Abstract
Purpose
The aim was to determine effects of diluent monomer and monocalcium phosphate monohydrate (MCPM) on polymerization kinetics and volumetric stability, apatite precipitation, strontium release and fatigue of novel dual-paste composites for vertebroplasty.
Materials and methods
Polypropylene (PPGDMA) or triethylene (TEGDMA) glycol dimethacrylates (25 wt%) diluents were combined with urethane dimethacrylate (70 wt%) and hydroxyethyl methacrylate (5 wt%). 70 wt% filler containing glass particles, glass fibers (20 wt%) and polylysine (5 wt%) was added. Benzoyl peroxide and MCPM (10 or 20 wt%) or N-tolyglycine glycidyl methacrylate and tristrontium phosphate (15 wt%) were included to give initiator or activator pastes. Commercial PMMA (Simplex) and bone composite (Cortoss) were used for comparison. ATR-FTIR was used to determine thermal activated polymerization kinetics of initiator pastes at 50–80°C. Paste stability, following storage at 4–37°C, was assessed visually or through mixed paste polymerization kinetics at 25°C. Polymerization shrinkage and heat generation were calculated from final monomer conversions. Subsequent expansion and surface apatite precipitation in simulated body fluid (SBF) were assessed gravimetrically and via SEM. Strontium release into water was assessed using ICP-MS. Biaxial flexural strength (BFS) and fatigue properties were determined at 37°C after 4 weeks in SBF.
Results
Polymerization profiles all exhibited an inhibition time before polymerization as predicted by free radical polymerization mechanisms. Initiator paste inhibition times and maximum reaction rates were described well by Arrhenius plots. Plot extrapolation, however, underestimated lower temperature paste stability. Replacement of TEGDMA by PPGDMA, enhanced paste stability, final monomer conversion, water-sorption induced expansion and strontium release but reduced polymerization shrinkage and heat generation. Increasing MCPM level enhanced volume expansion, surface apatite precipitation and strontium release. Although the experimental composite flexural strengths were lower compared to those of commercially available Simplex, the extrapolated low load fatigue lives of all materials were comparable.
Conclusions
Increased inhibition times at high temperature give longer predicted shelf-life whilst stability of mixed paste inhibition times is important for consistent clinical application. Increased volumetric stability, strontium release and apatite formation should encourage bone integration. Replacing TEGDMA by PPGDMA and increasing MCPM could therefore increase suitability of the above novel bone composites for vertebroplasty. Long fatigue lives of the composites may also ensure long-term durability of the materials.
Introduction
Osteoporotic fracture of the spine (osteoporotic vertebral fracture; OVF) causes severe pain, height loss, limited mobility, kyphosis, and reduced pulmonary function [1]. Non-surgical treatments such as analgesics and rehabilitation are commonly used but often fail to relieve severe pain in some patients [2, 3]. Hence, surgical managements that relieve severe pain rapidly such as vertebroplasty (VP) and balloon kyphoplasty (KP) are indicated. These procedures involve injection of a bone cement to stabilize fractures. Common complications of these treatments are cement leakage (up to 77%) leading to neurological deficits [4], adjacent vertebral fractures (12–15%) [5], and post-operative infection which can be a rare but serious complication [6].
Polymethyl methacrylate (PMMA) cement is the most commonly used bone cement for VP and KP. Limitations of this cement include poor controlled setting and viscosity that may increase the risk of cement leakage [7]. Further concerns are high polymerization shrinkage, heat generation, and risk of toxic unreacted monomers release [8]. These shortcomings may cause gap formation and local inflammation leading to fibrous encapsulation [9] reducing the integrity of the bone-cement interface. Furthermore, conventional PMMA cements also lack the ability to promote bone formation.
Two-paste injectable bone composites have been developed to address some limitations of the PMMA cements but various shortcomings remain. For example, the primary base monomer used has been bisphenol A-glycidyl methacrylate (Bis-GMA). This monomer is known to limit final monomer conversion of composites due to its limited mobility [10]. Additionally, the commercial composites contain TEGDMA as a diluent monomer, which is known to increase shrinkage and heat generation of dental composites due to its high density of methacrylate groups [10, 11]. Furthermore, the composites contain the tertiary amine DMPT (N,N-dimethyl-p-toluidine), which is highly toxic to human cells [10, 12]. Recently developed light-activated urethane dimethacrylate (UDMA)-based dental composites exhibited higher monomer conversion than Bis-GMA based commercial composites [10]. The same study also demonstrated that replacing TEGDMA by polypropylene glycol dimethacrylate (PPGDMA) increased monomer conversion and cytocompatibility of dental composites while polymerization shrinkage was reduced.
Following mixing, chemically-activated bone composites should ideally cure rapidly after a well-defined inhibition time that provides sufficient working time for injection. A potential problem, however, is unmixed paste instability due to thermal initiated polymerization arising upon storage [13]. Manufacturers usually recommend chemically-activated paste storage below 4°C [14]. This ideal storage condition, however, may be difficult to achieve in some circumstances. For example, medical products shipping to tropical regions can expose materials to fluctuating temperatures between—4 to 42°C and 10 to 40°C during air and marine transport respectively [15]. Stability may be estimated from inhibition times at elevated temperatures for unmixed pastes. Temperature dependence of these times is expected to be governed by Arrhenius type equations, be directly proportional to concentration of initiator (benzoyl peroxide, BP) and inversely proportional to inhibitor levels added to stabilise different monomers [16].
The addition of monocalcium phosphate (MCPM) and tri calcium/strontium phosphates (TCP/TSrP) into dental composites has been shown to promote hygroscopic expansion which could potentially balance polymerization shrinkage and relieve residual shrinkage stress [17, 18]. The addition of these reactive phosphates also enabled surface apatite formation which is known to correlate with in vivo bone bonding [19, 20]. Apatite formation can also be enhanced by the addition of polylysine (PLS) [17]. Furthermore, strontium can promote osteoblast proliferation and maturation whilst inhibiting osteoclast activities [21–23]. It will also enhance radiopacity [24], which may facilitate the surgical procedure and enable follow up with radiographs.
Injected bone composites should be able to withstand the fluctuating and repetitive loads during physical activities [25]. This may then help to prevent mechanical failure due to crack propagation induced by repetitive subcritical loads (fatigue failure). A recent study [26] indicated that high strength values of composites displayed under static loading were not directly related to fatigue performance. Fatigue of various materials was previously assessed by generating stress versus number of cycles curve (S-N curve) during simulated fatigue [27, 28]. At a given applied stress, the steep gradient of S-N plot was associated with a significant reduction in failure cycles [29]. Therefore, a low gradient rather than high gradient was preferable in terms of fatigue performance [30].
The aim of this study was to compare TEGDMA/UDMA versus PPGDMA/UDMA-based bone composites with added Ca/Sr phosphates (MCPM and TSrP) and polylysine (PLS). Initiator paste stability, kinetics of polymerization, final monomer conversions, polymerization shrinkage and heat generation, water sorption induced mass and volume changes, surface apatite formation, strontium release, and biaxial flexural strength/fatigue were assessed. The effect of MCPM levels (5 wt% versus 10 wt%) and type of diluent monomers (TEGDMA versus PPGDMA) were examined.
Materials and methods
Material paste preparation
Experimental bone composites were prepared using a powder to liquid mass ratio of 70: 30. The liquid phase (Table 1) contained urethane dimethacrylate (UDMA) (MW 479 g/mol, DMG, Hamburg, Germany), polypropylene glycol dimethacrylate (PPGDMA) (MW 600 g/mol, Polyscience, PA, USA) or triethylene glycol dimethacrylate (TEGDMA) (MW 286 g/mol, DMG, Hamburg, Germany), and hydroxyethyl methacrylate (HEMA, MW 130 g/mol) (DMG, Hamburg, Germany). To this was added either benzoyl peroxide (BP) (MW 242 g/mol Polyscience, PA, USA) for the initiator liquid or N-tolyglycine glycidyl methacrylate (NTGGMA) (MW 329 g/mol, Esschem, Seaham, UK) for the activator liquid.
Table 1. Components of liquid phases before mixing with the powder phase.
Upon mixing the composite, BP and NTGGMA concentrations will become 1.5 and 1 wt% respectively.
| Liquid phase/components | UDMA | PPGDMA /TEGDMA | HEMA | BP | NTGGMA |
|---|---|---|---|---|---|
| wt% of monomers | wt% of liquid | ||||
| Initiator liquid | 70 | 25 | 5 | 3 | 0 |
| Activator liquid | 70 | 25 | 5 | 0 | 2 |
Powder phase (Table 2) contained glass filler (particle diameter of 0.7 μm, DMG, Hamburg, Germany), glass fiber (30 μm in diameter and 150 μm in length, Mo-Sci, PA, USA), monocalcium phosphate monohydrate (MCPM) particle diameter of 53 μm, Himed, NY, USA), tristrontium phosphate (TSrP) (particle diameter of 10 μm, Sigma Aldrich, Gillingham, UK), and polylysine (PLS) (particle diameter of 20–40 μm, Handary, Brussel, Belgium).
Table 2. Components of powder phase.
Formulations with varying level MCPM (5, 10 wt%) and types of diluent monomer (PPGDMA, TEGDMA). The powder phase of each formulation was mixed with PPGDMA (PPG) or TEGDMA (TEG) liquid phases presented in Table 1. MCPM and TSrP in filler are halved after mixing initiator and activator paste.
| Formulations | Glass fillers | Glass fibers | MCPM | TSrP | PLS | |
|---|---|---|---|---|---|---|
| wt% | ||||||
| Initiator pastes | M5PPG/M5TEG | 65 | 20 | 10 | 0 | 5 |
| M10PPG/M10TEG | 55 | 20 | 20 | 0 | 5 | |
| Activator paste | 60 | 20 | 0 | 15 | 5 | |
Composite pastes were prepared at 23°C. Powders and monomers were weighed using a four-figure balance (OHAUS PA214, Pine Brook, USA). The powder phase was mixed with the liquid phase containing either initiator or activator using a planetary mixer (SpeedMixer, DAC 150.1 FVZ, Hauschild Engineering, Germany) at 2000 rpm for 2 min. The initiator and activator pastes were then poured into a double-barrel syringe (MIXPAC, SULZER, Switzerland) over a vibrator to reduce air entrapment. The syringe was left in an upright position for 24 hr at 23°C to allow the release of air bubbles. For stability studies, pastes were then stored at 4, 23, and 37°C for 1, 3, 6, 9, and 12 months to give “aged” pastes. Mixed experimental initiator and activator pastes were obtained using an automatic mixing tip attached to the double-barrel syringe and a mixing gun (MIXPAC Dispenser, SULZER, Switzerland). Commercial PMMA cement (Simplex) and bone composite (Cortoss) (Table 3), mixed as per manufacturer’s instructions within their use by date, were used as comparisons.
