Abstract
Background
Seasonal affective disorder (SAD) is a seasonal pattern of recurrent major depressive episodes that most commonly occurs during autumn or winter and remits in spring. The prevalence of SAD ranges from 1.5% to 9%, depending on latitude. The predictable seasonal aspect of SAD provides a promising opportunity for prevention. This review ‐ one of four reviews on efficacy and safety of interventions to prevent SAD ‐ focuses on light therapy as a preventive intervention. Light therapy is a non‐pharmacological treatment that exposes people to artificial light. Mode of delivery and form of light vary.
Objectives
To assess the efficacy and safety of light therapy (in comparison with no treatment, other types of light therapy, second‐generation antidepressants, melatonin, agomelatine, psychological therapies, lifestyle interventions and negative ion generators) in preventing SAD and improving patient‐centred outcomes among adults with a history of SAD.
Search methods
We searched Ovid MEDLINE (1950‐ ), Embase (1974‐ ), PsycINFO (1967‐ ) and the Cochrane Central Register of Controlled Trials (CENTRAL) to 19 June 2018. An earlier search of these databases was conducted via the Cochrane Common Mental Disorders Controlled Trial Register (CCMD‐CTR) (all years to 11 August 2015). Furthermore, we searched the Cumulative Index to Nursing and Allied Health Literature, Web of Science, the Cochrane Library, the Allied and Complementary Medicine Database and international trial registers (to 19 June 2018). We also conducted a grey literature search and handsearched the reference lists of included studies and pertinent review articles.
Selection criteria
For efficacy, we included randomised controlled trials (RCTs) on adults with a history of winter‐type SAD who were free of symptoms at the beginning of the study. For adverse events, we also intended to include non‐randomised studies. We intended to include studies that compared any type of light therapy (e.g. bright white light, administered by visors or light boxes, infrared light, dawn stimulation) versus no treatment/placebo, second‐generation antidepressants, psychological therapies, melatonin, agomelatine, lifestyle changes, negative ion generators or another of the aforementioned light therapies. We also planned to include studies that looked at light therapy in combination with any comparator intervention.
Data collection and analysis
Two review authors screened abstracts and full‐text publications, independently abstracted data and assessed risk of bias of included studies.
Main results
We identified 3745 citations after de‐duplication of search results. We excluded 3619 records during title and abstract review. We assessed 126 full‐text papers for inclusion in the review, but only one study providing data from 46 people met our eligibility criteria. The included RCT had methodological limitations. We rated it as having high risk of performance and detection bias because of lack of blinding, and as having high risk of attrition bias because study authors did not report reasons for dropouts and did not integrate data from dropouts into the analysis.
The included RCT compared preventive use of bright white light (2500 lux via visors), infrared light (0.18 lux via visors) and no light treatment. Overall, white light and infrared light therapy reduced the incidence of SAD numerically compared with no light therapy. In all, 43% (6/14) of participants in the bright light group developed SAD, as well as 33% (5/15) in the infrared light group and 67% (6/9) in the non‐treatment group. Bright light therapy reduced the risk of SAD incidence by 36%; however, the 95% confidence interval (CI) was very broad and included both possible effect sizes in favour of bright light therapy and those in favour of no light therapy (risk ratio (RR) 0.64, 95% CI 0.30 to 1.38; 23 participants, very low‐quality evidence). Infrared light reduced the risk of SAD by 50% compared with no light therapy, but the CI was also too broad to allow precise estimations of effect size (RR 0.50, 95% CI 0.21 to 1.17; 24 participants, very low‐quality evidence). Comparison of both forms of preventive light therapy versus each other yielded similar rates of incidence of depressive episodes in both groups (RR 1.29, 95% CI 0.50 to 3.28; 29 participants, very low‐quality evidence). Reasons for downgrading evidence quality included high risk of bias of the included study, imprecision and other limitations, such as self‐rating of outcomes, lack of checking of compliance throughout the study duration and insufficient reporting of participant characteristics.
Investigators provided no information on adverse events. We could find no studies that compared light therapy versus other interventions of interest such as second‐generation antidepressants, psychological therapies, melatonin or agomelatine.
Authors' conclusions
Evidence on light therapy as preventive treatment for people with a history of SAD is limited. Methodological limitations and the small sample size of the only available study have precluded review author conclusions on effects of light therapy for SAD. Given that comparative evidence for light therapy versus other preventive options is limited, the decision for or against initiating preventive treatment of SAD and the treatment selected should be strongly based on patient preferences.
Plain language summary
Light therapy for prevention of winter depression
Why is this review important?
Many people in northern latitudes suffer from winter blues, which occurs as a reaction to reduced sunlight. Three‐quarters of those affected are women. Lethargy, overeating, craving for carbohydrates and depressed mood are common symptoms. In some people, winter blues becomes depression, which seriously affects their daily lives. Up to two‐thirds experience depressive symptoms every winter.
Who will be interested in this review?
Anyone who has experienced winter depression, or who has relatives and friends who have experienced winter depression.
What questions does this review aim to answer?
In light of the seasonal pattern and the high rate of recurrence, beginning light therapy in early autumn (fall) when people are still free of depressive symptoms could help to prevent the onset of depressed mood. The goal of this review was to find out whether light therapy can prevent the onset of depression in winter when it is used in healthy people with a history of winter depression, and if it is safe. To date, this question has not been examined in a systematic way, but it is of importance for those who have suffered winter depression.
Which studies were included in the review?
We searched databases up to 19 June 2018 for studies on light therapy to prevent winter depression. Among 3745 records, we found one randomised controlled study including 46 people who received light therapy or no treatment. All individuals in these studies had a history of winter depression.
What does the evidence from the review reveal?
The quality of evidence for all outcomes was very low, so we can draw no conclusions about whether light therapy is effective in preventing winter depression. The included study provided no information on side effects of light therapy.
Doctors need to discuss with patients considering preventive treatment the advantages and disadvantages of light therapy and other potentially preventive treatments for winter depression, such as drug treatments, psychological therapies or lifestyle interventions. As no available studies have compared these treatments, treatment selection should be strongly based on patient preferences.
What should happen next?
The review authors recommend that future studies should directly compare light therapy versus other treatments, such as drug treatments, psychological therapies or lifestyle interventions to determine the best treatment for preventing winter depression.
Summary of findings
Background
Description of the condition
Seasonal affective disorder (SAD) is a seasonal pattern of recurrent major depressive episodes that most commonly occurs during autumn or winter and remits in spring or summer (Rosenthal 1984). In addition to the predictable seasonal pattern of depression, persons suffering from SAD commonly experience atypical symptoms including hypersomnia, carbohydrate craving with increased appetite and weight gain and extreme fatigue (Sohn 2005). Prevalence in the USA ranges from 1.5% in southern Florida to 9% in northern regions (Rosen 1990). In northern latitudes, the prevalence of SAD is estimated to be about 10% (Byrne 2008). SAD is a multifactorial condition. Chronobiological mechanisms related to circadian rhythms, melatonin, serotonin turnover and photoperiodism (length of dark hours relative to light hours in a 24‐hour period) are all thought to play a role in SAD (Ciarleglio 2011; Levitan 2007). A quintessential and especially harmful quality of this illness is its high risk of recurrence and persistence. Approximately two‐thirds of those diagnosed with SAD will face recurrence of these distressing symptoms the following winter (Rodin 1997). In the five to 11 years following initial diagnosis, 22% to 42% of people still suffer from SAD, and 33% to 44% develop a non‐seasonal pattern in subsequent episodes; the disorder resolves completely in only 14% to 18% of people (Magnusson 2005; Schwartz 1996). Indeed, many people who suffer from SAD experience this type of depression every year, which makes it particularly amenable to preventive treatment (Westrin 2007).
Description of the intervention
Various interventions such as second‐generation antidepressants, light therapy, melatonin or agomelatine, psychological therapies and lifestyle interventions have been used for prevention of SAD. Of those, light therapy (e.g. bright white light, dawn simulation) is a non‐pharmacological treatment that has proved effective and is often used as first‐line therapy for individuals with SAD (Terman 2005). It is commonly applied with use of a light box; however, application with a light visor (a portable head‐mounted light source) is possible (Pail 2011). To achieve an effect on circadian rhythms, the dosage of bright light given should be greater than the artificial lighting usually used in homes ‐ about 5000 lux per day (2500 lux for two hours, or 10,000 lux for 30 minutes) (Levitan 2005). Dawn simulation, on the other hand, increases light exposure from 0 to around 200 to 300 lux, over 1.5 to 2.5 hours (Golden 2005). A recent meta‐analysis found that the odds ratio (OR) for remission was similar to that of many pharmaceutical treatments for depression (OR 2.9, 95% confidence interval (CI) 1.6 to 5.4) (Golden 2005). People with SAD should be treated with light therapy units that are specifically designed to treat SAD with the goal of achieving strong response. Otherwise, these units may not provide adequate brightness and may not allow appropriate ultraviolet light filtration (Levitan 2005). It is not necessary to stare directly into the light (Pail 2011). However, it is important that the light meets the eye because it is hypothesised that the effect of light therapy is mediated through the eyes by retinal cells that are not part of the visual system (Pail 2011). Other forms of light therapy (e.g. infrared light) are available, but their effectiveness for people with SAD is not known.
How the intervention might work
Much research into the origin of SAD has focused on the role of circadian rhythms and melatonin (Lam 2006). Decreased seasonal exposure to light through phase shifts in circadian rhythms, resulting in alterations of serotonin metabolism, is thought to be a reason for development of SAD. The circadian system is influenced primarily by light, and exposure to light acts as a signal for the circadian clock (Quera‐Salva 2011). As a consequence, light therapy has been studied intensively as treatment for SAD (Partonen 1998). Underlying hypotheses on the pathophysiology of SAD, such as the depressogenic effects of melatonin, support the rationale for light therapy. Light therapy helps to suppress the release of melatonin and lengthens the photoperiod (Golden 2005). Timing, duration and intensity play an important role in light therapy. Studies have shown that light therapy is most effective when administered early in the day because the typical depressed person is phase‐delayed (Levitan 2005), and therapy administered early in the morning regulates the circadian pattern of melatonin secretion. However, in some people, evening light therapy may be more successful, because phase‐advanced individuals may benefit more from the corrective phase delay provided by evening light therapy (Lewy 1987; Lewy 2006). Seasonal recurrence of depressive episodes provides the rationale for light therapy provided as preventive treatment for SAD.
Why it is important to do this review
The predictable seasonal aspect of SAD provides a specific and promising opportunity for prevention. However, both people with SAD and clinicians face much uncertainty in their collaborative decisions about the choice of a preventive intervention (Westrin 2007). Although a recent Cochrane Review assessed the efficacy and risk of harms of light therapy compared with second‐generation antidepressants for short‐term treatment of SAD (Thaler 2011), to date no review has determined the efficacy, effectiveness and risk of harms of light therapy for preventing recurrent SAD.
Our findings are intended to provide insights into (1) available evidence on the benefits and harms of competing interventions in the prevention of SAD, with respect to patient‐centred outcomes, and (2) gaps in the evidence base that will inform future research needs.
This is one of four reviews of interventions to prevent SAD. The others focus on second‐generation antidepressants (Gartlehner 2015), agomelatine and/or melatonin (Kaminski‐Hartenthaler 2015), and psychological therapies (Forneris 2014), as preventive interventions.
Objectives
To assess the efficacy and safety of light therapy (in comparison with no treatment, other types of light therapy, second‐generation antidepressants, melatonin, agomelatine, psychological therapies, lifestyle interventions and negative ion generators) in preventing seasonal affective disorder (SAD) and improving patient‐centred outcomes among adults with a history of SAD.
Methods
Criteria for considering studies for this review
Types of studies
Efficacy (beneficial effects)
We included randomised controlled trials (RCTs; including cross‐over studies and cluster‐randomised trials) on light therapy for prevention of seasonal affective disorder (SAD).
Adverse effects
We planned to include the following.
RCTs (including cross‐over studies and cluster‐randomised trials) of light therapy for prevention of SAD.
Non‐randomised controlled studies, such as non‐randomised trials, prospective cohort studies or case‐control studies of light therapy for prevention of SAD.
Types of participants
Participant characteristics
Male and female adults (≥ 18 years of age) of all races, ethnicities and cultural groups, with a history of SAD, who do not fulfil the criteria for a current major depressive episode.
Diagnosis
We defined SAD according to the Diagnostic and Statistical Manual of Mental Disorders (DSM‐5; APA 2013) as a seasonal pattern of recurrent major depressive episodes. However, we restricted our focus to winter‐type SAD (i.e. major depression in the autumn/winter with full remission in the spring/summer), and we did not include people with bipolar disorder with a seasonal pattern. We included studies that used definitions from prior versions of the DSM (APA 1980; APA 1987; APA 2000).
Comorbidities
We excluded studies that enrolled participants with depressive disorder due to another medical condition. We planned to include populations at risk of SAD with common comorbidities (e.g. diabetes, cardiovascular disease) that are not the cause of the depressive episode.
Setting
We included studies conducted in all settings.
Subset data
We intended to include studies that provided data on subsets of participants of interest, as long as the subset met our eligibility criteria. We did not include studies with 'mixed' populations if investigators did not adequately stratify data with respect to our population of interest.
Types of interventions
Experimental interventions
We included the following forms of light therapy.
Bright white light.
Infrared light.
Dawn simulation.
We did not limit light therapy in terms of dosage, mode of delivery or duration. We intended to include combination therapies of light therapy with any of the comparator interventions listed below.
Comparator interventions
We planned to compare any light therapy with:
placebo, no treatment or waiting list;
another light therapy from the list above;
second‐generation antidepressants;
melatonin or agomelatine;
psychological therapies;
lifestyle interventions (e.g. exercising, making the environment sunnier (open blinds), spending regular time outside, adapting nutrition (consuming a low‐fat diet, reducing refined sugars)); and
negative ion generators.
We also planned to compare light therapy in combination with any of the comparator interventions listed above with placebo, no treatment or waiting list, or the same comparator intervention as monotherapy (see Data extraction and management).
Types of outcome measures
We included studies that met the above inclusion criteria regardless of whether they reported on the following outcomes. In consultation with clinical experts, we selected the following outcomes a priori.
Primary outcomes
The primary outcome for benefit was the incidence of SAD, measured as the proportion of participants with a score of 20 or higher on the Structured Interview Guide for the Hamilton Depression Rating Scale, Seasonal Affective Disorders (SIGH‐SAD; Williams 2002).
The primary outcome for harms was the overall rate of adverse events related to preventive interventions.
Secondary outcomes
Severity of the SAD episode or SAD‐related symptoms, as measured by a validated tool (e.g. Hamilton Depression Rating Scale; Hamilton 1960).
Quality of life, as measured by a validated quality of life tool (e.g. Short Form (SF)‐36; Ware 1992).
