Abstract
Background
People with chronic obstructive pulmonary disease (COPD) are at increased risk of pneumococcal disease, especially pneumonia, as well as acute exacerbations with associated morbidity and healthcare costs.
Objectives
To determine the efficacy of injectable pneumococcal vaccination for preventing pneumonia in persons with COPD.
Search methods
We searched the Cochrane Airways COPD Trials Register and the databases CENTRAL, MEDLINE and Embase, using prespecified terms. Searches are current to November 2016.
Selection criteria
We included randomised controlled trials (RCT) comparing injectable pneumococcal polysaccharide vaccine (PPV) or pneumococcal conjugated vaccine (PCV) versus a control or alternative vaccine type in people with COPD.
Data collection and analysis
We used standard Cochrane methodological procedures. For meta‐analyses, we subgrouped studies by vaccine type.
Main results
For this update, we added five studies (606 participants), meaning that the review now includes a total of 12 RCTs involving 2171 participants with COPD. Average age of participants was 66 years, male participants accounted for 67% and mean forced expiratory volume in one second (FEV1) was 1.2 L (five studies), 54% predicted (four studies). We assessed risks of selection, attrition and reporting bias as low, and risks of performance and detection bias as moderate.
Compared with control, the vaccine group had a lower likelihood of developing community‐acquired pneumonia (CAP) (odds ratio (OR) 0.59 , 95% confidence interval (CI) 0.41 to 0.85; six studies, n = 1372; GRADE: moderate), but findings did not differ specifically for pneumococcal pneumonia (Peto OR 0.26, 95% CI 0.05 to 1.31; three studies, n = 1158; GRADE: low). The number needed to treat for an additional beneficial outcome (NNTB) (preventing one episode of CAP) was 19 (95% CI 13 to 52). Mortality from cardiorespiratory causes did not differ between vaccine and control groups (OR 1.07, 95% CI 0.69 to 1.66; three studies, n = 888; GRADE: moderate), nor did all‐cause mortality differ (OR 1.00, 95% CI 0.72 to 1.40; five studies, n = 1053; GRADE: moderate). The likelihood of hospital admission for any cause, or for cardiorespiratory causes, did not differ between vaccine and control groups. Vaccination significantly reduced the likelihood of a COPD exacerbation (OR 0.60, 95% CI 0.39 to 0.93; four studies, n = 446; GRADE: moderate). The NNTB to prevent a patient from experiencing an acute exacerbation was 8 (95% CI 5 to 58). Only one study (n = 181) compared the efficacy of different vaccine types ‐ 23‐valent PPV versus 7‐valent PCV ‐ and reported no differences for CAP, all‐cause mortality, hospital admission or likelihood of a COPD exacerbation, but investigators described a greater likelihood of some mild adverse effects of vaccination with PPV‐23.
Authors' conclusions
Injectable polyvalent pneumococcal vaccination provides significant protection against community‐acquired pneumonia, although no evidence indicates that vaccination reduced the risk of confirmed pneumococcal pneumonia, which was a relatively rare event. Vaccination reduced the likelihood of a COPD exacerbation, and moderate‐quality evidence suggests the benefits of pneumococcal vaccination in people with COPD. Evidence was insufficient for comparison of different pneumococcal vaccine types.
Plain language summary
Do injectable pneumococcal vaccines prevent pneumonia in people with COPD?
We wanted to find out if pneumococcal vaccination for people with chronic obstructive pulmonary disease (COPD) reduces the risk of pneumonia and associated mortality. We found a total of 12 studies including 2171 participants. Evidence gathered in this review is current to December 2015.
Background
People with COPD are at increased risk of respiratory illness such as pneumonia due to a bacterium called Streptococcus pneumoniae, other community‐acquired pneumonias and acute COPD exacerbations. These illnesses increase mortality and are associated with increased healthcare costs.
Study characteristics
For this updated review, we identified five new studies (606 participants), bringing the total number of studies to 12, involving 2171 participants with COPD. The average age of participants was 66 years, 67% were male and participants had received a diagnosis of moderate to severe COPD. Eleven studies compared an injectable vaccine versus a control, and one study compared two different types of injectable vaccine.
Key results
People who were vaccinated were less likely to experience an episode of community‐acquired pneumonia; 19 people with COPD (95% confidence interval (CI) 13 to 52) would have to be vaccinated to prevent one episode of pneumonia. Vaccination made no difference in the risk of pneumococcal pneumonia due to S pneumoniae or in the chance of dying or of being admitted to hospital. People who were vaccinated were less likely to experience a COPD exacerbation; eight people with COPD (95% CI 5 to 58) would have to be vaccinated to prevent one person from having an acute exacerbation. We found no difference in effectiveness between the two types of injectable vaccine.
Quality of the evidence
Evidence in this review is generally independent and reliable, and we are moderately certain about the results.
Conclusions
In line with current guidance, this review suggests that all people with COPD should be given pneumococcal vaccination to provide some protection against community‐acquired pneumonia, and to reduce the chance of an acute exacerbation.
Summary of findings
Background
Description of the condition
Chronic obstructive pulmonary disease (COPD) is characterised by airflow obstruction that is not fully reversible. Data from 12 countries in the Burden of Lung Disease (BOLD) initiative show that more than 10% of adults have COPD at Stage II or higher, as defined by GOLD 2016. Prevalence and staging vary across countries between men and women (Buist 2007) and increase with age. Worldwide, COPD was the fifth‐ leading cause of death in 2011, and it was the seventh‐leading cause of lost disability‐adjusted life years (DALYs) (WHO 2013).
Exacerbations and comorbidities contribute to the variable natural history of COPD in individual patients (GOLD 2016). Exacerbations contribute to long‐term decline in lung function (Donaldson 2002) and reduced physical activity (Donaldson 2005). They have a profound and long‐lasting effect on quality of life (Groenewegen 2001; Seemungal 1998) and contribute to increased risk of death (Soler‐Cataluna 2005). Exacerbations are a major contributor to healthcare costs, especially for hospital admission (Wedzicha 2003).
The clinical onset of an acute exacerbation is defined according to symptoms, although definitions vary (Rodriguez‐Roisin 2000). Anthonisen defined type 1 exacerbations on the basis of three major symptoms: increased dyspnoea, sputum volume and sputum purulence. Type 2 exacerbations required two major symptoms, and type 3 exacerbations required one major symptom plus cough, wheeze or symptoms of an upper respiratory tract infection (Anthonisen 1987). A later definition required an increase in two 'major symptoms' of dyspnoea ‐ sputum volume and sputum purulence ‐ or an increase in one major symptom and in one 'minor symptom' for two days (wheeze, sore throat, cough or common cold symptoms) (Seemungal 2000). Researchers recently developed a standardised measure for assessing the frequency, severity and duration of exacerbations of COPD using patient‐reported outcomes as described in clinical studies (Leidy 2010).
Patients with COPD with persistent lower airway bacterial colonisation when stable are at increased risk of exacerbations (Bogaert 2004; Patel 2002). Infection is frequently detected during exacerbations; one study found that 48.4% of participants had viral causes and 54.7% had bacterial causes of infection (Papi 2006). Infection‐associated exacerbations required longer hospitalisation and resulted in greater impairment of lung function than exacerbations in which no infection was present (Papi 2006). Investigators in one study (Patel 2002) recovered Streptococcus pneumoniae (S pneumoniae) from the sputum of 33% of participants. Risk of exacerbations of COPD is increased among patients with pneumococcal colonisation (Bogaert 2004). Researchers have discovered an association between detection of S pneumoniae as a new organism in the sputum of patients with COPD and significantly increased risk of an exacerbation (Sethi 2002).
Pneumonia is usually a serious illness, and diagnosis is based on the presence of radiological infiltrates, symptoms (cough, expectoration, fever, dyspnoea, pleuritic pain, altered mental status), signs of pulmonary consolidation on auscultation and leukocytosis (Ochoa‐Gondar 2008). Community‐acquired pneumonia (CAP) is a major health problem among adults over 65 years of age (Welte 2009), and prevalence of 14 cases per 1000 person‐years (95% confidence interval (CI) 12.7 to 15.3) has been reported. Hospitalisation rate is high (75%), and in‐patient stays are often lengthy (mean 10.4 days) (Ochoa‐Gondar 2008). Overall mortality estimates are high: 6% in Canada, 20% in the USA and Spain, 13% in the UK and 8% in Sweden (File 2003; Mandell 2007). Patients with COPD who develop CAP have more severe pneumonia, are admitted to the intensive care unit more frequently and have significantly higher 30‐ and 90‐day mortality than non‐COPD patients (Molinos 2009; Restrepo 2006). S pneumoniae is the predominant pathogen among all patients with CAP (Mandell 2007) and among patients with COPD and CAP, for whom a 43% pneumococcal aetiology has been found (Lieberman 2002; Torres 1996). Progression from COPD to CAP has been shown to be strongly associated with the presence of S pneumoniae (57.3%), and other pathogens were predominant among exacerbations that did not progress to CAP (61.7%) (File 2009).
Description of the intervention
On the basis of differences in polysaccharide capsules, investigators have identified 91 different serotypes of S pneumoniae. Capsule polysaccharides have antiphagocytic activity, which affects the pathogenesis of invasive pneumococcal disease (IPD), including CAP (Postma 2012), and the incidence of IPD differs between serotypes. In the late 1970s, a 14‐valent pneumococcal polysaccharide vaccine (PPV‐14) was registered in the United States; this was replaced in the 1980s by a 23‐valent pneumococcal polysaccharide vaccine (PPV‐23) (Pneumovax/Pneumo 23) in the USA and Europe. This vaccine contains purified capsular antigens from 23 serotypes that cover 85% to 90% of cases of invasive pneumococcal disease among adults (ERS 2014). The vaccine induces T‐cell‐independent short‐lived B‐cell immune responses by causing B cells to differentiate into plasma cells, producing antibodies without producing memory B cells. The immunological antibody response is age‐ and serotype‐dependent and generally is lower among elderly people than in younger adults. A booster vaccination produces no memory response.
To enhance the immunogenicity of pneumococcal vaccines, researchers have developed conjugate vaccines. Polysaccharide antigens are chemically joined to a highly immunogenic protein carrier (such as tetanus or diphtheria toxoid). This process leads to the induction of B cell‐dependent and T cell‐dependent responses as well as a memory response to a booster dose of the vaccine. Healthcare providers have administered pneumococcal conjugate vaccine containing capsular polysaccharides from seven pneumococcal serotypes (PCV‐7) to young children since the 2000s, with a resulting striking decrease in invasive pneumococcal disease caused by vaccine serotypes. As children are the main reservoir of S pneumoniae (60% are carriers), a reduction in the carrier rate has had beneficial effects among children and a protective herd effect in adults (Moseley 2013).
Investigators are evaluating new conjugate vaccines, including 7‐valent (PCV‐7), 10‐valent (PCV‐10) and 13‐valent (PCV‐13) vaccines, for use in children and adults, although respiratory guidelines in Europe (ERS 2014) and Australia (COPDX 2016) recommend immunisation with the PPV‐23 polysaccharide pneumococcal vaccine for adults at risk of pneumococcal disease, including those with COPD. The PCV‐13 and the PCV‐10 are not recommended for patients with COPD in Australia (NHMRC 2013). Recommendations for age at immunisation and at revaccination vary depending on the guideline, with some recommending vaccination only for patients who are over 64 years of age, or for younger patients with severe COPD or comorbid conditions (GOLD 2016), and others recommending vaccination for all patients 50 years of age and older, along with revaccination five years later (NHMRC 2013).
How the intervention might work
Patients with COPD are able to mount a significant immune response to pneumococcal infection (Bogaert 2004); thus immunisation against pneumococcal infection may be effective in preventing bacterial growth in the airways of patients with COPD, in turn decreasing the occurrence of exacerbations and pneumonia.
Why it is important to do this review
Major COPD guidelines (COPDX 2016; ERS 2014; GOLD 2016; NICE 2010) have recommended pneumococcal vaccination, largely on the basis of results showing the efficacy of pneumococcal vaccination as reported by observational studies in general populations and by randomised controlled trials (RCTs) in people without COPD. Both a large indirect cohort study (Butler 1993) and a meta‐analysis (Fine 1994) of pneumococcal vaccination have confirmed protection against invasive bacteraemic disease, but efficacy remains to be assessed in the population with COPD, for which risks of CAP and of deterioration may be higher owing to exacerbations of the disease.
Objectives
To determine the efficacy of injectable pneumococcal vaccination for preventing pneumonia in persons with COPD.
Methods
Criteria for considering studies for this review
Types of studies
We included in this review only RCTs using injectable pneumococcal vaccines.
Types of participants
We included studies if participants were adults with a diagnosis of COPD, preferably based on objective diagnostic criteria: demonstration of airflow obstruction on spirometry, generally forced expiratory volume in one second (FEV1)/forced vital capacity (FVC) ratio less than 0.7 (GOLD 2016) and a significant smoking history. We included studies in which the proportion of participants with COPD was at least 80%, if the age of other participants matched that of participants with COPD.
Types of interventions
At least one injectable pneumococcal vaccine ‐ a pneumococcal polysaccharide vaccine or a pneumococcal conjugate vaccine or other vaccine type. The control group could be given placebo or no vaccination, or different types of pneumococcal vaccine for comparison.
Types of outcome measures
Primary outcomes
Pneumonia
Mortality, respiratory‐related and all‐cause
Healthcare utilisation, including hospital admissions and emergency department visits
Secondary outcomes
Acute exacerbations of COPD
Days of disability from respiratory illness variously defined as days in bed, days off work or days when the participant was unable to undertake normal activities
Lung function
Adverse effects of vaccination
Cost‐effectiveness of pneumococcal vaccination
Quality of life
Search methods for identification of studies
Electronic searches
We searched the Cochrane Airways Specialised Register up to 25 November 2016. The Information Specialist for the Group maintains the Cochrane Airways Specialised Register, which contains studies identified from several sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL), through the Cochrane Register of Studies Online (CRSO) (http://crso.cochrane.org/).
Weekly searches of MEDLINE Ovid SP.
Weekly searches of Embase Ovid SP.
Monthly searches of PsycINFO Ovid SP.
Monthly searches of the Cumulative Index to Nursing and Allied Health Literature (CINAHL) EBSCO.
Monthly searches of Allied and Complementary Medicine (AMED) EBSCO.
Handsearches of the proceedings of major respiratory conferences.
We identified studies included in the Trials Register by applying search strategies based on the scope of the Cochrane Airways Review Group. We have provided details of these strategies, as well as a list of handsearched conference proceedings, in Appendix 1. See Appendix 2 for search terms used to identify studies for this review.
We carried out additional searches of CENTRAL CRSO (searched 25 November 2016), MEDLINE Ovid (1946 to 23 November 2016) and Embase Ovid (1974 to 23 November 2016). We have listed in Appendix 3 the search strategies used for these databases. We applied no restrictions on language of publication.
Searching other resources
From full‐text papers obtained, we searched the bibliographic lists for additional articles. We also conducted a search of ClinicalTrials.gov (www.ClinicalTrials.gov) and the World Health Organization (WHO) trials portal (www.who.int/ictrp/en/) up to 25 November 2016 and pharmaceutical company clinical trial databases of companies manufacturing pneumococcal vaccines.
Data collection and analysis
Selection of studies
At least two review authors (JW, JT or RWB) assessed all potentially relevant trials for relevance by screening the full texts to independently select studies for inclusion and identified and recorded reasons for exclusion of ineligible studies. We resolved disagreements through discussion or, if required, we consulted a third review author. We identified and excluded duplicates and collated multiple reports of the same study, so that each study (rather than each report) was the unit of interest in the review. We recorded the selection process via a PRISMA (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses) flow diagram (Figure 2).
2.