Table 3. Components of commercial products.
| Commercial materials | Compositions | Suppliers |
|---|---|---|
| Simplex P (powder and liquid) | Liquid: DMPT, MMA, inhibitor | Stryker, Berkshire, UK |
| Powder: BP, PMMA, MMA-styrene copolymer beads (diameter of ~25 μm), barium sulphate (diameter ~ 2 μm) | ||
| Cortoss (double-barrel syringe) | Liquid phase: Bis-GMA, Bis-EMA, TEGDMA, BP, DMPT, inhibitor | |
| Powder phase: glass ceramic particles (combeite, diameter of ~ 5–30 μm) |
FTIR studies of composite pastes
Monomer conversion profiles
Monomer conversion profiles of pastes (n = 3) were obtained using FTIR (Perkin-Elmer Series 2000, Beaconsfield, UK) with a temperature controlled ATR attachment (3000 Series RS232, Specac Ltd., UK). Initiator pastes or mixed experimental bone composites and commercial materials were placed in a metal circlip (1 mm depth and 10 mm diameter) on the ATR diamond and covered with an acetate sheet. FTIR spectra between 700–4000 cm-1 of the bottom surfaces of the specimens were recorded every 4 s at a resolution of 4 cm-1. For unmixed pastes, spectra were recorded for up to 10 hours at 50, 60, 70 or 80 ± 1°C. With mixed pastes, spectra were obtained for 40 min at 25 ± 1°C.
Monomer conversion, Dc, and rate of polymerization, Rp, versus time were obtained from FTIR spectra using Eqs 1 and 2 [17].
| (1) |
| (2) |
Where ΔB0 and ΔBt were the absorbance of the C-O peak (1320 cm-1) above background level at 1335 cm-1 initially and after time t and dDc/dt was the gradient of conversion versus time. Furthermore, final monomer conversion, Dc,max, was calculated by linear extrapolation of conversion versus inverse time to zero.
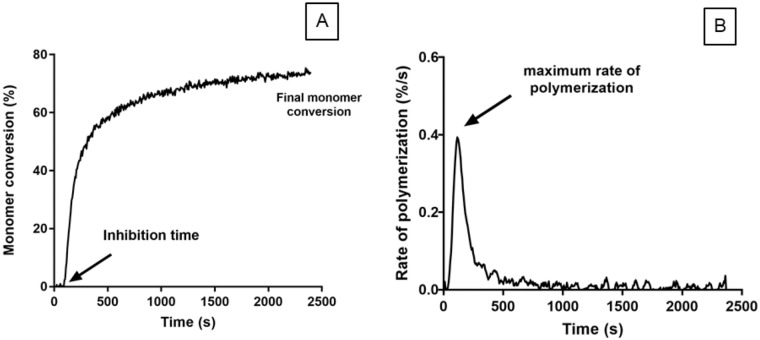
An example of a monomer conversion and reaction rate profile are shown in Fig 1A and 1B. These demonstrate a delay time (inhibition time) before rapid rise in monomer conversion (snap set). Maximum rate is observed between 10–40% conversion with the reaction slowing significantly thereafter.
Fig 1. Example profiles of A) polymerization and B) rate of polymerization of mixed M10PPG.
All mixed and unmixed pastes exhibited similar features for both profiles.
The standard mechanism of free radical polymerization of dimethacrylate monomers includes, initiation, inhibition, propagation, crosslinking and termination steps. Using these mechanisms, with the stationary state assumption that the concentration of free radicals is constant, gives the inhibition time as [16]:
| (3) |
[X] is the initial concentration of inhibitor and Ri the rate of initiation. Furthermore, rate of polymerization (Rp) can be described using the following equation[31].
| (4) |
[M] is the monomer concentration and kp and kt rate constants for propagation and termination steps. Combining Eqs 3 and 4, is therefore expected to be independent of rate of initiation and given by the following equation.
| (5) |
In the following, inhibition times were calculated by linear extrapolation of data between 10% and 40% monomer conversion back to 0% conversion. The gradient in this range was used to obtain the maximum rate of polymerization Rp,max and .
Thermally activated polymerization of unmixed initiator paste, activation energies and predicted shelf life
Pilot studies revealed that initiator pastes were more susceptible to heat than activator pastes and that modifying MCPM level had relatively minimal effect. Hence, initiator pastes of M10PPG and M10TEG were chosen to assess polymerization kinetics and thermally activated polymerization of the experimental bone composites. FTIR spectra of freshly mixed M10PPG and M10TEG initiator pastes (n = 1) were used to obtain their inhibition times, rates of polymerization and final conversions at temperatures of 50, 60, 70, and 80°C.
Inverse inhibition times and reaction rates are proportional to rate constants, k, whose temperature dependence, are generally described by Arrhenius type equations [32].
| (6) |
T is temperature in Kelvin and R the gas constant. A is a pre-exponential factor that is related to the frequency of molecular collisions between reacting species and Ea, the activation energy required for them to react.
Combining Eqs 3, 4 and 6, ln(1/ti) or lnRp,max versus 1/T are expected to be linear if Ea is temperature independent. In the following, these were plotted, and used to obtain activation energies for the initiation step and monomer conversion respectively. These plots were also extrapolated to estimate times of inhibition and 50% monomer conversion at 4, 23, and 37°C. These times provided estimates of when pastes might be expected to start polymerizing and solidify respectively.
Visually observed solidification of unmixed pastes and stability of mixed paste polymerization kinetics (observed shelf life)
To visually assess paste hardening with long-term storage, double-barrel syringes containing unmixed M10PPG and M10TEG initiator and activator pastes were stored at controlled temperatures of 4, 23, and 37°C. At 1 day, 1, 3, 6, 9 and 12 months, small portions were extruded to check for solidification. To assess stability of mixed paste polymerization kinetics at these times, for the 4°C stored samples a portion of the composite was mixed and polymerization kinetics at 25°C determined by FTIR-ATR (n = 3).
Polymerization kinetics, shrinkage and heat generation of freshly prepared and mixed pastes
To compare polymerization kinetics of different mixed composites, inhibition times, maximum reaction rates and final conversions of freshly prepared and immediately mixed experimental materials were compared with those for commercial PMMA (Simplex) and bone composite (Cortoss) cements. Additionally, for the experimental materials, polymerization volume shrinkage (φ) (%) and heat generation (ϵ) (kJ/cc) were calculated using the following equations.
| (7) |
| (8) |
where Mf, monomer mass fraction; Dc, monomer conversion (%); ρ, composite density (g/cm3); ni; the number of C = C bonds per molecule; Wi, molecular weight (g/mol) of each monomer; xi, mass fraction of each monomer in the liquid. These assume one mole of polymerizing C = C groups gives volumetric shrinkage of 23 cm3/mol (e) and generates 57 kJ of heat (ω) [33].
Properties of set discs prepared from fresh pastes
Polymerized disc preparation
To produce disc samples, freshly prepared and then mixed pastes were injected into metal circlips (1 mm in thickness and 10 mm in diameter). The samples were covered with an acetate sheet on top and bottom surfaces. The samples were left for 24 hr at 23°C to allow completion of polymerization. After removal from circlips, any excess was carefully trimmed. The set samples were subsequently immersed in a tube containing 10 mL of simulated body fluid (SBF) prepared according to BS ISO 23317:2012 [34] or deionized water at 37°C until the required test time.
Mass and volume changes
Mass and volume changes of set composite discs after immersion in SBF at 37°C for 0, 1, 6, 24, 48 hr and 1, 2, 3, 4, 5, 6 weeks were measured using a four-figure balance with a density kit (Mettler Toledo, Royston, UK). The percentage mass and volume change, ΔM and ΔV, were determined using the following equations [35].
| (9) |
| (10) |
where M0 and V0 is initial mass and volume, whilst Mt and Vt are mass and volume at time t after immersion.
Surface apatite formation
To assess ability of materials to promote surface apatite formation, disc specimens were immersed in SBF and incubated at 37°C for 1 week (n = 1). They were subsequently removed and sputtered with gold-palladium using a coating machine (Polaron E5000, East Sussex, UK) for 90 s at 20 mA. The specimens were examined under SEM (Phillip XL-30, Eindhoven, The Netherlands) operating with primary beam energy of 5 kV and a current of approximately 200 pA.
Strontium (Sr2+) release
Sr2+ release was measured from experimental bone composites discs (n = 3) immersed in 10 mL of deionized water. The specimens were incubated at 37°C for 4 weeks. The storage solution was collected and replaced with a fresh solution at 24 hr, 1, 2, 3, and 4 weeks. The collected solution was mixed with 2 vol% nitric acid (1:1 volume ratio). Calibration standards containing Sr2+ of 1 ppb, 2.5 ppb, 10 ppb, 25 ppb, 100 ppb, 250 ppb, and 1 ppm were prepared using the ICP-multi element standard solution XVI (Certipur Reference Materials, Merck KGaA, Germany). The cumulative Sr2+ release was calculated using the following equation.
| (11) |
where wSr is the initial amount of Sr2+ in the sample (g), St is the amount of Sr2+ released into storage solution (g) collected at time t (hr).
Biaxial flexural strength and fatigue life
To assess biaxial flexural strength (BFS) and fatigue performance of the materials, disc specimens were immersed in SBF and incubated at 37°C for 4 weeks (n = 25). Prior to fatigue testing, BFS of the composites was assessed using a “ball on ring” jig with a servo hydraulic testing frame (Zwick HC10, Zwick Testing Machine Ltd., Herefordshire, UK) equipped with a 1 kN load cell (n = 5) [27]. The specimens’ thickness was recorded using a digital vernier calliper. The sample was placed on a ring support (8 mm in diameter). The load was applied using a 4 mm diameter spherical ball indenter at 1 mm.min-1 crosshead speed. The failure stress was recorded in newton (N) and the BFS (S; Pa) was calculated using the following equation [17].
| (12) |
Where F is the load at failure (N), d is the specimen’s thickness (m), r is the radius of circular support (m), and v is Poison’s ratio (0.3).
