Abstract
Background:
Anthracycline chemotherapeutics, such as doxorubicin, are used widely in the treatment of numerous malignancies. The primary dose-limiting adverse effect of anthracyclines is cardiotoxicity that often presents as heart failure due to dilated cardiomyopathy years after anthracycline exposure. Recent data from animal studies indicate that anthracyclines cause cardiac atrophy. The timing of onset and underlying mechanisms are not well defined and the relevance of these findings to human disease is unclear.
Methods and Results:
Wild type mice were sacrificed one week after intraperitoneal administration of doxorubicin (1–25 mg/kg), revealing a dose-dependent decrease in cardiac mass (R2 = 0.64, p < 0.0001) and a significant decrease in cardiomyocyte cross-sectional area (336 ± 29 vs. 188 ± 14 μm2, p < 0.0001). Myocardial tissue analysis identified a dose-dependent upregulation of the ubiquitin ligase, muscle ring finger-1 (MuRF1, R2 = 0.91, p = 0.003) and a molecular profile of muscle atrophy. To investigate the determinants of doxorubicin-induced cardiac atrophy, we administered doxorubicin 20 mg/kg to mice lacking MuRF1 (MuRF1−/−) and wild type littermates. MuRF1−/− mice were protected from cardiac atrophy and exhibited no reduction in contractile function. To explore the clinical relevance of these findings, we analyzed cardiac MRI (CMR) data from 70 patients in the DETECT-1 cohort and found that anthracycline exposure was associated with decreased cardiac mass evident within one month and persisting to 6 months after initiation.
Conclusions:
Doxorubicin causes a subacute decrease in cardiac mass in both mice and humans. In mice, doxorubicin-induced cardiac atrophy is dependent upon MuRF1. These findings suggest that therapies directed at preventing or reversing cardiac atrophy might preserve the cardiac function of cancer patients receiving anthracyclines.
Subject terms: Animal models of human disease, Myocardial biology, Translational studies, Cardiomyopathy
INTRODUCTION
The anthracycline antibiotic doxorubicin (DOX) is a widely used and highly effective component of adjuvant chemotherapy for breast cancer and curative regimens for lymphomas, leukemias, and soft tissue sarcomas. The primary dose-limiting adverse effect of DOX is cardiotoxicity. Anthracycline-induced cardiomyopathy has become less frequent with contemporary dosing regimens and formulations but continues to be a meaningful source of morbidity and mortality.1 Cardiotoxicity related to DOX most commonly is detected years after therapeutic exposure and widely is considered to result from chronic maladaptive changes and remodeling. Chronic anthracycline cardiotoxicity typically manifests as dilated cardiomyopathy, with eccentric ventricular hypertrophy and increased cardiac mass, often associated with the clinical syndrome of heart failure.2
Acute and subacute cardiotoxic response to DOX exposure also occurs. Acute DOX cardiotoxicity is considered a rare clinical event, though numerous recent studies indicate that it is more common than previously thought (11%3 – 21%4) and predicts poor outcomes.5 The pathogenesis of acute DOX cardiotoxicity may be distinct from chronic cardiomyopathy,6 though the prevailing paradigm considers oxidative stress the central mechanism of injury, associated with mitochondrial dysfunction and cardiomyocyte death.7,8 Acute alterations in cardiac structure have not been studied carefully.
The perceived differences between acute and chronic DOX cardiotoxicity have implications on clinical management and prognosis, but also affect our understanding of experimental models of human disease. Acute DOX exposure is a commonly used, and widely criticized, mouse model of anthracycline cardiotoxicity. Critics contend that the model relies on high doses of DOX, and hence may not accurately model a clinically relevant human condition.9 The present study sought to clarify the early effects of anthracyclines on cardiac muscle mass in mice and humans.
Here we show that DOX causes dose-dependent cardiac atrophy in mice, beginning at relatively low dose exposure. Mice lacking Muscle Ring Finger-1 (MuRF1), a striated muscle- specific ubiquitin ligase that has been implicated as a mediator of cardiac atrophy in other mouse models,10 are resistant to DOX-induced cardiac atrophy and contractile dysfunction. Analysis of the DETECT-1 cardiac MRI cohort revealed that cancer patients treated with contemporary anthracycline regimens also undergo cardiac atrophy within one month of exposure.
METHODS
The data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure. Mice were 8–12 week-old male (or female, where specified) C57Bl6J for dose-response experiments. MuRF1−/− mice, congenic on a C57Bl6J background11 were compared with MuRF1+/+ littermates. Animal care and experimental protocols were approved by the UNC IACUC and complied with Guide for the Care and the use of Laboratory Animals. DOX was administered by single intraperitoneal injection. LAD ligations were performed as previously described.12 Echocardiograms were performed on awake, loosely restrained mice. The echocardiogram reader was blind to treatment condition. Mice were sacrificed by cervical dislocation after an overdose of isoflurane, and heart tissue was either perfused and fixed (immunohistochemistry) or immediately removed, flash frozen and processed for qRT-PCR, ATP assay, and immunoblotting. Hearts were fixed and sectioned according to standard procedures. Slides were stained with wheat-germ-agglutinin- Alexa Fluor 488 conjugate and counterstained with DAPI to visualize cardiomyocyte cross- sectional area.
The DETECT-1 cohort study was approved by the Wake Forest University School of Medicine Institutional Review Board and supported by the National Cancer Institute (R33CA12196).13 All subjects provided informed and witnessed consent for participation. Longitudinal change in LV mass was assessed in 70 anthracycline-treated patients who were imaged by cardiac MRI (CMR) a maximum of 4 times (at baseline, 1 month, 3 months, and 6 months).
For CMR data, the rate of change in left ventricular mass was analyzed by constructing a linear mixed model with random intercepts and time effects and statistical analyses were carried out using SAS 9.4 (SAS Institute; Cary, NC). All other comparisons were made using t-test (groups of 2), one-way ANOVA (groups of 3) with Tukey’s post-hoc analysis, or linear regression (GraphPad Prism; San Diego, CA). Results are presented as mean ± SEM, except as specified.
Complete experimental details are available in Supplemental Methods.
RESULTS
Doxorubicin induced a dose-related decrease in heart weight in wild type mice.