Study flow diagram.
Data extraction and management
Two review authors (JT, JW) independently extracted study details and used a data collection form to record the following study characteristics and outcome data.
Methods: study design, total duration of study, number of study centres and locations, study setting, duration and date of study.
Participants: N, mean age, age range, gender, withdrawals, inclusion criteria and exclusion criteria.
Interventions: study treatment, comparison, cointerventions.
Outcomes: primary and secondary outcomes specified and collected, time points reported.
Notes: funding for trial, trial registration, notable conflicts of interest of trial authors.
The first review author entered data into Review Manager (version 5.3) (RevMan 2014), and a second review author double‐checked the data. We checked that data were entered correctly by comparing data presented in the systematic review against information provided in the study reports.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias for each study (JW, JT), using criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Cochrane Handbook). We resolved disagreements by discussion or by consultation with another review author. We assessed risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias(es).
We graded each potential source of bias as high, low or unclear and provided a quote from the study report, together with a justification for our judgement, in the 'Risk of bias' table. We summarised risk of bias judgements across different studies for each of the domains listed. When information on risk of bias was related to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table.
When considering treatment effects, we took into account the risk of bias for studies that contributed to those outcomes.
Measures of treatment effect
We analysed dichotomous outcomes by using Mantel‐Haenszel odds ratios (ORs) with 95% confidence intervals (CIs). When events were rare, we employed the Peto odds ratio. We entered scale data with a consistent direction of effect. For continuous variables, we analysed data as mean differences (MDs) with 95% CIs. We used standardised mean differences (SMDs) with 95% CIs if investigators had used different scales of measurement for a specific outcome. The SMD is a statistic that expresses differences in means between treatment groups in units of the pooled standard deviation.
We undertook meta‐analyses only when this was meaningful, that is, when treatments, participants and the underlying clinical question were similar.
When skewed data were available (reported as medians and interquartile ranges), we described them narratively.
For 'time‐to‐event' outcomes such as log hazard ratios, we used the fixed‐effect generic inverse variance outcome to combine results. This method yields a weighted average of effect estimates of separate studies (Cochrane Handbook, Chapter 9). We calculated the number needed to treat for an additional beneficial outcome from the pooled OR and its CI, using baseline risk in the control group.
Unit of analysis issues
We used participants as the unit of analysis when analysing dichotomous data.
Dealing with missing data
We contacted investigators to obtain missing numerical outcome data when possible (e.g. when a study was identified as abstract only). When this was not possible, and missing data were thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results by performing a sensitivity analysis.
Assessment of heterogeneity
We used a Breslow‐Day test to assess heterogeneity for pooled effects when the null hypothesis was that all studies were evaluating the same effect; we considered a P value > 0.05 to indicate significant differences between studies. In addition, we used the I2 statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than to chance (Higgins 2003). We interpreted statistical heterogeneity as follows: 0% to 40% might not be important, 30% to 60% may represent moderate heterogeneity and 50% to 90% may represent substantial heterogeneity (Cochrane Handbook).
We assessed clinical and methodological heterogeneity by recording differences in study design and participant characteristics between individual studies. When we found substantial heterogeneity. we reported this and explored possible causes by conducting prespecified subgroup analyses.
Assessment of reporting biases
We tried to minimise reporting bias resulting from non‐publication of studies or from selective outcome reporting by using a broad search strategy, checking references of included studies and relevant systematic reviews and contacting study authors to ask for additional outcome data. We visually inspected funnel plots when 10 or more studies contributed to the analysis for a specific outcome.
Data synthesis
We combined studies to compare the following.
Comparison 1: pneumococcal polysaccharide vaccine, 23‐valent (PPSV‐23) OR 14‐valent (PPV‐14), versus control.
Comparison 2: 23‐valent pneumococcal polysaccharide vaccine (PPV‐23) versus 7‐valent diphtheria‐conjugated pneumococcal polysaccharide vaccine (PCV‐7).
We used a fixed‐effect model, but we performed a sensitivity analysis by using a random‐effects model if we detected unexplained heterogeneity. We presented the findings of our primary outcomes in a 'Summary of findings' table according to recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Cochrane Handbook) (generated with the use of GradePro software).
Subgroup analysis and investigation of heterogeneity
If heterogeneity was not sufficiently accounted for by study quality, we specified the following subgroup analyses a priori.
Vaccine type ‐ the number of capsular polysaccharide antigens used in the vaccine (more than 14 vs 14 or fewer).
Severity of COPD (assessed by lung function: mild = FEV1 50% to 79% predicted, moderate = FEV1 35% to 49% predicted, severe = FEV1< 35% predicted).
Setting of the study.
Match between strain of vaccine and infecting strains.
Age of participants.
Sensitivity analysis
In assessing heterogeneity, we considered possible causes associated with details of study design. We performed sensitivity analyses using random‐effects models versus a fixed‐effect model to examine risk of bias and other potential confounders, and to evaluate studies published only as abstracts.
Results
Description of studies
Results of the search
From searches for the original 2004 review, we included two studies (Davis 1987; Leech 1987), and in 2010, we included five additional studies (Alfageme 2006; Furumoto 2008; Steentoft 2006; Teramoto 2007 (published conference abstract); Ya Tseimakh 2006 (published conference abstract)). Through searches conducted for this 2016 review (current to 25 November 2016) (Figure 2), we identified 157 unique new citations, assessed 20 for eligibility, and added five to this review (Dransfield 2009; Kostinov 2014; Lin 2013; Trofimov 2010 (published conference abstract); Yilmaz 2013).
We have listed the reasons for exclusion of studies in the Characteristics of excluded studies table.
Included studies
For specific details of each study included in the review, see the Characteristics of included studies table.
We included in this review 12 RCTs of pneumococcal vaccines for a total of 2171 participants that provided outcome data for COPD. When studies included participants with other diagnoses, such as Furumoto 2008, we included only data for participants with COPD. Average duration of follow‐up was 14 months. Two studies (Steentoft 2006; Trofimov 2010) reported follow‐up for six months; three studies (Kostinov 2014; Lin 2013; Ya Tseimakh 2006) follow‐up for 12 months; four studies (Furumoto 2008; Leech 1987; Teramoto 2007; Yilmaz 2013) follow‐up for 24 months; two studies (Alfageme 2006; Davis 1987) follow‐up for 32 months and one study (Dransfield 2009) follow‐up for 48 months.
Study setting and participants
All studies were conducted in a community setting and were randomised, parallel‐group trials (Table 2). Participants (n = 2171) had a diagnosis of COPD that was based on spirometric criteria (Alfageme 2006; Dransfield 2009; Kostinov 2014; Steentoft 2006);clinical or spirometric criteria (Davis 1987); a clinical diagnosis of COPD (Furumoto 2008; Lin 2013; Teramoto 2007; Ya Tseimakh 2006; Yilmaz 2013); or a diagnosis not specified (Trofimov 2010). A common exclusion criterion was previous pneumococcal vaccination. The average age of study participants was 66 years, and the percentage of male participants was 67%(range 36% to 98%). When data could be extracted, the mean FEV1 was 1.2 L (five studies), 54% of predicted (four studies). Information on participants’ treatment with inhaled corticosteroids was available only for Dransfield 2009 (65%) and Lin 2013 (100%); in Steentoft 2006, 24% of participants were taking oral corticosteroids.
1. Comparison of studies.
| Study ID (n) | Vaccine 1 | Comparison | Setting/Follow‐up, months | Mean age/% male | Mean FEV1 (L) or % predicted | % AE 12 months | % ICS | % prior pneumonia | % current smokers |
| Alfageme 2006 (n = 600) | 23‐valent PPV | No vaccine | Seville, Spain/32 median | 69/98 | 1.2 ± 0.8 | NA | NA | 18 | 24 |
| Davis 1987 (n = 103) | 14‐valent PPV 0.5 mL SC | Saline 0.5 mL SC | New York, USA/24 to 32 | 63/NA | 1.4 ± 0.7 | NA | NA | 26 | 43 |
| Dransfield 2009 (n = 181) | 23‐valent PPV | 7‐valent PCV | USA 21 centres/48 | 64/37 | 45% | 15 | 65 | 45 | 36 |
| Furumoto 2008 (n = 55 with COPD ) | 14‐valent PPV + influenza | Influenza | Kyushu & Okinawa, Japan/24 | 69/64 | NA | NA | NA | NA | NA |
| Kostinov 2014 (Russian paper) (n = 200) | 23‐valent PPV | No vaccine | Russia/12 | 30‐70/36 | NA | NA | NA | NA | NA |
| Leech 1987 (n = 189) | 14‐valent PPV + influenza | Saline + influenza | Montreal Canada/24 | 68/71 | 0.95 ± 0.3 | NA | NA | NA | NA |
| Lin 2013 (abstract & poster) (n = 36) | 23‐valent PPV | Saline | Taipei, Taiwan/12 | 71/89 | 1% to 45% | > 50 | 100 (> 1500 mcg/d) | > 50 | 37 |
| Steentoft 2006 (n = 49) | 23‐valent PPV 0.5 mL SC | No vaccine | Denmark/6 | 65‐72/55 | 0.8 to 1.2 | NA | OCS 24% | NA | 46 |
| Teramoto 2007 (Abstract) (n = 196) | 23‐valent PPV | No vaccine | Japan/24 | 78/NA | NA | NA | NA | NA | NA |
| Trofimov 2010 (Russian paper) (n = 45) | 23‐valent PPV | No vaccine | Russia/6 | 55/67 | 62% | NA | NA | NA | NA |
| Ya Tseimakh 2006 (abstract) (n = 373) | 23‐valent PPV | No vaccine | Russia/6 | 69/57 | 62% | 100 | OCS not allowed | NA | 60 |
| Yilmaz 2013 (abstract & unpublished paper) (n = 144) | 23‐valent PPV | Placebo | Turkey & UK/24 | 65/93 | 1.4 L ± 0.6 | NA | NA | NA | NA |
AE = acute exacerbation of COPD.
ICS = inhaled corticosteroids.
OCS = oral corticosteroids.
PCV = diphtheria‐conjugated pneumococcal polysaccharide vaccine.
PPV = pneumococcal polysaccharide vaccine.
Intervention and comparison
Vaccine type
Investigators used a 23‐valent pneumococcal polysaccharide vaccine in Alfageme 2006, Dransfield 2009, Kostinov 2014, Lin 2013, Steentoft 2006, Teramoto 2007, Trofimov 2010, Ya Tseimakh 2006 and Yilmaz 2013, and a 14‐valent pneumococcal polysaccharide vaccine in Davis 1987, Furumoto 2008 and Leech 1987.
Treatment groups in Leech 1987 and Furumoto 2008 also received influenza vaccine.
Comparison
Control groups in Leech 1987 and Furumoto 2008 received the same influenza vaccine as the intervention group.
Control groups in Davis 1987, Lin 2013 and Yilmaz 2013 received a placebo injection.
Researchers in Alfageme 2006, Kostinov 2014, Steentoft 2006, Teramoto 2007, Trofimov 2010 and Ya Tseimakh 2006, withheld vaccine from the control group and did not administer a placebo.
Dransfield 2009 used a different vaccine in the comparison group ‐ a 7‐valent diphtheria‐conjugated pneumococcal polysaccharide vaccine.
In all studies, investigators administered injections subcutaneously.
Outcome measurement
Eight studies reported data on participants experiencing one or more episodes of pneumonia ‐ but not all episodes were confirmed as due to pneumococcal infection (Alfageme 2006; Davis 1987; Dransfield 2009; Furumoto 2008; Leech 1987; Lin 2013; Steentoft 2006; Teramoto 2007). The basis for the diagnosis of pneumonia was radiological AND included clinical symptoms/signs in Alfageme 2006, Davis 1987, Leech 1987 and Steentoft 2006; was radiological OR included clinical symptoms/signs in Furumoto 2008 and Lin 2013; and was self‐reported by participants in Dransfield 2009.
Excluded studies
Of 100 excluded citations, 35 were reviews/commentary articles, 41 were not of RCT design, 18 included non‐COPD participants or did not provide their data separately and six provided an intervention that was not an injectable pneumococcal vaccine. Individual reasons for exclusion of studies are listed in the Characteristics of excluded studies table.
Risk of bias in included studies
Review authors assessed the quality of the 12 studies included in the review against six criteria and provide a summary of results in Figure 3 and Figure 4.
3.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
4.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Allocation
Allocation generation
Overall risk of selection bias due to allocation generation was moderate. Six of the 12 studies did not report their methods for random sequence generation (Leech 1987; Lin 2013; Teramoto 2007; Trofimov 2010; Ya Tseimakh 2006; Yilmaz 2013). All of the remaining trials had low risk of bias. Methods for random sequence generation varied by study. Four studies used random number tables, one performed random number generation in blocks of 10 (Alfageme 2006) and another conducted randomisation centrally online (Dransfield 2009).
Allocation concealment
Overall risk of selection bias due to allocation concealment was moderate. However, nine of the 12 studies did not report their methods for allocation concealment (Alfageme 2006; Davis 1987; Kostinov 2014; Leech 1987; Lin 2013; Teramoto 2007; Trofimov 2010; Ya Tseimakh 2006; Yilmaz 2013). The remaining three had low risk of bias. Allocation concealment methods included third party randomisation and sequentially numbered, opaque, sealed envelopes.
Blinding
Overall risk of performance bias and detection bias was moderate, with three studies at particularly high risk of bias (Furumoto 2008; Trofimov 2010; Ya Tseimakh 2006). Two had low risk of bias (Alfageme 2006; Davis 1987), and nine could not be adequately assessed for risk.
Of the 12 studies, two were double‐blind (Davis 1987; Leech 1987), three were single‐blind (Alfageme 2006; Leech 1987; Yilmaz 2013), two were open‐label (Dransfield 2009; Trofimov 2010) and five did not describe the use of blinding. Among double‐blind trials, only Davis 1987 adequately described the method of blinding used. Of three single‐blind trials, Leech 1987 blinded participants, Alfageme 2006 blinded assessors and Yilmaz 2013 did not indicate who was blinded. We could not perform sensitivity analysis for Dransfield 2009, as it was the only study that compared PPSV‐23 versus PCV‐7. However, sensitivity analysis for the outcome of acute COPD exacerbation for Trofimov 2010 showed little change in the direction of effect.
Six of the 12 studies (Alfageme 2006; Kostinov 2014; Steentoft 2006; Teramoto 2007; Trofimov 2010; Ya Tseimakh 2006) did not use any form of placebo; Dransfield 2009 used PCV‐7 as a comparator. Sensitivity analysis for the primary outcome of pneumonia with exclusion of these studies showed a shift in effect direction, although the OR remained of no statistical significance (OR 0.78, 95% CI 0.16 to 3.68). For acute exacerbations of COPD, data showed no shift in effect direction nor in OR significance, with a wider CI (OR 0.41, 95% CI 0.18 to 0.92). We noted similar findings for all‐cause mortality (OR 0.95, 95% CI 0.48 to 1.86) and all‐cause hospital admissions (OR 0.80, 95% CI 0.21 to 3.13).
Incomplete outcome data
Overall risk of attrition bias was low. Six of the 12 studies managed to adequately address incomplete outcomes, with no unequal rates across groups and with adequate reasons provided for drop‐outs and losses to follow‐up (Alfageme 2006; Davis 1987; Dransfield 2009; Furumoto 2008; Kostinov 2014; Lin 2013).
Selective reporting
Overall risk of reporting bias was very low. Nine of the 12 studies adequately addressed all primary and secondary outcomes (Alfageme 2006; Davis 1987; Dransfield 2009; Furumoto 2008; Kostinov 2014; Leech 1987; Lin 2013; Steentoft 2006; Yilmaz 2013).
Other potential sources of bias
Of the 12 studies, 11 did not display other types of bias (Alfageme 2006; Davis 1987; Furumoto 2008; Kostinov 2014; Leech 1987; Lin 2013; Steentoft 2006; Teramoto 2007; Trofimov 2010; Ya Tseimakh 2006; Yilmaz 2013). The only study that displayed unclear risk was Dransfield 2009. As this study relied in part on self‐reported vaccination, some participants may have been misclassified as vaccine‐naive or previously vaccinated; or may have been enrolled within five years after the previous vaccination dose.
Effects of interventions
See: Table 1
Summary of findings for the main comparison. Pneumoccocal vaccination to prevent pneumonia in chronic obstructive pulmonary disease?
| Is pneumococcal vaccination effective in preventing pneumonia in chronic obstructive pulmonary disease? | ||||||
| Patient or population: patients with COPD Setting: community Intervention: pneumococcal vaccine Comparison: control | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with control | Risk with pneumococcal vaccine | |||||
| Pneumonia, community acquired, at least 1 episode Follow‐up: range 6 to 36 months | 148 per 1000 | 93 per 1000 (67 to 129) | OR 0.59 (0.41 to 0.85) | 1372 (6 RCTs) | ⊕⊕⊕⊝ Moderatea | Study limitations with lack of participant blinding and no use of placebo in 3 studies. NNTB to prevent 1 episode of CAP = 19 (95% CI 13 to 52) |
| Pneumococcal pneumonia, at least 1 episode Follow‐up: range 6 to 36 months | 11 per 1000 | 3 per 1000 (1 to 14) | OR 0.26 (0.05 to 1.31) | 1158 (3 RCTs) | ⊕⊕⊝⊝ Lowb,c | Very few confirmed episodes of pneumococcal pneumonia. Rate of pneumococcal CAP to total CAP from 2008 to 2013 varied from 17.1% to 37.3% of cases (Rodrigo 2015). |
| Death from cardiorespiratory causes Follow‐up: range 24 to 48 months | 98 per 1000 | 104 per 1000 (70 to 153) | OR 1.07 (0.69 to 1.66) | 888 (3 RCTs) | ⊕⊕⊕⊝ Moderated | |
| Death from all causes Follow‐up: range 12 to 48 months | 165 per 1000 | 165 per 1000 (125 to 217) | OR 1.00 (0.72 to 1.40) | 1053 (5 RCTs) | ⊕⊕⊕⊝ Moderated | |
| Hospital admission: any cause, at least 1 episode Follow‐up: range 6 to 12 months | 86 per 1000 | 65 per 1000 (29 to 140) | OR 0.74 (0.32 to 1.74) | 391 (3 RCTs) | ⊕⊕⊕⊝ Moderated | |
| COPD exacerbation: at least 1 episode Follow‐up: range 6 to 24 months | 608 per 1000 | 482 per 1000 (377 to591) | OR 0.60 (0.39 to 0.93) | 446 (4 RCTs) | ⊕⊕⊕⊝ Moderatea | Study limitations with lack of or unclear participant blinding in 3 studies. NNTB = 8 (95% CI 5 to 58); see Figure 1 |
| Lung function: FEV1 (L) Follow‐up: 12 months | Mean lung function: FEV1 (L) was 1.43 L | Mean lung function: FEV1 (L) in the intervention group was 0.12 L lower (7.17 lower to 6.93 greater) | ‐ | 142 (1 RCT) | ⊕⊕⊝⊝ Lowd,e | No difference in lung function seen at 3 or 24 months in 1 study |
| *The risk in the intervention group (and its 95% confidence interval) is based on assumed risk in the comparison group and relative effect of the intervention (and its 95% CI). CI: confidence interval; NNTB: number needed to treat for an additional beneficial outcome; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence. High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aStudy limitations increase risk of performance and detection bias.
bsubstantial heterogeneity present.
cWide confidence interval; few events in 2 studies, no events in 1 study.
dWide confidence interval; effect size includes the null.
eSingle study.
Comparison 1: pneumococcal polysaccharide vaccine, 23‐valent (PPSV‐23) OR 14‐valent (PPV‐14), versus control (11 studies; N = 2125)
Primary outcomes
Pneumonia
Analysis 1.1: likelihood of at least one episode of community‐acquired pneumonia (CAP): We found six relevant studies (n = 1372) with follow‐up ranging from six to 36 months. Results showed a statistically significant difference with lower likelihood for vaccine compared with control (subgrouped by vaccine number of serotypes) (OR 0.59, 95% 0.41 to 0.85) and no heterogeneity (Figure 5). Subgroup analysis of likelihood of CAP by lung function was possible only with data from Alfageme 2006 (Analysis 3.1) for participants with FEV1 < 40% predicted at baseline (OR 0.48, 95% CI 0.23 to 1.00) and for participants with FEV1 ≥ 40% predicted (OR 1.12, 95% CI 0.50 to 2.48). A test for subgroup differences was not statistically significant: Chi² = 2.36, df = 1 (P = 0.12), I² = 57.6%.
1.1. Analysis.

Comparison 1 Pneumococcal vaccine versus control, Outcome 1 Community‐acquired pneumonia: at least 1 episode.
5.

Forest plot of comparison: 1 Pneumococcal vaccine versus control, outcome: 1.1 Community‐acquired pneumonia: at least 1 episode.
3.1. Analysis.

Comparison 3 Analysis by follow‐up period/subgroup, Outcome 1 Pneumonia by lung function at baseline.
Analysis 1.2: rate of CAP per person‐year: For this outcome, we found one relevant trial with 12 months of follow up (n = 36). Investigators reported no significant differences between vaccine and control groups (risk ratio (RR) 0.37, 95% CI 0.12 to 1.14).
1.2. Analysis.

Comparison 1 Pneumococcal vaccine versus control, Outcome 2 Community‐acquired pneumonia: rate per person‐year.
Analysis 1.3: likelihood of at least one episode of pneumococcal pneumonia: We found three relevant trials with follow‐up ranging from six to 36 months (n = 1158). Results showed no significant differences between vaccine and control groups (subgrouped by vaccine number of serotypes) (Peto OR 0.26, 95% CI 0.05 to 1.31) (Figure 6). Heterogeneity was substantial: Chi² = 3.44, df = 1 (P = 0.06), I² = 71%; and the test for subgroup differences approached significance: Chi² = 3.44, df = 1 (P = 0.06), I² = 70.9%.
1.3. Analysis.

Comparison 1 Pneumococcal vaccine versus control, Outcome 3 Pneumococcal pneumonia: at least 1 episode.
6.
Forest plot of comparison: 1 Pneumococcal vaccine versus control, outcome: 1.4 Death from cardiorespiratory causes.
Mortality
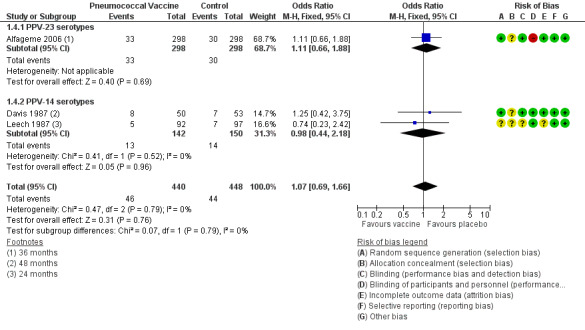
Analysis 1.4: death from cardiorespiratory causes: We found three relevant studies, with follow‐up ranging from 24 to 48 months (n = 888). Results showed no significant differences in likelihood between vaccine and control groups (subgrouped by vaccine number of serotypes) (OR 1.07, 95%CI 0.69 to 1.66) (Figure 6) and no heterogeneity.
1.4. Analysis.

Comparison 1 Pneumococcal vaccine versus control, Outcome 4 Death from cardiorespiratory causes.
Analysis 1.5: death from all causes: We found five relevant trials with follow‐up ranging from 12 to 48 months (n = 1053). Results revealed no significant differences in likelihood between vaccine and control groups (subgrouped by vaccine number of serotypes) (OR 1.00, 95% CI 0.72 to 1.40) and no heterogeneity.
1.5. Analysis.

Comparison 1 Pneumococcal vaccine versus control, Outcome 5 Death from all causes.
Healthcare utilisation
Analysis 1.6: likelihood of at least one episode of hospital admission for any cause: We found three relevant studies with follow‐up ranging from three to 12 months (n = 391). Results showed no significant differences in likelihood between vaccine and control groups (OR 0.74, 95% CI 0.32 to 1.74) and no heterogeneity. When we included the 24‐month follow‐up period for Yilmaz 2013, which was affected by a greater number of withdrawals (Analysis 3.2), the result was similar (OR 0.54, 95% 0.23 to 1.22).
1.6. Analysis.

Comparison 1 Pneumococcal vaccine versus control, Outcome 6 Hospital admission, any cause: at least 1 episode.
3.2. Analysis.

Comparison 3 Analysis by follow‐up period/subgroup, Outcome 2 Hospital admission, any cause: by follow‐up periods.
Analysis 1.7: rate of cardiorespiratory‐related hospital admissions: We found one relevant study (Leech 1987; n = 160) that reported no significant differences between vaccine and control groups for follow‐up between seven and 12 months (RR 0.89, 95% CI 0.51 to 1.58) nor any differences for longer follow‐up periods of 13 to 18 months and 19 to 24 months (Analysis 3.3).
1.7. Analysis.

Comparison 1 Pneumococcal vaccine versus control, Outcome 7 Hospital admission: cardiorespiratory‐related.
3.3. Analysis.

Comparison 3 Analysis by follow‐up period/subgroup, Outcome 3 Hospital admission, cardiorespiratory‐related: by follow‐up periods.
Analysis 1.8: rate of all‐cause hospital admissions: We found one relevant study with 12 months of follow‐up (n = 36). Results showed no significant differences between vaccine and control groups (RR 0.84, 95% CI 0.26 to 2.71).
1.8. Analysis.

Comparison 1 Pneumococcal vaccine versus control, Outcome 8 Hospital admission: all‐cause.
Analysis 1.9: likelihood of at least one emergency department (ED) visit for any cause: We found one relevant study (Yilmaz 2013) with follow‐up between three and 12 months (n = 142). Results showed statistically significant differences, with lower likelihood for vaccine compared with control (OR 0.26, 95% CI 0.07 to 0.91); results for a long‐term follow‐up period of 12 to 24 months were similar (Analysis 3.4). Another single study (Leech 1987) reported ED visits due to respiratory causes, upper respiratory tract infection (URTI), lower respiratory tract infection (LRTI) and pneumonia and described no significant differences with vaccination (Analysis 3.5).
1.9. Analysis.

Comparison 1 Pneumococcal vaccine versus control, Outcome 9 ED visit, any cause: at least 1 episode.
3.4. Analysis.

Comparison 3 Analysis by follow‐up period/subgroup, Outcome 4 Emergency department visit, any cause: by follow‐up period.
3.5. Analysis.

Comparison 3 Analysis by follow‐up period/subgroup, Outcome 5 Emergency visits (by cause).
Secondary outcomes
Analysis 1.10: likelihood of at least one episode of COPD exacerbation: For this outcome, we found four relevant studies (n = 446), with varying durations of follow‐up: six months for Steentoft 2006, 12 months for Kostinov 2014 and Yilmaz 2013 and 24 months for Furumoto 2008. Results showed a statistically significant difference with lower likelihood for vaccine than for control (OR 0.60, 95% CI 0.39 to 0.93) (Figure 7 and Figure 1), with no heterogeneity. When we used the 24‐month follow‐up period for Yilmaz 2013, which was affected by a greater number of withdrawals, the result was similar (Analysis 3.6) but showed greater heterogeneity (OR 0.53, 95% CI0.34 to 0.81; Chi² = 5.66, df = 3 (P = 0.13), I² = 47%).
1.10. Analysis.