For assessing fatigue performance, a sinusoidal load of 5 Hz [28] was applied to specimens using 80%, 70%, 60%, and 50% of mean BFS (n = 20, 5 specimens per each level of stress). The tests were continued until fracture occurred or the requisite number of load cycles (100,000 cycles) had been applied. BFS was plotted against cycles of failure to generate classical stress-number of cycle curve (S/N curve). Failure cycle from 1st to 5th samples were plotted against BFS which therefore gave five S/N curves. Mean of gradient from the plots was obtained (n = 5) and used to compare fatigue performance [30]. Furthermore, number of failure cycles (fatigue life) at stress level of 10 MPa was obtained by extrapolating the regression line. This value represents fatigue life of materials upon applying low stress that may be generated during normal movements such as flexion, lateral bending, or walking [36].
Statistical analysis
All values and errors reported throughout this study were mean (± standard deviation; SD). SPSS Statistics software (version 24 for Windows, IBM, USA) was used for statistical analysis. Homogeneity of variance was assessed using Levene’s test. When variances were equal, data were analyzed using one-way analysis of variance (ANOVA) followed by post-hoc Tukey’s test for multiple comparisons. Alternatively, the Kruskal-Wallis test, followed by multiple comparison using Dunnett’s T3 tests, was used if the variances were not equal [37]. Correlation between inhibition time and final monomer conversion with storage time was tested using Pearson’s correlation. The significance value was set at p = 0.05. Line fitting for regression analysis was undertaken using the Linest function in Microsoft Office Excel 2016 for Windows.
Factorial analysis was used to assess the effect of MCPM level and diluent monomers on properties of composites from freshly prepared pastes. A full factorial equation for two variables each at high (10 wt% MCPM, PPGDMA) and low levels (5 wt% MCPM, TEGDMA) can be fitted using the following equation [17].
| (13) |
Where a1 and a2 were the effect of each variable on the property P of the composites, < lnP > is the average value of lnP. The a1,2 term is an interaction effect. The percentage effect of each variable, Q, can be calculated using the following equation.
| (14) |
GH and G0 are the geometric average property for the samples with the variable at its high versus low value respectively. The effect of variable change was considered significant if the magnitude of ai was greater than both its calculated 95% CI and interaction terms.
Results
FTIR studies of composite pastes
Thermally activated polymerization of unmixed initiator paste, activation energies and predicted shelf life
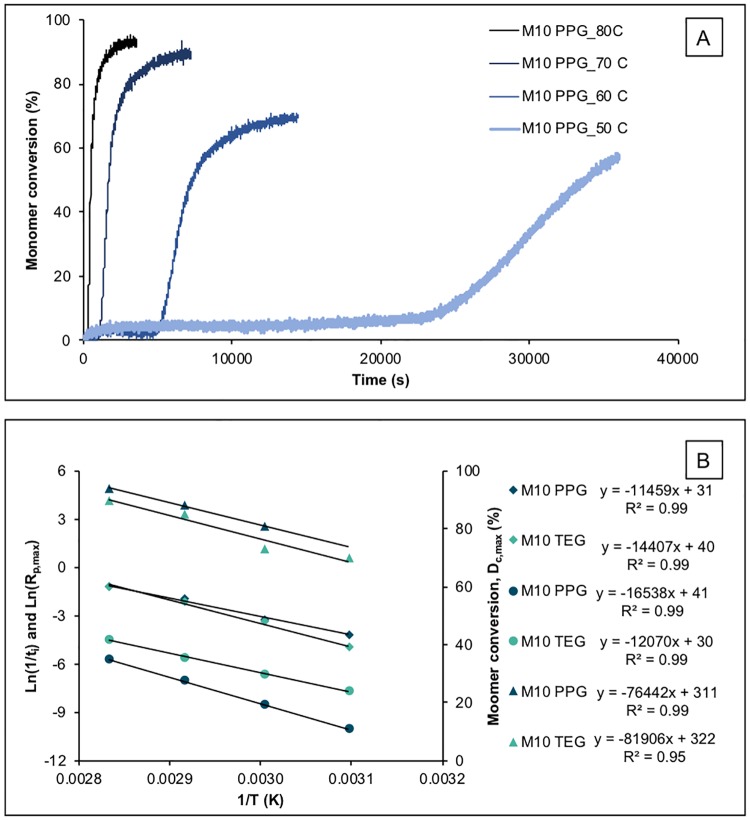
An example of M10PPG initiator paste conversion versus time and temperature is shown in Fig 2A. Upon raising temperature from 50 to 80°C, average ti decreased from 27,000 to 350 s, whilst Rp.max increased from 0.010 to 0.23%/s giving of 1.9 to 4.3%s-0.5. Profiles for M10TEG exhibited shorter inhibition times of 1,600 to 70 s, Rp.max of 0.011 to 0.44%/s and of 0.4 to 3.6%s-0.5.
Fig 2.
A) Example (n = 1) polymerization profiles of unmixed initiator paste of M10PPG at different temperatures. The paste contains 3 wt% BP and 20 wt% MCPM with no activator nor TSrP. B) Average (n = 1) inhibition time (ti; circles), Rp,max (diamond), and final monomer conversion (triangle) of M10TEG and M10PPG initiator pastes plotted as Ln(1/ti) or Ln(Rp,max) versus inverse of temperature (Arrhenius plots). Reaction rates at a given temperature were largely similar for M10TEG compared with M10PPG although delay times and final conversions were significantly and slightly reduced respectively.
ln(1/ti) and ln(Rp,max) versus inverse temperature (Fig 2B) gave linear plots (R2 > 0.99). M10PPG initiation and polymerization activation energies calculated from these were 137 and 95 kJ/mol whilst lnA terms were 41 and 31 respectively. For M10TEG, activation energies were 100 and 120 kJ/mol and lnA terms were 30 and 40 respectively. Extrapolation, gave M10PPG, ti of 3 days, 1 month, and 51 months at 37, 23, and 4°C respectively. Times for 50% conversion were comparable. Conversely, M10TEG ti was 3 hours, 18 hours and 12 days, whilst times for 50% conversion, were 19 hours, 7 days and 7 months at 37, 23, and 4°C respectively.
At 50% conversion, the reaction rates began to slow and tended to final conversions that declined linearly versus 1/T (Fig 2B). At 50°C, reaction level following the 10 hours of observation was too low with M10PPG to enable determination of final conversion. Between 80 and 60°C, however, final monomer conversion declined from 94 to 81% for M10PPG and from 90 to 73% for M10TEG.
Visual solidification of unmixed pastes and stability of mixed paste polymerization kinetics (observed shelf life)
Visual inspection indicated that, at 37°C, both M10PPG and M10TEG initiator pastes solidified in the syringes between 1 day and 1 month. At 25°C, M10PPG polymerized between 3 and 9 months whilst M10TEG initiator pastes solidified between 1 and 3 months. All initiator pastes stored at 4°C, however, remained fluid even after 12 months storage.
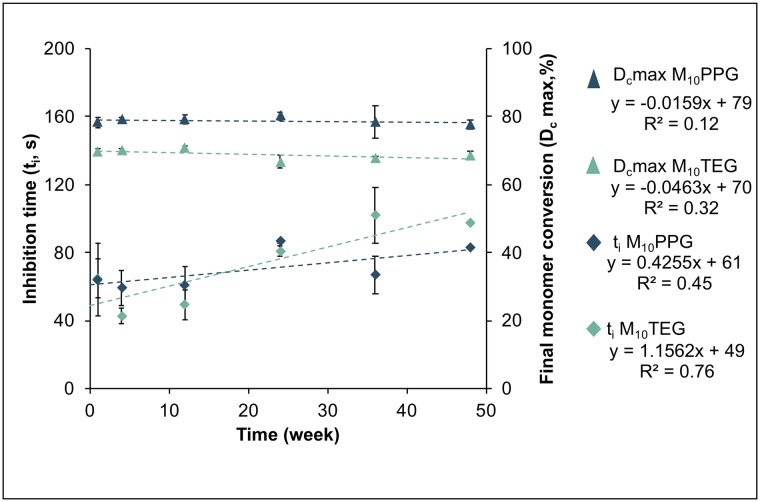
Fig 3 shows inhibition time and monomer conversion of mixed M10PPG and M10TEG pastes that had been stored at 4°C unmixed for up to 12 months. With M10PPG, inhibition time (74 s), polymerization rate (0.43%/s), (3.7%s-0.5) and final conversion (79%) exhibited only minor change with pre-aging of pastes. With M10TEG, and final conversion also remained constant at 2.1%s-0.5 and 70% respectively. M10TEG inhibition time, however, showed a significant increase from 53 s at 24 hr to 104 s at 12 months of pre-aging (R2 = 0.77, p = 0.02).
Fig 3. Inhibition time (diamond) and final monomer conversion (triangle) of the mixed experimental bone composites after storage unmixed at 4°C for up to 12 months.
Error bars are SD (n = 3 at each time point).
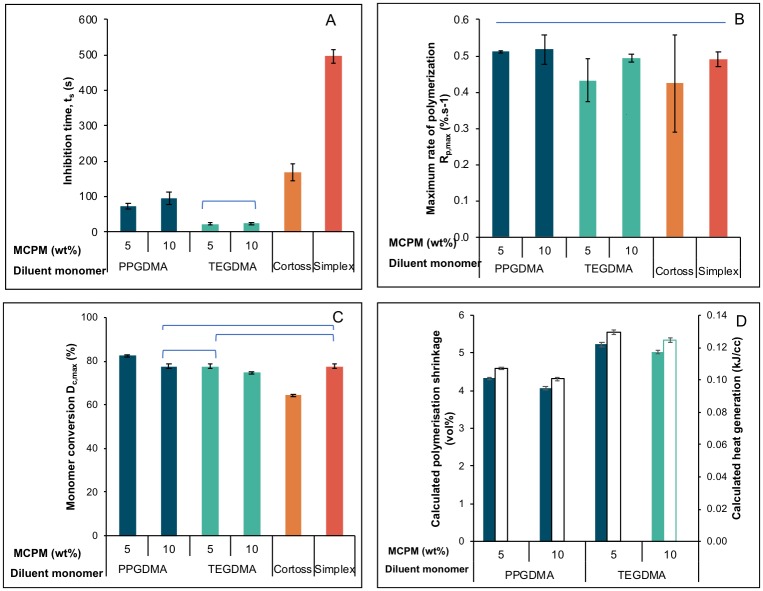
Polymerization kinetics of freshly prepared and mixed materials
Increasing MCPM level showed no significant effect on inhibition time and rate of polymerization. The shortest and longest inhibition times were observed with M10TEG (24 ± 4 s) and Simplex (496 ± 17 s) respectively (Fig 4A). Inhibition times of all experimental bone composites (24–96 s) were shorter than that of Simplex and Cortoss (169 ± 23 s). Average inhibition time of PPGDMA based composites (85 s) was longer than that of TEGDMA based composites (24 s). Factorial analysis indicated that replacing TEGDMA by PPGDMA increased inhibition time by ~ 250% whilst the effect of increasing MCPM level was negligible. Additionally, experimental composites exhibited comparable Rp,max to commercial materials (Fig 4B). Average for PPGDMA, TEGDMA, Cortoss and Simplex were 4.7, 2.5, 5.4 and 10.9%s-0.5 respectively.