Quality of interpersonal and social functioning, as measured by a validated tool (e.g. the Range of Impaired Functioning Tool (LIFE–RIFT; Leon 1999).
Proportion of participants with serious adverse events.
Rates of discontinuation of preventive intervention due to adverse events.
Overall rate of discontinuation.
Timing of outcome assessment
Depending on available data, we planned to synthesise outcomes at different time points (i.e. short‐term, medium‐term and long‐term) throughout an entire six‐month period of risk during an autumn‐winter season.
Hierarchy of outcome measures
Our main focus was patient‐centred outcomes (i.e. outcomes that patients notice and care about). If several measures assessed the same outcome, we consulted a priori with clinical experts regarding the validity and reliability of individual outcome measures and prioritised accordingly.
Search methods for identification of studies
The Cochrane Common Mental Disorders Group (CCMD) maintains two clinical trials registers at its editorial base in Bristol, UK: a references‐based register and a studies‐based register. The CCMD Specialised Register (CCMDCTR)‐References contains more than 39,000 reports of randomised controlled trials (RCTs) on common mental disorders. Approximately 60% of these references have been tagged to individual, coded trials. The coded trials are held in the Specialised CCMDCTR‐Studies Register, and records are linked between the two registers through the use of unique study ID tags. Coding of trials is based on the EU‐Psi coding manual and use of a controlled vocabulary (the CCMD Information Specialist can provide further details). Reports of trials for inclusion in the Group Registers are collated from routine (weekly), generic searches of MEDLINE (1950‐), Embase (1974‐) and PsycINFO (1967‐); quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL); and review‐specific searches of additional databases. Reports of trials are also obtained from international trials registers through trials portals of the World Health Organization (the International Clinical Trials Registry Platform (ICTRP)) and pharmaceutical companies and by handsearching of key journals, conference proceedings and other (non‐Cochrane) systematic reviews and meta‐analyses.
Details of CCDAN's generic search strategies (used to identify RCTs) can be found on the Group website, with an example of the core MEDLINE search displayed in Appendix 1. This register is current to June 2016 only.
Electronic searches
The searches for this review are up‐to‐date as of 19 June 2018. Details of all searches conducted between April 2013 and June 2018 are described below.
The Information Specialist with the Cochrane Common Mental Disorders Group (CCMD) ran an initial search of their Group's specialised registers (CCMD‐CTR‐Studies and CCMD‐CTR‐References) (all years to 12 April 2013) using terms for condition only. An updated search was performed on 11 August 2015, prior to the first publication of this review.
("seasonal affective disorder*" or "seasonal depression" or "seasonal mood disorder*" or "winter depression" or SIGH‐SAD*).
In addition, we conducted our own searches of the following electronic databases (to 26 May 2014) to ensure that no studies had been missed by the CCMD‐CTR specialised registers (Appendix 2).
International Pharmaceutical Abstracts.
Cumulative Index to Nursing and Allied Health Literature (CINAHL).
Web of Science (formerly Web of Knowledge: includes Web of Science, Current Contents Connect, Conference Proceedings Citation Index, BIOSIS, Derwent Innovations Index, Data Citation Index, SciELO Citation Index) (all available years).
The Cochrane Library.
Allied and Complementary Medicine Database (AMED).
We also searched international trial registries via the World Health Organization trials portal (ICTRP) and ClinicalTrials.gov to identify unpublished or ongoing studies.
We did not restrict searches by date, language and publication status.
In June 2018, CCMD's Information Specialist updated the search for studies on all of the databases listed above (Appendix 3), with the exception of International Pharmaceutical Abstracts. The search of these databases was necessary as the CCMD‐CTR was out of date at the time (current to June 2016 only).
Searching other resources
Grey literature
To detect additional studies, we checked the following sources.
IFPMA (International Federation of Pharmaceutical Manufacturers and Associations) Clinical Trials Portal.
OpenGrey.
National Institute of Health RePORTER.
Health Services Research Projects in Progress (HSRProj).
Hayes Inc. Health Technology Assessment.
The New York Academy of Medicine Grey Literature Index.
Conference Papers Index.
European Medicines Agency.
Drugs@FDA (Food and Drug Administration).
National Registry of Evidence‐Based Programs and Practices (NREPP) (no longer available online).
Reference lists
We handsearched the references of all included studies and pertinent review articles.
Correspondence
We contacted trialists and subject matter experts to ask for information on unpublished and ongoing studies, or to request additional trial data.
Data collection and analysis
Selection of studies
Two review authors independently screened the titles and abstracts of all studies identified by the searches. We retrieved full‐text copies of all studies that potentially met the inclusion criteria as determined by this initial assessment, and two review authors independently screened them to determine their eligibility.
If the two review authors did not reach consensus, they discussed disagreements and resolved them through consultation with a third party. We contacted study authors if relevant information was missing. We tracked all results in an EndNote X8 database.
We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Moher 2009) and Characteristics of included studies tables.
Data extraction and management
We used a data collection form. Two review authors independently extracted study characteristics and outcome data from included studies. We resolved discrepancies by reaching consensus or by involving another review author. We reported whether studies were detected by a search of databases of published studies, by handsearch or by a search of grey literature.
We extracted the following study characteristics.
Methods: study design, duration of study, details of any 'run‐in' period, duration of treatment period, number of study centres and locations, study settings, withdrawals and dates of studies.
Participants: number of participants, mean age, age range, proportion of women, number of prior depressive episodes, diagnostic criteria, inclusion criteria and exclusion criteria.
Interventions: intervention, comparison, concomitant interventions and excluded interventions.
Outcomes: primary and secondary outcomes specified and collected and time points reported.
Notes: funding for studies and notable conflicts of interest of study authors.
We noted in the Characteristics of included studies table if outcome data were not reported in a useable way. We resolved disagreements by reaching consensus or by involving a third person. One review author transferred data into the Review Manager file (Review Manager 2014). We double‐checked that data were entered correctly by comparing data presented in the systematic review versus the study reports. A second review author checked study characteristics for accuracy against the trial report.
Main planned comparisons
Light therapy versus placebo, no treatment or waiting list.
Light therapy versus other light therapy.
Light therapy versus second‐generation antidepressants.
Light therapy versus melatonin or agomelatine.
Light therapy versus psychological therapies.
Light therapy versus lifestyle intervention.
Light therapy versus negative ion generators.
Light therapy plus comparator intervention (as listed in Types of interventions) versus placebo or no treatment control group or waiting list.
Light therapy plus comparator intervention (as listed in Types of interventions) versus the same comparator intervention as monotherapy (e.g. light therapy plus psychological therapy versus psychological therapy alone).
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias of included randomised trials using the Cochrane 'Risk of bias' tool, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This tool includes assessment of random sequence generation; allocation concealment; blinding of participants, personnel and outcome assessors; incomplete outcome data; selective reporting; and other potential threats to validity. Specifically, we assessed attrition in these trials and reasons for attrition, particularly when attrition rates between two groups in a trial differed substantially. In addition, we assessed whether all relevant outcomes for the trial were reported in the published articles. We assigned each domain at high risk of bias, low risk of bias or unclear risk of bias.
For non‐randomised studies, we planned to use the Newcastle‐Ottawa Scale, involving selection of cases or cohorts and controls, adjustment for confounders, methods of outcome assessment, length of follow‐up and statistical analysis (Wells 2009). Review authors resolved discrepancies by reaching consensus or by consulting with a third review author.
Measures of treatment effect
We used data extracted from the original studies to construct 2 × 2 tables for dichotomous outcomes. When multiple studies allowed for quantitative analysis, we planned to calculate the risk ratio (RR) with 95% confidence intervals (CIs) for each outcome. We chose RR as an effect measure because for decision makers, RRs are easier to interpret than odds ratios (ORs), particularly when event rates are high.
We planned to pool continuous data using the mean difference (MD) if an outcome was measured on the same scale, or the standardised mean difference (SMD) if an outcome was measured on different scales. If available, we intended to use final measurements rather than changes from baseline to estimate differences between treatments. When it was considered necessary to use both change and postintervention scores within a comparison, we would have presented these by subgroup using the mean difference (MD) rather than the SMD.
For time‐to‐event data, we planned to calculate a pooled hazard ratio when this was available, or to dichotomise data at multiple time points into response/no response (e.g. at one week, at two weeks, at four weeks, etc.).
We intended to use the same time points as specified under 'Timing of outcomes assessment' in the section Types of outcome measures to form the basis for dichotomisation into response/no response.
For non‐randomised studies, we planned to use adjusted treatment effects if available.
Unit of analysis issues
Cluster‐randomised trials
To incorporate cluster‐randomised trials, we intended to reduce the size of each trial to its 'effective sample size'. If intracluster correlation coefficients had not been reported, we planned to find external estimates from similar studies. We intended to undertake sensitivity analysis to assess the impact of including such trials.
Cross‐over trials
To avoid carry‐over effects, we planned to include data only from the first period of cross‐over studies.
Studies with multiple treatment groups
For included trials that consisted of multiple treatment groups (e.g. differing dosing regimens of light therapy), we planned to include data for the treatment arms and to halve the data from the placebo arm, or to collapse the data for different doses into one group when this was clinically appropriate (Hansen 2009).
Dealing with missing data
We used intention‐to‐treat (ITT) analysis when data were missing for participants who dropped out of trials before completion. We calculated two ITT analyses: one assuming that all dropouts developed SAD, the other assuming that all dropouts stayed free of depressive symptoms. When data regarding an outcome of interest were not reported, we planned to contact the authors of publications to obtain missing results, as long as the study was published over the past 20 years. We documented all correspondence with trialists and reported responses in the full review.
Assessment of heterogeneity
We planned to use the Cochrane Chi2 test (Q‐test) to assess heterogeneity. A P value less than 0.10 is considered statistically significant. We planned to use the I2 statistic to estimate the degree of heterogeneity. This measure describes the percentage of total variation across studies that results from heterogeneity rather than from chance. We planned to interpret the importance of any heterogeneity in terms of its magnitude and direction of effects. We planned to not consider thresholds; instead we intended to adopt the overlapping bands, as suggested in the Cochrane Handbook for Systematic Reviews of Interventions. For example, we planned to consider an I2 value between 0% and 40% as probably not important, between 30% and 60% as representing moderate heterogeneity, between 50% and 90% as representing substantial heterogeneity, and between 75% and 100% as representing considerable heterogeneity (Higgins 2011).
Assessment of reporting biases
If we had found more than 10 studies, we planned to perform a funnel plot analysis. A funnel plot is a graph used to detect publication bias. We intended to look at whether the largest studies were near the average and small studies spread on both sides of the average. Variations from this assumption can indicate the existence of publication bias, but asymmetry may not necessarily be caused by publication bias. In addition, we planned to use Kendell's tau (Begg 1994), Egger's regression intercept (Egger 1997), and Fail‐Safe N (Rosenthal 1979), to assess reporting biases.
Data synthesis
We analysed data using Review Manager 5 software (Review Manager 2014). We planned to pool data for meta‐analysis when participant groups were similar, and when studies assessed the same treatments with the same comparator and had similar definitions of outcome measures over a similar duration of treatment.
In general, we planned to use random‐effects models to combine results because we did not expect the true effect to be the same for all included studies. We intended to also employ fixed‐effect models to determine differences in treatment effects between random‐effects and fixed‐effect results. Studies would have been weighted using the Mantel‐Haenszel method. We rated the strength of the evidence using the system developed by the GRADE Working Group (Guyatt 2011).
We planned to perform qualitative analysis of data on adverse effects by comparing crude rates. We planned to conduct quantitative analysis of the rates of adverse effects only if we located a sufficient number of prospective observational studies or randomised trials that gathered data on adverse effects that were suitable for pooling.
Subgroup analysis and investigation of heterogeneity
Sex, age, history of non‐seasonal major depressive episodes and psychiatric comorbidities are potential effect measure modifiers for prevention of SAD. Timing, duration, type and intensity of light therapy may also modify the preventive effect on SAD. If data were sufficient, we would have conducted subgroup analyses for the primary outcome measures. Subgroup analyses should be performed and interpreted with caution because multiple analyses could lead to false‐positive conclusions. We planned to conduct subgroup analyses based on:
men versus women;
history of non‐seasonal major depressive episodes versus no history of non‐seasonal major depressive episodes;
younger than 65 years of age versus 65 years of age older; and
Axis I, Axis II comorbidities versus no Axis I, Axis II comorbidities.
Sensitivity analysis
Sensitivity analyses were conducted to test the robustness of decisions made during the review process.
We planned to conduct sensitivity analyses:
excluding small studies (i.e. studies with fewer than 30 participants);
excluding studies with high risk of bias (i.e. studies that had been rated as high risk of bias in one or more domains);
excluding studies published only in abstract form;
with adjusted versus unadjusted results; and
excluding cluster‐randomised trials.
'Summary of findings' tables
We assessed the quality of the evidence using the GRADE approach and presented the results in Table 1, Table 2, Table 3 and Table 4 for our main comparisons and outcomes (as listed in Types of outcome measures). We did not expect to be able to stratify populations into low‐, medium‐ or high‐risk populations. For 'assumed risk', we used prevalence studies from countries in northern latitudes (e.g. Scandinavia, Canada, northern USA) in which SAD leads to substantial burden of disease.
Summary of findings for the main comparison. Bright white light therapy compared with no light therapy for prevention of SAD.
| Bright white light therapy compared with no light therapy for prevention of SAD | ||||||
| Patient or population: all participants were known SAD patients who had been successfully treated with conventional light therapy in previous winters Settings: this was an outpatient field study. Participants chose when (between 6 am and 9 am) and where they would use the visors Intervention: bright white light therapy Comparison: no light therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No light therapy | Light therapy | |||||
| Incidence of SAD (SIGH‐SAD score ≥ 20) (follow‐up 26 weeks) |
Low | RR 0.64 (0.30 to 1.38) | 23 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | ||
| 300 per 1000 | 192 per 1000 (90 to 414) | |||||
| Moderate | ||||||
| 500 per 1000 | 320 per 1000 (150 to 690) |
|||||
| High | ||||||
| 600 per 1000 | 276 per 1000 (210 to 966) |
|||||
| Incidence of severe SAD (SIGH‐SAD‐SR (≥ 40)) (follow‐up 26 weeks) |
Study population | RR 0.21 (0.03 to 1.75) | 23 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | ||
| 333 per 1000 | 70 per 1000 (10 to 583) | |||||
| Overall discontinuation (follow‐up 26 weeks) |
Study population | RR 2.22 (0.29 to 17.27) | 28 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | ||
| 100 per 1000 | 222 per 1000 (29 to 1000) | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio; SIGH‐SAD‐SR: Structured Interview Guide for the Hamilton Depression Rating Scale‐Seasonal Affective Disorders self‐rating version | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: we are very uncertain about the estimate | ||||||
aDowngraded two levels because of severe risk of bias due to non‐blinding and unclear randomisation process and allocation concealment; no intention‐to‐treat analysis was reported, outcomes were self‐rated, compliance throughout study duration was not checked and participant characteristics were not reported comprehensively. bDowngraded one level because of small sample size (lack of power and random error could have influenced results).