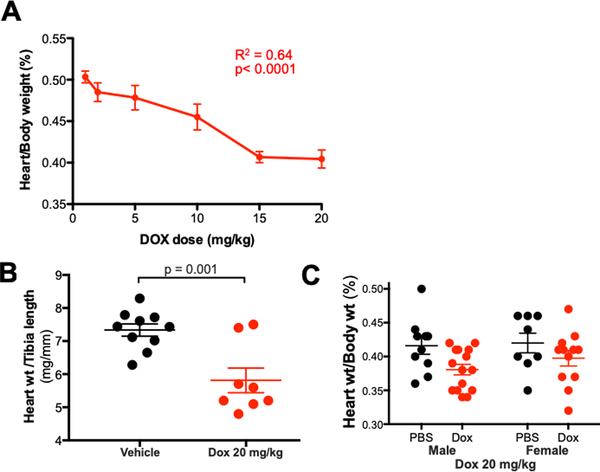
To test the effect of DOX on cardiac structure and function, we administered intraperitoneal vehicle or escalating doses of DOX (1, 2, 5, 10, 15, 20 mg/kg one time) to 8–12 week-old male wild type C57B6J mice. We detected a statistically significant decrease in body weight, as compared to vehicle-treated animals, only at the 20 mg/kg dose (Table 1). The average heart weight of mice treated with DOX 20 mg/kg (n=9) was 89 ± 3 mg, 20% lower than the average heart weight of vehicle-treated mice (111 ± 3 mg, n=26). Linear regression analysis indicated that heart weight indexed to body weight decreased in a dose-dependent fashion after DOX administration (p<0.0001, Y= −0.0052*X + 0.50, R2=0.64), suggesting that cardiac atrophy occurs out of proportion to cachexia or other systemic effects (Figure 1A). The decrease in indexed heart weight achieved statistical significance at 10 mg/kg and all higher doses. The relationship between indexed heart weight and Log2-DOX dose was even closer (p=0.007, R2=0.87, Supplemental Figure 1). Consistent with these findings, heart weight indexed to tibia length (which should be unaffected by systemic illness) was 21% lower in mice treated with DOX 20 mg/kg compared with vehicle-treated mice (Figure 1B). In light of a recent report that female mice are relatively resistant to DOX cardiotoxicity,14 we treated 8–12 week-old female C57B6 mice with vehicle or DOX 20 mg/kg and found no significant decrease in heart weight indexed to body weight (Figure 1C).
Table 1.
Morphometrics 7 days after doxorubicin administration in wild type C57Bl6/J mice
| Doxorubicin μg/kg IP (n) | Body weight final (g) | Body weight change (g) | Heart weight (mg) | Heart/body weight (%) |
|---|---|---|---|---|
| Vehicle (26) | 24.4 ± 0.8 | −0.1 ± 0.1 | 111 ± 3 | 0.46 ± 0.01 |
| 1 (6) | 21.7 ± 1.5 | +0.5 ± 0.2 | 108 ± 7 | 0.50 ± 0.01 |
| 2 (6) | 19.3 ± 0.5 | −0.3 ± 0.2 | 94 ± 5 | 0.49 ± 0.01 |
| 5 (6) | 23.2 ± 1.9 | −0.7 ± 0.2 | 111 ± 8 | 0.48 ± 0.02 |
| 10 (6) | 22.2 ± 1.1 | −0.6 ± 0.3 | 101 ± 4 | 0.46 ± 0.02 |
| 15 (6) | 20.8 ± 0.8 | −0.2 ± 0.1 | 84 ± 2 | 0.41 ± 0.01 |
| 20 (9) | 23.3 ± 1.1 | −2.1 ± 0.6 | 89 ± 3 | 0.40 ± 0.01 |
All values are mean ± SEM, n given in parentheses
Figure 1. Doxorubicin induces a dose-dependent decrease in cardiac mass.
C57Bl6J mice were given a single dose of intraperitoneal doxorubicin. (A) Heart weight indexed to body weight at each dose (n=9 for 20 mg/kg, n=6 for all other doses) (B) Heart weight indexed to tibia length; (C) Heart weight indexed to body weight for male and female mice that received doxorubicin 20 mg/kg.
Doxorubicin decreases cardiomyocyte size with only modest induction of cardiomyocyte death.
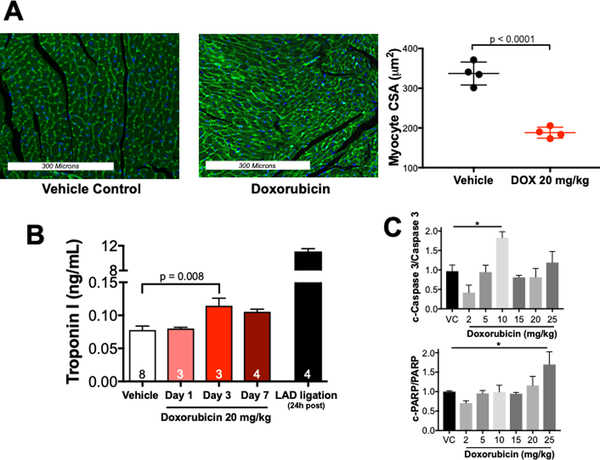
Heart weight decreases either due to a decrease in cell volume or number. To evaluate the effect of DOX on cardiomyocyte volume, we analyzed wheat germ agglutinin-stained sections of vehicle and DOX-treated mouse hearts 7 days after treatment with vehicle or DOX 20 mg/kg. DOX treatment was associated with a 44% decrease (336 ± 29 vs. 188 ± 14 μm2) in cardiomyocyte cross-sectional area (Figure 2A).
Figure 2. Doxorubicin causes cardiomyocyte atrophy in vivo.
Mice were sacrificed 7 days after receiving doxorubicin 20 mg/kg i.p. (A) Representative wheat germ agglutinin-Alexa Fluor 488 conjugate stained heart sections. Analysis of myocyte cross-sectional areas from 4 hearts (800 cross-sectional areas total, 200 cross sections per mouse over 4–6 histological sections). A Student’s t-test was used to determine significance between groups. (B) Serum Troponin I was measured by ELISA. (C) Summary densitometry of immunoblots for markers of apoptosis. n= 6 for VC, n = 3–4 for DOX-treated. Results were compared using a one-way ANOVA. c-Caspase 3 = cleaved caspase 3; c-PARP = cleaved poly ADP ribose polymerase, LAD = left anterior descending coronary artery.
To assess for DOX-induced cell death in vivo, we measured serum Troponin-I by ELISA (LifeDiagnostics) at 1, 3, and 7 days after DOX 20 mg/kg administration. We found a small and transient increase in serum Troponin-I in DOX-treated mice compared with vehicle (0.11 vs. 0.08 ng/mL) at Day 3, but this mild elevation was undetectable by Day 7 (Figure 2B). As a positive control for the ELISA, we measured TnI in the serum of mice (n=4) drawn 24 hours after myocardial infarction induced by left anterior descending coronary artery ligation. The average TnI in these mice was 11.1 ng/mL (Figure 2B)
To evaluate the contribution of apoptosis to DOX-induced cell death we used terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) and immublotted for classical apoptotic markers. TUNEL stains of fixed heart tissue revealed no evidence of apoptosis in either vehicle or DOX-treated mice (data not shown). There was a significant increase in cleaved caspase 3 only at 10 mg/kg and an increase in cleaved PARP only at 25 mg/kg (Figure 2C and Supplemental Figure 2). Collectively, these findings suggest that cardiomyocyte atrophy, rather than cell death, is the primary determinant of the subacute decrease in heart weight after DOX exposure.
Doxorubicin causes a dose-dependent decrease in contractile function that parallels cardiac atrophy.