Comparison 1 Pneumococcal vaccine versus control, Outcome 10 At least 1 COPD exacerbation.
7.

Forest plot of comparison: 1 Pneumococcal vaccine versus control, outcome: 1.1 At least 1 COPD exacerbation.
1.

In the control group, 608 out of 1000 people had one or more exacerbations over 6 to 24 months, compared with 482 (95% CI 377 to 591) out of 1000 for the active treatment group.
3.6. Analysis.

Comparison 3 Analysis by follow‐up period/subgroup, Outcome 6 At least 1 COPD exacerbation: varying follow‐up.
Analysis 1.11: COPD exacerbations: For this outcome, we found one relevant study with six months of follow‐up (n = 373). Results showed a significant difference between vaccine and control groups (mean difference (MD) ‐0.59 episodes, 95% CI ‐0.80 to ‐0.38).
1.11. Analysis.

Comparison 1 Pneumococcal vaccine versus control, Outcome 11 COPD exacerbation rate.
Analysis 1.12: rate of COPD exacerbations per person‐year: For this outcome, we found one relevant study with 12 months of follow‐up (n = 36). Results showed no significant differences between vaccine and control groups (RR 0.87, 95% CI 0.44 to 1.72).
1.12. Analysis.

Comparison 1 Pneumococcal vaccine versus control, Outcome 12 COPD exacerbations: rate/person‐year.
Lung function
Analysis 1.13: FEV1: We found one relevant study with follow‐up of 24 months (n = 144). Results showed no significant differences between vaccine and control groups for measurements taken at three, 12 and 24 months.
1.13. Analysis.

Comparison 1 Pneumococcal vaccine versus control, Outcome 13 Lung function: FEV1 (L).
Health‐related quality of life
Analysis 1.14: St George's Respiratory Questionnaire (SGRQ) overall score: We found one relevant study with follow‐up of 24 months (n = 144). Results showed no significant differences between vaccine and control groups for measurements taken at three, 12 and 24 months.
1.14. Analysis.

Comparison 1 Pneumococcal vaccine versus control, Outcome 14 Quality of life: SGRQ overall.
Adverse effects
No data were available for meta‐analysis. Adverse effects reported after vaccination in Ya Tseimakh 2006 included erythema and induration observed in 22% and fever and headache in 5%.
Leech 1987 stated that "there were no adverse reactions to pneumococcal vaccine", and study authors for Alfageme 2006 indicated that "no patient reported any local or systemic reaction to the vaccine".
Sensitivity analysis
In sensitivity analysis of the likelihood of community‐acquired pneumonia with removal of studies available only as conference abstracts, and with Teramoto 2007 and Ya Tseimakh 2006 excluded, effect size was lessened and became non‐significant and heterogeneity was eliminated, although the direction of effect remained the same (OR 0.80, 95% CI 0.51 to 1.25; four studies, n = 803).
Comparison 2: 23‐valent pneumococcal polysaccharide vaccine (PPV‐23) versus 7‐valent diphtheria‐conjugated pneumococcal polysaccharide vaccine (PCV‐7); (one study; N = 181)
Only one study (n = 181) compared 23‐valent pneumococcal polysaccharide vaccine (PPSV‐23) with 7‐valent diptheria‐conjugated pneumococcal polysaccharide vaccine (PCV‐7) (Dransfield 2009). The follow‐up period was 48 months. This study found no statistically significant differences in likelihood between the two vaccines in terms of:
Analysis 2.1: incidence of community‐acquired pneumonia (OR 1.01, 95% CI 0.40 to 2.56);
Analysis 2.2: all‐cause mortality (OR 1.83, 95% CI 0.5 to 6.50);
Analysis 2.3: hospital admission (OR 0.91, 95% CI 0.47 to 1.74); and
Analysis 2.4: COPD exacerbation (OR 1.07, 95% CI 0.60 to 1.91).
2.1. Analysis.

Comparison 2 Comparison PPV‐23 versus PCV‐7, Outcome 1 Community‐acquired pneumonia: at least 1 episode.
2.2. Analysis.

Comparison 2 Comparison PPV‐23 versus PCV‐7, Outcome 2 Death from all causes.
2.3. Analysis.

Comparison 2 Comparison PPV‐23 versus PCV‐7, Outcome 3 Hospital admission, any cause: at least 1 episode.
2.4. Analysis.

Comparison 2 Comparison PPV‐23 versus PCV‐7, Outcome 4 Acute exacerbation COPD.
We assessed short‐term adverse effects of vaccines by using a seven‐day diary (Analysis 2.5) and noted a statistically significant difference for PPSV‐23 compared with PCV‐7 in the likelihood of fatigue (OR 2.40, 95% CI 1.15 to 5.00) and redness or discolouration ≤ 15 cm (OR 3.52, 95% CI 1.51 to 8.21).
2.5. Analysis.