Summary of findings 2. Infrared light therapy compared with no light therapy for prevention of SAD.
| Infrared light therapy compared with no light therapy for prevention of SAD | ||||||
| Patient or population: all participants were known SAD patients who had been successfully treated with conventional light therapy in previous winters Settings: outpatient field study; participants chose when (between 6 am and 9 am) and where they would use the visors Intervention: infrared light therapy Comparison: no light therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No light therapy | Infrared light therapy | |||||
| Incidence of SAD (SIGH‐SAD score ≥ 20) (follow‐up 26 weeks) |
Low | RR 0.50 (0.21 to 1.17) | 24 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | ||
| 300 per 1000 | 150 per 1000 (63 to 351) |
|||||
| Moderate | ||||||
| 500 per 1000 | 250 per 1000 (105 to 585) |
|||||
| High | ||||||
| 600 per 1000 | 300 per 1000 (126 to 702) |
|||||
| Incidence of severe SAD (SIGH‐SAD‐SR (≥ 40)) (follow‐up 26 weeks) |
Study population | RR 0.20 (0.02 to 1.64) | 24 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | ||
| 333 per 1000 | 67 per 1000 (67 to 547) | |||||
| Overall discontinuation (follow‐up 26 weeks) |
Study population | RR 1.67 (0.20 to 13.98) | 28 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | ||
| 100 per 1000 | 167 per 1000 (20 to 1000) | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio; SIGH‐SAD‐SR: Structured Interview Guide for the Hamilton Depression Rating Scale‐Seasonal Affective Disorders self‐rating version | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: we are very uncertain about the estimate | ||||||
aDowngraded two levels because of severe risk of bias due to non‐blinding and unclear randomisation process and allocation concealment; no intention‐to‐treat analysis was reported, outcomes were self‐rated, compliance throughout study duration was not checked and participant characteristics were not reported comprehensively. bDowngraded one level because of small sample size (lack of power and random error could have influenced results).
Summary of findings 3. Light therapy compared with no light therapy for prevention of SAD.
| Light therapy (bright white or infrared) compared with no light therapy for prevention of SAD | ||||||
| Patient or population: all participants were known SAD patients who had been successfully treated with conventional light therapy in previous winters Settings: outpatient field study; participants chose when (between 6 am and 9 am) and where they would use the visors Intervention: light therapy Comparison: no light therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No light therapy | Infrared light therapy | |||||
| Incidence of SAD (SIGH‐SAD score ≥ 20) (follow‐up 26 weeks) |
Low | RR 0.57 (0.30 to 1.10) | 38 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | ||
| 300 per 1000 | 171per 1000 (90 to 330) |
|||||
| Moderate | ||||||
| 500 per 1000 | 285 per 1000 (150 to 550) |
|||||
| High | ||||||
| 600 per 1000 | 342 per 1000 (180 to 660) |
|||||
| Incidence of severe SAD (SIGH‐SAD‐SR (≥ 40)) (follow‐up 26 weeks) |
Study population | RR 0.21 (0.04 to 1.05) | 38 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | ||
| 333 per 1000 | 70 per 1000 (13 to 350) | |||||
| Overall discontinuation (follow‐up 26 weeks) |
Study population | RR 1.94 (0.27 to 14.01) | 46 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | ||
| 100 per 1000 | 194 per 1000 (27 to 1000) | |||||
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio; SIGH‐SAD‐SR: Structured Interview Guide for the Hamilton Depression Rating Scale‐Seasonal Affective Disorders self‐rating version | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: we are very uncertain about the estimate | ||||||
aDowngraded two levels because of severe risk of bias due to non‐blinding and unclear randomisation process and allocation concealment; no intention‐to‐treat analysis was reported, outcomes were self‐rated, compliance throughout study duration was not checked and participant characteristics were not reported comprehensively. bDowngraded one level because of small sample size (lack of power and random error could have influenced results).
Summary of findings 4. Bright white light therapy compared with infrared light therapy for prevention of SAD.
| Bright white light therapy compared with infrared light therapy for prevention of SAD | ||||||
| Patient or population: all participants were known SAD patients who had been successfully treated with conventional light therapy in previous winters Settings: outpatient field study; participants chose when (between 6 am and 9 am) and where they would use the visors Intervention: bright white light therapy Comparison: infrared light therapy | ||||||
| Outcomes | Risk in both groups | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk in this treatment group | Risk in this treatment group | |||||
| Infrared light therapy | Bright white light therapy | |||||
| Incidence of SAD (SIGH‐SAD score ≥ 20) (follow‐up 26 weeks) |
Study population | RR 1.29 (0.50 to 3.28) | 29 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | ||
| 333 per 1000 | 357 per 1000 | |||||
| Incidence of severe SAD (SIGH‐SAD‐SR (≥ 40)) (follow‐up 26 weeks) |
Study population | RR 1.07 (0.07 to 15.54) | 29 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | ||
| 67 per 1000 | 71 per 1000 | |||||
| Overall discontinuation (follow‐up 26 weeks) |
Study population | RR 1.33 (0.35 to 5.13) | 36 (1 RCT) | ⊕⊝⊝⊝ Very lowa,b | ||
| 167 per 1000 | 222 per 1000 | |||||
| CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio; SIGH‐SAD‐SR: Structured Interview Guide for the Hamilton Depression Rating Scale‐Seasonal Affective Disorders self‐rating version | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: we are very uncertain about the estimate | ||||||
aDowngraded two levels because of severe risk of bias due to non‐blinding and unclear randomisation process and allocation concealment; no intention‐to‐treat analysis was reported, outcomes were self‐rated, compliance throughout study duration was not checked and participant characteristics were not reported comprehensively. bDowngraded one level because of small sample size (lack of power and random error could have influenced results).
We used the GRADEpro Guideline Development Tool to rate the quality of evidence and to prepare the 'Summary of findings' tables (GRADEpro GDT 2015).
Results
Description of studies
Results of the search
We identified 3745 citations through electronic searches and reviews of reference lists after de‐duplication of search results. We excluded 3619 records during title and abstract reviews. We included 126 articles for full‐text review, of which one publication met eligibility criteria for this review. We excluded 125 articles because the intervention did not meet our eligibility criteria; in most studies, participants already had depressive symptoms when the therapeutic intervention was started. Our focus was on prevention. Therefore, we included only studies that included people with a history of seasonal affective disorder (SAD) who were free of symptoms at the beginning of the study. Another major reason for exclusion was that people had major depressive disorder rather than SAD, and that the study design did not match our inclusion criteria (e.g. studies without a control group). The PRISMA flow chart documents the disposition of the literature in this review (Figure 1). Under Excluded studies, we describe the reasons for excluding these studies in greater detail.
1.
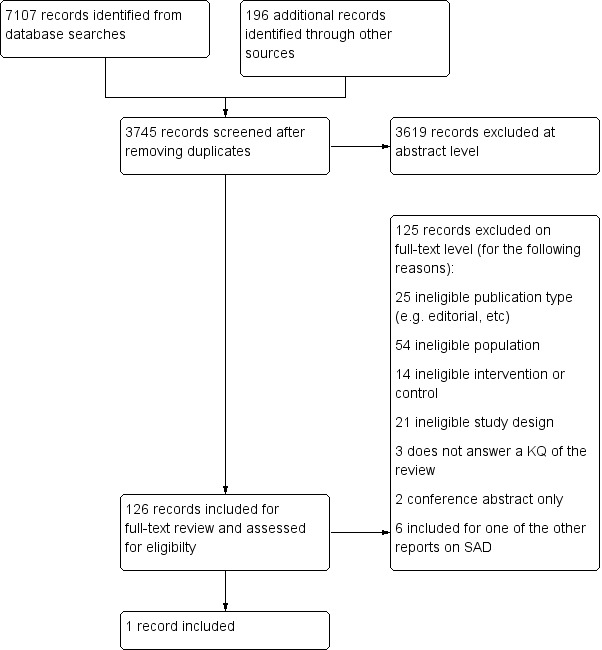
PRISMA flow diagram
Included studies
We included one randomised controlled trial (RCT) comparing three study arms for prevention of SAD: bright white visor light versus infrared visor light versus no light therapy (Meesters 1999). Bio Bright, Inc. sponsored the equipment, and study authors reported no other information on funding. We found no non‐randomised studies for assessment of risk of harms.
In the following section, we present study characteristics and results in greater detail (see also Characteristics of included studies).
Design
The included RCT was a single‐centre, non‐blinded study that was composed of two winter seasons (1993‐94 and 1994‐95) that started each year in October and ended in April.
Sample size
During both winter seasons, the study included a total of 46 participants with a history of SAD who had no depressive symptoms when the study started. Initially, investigators had recruited 50 outpatients (30 in the winter season of 1993‐94; 20 in the winter season of 1994‐95); however, four developed depression between the time of giving consent and the start of the study. Therefore, these patients were not included in the study.
Setting
The study was conducted in the Netherlands and included participants from an outpatient clinic. The intervention was implemented at participants' homes.
Participants
Participants were adult outpatients with a history of SAD who had been successfully treated with conventional light treatment in previous winter seasons. The participants were non‐depressed at the start of the study. All participants were free of drugs and were diagnosed with SAD according to the criteria of Rosenthal et al (Rosenthal 1984), and in keeping with an older version (1987) of the DSM (DSM‐III‐R). A total of 50 adults with a history of SAD gave consent to participate in the study. Four developed symptoms of depression before the study started. In all, 46 participants took part in the study and were randomly assigned to one of three study arms. Eighteen participants were randomly assigned to bright white visor light (2500 lux); four dropped out (no information on age or gender reported). Of the remaining 14 participants, 12 were women with a mean age of 39.5 years (standard deviation (SD) ± 9.3), and two were men with a mean age of 41.0 years (SD ± 12.7). Eigtheen participants were randomly assigned to the infrared visor light (0.18 lux); three dropped out (no information on age or gender reported). Of the remaining 15 participants in the infrared light group, 10 were women with a mean age of 36.6 years (SD ± 4.9), and five were men with a mean age of 35.4 years (SD ± 6.9). Ten participants were randomly assigned to no light exposure; one person dropped out (no information on age or gender reported). Of the remaining nine participants in the no treatment group, five were women with a mean age of 39.4 years (± 8.0), and four were men with a mean age of 47.5 years (SD ± 7.0). Study authors reported no other participant characteristics.
Interventions
Participants were randomly assigned to three study arms: bright white visor light (bright light inclusive infrared light), pure infrared visor light (visible light filtered out) and no light exposure. Participants in the bright white light therapy group used a bright white visor light. Those in the infrared light group were exposed to infrared light by means of a light visor equipped with a Kodak Wratten filter (type 89b, 720 mm). The light visors ‐ portable head‐mounted devices ‐ were manufactured by Bio Bright, Inc. and contained two krypton incandescent bulbs. Participants used them at home and were instructed to use them 30 minutes daily (except on Saturdays and Sundays) between 6:00 am and 9:00 am. As participants used the treatment at home on their own, they were able to choose the exact treatment time on their own and to integrate treatments into their daily routine. Participants in the control group received no type of light therapy (no visors were provided) and represented a waiting list control.
Outcomes
The primary outcome reported in this study was development of depression, according to a translated version of the Structured Interview Guide for the Hamilton Depression Rating Scale‐Seasonal Affective Disorders self‐rating version (SIGH‐SAD‐SR, 29‐item version) and according to the Beck Depression Inventory (BDI, 21‐item version). As the primary outcome for this Cochrane Review was defined using SIGH‐SAD (see Types of outcome measures), we focus only on the SIGH‐SAD‐SR data reported. According to SIGH‐SAD‐SR criteria, individuals with a score ≥ 20 were considered depressive. A secondary outcome of this Cochrane Review was severity of depression defined as "Severity of the SAD episode or SAD‐related symptoms, as measured by a validated tool (e.g. Hamilton Depression Rating Scale (Hamilton 1960))." Severity is usually reported as a continuous outcome, however in the included study it was dichotomised by the authors. Participants with a SIGH‐SAD‐SR score ≥ 40 were considered severely depressed. Therefore we reported this outcome as a dichotomous outcome in this review.
Researchers did not assess adverse events.
Excluded studies
Overall, we assessed 126 studies at full‐text level and excluded 125 of them. We excluded most studies because they were treatment ‐ not prevention ‐ studies and/or because they focused on major depression ‐ not on SAD. Some studies were intended to prevent SAD, but we had to exclude them for different reasons.
Two studies included participants with a history of SAD and investigated the preventive effects of light therapy (Meesters 1994; Partonen 1996). We excluded these studies because they had no control group and therefore did not meet our eligibility criteria. Two controlled studies investigated whether starting light therapy at an early stage of a depressive episode can prevent a full‐blown winter depressive episode (Meesters 1991; Meesters 1993). We excluded these studies because participants already had depressive symptoms when the study started. One study also investigated whether starting light therapy at an early stage of a depressive episode can prevent a full‐blown SAD; however, in this study participants already had symptoms when the study started, and a control group was missing (Terman 1994).
Characteristics of excluded studies show all records that narrowly missed the inclusion criteria for this systematic review and mention those studies that were included in the review on second‐generation antidepressants (Gartlehner 2015). In the Characteristics of excluded studies section, we explain why we did not include these studies in this review.
Ongoing studies
We identified no ongoing studies of interest.
Studies awaiting classification
We found no studies currently awaiting classification.
Risk of bias in included studies
For details of the risk of bias judgement, see Characteristics of included studies. We present graphical representations of the overall risk of bias in the included study in Figure 2 and Figure 3.
2.
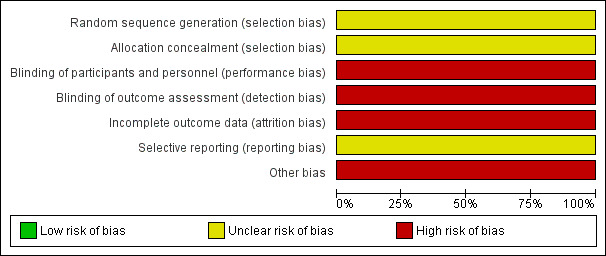
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across included studies.
3.
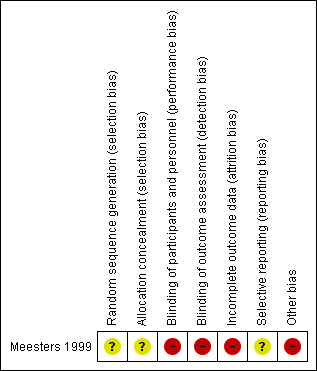
Risk of bias summary: review authors' judgements about each risk of bias item for the included study.