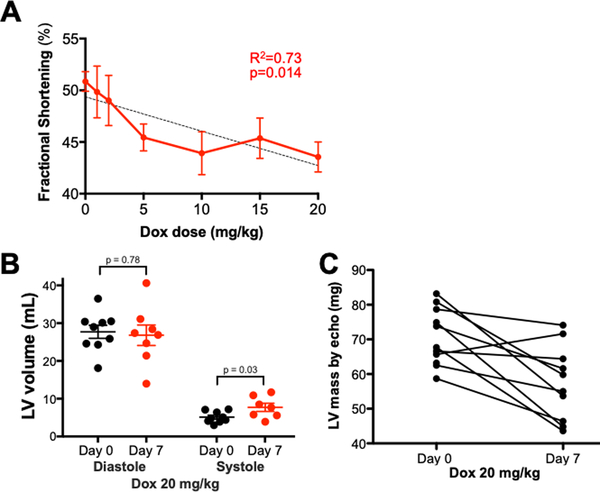
High dose anthracycline administration causes subacute cardiac dysfunction in male mice, though the dose threshold for injury is unclear. We used conscious echocardiography with blinded readers to assess whether cardiac atrophy after escalating doses of DOX is accompanied by functional deficits. Linear regression analysis indicated that DOX induced a dose-related decrease in fractional shortening (p=0.014, Y=−0.332*X + 49.3, R2=0.73), an echocardiographic indicator of contractile function (Figure 3A). The relationship between fractional shortening and Log2-DOX dose was even closer (p=0.005, R2=0.88, Supplemental Figure 3). Though chronic DOX cardiotoxicity is characterized by dilated cardiomyopathy, we found no subacute change in left ventricular diastolic diameter or volume, but a modest increase in left ventricular systolic diameter and volume (Day 7: 7.7 ± 1.1 mL vs. Day 0: 5.1 ± 0.5 mL, Figure 3B) and a decrease in calculated left ventricular mass (Day 7: 70.5 ± 2.4 mg vs. Day 0: 57.5 ± 3.4 mg, p=0.005, Figure 3C) consistent with atrophic hypocontractility.
Figure 3. Doxorubicin causes a dose-dependent decrease in contractile function on conscious echocardiography.
Mice were injected with intraperitoneal doxorubicin. Conscious echocardiography was performed at baseline and 7 days after injection. (A) Contractile function (fractional shortening) as a function of doxorubicin dose (n=15 for vehicle, n=9 for 20mg/kg, n=6 for all other doses) by logistic regression; (B) Left ventricular volumes at baseline and Day 7 by Student’s t-test; (C) Calculated left ventricular mass at baseline and on Day 7.
Doxorubicin induces a characteristic molecular profile of atrophy, including MuRF1 upregulation.
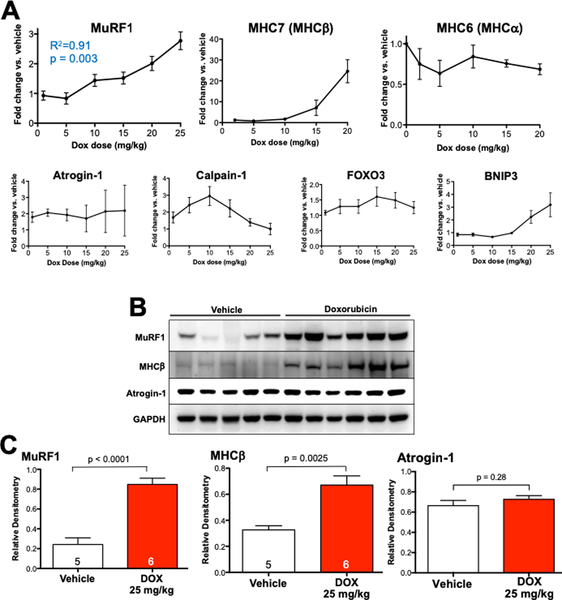
We next used quantitative RT-PCR to evaluate the molecular response to escalating doses of DOX, focusing on genes that classically are upregulated during cardiac atrophy.15 We found dose-related increases in the expression of striated muscle-specific ubiquitin ligase MuRF1 (p = 0.003, Y = 0.07597*X + 0.6219, R2 = 0.91), as well as its upstream regulator, BNIP3.16 Abundance of the ubiquitin ligase atrogin-1 and the neutral protease calpain-1 (Figure 4A, Supplemental Figure 4) were not predictably affected by DOX, contrary to previous reports.17, 18 Myosin heavy chain (MHC) isoform predominance underwent characteristic atrophic switching, from MHC-α (MYH6) to MHC-β (MYH7) and atrial natriuretic peptide (ANP) was increased (Figure 4A). Interestingly, we also identified a significant increase in both MuRF1 and atrogin-1 mRNA expression in the gastrocnemius muscle of mice treated with DOX 20 mg/kg (Supplemental Figure 5). MHC-β abundance was unchanged in gastrocnemius from DOX- treated mice, indicative of previously recognized differences in transcriptional regulation of MHC-β in cardiac and skeletal muscle atrophy.19
Figure 4. Doxorubicin increases cardiac expression of MuRF-1 and a molecular signature of atrophy in vivo.
Mice were sacrificed 7 days after intraperitoneal injection of doxorubicin or vehicle control. (A) Quantitative RT-PCR assayed molecular markers of atrophy at various DOX doses. Logistic regression was applied to MuRF1 results. (B) Heart lysates were immunoblotted and (C) summary densitometry was compared (vehicle control vs. DOX 25 mg/kg) by Student’s t-test.
Immunoblotting of the hearts of mice treated with vehicle or DOX 25 mg/kg revealed a nearly 4-fold upregulation of MuRF1 (densitometry relative to GAPDH 0.85 ± 0.06 vs. 0.24 ± 0.07, p < 0.0001), a 2-fold upregulation of MHC-β (densitometry relative to GAPDH 0.67 ± 0.07 vs. 0.33 ± 0.03, p = 0.0025) compared with roughly 30-fold mRNA upregulation and no change in atrogin- 1 protein abundance (Figure 4B).
Mice lacking MuRF1 (MuRF1−/−) mice are resistant to doxorubicin-induced cardiac atrophy and contractile dysfunction.
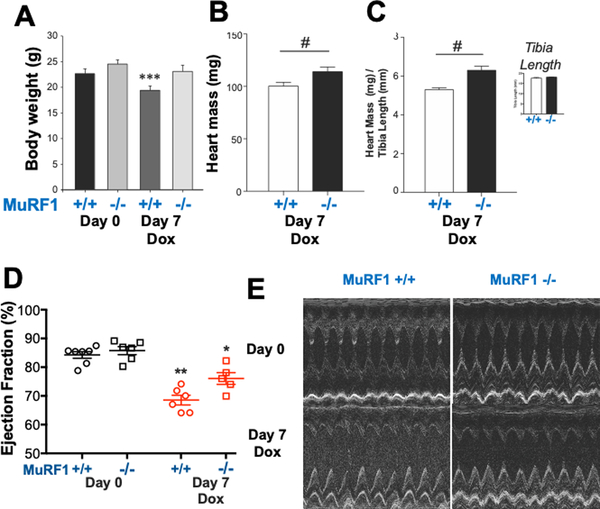
MuRF1 regulates cardiac atrophy due to dexamethasone and after reversal of transverse aortic constriction.10 Given that MuRF1 was increased in the setting of DOX-induced cardiac atrophy, we sought to determine whether this upregulation was causal using mice globally lacking MuRF1 (MuRF1−/−) on a C57-Bl6J background.11 Male MuRF1−/− mice and wild type (MuRF1+/+) littermates underwent conscious echocardiography prior to intraperitoneal administration of DOX 20 mg/kg or vehicle, then repeat echocardiography prior to sacrifice on Day 7 post-treatment. Body weight decreased in WT mice after DOX (22.6 ± 0.9 to 19.4 ± 0.9 g) but was unchanged in MuRF1−/− mice (Table 2, Figure 5A). Raw heart weight (Figure 5B) and heart weight indexed to tibia length (Figure 5C) were reduced in MuRF1+/+ but preserved in MuRF1−/− mice after DOX. Tibia length was identical in MuRF1−/− and MuRF1+/+ mice. Contractile function, as measured by ejection fraction, was decreased in MuRF1+/+, but not MuRF1−/− hearts (Figure 5D and E, Table 2). Collectively, these findings indicate that MuRF1 mediates subacute DOX-induced cardiac atrophy and contractile dysfunction.