Comparison 2 Comparison PPV‐23 versus PCV‐7, Outcome 5 Adverse effects.
We found no statistically significant differences for PPSV‐23 compared with PCV‐7 in the likelihood of headache (OR 1.59, 95% CI 0.61 to 4.18), fever (OR 0.66, 95% CI 0.14 to 3.10), pain (OR 1.36, 95% CI 0.66 to 2.82), localised swelling (OR 1.61, 95% CI 0.74 to 3.52), limitation in arm movement (OR 1.85, 95% CI 0.88 to 3.90) or redness or discolouration > 15 cm (OR 4.67, 95% CI 0.22 to 99.46).
Discussion
Summary of main results
For this systematic review update, a total of 12 randomised controlled trials (RCTs) (2171 participants) met our inclusion criteria. These investigators reported the effects of injectable pneumococcal polysaccharide vaccines (PPVs) in 2171 participants with chronic obstructive pulmonary disease (COPD). When compared with control for the primary outcome ‐ protection against community‐acquired pneumonia (CAP) ‐ results showed a lower likelihood with vaccine (odds ratio (OR) 0.59, 95% confidence interval (CI) 0.41 to 0.85; GRADE: moderate). The number needed to treat for an additional beneficial outcome (NNTB) to prevent one episode of CAP was 19 (95% CI 13 to 52). However, for pneumococcal pneumonia, researchers reported no significant difference with vaccination (Peto OR 0.26, 95% CI 0.05 to 1.31; GRADE: low), with only three studies (Alfageme 2006; Leech 1987; Ya Tseimakh 2006) measuring events and observing very few events. The difference in results between CAP and pneumococcal pneumonia may be related to both the paucity of events and non‐detection of pneumococcus.
We found no difference in mortality from cardiorespiratory causes between vaccine and control (OR 1.07, 95% CI 0.69 to 1.66; GRADE: moderate) in three studies (Alfageme 2006; Davis 1987; Leech 1987), nor in all‐cause mortality in five studies (Alfageme 2006; Davis 1987; Leech 1987; Lin 2013; Yilmaz 2013) (OR 1.00, 95% CI 0.72 to 1.40; GRADE: moderate).
The likelihood of hospital admission for any cause or for cardiorespiratory causes did not differ between vaccine and control groups; three studies reported admission for all causes (Kostinov 2014; Steentoft 2006; Yilmaz 2013) (OR 0.74, 95% CI 0.32 to 1.74; GRADE: moderate), and one study for cardiorespiratory‐related causes (Leech 1987) (risk ratio (RR) 0.89, 95% CI 0.51 to 1.58; GRADE: moderate). The likelihood of an emergency department visit for any cause was lower in one study (Yilmaz 2013) for vaccine than for control (OR 0.26, 95% CI 0.07 to 0.91; GRADE: moderate).
The likelihood of a COPD exacerbation (Figure 7) was significantly reduced (OR 0.60, 95% CI 0.39 to 0.93; GRADE: moderate) in four studies (Furumoto 2008; Kostinov 2014; Steentoft 2006; Yilmaz 2013). The NNTB to prevent one episode of acute exacerbation was 8 (95% CI 5 to 58), which represents a reduction in risk from 608/1000 for control to 482/1000 for vaccination (Figure 1). Three of these studies defined exacerbations of COPD as worsening respiratory symptoms beyond normal day‐to‐day variation, and the basis for exacerbations was not given in Kostinov 2014, as the definition was not based on any need for additional treatment, and we were not able to classify the severity of the exacerbations. Ya Tseimakh 2006 provided no definition of an exacerbation (published abstract only) and reported a lower exacerbation rate over six months (Analysis 1.11; mean difference (MD) ‐0.59, 95% CI ‐0.80 to ‐0.38). The rate of exacerbation in Lin 2013 was not lower with vaccination; this study assessed the effect of vaccination on moderate exacerbations of COPD (Burge 2003), defined as the requirement for treatment with parenteral corticosteroids with or without an antibiotic (Analysis 1.12; RR 0.87, 95% CI 0.44 to 1.72).
One study (Ya Tseimakh 2006) reported local adverse effects in the vaccination group only, with erythema occurring in 22% of vaccinated participants. Another study (Alfageme 2006) found no significant difference in lung function between vaccine and control groups.
No studies provided data on days of disability from respiratory illness or cost‐effectiveness of pneumococcal vaccination for meta‐analyses comparing vaccine and control.
A single study (Dransfield 2009) comparing 23‐valent pneumococcal polysaccharide vaccine and 7‐valent pneumococcal conjugate vaccine reported no differences in vaccination outcomes for CAP (OR 1.01, 95% CI 0.40 to 2.56), for mortality from all causes (OR 1.83, 95% CI 0.5 to 6.50), for hospital admission for any cause (OR 0.91, 95% CI 0.47 to 1.74) or for likelihood of experiencing a COPD exacerbation (OR 1.05, 95% CI 0.58 to 1.88). The likelihood of some mild adverse effects was higher with vaccination, with increased likelihood for PPV‐23 compared with PCV‐7 for fatigue (OR 2.40, 95% CI 1.15 to 5.00), local redness or discolouration ≤ 15 cm (OR 3.52, 95% CI 1.51 to 8.21) and limitation of arm movement (OR 1.85, 95% CI 0.88 to 3.90).
Overall completeness and applicability of evidence
Some studies described gender imbalance among participants; three studies included more than 80% male participants (Alfageme 2006; Lin 2013; Yilmaz 2013). Cigarette smoking is recognised as the single biggest risk factor in the development of COPD, and in some studies, gender imbalance reflects the imbalance among smokers or among participants treated in veterans' healthcare facilities. We examined studies for differences in baseline characteristics that might potentially confound results. Baseline forced expiratory volume in one second (FEV1) and FEV1/forced vital capacity (FVC) did not significantly differ across groups in all fully published studies nor in studies for which study authors supplied data. Influenza vaccination was similar in Furumoto 2008 (100% vaccination and control) and Yilmaz 2013 (62% vaccination, 52% control) ‐ two studies that contributed to analysis of COPD exacerbations, but Kostinov 2014 and Steentoft 2006 did not report influenza vaccine status.
Treatments given in control groups varied. In Furumoto 2008 and Leech 1987, intervention groups received both a pneumococcal polysaccharide vaccine and an influenza vaccine, and the control group received only the influenza vaccine. In Alfageme 2006, Kostinov 2014, Steentoft 2006,Teramoto 2007, Trofimov 2010 and Ya Tseimakh 2006, control groups did not receive a vaccine. Analysis by severity of COPD showed no significantly different effects for risk of pneumonia for severe compared with moderate airflow limitation.
Results may be compared with those reported by RCTs that did not provide separate data for participants with COPD. In several older studies, for example, Klastersky 1986, in which participants had bronchogenic carcinoma, investigators found a small advantage for vaccination regarding likelihood of pneumococcal infection, Gaillat 1985 found a lower likelihood of pneumonia but no effect on mortality among residents living in aged‐care facilities and Koivula 1997 found no reduction in pneumonia events overall but a protective effect of pneumococcal vaccination in persons at increased risk of pneumonia (age ≥ 70 years, heart disease, lung disease, bronchial asthma, alcoholism, institutionalised or permanently bedridden). Simberkoff 1986 showed no difference in pneumonia among high‐risk participants (age > 55, chronic renal, hepatic, cardiac or pulmonary disease; alcoholism; or diabetes mellitus). Ortqvist 1998, which included 21% of participants 50 to 85 years of age with COPD, found no reduction in risk of pneumonia, pneumococcal pneumonia or mortality with vaccination compared with placebo.
A recent large study (Bonten 2015) compared 13‐valent pneumococcal conjugate vaccine versus placebo in 84,496 participants over 65 years of age at 101 community‐based sites in the Netherlands, where pneumococcal vaccination in older adults was not routine. Risk of CAP in the PCV‐13 group compared with the placebo group was reduced by 37.7% (95% CI 14.3 to 55.1), and risk of invasive pneumococcal disease was reduced by 75.8% (95% CI 46.5 to 90.3) in modified intention‐to‐treat (ITT) analyses. Results are not available for participants with COPD, but overall, 12.3% of participants were current smokers, 4.9% reported a diagnosis of asthma and 25.4% had been given a diagnosis of heart disease.
A systematic review (Kew 2014) showed that people with COPD treated with inhaled corticosteroids (budesonide and fluticasone, delivered alone or in combination with a long‐acting beta agonist (LABA)) had increased risk of serious pneumonia resulting in hospitalisation. In this current review of effects of pneumococcal vaccines for preventing pneumonia, only three studies reported the proportion of participants using corticosteroids; Lin 2013 indicated that 100% of participants were taking inhaled corticosteroids, Steentoft 2006 revealed that 24% used oral corticosteroids in the comparison with control and Dransfield 2009 described use of inhaled corticosteroids by 65% of participants in comparisons of PPV‐23 versus PCV‐7. Subgroup analyses were not possible.
Clinical guidelines provided by internationally recognised respiratory societies have advocated use of pneumococcal vaccination in patients with COPD. Guidelines from the UK National Institute of Clinical Excellence (NICE) state that "pneumococcal vaccination and an annual influenza vaccination should be offered to all patients with COPD as recommended by the Chief Medical Officer" (NICE 2004). COPDX guidelines for Australia and New Zealand state that "pneumococcal immunisation (polyvalent covering 23 virulent serotypes) is recommended in people with COPD", and evidence for this recommendation is graded at level II (COPDX 2016). The Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines published jointly by the National Heart Lung and Blood Institute in the USA and the World Health Organization (WHO) advise that "pneumococcal vaccination should be offered to every COPD patient; vaccine appears to be more effective in older patients and those with more severe disease or cardiac comorbidity" (GOLD 2016).
The WHO (WHO 2012) has made recommendations for use of pneumococcal vaccines in children, which are influencing pneumococcal disease, carriage and herd protection. Pneumococcal conjugated vaccines PCV‐10 and PCV‐13 are licensed for prevention of invasive disease, pneumonia and acute otitis media caused by respective vaccine serotypes in children from six weeks to five years of age, with high vaccine efficacy. The WHO recommends that inclusion of PCVs be given priority in childhood immunisation programmes worldwide, especially in countries with under‐five‐mortality of > 50/1000 live births. Although herd effects of immunisation in children have reduced invasive pneumococcal disease (IPD), it is recommended that adults over 65 should be immunised.
The studies included in this review reported a low frequency of proven pneumococcal pneumonia; thus we acknowledge the possibility of a type 2 error, given the rare events reported. Investigators have found that the overall contribution of pneumococcal pneumonia to overall CAP varies (Rodrigo 2015); between 2008 and 2013, rates of 17.1% to 37.3% were reported.
A recent systematic review aimed to determine the incidence and burden of vaccine‐preventable pneumococcal disease in the adult population in the UK (Chalmers 2016). This study found a high burden of pneumococcal disease among adults, along with substantial ongoing changes in the epidemiology of pneumococcal disease. Among those > 65 years of age, the incidence of IPD in 2013‐2014 was 20.58 per 100,000 population. However, the incidence of PCV13 serotype IPD among people > 65 years of age was 10.33 per 100,000 population from 2008 to 2010, and fell to 3.72 per 100,000 in 2013‐2014. In this population, PCV‐7 serotypes were reduced from 4.58 per 100,000 in 2008 to 2010 to 0.53 per 100,000 population in 2013‐2014.
Quality of the evidence
We graded evidence showing beneficial effects on CAP (OR 0.62, 95% 0.43 to 0.89) and effects on mortality estimates (OR 1.07, 95% CI 0.69 to 1.66 for cardiorespiratory causes; OR 1.00, 95% CI 0.72 to 1.40 for all‐cause mortality) as having moderate quality. We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different. We graded evidence for the unchanged likelihood of hospital admission for any cause (OR 0.74, 95% CI 0.32 to 1.74) as having moderate quality. We graded the quality of evidence for the lower likelihood of an acute exacerbation of COPD (OR 0.25, 95% CI 0.16 to 0.38) as moderate; lack of participant and/or personnel blinding may have led to better general care and treatment for patients with COPD in the vaccinated group.
Potential biases in the review process
Methodological limitations
Twelve studies involving 2171 participants contributed data to this review. At the review level, we believe incomplete identification of studies was not an issue, and we found no evidence of publication bias. The average number of participants per study was 187, although individual studies reported from 36 to 600 participants; these relatively low numbers are probably too small, given the incidence of pneumococcal infection among study populations. It is likely that larger studies with participant numbers of around 1000 would be needed to demonstrate statistically significant effects.
Agreements and disagreements with other studies or reviews
A systematic review current to June 2012 (Moberley 2013) assessed the efficacy and effectiveness of PPVs in preventing pneumococcal disease or death among adults. In 18 RCTs involving 64,852 participants, investigators provided strong evidence of PPV efficacy against IPD (OR 0.26, 95% CI 0.14 to 0.45). They found efficacy against all‐cause pneumonia in low‐income (OR 0.54, 95% CI 0.43 to 0.67) but not in high‐income countries among the general population (OR 0.71, 95% CI 0.45 to 1.12) and among adults with chronic illness (OR 0.93, 95% CI 0.73 to 1.19). Study authors noted that vaccine efficacy against primary outcomes appeared poorer among adults with chronic illness, but small number of identified studies limited power to detect significant effects. This review also found no significant change in all‐cause mortality (OR 0.90, 95% CI 0.74 to 1.09).
Review authors have assessed evidence for effectiveness of pneumococcal vaccine in other chronic respiratory conditions; a systematic review of children and adults with bronchiectasis, current to November 2008, identified no eligible RCTs (Chang 2009). A systematic review, current to May 2014, conducted to assess the efficacy of pneumococcal vaccines in reducing morbidity among people with cystic fibrosis, also identified no relevant trials (Burgess 2014). A systematic review of the efficacy of pneumococcal vaccine in reducing mortality or morbidity from pneumococcal disease among patients with asthma (Sheikh 2002) found no evidence of effects on acute asthma exacerbations.
Studies using a retrospective, case‐control design that often included people with chronic lung conditions showed the efficacy of pneumococcal vaccination to be approximately 50% to 80% against invasive pneumococcal disease in high‐risk populations (Fedson 1994; Leophonte 2001). Prospective cohort studies have generally failed to show reductions in the risk of non‐bacteraemic infection, although Alfageme 2006 and Jackson 2003 demonstrated protection against bacteraemia. Regardless of design, most studies have found that the protective efficacy of vaccination is uniformly diminished in elderly and immunocompromised individuals. Although cohort studies are potentially easier to conduct logistically (Hak 2006), evidence from these studies is subject to limitations in generalisability (Hak 2006) and in interpretation (Jackson 2006).
Authors' conclusions
Implications for practice.
Moderate‐quality evidence derived from RCTs included in this review suggests that injectable polyvalent pneumococcal vaccines provide protection against community‐acquired pneumonia and reduce the likelihood of exacerbations of chronic obstructive pulmonary disease (COPD). Evidence was insufficient for comparisons of different pneumococcal vaccine types. Evidence in this review supports pneumococcal vaccination for people with COPD, as recommended by respiratory guidelines.
Implications for research.
Pneumococcal immunisation among children and older adults in many countries has reduced the incidence and changed the epidemiology of pneumococcal disease. Future randomised controlled trials restricted to people with COPD will be difficult to conduct with adequate power to detect significant effects, especially for rare events such as confirmed pneumococcal pneumonia.
Feedback
Questioning the certainty of main conclusion, 21 December 2017
Summary
Thank you for your comprehensive review on the potential benefit of pneumococcal vaccination in patients with COPD. We are concerned with your main conclusion, that "injectable polyvalent vaccination provides significant protection against community‐acquired pneumonia" (CAP), for a number of reasons.
Firstly, of the 12 studies with 2171 participants included in your review, only 6 studies (1372 participants) were included in the meta‐analysis of CAP (Analysis 1.1). It appears that five of the excluded trials evaluated the efficacy of pneumococcal vaccination compared to placebo or no comparator, and thus it seems reasonable to have CAP as a reported outcome. Given the effect size reported (63 versus 81 cases in the vaccine and control group, respectively), the missing data from these trials (425 patients), could significantly impact the results of Analysis 1.1. We are curious to know whether efforts were made to collect this data from the original authors. Moreover, the reporting of results from Furomoto 2008 appears discordant with your criteria to include studies that have at least 80% of participants with COPD, as the proportion was 33% (55/167) in the per‐protocol results of this paper.
Our review of Teramoto 2007, which contributed substantial weight to Analysis 1.1, raised significant doubt regarding the reliability of information from this abstract. For example, their objective was “to test the effect of tiotropium bromide on lung functions, respiratory muscle function and dyspnoea sensation during exercise,” a statement clearly inconsistent with the remainder of the abstract. We believe the results of the sensitivity analysis which excluded this paper (showing a non‐significant effect) merits greater emphasis as it represents a major caveat to your main conclusion.
We also have minor concerns regarding the reported number of CAP events in the placebo group cited from Davis 1987 for Analysis 1.1 and the number of deaths due to cardiorespiratory causes reported from Alfageme 2006 in Analysis 1.4. In both cases, the numbers reported in your meta‐analyses do not appear in the original publications. We believe that providing the source of this information would offer greater clarity to your readers.
Based on these concerns and the current available evidence presented in your review, we believe it remains inconclusive whether pneumococcal vaccination in COPD patients leads to a reduction in pneumonia. We suggest that this uncertainty be reflected in the final conclusion regarding the benefits for pneumococcal vaccine in COPD patients.
Elaine Tung, Priscilla Shum, Jinglin Tang
Reply
We thank the contributors for this feedback.
In five included studies no outcome data on CAP were available, either in publications or from requests to authors, when made. In more detail:
Kostinov 2014, Russian, abstract English. CAP not listed as outcome, no data available. Extraction performed by a Russian translator. No request sent to authors.
Leech 1987, reported Pneumococcal pneumonia, and hospitalisation for pneumonia, data in meta analyses 1.3 and 1.7. No request for CAP episodes made.
Lin 2013, listed Incidence of Pneumonia as Primary Outcome Measure in trial registration. Conference presentation supplied in English in June 2015‐stated “Pneumonia was diagnosed by primary clinician’s judgement” reported as Pneumonia episodes/person‐yr, included in analysis 1.2, as the rate ratio.
Trofimov 2010, published in Russian, abstract English. No primary or secondary outcomes for the review were listed as extracted by translator. No request sent to authors.
Yilmaz 2013, contact author Dr. Dilber Yilmaz supplied unpublished draft in May 2015. Pneumonia was not listed or reported.
Results from Furomoto 2008 are based only on the sub‐group of participants with COPD, 55 of 167 study total (standard method in Cochrane systematic reviews of studies in mixed respiratory disease populations). The criterion to include studies that have at least 80% of participants with COPD only applies where separate results for participants with COPD are not available.
The data for Teramoto 2007 were extracted from a published abstract. A request was sent September 2009, without response. We concluded it was highly likely that the objective was not accurately stated, possibly an error in English writing. Title was ‘Clinical efficacy of anti‐pneumococcal vaccination in elderly patients with COPD’. While the study does contribute significant weight to the overall result, so does that of Alfageme 2006, reflecting the size and event rates of the two studies. We reported no heterogeneity (Chi² = 5.52, df = 5 (P = 0.36); I² = 9%) and an effect size OR 0.62, 95% CI 0.43 to 0.89; 6 studies , n = 1372. With the removal of these 2 studies (which contributed 45% participants), the effect was lessened and non significant, but direction was the same (OR 0.80, 95% CI 0.51 to 1.25; 4 studies, n = 803). We believe this is consistent with a lack of power, resulting from the removal of participants, rather than a change in the effect.
Regarding the reported number of CAP events in the placebo group cited from Davis 1987 for Analysis 1.1 OR 0.62; 95% CI 0.43 to 0.89 [Heterogeneity: Chi² = 5.52, df = 5 (P = 0.36); I² = 9%], we thank the contributors for pointing this out. The figure for the placebo group for CAP at least 1 episode should be 7 (N=53) and with the corrected data outcome 1.1 is OR 0.59; 95% CI 0.41 to 0.85, Heterogeneity: Chi² = 5.67, df = 5 (P = 0.34); I² = 12%. This error had arisen between publication of this version 4 of the review Jan 2017 and version 3 in 10 Nov 2010, but has now been corrected.
Deaths due to cardiorespiratory causes in Alfageme 2006 Analysis 1.4 PPV group 33/298, placebo group 30/298, were from unpublished data. As the feedback contributor states this is not found in the publication. The paper data extraction form and correspondence are not available to the current corresponding review author. OR 1.07, 95%CI 0.69 to 1.66 changes to OR 0.98, 95%CI 0.44 to 2.18 if the data from Alfageme 2006 are not included
We hold that the review conclusions of moderate‐quality evidence, indicating that injectable polyvalent pneumococcal vaccines provide protection against community‐acquired pneumonia, are justified by the studies and data included.
R Wood‐Baker, J Walters, P Poole
Contributors
Authors of feedback: Elaine Tung, Priscilla Shum, Jinglin Tang, Lower Mainland Pharmacy Services, Vancouver, Canada.
Do you have any affiliation with or involvement in any organisation with a financial interest in the subject matter of your comment? I do not have any affiliation with or involvement in any organisation with a financial interest in the subject matter of my comment
Authors of reply: R Wood‐Baker, J Walters, P Poole.
What's new
| Date | Event | Description |
|---|---|---|
| 18 March 2019 | Amended | Corrected the conflict of interest statements for all authors. |
History
Protocol first published: Issue 1, 1999 Review first published: Issue 4, 2006
| Date | Event | Description |
|---|---|---|
| 19 March 2018 | Feedback has been incorporated | Correction of data error identified in feedback. Outcome 1.1 CAP 0.59 (0.41, 0.85). NNTB 19 (NNTB 13 to NNTB 52). |
| 17 January 2018 | Amended | Feedback added. |
| 23 November 2016 | New citation required and conclusions have changed | This update, which includes additional studies, now shows statistical significance in reducing the likelihood of community‐acquired pneumonia (odds ratio (OR) 0.62, 95% confidence interval (CI) 0.43 to 0.89), as well as statistical significance in reducing the likelihood of an acute exacerbation of chronic obstructive pulmonary disease (COPD) (OR 0.60, 95% CI 0.39 to 0.9). One included study (Dransfield 2009) compared 2 different vaccine types and found no significant differences for the primary outcomes. |
| 23 November 2016 | New search has been performed | Searches updated for this review identified 5 additional studies (Dransfield 2009; Kostinov 2014; Lin 2013; Teramoto 2007; Yilmaz 2013) that compared vaccine versus control and involved 606 participants. This review was last updated in 2010. The review now includes a total of 12 studies involving 2171 participants. |