Allocation
Study authors state that participants were randomly assigned to treatment groups. However, they do not provide detailed information about the randomisation scheme and do not explain how allocation concealment was achieved. The only participant characteristics reported are age and sex (only for the per‐protocol population). Study authors do not report details on participants' history of SAD nor on their prior experience with light therapy, socioeconomic status of included participants or whether treatment groups were similar in terms of participant characteristics.Therefore, we rated the risk for selection bias as unclear.
Blinding
Participants were not blinded to type of treatment. As participants were using a self‐reporting scale, they were assessing outcomes too. Therefore, we rated these domains as high risk.
Incomplete outcome data
Eight of 46 participants (17%) dropped out of the study (four in the bright light group, three in the infrared light group and one in the control group) and were not included in the analysis (no intention‐to‐treat (ITT)). Investigators reported no characteristics (e.g. age, sex) of participants who dropped out. Therefore, we rated the risk for attrition bias high.
Selective reporting
We could not identify a protocol for this study. Therefore, we rated this domain as unclear.
Other potential sources of bias
Participants self‐administered the intervention in their own homes. Whether they used the intervention properly is unknown. Therefore, we rated this domain as high.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Comparison 1. Light therapy versus placebo, no treatment or waiting list
One study including 46 participants contributed data for this comparison (Meesters 1999). In this study, investigators compared bright white visor light (2500 lux) (n = 18) and infrared visor light (0.18 lux) (n = 18) with no light exposure (n = 10). Because study authors reported only per‐protocol analyses, we additionally conducted two types of ITT analyses. In one, we assumed that participants who dropped out of the study were depressed; in the other, we assumed that participants who dropped out of the study were not depressed. We chose this approach because with eight dropouts in such a small study, assumptions about the dropouts can determine analytical results. Therefore, we decided to report per‐protocol results as well.
We conducted three comparisons as summarised in this section: bright light therapy versus no light therapy, infrared light therapy versus no light therapy and "light therapy" (both bright white light therapy and infrared light therapy) versus no light therapy.
We did not synthesise outcomes at different time points because data were presented only in figures and were not ready to include in analyses.
Primary outcomes
1.1 Incidence of SAD
We defined a priori the incidence of SAD as the proportion of participants with a SIGH‐SAD score of 20 or higher.
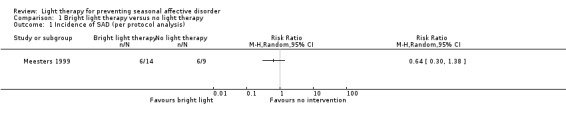
For the first comparison, risk of developing a depressive episode (SIGH‐SAD‐SR ≥ 20) was numerically smaller for participants in the bright light group than for those in the non‐treatment group. According to the per‐protocol analysis, 43% (6/14) of those in the bright light group developed a depressive episode compared with 67% (6/9) of participants in the non‐treatment group. The risk of developing a depressive episode during the winter was 36% smaller for individuals in the bright light group than for those in the non‐treatment group; however, the confidence interval (CI) was very broad, implying that with 95% confidence, the true effect lies within 0.30 to 1.38 (risk ratio (RR) 0.64, 95% CI 0.30 to 1.38; 23 participants, very low‐quality evidence; see Analysis 1.1).
1.1. Analysis.
Comparison 1 Bright light therapy versus no light therapy, Outcome 1 Incidence of SAD (per protocol analysis).
When we assumed that none of the participants who dropped out developed a depressive episode, ITT analysis showed that 33% (6/18) of individuals in the bright light group and 60% (6/10) of those in the non‐treatment group developed depression. The risk of developing depression under bright light therapy was 44% smaller than in the non‐treatment group; however, the broad CI suggests with 95% certainty that the true effect lies between 0.24 and 1.27 (RR 0.56, 95% CI 0.24 to 1.27; 28 participants; see Analysis 1.2).
1.2. Analysis.
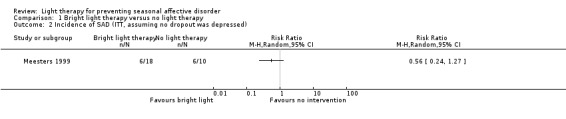
Comparison 1 Bright light therapy versus no light therapy, Outcome 2 Incidence of SAD (ITT, assuming no dropout was depressed).
If we assumed, on the other hand, that all dropouts developed depression, ITT analysis showed that 56% (10/18) of participants in the bright light group developed depression compared with 70% (7/10) in the non‐treatment group. The risk of developing depression was 21% smaller in the bright light group than in the non‐treatment group; however, the broad CI suggests with 95% certainty a possible true value between 0.44 and 1.42 (RR 0.79, 95% CI 0.44 to 1.42; 28 participants; see Analysis 1.3).
1.3. Analysis.
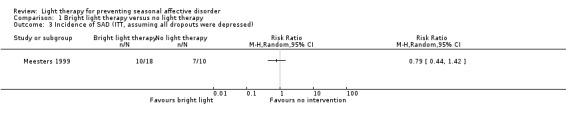
Comparison 1 Bright light therapy versus no light therapy, Outcome 3 Incidence of SAD (ITT, assuming all dropouts were depressed).
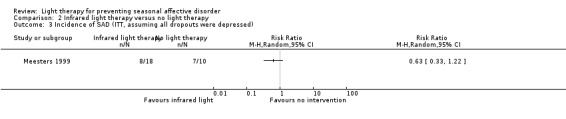
For the second comparison, a favourable effect of the light condition was observed in the infrared light group; however, CIs always included possible true values favouring infrared light and favouring no treatment, no matter what type of analysis we applied (per‐protocol: 33% (5/15) versus 67% (6/9); RR 0.50, 95% CI 0.21 to 1.17; 24 participants, very low‐quality evidence; see Analysis 2.1; ITT with no dropouts depressed: 28% (5/18) versus 60% (6/10); RR 0.46, 95% CI 0.19 to 1.14; 28 participants; see Analysis 2.2; ITT with all dropouts depressed: 44% (8/18) versus 70% (7/10); RR 0.63, 95% CI 0.33 to 1.22; 28 participants; see Analysis 2.3).
2.1. Analysis.
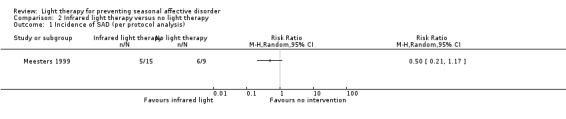
Comparison 2 Infrared light therapy versus no light therapy, Outcome 1 Incidence of SAD (per protocol analysis).
2.2. Analysis.
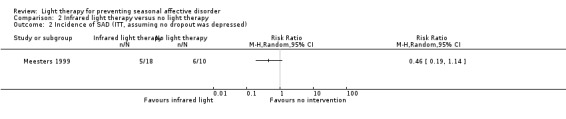
Comparison 2 Infrared light therapy versus no light therapy, Outcome 2 Incidence of SAD (ITT, assuming no dropout was depressed).
2.3. Analysis.
Comparison 2 Infrared light therapy versus no light therapy, Outcome 3 Incidence of SAD (ITT, assuming all dropouts were depressed).
For the third comparison, when we compared both types of light therapy given to one treatment arm, we observed a numerically favourable effect of light therapy versus no light therapy; however, CIs always included possible true values favouring light and no light therapy, no matter if we used per‐protocol or ITT analysis (per‐protocol: 38% (11/29) versus 67% (6/9); RR 0.57, 95% CI 0.30 to 1.10; 38 participants, very low‐quality of evidence; see Analysis 3.1; ITT with no dropouts depressed: 31% (11/36) versus 60% (6/10); RR 0.51, 95% CI 0.25 to 1.03; 46 participants; see Analysis 3.2; ITT with all dropouts depressed: 50% (18/36) versus 70% (7/10); RR 0.71, 95% CI 0.42 to 1.20; 46 participants; see Analysis 3.3).
3.1. Analysis.
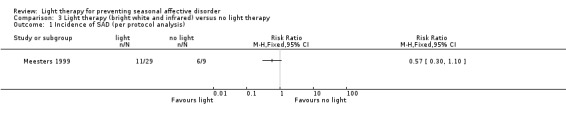
Comparison 3 Light therapy (bright white and infrared) versus no light therapy, Outcome 1 Incidence of SAD (per protocol analysis).
3.2. Analysis.
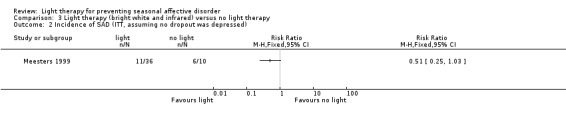
Comparison 3 Light therapy (bright white and infrared) versus no light therapy, Outcome 2 Incidence of SAD (ITT, assuming no dropout was depressed).
3.3. Analysis.
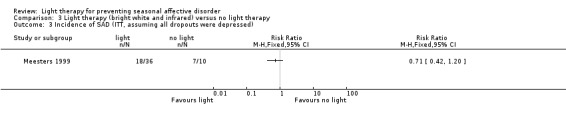
Comparison 3 Light therapy (bright white and infrared) versus no light therapy, Outcome 3 Incidence of SAD (ITT, assuming all dropouts were depressed).
1.2 Overall rate of adverse events
The included RCT reported no data on adverse events. We found no additional eligible evidence addressing this outcome.
Secondary outcomes
1.3 Severity of SAD or SAD‐related symptoms
For the first comparison, when we looked only at those participants who developed severe depression using SIGH‐SAD‐SR (≥ 40) as the criterion for severe depression, the broad CI included possible true values favouring bright light and favouring no treatment (per‐protocol: 7% (1/14) versus 33% (3/9); RR 0.21, 95% CI 0.03 to 1.75; 23 participants, very low‐quality evidence; see Analysis 1.4; ITT with no dropout depressed: 6% (1/18) versus 30% (3/10); RR 0.19, 95% CI 0.02 to 1.55; 28 participants; see Analysis 1.5; ITT with all dropouts depressed: 28% (5/18) versus 40% (4/10); RR 0.56, 95% CI 0.18 to 1.76; 28 participants; see Analysis 1.6).
1.4. Analysis.
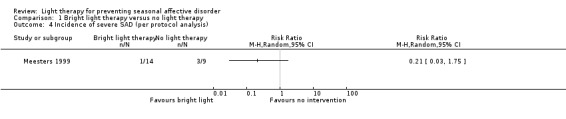
Comparison 1 Bright light therapy versus no light therapy, Outcome 4 Incidence of severe SAD (per protocol analysis).
1.5. Analysis.
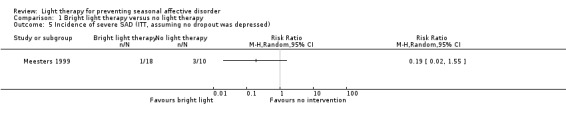
Comparison 1 Bright light therapy versus no light therapy, Outcome 5 Incidence of severe SAD (ITT, assuming no dropout was depressed).
1.6. Analysis.
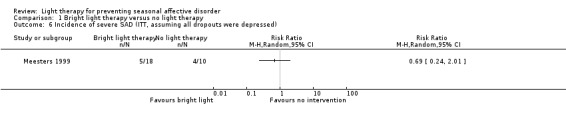
Comparison 1 Bright light therapy versus no light therapy, Outcome 6 Incidence of severe SAD (ITT, assuming all dropouts were depressed).
For the second comparison, infrared light therapy versus no light therapy the 95% CI was also very broad when we used SIGH‐SAD‐SR (per‐protocol: 7% (1/15) versus 33% (3/9); RR 0.20, 95% CI 0.02 to 1.64; 24 participants, very low‐quality evidence; see Analysis 2.4; ITT with no dropout depressed: 6% (1/18) versus 30% (3/10); RR 0.19, 95% CI 0.02 to 1.55; 28 participants; see Analysis 2.5; ITT with all dropouts depressed: 22% (4/18) versus 40% (4/10); RR 0.56, 95% CI 0.18 to 1.76; 28 participants; see Analysis 2.6).
2.4. Analysis.
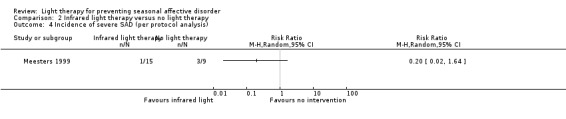
Comparison 2 Infrared light therapy versus no light therapy, Outcome 4 Incidence of severe SAD (per protocol analysis).
2.5. Analysis.
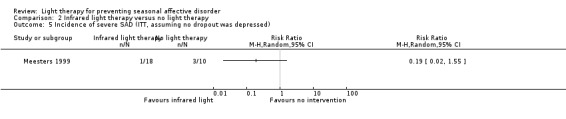
Comparison 2 Infrared light therapy versus no light therapy, Outcome 5 Incidence of severe SAD (ITT, assuming no dropout was depressed).
2.6. Analysis.
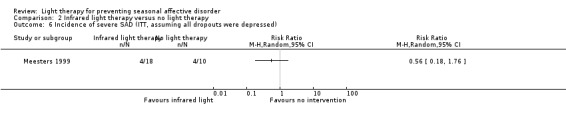
Comparison 2 Infrared light therapy versus no light therapy, Outcome 6 Incidence of severe SAD (ITT, assuming all dropouts were depressed).
For the third comparison, combining bright white light and infrared light versus "light therapy" and comparing it with no light therapy showed numerically favourable results for light therapy; however, again the 95% CI was very broad. In the per‐protocol analysis, the true effect could favour both light therapy and no light therapy (7% (2/29) versus 33% (3/9); RR 0.21, 95% CI 0.04 to 1.05; 38 participants, very low‐quality evidence; see Analysis 3.4). In the ITT analysis, when we assumed that all dropouts were not depressed, the 95% CI shows a true favourable effect of light therapy in reducing the risk of development of a severe depressive episode (6% (2/36) versus 30% (3/10); RR 0.63, 95% CI 0.24 to 1.61; 46 participants; see Analysis 3.5). However, when we assumed that all dropouts had developed a severe episode of depression, this was no longer the case (25% (9/36) versus 40% (4/10); RR 0.63, 95% CI 0.22 to 1.61; 46 participants; see Analysis 3.6).
3.4. Analysis.
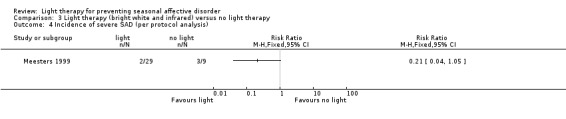
Comparison 3 Light therapy (bright white and infrared) versus no light therapy, Outcome 4 Incidence of severe SAD (per protocol analysis).
3.5. Analysis.
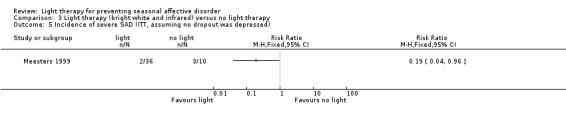
Comparison 3 Light therapy (bright white and infrared) versus no light therapy, Outcome 5 Incidence of severe SAD (ITT, assuming no dropout was depressed).