Table 2.
Conscious transthoracic echocardiography performed on 8 week-old MuRF1−/− and strain-matched MuRF1+/+ mice.
| Baseline MuRF1+/+ n=9 |
Baseline MuRF1−/− n=6 |
MuRF1+/+ DOX 20 mg/kg 7 Days n=8 |
MuRF1−/− DOX 20 mg/kg 7 Days n=5 |
|
|---|---|---|---|---|
| AWTD (mm) | 0.97 ± 0.06 | 0.94 ± 0.05 | 0.93 ± 0.08 | 0.95 ± 0.07 |
| AWTS (mm) | 1.72 ± 0.12 | 1.74 ± 0.12 | 1.30 ± 0.17 | 1.48 ± 0.20 |
| LVEDD | 3.24 ± 0.39 | 3.36 ± 0.32 | 2.98 ± 0.59 | 3.05 ± 0.25 |
| LVESD | 1.53 ± 0.24 | 1.55 ± 0.27 | 1.93 ± 0.45 | 1.73 ± 0.22 |
| PWTD (mm) | 0.94 ± 0.09 | 0.98 ± 0.07 | 0.87 ± 0.14 | 0.90 ± 0.16 |
| PWTS (mm) | 1.57 ± 0.15 | 1.67 ± 0.17 | 1.25 ± 0.22* | 1.44 ± 0.22 |
| EF (%) | 84.8 ± 3.0 | 85.8 ± 3.4 | 66.5 ± 5.4† | 76.1 ± 4.5* |
| FS (%) | 52.8 ± 3.0 | 54.2 ± 3.9 | 35.5 ± 4.0† | 43.6 ± 4.0‡ |
| LV Vol;d (ml) | 42.9 ± 11.7 | 46.5 ± 11.3 | 36.3 ± 18.3* | 36.9 ± 6.9 |
| LV Vol;s (ml) | 6.69 ± 2.88 | 6.87 ± 3.41 | 12.66 ± 8.29 | 8.94 ± 2.68 |
| Body Weight (g) | 22.6 ± 2.7 | 24.6 ± 2.0 | 19.4 ± 2.5† | 23.1 ± 2.7 |
Data represent means ± SD. A One-Way Analysis of Variance was performed, followed by an all pairwise multiple comparison procedure (Holm- Sidak method), * vs. Column 1 and Column 2, † vs. All other columns, ‡ vs. Column 1.
AWTD, anterior wall thickness in diastole; AWTS, anterior wall thickness in systole; LVEDD, left ventricular end-diastolic dimension; LVESD, left ventricular end-systolic dimension; PWTD, posterior wall thickness in diastole; PWTS, posterior wall thickness in systole; FS, fractional shortening, calculated as (LVEDD-LVESD)/LVEDD x 100; EF%, Ejection Fraction calculated as (end Simpson’s diastolic volume – end Simpson’s systolic volume)/end Simpson’s diastolic volume * 100.
Figure 5. MuRF1−/− mice are protected from doxorubicin-induced cardiac atrophy.
MuRF1−/− and littermate MuRF1+/+ mice were given doxorubicin 20 mg/kg i.p. and sacrificed after 7 days. (A) Body weight before doxorubicin (Day 0) and on the day of sacrifice (Day 7); (B) Heart mass on Day 7; (C) Heart mass indexed to tibia length on Day 7 (inset: tibia length). (D) Contractile function (ejection fraction) was measured using (E) conscious echocardiography. #p<0.05 by Student’s T-test. One-way Analysis of Variance followed by an all pairwise multiple comparison procedure (Holm-Sidak method) compared multiple groups. *p<0.05 vs. Column 1 and Column 2, **p<0.05 vs. All other columns, ***p<0.05 vs. Column 1.
Anthracycline exposure is associated with early cardiac atrophy in humans.
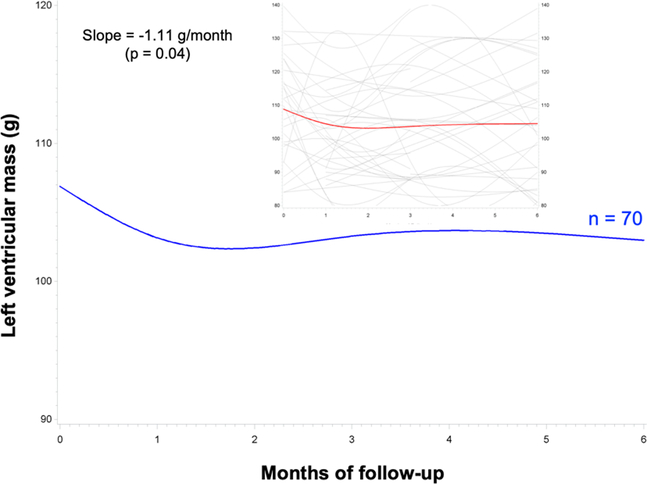
Recent reports have drawn attention to the role of cardiac atrophy in chronic anthracycline-induced cardiotoxicity.20, 21 The early effects of anthracyclines on human cardiac mass have not been published previously. To assess whether humans undergo subacute cardiac atrophy similar to the mice in our study, we queried the DETECT-1 cohort study data.13 DETECT-1 enrolled consecutive patients undergoing chemotherapy at a single academic cancer center and followed them with cardiac MRI (CMR) at baseline and 1, 3, and 6 months after initiation of chemotherapy. We identified 70 DETECT-1 subjects who received anthracycline-based chemotherapy (Table 3) and analyzed the rate of change in left ventricular mass by CMR, constructing a linear mixed model with random intercepts and time effects. We found a statistically significant decrease in cardiac mass that was evident 1 month after initiation of therapy and persisted throughout the entire 6-month follow-up (Figure 6). In this small sample, we did not find significant associations between cardiac mass and other measures of left ventricular performance, though the study was not powered to identify such associations.
Table 3.
