Abstract
Purpose
To describe our early experience with gene expression profiling (GEP) assessment for juxtafoveal, subfoveal, and peripapillary indeterminate high-risk melanocytic lesions to assist in making early treatment decisions in patients who did not feel comfortable with either close observation or definitive treatment.
Methods
A prospective cohort of patients with indeterminate lesions who underwent GEP were enrolled. Nonparametric statistical analysis was utilized given the small sample size.
Results
Fifteen patients were included in this series. Six (40%) were class 1A and 9 (60%) class 1B. Class 1A and 1B lesions had a median of three and four clinical risk factors, respectively (p = 0.27). There was no statistically significant difference for the largest basal diameter between the classes (p = 0.31); however, class 1B lesions were thicker than class 1A lesions (p = 0.03). None of the class 1A lesions showed definite growth or metastasis over a mean follow-up period of 17.1 ± 1.8 months from fine needle aspiration biopsy. All class 1B patients opted for plaque brachytherapy, and to date none of these patients have developed metastasis, with a mean follow-up of 18.7 ± 8.4 months.
Conclusion
There may be a role for GEP assessment in high-risk, indeterminate, posteriorly located choroidal lesions to assist in treatment planning.
Keywords: Gene expression profiling, Uveal melanoma, Plaque brachytherapy, Fine needle aspiration biopsy, Choroidal nevus
Introduction
Advances over the past few decades have given us powerful tools for controlling uveal melanoma locally and preventing enucleation; however, we have yet to develop an effective treatment to reduce the risk of metastatic disease or to effectively treat it when it occurs [1, 2, 3]. Waiting for growth to occur in small lesions identified as high risk by an ophthalmologist can increase the risk of metastasis even when controlling for tumor size (relative risk of 8.1 on univariate analysis and of 3.2 on multivariate analysis controlling for tumor size) [4, 5]. Therefore, high-risk melanocytic lesions ≤3 mm in thickness without any documentation of growth may be offered treatment [3, 5, 6].
Many clinical characteristics of choroidal melanocytic lesions have been studied and reported as risk factors for growth [7, 8, 9] and metastasis [5, 10]. In addition to clinical and histopathological criteria, chromosomal prognostic factors [11, 12, 13, 14] and gene expression profiling (GEP) classification [15, 16, 17, 18, 19, 20, 21, 22, 23, 24] have been validated as predictors of metastasis and melanoma-specific mortality. A recent multicenter prospective study of GEP [16] also compared the prognostic performance of GEP compared to TNM classification and chromosome 3 status (as detected by a validated assay which interrogates single nucleotide polymorphisms). That study concluded that that GEP was the most accurate prognostic marker of all factors analyzed. Since then, several additional studies [15, 16, 17, 18, 19, 20, 21, 22, 23, 24] have supported this finding.
The challenge in managing “indeterminate” small choroidal melanocytic lesions is determining which represent small melanomas and are therefore likely to grow and/or metastasize versus those that are actually atypical nevi or melanocytic proliferations that have no malignant potential despite traditional risk factors. If we could accurately and reliably predict the natural course of these lesions, early treatment of melanomas before they grow and become more likely to metastasize would save lives, and treatment-associated morbidity/visual loss would be avoided for patients with lesions that are less likely to grow or metastasize.
Therefore, our group has offered GEP assessment for juxtafoveal, subfoveal, and peripapillary high-risk melanocytic lesions that in our center would be offered treatment if they were peripherally located. Our early experience with this algorithm is presented.
Methods
All data were collected in a prospective and standardized fashion. The patients included in this study had posteriorly located choroidal pigmented melanocytic lesions and fit one of three groups: (1) a lesion that had three or more risk factors for growth with no prior follow-up and the patient felt uncomfortable proceeding with either close observation or treatment, (2) a lesion that was most likely a small melanoma that would be offered treatment in our center, but the patient felt uncomfortable proceeding with treatment for a variety of reasons (e.g., amblyopia in the other eye, fear of “radiation” or its complications, previous health care providers labeling the lesion as a benign “freckle”), or (3) a lesion that had demonstrated growth (either definite or equivocal) based on fundus photographs and/or ultrasound and the patient wanted additional information before choosing either to continue with close observation versus opting for early treatment. Amelanotic lesions were excluded. There were no additional exclusion criteria. The data regarding the 15 patients included in this study were extracted from our prospectively collected database. There were three main outcomes evaluated in this study. First, we wanted to evaluate our biopsy yield for GEP of these small lesions. Second, we wanted to assess whether biopsy results would be used by patients to aid in treatment (or observation) decision making. Third, we wanted to follow the outcomes based on GEP including vision complications from biopsy, growth in those who elected observation, and metastatic and survival data.