| 4 June 2014 | Amended | We included comparison of vaccine types. |
| 13 May 2010 | New citation required and conclusions have changed | We promoted pneumonia to a primary outcome for the 2010 update and added 'Risk of bias' tables. We included 3 new studies identified by searches run up to March 2010. Data for community‐acquired pneumonia changed the size of the effect estimate, although it remained not statistically significant. In the previous version of the review, the OR was 0.89 (95% CI 0.58 to 1.37). With the addition of new data, the pooled effect estimate was OR 0.72, 95% CI 0.51 to 1.01. |
| 31 July 2008 | Amended | We converted this review to new review format. |
| 21 July 2006 | New citation required and conclusions have changed | We made substantive amendments. |
Acknowledgements
We acknowledge the support of the Cochrane Airways Review Group in conducting searches, and of Toby Lasserson for extracting data for the original review. We also acknowledge the contributions of Punam Mangtani to the original review.
We are grateful to author Dr. Dilber Yilmaz who supplied an unpublished draft of the study findings for Yilmaz 2013 in May 2015. We are grateful to Liliya Eugenevna Ziganshina, Mansur Kutlubaev and Vladimir Rafalskiy for helping to translate non‐English publications, and to Ming‐Tzer Lin for supplying data.
Robert Granger, an author of the original review published in 2004, contributed to study selection; data extraction/entry, analysis and interpretation; and writing of the final review. Sabin Smith, an author on the review in 2009, contributed to study selection; data extraction/entry, analysis and interpretation; and drafting of the final review.
Chris J Cates was the Contact Editor for this review and commented critically on it.
The Background and Methods sections of this review are based on a standard template used by the Cochrane Airways Review Group.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Review Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, the National Health Service (NHS) or the Department of Health.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL (the Cochrane Library) | Monthly |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
COPD search
1. Lung Diseases, Obstructive/
2. exp Pulmonary Disease, Chronic Obstructive/
3. emphysema$.mp.
4. (chronic$ adj3 bronchiti$).mp.
5. (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).mp.
6. COPD.mp.
7. COAD.mp.
8. COBD.mp.
9. AECB.mp.
10. or/1‐9
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomised or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases.
Appendix 2. Search strategy to retrieve relevant trials from the CAGR
#1 MeSH DESCRIPTOR Pulmonary Disease, Chronic Obstructive Explode All
#2 MeSH DESCRIPTOR Bronchitis, Chronic
#3 (obstruct*) near3 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*)
#4 COPD:MISC1
#5 (COPD OR COAD OR COBD):TI,AB,KW
#6 #1 OR #2 OR #3 OR #4 OR #5
#7 MeSH DESCRIPTOR Pneumococcal Vaccines
#8 ((vaccin* or immuni*) and pneum*)
#9 Pneumovax
#10 Pnu‐Imune
#11 Pnu‐Immune
#12 Prevnar
#13 "Pneumo 23"
#14 #7 or #8 or #9 or #10 or #11 or #12 or #13
#15 #6 and #14
[In search line #4, MISC1 denotes the field in the record where the reference has been coded for condition, in this case, COPD]
Appendix 3. Search strategies
CENTRAL search
#1 MeSH descriptor Lung Diseases, Obstructive, this term only
#2 MeSH descriptor Pulmonary Disease, Chronic Obstructive explode all trees
#3 emphysema*
#4 chronic* near/3 bronchiti*
#5 (obstruct*) near/3 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*)
#6 COPD
#7 COAD
#8 COBD
#9 AECB
#10 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9)
#11 MeSH descriptor Pneumococcal Vaccines explode all trees
#12 pneum* near/3 (vaccin* or immuni*)
#13 Pneumovax or Pnu‐Imune or Pnu‐Immune or Prevnar or Prevenar or "Pneumo 23"
#14 (#11 OR #12 OR #13)
#15 (#10 AND #14)
MEDLINE search
1 exp Pulmonary Disease, Chronic Obstructive/
2 (obstruct$ adj3 (lung$ or respirat$ or pulmonar$) adj3 disease$).mp.
3 Bronchiti$.mp.
4 emphysema$.mp.
5 ((lung$ or thorax) adj3 hyperlucen$).mp.
6 (chronic adj5 obstruct$).mp.
7 (pulmonar$ or lung$ or airway$ or airflow$ or bronch$ or respirat$).mp.
8 6 and 7
9 (COPD or COAD).mp.
10 AECB.mp.
11 1 or 2 or 3 or 4 or 5 or 8 or 9 or 10
12 Pneumococcal Vaccines/
13 (pneum$ adj3 (vaccin$ or immuni$)).mp.
14 (Pneumovax or Pnu‐Imune or Pnu‐Immune or Prevnar or Prevenar or "Pneumo 23")
15 12 or 13 or 14
16 11 and 15
17 (clinical trial or controlled clinical trial or randomized controlled trial).pt.
18 (randomized or randomised).ab,ti.
19 placebo.ab,ti.
20 dt.fs.
21 randomly.ab,ti.
22 trial.ab,ti.
23 groups.ab,ti.
24 or/16‐22
25 Animals/
26 Humans/
27 24 not (24 and 25)
28 23 not 26
29 16 and 27
Embase search
1 Chronic Obstructive Lung Disease/
2 Emphysema/
3 exp Lung Emphysema/
4 Chronic Bronchitis/
5 (obstruct$ adj3 (lung$ or respirat$ or pulmonar$) adj3 disease$).mp.
6 Bronchiti$.mp.
7 emphysema$.mp.
8 ((lung$ or thorax) adj3 hyperlucen$).mp.
9 (chronic adj5 obstruct$).mp.
10 (pulmonar$ or lung$ or airway$ or airflow$ or bronch$ or respirat$).mp.
11 9 and 10
12 (COPD or COAD).mp.
13 AECB.mp.
14 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 11 or 12 or 13
15 Pneumococcus Vaccine/
16 (pneum$ adj3 (vaccin$ or immuni$)).mp.
17 (Pneumovax or Pnu‐Imune or Pnu‐Immune or Prevnar or Prevenar or "Pneumo 23")
18 15 or 16 or 17
19 14 and 18
20 Randomized Controlled Trial/
21 Controlled Study/
22 randomization/
23 Double Blind Procedure/
24 Single Blind Procedure/
25 Clinical Trial/
26 Crossover Procedure/
27 follow up/
28 exp prospective study/
29 or/19‐27
30 (clinica$ adj3 trial$).mp.
31 ((singl$ or doubl$ or trebl$ or tripl$) adj5 (mask$ or blind$ or method$)).mp.
32 exp Placebo/
33 placebo$.mp.
34 random$.mp.
35 (latin adj3 square$).mp.
36 exp Comparative Study/
37 ((control$ or prospectiv$ or volunteer$) adj3 (trial$ or method$ or stud$)).mp.
38 (crossover$ or cross‐over$).mp.
39 or/30‐38
40 29 or 39
41 exp ANIMAL/
42 Nonhuman/
43 Human/
44 41 or 42
45 44 not 43
46 40 not 45
47 19 and 46
Data and analyses
Comparison 1. Pneumococcal vaccine versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Community‐acquired pneumonia: at least 1 episode | 6 | 1372 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.41, 0.85] |
| 1.1 PPV‐23 serotypes | 5 | 1269 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.42, 0.89] |
| 1.2 PPV‐14 serotypes | 1 | 103 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.10, 1.72] |
| 2 Community‐acquired pneumonia: rate per person‐year | 1 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 2.1 PPV‐23 serotypes | 1 | 36 | Rate Ratio (Fixed, 95% CI) | 0.37 [0.12, 1.14] |
| 3 Pneumococcal pneumonia: at least 1 episode | 3 | 1158 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.26 [0.05, 1.31] |
| 3.1 PPV‐23 serotypes | 2 | 969 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.02, 0.78] |
| 3.2 PPV‐14 serotypes | 1 | 189 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.80 [0.15, 393.72] |
| 4 Death from cardiorespiratory causes | 3 | 888 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.69, 1.66] |
| 4.1 PPV‐23 serotypes | 1 | 596 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.66, 1.88] |
| 4.2 PPV‐14 serotypes | 2 | 292 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.44, 2.18] |
| 5 Death from all causes | 5 | 1053 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.72, 1.40] |
| 5.1 PPV‐23 serotypes | 3 | 761 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.69, 1.51] |
| 5.2 PPV‐14 serotypes | 2 | 292 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.48, 1.86] |
| 6 Hospital admission, any cause: at least 1 episode | 3 | 391 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.32, 1.74] |
| 6.1 PPV‐23 serotypes | 3 | 391 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.32, 1.74] |
| 7 Hospital admission: cardiorespiratory‐related | 1 | Rate Ratio (Random, 95% CI) | Totals not selected | |
| 7.1 PPV‐14 serotypes | 1 | Rate Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Hospital admission: all‐cause | 1 | Rate Ratio (Random, 95% CI) | Totals not selected | |
| 8.1 PPV‐23 serotypes | 1 | Rate Ratio (Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 ED visit, any cause: at least 1 episode | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10 At least 1 COPD exacerbation | 4 | 446 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.39, 0.93] |
| 10.1 PPV‐23 serotypes | 4 | 446 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.39, 0.93] |
| 11 COPD exacerbation rate | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 PPV‐23 serotypes | 1 | 373 | Mean Difference (IV, Fixed, 95% CI) | ‐0.59 [‐0.80, ‐0.38] |
| 12 COPD exacerbations: rate/person‐year | 1 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 12.1 PPV‐23 serotypes | 1 | 36 | Rate Ratio (Fixed, 95% CI) | 0.87 [0.44, 1.72] |
| 13 Lung function: FEV1 (L) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.3 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14 Quality of life: SGRQ overall | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 14.1 3 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.2 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 14.3 24 months | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Comparison PPV‐23 versus PCV‐7.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Community‐acquired pneumonia: at least 1 episode | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Death from all causes | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Hospital admission, any cause: at least 1 episode | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4 Acute exacerbation COPD | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5 Adverse effects | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.1 Fatigue | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.2 Headache | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.3 Fever | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.4 Pain | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.5 Redness or discolouration ≤ 15 cm | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.6 Redness or discolouration > 15 cm | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.7 Localised swelling | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5.8 Limitation of arm movement | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Analysis by follow‐up period/subgroup.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pneumonia by lung function at baseline | 1 | 596 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.71 [0.41, 1.22] |
| 1.1 FEV1 < 40% expected | 1 | 246 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.48 [0.23, 1.00] |
| 1.2 FEV1 ≥ 40% expected | 1 | 350 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.12 [0.50, 2.48] |
| 2 Hospital admission, any cause: by follow‐up periods | 3 | 377 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.23, 1.22] |
| 2.1 6‐12 months | 2 | 249 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.23, 2.12] |
| 2.2 12‐24 months | 1 | 128 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.11, 1.19] |
| 3 Hospital admission, cardiorespiratory‐related: by follow‐up periods | 1 | Rate Ratio (Random, 95% CI) | Subtotals only | |
| 3.1 7‐12 months | 1 | 160 | Rate Ratio (Random, 95% CI) | 0.89 [0.59, 1.36] |
| 3.2 13‐18 months | 1 | 150 | Rate Ratio (Random, 95% CI) | 1.22 [0.69, 2.16] |
| 3.3 19‐24 months | 1 | 112 | Rate Ratio (Random, 95% CI) | 0.70 [0.24, 1.99] |
| 4 Emergency department visit, any cause: by follow‐up period | 1 | Odds Ratio (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 3‐12 months | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 12‐24 months | 1 | Odds Ratio (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 Emergency visits (by cause) | 1 | Rate Ratio (Fixed, 95% CI) | Subtotals only | |
| 5.1 Due to URTI | 1 | Rate Ratio (Fixed, 95% CI) | 1.29 [0.68, 2.47] | |
| 5.2 Due to LRTI | 1 | Rate Ratio (Fixed, 95% CI) | 1.00 [0.75, 1.33] | |
| 5.3 Due to pneumonia | 1 | Rate Ratio (Fixed, 95% CI) | 0.99 [0.52, 1.88] | |
| 6 At least 1 COPD exacerbation: varying follow‐up | 4 | 432 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.34, 0.81] |
| 6.1 12 months | 2 | 249 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.41, 1.19] |
| 6.2 > 12‐24 months | 2 | 183 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.15, 0.63] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alfageme 2006.
| Methods | Setting of study: population‐based intervention Study design: RCT parallel Type of analysis: case available | |
| Participants | Total number of participants: 600 (4 lost to follow‐up; 2 from each group) Gender distribution: vaccine group M = 96.6%; control group M = 93.3% Mean age (years): vaccine group = 69; control group = 68 Age range: vaccine group = 62 to 73; control group = 61 to 73 Inclusion criterion: spirometric diagnosis of COPD Exclusion criteria: prior pneumococcal vaccination, pregnant, immunosuppressed, known neoplasia, renal insufficiency in dialysis, HIV infection, hypogammaglobulinaemia, anatomical and/or functional asplenia Diagnostic criteria (COPD): SEPAR criteria (Sociedad Espanola de Patologia Respiratoria, or Spanish Society of Respiratory Pathology), FEV1 < 80% and FEV1/FVC < 70%; severity of COPD: vaccine group FEV1 < 40% = 132; ≥ 40% = 166; control group FEV1 < 40% = 114; ≥ 40% = 184 Current smokers: vaccine group = 22%; control group = 26% Diagnostic criteria (pneumonia): clinical symptoms (lower respiratory tract infection with fever) and imaging findings (new infiltrate typical of pneumonia, which decreases during follow‐up). Pneumococcal pneumonia diagnosed with isolated S pneumoniae in blood, pleural fluid or bronchial samples. Microbiological diagnosis (pneumococcus): presence of pneumonia and isolation of S pneumoniae from sputum, broncho‐aspirate, blood, pleural fluid or CSF | |
| Interventions | Vaccine type: 23‐valent pneumococcal capsular polysaccharide Numbers in each group: intervention = 298; control (no intervention) = 298 Dose: 0.5 mL Pneumo‐23, Sanofi‐Pasteur MSD Delivery: subcutaneous injection in deltoid muscle Cointerventions: none Comparison: no vaccine Duration of study: vaccine group, median 980 days (range 20 to 1454); control group, median 978 days (range 21 to 1183) | |
| Outcomes | Types of outcomes measured: ‐ Acute exacerbations: definition: (1) increased dyspnoea, (2) increased sputum volume and (3) increased sputum purulence and (4) absence of newly appeared infiltration on a chest radiograph; 2 of the 3 respiratory symptoms present, or 1 of these and 1 additional symptom, such as fever with no other causes or increased cough; I = 30, C = 9 ‐ Pneumonia: definition: clinical symptoms (cough, sputum or fever) plus increased white blood cell count or serum C‐reactive protein and appearance of a new infiltration on chest radiograph; pneumonia‐free survival plot, log rank = 1.15, P = 0.28 (NS)) ‐ Number of hospital admissions (yes, all causes): I = 18, C = 6 ‐ Change in lung function: reported, but data cannot be used ‐ All‐cause mortality in year post vaccination: no | |
| Notes | C = control, I = intervention | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation code developed with a computer random number generator in block lengths of 20 (10 in each group) |
| Allocation concealment (selection bias) | Unclear risk | "They were then randomly assigned to the intervention group" Not stated if allocation was performed centrally or with the use of sealed opaque envelopes |
| Blinding (performance bias and detection bias) Assessors | Low risk | "The vaccination status of the patient was kept in a specific encrypted database and was not stated in the patients' clinical records. The main investigator of this study (IA) was the only person with access to this database, but this investigator did not participate in the follow‐up or in adjudicating the outcome events. This task was performed by the physicians conducting the follow‐up, who were unaware of the treatment group allocation of their patients. These investigators were committed not to ask patients about their vaccination status." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding measures used in the study "A considerable limitation of this study is the lack of a blind placebo comparison group." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Four patients (2 from each arm of the study) were lost to follow‐up and were excluded from final analyses. A minimum follow‐up period of 3 years was given for each participant, except 115, who died before the end of follow‐up. |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it appears that published reports include the prespecified outcomes |
| Other bias | Low risk | No other issues were noted. |
Davis 1987.
| Methods | Study design: randomised, double‐blind, placebo‐controlled trial
Method of randomisation: random number table. Participants studied for 1 to 48 months of treatment Study outcomes assessed by person blinded to Tx allocation? yes |
|
| Participants | Total number of participants: 103
Gender distribution: not stated
Mean age (years): intervention group = 64 ± 10, control group = 61 ± 10
Age range: not stated
Inclusion criterion: COPD (assessed by clinical and pulmonary function criteria)
Exclusion criteria:
‐ Reversible airflow obstruction in the absence of chronic bronchitis (cough 3 of 12 months for 3 consecutive years) or emphysema as judged clinically, radiologically and by lung function testing
‐ Malignant neoplasms
‐ Sickle cell disease
‐ Severe renal impairment
‐ Severe hepatic impairment
Diagnostic criteria (COPD): ATS standards
Severity of COPD: active: FEV1 (L) = 1.33 ± 0.61; FEV1/FVC = 52 ± 13; placebo: FEV1 (L) = 1.47 ± 0.75; FEV1/FVC = 55 ± 14
Smoking status: active: current = 53%, never n = 5; placebo: current = 33%, never n = 5
Diagnostic criteria (pneumonia): clinical and imaging findings in the presence of pneumococcus in sputum
Etiological diagnosis (pneumococcus): diagnosis only if pathogens isolated from blood or body fluids. Processed < 6 hours after collection
Microbiological methods described Baseline characteristics (smoking status): ‐ Current smokers: PLA: 27/53; VAX: 17/50 (P = 0.036 for difference); non‐smokers: PLA: 5; VAX: 5 |
|
| Interventions | Vaccine type: 14 pneumococcal capsular polysaccharide antigens Number in each group: intervention = 50; placebo = 53 Dose: 0.5 mL (50 mcg of each of the 14 capsular antigens) Delivery: subcutaneous injection Cointerventions: none Comparison: saline Duration of study: 24 to 32 months Participants followed up for 48 months (mean follow‐up in each arm: PLA: 32.2 months; VAX: 31.7 months) | |
| Outcomes | Incidence of pneumonia: community‐acquired pneumonia and putative pneumococcal pneumonia: clinical and imaging findings in the presence of pneumococcus in sputum Pneumonia‐free survival plots: no hazard ratio, P = 0.249 All‐cause mortality survival plots: no hazard ratio, P = 0.718 Antibody titers: not analysed Sputum flora: not analysed |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Study participants arranged in a double‐blind manner on the basis of a table of random numbers to a group receiving placebo or to a group receiving vaccine |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) Assessors | Low risk | Double‐blind study; study outcomes assessed by person blinded to Tx allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study; placebo injection given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number of withdrawals/losses to follow‐up similar in both groups |
| Selective reporting (reporting bias) | Low risk | Protocol not available but all outcomes specified in methods are reported |
| Other bias | Low risk | None noted |
Dransfield 2009.
| Methods | Design: randomised controlled trial, parallel group Setting: NHLBI COPD Clinical Research Network; 10 centres, USA Comments: study registered online (NCT00457977) and completed in May 2011 Author's name: Mark T. Dransfield Institution: National Heart, Lung and Blood Institute Email: mdransfield99@msn.com Address: University of Alabama at Birmingham and the Birmingham VA Medical Center, 422 THT, 1900 University Blvd, Birmingham, AL 35294, USA Follow‐up: 48 months |
|
| Participants | Inclusion criteria:
‐ > 40 yo male and female
‐ ≥ 10 pack‐year cigarette smoking history
‐ Clinical diagnosis of moderate to very severe COPD (defined as FEV1/FVC < 70% and FEV1 < 70% predicted)
‐ Never received PPSV‐23 OR did not receive PPSV‐23 during the 5 years before randomisation Exclusion criteria: ‐ Diagnosis of asthma ‐ Sensitivity to pneumococcal vaccination ‐ Bleeding disorder, chronic anticoagulation or the presence of conditions known to impair pneumococcal vaccine response ‐ Acute illness requiring antibiotics or steroids within the past month or not expected to survive 12 months N = 181; PPSV‐23 n = 90, PCV‐7 n = 91 No statistically significant differences between pretreatment groups was reported. Age, years (%): 64 (10); 63 (9) FEV1 (% predicted): 44.8 (15); 44.9 (15) ICS use %: 64; 66 Current smoker %: 36; 36 Pack‐years of smoking: 55 (27); 52 (28) Male %: 37; 38 LTOT %: 32; 23 Previous pneumonia %: 45; 44 Hospitalisation or unscheduled emergency visit (≤ 1 year before enrolment) %: 11; 18 Received systemic steroids and/or antibiotics %: 34; 38 Vaccine naive %: 42; 45 Years since last vaccination %: 8.4 ± 3.5; 7.6 ± 2.7 |
|
| Interventions | 23‐Valent pneumococcal polysaccharide vaccine (PPV‐23) 0.5 mL intramuscular 7‐Valent diphtheria‐conjugated pneumococcal polysaccharide vaccine (PCV‐7) 1.0 mL intramuscular |
|
| Outcomes | Vaccine responsiveness: antibody levels (IgG) not included in meta‐analysis Acute exacerbation COPD ‐ Pneumonia: self‐reported by participants; no diagnostic criteria described ‐ Hospitalisation ‐ Fatigue ‐ Headache ‐ Limitation of arm movement ‐ Redness or discolouration ≤ 15 cm ‐ Redness or discolouration > 15 cm ‐ Localised swelling |
|
| Notes | Sponsorship source: 639191; National Heart, Lung and Blood Institute of the National Institutes of Health (U10 HL074441, U10 HL074418, U10 HL074428, U10HL074409, U10 HL074407, U10 HL074422, U10 HL074416, U10 HL074408,U10 HL074439, U10 HL074431, U10 HL074424) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed after linking to the clinical trial co‐ordinating centre website and stratified by study centre. |
| Allocation concealment (selection bias) | Low risk | Independent third party allocation. Randomisation was performed after linking to the clinical trial co‐ordinating centre website and stratified by study centre |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | Open‐label trial with no blinding |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were not blinded, PPV group received influenza, control group received only influenza. Lack of blinding was not likely to affect measurement of dichotomous outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Approximately 15% of people in both groups were lost to follow up and exited the study early. Reasons for withdrawal were given. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported in methods and trial registration are available in publications. |
| Other bias | Unclear risk | Trial relied in part on self‐reported vaccination; some participants may have been misclassified as vaccine naive or previously vaccinated, or may have been enrolled < 5 years after previous PPSV‐23. |
Furumoto 2008.
| Methods | Study design: parallel‐group randomised controlled trial Location, number of centres: 13 hospitals in the district of Kyushu and Okinawa, Japan Duration of study: 2 years (November 2001 to April 2002) |
|
| Participants | Number screened: ≥ 383 potentially eligible patients with CLD contacted by researchers Number randomised: 191 (55 with COPD) Number completed: 167; intervention group n = 87, control group n = 80 Gender distribution: intervention = 69% male; control = 57.5% male Mean age (years): intervention = 67.8 (SD 9); control 70.1 (SD 9.5) Inclusion criteria: ‐ Patients with chronic lung disease (CLD) who previously experienced acute exacerbations and were able to comply with a schedule of monthly clinical visits ‐ Between 40 and 80 years of age ‐ Investigators selected participants. No diagnostic criteria for COPD were given. Exclusion criteria: ‐ Patients who were pregnant or were immunocompromised, with conditions such as active malignant disease, renal insufficiency in dialysis or HIV infection, hypogammaglobulinaemia or anatomical or functional asplenia, who had previously received 23‐valent PV (Pneumovax, Banyu, Japan) Baseline details: Participants with CLD included 55 with COPD (24 PV + IV, 31 IV), 50 with sequelae of pulmonary TB (33 PV + IV, 17 IV), 62 with other CLD (bronchiectasis 20, asthma 13, pneumoconiosis 14, interstitial pneumonia 9, diffuse panbronchiolitis 5, sarcoid 1) (30 PV + IV, 32 IV) |