Baseline characteristics of DETECT-1 patients treated with anthracyclines (n=70)
| Demographics | |
| Age (years) | 51 ± 14 |
| Women (%) | 44 (63%) |
| Cancer type | |
| AML | 16 (23%) |
| Breast | 29 (41%) |
| Hodgkin | 4 (6%) |
| Non-Hodgkin | 13 (19%) |
| Lymphoma | 6 (19%) |
| Metastatic, unknown primary | 2 (3%) |
| Medical history | |
| Body mass index | 28 ± 6 |
| Tobacco | 32 (46%) |
| Hypertension | 32 (46%) |
| Coronary artery disease | 5 (7%) |
| Cardiac MRI | |
| Ejection fraction (%) | 58 ± 7 |
| Left ventricular mass (gm) | 116 ± 31 |
Figure 6. Anthracyclines are associated with a subacute decrease in cardiac mass detected by cardiac MRI in cancer patients.
70 subjects from the DETECT-1 cohort analysis underwent cardiac MRI at baseline, then 1, 3, and 6 months after initiation of anthracycline-based chemotherapy. The rate of change in left ventricular mass by CMR was analyzed by constructing a linear mixed model with random intercepts and time effects using SAS 9.4. Inset shows full range of values.
DISCUSSION
We undertook these studies to explicate the role of cardiomyocyte atrophy in the early response to doxorubicin exposure. Clinically detected anthracycline-induced cardiotoxicity most commonly manifests as dilated cardiomyopathy with eccentric cardiac hypertrophy, though the potential for anthracyclines to cause late cardiomyocyte atrophy was recognized decades ago in human autopsy studies.22 These early case series were performed in an era of high-dose anthracycline treatment in subjects with overtly pathological response to therapy, hence may not be entirely relevant to contemporary practice. We employed systematic dose escalation assessment of DOX’s effect on cardiac mass to expand upon published mouse studies of anthracycline-mediated atrophy that used fixed dosing.23, 24 Our data indicate that indexed heart weight in mice decreases significantly at 10 mg/kg, a dose that allometrically scales to roughly 30 mg/m2 in humans25— within the limits of current dosing regimens. Our analysis of the DETECT-1 cohort demonstrates that subacute human cardiac atrophy occurs with contemporary anthracycline dosing regimens as well, in patients without a clinical diagnosis of cardiomyopathy or heart failure.
The mass of the post-natal heart decreases either due to decreased myocyte volume or myocyte attrition. Others have reported that DOX can cause cardiac atrophy in mice,24, 26 and here we present evidence that decreased cardiomyocyte cross-sectional area primarily accounts for the dose-dependent decrease in cardiac mass after DOX exposure. The prevailing paradigm considers cardiomyocyte death, through necrosis or apoptosis, to be central to anthracycline- mediated cardiac injury.27 However, numerous studies indicate that the contribution of cardiomyocyte death to DOX cardiotoxicity has been overstated historically (reviewed in 28), due in part to the supratherapeutic concentrations of DOX used for in vitro experiments. In particular, the contribution of in vivo apoptosis has been contested by compelling recent work demonstrating the very limited apoptotic capacity of the adult mouse heart in response to anthracyclines and irradiation.29 We find little convincing evidence for DOX-induced apoptosis in this study: TUNEL staining was entirely negative and immunoblotting for cleaved caspase 3 and cleaved PARP each were elevated at single discrepant doses.
Our present studies in mice and multiple recent human studies30, 31 indicate that the rise in troponin after contemporary anthracycline treatment is very modest and transient, well below a threshold that could be reasonably expected to decrease cardiac mass. Cardiac troponins are released into the serum either through loss of cardiomyocyte membrane integrity or through increased turnover of intracellular troponin by the ubiquitin-proteasome system,32 including ubiquitin ligases such as MuRF1. Indeed, troponin I is a direct target of MuRF1.33 Interestingly, cardiac atrophy due to other causes, including cancer34 and anorexia,35 is associated with elevated serum troponin and other biomarkers of cardiac damage. Collectively, these observations suggest that the early decrease in heart weight after anthracycline exposure likely is largely attributable to myofibrillar atrophy, rather than cardiomyocyte loss. Similarly, it seems possible that early declines in cardiac contractile function after DOX result mostly from depletion of cardiomyocyte contractile apparatus and insults to cardiomyocyte metabolism,7 rather than cell death. Our findings, coupled with these published data, inform our understanding of a commonly used rodent model of heart injury and may be relevant to anthracycline-induced cardiotoxicity in humans.
The central mechanistic finding of our studies is that DOX-induced cardiac atrophy is mediated by MuRF1. MuRF1 is a striated muscle-specific ubiquitin ligase expressed exclusively in skeletal and cardiac muscle. Ubiquitin ligases add ubiquitin to lysine residues in the side chains of proteins as a post-translational modification. Ubiquitin ligases are found in all eukaryotic cells and ubiquitination regulates the stability and activity of many proteins, targeting defective proteins to the proteasome for degradation. In cardiomyocytes, the ubiquitin proteasome system (UPS) regulates sarcomeric protein turnover to maintain normal heart function.36 Dysregulation of the UPS contributes to the pathobiology of heart failure.37
Upregulation of MuRF1, along with atrogin-1 and FOXO3a, accompanies disuse atrophy in skeletal muscle, though there is some controversy as to whether MuRF1 is a bona fide mediator, or merely a marker of atrophy.38, 39 Here we confirm previous reports that DOX increases MuRF1 mRNA abundance in mouse heart and skeletal muscle in vivo.40, 41 We extend these findings by showing that DOX administration causes a dose-dependent increase in expression of MuRF1 and molecular markers of atrophy (MHC-β and ANP) that parallels the decrease in heart weight. The hearts of mice globally lacking MuRF1 were protected from atrophy and contractile dysfunction after DOX exposure, suggesting that MuRF1 upregulation indeed participates in anthracycline- mediated cardiac atrophy, rather than merely serving as a molecular marker.
The role of MuRF1 in cardiac atrophy is complex; it serves both as a ubiquitin ligase and a regulator of transcription.10 MuRF1 specifically degrades numerous canonical myofibrillar proteins, including MHCα, MHCβ,42 and cardiac troponin-I.33 MuRF1 also regulates transcription of key cardiomyocyte genes, including cardiac myosin binding protein C.43 Considered more broadly, the molecular profiling of atrophy is similarly complex. Multiple etiologies of atrophy all lead to transcriptional induction of MHCβ, ANP, and α-skeletal actin (reviewed in15) collectively referred to as the “fetal gene program” for its resemblance to prenatal expression patterns. Upregulation of these same genes long has been associated with cardiac hypertrophy—the morphological converse of atrophy—suggesting the possibility that these changes may simply represent a transcriptional response to injury, rather than critical determinants of phenotype. Consistent with this paradigm, we show dose-dependent increases in “fetal genes” with an inverse relationship to heart size and myocyte cross-sectional area.