3.6. Analysis.
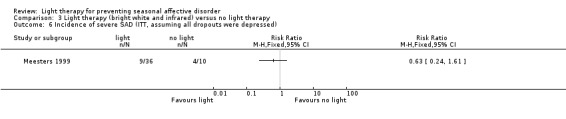
Comparison 3 Light therapy (bright white and infrared) versus no light therapy, Outcome 6 Incidence of severe SAD (ITT, assuming all dropouts were depressed).
1.4 Quality of life
We found no eligible evidence addressing this outcome.
1.5 Quality of interpersonal and social functioning
We found no eligible evidence addressing this outcome.
1.6 Proportion of participants with serious adverse events
We found no eligible evidence addressing this outcome.
1.7 Rates of discontinuation due to adverse events
We found no eligible evidence addressing this outcome.
1.8 Overall rate of discontinuation
Eight participants dropped out during the study period (four in the bright light group, three in the infrared light group and one in the control group) for the following reasons: one person failed to wear the light visor, one started to use medication, one in the "non‐treatment group" started with light therapy on her own and five stopped because they lacked motivation to keep scoring self‐rating scales when suffering from no symptoms, moving to a new home and other reasons that study authors did not describe in detail.
Additionally, researchers offered light treatment in the clinic to all participants who developed severe depression (SIGH‐SAD‐SR ≥ 40) and left the study at that moment. As this type of discontinuation is part of the main outcome, we did not take it into account when we compared discontinuation rates between groups receiving light therapy and those receiving no light therapy.
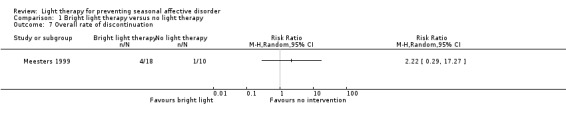
For the first comparison, four of 18 (22%) participants randomly assigned to bright light therapy dropped out during the study period compared with one person in the no treatment group (10%) (RR 2.22, 95% CI 0.29 to 17.27; 28 participants, very low‐quality evidence; see Analysis 1.7).
1.7. Analysis.
Comparison 1 Bright light therapy versus no light therapy, Outcome 7 Overall rate of discontinuation.
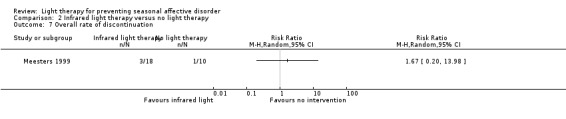
For the second comparison, the infrared light therapy group dropout rate was higher than that of the non‐treatment group (17% (3/18) versus 10% (1/10); RR 1.67, 95% CI 0.20 to 13.98; 28 participants, very low‐quality evidence; see Analysis 2.7), but the difference was small and the CI included possible true values favouring infrared light and favouring no treatment.
2.7. Analysis.
Comparison 2 Infrared light therapy versus no light therapy, Outcome 7 Overall rate of discontinuation.
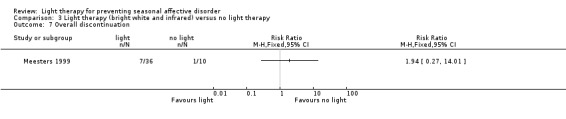
For the third comparison, both types of light therapy together versus no light therapy showed that the dropout rate in the light therapy group was numerically higher (19% (7/36) versus 10% (1/10); RR 1.94, 95% CI 0.27 to 14.01; 46 participants, very low‐quality evidence; see Analysis 3.7).
3.7. Analysis.
Comparison 3 Light therapy (bright white and infrared) versus no light therapy, Outcome 7 Overall discontinuation.
Comparison 2. Light therapy versus other light therapy
One study contributed data from 36 participants to this comparison; 18 were randomly assigned to bright white visor light, and 18 to infrared visor light (Meesters 1999). As explained in Comparison 1, we reported per‐protocol results as well as results derived from two types of ITT analyses. In one, we assumed that participants who dropped out of the study were depressed; in the other, we assumed that those who dropped out of the study were not depressed.
Primary outcomes
2.1 Incidence of SAD
In the bright light condition, the rate of participants developing depression (SIGH‐SAD‐SR ≥ 20) was numerically higher than that of participants in the infrared light therapy group; however, CIs always included possible true values favouring light and no light therapy, no matter whether we performed per‐protocol or ITT analysis (per‐protocol: 43% versus 33%; RR 1.29, 95% CI 0.50 to 3.28; 29 participants, very low‐quality evidence; see Analysis 4.1; ITT with no dropouts depressed: 33% versus 28%; RR 1.20, 95% CI 0.45 to 3.23; 36 participants; see Analysis 4.2; ITT with all dropouts depressed: 56% versus 44%; RR 1.25, 95% CI 0.65 to 2.42; 36 participants; see Analysis 4.3).
4.1. Analysis.
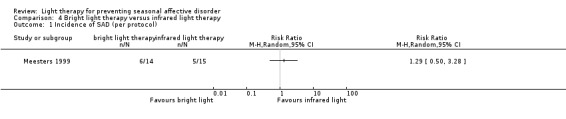
Comparison 4 Bright light therapy versus infrared light therapy, Outcome 1 Incidence of SAD (per protocol).
4.2. Analysis.
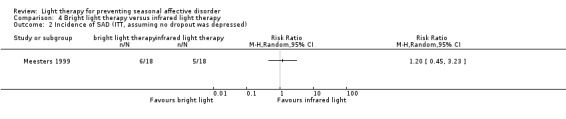
Comparison 4 Bright light therapy versus infrared light therapy, Outcome 2 Incidence of SAD (ITT, assuming no dropout was depressed).
4.3. Analysis.
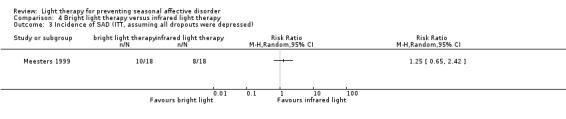
Comparison 4 Bright light therapy versus infrared light therapy, Outcome 3 Incidence of SAD (ITT, assuming all dropouts were depressed).
2.2 Overall rate of adverse events
We did not find any eligible evidence addressing this outcome.
Secondary outcomes
2.3 Severity of SAD or SAD‐related symptoms
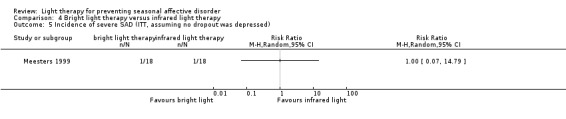
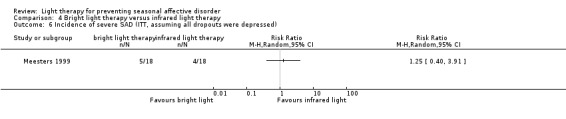
When we looked only at participants who developed severe depression (SIGH‐SAD‐SR ≥ 40), we observed no differences between individuals in the bright light therapy group and those in the infrared light therapy group ‐ both light conditions seemed to be similar (per‐protocol: 7% versus 7%; RR 1.07, 95% CI 0.07 to 15.54; 29 participants; see Analysis 4.4; ITT with no dropouts depressed: 6% versus 6%; RR 1.00, 95% CI 0.07 to 14.79; 36 participants; see Analysis 4.5; ITT with all dropouts depressed: 28% versus 22%; RR 1.25, 95% CI 0.40 to 3.91; 36 participants; see Analysis 4.6).
4.4. Analysis.
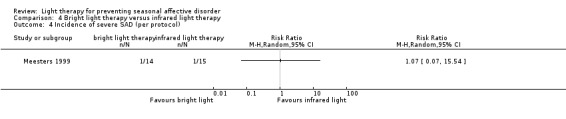
Comparison 4 Bright light therapy versus infrared light therapy, Outcome 4 Incidence of severe SAD (per protocol).
4.5. Analysis.
Comparison 4 Bright light therapy versus infrared light therapy, Outcome 5 Incidence of severe SAD (ITT, assuming no dropout was depressed).
4.6. Analysis.
Comparison 4 Bright light therapy versus infrared light therapy, Outcome 6 Incidence of severe SAD (ITT, assuming all dropouts were depressed).
2.4 Quality of life
We found no eligible evidence addressing this outcome.
2.5 Quality of interpersonal and social functioning
We found no eligible evidence addressing this outcome.
2.6 Proportion of participants with serious adverse events
We found no eligible evidence addressing this outcome.
2.7 Rates of discontinuation due to adverse events
We found no eligible evidence addressing this outcome.
2.8 Overall rate of discontinuation
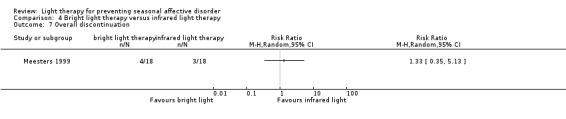
Discontinuation was similar in the two light therapy groups; 22% (4/18) of participants randomly assigned to bright light therapy dropped out during the study period compared with 17% (3/18) of those in the infrared light therapy group (RR 1.33, 95% CI 0.35 to 5.13; 36 participants, very‐low quality evidence; see Analysis 4.7).
4.7. Analysis.
Comparison 4 Bright light therapy versus infrared light therapy, Outcome 7 Overall discontinuation.
Comparison 3. Light therapy versus second‐generation antidepressants
We found no eligible studies assessing this comparison.
Comparison 4. Light therapy versus melatonin or agomelatine
We found no eligible studies assessing this comparison.
Comparison 5. Light therapy versus psychological therapy
We found no eligible studies assessing this comparison.
Comparison 6. Light therapy versus lifestyle intervention
We found no eligible studies assessing this comparison.
Comparison 7. Light therapy versus negative ion generators
We found no eligible studies assessing this comparison.
Comparison 8. Light therapy + comparator intervention (as listed under Types of interventions) versus placebo or no treatment control group or waiting list
We found no eligible studies assessing this comparison.
Comparison 9. Light therapy + comparator intervention (as listed under Types of interventions) versus the same comparator intervention as monotherapy (e.g. light therapy + psychological therapy versus psychological therapy alone)
We found no eligible studies assessing this comparison.
Subgroup analyses
Data were insufficient for subgroup analyses.
Sensitivity analyses
Data were insufficient for sensitivity analyses.
Reporting bias
As we identified only one study, statistical approaches to assessment of publication bias are not possible. It is unclear whether reporting bias is a problem of the included study, as we could not identify a protocol for the study.
Discussion
Summary of main results
Overall, bright white visor light and infrared visor light numerically reduced the incidence of seasonal affective disorder (SAD), as well as the incidence of severe SAD, as compared with no light therapy; however, 95% confidence intervals (CIs) were very broad and included both possible true effects favouring light therapy and possible true effects favouring no treatment. Comparison of both light conditions versus each other showed no differences in SAD incidence and in the incidence of severe SAD.
Overall, the number of dropouts (not including those who stopped the study because of depression) was similar between study arms.
Table 1, Table 2, Table 3 and Table 4 show that quality of evidence for all outcomes was very low.
Overall completeness and applicability of evidence
A major limitation of our report is that evidence is limited to one randomised controlled trial (RCT) with a very small number of participants (n = 46) that is rated as having high risk of bias in several domains. Therefore, results of this RCT can be influenced by lack of power and by random error.
The population included in this study that met our eligibility criteria was only loosely described (age, gender); therefore, it is difficult to say whether the results can be applied to a broad spectrum of people who experience SAD.
As no results on adverse events were reported, we cannot estimate potential harms of the intervention.
Quality of the evidence
We graded the quality of evidence for available efficacy outcomes (incidence of SAD, severity of SAD, overall discontinuation) as very low. Reasons for downgrading the quality of evidence included high risk of bias of the included study and imprecision due to lack of optimal information size. Other limitations of the included study were that outcomes were self‐rated, compliance throughout study duration was not checked, participant characteristics were not reported comprehensively, no safety data were reported (especially important because infrared light can cause such events as eye damage) and the wavelength of infrared light used was not specified, which is important because absorption of infrared light in the eye depends on the wavelength (Berg 1997). We did not downgrade for indirectness because we drew conclusions only on the efficacy of these two special types of light therapy (bright white visor light and infrared visor light). Drawing conclusions about light therapy per se is not possible because different types of administration of light therapy may lead to different results.
Potential biases in the review process
Our eligibility criteria included non‐randomised studies for assessment of harms because methods research indicates that rare but potentially serious adverse events are not covered well in RCTs. Despite extensive literature searches, we could not identify any non‐randomised studies. We identified only one RCT that was published in 1999 and did not report on adverse events. As the study was conducted 20 years ago, we did not contact the study author to ask for more detailed information. Other studies that investigated light therapy as treatment ‐ not as prevention ‐ have reported possible side effects from light therapy, including headache (13% to 21%), eye strain or visual disturbance (19% to 27%), nausea (7%), sweating (7%), sedation (6% to 7%) and agitation (6% to 13%) (Lam 1999). However, based on the evidence included in this systematic review, we do not know whether light therapy as preventive treatment is associated with side effects.
In this systematic review, we considered all types of bright white therapy, infrared light therapy and dawn simulation therapy to be eligible (no limitations on dosage, duration or mode of delivery) because we wanted to base our systematic review on all available evidence on preventive use of any type of light therapy. The included study investigated two types of light therapy that nowadays generally are not accepted as treatment for people with SAD (Meesters 1999). Three studies showed that bright white light delivered by visors is not more effective than placebo (Joffe 1993; Rosenthal 1993; Teicher 1995). Therefore, experts often do not consider visors as a legitimate light therapy intervention. This might explain why bright white light was not more effective than no light therapy in reducing the incidence of SAD in Meesters 1999. Nowadays, dim red light producing only a visually negligible lux of red light is often considered sham treatment rather than active light therapy. This must be considered when results of the included study are interpreted.
Finally, publication bias and selective outcomes reporting are potential limitations of any systematic review. Although we searched for grey and unpublished literature, the extent and impact of reporting biases of this body of evidence is impossible to determine.
Agreements and disagreements with other studies or reviews
One uncontrolled study included participants with a history of SAD and showed no preventive effects of light therapy on SAD when administered before the winter season (Meesters 1994). Another uncontrolled study observing participants who received light therapy before symptoms started showed a preventive effect ‐ none of the six individuals developed a depressive episode during the winter season, according to SIGH‐SAD‐SR (Partonen 1996). Two controlled studies showed that light therapy at an early stage of a depressive episode can prevent a full‐blown winter depressive episode (Meesters 1991; Meesters 1993). Another study showed that treating individuals with light therapy at an early stage could postpone a subsequent episode of SAD (Terman 1994).