|
| Interventions | Vaccine type: intervention pneumococcal polysaccharide vaccine (PV) and a trivalent, split virion, influenza vaccine (IV) containing A/NewCaledonia/20/99H1N1, A/Panama/2007/99H3N2 and B/Johannesburg/5/99 for the 2001/2002 season; for the 2002/2003 season, a vaccine containing A/NewCaledonia/20/99H1N1, A/Panama/2007/99H3N2 and B/Guangdong/7/97 Control: a trivalent, split virion, influenza vaccine containing A/NewCaledonia/20/99H1N1, A/Panama/2007/99H3N2 and B/Johannesburg/5/99 for the 2001/2002 season; for the 2002/2003 season, a vaccine containing A/NewCaledonia/20/99H1N1, A/Panama/2007/99H3N2 and B/Guangdong/7/97 but no PV |
|
| Outcomes | Primary outcomes:
‐ Time to first episode of pneumonia or to acute exacerbation (AE) after enrolment in the study: data not available for participants with COPD only ‐ Pneumonia: diagnostic criteria: clinical symptoms (cough, sputum or fever) plus increased WBC count or increased C‐reactive protein or appearance of new infiltration on CXR; data available for participants with COPD ‐ Exacerbations: definition: 2 or 3 of increased dyspnoea, increased sputum volume, increased sputum purulence plus absence of new infiltration on CXR, or 1 of these symptoms and 1 additional symptom plus absence of new infiltration on CXR; data available for participants with COPD. Kaplan–Meier survival curves for infectious acute exacerbation demonstrated a significant difference between the 2 groups (P = 0.041). ‐ Infectious acute exacerbation: defined by increase in WBC count or increased C‐reactive protein. Pneumoccal AE: defined as isolating sputum S pneumoniae. Participants examined monthly by study investigators. Asked to visit study hospital at any onset fever, cough or sputum, or if experiencing breathlessness during 2‐year period; data available for participants with COPD Secondary outcomes: ‐ Mortality data not available for participants with COPD alone |
|
| Notes | Data included only for participants with COPD | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly assigned in equal proportions to either group. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered, opaque, sealed envelopes were held by study administrators. |
| Blinding (performance bias and detection bias) Assessors | High risk | No attempt was made to blind clinical assessors to vaccine allocation in the study. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were not blinded; PPV group received influenza, and control group only received influenza. Lack of blinding was not likely to affect measurement of dichotomous outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | During 2‐year follow‐up period, 2 and 11 participants were lost from the PV + IV and IV groups, respectively. In addition, early termination of follow‐up occurred for 5 participants from the PV + IV group and for 6 participants from the IV group because they wanted to withdraw from the study. Subsequently, 87 participants in the PV + IV group and 80 in the IV group completed the analysis. |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it appears that published reports included prespecified outcomes. |
| Other bias | Low risk | None noted |
Kostinov 2014.
| Methods | Sponsorship source: not stated Country: Russia Setting: 2 centres: Mechnikov Research Institute of Vaccines and Sera, Ministry of Health Omsk; Polyclinic Tyumen Authors' names: Kostinov MP, Ryzhov AA, Magarshak OO, Zhirova SN, Protasov AD, Erofeev YUV, Miunova OV, TOlokonnikova IN, Liverko EV Institution: Mechnikov Research Institute of Vaccines and Sera, Moscow |
|
| Participants | Design: randomised controlled trial, parallel group Follow‐up: 12 months Inclusion criteria: ‐ Participants 30 to 55 years of age ‐ Diagnosis of COPD according to GOLD 2011 ‐ on the basis of patient history, complaints: cough, sputum production, shortness of breath worsening on exercising ‐ All patients had undergone spirometry and bronchodilator reversibility testing (400 mcg of salbutamol). ‐ FEV1 reversibility < 12% (or < 200 mL), ratio FEV1/FVC < 70% Exclusion criteria: ‐ Age < 30, > 50 ‐ Pneumococcal vaccination over past 3 years ‐ Acute infection (TB, active phase of chronic viral hepatitis), mental disorders, renal or hepatic insufficiency, neoplastic disease, chronic disease in exacerbations, hypersensitivity to vaccine components, severe complications of prior vaccinations, pregnancy, autoimmune disease Groups: PPSV‐23 n = 100; no vaccine n = 100 Age (years): 30 to 50 FEV1 % predicted: not known ICS use %: not known Current smoker %: not known Pack‐years of smoking: not known Male: 41 (41%); 31 (31%) LTOT: not known Previous pneumonia: not known Hospitalisation or unscheduled emergency visit (≤ 1 year before enrolment): 16; 6 |
|
| Interventions | Vaccine Pneumo‐23 (Sanofi, France), intramuscular 0.5 mL, once after signing of informed consent | |
| Outcomes | Acute exacerbation COPD Hospitalisation |
|
| Notes | Publication in Russian.Abstract in English. CAP was not listed in methods section as an outcome and no data was available. Extraction was performed by a Russian translator. .Liliya Eugenevna Ziganshina No request was sent to authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "patients were randomised into groups with the use of the method of serial (sequential) numbers" in translated text |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | No information provided, no reference to blinding |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No reference to blinding but no placebo given in control group |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number of participants randomised to treatment and number of participants analysed the same; no withdrawals reported |
| Selective reporting (reporting bias) | Low risk | Results for all outcome measures were reported. |
| Other bias | Low risk | No issues of concern in translation of paper |
Leech 1987.
| Methods | Setting: Montreal Chest Hospital (stable ambulatory population) Study design: randomised, double‐blind, placebo‐controlled trial Type of analysis: case available Follow‐up: 24 months |
|
| Participants | Total number of participants: 189 Gender distribution (male): vaccine = 66; placebo = 69 Mean age of participants (years): vaccine = 66 ± 9; placebo = 67 ± 9 Age range (years): 40 to 89 Inclusion criterion for active group: patients seen in outpatient clinic who had COPD (FEV1 < 1.5 L) Exclusion criteria: previous pneumococcal vaccination, asthma, cystic fibrosis or bronchiectasis Diagnostic criteria (COPD): not stated, other than prior diagnosis of COPD by physician Severity of COPD: vaccine group (mean) FEV1 = 0.94 L; FVC = 2.18 L/s; placebo group (mean) FEV1 = 0.96 L; FVC = 2.13 L/s Microbiological diagnosis (pneumococcus): not stated, although sputum cultured in 10% of participants N = 189 (VAX: 92; PLA: 97) Gender distribution: PLA = 69 M; VAX = 66 M Mean age: PLA = 67 (SD 9); VAX = 66 (SD 9); FEV1 (L): PLA = 0.96 (SD 0.30); VAX = 0.94 (SD 0.26); FVC: PLA = 2.13 (SD 0.64); VAX = 2.18 (SD 0.58) | |
| Interventions | Vaccine types: 14‐valent pneumococcal polysaccharide (in 1 arm) and influenza vaccination (in the other arm)
Numbers in each group: intervention = 92; placebo = 97
Dose: not stated
Delivery: injection
Cointerventions: none
Comparison: saline (in 1 arm) and influenza vaccination (in the other arm)
Follow‐up points: 6‐month intervals
Duration of study: 2 years Influenza vaccination (given at baseline, end of years 1 and 2, unless previous adverse reaction or declined) |
|
| Outcomes | Incidence of pneumonia: diagnostic criteria (pneumococcal pneumonia): pneumonia defined as symptoms of lower respiratory tract infection (fever, increased cough and change in colour or increase in quantity of sputum) and evidence of new infiltrate on chest x‐ray Upper respiratory tract infection (URTI): definition: symptoms of sore throat, runny nose, fever and increased cough without increase in quantity or change in colour of sputum Mortality (all‐cause) Hospital admission (all‐cause); length of hospital stay; emergency visits (all causes); hospital admissions, emergency visits to clinic or emergency department assessed by participant/family interview and chart review Adverse events (pneumococcal sepsis) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Makes reference to study participants "randomly assigned" to control or intervention group; however, does not make reference to method of sequence generation Participants stratified by age and FEV1 |
| Allocation concealment (selection bias) | Unclear risk | Information not available |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | Described as double‐blind study but no information on blinding of assessors given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study with placebo injection given |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | "A total of 23 patients (12%) could not be traced for follow‐up and were not included in the analysis of death rates. At each follow‐up interview some patients refused to answer questions and were not included in the analysis of hospital admissions and emergency visits. 59% followed up at 24 months" |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it appears that published reports include prespecified outcomes. |
| Other bias | Low risk | No other issues identified |
Lin 2013.
| Methods | Sponsorship source: not declared in trial registration Country: Taiwan (from March 2009 to May 2010) Setting: outpatient department of tertiary medical centre, Chest Division, Department of Internal Medicine, Far Eastern Memorial Hospital, New Taipei City, Taiwan Authors' names: Ming‐Tzer Lin1,2,3, Shih‐Lung Cheng4 Institution: Far Eastern Memorial Hospital, Taipei City, Taiwan Email: lightpool2010@gmail.com Addresses: Department of Internal Medicine, Hsiao Chung‐Cheng Hospital; Department of Internal Medicine, National Taiwan University Hospital; Graduate Institute of Epidemiology and Preventive Medicine, College of Public Health, National Taiwan University Design: randomised controlled trial, parallel group |
|
| Participants | Inclusion criterion:
‐ Diagnosis COPD (FEV1/FVC < 70% with exposure to smoking) with high daily dose of ICS (beclometasone equivalent dose > 1000 mcg/d) Exclusion criterion: ‐ Received PPSV‐23 in recent 5 years or immunosuppressed status Group differences: Demographic data were compatible between groups, except PPSV‐23 group had higher number of previous pneumonia episodes than control group (P = 0.038) PPSV‐23 n = 19, placebo n = 17 Age (years): 68.9 (9.2); 72.8 (6.7) FEV1 % predicted: 43.1 (12.3); 46.5 (11.1) ICS use %: 100% 2000 mcg BDP (1250 to 2000); 100% 1500 mcg BDP (1250 to 2000) Current smoker %: 10 (52%) ; 4 (24%) Pack‐years smoking: 57.8 (32.1); 62.7 (32.8) Male: 18 (95%); 14 (82%) Long‐term oxygen therapy: 3 (16%); 3 (18%) Previous pneumonia in past 1 year: 0; 0 Hospitalisation or unscheduled emergency visit (≤ 1 year before enrolment): 1 (0 to 3); 1 (0 to 2) Received systemic steroids and/or antibiotics: NA; NA Vaccine naïve: NA; NA Years since last vaccination: > 5: > 5 |
|
| Interventions | PPSV‐2323‐Valent pneumococcal polysaccharide vaccine 0.5 mL subcutaneously Placebo normal saline 0.5 mL subcutaneously |
|
| Outcomes | Acute exacerbation COPD (person‐years). Moderate exacerbation defined as an exacerbation treated with parenteral corticosteroids with or without an antibiotic Pneumonia in person‐years. Pneumonia was diagnosed according to primary clinician’s judgement Hospitalisation in person‐years Death Change in lung function (postbronchodilator FEV1, FVC) listed in trial registration but not reported in conference presentation |
|
| Notes | Data supplied as conference presentation ClinicalTrials.gov Identifier: sponsor: NCT01381367 Far Eastern Memorial Hospital: first received: 16 February 2009 Information provided by Far Eastern Memorial Hospital Last updated: June 24, 2011; last verified: June 2011 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as double‐blinded, randomised controlled trial. Method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Described as double‐blinded, randomised controlled trial. Allocation after enrolment, method not reported |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | Described as double‐blinded. Placebo used in control group. No details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Described as double‐blinded. Placebo used in control group. No details given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 36 patients recruited: 19 PPSV‐23/17 placebo. Outcome data for all participants reported |
| Selective reporting (reporting bias) | Low risk | All primary and important secondary outcomes listed in trial registration were available in poster report. Lung function not reported |
| Other bias | Low risk | Study not fully published, but poster presentation includes study methods and results. No other issues noted |
Steentoft 2006.
| Methods | Setting of study: hospital‐based Study design: RCT parallel: 1 control group with 3 levels of steroid load, block‐randomised to vaccine or to no vaccine Type of analysis: case available |
|
| Participants | Total number of participants: 49 Gender distribution: M = 27; F = 22 Mean age: control: 67.5 years Intervention: 65, 72 and 71 years for the 3 groups Age range (years): 47 to 86 Inclusion criterion: COPD Diagnostic criteria (COPD): COPD defined by GOLD guidelines (FEV1/FVC < 70%, FEV1 reversibility‐test < 200 mL) Exclusion criterion: prior pneumococcal vaccine Severity of COPD at baseline: ‐ Control: FEV1% = 50.2 ‐ Intervention: FEV1% = 48.2, 46.0 and 44.2 for the 3 groups Smoking status: ‐ Active: current = 46%, past = 54% ‐ Placebo: current = 58%, past = 42% Diagnostic criterion (pneumonia): radiologically verified, but no other criteria stated Etiological diagnosis (pneumococcus): not described |
|
| Interventions | Vaccine type: 23‐polyvalent pneumococcal vaccine Numbers in each group: ‐ Intervention = 37 ‐ Placebo = 12 Dose: 0.5 mL Delivery: subcutaneous injection Cointerventions: ‐ Three groups with various exposure patterns to oral prednisolone * No steroids 3 months before vaccination, then steroids for 4 weeks after vaccination * Long‐term steroid treatment, before and after vaccination * Vaccination after 4 weeks with steroid treatment, then no steroids after vaccination Groups 1 and 3 above received 37.5 mg starting dose of prednisolone, tapered to 0 during respective time frames Comparison: no vaccine Duration of study: 6 months |
|
| Outcomes | Types of outcomes measured:
‐ Acute exacerbations (definition: incidents with fever and expectoration)
‐ Pneumonia (definition: radiologically verified pneumonia)
‐ Number of hospital admissions (all‐cause)
‐ Improvement/worsening in lung function (reported, not analysed) ‐ Extra prednisone use: not analysed ‐ Extra beta agonist use: not analysed ‐ Antibiotics: not analysed ‐ Antibody titres post vaccination‐ not analysed |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were block‐randomised to vaccine or no vaccine. |
| Allocation concealment (selection bias) | Low risk | Third party held randomisation schedule. |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | Not stated for clinical diagnoses if outcomes assessed by person blinded to Tx allocation. Laboratory staff assessing antibody levels were blinded to allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo injection given in control group |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No data on withdrawals given |
| Selective reporting (reporting bias) | Low risk | The study protocol was not available, but it appears that published reports include prespecified outcomes. |
| Other bias | Low risk | None noted |
Teramoto 2007.
| Methods | Study design: parallel‐group randomised controlled trial Setting: Toyko, Japan Duration of study: 2 years No funding declared |
|
| Participants | Number screened: not available Number randomised: 196 Number completed: unclear Gender distribution: not reported Mean age and range (years): 77.8 (75.1 to 80.5) Inclusion criteria: elderly patients with COPD, diagnostic criteria not stated Exclusion criteria: not reported |
|
| Interventions | Intervention: 23‐valent pneumococcal polysaccharide vaccine Control: no vaccination Cointerventions: none Treatment period: single PPV vaccination administered to intervention group Follow‐up period: 2 years |
|
| Outcomes | Pneumonia: definition: radiographically proven community‐acquired pneumonia of pneumococcal or unknown aetiology. Survival plot for community acquired pneumonia: no significant difference reported | |
| Notes | Study available only as abstract publication. Study author contacted for details of study and outcome data 25/09/09, but no response received by 01/03/10 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No mention of allocation sequence generation method, although study described as randomised |
| Allocation concealment (selection bias) | Unclear risk | No description of method used |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | No mention regarding blinding of assessors |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo injection given in control group |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on withdrawals after randomisation |
| Selective reporting (reporting bias) | Unclear risk | One outcome reported in abstract. Other data not published yet |
| Other bias | Low risk | None noted |
Trofimov 2010.
| Methods | Sponsorship source: not known Country: Russia Setting: St Petersburg State Medical University Author's name: Tromifov VI Institution: ZH, Mikrobiol, Moscow Design: randomised controlled trial, parallel group |
|
| Participants | Inclusion criteria for types of participants recruited into the study were not reported. Exclusion criteria for types of participants recruited into the study were not reported. Group differences: groups comparable by age, sex, history and lung function PPSV‐23 n = 20: control n = 25 Male: 14/20 (70%); 16/25 (64%) Age (years): 56.38 (2.78); 52.75 (2.48) FEV1 % predicted: 55.8 (2.8); 67.7 (3.1) ICS use %: not known Current smoker %: not known LTOT: not known Previous pneumonia: not known Hospitalisation or unscheduled emergency visit (≤ 1 year before enrolment): not known Received systemic steroids and/or antibiotics: not known Vaccine naive: not known Years since last vaccination: not known Pack‐years of smoking: not known |
|
| Interventions | PPSV‐23: Vaccine Pneumo‐23 Injectable, route of delivery not reported, dosage not reported Control: standard treatment |
|
| Outcomes | Stable remission of disease during follow‐up of 6 months | |
| Notes | Paper in Russian. Abstract in English. No primary or secondary outcomes for the review were listed in methods section. Extraction was performed by Translator: Liliya Eugenevna Ziganshina 24/03/15 No request was sent to authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described in translated text as open randomised study, but no method specified |
| Allocation concealment (selection bias) | Unclear risk | No information on allocation |
| Blinding (performance bias and detection bias) Assessors | High risk | Open randomised study; no placebo |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open randomised study; placebo not given |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 45 participants randomised; no information on withdrawals |
| Selective reporting (reporting bias) | Unclear risk | Lung function, blood count, sputum cytology, immunological parameters and stability of disease were listed as outcomes. Results for lung function were not presented. All intervention groups were described as having 'stable remission of disease' and 20% in standard treatment group |
| Other bias | Low risk | None noted |
Ya Tseimakh 2006.
| Methods | Setting of study: Barnaul, Russia Study design: parallel‐group randomised controlled trial Duration of study: 6 months |
|
| Participants | Number screened: not available Number randomised: 373 Number completed: 373 Gender distribution: not available Mean age (years): intervention 57.9 ± 0.51; control 57.8 ± 0.95 Inclusion criteria: ‐ Patients with COPD (diagnostic criteria not stated) ‐ Age 18 to 70 years ‐ Frequency of exacerbations of COPD before beginning of studies ≥ 2 times per year Exclusion criterion: ‐ Patients with immunodeficiency, long‐term systemic glucocorticoids |
|
| Interventions | Intervention: pneumococcal polysaccharide vaccine 'Pneumo 23' Control: no vaccine. Cointerventions: none Treatment period: single vaccination given to control group Follow‐up period: 6 months |
|
| Outcomes | COPD exacerbations ‐ no definition given: reported mean rate with SD Acute respiratory infection (ARI): no definition given Adverse events (erythema, induration, fever, headache): % reported for vaccine group |
|
| Notes | Only interim results available, as abstract publication. Study authors contacted for 12‐month data without response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised controlled trial; no description of method used for randomisation |
| Allocation concealment (selection bias) | Unclear risk | No mention of allocation concealment method used; control group did not receive treatment |
| Blinding (performance bias and detection bias) Assessors | High risk | No blinding of participants or study personnel |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo injection given in control group |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information supplied regarding withdrawals |
| Selective reporting (reporting bias) | Unclear risk | Publication as abstract only; no response to request data |
| Other bias | Low risk | No other issues identified |
Yilmaz 2013.
| Methods | Sponsorship source: no information available Country: Turkey/UK Setting: tertiary hospital, conducted between July 2006 and October 2008 Comments: "Publication details abstract 2013; 187 (meeting abstracts): A2182. Unpublished data requested and supplied by author" Authors' names: Yilmaz D, Uzaslan E, Ege E Institution: Uludad University Medical Faculty, Bursa/St George's, London Email, Dilber Yılmaz Durmaz: drdilberyilmaz@gmail.com Address: St George's Hospital, University of London, Uludag University Medical Faculty, Bursa, Turkey Design: randomised controlled trial, parallel group Follow‐up: 24 months |
|
| Participants | Inclusion criteria:
‐ Clinical diagnosis of COPD ≥ 12 months before baseline visit
‐ Age ≥ 40
‐ Written informed consent
‐ Former or current smoker with a smoking history ≥ 10 pack‐years Exclusion criteria: ‐ Vaccination with PPV within previous 5 years ‐ Immune suppression ‐ Chronic renal failure ‐ Bronchiectasis ‐ Previous lung surgery ‐ Malignancy ‐ COPD exacerbation or pneumonia within previous 30 days ‐ Unstable cardiac disease ‐ Pregnancy or suspected pregnancy Group differences: PV and placebo group had no significant difference in terms of age, sex, GOLD stages, annual influenza vaccination, pneumonia history, number of exacerbations and pneumonia in the past 1 and 2 years (P > 0.05) PPSV‐23 n = 116; placebo n = 28 Group differences: no significant differences in baseline characteristics between the 2 groups. All differences between baseline characteristics were non‐significant Age (years): 65.3 ± 9.3; 64.9 ± 8.8 FEV1 (L): 1.48 (± 0.617); 1.408 (± 0.54) ICS use %: NA; NA Current smoker %: NA; NA Pack‐years of smoking: 48.6 ± 27.9; 40.6 ± 23.6 Male: 108/116 (93%); 26/28 (93%) LTOT: NA; NA Previous pneumonia: 74 (63%); 16 (57%) Hospitalisation or unscheduled emergency visit (≤ 1 year before enrolment): NA; NA Received systemic steroids and/or antibiotics: NA; NA Vaccine naive: 0; 0 Years since last vaccination: NA Mean FEV1 (mL): 1438 ± 617; 1408 ± 540 |
|
| Interventions | PPSV‐23: 23‐valent PPV (Pneumo 23, Lyon, France), dose NA, route not stated Placebo: not described |
|
| Outcomes | Acute exacerbation COPD (defined as an acute event characterised by worsening of respiratory symptoms beyond normal day‐to‐day variations) Courses of antibiotics Hospitalisation Death Emergency department visits FEV1 (L) SGRQ (St George's Respiratory Questionnaire) |
|
| Notes | Published as abstract; Dr. Dilber Yilmaz supplied an unpublished draft of the study findings in May 2015, following a request in Feb 2015. Pneumonia was not listed or reported as an outcome. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, prospective, single‐blind, 24‐month trial; no details on randomisation schedule |
| Allocation concealment (selection bias) | Unclear risk | Randomised, prospective, single‐blind, 24‐month trial; no details on allocation method. Unequal group numbers 3:1 (active:placebo) |