Informed consent was obtained from all patients prior to participation in the study. Following discussion regarding the current literature surrounding GEP, including the clinical scenarios in which it has previously been studied (i.e., for prognostication of “confirmed” tumors rather than to provide additional information to help guide treatment of indeterminate lesions), patients were offered either close observation (clinical assessment including direct ophthalmoscopy and B-scan ultrasonography every 3 months) or GEP through fine needle aspiration biopsy (FNAB). When available, prior fundus photographs were reviewed to determine whether or not the lesion had grown.
As all tumors were located post-equatorially, FNAB was performed via a trans-pars plana, transvitreal approach using a 27-gauge long needle attached to IV extension tubing on a 10-mL syringe. Under visualization with an indirect ophthalmoscope and a 20D lens, the needle was embedded in the thickest area of the tumor in most cases. If there appeared to be a more “active” area of the lesion (i.e., orange pigment), the biopsy was taken from this area. Suction was applied once the lesion was entered. And this had been achieved, the needle was quickly removed from the eye. The standard method consisted of first pushing air through the needle, followed by drawing up of buffer, and then re-expressing the extraction buffer into the provided specimen container. Definite growth was defined as an increase in basal dimensions by ≥250 μm over a 5-year period as measured on standard fundus photographs. This cutoff is in keeping with the median rate of nevus enlargement of 0.06 mm/year reported in a long-term follow-up study by Mashayekhi et al. [25]. Patients had demonstrated “equivocal growth” defined as a slight increase in size but not significant enough to be classified as definite growth.
It is critical to note that this protocol was only offered to patients who presented with classic features consistent with those of a melanocytic lesion. Lesions were defined as being posteriorly located if the posterior edge of the tumor was within 2 mm of either the optic nerve or the fovea. We ascribed the term “indeterminate” to indicate the relative diagnostic uncertainty for this group of patients who fall between the diagnoses of high-risk choroidal nevus and small choroidal melanoma. Lesion height was determined on B scan by measuring from the tumor apex to the posterior sclera. High risk was defined as three or more risk factors for growth (tumor thickness > 2.0 mm, posterior margin ≤3 mm to the optic nerve, subretinal fluid, visual symptoms, orange pigment, hollow on ultrasound, absent drusen or absent halo) [7, 8, 9]. Subretinal fluid was recorded as a risk factor if it was noted either clinically or on optical coherence tomography.
Visual acuity was obtained using a Snellen chart; acuities were converted to logarithm of the minimum angle of resolution (logMAR). Basic demographics including age, gender, laterality, tumor dimensions, and proximity to the fovea and optic nerve head were recorded. Due to the small sample size, nonparametric tests were utilized to compare values between groups. The Mann-Whitney U (Wilcoxon rank-sum) test was used to compare characteristics between class 1A and 1B tumors when the groups were independent and the Wilcoxon signed-rank test when the groups were not independent.
Results
Of the 15 patients included in this series, 11 (73%) were male. Fourteen of the 15 patients were AJCC 8th edition [26] stage I (T1a) and 1 patient was stage IIA (T2a). Two patients (13%) presented with visual symptoms (blurred/decreased vision and/or photopsia). The remaining 12 patients were picked up on routine examination and subsequently referred to the ocular oncol ogy service given the suspicious nature or possible early growth of their lesion. The mean (± SD) age at the time of GEP testing was 53 ± 11 years (range 39–83 years). Eleven patients were either followed prior to their GEP biopsy by our service or had reliable fundus photographs sent from their referring physician. The mean pre-GEP follow-up for these 11 patients was 4 ± 4 years (Table 1).
Table 1.