We could identify no systematic reviews on the effects of light therapy as preventive treatment for people with a history of SAD. A systematic review and meta‐analysis of light therapy as treatment for mood disorders showed that bright light therapy and dawn simulation were efficacious in reducing the severity of SAD symptoms (Golden 2005).
Authors' conclusions
Implications for practice.
The evidence base for light therapy as preventive treatment for seasonal affective disorder (SAD) in people with a history of SAD is limited. Methodological limitations and small sample size of the only available study precluded review author conclusions on the effects of light therapy for SAD. Given the lack of comparative evidence, the decision for or against initiating preventive treatment of SAD and the choice of treatment should be based strongly on patient preferences.
Implications for research.
Independently funded, high‐quality studies undertaken to explicitly investigate the efficacy and safety of established light therapies for the prevention of SAD, in which the research team can monitor how the intervention is implemented, are necessary. These studies must include a large sample (to reach at least 80% power), must begin the intervention before the first depressive symptoms occur and must investigate different ways of administering light therapy. Investigators should also investigate the combination of light therapy with a second‐generation antidepressant because some psychotropic medications may increase photosensitivity.
In this review, we have focused explicitly on prevention of SAD in people with a history of SAD who were free of depressive symptoms at the start of treatment. We suggest that future research on light therapy should include a systematic review on prophylactic long‐term effects of light therapy in individuals with SAD.
What's new
| Date | Event | Description |
|---|---|---|
| 15 April 2019 | Amended | Minor correction made to the PRISMA diagram. |
History
Protocol first published: Issue 9, 2014 Review first published: Issue 11, 2015
| Date | Event | Description |
|---|---|---|
| 15 March 2019 | New search has been performed | We updated the searches on 19 June 2018; we did not identify any new trials. |
| 15 March 2019 | New citation required but conclusions have not changed | Review updated |
Acknowledgements
We would like to thank Evelyn Auer and Sandra Hummel for providing administrative support during the course of this review. We would also like to thank Julia Hoffmann, and Jeffrey H Sonis for their support when conducting the former version of this Cochrane Review.
CRG Funding Acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Common Mental Disorders Group.
Disclaimer: views and opinions expressed herein are those of the review authors and do not necessarily reflect those of NIHR, National Health Service (NHS) or the Department of Health and Social Care.
Appendices
Appendix 1. CCMDCTR: Core MEDLINE search
The search strategy listed below is the weekly OVID Medline search which was used to inform the Group’s specialised register (to June 2016). It is based on a list of terms for all conditions within the scope of the Cochrane Common Mental Disorders Group plus a sensitive RCT filter.
OVID MEDLINE search strategy, used to inform the Cochrane Common Mental Disorders Group's Specialised Register A weekly search alert based on condition + RCT filter only 1. [MeSH Headings]: eating disorders/ or anorexia nervosa/ or binge‐eating disorder/ or bulimia nervosa/ or female athlete triad syndrome/ or pica/ or hyperphagia/ or bulimia/ or self‐injurious behavior/ or self mutilation/ or suicide/ or suicidal ideation/ or suicide, attempted/ or mood disorders/ or affective disorders, psychotic/ or bipolar disorder/ or cyclothymic disorder/ or depressive disorder/ or depression, postpartum/ or depressive disorder, major/ or depressive disorder, treatment‐resistant/ or dysthymic disorder/ or seasonal affective disorder/ or neurotic disorders/ or depression/ or adjustment disorders/ or exp antidepressive agents/ or anxiety disorders/ or agoraphobia/ or neurocirculatory asthenia/ or obsessive‐compulsive disorder/ or obsessive hoarding/ or panic disorder/ or phobic disorders/ or stress disorders, traumatic/ or combat disorders/ or stress disorders, post‐traumatic/ or stress disorders, traumatic, acute/ or anxiety/ or anxiety, castration/ or koro/ or anxiety, separation/ or panic/ or exp anti‐anxiety agents/ or somatoform disorders/ or body dysmorphic disorders/ or conversion disorder/ or hypochondriasis/ or neurasthenia/ or hysteria/ or munchausen syndrome by proxy/ or munchausen syndrome/ or fatigue syndrome, chronic/ or obsessive behavior/ or compulsive behavior/ or behavior, addictive/ or impulse control disorders/ or firesetting behavior/ or gambling/ or trichotillomania/ or stress, psychological/ or burnout, professional/ or sexual dysfunctions, psychological/ or vaginismus/ or Anhedonia/ or Affective Symptoms/ or *Mental Disorders/ 2. [Title/ Author Keywords]: (eating disorder* or anorexia nervosa or bulimi* or binge eat* or (self adj (injur* or mutilat*)) or suicide* or suicidal or parasuicid* or mood disorder* or affective disorder* or bipolar i or bipolar ii or (bipolar and (affective or disorder*)) or mania or manic or cyclothymic* or depression or depressive or dysthymi* or neurotic or neurosis or adjustment disorder* or antidepress* or anxiety disorder* or agoraphobia or obsess* or compulsi* or panic or phobi* or ptsd or posttrauma* or post trauma* or combat or somatoform or somati#ation or medical* unexplained or body dysmorphi* or conversion disorder or hypochondria* or neurastheni* or hysteria or munchausen or chronic fatigue* or gambling or trichotillomania or vaginismus or anhedoni* or affective symptoms or mental disorder* or mental health).ti,kf. 3. [RCT filter]: (controlled clinical trial.pt. or randomized controlled trial.pt. or (randomi#ed or randomi#ation).ab,ti. or randomly.ab. or (random* adj3 (administ* or allocat* or assign* or class* or control* or determine* or divide* or distribut* or expose* or fashion or number* or place* or recruit* or subsitut* or treat*)).ab. or placebo*.ab,ti. or drug therapy.fs. or trial.ab,ti. or groups.ab. or (control* adj3 (trial* or study or studies)).ab,ti. or ((singl* or doubl* or tripl* or trebl*) adj3 (blind* or mask* or dummy*)).mp. or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or randomized controlled trial/ or pragmatic clinical trial/ or (quasi adj (experimental or random*)).ti,ab. or ((waitlist* or wait* list* or treatment as usual or TAU) adj3 (control or group)).ab.) 4. (1 and 2 and 3)
Records are screened for reports of RCTs within the scope of the Cochrane Common Mental Disorders Group. Secondary reports of RCTs are tagged to the appropriate study record. Similar weekly search alerts are also conducted on OVID Embase and PsycINFO, using relevant subject headings (controlled vocabularies) and search syntax, appropriate to each resource.
Appendix 2. Database searches 2014
PubMed 26.05.2014
| Search | Query | Items found |
| #1 | Search "Seasonal Affective Disorder"[Mesh] | 1061 |
| #2 | Search "seasonal affective disorder"[All Fields] | 1415 |
| #3 | Search seasonal affective disorder* | 1451 |
| #4 | Search "seasonal depression"[All Fields] | 162 |
| #5 | Search seasonal mood disorder* | 10 |
| #6 | Search "winter depression" | 248 |
| #7 | Search SIGH‐SAD | 61 |
| #8 | Search (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7) | 1555 |
| #9 | Search (#8 AND 2013/05:2014[dp]) | 46 |
The Cochrane Library 26.05.2014
| ID | Search | Hits |
| #1 | seasonal affective disorder (Word variations have been searched) | 312 |
| #2 | winter blues (Word variations have been searched) | 25 |
| #3 | seasonal depression | 295 |
| #4 | seasonal mood disorder | 134 |
| #5 | winter depression | 256 |
| #6 | SIGH‐SAD | 39 |
| #7 | {or #1‐#6} Publication Date from 2013 to 2014 | 69 |
EMBASE 26.05.2014
| No. | Query | Results |
| #1 | 'seasonal affective disorder'/exp AND [humans]/lim AND [embase]/lim | 640 |
| #3 | 'seasonal affective disorder'/mj | 484 |
| #4 | #1 OR #3 | 831 |
| #5 | #4 AND [2013‐2014]/py | 79 |
PsycINFO, AMED, IPA, CINAHL (via EBSCO HOST) 26.05.2014
| # | Query | Limiters/Expanders | Last Run Via | Results |
| S1 | seasonal affective disorder | Limiters ‐ Published Date: 20130501‐ | Interface ‐ EBSCOhost Research Databases | 39 |
| Search modes ‐ Boolean/Phrase | Search Screen ‐ Advanced Search | |||
| Database ‐ PsycINFO;AMED ‐ The Allied and Complementary Medicine Database;CINAHL with Full Text;International Pharmaceutical Abstracts |
Web of Knowledge (via UNC) 28.05.2014
| Set | Results | |
| # 5 | 69 | #4 AND #1 |
| Timespan=2013‐2014 | ||
| Search language=English | ||
| # 4 | Approximately | #3 OR #2 |
| 1,107,073 | Timespan=2013‐2014 | |
| Search language=English | ||
| # 3 | Approximately | TOPIC: (treatment) |
| 975,242 | Timespan=2013‐2014 | |
| Search language=English | ||
| # 2 | Approximately | TOPIC: (prevention) |
| 188,628 | Timespan=2013‐2014 | |
| Search language=English | ||
| # 1 | 165 | TOPIC: ("seasonal affective disorder") |
| Timespan=2013‐2014 | ||
| Search language=English |
Appendix 3. Database searches 2018
Summary of searches (19 June 2018)
CCMD Register, n = 8
CENTRAL, n = 30
MEDLINE, n = 233
Embase, n = 301
PsycINFO, n = 154
International Pharmaceutical Abstracts, (database unavailable)
CINHAL, n = 77
Web of Knowledge, n = 489
AMED, n = 1
Total = 1293 Duplicates removed = 607 Number to screen = 686
Database search strategies
CCMD‐CTR (searched via Cochrane CRS) Date searched: Tuesday, 19th June 2018 (Register current to June 2016, only) Hits: 303 (8 in scope for this update) 1"seasonal affective disorder" AND INREGISTER (277) 2seasonal affective disorder* AND INREGISTER (280) 3"seasonal depression" AND INREGISTER34 4 seasonal mood disorder* AND INREGISTER (6) 5 "winter depression" AND INREGISTER (72) 6 SIGH‐SAD AND INREGISTER (48) 7 #1 OR #2 OR #3 OR #4 OR #5 OR #6 (303) Cochrane CENTRAL searched the Cochrane Library (Wiley interface) Data parameters: Issue 5 of 12, May 2018 Date searched: Tuesday, 19th June 2018 Hits: 363 (30 in scope for this update) #1 MeSH descriptor: [Seasonal Affective Disorder] explode all trees 172 #2 "seasonal affective disorder" 364 #3 seasonal affective disorder* 397 #4 "seasonal depression" 46 #5 seasonal mood disorder* 199 #6 "winter depression" 85 #7 SIGH‐SAD 55 #8 (#1 or #2 or #3 or #4 or #5 or #6 or #7) 452 Notes: Of 452 returned from searching The Cochrane Library, 363 were records from CENTRAL. Records dating pre‐2015 were visually inspected and manually removed.
Ovid MEDLINE(R) Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) Data parameters: 1946 to Present Date searched: Tuesday, 19th June 2018 Hits: 233 1 Seasonal Affective Disorder/1180 2 "seasonal affective disorder".ti,ab,kw,ot.1206 3 seasonal affective disorder*.ti,ab,kw,ot.1277 4 "seasonal depression".ti,ab,kw,ot.188 5 seasonal mood disorder*.ti,ab,kw,ot.12 6 "winter depression".ti,ab,kw,ot.272 7 SIGH‐SAD.ti,ab,kw,ot.78 8 (1 or 2 or 3 or 4 or 5 or 6 or 7) 1790 9 (2015* or 2016* or 2017* or 2018*).yr,ed. 4792024 10 (8 and 9) 233
Embase (Ovid Interface) Data parameters: 1974 to 2018 June 18 Date searched: Tuesday, 19th June 2018 Hits: 301 1 Seasonal Affective Disorder/ 1239 2 "seasonal affective disorder".ti,ab,kw,ot. 1528 3 seasonal affective disorder*.ti,ab,kw,ot. 1618 4 "seasonal depression".ti,ab,kw,ot. 246 5 seasonal mood disorder*.ti,ab,kw,ot. 23 6 "winter depression".ti,ab,kw,ot. 334 7 SIGH‐SAD.ti,ab,kw,ot. 92 8 (1 or 2 or 3 or 4 or 5 or 6 or 7) 2297 9 (2015* or 2016* or 2017* or 2018*).yr,ed. 4907927 10 (8 and 9) 301
PsycINFO (Ovid) Data parameters: 2002 to June Week 2 2018 Date searched: Tuesday, 19th June 2018 Hits: 154 1 Seasonal Affective Disorder/ 484 2 "seasonal affective disorder".ti,ab,kw,ot. 511 3 seasonal affective disorder*.ti,ab,kw,ot. 529 4 "seasonal depression".ti,ab,kw,ot. 94 5 seasonal mood disorder*.ti,ab,kw,ot. 6 6 "winter depression".ti,ab,kw,ot. 72 7 SIGH‐SAD.ti,ab,kw,ot. 53 8 (1 or 2 or 3 or 4 or 5 or 6 or 7) 690 9 (2015* or 2016* or 2017* or 2018*).yr,ed. 633488 10 (8 and 9) 154
CINAHL via EBSCOHost Data parameters: 1937‐Current Date searched: Tuesday, 19th June 2018 Hits: 77 S9 (S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7) Limiters: Published Date (20150101 ‐ 20180631) 77 S8 (S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7) 454 S7 TI SIGH‐SAD OR AB SIGH‐SAD 15 S6 TI "winter depression" OR AB "winter depression" 24 S5 TI seasonal mood disorder* OR AB seasonal mood disorder* 16 S4 TI "seasonal depression" OR AB "seasonal depression" 43 S3 TI Seasonal Affective Disorder* OR AB Seasonal Affective Disorder* 287 S2 TI "Seasonal Affective Disorder" OR AB "Seasonal Affective Disorder" 276 S1 (MM "Seasonal Affective Disorder") 365
Web of Science (Web of Science Core Collection, BIOSIS, Data citation Index, KCI Korean Journal Database, MEDLINE, Russian Science Citation Database, SciELO Citation Index)* Data parameters: 1900 to Present Date searched: Tuesday, 19th June 2018 Hits: 489 #8 TOPIC ((#6 OR #5 OR #4 OR #3 OR #2 OR #1) Refined by: PUBLICATION YEARS (2018 OR 2017 OR 2016 OR 2015) 489 #7 TOPIC (#6 OR #5 OR #4 OR #3 OR #2 OR #1) 3819 #6 TOPIC (SIGH‐SAD) 84 #5 TOPIC ("winter depression") 790 #4 TOPIC (seasonal mood disorder*) 1525 #3 TOPIC ("seasonal depression") 267 #2 TOPIC (Seasonal Affective Disorder*) 3355 #1 TOPIC ("Seasonal Affective Disorder") 3007
Notes: In the 2015 review, which these searches update, Web of Knowledge was searched. Web of Knowledge (containing Web of Science, Current Contents Connect, Conference Proceedings Citation Index, BIOSIS, Derwent Innovations Index, Data Citation Index, SciELO Citation Index) has been discontinued. This search was the closest representation of the previous search.