Fig 4.
A) inhibition time, B) maximum rate of polymerization, C) final monomer conversion for experimental and commercial products, and D) calculated polymerization shrinkage and heat generation for experimental composites tested at 25°C. Lines indicate no significant difference (p > 0.05). Error bars are SD (n = 3).
Final monomer conversions (Dc,max) of experimental bone composites were higher than that of Cortoss (64 ± 1%) (Fig 4C). M5PPG (82 ± 1%) showed significantly higher final monomer conversion than Simplex (78 ± 1%) (p < 0.05). Averaged final monomer conversion of PPGDMA-based bone composites (80%) was greater than that of TEGDMA-based composites (76%). Factorial analysis indicated that replacing TEGDMA by PPGDMA increased monomer conversion on average by 5%. Additionally, monomer conversion of the composites was increased by 5% upon decreasing MCPM level from 10 to 5 wt%.
Average calculated polymerization shrinkage and heat generation of TEGDMA-based experimental bone composites (5 vol% and 0.13 kJ/cc) were slightly higher than those of PPGDMA-based composites (4 vol% and 0.10 kJ/cc) (Fig 4D). Factorial analysis indicated that the calculated shrinkage and heat generation were increased by 22% upon replacing PPGDMA by TEGDMA.
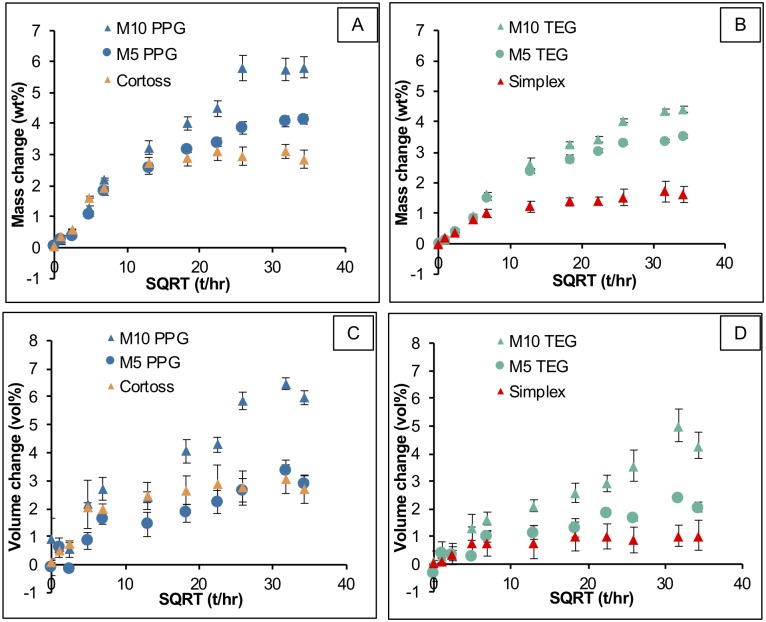
Mass and volume changes
Initial mass and volume change of materials increased linearly with square root of time consistent with diffusion-controlled water sorption. Simplex and Cortoss equilibrium values of mass change were 1.6 ± 0.1 wt% and 3.0 ± 0.1 wt% (Fig 5A and 5B). Mass changes at later times of 3.4 ± 0.1 wt% (M5TEG), 4.0 ± 0.1 wt% (M5PPG), 4.3 ± 0.2 wt% (M10TEG), and 5.8 ± 0.0 wt% (M10PPG) were obtained for the experimental materials. Replacing TEGDMA by PPGDMA and increasing MCPM level increased mass changes at late time by 34 ± 1% and 27 ± 3% respectively.
Fig 5. Mass and volume changes versus square root of time (hr) of all materials immersed in SBF for up to 6 weeks.
Error bars are SD (n = 3).
Volume change of Simplex and Cortoss reached maximum values at 1 week of 1.0 (± 0.1) and 2.8 (± 0.2) vol% (Fig 5C and 5D). Later time maximum values were 1.5 (± 0.1), 3.0 (± 0.3), 4.3 (± 0.7), 6.1 (± 0.3) vol% for M5TEG, M5PPG, M10TEG, and M10PPG respectively. Factorial analysis indicated that replacing TEGDMA by PPGDMA and rising MCPM level enhanced volume change at late time by 45 ± 15% and 109 ± 8% respectively.
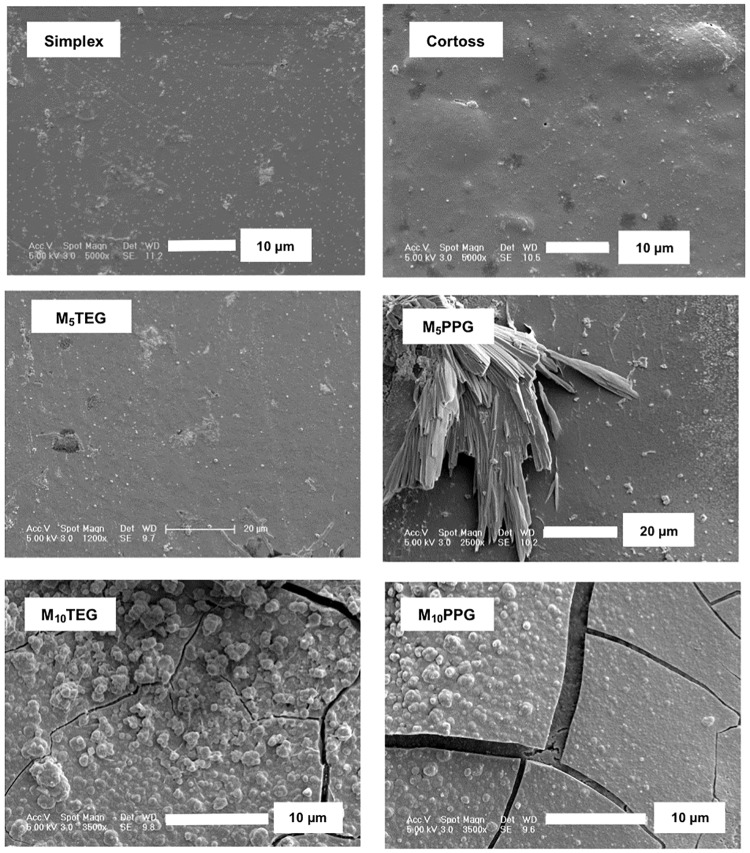
Surface apatite formation
At 1 week, no precipitates were observed on surfaces of M5TEG, Simplex, and Cortoss (Fig 6). Patchy crystals consistent with brushite were observed on some areas of M5PPG. Conversely, thin patchy surface apatite (~ 1 μm) layers partially covered surfaces of M10TEG and M10PPG.
Fig 6. Representative SEM images for each material after immersion in SBF for up to 7 days.
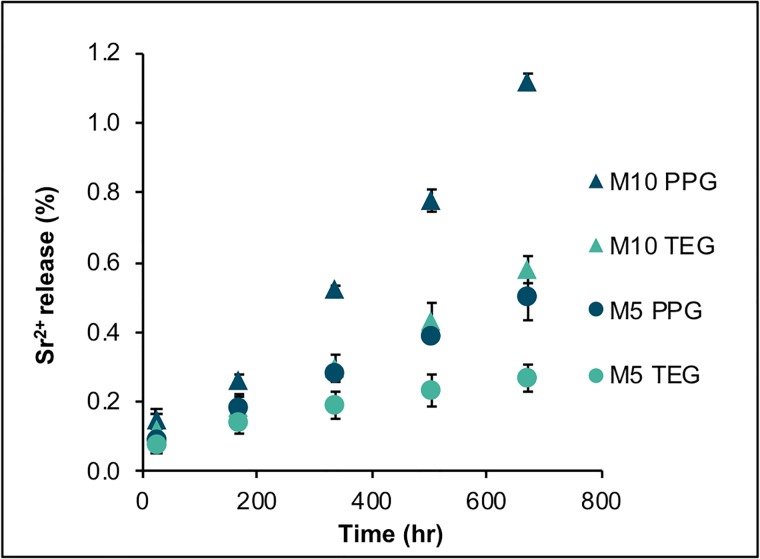
Strontium release
The cumulative release of Sr2+ increased linearly with time (hr) (Fig 7). Highest and lowest rate of Sr2+ release was 0.0015%.hr-1 and 0.0006%.hr-1 observed with M10PPG and M5TEG respectively. M10PPG exhibited the highest accumulative Sr2+ release at 4 weeks (1.12 ± 0.02%). Factorial analysis indicated that cumulative release of Sr2+ at 4 weeks was increased by 127 ± 14% upon increasing MCPM level. Additionally, the release was increased by 111 ± 42% upon replacing TEGDMA by PPGDMA.
Fig 7. Cumulative Sr2+ release versus hr from bone composites immersed in deionized water up to 4 weeks.
Error bars are SD (n = 3).
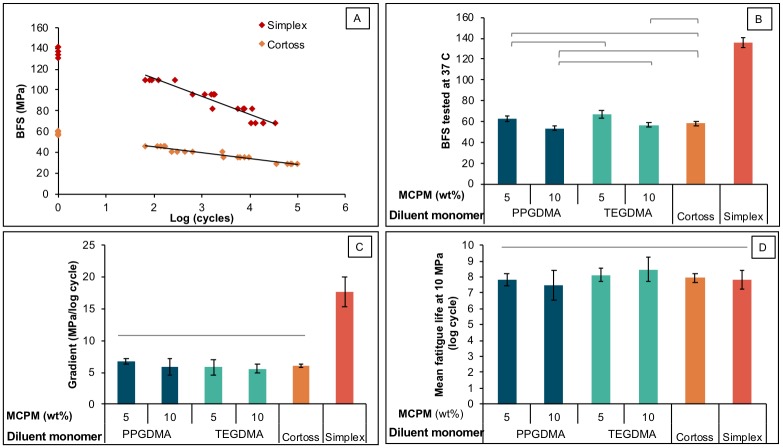
Biaxial flexural strength and fatigue
The highest and lowest BFS of materials tested in SBF at controlled temperature of 37°C was obtained from Simplex (137 ± 4 MPa) and M10PPG (54 ± 2 MPa) respectively (Fig 8A). M5PPG had a comparable BFS (63 ± 3 MPa) to M5TEG (67 ± 4 MPa). The BFS of M5TEG was significantly higher than that of M10PPG (54 ± 2 MPa), M10TEG (57 ± 2 MPa), and Cortoss (58 ± 2 MPa). Factorial analysis showed that BFS was increased by 18 ± 5% upon decreasing MCPM level, whilst the effect of diluent monomers was negligible.
