodents [2]. Studies suggest that the regulation of uptake of adipocyte long-chain fatty acids (LCFA) is an important control point for adiposity [8]. Walewski et al. [9] found that the administration of spexin significantly inhibited the uptake of LCFA into adipocytes indicating its direct effect on peripheral adipocytes. They speculated that spexin has a role in the normal regulation of function of obese white adipose tissue including uptake of LCFA and that the absence of spexin may be a major component of the hormonal dysregulation seen in obese fat and that the repletion of circulating spexin may help restore normal feeding behaviors and energy balance in obese animals and man. Subsequently, Kumar et al. [6] described lower circulating levels of spexin in obese children compared to normal weight children like in Walewski et al.'s study [9] They failed to find any relationship between spexin levels and insulin, glucose levels, adiponectin, leptin as well as C-reactive protein possibly due to the inclusion of normoglycemic uncomplicated obese adolescents who have a more favorable cardiometabolic profile compared to adults. However, Hodges et al. [5] did not find any difference in spexin levels between normal weight, obese with normal glucose tolerance, and obese with type 2 diabetic adolescents.
Reports on the role of spexin in glycemic parameters are controversial. The promising reports of the role of spexin in diabetes and obesity observed in obese and type 2 diabetic adults and rats was not confirmed in different patient populations. Two other cohorts evaluating adolescent subjects revealed controversial results in terms of spexin levels. As spexin is shown to be associated with obesity in most, but not all, studies, we investigated the role of spexin in glycemic parameters and whether the presence of diabetes affects the spexin levels without the presence of obesity or insulin resistance. Although spexin is secreted from adipose tissue, we found no correlation between spexin levels and BMI in normal weight type 1 diabetic patients. We assume that absence of insulin resistance may affect our findings, but there was no correlation between spexin levels and BMI in our obese type 2 diabetic patients either. Our findings are in line with Hodges et al.'s [5], depicting a lack of association between spexin levels and adiposity.
The processing and expression of spexin has been demonstrated in a human pancreatic cell line [10]. In our study cohort, the presence of both type 1 and type 2 diabetes resulted in lower levels of spexin. However, there was no correlation between glycemic parameters and spexin levels in both type 1 and type 2 diabetic patients and controls. Moreover, spexin levels were not correlated with glycemic parameters and BMI in patients with type 2 diabetes after age adjustment either. This may be a result of the functional endocrine pancreatic tissue expressing spexin which is particularly low in type 1 diabetics but cannot explain why type 2 diabetics have low spexin levels although insignificantly higher than type 1 diabetics. Since the design of the present study was to compare type 1 diabetic patients with age- and BMI-matched controls, it is not exactly appropriate to compare spexin levels in our cohort of type 2 diabetic patents, which may be the reason for this finding. However, spexin levels were not correlated with glycemic parameters in patients with type 2 diabetes after adjustment for age and BMI either. Our findings further support a lack of association between glycemic control and serum spexin levels.
Gu et al. [2] have found the highest expression of spexin in adrenal tissue. The administration of ACTH was reported to decrease adrenal levels of spexin mRNA in vivo, while the administration of dexamethasone increased the levels of spexin mRNA [11]. Spexin was found to have a direct involvement in the regulation of adrenocortical cell proliferation. Prolonged ACTH administration resulted in elevated levels of corticosterone but the levels of spexin mRNA remained either unchanged or lowered, suggesting that glucocorticoid hormones are not involved in the direct regulation of spexin gene expression [10]. It has been suggested that spexin may be involved in the regulation of the stress response [12]. Although we did not definitively show the association of glucocorticoids with spexin, we found no association between cortisol levels and spexin. Expression of spexin in the adrenal gland may be related to other adrenal hormones or cytokines. Further studies are needed to clarify this issue.
Conclusion
The presence of type 1 diabetes results in lower spexin levels independent of glucose, lipid parameters, and BMI. Its expression from pancreas apart from current glycemic control of the patients may be the main determinant of spexin levels in type 1 diabetic patients.