Though our findings directly implicate MuRF1 in DOX-induced cardiac atrophy, we cannot exclude contributions from other mediators. Previous studies found that DOX increases abundance of atrogin-1, another critical muscle-specific ubiquitin ligase, via activation of p38-MAP kinase in vitro in NRVMs exposed to DOX.18 We found no upregulation of either atrogin-1 mRNA or protein in our in vivo model of anthracycline cardiotoxicity, though further experiments in genetically altered mice would be required to fully exclude the involvement of atrogin-1 in DOX- induced cardiac atrophy. Similarly, some reports have shown that calpain-1 contributes to anthracycline-induced cardiotoxicity and skeletal muscle myopathy.23 However, other reports indicate that calpain abundance and activity are decreased after DOX exposure in vivo and in vitro.44 We found no clear relationship between DOX dose and calpain-1 expression, though we cannot entirely exclude a role for calpain-1 in anthracycline-induced cardiac atrophy.
It is possible that some of our findings, including the transience of the troponin elevation, result from the use of a single-dose model that may not accurately recapitulate clinical dosing strategies. We also acknowledge the inherent limitations in the use of inbred mouse strains for the prediction of anthracycline toxicity in humans45 and chose the widely-used C57BL/6 strain for our experiments to facilitate reproduction and extension of our results in other research labs. We hope that the inclusion of clinical data from the DETECT-1 cohort will enhance confidence in the relevance of our findings to the pathobiology of human anthracycline cardiotoxicity.
Skeletal sarcopenia related to chemotherapy is an independent risk factor in patients with multiple types of malignancies.46,47 The effects of cancer and its treatment on cardiac muscle mass are less well understood.48 One previous case series using cardiac MRI found that decreased cardiac mass was an independent predictor of adverse events in patients diagnosed with chronic anthracycline-induced cardiomyopathy presenting at a median of 88 months after conclusion of treatment.49 More recently, a study of 76 patients showed that decreased cardiac mass 6 months after anthracycline exposure was associated with worse heart failure symptoms.21 A separate study of 27 women used cardiac MRI to demonstrate a decrease in cardiomyocyte mass, consistent with cardiomyocyte atrophy, 351–700 days after anthracycline-containing chemotherapy.20 Our analysis of the DETECT-1 cohort extends these findings by identifying decreased cardiac mass within 1 month of anthracycline exposure—to our knowledge, the earliest demonstration of anthracycline-induced atrophy in humans. Coupled with the recent report that CMR ejection fraction decreases 3 months after treatment,50 our findings suggest that anthracyclines routinely have early adverse effects on the human heart at low contemporary doses. Mounting evidence indicates that cardiomyocyte atrophy contributes to this cardiotoxicity. Previous studies have shown that timely institution of neurohormonal antagonists after detection of anthracycline-induced heart injury can protect against the development of chronic anthracycline cardiomyopathy,51 suggesting that detection of early onset subclinical DOX cardiotoxicity may be actionable and clinically meaningful. Our findings indicate that cardiomyocyte atrophy is central to the pathogenesis of acute and subacute anthracycline- induced cardiotoxicity and suggest that therapies directed at preventing or reversing cardiac atrophy, including MuRF1 antagonists,52 might preserve the cardiac function of cancer patients receiving anthracyclines.
Supplementary Material
What is new?
The ubiquitin ligase, MuRF1, is upregulated in the hearts of mice treated with doxorubicin.
Mice lacking MuRF1 are resistant to doxorubicin-induced cardiac atrophy.
Human cardiac mass as measured by MRI decreases within one month of initiating anthracycline therapy.
What are the clinical implications?
Cardiac atrophy occurs soon after anthracycline exposure in humans and is associated with worse outcomes. Earlier surveillance with cardiac MRI could identify actionable cardiotoxicity.
Therapeutic inhibition of MuRF1 might blunt cardiac atrophy and mitigate anthracycline- induced cardiotoxicity.
Acknowledgments:
The authors wish to thank Dawud Hilliard (University of North Carolina- Lineberger Center Animal Histopathology Laboratory) for preparation of the histological specimens, Bentley Midkiff in the UNC Translational Pathology Laboratory for assistance in digitally scanning slides with Aperio VERSA Digital Pathology Scanner for quantitative analyses, and Brian Cooley in the UNC McAllister Heart Institute Animal Surgery Core.
Sources of funding: This work was supported by the National Institutes of Health (R01HL104129 to MSW; K08HL096836 and R01HL140067 to BCJ), Hugh A. McAllister Research Foundation (BCJ), and the American Heart Association (Post-Doctoral Fellowship to TLP and 17GRNT33710008 to BCJ).
Footnotes
Disclosures: None
REFERENCES
- 1.Bloom MW, Hamo CE, Cardinale D, Ky B, Nohria A, Baer L, Skopicki H, Lenihan DJ, Gheorghiade M, Lyon AR and Butler J. Cancer Therapy-Related Cardiac Dysfunction and Heart Failure: Part 1: Definitions, Pathophysiology, Risk Factors, and Imaging. Circulation Heart failure. 2016;9:e002661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mukku RB, Fonarow GC, Watson KE, Ajijola OA, Depasquale EC, Nsair A, Baas AS, Deng MC and Yang EH. Heart Failure Therapies for End-Stage Chemotherapy-Induced Cardiomyopathy. Journal of cardiac failure. 2016;22:439–48. [DOI] [PubMed] [Google Scholar]
- 3.Luminari S, Montanini A, Caballero D, Bologna S, Notter M, Dyer MJ, Chiappella A, Briones J, Petrini M, Barbato A, Kayitalire L and Federico M. Nonpegylated liposomal doxorubicin (MyocetTM) combination (R-COMP) chemotherapy in elderly patients with diffuse large B-cell lymphoma (DLBCL): results from the phase II EUR018 trial. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2010;21:1492–9. [DOI] [PubMed] [Google Scholar]
- 4.Tirelli U, Errante D, Van Glabbeke M, Teodorovic I, Kluin-Nelemans JC, Thomas J, Bron D, Rosti G, Somers R, Zagonel V and Noordijk EM. CHOP is the standard regimen in patients > or = 70 years of age with intermediate-grade and high-grade non-Hodgkin’s lymphoma: results of a randomized study of the European Organization for Research and Treatment of Cancer Lymphoma Cooperative Study Group. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 1998;16:27–34. [DOI] [PubMed] [Google Scholar]
- 5.Cardinale D, Sandri MT, Martinoni A, Tricca A, Civelli M, Lamantia G, Cinieri S, Martinelli G, Cipolla CM and Fiorentini C. Left ventricular dysfunction predicted by early troponin I release after high-dose chemotherapy. Journal of the American College of Cardiology. 2000;36:517–22. [DOI] [PubMed] [Google Scholar]