Fig 8.
A) BFS tested in SBF at 37°C, B) example plots of BFS versus log (cycle) (n = 20), C) gradients of S/N plots in positive values for clarity purpose, and D) extrapolated fatigue life at BFS of 10 MPa. Lines indicate no significant difference (p > 0.05) and error bars are SD (n = 5).
BFS versus logarithm of failure cycle number (S/N curve) gave straight line plots with negative gradients (Fig 8B). The most negative gradient was observed with Simplex (-17.7 ± 2.6 MPa/log cycle) (Fig 8C). The gradients for M5PPG (-6.7 ± 0.5 MPa/log cycle), M10PPG (-5.9 ± 1.5 MPa/log cycle), M5TEG (-5.9 ± 1.4 MPa/log cycle), M10TEG (-5.6 ± 0.8 MPa/log cycle) and Cortoss (-6.0 ± 0.3 MPa/log cycle) were comparable. Factorial analysis indicated that the effect of MCPM level and diluent monomers were negligible.
Extrapolated failure cycle values (fatigue life) at 10 MPa for the experimental composites (7.5–8.2 log cycle) were not significantly different from that for the commercial materials (7.8–7.9 log cycle) (Fig 8D). Additionally, factorial analysis showed that MCPM level and diluent monomer had no significant effect on the fatigue life.
Discussion
This study produced bone composites that are two-paste, chemical-cured versions of previously developed Ca/Sr and PLS-containing, single paste light-cured dental composites [17]. The main change involved light activated initiator (camphorquinone) replacement with a chemical activated initiator (BP). This enables the composite to cure chemically after mixing with a separate amine activator-containing paste instead of following light activation. Additionally, however, the tertiary amine activator N,N-dimethyl-p-toluidine (DMPT) was replaced by polymerizable NTGGMA to reduce the risk of toxic amine activator leaching. Furthermore, PLR was reduced from 4:1 to 2.3:1 to enable easy mixing of the initiator and activator-containing pastes through a fine mixing tip and enhanced flow within the vertebra. The effect of MCPM level and diluent monomers on various chemical and mechanical properties were examined.
FTIR studies of composite pastes
The shelf life of chemically-activated bone cement is affected by storage temperature, monomer type, inhibitor, initiator and activator levels [13]. Unmixed composite paste stability is crucial to avoid premature or thermal initiated polymerization during storage or shipment. Furthermore, polymerization kinetics following mixing must be stable and controllable to enable effective setting under clinical conditions.
In this study, a temperature-controlled FTIR-ATR system was employed to monitor polymerization kinetics. As paste at room temperature was placed on the hotter ATR plate and reaction kinetics are highly sensitive to temperature a potential error arises due to the time taken for the paste to reach the ATR temperature. This is reduced through use of thin samples. For the elevated temperature studies, this error was further minimized through ensuring inhibition times were more than 60 s. As reactions were monitored for up to 10 hours, this enabled a wide range of reaction temperatures and times. The reaction temperature for mixed pastes of 25°C mimics clinical conditions before injection into the vertebra and being just slightly above room temperature minimizes temperature variability errors.
In order to understand and predict kinetics of mixed and unmixed composite paste polymerization under different conditions, reaction mechanisms and theories were employed. The mechanism for dimethacrylate reaction used in the derivation of Eqs 3 to 5 included initiation, inhibition, propagation, crosslinking and bimolecular termination steps [38] which may be represented by
| Initiator dissociation | (15) | |
| Inhibition | (16) | |
| Propagation initiation | (17) | |
| Propagation | (18) | |
| Crosslinking initiation | (19) | |
| Termination | (20) |
A limitation of the theory is the possibility of inhibition via routes other than by the added inhibitor. This could include free radical loss by oxygen inhibition or upon contact with surfaces such as of the filler particles or the container that may contain inhibitors [39, 40]. The termination step may also occur via routes other than through bimolecular collision of two polymer free radicals [41]. Changes in relative importance of different mechanisms with reaction rate could cause errors in prediction of lower temperature stability. Lower temperature reaction rates predicted from Arrhenius plots were therefore compared with the stability and reaction kinetics of pastes that had been stored long-term.
Paste stability and inhibition times
The observation of a delay time prior to rapid polymerization is expected from kinetic theories for both thermal and amine activated reactions [15]. For initiator and mixed pastes, this will indicate their shelf-life and time available for injection into the body (working time) respectively. According to Eq 3, the inhibition time is proportional to the inhibitor concentration and inversely proportional to initiation rate. In initiator pastes, initiation rate is proportional to the benzoyl peroxide concentration. For amine activated reactions it is proportional to initiator and activator concentrations [15]. To increase initiator-paste stability and mixed paste working time, the inhibitor can therefore be increased, or the initiator and activator reduced.
Extrapolated Arrhenius plots predicted the greater low temperature stability of the PPGDMA compared with the TEGDMA initiator paste. Calculated shelf-lives, however, were ~10 times lower than those observed through long-term paste storage. This may be due to the alternative mechanisms of inhibition such as by oxygen or surface of the container when the rate of polymerization is slow. Calculated initiator paste inhibition times at 23°C were ~104 greater than those for the mixed pastes. Replacing half of the initiator by activator through paste mixing, therefore enabled rapid setting of the composite pastes.
The inhibitor in the supplied diluent TEGDMA monomer was 200 mM of MEHQ (4-methoxyphenol), whilst that of PPGDMA monomer was a mixture of 100 mM of MEHQ and 100 mM of BHT (butylated hydroxytoluene). A previous study has demonstrated that the addition of BHT enhanced the stabilisation effect of MEHQ [42] which might explain in part the observed lower inhibition times and stability of the TEGDMA initiator pastes.
The lower pre-exponential term and activation energy for the initiation step predicts faster free radical production with the TEGDMA initiator pastes at lower temperature but vice versa at high temperature. A possible explanation is that the smaller size of TEGDMA molecules reduces initial steric hindrance thereby lowering the activation energy for formation of free radicals when compared with UDMA[43]. Conversely the larger PPGDMA molecules are of comparable size to the bulk UDMA possibly giving more comparable activation energies for free radical formation. Higher concentrations of reacting molecules but slower monomer radical formation in the PPGDMA pastes might then explain the differences in reaction kinetics.
Polymerization rates
According to Eq 4, the rate of polymerization following the inhibition period is proportional to the rate of initiation. Preferably rate of polymerization following injection of a mixed paste should be rapid to prevent leakage from the injection site or subsequent release of monomers in the body. Rate of polymerization can potentially be raised by increasing the initiator and activator concentrations. The inhibitor may then additionally need to be raised to maintain the required working time and initiator paste stability. The rate of polymerization of the mixed pastes was comparable with that of the initiator pastes at 80°C and ~250 times that predicted for the unmixed initiator pastes at room temperature.
From Eq 5, might be expected to be constant. values for mixed pastes were comparable with those for the initiator pastes at the highest temperatures. A possible explanation for the decrease in for the initiator paste with decreasing temperature, however, could be alternative free radical inhibition and termination reactions when the reaction is slow. These alternative radical removal reactions might also result in loss of the benzoyl peroxide initiator upon storage. This could then explain the increase in inhibition time of mixed pastes following long-term TEGDMA initiator paste storage.
The higher pre-exponential term for the polymerization propagation step observed with the TEGDMA initiator pastes is to be expected if higher concentration of free radicals are generated. The higher activation energy for the propagation step may be due to the TEGDMA radicals requiring more energy than UDMA or PPGDMA radicals to react with UDMA monomer.
Maximum monomer conversions
Following 50% monomer conversion, the slowing of the dimethacrylate reaction rates can be explained by the propagation reaction that generates linear polymer chains, changing to a crosslinking process. The reaction will slow further when the conversion is sufficient to convert the material from a crosslinked rubber into a solid glassy polymer [44]. At elevated temperatures, higher conversion is required for this glass transition temperature to be reached.
Final conversions at room temperature for the PPGDMA and TEGDMA composites are comparable with values obtained using the same monomers but light activated polymerization [10]. Greater final conversion with the PPGDMA pastes could be a consequence of the longer flexible polypropylene glycol chain lowering the glass transition temperatures. Additionally, if the reaction is continuing at a fast rate when it solidifies, high concentrations of free radicals and localized heating could enable higher conversion. With the mixed fast reacting pastes, conversions at 25°C were comparable with those achieved at 60°C with the slower reacting initiator pastes.
Polymerization of freshly prepared materials
Working time of PMMA bone cements that require mixing powder with liquid and transfer to a syringe for vertebroplasty should be approximately 6 to 10 min [45]. Approximately 3 minutes is required for mixing. 4–8 mL of PMMA cement is generally sufficient to stabilize a fractured vertebra [46]. With an injection rate of 0.15 mL/s [47], an injection time of 0.5–1 minutes is then required to deliver bone cement through a cannula to an affected site. This must be undertaken before the paste viscosity becomes too high for injection. This change in viscosity occurs due to swelling of the beads in the monomer phase.
For a two-paste bone composite in double-barrel syringe, the mixing takes only a few seconds. Additionally, no change in rheological properties occurs following mixing, lower volumes are required to stabilise fractures, and less heat generation compared with PMMA cement [48]. These features are a distinct advance for the composites and enable significant shortening of the required working time.
The inhibition times measured from FTIR-ATR following mixing of both the powder-liquid PMMA cement (496 s) and two-paste Cortoss bone composite (169 s) are different to final setting times cited in the literature [49] (378 s for Simplex and 345 s for Cortoss). This may be a consequence of using a different method (surface indentation), volumes of material in the test and batch number or time after production. The inhibition time of freshly prepared TEGDMA based composite was too short (23 s) indicating that more inhibitor should be included. According to Eq 3, the inhibitor concentration would need to be increased 7 folds in order to bring the inhibition time up to that of Cortoss. This might additionally enhance the initiator paste shelf-life. With the PPGDMA paste, a doubling in inhibitor should give a similar inhibition time to that of Cortoss. From Eq 4, increased inhibitor should not affect the rates of polymerization. The similarities in experimental and commercial material reaction rates suggests this change would thus enable production of composites with “snap set” following sufficient working time for injection.