Baseline characteristics and GEP results
| Patient No. | Age, years | GEP result | Probability | LBD, mm | Height, mm | AJCC 8th edition T category (stage) | Distance to optic disc, mm | Distance to fovea, mm | Change over time | Time followed prior to GEP, years1 | Treatment | Follow-up from GEP, months |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 83 | 1B | 1.12 | 9 | 2.70 | T1a (I) | 1 | 1 | equivocal change | 2 | plaque | 40 |
| 2 | 47 | 1B | 0.85 | 2 | 2.08 | T1a (I) | 3 | 0.25 | definitive growth | 10 | plaque | 19 |
| 3 | 50 | 1A | 0.87 | 3 | 2.20 | T1a (I) | 2 | 1 | not followed prior to GEP | N/A | observation | 14 |
| 4 | 36 | 1B | 0.97 | 3 | 2.09 | T1a (I) | 1 | 1 | no growth | 2 | plaque | 22 |
| 5 | 65 | 1B | 1.04 | 8 | 2.08 | T1a (I) | 2.5 | 0 | no growth | 2 | plaque | 20 |
| 6 | 50 | 1A | 1.04 | 7 | 2.42 | T1a (I) | 4.5 | 2 | no growth | 0.7 | TTT | 20 |
| 7 | 53 | 1A | 0.97 | 3 | 1.89 | T1a (I) | 0 | 2 | not followed prior to GEP | N/A | observation | 18 |
| 8 | 59 | 1A | 0.63 | 5.5 | 1.76 | T1a (I) | 1 | 0.5 | equivocal change | 0.5 | observation | 18 |
| 9 | 48 | 1B | 1.06 | 13 | 2.04 | T2a (II) | 0 | 7 | equivocal change | 2 | plaque | 18 |
| 10 | 40 | 1A | 1.15 | 3 | flat | T1a (I) | 0 | 1 | no growth | 0.3 | observation | 17 |
| 11 | 46 | 1A | 0.77 | 9 | flat | T1a (I) | 2 | 2 | no growth | 8 | observation | 17 |
| 12 | 56 | 1B | 1.02 | 8 | 2.00 | T1a (I) | 1 | 0.5 | equivocal change | 4 | plaque | 17 |
| 13 (choroid) | 56 | 1B | 0.65 | 9 | 3.00 | T1a (I) | 3 | 0 | not followed prior to GEP | N/A | plaque | 12 |
| 14 | 47 | 1B | 0.39 | 4 | 1.80 | T1a (I) | 5 | 2.5 | not followed prior to GEP | N/A | plaque | 12 |
| 15 | 61 | 1B | 0.66 | 6 | 2.38 | T1a (I) | 5 | 2.0 | no growth | 10 | plaque | 12 |
GEP, gene expression profiling; LBD, largest basal diameter; N/A, not available; TTT, transpupillary thermotherapy.
Outside fundus photographs were used to determine whether a lesion had changed over time, provided they were of sufficient quality to make a reliable judgement.
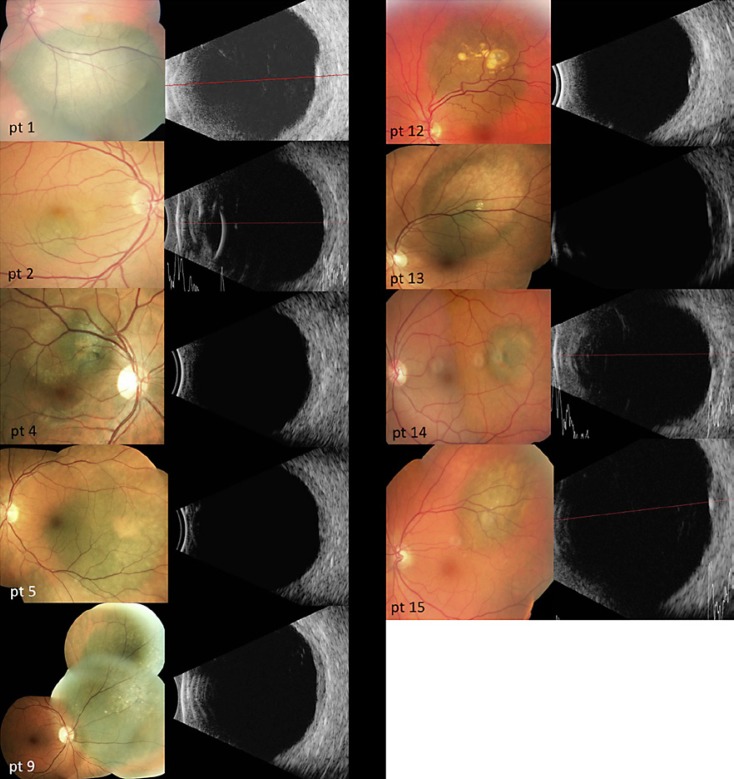
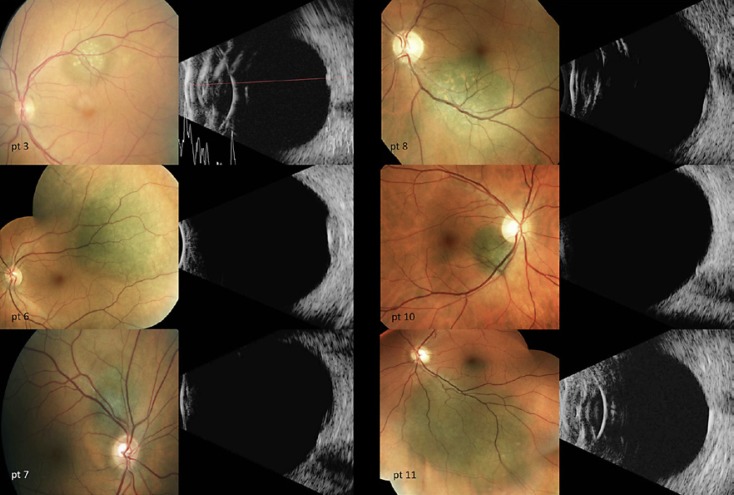
The mean distance from the fovea was 1.3 ± 1.1 mm (range 0–4 mm), with a mean distance from the optic nerve head of 2.1 ± 1.7 mm (range 0–3 mm). The mean largest basal diameter (LBD) was 6.2 ± 3.2 mm (range 2–13 mm). The mean tumor height, as measured by contact A or B scans, was 2.1 ± 0.5 mm, ranging from 0.85 to 3.0 mm (Table 1). The individual tumor risk factors for growth/metastasis can be seen in Table 2, and corresponding color fundus photographs and contact B scans of all patients included in this study can be seen in Figure 1 (class 1A lesions) and Figure 2 (class 1B lesions).