Allied and Complementary Medicine Database (AMED) Data parameters: 1985 to June 2018 Date searched: Tuesday, 19th June 2018 Hits: 1 1 Seasonal Affective Disorder/ 0 2 "seasonal affective disorder".ti,ab,kw,ot. 28 3 seasonal affective disorder*.ti,ab,kw,ot. 28 4 "seasonal depression".ti,ab,kw,ot. 2 5 seasonal mood disorder*.ti,ab,kw,ot. 0 6 "winter depression".ti,ab,kw,ot. 4 7 SIGH‐SAD.ti,ab,kw,ot. 2 8 (1 or 2 or 3 or 4 or 5 or 6 or 7) 32 9 (2015* or 2016* or 2017* or 2018*).yr,ed. 22289 10 (8 and 9) 1
Trials registers WHO International Clinical Trials Registry Platform (ICTRP) searched via: http://apps.who.int/trialsearch/Default.aspx search date: Tuesday, 19th June 2018 seasonal n = 113 records for 49 trials. These records were visually inspected and 20 records were retained for screening SIGH‐SAD n = 0 ClinicalTrials.Gov searched via: https://www.clinicaltrials.gov/ct2/home search date: Tuesday, 19th June 2018 Records were visually inspected and records 2015‐current when exported to Endnote. Search field: Condition or Disease seasonal affective n = 3 seasonal depression n = 3 (being duplicates of the above) SIGH‐SAD n = 0
Data and analyses
Comparison 1. Bright light therapy versus no light therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of SAD (per protocol analysis) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Incidence of SAD (ITT, assuming no dropout was depressed) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Incidence of SAD (ITT, assuming all dropouts were depressed) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Incidence of severe SAD (per protocol analysis) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Incidence of severe SAD (ITT, assuming no dropout was depressed) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 Incidence of severe SAD (ITT, assuming all dropouts were depressed) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7 Overall rate of discontinuation | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 2. Infrared light therapy versus no light therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of SAD (per protocol analysis) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Incidence of SAD (ITT, assuming no dropout was depressed) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Incidence of SAD (ITT, assuming all dropouts were depressed) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Incidence of severe SAD (per protocol analysis) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Incidence of severe SAD (ITT, assuming no dropout was depressed) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 Incidence of severe SAD (ITT, assuming all dropouts were depressed) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7 Overall rate of discontinuation | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 3. Light therapy (bright white and infrared) versus no light therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of SAD (per protocol analysis) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Incidence of SAD (ITT, assuming no dropout was depressed) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Incidence of SAD (ITT, assuming all dropouts were depressed) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Incidence of severe SAD (per protocol analysis) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Incidence of severe SAD (ITT, assuming no dropout was depressed) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Incidence of severe SAD (ITT, assuming all dropouts were depressed) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Overall discontinuation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 4. Bright light therapy versus infrared light therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of SAD (per protocol) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Incidence of SAD (ITT, assuming no dropout was depressed) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 3 Incidence of SAD (ITT, assuming all dropouts were depressed) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 4 Incidence of severe SAD (per protocol) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 5 Incidence of severe SAD (ITT, assuming no dropout was depressed) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 6 Incidence of severe SAD (ITT, assuming all dropouts were depressed) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 7 Overall discontinuation | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Meesters 1999.
| Methods | Single‐centre, non‐blinded, randomised controlled trial (duration: 2 winter seasons 1993‐94 and 1994‐95, October‐April each season) conducted in the Netherlands. 8 dropouts (4 bright white visor light, 3 infrared visor light, 1 no light exposure) | |
| Participants | 46 adult outpatients with a history of SAD who were without symptoms at the beginning of the study and were free of drugs Bright white visor light group: n = 18, but participant characteristics of only 14 participants reported: 2 men, mean age 41 years (± 12.7), 12 women, mean age 39.5 years (± 9.3) Infrared visor light group: n = 18, but participant characteristics of only 15 participants reported: 5 men, mean age 35.4 years (± 6.9), 10 women, mean age 36.6 years (± 4.9) No light exposure group: n = 10, but participant characteristics of only 9 participants reported: 4 men, mean age 47.5 years (± 7), 5 women, mean age 39.4 years (± 8) No information about number of prior depressive episodes nor other participant characteristics |
|
| Interventions | Bright white visor light (n = 18; 30 minutes/d in the morning except on weekends) versus infrared visor light (n = 18; 30 minutes/d in the morning except on weekends) versus no light exposure (n = 10) from October until April | |
| Outcomes | Development of depression (BDI ≥ 13, SIGH‐SAD‐SR ≥ 20), development of severe depression (BDI ≥ 22, SIGH‐SAD‐SR ≥ 40) | |
| Notes | Study was not funded by pharmaceutical industry; however, equipment was sponsored by Bio Bright, Inc. Study was identified by searches of electronic databases |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about generation of random sequence provided |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded to treatment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | We assume that the outcome assessment was performed by the participants themselves: "patients stopped participating because of reasons unrelated to their illness, such as a lack of motivation to keep scoring self‐rating scales when in a healthy condition" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 17% of dropouts were not taken into account in the data analysis. No participant characteristics (e.g. age, sex) were reported for these 8 dropouts |
| Selective reporting (reporting bias) | Unclear risk | We could not identify a protocol for this study. Therefore, we rated this domain unclear |
| Other bias | High risk | Intervention was implemented by participants on their own at their homes |
BDI: Beck Depression Inventory SAD: Seasonal affective disorder SIGH‐SAD‐SR: Hamilton Depression Rating Scale‐Seasonal Affective Disorders self‐rating version
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Fleer 2014 | The TAU (treatment as usual) group received an invitation to light therapy when first depressive symptoms occurred. Therefore, this study did not fulfil the eligibility criteria for our review which focuses on prevention of SAD with light therapy, beginning the light therapy when participants are still free of symptoms. |
| Graw 1997 | SAD participants were treated with light therapy when they suffered a depressive episode. They were interviewed again 2 to 5 years later. Treatment started when participants already had depressive symptoms ‐ not when they were free of symptoms; therefore, the study was not relevant for this systematic review. |
| Kasper 1988 | Study included participants without depressive symptoms, but also without a history of SAD. As the study investigated preventive effects of light therapy on healthy participants, it was not relevant for this systematic review. |
| Kjellman 1997 | Conference abstract |
| Lafer 1994 | Study investigated treatment ‐ not prevention ‐ of SAD |
| Meesters 1991 | Study investigated whether starting light therapy at an early stage of a depressive episode can prevent a full‐blown winter depressive episode. Included participants already had depressive symptoms when the study started; therefore, the study was not relevant for this systematic review. |
| Meesters 1993 | Study investigated whether starting light therapy at an early stage of a depressive episode can prevent a full‐blown winter depressive episode. Included participants already had depressive symptoms when the study started; therefore, the study was not relevant for this systematic review. |
| Meesters 1994 | Study included participants with a history of SAD and investigated preventive effects of light therapy. However, the study included only 1 intervention group and no control group, therefore, the study was not relevant for this systematic review. |
| Meesters 1995 | Study investigated treatment ‐ not prevention ‐ of SAD |
| Most 2010 | Study investigated prevention of depression and sleep disturbances in the elderly with light therapy. Included participants in this study were diagnosed with major depressive disorder without a seasonal pattern. Therefore, the study was not relevant for this systematic review. |
| NCT00076245 | Study investigated acute and long‐term efficacy of cognitive‐behavioural therapy for SAD alone and in combination with light therapy as compared with solo light therapy. Included participants already had symptoms when interventions were started; therefore, the study was not relevant for this systematic review. |
| NCT01714050 | Study investigated relapse prevention of SAD and compared cognitive‐behavioural therapy, light therapy and a combination of these ‐ not prevention of SAD. Included participants already had symptoms when the study started. |
| Norden 2000 | Study investigated dawn simulation in participants with subsyndromal winter depression. Participants had no history of SAD. |
| Partonen 1995 | Study investigated recurrence ‐ not prevention ‐ of SAD. Included participants already had symptoms when the study started. |
| Partonen 1996 | Study included participants with a history of SAD and investigated preventive effects of light therapy. However, the study included only 1 intervention group and no control group; therefore, the study was not relevant for this systematic review. |
| Rohan 2007 | Study investigated acute cognitive‐behavioural therapy for SAD alone and in combination with light therapy as compared with solo light therapy. This was a treatment study ‐ not a prevention study. |
| Rohan 2009 | Study investigated recurrence of SAD after 1 year of cognitive‐behavioural therapy, light therapy and a combination of these. Included participants already had depressive symptoms when the study started; therefore, it was not relevant for this systematic review. |
| Rohan 2013 | Study investigated relapse prevention of SAD and compared cognitive‐behavioural therapy, light therapy and a combination of these ‐ not prevention of SAD. Included participants already had symptoms when the study started. |
| Terman 1994 | Study investigated whether starting light therapy at an early stage of a depressive episode can prevent a full‐blown winter depressive episode. Included participants already had depressive symptoms when the study started, therefore, the study was not relevant for this systematic review. |
| Thorell 1999 | Study investigated relapse prevention ‐ not prevention of SAD. Included participants already had symptoms when the study started. |
| WELL 100006 | Study investigated preventive effects of bupropion XL in participants with a history of SAD. It is included in the systematic review on efficacy and safety of second‐generation antidepressants; however, as it does not investigate efficacy nor safety of light therapy as preventive treatment, it is not relevant for this systematic review. |
| WELL AK130936 | Study investigated preventive effects of bupropion XL in participants with a history of SAD. It is included in the systematic review on efficacy and safety of second‐generation antidepressants; however, as it does not investigate efficacy nor safety of light therapy as preventive treatment, it is not relevant for this systematic review. |
| WELL AKA130930 | Study investigated preventive effects of bupropion XL in participants with a history of SAD. It is included in the systematic review on efficacy and safety of second‐generation antidepressants; however, as it does not investigate efficacy nor safety of light therapy as preventive treatment, it is not relevant for this systematic review. |
| Wirz‐Justice 1990 | Study investigated treatment ‐ not prevention ‐ of SAD. Included participants already had symptoms when the study started. |
SAD: seasonal affective disorder
Differences between protocol and review
In the protocol, we planned to contact the authors of publications to request missing results. As the only included study was published more than 20 years ago, we did not contact the study author.
Contributions of authors
BN and GG drafted and revised the review text; MvN ran grey literature searches. BG, DW and CF provided clinical expertise for the Background section. BN, GG, BG, CF, AG, LM, JW, LL screened records on title/abstract and full‐text level. BN, AG extracted data, BN, GG assessed the risk of bias of the included study and conducted the GRADE assessment. All authors reviewed the manuscript and provided feedback on individual drafts.
Sources of support
Internal sources
Internal funds of Cochrane Austria, Austria, Other.
External sources
No sources of support supplied
Declarations of interest
Barbara Nussbaumer‐Streit ‐ no conflict of interest
Catherine A Forneris ‐ no conflict of interest
Laura C Morgan ‐ no conflict of interest
Megan G Van Noord ‐ no conflict of interest
Bradley N Gaynes ‐ no conflict of interest
Amy Greenblatt ‐ no conflict of interest
Jörg Wipplinger ‐ no conflict of interest
Linda J Lux ‐ no conflict of interest
Dietmar Winkler ‐ has received lecture fees from Angelini Pharmaceuticals, Lundbeck Pharmaceuticals and Pro Mente Austria and has received authorship honoraria from Medizin Medien Austria.
Gerald Gartlehner ‐ no conflict of interest
Edited (no change to conclusions)
References
References to studies included in this review
Meesters 1999 {published data only}
- Meesters Y, Beersma DG, Bouhuys AL, Hoofdakker RH. Prophylactic treatment of seasonal affective disorder (SAD) by using light visors: bright white or infrared light?. Biological Psychiatry 1999;46:239‐46. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Fleer 2014 {published data only}
- Fleer J, Schroevers M, Panjer V, Geerts E, Meesters Y. Mindfulness‐based cognitive therapy for seasonal affective disorder: A pilot study. Journal of Affective Disorder 2014;168:205‐9. [DOI] [PubMed] [Google Scholar]
Graw 1997 {published data only}
- Graw P, Gisin B, Wirz‐Justice A. Follow‐up study of seasonal affective disorder in Switzerland. Psychopathology 1997;30:208‐14. [DOI] [PubMed] [Google Scholar]
Kasper 1988 {published data only}
- Kasper S, Rogers SL, Yancey AL, Schulz PM, Skwerer RG, Rosenthal NE. Phototherapy in subsyndromal seasonal affective disorder (S‐SAD) and "diagnosed" controls. Pharmacopsychiatry 1988;21:428‐9. [DOI] [PubMed] [Google Scholar]
Kjellman 1997 {published data only}
- Kjellman B, Lindwall‐Sundel K, Stain‐Malmgren R. The effect of prophylactic light therapy in SAD. Society Light Treatment Biological Rhythms 1997;9:24. [Google Scholar]
Lafer 1994 {published data only}
- Lafer B, Sachs GS, Labbate LA, Thibault A, Rosenbaum JF. Phototherapy for seasonal affective disorder: a blind comparison of three different schedules. American Journal of Psychiatry 1994;151:1081‐3. [DOI] [PubMed] [Google Scholar]
Meesters 1991 {published data only}
- Meesters Y, Lambers PA, Jansen JH, Bouhuys AL, Beersma DG, Hoofdakker RH. Can winter depression be prevented by light treatment?. Journal of Affective Disorders 1991;23:75‐9. [DOI] [PubMed] [Google Scholar]
Meesters 1993 {published data only}
- Meesters Y, Jansen JH, Beersma DG, Bouhuys AL, Hoofdakker RH. Early light treatment can prevent an emerging winter depression from developing into a full‐blown depression. Journal of Affective Disorders 1993;29:41‐7. [DOI] [PubMed] [Google Scholar]
Meesters 1994 {published data only}
- Meesters Y, Jansen JH, Beersma DG, Bouhuys AL, Hoofdakker RH. An attempt to prevent winter depression by light exposure at the end of September. Biological Psychiatry 1994;35:284‐6. [DOI] [PubMed] [Google Scholar]
Meesters 1995 {published data only}
- Meesters Y, Jansen JH, Beersma DG, Bouhuys AL, Hoofdakker RH. Light therapy for seasonal affective disorder. The effects of timing. British Journal of Psychiatry 1995;166:607‐12. [DOI] [PubMed] [Google Scholar]
Most 2010 {published data only}
- Most EI, Scheltens P, Someren EJ. Prevention of depression and sleep disturbances in elderly with memory‐problems by activation of the biological clock with light ‐ a randomized clinical trial. Trials 2010;11:11‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00076245 {published data only}
- NCT00076245. Cognitive behavioral approaches to seasonal depression. clinicaltrials.gov/ct2/show/NCT00076245 (first received 19 January 2004).