| Blinding (performance bias and detection bias) Assessors | Unclear risk | Described as prospective, single‐blind, 24‐month trial; no details on which group was blinded |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Described as prospective, single‐blind trial. No indication that placebo injection was used in control group |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Study authors report that all participants were followed to the end of 2 years. |
| Selective reporting (reporting bias) | Low risk | All results were reported. Results for all outcomes listed in methods were reported. |
| Other bias | Low risk | No other issues identified |
AE: acute exacerbation; ARI; acute respiratory infection; ATS: American Thoracic Society; C; control; CLD: chronic lung disease; COPD: chronic obstructive pulmonary disease; CSF: cerebrospinal fluid; CXR: chest x‐ray; F: female; FEV1: forced expiratory volume in 1 second; FVC: forced vital capacity; GOLD: Global Initiative for Chronic Obstructive Lung Disease; HIV: human immunodeficiency virus; I: intervention; ICS: inhaled corticosteroids; IgG: immunoglobulin G; IV: influenza vaccine; LTOT: long‐term oxygen therapy; M: male; MSD: Merck Sharpe and Dohme; NHLBI: National Heart, Lung and Blood Institute; PCV: pneumococcal conjugated vaccine; PLA: placebo; PPSV: pneumococcal polysaccharide vaccine; PV: polysaccharide vaccine; RCT: randomised controlled trial; S pneumoniae: Streptococcus pneumoniae; SD: standard deviation; TB: tuberculosis; Tx: treatment; VA: Veterans Administration; VAX: vaccination; WBC: white blood cell
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aboussouan 1996 | Review article |
| Austrian 1976 | Participants are unlikely to have had COPD, and certainly no results are available for persons with COPD. |
| Austrian 1981 | Review article |
| Austrian 1984 | Editorial |
| Bacle 1997 | Review article |
| Bentley 1981 | Review article |
| Bolan 1986 | Not an RCT |
| Broome 1981 | Review article |
| Butler 1992 | Retrospective analysis of vaccine efficacy |
| Butler 1993 | Retrospective analysis of vaccine efficacy |
| Chang 2012 | Cohort study |
| Chodosh 1991 | Review article |
| Christenson 2001 | Prospective study (not an RCT) |
| Dilokthornsakul 2014 | Study observed the association between pneumococcal vaccine and thrombocytopaenia in participants with COPD. Not an efficacy study of pneumococcal vaccinations in participants with COPD |
| Douglas 1979 | Review article |
| Douglas 1984 | Study carried out in children 6 to 54 months |
| Ekwurzel 1938 | Excluded, as participants unlikely to have had COPD ("youthful group, 80% being under 25 years of age") |
| Ewig 1999 | Review article |
| Farr 1995 | Matched case‐controlled study |
| Fedson 1989 | Review article |
| Fedson 1994 | Review article |
| Fedson 1999 | Review article |
| Felton 1938 | Cohort observation study |
| Ferguson 1993 | Review article |
| Filice 1990 | Review article |
| Fine 1994 | Meta‐analysis |
| Forrester 1987 | Case‐controlled study |
| Foschino 1995 | Oral immunomodulator (not injectable vaccine) |
| Gable 1990 | Retrospective cohort study |
| Gaillat 1985 | No data available for participants with COPD |
| Gaillat 2009 | Narrative review |
| Gardner 1993 | Review article |
| Greenberg 2014 | Wrong patient population. Participants were not patients with COPD. |
| Gross 2010 | Narrative review |
| Hak 1998 | Prospective cohort study |
| Halasa 2001 | Injectable vaccine includes antigen from pneumococcus and other bacteria (written in Polish language). |
| Han 2011 | Narrative review |
| Hilleman 1981 | Review article |
| Hirschmann 1981 | Review article |
| Hirschmann 1994 | Commentary |
| Horwood 2002 | Review article |
| Hughes 2011 | Cross‐sectional study of predictors of colonisation of Pneumococcus bacterium in participants with COPD |
| Hung 2010 | Prospective cohort study |
| Jackson 2003 | Retrospective cohort study |
| Jimenez‐Garcia 2007 | Descriptive study of influenza and pneumococcal vaccination coverage among participants suffering from COPD |
| Jonsson 2002 | Study compares 23 valent pneumococcal vaccine or type 6B polysaccharide conjugated to tetanus toxoid in participants with COPD vs healthy adult controls |
| Kaiser 1974 | Retrospective analysis of isolates |
| Kaufman 1941 | Participants not adequately randomised. Participants allocated to active treatment by volunteering 1 year followed by by alternate allocation in the subsequent year |
| Kaufman 1947 | Likely to have included participants with COPD, given the age range of those involved in the study (80% > 60 years), although inclusion of persons with COPD was not explicitly stated. Request was made to originating institutions to provide relevant analyses of COPD subgroup, but no response was obtained. |
| Klastersky 1986 | No data available for participants with COPD |
| Klein 1983 | Trial of immunisation rates |
| Koivula 1997 | No data available for participants with COPD |
| Kraus 1985 | Study of antibody responses |
| LaForce 1989 | Review article |
| Lai 2007 | Experimental study of antibody responses to a 23‐valent pneumococcal polysaccharide vaccine and clinical outcome in Taiwanese participants with COPD |
| Landesman 1983 | Study of antibody responses |
| Larsson 1998 | Review article |
| Lee 2007 | Retrospective cohort study |
| Leophonte 2001 | Review article |
| MacIntyre 2010 | Study assessed safety of concomitant zoster vaccination with pneumococcal vaccination in healthy participants without COPD. |
| MacLeod 1945 | CCT in young adults; COPD unlikely |
| Madison 1998 | Review article |
| Meyer 2006 | Comparison of Pneumovax given by inhalation, alveolar vaccination or bronchial vaccination vs standard intramuscular vaccination. No placebo control |
| Monso 2003 | Commentary |
| Nichol 1999 | Retrospective cohort control study |
| Ochoa‐Gondar 2008 | Prospective cohort study |
| Orcel 1994 | Oral immunomodulator (not injectable vaccine) |
| Ortqvist 1998 | No data available for participants with COPD |
| Patrick 1981 | Cost/benefit analysis |
| Preheim 1978 | Case report |
| Ricci 2014 | Wong intervention. Study assessed efficacy of a sublingual pneumococcal vaccination. |
| Riley 1977 | No data available for participants with COPD |
| Rochemaure 1988 | Antigens for this oral immunomodulator are taken from Klebsiella pneumoniae and Escherichia coli (not Streptococcus pneumoniae). |
| Saag 1998 | Survey |
| Schenkein 2008 | Narrative review of pneumococcal vaccination in COPD. Not an RCT |
| Schnelle 2010 | Not an RCT |
| Schwartz 1982 | Review article |
| Sehatzadeh 2012 | Meta‐analysis |
| Shapiro 1984 | Case‐controlled study |
| Shapiro 1987 | Correspondence |
| Shapiro 1991 | Case‐controlled study |
| Sheikh 1999 | Asthma study |
| Simberkoff 1986 | No data available for participants with COPD |
| Simberkoff 1993 | Review article |
| Sims 1988 | Case‐controlled study |
| Sisk 1986 | Cost/benefit analysis; no data on efficacy |
| Smit 1977 | Participants were young adult novice miners, with no indication of chronic lung disease. Wrote to study authors for further information, but received no response (Oct 2004) |
| Sumitani 2008 | Not an RCT. Observational study; participants immunised with influenza vaccine (I‐V) and 23‐valent pneumococcal vaccine (P‐V) |
| Van Amptin 1998 | Retrospective study of patients hospitalised with infection |
| Vila‐Corcoles 2012 | Case‐controlled study |
| Watanuki 2007 | Cohort follow‐up study. Study author request for study details 12/05/09, but no response received |
| Wencker 1999 | Alpha 1 antitrypsin deficiency |
| Wenzel 1976 | Inappropriate intervention including mycoplasma rather than Streptococcus pneumoniae |
| WHO 1999 | Position paper |
| WHO 1999b | Review article |
| Wiebel 1977 | Antibody response study |
| Willems 1980 | Non‐randomised cost‐effectiveness study |
| Williams 1986 | Review article |
| Wright 1914 | Participants were young (otherwise healthy) mining labourers with no indication of having COPD |
CCT: case‐controlled trial; COPD: Chronic obstructive pulmonary disease; RCT: randomised controlled trial.
Differences between protocol and review
Review authors promoted pneumonia from a secondary to a primary outcome in the 2010 update. For the 2016 version of the review, review authors have changed the title to highlight the focus on the clinically relevant outcome of pneumonia. We have updated the Background by including information on new vaccines and guidelines. Studies comparing different types of vaccines have been conducted since the 2010 update, and we have included one of them (Dransfield 2009). Since the last update (in 2010), we have added new standard Cochrane headings and tables assessing risk of bias and providing a summary of findings.
Contributions of authors
Julia AE Walters: author of the review in 2009 and 2016: contributed to selection of studies; data extraction, analysis and interpretation; and writing of final review versions.
Joanne Ngie Qing Tang: author of the review in 2016: contributed to selection of studies; data extraction, analysis and interpretation; and writing of final review versions.
Phillippa Poole: developed the original protocol; edited and reviewed update drafts in 2004, 2009 and 2016.
Richard Wood‐Baker: developed original protocol; contributed to review versions in 2004 and 2009 through study selection; data extraction/entry, analysis and interpretation; and writing of the review; reviewed Results and Conclusions in update drafts in 2016.
Sources of support
Internal sources
-
RWB, JT, JAEW, Australia.
University of Tasmania
External sources
-
JAEW, Australia.
NHMRC Translation funding for Cochrane Airways Australia satellite
-
JT, Australia.
Cochrane scholarship award, Asthma Foundation Tasmania
Declarations of interest
Julia Walters: none known.
Joanne Tang: none known. Co‐recipient of the Cochrane Airways Australia Satellite Scholarship that required a completion/update and presentation of a Cochrane Review.
Phillippa Poole: none known.
Richard Wood‐Baker: none known.
Edited (no change to conclusions)
References
References to studies included in this review
Alfageme 2006 {published data only (unpublished sought but not used)}
- Alfageme I, Reyes N, Vazquez R, Perez J, Munoz, Hernandez M, et al. Clinical efficacy of pneumococcal vaccine in COPD patients. Preliminary results. Chest 2004;126(4 Suppl):837S. [Google Scholar]
- Alfageme I, Vazquez R, Reyes N, Munoz J, Fernandez A, Hernandez M, et al. Clinical efficacy of anti‐pneumococcal vaccination in patients with COPD. Thorax 2006;61:189‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davis 1987 {published data only}
- Davis AL, Aranda C, Christianson L, Schiffman G. Effects of 14‐valent pneumococcal capsular polysaccharide vaccine in patients with COPD. Chest 1984;85(Suppl):82‐3. [DOI: 10.1378/chest.85.6_Supplement.82S-a] [DOI] [Google Scholar]
- Davis AL, Aranda CP, Schiffman G, Christianson LC. Pneumococcal infection and immunologic response to pneumococcal vaccine in chronic obstructive pulmonary disease. A pilot study. Chest 1987;92(2):204‐12. [DOI] [PubMed] [Google Scholar]
Dransfield 2009 {published data only}
- Dransfield MT, Harnden S, Burton RL, Albert RK, Bailey WC, Casaburi R, et al. Long‐term comparative immunogenicity of protein conjugate and free polysaccharide pneumococcal vaccines in chronic obstructive pulmonary disease. Clinical Infectious Diseases 2012;55:e35‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dransfield MT, Nahm MH, Han MK, Harnden S, Criner GJ, Martinez FJ, et al. Superior immune response to protein‐conjugate versus free pneumococcal polysaccharide vaccine in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2009;180(6):499‐505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00457977. Comparing two pneumococcal vaccines in adults with chronic obstructive pulmonary disease (PNEUMO). https://clinicaltrials.gov/ct2/show/NCT00457977 (accessed 1 August 2016).
Furumoto 2008 {published data only}
- Furumoto A, Ohkusa Y, Chen M, Kawakami K, Masaki H, Sueyasu Y, et al. Additive effect of pneumococcal vaccine and influenza vaccine on acute exacerbation in patients with chronic lung disease. Vaccine 2008;26(33):4284‐9. [DOI] [PubMed] [Google Scholar]
Kostinov 2014 {published data only}
- Kostinov MP, Ryzhov AA, Magarshak OO, Zhirova SN, Protasov AD, Erofeev IuV, et al. [The clinical aspects of efficiency of the prevention of pneumococcal infection with vaccines in chronic obstructive pulmonary disease patients living in the West Siberian Region]. [Russian]. Terapevticheskii Arkhiv 2014;86(3):28‐33. [PubMed] [Google Scholar]
Leech 1987 {published data only}
- Leech JA, Gervais A, Ruben FL. Efficacy of pneumococcal vaccine in severe chronic obstructive pulmonary disease. Canadian Medical Association Journal 1987;136(4):361‐5. [PMC free article] [PubMed] [Google Scholar]
Lin 2013 {unpublished data only}
- NCT01381367. PPSV23 pneumococcal vaccine in chronic obstructive pulmonary disease (COPD). https://clinicaltrials.gov/ct2/show/NCT01381367 (accessed 1 August 2016).
Steentoft 2006 {published data only}
- Steentoft J, Konradsen HB, Hilskov J, Giglason G, Andersen JR. Response to pneumococcal vaccine in chronic obstructive lung disease ‐ the effect of ongoing, systemic steroid treatment. Vaccine 2006;24:1408‐12. [DOI] [PubMed] [Google Scholar]
Teramoto 2007 {published data only (unpublished sought but not used)}
- Teramoto S, Yamamoto H, Yamaguchi Y, Hanaoka Y, Ishil M, Ouchi Y, et al. Clinical efficacy of anti‐pneumococcal vaccination in elderly patients with COPD [Abstract]. American Thoracic Society International Conference; 2007 May 18‐23; San Francisco. 2007:A137.
Trofimov 2010 {published data only}
- Trofimov VI, Shaporova NL, Marchenko VN, Smirnova IaA. [Effect of pneumo 23 vaccine administration in patients with chronic obstructive pulmonary disease of intermediate severity]. [Russian]. Zhurnal Mikrobiologii, Epidemiologii, i Immunobiologii 2010;4:41‐4. [PubMed] [Google Scholar]
Ya Tseimakh 2006 {published data only (unpublished sought but not used)}
- Ya Tseimakh I, Martynenko I, Paraeva S. Prophylactic efficacy of pneumococcal vaccination for chronic obstructive pulmonary disease (COPD) [Abstract]. European Respiratory Journal 2006;28(Suppl 50):178s [P1091]. [Google Scholar]
Yilmaz 2013 {published and unpublished data}
- Yilmaz D, Uzaslan E, Ege E. Impact of pneumococcal polysaccharide vaccine on acute exacerbation and quality of life in COPD patients (Abstract). American Journal of Respiratory and Critical Care Medicine 2013;187(Meeting Abstracts):A2182. [Google Scholar]
References to studies excluded from this review
Aboussouan 1996 {published data only}
- Aboussouan LS. Acute exacerbations of chronic bronchitis: focusing management for optimum results. Postgraduate Medicine 1996;99(4):89‐104. [PubMed] [Google Scholar]
Austrian 1976 {published data only}
- Austrian R, Douglas RM, Schiffman C. Prevention of pneumococcal pneumonia by vaccination. Transactions of the Association of American Physicians 1976;89:184‐94. [PubMed] [Google Scholar]
Austrian 1981 {published data only}
- Austrian R. Some observations on the pneumococcus and on the current status of pneumococcal disease and its prevention. Reviews of Infectious Diseases 1981;3(Suppl):S1‐17. [DOI] [PubMed] [Google Scholar]
Austrian 1984 {published data only}
- Austrian R. A reassessment of pneumococcal vaccine. New England Journal of Medicine 1984;310:651‐3. [DOI] [PubMed] [Google Scholar]
Bacle 1997 {published data only}
- Bacle A, Diot P, Lemarie E. Anti‐pneumococci vaccine: justifications and results. Revue de Pneumologie Clinique 1997;53(3):128‐37. [PubMed] [Google Scholar]
Bentley 1981 {published data only}
- Bentley DW. Pneumococcal vaccine in the institutionalized elderly: review of past and recent studies. Reviews of Infectious Diseases 1981;3(Suppl):S61‐70. [DOI] [PubMed] [Google Scholar]
Bolan 1986 {published data only}
- Bolan G, Broome CV, Facklam RR, Plikaytis BD, Fraser DW, Schlech WF. Pneumococcal vaccine efficacy in selected populations in the United States. Annals of Internal Medicine 1986;104:1‐6. [DOI] [PubMed] [Google Scholar]
Broome 1981 {published data only}
- Broome CV. Efficacy of pneumococcal polysaccharide vaccines. Reviews of Infectious Diseases 1981;3(Suppl):S82‐8. [DOI] [PubMed] [Google Scholar]
Butler 1992 {published data only}
- Butler JC, Breiman RF, Campbell JF, et al. Efficacy of the pneumococcal vaccine: Is once enough?. 32nd Interscience Conference on Antimicrobial Agents and Chemotherapy; 1992 11‐14 Oct; Anaheim. 1992.
Butler 1993 {published data only}
- Butler JC, Breiman RF, Campbell JF, Lipman HB, Broome C, Facklam RR. Pneumococcal polysaccharide vaccine efficacy. JAMA 1993;270:1826‐31. [PubMed] [Google Scholar]
Chang 2012 {published data only}
- Chang YC, Chou YJ, Liu JY, Yeh TF, Huang N. Additive benefits of pneumococcal and influenza vaccines among elderly persons aged 75 years or older in Taiwan‐a representative population‐based comparative study. Journal of Infection 2012;65(3):231‐8. [DOI] [PubMed] [Google Scholar]
Chodosh 1991 {published data only}
- Chodosh S. Treatment of acute exacerbations of chronic bronchitis: state of the art. American Journal of Medicine 1991;91(Suppl 6A):87S‐91S. [DOI] [PubMed] [Google Scholar]
Christenson 2001 {published data only}
- Christenson B, Lundbergh P, Hedlund J, Ortqvist A. Effects of a large‐scale intervention with influenza and 23‐valent pneumococcal vaccines in adults aged 65 years of older: a prospective study. Lancet 2001;357(9261):1008‐11. [DOI] [PubMed] [Google Scholar]
Dilokthornsakul 2014 {published data only}
- Dilokthornsakul P, Lee TA. The association between pneumococcal vaccine and thrombocytopenia in elderly patients with chronic obstructive pulmonary disease: a case‐crossover study. 30th International Conference on Pharmacoepidemiology and Therapeutic Risk Management; 2014 Oct 24‐27; Taipei. 2014.
Douglas 1979 {published data only}
- Douglas RM, Riley ID. Pneumococcal disease and its prevention with polyvalent pneumococcal polysaccharide vaccines ‐ a review. Australian and New Zealand Journal of Medicine 1979;9:327‐38. [DOI] [PubMed] [Google Scholar]
Douglas 1984 {published data only}
- Douglas RM, Miles HB. Vaccination against Streptococcus pneumoniae in childhood: lack of demonstrable benefit in young Australian children. Journal of Infectious Diseases 1984;149(6):861‐9. [DOI] [PubMed] [Google Scholar]
Ekwurzel 1938 {published data only}
- Ekwurzel GM, Simmons JS, Dublin LI, Felton LD. Studies on immunizing substances in pneumococci. Public Health Reports 1938;53:1877‐93. [Google Scholar]
Ewig 1999 {published data only}
- Ewig S, Soler N, Torres A. Chronic obstructive pulmonary disease and infection: from stable patients to pneumonia. Clinical Pulmonary Medicine 1999;6(1):1‐8. [Google Scholar]
Farr 1995 {published data only}
- Farr BM, Johnston BL, Cobb DK, Fisch MJ, Germanson TP, Adal KA. Preventing pneumococcal bacteremia in patients at risk. Archives of Internal Medicine 1995;155:2336‐40. [PubMed] [Google Scholar]
Fedson 1989 {published data only}
- Fedson D, Henrichsen J, Makela H, Austrian R. Immunization of elderly people with polyvalent pneumococcal vaccine. Infection 1989;617(6):437‐41. [DOI] [PubMed] [Google Scholar]
Fedson 1994 {published data only}
- Fedson DS, Shapiro ED, LaForce FM, Mufson MA, Musher DM, Spika JS, et al. Pneumococcal vaccine after 15 years of use. Another view. Archives of Internal Medicine 1994;154(22):2531‐5. [PubMed] [Google Scholar]
Fedson 1999 {published data only}
- Fedson DS. The clinical effectiveness of pneumococcal vaccination: a brief review. Vaccine 1999;17:S85‐90. [DOI] [PubMed] [Google Scholar]
Felton 1938 {published data only}
- Felton LD, Ekwurtzel GM, Simmons JS, Dublin LI. Studies on immunizing substances in pneumococci. Public Health Reports 1938;53:1877. [Google Scholar]
Ferguson 1993 {published data only}
- Ferguson GT, Cherniack RM. Management of chronic obstructive pulmonary disease. New England Journal of Medicine 1993;328(17):1017‐22. [DOI] [PubMed] [Google Scholar]
Filice 1990 {published data only}
- Filice GA. Pneumoccal vaccines and public health policy. Archives of Internal Medicine 1990;150:1373‐5. [PubMed] [Google Scholar]
Fine 1994 {published data only}
- Fine MJ, Smith MA, Carson CA, Meffe F, Snakey SS, Weissfeld LA, et al. Efficacy of pneumococcal vaccination in adults. A meta‐analysis of randomized controlled trials. Archives of Internal Medicine 1994;154(23):2666‐77. [DOI] [PubMed] [Google Scholar]
Forrester 1987 {published data only}
- Forrester HL, Jahnigen DW, LaForce FM. Inefficacy of pneumococcal vaccine in a high‐risk population. American Journal of Medicine 1987;83(3):425‐30. [DOI] [PubMed] [Google Scholar]
Foschino 1995 {published data only}
- Foschino BMP, Resta O, Cassano A, Altieri A, Guido P, Piti A, et al. Infectious exacerbations of chronic pulmonary diseases. Therapeutic effectiveness of immunomodulants [Riacutizzazioni delle broncopneumopatie croniche]. Minerva Pneumologica 1995;43(2):39‐44. [Google Scholar]
Gable 1990 {published data only}
- Gable CB, Holzer SS, Engelhart L, Friedman RB, Smeltz F, Schroeder D, et al. Pneumococcal vaccine: efficacy and associated cost savings. JAMA 1990;264:2910‐5. [DOI] [PubMed] [Google Scholar]
Gaillat 1985 {published data only}
- Gaillat J, Zmirou D, Mallaret MR, Rouhan D, Stahl JP, Delormas P, et al. Clinical trial of an antipneumococcal vaccine in elderly subjects living in institutions [Essai clinique du vaccin antipneumococcique chez des personnes agées vivant en institution]. Revue d'Epidemiologie et de Sante Publique 1985;33(6):437‐44. [PubMed] [Google Scholar]
Gaillat 2009 {published data only}
- Gaillat J. Should patients with chronic obstructive pulmonary disease be vaccinated against pneumococcal diseases?. Expert Review of Respiratory Medicine 2009;3(6):585‐96. [DOI] [PubMed] [Google Scholar]
Gardner 1993 {published data only}
- Gardner P, Schaffner W. Immunization of adults. New England Journal of Medicine 1993;328(17):1252‐8. [DOI] [PubMed] [Google Scholar]
Greenberg 2014 {published data only}
- Greenberg RN, Gurtman A, Frenck RW, Strout C, Jansen KU, Trammel J, et al. Sequential administration of 13‐valent pneumococcal conjugate vaccine and 23‐valent pneumococcal polysaccharide vaccine in pneumococcal vaccine‐naive adults 60‐64 years of age. Vaccine 2014;32(20):2364‐74. [DOI] [PubMed] [Google Scholar]
Gross 2010 {published data only}
- Gross NJ. The COPD pipeline VII. COPD 2010;7(6):438‐40. [DOI] [PubMed] [Google Scholar]
Hak 1998 {published data only}
- Hak E, Essen GA, Buskens E, Stalman W, Melker RA. Is immunising all patients with chronic lung disease in the community against influenza cost effective? Evidence from a general practice based clinical prospective cohort study in Utrecht, The Netherlands. Journal of Epidemiology and Community Health 1998;52(2):120‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Halasa 2001 {published data only}
- Halasa J, Halasa M, Wojciechowska W, Podkowinska I, Kucharska E. Clinical efficacy of autovaccine in the treatment of infectious nonatopic asthma and COPD ‐ double blind placebo controlled trial [Ocena efektow leczenia autoszczepionka astmy nieatopowej infekcyjnej i POChP ‐ badanie z zastosowaniem podwojnie slepej proby z placebo]. Alergia Astma Immunologia 2001;6(2):109‐13. [Google Scholar]
Han 2011 {published data only}
- Han MK, Martinez FJ. Pharmacotherapeutic approaches to preventing acute exacerbations of chronic obstructive pulmonary disease. Proceedings of the American Thoracic Society 2011;8(4):356‐62. [DOI] [PubMed] [Google Scholar]
Hilleman 1981 {published data only}
- Hilleman MR, Carlson AJ Jr, McLean AA, Vella PP, Weibel RE, Woodhour AF. Streptococcus pneumoniae polysaccharide vaccine: age and dose responses, safety, persistence of antibody, revaccination, and simultaneous administration of pneumococcal and influenza vaccines. Reviews of Infectious Diseases 1981;3(Suppl):S31‐42. [DOI] [PubMed] [Google Scholar]
Hirschmann 1981 {published data only}
- Hirschmann JV, Lipsky BA. Pneumococcal vaccine in the United States: a critical analysis. JAMA 1981;246:1428‐32. [PubMed] [Google Scholar]
Hirschmann 1994 {published data only}
- Hirschmann JF. The pneumococcal vaccine after 15 years of use. Archives of Internal Medicine 1994;154:373‐7. [PubMed] [Google Scholar]
Horwood 2002 {published data only}
- Horwood F, Macfarlane J. Pneumococcal and influenza vaccination: current situation and future prospects. Thorax 2002;57(Suppl II):ii24‐ii30. [PMC free article] [PubMed] [Google Scholar]
Hughes 2011 {published data only}
- Hughes JM, Dransfield MT. Predictors of colonisation of pneumococcus in patients with COPD (Abstract). http://www.atsjournals.org/doi/pdf/10.1164/ajrccm‐conference.2011.183.1_MeetingAbstracts.A4565 (accessed 1 August 2016).
Hung 2010 {published data only}
- Hung IF, Leung AY, Chu DW, Leung D, Cheung T, Chan CK, et al. Prevention of acute myocardial infarction and stroke among elderly persons by dual pneumococcal and influenza vaccination: a prospective cohort study. Clinical Infectious Diseases 2010;51(9):1007‐16. [DOI] [PubMed] [Google Scholar]