High final monomer conversion is required for good physical/mechanical composite properties in addition to the low risk of toxic monomer leaching [50, 51]. The final monomer conversion of Cortoss in the current study was lower than that previously obtained (80%) using differential scanning calorimetry (DSC) [52]. DSC, however, has given higher final conversion compared to FTIR in other studies [53, 54]. Lower monomer conversion of Cortoss compared with experimental bone composites could be due to different primary base monomers. Generally, high glass transition temperature (Tg) monomers give low final monomer conversion [18, 55]. Primary base monomer of Cortoss is Bis-GMA (Tg = -7.7°C), whereas that of the experimental bone composites is UDMA (Tg = -35.3°C) [55].
Monomer conversion of Simplex (77%) in the current study is in good agreement with that obtained from published studies (70%) [56, 57]. Simplex contains the monomethacrylate, methyl methacrylate (MMA), which unlike dimethacrylates, gives only linear chains and no crosslinking reaction. Consequently, complete polymerization of all methacrylate groups is required to prevent monomer leaching. Conversely, with dimethacrylate-containing composites, 50% conversion may be sufficient to bind all monomers within the resin matrix [18]. Hence 70–80% observed conversion with the experimental bone composites is expected to reduce the risk of unreacted monomer release and potentially lead to improved cytocompatibility [10].
Polymerization heat generation and shrinkage
The lower concentration of double bonds per mole of PPGDMA contributed to the lower calculated polymerization shrinkage and heat generation of PPGDMA-based composites compared to the TEGDMA-based composites. The shrinkage of experimental bone composites in the current study was comparable to that of Cortoss (5 vol%) [58] but lower than that of PMMA bone cement (6–7 vol%) [59]. Additionally, as heat generation is proportional to shrinkage and a lower volume of composites is required to stabilize vertebral fractures [48], the composites should cause less thermal damage than Simplex upon placement. These properties may help to minimize gap or fibrous capsule formation and improve interfacial integrity at the bone-composite interface.
Mass and volume changes
Mass increase due to water sorption of Simplex reached equilibrium within 1 week which is in accordance with a published study [60]. Cortoss exhibited greater mass increase compared to Simplex due probably to the lower monomer conversion, higher flexibility of polymer network, and hydrophilicity of bioactive glass contained in the composite [61].
The volume increase of Simplex in the current study (~ 2 vol %) was lower than the polymerization shrinkage reported from a published study (6–7 vol%) [59]. This mismatch between shrinkage and expansion may cause gap formation and induce fibrous encapsulation at the bone-cement interfaces. This poor material-bone integration may impair load transfer mechanisms leading to re-fracture or progression of cracks toward adjacent vertebra [62].
For experimental bone composites, their mass and volume changes were governed primarily by MCPM level and type of diluent monomer. Raising MCPM level enhanced water uptake leading to the increase of mass and volume as was previously observed with dental composites [18, 35]. Low crosslinking density due to the high molecular weight of PPGDMA could promote water diffusion, thereby increasing the mass and volume changes of the PPGDMA-based composites. For PPGDMA formulations, therefore, 5 to 10 wt% of MCPM was sufficient to enable hygroscopic expansion comparable with the calculated polymerization shrinkage. TEGDMA formulations, however, may requires greater than 5–10 wt% of MCPM to allow expansion to compensate polymerization shrinkage. These expansions are expected to relieve shrinkage stress and minimize gaps at composite-bone interface. This could potentially help to improve interfacial integrity and load transfer and reduce recurrent fracturing of the treated vertebra.
Surface apatite formation
The apatite-forming ability in SBF has been adopted as a method for the determination of the bone bonding potential in biomaterials prior to any animal testing which requires large expenses and resources. It is proposed that the formation of surface apatite is associated with the ability of materials to promote in vivo bone bonding [63]. Other studies with MCPM-containing composites demonstrated that the level of apatite precipitation increased proportionally to time [64]. Mineral release is also expected to promote mineralization of newly formed bone [65].
When surface MCPM dissolves, it disproportionates into phosphoric acid and dicalcium phosphate. Under acidic conditions, the later will precipitate as brushite. If the acid is neutralized by buffering ions in the SBF, the brushite can transform into apatite [66]. Increasing MCPM level from 5 to 10 wt% encouraged this transformation and greater precipitation of surface apatite after immersion in SBF for 1 week. Replacing TEGDMA by PPGDMA, however, provided no obvious advantageous effect on surface apatite precipitation. In Cortoss, bioactive glass was added in an attempt to provide mineralization and enhance bonding with bone [49]. Surface apatite was however not seen after SBF immersion for 1 week. This could be due to the slower dissolution of its calcium phosphate containing glass (combeite) [67] when compared with MCPM.
Strontium ion release
Strontium release is of interest due to its potential beneficial effects for bone repair including increase of osteoblast proliferation and reduction of osteoclastic activities [22, 23, 68]. The observed linear release of Sr2+ from experimental bone composites suggests it is not diffusion-controlled. It is possible that the level of strontium release was dependent upon its release from the surface following water enhancing polymer expansion. This would explain the increase of strontium release observed upon using PPGDMA and rising MCPM level. The results gave the effect of replacing TEGDMA by PPGDMA on Sr2+ release as less than the effect upon doubling MCPM level. It is hypothesized that in addition to increased water sorption, MCPM may produce phosphoric acid that reacts with the tristrontium phosphate to form distrontium phosphate of higher aqueous solubility and thereby higher Sr ion release.
The release of Sr2+ would be enhanced upon increasing surface area. A previous study showed that a bone composite provided greater interfacial stability at bone/material interface than a PMMA cement [69]. This was attributed to possibly a faster bone response, improved bone binding to mineral precipitation around the composite, and/or more effective penetration of the composite into porous bone. The greater penetration could give a large surface and encourage greater localized release of strontium to the surrounding osteoporotic vertebra. This may potentially help to increase bone mass and improve mechanical properties of the vertebra, thereby decreasing the risk of recurrent fractures. The release of strontium that increases linearly with time may also enable constant drug release which may be considered beneficial.
Biaxial flexural strength (BFS) and fatigue
The mean BFS values obtained from Simplex and Cortoss were consistent with those reported in a previous study [49]. Mean BFS of experimental bone composites was also comparable to that of Cortoss. Increasing hydrophilic contents and flexibility of polymer networks usually reduces strength of composites. Results from the current study showed that increasing MCPM level and replacing TEGDMA by more flexible PPGDMA had no significant effect on the strength and fatigue of the composites. This might be due to the low level of MCPM used and the enhanced monomer conversion from PPGDMA. Despite the fact that specimens were aged for 4 weeks, the mean BFS values of all experimental bone composites were greater than the 24 hr flexural strength of 50 MPa required by ISO 5833: Implants for surgery—Acrylic resin cements [70].
The highest gradient of S-N curve was observed with Simplex. This could be due to the lack of reinforcing glass fillers or glass fiber to retard crack propagation. Additionally, pores caused by the poor integration between BaSO4 particles and polymer matrix could also act as crack initiators [71]. It is assumed that the lower gradient of S-N curve of Cortoss and experimental bone composites compared with Simplex may result from the beneficial effects of absorbed water that could improve fatigue resistance. The water can plasticize resin matrix and increase polymer chain mobility which could enhance crack tip blunting [72]. For experimental bone composites, releasing of active ingredients may leave voids behind but the contained fibers could help to bridge the voids and slow down crack initiation [73].
In physiologic conditions, the injected bone cements are expected to penetrate through porous bone and cracks forming irregular shapes depending on the morphology of fractures [74]. Hence, the injected cement may be subjected to various stresses including torsion, flexion, and compression. A finite element analysis demonstrated that the maximum stresses generated in the injected cement after vertebroplasty may range from 5 to 15 MPa [36]. In the current study, a representative flexural stress of 10 MPa was used to extrapolate number of failure cycles thereby allowing comparison of fatigue life amongst materials. The predicted failure cycle upon applying this flexural stress of experimental composites was comparable to that commercial products (~ 108 cycles). This may ensure a long-term mechanical performance of experimental bone composites.
Conclusions
Replacing diluent TEGDMA by PPGDMA provided beneficial effects such as increased inhibition time, increased final monomer conversion, and decreased calculated polymerization shrinkage and heat generation for the experimental bone composites. PPGDMA also promoted hygroscopic expansion to compensate polymerization shrinkage and enhanced strontium release. Additionally, no detrimental effect on mechanical properties of the composites was observed upon replacing TEGDMA by PPGDMA. Increasing MCPM level enhanced hygroscopic expansion, surface apatite formation, and strontium release. Increasing these reactive fillers reduced static strength of the composites but did not significantly reduce fatigue resistance of the composites.
Supporting information
Experimental and commercial bone composites raw data.