Table 2.
Clinical risk factors for growth of all lesions
| Patient No. | GEP result | Height >2 mm | Presence of subretinal fluid1 | Presence of orange pigment | Absence of drusen | Visual symptoms | Proximity to optic disc margin <3 mm | Acoustically hollow on ultrasound | Presence of halo | Number of risk factors of all 8 | Number of risk factors of 5 (TFSOM) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1B | + | – | – | + | – | + | – | – | 3 | 2 |
| 2 | 1B | + | + | + | + | – | + | – | – | 5 | 4 |
| 3 | 1A | + | – | + | – | – | + | – | – | 3 | 3 |
| 4 | 1B | + | + | + | + | + | + | – | – | 6 | 5 |
| 5 | 1B | + | – | + | – | – | + | + | – | 4 | 3 |
| 6 | 1A | + | – | + | + | – | – | + | – | 4 | 2 |
| 7 | 1A | – | + | + | – | – | + | – | – | 3 | 3 |
| 8 | 1A | – | + | + | + | – | + | + | – | 5 | 3 |
| 9 | 1B | + | – | – | + | – | + | – | – | 3 | 2 |
| 10 | 1A | – | + | + | + | – | + | – | – | 4 | 3 |
| 11 | 1A | – | – | – | + | – | + | – | – | 2 | 1 |
| 12 | 1B | + | – | – | + | – | + | – | – | 3 | 2 |
| 13 | 1B | + | – | – | + | + | + | – | – | 4 | 3 |
| 14 | 1B | – | + | + | + | – | – | + | + | 5 | 2 |
| 15 | 1B | + | + | + | – | – | – | + | + | 5 | 3 |
GEP, gene expression profiling.
Presence of subretinal fluid was determined by optical coherence tomography over the lesion.
Fig. 1.
Color fundus photographs and B-scan ultrasonography of all 6 class 1A lesions.
Fig. 2.
Color fundus photographs and B-scan ultrasonography of all 9 class 1B patients.
Six of the 15 patients (40%) included in our study had a class 1A GEP and 9 were GEP class 1B (60%). The class 1B tumors tended to be larger (mean LBD 6.9 vs. 5.1 mm for class 1A) and had a greater number of clinical risk factors for growth (class 1B mean 4.1 vs. class 1A mean 3.5); however, neither of these differences reached statistical significance in this small study. Interestingly, most class 1B versus 1A tumors being ≥2.0 mm thick (89 vs. 33%, respectively) and the mean tumor height between the groups (class 1A = 1.8 mm, class 1B = 2.2 mm) were statistically significantly different (p = 0.03). The number of clinical risk factors between the class 1A and 1B tumors was not statistically significantly different (p = 0.27 and p = 0.70 (Table 3). There was no statistically significant relationship between the LBD (p = 0.31) and the GEP probability value. A successful GEP classification was possible in all 15 cases and all discriminant scores were ≥0.100, indicating normal confidence.
Table 3.
Comparison of total number of risk factors, tumor thickness as measured by contact A and B ultrasound, and LBD between class 1A and 1B tumors
| Class 1A (n = 6) | Class 1B (n = 9) | p value1 | |
|---|---|---|---|
| Risk factors of all 82 | 3.5 (3.0)±0.8 | 4.1 (4.0)±0.9 | 0.27 |
| Risk factors for 53 | 2.5 (1.8)±1.1 | 2.9 (3.0)±1.1 | 0.70 |
| Tumor thickness, mm | 1.8±0.5 | 2.2±0.4 | 0.03 |
| LBD, mm | 5.1±0.5 | 6.9±3.5 | 0.31 |
Values are presented as mean (median) ± SD or mean ± SD. LBD, largest basal diameter.