- 6.Octavia Y, Tocchetti CG, Gabrielson KL, Janssens S, Crijns HJ and Moens AL. Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. Journal of molecular and cellular cardiology. 2012;52:1213–25. [DOI] [PubMed] [Google Scholar]
- 7.Tokarska-Schlattner M, Zaugg M, Zuppinger C, Wallimann T and Schlattner U. New insights into doxorubicin-induced cardiotoxicity: the critical role of cellular energetics. Journal of molecular and cellular cardiology. 2006;41:389–405. [DOI] [PubMed] [Google Scholar]
- 8.Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF and Yeh ET. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nature medicine. 2012;18:1639–42. [DOI] [PubMed] [Google Scholar]
- 9.Robert J Preclinical assessment of anthracycline cardiotoxicity in laboratory animals: predictiveness and pitfalls. Cell biology and toxicology. 2007;23:27–37. [DOI] [PubMed] [Google Scholar]
- 10.Willis MS, Rojas M, Li L, Selzman CH, Tang RH, Stansfield WE, Rodriguez JE, Glass DJ and Patterson C. Muscle ring finger 1 mediates cardiac atrophy in vivo. American journal of physiology Heart and circulatory physiology. 2009;296:H997–H1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willis MS, Ike C, Li L, Wang DZ, Glass DJ and Patterson C. Muscle ring finger 1, but not muscle ring finger 2, regulates cardiac hypertrophy in vivo. Circulation research. 2007;100:456–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bode MF, Auriemma AC, Grover SP, Hisada Y, Rennie A, Bode WD, Vora R, Subramaniam S, Cooley B, Andrade-Gordon P, Antoniak S and Mackman N. The factor Xa inhibitor rivaroxaban reduces cardiac dysfunction in a mouse model of myocardial infarction. Thromb Res. 2018;167:128–134. [DOI] [PubMed] [Google Scholar]
- 13.Drafts BC, Twomley KM, D’Agostino R Jr, Lawrence J, Avis N, Ellis LR, Thohan V, Jordan J, Melin SA, Torti FM, Little WC, Hamilton CA and Hundley WG. Low to moderate dose anthracycline-based chemotherapy is associated with early noninvasive imaging evidence of subclinical cardiovascular disease. JACC Cardiovasc Imaging. 2013;6:877–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moulin M, Piquereau J, Mateo P, Fortin D, Rucker-Martin C, Gressette M, Lefebvre F, Gresikova M, Solgadi A, Veksler V, Garnier A and Ventura-Clapier R. Sexual dimorphism of doxorubicin-mediated cardiotoxicity: potential role of energy metabolism remodeling. Circulation Heart failure. 2015;8:98–108. [DOI] [PubMed] [Google Scholar]
- 15.Baskin KK and Taegtmeyer H. Taking pressure off the heart: the ins and outs of atrophic remodelling. Cardiovascular research. 2011;90:243–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao DJ, Jiang N, Blagg A, Johnstone JL, Gondalia R, Oh M, Luo X, Yang KC, Shelton JM, Rothermel BA, Gillette TG, Dorn GW and Hill JA. Mechanical unloading activates FoxO3 to trigger Bnip3-dependent cardiomyocyte atrophy. J Am Heart Assoc. 2013;2:e000016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Campos EC, O’Connell JL, Malvestio LM, Romano MM, Ramos SG, Celes MR, Prado CM, Simoes MV and Rossi MA. Calpain-mediated dystrophin disruption may be a potential structural culprit behind chronic doxorubicin-induced cardiomyopathy. Eur J Pharmacol. 2011;670:541–53. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto Y, Hoshino Y, Ito T, Nariai T, Mohri T, Obana M, Hayata N, Uozumi Y, Maeda M, Fujio Y and Azuma J. Atrogin-1 ubiquitin ligase is upregulated by doxorubicin via p38-MAP kinase in cardiac myocytes. Cardiovascular research. 2008;79:89–96. [DOI] [PubMed] [Google Scholar]
- 19.Stevenson EJ, Giresi PG, Koncarevic A and Kandarian SC. Global analysis of gene expression patterns during disuse atrophy in rat skeletal muscle. The Journal of physiology. 2003;551:33–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ferreira de Souza T, Quinaglia ACST, Osorio Costa F, Shah R, Neilan TG, Velloso L, Nadruz W, Brenelli F, Sposito AC, Matos-Souza JR, Cendes F, Coelho OR, Jerosch-Herold M and Coelho-Filho OR. Anthracycline Therapy Is Associated With Cardiomyocyte Atrophy and Preclinical Manifestations of Heart Disease. JACC Cardiovasc Imaging. 2018;11:1045–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jordan JH, Castellino SM, Melendez GC, Klepin HD, Ellis LR, Lamar Z, Vasu S, Kitzman DW, Ntim WO, Brubaker PH, Reichek N, D’Agostino RB, Jr. and Hundley WG. Left Ventricular Mass Change After Anthracycline Chemotherapy. Circulation Heart failure. 2018;11:e004560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferrans VJ. Overview of cardiac pathology in relation to anthracycline cardiotoxicity. Cancer treatment reports. 1978;62:955–61. [PubMed] [Google Scholar]
- 23.Min K, Kwon OS, Smuder AJ, Wiggs MP, Sollanek KJ, Christou DD, Yoo JK, Hwang MH, Szeto HH, Kavazis AN and Powers SK. Increased mitochondrial emission of reactive oxygen species and calpain activation are required for doxorubicin-induced cardiac and skeletal muscle myopathy. The Journal of physiology. 2015;593:2017–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li L, Takemura G, Li Y, Miyata S, Esaki M, Okada H, Kanamori H, Khai NC, Maruyama R, Ogino A, Minatoguchi S, Fujiwara T and Fujiwara H. Preventive effect of erythropoietin on cardiac dysfunction in doxorubicin-induced cardiomyopathy. Circulation. 2006;113:535–43. [DOI] [PubMed] [Google Scholar]
- 25.Nair AB and Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016;7:27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rasanen M, Degerman J, Nissinen TA, Miinalainen I, Kerkela R, Siltanen A, Backman JT, Mervaala E, Hulmi JJ, Kivela R and Alitalo K. VEGF-B gene therapy inhibits doxorubicin- induced cardiotoxicity by endothelial protection. Proceedings of the National Academy of Sciences of the United States of America. 2016;113:13144–13149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen B, Peng X, Pentassuglia L, Lim CC and Sawyer DB. Molecular and cellular mechanisms of anthracycline cardiotoxicity. Cardiovascular toxicology. 2007;7:114–21. [DOI] [PubMed] [Google Scholar]
- 28.Kankeu C, Clarke K, Passante E and Huber HJ. Doxorubicin-induced chronic dilated cardiomyopathy-the apoptosis hypothesis revisited. J Mol Med (Berl). 2017;95:239–248. [DOI] [PubMed] [Google Scholar]
- 29.Sarosiek KA, Fraser C, Muthalagu N, Bhola PD, Chang W, McBrayer SK, Cantlon A, Fisch S, Golomb-Mello G, Ryan JA, Deng J, Jian B, Corbett C, Goldenberg M, Madsen JR, Liao R, Walsh D, Sedivy J, Murphy DJ, Carrasco DR, Robinson S, Moslehi J and Letai A. Developmental Regulation of Mitochondrial Apoptosis by c-Myc Governs Age- and Tissue- Specific Sensitivity to Cancer Therapeutics. Cancer cell. 2017;31:142–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Avila MS, Ayub-Ferreira SM, de Barros Wanderley MR Jr., das Dores Cruz F, Goncalves Brandao SM, Rigaud VOC, Higuchi-Dos-Santos MH, Hajjar LA, Kalil Filho R, Hoff PM, Sahade M, Ferrari MSM, de Paula Costa RL, Mano MS, Bittencourt Viana Cruz CB, Abduch MC, Lofrano Alves MS, Guimaraes GV, Issa VS, Bittencourt MS and Bocchi EA. Carvedilol for Prevention of Chemotherapy-Related Cardiotoxicity: The CECCY Trial. Journal of the American College of Cardiology. 2018;71:2281–2290. [DOI] [PubMed] [Google Scholar]