(XLSX)
Acknowledgments
DMG has supplied monomers and fillers. SULZER supplied syringes and mixing tips. Dr. Graham Palmer, Dr. George Georgio, and Dr. Nicola Mordan provided technical support.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was supported by: 1) PP: The Royal Thai Government, Ministry of Sciences and Technology (http://www.most.go.th); 2) WX and AY: The National Institute for Health Research, University College London Hospitals, Biomedical Research Centre (NIHR UCL BRC: BRC522a/OH/AY/110380)(www.uclhospitals.brc.nihr.ac.uk); National Institute for Health Research (NIHR) (NIHR: II-LB-0214-20002)(https://www.nihr.ac.uk); The Engineering and Physical Sciences Research Council (EPSRC: EP/I022341/1) (https://epsrc.ukri.org); Wellcome Trust (ISSF/FHCE/0079) (https://wellcome.ac.uk). This paper presents independent research in part funded by the National Institute for Health Research (NIHR) under its Invention for Innovation (i4i) Programme (Grant Reference Number II-LB-0214-20002). The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Mehbod A, Aunoble S, Le Huec JC. Vertebroplasty for osteoporotic spine fracture: prevention and treatment. Eur Spine J. 2003;12 Suppl 2:S155–62. 10.1007/s00586-003-0607-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benzel EC. Vertebroplasty and Kyphoplasty Spine Surgery. 3 ed Philadelphia: Elsevier Saunders; 2012. p. 1253–62. [Google Scholar]
- 3.Lin J, Hsieh Y-C, Chien L-N, Tsai W-L, Chiang YH. Vertebroplasty Associated with a Lower Risk of Mortality and Morbidity of Aged Patients with Painful Vertebral Compression Fractures: A Population-Based Propensity Score Matching Cohort Study in Taiwan. Spine J. 2016;16(10):S262 10.1016/j.spinee.2016.07.352 [DOI] [PubMed] [Google Scholar]
- 4.Tome-Bermejo F, Pinera AR, Duran-Alvarez C, Lopez-San Roman B, Mahillo I, Alvarez L. Identification of Risk Factors for the Occurrence of Cement Leakage During Percutaneous Vertebroplasty for Painful Osteoporotic or Malignant Vertebral Fracture. Spine. 2014;39(11):E693–E700. 10.1097/BRS.0000000000000294 [DOI] [PubMed] [Google Scholar]
- 5.Li YA, Lin CL, Chang MC, Liu CL, Chen TH, Lai SC. Subsequent vertebral fracture after vertebroplasty: incidence and analysis of risk factors. Spine (Phila Pa 1976). 2012;37(3):179–83. 10.1097/BRS.0b013e3181f72b05 [DOI] [PubMed] [Google Scholar]
- 6.Abdelrahman H, Siam AE, Shawky A, Ezzati A, Boehm H. Infection after vertebroplasty or kyphoplasty. A series of nine cases and review of literature. Spine J. 2013;13(12):1809–17. 10.1016/j.spinee.2013.05.053 [DOI] [PubMed] [Google Scholar]
- 7.Hoppe S, Wangler S, Aghayev E, Gantenbein B, Boger A, Benneker LM. Reduction of cement leakage by sequential PMMA application in a vertebroplasty model. Eur Spine J. 2016;25(11):3450–5. 10.1007/s00586-015-3920-3 [DOI] [PubMed] [Google Scholar]
- 8.Lewis G. Properties of nanofiller-loaded poly (methyl methacrylate) bone cement composites for orthopedic applications: a review. J Biomed Mater Res B Appl Biomater. 2017;105(5):1260–84. 10.1002/jbm.b.33643 [DOI] [PubMed] [Google Scholar]
- 9.Robo C, Hulsart-Billstrom G, Nilsson M, Persson C. In vivo response to a low-modulus PMMA bone cement in an ovine model. Acta Biomater. 2018;72:362–70. 10.1016/j.actbio.2018.03.014 [DOI] [PubMed] [Google Scholar]
- 10.Walters NJ, Xia W, Salih V, Ashley PF, Young AM. Poly(propylene glycol) and urethane dimethacrylates improve conversion of dental composites and reveal complexity of cytocompatibility testing. Dent Mater. 2016;32(2):264–77. 10.1016/j.dental.2015.11.017 [DOI] [PubMed] [Google Scholar]
- 11.Goncalves F, Kawano Y, Pfeifer C, Stansbury JW, Braga RR. Influence of BisGMA, TEGDMA, and BisEMA contents on viscosity, conversion, and flexural strength of experimental resins and composites. Eur J Oral Sci. 2009;117(4):442–6. 10.1111/j.1600-0722.2009.00636.x [DOI] [PubMed] [Google Scholar]
- 12.Nomura Y, Teshima W, Kawahara T, Tanaka N, Ishibashi H, Okazaki M, et al. Genotoxicity of dental resin polymerization initiators in vitro. J Mater Sci Mater Med. 2006;17(1):29–32. 10.1007/s10856-006-6326-2 [DOI] [PubMed] [Google Scholar]
- 13.Shim JB, Warner SJ, Hasenwinkel JM, Gilbert JL. Analysis of the shelf life of a two-solution bone cement. Biomaterials. 2005;26(19):4181–7. 10.1016/j.biomaterials.2004.10.027 [DOI] [PubMed] [Google Scholar]
- 14.Cardoso SA, Oliveira HL, Münchow EA, Carreño NLV, Gonini A Junior, Piva E. Effect of shelf-life simulation on the bond strength of self-etch adhesive systems to dentin. Appl Adhes Sci. 2014;2(1):26 10.1186/s40563-014-0026-9 [DOI] [Google Scholar]
- 15.Nakamura T, Yamaji T, Takayama K. Effects of packaging and heat transfer kinetics on drug-product stability during storage under uncontrolled temperature conditions. J Pharm Sci. 2013;102(5):1495–503. 10.1002/jps.23486 [DOI] [PubMed] [Google Scholar]
- 16.Capek I. Effect of Hydroquinone on the Kinetics of Emulsion Polymerization of Butyl Acrylate. Chemical Papers-Chemicke Zvesti. 1989;43(4):527–35. [Google Scholar]
- 17.Panpisut P, Liaqat S, Zacharaki E, Xia W, Petridis H, Young AM. Dental composites with calcium/strontium phosphates and polylysine. PLoS One. 2016;11(10):e0164653 10.1371/journal.pone.0164653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aljabo A, Xia W, Liaqat S, Khan M, Knowles J, Ashley P, et al. Conversion, shrinkage, water sorption, flexural strength and modulus of re-mineralizing dental composites. Dent Mater. 2015;31(11):1279–89. 10.1016/j.dental.2015.08.149 [DOI] [PubMed] [Google Scholar]
- 19.LeGeros RZ. Calcium phosphate-based osteoinductive materials. Chem Rev. 2008;108(11):4742–53. 10.1021/cr800427g [DOI] [PubMed] [Google Scholar]
- 20.Kokubo T, Kim H-M, Kawashita M. Novel bioactive materials with different mechanical properties. Biomaterials. 2003;24(13):2161–75. 10.1016/S0142-9612(03)00044-9 [DOI] [PubMed] [Google Scholar]
- 21.Schumacher M, Gelinsky M. Strontium modified calcium phosphate cements—approaches towards targeted stimulation of bone turnover. J Mater Chem B. 2015;3(23):4626–40. 10.1039/C5TB00654F [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Rawlinson SC, Hill RG, Fortune F. Strontium-substituted bioactive glasses in vitro osteogenic and antibacterial effects. Dent Mater. 2016;32(3):412–22. 10.1016/j.dental.2015.12.013 [DOI] [PubMed] [Google Scholar]
- 23.Montesi M, Panseri S, Dapporto M, Tampieri A, Sprio S. Sr-substituted bone cements direct mesenchymal stem cells, osteoblasts and osteoclasts fate. PLoS One. 2017;12(2):e0172100 10.1371/journal.pone.0172100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shahid S, Hassan U, Billington RW, Hill RG, Anderson P. Glass ionomer cements: effect of strontium substitution on esthetics, radiopacity and fluoride release. Dent Mater. 2014;30(3):308–13. 10.1016/j.dental.2013.12.003 [DOI] [PubMed] [Google Scholar]
- 25.Wilke HJ, Mehnert U, Claes LE, Bierschneider MM, Jaksche H, Boszczyk BM. Biomechanical evaluation of vertebroplasty and kyphoplasty with polymethyl methacrylate or calcium phosphate cement under cyclic loading. Spine. 2006;31(25):2934–41. 10.1097/01.brs.0000248423.28511.44 [DOI] [PubMed] [Google Scholar]
- 26.Belli R, Petschelt A, Lohbauer U. Are linear elastic material properties relevant predictors of the cyclic fatigue resistance of dental resin composites? Dent Mater. 2014;30(4):381–91. 10.1016/j.dental.2014.01.009 [DOI] [PubMed] [Google Scholar]
- 27.Pittayachawan P, McDonald A, Petrie A, Knowles JC. The biaxial flexural strength and fatigue property of Lava Y-TZP dental ceramic. Dent Mater. 2007;23(8):1018–29. 10.1016/j.dental.2006.09.003 [DOI] [PubMed] [Google Scholar]
- 28.Harmata AJ, Uppuganti S, Granke M, Guelcher SA, Nyman JS. Compressive fatigue and fracture toughness behavior of injectable, settable bone cements. J Mech Behav Biomed Mater. 2015;51:345–55. 10.1016/j.jmbbm.2015.07.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah DU, Schubel PJ, Clifford MJ, Licence P. Fatigue life evaluation of aligned plant fibre composites through S-N curves and constant-life diagrams. Compos Sci Technol. 2013;74:139–49. 10.1016/j.compscitech.2012.10.015 [DOI] [Google Scholar]
- 30.Koster U, Jaeger R, Bardts M, Wahnes C, Buchner H, Kuhn KD, et al. Creep and fatigue behavior of a novel 2-component paste-like formulation of acrylic bone cements. J Mater Sci Mater Med. 2013;24(6):1395–406. 10.1007/s10856-013-4909-2 [DOI] [PubMed] [Google Scholar]
- 31.Odian G. Radical Chain Polymerization Principles of Polymerization. New Jersey: John Wiley & Sons; 2004. p. 198–349. [Google Scholar]
- 32.Sideridou ID, Achilias DS, Karava O. Reactivity of Benzoyl Peroxide/Amine System as an Initiator for the Free Radical Polymerization of Dental and Orthopaedic Dimethacrylate Monomers: Effect of the Amine and Monomer Chemical Structure. Macromolecules. 2006;39(6):2072–80. 10.1021/ma0521351 [DOI] [Google Scholar]
- 33.Dewaele M, Truffier-Boutry D, Devaux J, Leloup G. Volume contraction in photocured dental resins: the shrinkage-conversion relationship revisited. Dent Mater. 2006;22(4):359–65. 10.1016/j.dental.2005.03.014 [DOI] [PubMed] [Google Scholar]
- 34.British Standard. BS ISO 23317:2012 Implants for surgery. In vitro evaluation for apatite-forming ability of implant materials. Switzerland: BSI Standards Limited; 2012.