A Mann-Whitney U test was performed with α = 0.05.
Risk factors included in this analysis: tumor thickness >2 mm (T), distance to optic disc margin <3 mm (M), presence of orange pigment (O), presence of subretinal fluid (F), symptoms (S), absence of drusen, acoustic hollowness on ultrasound, and presence of halo.
Risk factors include TFSOM.
Prior to FNAB, the mean logMAR visual acuity of all patients included in the study was 0.09 ± 0.13 (20/25). One patient suffered a complication from FNAB. In this case, the patient was unaware that he was anticoagulated on clopidogrel. Unfortunately, he developed a significant subretinal hemorrhage resulting in retinal detachment, requiring pars plana vitrectomy and tamponade with intraocular gas. He went on to undergo plaque brachytherapy for his subfoveal GEP class 1B lesion (patient No. 1). His final visual acuity was count fingers at 3 feet.
Patient No. 13 had two noncongruous intraocular tumors, one involving the ciliary body and one a choroidal lesion adjacent to the optic nerve. As such, this patient had two unique GEP samples sent. His ciliary body tumor yielded a class 2 GEP, and his choroidal lesion was found to be class 1B. Only the data regarding this patients' choroidal lesion were included in the analysis. The ciliary body tumor was excluded from all analysis as it did not fit the inclusion criteria of being a posteriorly located choroidal lesion, to which we applied this algorithm. The ciliary body lesion measured 4.3 mm in thickness and had classic melanocytic clinical, ultrasonographic, and cytologic findings necessitating treatment. We are still awaiting BAP1 testing for this patient.
Overall, the 15 patients included in this study were followed for a mean (± SD) of 18.1 ± 6.7 months since FNAB, with the class 1A patients being followed for slightly less time than the class 1B patients (17.1 ± 1.8 vs. 18.7 ± 8.4 months, respectively). Five of the 6 patients with GEP class 1A tumors were comfortable proceeding with close observation after receiving their GEP result. One requested to be treated with transpupillary thermotherapy (patient No. 6). None of the 5 patients with GEP class 1A who were observed showed definite growth or metastasis over a mean follow-up period of 17.1 ± 1.8 months. Of the 5 class 1A patients who were observed following FNAB, there was no statistically significant difference in pre- and post-FNAB logMAR visual acuity with a mean pre-FNAB visual acuity of 20/21 (logMAR 0.03 ± 0.05) and a post-FNAB visual acuity of 20/22 (logMAR 0.05 ± 0.05) (p = 0.32).
All of the patients whose choroidal lesions exhibited class 1B GEP elected to be treated with plaque brachytherapy rather than close observation. To date, none of the GEP class 1B patients included in this series have developed metastasis or local treatment failure (growth post radiation), and all are alive with a mean follow-up of 18.7 ± 8.4 months from GEP to the current date.
Discussion
As a greater proportion of smaller tumors are being treated, recent research has demonstrated that in addition to a class 2 gene expression profile, LBD is also an independent statistically significant predictor of survival [18, 19]. This suggests that metastasis is not only a product of a tumor's RNA activity, but also a result of tumor size [18, 19, 27]. Therefore, treating genetically “aggressive” tumors before their size increases may improve survival. In this context, knowledge of a tumor's RNA activity may add useful information for patients to consider when deciding whether to elect early treatment or rather to proceed with close observation until definitive growth of their lesion is documented.
The idea of offering FNAB to patients with indeterminate high-risk lesions is not new. Augsburger et al. [28] proposed utilizing cytology through an FNAB of these high-risk indeterminate lesions to provide further information on the malignancy potential of the lesion. However, there are some limitations to using cytology for this purpose, including low yield [28], the subjective component of cytologic assessment of melanocytic lesions [29, 30, 31], and the difficulty in distinguishing between low-grade melanomas and nevi [32]. Augsburger et al. [28] found that in 35% of cases the aspirate was insufficient for cytologic diagnosis. Of the 65% of cases which yielded sufficient aspirate for cytodiagnosis, 47.1% were classified as melanomas; 11.8% of these were classified as intermediate lesions and 5.9% were classified as benign nevi on cytopathology. Following FNAB, 12 patients with insufficient aspirates and the 4 patients with intermediate cells were classified as “nevus versus melanoma.” With a median follow-up of 2.6 years, the lesions of 8 (50%) of the patients in the “nevus versus melanoma” group subsequently grew, were reclassified as small melanomas, and received treatment.
