The list of signaling pathways currently understood to be influenced by bile acids (BAs) include farnesoid X receptor (FXR) in the liver and intestine; and the G-protein coupled receptor, Takeda G-protein-coupled receptor 5 (TGR5), in the intestine, adipose tissue, pancreas, and skeletal muscle (Fig. 1). Both cold temperature and TGR5 activation induce brown adipose tissue (BAT) thermogenesis.(1,2) However, little is known about the role of the BA production in response to cold-induced thermogenesis. Interestingly, variations in the intestinal microbiome have been found to be associated with effects on energy homeostasis, and vice versa the gut microbiome has been reported to be altered with cold exposure.(3) Thus, BA and the intestinal microbiome share a significant bidirectional relationship (Fig. 1) and, taken together, a plausible hypothesis may be that BAs have a role in cold-related changes to the microbiome. A recent publication in Nature Medicine(4) provides data to answer this very hypothesis.
FIG. 1.
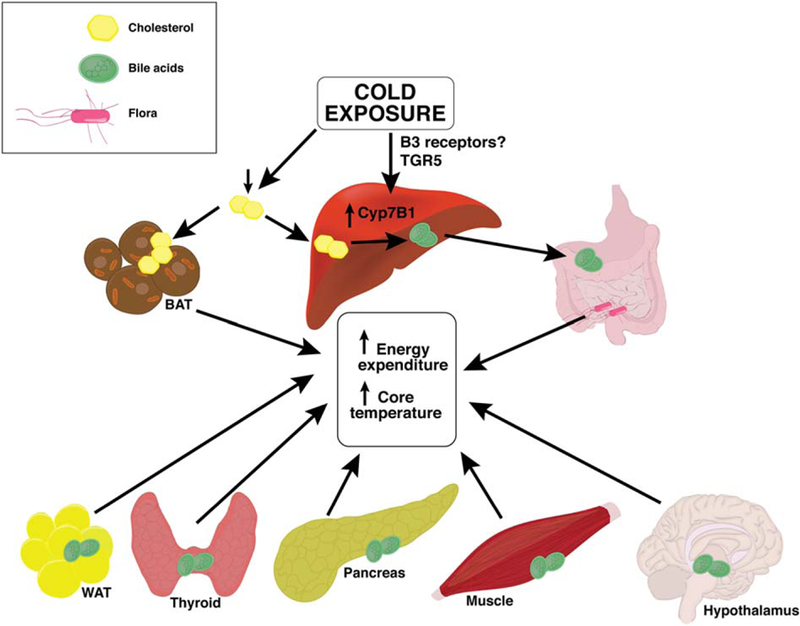
Cold exposure leads to a reduction in plasma cholesterol, increase in liver and brown adipose tissue (BAT) cholesterol uptake, Cyp7B1 up-regulation, and increased liver and fecal bile acids (BA) leading to a change in the microbiome. BA have target/receptors in the white adipose tissue (WAT), thyroid, pancreas, muscle, and hypothalamus that, when activated, lead to biochemical changes causing changes in energy homeostasis and temperature regulation as well. These effects collectively lead to an increase in energy metabolism and rise in the core temperature.
The first interesting observation in this article is that the alternative BA synthesis pathway is activated in lower temperatures, such that the livers of the animals exposed to cold showed significant increase in BAs with increased expression of the alternative BA synthesis pathway enzyme, Cyp7b1. Furthermore, the activation of the alternative BA synthesis pathway observed in the cold was thought to be independent of FXR regulation. Cyp7b1 induction was thought responsible for the cold-induced elevation in hepatic BAs, given that hepatic BA content elevation was further exaggerated by adeno-associated virus (AAV) overexpression of Cyp7b1 and blunted in Cyp7b1–/– mice. AAV Cyp7b1 overexpression resulted in higher core body temperatures, up-regulation of thermogenic genes, higher mitochondrial uncoupling protein 1 (UCP1) amount in the adipose tissue, and higher oxygen consumption while the opposite was found in Cyp7b1–/– mice, confirming the role of BAs derived from the alternative synthesis pathway in thermogenesis under cold stress.
The second noteworthy finding in this article was that cold leads to a Cyp7b1 pathway-mediated increase in fecal BAs and a specific gut floral profile. This cold-induced increase in BA was abrogated in Cyp7b1–/– mice, but enhanced by AAV Cyp7b1 overexpression. Using radioactive tracer, the investigators further showed that cholesterol to BA conversion in the liver rose in the cold while blocking cholesterol absorption in the intestine by ezetimibe (NPC1L1 inhibitor, ez), cold-induced rise in hepatic BAs was sustained; however, the rise in fecal BA excretion was not. Of note, more fecal taurine was observed in mice exposed to cold.
The third dimension of this article is that a rise in fecal BAs may have a role in the cold-related microbiome variation. Regardless of diet component (adding cholesterol) or diabetic back ground (db/db), cold causes a change in the microbiome profile. This change was shown to be related to the degree of rise in fecal BAs, regardless of the mechanism that leads to this rise. Low to no fecal BA such as that observed with ez treatment, or in MDR2 (Abcb4) knockout mice, did not have an altered gut microbiome under cold stimulus.
To summarize, this interesting work shows that under cold stimulus, hepatic cholesterol conversion to BA is increased by activation of the alternative pathway that, in turn, leads to a significant increase in fecal BA excretion, contributing to alterations in the intestinal microbiome.