Jackson 2003 {published data only}
- Jackson L, Neuzil K, Yu O, Benson P, Barlow W, Adams A, et al. Effectiveness of pneumococcal polysaccharide vaccine in older adults. New England Journal of Medicine 2003;348(18):1747‐55. [DOI] [PubMed] [Google Scholar]
Jimenez‐Garcia 2007 {published data only}
- Jimenez‐Garcia R, Arinez‐Fernandez MC, Hernandez‐Barrera V, Garcia‐Carballo MM, Miguel AG, Carrasco‐Garrido P. Compliance with influenza and pneumococcal vaccination among patients with chronic obstructive pulmonary disease consulting their medical practitioners in Catalonia, Spain. Journal of Infection 2007;54(1):65‐74. [DOI] [PubMed] [Google Scholar]
Jonsson 2002 {published data only}
- Jonsson S, Vidarsson G, Valdimarsson H, Schiffman G, Schneerson R, Jonsdottir I. Vaccination of COPD patients with a pneumococcus type 6B tetanus toxoid conjugate vaccine. European Respiratory Journal 2002;20(4):813‐8. [DOI] [PubMed] [Google Scholar]
Kaiser 1974 {published data only}
- Kaiser AB, Schaffner W. Prospectus: the prevention of bacteremic pneumococcal pneumonia. A conservative appraisal of vaccine intervention. JAMA 1974;230(3):404‐8. [PubMed] [Google Scholar]
Kaufman 1941 {published data only}
- Kaufman P. Studies on old age pneumonia II. Prophylactic effect of pneumococcus polysaccharide against pneumonia. Archives of Internal Medicine 1941;67:304‐19. [Google Scholar]
Kaufman 1947 {published data only}
- Kaufman P, Kaeffely A, Klingst SK, O'Brien C, Stein H. Pneumonia in old age: active immunization against pneumonia with pneumococcus polysaccharide: results of a six‐year study. Archives of Internal Medicine 1947;79:518‐31. [DOI] [PubMed] [Google Scholar]
Klastersky 1986 {published data only}
- Klastersky J, Mommen P, Cantraine F, Safary A. Placebo controlled pneumococcal immunisation in patients with bronchogenic carcinoma. European Journal of Cancer and Clinical Oncology 1986;22:807‐13. [DOI] [PubMed] [Google Scholar]
Klein 1983 {published data only}
- Klein RS, Adachi N. Pneumococcal vaccine in the hospital. Improved use and implications for high‐risk patients. Archives of Internal Medicine 1983;143(10):1878‐81. [DOI] [PubMed] [Google Scholar]
Koivula 1997 {published data only}
- Koivula I, Sten M, Leinonen M, Makela PH. Clinical efficacy of pneumococcal vaccine in the elderly: a randomized, single‐blind population‐based trial. American Journal of Medine 1997;103:281‐90. [DOI] [PubMed] [Google Scholar]
Kraus 1985 {published data only}
- Kraus C, Fischer S, Ansorg R, Huttemann U. Pneumococcal antibodies (IgG, IgM) in patients with chronic obstructive lung disease 3 years after pneumococcal vaccination. Medical Microbiology and Immunology 1985;174(1):51‐8. [DOI] [PubMed] [Google Scholar]
LaForce 1989 {published data only}
- LaForce LM. Pneumococcal vaccine. Seminars in Respiratory Infections 1989;4(4):293‐8. [PubMed] [Google Scholar]
Lai 2007 {published data only}
- Lai CC, Lee LN, Yu CJ, Hsueh PR, Yang PC, Kuo SH, et al. Antibody responses to pneumococcal polysaccharide vaccine in Taiwanese patients with chronic obstructive pulmonary disease. Journal of the Formosan Medical Association 2007;106(3):196‐203. [DOI] [PubMed] [Google Scholar]
Landesman 1983 {published data only}
- Landesman SH, Smith PM, Schiffman G. Pneumococcal vaccine in elderly patients with COPD. Chest 1983;84:433‐5. [DOI] [PubMed] [Google Scholar]
Larsson 1998 {published data only}
- Larsson L. Use of antibiotics, antioxidants, mucolytics and vaccines in the therapy of chronic obstructive pulmonary disease. European Respiratory Monograph 1998;3(7):163‐8. [Google Scholar]
Lee 2007 {published data only}
- Lee TA, Weaver FM, Weiss KB. Impact of pneumococcal vaccination on pneumonia rates in patients with COPD and asthma. Journal of General Internal Medicine 2007;22(1):62‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leophonte 2001 {published data only}
- Leophonte P, Neukirch F. Anti‐pneumococci vaccination: role and indications in the prevention of community acquired infections of the lower respiratory tract. Medecine et Maladies Infectieuses 2001;31(4):181‐94. [Google Scholar]
MacIntyre 2010 {published data only}
- MacIntyre CR, Egerton T, McCaughey M, Parrino J, Campbell BV, Su SC, et al. Concomitant administration of zoster and pneumococcal vaccines in adults >60 years old. Human Vaccines 2010;6(11):894‐902. [DOI] [PubMed] [Google Scholar]
MacLeod 1945 {published data only}
- MacLeod CM, Hodges RG, Heidelberger M, Bern Hard NG. Prevention of pneumococcal pneumonia by immunisation with specific capsular polysaccharide. Journal of Experimental Medicine 1945;82:445‐65. [PMC free article] [PubMed] [Google Scholar]
Madison 1998 {published data only}
- Madison JM, Irwin RS. Chronic obstructive pulmonary disease. Lancet 1998;352(9126):467‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meyer 2006 {published data only}
- Meyer P, Menzel M, Muellinger B, Weber N, Haeussinger K, Ziegler‐Heitbrock L. Inhalative vaccination with pneumococcal polysaccharide in patients with chronic obstructive pulmonary disease. Vaccine 2006;24(31‐32):5832‐8. [DOI] [PubMed] [Google Scholar]
Monso 2003 {published data only}
- Monso E. Vaccination and antioxidant therapy [Vacunacion y tratamiento antioxidante]. Archivos de Bronconeumologia 2003;39(Suppl 3):31‐3. [Google Scholar]
Nichol 1999 {published data only}
- Nichol KL, Baken L, Wuorenma J, Nelson A. The health and economic benefits associated with pneumococcal vaccination of elderly persons with chronic lung disease. Archives of Internal Medicine 1999;159(20):2437‐42. [DOI] [PubMed] [Google Scholar]
Ochoa‐Gondar 2008 {published data only}
- Ochoa‐Gondar O, Vila‐Corcoles A, Ansa X, Rodriguez‐Blanco T, Salsench E, Diego C, et al. Effectiveness of pneumococcal vaccination in older adults with chronic respiratory diseases: results of the EVAN‐65 study. Vaccine 2008;26(16):1955‐62. [DOI] [PubMed] [Google Scholar]
- Ochoa‐Gondar O, Vila‐Corcoles A, Diego C, Arija V, Maxenchs M, Grive M, et al. The burden of community‐acquired pneumonia in the elderly: the Spanish EVAN‐65 Study. BMC Public Health 2008;8(1):222. [DOI] [PMC free article] [PubMed] [Google Scholar]
Orcel 1994 {published data only}
- Orcel B, Delclaux B, Baud M, Derenne JP. Oral immunization with bacterial extracts for protection against acute bronchitis in elderly institutionalized patients with chronic bronchitis. European Respiratory Journal 1994;7(3):446‐52. [DOI] [PubMed] [Google Scholar]
Ortqvist 1998 {published data only}
- Ortqvist A, Hedlund J, Burman LA, Elbel E, Hofer M, Leinonen M, et al. Randomised trial of 23‐valent pneumococcal capsular polysaccharide vaccine in prevention of pneumonia in middle‐aged and elderly people. Swedish Pneumococcal Vaccination Study Group. Lancet 1998;351(9100):399‐403. [DOI] [PubMed] [Google Scholar]
Patrick 1981 {published data only}
- Patrick KM, Woolley FR. A cost‐benefit analysis of immunisation of pneumococcal pneumonia. JAMA 1981;245:473‐7. [PubMed] [Google Scholar]
Preheim 1978 {published data only}
Ricci 2014 {published data only}
- Ricci R, Palmero C, Bazurro G, Riccio AM, Garelli V, Marco E, et al. The administration of a polyvalent mechanical bacterial lysate in elderly patients with COPD results in serological signs of an efficient immune response associated with a reduced number of acute episodes. Pulmonary Pharmacology & Therapeutics 2014;27(1):109‐13. [DOI] [PubMed] [Google Scholar]
Riley 1977 {published data only}
- Riley RD, Tarr PI, Andrews M. Immunisation with a polyvalent pneumococcal vaccine: reduction of adult respiratory mortality in a New Guinea Highlands community. Lancet 1977;1:1338‐41. [DOI] [PubMed] [Google Scholar]
Rochemaure 1988 {published data only}
- Rochemaure J, Lehert PH, Sauvaget J, Robillard M, Betbeder‐Matibet A. Reduction with an immunomodulator of the infection rate in chronic bronchitis [Reduction par un immunomodulateur de taux d'infections respiratoires dans la bronchite chronique]. Revue de Pneumologie Clinique 1988;44(1):43‐7. [PubMed] [Google Scholar]
Saag 1998 {published data only}
- Saag KG, Doebbeling BN, Rohrer JE, Kolluri S, Peterson R, Hermann ME, et al. Variation in tertiary prevention and health service utilization among the elderly. The role of urban‐rural residence and supplemental insurance. Medical Care 1998;36(7):965‐76. [DOI] [PubMed] [Google Scholar]
Schenkein 2008 {published data only}
- Schenkein JG, Nahm MH, Dransfield MT. Pneumococcal vaccination for patients with COPD: current practice and future directions. [Review] [53 refs]. Chest 2008;133(3):767‐74. [DOI] [PubMed] [Google Scholar]
Schnelle 2010 {published data only}
- Schnelle H, Eeagan TL, Gulsvik A, Nielsen R. Predictors of COPD exacerbation in hospital patients, population based cases and controls. American Journal of Respiratory and Critical Care Medicine 2010;A41:A1507. [Google Scholar]
Schwartz 1982 {published data only}
- Schwartz JS. Pneumococcal vaccine: clinical efficacy and effectiveness. Annals of Internal Medicine 1982;96:208‐20. [DOI] [PubMed] [Google Scholar]
Sehatzadeh 2012 {published data only}
- Sehatzadeh S. Influenza and pneumococcal vaccinations for patients with chronic obstructive pulmonary disease (COPD): an evidence‐based review. Ontario Health Technology Assessment Series 2012;12(3):1‐64. [PMC free article] [PubMed] [Google Scholar]
Shapiro 1984 {published data only}
- Shapiro ED, Clemens JD. A controlled evaluation of the protective efficacy of pneumococcal vaccine for patients at high risk of serious pneumococcal infections. Annals of Internal Medicine 1984;101:325‐30. [DOI] [PubMed] [Google Scholar]
Shapiro 1987 {published data only}
- Shapiro ED. Pneumococcal vaccine failure. New England Journal of Medicine 1987;316(20):1272‐3. [DOI] [PubMed] [Google Scholar]
Shapiro 1991 {published data only}
- Shapiro ED, Berg AT, Austrian R, Schroeder D, Parcells V, Margollis A, et al. The protective efficacy of polyvalent pneumococcal polysaccharide vaccine. New England Journal of Medicine 1991;323:1453‐60. [DOI] [PubMed] [Google Scholar]
Sheikh 1999 {published data only}
- Sheikh A. Evidence‐based problem solving. What is the efficacy of pneumococcal vaccination in people with asthma?. Asthma in General Practice 1999;7(2):21‐2. [Google Scholar]
Simberkoff 1986 {published data only}
- Simberkoff MS, Cross AP, Al‐Ibrahim M, Baltch AL, Geiseler PJ, Nadler J, et al. Efficacy of pneumococcal vaccine in high risk‐patients. Results of a Veterans Administration cooperative study. New England Journal of Medicine 1986;315(21):1318‐27. [DOI] [PubMed] [Google Scholar]
Simberkoff 1993 {published data only}
- Simberkoff MS. Pneumococcal vaccine in the prevention of community‐acquired pneumonia: a skeptical view of cost‐effectiveness. Seminars in Respiratory Infection 1993;8(4):294‐9. [PubMed] [Google Scholar]
Sims 1988 {published data only}
- Sims RV, Steinmann WC, McConville JH, King LR, Zwick WC, Schwartz JS. The clinical effectiveness of pneumococcal vaccine in the elderly. Annals of Internal Medicine 1988;108(5):653‐7. [DOI] [PubMed] [Google Scholar]
Sisk 1986 {published data only}
- Sisk JE, Riegelman RK. Cost effectiveness of vaccination against pneumococcal pneumonia: an update. Annals of Internal Medicine 1986;104:79‐86. [DOI] [PubMed] [Google Scholar]
Smit 1977 {published data only}
- Smit P, Oberholzer D, Hayden‐Smith S, Koornhof HJ, Hilleman MR. Protective efficacy of pneumococcal polysaccharide vaccines. JAMA 1977;238:2613‐6. [PubMed] [Google Scholar]
Sumitani 2008 {published data only}
- Sumitani M, Tochino Y, Kamimori T, Fujiwara H, Fujikawa T. Additive inoculation influenza vaccine and 23‐valent pneumococcal polysaccharide vaccine to prevent lower respiratory tract infections in chronic respiratory disease patients. Internal Medicine 2008;47(13):1189‐97. [DOI] [PubMed] [Google Scholar]
Van Amptin 1998 {published data only}
- Amptin JMA, Bouter KP, Diepersloot RJA, Overbeek BP, Netten P, Erkelens DW. Pneumococcal bacteraemia: incidence, outcome and predisposing factors. European Journal of Internal Medicine 1998;9:145‐50. [Google Scholar]
Vila‐Corcoles 2012 {published data only}
- Vila‐Corcoles A, Ochoa‐Gondar O, Rodriguez‐Blanco T, Gutierrez‐Perez A, Vila‐Rovira A. Clinical effectiveness of 23‐valent pneumococcal polysaccharide vaccine against pneumonia in patients with chronic pulmonary diseases: a matched case‐control study. Human Vaccines & Immunotherapeutics 2012;8(5):639‐44. [DOI] [PubMed] [Google Scholar]
Watanuki 2007 {published data only}
- Watanuki Y, Miyazawa N, Kudo M, Inoue S, Goto H, Kaneko T, et al. Effects of pneumococcal vaccine in patients with chronic respiratory disease [Abstract]. European Respiratory Journal 2007;30(Suppl 51):224s [E1368]. [Google Scholar]
Wencker 1999 {published data only}
- Wencker M, Konietzko N. Alpha‐1‐protease inhibitor deficiency and pulmonary emphysema as viewed by pulmonary specialists in private practice [Alpha‐1‐Proeinaseninhibitor‐Mangel und Lungenemphysem aus der Sicht des niedergelassenen Pneumologen]. Atemwegs‐und Lungenkrankheiten 1999;25(2):89‐95. [Google Scholar]
Wenzel 1976 {published data only}
- Wenzel P, Craven R, Davies J, Hendley J, Hamory B, Gwaltney J. Field trial of an inactivated Mycoplasma pneumoniae vaccine. Journal of Infectious Diseases 1976;134(6):571‐6. [DOI] [PubMed] [Google Scholar]
WHO 1999 {published data only}
- World Health Organization. Pneumococcal vaccines: World Health Organization position paper. Weekly Epidemiological Record 1999;74(23):177‐83. [Google Scholar]
WHO 1999b {published data only}
- World Health Organization. Pneumococcal vaccines: World Health Organization position paper. Canada Communicable Disease Report 1999;25(17):150‐1. [PubMed] [Google Scholar]
Wiebel 1977 {published data only}
- Wiebel RE, Vella PP, McLean AA. Studies in human subjects of polyvalent pneumococcal vaccines. Proceedings of the Society for Experimental Biology and Medicine 1977;156:144‐50. [DOI] [PubMed] [Google Scholar]
Willems 1980 {published data only}
- Willems JS, Sanders CR, Riddiough MA. Cost effectiveness of vaccination against pneumococcal pneumonia. New England Journal of Medicine 1980;303:553‐9. [DOI] [PubMed] [Google Scholar]
Williams 1986 {published data only}
- Williams JH, Moser KM. Pneumococcal vaccine and patients with chronic lung disease. Annals of Internal Medicine 1986;104(1):106‐9. [DOI] [PubMed] [Google Scholar]
Wright 1914 {published data only}
- Wright AE, Morgan WP, Colebrook L, Dodgson RW. Prophylactic inoculation against pneumococcus infections. Lancet 1914;183(4714):87‐95. [Google Scholar]
Additional references
Anthonisen 1987
- Anthonisen NR, Manfreda J, Warren CP, Hershfield ES, Harding GK, Nelson NA. Antibiotic therapy in exacerbations of chronic obstructive pulmonary disease. Annals of Internal Medicine 1987;106(2):196‐204. [DOI] [PubMed] [Google Scholar]
Bogaert 2004
- Bogaert D, Valk P, Ramdin R, Sluijter M, Monninkhof E, Hendrix R, et al. Host‐pathogen interaction during pneumococcal infection in patients with chronic obstructive pulmonary disease. Infection and Immunity 2004;72(2):818‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bonten 2015
- Bonten MJM, Huijts SM, Bolkenbaas M, Webber C, Patterson S, Gault S, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. New England Journal of Medicine 2015;372:1114‐25. [DOI] [PubMed] [Google Scholar]
Buist 2007
- Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, et al. International variation in the prevalence of COPD (the BOLD study): a population‐based prevalence study. The Lancet 2007;370(9589):741‐50. [DOI] [PubMed] [Google Scholar]
Burge 2003
- Burge S, Wedzicha JA. COPD exacerbations: definitions and classifications. European Respiratory Journal 2003;21:46s‐53s. [DOI] [PubMed] [Google Scholar]
Burgess 2014
- Burgess L, Southern KW. Pneumococcal vaccines for cystic fibrosis. Cochrane Database of Systematic Reviews 2014, Issue 8. [DOI: 10.1002/14651858.CD008865.pub3] [DOI] [PubMed] [Google Scholar]
Chalmers 2016
- Chalmers JD, Campling J, Dicker A, Woodhead M, Madhava H. A systematic review of the burden of vaccine preventable pneumococcal disease in UK adults. BMC Pulmonary Medicine 2016;16:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chang 2009
- Chang CC, Singleton RJ, Morris PS, Chang AB. Pneumococcal vaccines for children and adults with bronchiectasis. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD006316.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cochrane Handbook
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org. [Google Scholar]
COPDX 2016
- COPD Guidelines Steering Committee. The COPD‐X plan: Australian and New Zealand guidelines for the management of chronic obstructive pulmonary disease, March 2016. http://www.copdx.org.au/ (accessed 2 July 2016).
Donaldson 2002
- Donaldson GC, Seemungal TA, Bhowmik A, Wedzicha JA. Relationship between exacerbation frequency and lung function decline in chronic obstructive pulmonary disease. Thorax 2002;57(10):847‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
Donaldson 2005
- Donaldson GC, Wilkinson TMA, Hurst JR, Perera WR, Wedzicha JA. Exacerbations and time spent outdoors in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2005;171:446‐52. [DOI] [PubMed] [Google Scholar]
ERS 2014
- European Respiratory Society. Immunisation against respiratory diseases. http://www.erswhitebook.org/chapters/immunisation‐against‐respiratory‐diseases/streptococcus‐pneumoniae/ (accessed 25 November 2014).
File 2003
- File TM Jr. Community‐acquired pneumonia. The Lancet 2003;362(9400):1991‐2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
File 2009
- File TM Jr, Monte SV, Schentag JJ, Paladino JA, Klugman KP, Lavin B, et al. A disease model descriptive of progression between chronic obstructive pulmonary disease exacerbations and community‐acquired pneumonia: roles for underlying lung disease and the pharmacokinetics/pharmacodynamics of the antibiotic. International Journal of Antimicrobial Agents 2009;33(1):58‐64. [DOI] [PubMed] [Google Scholar]
GOLD 2016
- GOLD Executive Committee. Global strategy for diagnosis, management, and prevention of COPD. http://www.goldcopd.com (accessed 2 July 2016).
Groenewegen 2001
- Groenewegen K, Schols A, Wouters EFM. Prognosis after hospitalization for acute exacerbations of COPD. European Respiratory Journal 2001;8(Suppl 33):209s. [Google Scholar]
Hak 2006
- Hak E, Hoes AW, Nordin JIM, Nichol KL. Benefits of influenza vaccine in US elderly ‐ appreciating issues of confounding bias and precision. International Journal of Epidemiology 2006;35(3):800‐2. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. British Medical Journal 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jackson 2006
- Jackson LA, Jackson ML, Nelson JC, Neuzil KM, Weiss NS. Evidence of bias in estimates of influenza vaccine effectiveness in seniors. International Journal of Epidemiology 2006;35(2):337‐44. [DOI] [PubMed] [Google Scholar]
Kew 2014
- Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2014;3:CD010115.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Leidy 2010
- Leidy NK, Wilcox TK, Jones PW, Murray L, Winnette R, Howard K, et al. Development of the EXAcerbations of Chronic Obstructive Pulmonary Disease Tool (EXACT): a patient‐reported outcome (PRO) measure. Value in Health 2010;13(8):965‐75. [DOI] [PubMed] [Google Scholar]
Lieberman 2002
- Lieberman D, Gelfer Y, Varshavsky R, Dvoskin B, Leinonen M, Friedman MG. Pneumonic vs nonpneumonic acute exacerbations of COPD. Chest 2002;122(4):1264‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mandell 2007
- Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community‐acquired pneumonia in adults. Clinical Infectious Diseases 2007;44(Suppl 2):S27‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moberley 2013
- Moberley S, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD000422.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Molinos 2009
- Molinos L, Clemente MG, Miranda B, Alvarez C, Busto B, Cocina BR, et al. Community‐acquired pneumonia in patients with and without chronic obstructive pulmonary disease. Journal of Infection 2009;58(6):417‐24. [DOI] [PubMed] [Google Scholar]
Moseley 2013
- Moseley AM, Dransfield MT. Pneumococcal vaccination in adults: recent evidence from clinical trials and observational studies. Clinical Investigation 2013;3(9):887‐97. [Google Scholar]
NHMRC 2013
- NHMRC: Australian Technical Advisory Group on Immunisation. The Australian Immunisation Handbook. 10th Edition. Canberra: Australian Government Department of Health, 2013. [Google Scholar]
NICE 2004
- National Institute of Clinical Excellence. COPD: national clinical guideline on management of chronic obstructive pulmonary disease in adults in primary and secondary care. Thorax 2004;59(Suppl 1):181‐272. [PMC free article] [PubMed] [Google Scholar]
NICE 2010
- National Clinical Guideline Centre. Chronic Obstructive Pulmonary Disease: Management of Chronic Obstructive Pulmonary Disease in Adults in Primary and Secondary Care. London: National Clinical Guideline Centre, 2010. www.nice.org.uk/guidance/cg101. [Google Scholar]
Papi 2006
- Papi A, Bellettato CM, Braccioni F, Romagnoli M, Casolari P, Caramori G, et al. Infections and airway inflammation in chronic obstructive pulmonary disease severe exacerbations. American Journal of Respiratory & Critical Care Medicine 2006;173(10):1114‐21. [DOI] [PubMed] [Google Scholar]
Patel 2002
- Patel IS, Seemungal TAR, Wilks M, Lloyd‐Owen SJ, Donaldson GC, Wedzicha JA. Relationship between bacterial colonisation and the frequency, character, and severity of COPD exacerbations. Thorax 2002;57:759‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Postma 2012
- Postma DF, Werkhoven CH, Huijts SM, Bolkenbaas M, Oosterheert JJ, Bonten MJ. New trends in the prevention and management of community‐acquired pneumonia. Netherlands Journal of Medicine 2012;70(8):337‐48. [PubMed] [Google Scholar]
Restrepo 2006
- Restrepo MI, Mortensen EM, Pugh JA, Anzueto A. COPD is associated with increased mortality in patients with community‐acquired pneumonia. European Respiratory Journal 2006;28(2):346‐51. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre. Review Manager (RevMan). Version 5.3. Copenhagen: The Cochrane Collaboration, 2014.
Rodrigo 2015
- Rodrigo C, Bewick T, Sheppard C, Greenwood S, McKeever TM, Trotter CL, et al. Impact of infant 13‐valent pneumococcal conjugate vaccine on serotypes in adult pneumonia. European Respiratory Journal 2015;45(6):1632‐41. [DOI] [PubMed] [Google Scholar]
Rodriguez‐Roisin 2000
- Rodriguez‐Roisin R. Toward a consensus definition for COPD exacerbations. Chest 2000;117(90052):398S‐401S. [DOI] [PubMed] [Google Scholar]
Seemungal 1998
- Seemungal TAR, Donaldson GC, Paul EA, Bestall JC, Jeffries DJ, Wedzicha JA. Effect of exacerbation on quality of life in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 1998;157:1418‐22. [DOI] [PubMed] [Google Scholar]
Seemungal 2000
- Seemungal T, Donaldson G, Bhowmik A, Jefries D, Wedizicha J. Time course and recovery of exacerbations in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2000;161(5):1608‐13. [DOI] [PubMed] [Google Scholar]
Sethi 2002
- Sethi S, Evans N, Grant BJB, Murphy TF. New strains of bacteria and exacerbations of chronic obstructive pulmonary disease. New England Journal of Medicine 2002;347(7):465‐71. [DOI] [PubMed] [Google Scholar]
Sheikh 2002
- Sheikh A, Alves B, Dhami S. Pneumococcal vaccine for asthma. Cochrane Database of Systematic Reviews 2002, Issue 1. [DOI: 10.1002/14651858.CD002165] [DOI] [PubMed] [Google Scholar]
Soler‐Cataluna 2005
- Soler‐Cataluna JJ, Martinnez‐Garcia MA, Roman Sanchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 2005;60(11):925‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Torres 1996
- Torres A, Dorca J, Zalacain R, Bello S, El‐Ebiary M, Molinos L, et al. Community‐acquired pneumonia in chronic obstructive pulmonary disease: a Spanish multicenter study. American Journal of Respiratory and Critical Care Medicine 1996;154(5):1456‐61. [DOI] [PubMed] [Google Scholar]
Wedzicha 2003
- Wedzicha JA, Donaldson GC. Exacerbations of chronic obstructive pulmonary disease. Respiratory Care 2003;48(12):1204‐15. [PubMed] [Google Scholar]
Welte 2009
- Welte T, Kohnlein T. Global and local epidemiology of community‐acquired pneumonia: the experience of the CAPNETZ Network. Seminars in Respiratory and Critical Care Medicine 2009;30(2):127‐35. [DOI] [PubMed] [Google Scholar]
WHO 2012
- World Health Organization. WHO position paper on pneumococcal vaccines 2012. http://www.who.int/immunization/position_papers/PP_pneumococcal_April_2012_summary.pdf (accessed 8 April 2014).
WHO 2013
- World Health Organization. Global health estimates ‐ years 2000‐2011. http://www.who.int/healthinfo/global_burden_disease/en/ (accessed 8 April 2014).