When discussing the GEP result with the patient in this study, the treating physician again reviewed the risks and benefits associated with plaque brachytherapy versus close observation. In addition, the difference in the “case mix” between the validation studies on GEP and these cases was re-explained and discussed. When empowered with the knowledge that their lesion was found to have a class 1B GEP, all patients preferred to proceed with radiation treatment without waiting for definitive documentation of growth. Although the exact risks per case vary, the “average” patient's decision appeared to be based primarily on the knowledge of two key points: (1) they had a > 50% chance of demonstrating tumor growth if observed (based on having ≥3 clinical risk factors), and (2) their expected 5-year metastasis-free survival if observed until other events which would traditionally prompt treatment with plaque brachytherapy (such as definitive growth) occurred would be approximately 79% [33]. Of note, several series have documented excellent outcomes with treatment of small melanomas using palladium [34], iodine 125 [35], and ruthenium [36], even for posteriorly located lesions.
The primary weaknesses of our study are the small sample size and the short follow-up time that did not provide sufficient power to properly compare the subgroups or allow for long-term assessment of metastasis. Additionally, one must keep in mind that we did not perform concurrent cytopathology, as is performed in some centers. As such, it is important that this algorithm be used in the correct patient population (those for whom there is no uncertainty regarding the underlying choroidal melanocytic origin of their lesion) as a GEP result will be provided irrespective of the tissue sampled [37]. Furthermore, this treatment algorithm is challenging to explain to a patient, and therefore may not be suitable for all patients. Understanding the limitations of current research in terms of its external generalizability (case mix) for predicting future survival in this subgroup of patients is difficult, but we felt that all of the patients in this series had a good grasp of the information and were well informed. Finally, one must keep in mind that discordant GEP classification within a lesion is possible [38] and that a class 1A result does not obviate the need for ongoing close follow-up. In the near future, incorporation of newer genetic tests, such as PRAME testing, may further improve the accuracy and prognostication information derived from our use of GEP in this patient population, as it has been reported that 0% of class 1 PRAME-negative patients developed metastasis within 5 years [39].
Conclusion
GEP assessment via FNAB for a highly selective group of patients with high-risk melanocytic lesions of the posterior pole was helpful in providing further information to aid in treatment decision making in a cohort of patients that did not feel comfortable continuing with either close observation or definitive treatment.
Statement of Ethics
Ethics approval for a prospective database containing standardized information of all ocular oncology patients seen at our center was obtained from the Health Research Ethics Board for Alberta – Cancer Committee. Consent was obtained from all participants to have demographic data, tumor characteristics, and outcomes collected.
Disclosure Statement
No funding was received for this study. None of the authors have any financial disclosures to make or proprietary interests to declare.
References
- 1.Harbour JW, Chao DL. A molecular revolution in uveal melanoma: implications for patient care and targeted therapy. Ophthalmology. 2014;121:1281–1288. doi: 10.1016/j.ophtha.2013.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pereira PR, Nakao A, Lim L, et al. Current and emerging treatment options for uveal melanoma. Clin Ophthalmol. 2013;7:1669–1682. doi: 10.2147/OPTH.S28863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weis E, Salopek TG, McKinnon JG, et al. Management of uveal melanoma: a consensus-based provincial clinical practice guideline. Curr Oncol. 2016;23:57–64. doi: 10.3747/co.23.2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shields C, Shields J, Kiratli H, De Potter P, Cater J. Risk factors for growth and metastasis of small choroidal melanocytic lesions. Ophthalmology. 1995;102:1351–1361. [PubMed] [Google Scholar]