- 31.Cardinale D, Ciceri F, Latini R, Franzosi MG, Sandri MT, Civelli M, Cucchi G, Menatti E, Mangiavacchi M, Cavina R, Barbieri E, Gori S, Colombo A, Curigliano G, Salvatici M, Rizzo A, Ghisoni F, Bianchi A, Falci C, Aquilina M, Rocca A, Monopoli A, Milandri C, Rossetti G, Bregni M, Sicuro M, Malossi A, Nassiacos D, Verusio C, Giordano M, Staszewsky L, Barlera S, Nicolis EB, Magnoli M, Masson S, Cipolla CM and Investigators I-OS. Anthracycline-induced cardiotoxicity: A multicenter randomised trial comparing two strategies for guiding prevention with enalapril: The International CardioOncology Society-one trial. European journal of cancer. 2018;94:126–137. [DOI] [PubMed] [Google Scholar]
- 32.Park KC, Gaze DC, Collinson PO and Marber MS. Cardiac troponins: from myocardial infarction to chronic disease. Cardiovascular research. 2017;113:1708–1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kedar V, McDonough H, Arya R, Li HH, Rockman HA and Patterson C. Muscle-specific RING finger 1 is a bona fide ubiquitin ligase that degrades cardiac troponin I. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:18135–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pavo N, Raderer M, Hulsmann M, Neuhold S, Adlbrecht C, Strunk G, Goliasch G, Gisslinger H, Steger GG, Hejna M, Kostler W, Zochbauer-Muller S, Marosi C, Kornek G, Auerbach L, Schneider S, Parschalk B, Scheithauer W, Pirker R, Drach J, Zielinski C and Pacher R. Cardiovascular biomarkers in patients with cancer and their association with all- cause mortality. Heart. 2015;101:1874–80. [DOI] [PubMed] [Google Scholar]
- 35.Zastrow A, Wolf J, Giannitsis E, Katus H, Herzog W, Friederich HC and Mussler C. Elevated myocardial enzymes and natriuretic peptides in anorexia nervosa: prototypic condition for the pathophysiology of cachexia? Cardiology. 2011;118:256–9. [DOI] [PubMed] [Google Scholar]
- 36.Pagan J, Seto T, Pagano M and Cittadini A. Role of the ubiquitin proteasome system in the heart. Circulation research. 2013;112:1046–58. [DOI] [PubMed] [Google Scholar]
- 37.Willis MS, Bevilacqua A, Pulinilkunnil T, Kienesberger P, Tannu M and Patterson C. The role of ubiquitin ligases in cardiac disease. Journal of molecular and cellular cardiology. 2014;71:43–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bodine SC and Baehr LM. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. American journal of physiology Endocrinology and metabolism. 2014;307:E469–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Atherton PJ, Greenhaff PL, Phillips SM, Bodine SC, Adams CM and Lang CH. Control of skeletal muscle atrophy in response to disuse: clinical/preclinical contentions and fallacies of evidence. American journal of physiology Endocrinology and metabolism. 2016;311:E594–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kavazis AN, Smuder AJ and Powers SK. Effects of short-term endurance exercise training on acute doxorubicin-induced FoxO transcription in cardiac and skeletal muscle. Journal of applied physiology. 2014;117:223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sishi BJ, Bester DJ, Wergeland A, Loos B, Jonassen AK, van Rooyen J and Engelbrecht AM. Daunorubicin therapy is associated with upregulation of E3 ubiquitin ligases in the heart. Experimental biology and medicine. 2012;237:219–26. [DOI] [PubMed] [Google Scholar]
- 42.Fielitz J, Kim MS, Shelton JM, Latif S, Spencer JA, Glass DJ, Richardson JA, Bassel-Duby R and Olson EN. Myosin accumulation and striated muscle myopathy result from the loss of muscle RING finger 1 and 3. The Journal of clinical investigation. 2007;117:2486–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mearini G, Gedicke C, Schlossarek S, Witt CC, Kramer E, Cao P, Gomes MD, Lecker SH, Labeit S, Willis MS, Eschenhagen T and Carrier L. Atrogin-1 and MuRF1 regulate cardiac MyBP-C levels via different mechanisms. Cardiovascular research. 2010;85:357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Y, Zheng D, Wei M, Ma J, Yu Y, Chen R, Lacefield JC, Xu H and Peng T. Over- expression of calpastatin aggravates cardiotoxicity induced by doxorubicin. Cardiovasc Res. 2013;98:381–90. [DOI] [PubMed] [Google Scholar]
- 45.Jensen BC and McLeod HL. Pharmacogenomics as a risk mitigation strategy for chemotherapeutic cardiotoxicity. Pharmacogenomics. 2013;14:205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nakamura N, Hara T, Shibata Y, Matsumoto T, Nakamura H, Ninomiya S, Kito Y, Kitagawa J, Kanemura N, Goto N, Shiraki M, Miyazaki T, Takeuchi T, Shimizu M and Tsurumi H. Sarcopenia is an independent prognostic factor in male patients with diffuse large B-cell lymphoma. Annals of hematology. 2015;94:2043–53. [DOI] [PubMed] [Google Scholar]
- 47.Shachar SS, Williams GR, Muss HB and Nishijima TF. Prognostic value of sarcopenia in adults with solid tumours: A meta-analysis and systematic review. European journal of cancer. 2016;57:58–67. [DOI] [PubMed] [Google Scholar]
- 48.Murphy KT. The pathogenesis and treatment of cardiac atrophy in cancer cachexia. American journal of physiology Heart and circulatory physiology. 2016;310:H466–77. [DOI] [PubMed] [Google Scholar]
- 49.Neilan TG, Coelho-Filho OR, Pena-Herrera D, Shah RV, Jerosch-Herold M, Francis SA, Moslehi J and Kwong RY. Left ventricular mass in patients with a cardiomyopathy after treatment with anthracyclines. The American journal of cardiology. 2012;110:1679–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jolly MP, Jordan JH, Melendez GC, McNeal GR, D’Agostino RB Jr. and Hundley WG. Automated assessments of circumferential strain from cine CMR correlate with LVEF declines in cancer patients early after receipt of cardio-toxic chemotherapy. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2017;19:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cardinale D, Colombo A, Bacchiani G, Tedeschi I, Meroni CA, Veglia F, Civelli M, Lamantia G, Colombo N, Curigliano G, Fiorentini C and Cipolla CM. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131:1981–8. [DOI] [PubMed] [Google Scholar]
- 52.Bowen TS, Adams V, Werner S, Fischer T, Vinke P, Brogger MN, Mangner N, Linke A, Sehr P, Lewis J, Labeit D, Gasch A and Labeit S. Small-molecule inhibition of MuRF1 attenuates skeletal muscle atrophy and dysfunction in cardiac cachexia. J Cachexia Sarcopenia Muscle. 2017;8:939–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.