- 35.Mehdawi IM, Pratten J, Spratt DA, Knowles JC, Young AM. High strength re-mineralizing, antibacterial dental composites with reactive calcium phosphates. Dent Mater. 2013;29(4):473–84. 10.1016/j.dental.2013.01.010 [DOI] [PubMed] [Google Scholar]
- 36.Rohlmann A, Boustani HN, Bergmann G, Zander T. A probabilistic finite element analysis of the stresses in the augmented vertebral body after vertebroplasty. Eur Spine J. 2010;19(9):1585–95. 10.1007/s00586-010-1386-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shingala MC, Rajyaguru A. Comparison of post hoc tests for unequal variance. In J New Technol 2015;2(5):22–33. [Google Scholar]
- 38.Achilias DS, Sideridou ID. Kinetics of the Benzoyl Peroxide/Amine Initiated Free-Radical Polymerization of Dental Dimethacrylate Monomers: Experimental Studies and Mathematical Modeling for TEGDMA and Bis-EMA. Macromolecules. 2004;37(11):4254–65. 10.1021/ma049803n [DOI] [Google Scholar]
- 39.Lee TY, Guymon CA, Jönsson ES, Hoyle CE. The effect of monomer structure on oxygen inhibition of (meth)acrylates photopolymerization. Polymer. 2004;45(18):6155–62. 10.1016/j.polymer.2004.06.060 [DOI] [Google Scholar]
- 40.Gauthier MA, Stangel I, Ellis TH, Zhu XX. Oxygen inhibition in dental resins. J Dent Res. 2005;84(8):725–9. 10.1177/154405910508400808 [DOI] [PubMed] [Google Scholar]
- 41.Nakamura Y, Yamago S. Termination Mechanism in the Radical Polymerization of Methyl Methacrylate and Styrene Determined by the Reaction of Structurally Well-Defined Polymer End Radicals. Macromolecules. 2015;48(18):6450–6. 10.1021/acs.macromol.5b01532 [DOI] [Google Scholar]
- 42.Belbakra Z, Cherkaoui ZM, Allonas X. Photocurable polythiol based (meth)acrylate resins stabilization: New powerful stabilizers and stabilization systems. Polym Degrad Stabil. 2014;110:298–307. 10.1016/j.polymdegradstab.2014.09.012 [DOI] [Google Scholar]
- 43.Srivastava R, Wolska J, Walkowiak-Kulikowska J, Koroniak H, Sun YY. Fluorinated bis-GMA as potential monomers for dental restorative composite materials. Eur Polym J. 2017;90:334–43. 10.1016/j.eurpolymj.2017.03.027 [DOI] [Google Scholar]
- 44.Ye S, Cramer NB, Bowman CN. Relationship between Glass Transition Temperature and Polymerization Temperature for Cross-Linked Photopolymers. Macromolecules. 2011;44(3):490–4. 10.1021/ma101296j [DOI] [Google Scholar]
- 45.Lewis G. Injectable bone cements for use in vertebroplasty and kyphoplasty: state-of-the-art review. J Biomed Mater Res B Appl Biomater. 2006;76(2):456–68. 10.1002/jbm.b.30398 [DOI] [PubMed] [Google Scholar]
- 46.Hadley C, Awan OA, Zoarski GH. Biomechanics of vertebral bone augmentation. Neuroimaging Clin N Am. 2010;20(2):159–67. 10.1016/j.nic.2010.02.002 [DOI] [PubMed] [Google Scholar]
- 47.Loeffel M, Ferguson SJ, Nolte L-P, Kowal JH. Vertebroplasty: Experimental Characterization of Polymethylmethacrylate Bone Cement Spreading as a Function of Viscosity, Bone Porosity, and Flow Rate. Spine. 2008;33(12):1352–9. 10.1097/BRS.0b013e3181732aa9 [DOI] [PubMed] [Google Scholar]
- 48.Middleton ET, Rajaraman CJ, O’Brien DP, Doherty SM, Taylor AD. The safety and efficacy of vertebroplasty using Cortoss cement in a newly established vertebroplasty service. Br J Neurosurg. 2008;22(2):252–6. 10.1080/02688690701824354 [DOI] [PubMed] [Google Scholar]
- 49.Boyd D, Towler MR, Wren A, Clarkin OM. Comparison of an experimental bone cement with surgical Simplex P, Spineplex and Cortoss. J Mater Sci Mater Med. 2008;19(4):1745–52. 10.1007/s10856-007-3363-4 [DOI] [PubMed] [Google Scholar]
- 50.Finan L, Palin WM, Moskwa N, McGinley EL, Fleming GJ. The influence of irradiation potential on the degree of conversion and mechanical properties of two bulk-fill flowable RBC base materials. Dent Mater. 2013;29(8):906–12. 10.1016/j.dental.2013.05.008 [DOI] [PubMed] [Google Scholar]
- 51.Zhang L, Weir MD, Chow LC, Antonucci JM, Chen J, Xu HH. Novel rechargeable calcium phosphate dental nanocomposite. Dent Mater. 2016;32(2):285–93. 10.1016/j.dental.2015.11.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pomrink GJ, DiCicco MP, Clineff TD, Erbe EM. Evaluation of the reaction kinetics of CORTOSS, a thermoset cortical bone void filler. Biomaterials. 2003;24(6):1023–31. [DOI] [PubMed] [Google Scholar]
- 53.Esposito Corcione C, Malucelli G, Frigione M, Maffezzoli A. UV-curable epoxy systems containing hyperbranched polymers: Kinetics investigation by photo-DSC and real-time FT-IR experiments. Polym Test. 2009;28(2):157–64. 10.1016/j.polymertesting.2008.11.002 [DOI] [Google Scholar]
- 54.Esposito Corcione C, Frigione M, Maffezzoli A, Malucelli G. Photo—DSC and real time—FT-IR kinetic study of a UV curable epoxy resin containing o-Boehmites. Eur Polym J. 2008;44(7):2010–23. 10.1016/j.eurpolymj.2008.04.030 [DOI] [Google Scholar]
- 55.Sideridou I, Tserki V, Papanastasiou G. Effect of chemical structure on degree of conversion in light-cured dimethacrylate-based dental resins. Biomaterials. 2002;23(8):1819–29. 10.1016/S0142-9612(01)00308-8 [DOI] [PubMed] [Google Scholar]
- 56.Vallo CI. Theoretical prediction and experimental determination of the effect of mold characteristics on temperature and monomer conversion fraction profiles during polymerization of a PMMA-based bone cement. J Biomed Mater Res. 2002;63(5):627–42. 10.1002/jbm.10334 [DOI] [PubMed] [Google Scholar]
- 57.Ali U, Karim KJBA, Buang NA. A Review of the Properties and Applications of Poly (Methyl Methacrylate) (PMMA). Polym Rev (Phila Pa). 2015;55(4):678–705. [Google Scholar]
- 58.Khan MA, Walters NJ, Young AM. Fibre-reinforced injectable orthopedic composites with improved toughness and cell compatibility. Pioneering the Future of Biomaterials; Denver Colorado2014.
- 59.Kuehn KD, Ege W, Gopp U. Acrylic bone cements: composition and properties. Orthop Clin North Am. 2005;36(1):17–28, v 10.1016/j.ocl.2004.06.010 [DOI] [PubMed] [Google Scholar]
- 60.Ayre WN, Denyer SP, Evans SL. Ageing and moisture uptake in polymethyl methacrylate (PMMA) bone cements. J Mech Behav Biomed Mater. 2014;32:76–88. 10.1016/j.jmbbm.2013.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bae H, Shen M, Maurer P, Peppelman W, Beutler W, Linovitz R, et al. Clinical experience using Cortoss for treating vertebral compression fractures with vertebroplasty and kyphoplasty: twenty four-month follow-up. Spine. 2010;35(20):E1030–E6. 10.1097/BRS.0b013e3181dcda75 [DOI] [PubMed] [Google Scholar]
- 62.Wang JL, Chiang CK, Kuo YW, Chou WK, Yang BD. Mechanism of fractures of adjacent and augmented vertebrae following simulated vertebroplasty. J Biomech. 2012;45(8):1372–8. 10.1016/j.jbiomech.2012.03.003 [DOI] [PubMed] [Google Scholar]
- 63.Huang L, Zhou B, Wu H, Zheng L, Zhao J. Effect of apatite formation of biphasic calcium phosphate ceramic (BCP) on osteoblastogenesis using simulated body fluid (SBF) with or without bovine serum albumin (BSA). Mater Sci Eng C Mater Biol Appl. 2017;70(Pt 2):955–61. 10.1016/j.msec.2016.05.115 [DOI] [PubMed] [Google Scholar]
- 64.Aljabo A, Abou Neel EA, Knowles JC, Young AM. Development of dental composites with reactive fillers that promote precipitation of antibacterial-hydroxyapatite layers. Mater Sci Eng C Mater Biol Appl. 2016;60:285–92. 10.1016/j.msec.2015.11.047 [DOI] [PubMed] [Google Scholar]
- 65.Bellows CG, Aubin JE, Heersche JN. Initiation and progression of mineralization of bone nodules formed in vitro: the role of alkaline phosphatase and organic phosphate. Bone Miner. 1991;14(1):27–40. 10.1016/0169-6009(91)90100-E [DOI] [PubMed] [Google Scholar]
- 66.Dorozhkin SV. Calcium Orthophosphate Cements and Concretes. Materials. 2009;2(1):221–91. 10.3390/ma2010221 [DOI] [Google Scholar]
- 67.Lieberman IH, Togawa D, Kayanja MM. Vertebroplasty and kyphoplasty: filler materials. Spine J. 2005;5(6 Suppl):305S–16S. 10.1016/j.spinee.2005.02.020 [DOI] [PubMed] [Google Scholar]
- 68.Schumacher M, Wagner AS, Kokesch-Himmelreich J, Bernhardt A, Rohnke M, Wenisch S, et al. Strontium substitution in apatitic CaP cements effectively attenuates osteoclastic resorption but does not inhibit osteoclastogenesis. Acta Biomater. 2016;37:184–94. 10.1016/j.actbio.2016.04.016 [DOI] [PubMed] [Google Scholar]
- 69.Erbe EM, Clineff TD, Gualtieri G. Comparison of a new bisphenol-a-glycidyl dimethacrylate-based cortical bone void filler with polymethyl methacrylate. Eur Spine J. 2001;10 (Suppl 2):S147–52. 10.1007/s005860100288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.British Standard. BS ISO 5833:2002 Implants for surgery. Acrylic resin cements: BSI Standards Limited; 2002.
- 71.Kurtz SM, Villarraga ML, Zhao K, Edidin AA. Static and fatigue mechanical behavior of bone cement with elevated barium sulfate content for treatment of vertebral compression fractures. Biomaterials. 2005;26(17):3699–712. 10.1016/j.biomaterials.2004.09.055 [DOI] [PubMed] [Google Scholar]
- 72.Schmitt S, Krzypow DJ, Rimnac CM. The effect of moisture absorption on the fatigue crack propagation resistance of acrylic bone cement. Biomed Tech (Berl). 2004;49(3):61–5. 10.1515/BMT.2004.012 [DOI] [PubMed] [Google Scholar]
- 73.Kane RJ, Yue W, Mason JJ, Roeder RK. Improved fatigue life of acrylic bone cements reinforced with zirconia fibers. J Mech Behav Biomed Mater. 2010;3(7):504–11. 10.1016/j.jmbbm.2010.05.007 [DOI] [PubMed] [Google Scholar]
- 74.Chen H, Kubo KY. Bone three-dimensional microstructural features of the common osteoporotic fracture sites. World J Orthop. 2014;5(4):486–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Experimental and commercial bone composites raw data.
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.