- 5.Shields C, Furuta M, Thangappan A, et al. Metastasis of uveal melanoma millimeter-by-millimeter in 8033 consecutive eyes. Arch Ophthalmol. 2009;127:989–998. doi: 10.1001/archophthalmol.2009.208. [DOI] [PubMed] [Google Scholar]
- 6.Simpson ER, Gallie B, Laperrierre N, et al. The American Brachytherapy Society consensus guidelines for plaque brachytherapy of uveal melanoma and retinoblastoma. Brachytherapy. 2014;13:1–14. doi: 10.1016/j.brachy.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Shields CL, Cater J, Shields J, Singh D, Santos MC, Carvalho C. Combination of clinical factors predictive of growth of small choroidal melanocytic tumors. Arch Ophthalmol. 2000;118:360–364. doi: 10.1001/archopht.118.3.360. [DOI] [PubMed] [Google Scholar]
- 8.Factors predictive of growth and treatment of small choroidal melanoma COMS Report No. 5. The Collaborative Ocular Melanoma Study Group. Arch Ophthalmol. 1997;115:1537–1544. doi: 10.1001/archopht.1997.01100160707007. [DOI] [PubMed] [Google Scholar]
- 9.Shields C, Furuta M, Berman E, et al. Choroidal nevus transformation into melanoma: analysis of 2514 consecutive cases. Arch Ophthalmol. 2009;127:981–987. doi: 10.1001/archophthalmol.2009.151. [DOI] [PubMed] [Google Scholar]
- 10.Shields CL, Shields JA, Kiratli H, De Potter P, Cater JR. Risk factors for growth and metastasis of small choroidal melanocytic lesions. Trans Am Ophthalmol Soc. 1995;93:259–275. discussion 275–279. [PMC free article] [PubMed] [Google Scholar]
- 11.Dopierala J, Damato BE, Lake SL, Taktak AFG, Coupland SE. Genetic heterogeneity in uveal melanoma assessed by multiplex ligation-dependent probe amplification. Invest Ophthalmol Vis Sci. 2010;51:4898–4905. doi: 10.1167/iovs.09-5004. [DOI] [PubMed] [Google Scholar]
- 12.Prescher G, Becher R. Nonrandom chromosomal abnormalities in primary uveal melanoma. J Natl Cancer Inst. 1990;82:1765–1769. doi: 10.1093/jnci/82.22.1765. [DOI] [PubMed] [Google Scholar]
- 13.Prescher G, Bornfeld N, Hirche H, Horsthemke B, Becher R. Prognostic implications of monosomy 3 in uveal melanoma. Lancet. 1993;347:1222–1225. doi: 10.1016/s0140-6736(96)90736-9. [DOI] [PubMed] [Google Scholar]
- 14.Bronkhorst IHG, Maat W, Jordanova ES, et al. Effect of heterogeneous distribution of monosomy 3 on prognosis in uveal melanoma. Arch Pathol Lab Med. 2011;135:1042–1047. doi: 10.5858/2010-0477-OAR1. [DOI] [PubMed] [Google Scholar]
- 15.Correa ZM, Augsburger JJ. Sufficiency of FNAB aspirates of posterior uveal melanoma for cytologic versus GEP classification in 159 patients, and relative prognostic significance of these classifications. Graefes Arch Clin Exp Ophthalmol. 2014;252:131–135. doi: 10.1007/s00417-013-2515-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Onken MD, Worley LA, Char DH, et al. Collaborative Ocular Oncology Group report number 1: prospective validation of a multi-gene prognostic assay in uveal melanoma. Ophthalmology. 2012;119:1596–1603. doi: 10.1016/j.ophtha.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Decatur CL, Ong E, Garg N, et al. Driver mutations in uveal melanoma: associations with gene expression profile and patient outcomes. JAMA Ophthalmol. 2016;134:728–733. doi: 10.1001/jamaophthalmol.2016.0903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Corrêa ZM, Augsburger JJ. Independent prognostic significance of gene expression profile class and largest basal diameter of posterior uveal melanomas. Am J Ophthalmol. 2016;162:20–27. doi: 10.1016/j.ajo.2015.11.019. e1. [DOI] [PubMed] [Google Scholar]
- 19.Walter SD, Chao DL, Feuer W, Schiffman J, Char DH, Harbour JW. Prognostic implications of tumor diameter in association with gene expression profile for uveal melanoma. JAMA Ophthalmol. 2016;134:734–740. doi: 10.1001/jamaophthalmol.2016.0913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrausch U, Martus P, Tonnies H, et al. Significance of gene expression analysis in uveal melanoma in comparison to standard risk factors for risk assessment of subsequent metastases. Eye. 2008;22:997–1007. doi: 10.1038/sj.eye.6702779. [DOI] [PubMed] [Google Scholar]
- 21.Gill HS, Char DH. Uveal melanoma prognostication: from lesion size and cell type to molecular class. Can J Ophthalmol. 2012;47:246–253. doi: 10.1016/j.jcjo.2012.03.038. [DOI] [PubMed] [Google Scholar]