NCT01714050 {published data only}
- NCT01714050. Cognitive‐behavioral therapy vs. light therapy for preventing SAD recurrence. clinicaltrials.gov/ct2/show/NCT01714050 (first received 25 October 2012).
Norden 2000 {published data only}
- Norden MJ, Avery DH. Dawn simulation for subsyndromal winter depression. American Psychiatric Association. Abstracts. Paper No. 109A 2000.
Partonen 1995 {published data only}
- Partonen T, Lonnqvist J. The influence of comorbid disorders and of continuation light treatment on remission and recurrence in winter depression. Psychopathology 1995;28:256‐62. [DOI] [PubMed] [Google Scholar]
Partonen 1996 {published data only}
- Partonen T, Lonnqvist J. Prevention of winter seasonal affective disorder by bright‐light treatment. Psychological Medicine 1996;26:1075‐80. [DOI] [PubMed] [Google Scholar]
Rohan 2007 {published data only}
- Rohan KJ, Roecklein KA, Tierney Lindsey K, Johnson LG, Lippy RD, Lacy TJ, et al. A randomized controlled trial of cognitive‐behavioral therapy, light therapy, and their combination for seasonal affective disorder. Journal of Consulting and Clinical Psychology 2007;75:489‐500. [DOI] [PubMed] [Google Scholar]
Rohan 2009 {published data only}
- Rohan KJ, Roecklein KA, Lacy TJ, Vacek PM. Winter depression recurrence one year after cognitive‐behavioral therapy, light therapy, or combination treatment. Behavioral Therapy 2009;40:225‐38. [DOI] [PubMed] [Google Scholar]
Rohan 2013 {published data only}
- Rohan KJ, Evans M, Mahon JN, Sitnikov L, Ho S, Nillni YI, et al. Cognitive‐behavioral therapy vs. light therapy for preventing winter depression recurrence: study protocol for a randomized controlled trial. Trials 2013;14:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Terman 1994 {published data only}
- Terman JS, Terman M, Amira L. One‐week light treatment of winter depression near its onset: the time course of relapse. Depression and Anxiety 1994;2:20‐31. [Google Scholar]
Thorell 1999 {published data only}
- Thorell LH, Kjellman B, Arned M, Lindwall‐Sundel K, Walinder J, Wetterberg L. Light treatment of seasonal affective disorder in combination with citalopram or placebo with 1‐year follow‐up. International Clinical Psychopharmacology 1999;14 Suppl 2:S7‐11. [PubMed] [Google Scholar]
WELL 100006 {published data only}
- GlaxoSmithKline. A 7‐month, multicenter, randomized, double‐blind, placebo‐controlled comparison of 150‐300mg/day of extended‐release bupropion hydrochloride (WELLBUTRIN XL) and placebo for the prevention of seasonal affective disorder in subjects with a history of seasonal affective disorder followed by an 8‐week observational follow‐up phase. www.gsk‐studyregister.com/study/AK130936 2003.
- Modell JG, Rosenthal NE, Harriett AE, Krishen A, Asgharian A, Foster VJ, et al. Seasonal affective disorder and its prevention by anticipatory treatment with bupropion XL. Biological Psychiatry 2005;58:658‐67. [DOI] [PubMed] [Google Scholar]
WELL AK130936 {published data only}
- GlaxoSmithKline. A 7‐Month, Multicenter, Randomized, Double‐Blind, Placebo‐Controlled Comparison of 150‐300mg/day of Extended‐Release Bupropion Hydrochloride (WELLBUTRIN XL) and Placebo for the Prevention of Seasonal Affective Disorder in Subjects with a History of Seasonal Affective Disorder Followed by an 8‐Week Observational Follow‐up Study. GSK ‐ Clinical Study Register [www.gsk‐clinicalstudyregister.com] 2004.
- Modell JG, Rosenthal NE, Harriett AE, Krishen A, Asgharian A, Foster VJ, et al. Seasonal affective disorder and its prevention by anticipatory treatment with bupropion XL. Biological Psychiatry 2005;58:658‐67. [DOI] [PubMed] [Google Scholar]
WELL AKA130930 {published data only}
- GlaxoSmithKline. A 7‐Month, Multicenter, Randomized, Double‐Blind, Placebo‐Controlled Comparison of 150‐300mg/day of Extended‐Release Bupropion Hydrochloride (WELLBUTRIN XL) and Placebo for the Prevention of Seasonal Affective Disorder in Subjects with a History of Seasonal Affective Disorder Followed by an 8‐Week Observational Follow‐up Phase. GSK ‐ Clinical Study Register [www.gsk‐clinicalstudyregister.com] 2003.
- Modell JG, Rosenthal NE, Harriett AE, Krishen A, Asgharian A, Foster VJ, et al. Seasonal affective disorder and its prevention by anticipatory treatment with bupropion XL. Biological Psychiatry 2005;58:658‐67. [DOI] [PubMed] [Google Scholar]
Wirz‐Justice 1990 {published data only}
- Wirz‐Justice A, Graw P, Krauchi K, Gisin B, Arendt J, Aldhous M, et al. Morning or night‐time melatonin is ineffective in seasonal affective disorder. Journal of Psychiatric Research 1990;24:129‐37. [DOI] [PubMed] [Google Scholar]
Additional references
APA 1980
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd Edition. Washington, DC: American Psychiatric Association, 1980. [Google Scholar]
APA 1987
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 3rd Edition. Washington, DC: American Psychiatric Association, 1987. [Google Scholar]
APA 2000
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington, DC: American Psychiatric Association, 2000. [Google Scholar]
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th Edition. Washington, DC: American Psychiatric Association, 2013. [Google Scholar]
Begg 1994
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088‐101. [PubMed] [Google Scholar]
Berg 1997
- Berg TJ, Spekreijse H. Near infrared light absorption in the human eye media. Vision Research 1997;37:249‐53. [DOI] [PubMed] [Google Scholar]
Byrne 2008
- Byrne B, Brainard GC. Seasonal affective disorder and light therapy. Sleep Medicine Clinics 2008;3(2):307‐15. [Google Scholar]
Ciarleglio 2011
- Ciarleglio CM, Resuehr HES, McMahon DG. Interactions of the serotonin and circadian systems: nature and nurture in rhythms and blues. Neuroscience 2011;197:8‐16. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Forneris 2014
- Forneris CA, Nussbaumer B, Kaminski‐Hartenthaler A, Morgan LC, Gaynes BN, Sonis JH, et al. Psychological therapies for preventing seasonal affective disorder. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD011270.pub2] [DOI] [PubMed] [Google Scholar]
Gartlehner 2015
- Gartlehner G, Nussbaumer B, Gaynes BN, Forneris CA, Morgan LC, Kaminski‐Hartenthaler A, et al. Second‐generation antidepressants for preventing seasonal affective disorder in adults. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD011268.pub2] [DOI] [PubMed] [Google Scholar]
Golden 2005
- Golden RN, Gaynes BN, Ekstrom RD, Hamer RM, Jacobsen FM, Suppes T, et al. The efficacy of light therapy in the treatment of mood disorders: a review and meta‐analysis of the evidence. American Journal of Psychiatry 2005;162(4):656‐62. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [DOI] [PubMed] [Google Scholar]
Hamilton 1960
- Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry 1960;23:56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hansen 2009
- Hansen RA, Moore CG, Dusetzina SB, Leinwand BI, Gartlehner G, Gaynes B. Controlling for drug dose in systematic review and meta‐analysis: a case study of effect of antidepressant dose. Medical Decision Making 2009;29(1):91‐103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Joffe 1993
- Joffe RT, Moul DE, Lam RW, Levitt AJ, Teicher MH, Lebeque B, et al. Light visor treatment for seasonal affective disorder: a multicenter study. Psychiatry Research 1993;46(1):29‐39. [DOI] [PubMed] [Google Scholar]
Kaminski‐Hartenthaler 2015
- Kaminski‐Hartenthaler A, Nussbaumer B, Forneris CA, Morgan LC, Gaynes BN, Sonis JH, et al. Melatonin and agomelatine for preventing seasonal affective disorder. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD011271.pub2] [DOI] [PubMed] [Google Scholar]
Lam 1999
- Lam RW, Levitt AJ, editor(s). Canadian Consensus Guidelines for the Treatment of Seasonal Affective Disorder. Clinical & Academic Publishing, 1999. [Google Scholar]
Lam 2006
- Lam RW, Levitt AJ, Levitan RD, Enns MW, Morehouse R, Michalak EE, et al. The Can‐SAD study: a randomized controlled trial of the effectiveness of light therapy and fluoxetine in patients with winter seasonal affective disorder. American Journal of Psychiatry 2006;163(5):805‐12. [DOI] [PubMed] [Google Scholar]
Leon 1999
- Leon AC, Solomon DA, Mueller TI, Turvey CL, Endicott J, Keller MB. The Range of Impaired Functioning Tool (LIFE‐RIFT): a brief measure of functional impairment. Psychological Medicine 1999;29(4):869‐78. [DOI] [PubMed] [Google Scholar]
Levitan 2005
- Levitan RD. What is the optimal implementation of bright light therapy for seasonal affective disorder (SAD)?. Journal of Psychiatry and Neuroscience 2005;30:72. [PMC free article] [PubMed] [Google Scholar]
Levitan 2007
- Levitan RD. The chronobiology and neurobiology of winter seasonal affective disorder. Dialogues in Clinical Neuroscience 2007;9(3):315‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lewy 1987
- Lewy AJ, Sack RL, Miller LS, Hoban TM. Antidepressant and circadian phase‐shifting effects of light. Science 1987;235:352‐4. [DOI] [PubMed] [Google Scholar]
Lewy 2006
- Lewy AJ, Lefler BJ, Emens JS, Bauer VK. The circadian basis of winter depression. Proceedings of the National Academy of Sciences of the United States of America 2006;103:7414‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Magnusson 2005
- Magnusson A, Partonen T. The diagnosis, symptomatology, and epidemiology of seasonal affective disorder. CNS Spectrums 2005;10(8):625‐34. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. The PRISMA Group. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: The PRISMA Statement. PLoS Medicine 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pail 2011
- Pail G, Huf W, Pjrek E, Winkler D, Willeit M, Praschak‐Rieder N, et al. Bright‐light therapy in the treatment of mood disorders. Neuropsychobiology 2011;64:152‐62. [DOI] [PubMed] [Google Scholar]
Partonen 1998
- Partonen T, Lonnqvist J. Seasonal affective disorder. Lancet 1998;352:1369‐74. [DOI] [PubMed] [Google Scholar]
Quera‐Salva 2011
- Quera‐Salva MA, Hartley S, Barbot F, Alvarez JC, Lofaso F, Guilleminault C. Circadian rhythms, melatonin and depression. Current Pharmaceutical Design 2011;17:1459‐70. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rodin 1997
- Rodin I, Thompson C. Seasonal affective disorder. Advances in Psychiatric Treatment 1997;3:352‐9. [Google Scholar]
Rosen 1990
- Rosen LN, Targum SD, Terman M, Bryant MJ, Hoffman H, Kasper SF. Prevalence of seasonal affective disorder at four latitudes. Psychiatry Research 1990;31:131‐44. [DOI] [PubMed] [Google Scholar]
Rosenthal 1979
- Rosenthal R. The "file‐drawer problem" and tolerance for null results. Psychological Bulletin 1979;86:638‐41. [Google Scholar]
Rosenthal 1984
- Rosenthal NE, Sack DA, Gillin JC, Lewy AJ, Goodwin FK, Davenport Y, et al. Seasonal affective disorder. A description of the syndrome and preliminary findings with light therapy. Archives of General Psychiatry 1984;41(1):72‐80. [DOI] [PubMed] [Google Scholar]
Rosenthal 1993
- Rosenthal NE, Moul DE, Hellekson CJ, Oren DA, Frank A, Brainard GC, et al. A multicenter study of the light visor for seasonal affective disorder: no difference in efficacy found between two different intensities. Neuropsychopharmacology 1993;8(2):151‐60. [DOI] [PubMed] [Google Scholar]
Schwartz 1996
- Schwartz PJ, Brown C, Wehr TA, Rosenthal NE. Winter seasonal affective disorder: a follow‐up study of the first 59 patients of the National Institute of Mental Health Seasonal Studies Program. American Journal of Psychiatry 1996;153(8):1028‐36. [DOI] [PubMed] [Google Scholar]
Sohn 2005
- Sohn CH, Lam RW. Update on the biology of seasonal affective disorder. CNS Spectrums 2005;10(8):635‐46. [DOI] [PubMed] [Google Scholar]
Teicher 1995
- Teicher MH, Gold CA, Oren DA, Schwartz PJ, Luetke C, Brown C, et al. The phototherapy light visor: more to it than meets the eye. American Journal of Psychiatry 1995;152(8):1197‐202. [DOI] [PubMed] [Google Scholar]
Terman 2005
- Terman M, Terman JS. Light therapy for seasonal and nonseasonal depression: efficacy, protocol, safety, and side effects. CNS Spectrums 2005;10:647‐63. [DOI] [PubMed] [Google Scholar]
Thaler 2011
- Thaler K, Delivuk M, Chapman A, Gaynes BN, Kaminski A, Gartlehner G. Second‐generation antidepressants for seasonal affective disorder. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD008591.pub2] [DOI] [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473‐83. [PubMed] [Google Scholar]
Wells 2009
- Wells GA, Shea B, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. www.ohri.ca/programs/clinical_epidemiology/oxford.asp 2009.
Westrin 2007
- Westrin A, Lam RW. Long‐term and preventative treatment for seasonal affective disorder. CNS Drugs 2007;21(11):901‐9. [DOI] [PubMed] [Google Scholar]
Williams 2002
- Williams JBW, Link MJ, Rosenthal NE, Terman M. Structured Interview Guide for the Hamilton Depression Rating Scale ‐ Seasonal Affective Disorder version (SIGH‐SAD). New York: New York State Psychiatric Institute, 2012. [Google Scholar]
References to other published versions of this review
Nussbaumer 2014
- Nussbaumer B, Kaminski‐Hartenthaler A, Forneris CA, Morgan LC, Sonis JH, Gaynes BN, et al. Light therapy for preventing seasonal affective disorder. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD011269] [DOI] [PubMed] [Google Scholar]
Nussbaumer 2015
- Nussbaumer B, Kaminski‐Hartenthaler A, Forneris CA, Morgan LC, Sonis JH, Gaynes BN, et al. Light therapy for preventing seasonal affective disorder. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD011269.pub2] [DOI] [PubMed] [Google Scholar]