Having said that, the article does not completely address certain key issues. First, the mechanism behind cold-induced Cyp7b1 induction and that of the beneficial effects to the organism from the microbiome profile alteration are still left unclear (Fig. 1). Another factor that warrants further perusal is that BA species and conjugates differ between humans and mice. Case in point is the possible role of taurine, given that although taurine is the major amino acid used for BA conjugation in mice, it is not so for humans. In addition, major developmental expression and role differences related to Cyp7A1 and Cyp7B1 exist between humans and mice (humans with Cyp7B1 deficiency require liver transplantation). Thus, these subtle, yet important, differences import the need for further human validation studies. Another thought-provoking observation in the literature is the difference in cold-induced thermogenesis related to the sex of the animal attributed to hormonal differences. Cyp7b1 is observed to be expressed more in males.(5) Given that the experiments in this article were focused on only male mice, it will be of value to investigate whether there are any differences in the pathway studied between male and females. Muscles possess TGR5 expression and play a role in thermogenesis. It will be worth knowing whether the BAs/muscle talk contributes to cold-induced energy homoeostasis. A recent study reports that Tgr5 plays a role in regulation of Cyp7b1 expression β3 adrenergic receptor and may be activated by TGR5 agonists. Whether TGR5 activation occurs in the model illustrated by this article is yet to be seen.(6) Altogether, this article lends itself to multiple future lines of investigation, which we have tried to summarize (Fig. 1).
Broadening the knowledge of the BA role in energy balance expands their many possible future therapeutic applications. BA signaling might be the real player behind the beneficial effects achieved by bariatric surgery of improvement in nonalcoholic fatty liver disease (NAFLD) and obesity. Suppression of intestinal bile acid uptake by inhibition of apical sodium-dependent BA transporter in mice was shown to improve glucose tolerance, decrease liver lipid synthesis, and improve NAFLD activity score.(7) In the pancreas, TGR5 activation in cell cultures leads to augmentation of insulin secretion from β cells and stimulation of glucagon-like peptide-1 synthesis from α cells, leading to improved glucose tolerance and insulin sensitivity.(8) Furthermore, BAs are key in thyroid-related metabolic actions, because they increase energy expenditure through TGR5 activation leading to downstream conversion of T4 to T3; an observation confirmed in both mice and humans.(1) BA synthesis is increased in hyperthyroidism. Additionally, BAs may play a part in stimulation of thyroid-stimulated hormone (TSH) secretion through thyrotropin-releasing hormone/hypothalamus given that TGR5 expression is prominent in the hypothalamus. In gastric bypass, TSH inversely correlated with BA levels.(2) These are only a few examples of the integral multiorgan functions these simple molecules have in regulation of whole-body metabolism. Studying BA metabolic pathways in sickness and health can illuminate tremendous treatment options.
In summary, this breakthrough article reports that in mice exposed to cold stimulus, cholesterol clearance is enhanced and its metabolism is directed toward the alternative BA synthesis pathway, increasing thermogenesis capacity of BAT and leading to a cold-induced gut microbiome distinct pattern through increased fecal bile acid abundance, though many more questions arise as they do when a new area of research is uncovered!
Footnotes
Potential conflict of interest: Dr. Kohli consults for Intercept and advises for Alexion.
Contributor Information
Nisreen Soufi, Division of Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, Children’s Hospital Los Angeles, Los Angeles, CA
Rohit Kohli, Division of Gastroenterology, Hepatology and Nutrition, Department of Pediatrics, Children’s Hospital Los Angeles, Los Angeles, CA
REFERENCES
- 1 ).Ockenga J, Valentini L, Schuetz T, Wohlgemuth F, Glaeser S, Omar A, et al. Plasma bile acids are associated with energy expenditure and thyroid function in humans. J Clin Endocrinol Metab 2012;97:535–542. [DOI] [PubMed] [Google Scholar]
- 2 ).Kohli R, Bradley D, Setchell KD, Eagon JC, Abumrad N, Klein S. Weight loss induced by Roux-en-Y gastric bypass but not laparoscopic adjustable gastric banding increases circulating bile acids. J Clin Endocrinol Metab 2013;98:E708–E712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3 ).Zietak M, Kovatcheva-Datchary P, Markiewicz LH, Stahlman M, Kozak LP, Backhed F. Altered microbiota contributes to reduced diet-induced obesity upon cold exposure. Cell Metab 2016;23:1216–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4 ).Worthmann A, John C, Ruhlemann MC, Baguhl M, Heinsen FA, Schaltenberg N, et al. Cold-induced conversion of cholesterol to bile acids in mice shapes the gut microbiome and promotes adaptive thermogenesis. Nat Med 2017; 23:839–849. [DOI] [PubMed] [Google Scholar]
- 5 ).Li-Hawkins J, Lund EG, Bronson AD, Russell DW. Expression cloning of an oxysterol 7alpha-hydroxylase selective for 24-hydroxycholesterol. J Biol Chem 2000;275:16543–16549. [DOI] [PubMed] [Google Scholar]
- 6 ).Donepudi AC, Boehme S, Li F, Chiang JY. G-protein-coupled bile acid receptor plays a key role in bile acid metabolism and fasting-induced hepatic steatosis in mice. HEPATOLOGY 2017;65: 813–827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7 ).Rao A, Kosters A, Mells JE, Zhang W, Setchell KD, Amanso AM, et al. Inhibition of ileal bile acid uptake protects against non-alcoholic fatty liver disease in high-fat diet-fed mice. Sci Transl Med 2016;8:357ra122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8 ).Kumar DP, Asgharpour A, Mirshahi F, Park SH, Liu S, Imai Y, et al. Activation of transmembrane bile acid receptor tgr5 modu-lates pancreatic islet alpha cells to promote glucose homeostasis. J Biol Chem 2016;291:6626–6640. [DOI] [PMC free article] [PubMed] [Google Scholar]