- 22.van Gils W, Lodder EM, Mensink HW, et al. Gene expression profiling in uveal melanoma: two regions on 3p related to prognosis. Invest Ophthalmol Vis Sci. 2008;49:4254–4262. doi: 10.1167/iovs.08-2033. [DOI] [PubMed] [Google Scholar]
- 23.Onken MD, Worley LA, Ehlers JP, Harbour JW. Advances in brief gene expression profiling in uveal melanoma reveals two molecular classes and predicts metastatic death. Cancer Res. 2004;64:7205–7209. doi: 10.1158/0008-5472.CAN-04-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Worley LA, Onken MD, Person E, et al. Transcriptomic versus chromosomal prognostic markers and clinical outcome in uveal melanoma. Clin Cancer Res. 2007;13:1466–1472. doi: 10.1158/1078-0432.CCR-06-2401. [DOI] [PubMed] [Google Scholar]
- 25.Mashayekhi A, Siu S, Shields CL, Shields JA. Slow enlargement of choroidal nevi: a long-term follow-up study. Ophthalmology. 2011;118:382–388. doi: 10.1016/j.ophtha.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 26.Amin MB, Edge S, Greene F, Byrd DR, Brookline RK, Washington MK, Gershenwald JE, Compton CC, Hess KR, Sullivan DC, Jessup JM, Brierley JD, Gaspar LE, Schilsky RL, Balch CM, Winchester DP, Asare EA, Madera M, Gress DM, Meyer LR. ed 8. New York: Springer International Publishing; 2017. AJCC Cancer Staging Manual. [Google Scholar]
- 27.Kujala E, Damato B, Coupland SE, Desjardins L, Bechrakis NE, Grange JD, Kivelä T. Staging of ciliary body and choroidal melanomas based on anatomic extent. J Clin Oncol. 2013;31:2825–2831. doi: 10.1200/JCO.2012.45.2771. [DOI] [PubMed] [Google Scholar]
- 28.Augsburger JJ, Correa ZM, Schneider S, et al. Diagnostic transvitreal fine-needle aspiration biopsy of small melanocytic choroidal tumors in nevus versus melanoma category. Trans Am Ophthalmol Soc. 2002;100:224–225. [PMC free article] [PubMed] [Google Scholar]
- 29.Callender GR. Malignant melanotic tumors of the eye: a study of histologic types in 111 cases. Trans Am Acad Ophthalmol Otolaryngol. 1931;36:131–142. [Google Scholar]
- 30.McLean I, Foster W, Zimmerman L, Gamel J. Modifications of Callender's classification of uveal melanoma at the Armed Forces Institute of Pathology. Am J Ophthalmol. 1983;96:502–509. doi: 10.1016/s0002-9394(14)77914-0. [DOI] [PubMed] [Google Scholar]
- 31.Gamel JW, McLean IW. Quantitative analysis of the Callender classification of uveal melanoma cells. Arch Ophthalmol. 1977;95:686–691. doi: 10.1001/archopht.1977.04450040152024. [DOI] [PubMed] [Google Scholar]
- 32.Eagle RC. The pathology of ocular cancer. Eye (Lond) 2013;27:128–136. doi: 10.1038/eye.2012.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Castle biosciences patient report http://www.myuvealmelanoma.com/wp-content/uploads/2016/10/DDx-UM_Sample_LabReport_V4.4.pdf (accessed April 18, 2018)
- 34.Semenova E, Finger PT. Palladium-103 radiation therapy for small choroidal melanoma. Ophthalmology. 2013;120:2353–2357. doi: 10.1016/j.ophtha.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 35.Murray TG, Markoe AM, Gold AS, et al. Long-term followup comparing two treatment dosing strategies of (125) I plaque radiotherapy in the management of small/medi um posterior uveal melanoma. J Ophthalmol. 2013;2013:517032. doi: 10.1155/2013/517032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salkola S, Heikkonen J, Eskelin S, Kivela T. Management of choroidal melanomas less than 10 mm in largest basal diameter with a 10 mm ruthenium plaque. Retina. 2014;34:2110–2120. doi: 10.1097/IAE.0000000000000202. [DOI] [PubMed] [Google Scholar]
- 37.Klufas MA, Itty S, McCannel CA, Glasgow BJ, Moreno C, McCannel TA. Variable results for uveal melanoma-specific gene expression profile prognostic test in choroidal metastasis. JAMA Ophthalmol. 2015;133:1073–1076. doi: 10.1001/jamaophthalmol.2015.1790. [DOI] [PubMed] [Google Scholar]
- 38.Augsburger JJ, Correa ZM, Augsburger BD. Frequency and implications of discordant gene expression profile class in posterior uveal melanomas sampled by fine needle aspiration biopsy. Am J Ophthalmol. 2015;159:248–256. doi: 10.1016/j.ajo.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Field MG, Decatur CL, Kurtenbach S, et al. PRAME as an independent biomarker for metastasis in uveal melanoma. Clin Cancer Res. 2016;22:1234–1242. doi: 10.1158/1078-0432.CCR-15-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]