Statement of Ethics
All procedures used were in accordance with the guidelines of the Helsinki Declaration on Human Experimentation.
Disclosure Statement
The authors report no conflicts of interest.
References
- 1.Porzionato A, Rucinski M, Macchi V, Stecco C, Malendowicz LK, De Caro R. Spexin expression in normal rat tissues. J Histochem Cytochem. 2010 Sep;58((9)):825–37. doi: 10.1369/jhc.2010.956300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gu L, Ma Y, Gu M, Zhang Y, Yan S, Li N, et al. Spexin peptide is expressed in human endocrine and epithelial tissues and reduced after glucose load in type 2 diabetes. Peptides. 2015 Sep;71:232–9. doi: 10.1016/j.peptides.2015.07.018. [DOI] [PubMed] [Google Scholar]
- 3.Kim DK, Yun S, Son GH, Hwang JI, Park CR, Kim JI, et al. Coevolution of the spexin/galanin/kisspeptin family: spexin activates galanin receptor type II and III. Endocrinology. 2014 May;155((5)):1864–73. doi: 10.1210/en.2013-2106. [DOI] [PubMed] [Google Scholar]
- 4.Fang P, Yu M, Shi M, He B, Zhang Z, Bo P. The neuropeptide galanin benefits insulin sensitivity in subjects with type 2 diabetes. Curr Protein Pept Sci. 2013 Dec;14((8)):669–73. [PubMed] [Google Scholar]
- 5.Hodges SK, Teague AM, Dasari PS, Short KR. Effect of obesity and type 2 diabetes, and glucose ingestion on circulating spexin concentration in adolescents. Pediatr Diabetes. 2018 Mar;19((2)):212–6. doi: 10.1111/pedi.12549. [DOI] [PubMed] [Google Scholar]
- 6.Kumar S, Hossain J, Nader N, Aguirre R, Sriram S, Balagopal PB. Decreased circulating levels of spexin in obese children. J Clin Endocrinol Metab. 2016 Jul;101((7)):2931–6. doi: 10.1210/jc.2016-1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Diabetes Association Standards of medical care in diabetes 2017. Diabetes Care. 2017;40:48–56. [Google Scholar]
- 8.Petrescu O, Fan X, Gentileschi P, Hossain S, Bradbury M, Gagner M, et al. Long-chain fatty acid uptake is upregulated in omental adipocytes from patients undergoing bariatric surgery for obesity. Int J Obes. 2005 Feb;29((2)):196–203. doi: 10.1038/sj.ijo.0802868. [DOI] [PubMed] [Google Scholar]
- 9.Walewski JL, Ge F, Lobdell H, 4th, Levin N, Schwartz GJ, Vasselli JR, et al. Spexin is a novel human peptide that reduces adipocyte uptake of long chain fatty acids and causes weight loss in rodents with diet-induced obesity. Obesity (Silver Spring) 2014 Jul;22((7)):1643–52. doi: 10.1002/oby.20725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rucinski M, Porzionato A, Ziolkowska A, Szyszka M, Macchi V, De Caro R, et al. Expression of the spexin gene in the rat adrenal gland and evidences suggesting that spexin inhibits adrenocortical cell proliferation. Peptides. 2010 Apr;31((4)):676–82. doi: 10.1016/j.peptides.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 11.Sonmez K, Zaveri NT, Kerman IA, Burke S, Neal CR, Xie X, et al. Evolutionary sequence modeling for discovery of peptide hormones. PLOS Comput Biol. 2009 Jan;5((1)):e1000258. doi: 10.1371/journal.pcbi.1000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mirabeau O, Perlas E, Severini C, Audero E, Gascuel O, Possenti R, et al. Identification of novel peptide hormones in the human proteome by hidden Markov model screening. Genome Res. 2007 Mar;17((3)):320–7. doi: 10.1101/gr.5755407. [DOI] [PMC free article] [PubMed] [Google Scholar]